Abstract
Purpose:
Vasopressin, used as a catecholamine adjunct, is a vasoconstrictor that may be detrimental in some hemodynamic profiles, particularly left ventricular (LV) systolic dysfunction. This study tested the hypothesis that echocardiographic parameters differ between patients with a hemodynamic response after vasopressin initiation and those without a response.
Methods:
This retrospective, single-center, cross-sectional study included adults with septic shock receiving catecholamines and vasopressin with an echocardiogram performed after shock onset but before vasopressin initiation. Patients were grouped by hemodynamic response, defined as decreased catecholamine dosage with mean arterial pressure ≥65 mmHg six hours after vasopressin initiation, with echocardiographic parameters compared. LV systolic dysfunction was defined as LV ejection fraction (LVEF) <45%.
Results:
Of 129 included patients, 72 (56%) were hemodynamic responders. Hemodynamic responders, versus non-responders, had higher LVEF (61% [55%,68%] vs. 55% [40%,65%]; p=0.02) and less-frequent LV systolic dysfunction (absolute difference −16%; 95% CI −30%,−2%). Higher LVEF was associated with higher odds of hemodynamic response (for each LVEF 10%, response OR 1.32; 95% CI 1.04–1.68). Patients with LV systolic dysfunction, versus without LV systolic dysfunction, had higher mortality risk (HR(t)=e[0.81−0.1*t]; at t=0, HR 2.24; 95% CI 1.08–4.64).
Conclusions:
Pre-drug echocardiographic profiles differed in hemodynamic responders after vasopressin initiation versus non-responders.
Keywords: shock, septic, vasoconstrictor agents, vasopressin, norepinephrine, echocardiography
Summary:
In septic shock patients receiving adjunctive vasopressin, pre-drug echo parameters differed between hemodynamic responders and non-responders. Higher LVEF was associated with higher response odds. Echo may provide useful information to optimize adjunctive vasopressors.
Introduction
Septic shock is the most common cause of circulatory shock, with a 33% mortality rate in North America despite decades of therapeutic investigation.[1, 2] Trials of adjunctive vasopressors in patients with septic shock have failed to show a patient-centered outcome benefit over norepinephrine alone.[3–5] The lack of positive studies may be due to diverse hemodynamics in the patient population leading to differential response based on drug pharmacology. Septic shock is characterized by systemic vasodilation with cardiac output traditionally described as preserved or increased.[6] However, studies have shown heterogeneous hemodynamic profiles that are influenced by therapies such as intravenous fluids and vasopressors.[7, 8] Left ventricular (LV) systolic dysfunction, LV hyperkinesia, LV diastolic dysfunction, and right ventricular (RV) dysfunction are prevalent in patients with early septic shock and may have prognostic implications.[7–14] Because of possible complex hemodynamics, echocardiography is recommended to determine the hemodynamic profile in patients with circulatory shock with an unclear diagnosis or inadequate response to initial therapy.[15, 16] Despite increasing utilization of bedside echocardiography for shock management, its usefulness for vasoactive therapy decision-making is unclear.[16]
Vasopressin is commonly administered as a norepinephrine adjunct in septic shock.[17, 18] Both norepinephrine and vasopressin are vasoconstrictors that cause systemic vasoconstriction and increase venous return.[19, 20] Yet, only half of vasopressin recipients have a blood pressure response.[21, 22] Unlike norepinephrine, where cardiac output is preserved or modestly increased due to its adrenergic β1-agonism, vasopressin may decrease cardiac output because it does not have direct inotropic properties.[20, 23] Therefore, therapeutic response to vasopressin may differ based on pre-existing ventricular function.[24–27] Particularly concerning is LV systolic dysfunction, where vasopressin may further decrease cardiac output.[28] Studies have evaluated factors associated with hemodynamic response after vasopressin initiation and outcomes in septic shock, but the role of underlying cardiac function in hemodynamic response is currently unknown. We designed this study to evaluate pre-drug echocardiographic parameters in patients with septic shock receiving adjunctive vasopressin. We hypothesized that pre-drug echocardiographic profiles would differ between hemodynamic responders and non-responders, specifically with hemodynamic responders having a lower prevalence of LV systolic dysfunction.
Material and Methods
This single-center, retrospective, cross-sectional study evaluated ICU patients at Cleveland Clinic from January 2012 to November 2017. Electronic health records of patients ≥18 years were screened for septic shock presence.[29] All identified patients also met the Sepsis-3 septic shock definition.[30] Identified patients initiated on vasopressin as a catecholamine vasopressor adjunct and had a transthoracic echocardiogram performed were included. Most routinely used echocardiographic parameters are dependent on myocardial loading conditions and change dynamically in septic shock, especially with therapies such as vasopressors and inotropes.[7, 12] To minimize confounding of echocardiographic parameters we only included patients with an echocardiogram performed during catecholamine administration and within 72 hours prior to vasopressin initiation. Those in whom the vasopressin dosage was titrated within the first six hours of therapy, and those with echocardiogram images inadequate for assessment were excluded. All patients meeting study inclusion criteria without meeting an exclusion criterion during the study time frame were included. Only the first episode of exposure to vasopressin per patient was evaluated. All patients were managed in accord with the Surviving Sepsis Campaign guidelines available at the time of treatment.[31, 32]
Comprehensive transthoracic echocardiogram images were obtained by registered cardiac sonographers and evaluated according to guidelines.[33–35] Parameters of LV systolic function (LVEF), LV diastolic function (peak early diastolic velocity of the septal mitral annulus [e’] and mitral LV inflow peak early velocity [E]), and RV function (semi-quantitative RV dilation and RV systolic function, tricuspid annular plane systolic excursion [TAPSE], and tricuspid lateral annulus peak systolic velocity [TAPSV]; also known as RV S’) were systematically measured.[33–35] LV systolic dysfunction was defined as LVEF <45%.[7, 12] LV hyperkinesia was defined as LVEF >70%.[36] LV diastolic dysfunction was defined as septal e’ <7 cm/sec with septal E/e’ >15.[35] A multimodal technique was used to define RV dysfunction based on adapted recommendations from the American Society of Echocardiography.[33, 34] Patients were adjudicated to have RV dysfunction if one or more of the following were present: RV dilation, RV systolic dysfunction, TAPSE <17 mm, or TAPSV <9.5 cm/sec.[13, 33, 34, 37]
In the primary analysis patients were grouped by their outcome of hemodynamic response or non-response. A positive hemodynamic response was defined as a decrease from baseline in norepinephrine-equivalent (NEQ) catecholamine dose with a mean arterial pressure (MAP) ≥65 mm Hg at six hours after initiation of vasopressin.[21, 38] Patients who died within six hours of vasopressin initiation were adjudicated as non-responders. Exposure variables of interest were echocardiographic parameters prior to vasopressin initiation; the primary exposure of interest was LVEF. First, the distribution of echocardiographic parameters by hemodynamic response were evaluated graphically with density plots. Next, parameters were categorized according to the above definitions for LV systolic dysfunction, LV hyperkinesia, LV diastolic dysfunction, and RV dysfunction. Frequencies of ventricular dysfunction within hemodynamic response groups were compared with between-outcome absolute differences. To complement the primary analysis, the data were also reoriented to group patients into cohorts of pre-drug ventricular dysfunction or no dysfunction for each of the four categories. The frequency of hemodynamic response within each dysfunction cohort was evaluated with between-cohort absolute differences. The LV systolic dysfunction cohort analysis also included evaluation of secondary outcomes of 28-day mortality; the ratio of mean arterial pressure to norepinephrine-equivalent catecholamine dose (MAP/NEQ) at six hours after vasopressin initiation;[39] and days alive and free of the ICU, vasopressors, mechanical ventilation, and renal replacement therapy.[40] Exposure time for 28-day mortality was defined as the time from vasopressin initiation until death within 28 days, with censoring of alive patients at 28 days. In a sensitivity analysis blood pressure improvement after vasopressin initiation was defined as an increase in MAP/NEQ at six hours after vasopressin initiation. Patients were grouped by outcomes of increase or decrease in MAP/NEQ at six hours after vasopressin initiation, and analyses subsequently conducted as outlined for the primary analysis.
Categorical data are described as n (%) and continuous data are described as median (interquartile range). The association of LVEF with hemodynamic response was assessed with multivariable logistic regression. Variables considered for confounder adjustment in the model were based on previous literature and clinical expertise.[21, 37, 41, 42] A directed acyclic graph was utilized for model variable selection, with arterial pH, mechanical ventilation, and sequential organ failure assessment (SOFA) score included as confounders for the effect of LVEF on hemodynamic response after vasopressin initiation (eFigure 1). Analyses were performed using Stata (version 14.2; StataCorp; College Station, TX) and R (version 4.1.2; R Foundation for Statistical Computing; Vienna, Austria) based on an overall significance level of 0.05. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE reporting guideline and was approved by the Cleveland Clinic Institutional Review Board with a waiver of informed consent (approval number 20–1250 on 3 December 2020). Additional method details and the STROBE checklist are included in the Supplementary Material.
Results
Patient population
A total of 4397 patients with septic shock were assessed for eligibility. Of the screened patients, 4023 did not meet inclusion criteria and 245 were excluded, resulting in 129 patients included in the study. (eFigure 2) Included patients were most frequently admitted to a medical ICU and severely ill. (Table 1 and eTable1) At the time of vasopressin initiation, 17.7 (8.7, 33.1) hours had elapsed from catecholamine initiation and patients were receiving NEQ dose 25 (18, 40) mcg/min with lactate 3.0 (2.2, 6.0) mmol/L. Hemodynamic response was observed in 72 patients (56%), with responders, compared with non-responders, having higher MAP (70 [65, 78] mm Hg vs. 64 [59, 72] mm Hg, p<0.01) and arterial pH (7.35 [7.29, 7.39] vs. 7.32 [7.24, 7.36], p=0.04) at vasopressin initiation. (Table 1)
Table 1.
Patient Characteristics
| Characteristic | Total (N=129) | Hemodynamic Response (n=72) | Hemodynamic Non-Response (n=57) | p |
|---|---|---|---|---|
| At ICU Admission | ||||
| Age (years) | 61.0 (53.0, 69.0) | 62.0 (52.5, 69.5) | 61.0 (53.0, 69.0) | 0.88 |
| Male sex | 63 (48.8) | 27 (47.4) | 36 (50.0) | 0.86 |
| Body weight (kg) | 86.6 (70.5, 107.0) | 84.3 (70.0, 106.8) | 87.7 (76.0, 107.5) | 0.55 |
| Premorbid LVEF (%)a | 60 (55, 60) | 60 (55, 66) | 60 (50, 66) | 0.95 |
| APACHE III | 108 (83, 130) | 110 (84, 131) | 107 (82, 128) | 0.54 |
| At Catecholamine Initiation | ||||
| Broad spectrum antibiotic receipt | 120 (93.0) | 66 (91.7) | 54 (94.7) | 0.73 |
| Lactate (mmol/L) | 4.2 (3.1, 7.8) | 4.2 (3.0, 6.8) | 4.2 (3.1, 9.0) | 0.46 |
| At Echocardiogram Performance | ||||
| Hours elapsed from catecholamine start | 6.2 (2.2, 13.8) | 8.0 (2.6, 15.2) | 4.5 (1.9, 10.7) | 0.31 |
| SAP (mm Hg) | 99 (89, 110) | 101 (91, 112) | 97 (89, 108) | 0.26 |
| DAP (mm Hg) | 52 (48, 59) | 52 (48, 60) | 52 (48, 56) | 0.54 |
| MAP (mm Hg) | 68 (63, 76) | 67 (63, 75) | 69 (63, 77) | 0.30 |
| Norepinephrine-equivalent dose (mcg/min) | 16 (10, 25) | 16 (10, 25) | 17 (9, 25) | 0.89 |
| MAP/NEQ (mm Hg/[mcg/kg/min]) | 366 (222, 671) | 394 (218, 767) | 341 (229, 650) | 0.83 |
| At Vasopressin Initiation | ||||
| Hours elapsed from echocardiogram | 7.6 (3.2, 19.5) | 10.7 (3.7, 25.0) | 6.2 (2.1, 13.6) | 0.01 |
| Hours elapsed from catecholamine start | 17.7 (8.7, 33.1) | 20.0 (10.0, 39.4) | 12.3 (7.0, 28.2) | 0.05 |
| SAP (mm Hg) | 101 (93, 113) | 105 (97, 116) | 98 (87, 110) | <0.01 |
| DAP (mm Hg) | 51 (46, 57) | 53 (48, 61) | 49 (43, 56) | 0.04 |
| MAP (mm Hg) | 68 (62, 76) | 70 (65, 78) | 64 (59, 72) | <0.01 |
| Norepinephrine-equivalent dose (mcg/min) | 25 (18, 40) | 27 (20, 40) | 22 (15, 40) | 0.27 |
| MAP/NEQ (mm Hg/[mcg/kg/min]) | 242 (151, 360) | 241 (147, 350) | 242 (156, 376) | 0.81 |
| Lactate (mmol/L) | 3.0 (2.2, 6.0) | 3.0 (2.1, 5.1) | 3.4 (2.2, 9.3) | 0.35 |
| Arterial pH | 7.34 (7.26, 7.38) | 7.35 (7.29, 7.39) | 7.32 (7.24, 7.36) | 0.04 |
| SOFA | 14 (12, 17) | 15 (12, 17) | 14 (12, 17) | 0.43 |
| Mechanical ventilation | 98 (76.0) | 58 (80.6) | 40 (70.2) | 0.21 |
| At Hemodynamic Response Adjudication (6 Hours After Vasopressin Initiation)b | ||||
| SAP (mm Hg) | 111 (101, 127) | 114 (105, 130) | 105 (94, 123) | <0.01 |
| DAP (mm Hg) | 60 (53, 67) | 61 (56, 68) | 56 (49, 65) | 0.03 |
| MAP (mm Hg) | 79 (72, 89) | 81 (76, 86) | 75 (68, 85) | <0.01 |
| Norepinephrine-equivalent dose (mcg/min) | 20 (10, 30) | 15 (7, 22) | 27 (15, 60) | <0.01 |
| Norepinephrine-equivalent dose change after vasopressin initiation (mcg/min) | −6 (−14, 2) | −10 (−17, −8) | 5 (0, 29) | <0.01 |
| MAP/NEQ (mm Hg/[mcg/kg/min]) | 231 (128, 374) | 290 (194, 436) | 159 (103, 263) | <0.01 |
| Vasopressin dose (units/min) | 0.03 (0.03, 0.04) | 0.03 (0.03, 0.04) | 0.03 (0.03, 0.03) | 0.58 |
Data presented as n (%) or median (interquartile range). Additional patient characteristics, including norepinephrine-equivalent doses in mcg/kg/min, can be found in eTable 1 in the Supplementary Materials.
N=61.
N=128. APACHE = acute physiologic and chronic health evaluation, DAP = diastolic arterial pressure, LVEF = left ventricular ejection fraction, MAP = mean arterial blood pressure, MAP/NEQ = mean arterial pressure/norepinephrine-equivalent catecholamine dose, SAP = systolic arterial pressure, SOFA = sequential organ failure assessment
Echocardiographic profiles prior to vasopressin initiation
Echocardiograms were performed 6.2 (2.2, 13.8) hours after catecholamine start and 7.6 (3.2, 19.5) hours prior to vasopressin initiation. At the time of echocardiogram, patients were receiving NEQ dose 16 (10, 25) mcg/min, and most were receiving norepinephrine monotherapy (n=108; 84%) and mechanically ventilated (n=89; 69%). Compared with hemodynamic non-responders, responders had higher LVEF, TAPSE, and TAPSV. (eTable 1) (Figure 1) After adjusting for arterial pH, mechanical ventilation, and SOFA with multivariable logistic regression, higher LVEF was independently associated with higher odds of hemodynamic response (for each LVEF 10%, response OR 1.32; 95% CI 1.04–1.68). Categorizing echocardiographic parameters into ventricular dysfunctions revealed 33 patients (26%) had no ventricular dysfunction, 62 (48%) had a single ventricular dysfunction, and 34 (26%) had multiple ventricular dysfunctions. (eFigure 3) Hemodynamic responders, compared with non-responders, had a lower frequency of LV systolic dysfunction (absolute difference −16% [95% CI −30% to −2%]). No between-group difference was detected in the frequency of LV hyperkinesia (absolute difference 3% [95% CI −10% to 15%]), LV diastolic dysfunction (absolute difference 1% [95% CI −12% to 14%]), or RV dysfunction (absolute difference −3% [95% CI −20% to 14%]). (Figure 2) Results were similar in the sensitivity analysis of patients grouped by outcome of MAP/NEQ increase or decrease at six hours after vasopressin initiation; patients with a MAP/NEQ increase had higher LVEF and less frequent LV systolic dysfunction (absolute difference −18% [95% CI −31% to −4%]). (eTable 2, eFigure 4, eFigure 5)
Figure 1.
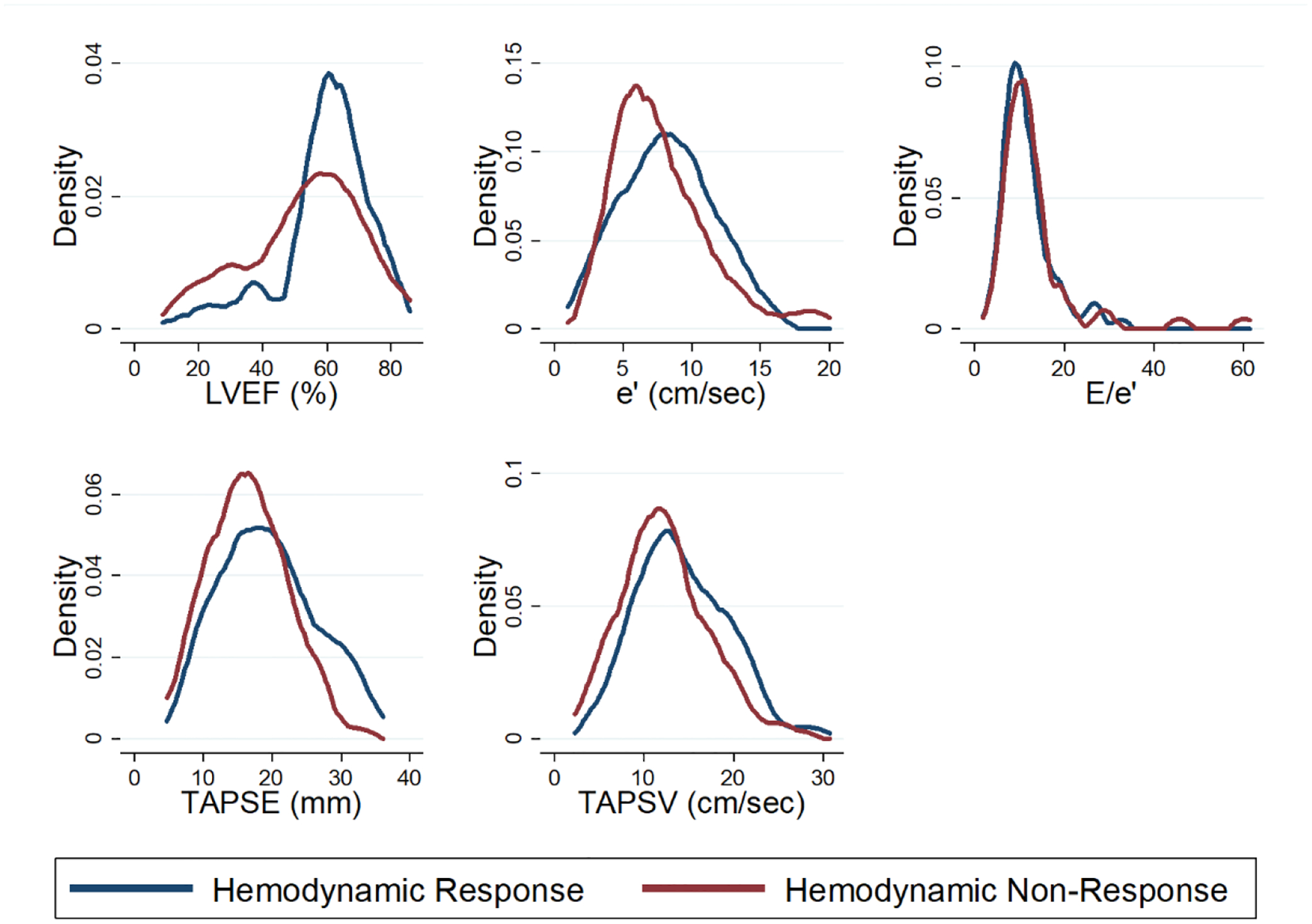
Distribution of Echocardiographic Parameters by Hemodynamic Response
Density plots representing the distribution of echocardiographic parameters by hemodynamic response group after vasopressin initiation. Hemodynamic responders had higher LVEF (p=0.02), TAPSE (p=0.03), and TAPSV (p=0.02). No between-group difference was detected in the analysis of e’ and E/e’. N=110 for TAPSE, N=123 for TAPSV. e’ = peak early diastolic velocity of the mitral annulus, E = mitral left ventricular inflow peak early velocity, LVEF = left ventricular ejection fraction, TAPSE = tricuspid annular plane systolic excursion, TAPSV = tricuspid lateral annulus peak systolic velocity (also known as RV S’)
Figure 2.
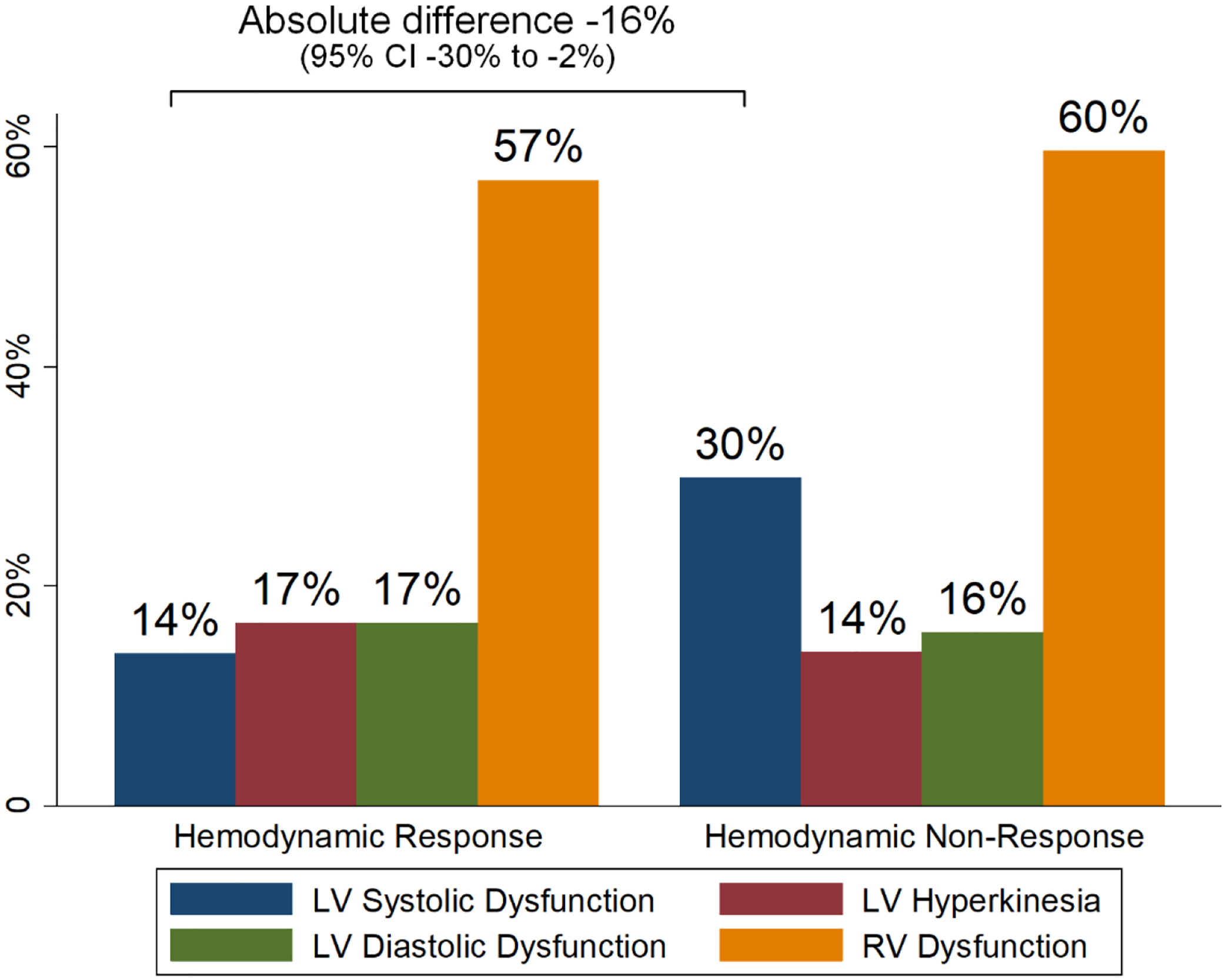
Ventricular Dysfunction Categories by Hemodynamic Response
Proportion of patients with pre-drug categorized ventricular dysfunctions within hemodynamic response and hemodynamic non-response groups. Hemodynamic responders, compared with hemodynamic non-responders, less frequently had pre-drug LV systolic dysfunction (absolute difference −16% [−30% to −2%]). No between-group difference was detected in the comparison of the frequency of other pre-drug ventricular dysfunction categories. LV = left ventricular, RV = right ventricular
Ventricular dysfunction cohorts
When the data were reoriented to describe ventricular dysfunction cohorts prior to vasopressin initiation, patients with LV systolic dysfunction, compared to patients without LV systolic dysfunction, were less frequently hemodynamic responders (n/N=10/27 [37%] vs. n/N=62/102 [61%], absolute difference −24% [95% CI −44% to −3%]). No difference was detected in hemodynamic response frequency in patients with other ventricular dysfunctions categories. (Figure 3A–D) After adjusting for APACHE III, SOFA, and lactate at vasopressin initiation, LV systolic dysfunction was associated with higher risk for 28-day mortality with a time-varying effect (HR(t)=e[0.81−0.1*t]; at t=0, HR 2.24; 95% CI 1.08–4.64). (Figure 4) A difference was not detected between patients with and without LV systolic dysfunction in days alive and free of the ICU, vasopressors, mechanical ventilation, or renal replacement therapy. (eTable 3)
Figure 3A-D.
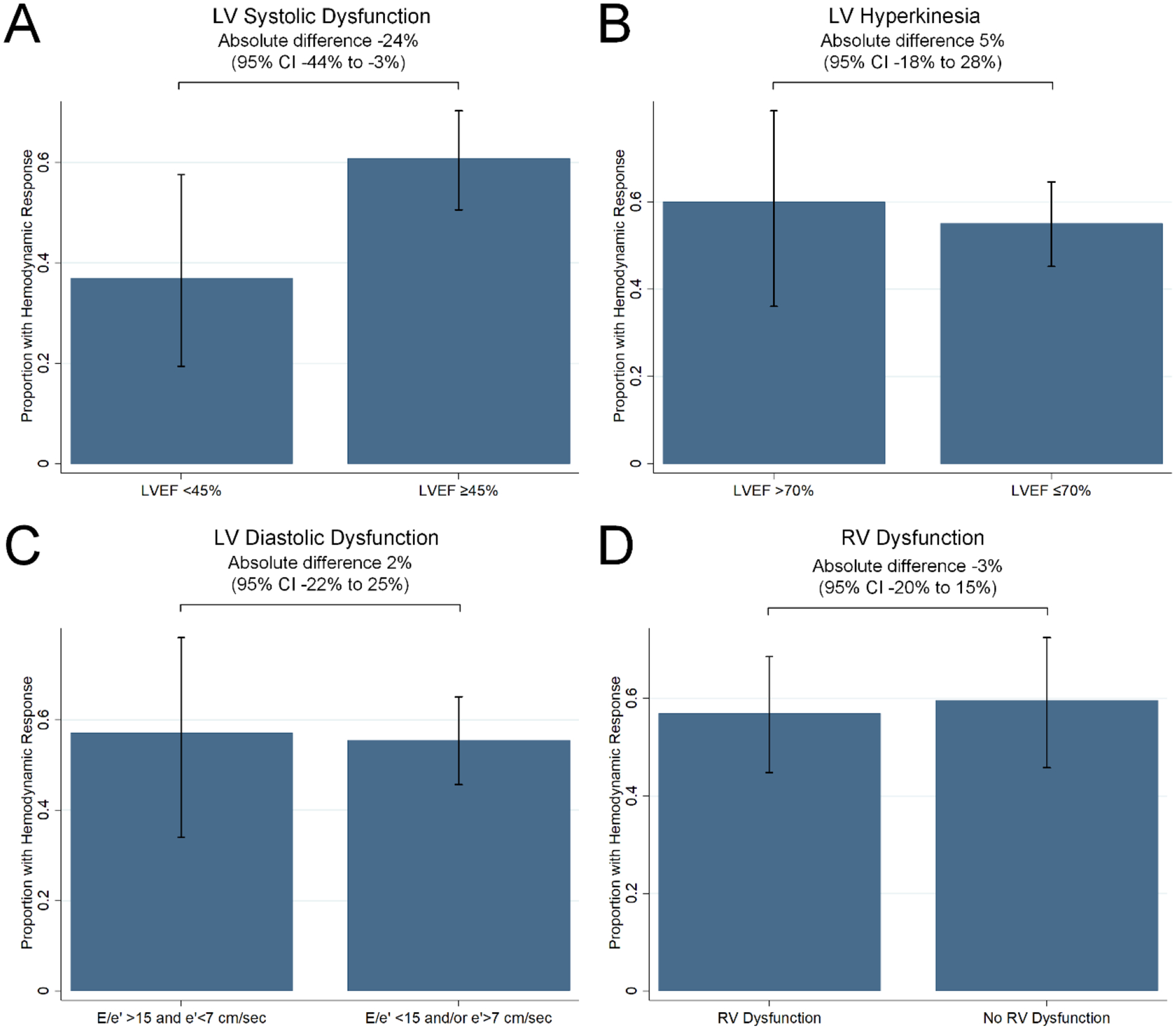
Hemodynamic Response by Ventricular Dysfunction Categories
Proportion of patients with hemodynamic response after vasopressin initiation within pre-drug categorized (A) LV systolic dysfunction, (B) LV hyperkinesia, (C) LV diastolic dysfunction, and (D) RV dysfunction categories. Patients with pre-vasopressin LV systolic dysfunction, compared with patients without LV systolic dysfunction, less frequently had hemodynamic response (absolute difference 24% [−44% to −3%]). No difference was detected in the comparison of hemodynamic response frequency within other ventricular dysfunction categories. e’ = peak early diastolic velocity of the mitral annulus, E = mitral left ventricular inflow peak early velocity, LV = left ventricular, LVEF = left ventricular ejection fraction, RV = right ventricular
Figure 4.
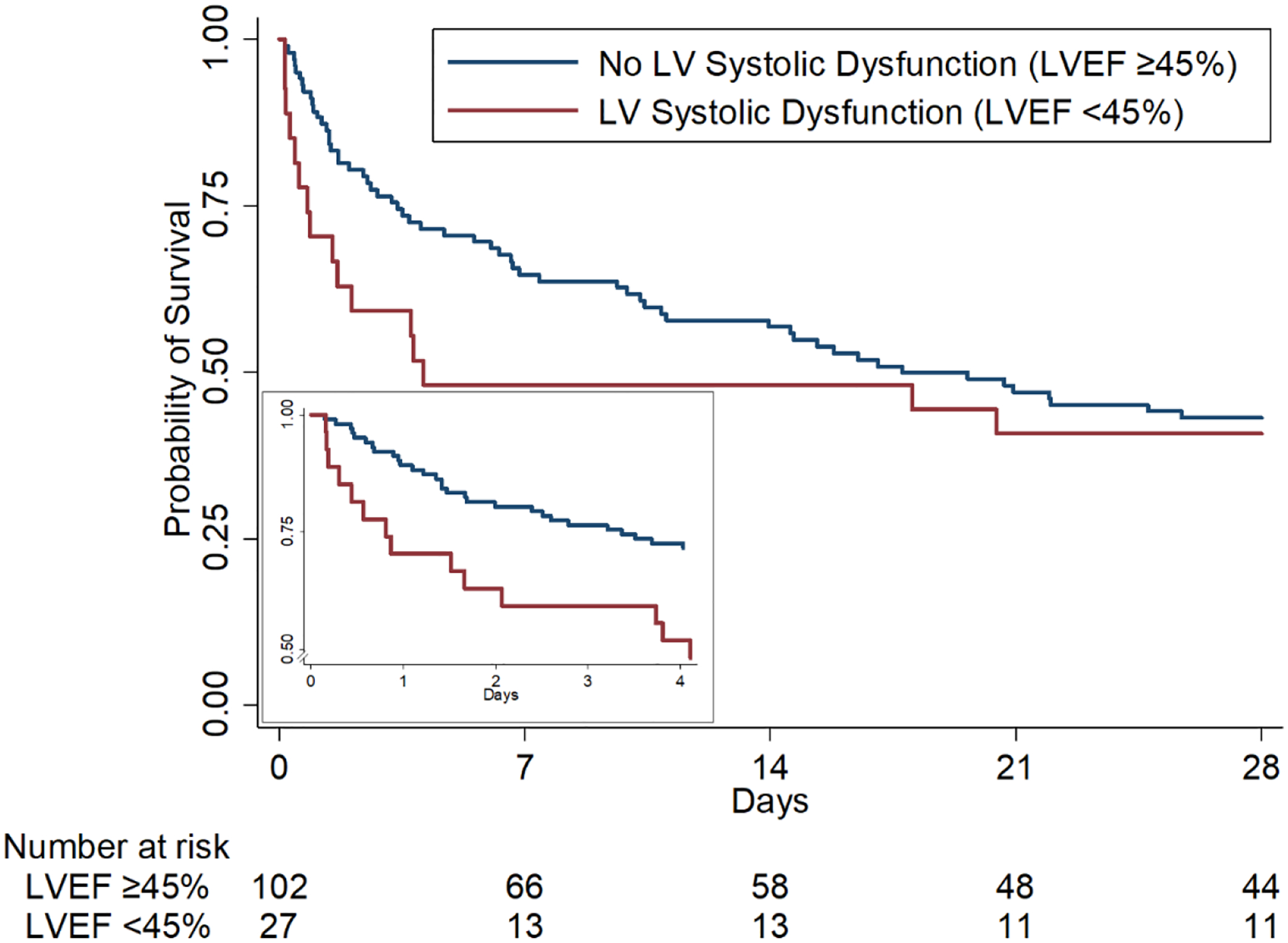
Kaplan-Meier Curves for 28-Day Survival by Left Ventricular Systolic Dysfunction Group
Kaplan-Meier estimated probability of survival in patients with pre-vasopressin LV systolic dysfunction (red) and no LV systolic dysfunction (blue). Compared to those without LV systolic dysfunction, patients with LV systolic dysfunction receiving adjunctive vasopressin had higher risk for 28-day mortality with a time-varying effect (HR(t)=e[0.81−0.1*t]; at t=0, HR 2.24; 95% CI 1.08–4.64). Insert: Kaplan-Meier estimated probability of survival over the first four days after vasopressin initiation. Four days was utilized as the exposure time for the insert because over 85% of patients received vasopressin for four days or less. LV = left ventricular, LVEF = left ventricular ejection fraction.
Discussion
In this study of patients with septic shock receiving adjunctive vasopressin, we found pre-vasopressin echocardiographic parameters differed between hemodynamic responders and non-responders 6 hours after vasopressin initiation. Consistent with our a priori hypothesis, hemodynamic responders had higher LVEF and a lower prevalence of LV systolic dysfunction. Higher LVEF was independently associated with higher odds of hemodynamic response. A sensitivity analysis of blood pressure improvement defined by MAP/NEQ increase after vasopressin initiation corroborated and complemented the findings in our primary analysis. When the data were reoriented into ventricular dysfunction cohorts in order to inform bedside decision-making, patients with baseline LV systolic dysfunction less frequently demonstrated hemodynamic response after vasopressin initiation. Further, compared with patients without LV systolic dysfunction, patients with LV systolic dysfunction had higher mortality. Although arterial pressures (which can influence LVEF and assessment of LV systolic dysfunction) were lower in hemodynamic non-responders at the time of vasopressin initiation, we did not detect a difference between hemodynamic responders and non-responders in NEQ or MAP/NEQ at the time of echocardiogram or at vasopressin initiation. This suggests differential ventricular loading conditions are unlikely the main explanation for our findings. Our novel study is the first to evaluate the association of echocardiographic profiles prior to adjunctive vasopressin with blood pressure response and patient outcomes, and provides valuable data to clinicians contemplating the initiation of adjunctive vasopressin in patients with septic shock.
Vasopressin is suggested as a norepinephrine adjunct in patients with septic shock.[17] While meta-analyses have demonstrated a mortality benefit of vasopressin analogues in the broader category of vasodilatory/distributive shock, vasopressin has not been associated with a mortality improvement specifically in patients with septic shock.[43, 44] These seemingly discrepant findings may be due to variable hemodynamic profiles observed in patients with septic shock, with the notable occurrence of sepsis-induced myocardial dysfunction.[8, 45] For the average patient with septic shock, adjunctive low-dosage vasopressin does not change cardiac index. In a post-hoc analysis of the Vasopressin And Septic Shock Trial (VASST), no difference in cardiac index over time was detected between patients allocated to adjunctive vasopressin with norepinephrine versus norepinephrine monotherapy.[30] Patients with chronic heart failure (New York Heart Association class III and IV) were excluded from the trial, though, and included patients on average had a baseline elevated cardiac index. Additionally, the hemodynamic analysis evaluated the average treatment effect in the study, and did not assess for heterogeneity of treatment effect by baseline cardiac index. Because of vasopressin’s pharmacology, it may have differential pharmacodynamic effects based on a patient’s underlying hemodynamic profile.[24–27] Animal models of heart failure have demonstrated decreased cardiac output after vasopressin initiation.[46, 47] Also, in a study of patients with compensated heart failure with reduced ejection fraction, vasopressin 0.1–0.8 pmol/kg/min (0.005–0.042 units/min for an 80 kg patient) decreased cardiac output and stroke volume compared with baseline.[28] Our study showed an incremental decrease in hemodynamic response odds after vasopressin initiation with every 10% decrease in LVEF, signifying the impact of vasopressin initiation on the outcome to be exacerbated as LV dysfunction becomes more substantial. Although a previous meta-analysis did not detect a difference in LV function between sepsis survivors and non-survivors,[10] recent studies have found higher mortality in patients with septic shock and a cluster of features of LV systolic dysfunction (low LVEF and low cardiac index),[8] and those with LVEF <25%.[48] These data suggest patients with septic shock and LV systolic dysfunction are a vulnerable population and vasopressors must be selected judiciously. We hypothesize patients in our study with LV systolic dysfunction had a (further) decrease in cardiac output after vasopressin initiation, leading to less frequent hemodynamic response and higher mortality.[49] Future studies should evaluate this hypothesis. Our data support echocardiography as a valuable bedside tool to rule out LV systolic dysfunction prior to adjunctive vasopressin initiation in patients with septic shock in order to minimize drug-related harm.
We did not detect a difference between hemodynamic responders and non-responders in the frequency of LV diastolic dysfunction or RV dysfunction. To our knowledge, vasopressin has not been previously evaluated in either animal models or patients with septic shock and LV diastolic dysfunction. Because vasopressin typically decreases heart rate, we hypothesized hemodynamic responders would more frequently have LV diastolic dysfunction, with improved blood pressure due to increased ventricular filling time.[30, 50] Since we did not detect differences between hemodynamic responders and non-responders in E/e’, e’, or categorized LV diastolic dysfunction, our data suggest LV diastolic dysfunction is not a major contributor to hemodynamic response after vasopressin initiation in septic shock. In light of a number of case series describing beneficial effects of vasopressin-induced vasodilation of the pulmonary vasculature, a systematic review suggested low-dosage vasopressin for patients with vasodilatory shock and RV dysfunction.[51, 52] While vasopressin was beneficial in a rat model of pulmonary vasoconstriction, in a canine model of acute pulmonary hypertension vasopressin increased pulmonary vascular resistance, decreased cardiac output, and decreased RV contractility.[53, 54] Although we did not detect a between-group difference in categorized RV dysfunction, TAPSE and TAPSV were higher in hemodynamic responders, consistent with better baseline RV function.[55] Our data do not support preferential use of vasopressin when signs of RV dysfunction are present in patients with septic shock; further research in this area is needed.
Our study has a number of strengths. First, we conscientiously designed the study to ensure echocardiograms were performed during catecholamine vasopressor administration and in close proximity to vasopressin initiation, without reliance on premorbid information. As demonstration of the importance of our study design, we did not detect a difference in premorbid LVEF or the frequency of heart failure history between hemodynamic responders and non-responders, but there was a between-group difference in LVEF and the frequency of LV systolic dysfunction during septic shock. Additionally, we performed multiple clinically-relevant analyses to both better understand hemodynamic response after vasopressin initiation and inform bedside decision-making. The study is limited by its single-center nature and relatively small sample size. Further, our study design to ensure comprehensive echocardiogram timing during septic shock but prior to vasopressin initiation may have led to a selection bias for the study sample. This is exampled by our reported hemodynamic response frequency of 56%, which is higher than previously-reported frequencies of 40–45% utilizing the same definition for hemodynamic response at 6 hours after vasopressin initiation.[21, 22] Whether echocardiographic parameters differ in hemodynamic responders vs. non-responders in a population of patients with a lower hemodynamic response frequency is unclear. Also, because invasive or semi-invasive hemodynamic parameters, such as cardiac output/index, were not available the hemodynamic profile of included patients is incomplete. Yet, we designed this study to specifically evaluate echocardiographic parameters because they are commonly utilized at the bedside for shock diagnosis and management.[15, 16] Additionally, assessment of hemodynamic response at six hours after vasopressin initiation may lead to misclassification of response. Future studies should elucidate the optimal time point for assessing hemodynamic response after vasopressin initiation and the best definition of this endpoint (reflective of improved organ and tissue perfusion). Further, our study was not designed to evaluate the mechanism for hemodynamic non-response, which could include those of a cardiac nature (such as LVEF worsening due to increased afterload, or new myocardial ischemia) or a failure of vasopressin to improve arterial vasoconstriction. Lastly, because not all patients had a premorbid echocardiogram available for analysis, we are unable to distinguish between pre-existing ventricular dysfunction and sepsis-induced changes. This differentiation may have important prognostic and treatment implications.[9, 56] However, regardless of the temporality, our findings suggest echocardiographic findings during septic shock can guide adjunctive vasoactive therapy decision-making. Future studies with a larger sample size should use statistical clustering methods to distill redundant information and isolate important hemodynamic (echocardiographic, etc.) parameters for hemodynamic response after vasopressin initiation.
Conclusions
Hemodynamic responders and non-responders 6 hours after vasopressin initiation had different pre-drug echocardiographic profiles. Patients with septic shock administered adjunctive vasopressin with concomitant LV systolic dysfunction had less frequent hemodynamic response and higher mortality. In patients with septic shock not responding to initial catecholamines, echocardiography may provide useful information to optimize adjunctive vasopressor therapy and minimize drug-related harm.
Supplementary Material
Highlights.
Hemodynamic responders, compared with non-responders, had higher LVEF (61% vs. 55%) and less-frequent LV systolic dysfunction (absolute difference −16%; 95% CI −30% to −2%)
Higher LVEF was associated with higher odds of hemodynamic response (for each LVEF 10%, response OR 1.32; 95% CI 1.04–1.68)
Patients receiving vasopressin with LV systolic dysfunction had higher mortality risk compared to those without LV systolic dysfunction (HR(t)=e[0.81−0.1*t]; at t=0, HR 2.24; 95% CI 1.08–4.64)
Echocardiography may provide useful information to optimize adjunctive vasopressor therapy in patients with septic shock
Acknowledgements
The authors thank Lu Wang, MS, for her independent statistical review.
Conflicts of Interest and Source of Funding:
PC disclosed he received consulting and speaking fees from Philips. All other authors have disclosed that they do not have any conflicts of interest. Supported by the National Institutes of Health, National Institute of General Medical Sciences (VV: R01GM099807 and SRB: K08GM147806). The funding source had no role in study design; data collection, analysis, or interpretation; writing the report; or the decision to submit the report for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- APACHE
acute physiologic and chronic health evaluation
- e’
peak early diastolic velocity of the mitral annulus
- E
mitral left ventricular inflow peak early velocity
- ICU
intensive care unit
- LVEF
left ventricular ejection fraction
- MAP
mean arterial blood pressure
- MAP/NEQ
mean arterial pressure/norepinephrine-equivalent catecholamine dose
- RV
right ventricular
- SOFA
sequential organ failure assessment
- TAPSE
tricuspid annular plane systolic excursion
- TAPSV
tricuspid lateral annulus peak systolic velocity (also known as RV S’)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article was presented at SCCM Congress, April 2022, virtual meeting.
Author Contributions
Siddharth Dugar: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – reviewing and editing
Matthew T. Siuba: Conceptualization, Data curation, Investigation, Methodology, Writing – reviewing and editing
Gretchen L. Sacha: Data curation, Investigation, Methodology, Writing – reviewing and editing
Ryota Sato: Data curation, Investigation, Methodology, Writing – reviewing and editing
Ajit Moghekar: Data curation, Investigation, Methodology, Supervision, Writing – reviewing and editing
Patrick Collier: Data curation, Investigation, Methodology, Writing – reviewing and editing
Richard A. Grimm: Data curation, Investigation, Methodology, Supervision, Writing – reviewing and editing
Vidula Vachharajani: Data curation, Investigation, Methodology, Supervision, Writing – reviewing and editing
Seth R. Bauer: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft
References
- [1].Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013;369(18):1726–34. [DOI] [PubMed] [Google Scholar]
- [2].Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care 2019;23(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008;358(9):877–87. [DOI] [PubMed] [Google Scholar]
- [4].Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med 2017;377(5):419–30. [DOI] [PubMed] [Google Scholar]
- [5].Laterre PF, Berry SM, Blemings A, Carlsen JE, François B, Graves T, et al. Effect of Selepressin vs Placebo on Ventilator- and Vasopressor-Free Days in Patients With Septic Shock: The SEPSIS-ACT Randomized Clinical Trial. JAMA 2019;322(15):1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rabuel C, Mebazaa A. Septic shock: a heart story since the 1960s. Intensive Care Med 2006;32(6):799–807. [DOI] [PubMed] [Google Scholar]
- [7].Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 2008;36(6):1701–6. [DOI] [PubMed] [Google Scholar]
- [8].Geri G, Vignon P, Aubry A, Fedou AL, Charron C, Silva S, et al. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med 2019;45(5):657–67. [DOI] [PubMed] [Google Scholar]
- [9].Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 1984;100(4):483–90. [DOI] [PubMed] [Google Scholar]
- [10].Huang SJ, Nalos M, McLean AS. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit Care 2013;17(3):R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sanfilippo F, Corredor C, Arcadipane A, Landesberg G, Vieillard-Baron A, Cecconi M, et al. Tissue Doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: a systematic review and meta-analysis. Br J Anaesth 2017;119(4):583–94. [DOI] [PubMed] [Google Scholar]
- [12].Boissier F, Razazi K, Seemann A, Bedet A, Thille AW, de Prost N, et al. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med 2017;43(5):633–42. [DOI] [PubMed] [Google Scholar]
- [13].Kim J-s, Kim Y-J, Kim M, Ryoo SM, Kim WY. Association between right ventricle dysfunction and poor outcome in patients with septic shock. Heart 2020;106(21):1665–71. [DOI] [PubMed] [Google Scholar]
- [14].Chotalia M, Ali M, Hebballi R, Singh H, Parekh D, Bangash MN, et al. Hyperdynamic Left Ventricular Ejection Fraction in ICU Patients With Sepsis. Crit Care Med 2022;50(5):770–9. [DOI] [PubMed] [Google Scholar]
- [15].Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40(12):1795–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vieillard-Baron A, Millington SJ, Sanfilippo F, Chew M, Diaz-Gomez J, McLean A, et al. A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med 2019;45(6):770–88. [DOI] [PubMed] [Google Scholar]
- [17].Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med 2021;49(11):e1063–e143. [DOI] [PubMed] [Google Scholar]
- [18].Sacha GL, Kiser TH, Wright GC, Vandivier RW, Moss M, Burnham EL, et al. Association Between Vasopressin Rebranding and Utilization in Patients With Septic Shock. Crit Care Med 2022;50(4):644–54. [DOI] [PubMed] [Google Scholar]
- [19].Monnet X, Jabot J, Maizel J, Richard C, Teboul JL. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med 2011;39(4):689–94. [DOI] [PubMed] [Google Scholar]
- [20].Annane D, Ouanes-Besbes L, de Backer D, Du B, Gordon AC, Hernández G, et al. A global perspective on vasoactive agents in shock. Intensive Care Med 2018;44(6):833–46. [DOI] [PubMed] [Google Scholar]
- [21].Sacha GL, Lam SW, Duggal A, Torbic H, Bass SN, Welch SC, et al. Predictors of response to fixed-dose vasopressin in adult patients with septic shock. Ann Intensive Care 2018;8(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sacha GL, Lam SW, Wang L, Duggal A, Reddy AJ, Bauer SR. Association of Catecholamine Dose, Lactate, and Shock Duration at Vasopressin Initiation With Mortality in Patients With Septic Shock. Crit Care Med 2022;50(4):614–23. [DOI] [PubMed] [Google Scholar]
- [23].Albanèse J, Leone M, Delmas A, Martin C. Terlipressin or norepinephrine in hyperdynamic septic shock: a prospective, randomized study. Crit Care Med 2005;33(9):1897–902. [DOI] [PubMed] [Google Scholar]
- [24].Bauer SR, Lam SW. Arginine vasopressin for the treatment of septic shock in adults. Pharmacotherapy 2010;30(10):1057–71. [DOI] [PubMed] [Google Scholar]
- [25].Bauer SR, Sacha GL, Lam SW. Safe Use of Vasopressin and Angiotensin II for Patients with Circulatory Shock. Pharmacotherapy 2018;38(8):851–61. [DOI] [PubMed] [Google Scholar]
- [26].Sacha GL, Bauer SR, Lat I. Vasoactive Agent Use in Septic Shock: Beyond First-Line Recommendations. Pharmacotherapy 2019;39(3):369–81. [DOI] [PubMed] [Google Scholar]
- [27].Jones TW, Smith SE, Van Tuyl JS, Newsome AS. Sepsis With Preexisting Heart Failure: Management of Confounding Clinical Features. J Intensive Care Med 2021;36(9):989–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goldsmith SR, Francis GS, Cowley AW, Goldenberg IF, Cohn JN. Hemodynamic effects of infused arginine vasopressin in congestive heart failure. J Am Coll Cardiol 1986;8(4):779–83. [DOI] [PubMed] [Google Scholar]
- [29].United States Centers for Disease Control and Prevention. Hospital toolkit for adult sepsis surveillance, https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Aug-2018_508.pdf; 2018. [Accessed August 31, 2022].
- [30].Gordon AC, Wang N, Walley KR, Ashby D, Russell JA. The cardiopulmonary effects of vasopressin compared with norepinephrine in septic shock. Chest 2012;142(3):593–605. [DOI] [PubMed] [Google Scholar]
- [31].Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41(2):580–637. [DOI] [PubMed] [Google Scholar]
- [32].Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45(3):486–552. [DOI] [PubMed] [Google Scholar]
- [33].Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- [34].Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23(7):685–713; quiz 86–8. [DOI] [PubMed] [Google Scholar]
- [35].Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29(4):277–314. [DOI] [PubMed] [Google Scholar]
- [36].Paonessa JR, Brennan T, Pimentel M, Steinhaus D, Feng M, Celi LA. Hyperdynamic left ventricular ejection fraction in the intensive care unit. Critical Care 2015;19(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vallabhajosyula S, Kumar M, Pandompatam G, Sakhuja A, Kashyap R, Kashani K, et al. Prognostic impact of isolated right ventricular dysfunction in sepsis and septic shock: an 8-year historical cohort study. Ann Intensive Care 2017;7(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bauer SR, Sacha GL, Siuba MT, Wang L, Wang X, Scheraga RG, et al. Vasopressin Response and Clinical Trajectory in Septic Shock Patients. J Intensive Care Med 2023;38(3):273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bosch NA, Teja B, Wunsch H, Walkey AJ. Characterization and validation of a novel measure of septic shock severity. Intensive Care Med 2020;46(1):135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Russell JA, Lee T, Singer J, De Backer D, Annane D. Days alive and free as an alternative to a mortality outcome in pivotal vasopressor and septic shock trials. J Crit Care 2018;47:333–7. [DOI] [PubMed] [Google Scholar]
- [41].Bauer SR, Sacha GL, Siuba MT, Lam SW, Reddy AJ, Duggal A, et al. Association of Arterial pH With Hemodynamic Response to Vasopressin in Patients With Septic Shock: An Observational Cohort Study. Crit Care Explor 2022;4(2):e0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann Am Thorac Soc 2019;16(1):22–8. [DOI] [PubMed] [Google Scholar]
- [43].McIntyre WF, Um KJ, Alhazzani W, Lengyel AP, Hajjar L, Gordon AC, et al. Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis. JAMA 2018;319(18):1889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nagendran M, Russell JA, Walley KR, Brett SJ, Perkins GD, Hajjar L, et al. Vasopressin in septic shock: an individual patient data meta-analysis of randomised controlled trials. Intensive Care Med 2019;45(6):844–55. [DOI] [PubMed] [Google Scholar]
- [45].Walley KR. Sepsis-induced myocardial dysfunction. Curr Opin Crit Care 2018;24(4):292–9. [DOI] [PubMed] [Google Scholar]
- [46].Arnolda L, McGrath BP, Cocks M, Johnston CI. Vasoconstrictor role for vasopressin in experimental heart failure in the rabbit. J Clin Invest 1986;78(3):674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Müller S, How OJ, Hermansen SE, Stenberg TA, Sager G, Myrmel T. Vasopressin impairs brain, heart and kidney perfusion: an experimental study in pigs after transient myocardial ischemia. Crit Care 2008;12(1):R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dugar S, Sato R, Chawla S, Young J, Wang X, Grimm R, et al. Is Left Ventricular Systolic Dysfunction Associated With Increased Mortality Among Patients With Sepsis and Septic Shock? Chest 2023. [DOI] [PubMed] [Google Scholar]
- [49].Ky B, French B, May Khan A, Plappert T, Wang A, Chirinos JA, et al. Ventricular-Arterial Coupling, Remodeling, and Prognosis in Chronic Heart Failure. J Am Call Cardiol 2013;62(13):1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Leite-Moreira AF. Current perspectives in diastolic dysfunction and diastolic heart failure. Heart 2006;92(5):712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Evora PR, Pearson PJ, Schaff HV. Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery. V1-receptor-mediated production of nitric oxide. Chest 1993;103(4):1241–5. [DOI] [PubMed] [Google Scholar]
- [52].Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 2010;14(5):R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Eichinger MR, Walker BR. Enhanced pulmonary arterial dilation to arginine vasopressin in chronically hypoxic rats. Am J Physiol 1994;267(6 Pt 2):H2413–9. [DOI] [PubMed] [Google Scholar]
- [54].Leather HA, Segers P, Berends N, Vandermeersch E, Wouters PF. Effects of vasopressin on right ventricular function in an experimental model of acute pulmonary hypertension. Crit Care Med 2002;30(11):2548–52. [DOI] [PubMed] [Google Scholar]
- [55].Hockstein MA, Haycock K, Wiepking M, Lentz S, Dugar S, Siuba M. Transthoracic Right Heart Echocardiography for the Intensivist. J Intensive Care Med 2021;36(9):1098–109. [DOI] [PubMed] [Google Scholar]
- [56].Vindhyal MR, Lu L, Ranka S, Acharya P, Shah Z, Gupta K. Impact of Underlying Congestive Heart Failure on In-Hospital Outcomes in Patients with Septic Shock. J Intensive Care Med 2022;37(7):965–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


