Abstract
Background
Dementia, a global health priority, has no current cure. Around 50 million people worldwide currently live with dementia, and this number is expected to treble by 2050. Some health conditions and lifestyle behaviours can increase or decrease the risk of dementia and are known as 'predictors'. Prognostic models combine such predictors to measure the risk of future dementia. Models that can accurately predict future dementia would help clinicians select high‐risk adults in middle age and implement targeted risk reduction.
Objectives
Our primary objective was to identify multi‐domain prognostic models used in middle‐aged adults (aged 45 to 65 years) for predicting dementia or cognitive impairment. Eligible multi‐domain prognostic models involved two or more of the modifiable dementia predictors identified in a 2020 Lancet Commission report and a 2019 World Health Organization (WHO) report (less education, hearing loss, traumatic brain injury, hypertension, excessive alcohol intake, obesity, smoking, depression, social isolation, physical inactivity, diabetes mellitus, air pollution, poor diet, and cognitive inactivity). Our secondary objectives were to summarise the prognostic models, to appraise their predictive accuracy (discrimination and calibration) as reported in the development and validation studies, and to identify the implications of using dementia prognostic models for the management of people at a higher risk for future dementia.
Search methods
We searched MEDLINE, Embase, PsycINFO, CINAHL, and ISI Web of Science Core Collection from inception until 6 June 2022. We performed forwards and backwards citation tracking of included studies using the Web of Science platform.
Selection criteria
We included development and validation studies of multi‐domain prognostic models. The minimum eligible follow‐up was five years. Our primary outcome was an incident clinical diagnosis of dementia based on validated diagnostic criteria, and our secondary outcome was dementia or cognitive impairment determined by any other method.
Data collection and analysis
Two review authors independently screened the references, extracted data using a template based on the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS), and assessed risk of bias and applicability of included studies using the Prediction model Risk Of Bias ASsessment Tool (PROBAST). We synthesised the C‐statistics of models that had been externally validated in at least three comparable studies.
Main results
We identified 20 eligible studies; eight were development studies and 12 were validation studies. There were 14 unique prognostic models: seven models with validation studies and seven models with development‐only studies. The models included a median of nine predictors (range 6 to 34); the median number of modifiable predictors was five (range 2 to 11). The most common modifiable predictors in externally validated models were diabetes, hypertension, smoking, physical activity, and obesity. In development‐only models, the most common modifiable predictors were obesity, diabetes, hypertension, and smoking. No models included hearing loss or air pollution as predictors. Nineteen studies had a high risk of bias according to the PROBAST assessment, mainly because of inappropriate analysis methods, particularly lack of reported calibration measures. Applicability concerns were low for 12 studies, as their population, predictors, and outcomes were consistent with those of interest for this review. Applicability concerns were high for nine studies, as they lacked baseline cognitive screening or excluded an age group within the range of 45 to 65 years.
Only one model, Cardiovascular Risk Factors, Ageing, and Dementia (CAIDE), had been externally validated in multiple studies, allowing for meta‐analysis. The CAIDE model included eight predictors (four modifiable predictors): age, education, sex, systolic blood pressure, body mass index (BMI), total cholesterol, physical activity and APOEƐ4 status. Overall, our confidence in the prediction accuracy of CAIDE was very low; our main reasons for downgrading the certainty of the evidence were high risk of bias across all the studies, high concern of applicability, non‐overlapping confidence intervals (CIs), and a high degree of heterogeneity.
The summary C‐statistic was 0.71 (95% CI 0.66 to 0.76; 3 studies; very low‐certainty evidence) for the incident clinical diagnosis of dementia, and 0.67 (95% CI 0.61 to 0.73; 3 studies; very low‐certainty evidence) for dementia or cognitive impairment based on cognitive scores. Meta‐analysis of calibration measures was not possible, as few studies provided these data.
Authors' conclusions
We identified 14 unique multi‐domain prognostic models used in middle‐aged adults for predicting subsequent dementia. Diabetes, hypertension, obesity, and smoking were the most common modifiable risk factors used as predictors in the models. We performed meta‐analyses of C‐statistics for one model (CAIDE), but the summary values were unreliable. Owing to lack of data, we were unable to meta‐analyse the calibration measures of CAIDE. This review highlights the need for further robust external validations of multi‐domain prognostic models for predicting future risk of dementia in middle‐aged adults.
Keywords: Humans, Middle Aged, Cognitive Dysfunction, Cognitive Dysfunction/complications, Cognitive Dysfunction/diagnosis, Dementia, Dementia/complications, Dementia/etiology, Hypertension, Hypertension/complications, Obesity, Obesity/complications, Prognosis
Plain language summary
What tools exist to assess the presence of multiple risk factors for dementia in middle‐aged people, and can they correctly predict future dementia?
Key messages
• We found 14 tools used in middle‐aged people to predict future dementia.
• Seven studies tested a prediction tool named Cardiovascular Risk Factors, Ageing, and Dementia (CAIDE).
• The benefits of using these tools to predict dementia later in life are unclear, because the studies provided little high‐quality evidence.
What is dementia?
Dementia refers to a group of brain conditions that commonly affect older people and lead to progressive problems with memory, problem‐solving, or performing everyday activities. People with certain health conditions or behaviours in middle age – such as high blood pressure, excessive alcohol intake, smoking, depression, low levels of exercise, or poor diet – have a higher chance of developing dementia in later life. We classify these health conditions or behaviours as 'modifiable risk factors' for dementia, because measures such as lifestyle changes can reduce them.
What are prediction tools?
To develop prediction tools, researchers observe a group of people over years to see how many with such risk factors develop dementia. The tools assign a higher risk score to people who have a higher chance of getting dementia later in life, based on the presence or absence of risk factors in middle age.
Why do we use tools that assess risk factors to predict future dementia?
Currently, about 50 million people across the world have dementia, and without adequate preventive measures, that number is expected to triple by 2050. If we control risk factors in middle age, we may avert or delay the future development of dementia or reduce dementia severity. Preventive tools help select people who are best suited to lifestyle modification programmes aimed at regulating risk factors.
What did we want to find out?
We wanted to find out what tools are available for middle‐aged adults (aged 45 to 65 years), and how well they predict dementia later in life (at least five years after the initial assessment). We looked for tools that included risk factors widely accepted to be linked to dementia onset.
What did we do?
We searched for studies that evaluated tools used in middle‐aged adults to identify those at high risk of dementia later in life. We investigated how well these tools predicted future dementia based on an accuracy value. If the accuracy value is more than a recommended standard of 0.75, we can say that the tool is accurate at predicting future dementia. It is also important to establish that a tool developed in one group of people (in the original development study) can accurately predict dementia in another group of people (in validation studies); only then can it be applied in routine healthcare practice. We compared and summarised the results of the studies.
What did we find?
We found 20 studies that described 14 different tools for dementia prediction. The tools included between two and 11 modifiable risk factors for dementia. Seven of the tools featured in two or more studies and were considered validated. Seven studies used a tool called Cardiovascular Risk Factors, Ageing, and Dementia (CAIDE). The CAIDE tool included current measures of a person's blood pressure, weight and height, cholesterol level, and frequency of exercise to predict future dementia. The combined accuracy value across the studies was 0.71, not high enough for us to consider CAIDE a reliable tool for predicting future dementia.
What are the limitations of the evidence?
Half (seven) of the tools were used in a single study, so we were unable to measure how well they predicted future dementia. Most studies provided too little information for us to assess accuracy values.
How up to date is this evidence?
The evidence is up‐to‐date to June 2022.
Summary of findings
Summary of findings 1. Summary of findings: CAIDE.
| Outcomes | Number of studies, participants, events | Summary measures | Certainty of the evidence (GRADE)a |
|
Incident clinical dementia |
3 studies 24,147 participants 3492 dementia cases |
Discrimination: C‐statistic 0.71 (95% CI 0.66 to 0.76) Calibration: NR |
Very Lowb |
| Dementia or cognitive impairmentc | 3 studies 8565 participants 524 dementia cases |
Discrimination: C‐statistic 0.67 (95% CI 0.61 to 0.73) Calibration: NR |
Very Lowd |
| CAIDE: Cardiovascular Risk Factors, Aging, and Incidence of Dementia; CI: confidence interval; NR: not reported. | |||
a Guidance on applying the GRADE framework specifically to prognostic models is not yet available, but we applied GRADE judgements and provided detailed text descriptions as per our methods. b Text descriptions of the GRADE judgements:
- Risk of bias: we downgraded the evidence by one level owing to high risk of bias across multiple domains.
- Inconsistency: we downgraded the evidence by one level owing to the high degree of heterogeneity.
- Imprecision: the confidence intervals overlapped between studies and were close to the threshold of 0.75.
- Indirectness: we downgraded the evidence by one level owing to applicability concerns (Exalto 2014; Fayosse 2020).
- Publication bias: there were too few studies to create a funnel plot.
- The overall GRADE rating was of very low‐certainty
c Secondary outcome based on any cognitive tests as reported by study authors. d Text descriptions of the GRADE judgements:
- Risk of bias: we downgraded the evidence by one level owing to high risk of bias across multiple domains.
- Inconsistency: we downgraded the evidence by one level owing to the high degree of heterogeneity.
- Imprecision: we downgraded the evidence by one level as there was no overlapping of the confidence intervals between the studies.
- Indirectness: we downgraded the evidence by one level owing to applicability concerns (Chosy 2019; Virta 2013)
- Publication bias: there were too few studies to create a funnel plot.
- The overall GRADE rating was of very low‐certainty.
Background
Description of the health condition and context
Dementia, or major neurocognitive disorder, refers to a significant decline from a previous level of performance in at least one domain of cognitive function, to the extent that the cognitive impairment interferes with the person's capacity to carry out everyday activities unaided (Russo 2017). Dementia is an umbrella term for several different brain disorders that affect cognitive function. The most common forms are dementia due to Alzheimer's disease, vascular dementia, alcohol‐related dementia, and dementia with Lewy bodies (Longo 2018). Alzheimer's disease accounts for up to 75% of all dementia cases (Menéndez 2014).
Dementia is a global public health priority. Approximately 50 million people are currently living with dementia worldwide (Livingston 2017). This number is expected to increase drastically as the world population shifts to an older demographic, as most types of dementia occur primarily in older adults. Early‐onset dementia (which affects people aged under 65 years) accounts for 6.9% to 45.3% of overall dementia cases in different countries (Vieira 2013). The global prevalence of dementia in adults over 60 years of age varies from 5% to 7% in most regions of the world (Prince 2013). By 2050, one in six people in the world will be over 65 years of age, and the number of people living with dementia is projected to reach 152 million (Livingston 2017). This is likely to create a considerable strain in the future, as people living with dementia have an increasing reliance on health and aged care services, including physician visits, allied health visits, admission to hospital, and long‐term care (Halima 2018).
A growing evidence base shows that dementia has a long and complicated preclinical phase in which many factors play a role. The multiple risk factors for dementia can be grouped broadly into 'modifiable' and 'non‐modifiable' risk factors. The Lancet Commission identified 12 potentially modifiable risk factors that account for around 40% of worldwide cases of dementia (Livingston 2020). This investigation demonstrated that around 7% of the population attributable fraction of dementia was associated with modifiable risk factors present in early life (under 45 years), 15% in midlife (45 to 65 years), and 18% in later life (over 65 years).
The modifiable risk factors were as follows.
Less early‐life education
Midlife hypertension, obesity, hearing loss, traumatic brain injury, and excessive alcohol intake
Later‐life smoking, depression, physical inactivity, social isolation, type 2 diabetes mellitus, and air pollution
Non‐modifiable risk factors for dementia include increasing age, presence of the Apolipoprotein E (ApoE) Ɛ4 allele, and family history of dementia.
In the absence of any disease‐modifying treatments for dementia, health improvement measures at the population and individual level constitute a potential means of preventing or delaying the onset of dementia. As several different factors appear to influence the development of dementia, risk assessment (e.g. prognostic models) and risk reduction measures will likely need to be multi‐domain in nature. Research suggests that an achievable 10% to 25% reduction in critical modifiable risk factors for dementia could prevent 1.1 to 3.0 million Alzheimer's disease cases internationally (Livingston 2017). As 15% of the population attributable fraction of dementia worldwide is associated with modifiable risk factors in midlife, risk reduction measures in people aged 45 to 65 years could have important beneficial effects. Furthermore, it may be possible to take action in midlife to prevent or reduce the prevalence of some later‐life risk factors. Although there are several models for predicting dementia in older adults, fewer are aimed at middle‐aged adults (Hou 2019).
Description of the prognostic models
Prognostic models combine several characteristics (e.g. related to the person, disease, or treatment), known as predictors, to predict an outcome (Moons 2018). Dementia prognostic models may assess a variety of different predictors (e.g. hypertension, diabetes, obesity, low education), and provide a numerical representation of the likelihood that an individual will develop dementia within a specified time frame (Goerdten 2019). No gold standard prognostic model exists for dementia, and it remains unclear whether there is a right time or a right population for dementia prognostication (PHG Foundation 2019).
Because available prognostic models for predicting dementia are based on data from prospective studies in a variety of settings, they differ in their predictive ability (Tang 2015). The application of prognostic models can vary according to the target population (e.g. middle‐aged versus older adults). The ideal prognostic model should have satisfactory predictive accuracy and validity for the intended population. The most common predictive accuracy measures are discrimination and calibration. Discrimination is quantified by the C‐statistic (concordance statistic) or area under the receiver operating characteristics curve (AUC). Calibration is quantified by the ratio between the observed and expected outcomes of the model, which helps to establish whether the prediction under‐ or over‐estimates the overall risk (Debray 2017).
Prognostic model validation is an important step after model development. Internal and external validations are required to ensure that a model works within and outside the development data set. Internal validation involves methods such as cross‐validation, split‐sample validation, or bootstrap resampling validation (Steyerberg 2014). External validation tests the model in an entirely different dataset (fully independent validation) or the same setting at a different point in time (temporal validation). This helps to ascertain the performance parameters in a new population (Steyerberg 2019). Because most available prognostic models for dementia lack external validation, they have limited clinical utility in health practices (Hou 2019).
The Cardiovascular Risk Factors, Ageing, and Dementia (CAIDE) risk score was the first dementia risk score to be developed in middle‐aged people (Kivipelto 2006). It is a multi‐domain model based on a large cohort of Finnish participants and including a combination of demographic characteristics and health indices. Subsequent studies have validated the CAIDE model in multiple cohorts and different ethnicities (Chosy 2019; Reijmer 2011; Stephan 2020; Virta 2013). There are other commonly used dementia prognostic models for older people (Table 2), or for populations including both middle‐aged and older people (Licher 2019; Walters 2016).
1. Examples of dementia prediction models, their predictors, and performance measures.
| Model | Development age group | Predictors (modifiable risk factors underlined) |
C‐statistic (development cohort) |
|
CAIDE (Kivipelto 2006) |
39–64 years | Age, sex, education, cholesterol, BMI, blood pressure, presence of APOE4 allele | 0.77 |
|
ANU‐ADRI (Andrews 2017) |
Developed through literature review, included all age groups | Age, sex, education,BMI, diabetes mellitus, depression, cholesterol, traumatic brain injury, smoking, alcohol intake, physical activity, cognitive activity,fish intake, social engagement, pesticide exposure | 0.74 |
|
BDSI (Barnes 2014) |
> 65 years | Age, education, history of stroke, diabetes, BMI, depressive symptoms, assistance needed with money or medication | 0.68 to 0.78 |
|
DRS (Walters 2016) |
60–95 years | Age, sex, depression, anxiety,transient ischaemic attack, atrial fibrillation, BMI, systolic blood pressure, lipid ratio, aspirin use, antihypertensive use, NSAID use, smoking, alcohol intake, local area deprivation | 0.84 |
ANU‐ADRI: Australian National University Alzheimer's Disease Risk Index; BDSI: Brief Dementia Screening Indicator; BMI: body mass index; CAIDE: Cardiovascular Risk Factors, Aging, and Dementia; DRS: Dementia Risk Score; NSAID: non‐steroidal anti‐inflammatory drug.
Why is it important to do this review?
The estimated cost of dementia was USD 818 billion in 2015, when around 47 million people were living with the disease (Livingston 2017). The prevalence is expected to treble by 2050, making dementia the single most significant cause of disability in older adults. A one‐year delay in dementia onset would reduce the number of people living with dementia in 2050 by around nine million (Livingston 2017). The ability to accurately predict the risk of later‐life dementia will assist governments and health policymakers in planning for future disease burdens and healthcare costs. Reliable and timely prognostication, particularly involving modifiable predictors, may allow policymakers to redirect targeted efforts and funding to specific population risk factors, and help clinicians make decisions about midlife risk management for people at risk of future dementia.
Midlife (45 to 65 years) is an important age for dementia risk assessment, as positive changes in modifiable risk factors for dementia during this phase of life have the potential to reduce dementia incidence later in life. Preclinical brain changes occur decades before clinical symptoms and usually remain undiagnosed (Virta 2013). Assessment of the pooled risk of an individual may be helpful in prioritising interventions to reduce the progression of such brain changes, or at least to reduce their severity.
In 2010, a review of screening methods for dementia identified 25 models for dementia prediction and inferred that multi‐domain models are more effective than single‐domain models (Stephan 2010). In 2015, a review of dementia risk prediction models identified a need to subtype the models based on age and setting (Tang 2015). A 2019 systematic review of models for predicting the risk of dementia identified only four studies addressing midlife risk (Hou 2019). However, the search strategy was limited to PubMed, there was no attempt to meta‐analyse the data, and the review did not use the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS; Moons 2014).
Against this background, our review will include a comprehensive search and a formal synthesis of prognostic models applied in midlife populations.
Objectives
Primary objective
To identify multi‐domain prognostic models that have been used in middle‐aged adults (aged 45 to 65 years) for predicting dementia or cognitive impairment over a period of at least five years. Eligible multi‐domain prognostic models involved two or more of the dementia predictors identified by the Lancet Commission in 2020 and the World Health Organization (WHO) in 2019 (i.e. less education, hearing loss, traumatic brain injury, hypertension, excessive alcohol intake, obesity, smoking, depression, social isolation, physical inactivity, diabetes mellitus, air pollution, poor diet, and cognitive inactivity).
Secondary objectives
To descriptively summarise the characteristics of these prognostic models.
To appraise the predictive accuracy (discrimination and calibration) of these models in their development and validation studies.
To identify implications of the use of dementia prognostic models for the management of people at risk of dementia.
Objectives in PICOTS format
| Population | Middle‐aged adults (45 to 65 years) with no history of cognitive impairment or dementia at baseline. We will include cohorts with a mean/median age between 45 and 65 years. |
| Index | Multi‐domain prognostic models involving two or more of the following modifiable predictors of dementia: less education, hearing loss, traumatic brain injury, hypertension, excessive alcohol intake, obesity, smoking, depression, social isolation, physical inactivity, diabetes mellitus, air pollution, poor diet, and cognitive inactivity. |
| Comparator | No comparator |
| Outcome |
|
| Time | At least five years of follow‐up |
| Setting | All settings |
Methods
Criteria for considering studies for this review
Types of studies
We included non‐randomised cohort studies (both prospective and retrospective), registries, nested case‐control studies, and prognostic studies based on randomised controlled trial data. Eligible study types included the following.
Prognostic model development studies without external validation
Prognostic model development studies with external validation
External model validation studies with or without model updating
We defined internal validation as the use of resampling (e.g. bootstrapping or cross‐validation) or split‐sample (e.g. data randomly or non‐randomly split into two groups, one for prediction and one for validation). External validation was fully independent validation using separate data (e.g. from a different study)(Moons 2015). The external validation should have the same outcome as the development study.
Target population
The target population for this review was middle‐aged adults aged between 45 and 65 years, with no history of cognitive impairment or dementia (either self‐reported or medically documented). We included all studies where the baseline mean or median age of the cohort was between 45 and 65 years. All settings were valid.
Types of prognostic models
Eligible prognostic models were multi‐domain in nature. For this review, we defined multi‐domain as including at least two of the following modifiable predictors for dementia
Less education
Hearing loss
Traumatic brain injury
Hypertension
Excessive alcohol intake
Obesity
Smoking
Depression
Social isolation
Physical inactivity
Diabetes mellitus
Air pollution
Poor diet
Cognitive inactivity
This list is based on a 2020 report on dementia prevention, intervention, and care by the Lancet Commission (Livingston 2020), and the WHO 2019 report on risk reduction of cognitive decline and dementia (WHO 2019). While modifying some of these predictors would require population‐level intervention (e.g. traumatic brain injury), we classified all as modifiable for this review.
Types of outcomes
The primary outcome was an incident clinical diagnosis of dementia of any subtype, according to standard classification systems such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases (ICD). The diagnosis of subtypes of dementia could also be based on specific diagnostic criteria (e.g. National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) criteria for Alzheimer's disease (McKhann 2011), McKeith criteria for Dementia with Lewy Body (McKeith 2017), Lund criteria for frontotemporal dementias (England 1994), or National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS‐AIREN) criteria for vascular dementia (Roman 1993)).
The secondary outcome was incident dementia or cognitive impairment assessed using any other method, as defined by the study authors (e.g. cognitive scores). We assessed the implications of the method of diagnosis in the applicability assessment (e.g. cut‐offs that define dementia or cognitive impairment based on the sensitivity and specificity of cognitive scores in previously published literature). Validation studies could use a dementia incidence or cognitive impairment outcome other than that used in the model's development study.
The minimum follow‐up duration for the outcome of dementia was five years from the baseline assessment of predictors.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception to 6 June 2022.
MEDLINE (OvidSP)
Embase (OvidSP)
PsycINFO (OvidSP)
CINAHL (Cumulative Index to Nursing and Allied Health Literature; EBSCOhost)
ISI Web of Science Core Collection
We included articles published in any language, though the title and abstract had to be in English. Appendix 1 shows the search strategies.
Searching other resources
In addition to searching electronic databases, we handsearched the reference lists of relevant systematic reviews and performed forwards and backwards citation tracking of all included studies using the Web of Science platform.
Data collection and analysis
Selection of studies
The Information Specialist for the Cochrane Dementia and Cognitive Improvement Group deduplicated the initial results and performed an initial screening. Two review authors (GMG and AJC) independently screened the titles and abstracts, grouping records into two categories: 'retrieve' (further split into 'eligible'/'potentially eligible'/'unclear' subgroups) and 'do not retrieve'. The same two review authors assessed the full‐text articles of the retrieved records against our eligibility criteria. They consulted a third reviewer (JG and TJQ) in case of disagreement. We collated multiple reports of single studies to avoid duplications. We contacted investigators of studies with unclear eligibility for additional information. We recorded the selection process in a PRISMA flow diagram and provided justifications for excluding studies in a Characteristics of excluded studies table.
The CHARMS checklist guided the extraction of study details (Moons 2014; see Appendix 2). Following pilot testing of the Microsoft Office Excel 2019 data extraction sheet on three studies (Exalto 2014; Kivipelto 2006; Park 2019), two reviewers (GMG and MRS) independently extracted data from all included studies. A third review author (AJC) checked the accuracy of data extraction in a random sample of 10% of included studies.
Assessment of risk of bias and applicability concerns in included studies
We used the Prediction model Risk Of Bias ASsessment Tool (PROBAST) to evaluate risk of bias and applicability concerns for primary studies that developed or validated multi‐variable prognosis models (Moons 2019; Wolff 2019).
Two review authors (GMG and MRS) independently assessed risk of bias and applicability concerns and assigned ratings of low, unclear or high (see Appendix 2). The same review authors performed the GRADE assessments independently and in duplicate. A third review author (AJC) resolved any disagreements by arbitration and checked the risk of bias and applicability assessment results in a random sample of 10% of included studies.
We assigned an overall low risk of bias rating to studies if all the risk domains were at low risk. If at least one domain was at high risk, we judged the study at overall high risk of bias. If at least one domain was at unclear risk and none at high risk, the overall rating was unclear risk. We applied the same criteria to judge overall applicability concerns.
Extraction of measures of predictive performance
The predictive performance measures of the models helped to assess the strength of prediction for the outcome over a time period. For dichotomous outcomes, we sought discrimination values in the form of concordance statistics (C‐statistics). For survival analysis (time‐to‐event analysis), the usual discrimination value was Harell's C‐ statistic. The performance measures comparison also included variance in the form of standard errors (SEs) or confidence intervals (CIs), as reported in the studies (Debray 2017).
We also reported the following metrics, where available.
Sensitivity and specificity
Positive predictive value (PPV)
Negative predictive value (NPV)
Positive or negative likelihood ratio
Net reclassification index (NRI)
Integrated discrimination improvement (IDI)
The calibration measure comparison between articles used either an observed/expected ratio of the outcomes or the metrics in the calibration plots.
Dealing with missing data
We attempted to contact study authors in case of missing or unclear data. Where articles did not report values of predictive accuracy (e.g. calibration and discrimination), we used transforming equations to derive them, where possible (Debray 2017; Parmar 1998; Tierney 2007).
Assessment of heterogeneity
We investigated heterogeneity in validation studies for each model. Where we meta‐analysed data from validation studies, we assessed the degree of heterogeneity by visually inspecting the forest plot and using the Chi2 test. To quantify heterogeneity, we used the I2 statistic, which ranges from 0% to 100% and represents the percentage of variation between the sample estimates that is due to heterogeneity rather than sampling error. (Higgins 2002). We considered heterogeneity to be significant if the I2 value was above 50% (Debray 2017; Sedgwick 2012; Snell 2016; Snell 2018).
Assessment of reporting biases
If we had included at least 10 studies in any meta‐analysis, we would have used a funnel plot to assess publication bias (Debray 2018). This graph would plot the predictive performance measures (discrimination or calibration) of the models and their SEs. In the presence of reporting biases, the funnel plot has a skewed and asymmetrical shape. In addition to visual inspection, we planned to conduct a formal Egger's statistical test to objectively identify asymmetry in the funnel plot (Sedgwick 2013).
Data Synthesis
Data synthesis and meta‐analysis approaches
We summarised the relevant details of each model in tabular form. Each model with at least two studies has a separate table showing the characteristics of the included studies on development and validation. If a model had been externally validated in multiple studies (at least three) and different populations, we conducted a meta‐analysis of the performance measures using a random‐effects model, assuming significant heterogeneity. In the absence of sufficient data for a meta‐analysis, we used a narrative synthesis.
We reported the summary C‐statistic, summary calibration plot, and summary observed/expected ratio, along with their 95% CIs (Debray 2014; Riley 2010). The bivariate analysis of hierarchical models estimated the summary values of sensitivity, specificity, predictive values, and likelihood ratios across the different studies in each model (Reitsma 2005). We planned to list the NRI and the IDI of each model without any analysis. We performed log‐transformation of certain statistical measures (e.g. C‐statistics) before meta‐analysis. We carried out the statistical analysis using Statistical Analysis Software (SAS) version 9.4.
We planned to review the clinical utility/usability of each model where information was available. This included the administration time, ease of collecting the required information (e.g. through self‐reporting versus clinical investigation), and electronic form versus hard copy. We also planned to compare the usability of models in different settings (e.g. high‐income countries versus low‐income countries, prediction for populations versus individuals).
Subgroup analysis and investigation of heterogeneity
We anticipated a high degree of heterogeneity in the following factors.
Measurement of predictors
Type of dementia
Diagnostic method
Geographical location of the validation study
It was essential to understand how performance metrics differed across validation studies according to these variables. We did not expect to find two different models with comparable predictors that would allow for meta‐analysis across models. Even if we were to find similar models, the case‐mix variation and the difference in the predictors used in each model would make it difficult to synthesise data and calculate a general summary value of model performance. We planned to perform meta‐regression, a standard random‐effects meta‐analysis model, by including study‐level covariates to formally test for subgroup differences if we found at least 10 eligible validation studies (Snell 2016; Snell 2018).
Sensitivity analysis
We planned to conduct sensitivity analysis to check the effect on summary measures of studies with high risk of bias or applicability concerns ratings, or of different study designs (prospective versus retrospective). We planned to create a multi‐variate meta‐analysis that combined both the values of calibration and discrimination, if sufficient studies were available (at least 10), and estimate the 95% CI using restricted maximum likelihood estimation (REML) and the Hartung‐Knapp‐Sidik‐Jonkman (HKSI) method (Debray 2017).
Conclusions and summary of findings
We prepared a summary of findings table for each dementia risk model that had been externally validated in two or more studies.
The GRADE framework defines the certainty of the evidence for the effect estimates (Guyatt 2011). This framework, normally used for therapeutic intervention studies, has similar use in prognostic studies (Foroutan 2020). With GRADE, the general approach involves initially considering the evidence to have a high level of certainty, and downgrading for any concerns related to the following five domains (Iorio 2015); assessment methods for this review are presented in brackets after each domain.
Risk of bias (PROBAST tool)
Inconsistency (based on variation in the point estimates, CI estimates, and thresholds of clinical relevance of the performance metrics (e.g. calibration and discrimination))
Imprecision (based on CI width for the performance metrics' summary estimates and its relation to the clinical utility threshold)
Indirectness (PROBAST tool applicability concerns)
Publication bias (funnel plot assessment)
We planned to adopt the most recent guideline for upgrading the studies (e.g. based on optimal effect size, presence of a dose‐response gradient, or both), but none were available at the time of assessment.
For each outcome we assigned one of four certainty levels: high, moderate, low, or very low. We justified our GRADE judgements in footnotes to the summary of findings table.
We reported the inference from the review based on the PRISMA statement (Page 2021). We considered the recommendations in the TRIPOD statement (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) specific to the field of prognostic studies (Collins 2015; Moons 2015).
Results
Results of the search
The database search yielded 37,032 titles (26,179 after deduplication). The Cochrane Dementia and Cognitive Improvement group conducted first‐pass screening to remove titles that were obviously off‐topic. We then screened 7351 abstracts and retrieved 117 full‐text articles (including two from citation tracking). We excluded 92 references that did not meet our inclusion criteria, and categorising four studies as awaiting classification (three were only available as abstracts (Anca 2018; Kerut 2018; Khalid 2020), and the fourth did not clearly define the baseline age of the cohort (Rawtaer 2016)). One article was a protocol for the development of a new model; we categorised it as an ongoing study (Fisher 2017). We recorded our reasons for exclusion of ineligible articles in the Characteristics of excluded studies table and in Figure 1. We included 20 studies in the review: eight were model development studies (Cremers 2020; Ibarrondo 2022; Kim 2019; Kivipelto 2006; Li 2018a; Park 2019; Schiepers 2017; Walters 2016), and 12 were model validation studies (Andrews 2017; Chosy 2019; Deckers 2020; Exalto 2014; Fayosse 2020; Li 2018b; McGrath 2022; Reijmer 2011; Schaich 2021; Tynkkynen 2017; Virta 2013; Vos 2017). Figure 1 shows the study selection process as a PRISMA diagram.
1.

PRISMA diagram showing study selection process.
The 20 included studies described 14 unique prognostic models (see Table 3). All studies had a retrospective cohort design.
2. Development and validation studies of dementia prediction models .
| Model | Development study | External validation study | ||
| Reference ID | Cohort | Reference ID | Cohort | |
| ASCVD‐PCE | Goff 2014a | ARIC (Atherosclerosis Risk in Communities) study, Cardiovascular Health Study, CARDIA (Coronary Artery Risk Development in Young Adults) study, Framingham Original and Offspring Study cohorts | Schaich 2021 | Multi‐Ethnic Study of Atherosclerosis (MESA) |
| ANU‐ADRI |
Anstey 2013b |
Developed through a systematic review | Andrews 2017 | Personality and Total Health (PATH) |
| CAIDE | Kivipelto 2006 | CAIDE study | Chosy 2019 | Honolulu‐Asia Aging Study (HAAS) |
| Exalto 2014 | Kaiser Permanente Medical Care Program of Northern California (KPNC) | |||
| Fayosse 2020 | Whitehall II study | |||
| Reijmer 2011 | Hoorn Study | |||
| Schaich 2021 | Multi‐Ethnic Study of Atherosclerosis (MESA) |
|||
| Tynkkynen 2017 | National FINRISK study | |||
| Virta 2013 | Finnish Twin Cohort | |||
| DSI | Cremers 2020 | Rotterdam Study (RS) | NI | — |
| FRS | D’Agostino 2008a | The Framingham Heart Study | Fayosse 2020 | Whitehall II study |
| FINDRISC | Lindstrom 2003a | National population register and FINRISK study | Fayosse 2020 | Whitehall II study |
| FSRP | Wolf 1991a | The Framingham Heart Study | McGrath 2022 | The Framingham Heart Study and Framingham Heart Study Offspring cohort |
| Schaich 2021 | Multi‐Ethnic Study of Atherosclerosis (MESA) |
|||
| LIBRA | Schiepers 2017 | Maastricht Ageing Study | Deckers 2020 | CAIDE study |
| Vos 2017 | The Development of Screening Guidelines and Clinical Criteria of Predementia Alzheimer's Disease (DESCRIPA) Study | |||
| Model developed from the Framingham Heart Study Offspring cohort | Li 2018a | Framingham Heart Study Offspring cohort | NI | — |
| Model developed from the European Prospective Investigation into Cancer and Nutrition (EPIC)‐Spain cohort | Ibarrondo 2022 | European Prospective Investigation into Cancer and Nutrition (EPIC)‐Spain cohort | NI | — |
| Model developed from the Taiwan National Diabetes Care Management Program | Li 2018b | Taiwan National Diabetes Care Management Program | NI | — |
| Model developed from The Health Improvement Network (THIN) database | Walters 2016 | The Health Improvement Network (THIN) database | NI | — |
| Model developed from the Korean health examination database | Park 2019 | Korean health examination database | NI | — |
| Model developed from the South Korean national health examination cohort | Kim 2019 | South Korean national health examination cohort | NI | — |
ANU‐ADRI: Australian National University Alzheimer's Disease Risk Index; ASCVD‐PCE: Atherosclerotic Cardiovascular Disease Pooled Cohort Equation; CAIDE: Cardiovascular Risk Factors, Aging, and Dementia; DSI: Disease State Index; FRS: Framingham Risk Score; FSRP: Framingham Stroke Risk Profile; FINDRISC: Finnish Diabetes Risk Score; LIBRA: LIfestyle for BRAin Health; NI: none identified.
aIneligible for inclusion in this review due to wrong outcome. bIneligible for inclusion in this review dur to wrong population age.
Seven of the 14 models had been externally validated. The following two models had development and external validation studies that were eligible for this review.
Cardiovascular Risk Factors, Ageing, and Dementia (CAIDE; Chosy 2019; Exalto 2014; Fayosse 2020; Kivipelto 2006; Reijmer 2011; Schaich 2021; Tynkkynen 2017; Virta 2013)
LIfestyle for BRAin Health (LIBRA; Deckers 2020; Schiepers 2017; Vos 2017)
For the following five models, only the external validation studies were eligible for this review.
Australian National University Alzheimer's Disease Risk Index (ANU‐ADRI) was developed in an older age group (Anstey 2013); Andrews 2017 validated the model in middle‐aged people.
Atherosclerotic Cardiovascular Disease Pooled Cohort Equation (ASCVD‐PCE) risk score was developed for cardiovascular outcomes (Goff 2014); Schaich 2021 validated the model for dementia.
Framingham Risk Score (FRS) was developed for cardiovascular outcomes (D’Agostino 2008); Fayosse 2020 validated the model for dementia.
Finnish Diabetes Risk Score (FINDRISC) was developed for the outcome of diabetes (Lindstrom 2003); Fayosse 2020 validated the model for dementia.
Framingham Stroke Risk Profile (FSRP) was developed for the outcome of cerebrovascular accident (Wolf 1991); McGrath 2022 and Schaich 2021 validated the model for dementia.
The following seven models only had development studies.
Model developed from the Framingham Heart Study Offspring cohort (Li 2018a)
Disease State Index (DSI; Cremers 2020)
Model developed from the European Prospective Investigation into Cancer and Nutrition (EPIC)‐Spain cohort (Ibarrondo 2022)
Model developed from the Taiwan National Diabetes Care Management Program (Li 2018b)
Model developed from The Health Improvement Network (THIN) database (Walters 2016)
Model developed from the Korean health examination database (Park 2019)
Model developed from the South Korean national health examination cohort (Kim 2019)
Predictors included in the prognostic models
The 14 identified prognostic models comprised a median of nine predictors (range six to 34); the median, of which a median of five (range two to 11) were modifiable predictors (see Characteristics of included studies).
Five of our prespecified modifiable predictors (diabetes, hypertension, smoking, physical activity, and obesity) were included in at least four of the seven models with validation (see Table 4). The most common modifiable predictors in the development‐only models were obesity, diabetes, hypertension, and smoking (see Table 4). Two of our prespecified modifiable predictors (hearing loss and air pollution) featured in no models.
3. Predictors reported in the final models. .
| Prognostic models | Less education | Traumatic brain injury | Hypertension | Alcohol intake | Obesity | Smoking | Depression | Social isolation | Physical activity | Diabetes mellitus | Diet | Cognitive activity |
| Models with validation (validation studies) | ||||||||||||
| ANU‐ADRI (Andrews 2017) | Yes | Yes | — | Yes | Yes | Yes | Yes | Yes | Yes | Yes | — | Yes |
| ASCVD‐PCE (Schaich 2021) | — | — | Yes | — | — | Yes | — | — | — | Yes | — | — |
| CAIDE (Chosy 2019; Exalto 2014; Fayosse 2020; Kivipelto 2006; Reijmer 2011; Schaich 2021; Virta 2013; Tynkkynen 2017) | Yes | Yesa | Yes | — | Yes | Yesa | Yesa | — | Yesb | Yesa | — | — |
| FINDRISC (Fayosse 2020) | — | — | Yes | — | Yes | — | — | — | Yes | Yes | Yes | — |
| FRS (Fayosse 2020) | — | — | Yes | — | — | Yes | — | — | — | Yes | — | — |
| FSRP (McGrath 2022; Schaich 2021) | — | — | Yes | — | — | Yes | — | — | — | Yes | — | — |
| LIBRA (Deckers 2020; Schiepers 2017; Vos 2017) | Yes9c | — | Yes | Yes | Yes | Yes | Yes | — | Yes | Yes | Yes10d | Yes11e |
| Development‐only models | ||||||||||||
| Cremers 2020 | Yes | — | Yes | Yes | Yes | Yes | — | — | — | Yes | — | — |
| Ibarrondo 2022 | Yes | — | Yes | — | Yes | Yes | — | — | Yes | Yes | Yes | — |
| Kim 2019 | — | — | Yes | — | Yes | Yes | Yes | — | Yes | Yes | — | — |
| Li 2018a | — | — | — | Yes | Yes | — | — | — | — | — | — | — |
| Li 2018b | — | — | — | — | Yes | — | — | — | — | Yes | — | — |
| Park 2019 | — | — | Yes | — | Yes | Yes | Yes | — | Yes | Yes | — | — |
| Walters 2016 | — | — | Yes | Yes | Yes | Yes | Yes | — | — | Yes | — | — |
ANU‐ADRI: Australian National University Alzheimer's Disease Risk Index; ASCVD‐PCE: Atherosclerotic Cardiovascular Disease Pooled Cohort Equation; CAIDE: Cardiovascular Risk Factors, Aging, and Dementia; FINDRISC: Finnish Diabetes Risk Score; FRS: Framingham Risk Score; FSRP: Framingham Stroke Risk Profile; LIBRA: LIfestyle for BRAin Health.
a Reported only in Exalto 2014. b Physical activity was not available for Exalto 2014 or Tynkkynen 2017. c Reported only in Schiepers 2017 and Vos 2017. d Reported only in Deckers 2020 and Schiepers 2017. e Reported only in Schiepers 2017.
We accepted all definitions of predictors reported in individual studies (Appendix 3 presents the definitions in a table). The predictors with the most variable definitions across studies were depression, alcohol intake, diabetes, hypertension, and physical activity. For depression, measurement methods ranged from single‐item questions such as "Do you often feel unhappy or depressed?" (Exalto 2014), to a multitude of instruments that define depressive symptoms or diagnosis of depression (Vos 2017). Studies assessed alcohol intake based on drinks per week, collecting this information through self‐reporting or medical records, and applying different standards/cut‐offs. To define diabetes, studies used: self‐report (Andrews 2017); medical history (Park 2019; Vos 2017; Walters 2016); or objective measures with cut‐offs, and use of anti‐diabetic medications (Cremers 2020; Exalto 2014). For hypertension, there were variations across studies in the site of measurement, the number of readings per participant, and the assessor (Appendix 3). Definitions of physical activity were based on measures of frequency (Deckers 2020; Fayosse 2020); or intensity, duration, and metabolic equivalents of exercise (Schaich 2021; Virta 2013).
Models also used modifiable predictors other than those included in the WHO and Lancet Commission report. These predictors included cholesterol level (Chosy 2019; Cremers 2020; Deckers 2020; Fayosse 2020; Ibarrondo 2022; Kim 2019; Kivipelto 2006; Reijmer 2011; Schaich 2021; Schiepers 2017; Tynkkynen 2017; Virta 2013; Vos 2017; Walters 2016), pesticide exposure (Andrews 2017), waist circumference (Fayosse 2020), and sleep deprivation (Li 2018a).
Measurement of the outcome
Fifteen studies reported our primary outcome of incident clinical diagnosis of dementia based on clinical examination or recorded diagnosis in administrative databases (Andrews 2017; Deckers 2020; Exalto 2014; Fayosse 2020; Ibarrondo 2022; Kim 2019; Kivipelto 2006; Li 2018a; Li 2018b; McGrath 2022; Park 2019; Schiepers 2017; Tynkkynen 2017; Vos 2017; Walters 2016). Five studies reported our secondary outcome of incident dementia or cognitive impairment based on neuropsychological cognitive tests (Chosy 2019; Cremers 2020; Reijmer 2011; Schaich 2021; Virta 2013).
Characteristics and performance of the models
We have described the characteristics of included studies per model and per predicted outcome (i.e. primary or secondary).
Models with validation studies
Cardiovascular Risk Factors, Ageing, and Dementia (CAIDE)
Kivipelto 2006 developed the CAIDE model from a population‐based study of 1409 individuals, with a mean follow‐up time of 20.9 years. The study derived two models with seven and eight predictors (five modifiable). Model 1 included age, education, sex, systolic blood pressure, body mass index (BMI), total cholesterol, and physical activity. Model 2 included apolipoprotein E ɛ4 allele (APOEɛ4) status in addition to all the model 1 predictors. Four studies used model 2 (Chosy 2019; Schaich 2021; Tynkkynen 2017; Virta 2013), and three studies used model 1 (Exalto 2014; Fayosse 2020; Reijmer 2011). The study authors converted the beta coefficients into a risk score for easier use in the clinical setting.
We found seven external validation studies that used CAIDE; three predicted our primary outcome (Exalto 2014; Fayosse 2020; Tynkkynen 2017), and four predicted our secondary outcome (Chosy 2019; Reijmer 2011; Schaich 2021; Virta 2013).
Primary outcome
The validation studies for incident clinical diagnosis of dementia included 24,147 individuals from the USA, UK, and Finland (Exalto 2014; Fayosse 2020; Tynkkynen 2017; see Table 5). The mean follow‐up ranged from 16.1 years to 36.9 years. All studies used electronic medical records to identify eligible cases based on ICD codes. Exalto 2014 employed a further confirmatory dementia diagnosis by a medical specialist in a memory clinic for the possible dementia cases. Tynkkynen 2017 used drug reimbursement for cholinesterase inhibitors or memantine to calculate incident dementia cases, and reported the number of Alzheimer's disease cases separately. The modelling methods were Cox proportional hazards and logistic regression. The C‐statistics ranged from 0.65 to 0.74. Tynkkynen 2017 updated the model using the measures of cardiac troponin and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), and Exalto 2014 added the effects of central obesity, depressed mood, diabetes, head trauma, poor lung function, and smoking to the CAIDE score. The addition of these new predictors did not improve the C‐statistics. Exalto 2014 measured calibration with the Hosmer‐Lemeshow goodness‐of‐fit test, and Fayosse 2020 used the Greenwood‐Nam‐D'Agostino test (an extension of the Hosmer‐Lemeshow test). Exalto 2014 reported that the addition of smoking status to the model improved calibration, but that overall calibration was poor.
4. Summary of characteristics of included studies (CAIDE).
| Study, country, outcome type | Follow‐up in years, mean (SD) | Age in years, mean (SD) | Sex, % women | Sample size (number of events:number of predictors) |
Number of predictors (modifiable) |
Discrimination, C‐statistic (95% CI) |
| Development study | ||||||
| Kivipelto 2006, Finland, primary | 20.9 (4.9) | 50.4 (6.0) | 62% | 1409 (7) |
8 (4) | 0.78 (0.72 to 0.84) |
| Validation study with a primary outcome | ||||||
| Exalto 2014, USA, primary | 36.9 (4.1) | 46.1 (4.3) | 49%, 60%a | 9480 (230) |
12 (7) | 0.74 (NR) |
| Fayosse 2020, UK, primary | 23.5 (4.0) | 50 (39‐63)b | 30.8% | 7553 (45) |
7 (4) | 0.71 (0.69 to 0.73) |
| Tynkkynen 2017, Finland, primary | 16.1 (0.12)b | 47.9 (13.2) |
48.9% | 7114 (45) |
9 (3) |
0.65 (NR) |
| Validation study with a secondary outcome | ||||||
| Chosy 2019, USA, secondary | 25.2 (1.1) | 52.3 (4.4), 58.4 (5.4)c | 0% | 3582 (23) |
8 (4) | 0.64 (0.62 to 0.67) |
| Reijmer 2011, Netherlands, secondary | 15 (NR) | 55 (3.7) | 49% | 322 (NR) |
7 (5) | 0.63 (0.53 to 0.73), 0.72 (0.61 to 0.82)d |
| Schaich 2021, USA, secondary | 15.7 (0.7) | 60.1 (9.4) | 53.3% | 4392 (27) |
8 (4) | 0.63 (0.59 to 0.66) |
| Virta 2013, Finland, secondary | 22.6 (2.3) | 51.7 (6.1) | 48.9% | 591 (14) |
8 (4) | 0.75 (0.70 to 0.81), 0.74 (0.69 to 0.79)e |
CI: confidence interval; NR: not reported; SD: standard deviation.
a Sex reported separately for people with and without dementia. b Median (interquartile range). c Age reported separately for people without and with dementia. d C‐statistics for information processing speed and abstract reasoning, respectively. e For models with and without the predictor apolipoprotein E ε4 allele, respectively.
Secondary outcomes
The four validation studies for incident dementia or cognitive impairment included a total of 8887 individuals from the USA, Netherlands, and Finland (Chosy 2019; Reijmer 2011; Schaich 2021; Virta 2013; see Table 5). Follow‐up ranged from 15 years to 25 years. We identified two different categories of cognitive impairment measurement: three studies used standard cognition screening tools, namely telephone assessment of dementia (TELE; Virta 2013) and Cognitive Abilities Screening Instrument (CASI; Chosy 2019; Schaich 2021); while Reijmer 2011 used cognitive domain‐specific z scores. Schaich 2021 also used other cognitive tools, including a measure of global cognitive performance, a test of processing speed (Digit Symbol Coding; DSC), and a test of working memory (Digit Span; DS).
Based on these tools, the studies adopted the following definitions of cognitive impairment.
Virta 2013 used a TELE score cut‐off of 16 points to classify participants as cognitively healthy or impaired.
Chosy 2019 considered a Cognitive Abilities Screening Instrument (CASI) score below 60 indicative of severe cognitive impairment.
Schaich 2021 measured the association between a one‐standard deviation (SD) increase in risk scores and a one‐SD decline in CASI performance.
Reijmer 2011 defined impairment in a particular cognitive domain as a z‐score of less than or equal to 1.5 on at least one measure included in that domain.
All studies used logistic regression as the modelling method. The C‐statistics ranged from 0.63 to 0.75. Schaich 2021 reported a calibration slope of 1.05 (95% CI 0.71 to 1.40) and an intercept of −0.04 (95% CI −0.18 to 0.10).
Three studies stratified the risk scores. Two studies used a cut‐off to classify scores into high and low risk (nine points in Chosy 2019; 10 points in Reijmer 2011). Virta 2013 divided the risk scores into quartiles. Scores above 9 (in models without APOEƐ4) or 10 (in models with APOEƐ4) were associated with significantly higher odds for the secondary outcome.
LIfestyle for BRAin Health (LIBRA)
Deckers 2015 (not an included study) selected the predictors of the LIBRA model through an extensive literature review, and Schiepers 2017 (the development study) assessed the model's predictive performance in the 12‐year longitudinal Maastricht Ageing Study (MAAS). Schiepers 2017 randomly selected participants from the family practice register and included those aged over 50 years, excluding people with Mini‐Mental State Examination (MMSE) scores of 24 or below, or with any baseline neurological pathology. The study involved 949 participants with 16 years of follow‐up for dementia and 12 years for cognitive impairment. It used 14 predictors (of which 10 were modifiable): age, sex, education, alcohol consumption, history of cardiovascular disease, physical activity, renal function, diabetes, cholesterol, smoking, obesity, hypertension, depression, and cognitive activity. Schiepers 2017 used the DSM‐IV definition of all‐cause dementia as the primary outcome. It also measured a secondary outcome of cognitive impairment, determined using three cognitive tests: the visual verbal word learning task, the Stroop colour‐word interference test, and the letter‐digit substitution test. People scoring below 1.5 SD of the total test scores were considered to have cognitive impairment.
Primary outcome
We identified two external validation studies for the primary outcome (Deckers 2020; Vos 2017; see Table 6). The validation studies of incident clinical dementia included 4280 participants from Finland, France, Italy, the Netherlands, and Sweden. The method of dementia diagnosis was detailed neuropsychological assessment satisfying DSM‐IV criteria. Mean follow‐up ranged from 8.1 years to 20.9 years. Notably, the validation studies used fewer variables than the development model. The modelling method was Cox proportional hazards, and the C‐statistics ranged from 0.57 to 0.75. Deckers 2020 updated the model with diet, achieving marginal improvement in the C‐statistic.
5. Summary of characteristics of included studies (LIBRA).
| Study; country; outcome type | Follow‐up in years, mean (SD) | Age in years, mean (SD) | Sex, % women | Sample size (number of events:number of predictors) |
Number of predictors (modifiable) |
Discrimination, C‐statistic |
| Development study | ||||||
| Schiepers 2017; Netherlands; primary and secondary | 16 (NR), 12 (NR)a |
65 (8.7) | 49% | 949 (4), 746 (6)a | 15 (10) | 0.75 (95% CI 0.69 to 0.80), 0.57 (95% CI 0.51 to 0.63)a |
| Validation studies | ||||||
| Deckers 2020; Finland; primary and secondary | 20.9 (4.9) |
47.8 (4.7) |
61%, 60.5%, 58.3%b | 1024 (7, 13)a | 11 (8) | Range 0.65 to 0.75 |
| Vos 2017; Italy, France, Netherlands, Sweden; primary | 8.1 (3.5) |
65 (4) | 51% | 3256 (21) | 9 (8) | 0.57 (SE 0.03) |
CI: confidence interval; NR: not reported; SD: standard deviation; SE: standard error
a For the primary and secondary outcome, respectively. b Percentage of women among participants with normal cognitive function/with cognitive impairment/with dementia.
Secondary outcome
We identified one external validation study for the secondary outcome (Deckers 2020; see Table 6). Deckers 2020 used clinical diagnosis of mild cognitive impairment as a separate outcome based on DSM‐IV criteria. The study involved 1024 participants from Finland, mean follow‐up was 20.9 years, and the C‐statistic ranged from 0.65 to 0.75 (depending on inclusion of diet and adjustment for sex and education). There were no reported measures of calibration.
Models externally validated for a different use (where development study was ineligible for inclusion in this review)
Australian National University Alzheimer's Disease Risk Index (ANU‐ADRI)
The ANU‐ADRI development study involved multiple systematic reviews of variables related to dementia; the study authors derived a score based on the various reported beta coefficients (Anstey 2013). There were 15 candidate predictors: nine risk factors (five of which are modifiable) and six protective factors (all modifiable). The risk factors were age, sex, BMI, presence of diabetes, symptoms of depression, cholesterol, history of traumatic brain injury, smoking, and pesticide exposure. The protective factors were educational level, alcohol consumption, social engagement, physical activity, involvement in cognitively stimulating activities, and fish intake. Anstey 2013 did not meet our inclusion criteria as it focused on an older age group.
Primary outcome
One study drew 2078 people aged 60 to 64 years from the Australian Personality and Total Health (PATH) cohort (Andrews 2017; see Table 7). Mean follow‐up was 9.6 years. The study reported a combined C‐statistic of 0.60 (SE 0.05) for minimal cognitive impairment or dementia based on cognitive tests (MMSE, California verbal learning test, symbol digital modalities test, Purdue pegboard both hands, simple reaction time, or memory and cognition questionnaire) and a neurologist review of the cases.
6. Summary of characteristics of models externally validated for a different use.
| Study, country, outcome type | Follow‐up in years, mean (SD) | Age in years | Sex, % women | Sample size (number of events: number of predictors) |
Number of predictors (modifiable) |
Discrimination, C‐statistic (variance measure) |
| ANU‐ADRI, Australia, primary outcome | 9.6 (NR) | Range 60–64 | 48.5% | 2078 (7) | 12 (10) | 0.60 (SE 0.05) ** |
| ASCVD‐PCE, USA, secondary outcome | 15.7 (0.7) | Mean 60.1 (SD 9.4) | 53.3% | 4392 (24) | 9 (3) | 0.65 (95% CI 0.61 to 0.68) |
| FINDRISC, UK, primary outcome | 23.5 (4) | Median 50 (IQR 39–63) *** | 30.8% | 7553 (39) | 8 (5) | 0.63 (95% CI 0.60 to 0.65) |
| FRS, UK, primary outcome | 23.5 (4) | Median 50 (IQR 39–63) *** | 30.8% | 7553 (53) | 6 (3) | 0.71 (95% CI 0.69 to 0.74) |
| FSRP (McGrath 2022), USA, primary outcome | 10 (NR) | 95% CI 52.5 to 57.5# | 57.2% | 3735 (10) | 7 (3) | 0.61 (SE 0.04) ** |
| FSRP (Schaich 2021), USA, secondary outcome | 15.7 (0.7) | Mean 60.1 (SD 9.4) | 53.3% | 4392 (24) | 9 (3) | 0.65 (95% CI 0.61 to 0.69) |
ANU‐ADRI: Australian National University Alzheimer's Disease Risk Index; ASCVD‐PCE: Atherosclerotic Cardiovascular Disease Pooled Cohort Equation; CI: confidence interval; FINDRISC: Finnish Diabetes Risk Score; FRS: Framingham Risk Score; FSRP: Framingham Stroke Risk Profile; NR: not reported; SD: standard deviation.
Secondary outcome
No studies evaluated the accuracy of the ANU‐ADRI for predicting incident dementia or cognitive impairment determined by any method other than those described for the primary outcome (See 'Types of outcomes' in Methods section).
Atherosclerotic Cardiovascular Disease Pooled Cohort Equation (ASCVD‐PCE)
The data for the development of ASCVD‐PCE included participants from multi‐ethnic backgrounds and from different geographical areas (Goff 2014). The aim of the development study was to predict 10‐year risk of first atherosclerotic cardiovascular event in non‐Hispanic African Americans and non‐Hispanic white people aged 40 to 79 years. The predictors in the model were age, race, sex, systolic blood pressure, total and HDL cholesterol, antihypertensive medication use, diabetes mellitus, and smoking status. The C‐statistic ranged from 0.71 (African American men) to 0.8 (African American women). Goff 2014 was ineligible for inclusion in this review because it did not measure dementia as an outcome.
Primary outcome
No studies evaluated the accuracy of the ASCVD‐PCE for predicting incident clinical diagnosis of dementia of any subtype according to standard classification systems or based on the diagnostic criteria set out in 'Types of outcomes' (Methods section).
Secondary outcome
Schaich 2021 evaluated the accuracy of the ASCVD‐PCE for predicting cognitive performance, as there was insufficient evidence related to the strength of association between vascular risk scores and dementia in wider ethnic groups (see Table 7). Schaich 2021 used the Multi‐Ethnic Study of Atherosclerosis (MESA) cohort and included 4392 people with a mean age of 60.1 years, following them for 15 years. The C‐statistic was 0.65 (95% CI 0.61 to 0.68) for the outcome of cognitive decline, defined as a one‐SD reduction in the baseline CASI score.
Finnish Diabetes Risk Score (FINDRISC)
Lindstrom 2003 developed and validated this model for predicting new cases of drug‐treated type 2 diabetes mellitus in participants sampled from the National population register of Finland. The predictors in the model were age; BMI; waist circumference; history of antihypertensive drug treatment and high blood glucose; physical activity; and daily consumption of fruits, berries, or vegetables. The AUC was 0.85 for the development cohort and 0.86 for the validation cohort. Lindstrom 2003 was ineligible for inclusion in this review because it did not measure dementia as an outcome.
Primary outcome
Fayosse 2020 used the FINDRISC model to predict the development of dementia in 7553 participants with a mean age of 50 years, followed up for 23.5 years (see Table 7). The C‐statistic of the model was 0.63 (95% CI 0.60 to 0.65), and the calibration, based on the Hosmer‐Lemeshow goodness‐of‐fit test, was poor.
Secondary outcome
No studies evaluated the accuracy of the FINDRISC for predicting incident dementia or cognitive impairment determined by any method other than those described for the primary outcome (See 'Types of outcomes' in Methods section).
Framingham Risk Score (FRS)
The FRS development study aimed to predict a composite outcome of cardiovascular events in adults with a mean age of 49 years (D’Agostino 2008). The predictors in the model were age, total and high‐density lipoprotein cholesterol, systolic blood pressure, treatment for hypertension, smoking status, and diabetes mellitus. The C‐statistic of the model was 0.76 for men and 0.79 for women. D’Agostino 2008 was ineligible for inclusion in this review because it did not measure dementia as an outcome.
Primary outcome
Fayosse 2020 assessed the predictive performance of FRS in Whitehall II study participants aged 39 to 63 years, followed up for 23.5 years, for the incidence of dementia (see Table 7). The study authors collected the outcome using three electronic health records available in the UK (National Hospital Episode statistics, the Mental Health Services dataset, and the mortality register). The overall C‐statistic for the model was 0.71 (95% CI 0.69 to 0.74). Fayosse 2020 also assessed calibration power in plots of observed and predicted dementia rate per 1000 person‐years in deciles of the predictor, finding that the age‐alone model gave similar performance to the complete FRS model.
Secondary outcome
No studies evaluated the accuracy of the FRS for predicting incident dementia or cognitive impairment determined by any method other than those described for the primary outcome (See 'Types of outcomes' in Methods section).
Framingham Stroke Risk Profile (FSRP)
Wolf 1991 developed the FSRP to predict 10‐year stroke risk in individuals aged over 55 years. The predictors in the model were age, sex, systolic blood pressure, antihypertensive use, prevalent cardiovascular disease, current smoking, left ventricular hypertrophy, history of atrial fibrillation, and diabetes mellitus. Wolf 1991 was ineligible for inclusion in this review because it did not measure dementia as an outcome.
Primary outcome
In McGrath 2022, a dementia review committee determined the outcome of dementia based on neurological examination records and neuropsychological and neuroimaging test results (see Table 7). The cohort of 3735 participants included people from the original Framingham cohort and the offspring cohort. The duration of follow‐up was 10 years. McGrath 2022 examined the predictive capacity of the FSRP at different time points, starting from 55 years of age. The number of outcomes at 10‐year follow‐up of the 55‐year‐old cohort was 72/3735. The study used Cox proportional hazards model, obtaining a C‐statistic of 0.61 (SE 0.04)
Secondary outcome
Schaich 2021 assessed the predictive power of the FSRP in a multi‐ethnic cohort of 4392 participants drawn from the Multi‐Ethnic Study of Atherosclerosis (MESA; see Table 7). The study analysed the association between an increase in risk score and a one‐SD decrease in cognitive scores after 15 years. The study reported 218 dementia cases during follow‐up. It used logistic regression and obtained a C‐statistic of 0.66 (95% CI 0.61 to 0.69). The calibration slope was 1.05 (95% CI 0.71 to 1.40) and the intercept was −0.04 (95% CI −0.18 to 0.10).
Development‐only models
We found seven models with no external validation study (see Table 8). All the studies validated the models internally using techniques such as split‐sampling, cross‐validation, bootstrapping, or decision tree classification. Six models measured our primary outcome (Ibarrondo 2022; Kim 2019; Li 2018a; Li 2018b; Park 2019; Walters 2016), and one measured our secondary outcome (Cremers 2020). The number of predictors in these models ranged from six to 34 (two to seven of which were modifiable). Follow‐up ranged from five to 30 years. The modelling methods were Cox proportional hazards, non‐parametric models of Fine and Gray, and machine learning methods (namely decision tree and supervised machine learning). Mean C‐statistics ranged from 0.65 to 0.90. Four studies reported calibration as slope, intercept, or a calibration plot (Ibarrondo 2022; Kim 2019; Park 2019; Walters 2016). The reported calibration slopes were 0.96 (Kim 2019), 0.99 (Park 2019), and 0.98 (Walters 2016); and the reported intercepts were 0.001 (Kim 2019) and 0.002 (Park 2019). Li 2018b reported calibration based on the Hosmer Lemeshow goodness‐of‐fit test.
7. Characteristics of included studies without external validation.
| Study, country, outcome type | Follow‐up in years, mean (SD) | Age in years, mean (SD) | Sex, % females | Sample size (number of events:number of predictors) |
Number of predictors (modifiable) |
Discrimination, C‐statistics (95% CI) |
| Ibarrondo 2022, Spain, primary | 21.6 (3.4) | 49.08 (7.75) | 43% | 25,015 (62) |
12 (7) | 0.65 (0.61 to 0.68) |
| Li 2018a, USA, primary | 30 (NR) | 40–65* | 50.4% | 904 (37) |
6 (2) | 0.79, 0.89b |
| Li 2018b, Taiwan, primary | 8.09 (NR) | 64.3 (8.53) | 55.2% | 9180 (34) |
11 (2) | 0.75 (0.73 to 0.77) |
| Park 2019, South Korea, primary | 10.24 (1.73) | 51.39 (8.12) | 45.2% | 141,910 (608) |
11 (6) | 0.81 (0.81 to 0.82) |
| Walters 2016, UK, primary | 5 (3.2‐5)a | 65.6 (6.11) | 51.8% | 226,140 (141) |
12 (6) | 0.84 (0.81 to 0.87) |
| Kim 2019, South Korea, primary | 10.39 (1.44) | 52.53 (9.35) | 45.6% | 95,969 (389) | 15 (6) | 0.84 (0.83 to 0.85), 0.87 (0.86 to 0.88), 0.90 (0.90 to 0.90)c |
| Cremers 2020, Netherlands, secondary | 5.7 (0.6) | 60.9 (9.1) | 55.6% | 2542 (3) |
34 (6) | 0.77 (0.72 to 0.82) |
CI: confidence interval; NR: not reported; SD: standard deviation.
a Data presented as range. b Decision tree model and random forest model, respectively. c Baseline model, model with average values of predictors, and model developed using deep learning method, respectively.
Excluded studies
We excluded 92 studies during full‐text screening: 51 did not describe prognostic model development or validation (17 reported associations rather than prediction of an outcome, and five were systematic reviews), 24 did not use a middle‐aged population, 10 had a follow‐up period shorter than five years, three involved models that were not multi‐domain in nature, two did not measure dementia or cognitive impairment as an outcome, and two included cohorts with baseline cognitive impairment (see Figure 1 and Characteristics of excluded studies).
Reporting deficiencies in studies of models with validation
Two studies did not report the SD of the follow‐up duration (McGrath 2022; Reijmer 2011).
One study did not report the number of dementia cases at the end of follow‐up (Reijmer 2011).
Three studies did not report the number of participants with missing data or mention whether they had used complete case analysis (Deckers 2020; Reijmer 2011; Schiepers 2017).
Four studies did not report the handling of missing data (Deckers 2020; Reijmer 2011; Schiepers 2017; Vos 2017).
Three studies that performed imputation of the missing samples did not specify the pooling method used to derive model performance (Andrews 2017; Schaich 2021; Tynkkynen 2017).
Five studies did not report the modelling assumptions for the Cox proportional hazards regression model (Andrews 2017; Exalto 2014; McGrath 2022; Schiepers 2017; Vos 2017).
Ten studies did not report the calibration slope (Andrews 2017; Chosy 2019; Deckers 2020; Kivipelto 2006; McGrath 2022; Reijmer 2011; Schiepers 2017; Tynkkynen 2017; Virta 2013; Vos 2017).
Twelve studies did not report the calibration intercept (Andrews 2017; Chosy 2019; Deckers 2020; Exalto 2014; Fayosse 2020; Kivipelto 2006; McGrath 2022; Reijmer 2011; Schiepers 2017; Tynkkynen 2017; Virta 2013; Vos 2017).
Three studies did not report the SE of the overall C‐statistics (Deckers 2020; Exalto 2014; Tynkkynen 2017).
Five studies did not report the risk stratification of the scores (Andrews 2017; Fayosse 2020; McGrath 2022; Schaich 2021; Tynkkynen 2017).
Two development studies did not report any form of internal validation (Kivipelto 2006; Schiepers 2017).
Four studies that updated models with additional factors did not report the updated sum score for further validation (Deckers 2020; Exalto 2014; McGrath 2022; Tynkkynen 2017).
Eleven studies did not report the comparison between the validation cohort and the model's development cohort (Andrews 2017; Chosy 2019; Deckers 2020; Exalto 2014; Fayosse 2020; McGrath 2022; Reijmer 2011; Schaich 2021; Tynkkynen 2017; Virta 2013; Vos 2017).
The development study of the LIBRA model did not report the entire model equation (Schiepers 2017).
Twelve studies did not report the sample size calculation (Andrews 2017; Chosy 2019; Deckers 2020; Exalto 2014; Fayosse 2020; Kivipelto 2006; McGrath 2022; Reijmer 2011; Schaich 2021; Schiepers 2017; Tynkkynen 2017; Vos 2017), and no studies estimated sample size based on the target prediction performance.
Reporting deficiencies in studies of development‐only models
Two studies did not report the SD of follow‐up duration (Li 2018a, Li 2018b)
Three studies did not report the number of participants with missing data (Cremers 2020; Li 2018a; Park 2019).
Four studies did not report the missing data in each of the predictors (Ibarrondo 2022; Kim 2019; Li 2018a; Park 2019).
Four studies that performed imputation of the missing samples did not specify the pooling method used to derive model performance (Ibarrondo 2022; Kim 2019; Li 2018b; Park 2019).
Two studies did not report the modelling assumptions for the Cox proportional hazards regression model (Li 2018b; Park 2019).
No studies reported no form of shrinkage analysis.
Two studies reported no form of calibration (Cremers 2020; Li 2018a).
One study did not report the SD of the C‐statistic (Li 2018a).
Three studies did not report any form of risk stratification (Cremers 2020; Kim 2019; Li 2018a).
Five studies did not report the final model in the form of a usable sum score (Cremers 2020; Kim 2019; Li 2018a; Park 2019; Walters 2016).
Four studies that did not use a machine learning method did not report the entire model equation (Ibarrondo 2022; Kim 2019; Li 2018b; Park 2019).
Six studies did not mention sample size calculation (Cremers 2020; Ibarrondo 2022; Kim 2019; Li 2018a; Li 2018b; Park 2019).
Risk of bias and applicability concerns assessment of included studies
Refer to the Characteristics of included studies table.
Models with validation studies
Cardiovascular Risk Factors, Ageing, and Dementia (CAIDE)
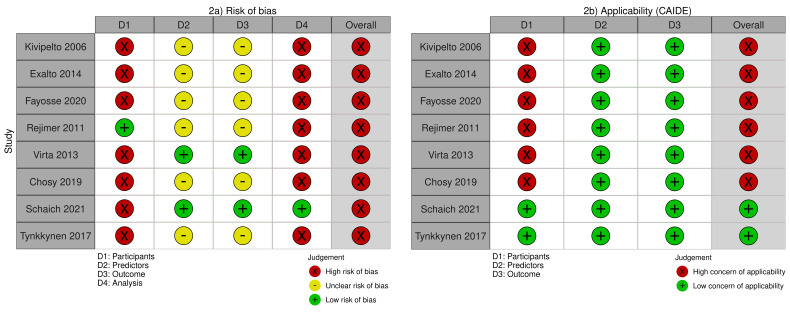
We rated the overall risk of bias for the development study as high due to concerns in the participants and analysis domains (Kivipelto 2006; see Figure 2). The study did not document any baseline cognitive assessment (or exclusion of dementia cases), resulting in high risk of bias and high concern for applicability in the participants domain. Our response to PROBAST signalling question 2.2 ("Were predictor assessments made without knowledge of outcome data?") and 3.5 ("Was the outcome determined without knowledge of predictor information?") was 'unclear', because the study provided insufficient information for us to make a judgement. There were concerns related to a low events/predictors ratio (due to a low number of dementia diagnoses at the end of follow‐up), inappropriate categorisation of continuous predictors, absence of information regarding the handling of missing data, inclusion of predictors based on univariate analysis, and absence of calibration and internal validation.
2.
Figure 2a and 2b. PROBAST risk of bias and applicability assessment of the CAIDE model.
Our risk of bias and applicability concerns ratings were mostly high for the participants domain, low or unclear for predictors and outcomes, and high for analysis (risk of bias only). Consequently, the overall risk of bias rating was high for all studies, and the overall applicability concerns rating was high for most studies. Reasons for a high risk of bias rating in the participants domain included no reported exclusion of dementia cases at baseline, no reported baseline cognitive screening, and exclusion of high‐risk individuals (e.g. people with other cardiovascular diseases). We assigned a high rating for applicability concerns if there was no baseline exclusion of dementia cases. Only Schaich 2021 and Virta 2013 stated that the predictors were assessed without knowledge of the outcome and the outcome determined without knowledge of the predictors, so the remaining studies were at unclear risk of bias for these domains. The main reasons for our high risk of bias rating in the analysis domain was low events/predictors ratio, handling of missing data, and absence of calibration.
LIfestyle for BRAin Health (LIBRA)
We rated the risk of bias in the development study as low for participants, unclear for predictors and outcome, and high for analysis (Schiepers 2017; see Figure 3). Reasons for these ratings included low event/predictor ratio, unnecessary categorisation of continuous predictors, incorrect handling of missing data, and absence of calibration and internal validation.
3.
Figure 3a and 3b. PROBAST risk of bias and applicability assessment of the LIBRA model.
For the validation studies, our risk of bias ratings for the predictors and outcome domains were unclear owing to a lack of information regarding the recording of outcomes without knowledge of the predictor data (Deckers 2020; Vos 2017). The high risk of bias in the analysis domain was due to incorrect handling of missing data, absence of calibration, and a low number of outcome events.
Our applicability concerns ratings were low for all studies in all domains.
Models externally validated for a different use (where the development study was ineligible for inclusion in this review)
Australian National University Alzheimer's Disease Risk Index (ANU‐ADRI)
We rated the validation study of ANU‐ADRI at high risk of bias because it excluded high‐risk individuals, reported few events, and did not report calibration (Andrews 2017; see Figure 4). The rating of the predictors and outcome domains were unclear, owing to a lack of information regarding the recording of outcomes without knowledge of the predictor data.
4.
Figure 4a and 4b. PROBAST risk of bias and applicability assessment of ANU‐ADRI, ASCVD‐PCE, FRS, and FSRP.
Our overall rating for applicability concerns was high, as the study only included people aged 60 to 64 years.
Atherosclerotic Cardiovascular Disease Pooled Cohort Equation (ASCVD‐PCE)
We rated the validation study of ASCVD‐PCE at high risk of bias for the participants domain, as it excluded individuals with cardiovascular disease at baseline (Schaich 2021). Our risk of bias rating for the analysis domain was low because the study reported a sufficient number of outcomes, reported calibration, and handled missing data as recommended (Figure 4). The study authors responded to our email request and shared the calibration of the model, stating that the predictors were blinded from the outcome analysis. Our applicability concerns ratings were low for all domains.
Finnish Diabetes Risk Score (FINDRISC) and Framingham Risk Score (FRS)
The validation study of the FRS and FINDRISC did not report baseline exclusion of dementia cases, so our risk of bias and application concerns ratings were high (Fayosse 2020). The ratings for predictors and outcome were unclear because Fayosse 2020 did not state whether the outcome was determined without knowledge of the predictor data (Figure 4). Risk of bias for the analysis domain was high owing to incorrect handling of missing data and absence of calibration. Our applicability concerns rating for participants was high because the study excluded people aged 56 to 65 years.
Framingham Stroke Risk Profile (FSRP)
McGrath 2022 excluded people with coronary heart disease and thus selected a low‐risk group as the cohort. It also excluded people aged under 55 years, so the model may not be suitable for people aged 45 to 54 years. As a result, risk of bias and applicability concerns were high for the participants domain. Our risk of bias rating for predictors and outcome was unclear owing to a lack of information regarding the recording of outcomes independent of the predictor data (Figure 4). We rated the analysis domain at high risk of bias because there were few events and no reported calibration. Our ratings for Schaich 2021 are as described for the ASCVD‐PCE model.
Development‐only models
All studies excluded participants with dementia at baseline, so risk of bias for the participant domain was low in all cases (Figure 5). Our applicability concerns ratings for all domains were low for all studies except Li 2018b, which excluded people aged under 50 years. For the predictors and outcome domains, risk of bias was unclear owing to a lack of information regarding the recording of outcomes independent of the predictor data. Cremers 2020 included predictors in the outcome, so we rated it at high risk of bias for the predictor domain. We rated all studies except Kim 2019 and Walters 2016 at high risk of bias for the analysis domain for the following reasons.
5.
Figure 5a and 5b. PROBAST risk of bias and applicability assessment of development‐only models.
Few outcome events (Cremers 2020)
Unnecessary categorisation of continuous predictors (Ibarrondo 2022; Li 2018a; Li 2018b; Park 2019)
Not avoiding univariate analysis‐based selection of predictors (Li 2018b)
Absence of calibration (Cremers 2020; Li 2018a; Li 2018b)
Prediction performance of the models with at least three external validation studies per outcome
Refer to the Table 1.
Discrimination performance
CAIDE and incident clinical diagnosis of dementia of any subtype
We included three external validation studies in the random‐effects meta‐analysis of C‐statistics (Exalto 2014; Fayosse 2020; Tynkkynen 2017). The total number of participants was 24,147, and there were 3492 reported dementia cases during the overall observation period (mean duration 16 to 36 years). Exalto 2014 and Tynkkynen 2017 did not report the SD for the C‐statistics. We used the SE of the C‐statistic of three ethnicities reported by Exalto 2014, and the SD of models with and without additional predictors reported by Tynkkynen 2017, to find the summary SE. The summary C‐statistic of the three studies was 0.71 (95% CI 0.66 to 0.76; very low‐certainty evidence; Figure 6). We downgraded the certainty of the evidence by one level due to high risk of bias across multiple domains, by one level for inconsistency due to a high degree of heterogeneity (I2 = 91%), and by one level for applicability concerns. Refer to Appendix 4 for the complete GRADE explanations.
6.
Discrimination for the CAIDE predicting the incident clinical diagnosis of dementia of any subtype.
CAIDE and incident dementia or cognitive impairment assessed using cognitive scores
We included three external validation studies of the CAIDE model in the meta‐analysis of prediction performance (Chosy 2019; Schaich 2021; Virta 2013). The total sample size was 8565, and there were 524 reported events during the overall observation period (mean duration 15.7 to 25.2 years). The summary C‐statistic of the three studies was 0.67 (95% CI 0.61 to 0.73; very‐low‐certainty evidence; Figure 7). We downgraded the certainty of the evidence by one level due to high risk of bias across multiple domains, by one level for inconsistency due to a high degree of heterogeneity (I2 = 88%), and by one level for indirectness due to applicability concerns. Refer to Appendix 4 for the complete GRADE explanations. We did not include Reijmer 2011 in the meta‐analysis because it reported C‐statistics for information processing speed and abstract reasoning only.
7.
Discrimination for the CAIDE predicting incident dementia or cognitive impairment assessed using cognitive scores.
Calibration performance
There were insufficient studies reporting calibration performance for meta‐analysis.
Discussion
Summary of main results
We identified 14 multi‐domain prognostic models with two or more potentially modifiable predictors used in middle‐aged adults for predicting subsequent dementia. Seven models were externally validated and seven were development‐only models. The 14 models included a median of nine predictors (range six to 34); the median number of modifiable predictors was five (range two to 11). The most common modifiable predictors were diabetes, hypertension, obesity, and smoking. One model (CAIDE) had been externally validated in at least three cohorts of middle‐aged adults, and we were able to synthesise the reported C‐statistics in a meta‐analysis. We could not estimate the summary values of calibration owing to lack of data.
Cardiovascular Risk Factors, Aging, and Dementia (CAIDE)
The summary C‐statistic of CAIDE was 0.71 (95% CI 0.66 to 0.76) for incident clinical dementia (three studies) and 0.67 (95% CI 0.61 to 0.73) for incident dementia or cognitive impairment assessed using cognitive scores (three studies). C‐statistic values over 0.75 indicate that the model has good discrimination (i.e. ability to discriminate between people with and without the outcome during follow‐up; Debray 2017). CAIDE did not achieve this status. A previous systematic review classified discrimination values into three categories: high for values from 0.9 to 1, moderate for 0.7 to 0.9, and low for values below 0.7 (Hou 2019). Under this classification, CAIDE had moderate discrimination for incident dementia. The lower summary C‐statistic for the secondary outcome compared to the primary outcome may be due to variation in the individual diagnostic accuracies of the cognitive tests used in these studies.
The performance standards required for risk prediction also depend on the model's intended context and purpose. There is no recommended C‐statistic cut‐off for dementia prognostic models. The FRS model for cardiovascular risk prediction is considered to have good discrimination, although its reported accuracy ranges from 0.64 to 0.74 (Damen 2019). At present, most dementia prognostic models are designed to classify participants as high risk or low risk to determine their suitability for risk reduction interventions. Only two validation studies reported models' sensitivity, specificity, and thresholds for risk stratification (Chosy 2019; Reijmer 2011). Chosy 2019 reported a PPV of 14% at a cut‐off of 10, suggesting that the model may incorrectly categorise high‐risk individuals as low‐risk.
CAIDE performed better in the development study compared to the validation studies. This may be due to overfitting of the model to its development data and a small sample size, which makes the sample unrepresentative and more homogenous. The CAIDE development study did not report use of any methods for addressing the overfitting, such as internal validation or shrinkage analysis. In addition, model performance in the validation studies may be poor because there were few outcome events compared to the total number of predictors. As the events/predictors ratio in Virta 2013 was below 1:20, the usual analytical methods may have made inaccurate predictions.
We observed a high degree of heterogeneity in the meta‐analysis, which may be due to differences in participant characteristics (case‐mix variation), study characteristics (follow‐up time or outcome definition), model characteristics (number of predictors), statistical analysis, and risk of bias. The statistical heterogeneity may also be related to differences in predictor definitions between development and validation studies. There was a lack of harmonisation in the handling of predictors between the studies. For example, blood pressure was measured with participants in a seated position in the development study (Kivipelto 2006), and in supine position in one validation study (Exalto 2014). Validation studies should use the same definitions and cut‐off points as the development study wherever possible. The studies reported no sensitivity checks for assessing the impact of the difference in definitions, and there were too few studies to perform subgroup analyses of different predictor definitions.
Even in models with good discrimination, miscalibration can result in systematic over‐ or underestimation of risk estimates. The recommended calibration slope is 1, with an intercept of 0 (Calster 2019). The calibration intercept of Schaich 2021 was negative, which suggests the model overestimates the risk of future dementia, and the slope was greater than 1, which means the risk estimates given by the model may be too moderate. Exalto 2014 and Fayosse 2020 reported calibration using Hosmer‐Lemeshow goodness‐of‐fit, but this method alone is not considered appropriate for assessing calibration as the resulting P value is not indicative of the presence, nor size, of any miscalibration (Moons 2019). In addition, Fayosse 2020 reported an overall miscalibration for the model.
We cannot establish the generalisability and usefulness of CAIDE in clinical practice, as fewer than 10 studies provided data suitable for meta‐analysis of the primary and secondary outcomes, and the studies were conducted only in the USA, UK, and Finland. We cannot recommend inclusion of the model in clinical pathways at present owing to the uncertainty of the evidence; however, addressing the modifiable predictors in these models will likely have general health benefits regardless of dementia risk.
Other models with validation
LIBRA included more predictors but had a lower C‐statistic in the development study compared to CAIDE. This may be due to the difference in mean age (15 years older in LIBRA). Only Deckers 2020 validated the model in a younger age group, and it reported few outcome events. Our applicability concerns ratings were lower for the LIBRA studies compared to the CAIDE studies. As no studies reported calibration, we are uncertain about the error of the risk estimates given by LIBRA.
The development studies of FRS, ASCVD‐PCE, and FINDRISC reported higher C‐statistics compared to their validation studies. FRS had a moderate C‐statistic, whereas all other models that had been validated for a different use had poor discrimination. This may be because the FRS study had a larger sample size and higher ratio of events to predictors compared with the other studies. However, the calibration measure showed that the model lacked goodness‐of‐fit. FRS had the fewest predictors (three), which may limit its sensitivity to risk modifications.
ANU‐ADRI was the only model to include only self‐reported predictors. It had the highest number of modifiable predictors, but the lowest C‐statistic of all models with validation. Because the model was developed for use in older adults, it may be less reliable for predicting subsequent dementia in middle‐aged adults.
Development‐only models
We identified seven models without external validation; six reported a C‐statistic above 0.75 (Cremers 2020; Kim 2019; Li 2018a; Li 2018b; Park 2019; Walters 2016). The models described in Kim 2019, Park 2019 and Walters 2016 had a slope below 1, which suggests that the model‐predicted risk may be too extreme. If we use models with a slope below 1 for risk stratification, values assigned to people at high risk may be too high, and values assigned to people at low risk may be too low (Stevens 2020).
To ensure reliability, researchers should perform internal validation when developing a prognostic model. In five studies, the authors created a random development and validation data set from the original data as an internal validation method (Kim 2019; Li 2018a; Li 2018b; Park 2019; Walters 2016). This split‐sample technique is reliable only when the sample size is very large (ratio of outcome events to predictors above 40), and was ideal for Li 2018b, Park 2019, and Walters 2016; otherwise, a bootstrapping method is the better choice (Steyerberg 2001). Cremers 2020 applied a two‐fold cross‐validation method, using a random 50 per cent of the data to develop the model, and estimating its predictive performance in another randomly selected 50 per cent of the data from the same cohort. In bootstrapping, a set of samples are randomly drawn with replacement from the original dataset, with each sample being of the same size as the original dataset. The model is then developed and tested on each of these bootstrap samples, and the prediction accuracy is tested in the original dataset (Moons 2019). Ibarrondo 2022 used this method.
Three studies used machine learning methods for model development (Cremers 2020; Kim 2019; Li 2018a). Machine learning is a branch of artificial intelligence (AI) that identifies patterns in the source data. It is a flexible method for capturing associations from complex data with many predictors (e.g. Cremers 2020 had 34 predictors). However, unlike with traditional models, it can be difficult to present machine learning models as an equation or score. As no eligible validation studies used machine learning‐based tools, their clinical usability and practicality remain unclear. There are no specific reporting guidelines for machine learning‐based prognostic studies; an AI‐specific TRIPOD checklist and PROBAST tool is currently under development (Collins 2021).
Overall completeness, certainty of the evidence, and study limitations
Overall completeness of the data
There were serious gaps in the reporting of model development and validation studies. For models with external validation, only one study reported the calibration slope, intercept, or ratio of observed to expected events. Three studies did not report 95% CIs of C‐statistics. Only five of the seven development‐only models reported some form of calibration, and one study did not report the 95% CI of the C‐statistic. Although the C‐statistic is essential, clinicians often find it difficult to interpret and may prefer calibration values.
We could not calculate the ratio of outcome events to predictors for one study, as it did not report the number of outcome events. This ratio determines whether the model can achieve an expected predictive performance. Fourteen studies had missing data (either participant level data at follow‐ups or absence of entire data on some predictors) and so may have underestimated models' predictive performance. Eight studies excluded participants or predictors with missing data from the analysis instead of using recommended imputation methods. Most studies did not compare the characteristics of excluded versus included participants and the distribution of relevant variables. Seven development studies did not report the model equation, which is essential for future model validations.
Our inclusion of articles over 14 years (2006 to 2022) may help to explain many reporting deficiencies, as the methodological framework of prognostic model research is constantly evolving.
It is important to account for ethnic diversity in model validation. While three models (ASCVD‐PCE, CAIDE, and FSRP) were validated in a representative sample from a multi‐ethnic population (Exalto 2014; Schaich 2021), and one model used ethnicity as a predictor (ASCVD‐PCE), more extensive validation efforts are required to clarify the interplay of ethnicity with modifiable risk factors and its impact on model performance.
No studies assessed the predictive performance of any model in low‐income or middle‐income countries, or the clinical usefulness and cost‐effectiveness of any model. Issues of equity and access to healthcare systems in low‐ and middle‐income countries cannot be underestimated. Certain predictors, such as education and air pollution, may be more relevant in low‐ and middle‐income countries compared to high‐income countries. It may be challenging to apply models with certain predictors (e.g. APOEε4) in a resource‐poor setting. ANU‐ADRI was the only model to include only self‐reportable predictors. Such models will be beneficial for assessing dementia risk in a cost‐effective manner, especially in low‐ or middle‐income settings.
Certainty of the evidence
We evaluated the certainty of the evidence for the primary and secondary outcome in the only model with at least three external validation studies (CAIDE). We used the available GRADE description for overall prognosis studies (Iorio 2015), as there is no official GRADE guidance related to discriminative performance, and we were unable to apply GRADE for calibration owing to insufficient data (Foroutan 2022). Overall, there were concerns related to risk of bias and inconsistency, resulting in very low‐certainty evidence ratings for CAIDE. Most studies were at high risk of bias for the analysis domain. The C‐statistic estimates were inconsistent and thus unreliable. We could not assess publication bias using a funnel plot because there were too few studies.
Limitations of prognostic model studies
Most PROBAST ratings for the external validation studies were high because of concerns in the analysis domain related to lack of calibration measures, poor handling of missing data, univariate mode of predictor selection, lack of appropriate internal validation, and unnecessary categorisation of continuous predictors. Development and external validations studies of the same model often used different predictor definitions. The development‐only models varied in terms of reporting quality, the predictors involved, and the applicable settings. We cannot assess the clinical application of these models until they are externally validated.
In studies with longer follow‐up, a type of survival bias, where participants have to be alive to be included throughout, can affect model performance (Austin 2016). In addition, no models evaluated the time‐varying effect of the risk and protective factors, as they generally only involved one‐time collection of the predictor values and a single time point of measurement for the outcome. Participants' adherence to medications, life‐style modifications, and level of control of metabolic diseases can vary over time, increasing or decreasing their risk of dementia.
Limitations of the current review
We included only prognostic models with at least two of the modifiable risk factors listed in the Lancet Commission and WHO reports (Livingston 2020; WHO 2019). Models with fewer than two of these risk factors were ineligible, even if they included other potentially relevant risk factors or protective factors for dementia. As a result, we may have excluded studies that focused on newer relevant predictors, such as anticholinergic burden (Taylor 2021). Our definition of modifiable predictors included some risk factors that can only be modified at a societal level (e.g. air pollution). Other predictors, such as traumatic brain injury, have preventable aspects at a societal level (e.g. constructing better roads) and an individual level (e.g. wearing a helmet and following traffic rules).
We restricted our review to prognostic models used in middle‐aged cohorts; consequently, some development studies of models included in this review (e.g. ANU‐ADRI) and additional external validation studies of models included in this review were ineligible for inclusion.
We applied a minimum follow‐up duration of five years for the outcome of dementia. We may have identified additional studies if we considered a shorter follow‐up duration; however, because we are focusing on middle‐aged adults, and dementia typically occurs in older age, we felt that this time period was appropriate.
While we considered articles published in any language, the title and abstract had to be in English for screening purposes, and we may have missed some studies published in non‐English journals. Our selection was also specific to middle‐aged people, whereas most available models are for older adults.
Agreements and disagreements with other studies or reviews
We identified two systematic reviews that focused on a similar objective (Hou 2019; Tang 2015). Hou 2019 reviewed 61 articles that described dementia prognostic models, but most models were for older populations (39/61). As in our review, all included studies had to report measures of predictive performance. Hou 2019 identified only four studies that applied the CAIDE model in a middle‐aged cohort (versus eight studies in our review), possibly because the search was limited to PubMed and the search strategy was less comprehensive. Of the four studies included in Hou 2019, we included three in our review (Exalto 2014; Kivipelto 2006; Tynkkynen 2017), and excluded one, as it was not a multi‐domain model (Vuoksimaa 2016). Hou 2019 did not meta‐analyse any data. Tang 2015 applied no age restrictions and identified 21 studies, only four of which focused on middle‐aged adults. They conducted a quality assessment based on the Newcastle Ottawa scale. Tang 2015 did not perform meta‐analysis owing to the large variability observed across the studies. Consistent with our review, Hou 2019 and Tang 2015 recommended improvement in the methodology of model development, reporting of calibration, and further validation of existing models.
Another difference between this review and the previous reviews is that we used a predefined list of predictors as an inclusion criterion. Hou 2019 and Tang 2015 were less restrictive with regard to population age or length of follow‐up. Both systematic reviews were published before the PROBAST tool became available, so they lacked a rigorous assessment of risk of bias and applicability. Ours is the first systematic review to focus on prognostic models with relevant modifiable predictors for assessing future dementia risk in middle‐aged people.
Authors' conclusions
Implications for research
Our review identified 14 multi‐domain prognostic models used to predict risk of future dementia in middle‐aged adults, based on two or more of the modifiable risk factors identified by the World Health Organization (WHO) and Lancet Commission reports. Twelve of the 14 modifiable predictors of interest were present in the models. Future studies could investigate the remaining two predictors (hearing loss and air pollution). If evidence emerges in relation to new modifiable predictors, these should also be considered for integration into new or existing prognostic models.
Six models had too few validation studies for meta‐analysis, and seven models had no eligible external validation studies. Future research should validate these existing prognostic models in diverse cohorts (e.g. other ethnicities, low‐ and middle‐income countries).
Future studies should apply rigorous methods and report results using the CHARMS (CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies) or TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) checklist. Researchers could consider additional checklists, such as PROBAST (Prediction model Risk Of Bias ASsessment Tool) for artificial intelligence, based on the specific prediction methods. The minimum reported information in future prognostic model studies should include baseline demographics, inclusion/exclusion criteria, number of outcome events, follow‐up time, sample size calculation, handling of missing data, discrimination, calibration (intercept and slope), and confidence intervals. In development studies, internal validation improves model performance reliability. When externally validating existing models, researchers should describe possible differences between the definitions of predictors in the development and validation studies and conduct sensitivity analyses to assess the impact of these different definitions where possible.
This review highlighted the need to apply prognostic models in clinical practice and evaluate their cost‐effectiveness.
Implications for practice
We described seven multi‐domain prognostic models (i.e. models involving two or more of the modifiable risk factors identified by the WHO and Lancet Commission reports) that had been externally validated in middle‐aged adults for the prediction of subsequent dementia (CAIDE, LIBRA, ANU‐ADRI, ASCVD‐PCE, FRS, FINDRISC, and FSRP). There were sufficient validation studies of CAIDE to enable meta‐analysis, but the summary discriminative performance of the model was unreliable for prediction of dementia. Researchers could make use of prognostic models to identify participants for randomised controlled trials of dementia risk reduction interventions. Risk scores derived from prognostic models could also help to encourage adherence to lifestyle modification programmes if participants receive information on risk score changes over time. Prognostic models assist in long‐term planning of health and social services. Several modifiable predictors included in the prognostic models are also associated with incidence of cardiovascular disease. This highlights the importance of addressing these modifiable risk factors at the community level.
History
Protocol first published: Issue 6, 2021
Acknowledgements
We thank Ms Sue Marcus, Managing Editor, Cochrane Dementia and Cognitive Improvement Group (CDCIG), for providing valuable support in the preparation of this review.
We thank Anna Noel‐Storr for her assistance in developing the search strategy, conducting the database search (8 June 2021 and 6 June 2022), and conducting first pass screening of retrieved studies
We would like to thank peer reviewers Nina Kreuzberger, Janice Ranson and Carrie Stewart and consumer reviewers Cahtie Hofstetter and Shibley Rahman for their comments and feedback.
We would like to thank Julia Turner for copy editing this review.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946‐present (Ovid SP) [Date of most recent search: 06 June 2022] |
1. Cohort Studies/ 2. Follow‐Up Studies/ 3. Incidence/ 4. Incidental Findings/ 5. Longitudinal Studies/ 6. Predictive Value of Tests/ 7. Prognosis/ 8. Risk Assessment/ 9. Risk Score/ 10. Risk Factors/ 11. course*.ti,ab. 12. cohort.ti,ab. 13. (decision* and model*).ti,ab. 14. natural history.ti,ab. 15. follow‐up stud*.ti,ab. 16. prognos*.ti,ab. 17. predict*.ti. 18. relative risk.ti,ab. 19. (risk profile or (risk adj2 model*)).ti,ab. 20. validat*.ti. 21. scor*.ti,ab. 22. or/1‐21 23. Middle Aged/ 24. middle age.ti,ab. 25. middle aged.ti,ab. 26. midlife.ti,ab. 27. mid‐life.ti,ab. 28. nondemented.ti,ab. 29. non‐demented.ti,ab. 30. (cognitively healthy or cognitively normal).ti,ab. 31. or/23‐30 32. Air Pollution/ 33. Alcoholics/ 34. Life Style/ 35. Blood Pressure/ 36. Body Weight/ 37. Body Mass Index/ 38. Brain Injuries, Traumatic/ 39. Cognitive Reserve/ 40. Depression/ 41. Diabetes Mellitus/ 42. Diet/ 43. Educational Status/ 44. Exercise/ 45. Hypertension/ 46. Nutritional status/ 47. Obesity/ 48. Smoking/ 49. Smoking Cessation/ 50. Social Isolation/ 51. Social Marginalization/ 52. air pollution.ti,ab. 53. alcohol*.ti,ab. 54. body weight.ti,ab. 55. brain injur*.ti,ab. 56. (cognit* activit* or cognitive train* or cognitive stimulation).ti,ab. 57. cognitive reserve.ti,ab. 58. depression.ti,ab. 59. depressive.ti,ab. 60. depressed.ti,ab. 61. diet*.ti,ab. 62. diabetes.ti,ab. 63. education*.ti,ab. 64. hypertensi*.ti,ab. 65. isolated.ti,ab. 66. life style.ti,ab. 67. lifestyle.ti,ab. 68. lonely.ti,ab. 69. loneliness.ti,ab. 70. nutrition*.ti,ab. 71. obesity.ti,ab. 72. overweight.ti,ab. 73. physical activit*.ti,ab. 74. physical exercis*.ti,ab. 75. sedentary.ti,ab. 76. social isolation.ti,ab. 77. smoking.ti,ab. 78. smoker.ti,ab. 79. TBI.ti,ab. 80. or/32‐79 81. exp Dementia/ 82. dement*.mp. 83. alzheimer*.mp. 84. (VaD or VCI or "vascular cognit* impair*").mp. 85. (lewy* adj2 bod*).mp. 86. (LBD or DLB).mp. 87. (FTD or FTLD or frontotemp* or "fronto‐temp*").mp. 88. neurocognitive*.mp. 89. or/81‐88 90. CAIDE.ti,ab. 91. ANU‐ADRI.ti,ab. 92. BDSI.ti,ab. 93. (DRS and dement*).ti,ab. 94. LIBRA.ti,ab. 95. "Cardiovascular Risk Factors, Aging and Dementia".ti,ab. 96. "Alzheimer's Disease Risk Index".ti,ab. 97. "Brief Dementia Screening Indicator".ti,ab. 98. "Dementia Risk Score".ti,ab. 99. "Lifestyle for Brain Health".ti,ab. 100. or/90‐99 101. 22 and 31 and 80 and 89 102. 100 or 101 |
Jun 2021: 8991 Jun 2022: 722 |
| Embase 1974‐2022 June 05 (Ovid SP) [Date of most recent search: 06 June 2022] |
1. Cohort Studies/ 2. Follow‐Up Studies/ 3. Incidence/ 4. Incidental Findings/ 5. Longitudinal Studies/ 6. Predictive Value of Tests/ 7. Prognosis/ 8. Risk Assessment/ 9. Risk Score/ 10. Risk Factors/ 11. course*.ti,ab. 12. cohort.ti,ab. 13. (decision* and model*).ti,ab. 14. natural history.ti,ab. 15. follow‐up stud*.ti,ab. 16. prognos*.ti,ab. 17. predict*.ti. 18. relative risk.ti,ab. 19. (risk profile or (risk adj2 model*)).ti,ab. 20. validat*.ti. 21. scor*.ti,ab. 22. or/1‐21 23. Middle Aged/ 24. middle age.ti,ab. 25. middle aged.ti,ab. 26. midlife.ti,ab. 27. mid‐life.ti,ab. 28. nondemented.ti,ab. 29. non‐demented.ti,ab. 30. (cognitively healthy or cognitively normal).ti,ab. 31. or/23‐30 32. Air Pollution/ 33. Alcoholics/ 34. Life Style/ 35. Blood Pressure/ 36. Body Weight/ 37. Body Mass Index/ 38. Brain Injuries, Traumatic/ 39. Cognitive Reserve/ 40. Depression/ 41. Diabetes Mellitus/ 42. Diet/ 43. Educational Status/ 44. Exercise/ 45. Hypertension/ 46. Nutritional status/ 47. Obesity/ 48. Smoking/ 49. Smoking Cessation/ 50. Social Isolation/ 51. Social Marginalization/ 52. air pollution.ti,ab. 53. alcohol*.ti,ab. 54. body weight.ti,ab. 55. brain injur*.ti,ab. 56. (cognit* activit* or cognitive train* or cognitive stimulation).ti,ab. 57. cognitive reserve.ti,ab. 58. depression.ti,ab. 59. depressive.ti,ab. 60. depressed.ti,ab. 61. diet*.ti,ab. 62. diabetes.ti,ab. 63. education*.ti,ab. 64. hypertensi*.ti,ab. 65. isolated.ti,ab. 66. life style.ti,ab. 67. lifestyle.ti,ab. 68. lonely.ti,ab. 69. loneliness.ti,ab. 70. nutrition*.ti,ab. 71. obesity.ti,ab. 72. overweight.ti,ab. 73. physical activit*.ti,ab. 74. physical exercis*.ti,ab. 75. sedentary.ti,ab. 76. social isolation.ti,ab. 77. smoking.ti,ab. 78. smoker.ti,ab. 79. TBI.ti,ab. 80. or/32‐79 81. exp Dementia/ 82. dement*.mp. 83. alzheimer*.mp. 84. (VaD or VCI or "vascular cognit* impair*").mp. 85. (lewy* adj2 bod*).mp. 86. (LBD or DLB).mp. 87. (FTD or FTLD or frontotemp* or "fronto‐temp*").mp. 88. neurocognitive*.mp. 89. or/81‐88 90. CAIDE.ti,ab. 91. ANU‐ADRI.ti,ab. 92. BDSI.ti,ab. 93. (DRS and dement*).ti,ab. 94. LIBRA.ti,ab. 95. "Cardiovascular Risk Factors, Aging and Dementia".ti,ab. 96. "Alzheimer's Disease Risk Index".ti,ab. 97. "Brief Dementia Screening Indicator".ti,ab. 98. "Dementia Risk Score".ti,ab. 99. "Lifestyle for Brain Health".ti,ab. 100. or/90‐99 101. 22 and 31 and 80 and 89 102. 100 or 101 |
Jun 2021: 10046 Jun 2022: 1539 |
| PSYCINFO 1806‐June week 1 2022 (Ovid SP) [Date of most recent search: 06 June 2022] |
1. exp Longitudinal Studies/ 2. exp Prediction/ 3. exp Prognosis/ 4. exp Risk Assessment/ 5. exp Risk Factors/ 6. course*.ti,ab. 7. cohort.ti,ab. 8. (decision* and model*).ti,ab. 9. natural history.ti,ab. 10. follow‐up stud*.ti,ab. 11. prognos*.ti,ab. 12. predict*.ti. 13. relative risk.ti,ab. 14. (risk profile or (risk adj2 model*)).ti,ab. 15. validat*.ti. 16. scor*.ti,ab. 17. or/1‐16 18. middle age.ti,ab. 19. middle aged.ti,ab. 20. midlife.ti,ab. 21. mid‐life.ti,ab. 22. nondemented.ti,ab. 23. non‐demented.ti,ab. 24. (cognitively healthy or cognitively normal).ti,ab. 25. or/18‐24 26. Pollution/ 27. Health Behavior/ or Lifestyle/ 28. Blood Pressure/ 29. Body Weight/ 30. Body Mass Index/ 31. Traumatic Brain Injury/ 32. Cognitive Reserve/ 33. "Depression (Emotion)"/ 34. Diabetes Mellitus/ 35. Diets/ 36. Educational Attainment Level/ or Educational Background/ 37. Exercise/ 38. Hypertension/ 39. Nutritional Deficiencies/ or Nutrition/ 40. Obesity/ 41. Tobacco Smoking/ 42. Smoking Cessation/ 43. Social Isolation/ 44. At Risk Populations/ or Marginalization/ 45. air pollution.ti,ab. 46. alcohol*.ti,ab. 47. body weight.ti,ab. 48. brain injur*.ti,ab. 49. (cognit* activit* or cognitive train* or cognitive stimulation).ti,ab. 50. cognitive reserve.ti,ab. 51. depression.ti,ab. 52. depressive.ti,ab. 53. depressed.ti,ab. 54. diet*.ti,ab. 55. diabetes.ti,ab. 56. education*.ti,ab. 57. hypertensi*.ti,ab. 58. isolated.ti,ab. 59. life style.ti,ab. 60. lifestyle.ti,ab. 61. lonely.ti,ab. 62. loneliness.ti,ab. 63. nutrition*.ti,ab. 64. obesity.ti,ab. 65. overweight.ti,ab. 66. physical activit*.ti,ab. 67. physical exercis*.ti,ab. 68. sedentary.ti,ab. 69. social isolation.ti,ab. 70. smoking.ti,ab. 71. smoker.ti,ab. 72. TBI.ti,ab. 73. or/26‐72 74. exp Dementia/ 75. dement*.mp. 76. alzheimer*.mp. 77. (VaD or VCI or "vascular cognit* impair*").mp. 78. (lewy* adj2 bod*).mp. 79. (LBD or DLB).mp. 80. (FTD or FTLD or frontotemp* or "fronto‐temp*").mp. 81. neurocognitive*.mp. 82. or/74‐81 83. CAIDE.ti,ab. 84. ANU‐ADRI.ti,ab. 85. BDSI.ti,ab. 86. (DRS and dement*).ti,ab. 87. LIBRA.ti,ab. 88. "Cardiovascular Risk Factors, Aging and Dementia".ti,ab. 89. "Alzheimer's Disease Risk Index".ti,ab. 90. "Brief Dementia Screening Indicator".ti,ab. 91. "Dementia Risk Score".ti,ab. 92. "Lifestyle for Brain Health".ti,ab. 93. or/83‐92 94. 17 and 25 and 73 and 82 95. 93 or 94 |
Jun 2021: 2088 Jun 2022: 181 |
| 4. Web of Science core collection [Date of most recent search: 06 June 2022] |
Search 1 "cohort study" OR "follow‐up study" OR "risk profile" OR "relative risk" OR prognosis OR prognostic OR "disease course" OR "risk factor*" OR "risk assessment" OR incidence OR "longitudinal study" OR "predictive value" OR "decision model" "middle‐aged" OR "middle age" OR midlife OR "mid‐life" OR nondemented OR "non demented" OR "cognitively healthy" OR "cognitively normal" pollution OR diet OR "body weight" OR obesity OR depression OR exercise OR smoking OR alcoholic OR hypertension OR isolation OR marginalisation OR marginalization OR "brain injury" OR "cognitive reserve" OR diabetes OR "nutritional status" OR education dementia OR alzheimer* OR neurocognitive* OR "lewy bod" OR frontotemp* OR "fronto‐temp*" Search 2 (CAIDE OR ANU‐ADRI OR BDSI OR (DRS and dement*) OR LIBRA OR "Cardiovascular Risk Factors, Aging and Dementia" OR "Alzheimer's Disease Risk Index" OR "Brief Dementia Screening Indicator" OR "Dementia Risk Score" OR "Lifestyle for Brain Health") |
Jun 2021: 2456 + 1440 Jun 2022: 171 |
| 5. CINAHL (EBSCOhost) [Date of most recent search: 06 June 2022] |
S1 (MH "Prospective Studies") S2 (MH "Postexposure Follow‐Up") S3 (MH "Incidence") S4 (MH "Predictive Value of Tests") OR (MH "Predictive Validity") S5 (MH "Prognosis") S6 (MH "Risk Assessment") S7 (MH "Risk Factors") S8 TX "disease course" S9 AB cohort* S10 decision* AND model* S11 "natural history" S12 follow‐up stud* S13 prognos* S14 TI predict* S15 "relative risk" S16 "risk profile" S17 "risk model*" S18 TI validat* S19 AB score* S20 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 S21 (MH "Middle Age") S22 TX "middle age" S23 TX "middle aged" S24 TX midlife S25 TX "mid‐life" S26 TX nondemented S27 TX non‐demented S28 TX "cognitively healthy" S29 TX "cognitively normal" OR "normal cognition" S30 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 S31 (MH "Air Pollution") OR (MH "Air Pollutants, Environmental") OR (MH "Air Pollutants, Occupational") S32 (MH "Alcoholics") OR (MH "Alcohol Amnestic Disorder") S33 (MH "Life Style") OR (MH "Life Style, Sedentary") S34 (MH "Blood Pressure") S35 (MH "Body Weight") S36 (MH "Body Mass Index") S37 (MH "Brain Injuries") S38 (MH "Depression") S39 (MH "Diabetes Mellitus") OR (MH "Diabetes Mellitus, Type 2") OR (MH "Diabetes Mellitus, Type 1") S40 (MH "Diet") S41 (MH "Educational Status") OR (MH "Health Status Disparities") S42 (MH "Exercise") S43 (MH "Hypertension") S44 (MH "Nutritional Status") S45 (MH "Obesity") S46 (MH "Smoking") OR (MH "Smoking Cessation") S47 (MH "Social Isolation") S48 (MH "Social Identity") OR (MH "Social Environment") S49 TX "air pollution" S50 TX alcohol* S51 TX "body weight" S52 TX "brain injur*" S53 TX "cognit* activit*" OR "cognitive train*" OR "cognitive stimulation" S54 TX "cognitive reserve" S55 TX depression S56 TX depressive S57 TX depressed S58 TX diabetes S59 TX diet* S60 TX education* S61 TX hypertensi* S62 TX isolated S63 TX "life style" S64 TX lifestyle S65 TX lonely S66 TX loneliness S67 TX nutrition* S68 TX obesity S69 TX overweight S70 TX physical activit* S71 TX "physical exercis*" S72 TX sedentary S73 TX "social* isolat*" S74 TX smoking OR smoker S75 TX TBI S76 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 S77 (MH "Dementia+") S78 TX dement* S79 TX alzheimer* S80 TX VaD or VCI or "vascular cognit* impair*" S81 TX "lewy* bod*" S82 TX LBD OR DLB S83 TX FTD OR FTLD OR frontotemp* OR "fronto‐temp*" S84 TX neurocognitive* S85 S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83 OR S84 S86 TX CAIDE S87 TX ANU‐ADRI S88 TX BDSI S89 TX DRS AND dement* S90 TX LIBRA S91 TX "Cardiovascular Risk Factors, Aging and Dementia" S92 TX "Alzheimer's Disease Risk Index" S93 TX "Brief Dementia Screening Indicator" S94 TX "Dementia Risk Score" S95 TX "Lifestyle for Brain Health" S96 S86 OR S87 OR S88 OR S89 OR S90 OR S91 OR S92 OR S93 OR S94 OR S95 S97 S20 AND S30 AND S76 AND S85 S98 S96 OR S97 |
Jun 2021: 9173 Jun 2022: 225 |
| TOTAL before de‐duplication | Jun 2021: 34,194 Jun 2022: 2828 TOTAL: 37,032 |
|
Appendix 2. Relevant items to extract from individual studies in a systematic review of prediction models for purposes of description or assessment of the risk of bias or applicability
Relevant items to extract from individual studies in a systematic review of prediction models for purposes of description or assessment of the risk of bias or applicability (Moons 2014).
| Domain | Key items | General | Applicability | Risk of bias |
| Source of data | Source of data (e.g. cohort, case‐control, randomised trial participants, or registry data) | X | X | |
| Participants | Participant eligibility and recruitment method (e.g. consecutive participants, location, number of centres, setting, inclusion and exclusion criteria) | X | X | X |
| Participant description | X | X | ||
| Details of treatments received, if relevant | X | X | ||
| Study dates | X | X | ||
| Outcomes to be predicted | Definition and method for measurement of outcome | X | X | |
| Was the same outcome definition (and method for measurement) used in all participants? | X | |||
| Type of outcome (e.g. single or combined endpoints) | X | X | ||
| Was the outcome assessed without knowledge of the candidate predictors (i.e. blinded)? | X | |||
| Were candidate predictors part of the outcome (e.g. in panel or consensus diagnosis)? | X | |||
| Time of outcome occurrence or summary of duration of follow‐up | X | |||
| Candidate predictors | Number and type of predictors (e.g. demographics, participant history, physical examination, additional testing, disease characteristics) | X | ||
| Definition and method for measurement of candidate predictors | X | X | ||
| Timing of predictor measurement (e.g. at participant presentation, at diagnosis, at treatment initiation) | X | |||
| Were predictors assessed blinded for outcome, and for each other (if relevant)? | X | |||
| Handling of predictors in the modelling (e.g. continuous, linear, non‐linear transformations or categorised) | X | |||
| Sample size | Number of participants and number of outcomes/events | X | ||
| Number of outcomes/events in relation to the number of candidate predictors (Events Per Variable) | X | |||
| Missing data | Number of participants with any missing value (include predictors and outcomes) | X | X | |
| Number of participants with missing data for each predictor | X | X | ||
| Handling of missing data (e.g. complete‐case analysis, imputation, or other methods) | X | |||
| Model development | Modelling method (e.g. logistic, survival, neural networks, or machine learning techniques) | X | ||
| Modelling assumptions satisfied | X | |||
| Method for selection of predictors for inclusion in multi‐variable modelling (e.g. all candidate predictors, pre‐selection based on unadjusted association with the outcome) | X | |||
| Method for selection of predictors during multi‐variable modelling (e.g. full model approach, backward or forward selection) and criteria used (e.g. P value, Akaike Information Criterion) | X | |||
| Shrinkage of predictor weights or regression coefficients (e.g. no shrinkage, uniform shrinkage, penalised estimation) | X | X | ||
| Model performance | Calibration (calibration plot, calibration slope, Hosmer‐Lemeshow test) and Discrimination (C‐statistic, D‐statistic, log‐rank) measures with confidence intervals | X | ||
| Classification measures (e.g. sensitivity, specificity, predictive values, net reclassification improvement) and whether a priori cut points were used | X | |||
| Model Evaluation | Method used for testing model performance: development dataset only (random split of data, resampling methods, e.g. bootstrap or cross‐validation, none) or separate external validation (e.g. temporal, geographical, different setting, different investigators) | X | ||
| In case of poor validation, whether model was adjusted or updated (e.g. intercept recalibrated, predictor effects adjusted, or new predictors added) | X | X | ||
| Results | Final and other multi‐variable models (e.g. basic, extended, simplified) presented, including predictor weights or regression coefficients, intercept, baseline survival, model performance measures (with standard errors or confidence intervals) | X | X | |
| Any alternative presentation of the final prediction models, e.g. sum score, nomogram, score chart, predictions for specific risk subgroups with performance | X | X | ||
| Comparison of the distribution of predictors (including missing data) for development and validation datasets | X | |||
| Interpretation and Discussion | Interpretation of presented models (confirmatory, i.e. model useful for practice versus exploratory, i.e. more research needed) | X | X | |
| Comparison with other studies, discussion of generalisability, strengths and limitations | X | X | ||
| Author correspondence | Details of correspondence with study authors for additional information or clarification of queries |
Appendix 3. The predictor terms used in the included studies for key domains of predictors.
| Predictor | Study/studies | Definitiona |
| Less education | Andrews 2017; Chosy 2019; Exalto 2014; Fayosse 2020; Kivipelto 2006; Reijmer 2011 | Self‐reported number of years of education |
| Cremers 2020 | Categorised into 7 categories, ranging from primary education only to university level | |
| Schaich 2021 | ≥ high school, 9 to 11 years, less than 9 years | |
| Schiepers 2017 | Educational level categorised into 'low' (< 8 years of formal education) and 'average/high (≥ 8 years) | |
| Virta 2013 | Years of formal schooling (0–6 years, 7–9 years, ≥ 10 years) | |
| Traumatic brain injury | Andrews 2017; Exalto 2014 | Self‐reported history of traumatic brain injury with loss of consciousness |
| Hypertension | Chosy 2019 | Direct measurement of systolic BP |
| Cremers 2020 | BP was measured twice. Antihypertensive medication was obtained by using questionnaires and by checking the medication cabinets of the participants. | |
| Deckers 2020 | Hypertension defined as systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg, measured by a trained nurse | |
| Exalto 2014 | Systolic BP measured according to standard procedures in the supine position | |
| Fayosse 2020 | Systolic BP was taken as the average of 2 measurements (Hawksley random‐zero sphygmomanometer) with the participant in a sitting position after 5 minutes' rest. Treated hypertension was determined using chapters 2.2 to 2.6 of the British National Formulary. | |
| Kivipelto 2006 | Systolic and diastolic BP was measured from the right arm of participants after they had been seated for 5 minutes. | |
| McGrath 2022 | Systolic BP defined as the mean of 2 physician‐recorded measurements taken from the participant's left arm, with the participant sitting. Antihypertensive medication use was self‐reported and verified using prescription data and pill bottles when available. | |
| Park 2019 | Direct measurement of systolic BP and diastolic BP (prehypertensive, stage I, and stage II) | |
| Reijmer 2011 | Systolic and diastolic BP measured in the right arm with a random‐zero sphygmomanometer while participants were sitting; investigators calculated the mean of duplicate measurements. | |
| Schaich 2021 | Resting brachial systolic BP measured using an automated Dinamap Monitor Pro 100 device (GE Medical Systems Ltd, Buckinghamshire, UK) with participant seated; investigators took 3 measurements and used the mean of the 2nd and 3rd measurements. | |
| Schiepers 2017 | High BP (i.e. mean systolic BP ≥ 140 mmHg or mean diastolic BP ≥ 90 mmHg) or use of antihypertensive medication | |
| Tynkkynen 2017 | BP measured twice from the right arm after 5 minutes of sitting using a mercury sphygmomanometer | |
| Virta 2013 | Information on blood pressure and cholesterol level was enquired with the question "Has a nurse or a doctor measured your blood pressure/has your cholesterol level been measured during the last 5 years?" The answer options included: has not been measured, subject doesn't recall if has been measured, found normal, and found elevated. Blood pressure was taken to be high even if it had been found to be only slightly elevated. Also subjects who reported physician‐diagnosed hypertension or use of antihypertensive. | |
| Vos 2017 | Based on the medical history or if systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg | |
| Alcohol intake | Andrews 2017 | Calculated according to National Health and Medical Research Council 2001 guidelines using number of drinks per week, with light to moderate intake in males being 0.25–20.5 drinks per week and in females being 0.25–13.5 drinks per week |
| Deckers 2020 | Low‐to‐moderate alcohol consumption was based on categorised frequency of alcohol use. | |
| Li 2018a | Persons who self‐reported consumption of beer, wine, or cocktail were classified as consumers of alcohol. | |
| Schiepers 2017 | Alcohol consumption measured in standard units per week was categorised into 'low/moderate' (< 14 units/week) or 'other'. The latter category included both non‐drinkers and individuals drinking at least 14 units of alcohol per week. | |
| Vos 2017 | Low/moderate alcohol use – based on medical history, ≤ 40 g/week, < 1 glass wine or 1 beer/day, < 14 glasses/week, ≤ 0.25 L wine per day | |
| Walters 2016 | History of heavy alcohol use (> 56 units/week for men, 49 units/week for women), or a read‐code entry in their medical records indicating an alcohol problem | |
| Obesity | Chosy 2019; Cremers 2020; Park 2019; Reijmer 2011; Schaich 2021 | BMI as direct measurement by health professionals |
|
Deckers 2020; Schiepers 2017; Virta 2013 |
BMI threshold of 30 Kg/m2 | |
| Li 2018a | BMI threshold of 27 Kg/m2 | |
| Fayosse 2020 | Weight was measured in underwear to the nearest 0.1 kg on digital Soehnle electronic scales (Leifheit AS, Nassau, Germany). With the participant standing erect in bare feet with head in the Frankfurt plane, height was measured to the nearest 1 mm using a stadiometer. BMI (kg/m2). | |
| Smoking | Andrews 2017; Fayosse 2020; Schaich 2021 | Self‐reported smoking status as current smoker, past smoker, or never‐smoker |
| Deckers 2020 | Participants were divided into ever smokers and never‐smokers. |
|
| Li 2018a | Smoking status classified as either smoker or non‐smoker at the time of examination | |
| Park 2019 | Current smoking status (currently smoking or has smoked > 100 cigarettes in their life) | |
| Schiepers 2017 | Current smoking ('yes' or 'no') | |
| Vos 2017 | Current smoking based on medical history | |
| Walters 2016 | Smoking status up to 5 years prior to baseline (current, non‐smoker, or ex‐smoker) | |
| McGrath 2022 | Participant self‐reported smoking within the previous 12 months at the time of risk factor measurement | |
| Depression | Andrews 2017 | PHQ‐9, following the coding algorithm provided in the PHQ‐9 instruction manual, with a score > 10 used as a cut‐off |
| Deckers 2020 | A cut‐off point for depressive symptoms was created based on the sum of scores for 2 questions related to feelings of hopelessness. | |
| Exalto 2014 | Based on the question "Do you often feel unhappy or depressed?" | |
| Park 2019 | Recorded diagnosis of depression | |
| Schiepers 2017 | Depressive state measured with the Dutch version of the depression subscale of the SCL90 | |
| Vos 2017 | Based on medications and the following instruments: MADRS, GDS, CESD, or SCL90 | |
| Walters 2016 | Current (in 12 months prior to baseline) depression diagnosis/treatment with antidepressant medication | |
| Social isolation | Andrews 2017 | Constructed from 4 domains for marital status, size of social network, quality of social network, level of social activities |
| Walters 2016 | Local area deprivation score – quintiles of Townsend Index | |
| Physical activity | Andrews 2017 | Combined self‐reported number of hours performing mild, moderate, and vigorous activities, weighted by multiples of 1, 2 and 3, respectively |
| Chosy 2019 | Self‐reported average time spent at different levels of physical activity (none to heavy) weighted by the level of oxygen consumption associated with each activity. | |
| Deckers 2020 | Persons who engaged in physical activity ≥ twice/week, lasting ≥ 20–30 minutes each occasion, and causing sweating and breathlessness, were regarded as physically active. | |
| Fayosse 2020 | Duration in moderate to vigorous physical activity, ≥ 4 hours/week | |
| Kivipelto 2006 | Participants were regarded as being active or inactive on the basis of the frequency of leisure time physical activity. Those who were engaged in physical activity ≥ twice/week, lasting ≥ 20–30 min each time, and causing sweating and breathlessness, were regarded as active, and others as inactive. | |
| Li 2018a | More physical activity defined by those who reported engaging in more moderate (or greater) activity than slight (or none) activity on a daily basis | |
| Park 2019 | Regular exercise (> 30 minutes a week) | |
| Reijmer 2011 | Regular sport activity | |
| Schiepers 2017 | Physical activity recorded as 'average number of hours up and about per day'. Participants in the lowest terciles were classified as physically inactive. | |
| Virta 2013 | Subjects exercising ≥ 6 times/month for a mean duration of 30 min with a mean intensity corresponding to at least vigorous walking were classified as conditioners. Those not partaking in leisure time physical activity were classified as sedentary. Other subjects were classified as occasional exercisers. In addition, the subjects were divided into quartiles according to their leisure time activity MET index values. | |
| Schaich 2021 | Physical activity (kcal/MET/week) assessed using a survey | |
| Diabetes mellitus | Andrews 2017 | Self‐reported history of diabetes |
| Park 2019; Vos 2017; Walters 2016 | Recorded medical diagnosis of diabetes | |
| Cremers 2020 | Fasting serum glucose level (> 7.0 mmol/L) or, if unavailable, non‐fasting serum glucose level (≥ 11.1 mmol/L) or the use of anti‐diabetic medication | |
| Deckers 2020 | Self‐reports of diagnoses made by a physician and diagnoses from the Finnish Hospital Discharge Register | |
| Exalto 2014 | Diabetes mellitus was defined by having 1 of the following: 1) self‐report of physician‐diagnosed diabetes mellitus, 2) use of insulin, or oral hypoglycaemic agents, 3) a fasting glucose level ≥ 140 mg/dL, or a non‐fasting glucose level ≥ 200 mg/dL. | |
| Fayosse 2020 | Diabetes was defined by fasting glucose ≥ 7.0 mmol/L or reported doctor‐diagnosed diabetes or the use of diabetes medication. | |
| Li 2018b | Duration of diabetes, variation in Fasting Plasma Glucose, variation in HbA1c, antidiabetic medication use. | |
| Schaich 2021 | Diabetes was defined as fasting glucose ≥ 126 mg/dL or use of hypoglycaemic medication. | |
| McGrath 2022 | Diabetes mellitus was defined as a fasting blood glucose ≥ 7 mmol/L, random blood glucose ≥ 11.1 mmol/L, or use of insulin or oral hypoglycaemic medications. | |
| Diet | Deckers 2020 | Adherence to a healthy diet was based on cut‐offs of the CAIDE Healthy Diet Index. |
| Fayosse 2020 | Frequency of fruit and vegetables (8‐point scale categorised as 'less than daily' or 'daily' | |
| Cognitive activity | Andrews 2017 | Assessed as the number of cognitive activities undertaken in the last 6 months, comprising reading, writing, playing games or attending cultural events |
| Schiepers 2017 | Cognitive activity was measured by calculating the average number of hours per week spent on reading or mental exercise (e.g. gaming). Sum scores in the highest terciles indicated high cognitive activity. |
BMI: Body Mass Index; BP: Blood Pressure; CAIDE: Cardiovascular risk factors, Ageing and Dementia; CESD: Centre for Epidemiological Studies Depression; GDS: Geriatric Depression Scale; MADRS: Montgomery‐Asberg Depression Rating Scale; MET: metabolic equivalent; PHQ‐9: Patient Health Questionnaire; SCL90: Symptom Checklist‐90.
a As reported by study authors. We have included the definition only if the study described the method of measurement or at least the cut‐off used for the predictor.
Appendix 4. GRADE description of the prognostic models.
GRADE assessment for CAIDE for incident clinical diagnosis of dementia of any subtype
Risk of bias: we downgraded the certainty of the evidence by one level due to high risk of bias across multiple domains.
Inconsistency: we downgraded the certainty of the evidence by one level due to a high degree of heterogeneity.
Imprecision: the confidence intervals overlapped across the studies and were close to the threshold of 0.75.
Indirectness: we downgraded the certainty of the evidence by one level due to applicability concerns (Exalto 2014; Fayosse 2020).
Publication bias: there were too few studies to draw a funnel plot.
The overall GRADE rating was of very low certainty.
GRADE assessment for CAIDE for incident dementia or cognitive impairment assessed using cognitive scores.
Risk of bias: we downgraded the certainty of the evidence by one level due to high risk of bias across multiple domains.
Inconsistency: we downgraded the certainty of the evidence by one level due to a high degree of heterogeneity.
Imprecision: we downgraded the certainty of the evidence by one level as there was no overlapping of the confidence intervals.
Indirectness: we downgraded the certainty of the evidence by one level due to applicability concerns (Chosy 2019; Virta 2013)
Publication bias: there were too few studies to draw a funnel plot.
The overall GRADE rating was of very low certainty.
Appendix 5. Summary of PROBAST tool for risk of bias and applicability assessment.
See Moons 2019.
| Participants | Predictors | Outcome | Analysis |
Signalling questionsa:
|
Signalling questionsa:
|
Signalling questionsa:
|
Signalling questionsa:
|
| Risk of bias introduced by selection of participantsb | Risk of bias introduced by predictors or their assessmentb | Risk of bias introduced by the outcome or its determinationb | Risk of bias introduced by the analysisb |
| Concern that the included participants and setting do not match the review questionb | Concern that the definition, assessment or timing of predictors in the model do not match the review questionb | Concern that the outcome, its definition, timing or determination do not match the review questionb | — |
| Footnotes: a Possible responses are yes, probably yes, probably no, no, and no information. b Possible judgements, based on the answers to the signalling questions, are low, high, and unclear. | |||
Data and analyses
Comparison 1. Meta‐analysis of C‐statistics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Discrimination value (CAIDE with primary outcome) | 3 | C‐statistics (IV, Random, 95% CI) | 0.71 [0.66, 0.76] | |
| 1.2 Discrimination value (CAIDE with secondary outcome) | 3 | C‐statistics (IV, Random, 95% CI) | 0.67 [0.61, 0.73] |
1.1. Analysis.

Comparison 1: Meta‐analysis of C‐statistics, Outcome 1: Discrimination value (CAIDE with primary outcome)
1.2. Analysis.

Comparison 1: Meta‐analysis of C‐statistics, Outcome 2: Discrimination value (CAIDE with secondary outcome)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrews 2017.
| Study characteristics | ||
| General Information | Model name: ANU‐ADRI Type of study: external validation Aim of the study: to investigate the association between the ANU‐ADRI and a late‐onset Alzheimer's disease genetic risk score and incident MCI/dementia, and extend the model using multi‐state models (MSMs) to account for backward transitions between cognitive states (i.e. cognitive recovery) and competing risks (i.e. dementia and death). Data source: Personality and Total Health (PATH) Through Life project Duration of follow‐up: mean 9.6 years |
|
| Participants | Country: Australia Age: 60–64 years (eligible range) Sex: 48.5% women Inclusion criteria: people aged 60–64 years at baseline were assessed at 4‐year intervals for 12 years. Exclusion criteria: non‐"Caucasian" ethnicity (presumed to mean non‐white); self‐reported history of stroke, transient ischaemic attack, epilepsy, brain tumours or brain infection. |
|
| Predictors | Number of predictors: 12 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: clinical diagnosis for MCI and dementia if:
Participants were assessed at 4‐year intervals for 12 years. At wave 4, participants were selected for review if:
|
|
| Missing data | Number of participants with missing data: reported Handling of missing data: imputation using random forest algorithm |
|
| Analysis | Number of participants: 2078 Number of events: dementia: 85; MCI: 196 EPV: 7 Modelling method: Cox proportional hazards Selection of predictors during modelling: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): high concern (only included participants aged 60–64 years) Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: high concern |
|
| Notes | Funding: "the study was supported by the Dementia Collaborative Research Centres and National Health and Medical Research Council (NHMRC) grants 973302, 179805, 1002160 and 1002560. JIV was supported by the Eccles Scholarship in Medical Sciences, the Fenner Merit Scholarship and The Australian National University High Degree Research scholarships. NC is funded by Research Fellowship number 12010227. KJA is funded by NHMRC Research Fellowship number 1002560. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript." Conflicts of interest: "the authors declare that they have no competing interests." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | No | Excluded people with stroke and transient Ischaemic attack |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Chosy 2019.
| Study characteristics | ||
| General Information | Model name: CAIDE Type of study: external validation Aim of the study: to test the predictive capacity of the CAIDE dementia risk score among a cohort of Japanese‐American men Data source: Honolulu‐Asia Ageing Study (HAAS) Duration of follow‐up: mean 25.2 (SD 1.1) |
|
| Participants | Country: USA Age: mean 53.1 years Sex: only men Inclusion criteria: cohort of Japanese men who agreed to participate in 4th re‐examination to assess their cognitive function Exclusion criteria: missing data |
|
| Predictors | Number of predictors: 8 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: secondary outcome Definition and method of measurement: Cognitive Abilities Screening Instrument (CASI) score < 60 indicated severe cognitive impairment |
|
| Missing data | Number of participants with missing data: 20% Handling of missing data: complete case analysis |
|
| Analysis | Number of participants: 3582 Number of events: 189 EPV: 23 Modelling method: logistic regression Selection of predictors for model inclusion: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): high concern (no mention of cognitive screening or exclusion of baseline dementia cases) Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: high concern |
|
| Notes | Funding: "This work was supported by the National Institute on Ageing (NIA) grants UF1AG053983 and UF1AG057707; Intramural Research Program, NIA; and the Office of the Assistant Secretary of Defence for Health Affairs under Award No. W81XWH‐15‐1‐0431. The content in this article does not necessarily reflect the official views of the United States Government." Conflicts of interest: "The authors have no conflicts of interest to declare." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | No | No mention of cognitive screening or exclusion of baseline dementia cases. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without the knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Cremers 2020.
| Study characteristics | ||
| General Information | Model name: Disease State Index Type of study: development Aim of the study: to validate whether the cognitive decline in the general population can be predicted with multi‐variate data using a previously proposed supervised classification method Data source: 3 cohorts from the Rotterdam study (RS) Duration of follow‐up: mean 5.7 years (SD 0.6) |
|
| Participants | Country: Netherlands Age: mean 60.9 years (SD 9.1) Sex: 55.6% women Inclusion criteria: only people with cognitive assessment at both baseline and follow‐up were included in the analysis. Exclusion criteria: baseline dementia, MCI, or MRI‐defined cortical infarcts |
|
| Predictors | Number of predictors: 34 Selection of predictors: included all available factors that correlated with future dementia Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: secondary outcome Definition and method of measurement: cognitive decline defined by the general cognitive factor (g‐factor) from the follow‐up visit minus the g‐factor from the baseline visit, resulting in a delta g‐factor. The cognitive tests used to create the g‐factor were colour‐word interference subtask of the Stroop test (information processing speed and executive functioning), Letter Digit Substitution Test (executive function), verbal fluency test (executive functioning), delayed recall score of the 15‐word verbal learning test (memory), and Purdue pegboard test. |
|
| Missing data | Number of participants with missing data: NR Handling of missing data: classifier method |
|
| Analysis | Number of participants: 2542 Number of events: 127 EPV: 3 Modelling method: supervised machine learning Selection of predictors during modelling: included all the available factors that correlated with future dementia Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "This study was supported by the European Union Seventh Framework Program (FP7/2007‐2013) under grant agreement no. 601055, VPH‐Dare@IT (FP7‐ICT‐2011‐9‐601055) and the EuroPOND initiative, which is funded by the European Union's Horizon 2020 Research and Innovation Program under grant agreement no. 666992. None of the funding sources influenced the design and conduct of the study, collection, management, analysis, interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication." Conflicts of interest: "JL was employed by the company Combinostics, which develops advanced tools for data‐driven diagnostics. WN was employed by the company Quantib B.V, which develops software to extract quantitative imaging biomarkers from medical imaging data. GK received research grants from GE Healthcare, Siemens AG and Bayer Health Care, Bracco, €40,000 per year consultancy fee. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Data source and inclusion criteria were probably appropriate. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without the knowledge of the outcome data. |
| Domain 3: Outcome | No | Predictors not excluded from definition of the outcome. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Deckers 2020.
| Study characteristics | ||
| General Information | Model name: LIBRA Type of study: external validation Aim of the study: to investigate the predictive validity of the LIBRA score in the longitudinal population‐based Cardiovascular Risk Factors, Ageing and Dementia (CAIDE) study Data source: Finnish CAIDE population‐based study Duration of follow‐up: mean 20.9 years (SD 4.9) |
|
| Participants | Country: Finland Age: mean 47.8 years (SD 4.7) Sex: 58.3%–61% women Inclusion criteria: participants with ≥ 1 re‐examination in midlife and completed cognitive assessments Exclusion criteria: dementia or MCI |
|
| Predictors | Number of predictors: 11 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: "cognitive status assessed with a 3‐step protocol (screening, clinical, and differential diagnostic phase). A review board consisting of a senior neurologist, senior neuropsychologist, study physician, and study neuropsychologist ascertained the final diagnosis based on all available information." |
|
| Missing data | Number of participants with missing data: NR Handling of missing data: NR |
|
| Analysis | Number of participants: 1024 Number of events: dementia: 84; MCI: 151 EPV: 7–13 Modelling method: Cox proportional hazards Selection of predictors during modelling: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "Academy of Finland, Grant/Award Numbers: 278457, 287490, 317465 and 319318; Alzheimerfonden Sweden; Center for Innovative Medicine (CIMED), Sweden; Knut and Alice Wallenberg Foundation Sweden; European Union Seventh Framework Programme, Grant/Award Number: 601055; Stiftelsen Stockholms sjukhem Sweden; Region Stockholm (ALF, NSV); Swedish Research Council." Conflicts of interest: NR |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Data source and inclusion criteria were probably appropriate. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Exalto 2014.
| Study characteristics | ||
| General Information | Model name: CAIDE Type of study: external validation Aim of the study: to validate externally the CAIDE risk score in a large, diverse population of members of an integrated health care delivery system in the USA; and to try to improve the predictability with the testing of additional midlife risk factors Data source: Kaiser Permanente Health Survey Duration of follow‐up: mean 36.9 years (SD 4.1) |
|
| Participants | Country: USA Age: 40–55 years (eligible range) Sex: 49%–60% women Inclusion criteria: Kaiser Permanente members aged 40–55 years who participated between 1964 and 1973 Exclusion criteria: missing data on education, BMI, systolic blood pressure, cholesterol, diabetes mellitus, depressed mood, pulmonary function, or race |
|
| Predictors | Number of predictors: 6 Selection of predictors: based on the development study and additional predictors Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: codes for possible dementia (according to ICD‐9) or specialist‐confirmed dementia (confirmed by a medical specialist in a memory clinic or neurology department) collected from electronic medical records stored in database of diagnoses from all outpatient encounters at Kaiser Permanente medical centres and clinic diagnoses |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: complete case analysis |
|
| Analysis | Number of participants: 9480 Number of events: 2767 EPV: 230 Modelling method: Cox proportional hazards and logistic regression Selection of predictors during modelling: based on the development study and additional predictors Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): high concern (study did not include people aged 56–65 years) Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: high concern |
|
| Notes | Funding: "This work is supported by Kaiser Permanente Community Benefits Grant (R. A. Whitmer as principal investigator) and a Fulbright fellowship (L. G. Exalto)." Conflict of interest: NR |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | No | No mention of cognitive screening or exclusion of baseline dementia cases. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration plot, slope and intercept not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Fayosse 2020.
| Study characteristics | ||
| General Information | Model name: CAIDE, FRS, and FINDRISC Type of study: external validation Aim of the study: to compare CAIDE to FRS and FINDRISC diabetes score as predictors of dementia and to assess the role of age in their associations with dementia Data source: the Whitehall II study Duration of follow‐up: mean 23.5 years (SD 4.0) |
|
| Participants | Country: UK Age: mean 50 years (range 39 to 63) Sex: 30.8% women Inclusion criteria: participants aged 35–55 years at recruitment Exclusion criteria: no response to study invitation; death |
|
| Predictors | Number of predictors: CAIDE: 7; FRS: 6; FINDRISC: 8 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: dementia diagnoses (ICD‐10 codes F00–F03, F05.1, G30, and G31) recorded in 3 registers (Hospital Episode Statistics, the Mental Health Services Data Set, and the mortality register) |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: complete case analysis |
|
| Analysis | Number of participants: 7553 Number of events: 318 EPV: CAIDE: 45; FRS: 53; FINDRISC: 39 Modelling method: Cox proportional hazards Selection of predictors: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): high concern (no mention of cognitive screening or exclusion of baseline dementia cases) Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: high concern |
|
| Notes | Funding: "The Whitehall II study has been supported by grants from the National Institute on Ageing, NIH (R01AG056477, RF1AG062553), UK Medical Research Council (R024227, S011676), and the British Heart Foundation (RG/16/11/32334). MK was additionally supported by NordForsk, Academy of Finland (311492) and Helsinki Institute of Life Science." Conflicts of interest: "The authors declare that they have no competing interests." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | No | No mention of cognitive screening or exclusion of baseline dementia cases. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration plot, slope and intercept not reported |
| Overall judgement | No | ≥ 1 domain at high risk. |
Ibarrondo 2022.
| Study characteristics | ||
| General Information | Model name: European Prospective Investigation into Cancer and Nutrition (EPIC)‐Spain cohort Type of study: development Aim of the study: to develop competing risk models to predict the late risk of dementia based on risk factors at middle age using data from the EPIC‐Spain cohort Data source: EPIC‐Spain cohort Duration of follow‐up: mean 21.61 years (SD 3.45) |
|
| Participants | Country: Spain Age: mean 49.08 years (SD 7.75) Sex: 43% women Inclusion criteria: participants from the EPIC database Exclusion criteria: inability to participate due to impaired mental capacity |
|
| Predictors | Number of predictors: 12 Selection of predictors: included all the available factors that correlated with a future dementia Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: incident cases of dementia (ICD‐9a and ICD‐10a definition) collected from electronic health records |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: complete case (but multiple imputation for some missing candidate predictors) |
|
| Analysis | Number of participants: 25,015 Number of events: 755 EPV: 62 Modelling method: Fine‐Gray non‐parametric models Selection of predictors during modelling: included all the available factors that correlated with a future dementia Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "The EPIC study received financial support from the International Agency for Research on Cancer (AEP/93/06), the European Commission (SO‐97‐ 200302‐05F02, SP23‐CT‐2005‐006438), the Health Research Fund (FIS) of the Spanish Ministry of Health, the Red Temática de Investigación Cooperativa de Centros de Cáncer (RTICCC C03/10, RD06/0020), the CIBER de Epidemiología y Salud Pública (CIBERESP), the participating Regional Governments of Andalusia, Asturias, Basque Country, Murcia (no. 6236), and Navarra, and the Catalan Institute of Oncology (ICO). The EPIC‐Murcia study received partial funding from the Fundación Seneca (19487/PI/14), the Murcia Biomedical Research Institute (IMIB)‐FFIS and the Spanish Biomedical Research Network Center (CIBER) (BOE‐A‐2020‐ 6018)." Conflict of interest: "Nothing to Disclose" |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Data source and inclusion criteria were probably appropriate. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Unnecessary categorisation of continuous predictors. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Kim 2019.
| Study characteristics | ||
| General Information | Model name: model developed from the South Korean national health examination cohort Type of study: development Aim of the study: to measure the accuracy of conventional hazards regression models and a deep learning Recurrent Neural Network/Long Short‐Term Memory model to predict all‐cause dementia and Alzheimer's disease using a nationwide periodic health examination dataset Data source: South Korean national health examination cohort Duration of follow‐up: mean 10.39 years (SD 1.44) |
|
| Participants | Country: South Korea Age: mean 52.53 years (SD 9.35) Sex: 45.6% women Inclusion criteria: age 40–79 years; baseline for the dataset was 2002–2003. Exclusion criteria: all‐cause dementia or death at baseline; no further health examinations after the baseline year |
|
| Predictors | Number of predictors: 15 Selection of predictors: included all the available factors that correlated with a future dementia Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: instances of all‐cause dementia (ICD‐10 codes F00–F03 and G30) and Alzheimer's disease (ICD‐10 codes F00 and G30) |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: multiple imputation |
|
| Analysis | Number of participants: 95,969 Number of events: 5456 EPV: 389 Modelling method: Cox proportional hazards and deep learning Selection of predictors for model inclusion: included all the available factors that correlated with a future dementia Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "This work was supported by the Institute for Information & Communications Technology Promotion (IITP) grant funded by the Korea government (MSIT; 2017‐0‐00255, autonomous digital companion framework and application)." Conflicts of interest: "M‐HC is an employee of Selvas AI, Inc. The agency (Selvas AI, Inc) had no role in the study design, data collection and analyses, or manuscript preparation. The other authors have no conflicts of interest to declare." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Data source and inclusion criteria were probably appropriate. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | Yes | Analysis method was appropriate. |
| Overall judgement | Unclear | ≥ 1 domain at unclear risk. |
Kivipelto 2006.
| Study characteristics | ||
| General Information | Model name: CAIDE Type of study: development Aim of the study: to develop a simple method for predicting the risk of late‐life dementia in middle‐aged people on the basis of their risk profiles Data source: North Karelia Project and the FINMONICA study Duration of follow‐up: mean 20.9 years (SD 4.9). |
|
| Participants | Country: Finland Age: mean 50.4 years (SD 6.0) Sex: 62% women Inclusion criteria: individuals aged 65–79 years at the end of 1997 who were living in two geographically defined areas in or close to the towns of Kuopio and Joensuu in eastern Finland Exclusion criteria: non‐attendance of the second clinical phase |
|
| Predictors | Number of predictors: 8 Selection of predictors: included all the available factors that correlated with a future dementia Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: dementia diagnosis determined through 3‐step cognitive status assessment (screening, clinical diagnosis, and differential diagnosis). Participants with MMSE score ≤ 24 at the screening phase were referred for further examinations, including thorough neurological and cardiovascular examinations and a detailed neuropsychological examination. The differential diagnostic phase included brain MRI, blood tests, cerebrospinal fluid analysis, and an electrocardiogram. |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: complete case analysis |
|
| Analysis | Number of participants: 1409 Number of events: 61 EPV: 7 Modelling method: logistic regression Selection of predictors during modelling: univariate analysis Performance measure reported:
|
|
| PROBAST: applicability | Domain 1 (participant selection): high concern (no mention of cognitive screening or exclusion of baseline dementia cases) Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: high concern |
|
| Notes | Funding: "This study was supported by the Ageing Program of the Academy of Finland, EVO‐grants of Kuopio University Hospital (5772708, 5772720), and the Alzheimer's Association, USA. MK was supported by Academy of Finland grants 103334 and 206951, Swedish Council for Working Life and Social Research, and the Gamla Tjänarinnor Foundation. TN was supported by the Helsingin Sanomain 100‐vuotissäätiö." Conflicts of interest: "We have no conflicts of interest." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | No | No mention of cognitive screening or exclusion of baseline dementia cases. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported and predictors selected based on univariate analysis. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Li 2018a.
| Study characteristics | ||
| General Information | Model name: model developed from the Framingham Heart Study Offspring cohort Type of study: development Aim of the study: to highlight associations between easily obtainable lifestyle risk factors in midlife and dementia in later adulthood Data source: Framingham Heart Study Offspring cohort Duration of follow‐up: 30 years |
|
| Participants | Country: USA Age: 40–65 years (eligible range) Sex: 50% women Inclusion criteria: individuals aged 40–65 years at the time of the second Offspring examination Exclusion criteria: dementia at baseline |
|
| Predictors | Number of predictors: 6 Selection of predictors: included all the available factors that correlated with a future dementia Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: cognitive status has been monitored in the original cohort since 1975, when comprehensive neuropsychological testing was performed. At that time, participants with low cognitive scores (the lowest 10%) also underwent neurologic assessment. |
|
| Missing data | Number of participants with missing data: NR Handling of missing data: NR |
|
| Analysis | Number of participants: 904 Number of events: 227 EPV: 37 Modelling method: machine learning (decision tree and random forest) Selection of predictors during modelling: the predictors were included based on "Mean Decrease Accuracy" Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "This work is supported by the Milstein Medical Asian American Partnership Foundation Irma & Paul Milstein Program for Senior Health Fellowship; Framingham Heart Study's National Heart, Lung, and Blood Institute contract (N01‐HC‐25195; HHSN268201500001I); by grants (R01 AG016495, R01‐AG008122, R01‐AG033040) from the National Institute on Ageing; and by a grant (R01‐NS017950) from the National Institute of Neurological Disorders and Stroke." Conflicts of interest: "Nothing to disclose." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Data source and inclusion criteria were probably appropriate. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Li 2018b.
| Study characteristics | ||
| General Information | Model name: dementia risk score Type of study: development and validation Aim of the study: to develop a prediction model for dementia in people with type 2 diabetes in China Data source: Taiwan National Diabetes Care Management Program (NDCMP) Duration of follow‐up: mean 8.09 years |
|
| Participants | Country: Taiwan Age: mean 64.3 years (SD 8.5) Sex: 55.2% women Inclusion criteria: Chinese people with type 2 diabetes enrolled in Taiwan National Diabetes Care Management Program (NDCMP) from 2002–2004 Exclusion criteria: age < 50 years; < 2 records within 1 year of follow‐up to estimate fasting plasma glucose and HbA1c variability; missing data for any actor considered in the study |
|
| Predictors | Number of predictors: 11 Selection of predictors: included all the available factors that correlated with a future dementia Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: dementia, assessed using inpatient/outpatient claim data (ICD‐9‐CM code 290). Dementia cases included people with ≥ 3 ambulatory claims or ≥ 1 inpatient care claim. |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: multiple imputation |
|
| Analysis | Number of participants: 9180 Number of events: 375 EPV: 34 Modelling method: Cox proportional hazards Selection of predictors during modelling: backward elimination Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): high concern (study excluded people aged < 50 years) Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: high concern |
|
| Notes | Funding: "This study was supported primarily by the Bureau of National Health Insurance (DOH94‐NH‐1007), the Ministry of Science and Technology of Taiwan (NSC 101‐2314‐B‐039 ‐017‐MY3, NSC 102‐2314‐B‐039‐005‐ MY2, MOST 104‐2314‐B‐039‐016, MOST 105‐2314‐B‐ 039‐021‐MY3 and MOST 105‐2314‐B‐039‐025 ‐MY3), the Ministry of Health and Welfare, Taiwan (MOHW107‐TDU‐B‐212‐123004) and China Medical University (CMU104‐S‐17)." Conflicts of interest: "The authors declare no financial or other conflicts of interest." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Data source and inclusion criteria were probably appropriate. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported and predictors selected based on univariate analysis. |
| Overall judgement | No | ≥ 1 domain at high risk. |
McGrath 2022.
| Study characteristics | ||
| General Information | Model name: FSRP Type of study: external validation Aim of the study: to find the association between the FSRP and its components at 5 time points across mid‐to‐later life and 10‐year risk of incident dementia, and to identify the most important vascular predictors for inclusion in age‐specific dementia risk scores Data source: Framingham original cohort and offspring cohort Duration of follow‐up: 10 years |
|
| Participants | Country: USA Age: range 52.5–57.5 years Sex: 57.2% women Inclusion criteria: attendance of Framingham Heart Study examination within 2.5 years of the index age (55 years) Exclusion criteria: Age ≤ 55 years; history of a coronary heart disease, stroke, cancer, or an abnormal ECG |
|
| Predictors | Number of predictors: 7 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: diagnosis of dementia based on a review of all available neurological examination records, neuropsychological assessments, neuroimaging investigations, hospital/nursing home/outpatient clinic records, family interviews, and autopsy results (when available) by a dementia review committee |
|
| Missing data | Number of participants with missing data: NR Handling of missing data: complete case |
|
| Analysis | Number of participants: 3735 Number of events: 72 EPV: 10 Modelling method: Cox proportional hazards regression Selection of predictors during modelling: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): high concern (study excluded people aged < 50 years) Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: high concern |
|
| Notes | Funding: "This research was supported by the Health Research Board of Ireland (CSF‐2020‐011) and the Alzheimer's Association (AACSF‐18‐566570). The Framingham Heart Study is supported by the NHLBI (contract no. N01‐HC‐25195, no. HHSN268201500001, and no. 75N92019D00031‐0‐75). This research was also supported by NHLBI grants R01 HL60040 and R01 HL70100, grants from the National Institute on Ageing (R01 AG054076, R01 AG049607, R01 AG033193, R01 AG059421, R01 AG066524, U01 AG049505, U01 AG052409) and grants from the National Institute of Neurological Disorders and Stroke (R01 NS017950 and UH2 NS100605)." Conflicts of interest: "The authors report no disclosures relevant to the manuscript." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | No | Study excluded people with history of coronary heart disease, stroke, cancer, or an abnormal ECG. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Park 2019.
| Study characteristics | ||
| General Information | Model name: model developed from the Korean health examination database Type of study: development Aim of the study: to develop a dementia prediction model using health examination data from National Health Insurance Service (NHIS‐HEALS) for middle‐aged Koreans Data source: National Health Insurance Service (NHIS‐HEALS) Duration of follow‐up: mean 10.2 years (SD 1.7) |
|
| Participants | Country: South Korea Age: mean 51.3 years (SD 8.1) Sex: 45.2% women Inclusion criteria: age 40–69 years Exclusion criteria: diagnosis of dementia at baseline; death within first 2 years of observation |
|
| Predictors | Number of predictors: 11 Selection of predictors: included all the available factors that correlated with a future dementia Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: incidence of all‐cause dementia according to ICD‐10 definitions |
|
| Missing data | Number of participants with missing data: NR Handling of missing data: multiple imputation using fully conditional specification |
|
| Analysis | Number of participants: 141,910 Number of events: 6688 EPV: 1409 Modelling method: Cox proportional hazards Selection of predictors during modelling: included all the available factors that correlated with a future dementia Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "This research was supported by the Leading Foreign Research Institute Recruitment Program (No. 2012027176 to H. Chang) and the Basic Science Research Program (No. 2017R1A2B3008214 to E. Lee) through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Korean Mental Health Technology R& Project, Ministry of Health & Welfare, Republic of Korea [HM15C0995 to E. Lee]." Conflicts of interest: "The authors have declared that no competing interests exist." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Appropriate data source and inclusion of participants. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Unnecessary categorisation of continuous predictors. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Reijmer 2011.
| Study characteristics | ||
| General Information | Model name: CAIDE Type of study: external validation Aim of the study: to assess whether the CAIDE risk score predicts cognitive impairment that does not meet the criteria of dementia Data source: population‐based Hoorn study Duration of follow‐up: 15 years |
|
| Participants | Country: Netherlands Age: mean 55.0 years (SD 3.7) Sex: 49% women Inclusion criteria: age 50–64 years at baseline Exclusion criteria: age ≥ 65 years at baseline; unreliable assessment of cognitive functioning |
|
| Predictors | Number of predictors: 7 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: secondary outcome Definition and method of measurement: cognitive status, measured with a neuropsychological test battery consisting of 12 verbal and nonverbal tasks. Cognitive test scores were standardised into z‐scores. Impairment in a particular cognitive domain was defined as a z‐score ≤ 1.5 on ≥ 1 measure included in that domain |
|
| Missing data | Number of participants with missing data: NR Handling of missing data: complete case analysis |
|
| Analysis | Number of participants: 322 Number of events: NR EPV: NR Modelling method: logistic regression Selection of predictors during modelling: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): high concern (study excluded people aged ≥ 65 years) Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: high concern |
|
| Notes | Funding: "This study was supported by grant 2003.01.004 from the Dutch Diabetes Research Foundation. The research of G.J.B. and R.P.C.K. is supported by a high potential grant from Utrecht University." Conflicts of interest: "None of the authors have indicated a conflict of interest." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Data source and inclusion criteria were probably appropriate. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Schaich 2021.
| Study characteristics | ||
| General Information | Model names: CAIDE, FSRP, ASCVD‐PCE Type of study: external validation Aim of the study: to compare the CAIDE, FSRP, and ASCVD‐PCE risk scores in relation to future global cognitive performance, processing speed, working memory, and odds of global cognitive decline over 6 years in a multi‐ethnic population of middle‐aged and older adults in the USA Data source: Multi‐Ethnic Study of Atherosclerosis (MESA) Duration of follow‐up: mean 15.7 years (SD 0.7) |
|
| Participants | Country: USA Age: mean 60.1 years (SD 9.4) Sex: 53.3% women Inclusion criteria: no clinical cardiovascular disease at baseline Exclusion criteria: incomplete examinations; clinically recognised dementia at baseline |
|
| Predictors | Number of predictors: CAIDE: 8; FSRP: 9; ASCVD‐PCE: 9 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: secondary outcome Definition and method of measurement: 3 standardised cognitive tests, namely Cognitive Abilities Screening Instrument (CASI, version 2; a measure of global cognitive performance), Digit Symbol Coding (DSC; a test of processing speed); and Digit Span (DS; a test of working memory) |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: multiple imputation |
|
| Analysis | Number of participants: 4392 Number of events: 218 EPV: CAIDE: 27; FSRP: 24; ASCVD‐PCE: 24 Modelling method: logistic regression Selection of predictors during modelling: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "This work was supported by the National Institute on Ageing (grant numbers R03‐AG064569 to C.L.S.; P30‐AG049638 to S.C.; R01‐AG058969 and R01‐AG054069 to T.M.H.; P30‐AG059303 and K24‐AG045334 to J.A.L.; R01‐AG055606 to S.R.R.; and RF1‐AG054474 to J.D.) and the National Heart, Lung, and Blood Institute (grant numbers F32‐HL146075 to C.L.S. and R01‐HL127659 to S.R.H.). Multi‐Ethnic Study of Atherosclerosis is supported by the National Heart, Lung, and Blood Institute (contract numbers 75N92020D00001, HHSN268201500003I, N01‐HC‐95159, 75N92020D00005, N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168 and N01‐HC‐95169) and the National Center for Advancing Translational Sciences (grant numbers UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420)." Conflicts of interest: "J.A.L. is a consultant to vTv Therapeutics and receives a stipend from Wolters Kluwer as Editor‐in‐Chief of the journal Alzheimer's Disease and Associated Disorders. Remaining authors declare no conflicts of interest." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | No | Participants were free of clinical cardiovascular disease at baseline. |
| Domain 2: Predictors | Yes | Predictors were probably defined and assessed in the right way, and the predictors are available for when the model is put to use. |
| Domain 3: Outcome | Yes | Investigators used a suitably defined standard outcome without including any predictor information, determined the outcome similarly for the participants. They probably determined the outcome without knowledge of predictor data and following a reasonable time interval. |
| Domain 4: Analysis | Yes | Analysis is appropriate. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Schiepers 2017.
| Study characteristics | ||
| General Information | Model name: LIBRA Type of study: development Aim of the study: to develop a new prediction model for dementia by focusing exclusively on modifiable risk factors Data source: the Maastricht Ageing Study Duration of follow‐up: 16 years for dementia; 12 years for cognitive impairment |
|
| Participants | Country: Netherlands Age: mean 65.0 years (SD 8.7) Sex: 49.1% women Inclusion criteria: age ≥ 50 years at baseline; legal competence; MMSE score > 24 Exclusion criteria: chronic neurological pathology; psychiatric disorders; mental retardation; psychotropic drug use |
|
| Predictors | Number of predictors: 15 Selection of predictors: included all the available factors that correlated with a future dementia Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: dementia status was based on DSM‐4 criteria in all participants up to 16 years after the start of the study, using both the International Classification of Primary Care coded information from individual patient records and medical and cognitive data obtained in the Maastricht Ageing Study. Participants who scored 1.5 SD below the mean on any of 3 cognitive tests (the visual verbal word learning task, the Stroop colour‐word interference test, and the letter‐digit substitution test) were classified as cognitively impaired. |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: complete case analysis |
|
| Analysis | Number of participants: dementia: 949; cognitive impairment: 746 Number of events: dementia: 61; cognitive impairment: 91 EPV: 4–6 Modelling method: Cox proportional hazards Modelling assumptions: NR Selection of predictors during modelling: included all the available factors that correlated with a future dementia Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "This study is part of the In‐MINDD (Innovative Midlife Intervention for Dementia Deterrence) project, which is funded by the European Community's Framework Programme Seven (FP7), contract no. 304979." Conflicts of interest: "none declared" |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Appropriate data source and probably appropriate inclusion of participants. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Tynkkynen 2017.
| Study characteristics | ||
| General Information | Model names: CAIDE Type of study: external validation Aim of the study: to examine whether high‐sensitivity cardiac troponin I and N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) are associated with incident dementia and Alzheimer's disease independently of each other Data source: the National FINRISK 1997 Study Duration of follow‐up: mean 16.1 years (SD 0.12) |
|
| Participants | Country: Finland Age: mean 47.9 years (SD 13.2) Sex: 48.9% Inclusion criteria: age 25–74 years Exclusion criteria: prevalent dementia; prevalent ischaemic and haemorrhagic stroke; prevalent congestive heart failure |
|
| Predictors | Number of predictors: 9 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: participants classified as having incident dementia (including Alzheimer's disease) were those entitled to a special reimbursement for anticholinesterase inhibitors or memantine, those who had purchased anticholinesterase inhibitors or memantine ≥ 3 times during the follow‐up, and those with a hospitalisation or death with ICD‐10 codes F00, F01, F02, F03, or G30. |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: multiple imputation |
|
| Analysis | Number of participants: 7114 Number of events: 407 EPV: 45 Modelling method: Cox proportional hazards regression Selection of predictors during modelling: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "This work has been supported by the European Union Seventh Framework Programme (FP7/2007‐2013) under grant agreement No. HEALTH‐F2‐2011‐278913 (BiomarCaRE). Salomaa V. was supported by the Finnish Foundation for Cardiovascular Research. Tynkkynen J. was supported by North Karelian central hospital for conducting this paper as part of his doctoral thesis." Conflicts of interest: "On behalf of all authors, the corresponding author states that there is no conflict of interest. All the participants gave a written informed consent and the survey was conducted in accordance with the Declaration of Helsinki. Ethics approval was received from the Ethics Committee of the National Public Health Institute." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | No | The study excluded cases of prevalent ischaemic and haemorrhagic stroke and prevalent congestive heart failure from the analysis. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Virta 2013.
| Study characteristics | ||
| General Information | Model name: CAIDE Type of study: external validation Aim of the study: to assess the validity of the CAIDE Study risk score in predicting cognitive impairment Data source: the Finnish Twin cohort Duration of follow‐up: mean 22.6 years (SD 2.3) |
|
| Participants | Country: Finland Age: mean 51.7 years (SD 6.1) Sex: 48.9% women Inclusion criteria: same‐sexed twin pairs of known zygosity born in Finland before 1958 with both co‐twins alive in 1967. The monozygotic (MZ) twin pairs aged 65 years with both co‐twins alive were asked to participate in a telephone interview evaluating cognitive performance between 1999 and 2001, and the corresponding dizygotic (DZ) twins irrespective of twin status between 2003 and 2007. The MZ twins were contacted first to identify MZ twin pairs discordant in cognitive function. Exclusion criteria: missing questionnaire data on any of the required variables |
|
| Predictors | Number of predictors: 8 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: secondary outcome Definition and method of measurement: the telephone assessment for dementia (TELE) assesses cognitive function using questions or tasks that test orientation, long‐term memory, short‐term memory (immediate and delayed recall of word list), attention, abstraction, calculation, language repetition, and non‐verbal praxis. Based on the TELE score, the subjects were classified as being cognitively healthy, having mild impairment in cognitive function, or having moderate to severe cognitive impairment. |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: complete case analysis |
|
| Analysis | Number of participants: 591,439 Number of events: 117 EPV: 14 Modelling method: logistic regression Selection of predictors during modelling: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): high concern (no mention of cognitive screening or exclusion of baseline dementia cases) Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: high concern |
|
| Notes | Funding: "This study was financially supported by the Academy of Finland (project #205954), the Sigrid Juselius Foundation and Clinical grants of Turku University Hospital (EVO). The Finnish Twin Cohort study is part of the Academy of Finland Center of Excellence in Complex Disease Genetics (grant #s 213506, 129680)." Conflicts of interest: "The authors declare that they have no conflict of interest." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | No | No mention of cognitive screening or exclusion of baseline dementia cases. |
| Domain 2: Predictors | Yes | Predictors probably defined and assessed in the right way, and the predictors are available for when the model is put to use. |
| Domain 3: Outcome | Yes | Investigators used a suitably defined standard outcome without including any predictor information, and they determined the outcome similarly for all participants. The investigators probably determined the outcome without knowledge of the predictor data and following a reasonable time interval. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Vos 2017.
| Study characteristics | ||
| General Information | Model name: LIBRA index Type of study: external validation Aim of the study: to investigate the predictive validity of the LIBRA index for incident dementia in midlife, late life, and the oldest age group in a large multi‐centre European population‐based cohort Data source: European population‐based DESCRIPA study Duration of follow‐up: mean 8.1 years (SD 3.5) |
|
| Participants | Country: Sweden Italy, Netherlands, France Age: mean 65.0 years (SD 4.0) Sex: 51% women Inclusion criteria: age ≥ 55 years; good subjective general health; direct or indirect baseline measure of ≥ 7 of 9 modifiable risk factors; information on educational level; ≥ 1 clinical follow‐up Exclusion criteria: dementia at baseline |
|
| Predictors | Number of predictors: 9 Selection of predictors: based on the development study Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: incident dementia according to the DSM (3rd edition, revised) or, for the Longitudinal Aging Study Amsterdam cohort, defined as impairment in multiple cognitive domains |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: NR |
|
| Analysis | Number of participants: 3256 Number of events: 190 EPV: 21 Modelling method: Cox proportional hazards Selection of predictors during modelling: based on the development study Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "This research was supported by the European Union Fifth Framework Programme under grant agreement No. QLRT‐2001‐2455 (DESCRIPA), European Union Seventh Framework Programme (FP7/2007‐2013) under grant agreement No. 304979 (In‐MINDD), and EU/EFPIA Innovative Medicines Initiative Joint Undertaking under European Medical Information Framework grant agreement No. 115372 (EMIF‐AD)." Conflict of interest: "Jean‐François Dartigues Grants Agency: ROCHE Dates: 2016‐2017 Agency: IPSEN Dates: 2016 Ingmar Skoog Consulting Fees: Takeda consultation and speakers Bureau. 2016: 9,000 Euro Lecture Fees: Takeda, approximately Three times per year Stephanie Vos Grants Agency: Janssen Pharmaceutica Dates: 12/2015" |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Appropriate data source and probably appropriate inclusion of participants. |
| Domain 2: Predictors | Unclear | No information on whether investigators assessed predictors without knowledge of the outcome data. |
| Domain 3: Outcome | Unclear | No information on whether investigators determined the outcome without knowledge of the predictor data. |
| Domain 4: Analysis | No | Calibration not reported. |
| Overall judgement | No | ≥ 1 domain at high risk. |
Walters 2016.
| Study characteristics | ||
| General Information | Model name: dementia risk score Type of study: development Aim of the study: to develop and validate a 5‐year dementia risk score using routinely collected data from a large nationally representative primary care database in the UK Data source: The Health Improvement Network (THIN) cohort Duration of follow‐up: 5 years (IQR 3.27–5) |
|
| Participants | Country: UK Age: mean 65.6 years (SD 6.11) Sex: 51.8% women Inclusion criteria: age 60–95 years, contributing to the THIN database between 1 January 1 2000, and 31 December 2011 Exclusion criteria: recorded dementia, cognitive impairment, or symptoms of confusion prior to study entry; exclusion diagnosis indicating specific subtypes of dementia syndrome |
|
| Predictors | Number of predictors: 12 Selection of predictors: included all the available factors that correlated with a future dementia Definition of predictors: see Appendix 3 |
|
| Outcomes | Type of outcome: primary outcome Definition and method of measurement: newly recorded dementia diagnoses, including Alzheimer's disease, vascular dementia, and unspecified or mixed dementia, excluding dementia diagnoses associated with Parkinson's disease, Lewy body dementia, Huntingdon, Picks, HIV, and drug‐induced and alcohol‐related dementia |
|
| Missing data | Number of participants with missing data: reported Handling of missing data: multiple imputation using a fully conditional specification method |
|
| Analysis | The number of participants: 226,140 The number of events: 1699 EPV: 141 Modelling method: Cox proportional hazards Selection of predictors during modelling: included all the available factors that correlated with a future dementia Performance measures:
|
|
| PROBAST: applicability | Domain 1 (participant selection): low concern Domain 2 (predictors): low concern Domain 3 (outcome): low concern Overall applicability: low concern |
|
| Notes | Funding: "The Predicting dementia risk in primary care: development and validation of a risk score using routinely collected data study is funded by the National Institute for Health Research (NIHR) School for Primary Care Research (SPCR), UK (http://www.nihr.ac.uk/funding/school‐for‐primary‐care‐research.htm). RO was funded by the NIHR UCLH/UCL Biomedical Research Centre. This article presents independent research funded by the NIHR, UK. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The funder has had no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the manuscript, or decision to submit for publication." Conflict of interest: "The authors declare that they have no competing interests." |
|
| Item | Authors' judgement | Support for judgement |
| Domain 1: Participant selection | Yes | Appropriate data source and appropriate inclusion of participants. |
| Domain 2: Predictors | No | Model derived from clinical data, so outcome not formally blinded to the assessment of risk factors. |
| Domain 3: Outcome | No | Model derived from clinical data, so outcome not formally blinded to the assessment of risk factors. |
| Domain 4: Analysis | Yes | Reported measures of calibration and discrimination. |
| Overall judgement | No | ≥ 1 domain at high risk. |
ANU‐ADRI: Australian National University Alzheimer's Disease Risk Index; ASCVD‐PCE: AtheroSclerotic CardioVascular Disease Pooled Cohort Equation; AUC: area under the receiver operating characteristics curve; BMI: body mass index; CAIDE: Cardiovascular Risk Factors, Ageing, and Dementia; CI: confidence interval; DSM: Diagnostic and Statistical Manual of Mental Disorders; ECG: electrocardiogram; EPV: events per variable or ratio of outcome events to candidate predictors; EPIC: European Prospective Investigation into Cancer and Nutrition; FINDRISC: Finnish Diabetes Risk Score; FRS: Framingham Risk Score; ICD: International Statistical Classification of Diseases and Related Health Problems; IQR: interquartile range; LIBRA: LIfestyle for BRAin Health; MACQ: Memory and Cognition Questionnaire; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; MRI: magnetic resonance imaging; NR: not reported; SDMT: Symbol Digit Modalities Test; SD: standard deviation; SE: standard error; SNAC: Swedish National Study on Ageing and Care.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2016 | Not a prognostic model. |
| Almeida 2021 | Not a prognostic model. |
| Anstey 2014 | Not a middle‐aged cohort. |
| Aschwanden 2020 | Not a middle‐aged cohort. |
| Ashby 2020 | Not a prognostic model, not multi‐domain, and inadequate follow‐up. |
| Atkins 2019 | Not a prognostic model, not multi‐domain. |
| Baird 2022 | Participants had baseline cognitive impairment. |
| Barnes 2014 | Not a middle‐aged cohort. |
| Ben 2020 | Inadequate follow‐up. |
| Beydoun 2020 | Not a prognostic model. |
| Borenstein 2014 | Not a prognostic model. |
| Borges 2017 | Inadequate follow‐up and outcome did not match our inclusion criteria. |
| Brewster 2012 | Not a prognostic model. |
| Calvin 2020 | The outcome was not dementia or cognitive impairment. |
| Cherbuin 2019 | Predictive performance measures not reported. |
| Colpo 2016 | Not a middle‐aged cohort. |
| Copenhaver 2021 | Not a middle‐aged cohort. |
| Dallora 2020 | Not a middle‐aged cohort. |
| Debette 2011 | Not a prognostic model. |
| Deckers 2019a | Predictive performance measures not reported. |
| Deckers 2019b | Not a prognostic model. |
| DeFina 2013 | Not a prognostic model. |
| DeRight 2014 | Not a middle‐aged cohort. |
| DeRight 2015 | Not a development or validation study of a model. |
| Downer 2016 | Not a middle‐aged cohort. |
| Ecay 2018 | Predictive performance measures not reported. |
| Enache 2016 | Inadequate follow‐up and outcome does not match our inclusion criteria. |
| Gonzalez 2018 | Not a prognostic model. |
| Gourley 2019 | Inadequate follow‐up. |
| Gourley 2020 | Not a prognostic model. |
| Graves 2019 | Not a middle‐aged cohort. |
| Gupta 2015 | Not a prognostic model. |
| Hakansson 2011 | Not multi‐domain. |
| Hazzouri 2013 | Not a prognostic model. |
| Heger 2019 | Predictive performance measures not reported. |
| Hessler 2016 | Not a middle‐aged cohort. |
| Hou 2019 | Not a development or validation study. |
| Huang 2020 | Not a middle‐aged cohort and inadequate follow‐up. |
| Iso 2021 | Predictive performances not reported. |
| Jacqmin 2014 | Not a middle‐aged cohort and not multi‐domain. |
| Juul 2020 | Predictive performance measures not reported. |
| Kaffashian 2011 | Not a prognostic model. |
| Kaffashian 2013a | Predictive performance measures not reported. |
| Kaffashian 2013b | Predictive performance measures not reported. |
| Kalmijn 2000 | Predictive performance measures not reported. |
| Kamat 2010 | Not a prognostic model and not a middle‐aged cohort. |
| Lee 2018 | Predictive performance measures not reported. |
| Li 2017 | Not a prognostic model and inadequate follow‐up. |
| Li 2018c | Not a middle‐aged cohort. |
| Liang 2020 | Not a prognostic model. |
| Licher 2018 | Not a middle‐aged cohort. |
| Licher 2019 | Not a middle‐aged cohort. |
| Ling 2022 | Follow‐up less than 5 years. |
| Lins 2017 | Inadequate follow‐up. |
| Llewellyn 2008 | Predictive performance measures not reported. |
| Lu 2020 | The participants had baseline cognitive impairment. |
| Malik 2021 | Predictive performance measures not reported. |
| Maria 2014 | Not a development or validation study. |
| Mehta 2016 | Not a middle‐aged cohort. |
| Novotni 2019 | Not a prognostic model. |
| Ogunmoroti 2016 | Predictive performance measures not reported. |
| Pase 2016a | Predictive performance measures not reported. |
| Pase 2016b | Not a middle‐aged cohort. |
| Pekkala 2017 | Not a middle‐aged cohort. |
| Pettigrew 2020 | Not a prognostic model. |
| Phongpreecha 2020 | Inadequate follow‐up and not multi‐domain. |
| Rabin 2011 | Not a prognostic model. |
| Reem 2019 | Not a prognostic model. |
| Rundek 2020 | Predictive performance measures not reported. |
| Sabia 2009 | Predictive performance measures not reported. |
| Sabia 2019 | Predictive performance measures not reported. |
| Sacktor 2017 | Inadequate follow‐up. |
| Salvado 2019 | Inadequate follow‐up and not multi‐domain. |
| Senanarong 2017 | Not a middle‐aged cohort. |
| Solomon 2014 | Not a prognostic model. |
| Solomon 2015 | Not a development or validation study. |
| Song 2011 | Not a middle‐aged cohort. |
| Stephen 2017 | Not a prognostic model. |
| Stephen 2020 | Not a prognostic model. |
| Tang 2015 | Not a development or validation study. |
| Tang 2020 | Not a middle‐aged cohort. |
| Tierney 2010 | Not a middle‐aged cohort. |
| Torres 2020 | Inadequate follow‐up. |
| Tortelli 2017 | Not a middle‐aged cohort. |
| Van 2019 | Not a prognostic model. |
| Viswanathan 2015 | Not a middle‐aged cohort. |
| Vuoksimaa 2016 | Not multi‐domain. |
| Vuorinen 2015 | Outcome not matching our inclusion criteria. |
| Wang 2016 | Not multi‐domain. |
| Yaffe 2020 | Not a prognostic model. |
| Yang 2021 | Not a prognostic model. |
| Yates 2014 | Not a prognostic model. |
Characteristics of studies awaiting classification [ordered by study ID]
Anca 2018.
| Notes | Model name: CAIDE Type of study: external validation Aim of the study: predictive capacity of CAIDE in general adult population Data source: NR Study design: prospective Type of sampling: NR Study dates: NR Duration of follow‐up: NR Participants Setting: NR Country: Romania Number of centres: NR Age: NR Sex: NR Inclusion criteria: NR Exclusion criteria: NR Predictors Number of predictors: NR Type of predictors: NR Outcomes Type of outcome: NR Definition and method of measurement: evaluation of cognitive status every year Type of outcome: NR Missing data Number of participants with missing data: NR Handling of missing data: NR Analysis Number of participants: NR Number of events: NR EPV: NR Modelling method: NR Modelling assumptions: NR Selection of predictors during modelling: NR Criteria of predictors during modelling: NR Performance measures:
Notes Funding: NR Conflict of interest: NR |
Kerut 2018.
| Notes | Model name: CAIDE Type of study: external validation Aim of the study: the performance of the CAIDE risk score and CAIDE plus clinical factors in predicting short‐term incident cognitive impairment amongst the African American Genetic Epidemiology Network of Arteriopathy (GENOA) cohort Data source: Genetic Epidemiology Network of Arteriopathy (GENOA) cohort Study design: retrospective Type of sampling: NR Study dates: NR Duration of follow‐up: 7 years Participants Setting: NR Country: USA Number of centres: NR Age: mean 62.3 years Sex: 74% women Inclusion criteria: cognitively unimpaired African Americans at visit 2 (MMSE > 20 and global cognition z‐score > −1.5), when CAIDE risk score components (age, education, sex, BMI, systolic blood pressure, cholesterol, and physical activity) were assessed, who also underwent cognitive exams at visit 3 (mean of 7 years later) Exclusion criteria: NR Predictors Number of predictors: 7 Type of predictors: participant demographics, history, examination Outcomes Type of outcome: secondary outcome Definition and method of measurement: incident cognitive impairment (follow‐up MMSE ≤ 20, z‐score ≤ −1.5, MMSE decline ≥1 point/year, or z‐score decline ≥0.1/year) Type of outcome: combined Missing data Number of participants with missing data: NR Handling of missing data: NR Analysis Number of participants: 587 Number of events: 107 EPV: 15 Modelling method: NR Modelling assumptions: NR Selection of predictors during modelling: NR Criteria of predictors during modelling: NR Performance measures:
Notes Funding: NR Conflict of interest: NR |
Khalid 2020.
| Notes | Model name: Diabetes‐Specific Dementia risk score (DSDRS) Type of study: development Aim of the study: to test the performance of individual and combined predictors of diabetes‐specific dementia risk score (DSDRS) in the UK‐based Edinburgh Type 2 Diabetes Study (ET2DS) Data source: UK‐based Edinburgh Type 2 Diabetes Study (ET2DS) Study design: retrospective Type of sampling: NR Study dates: NR Duration of follow‐up: 10 years Participants Setting: NR Country: Scotland Number of centres: NR Age: 60–75 years (eligible range) Sex: NR Inclusion criteria: NR Exclusion criteria: NR Predictors Number of predictors: NR Type of predictors: NR Outcomes Type of outcome: NR Definition and method of measurement of outcome: NR Missing data Number of participants with missing data: NR Handling of missing data: NR Analysis Number of participants: 1066 Number of events: 100 EPV: NR Modelling method: logistic regression Modelling assumptions: NR Selection of predictors during modelling: NR Criteria of predictors during modelling: NR Performance measures:
Notes Funding: NR Conflict of interest: NR |
Rawtaer 2016.
| Notes | Model names: CAIDE and SLAS (Singapore Longitudinal Ageing Study) Type of study: development and external validation Aim of the study: to produce a risk score that best predicts incident neurocognitive disorder amongst Chinese elderly and to validate this score against the modified risk score derived from the CAIDE study Data source: Singapore Longitudinal Ageing Study (SLAS) Study design: retrospective cohort Type of sampling: NR Study dates: 2003–2010 Duration of follow‐up: 5 years Participants Setting: NR Country: Singapore Number of centres: NR Age: unclear whether baseline average age is between 45 and 65 years. Sex: 55.6 % women among participants without neurocognitive disorders, and 62.1% women among participants without neurocognitive disorders Inclusion criteria: baseline MMSE score ≥ 26; complete information on cognitive status (normal cognitive function or neurocognitive disorder) at follow‐up Exclusion criteria: NR Predictors Number of predictors: 7 Type of predictors: participant demographics, physical examination, additional tests Outcomes Type of outcome: secondary outcome Definition and method of measurement: cognitive function was assessed by a modified version of the MMSE [11] and the Clinical Dementia Rating Scale (CDR) performed by trained research staff. Incident neurocognitive disorder was defined as a global CDR score ≥ 0.5 Missing data Number of participants with missing data: NR Handling of missing data: NR Analysis Number of participants: 957 Number of events: 72 EPV: 8 Modelling method: logistic regression Modelling assumptions: NR Selection of predictors during modelling: univariate analysis Criteria of predictors during modelling: P value Performance measure reported:
Notes Funding: This work was supported by: the Biomedical Research Council, Agency for Science, Technology and Research in Singapore (grant numbers 03/1/21/17/214, 340 08/1/21/19/567); the Virtual Institute for the Study of Ageing, National University of Singapore (grant number VG‐8); the Alice Lim Memorial Fund, Singapore (Alice Lim Award 2010). Support in kind was provided by the Presbyterian Welfare Services Sarah Centre, the Henderson Senior Citizens’ Home, National Trade Union Congress Eldercare Co‐op Ltd, the Presbyterian Community Services (Sarah Seniors Activities Centre), the Training and Research Academy at Jurong Point, the Geylang East Home for the Aged, and the Thye Hua Kwan Moral Society. Conflicts of interest: The authors declare that they have no conflicts of interest. |
AUC: area under the receiver operating characteristics curve; BMI: body mass index; CAIDE: Cardiovascular Risk Factors, Ageing, and Dementia; DSDRS: Diabetes‐Specific Dementia risk score; EPV: events per variable; MMSE: Mini‐Mental State Examination; NCD: neurocognitive disorder; NR: not reported; SLAS: Singapore Longitudinal Ageing Study
Characteristics of ongoing studies [ordered by study ID]
Fisher 2017.
| Study name | Dementia Population Risk Tool (DemPoRT) |
| Starting date | Protocol published in 2017 |
| Contact information | Dr Peter Tanuseputro;ptanuseputro@ ohri. ca |
| Notes | Model names: Dementia Population Risk Tool (DemPoRT) Type of study: development with internal validation Aim of the study: to develop and validate the Dementia Population Risk Tool (DemPoRT) using predictors from large population‐based community health surveys that are individually linked to routinely collected health administration data in Ontario to predict and project dementia incidence at the population level. Data source: respondents of the Canadian Community Health Survey (CCHS) Study design: retrospective Type of sampling: NR Participants Setting: Country: Canada Number of centres: NR Age: > 55 years Sex: 2 DemPoRT models, 1 for males and 1 for females, will be derived and validated. Inclusion criteria: derivation cohort will consist of eligible respondents of the 2001, 2003, 2005, and 2007 CCHS, while validation cohorts will consist of respondents to the 2008/2009 cycle. Exclusion criteria: > 55 years of age at survey administration; self‐reported history of dementia; lack of eligibility for Ontario’s universal health insurance Predictors Number of predictors: 32 Type of predictors: participant demographics and history Outcomes Type of outcome: primary outcome Definition and method of measurement of outcome: linkage of survey respondents to population‐level administrative healthcare databases Type of outcome: combined Missing data Handling of missing data: multiple imputation Analysis Number of participants: 18,764 respondents in male derivation cohort, 25,288 respondents in female derivation cohort; validation cohorts will consist of approximately 4600 males and 6300 females. Number of events: investigators expect approximately 225 events for men and 400 for women in the validation cohort. Modelling method: Cox proportional hazards regression Modelling assumptions: Schoenfeld residuals Selection of predictors: extensive literature review Performance measures to be reported: calibration and discrimination Notes Funding: This work is supported by the Canadian Institutes of Health Research operating grant MOP142237. SF is supported by a doctoral award from the Canadian Institutes of Health Research. This project is supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The sponsors have no role in the design or conduct of the study; in the collection, analysis or interpretation of the data; or in the preparation, review or approval or the manuscript. The options, results and conclusions reported are those of the authors and are independent of the funding source. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Conflict of interest: none declared. |
NR: not reported.
Differences between protocol and review
See Mohanannair Geethadevi 2021 (review protocol).
We chose to clarify the methods regarding the operational definitions of 'internal validation' and 'external validation' studies. This included separating the 'types of prognostic models' section from the 'types of studies' section.
We chose to refine the inclusion criteria to only include studies that reported prediction performance measures, rather than just associations (similar to previous reviews (Hou 2019; Tang 2015)).
We provided additional details on our forward and backwards citation tracking process via Web of Science.
We simplified the description of the PROBAST tool to two steps under 'Assessment of risk of bias in included studies'.
We could not assess the clinical utility/usability of the models as no included studies provided this information.
We did not report the summary calibration plot or the summary observed/expected ratio as most studies did not report these data.
We did not use transforming equations owing to lack of information.
We could not perform sensitivity analysis owing to lack of data.
We could not perform multi‐variate meta‐analysis owing to lack of data.
We did not construct a funnel plot to assess the publication bias of the selected studies because we included fewer than 10 studies per model.
We reported risk of bias and applicability using the robvis data visualization tool (McGuinness 2021).
Contributions of authors
Screening of the articles: GMG, AJC Assessment of studies for inclusion: GMG, TQ, JG, AJC Data extraction and risk of bias assessment: GMG, MRS, AJC Data entry into RevMan: GMG Data analysis: GMG in consultation with TQ and AJC Disagreement resolution: TQ, JG, AJC Drafting the manuscript: GMG, AJC Reviewing the manuscript: all authors
Sources of support
Internal sources
-
Monash University, Australia
GMG was supported by Graduate Scholarship and International Tuition Scholarship from Monash University.
-
National Health and Medical Research Council (NHMRC), Australia
AJC was support by an NHMRC Emerging Leadership Fellowship (administered by Monash University)
External sources
-
NIHR, UK
This review was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
GMG: none known TQ: none known JG: JG has received investigator‐initiated research grants from Boehringer Ingelheim (2014), from Pfizer through the Global Research Awards for Nicotine Dependence (2017), and from GlaxoSmithKline (GSK) through Medical Education Grants (2018) for unrelated projects. He has also provided consultancy services to GSK (review of educational materials – 2018) and Pfizer (delivering education sessions as part of CPD – 2019; not for promoting any particular product or molecule). These grants were largely interdisciplinary and involved multiple investigators. JG has not used any part of the funding for his salary or for other personal benefits. He has a tenured academic appointment at Monash University. These funds were paid to his employer (Monash University) and were used to support staff working on those projects or for professional development (e.g. conference attendance). KA: KA is an advisor to the Staying Sharp Platform of the American Association of Retired Persons and a member of the governance committee of the Global Council on Brain Health. KA received an honorarium from the Canadian Network on Neurodegeneration and Ageing for giving a keynote address at their conference on dementia prevention. JSB: JSB has received grant funding or consulting funds from the National Health and Medical Research Council (NHMRC), Medical Research Future Fund (MRFF), Victorian Government Department of Health and Human Services, Dementia Australia Research Foundation, Yulgilbar Foundation, Aged Care Quality and Safety Commission, Dementia Centre for Research Collaboration, Pharmaceutical Society of Australia, GlaxoSmithKline Supported Studies Programme, Amgen, and several aged care provider organisations unrelated to this work. All grants and consulting funds were paid to the employing institution. MRS: none known AJC: none known
New
References
References to studies included in this review
Andrews 2017 {published data only}
- Andrews SJ, Eramudugolla R, Velez JI, Cherbuin N, Easteal S, Anstey KJ. Validating the role of the Australian National University Alzheimer's Disease Risk Index (ANU-ADRI) and a genetic risk score in progression to cognitive impairment in a population-based cohort of older adults followed for 12 years. Alzheimer's Research & Therapy 2017;9(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chosy 2019 {published data only}
- Chosy EJ, Edland SD, Gross N, Meyer MJ, Liu CY, Launer LJ, et al. The CAIDE Dementia Risk Score and the Honolulu-Asia Aging Study. Dementia and Geriatric Cognitive Disorders 2019;48(3):164-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cremers 2020 {published data only}
- Cremers GM, Huizinga W, Niessen WJ, Krestin GP, Poot DH, Ikram MA, et al. Predicting global cognitive decline in the general population using the Disease State Index. Frontiers in Aging Neuroscience 2020;11:379. [DOI: 10.3389/fnagi.2019.00379] [DOI] [PMC free article] [PubMed]
Deckers 2020 {published data only}
- Deckers K, Barbera M, Köhler S, Ngandu T, Van BM, Rusanen M, et al. Long-term dementia risk prediction by the LIBRA score: a 30-year follow-up of the CAIDE study. International Journal of Geriatric Psychiatry 2020;35(2):195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Exalto 2014 {published data only}
- Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimer's & Dementia 2014;10(5):562-70. [DOI] [PubMed] [Google Scholar]
Fayosse 2020 {published data only}
- Fayosse A, Nguyen DP, Dugravot A, Dumurgier J, Tabak AG, Kivimäki M, et al. Risk prediction models for dementia: role of age and cardiometabolic risk factors. BMC Medicine 2020;18(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibarrondo 2022 {published data only}
- Ibarrondo O, Huerta Jose M, Amiano P, Andreu ME, Mokoroa O, Ardanaz E, et al. Dementia risk score for a population in Southern Europe calculated using competing risk models. Journal of Alzheimer's Disease 2022;86(4):1751-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2019 {published data only}
- Kim WJ, Sung JM, Sung D, Chae MH, An SK, Namkoong K, et al. Cox proportional hazard regression versus a deep learning algorithm in the prediction of dementia: an analysis based on periodic health examination. JMIR Medical Informatics 2019;7(3):e13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kivipelto 2006 {published data only}
- Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurology 2006;5(9):735-41. [DOI] [PubMed] [Google Scholar]
Li 2018a {published data only}
- Li J, Ogrodnik M, Kolachalama VB, Lin H, Au R. Assessment of the mid-life demographic and lifestyle risk factors of dementia using data from the Framingham Heart Study offspring cohort. Journal of Alzheimer's Disease 2018;63:1119-27. [DOI] [PubMed] [Google Scholar]
Li 2018b {published data only}
- Li CI, Li TC, Liu CS, Liao LN, Lin WY, Lin CH, et al. Risk score prediction model for dementia in patients with type 2 diabetes. European Journal of Neurology 2018;25(7):976-83. [DOI] [PubMed] [Google Scholar]
McGrath 2022 {published data only}
- McGrath ER, Beiser AS, O'Donnell A, Himali JJ, Pase MP, Satizabal CL, et al. Determining vascular risk factors for dementia and dementia risk prediction across mid- to later-life: the Framingham Heart Study. Neurology 2022;99:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2019 {published data only}
- Park KM, Sung JM, Kim WJ, An SK, Namkoong K, Lee E, et al. Population-based dementia prediction model using Korean public health examination data: a cohort study. PLOS One 2019;14(2):e0211957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reijmer 2011 {published data only}
- Reijmer YD, Van BE, Van SS, Dekker JM, Nijpels G, Stehouwer DA, et al. Dementia risk score predicts cognitive impairment after a period of 15 years in a nondemented population. Dementia and Geriatric Cognitive Disorders 2011;31(2):152-7. [DOI] [PubMed] [Google Scholar]
Schaich 2021 {published and unpublished data}
- Schaich CL, Yeboah J, Espeland MA, Baker LD, Ding J, Hayden KM, et al. Association of vascular risk scores and cognitive performance in a diverse cohort: the Multi-Ethnic Study of Atherosclerosis. Journal of Gerontology: Medical Sciences 2021;77(6):1208-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiepers 2017 {published data only}
- Schiepers JG, Köhler S, Deckers K, Irving K, O'Donnell CA, Van AM, et al. Lifestyle for Brain Health (LIBRA): a new model for dementia prevention. International Journal of Geriatric Psychiatry 2018;33(1):167-75. [DOI] [PubMed] [Google Scholar]
Tynkkynen 2017 {published data only}
- Tynkkynen J, Hernesniemi JA, Laatikainen T, Havulinna AS, Salo P, Blankenberg S, et al. High-sensitivity cardiac troponin I and NT-proBNP as predictors of incident dementia and Alzheimer’s disease: the FINRISK Study. Journal of Neurology 2016;264(3):503-11. [DOI] [PubMed] [Google Scholar]
Virta 2013 {published and unpublished data}
- Virta JJ, Heikkilä K, Perola M, Koskenvuo M, Räihä I, Rinne JO, et al. Midlife cardiovascular risk factors and late cognitive impairment. European Journal of Epidemiology 2013;28(5):405-16. [DOI] [PubMed] [Google Scholar]
Vos 2017 {published data only}
- Vos JB, Boxtel PJ, Schiepers JG, Deckers K, De VM, Carrière I, et al. Modifiable risk factors for prevention of Dementia in Midlife, Late Life and the Oldest-Old: validation of the LIBRA index. Journal of Alzheimer's Disease 2017;58:537-47. [DOI] [PubMed] [Google Scholar]
Walters 2016 {published and unpublished data}
- Walters K, Hardoon S, Petersen I, Iliffe S, Omar RZ, Nazareth I, et al. Predicting dementia risk in primary care: development and validation of the Dementia Risk Score using routinely collected data. BMC Medicine 2016;14(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 2016 {published data only}
- Adams ML, Grandpre J. Dose-response gradients between a composite measure of six risk factors and cognitive decline and cardiovascular disease. Preventive Medicine 2016;91:329-34. [DOI] [PubMed] [Google Scholar]
Almeida 2021 {published data only}
- Almeida MP, Steptoe A, Cadar D. Markers of cognitive reserve and dementia incidence in the English Longitudinal Study of Ageing. British Journal of Psychiatry 2021;218(5):243-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anstey 2014 {published data only}
- Anstey KJ, Cherbuin N, Herath PM, Qiu C, Kuller LH, Lopez OL, et al. A self-report risk index to predict occurrence of dementia in three independent cohorts of older adults: the ANU-ADRI. PLOS One 2014;9(1):e86141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aschwanden 2020 {published data only}
- Aschwanden D, Aichele S, Ghisletta P, Terracciano A, Kliegel M, Sutin AR, et al. Predicting cognitive impairment and dementia: a machine learning approach. Journal of Alzheimer's Disease 2020;75:728-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashby 2020 {published data only}
- Ashby MK, Willie TD, Eldemire SD. Proportion of dementia explained by five key factors in Jamaica. Journal of Alzheimer's Disease 2020;78:603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2019 {published data only}
- Atkins JL, Delgado J, Pilling LC, Bowman K, Masoli AH, Kuchel GA, et al. Impact of low cardiovascular risk profiles on geriatric outcomes: Evidence from 421,000 participants in two cohorts. Journals of Gerontology: Medical Sciences 2019;74(3):350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baird 2022 {published data only}
- Baird K, Baillon S, Lau SL, Storey M, Lindesay J, Velayudhan L. Predictive factors for conversion to dementia in individuals with early-onset mild cognitive impairment. Dementia and Geriatric Cognitive Disorders 2022;50(6):548-53. [DOI] [PubMed] [Google Scholar]
Barnes 2014 {published data only}
- Barnes DE, Beiser AS, Lee A, Langa KM, Koyama A, Preis SR, et al. Development and validation of a brief dementia screening indicator for primary care. Alzheimer's & Dementia 2014;10(6):656-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ben 2020 {published data only}
- Ben MZ, Haas K, Black CM, Khandker RK, Chandrasekaran V, Lipton R, et al. Predicting dementia with routine care EMR data. Artificial Intelligence in Medicine 2020;102:101771. [DOI] [PubMed] [Google Scholar]
Beydoun 2020 {published data only}
- Beydoun MA, Kivimaki M. Midlife obesity, related behavioral factors, and the risk of dementia in later life. Neurology 2020;94(2):53-4. [DOI] [PubMed] [Google Scholar]
Borenstein 2014 {published data only}
- Borenstein A, Mortimer J, Larson E. Factor scores for brain reserve, Alzheimer and vascular pathology are independent risk factors for dementia in a population-based cohort study: the Kame Project. Neurology 2014;82 Suppl 10:58. [Google Scholar]
Borges 2017 {published data only}
- Borges MK, Ferrari AJ, De CV. Validity and reliability of the Brazilian Portuguese version of the ANU-ADRI. Alzheimer's & Dementia 2017;13:P1199-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brewster 2012 {published data only}
- Brewster P, Tuokko H, MacDonald S. Inter-test variability contributes independently to the five-year prediction of Alzheimer's disease in nondemented older adults. Alzheimer's & Dementia 2012;8:P369. [DOI] [PubMed] [Google Scholar]
Calvin 2020 {published data only}
- Calvin CM, deBoer C, Raymont V, Gallacher J, Koychev I. Prediction of Alzheimer's disease biomarker status defined by the 'ATN framework' among cognitively healthy individuals: results from the EPAD longitudinal cohort study. Alzheimer's Research and Therapy 2020;12(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherbuin 2019 {published data only}
- Cherbuin N, Shaw ME, Walsh E, Sachdev P, Anstey KJ. Validated Alzheimer’s Disease Risk Index (ANU-ADRI) is associated with smaller volumes in the default mode network in the early 60s. Brain Imaging and Behavior 2019;13(1):65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colpo 2016 {published data only}
- Colpo M, Mossello E, DelPanta V, Sini M, Fedecostante M, Bandinelli S. Predicting the risk of cognitive decline in old age: the ANU-ADRI score in the INCHIANTI study. Gerontologist 2016;56 Suppl 3:439. [Google Scholar]
Copenhaver 2021 {published data only}
- Copenhaver MM, Sanborn V, Shrestha R, Mistler CB, Sullivan MC, Gunstad J. Developing a cognitive dysfunction risk score for use with opioid-dependent persons in drug treatment. Drug and Alcohol Dependence 2021;224:108726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dallora 2020 {published data only}
- Dallora AL, Minku L, Mendes E, Rennemark M, Anderberg P, Sanmartin BJ. Multifactorial 10-year prior diagnosis prediction model of dementia. International Journal of Environmental Research and Public Health 2020;17(18):6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Debette 2011 {published data only}
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011;77(5):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deckers 2019a {published data only}
- Deckers K, Nooyens A, Van BM, Verhey F, Verschuren M, Köhler S. Gender and educational differences in the association between lifestyle and cognitive decline over 10 years: the Doetinchem Cohort Study. Journal of Alzheimer's Disease 2019;70(s1):S31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deckers 2019b {published data only}
- Deckers K, Cadar D, Van PJ, Verhey RJ, Steptoe A, Köhler S. Modifiable risk factors explain socioeconomic inequalities in dementia risk: evidence from a population-based prospective cohort study. Journal of Alzheimer's Disease 2019;71:557-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeFina 2013 {published data only}
- DeFina LF, Willis BL, Radford NB, Gao A, Leonard D, Haskell WL, et al. The association between midlife cardiorespiratory fitness levels and later-life dementia. Annals of Internal Medicine 2013;158(3):162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeRight 2014 {published data only}
- DeRight, Jonathan. Detection of dementia risk in primary care: preliminary investigation of a composite dementia risk score in veterans. New York (USA): Syracuse University, 2014. [Google Scholar]
DeRight 2015 {published data only}
- DeRight J, Jorgensen RS, Cabral MJ. Composite cardiovascular risk scores and neuropsychological functioning: a meta-analytic review. Annals of Behavioral Medicine 2015;49(3):344-57. [DOI] [PubMed] [Google Scholar]
Downer 2016 {published data only}
- Downer B, Veeranki SP, Wong R. A late life risk index for severe cognitive impairment in Mexico. Journal of Alzheimer's Disease 2016;52:191-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ecay 2018 {published data only}
- Ecay TM, Estanga A, Tainta M, Izagirre A, Garcia SM, Villanua J, et al. Increased CAIDE dementia risk, cognition, CSF biomarkers, and vascular burden in healthy adults. Neurology 2018;91(3):e217. [DOI] [PubMed] [Google Scholar]
Enache 2016 {published data only}
- Enache D, Solomon A, Cavallin L, Kåreholt, Kramberger MG, Aarsland D, et al. CAIDE dementia risk score and biomarkers of neurodegeneration in memory clinic patients without dementia. Neurobiology of Aging 2016;42:124-31. [DOI] [PubMed] [Google Scholar]
Gonzalez 2018 {published data only}
- Gonzalez HM, Tarraf W, Harrison K, Windham BG, Tingle J, Alonso A, et al. Midlife cardiovascular health and 20-year cognitive decline: Atherosclerosis Risk in Communities Study results. Alzheimer's & Dementia 2018;14(5):579-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gourley 2019 {published data only}
- Gourley D, Pasha EP, Kaur SS, Haley AP, Tanaka H. CAIDE dementia risk score indicates cortical thinning in low-risk, middle-aged adults. Journal of Federation of American Societies for Experimental Biology 2019;33(S1):737. [Google Scholar]
Gourley 2020 {published data only}
- Gourley D, Pasha EP, Kaur SS, Haley AP, Tanaka H. Association of dementia and vascular risk scores with cortical thickness and cognition in low-risk middle-aged adults. Alzheimer's Disease and Associated Disorders 2020;34(4):313-7. [DOI] [PubMed] [Google Scholar]
Graves 2019 {published data only}
- Graves KG, May HT, Jacobs V, Knowlton KU, Muhlestein JB, Lappe DL, et al. CHA2DS2-VASc scores and intermountain mortality risk scores for the joint risk stratification of dementia among patients with atrial fibrillation. Heart Rhythm 2019;16(1):3-9. [DOI] [PubMed] [Google Scholar]
Gupta 2015 {published data only}
- Gupta A, Preis SR, Beiser A, Devine S, Hankee L, Seshadri S, et al. Mid-life cardiovascular risk impacts memory function: the Framingham Offspring Study. Alzheimer Disease & Associated Disorders 2015;29(2):117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hakansson 2011 {published data only}
- Hakansson K, Helkala EL, Soininen H, Nissinen A, Mohammed A, Winblad B, et al. Perceived marital problems in midlife are associated with cognitive health in later life. Alzheimer's & Dementia 2011;7:S595. [Google Scholar]
Hazzouri 2013 {published data only}
- Hazzouri ZA, Haan MN, Neuhaus JM, Pletcher M, Peralta CA, López L, et al. Cardiovascular risk score, cognitive decline, and dementia in older Mexican Americans: the role of sex and education. Journal of the American Heart Association 2013;2(2):e004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heger 2019 {published data only}
- Heger I, Deckers K, Schram MT, Stehouwer DA, Schaper NC, Dagnelie P, et al. Associations of the Lifestyle For Brain Health (LIBRA) index with structural brain changes and cognition: results from the Maastricht study. Alzheimer's & Dementia 2019;15:P890-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hessler 2016 {published data only}
- Hessler JB, Ander KH, Brönner M, Etgen T, Förstl H, Poppert H, et al. Predicting dementia in primary care patients with a cardiovascular health metric: a prospective population-based study. BMC Neurology 2016;16:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hou 2019 {published data only}
- Hou XH, Feng L, Zhang C, Cao XP, Tan L, Yu JT. Models for predicting risk of dementia: a systematic review. Journal of Neurology, Neurosurgery and Psychiatry 2019;90(4):373. [DOI] [PubMed] [Google Scholar]
Huang 2020 {published data only}
- Huang S, Qi GL, Amzal B. Prediction of Alzheimer's disease from asymptomatic stages using Machine learning (ML) models. Value in Health 2020;23:S274. [Google Scholar]
Iso 2021 {published data only}
- Iso MP, Kaprio J, Lindgren N, Rinne JO, Vuoksimaa E. Middle-age dementia risk scores and old-age cognition: a quasi-experimental population-based twin study with over 20-year follow-up. Journal of Neurology, Neurosurgery and Psychiatry 2021;92(3):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacqmin 2014 {published data only}
- Jacqmin GH, Blanche P, Chary E, Loubère L, Amieva H, Dartigues JF. Prognostic score for predicting risk of dementia over 10 years while accounting for competing risk of death. American Journal of Epidemiology 2014;180(8):790-98. [DOI] [PubMed] [Google Scholar]
Juul 2020 {published data only}
- Juul RI, Rasmussen KL, Nordestgaard BG, Tybjærg HA, Frikke SR. Impact of cardiovascular risk factors and genetics on 10-year absolute risk of dementia: risk charts for targeted prevention. European Heart Journal 2020;41(41):4024-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaffashian 2011 {published data only}
- Kaffashian S, Dugravot A, Nabi H, Batty GD, Brunner E, Kivimäki M, et al. Predictive utility of the Framingham general cardiovascular disease risk profile for cognitive function: evidence from the Whitehall II study. European Heart Journal 2011;32(18):2326-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaffashian 2013a {published data only}
- Kaffashian S, Dugravot A, Brunner EJ, Sabia S, Ankri J, Kivimäki M, et al. Midlife stroke risk and cognitive decline: a 10-year follow-up of the Whitehall II cohort study. Alzheimer's & Dementia 2013;9(5):572-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaffashian 2013b {published data only}
- Kaffashian S, Dugravot A, Elbaz A, Shipley MJ, Sabia S, Kivimäki M, et al. Predicting cognitive decline. Neurology 2013;80(14):1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalmijn 2000 {published data only}
- Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. Arteriosclerosis, Thrombosis, and Vascular Biology 2000;20(10):2255-60. [DOI] [PubMed] [Google Scholar]
Kamat 2010 {published data only}
- Kamat SM, Kamat AS, Grossberg GT. Dementia risk prediction: are we there yet? Clinics in Geriatric Medicine 2010;26(1):113-23. [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee SH, Han K, Cho H, Park YM, Kwon HS, Kang G, et al. Variability in metabolic parameters and risk of dementia: a nationwide population-based study. Alzheimer's Research & Therapy 2018;10(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li SS, Zheng J, Mei B, Wang HY, Zheng M, Zheng K. Correlation study of Framingham risk score and vascular dementia: an observational study. Medicine 2017;96(50):e8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2018c {published data only}
- Li J, Ogrodnik M, Devine S, Auerbach S, Wolf PA, Au R. Practical risk score for 5-, 10-, and 20-year prediction of dementia in elderly persons: Framingham Heart Study. Alzheimer's & Dementia 2018;14(1):35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2020 {published data only}
- Liang Y, Ngandu T, Laatikainen T, Soininen H, Tuomilehto J, Kivipelto M, et al. Cardiovascular health metrics from mid- to late-life and risk of dementia: a population-based cohort study in Finland. PLOS Medicine 2020;17(12):e1003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Licher 2018 {published data only}
- Licher S, Leening JG, Yilmaz P, Wolters FJ, Heeringa J, Bindels JE, et al. Development and validation of a dementia risk prediction model in the general population: an analysis of three longitudinal studies. American Journal of Psychiatry 2018;176(7):543-51. [DOI] [PubMed] [Google Scholar]
Licher 2019 {published data only}
- Licher S, Ahmad S, Karamujić H, Voortman T, Leening JG, Ikram MA, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nature Medicine 2019;25(9):1364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ling 2022 {published data only}
- Ling TC, Chang CC, Li CYi, Sung JM, Sun CY, Tsai KJ, et al. Development and validation of the dialysis dementia risk score: a retrospective, population-based, nested case-control study. European Journal of Neurology 2022;29(1):59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lins 2017 {published data only}
- Lins A, Muniz M, Garcia A, Gomes A, Cabral R, Bastos FC. Using artificial neural networks to select the parameters for the prognostic of mild cognitive impairment and dementia in elderly individuals. Computer Methods and Programs in Biomedicine 2017;152:93-104. [DOI] [PubMed] [Google Scholar]
Llewellyn 2008 {published data only}
- Llewellyn DJ, Lang IA, Xie J, Huppert FA, Melzer D, Langa KM. Framingham Stroke Risk Profile and poor cognitive function: a population-based study. BMC Neurology 2008;8(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2020 {published data only}
- Lu X, Chen J, Shu H, Wang Z, Shi Y, Yuan Y, et al. Predicting conversion to Alzheimer's disease among individual high-risk patients using the characterizing AD risk events index model. CNS Neuroscience and Therapeutics 2020;26(7):720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malik 2021 {published data only}
- Malik R, Georgakis MK, Neitzel J, Rannikmäe K, Ewers M, Seshadri S, et al. Midlife vascular risk factors and risk of incident dementia: longitudinal cohort and Mendelian randomization analyses in the UK Biobank. Alzheimer's Dementia 2021;17(9):1422-31. [DOI] [PubMed] [Google Scholar]
Maria 2014 {published data only}
- Maria P, Muriel R, Kate I. Towards a profile of dementia risk and online supports for dementia risk reduction: translating findings from a robust model based on modifiable risk factors. In: Irish Journal of Medical Science. Vol. 183. 2014:269-387.
Mehta 2016 {published data only}
- Mehta HB, Mehta V, Tsai CL, Chen H, Aparasu RR, Johnson ML. Development and validation of the RxDx-Dementia Risk Index to predict dementia in patients with Type 2 Diabetes and hypertension. Journal of Alzheimer's Disease 2016;49:423-32. [DOI] [PubMed] [Google Scholar]
Novotni 2019 {published data only}
- Novotni G, Jakimovska M, Novotni A, Aleksovski V. Predictive dementia risk score in cognitively normal adult children of sporadic Alzheimer’s disease patients. European Journal of Neurology 2019;26(S1):357. [Google Scholar]
Ogunmoroti 2016 {published data only}
- Ogunmoroti O, Allen NB, Cushman M, Michos ED, Rundek T, Rana JS, et al. Association between Life's Simple 7 and noncardiovascular disease: the multi-ethnic study of atherosclerosis. Journal of the American Heart Association 2016;5(10):e003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pase 2016a {published data only}
- Pase M, Beiser A, Enserro D, Xanthakis V, Aparicio HJ, Satizabal C, et al. Adherence to ideal cardiovascular health guidelines prevents vascular brain injury, dementia and cognitive decline. Neurology 2016;86 Suppl 16:1201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pase 2016b {published data only}
- Pase MP, Beiser A, Enserro D, Xanthakis V, Aparicio H, Satizabal CL, et al. Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke 2016;47(5):1201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pekkala 2017 {published data only}
- Pekkala T, Hall A, Lötjönen J, Mattila J, Soininen H, Ngandu T, et al. Development of a late-life dementia prediction index with supervised machine learning in the population-based CAIDE study. Journal of Alzheimer's Disease 2017;55:1067-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pettigrew 2020 {published data only}
- Pettigrew C, Soldan A, Wang J, Wang MC, Arthur K, Moghekar A, et al. Association of midlife vascular risk and AD biomarkers with subsequent cognitive decline. Neurology 2020;95(23):e3093-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phongpreecha 2020 {published data only}
- Phongpreecha T, Cholerton B, Mata IF, Zabetian CP, Poston KL, Aghaeepour N, et al. Multivariate prediction of dementia in Parkinson’s disease. Parkinson's Disease 2020;6(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabin 2011 {published data only}
- Rabin L, Wang C, Katz M, Derby C, Lipton R. Comparison of statistical approaches to optimize scoring of participant/informant reports to predict future dementia. Alzheimer's & Dementia 2011;7:S261. [Google Scholar]
Reem 2019 {published data only}
- Reem BH, Tomas EW. A machine learning approach towards detecting dementia based on its modifiable risk factors. International Journal of Advanced Computer Science and Applications 2019;10(8):148-58. [Google Scholar]
Rundek 2020 {published data only}
- Rundek T, Gardener H, Dias AS, Loewenstein DA, Duara R, et al. Global Vascular Risk Score and CAIDE dementia risk score predict cognitive function in the Northern Manhattan study. Journal of Alzheimer's Disease 2020;73:1221-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabia 2009 {published data only}
- Sabia S, Nabi H, Kivimaki M, Shipley MJ, Marmot MG, Singh MA. Health behaviors from early to late midlife as predictors of cognitive function: the Whitehall II study. American Journal of Epidemiology 2009;170(4):428-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabia 2019 {published data only}
- Sabia S, Fayosse A, Dumurgier J, Schnitzler A, Empana JP, Ebmeier KP, et al. Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 year follow-up of Whitehall II cohort study. BMJ 2019;366:l4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sacktor 2017 {published data only}
- Sacktor N, Soldan A, Grega M, Farrington L, Cai Q, Wang MC, et al. The BIOCARD index: a summary measure to predict onset of mild cognitive impairment. Alzheimer Disease & Associated Disorders 2017;31(2):114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salvado 2019 {published data only}
- Salvado G, Brugulat SA, Sudre CH, Grau RO, Suárez CM, Falcon C, et al. Spatial patterns of white matter hyperintensities associated with Alzheimer’s disease risk factors in a cognitively healthy middle-aged cohort. Alzheimer's Research & Therapy 2019;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Senanarong 2017 {published data only}
- Senanarong V, Hunnangkul S, Harnphadungkit K. Dementia risk score from Thai population study. Journal of the Neurological Sciences 2017;381:118-9. [Google Scholar]
Solomon 2014 {published data only}
- Solomon A, Ngandu T, Soininen H, Hallikainen MM, Kivipelto M, Laatikainen T. Validity of dementia and Alzheimer's disease diagnoses in Finnish national registers. Alzheimer's & Dementia 2014;10(3):303-9. [DOI] [PubMed] [Google Scholar]
Solomon 2015 {published data only}
- Solomon A, Soininen H, Kivipelto M. Dementia risk estimation tools and their use in clinical trials. In: Neurodegenerative Diseases. Vol. 15 Suppl 1. 2015:178.
Song 2011 {published data only}
- Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology 2011;77(3):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephen 2017 {published data only}
- Stephen R, Liu Y, Ngandu T, Rinne JO, Kemppainen N, Parkkola R, et al. Associations of CAIDE dementia risk score with MRI, PIB-PET measures, and cognition. Journal of Alzheimer's Disease 2017;59(2):695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephen 2020 {published data only}
- Stephen R, Soininen H. Biomarker validation of a dementia risk prediction score. Nature Reviews Neurology 2020;16(3):135-6. [DOI] [PubMed] [Google Scholar]
Tang 2015 {published data only}
- Tang YH, Harrison SL, Errington L, Gordon MF, Visser PJ, Novak G, et al. Current developments in dementia risk prediction modelling: an updated systematic review. PLOS One 2015;10(9):e0136181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2020 {published data only}
- Tang YH, Price CI, Robinson L, Exley C, Desmond DW, Kohler S, et al. Assessing the predictive validity of simple dementia risk models in harmonized stroke cohorts. Stroke 2020;51(7):2095-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2010 {published data only}
- Tierney MC, Moineddin R, McDowell I. Prediction of all-cause dementia using neuropsychological tests within 10 and 5 Years of diagnosis in a community-based sample. Journal of Alzheimer's Disease 2010;22:1240-31. [DOI] [PubMed] [Google Scholar]
Torres 2020 {published data only}
- Torres S, Alexander A, O’Bryant S, Medina LD. Cognition and the predictive utility of three risk scores in an ethnically diverse sample. Journal of Alzheimer's Disease 2020;75:1049-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tortelli 2017 {published data only}
- Tortelli R, Lozupone M, Guerra V, Barulli MR, Imbimbo BP, Capozzo R, et al. Midlife metabolic profile and the risk of late-life cognitive decline. Journal of Alzheimer's Disease 2017;59:130-121. [DOI] [PubMed] [Google Scholar]
Van 2019 {published data only}
- Van EA, Joosten H, Gansevoort RT, Slaets PJ, Izaks GJ. Treatable vascular risk and cognitive performance in persons aged 35 years or older: longitudinal study of six years. Journal of Prevention of Alzheimer's Disease 2019;6(1):42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viswanathan 2015 {published data only}
- Viswanathan A, Macklin EA, Betensky R, Hyman B, Smith EE, Blacker D. The influence of vascular risk factors and stroke on cognition in late life: analysis of the NACC cohort. Alzheimer Disease & Associated Disorders 2015;29(4):287-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vuoksimaa 2016 {published data only}
- Vuoksimaa E, Rinne JO, Lindgren N, Heikkilä K, Koskenvuo M, Kaprio J. Middle age self-report risk score predicts cognitive functioning and dementia in 20–40 years. Alzheimer's & Dementia 2016;4:118-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vuorinen 2015 {published data only}
- Vuorinen M, Spulber G, Damangir S, Niskanen E, Ngandu T, Soininen H, et al. Midlife CAIDE dementia risk score and dementia-related brain changes up to 30 years later on magnetic resonance imaging. Journal of Alzheimer's Disease 2015;44:101-93. [DOI] [PubMed] [Google Scholar]
Wang 2016 {published data only}
- Wang C, Ji X, Wu X, Tang Z, Zhang X, Guan S, et al. Frailty in relation to the risk of Alzheimer’s disease, dementia, and death in older Chinese adults: a seven-year prospective study. Journal fo Nutrition Health and Ageing 2016;21(6):648-54. [DOI] [PubMed] [Google Scholar]
Yaffe 2020 {published data only}
- Yaffe K, Bahorik AL, Hoang TD, Forrester S, Jacobs DR, Lewis CE, et al. Cardiovascular risk factors and accelerated cognitive decline in midlife. Neurology 2020;95(7):e839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2021 {published data only}
- Yang Y, Lv C, Li He, Chen K, Li X, Chen Y, et al. Community-based model for dementia risk screening: the Beijing Aging Brain Rejuvenation Initiative (BABRI) brain health system. Journal of the American Medical Directors Association 2021;22(7):1500-6. [DOI] [PubMed] [Google Scholar]
Yates 2014 {published data only}
- Yates P, Villemagne VL, Robertson J, Li C, Dennerstein L, Szoeke C, et al. Midlife vascular risk, apolipoprotein E4, and amyloid status 20 years later: results from the women's healthy ageing project. Alzheimer's & Dementia 2014;10:P228. [Google Scholar]
References to studies awaiting assessment
Anca 2018 {published data only}
- Anca IP, Irina MA, Ioana DA, Gabriel IP, Anna MH. Is CAIDE score an efficient tool in the prevention of Alzheimer's disease in general population? In: European Geriatric Medicine. Vol. 9. 2018:S172.
Kerut 2018 {published data only}
- Kerut SE, Lirette S, Abi SR, Turner ST, Benjamin EJ, Fornage M. CAIDE risk score prediction of short-term incident cognitive impairment in African Americans. Journal of American Geriatric Society 2018;66(S2):S290. [Google Scholar]
Khalid 2020 {published data only}
- Khalid W, Sluiman A, Terrera GM, Strachan M, Price J. Performance of dementia specific risk predictors in Edinburgh Type 2 Diabetes Study. Diabetic Medicine 2020;37(S1):46. [Google Scholar]
Rawtaer 2016 {published data only}
- Rawtaer I, Feng L, Yuen HK, Li J, Chong MS, Lim WS, et al. A risk score for the prediction of neurocognitive disorders among community-dwelling Chinese older adults. Dementia and Geriatric Cognitive Disorders 2016;41(5):348-58. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Fisher 2017 {published data only}
- Fisher S, Hsu A, Mojaverian N, Taljaard M, Huyer G, Manuel DG, et al. Dementia Population Risk Tool (DemPoRT): study protocol for a predictive algorithm assessing dementia risk in the community. BMJ Open 2017;7(10):e018018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Andrews 2017
- Andrews SJ, Eramudugolla R, Velez JI, Cherbuin N, Easteal S, Anstey KJ. Validating the role of the Australian National University Alzheimer's Disease Risk Index (ANU-ADRI) and a genetic risk score in progression to cognitive impairment in a population-based cohort of older adults followed for 12 years. Alzheimer's Research and Therapy 2017;9(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anstey 2013
- Anstey KJ, Cherbuin N, Herath PM. Development of a new method for assessing global risk of Alzheimer's disease for use in population health approaches to prevention. Prevention Science 2013;14(4):411-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Austin 2016
- Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133(6):601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2014
- Barnes DE, Beiser AS, Lee A, Langa KM, Koyama A, Preis SR, et al. Development and validation of a brief dementia screening indicator for primary care. Alzheimer's Dementia 2014;10(6):e656665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calster 2019
- Calster B, McLernon DJ, van SM, Wynants L, Steyerberg EW, Bossuyt P, et al. Calibration: the Achilles heel of predictive analytics. BMC Medicine 2019;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chosy 2019
- Chosy EJ, Edland SD, Gross N, Meyer MJ, Liu CY, Launer LJ, et al. The CAIDE dementia risk score and the Honolulu-Asia aging study. Dementia and Geriatric Cognitive Disorders 2019;48(3-4):164-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2015
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Medicine 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
Collins 2021
- Collins GS, Dhiman P, Andaur CL, Ma J, Hooft L, Reitsma JB, et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open 2021;11(7):e048008-e048008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Damen 2019
- Damen JA, Pajouheshnia R, Heus P, Moons GM, Reitsma JB, Scholten PM, et al. Performance of the Framingham risk models and pooled cohort equations for predicting 10-year risk of cardiovascular disease: a systematic review and meta-analysis. BMC Medicine 2019;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Debray 2014
- Debray TP, Koffijberg H, Nieboer D, Vergouwe Y, Steyerberg EW, Moons KG. Meta-analysis and aggregation of multiple published prediction models. Statistics in Medicine 2014;33(14):2341-62. [DOI] [PubMed] [Google Scholar]
Debray 2017
- Debray TP, Damen JA, Snell KI, Ensor J, Hooft L, Reitsma JB, et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ 2017;356:i6460. [DOI] [PubMed] [Google Scholar]
Debray 2018
- Debray PA, Moons KG, Riley RD. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: a comparison of new and existing tests. Research Synthesis Methods 2018;9(1):41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deckers 2015
- Deckers K, Boxtel MP, Schiepers OJ, Vugt M, Muñoz Sánchez JL, Anstey KJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. International Journal of Geriatric Psychiatry 2015;30(3):234-46. [DOI] [PubMed] [Google Scholar]
D’Agostino 2008
- D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General Cardiovascular Risk Profile for Use in Primary Care. Circulation 2008;117(6):743-53. [DOI] [PubMed] [Google Scholar]
England 1994
- England B, Brun A, Gustafson L, Passant U. Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. Journal of Neurology, Neurosurgery and Psychiatry 1994;57(4):416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foroutan 2020
- Foroutan F, Guyatt G, Zuk V, Vandvik PO, Alba AC, Mustafa R, et al. GRADE Guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. Journal of Clinical Epidemiology 2020;121:62-70. [DOI] [PubMed] [Google Scholar]
Foroutan 2022
- Foroutan F, Guyatt G, Trivella M, Kreuzberger N, Skoetz N, Riley RD, et al. GRADE concept paper 2: concepts for judging certainty on the calibration of prognostic models in a body of validation studies. Journal of Clinical Epidemiology 2022;143:202-11. [DOI] [PubMed] [Google Scholar]
Goerdten 2019
- Goerdten J, Cukic I, Danso SO, Carriere I, Muniz TG. Statistical methods for dementia risk prediction and recommendations for future work: a systematic review. Alzheimer's Dementia 2019;5:563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goff 2014
- Goff DC, Lloyd DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA Guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Journal of the American College of Cardiology 2014;63(25, Part B):2935-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380-2. [DOI] [PubMed] [Google Scholar]
Halima 2018
- Halima D, Stephanie W, David R, Jin H, Amber W, Betty B, et al. Health services utilization in older adults with dementia receiving care coordination: the MIND at Home trial. Health Services Research 2018;53(1):556-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins PT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
Hou 2019
- Hou XH, Feng L, Zhang C, Cao XP, Tan L, Yu JT. Models for predicting risk of dementia: a systematic review. Journal of Neurology, Neurosurgery and Psychiatry 2019;90(4):373-9. [DOI] [PubMed] [Google Scholar]
Iorio 2015
- Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350(7):870. [DOI] [PubMed] [Google Scholar]
Kivipelto 2006
- Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurology 2006;5(9):735-41. [DOI] [PubMed] [Google Scholar]
Licher 2019
- Licher S, Leening JG, Yilmaz P, Wolters FJ, Heeringa J, Bindels PJ, et al. Development and validation of a dementia risk prediction model in the general population: an analysis of three longitudinal studies. American Journal of Psychiatry 2019;176(7):543-51. [DOI] [PubMed] [Google Scholar]
Lindstrom 2003
Livingston 2017
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390(10113):2673-4. [DOI] [PubMed] [Google Scholar]
Livingston 2020
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396(10248):413-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Longo 2018
- Longo DL, Fauci AS, Kasper DL, Hauser SL, Loscalzo J, Jameson J, editor(s). Harrison's Principles of Internal Medicine. 20th edition. New York (NY): McGraw-Hill Education, 2018. [Google Scholar]
McGuinness 2021
- McGuinness LA, Higgins PT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 2021;12(1):55-61. [DOI] [PubMed] [Google Scholar]
McKeith 2017
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89(1):88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia 2011;7(3):263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menéndez 2014
- Menéndez González M, editor. Atlas of Biomarkers for Alzheimer's disease. New York (NY): Springer, 2014. [Google Scholar]
Moons 2014
- Moons KG, Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLOS Medicine 2014;11(10):e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moons 2015
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg WE, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): explanation and elaboration. Annals of Internal Medicine 2015;162(2):W1-73. [DOI] [PubMed] [Google Scholar]
Moons 2018
- Moons KG, Hooft L, Williams K, Hayden JA, Damen JA, Riley RD. Implementing systematic reviews of prognosis studies in Cochrane. Cochrane Database of Systematic Reviews 2018;10:ED000129. [10.1002/14651858.ED000129.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moons 2019
- Moons KG, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Annals of Internal Medicine 2019;170(1):W1-33. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar KB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
PHG Foundation 2019
- PHG Foundation. Dementia risk prediction models; What do policy makers need to know? Available from www.phgfoundation.org/media/84/download/dementia-risk-prediction-models.pdf 2019.
Prince 2013
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and meta-analysis. Alzheimer's Dementia 2013;9(1):e63-75. [DOI] [PubMed] [Google Scholar]
Reijmer 2011
- Reijmer YD, den Berg E, Sonsbeek S, Dekker JM, Nijpels G, Stehouwer CD, et al. Dementia risk score predicts cognitive impairment after a period of 15 years in a non-demented population. Dementia and Geriatric Cognitive Disorders 2011;31(2):152-7. [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes WS, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982-90. [DOI] [PubMed] [Google Scholar]
Riley 2010
- Riley RD, Steyerberg EW. Meta-analysis of a binary outcome using individual participant data and aggregate data. Research Synthesis Methods 2010;1(1):2-19. [DOI] [PubMed] [Google Scholar]
Roman 1993
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu CK, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993;43(2):250-60. [DOI] [PubMed] [Google Scholar]
Russo 2017
- Russo JA, Coker JK, King JH, editor(s). DSM-5® and family systems. New York (NY): Springer, 2017. [Google Scholar]
Sedgwick 2012
- Sedgwick P. Meta-analyses: tests of heterogeneity. BMJ 2012;344:e3971. [Google Scholar]
Sedgwick 2013
- Sedgwick P. Meta-analyses: how to read a funnel plot. BMJ 2013;346:1342. [DOI] [PubMed] [Google Scholar]
Snell 2016
- Snell KI, Hua H, Debray TP, Ensor J, Look MP, Moons KG, et al. Multivariate meta-analysis of individual participant data helped externally validate the performance and implementation of a prediction model. Journal of Clinical Epidemiology 2016;69:40-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snell 2018
- Snell KI, Ensor J, Debray TP, Moons KG, Riley RD. Meta-analysis of prediction model performance across multiple studies: which scale helps ensure between-study normality for the C-statistic and calibration measures? Statistical Methods in Medical Research 2018;27(11):3505-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephan 2010
- Stephan BC, Kurth T, Matthews FE, Brayne C, Dufouil C. Dementia risk prediction in the population: are screening models accurate? Nature Reviews Neurology 2010;6(6):318-26. [DOI] [PubMed] [Google Scholar]
Stephan 2020
- Stephan BC, Pakpahan E, Siervo M, Licher S, Muniz TG, Mohan D, et al. Prediction of dementia risk in low-income and middle-income countries (the 10/66 Study): an independent external validation of existing models. Lancet Global Health 2020;8(4):e524-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2020
- Stevens RJ, Poppe KK. Validation of clinical prediction models: what does the "calibration slope" really measure? Journal of Clinical Epidemiology 2020;118:93-9. [DOI] [PubMed] [Google Scholar]
Steyerberg 2001
- Steyerberg EW, Harrell FE, Borsboom JM, Eijkemans JC, Vergouwe Y, Habbema DF. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. Journal of Clinical Epidemiology 2001;54(8):774-81. [DOI] [PubMed] [Google Scholar]
Steyerberg 2019
- Steyerberg EW, editor. Clinical prediction models: a practical approach to development, validation, and updating. 2nd edition. Cham: Springer International Publishing, 2019. [Google Scholar]
Steyerberg 2014
- Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. European Heart Journal 2014;35(29):1925-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2015
- Tang EY, Harrison SL, Errington L, Gordon MF, Visser PJ, Novak G, et al. Current developments in dementia risk prediction modelling: an updated systematic review. PLOS One 2015;10(9):e0136181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2021
- Taylor-RM, Edwards S, Noel-Storr AH, McCleery J, Myint PK, Soiza R, et al. Anticholinergic burden (prognostic factor) for prediction of dementia or cognitive decline in older adults with no known cognitive syndrome. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013540. [DOI: 10.1002/14651858.CD013540.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vieira 2013
- Vieira RT, Caixeta L, Machado S, Silva AC, Nardi AE, Arias CO, et al. Epidemiology of early-onset dementia: a review of the literature. Clinical Practice and Epidemiology in Mental Health 2013;9:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Virta 2013
- Virta JJ, Heikkila K, Perola M, Koskenvuo M, Raiha I, Rinne JO, et al. Midlife cardiovascular risk factors and late cognitive impairment. European Journal of Epidemiology 2013;28(5):405-16. [DOI] [PubMed] [Google Scholar]
Walters 2016
- Walters K, Hardoon S, Petersen I, Iliffe S, Omar RZ, Nazareth I, et al. Predicting dementia risk in primary care: development and validation of the Dementia Risk Score using routinely collected data. BMC Medicine 2016;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2019
- World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines. www.who.int/publications/i/item/9789241550543 (accessed 20 April 2021).
Wolf 1991
- Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22(3):312-8. [DOI] [PubMed] [Google Scholar]
Wolff 2019
- Wolff RF, Moons KG, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Annals of Internal Medicine 2019;170(1):51-8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mohanannair Geethadevi 2021
- Mohanannair Geethadevi G, Quinn TJ, George J, Anstey K, Bell JS, Cross AJ. Multi-domain prognostic models used in middle aged adults without known cognitive impairment for predicting subsequent dementia. Cochrane Database of Systematic Reviews 2021, Issue 6. Art. No: CD014885. [DOI: 10.1002/14651858.CD014885] [DOI] [PMC free article] [PubMed] [Google Scholar]