Background/Summary
Cord management in non-vigorous newborns remains up for debate, as limited studies have validated strategies in this high-risk population. While multiple national and international governing bodies now recommend the routine practice of delayed cord clamping (DCC) in vigorous neonates, these organizations have not reached a consensus on the appropriate approach in non-vigorous neonates.1 Benefits of placental transfusion are greatly needed amongst non-vigorous neonates who are at risk of asphyxiation-associated mortality and morbidities, but the need for immediate resuscitation complicates matters. This chapter discusses the physiological benefits of placental transfusion for non-vigorous neonates and reviews the available literature on different umbilical cord management strategies for this population.
Benefits of placental transfusion in non-vigorous newborns
High risk neonates who are non-vigorous at birth may have experienced volume or blood loss in the peripartum period and therefore could benefit from volume transfer from the placenta (Figure 1). Placental transfusion from DCC has been historically known to improve red blood cell volume and provides up to 30% of placental volume.2–6 Even preterm neonates benefit from higher blood volumes following DCC.7,8 Additionally, iron stores are improved both short term and long term (up to 12 months after birth), as shown in a randomized control trial of 540 neonates undergoing DCC versus early cord clamping (ECC).9–11 Improved iron stores have been linked to better neurodevelopmental outcomes, while iron deficiency has been associated with cognitive impairment, behavioral disruptions, and slower motor development.12–14 Non-vigorous neonates are at risk for significant neurologic injuries from asphyxia, so improving iron stores could be protective against such long-term complications.
Figure 1.
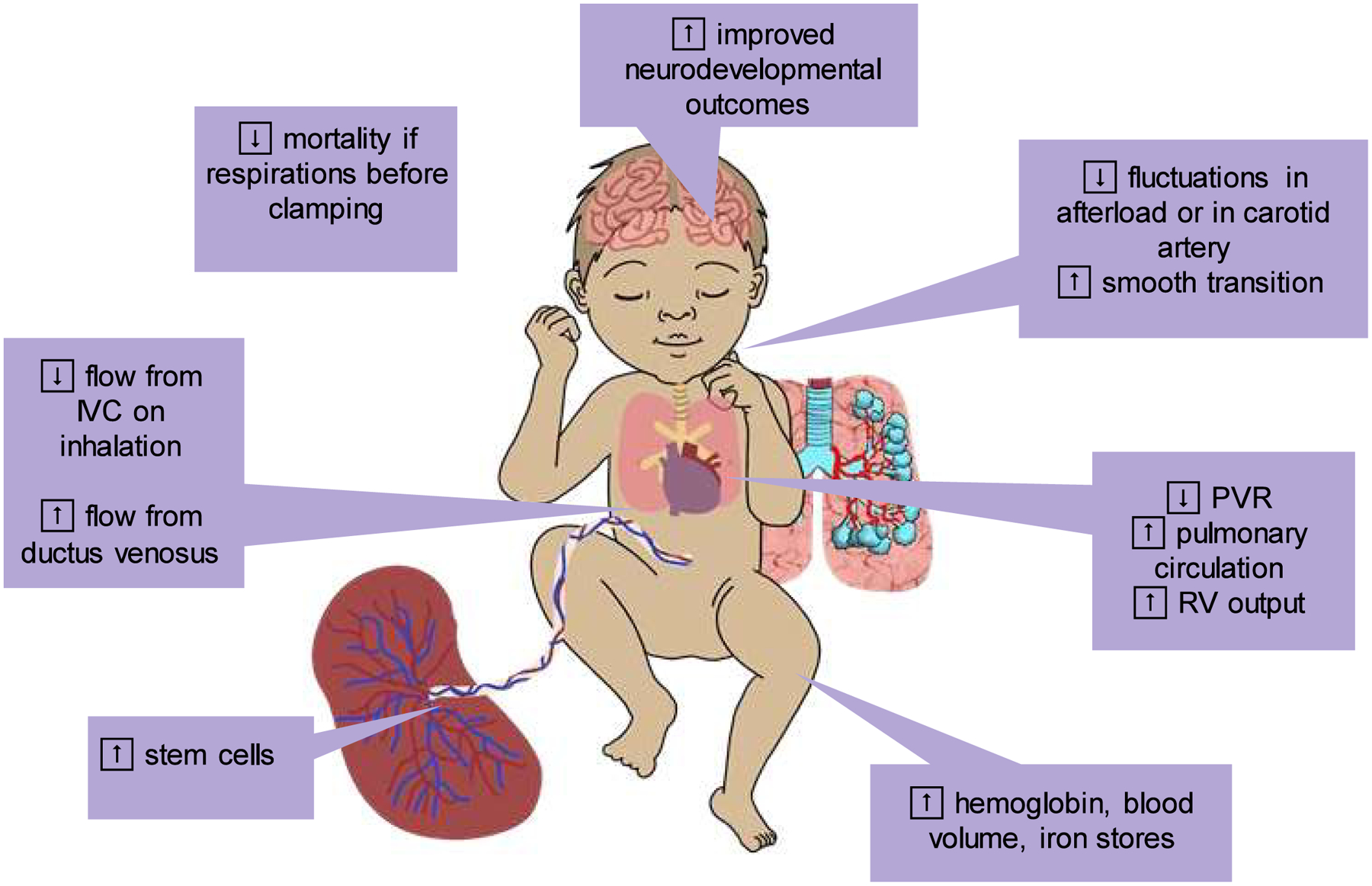
Benefits of Delayed Cord Clamping. Delayed cord clamping (DCC) contributes blood volume from the placenta to the neonate, which increases iron stores, hemoglobin levels, and pluripotent progenitor cell populations. Furthermore, DCC improves pulmonary blood flow and enable a smoother hemodynamic transition at birth. Neurodevelopmental injuries and mortality are reduced by DCC as compared to early cord clamping. Abbreviations: DCC = delayed cord clamping, IVC = inferior vena cava, PVR = pulmonary vascular resistance, RV = right ventricle.
Beyond improving red blood cell volumes and iron stores, DCC can ensure smoother transitions from fetal to neonatal physiology.15,16 Hemodynamic transition is often disrupted in non-vigorous neonates, due to decreased heart rates, poor oxygen delivery, and often times severe acidemia.17 Animal models have demonstrated that ECC can lead to abrupt increases in afterload, large swings in carotid artery pressures, decreased heart rates, and decreased right ventricular outputs, whereas DCC improved post-delivery cardiac function.18 The benefits of DCC on hemodynamic stability and neonatal transition is further compounded by respirations. A prospective observational study demonstrated increased rates of mortality and NICU admission with increased duration between the onset of breathing after cord clamping.19
Animal models have been used to elucidate the physiology behind DCC, respirations, and transitional hemodynamics. A fetal sheep model showed that breathing movements drop pulmonary vascular resistance (PVR).20 A lower PVR permits improved pulmonary circulation while the baby receives substantial blood volume from placental transfusion, which then improves left ventricular preload and subsequently improved cardiac output.21 Moreover, increased pulmonary circulation enables improved oxygen delivery to the lungs, which further leads to pulmonary vasodilation and further decreases of PVR.22 In preterm lambs, ventilation before cord clamping improves cerebral oxygenation.23 This “physiologically based” cord clamping approach (waiting to clamp until after ventilation is established) was shown in near-term sheep models to reduce cerebrovascular injury, as measured by markers of blood-brain barrier injury.24 Brouwer et al. explored the effects of breathing on venous return during delayed cord clamping in a small observational study of 15 term neonates using echocardiography and found that inhalation collapses the inferior vena cava (IVC) and limits venous return through the IVC while allowing greater blood flow from the ductus venosus and hepatic vein, thereby increasing placental blood transfer.25
Non-vigorous term and near-term neonates can be likened to extremely preterm neonates, due to their risk for hemodynamic instability and, in turn, risk for central nervous system injury. Lability in blood pressures, heart rates, and cardiac output puts preterm neonates at high risk of long-term disabilities and morbidities. Preterm neonates are at risk of intraventricular hemorrhages (IVH), due to the friability of germinal matrix vasculature as well as a lack of cerebral autoregulation.26 Early echocardiography in preterm neonates following delivery also demonstrated an association between lower superior vena cava flow (a surrogate marker of cardiac output and cerebral perfusion) and higher IVH risk.27 DCC, as compared to ECC, has been shown in preterm neonates to reduce in-hospital mortality and overall IVH.28–30 Asphyxiated term and near-term neonates are at risk for hypoxic ischemic encephalopathy, and this injury could be aggravated by swings in cardiac output, as watershed regions in the brain could suffer ischemia-reperfusion injury.31
Placental transfusion also enables the transfer of multipotent stem cells and progenitor cell populations.32–34 The umbilical cord is rich with hematopoietic progenitor cells that exhibit proliferative capabilities beyond those of bone marrow hematopoietic progenitor cells.35 Umbilical cord multipotent stem cells and progenitor cells have been implicated in repairing and protecting various organs against injuries, including the lung and brain.36 Specifically, animal models have demonstrated that exogenous introduction of human umbilical progenitor cells is therapeutic for cerebral palsy and traumatic brain injuries. A randomized control trial of 103 term and near-term neonates demonstrated that neonates undergoing DCC had greater CD34+ progenitor cell counts in circulating blood than did their counterparts who underwent ECC.37
Delayed cord clamping comes with few adverse effects. The most notable concerns through the years have included a risk for hyperbilirubinemia, secondary to placental transfusion, but this concern has been contested by randomized control trials showing no increased risk of jaundice either early or late (after 4 weeks of life) when babies undergo DCC versus ECC.38,39
Limitations with non-vigorous newborns
Though the benefits of DCC are plentiful in non-vigorous neonates, the application of this strategy is often thwarted by the need for immediate resuscitation. The safety of DCC in non-vigorous neonates is not well studied, as many randomized control trials studying cord management techniques often exclude infants in need of immediate resuscitation, or are deemed “too unstable” to meet inclusion criteria.40 Epidemiologic studies have demonstrated better outcomes, when neonates can establish spontaneous respirations prior to cord clamping.41 Non-vigorous neonates are less likely to achieve spontaneous respirations without significant resuscitation, and DCC can delay this. Variations in DCC practices are being explored to account for these limitations.
Resuscitation with intact cord
One approach to performing DCC without delaying resuscitation is to start resuscitation while the neonate is still connected to the placenta via an intact umbilical cord. A pilot study randomized non-vigorous term neonates to DCC for 1 minute or to DCC for at least 5 minutes with the option to provide active resuscitation on a trolley while the umbilical cord remains intact. The 5 minute infants had greater cerebral oxygenation and blood pressures.11 The Baby-DUCC pilot study demonstrated the feasibility of delaying cord clamping in term and near-term neonates until spontaneous or assisted ventilation is established.42 Resuscitation with an intact cord is feasible in preterm infants.43,44 Applying immediate resuscitation during DCC not only eliminates the concerns for delayed resuscitation, but also allows lengthening of the duration of DCC. A lamb model showed active umbilicoplacental blood flow for upwards of one hour while the lamb received resuscitation with an intact cord.45 Another animal model demonstrated that DCC with resuscitation (DCC-R) improved pulmonary blood flow, compared to DCC alone.46
Despite the promise of resuscitation during DCC, no trials to date have shown differences in mortality or major morbidities in neonates undergoing DCC versus DCC-R. In one study comparing DCC to DCC-R in preterm neonates, it was noted that in both groups, neonates were achieving either spontaneous respirations or established assisted ventilation in similar amounts of time prior to cord clamping, suggesting that the two groups did not actually differ in time to achieving lung recruitment prior to cord clamping.43 Moreover, animal models that associate DCC-R with improved outcomes compared to DCC anesthetize their animals for invasive ventilation via endotracheal tube and thereby overcoming the difficulties of resuscitating with non-invasive modalities (i.e., face mask) against a closed glottis.47
Resuscitation with intact cord requires practical considerations, including the need for special equipment.48 The need to be close to the mother, either near the introitus or near surgical incision site, poses logistical challenges to the resuscitation team. Resuscitation trolleys are available with a variety of features, including oxygen, air flow, heat source, monitoring capability, portable battery packs, and more.Of these, only one is approved by the US Food and Drug Administration, but additional studies have been done and more are underway to validate the use of these trolleys.49–52
There are limited data on the long-term outcomes of neonates who underwent DCC-R. The CORD pilot study showed a trend towards reduced mortality and death or NDI at two-year follow-up, but this study had an alarmingly low protocol adherence rate.53 The NepCordIII study, which took place in Nepal where they utilize the Helping Babies Breathe resuscitation program, conducted a two-year phone interview follow up and found that neurodevelopmental outcomes of non-vigorous term and late preterm infants who underwent DCC-R were better than that of infants who got ECC.54,55
Umbilical cord milking (UCM) to hasten resuscitation
An alternative to DCC for placental transfusion in non-vigorous neonates is umbilical cord milking (UCM) (Figure 2). This cord management strategy allows the rapid transferring of placental blood volume to the neonate without significantly delaying resuscitation. UCM is comparable to DCC for short term improvements in hematologic outcomes in vigorous term and late preterm neonates.56,57 Many non-vigorous term and near-term neonates are born by cesarean section under emergent or urgent circumstances, and are at risk of lower placental transfusion than in neonates born by vaginal delivery.58 It is therefore paramount to consider how to improve placental blood transfer at the time of delivery as to not further risk anemia or volume loss in this high-risk population. Neonates born via cesarean section have improved hemoglobin levels after UCM than with ECC.59–61 A systematic review and meta-analysis found that UCM, either with a cut or intact cord, are both superior to ECC.62 A small prospective cohort trial found that more blood can be transferred from intact-cord UCM versus cut-cord UCM.63 In fact, it has been suggested that UCM is superior to DCC in preterm cesarean section deliveries, as the neonates resulted in higher hemoglobin levels, blood pressures, temperatures, and urine outputs.64,65
Figure 2.

Comparison between Umbilical Cord Milking and Delayed Cord Clamping with Resuscitation. Both umbilical cord milking (UCM) and delayed cord clamping with resuscitation (DCC-R) can provide placental transfusion to a non-vigorous neonate. Each method has its merits. DCC-R ensures either spontaneous or assisted respirations occur before the cord is clamped, while UCM is easier to perform as no special equipment is needed. Abbreviations: DCC-R = delayed cord clamping with resuscitation, IVH = intraventricular hemorrhage, UCM = umbilical cord milking.
While volume transfer with UCM is promising, there are concerns that UCM is too rapid and could cause rapid rise and fall in intravascular pressures. In preterm neonates, the lack of cerebral autoregulation puts them at high risk of intraventricular hemorrhage.66–68 Preterm lamb models getting UCM showed marked hemodynamic fluctuations and disturbances when compared to DCC-R.69 Moreover, a randomized control trial of preterm neonates showed an association between UCM and IVH in neonates less than 28 weeks’ gestation.70 Despite these concerns, the negative effects of UCM on rapid hemodynamic shifts may be offset by spontaneous or assisted respirations. When comparing DCC-R with UCM or UCM with ventilation (UCM-V) in a preterm asphyxiated animal model, it was found that UCM-V had greater fetoplacental volume transfers and had less carotid flow fluctuations than did the UCM infants.46 Term neonates may also be at much lower risk of intracranial injury secondary to UCM, as they have more robust cerebral autoregulation than do extremely preterm neonates.71 In one randomized control trial of 200 term neonates, there were no differences in MCA flow velocities measured by doppler between the UCM and ECC groups.72
Girish et al. demonstrated the feasibility of performing UCM on term and late preterm non-vigorous neonates through a small feasibility trial.73 The MINVI Trial (Milking in NonVigorous Infants Trial) was a cluster-randomized crossover study of non-vigorous late preterm and term neonates of 35 weeks’ gestation and higher, comparing UCM to ECC. UCM resulted in fewer occurrences of moderate to severe hypoxic-ischemic encephalopathy, fewer patients requiring therapeutic hypothermia, and higher hemoglobin levels than did ECC controls. There were no data suggesting UCM caused any harm in this population. This was the first randomized controlled trial to demonstrate the safety and advantages of UCM over ECC in non-vigorous late preterm and term neonates.74
UCM may be advantageous to DCC with regards to stem cell transfer. Umbilical cord blood following milking has greater mesenchymal stromal cell and hematopoietic stem cell populations. This blood from a “milked” cord had greater ability to rescue and reconstitute the bone marrow of irradiated mice, suggesting that UCM may have improved delivery of pluripotent cells of great regenerative potential.75 Preterm neonates born to mothers with placental insufficiency had greater numbers of CD34+ pluripotent cells in circulation following UCM.76
Data on long term outcomes following UCM remain sparse. As aforementioned, improved iron stores are associated with improved neurodevelopmental outcomes. There is evidence that greater ferritin stores allow for better myelination of the brain at four months of age.77 Two randomized control trials studying preterm neonates randomized to DCC or UCM shows that the UCM group had higher language and cognitive scores at two to three years of age than did infants randomized to DCC.78,79
An ongoing trial in India (CORDMILK, Comparative Outcomes Related to Delivery-room Cord Milking In Low-resourced Countries, NCT03647394) is a cluster-crossover trial comparing UCM versus ECC in non-vigorous newborns ≥35 weeks’ gestation. The primary outcomes of interest are moderate to severe HIE or in-hospital death and survival without moderate to severe neurodevelopmental impairment at 2 years of age., Secondary outcomes include NICU admission, neurologic exam at the time of discharge, and brain magnetic resonance imaging (MRI) findings.
Conclusions
Currently, no consensus statements exist on the optimal cord management strategy in non-vigorous neonates. DCC offers great benefits but may delay resuscitation. DCC-R helps to remove barriers to performing resuscitation while allowing for placental transfusion, but can be logistically challenging. Recent studies suggest a role for UCM in non-vigorous term and near-term neonates to facilitate placental transfusion without compromising timely resuscitation. Additional studies are needed to determine if resuscitation with an intact cord or UCM should be adopted for non-vigorous neonates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no disclosures
Contributor Information
Jenny Koo, Sharp Mary Birch Hospital for Women and Newborns. Sharp Neonatal Research Institute. San Diego, CA 92127.
Zubair H Aghai, Thomas Jefferson University/Nemours, Philadelphia, Pennsylvania..
Anup Katheria, Sharp Mary Birch Hospital for Women and Newborns. Sharp Neonatal Research Institute. San Diego, CA 92127.
References:
- 1.Committee Opinion No. 684: Delayed Umbilical Cord Clamping After Birth. Obstet Gynecol. Jan 2017;129(1):e5–e10. doi: 10.1097/aog.0000000000001860 [DOI] [PubMed] [Google Scholar]
- 2.Colozzi AE. Clamping of the Umbilical Cord. New England Journal of Medicine. 1954/April/15 1954;250(15):629–632. doi: 10.1056/nejm195404152501502 [DOI] [PubMed] [Google Scholar]
- 3.Whipple GA, Sisson TRC, Lund CJ. Delayed Ligation of the Umbilical Cord. Obstetrics & Gynecology. 1957/December 1957;10(6):603–610. doi: 10.1097/00006250-195712000-00002 [DOI] [PubMed] [Google Scholar]
- 4.Lanzkowsky P Effects of early and late clamping of umbilical cord on infant’s haemoglobin level. British medical journal. 1960;2(5215):1777–1782. doi: 10.1136/bmj.2.5215.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao A, Hirvensalo M, Lind J. PLACENTAL TRANSFUSION-RATE AND UTERINE CONTRACTION. The Lancet. 1968/February 1968;291(7539):380–383. doi: 10.1016/s0140-6736(68)91352-4 [DOI] [PubMed] [Google Scholar]
- 6.Yao A DISTRIBUTION OF BLOOD BETWEEN INFANT AND PLACENTA AFTER BIRTH. The Lancet. 1969/October 1969;294(7626):871–873. doi: 10.1016/s0140-6736(69)92328-9 [DOI] [PubMed] [Google Scholar]
- 7.Aladangady N Infants’ Blood Volume in a Controlled Trial of Placental Transfusion at Preterm Delivery. PEDIATRICS. 2006/January/01 2006;117(1):93–98. doi: 10.1542/peds.2004-1773 [DOI] [PubMed] [Google Scholar]
- 8.Strauss RG, Mock DM, Johnson KJ, et al. A randomized clinical trial comparing immediate versus delayed clamping of the umbilical cord in preterm infants: short-term clinical and laboratory endpoints. Transfusion. 2008;48(4):658–665. doi: 10.1111/j.1537-2995.2007.01589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson O, Hellstrom-Westas L, Andersson D, Domellof M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ (Clinical research ed). 2011;343:d7157–d7157. doi: 10.1136/bmj.d7157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Evidence-Based Child Health: A Cochrane Review Journal. 2014/June 2014;9(2):303–397. doi: 10.1002/ebch.1971 [DOI] [PubMed] [Google Scholar]
- 11.Katheria AC, Brown MK, Faksh A, et al. Delayed Cord Clamping in Newborns Born at Term at Risk for Resuscitation: A Feasibility Randomized Clinical Trial. The Journal of Pediatrics. 2017/August 2017;187:313–317.e1. doi: 10.1016/j.jpeds.2017.04.033 [DOI] [PubMed] [Google Scholar]
- 12.Andersson O, Lindquist B, Lindgren M, Stjernqvist K, Domellof M, Hellstrom-Westas L. Effect of Delayed Cord Clamping on Neurodevelopment at 4 Years of Age. JAMA Pediatrics. 2015/July/01 2015;169(7):631. doi: 10.1001/jamapediatrics.2015.0358 [DOI] [PubMed] [Google Scholar]
- 13.Gunnarsson BS, Thorsdottir I, Palsson G, Gretarsson SJ. Iron status at 1 and 6 years versus developmental scores at 6 years in a well-nourished affluent population. Acta Paediatr. Mar 2007;96(3):391–5. doi: 10.1111/j.1651-2227.2007.00086.x [DOI] [PubMed] [Google Scholar]
- 14.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. Feb 2001;131(2s-2):649S–666S; discussion 666S-668S. doi: 10.1093/jn/131.2.649S [DOI] [PubMed] [Google Scholar]
- 15.Bhatt S, Polglase GR, Wallace EM, Te Pas AB, Hooper SB. Ventilation before Umbilical Cord Clamping Improves the Physiological Transition at Birth. Frontiers in pediatrics. 2014;2:113–113. doi: 10.3389/fped.2014.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niermeyer S, Velaphi S. Promoting physiologic transition at birth: re-examining resuscitation and the timing of cord clamping. Semin Fetal Neonatal Med. Dec 2013;18(6):385–92. doi: 10.1016/j.siny.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 17.Hutchon DJ. Immediate cord clamping may increase neonatal acidaemia. Bjog. Aug 2008;115(9):1190–1. doi: 10.1111/j.1471-0528.2008.01797.x [DOI] [PubMed] [Google Scholar]
- 18.Bhatt S, Alison BJ, Wallace EM, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. The Journal of physiology. 2013;591(8):2113–2126. doi: 10.1113/jphysiol.2012.250084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ersdal HL, Linde J, Mduma E, Auestad B, Perlman J. Neonatal Outcome Following Cord Clamping After Onset of Spontaneous Respiration. PEDIATRICS. 2014/July/14 2014;134(2):265–272. doi: 10.1542/peds.2014-0467 [DOI] [PubMed] [Google Scholar]
- 20.Polglase GR, Wallace MJ, Grant DA, Hooper SB. Influence of fetal breathing movements on pulmonary hemodynamics in fetal sheep. Pediatr Res. Dec 2004;56(6):932–8. doi: 10.1203/01.PDR.0000145254.66447.C0 [DOI] [PubMed] [Google Scholar]
- 21.Kluckow M, Hooper SB. Using physiology to guide time to cord clamping. Seminars in Fetal and Neonatal Medicine. 2015/August 2015;20(4):225–231. doi: 10.1016/j.siny.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 22.Hooper SB, Polglase GR, te Pas AB. A physiological approach to the timing of umbilical cord clamping at birth. Arch Dis Child Fetal Neonatal Ed. Jul 2015;100(4):F355–60. doi: 10.1136/archdischild-2013-305703 [DOI] [PubMed] [Google Scholar]
- 23.Polglase GR, Dawson JA, Kluckow M, et al. Ventilation onset prior to umbilical cord clamping (physiological-based cord clamping) improves systemic and cerebral oxygenation in preterm lambs. PloS one. 2015;10(2):e0117504–e0117504. doi: 10.1371/journal.pone.0117504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polglase GR, Blank DA, Barton SK, et al. Physiologically based cord clamping stabilises cardiac output and reduces cerebrovascular injury in asphyxiated near-term lambs. Arch Dis Child Fetal Neonatal Ed. Nov 2018;103(6):F530–F538. doi: 10.1136/archdischild-2017-313657 [DOI] [PubMed] [Google Scholar]
- 25.Brouwer E, Knol R, Kroushev A, et al. Effect of breathing on venous return during delayed cord clamping: an observational study. Arch Dis Child Fetal Neonatal Ed. Jan 2022;107(1):65–69. doi: 10.1136/archdischild-2020-321431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballabh P Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. Jan 2010;67(1):1–8. doi: 10.1203/PDR.0b013e3181c1b176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed. May 2000;82(3):F188–94. doi: 10.1136/fn.82.3.f188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarnow-Mordi W, Morris J, Kirby A, et al. Delayed versus Immediate Cord Clamping in Preterm Infants. N Engl J Med. December 21 2017;377(25):2445–2455. doi: 10.1056/NEJMoa1711281 [DOI] [PubMed] [Google Scholar]
- 29.Fogarty M, Osborn DA, Askie L, et al. Delayed Versus Early Umbilical Cord Clamping for Preterm Infants: A Systematic Review and Meta-Analysis. Obstetric Anesthesia Digest. 2018/December 2018;38(4):179–180. doi: 10.1097/01.aoa.0000547275.86917.a2 [DOI] [PubMed] [Google Scholar]
- 30.Rabe H, Gyte GML, Diaz-Rossello JL, Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database of Systematic Reviews. 2019;(9)doi: 10.1002/14651858.CD003248.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin X, Cheng J, Zhong Y, et al. Mechanism and Treatment Related to Oxidative Stress in Neonatal Hypoxic-Ischemic Encephalopathy. Front Mol Neurosci. 2019;12:88. doi: 10.3389/fnmol.2019.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen N, Hudson JE, Walczak P, et al. Human umbilical cord blood progenitors: the potential of these hematopoietic cells to become neural. Stem Cells. 2005;23(10):1560–70. doi: 10.1634/stemcells.2004-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson N, Divers R, Kedar R, Mehindru A, Borlongan MC, Borlongan CV. Discarded Wharton jelly of the human umbilical cord: a viable source for mesenchymal stromal cells. Cytotherapy. Jan 2015;17(1):18–24. doi: 10.1016/j.jcyt.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawton C, Acosta S, Watson N, et al. Enhancing endogenous stem cells in the newborn via delayed umbilical cord clamping. Neural Regen Res. Sep 2015;10(9):1359–62. doi: 10.4103/1673-5374.165218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. Jul 25 2013;122(4):491–8. doi: 10.1182/blood-2013-02-453175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alatyyat SM, Alasmari HM, Aleid OA, Abdel-Maksoud MS, Elsherbiny N. Umbilical cord stem cells: Background, processing and applications. Tissue Cell. Aug 2020;65:101351. doi: 10.1016/j.tice.2020.101351 [DOI] [PubMed] [Google Scholar]
- 37.Okulu E, Haskologlu S, Guloglu D, et al. Effects of Umbilical Cord Management Strategies on Stem Cell Transfusion, Delivery Room Adaptation, and Cerebral Oxygenation in Term and Late Preterm Infants. Front Pediatr. 2022;10:838444. doi: 10.3389/fped.2022.838444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ya§artekin Y, Sarici SO, Ozcan M, et al. Investigation of the relationship between cord clamping time and risk of hyperbilirubinemia. The Turkish Journal of Pediatrics. 2020;62(5):756. doi: 10.24953/turkjped.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 39.Rana N, Ranneberg LJ, Malqvist M, Kc A, Andersson O. Delayed cord clamping was not associated with an increased risk of hyperbilirubinaemia on the day of birth or jaundice in the first 4 weeks. Acta Paediatrica. 2019/July/15 2019;109(1):71–77. doi: 10.1111/apa.14913 [DOI] [PubMed] [Google Scholar]
- 40.Katheria AC. Neonatal Resuscitation with an Intact Cord: Current and Ongoing Trials. Children (Basel). Apr 22 2019;6(4)doi: 10.3390/children6040060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philip AGS, Teng SS. Role of Respiration in Effecting Placental Transfusion at Cesarean Section. Neonatology. 1977;31(3–4):219–224. doi: 10.1159/000240963 [DOI] [PubMed] [Google Scholar]
- 42.Blank DA, Badurdeen S, Omar F Kamlin C, et al. Baby-directed umbilical cord clamping: A feasibility study. Resuscitation. 2018/October 2018;131:1–7. doi: 10.1016/j.resuscitation.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 43.Katheria A, Poeltler D, Durham J, et al. Neonatal Resuscitation with an Intact Cord: A Randomized Clinical Trial. The Journal of pediatrics. 2016;178:75–80.e3. doi: 10.1016/j.jpeds.2016.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duley L, Dorling J, Pushpa-Rajah A, et al. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Archives of disease in childhood Fetal and neonatal edition. 2018;103(1):F6–F14. doi: 10.1136/archdischild-2016-312567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Duc K, Aubry E, Mur S, et al. Changes in Umbilico-Placental Circulation during Prolonged Intact Cord Resuscitation in a Lamb Model. Children (Basel). Apr 26 2021;8(5)doi: 10.3390/children8050337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandrasekharan P, Gugino S, Koenigsknecht C, et al. Placental transfusion during neonatal resuscitation in an asphyxiated preterm model. Pediatr Res. Sep 2022;92(3):678–684. doi: 10.1038/s41390-022-02086-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crawshaw JR, Kitchen MJ, Binder-Heschl C, et al. Laryngeal closure impedes non-invasive ventilation at birth. Archives of disease in childhood Fetal and neonatal edition. 2018;103(2):F112–F119. doi: 10.1136/archdischild-2017-312681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koo J, Katheria A. Cardiopulmonary Resuscitation with an Intact Umbilical Cord. Neoreviews. Jun 01 2022;23(6):e388–e399. doi: 10.1542/neo.23-6-e388 [DOI] [PubMed] [Google Scholar]
- 49.Katheria A, Lee HC, Knol R, Irvine L, Thomas S. A review of different resuscitation platforms during delayed cord clamping. J Perinatol. July 2021;41(7):1540–1548. doi: 10.1038/s41372-021-01052-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Irvine L, Kowal D, Soraisham A, S C, A S, Rabi Y. Integrated neonatal support on placental circulation with resuscitation (INSPiRe): a feasibility study. presented at: Proceedings of the 10th American Pediatrics Healthcare & Pediatric Infectious Diseases Congress Toronto, Canada; 2017; [Google Scholar]
- 51.Knol R, Brouwer E, Klumper FJCM, et al. Effectiveness of Stabilization of Preterm Infants With Intact Umbilical Cord Using a Purpose-Built Resuscitation Table-Study Protocol for a Randomized Controlled Trial. Front Pediatr. 2019;7:134. doi: 10.3389/fped.2019.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knol R, Brouwer E, van den Akker T, et al. Physiological-based cord clamping in very preterm infants - Randomised controlled trial on effectiveness of stabilisation. Resuscitation. 02 January 2020;147:26–33. doi: 10.1016/j.resuscitation.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 53.Armstrong-Buisseret L, Powers K, Dorling J, et al. Randomised trial of cord clamping at very preterm birth: outcomes at 2 years. Archives of disease in childhood Fetal and neonatal edition. 2020;105(3):292–298. doi: 10.1136/archdischild-2019-316912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andersson O, Rana N, Ewald U, et al. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 minutes of birth (Nepcord III) - a randomized clinical trial. Matern Health Neonatol Perinatol. 2019;5:15. doi: 10.1186/s40748-019-0110-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isacson M, Gurung R, Basnet O, Andersson O, Kc A. Neurodevelopmental outcomes of a randomised trial of intact cord resuscitation. Acta Paediatr. February 2021;110(2):465–472. doi: 10.1111/apa.15401 [DOI] [PubMed] [Google Scholar]
- 56.Siddall RS, Crissey RR, Knapp WL. Effect on cesarean section babies of stripping or milking of the umbilical cords. American Journal of Obstetrics and Gynecology. 1952/May 1952;63(5):1059–1064. doi: 10.1016/0002-9378(52)90546-2 [DOI] [PubMed] [Google Scholar]
- 57.Mercer JS, Erickson-Owens DA. Rethinking placental transfusion and cord clamping issues. J Perinat Neonatal Nurs. Jul-Sep 2012;26(3):202–17; quiz 218–9. doi: 10.1097/JPN.0b013e31825d2d9a [DOI] [PubMed] [Google Scholar]
- 58.Zhou YB, Li HT, Zhu LP, Liu JM. Impact of cesarean section on placental transfusion and iron-related hematological indices in term neonates: a systematic review and meta-analysis. Placenta. Jan 2014;35(1):1–8. doi: 10.1016/j.placenta.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 59.Oh W Cord milking at delivery improves the iron status of term infants at 6 weeks. Evidence Based Medicine 2013/May/04 2013;18(6):e58–e58. doi: 10.1136/eb-2013-101294 [DOI] [PubMed] [Google Scholar]
- 60.Upadhyay A, Gothwal S, Parihar R, et al. Effect of Umbilical Cord Milking in Term and Near Term Infants. Obstetrical & Gynecological Survey. 2013/May 2013;68(5):333–334. doi: 10.1097/01.ogx.0000430374.84251.54 [DOI] [Google Scholar]
- 61.Erickson-Owens DA, Mercer JS, Oh W. Umbilical cord milking in term infants delivered by cesarean section: a randomized controlled trial. J Perinatol. Aug 2012;32(8):580–4. doi: 10.1038/jp.2011.159 [DOI] [PubMed] [Google Scholar]
- 62.Jeevan A, Ananthan A, Bhuwan M, Balasubramanian H, Rao S, Kabra NS. Umbilical cord milking versus delayed cord clamping in term and late-preterm infants: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. Dec 2022;35(25):5478–5488. doi: 10.1080/14767058.2021.1884676 [DOI] [PubMed] [Google Scholar]
- 63.McAdams RM, Fay E, Delaney S. Whole blood volumes associated with milking intact and cut umbilical cords in term newborns. Journal of Perinatology. 2017/December/12 2017;38(3):245–250. doi: 10.1038/s41372-017-0002-x [DOI] [PubMed] [Google Scholar]
- 64.Katheria AC, Sauberan JB, Akotia D, Rich W, Durham J, Finer NN. A Pilot Randomized Controlled Trial of Early versus Routine Caffeine in Extremely Premature Infants. Am J Perinatol. Jul 2015;32(9):879–86. doi: 10.1055/s-0034-1543981 [DOI] [PubMed] [Google Scholar]
- 65.Rabe H, Jewison A, Fernandez Alvarez R, et al. Milking Compared With Delayed Cord Clamping to Increase Placental Transfusion in Preterm Neonates. Obstetrics & Gynecology. 2011/February 2011;117(2):205–211. doi: 10.1097/aog.0b013e3181fe46ff [DOI] [PubMed] [Google Scholar]
- 66.Al-Wassia H, Shah PS. Efficacy and Safety of Umbilical Cord Milking at Birth. JAMA Pediatrics. 2015/January/01 2015;169(1):18. doi: 10.1001/jamapediatrics.2014.1906 [DOI] [PubMed] [Google Scholar]
- 67.Backes CH, Rivera B, Haque U, Copeland K, Hutchon D, Smith CV. Placental transfusion strategies in extremely preterm infants: The next piece of the puzzle. J Neonatal Perinatal Med Jan 1 2014;7(4):257–67. doi: 10.3233/npm-14814034 [DOI] [PubMed] [Google Scholar]
- 68.Ghavam S, Batra D, Mercer J, et al. Effects of placental transfusion in extremely low birthweight infants: meta-analysis of long- and short-term outcomes. Transfusion. Apr 2014;54(4):1192–8. [DOI] [PubMed] [Google Scholar]
- 69.Blank DA, Polglase GR, Kluckow M, et al. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Arch Dis Child Fetal Neonatal Ed. Dec 5 2017;doi: 10.1136/archdischild-2017-314005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katheria A, Reister F, Essers J, et al. Association of Umbilical Cord Milking vs Delayed Umbilical Cord Clamping With Death or Severe Intraventricular Hemorrhage Among Preterm Infants. JAMA. 2019;322(19):1877–1886. doi: 10.1001/jama.2019.16004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhee CJ, da Costa CS, Austin T, Brady KM, Czosnyka M, Lee JK. Neonatal cerebrovascular autoregulation. Pediatr Res. Nov 2018;84(5):602–610. doi: 10.1038/s41390-018-0141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jaiswal P, Upadhyay A, Gothwal S, Chaudhary H, Tandon A. Comparison of Umbilical Cord Milking and Delayed Cord Clamping on Cerebral Blood Flow in Term Neonates. Indian J Pediatr. May 27 2015;doi: 10.1007/s12098-015-1734-2 [DOI] [PubMed] [Google Scholar]
- 73.Girish M, Jain V, Dhokane R, Gondhali SB, Vaidya A, Aghai ZH. Umbilical cord milking for neonates who are depressed at birth: a randomized trial of feasibility. J Perinatol. Sep 2018;38(9):1190–1196. doi: 10.1038/s41372-018-0161-4 [DOI] [PubMed] [Google Scholar]
- 74.Katheria AC, Clark E, Yoder B, et al. Umbilical cord milking in nonvigorous infants: a cluster-randomized crossover trial. Am J Obstet Gynecol. Aug 13 2022;doi: 10.1016/j.ajog.2022.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katheria AC, Amino R, Konop JM, et al. Stem Cell Composition of Umbilical Cord Blood Following Milking Compared with Delayed Clamping of the Cord Appears Better Suited for Promoting Hematopoiesis. The Journal of Pediatrics. 2020/January/01/ 2020;216:222–226. doi: 10.1016/j.jpeds.2019.07.043 [DOI] [PubMed] [Google Scholar]
- 76.Nagy M, Nasef N, Gibreel A, et al. Impact of Umbilical Cord Milking on Hematological Parameters in Preterm Neonates With Placental Insufficiency. Original Research. Frontiers in Pediatrics. 2022-March-04 2022;9doi: 10.3389/fped.2021.827219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mercer JS, Erickson-Owens DA, Deoni SCL, et al. Effects of Delayed Cord Clamping on 4-Month Ferritin Levels, Brain Myelin Content, and Neurodevelopment: A Randomized Controlled Trial. J Pediatr. Dec 2018;203:266–272.e2. doi: 10.1016/j.jpeds.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katheria A, Garey D, Truong G, et al. A Randomized Clinical Trial of Umbilical Cord Milking vs Delayed Cord Clamping in Preterm Infants: Neurodevelopmental Outcomes at 22–26 Months of Corrected Age. J Pediatr. Mar 2018;194:76–80. doi: 10.1016/j.jpeds.2017.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rabe H, Sawyer A, Amess P, Ayers S. Neurodevelopmental Outcomes at 2 and 3.5 Years for Very Preterm Babies Enrolled in a Randomized Trial of Milking the Umbilical Cord versus Delayed Cord Clamping. Neonatology. 2016;109(2):113–9. doi: 10.1159/000441891 [DOI] [PubMed] [Google Scholar]


