Abstract
Background and objectives
Evidence of immune response to COVID-19 vaccine in psoriasis patients on biological agents is lacking. This study aimed to evaluate SARS-CoV-2 antibody levels following vaccination with CoronaVac or Pfizer/BioNTech mRNA in patients using biological agents or methotrexate, high-titer antibody levels achievement rate, and impact of medications on immunogenicity.
Methods
This noninterventional, prospective cohort study included 89 patients and 40 controls vaccinated with two doses of inactivated (CoronaVac) or Pfizer/BioNTech mRNA vaccines. Anti-spike and neutralising antibodies were analysed before and three to six weeks after the second dose. Adverse effects and symptomatic COVID-19 were assessed.
Results
Median anti-spike and neutralising antibody titers after CoronaVac were significantly lower in patients than controls (57.92 U/mL vs 125.4 U/mL, and 1/6 vs 1/32, respectively, p < 0.05). Patients were less likely to achieve high-titer anti-spike antibody levels (25.6 % vs 50 %). Infliximab was associated with attenuated vaccine response.
Pfizer/BioNTech vaccine induced comparable median anti-spike (2,080 U/mL vs 2,976.5 U/mL,) and neutralising antibody levels (1/96 vs 1/160) in patients and controls, respectively (p > 0.05). High-titer anti-spike and neutralising antibodies development rates were comparable among patients and controls (95.2 % vs 100 %, and 30.4 % vs 50.0 %, respectively, p > 0.05).
Nine (10.1 %) COVID-19 cases- all mild - were identified. Psoriasis flare was seen in 6.74 %, mostly after Pfizer/BioNTech vaccine.
Conclusion
Psoriasis patients treated with biological agents and methotrexate developed similar response to mRNA vaccine but weaker response to inactivated vaccine. Infliximab reduced response to the inactivated vaccine. Adverse effects were more frequent with mRNA vaccine, but none was severe.
Keywords: Psoriasis, Biologics, COVID-19, Vaccination, antiTNF, anti-IL17
1. Introductıon
Vaccination is the mainstay for the prevention of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus transmission. Although various vaccines have been demonstrated to reduce the rate of COVID-19 infection, the severity of the disease and its associated mortality in a healthy population, the data on the vaccines’ immunogenicity and the efficacy in patients with immune-mediated inflammatory disorders (IMIDs) are sparse [1], [2], [3]. In patients with IMIDs, including psoriasis, there are concerns about the possibility of achieving attenuated vaccine-induced immune response due to either the underlying disease or the immune modifying medications being used by the patients [1], [4]. Several studies have reported an attenuated immune response to COVID-19 vaccines in patients with autoimmune inflammatory rheumatic diseases due to intrinsic immune dysfunction [5], [6], [7]. In addition, an impaired immune response in patients using methotrexate and infliximab has been shown in various studies [1]. Although psoriasis is the most common IMID, affecting 2–3 % of the population, evidence of the response of patients with psoriasis to a two-dose COVID-19 vaccine regimen is lacking. Current COVID-19 vaccination recommendations for patients with psoriasis are mainly based on the extrapolation of data derived from studies involving autoimmune inflammatory rheumatic diseases, which differ from psoriasis in terms of disease characteristics, comorbidities and treatments [8].
As of December 2021, the World Health Organization (WHO) had issued an emergency use for nine vaccines, with efficacies ranging from 50 % to 95 % against SARS-Cov-2 infection [9], [10]. Messenger RNA (mRNA) vaccines, such as Pfizer-BioNTech and Moderna vaccines, have been investigated for their efficacy in various populations with underlying disorders [1], [11]. However, the efficacy and safety of inactivated vaccines – CoronaVac and Sinopharm, which account for nearly half of the 7.3 billion COVID-19 vaccine doses delivered globally at the beginning of the pandemic – in specific populations are uncertain due to scarcity of data [12]. Thus, we aimed to investigate immune responses to CoronaVac (inactivated whole-virion) and Pfizer-BioNTech vaccines in patients with psoriasis who are using biologic agents or methotrexate, as well as evaluate the impact of the biologic agents on immunogenicity, vaccine efficacy and adverse effects.
2. Materıals and methods
2.1. Study design and participants
This is a noninterventional, prospective cohort study, and the participants were enrolled between February and September 2021. Patients with psoriasis aged > 18 years using methotrexate or biologic agents, antitumour necrosis factor-alpha (anti-TNF), anti-IL17, anti-IL12/23 and anti-IL-36r, as well as controls who applied to our hospital for COVID-19 vaccination, all vaccinated with the two doses of CoronaVac or Pfizer-BioNTech mRNA vaccine, were selected for this study. Exclusion criteria included previous COVID-19 history or evidence of infection based on baseline serum IgG reactivity, concomitant immunosuppressive use or immunodeficiency diseases, pregnancy and breastfeeding. Apart from immunodeficiency, any comorbidity unlikely to have any influence on antibody responses such as hypertension, hyperlipidemia and diabetes were not among the exclusion criteria. The age, sex, body mass index and smoking and treatment status of the participants were recorded.
2.2. Setting
By the time vaccines were available, Turkish national vaccination programme included two doses of CoronaVac (which has received emergency use approval from the WHO in many countries, including Turkey) or Pfizer-BioNTech mRNA vaccine [13], [14]. Urgent vaccination was prioritised in patients with IMIDs, including psoriasis, treated with immunomodulatory/immunosuppressive agents.
2.3. Procedures
Prevaccine sera were obtained to screen serological evidence of infection, and those who had SARS-CoV-2 specific antibodies were excluded. According to the initial national vaccination recommendations, all study participants were administered the two-dose regimen of either CoronaVac or Pfizer-BioNTech vaccine three to four weeks apart. Three to six weeks after the second dose of either vaccine, venous blood samples were drawn. Serological response to the vaccine was investigated by analysing the levels of SARS-CoV-2 spike protein and neutralising antibodies; the latter is predictive of immune protection from symptomatic SARS-CoV-2 infection [15]. Seroconversion rates and total antibody titers against SARS-CoV-2 spike protein were quantified using the Roche Elecsys Anti-SARS-CoV-2 S immunoassay (Roche Diagnostics Limited, Burgess Hill, UK) and had clinical specificity and sensitivity of 99.8 % and 97.6 %, respectively [16]. An antibody titer ≥ 0.8 U/mL was considered reactive, and a high titer was defined as ≥ 132 U/mL, which is the convalescent plasma donorship threshold defined by the FDA for this kit [17].
The neutralising antibody levels were analysed as described previously [18]. Reciprocals of serum dilutions inhibiting at least 50 % of virus infectivity were expressed as the antibody titer. A high titer was defined as a titer of ≥ 1/160, which is the FDA recommendation titer of convalescent plasma donorship [19].
2.4. Safety and efficacy of the vaccines
Systemic and local adverse effects were queried, and psoriasis flare was assessed three to six weeks after the second dose of either vaccine. To assess the vaccines’ efficacy, the patients were asked through a phone call in December 2021 if they experienced symptomatic COVID-19 till that time, at least 2weeks after the second dose of the vaccine. Any positive response was verified by checking the electronic health records, and PCR confirmed cases were recorded as definite cases of COVID-19. Outcomes of COVID-19 were also investigated.
2.5. Outcomes
The primary outcomes were the neutralising and anti-SARS-CoV-2 spike protein antibody levels following two doses of either CoronaVac or Pfizer-BioNTech mRNA vaccine in patients with psoriasis who were using biologic agents or methotrexate. The secondary outcomes were as follows:
-
•
The proportion of individuals with a neutralising antibody titer ≥ 1/160 and an anti-SARS-CoV-2 spike protein titer ≥ 132 U/ml
-
•
The proportion of individuals with neutralising antibody titers above 1/20, which was the cut-off value determined for the present study since protective titer was uncertain
-
•
The impact of medications on immunogenicity
-
•
The incidence of symptomatic COVID-19 cases confirmed by RT-PCR at least 14 days after the second dose of the vaccine
-
•
The incidence of vaccine-related adverse effects and psoriasis flare
2.6. Statistical analysis
Descriptive data were presented as numbers, percentages and medians (25th–75th percentiles). Categorical variables were compared using the chi-square and Fisher’s exact tests. Continuous variables between two independent groups were analysed with the Mann-Whitney U test since the data did not follow a normal distribution. The Wilcoxon test was used to compare continuous variables in two repeated (before and after the vaccination) samples. The level of statistical significance was set as p < 0.05.
2.7. Ethical approval
This study was approved by the Clinical Research Ethics Committee of Marmara University and the Ministry of Health (authorization codes: 09.2021.572 and 2021-4-17T21-22-22).
2.8. Funding
The Turkish Society of Dermatovenereology provided the funding for this study.
3. Results
3.1. Demographics
Among 183 individuals that were screened, 89 patients with psoriasis and 40 controls with a median age of 46 and 47 years, respectively, were included (Fig. 1 ). The groups were similar in sex, age, body mass index and vaccine type (p > 0.05). More of the patients with psoriasis were smokers compared to the controls (p = 0.029). At the time of vaccination, 33.7 % (n = 30), 31.5 % (n = 28), 27 % (n = 24), 1.1 % (n = 1), 1.1 % (n = 1) and 5.6 % (n = 5) were using anti-TNFα, ustekinumab, anti-IL17, risankizumab, spesolimab and methotrexate, respectively. The demographic features, treatment and vaccination status of the study population are shown in Table 1 .
Fig. 1.
Flow diagram of the study.
Table 1.
Demographic features of the study population.
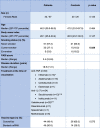 |
All but 3 patients were using biologics at the recommended dose and interval: *Infliximab infusions were done every 3 months in 1 patient, **secukinumab was used every 2 weeks in 1 patient, §1 patient used leflunomide concomittantly with secukinumab, ***ustekinumab was used every 14 weeks in 1 patient.
3.2. Seropositivity with CoronaVac
All the participants (n = 70, 44 psoriasis and 26 controls) developed seropositivity (100 %). The median (25th–75th percentiles) anti-SARS-CoV-2 spike antibody titers of the patients with psoriasis and the controls were 57.92 U/mL (8.6–152.8 U/mL) and 125.4 U/ml (54.6–347.5U/mL), respectively. The patients with psoriasis developed significantly lower antibody levels than the controls (p = 0.012). High titer anti-SARS-CoV-2 spike antibodies (≥132 U/mL) qualifying for convalescent plasma donation were found among 25.6 % and 50 % of the patients with psoriasis and the controls, respectively (p = 0.039) (Fig. 2 ).
Fig. 2.
Proportion of subjects in each group achieving above threshold levels of antispike and neutralising antibodies for plasma donation.
Neutralising antibody titers were studied in 37 patients and 21 controls. The median (25th–75th percentiles) titers were 1/6 (1/4–1/24) and 1/32 (1/12–1/48) for the patients with psoriasis and the controls, respectively. The patients had significantly lower neutralising antibody titers than the controls (p = 0.005). Also, a lower proportion of the patients (11/37, 29.7 %) had neutralising antibody levels above 1/20 compared to the controls (14/21, 66.7 %) (p = 0.006). None of the patients but one of the controls (4.8 %) achieved suitable antibody levels for convalescent plasma donation (Fig. 2). The serologic responses are shown in Table 2 .
Table 2.
Antispike and neutralising antibody levels in patients under biologics and controls.
 |
*p < 0.05 compared to controls. Cells containing < 5 patients were not included in the table.
3.3. Seropositivity with Pfizer-BioNTech mRNA vaccine
All the participants (n = 59, 45 psoriasis and 14 controls) developed seropositivity (100 %). The median (25th–75th percentiles) anti-SARS-CoV-2 spike antibody titers of patients with psoriasis and controls vaccinated with Pfizer-BioNTech mRNA vaccine were 2,080 U/mL (708.8–4,765.25 U/mL) and 2,976.5 U/mL (1,723.5–4,564.75 U/mL), respectively. The difference between the groups was statistically insignificant (p = 0.438). High-titer SARS-CoV-2 anti-spike antibodies (≥132 U/mL) suitable for convalescent plasma donation were found in 95.2 % and 100 % of the patients and the controls, respectively (p = 0.406) (Fig. 2). The use of ustekinumab (35.5 %), IL-17 inhibitors (33.3 %) and anti-TNFα (28.9 %) had comparable odds for developing high-titer seropositivity.
Among 46 patients with psoriasis and 14 controls who had neutralising antibody assay, the median (25th–75th percentiles) titers were 1/96 (32–288) and 1/160 (64–288), respectively (p = 0.424). Also, the frequency of developing neutralising antibody levels above 1/20 was similar among the groups (84.8 % vs 85.7 %, p = 0.93). Following vaccination, 30.4 % and 50 % of the patients with psoriasis and the controls achieved high neutralising antibody levels (≥1/160), qualifying for convalescent plasma donation. Intergroup difference was insignificant (p = 0.120) (Fig. 2). Regarding the treatment, patients using ustekinumab performed best (40 %), followed by those using anti-TNFα (18.2 %) and IL-17 inhibitors (10 %) in terms of high-titer seropositivity.
3.4. Impact of immunomodulatory treatments on seropositivity and protection
Pfizer/BioNTech mRNA vaccine-induced remarkably higher antibody responses than CoronaVac in both groups (Fig. 3, Fig. 4 ). In addition, the immunogenicity of the Pfizer/BioNTech mRNA vaccine among patients using various biological agents and methotrexate was not different from the controls. In contrast, administration of Coronavac was related to significantly weakened anti-SARS-CoV-2 spike (p < 0.001) and neutralising (p = 0.002) antibody responses in patients using infliximab compared to the controls (Table 2).
Fig. 3.
Antispike antibody titers after 2 doses of each vaccine in patients and controls.
Fig. 4.
Neutralising antibody titers after 2 doses of each vaccine in patients and controls.
Eighty-nine psoriasis cases were followed-up for a mean of 191 days (SD = ± 30.61, range = 100–280). Notably, nine (10.1 %) COVID-19 cases – three vaccinated with CoronaVac, five vaccinated with Pfizer-BioNTech mRNA, and one with two doses of CoronaVac and a subsequent dose of Pfizer-BioNTech mRNA vaccine were identified, and all had mild disease (Table 3 ).
Table 3.
Vaccination and treatment status of psoriasis patients developing COVID-19.
| Patient no | Vaccine Type | Medication | anti-SARS-CoV-2 Spike antibody titer (U/mL) |
Neutralizing antibody titer (1/) – |
|---|---|---|---|---|
| 1 | CoronaVac | USTEKINUMAB | NA | 36.84 |
| 2 | CoronaVac | IXEKIZUMAB | 32 | 396.3 |
| 3 | CoronaVac | METHOTREXATE | 64 | 123.4 |
| 4 | BioNtech mRNA | USTEKINUMAB | 24 | 796.5 |
| 5 | BioNtech mRNA | ADALIMUMAB | 8 | 709.1 |
| 6 | BioNtech mRNA | SECUKINUMAB | 32 | 1375 |
| 7 | BioNtech mRNA | USTEKINUMAB | 128 | 2398 |
| 8 | BioNtech mRNA | USTEKINUMAB | 48 | 1206 |
| 9 | *Both | IXEKIZUMAB | 4 | 57.9 |
Two doses of CoronaVac and one dose of BioNtech mRNA, NA: not available.
3.5. Adverse events
Adverse effects were infrequent and mild. CoronaVac was associated with a lower frequency of adverse effects. Local pain, erythema and oedema were found among 25 (28 %) patients with psoriasis and 8 (20 %) controls, 5 and 28 of them were administered CoronaVac and Pfizer-BioNTech mRNA vaccines, respectively. Systemic adverse effects (headaches, fever, fatigue) were observed among 14 (15.7 %) patients with psoriasis and 2 controls (5 %) and were caused by CoronaVac and Pfizer-BioNTech mRNA vaccines in 2 and 15 participants, respectively.
Psoriasis flare was seen in 6.74 % (6/89) of the patients, five of whom had generalised plaque psoriasis. The flare was seen 2–4 weeks following vaccination with either dose. CoronaVac caused flare in one patient, while Pfizer-BioNTech mRNA caused flare in four patients. Another patient with generalised pustular psoriasis (GPP), who had stable disease, developed flare seven days after the second dose of Pfizer-BioNTech mRNA vaccine, with a GPP severity score of 10, indicating a moderate severity [20]. The adverse events are shown in Table 4 . All the patients were managed by adjuvant topical agents or up-dosing of biologic medication.
Table 4.
Adverse effects of Coronavac and Biontech mRNA vaccines.
| Coronavac (n = 70) | Biontech mRNA (n = 59) | Total | |
|---|---|---|---|
| Local adverse effects | 5/70 (7.1 %) | 28/59 (47.5 %) | 33/129 (25.6 %) |
| Systemic adverse effects | 2/70 (2.9 %) | 15/59 (25.4 %) | 17/129 (13.2 %) |
| Psoriasis flare | 1/44 (2.3 %) | 5/45 (11.1 %) | 6/89 (6.7 %) |
4. Dıscussıon
Our study found that a two-dose vaccine schedule of both CoronaVac and Pfizer-BioNTech mRNA vaccines resulted in an immune response in all the participants, while the mRNA vaccine induced remarkably higher immunogenicity. We demonstrated a diminished serologic response to CoronaVac in patients with psoriasis compared to the controls, whereas vaccination with Pfizer/BioNTech mRNA induced a robust response in both groups. Both vaccines provided good efficacy against symptomatic SARS-CoV-2 infection along with a good safety profile in patients with psoriasis. Although breakthrough infections were seen in 10.1 % of the patients, they were mild, and none required hospitalisation. Adverse effects were remarkably high among the participants who received the Pfizer/BioNTech mRNA vaccine. Further, psoriasis flare was induced mostly by Pfizer/BioNTech mRNA.
The immune response to a recommended two-dose regimen of the Pfizer/BioNTech mRNA vaccine was strikingly higher than CoronaVac and comparable among the study groups. The rate of patients that developed high titer SARS-CoV-2 anti-spike and neutralising antibodies were 95.2 % and 30.4 %, respectively, indicating that patients with psoriasis can serologically respond to the Pfizer/BioNTech mRNA vaccine like the controls. Since our study investigated the immunogenicity of COVID-19 vaccines in patients with psoriasis following a two-dose regimen, the studies we could compare our results with are limited. Recently, Mahil et al. reported a weakened immune response in patients with psoriasis receiving biologics or methotrexate following the first dose of the Pfizer/BioNTech mRNA vaccine [21]. Nevertheless, patients with autoimmune diseases who failed to mount an antibody response to the first Pfizer/BioNTech mRNA dose have been shown to respond similarly to comparators following the second dose [22]. In a study with a similiar design to ours, Marovt et al investigated anti-spike antibody response to BNT162b2 (Pfizer/BioNTech) vaccine 4 weeks after the second dose in 32 patients with psoriasis treated with various biologics and 22 controls. Although seroconversion rates were similar, psoriasis patients had significantly lower antibody titers than controls with no difference across TNF, IL-12/23, IL-17 and IL-23 inhibitor users [23]. However, in a different study by Megna et al, IgG antibodies to COVID-19 spike protein was detected at approximately 4 weeks after the second dose of mRNA vaccine in 44 patients with psoriasis under biologics and 57 controls and no statistically significant difference was found in mean antibody levels between the groups [24]. In another study, Graceffa et al investigated anti-SARS-CoV-2 spike IgG titers following three doses of BNT162b2 in 45 psoriatic patients on biologic treatments and 45 controls. Similarly, a statistically significant increase in antibody titers with no significant differences between patients and controls were reported. Concomittant use of methotrexate was shown to negatively influence the vaccine response [25].
Several studies have shown lower seropositivity rates following two doses of COVID-19 mRNA vaccines in patients with IMIDs [1], [26]. Although methodological heterogeneity and the inclusion of a small number of patients with psoriasis in these studies make it difficult to generalise the results, the data point to a better response in patients with psoriasis and psoriatic arthritis compared to autoimmune rheumatologic inflammatory diseases [26]. We found high seroconversion rates but lower anti-spike and neutralising antibodies’ levels in patients with psoriasis compared to the controls after CoronaVac administration. Further, the chances of developing high antibody levels were lower in patients with psoriasis who received CoronaVac. These findings indicate a weak immunogenicity of the inactivated vaccine in patients with psoriasis. Our results are consistent with the findings of Seyahi et al., who found lower anti-spike antibody responses in patients with IMIDs following two doses of CoronaVac [27]. A weaker immunogenicity of inactivated COVID-19 vaccines than the mRNA counterparts is well documented [28]. National Psoriasis Foundation had recommended that patients with psoriatic disease receive an mRNA-based COVID-19 vaccine as soon as it became available [29]. Nevertheless, due to the limited availability of the mRNA vaccine, several vaccines approved for emergency use by WHO are being used widely. Booster doses, as recommended by the Center for Disease Control, can be applied when possible to overcome this challenge.
Treatment with biologic agents had variable effects on immunogenicity, with adalimumab, secukinumab and ixekizumab not impairing the serological response to either vaccine. Nevertheless, patients using infliximab had significantly weakened anti-SARS-CoV-2 spike and neutralising antibody responses following CoronaVac administration. Current data are inconclusive on the impact of biological agents on vaccine response. Several studies have shown an impaired vaccine response in patients using rituximab, methotrexate and JAK inhibitors and satisfactory responses in those using anti-TNFα, anti-IL-12/23 and anti-IL-17 agents [30]. In contrast, altered responses to anti-TNFα agents have been shown in various studies. In a survey of 4685 patients with inflammatory bowel disease who were using infliximab, significantly attenuated serological responses to SARS-CoV-2 infection were found [31]. Mahil et al. reported lower levels of anti-spike antibodies following one dose of mRNA-based COVID-19 vaccine in 84 patients with psoriasis who were receiving TNF inhibitors (79 %) and IL-23 inhibitors (83 %) compared to the controls (100 %) [21]. Furthermore, lower antibody titers that decayed faster after two doses of COVID-19 vaccine among patients using infliximab have been reported [32]. Impaired and shorter serological response is claimed to lead to chronic nasopharyngeal colonisation that may act as a reservoir for persistent transmission and the evolution of new SARS-CoV-2 variants. As the pandemic is ongoing and new variants can evade vaccine-based immunity, the use of infliximab should be carefully assessed in patients with psoriasis who can be treated with other biologics with a better safety profile [33].
We found a breakthrough infection rate of 10.1 % within a mean follow-up period of 191 days among the patients with psoriasis. In a recent study conducted in Israel by Bergwerk et al., COVID-19 breakthrough infections among health care workers that were fully vaccinated with two doses of Pfizer/BioNTech mRNA vaccine were investigated. The authors reported a breakthrough infection rate of 2.6 % among 1497 individuals who underwent RT-PCR testing during the four months after the second vaccine dose. Even though they captured asymptomatic cases, which constituted 33 % of all their cases, the rate reported by Bergwerk et al. is remarkably lower than ours [34]. One explanation may be the impairment of T-cell function, which is essential for antiviral immunity, due to anticytokine or methotrexate treatments in patients with psoriasis. A recent study showed that patients using biologics had a significantly lower T-cell response to SARS-Cov-2 compared to the controls (74 % and 100 %, respectively) [21]. Another explanation may be the weaker response induced by CoronaVac, which was used to vaccinate 49.3 % of the patients with psoriasis in this study.
Vaccine-related adverse events were significantly more common with the Pfizer/BioNTech mRNA vaccine than the CoronaVac vaccine. In line with our findings, a recent phase 4 study of CoronaVac among patients with autoimmune rheumatic diseases revealed less frequent adverse effects with CoronaVac compared to the reported frequency with mRNA vaccines [6]. Among the adverse effects observed in this study, local reactions such as pain and redness and constitutional symptoms such as fatigue, headaches and fever were the most common. No anaphylaxis or life-threatening adverse effects were seen. These findings are consistent with clinical studies and real-world data, reassuring the safety of the vaccines [5], [35]. Nonetheless, studies aiming to investigate the long-term safety of available vaccines, especially mRNA vaccines, are needed.
We found psoriasis flare among 6.74 % of our patients. The evidence of the impact of COVID-19 vaccines on psoriasis severity is limited to a few case reports, and our study is the first to investigate psoriasis flare prospectively in a representative cohort exposed to different vaccines. Recently, plaque psoriasis following the first or second dose of mRNA and Oxford‐AstraZeneca/Covishield vaccines was reported [36], [37]. Also, two cases with the exacerbation of GPP with mRNA and CoronaVac have been reported [38], [39]. Although we have seen a higher frequency of psoriasis flare following Pfizer/BioNTech mRNA vaccination, the number of events is small to make a comparison between vaccines. Given that the COVID-19 pandemic is ongoing and new variants are emerging, the risk of psoriasis flare should be given close attention.
4.1. Strengths and limitations
Our sample size was limited to make definitive conclusions on the impact of different biologics on seropositivity, and the persistence of antibodies in the long term was not investigated. Also, since only symptomatic cases of COVID-19 were analysed, the reported protective efficacy of the vaccines is limited to symptomatic patients. Another limitation is not testing cell mediated immunity. Nevertheless, excluding previous COVID-19 exposure, including a well-matched control group, using highly reproducible immunogenicity assays, analysing the response to two different vaccines with different technologies and assessing clinical efficacy and adverse effects are the main strengths of this study.
5. Conclusion
This study provides evidence for serological response in all patients with psoriasis who are being treated with biological agents and methotrexate following a two-dose mRNA vaccine regimen and a weaker response with the inactivated vaccine. Infliximab use was found to reduce immunogenicity among the patients. Breakthrough infections, which carry an infectious potential, were common among the patients. Side effects and disease flare were rare and nonserious, reassuring vaccine safety in this vulnerable group. These findings support the recommendation of the National Psoriasis Foundation that all patients with psoriasis should be vaccinated without withholding their biological treatment. Vaccination should be encouraged as part of routine clinical care, and further studies aiming to investigate the longevity of vaccine-elicited immunity should be carried out.
Funding source
Turkish Society of Dermatovenereology funded the study with no involvement in study design, data collection, data analysis, manuscript preparation and/or publication decisions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank The Turkish Society of Dermatovenereology for funding the study (001.2021) and Dr. Fahriye Saraç from Pendik Veterinary Control Institute for her technical support. We also thank our nurses in the Vaccination Unit for their technical support.
Data availability
Data will be made available on request.
References
- 1.Jena A., Mishra S., Deepak P., Kumar-M P., Sharma A., Patel Y.I., et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun Rev. 2022;21(1):102927. doi: 10.1016/j.autrev.2021.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanriover M.D., Doğanay H.L., Akova M., Güner H.R., Azap A., Akhan S., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros-Ribeiro A.C., Aikawa N.E., Saad C.G.S., Yuki E.F.N., Pedrosa T., Fusco S.R.G., et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744–1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 7.Mahil S.K., Bechman K., Raharja A., Domingo-Vila C., Baudry D., Brown M.A., et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol. 2022;4:e42–e52. doi: 10.1016/S2665-9913(21)00333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelfand J.M., Armstrong A.W., Bell S., Anesi G.L., Blauvelt A., Calabrese C., et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: Version 2-Advances in psoriatic disease management, COVID-19 vaccines, and COVID-19 treatments. J Am Acad Dermatol. 2021;84:1254–1268. doi: 10.1016/j.jaad.2020.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-19 vaccines WHO EUL issued. Available at: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued.
- 10.Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27:205–211. doi: 10.1038/s41591-021-01230-y. [DOI] [PubMed] [Google Scholar]
- 11.Simon D., Tascilar K., Fagni F., Krönke G., Kleyer A., Meder C., et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2021;80:1312–1316. doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallapaty S. China's COVID vaccines have been crucial - now immunity is waning. Nature. 2021;598(7881):398–399. doi: 10.1038/d41586-021-02796-w. [DOI] [PubMed] [Google Scholar]
- 13.Republic of Turkey Ministry of Health COVID-19 Vaccination Information Platform. Available at: https://covid19asi.saglik.gov.tr/?_Dil=2.
- 14.World Health Organization COVID-19 Pandemic. Available at: https://www.who.int.
- 15.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 16.Schaffner A., Risch L., Aeschbacher S., Risch C., Weber M.C., Thiel S.L., et al. Characterization of a pan-immunoglobulin assay quantifying antibodies directed against the receptor binding domain of the SARS-CoV-2 S1-subunit of the spike protein: a population-based study. J Clin Med. 2020;9(12):3989. doi: 10.3390/jcm9123989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Shaughnessy JA. Convalescent plasma EUA letter of authorization 12282021. Available at: https://www.fda.gov/media/141477/download.
- 18.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Investigational COVID-19 convalescent plasma guidance for industry. Available at: https://www.fda.gov/media/136798/download.
- 20.Fujita H., Terui T., Hayama K., Akiyama M., Ikeda S., Mabuchi T., et al. Japanese guidelines for the management and treatment of generalized pustular psoriasis: The new pathogenesis and treatment of GPP. J Dermatol. 2018;45:1235–1270. doi: 10.1111/1346-8138.14523. [DOI] [PubMed] [Google Scholar]
- 21.Mahil S.K., Bechman K., Raharja A., Domingo-Vila C., Baudry D., Brown M.A., et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boekel L., Steenhuis M., Hooijberg F., Besten Y.R., van Kempen Z.L.E., Kummer L.Y., et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3:e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marovt M., Deželak P., Ekart R., Marko P.B. Immune response to SARS-CoV-2 mRNA vaccine in patients with psoriasis treated with biologics. Clin Exp Dermatol. 2022;47:2041–2043. doi: 10.1111/ced.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Megna M., Potestio L., Battista T., Camela E., Genco L., Noto M., et al. Immune response to COVID-19 mRNA vaccination in patients with psoriasis undergoing treatment with biologics. Clin Exp Dermatol. 2022;47:2310–2312. doi: 10.1111/ced.15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graceffa D., Sperati F., Bonifati C., Spoletini G., Lora V., Pimpinelli F., et al. Immunogenicity of three doses of anti-SARS-CoV-2 BNT162b2 vaccine in psoriasis patients treated with biologics. Front Med (Lausanne) 2022;9:961904. doi: 10.3389/fmed.2022.961904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakuraba A., Luna A., Micic D. Serologic response to coronavirus disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis. Gastroenterology. 2022;162:88–108.e9. doi: 10.1053/j.gastro.2021.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seyahi E., Bakhdiyarli G., Oztas M., Kuskucu M.A., Tok Y., Sut N., et al. Antibody response to inactivated COVID-19 vaccine (CoronaVac) in immune-mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int. 2021;41:1429–1440. doi: 10.1007/s00296-021-04910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotshild V., Hirsh-Raccah B., Miskin I., Muszkat M., Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11(1):22777. doi: 10.1038/s41598-021-02321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Psoriasis Foundation. Covid-19 Task Force Guidance Statements. Available at: https://www.psoriasis.org/covid-19-task-force-guidance-statements/.
- 30.Friedman M.A., Curtis J.R., Winthrop K.L. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1255–1265. doi: 10.1136/annrheumdis-2021-221244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy N.A., Goodhand J.R., Bewshea C., Nice R., Chee D., Lin S., et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 32.Lin S, Kennedy N, Saifuddin A et al. Covid-19 vaccine-induced antibodies are attenuated and decay rapidly in infliximab treated patients; 2021. Doi: 10.21203/rs.3.rs-755879/v1.
- 33.Araf Y., Akter F., Tang Y.-D., Fatemi R., Parvez M.S.A., Zheng C., et al. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94:1825–1832. doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G., Li X., Sun M., Zhou Y., Yin M., Zhao B., et al. COVID-19 mRNA vaccines are generally safe in the short term: a vaccine vigilance real-world study says. Front Immunol. 2021;12:669010. doi: 10.3389/fimmu.2021.669010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krajewski P.K., Matusiak Ł., Szepietowski J.C. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J Eur Acad Dermatol Venereol. 2021;35:e632–e634. doi: 10.1111/jdv.17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagrani P., Jindal R., Goyal D. Onset/flare of psoriasis following the ChAdOx1 nCoV-19 Corona virus vaccine (Oxford-AstraZeneca/Covishield): Report of two cases. Dermatol Ther. 2021;34(5):e15085. doi: 10.1111/dth.15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yatsuzuka K., Murakami M., Kuroo Y., Fukui M., Yoshida S., Muto J., et al. Flare-up of generalized pustular psoriasis combined with systemic capillary leak syndrome after coronavirus disease 2019 mRNA vaccination. J Dermatol. 2022;49:454–458. doi: 10.1111/1346-8138.16271. [DOI] [PubMed] [Google Scholar]
- 39.Onsun N., Kaya G., Işık B.G., Güneş B. A generalized pustular psoriasis flare after CoronaVac COVID-19 vaccination: Case report. Health Promot Perspect. 2021;11:261–262. doi: 10.34172/hpp.2021.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.






