Abstract
Background and purpose:
Radiation therapy for glioblastoma (GBM) typically involves large target volumes. The aim of this study was to examine the recurrence pattern of GBM following modern radiochemotherapy according to EORTC guidelines and provide dose and distance information for the choice of optimal target volume margins.
Materials and methods:
In this study, the recurrences of 97 GBM patients, treated with radiochemotherapy from 2013 to 2017 at the Medical Center - University of Freiburg, Germany were analysed. Dose and distance based metrices were used to derive recurrence patterns.
Results:
The majority of recurrences (75 %) occurred locally within the primary tumor area. Smaller had a higher rate of distant recurrences. Larger treated volumes did not show a clinical benefit regarding progression- free and overall survival.
Conclusion:
The identified recurrence pattern suggests that adjustments or reductions in target volume margins are feasible and could result in similar survival rates, potentially combined with a lower risk of side effects.
Keywords: Glioblastoma, Target volume, Radiotherapy, Recurrence
Glioblastoma (GBM) is one of the most lethal cancer types with median survival prognosis rates of only 9 months and up to 15–16 months if the standard of care, consisting in maximal surgical composing of the surgical resection and adjuvant radiochemotherapy, is performed [1].
Radiation therapy is thus one of the three main pillars in the treatment of primary GBM and is classically performed with a total dose of 60 Gy administered in 2 Gy fractions, 5 times per week. One of the crucial elements in its implementation is the definition of target volumes. There are two major guidelines to delineate the clinical target volume (CTV) in GBM. The European Organization for Research and Treatment of Cancer (EORTC) recommends to deliver the whole dose in one phase (2 Gy per fraction, ad 60 Gy). The gross tumor volume () consists of the resection cavity and any residual enhanced tumor. Further, the CTV is defined as an isotropic margin of 2 cm around the . The Radiotherapy and Oncology Group (RTOG) suggests to follow a two phase approach. During the first phase, a larger volume called GTV1 (46 Gy) consisting of the surgical cavity, residual tumor and surrounding edema (visible on T2 Flair) is used for the CTV generation. In a second step, a smaller volume called GTV2 (additional 14 Gy) cavity and residual tumor is used. The CTV1/2 for both GTV1 and GTV2 are constructed using a margin of 2 cm each. Correction may be necessary for CTV generation near anatomical barriers like the skull, falx, ventricles, brainstem and optic chiasm [2].
The resulting volumes are generally large and encompass a considerable volume of normal brain tissue, increasing the risk of local inflammation and radiation necrosis, with consecutive neurotoxicity and significant effects on quality of life. Resulting adverse events such as headaches, nausea, fatigue, seizures, functional, neurocognitive and/or psychosocial impairment are therefore more frequent with larger irradiated volumes. [3].
Whether or not the 2 cm-large margins around the are necessary is decided based on tumor infiltration and recurrence patterns. Previous publications have already performed a dose-based evaluation (e.g. [4,5,6]) using Dose Volume Histogram (DVH)-based approaches to evaluate recurrence locations and proved that most recurrences occur within the irradiation field. This analysis aims to consider not only the dose distribution, but also the actual distance between recurrence and initial tumor site in order to provide information on tumor growth and choice of optimal CTV-margins. We hypothesize that a smaller margin, related to less side effects and similar tumor control, might be the better option to treat GBM patients.
Material and methods
Cases
A total of 97 patients were retrospectively analyzed. All patients received a dose of 60 Gy in 5 × 2 Gy fractions/week, prescribed to an EORTC-based treatment volume. The target volume delineation aimed to include peri- tumoral edema in the PTV, but did not involve an expansion of the T2 Flair-volume with 2 cm, as recommended by the RTOG.
All patients were treated consecutively at the Medical Center University of Freiburg between January 2013 and December 2017 and underwent either 3D conformal radiotherapy (3D CRT), intensity modulated radiation therapy delivered with static beams (IMRT) or dynamic arcs (VMAT), or a combination of these techniques. Chemotherapy with temozolomide was administered parallel and adjuvant to irradiation according to the Stupp protocol [7].
Patients were followed-up with MRI investigations six-eight weeks after treatment and every three months thereafter. Tumor progression was diagnosed after discussion in an interdisciplinary team and involved confirmation through sequential MRI investigations, amino acid PET or resection with evidence of tumor. The recurrences at the date of first progression following treatment (in total ) were manually segmented by an experienced radiation oncologist.
For all patients, common demographic data (age and sex) and clinical data (survival, region of primary tumor, side, IDH and MGMT status, and extent of surgery) were available (see Table 1).
Table 1.
Demographic and clinical data of 97 patients included in this study treated at the Medical Center - University of Freiburg from 2013 to 2017.
| Demographics | |
|---|---|
|
| |
| female | 37 / 97 |
| male | 60 / 97 |
| age (diagnosis) | 58 +/− 10 |
| Survival [months] | |
| PFS | 7 |
| OS | 16 |
| Occurrence of primary tumor | |
| left | 40 / 97 |
| right | 47 / 97 |
| both sides | 10 / 97 |
| Resection type | |
| complete | 32 / 97 |
| partial | 52 / 97 |
| biopsy | 13 / 97 |
| IDH | |
| wildtype | 89 / 97 |
| mutated | 6 / 97 |
| unknown | 2 / 97 |
| MGMT | |
| yes | 24 / 97 |
| no | 45 / 97 |
| unknown | 28 / 97 |
| Location of primary tumor | |
| temporal | 35 / 97 |
| occipital | 2 / 97 |
| frontal | 20 / 97 |
| parietal | 9 / 97 |
| midline/butterfly | 10 / 97 |
| cerebellum | 1 / 97 |
| multiple lobes | 20 / 97 |
PFS: median progression free survival, OS: median overall survival, IDH: Isocitrate dehydrogenase, MGMT: O6 – methylguanine – DNAmethyl – transferase.
Recurrence distance analysis
The principle of the distance analysis is shown in Fig. 1 (a). The first step is the calculation of the center of mass () for each segmented recurrence (green dot within the recurrence). For simplification we assume tumor growth to be isotropic, thus the is considered as a “starting point” of the occurring recurrence. With respect to patients containing more than a single recurrence, all are calculated separately for the different recurrences. For each recurrence volume, the shortest euclidean distance II • II between its , , and the closest point within the point cloud is calculated in R3 using the following formula:
| (1) |
Fig. 1.

a: Schematical representation of the distance recurrence analysis. For illustration reasons the image is shown in R2 (instead of R3 - further details see text). b: Example of the marginal analysis (for illustration reasons the image is shown in R2 (instead of R3). White: . Light-grey: covered with 57 Gy. Dark-grey: Volume created by the marginal analysis using .
Each recurrence was also masked to the dose matrix and various other metrics were calculated (see Section 2.4).
High dose margin analysis
To evaluate the distance between the boundary and high dose volume (which is the volume receiving 95 % of the prescribed dose-95Dpre) we expand the set gradually in R3 leading to . is the expansion which is done using binary dilation. We increase , which can be interpreted as a margin until we capture all points of . Another condition is that we want to find the lowest possible.
| (2) |
Eq. (2) creates margins which are isotropic. As mentioned before margins are corrected by anatomical barriers in practice (see Fig. 1 (b)). This is not a limitation using this analysis as we don’t evaluate the resulting volume but solely the resulting distance. Thus an overestimation (e.g. in Fig. 1 (b) - upper part) has no effect on the analysis in general as we quantify the distance needed to capture .
To avoid too large due to single high dose voxels we softened up Eq. (2) in a way that not the full set of but 90 % of all voxels need to be captured.
To evaluate the effect of the high dose margins with respect to survival all patients were divided into two groups: I. smaller and II. larger or equal to the median of all . Subsequently Kaplan Meier Estimator of both groups were generated and compared using log-rank tests [8].
Metrics
Characteristics of recurrence patterns
In order to get an overview about the recurrence patterns, typical metrics have been utilized to describe them in both qualitative and quantitative ways.
All patients have been evaluated with respect to the frequency (single, multiple) and location of recurrences based on Lee et al. [4]. The method classifies recurrences as.
“Central” if at least 95% of Vrecur receive 0.95 Dpre,
“In-field” if at least 80% but less than 95% of Vrecur receive 0.95 Dpre, and.
“Marginal” if at least 20% but less than 80% of Vrecur receive 0.95Dpre, where Vrecur stands for the volume of the recurrence and Dpre stands for the prescribed dose (60 Gy). Other recurrences are considered as “Outside” (for examples see Fig. 2).
Fig. 2.
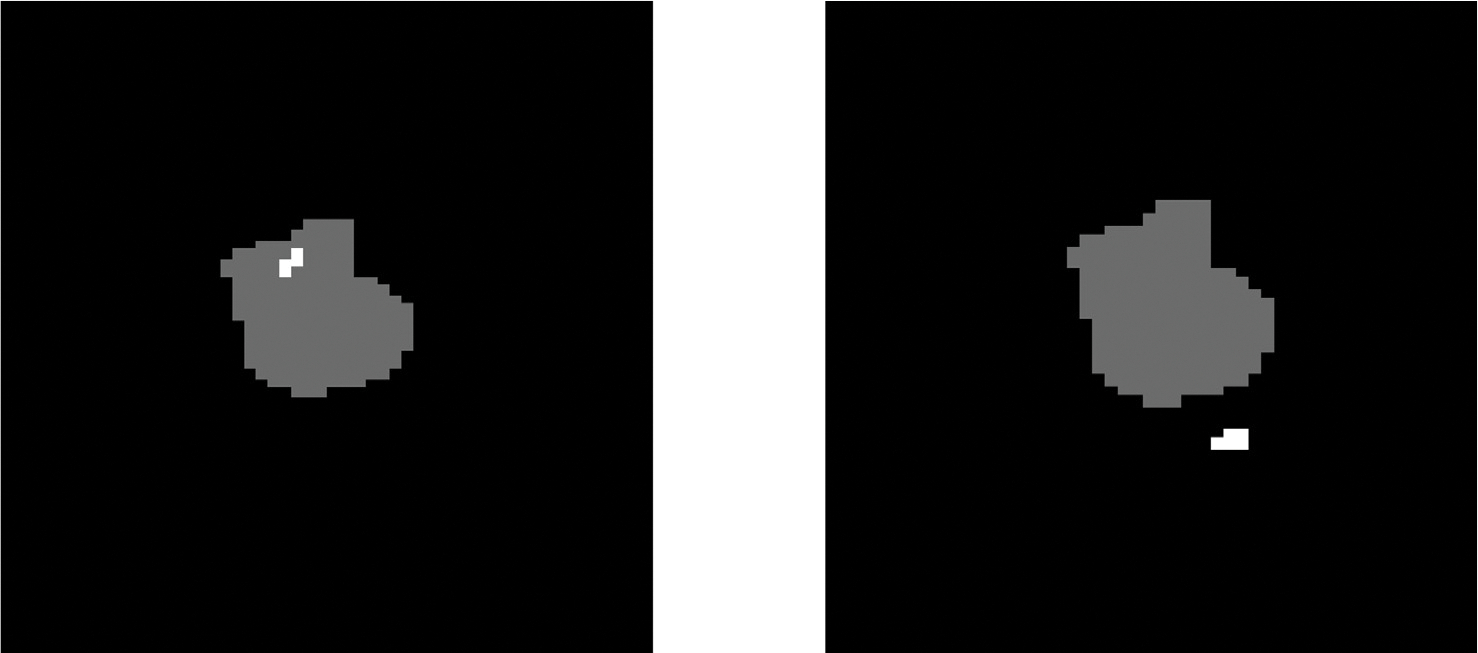
Examples for locations of recurrences (white) with respect to the (grey). Left: central recurrence within the . Right: marginal recurrence.
Dose-dependent metrices
For all masked doses the minimum, maximum, median, mean and standard deviation metrices were calculated using the standard numpy functions.
Volume-related metric
To measure the overlap of recurrence and , a volume-related metric was calculated as the intersection of the recurrence volume with the , relative to the volume of the recurrence:
| (3) |
Results
In this study 97 patients have been observed. The majority of patients (66) suffered a unifocal recurrence. Still, 31 patients developed a multifocal recurrence (23 patients had 2 lesions, 5 patients 3 lesions, and 3 patients 5 individual lesions). With respect to the classification based on Lee et al. [4] 99 recurrences occurred locally, 8 in-field, 11 marginally and 24 outside.
Fig. 3 shows Kaplan Meier survival curves of time to progression (left) and time to death (right) (see 2.3). The median high dose margin is 28 mm, which matches with target definition in practice (20 mm CTV margin plus additional 5 mm creating the PTV compensating potential position and treatment device uncertainties). High dose margins above 30 mm indicate treatment volumes receiving larger high dose volumes as usual (e.g. peritumoral edemas, which are also part of the PTV, but not ). According to log-rank tests both groups show no difference ( - Time to Progression and - Time to Death).
Fig. 3.
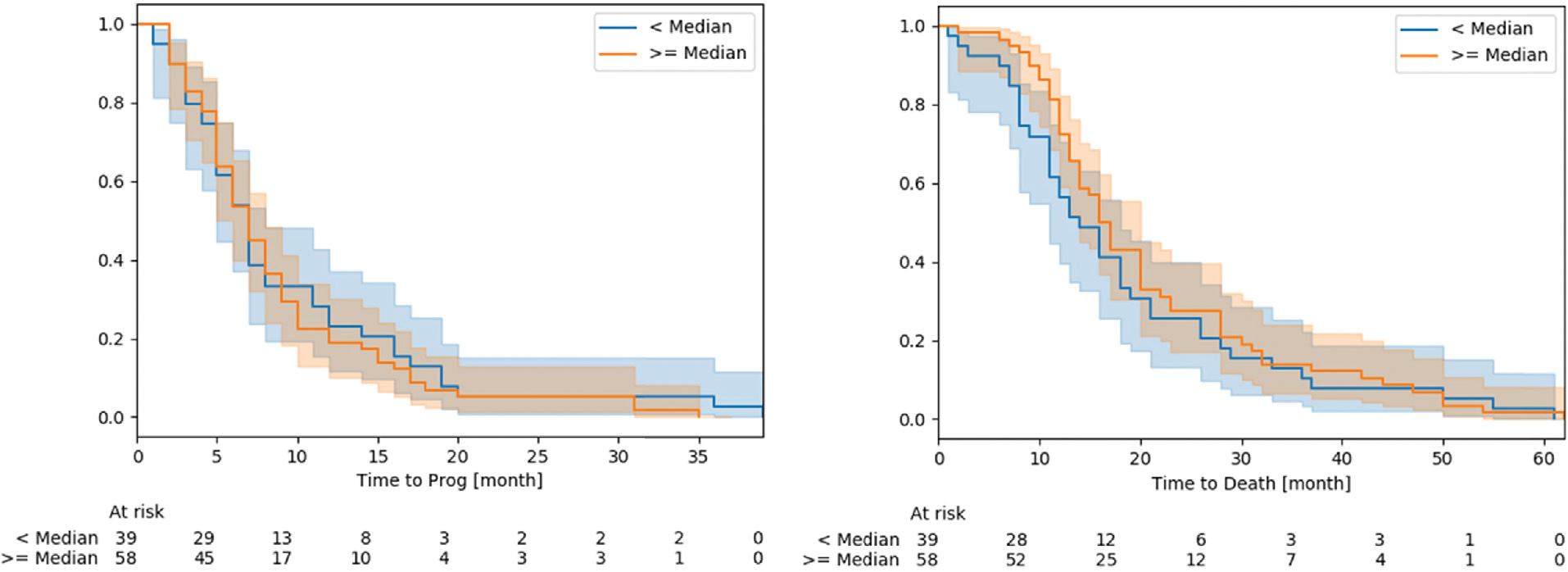
Kaplan Meier survival curves of Time to progression (left) and time to death (right), measured in months for both groups (smaller and larger or equal to the median . Shaded areas are the respective 95 % confidence levels. For details see text.
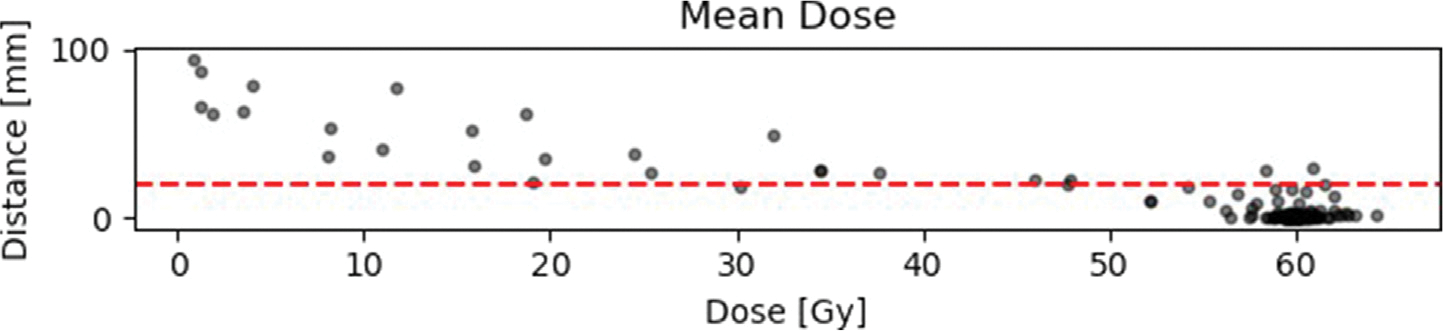
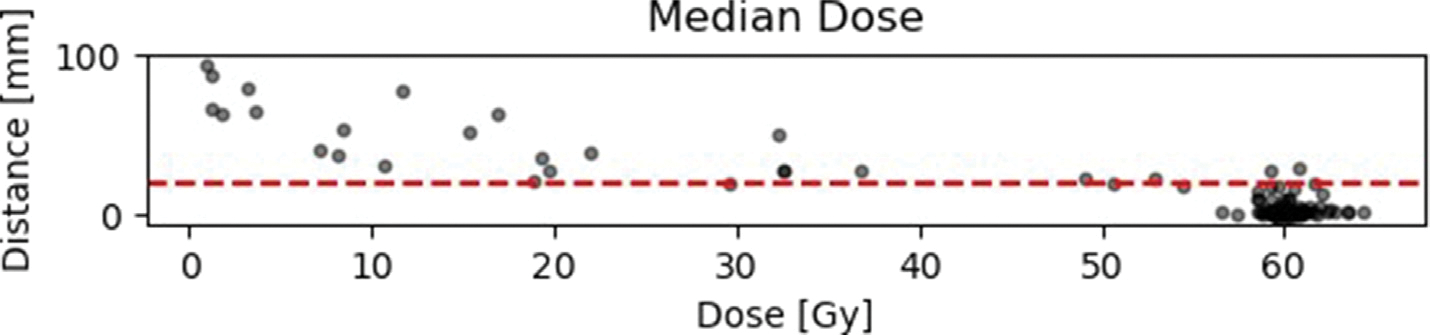
Fig. 4 (a), A.5 and A.6 show the results of the distance-dose analysis in scatter plots. Each dot represents an individual recurrence. The distance plotted along the y-axis is the distance determined in Subsection.
Fig. 4.
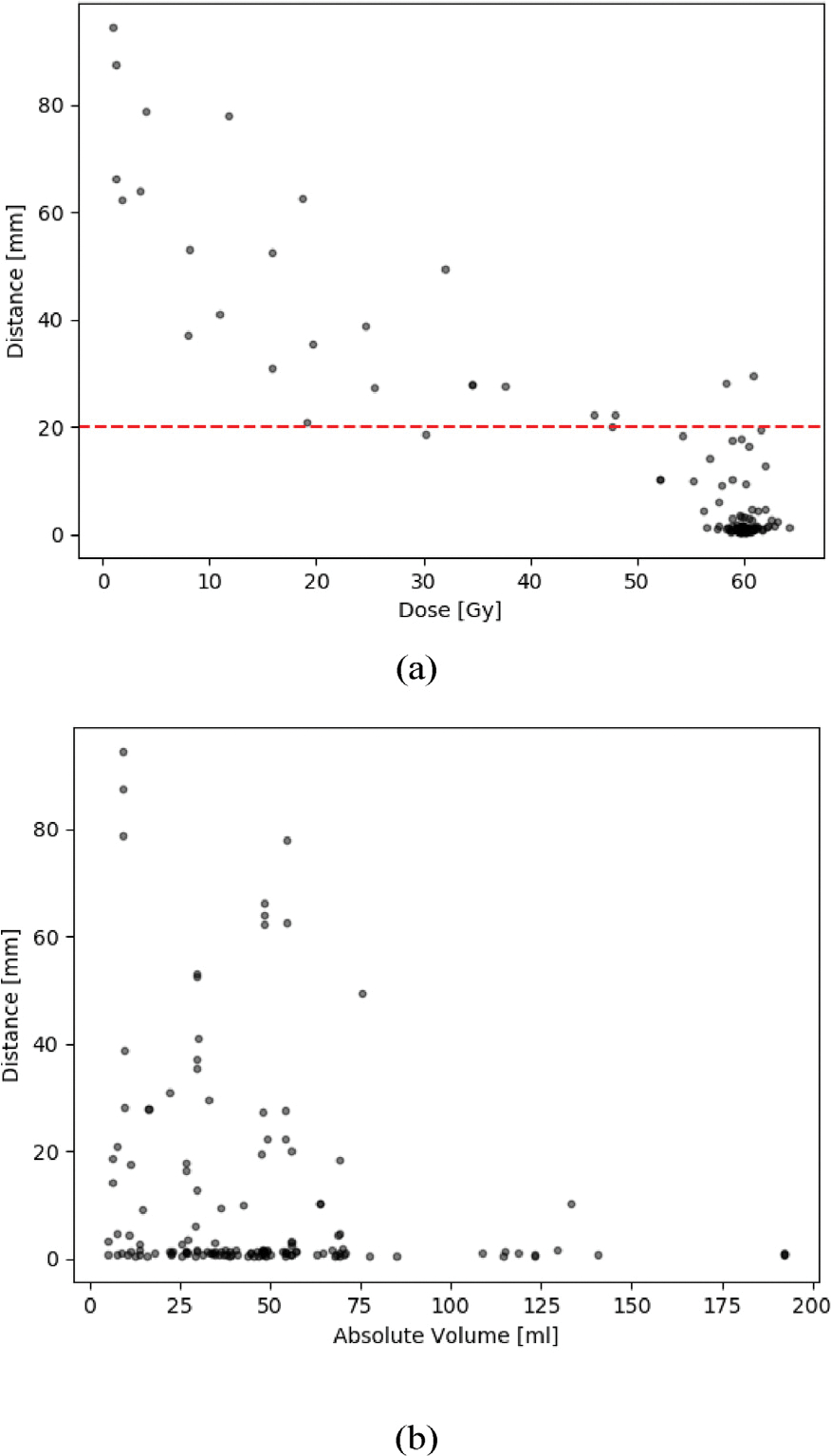
a: Results of the distance-dose analysis with respect to the mean dose. The horizontal red line indicates the suggested 2 cm margin in literature (for details and literature see 1). b: Here the of the recurrences from the is plotted as a function of the absolute Volume measured in ml.
2.2 and is measured in mm. The dose along the x-axis is the calculated mean dose of the whole recurrence volume and not for an individual point in the recurrence. Other metrices like the min, max, median and standard deviation can be found in the appendix (see Appendix A).
Fig. A.7 shows the relative volume RV of the recurrence overlapping with the (see Section 2.4.3). A value of 1 means that the whole recurrence is within the . 0 means that there is no overlap at all. 109 of 142 individual recurrences had an overlap with the .
Fig. 4 (b) shows the absolute volume of the with respect to the distance of each recurrence. Table 2 bins the data of Fig. 4 (b) into three groups according to the volume of the with respect to the ratio of distant recurrences. In patients with smaller than or equal to 30 ml, 26.5 % of recurrences occurred more than 2 cm away from the .
Table 2.
Interval based evaluation of data plotted in Fig. 4 (b).
| [ml] |
|
||
|---|---|---|---|
| ≤ 20mm | > 20mm | ||
|
| |||
| 0–30 | 49 | 36 (73.5 %) | 13 (26.5 %) |
| 31 – 60 | 63 | 50 (80.9 %) | 13 (19.1 %) |
| >60 | 30 | 29 (96.7 %) | 1 (3.3 %) |
with: : ratio of recurrences with a distance of more and less equal 20 mm. A chi-square test of independence showed that there was a significant association between volume and number of distant recurrences (X2(2, ) = 6.70, ).
There was a significant correlation between MGMT-positive status and improved overall survival (log-rank test: ), however no significant benefit regarding progression free survival.
Smaller size (median split procedure) was associated with improved progression free survival (log-rank test: ), but not with overall survival ().
Discussion
Most of the recurrences occurred within the 2 cm margin () around the , with a high percentage arising directly from the . 77 % of all recurrences had an overlap with the (), suggesting a progression of remaining tumor cell clusters. 75 % of all recurrences are considered to be central or in-field from the dose distribution point of view. Only 27 recurrences (19 %) occurred at distances larger than 2 cm from the . Thus, the predominant local type of relapse matches the information provided by previous studies (e.g.[9]).
It is interesting that the distance and frequency of distant recurrences in- creased when the volume of the decreased () (see Fig. 4 (b)). Intuitively, larger volumes should have been related to a higher rate of recurrences (both local and distant), but that doesn’t seem to be the case. Larger lead in the vast majority of cases included in this study to local recurrences. This behavior might have various reasons. One could be that the maximal distance of the recurrence to the is limited to less remaining healthy brain for larger . But also the position of the within the brain may affect the feasible distance. Furthermore, patients with larger initial tumors and thus larger might experience recurrences earlier as compared to patients with smaller . In Contrast, patients with smaller preoperative tumor volumes and/or MGMT-positive status would tend to develop recurrences later and distantly [10], which was also confirmed in our data.
Another reason could be that the smaller volumes were under-estimated during contouring. But still it is noticeable that especially the smallest of 10 to 20 ml provided the high frequency of distant recurrences (and also the most distant ones). From the data in Fig. 4 (b) we can thus assume that larger lead to local recurrences and smaller tend to relapse distantly. Taking into consideration that the mechanisms of distant relapse are different from the ones of direct tissue invasion, predicting and covering all possible distant locations cannot be performed by using larger margins. Therefore, small margins appear to be appropriate and sufficient for both small and large .
Furthermore, the margin analysis shows that patients treated with larger high dose margins neither had a benefit in progression free nor overall survival. Log-rank tests do not show a statistically significant difference between both groups (smaller and larger high dose margin) with respect to both time to progression and death. Subsequently, a modification of CTV margins com- pared to the ones used in the landmark EORTC trial seems feasible. Several small trials have already investigated alternative CTV delineation methods involving smaller volumes and have not found any detrimental consequences on outcome [11,5,12,13,14,15]. Also larger volumes, such as the RTOG volume, yielded similar results in clinical trials [16].
These favorable data are mainly due to the predominantly local recurrence pattern of GBM. In our cohort, 70 % of recurrent lesions were found centrally within the irradiation field.
The microscopic extension of GBM cells was shown to be the highest among gliomas, but also very heterogeneous between individuals [17]. While it is unlikely to reach distant recurrences by solely increasing the margin of the CTV, marginal recurrences (11 out of 142 in our study) are those which could potentially benefit from an adaption in target volume definition. One option might be a method to generate an anisotropic CTV margin. While individual pathological assessments are certainly the gold standard, they are not feasible in clinical practice. Non-invasive methods are thus warranted. We hypothesize that an anisotropic margin, depending on quantitative surrogates (e.g. using radiomics) could be beneficial. In literature there are also promising approaches to model glioma growth using fractional anisotropy derived from Diffusion Tensor Imaging (DTI) (e.g. [18]) or probabilistic methods using deep neural networks (e.g. [19,20]).
It seems to be counterintuitive that there are also a few recurrences which are quite close to the (<5mm) but have no overlap (see Fig. A.7). This is due to some very small volume recurrences a few millimeters away from the (see Fig. A.8). It’s also worth to mention that there is some uncertainty paired with the data. For some patients, radiation oncologist don’t follow the 2 cm approach strictly as it would result in a too large CTV. / PTV. Also there is some uncertainty in the delineation of the . One solution for these problems might be the use of automative segmentation algorithms (e.g. [21]).
In Fig. 4 (a) it is interesting to see that the COM of most recurrences is very close to or lies within the contour. The CTV and PTV margins thus provide up to 3 cm (depending on institutional margins for positioning and treatment planning) additional high dose area, which might not be necessary and associated with more side effects. Especially considering the importance for quality of life of maintaining cognitive functions, smaller volumes would allow a better protection of the hippocampus and of other sensitive structures such as the prefrontal or the inferior parietal cortex [22,23,24].
Conclusion
Most of the recurrences occurred locally (75 %), being central or in-field. The size to distant recurrence frequency relationship needs to be investigated further. Following the hypothesis, a CTV margin depending on its size could be a treatment option to obtain similar (or even improved) tumor control while having less side effects. Using smaller margins the dose reduction to healthy tissue and organs at risk (e.g. hippocampal-sparing) is more feasible. Furthermore the risk of radionecrosis is reduced.
Fig. A5.
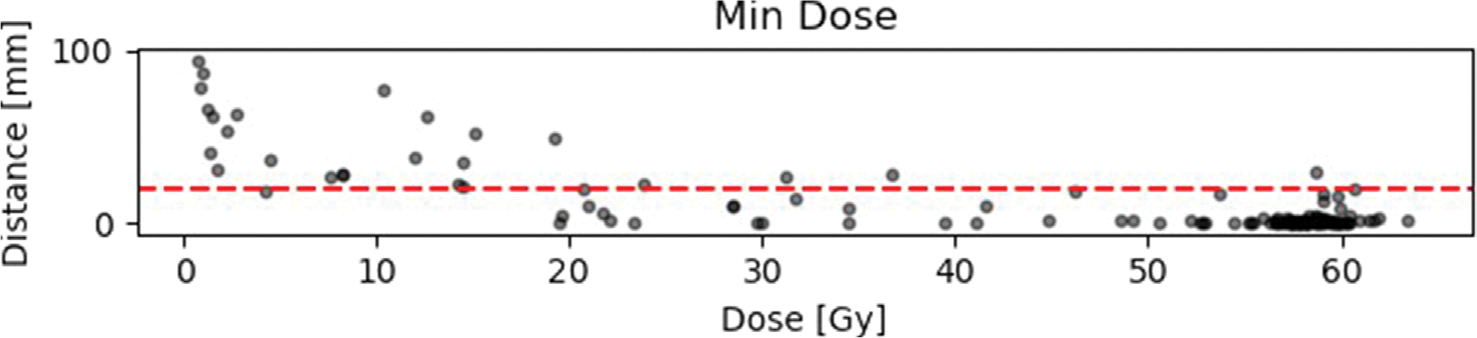
Results of the distance-dose analysis with respect to minimum, maximum and mean doses. The horizontal red line is equivalent to the suggested 2 cm margin in literature (for details and literature see 1).
Fig. A6.
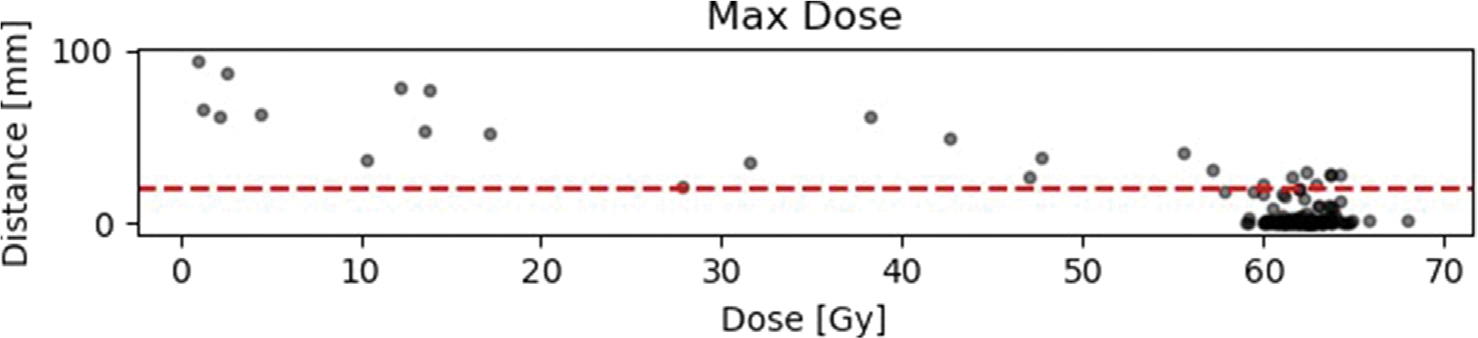
Results of the distance-dose analysis with respect to median, standard deviation and relative volume RV. The horizontal red line is equivalent to the suggested 2 cm margin in literature (for details and literature see 1).
Fig. A7.
Results of the distance-dose analysis with respect to the overlapping relative volume. The horizontal red line indicates the recommended 2 cm margin in literature (for details and literature see 1).
Fig. A8.
Two dimensional slice showing the recurrence (white), the COM of which is 4 mm away from the (grey) surface.
Footnotes
References
- [1].Bi WL, Beroukhim R, Beating the odds: extreme long-term survival with glioblastoma, Neuro-Oncology 16 (2014) 1159–1160. doi: 10.1093/neuonc/nou166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Niyazi M, Brada M, Chalmers AJ, Combs SE, Erridge SC, Fiorentino A, et al. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother Oncol 2016;118:35–42. 10.1016/j.radonc.2015.12.003. [DOI] [PubMed] [Google Scholar]
- [3].Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol 2012;2:73. 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee SW, Fraass BA, Marsh LH, Herbort K, Gebarski SS, Martel MK, Radany EH, Lichter AS, Sandler HM, Patterns of failure following high-dose 3-d conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study., International journal of radiation oncology, biology, physics 43 (1999) 79–88. doi: 10.1016/s0360-3016(98)00266-1. [DOI] [PubMed] [Google Scholar]
- [5].McDonald MW, Shu H-K-G, Curran WJ, Crocker IR. Pattern of failure after limited margin radiotherapy and temozolomide for glioblas- toma. Int J Radiat Oncol Biol Phys 2011;79:130–6. 10.1016/j.ijrobp.2009.10.048. [DOI] [PubMed] [Google Scholar]
- [6].Lotar Cordova A, Almeida TVR, Silva C. M. d., Piedade PA, Almeida CM, Bezzera Lima CG, Dutra C, Ferreira RM, Linhares MN, Denyak V, Evaluation of high-grade astrocytoma recurrence patterns after radiotherapy in the era of temozolomide: A single institution ex- perience., Reports of practical oncology and radiotherapy : journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology 23 (2018) 154–160. doi: 10.1016/j.rpor.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. 10.1056/nejmoa043330. [DOI] [PubMed] [Google Scholar]
- [8].Davidson-Pilon C lifelines: survival analysis in python. J Open Source Softw 2019;4:1317. 10.21105/joss.01317. [DOI] [Google Scholar]
- [9].Bette S, Barz M, Huber T, Straube C, Schmidt-Graf F, Combs SE, et al. Retrospective analysis of radiological recurrence patterns in glioblastoma, their prognostic value and association to postoperative infarct volume. Sci Rep 2018;8:4561. 10.1038/s41598-018-22697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tejada S, D´ ıez-Valle R, Aldave G, Marigil M, de Gallego J, Domınguez PD, Factors associated with a higher rate of distant failure after primary treatment for glioblastoma, Journal of Neuro-Oncology 116 (2013) 169–175. doi: 10.1007/s11060-013-1279-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zheng L, Zhou Z-R, Yu Q, Shi M, Yang Y, Zhou X, et al. The definition and delineation of the target area of radiotherapy based on the recurrence pattern of glioblastoma after temozolomide chemoradiotherapy. Front Oncol 2021;10. 10.3389/fonc.2020.615368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dobelbower MC, Burnett Iii OL, Nordal RA, Nabors LB, Markert JM, Hyatt MD, Fiveash JB, Patterns of failure for glioblastoma multiforme following concurrent radiation and temozolomide., Journal of medical imaging and radiation oncology 55 (2011) 77–81. doi: 10.1111/j.1754-9485.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- [13].Kumar N, Kumar R, Sharma SC, Mukherjee KK, Khandelwal N, Kumar R, et al. Clin-radiation therapy rt-09: To compare the treatment outcomes of two different tar- get volume delineation guidelines (rtog vs md anderson) in glioblastoma multiforme patients: a prospective randomized study. Neuro Oncol 2012;14:vi133–41. 10.1093/neuonc/nos238. [DOI] [Google Scholar]
- [14].Paulsson AK, McMullen KP, Peiffer AM, Hinson WH, Kearns WT, Johnson AJ, et al. Limited margins using modern radio- therapy techniques does not increase marginal failure rate of glioblastoma. Am J Clin Oncol 2014;37:177–81. 10.1097/coc.0b013e318271ae03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, and JF, Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the united states, Clinical Cancer Research 16 (2010) 2443–2449. doi: 10.1158/1078-0432.ccr-09-3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 2013;31:4085–91. 10.1200/jco.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nie S, Zhu Y, Yang J, Xin T, Xue S, Zhang X, et al. Determining optimal clinical target volume margins in high-grade glioma based on microscopic tumor extension and magnetic resonance imaging. Radiat Oncol 2021;16. 10.1186/s13014-021-01819-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Metz M-C, Molina-Romero M, Lipkova J, Gempt J, Liesche- Starnecker F, Eichinger P, et al. Predicting glioblastoma recurrence from preoperative MR scans using fractional-anisotropy maps with free-water suppression. Cancers 2020;12:728. 10.3390/cancers12030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Petersen J, Jäger PF, Isensee F, Kohl SAA, Neuberger U, Wick W, Debus J, Heiland S, Bendszus M, Kickingereder P, Maier-Hein KH, Deep probabilistic modeling of glioma growtharXiv:http://arxiv.org/abs/1907.04064v1
- [20].Ezhov I, Lipkova J, Shit S, Kofler F, Collomb N, Lemasson B, Barbier E, Menze B, Neural parameters estimation for brain tumor growth modelingarXiv: http://arxiv.org/abs/1907.00973v1
- [21].Shusharina N, Söderberg J, Edmunds D, Löfman F, Shih H, Bortfeld T, Automated delineation of the clinical target volume using anatomically constrained 3d expansion of the gross tumor volume., Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 146 (2020) 37–43. doi: 10.1016/j.radonc.2020.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].F’evre CL, Cheng X, Loit M-P, Keller A, Cebula H, An- toni D, Thiery A, Constans J-M, Proust F, Noel G, Role of hippocampal location and radiation dose in glioblastoma patients with hippocampal atrophy, Radiation Oncology 16 (jun 2021). doi: 10.1186/s13014-021-01835-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Acevedo-Vergara K, Perez-Florez M, Ramirez A, Torres-Bayona S, Dau A, Salva S, et al. Cognitive deficits in adult patients with high-grade glioma: a systematic review. Clin Neurol Neurosurg 2022;219:. 10.1016/j.clineuro.2022.107296107296. [DOI] [PubMed] [Google Scholar]
- [24].Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 2016;13:52–64. 10.1038/nrneurol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]


