Abstract
Haploidentical hematopoietic cell transplantation (HCT) with post-transplant cyclophosphamide (PTCy) graft-versus-host-disease (GVHD) prophylaxis is associated with inferior overall survival (OS) compared to HLA-matched unrelated donor (MUD) HCT with PTCy prophylaxis in patients undergoing reduced-intensity conditioning (RIC). Given prognostic implications of donor age, we investigated the differences in outcomes of patients with acute myeloid leukemia (AML, n=775) undergoing RIC-HCT with a younger MUD (donor age <35 years, n=84) versus younger haploidentical (donor age <35 years, n=302) versus an older haploidentical (≥35 years, n=389) donor. The older MUD group was excluded due to small numbers. Patients in the younger haploidentical group (median age 59.5 years) were somewhat younger than the younger MUD (median 66.8 years) and the older haploidentical (median 64.7 years) groups. More patients in the MUD group received peripheral blood grafts (82%) compared to the haploidentical groups (55-56%). In multivariate analysis, as compared to the younger MUD group, the younger haploidentical [hazard ratio (HR) 1.95, 95% confidence interval (CI) 1.22-3.12, p=0.005)] and the older haploidentical (HR 2.36, 95% CI 1.50-3.71, p<0.001) groups had a significantly inferior OS, and the younger haploidentical (HR 3.72, 95% CI 1.39-9.93, p=0.009) and older haploidentical group (HR 6.91, 95% CI 2.75-17.39, p<0.001) had a significantly higher risk of NRM. The older haploidentical group had a significantly higher risk of grade II-IV acute GVHD (HR 2.29, 95% CI 1.38-3.80, p=0.001) and grade III-IV acute GVHD (HR 2.70, 95% CI 1.09-6.71, p=0.03). There were no significant differences in chronic GVHD or relapse between the groups. Among adult AML patients in CR undergoing RIC-HCT with PTCy prophylaxis, a young MUD may be preferred over a younger haploidentical donor.
Keywords: matched unrelated donor, haploidentical donor, acute myeloid leukemia, AML, donor age, relapse, survival, older donor, younger donor
Background
The use of post-transplant cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis, which is standard practice with haploidentical hematopoietic cell transplantations (HCT), is now becoming the standard of care for patients undergoing HLA-matched unrelated donor (MUD) HCT with reduced-intensity conditioning (RIC).1,2 An analysis of the Center for International Blood and Marrow Transplant Research (CIBMTR) data comparing haploidentical versus MUD HCT with PTCy prophylaxis showed a significantly higher risk of acute GVHD and non-relapse mortality (NRM) and inferior overall survival (OS) in the haploidentical group in patients who received RIC. No survival differences were seen in those receiving myeloablative conditioning (MAC).3 While there were some differences between groups, this remains the only data currently to guide the decision-making on MUD versus haploidentical donors when both transplantation platforms would use PTCy. However, despite abundant evidence indicating the undesirable effects of increasing donor age on HCT outcomes,4-16 donor age was not accounted for in this study. In the prior study, the median donor age was 29.9 years (range, 18.6-59.6) in the MUD group and 37.5 years (range, 9.5-74.1) in the haploidentical group. The wide range of the donor age in the haploidentical group (who are most often either siblings or children) raises the question of whether the inferior outcomes of the haploidentical HCT were related to the older donor age. With a hypothesis that a younger haploidentical donor would lead to comparable OS as a younger MUD, and that older donors would be associated with inferior OS than younger donors, we compared the HCT outcomes of MUD vs haploidentical donors categorized as either young or old using the existing CIBMTR dataset of AML patients receiving RIC HCT.3 As a vast majority of unrelated donors are usually young (about 80-85% being ≤39 years age),17 we restricted the MUD group to younger donors only.
Methods
Patients with AML who underwent RIC were selected. All patients received T-cell replete grafts and received PTCy with calcineurin-inhibitor (CNI) and mycophenolate mofetil (MMF) for GVHD prophylaxis. Exclusion criteria3 were patients beyond second complete remission or with relapsed disease, secondary AML transformed from MDS, receipt of in vivo T-cell depletion or CD34+-selected grafts, and patients with missing donor age. Donors ≥35 years were defined as “older” and those <35 years were labeled as “younger” donors based on several previous publications showed a survival advantage with donors younger than 30-36 years than older donors.4,6,7,10-12
Statistical Analysis
The primary outcome of interest was OS, defined as the time from HCT to death from any cause. Secondary outcomes included grades II-IV and III-IV acute GVHD (aGVHD)18, chronic GVHD (cGVHD)19, relapse, and non-relapse mortality (NRM) – defined as death without recurrence of underlying malignancy, incidence of bacterial, fungal, and viral infections, and causes of death (COD). We also performed exploratory pairwise comparisons of younger haploidentical vs older haploidentical groups, and subgroup analyses restricted to patients who received peripheral blood (PB) grafts (because of differences in the graft source between the groups). Baseline characteristics were summarized using descriptive statistics with median and interquartile range (IQR) for continuous variables and numbers and percentages for categorical variables. The Kaplan-Meier method was used to estimate OS probability. The cumulative incidence estimate for NRM was calculated using relapse as a competing risk, while the cumulative incidence estimate for relapse was calculated using NRM as a competing risk. Multivariable Cox proportional hazards regression analysis for outcomes (OS, relapse, NRM, aGVHD, and cGVHD), and the Fine-Gray model for competing risks outcomes were used. A backward stepwise model selection was used to identify all significant variables from those in Table 1 for all outcomes. As donor type and age was the primary variable of interest, it was forced into all multivariate analyses. Factors significant at a 5% level were kept in the final model. No correction for multiple testing was applied. Interactions of the main test variable (donor) and the adjusted variables were tested in the Cox regression analysis. All adjusted variables were also tested for the proportional hazards (PH) assumption by assessing graphically and by including a time interaction of the variable(s) in the model. Any violations were adjusted through stratification. If the main variable (donor) violated the PH assumption, then models were constructed by dividing the post-HCT course into two time periods where the hazards crossed, using the maximized partial likelihood method to find the most appropriate breakpoint. All events were censored at 3 years, which was approximately the 75th percentile follow-up period among survivors in all groups. All statistical analyses were performed using STATA/MP 17.0 (College Station, TX: StataCorp LLC.). As the data analysis was conducted by the corresponding author at The MD Anderson Cancer Center, the local Institutional Review Board approved this study (protocol: 2022-0684), which was conducted in accordance with the Declaration of Helsinki.
Table 1:
Baseline Characteristics
| Young MUD (donor <35 years) |
Young Haplo (donor <35 years) |
Old Haplo (donor ≥35 years) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| N=84 | N=302 | N=389 | |||||
| Patient age; median (IQR) | 66.8 (61.9-70.5) | 59.5 (52.6 - 64.2) | 64.7 (55.6 - 69.5) | <0.01 | |||
| Donor age; median (IQR) | 25.4 (23.4-28.4) | 28.3 (24.2-32.1) | 45 (39.9-52.1) | <0.01 | |||
| Sex, n (%) | 0.91 | ||||||
| Male | 47 | 56% | 162 | 54% | 214 | 55% | |
| Female | 37 | 44% | 140 | 46% | 175 | 45% | |
| Graft, n (%) | <0.01 | ||||||
| Peripheral blood | 69 | 82% | 165 | 55% | 219 | 56% | |
| Bone marrow | 15 | 18% | 137 | 45% | 170 | 44% | |
| Disease risk index*, n (%) | 0.99 | ||||||
| Low-intermediate | 75 | 91% | 275 | 91% | 354 | 92% | |
| High-very high | 7 | 9% | 26 | 9% | 32 | 8% | |
| HCT-CI, n (%) | 0.03 | ||||||
| 0-2 | 37 | 44% | 169 | 56% | 181 | 47% | |
| ≥3 | 47 | 56% | 133 | 44% | 208 | 53% | |
| Race, n (%) | <0.01 | ||||||
| Caucasian | 80 | 95% | 214 | 71% | 282 | 72% | |
| Other | 4 | 5% | 88 | 29% | 107 | 28% | |
| KPS, n (%) | 0.62 | ||||||
| <90 | 48 | 57% | 165 | 55% | 198 | 52% | |
| ≥90 | 36 | 43% | 133 | 45% | 180 | 48% | |
| Recipient CMV status, n (%) | 0.36 | ||||||
| Seropositive | 24 | 29% | 97 | 32% | 105 | 27% | |
| Seronegative | 60 | 71% | 205 | 68% | 282 | 73% | |
| Conditioning Regimen | <0.01 | ||||||
| Flu/Cy/TBI | 28 | 33% | 254 | 84% | 349 | 90% | |
| Flu/Mel | 29 | 35% | 11 | 4% | 17 | 4% | |
| Flu/Bu | 19 | 23% | 3 | 1% | 2 | <1% | |
| Flu/TBI | 2 | 2% | 8 | 2% | 7 | 2% | |
| Mel/TBI | 6 | 7% | 26 | 9% | 14 | 4% | |
| Time from diagnosis, months | |||||||
| <6 months | 50 | 60% | 180 | 60% | 227 | 58% | |
| >6 months | 34 | 40% | 122 | 40% | 162 | 42% | |
| Year of HCT, n (%) | 0.09 | ||||||
| 2011-2014 | 13 | 16% | 19 | 13% | 74 | 19% | |
| 2015-2018 | 71 | 84% | 263 | 87% | 315 | 81% | |
| Follow-up among survivors in months, median (IQR) | 24.8 (12.2-37.5) | 24.1 (12.2-36.7) | 24.3 (12.1-40.6) | 0.76 | |||
Abbreviations: CMV, cytomegalovirus; HCT, hematopoietic cell transplantation; haplo, haploidentical; HCT-CI, Hematopoietic Cell Transplantation-Specific Comorbidity Index; IQR, interquartile range; KPS, Karnofsky Performance Scale; MUD, matched unrelated donor.
DRI: favorable cytogenetics (low risk), intermediate cytogenetics (intermediate risk) and adverse cytogenetics (high risk)
Results
We studied 775 patients: younger MUD (n=84), younger haploidentical (n=302), and older haploidentical (n=389) groups (Table 1). The median patient age at the time of HCT was 66.8 years vs 59.5 years vs 64.7 years, respectively, and the median donor age was 25.4 years vs 28.3 years vs 45 years, respectively. A majority of patients were males in all groups (56% vs 54% vs 55%, respectively). Peripheral blood (PB) was the graft source used in the majority of patients in all groups, but it was used more commonly in the MUD group (82% vs 55% vs 56%, respectively). More than 90% of patients had low-intermediate DRI in all groups. Fewer patients in the younger haploidentical group (44%) had an HCT-CI score of ≥3 as compared to the younger MUD (56%) or older haploidentical (53%) groups. As expected, there were significantly more non-Caucasian patients in both the haploidentical [younger (29%) and older (28%)] groups than in the younger MUD (5%) group. There were no significant differences in the Karnofsky Performance Scale, cytomegalovirus serostatus, time from diagnosis to HCT, or the year of transplant between the groups. Most patients in the younger haploidentical (84%) and older haploidentical (90%) groups received fludarabine, cyclophosphamide, and total body irradiation regimen as compared to the younger MUD group (33%). The median follow-up among survivors was approximately 24 months in all groups.
Survival
The estimated 3-year OS was 68.5% (younger MUD) vs 50.8% (younger haploidentical) vs 42.2% (older haploidentical). The univariate analyses are shown in supplemental table 1. In multivariate analysis, the younger haploidentical (HR 1.95, 95% CI 1.22-3.12, p=0.005), as well as the older haploidentical group (HR 2.36, 95% CI 1.50-3.71, p<0.001), had a significantly inferior OS as compared to the younger MUD group. [Table 2, Figure 1]. Similar findings were noted in the analysis restricted to patients who received PB grafts [supplemental table 2].
Table 2:
Outcomes
| Univariate analysis | Multivariate analysis* | ||||||
|---|---|---|---|---|---|---|---|
| Estimates | 95 % CI | HR | 95 % CI | P-value | |||
| Overall Survival, 3 years | |||||||
| Young MUD | 68.5 | 55.6 | 78.4 | Ref | |||
| Young Haplo | 50.8 | 43.9 | 57.2 | 1.95 | 1.22 | 3.12 | 0.005 |
| Old Haplo | 42.2 | 36.5 | 47.9 | 2.36 | 1.50 | 3.71 | <0.001 |
| Relapse, 3 years | |||||||
| Young MUD | 36.4 | 24.8 | 48.2 | Ref | |||
| Young Haplo | 51.3 | 44.3 | 58.0 | 1.16 | 0.74 | 1.83 | 0.52 |
| Old Haplo | 41.1 | 35.8 | 46.4 | 0.95 | 0.61 | 1.48 | 0.82 |
| NRM, 3 years | |||||||
| Young MUD | 8.0 | 2.8 | 16.9 | Ref | |||
| Young Haplo | 11.0 | 7.5 | 15.2 | 3.72 | 1.39 | 9.93 | 0.009 |
| Old Haplo | 22.0 | 17.7 | 26.6 | 6.91 | 2.75 | 17.39 | <0.001 |
| Grade II-IV aGVHD, day 100 | |||||||
| Young MUD | 20.2 | 12.4 | 29.4 | Ref | |||
| Young Haplo | 25.8 | 21.0 | 30.9 | 1.67 | 0.98 | 2.83 | 0.06 |
| Old Haplo | 33.7 | 29.0 | 38.4 | 2.29 | 1.38 | 3.80 | 0.001 |
| Grade III-IV aGVHD, day 100 | |||||||
| Young MUD | 1.3 | 0.1 | 6.0 | Ref | |||
| Young Haplo | 7.8 | 5.1 | 11.3 | 2.06 | 0.81 | 5.22 | 0.13 |
| Old Haplo | 11.0 | 8.1 | 14.4 | 2.70 | 1.09 | 6.71 | 0.03 |
| Chronic GVHD, 2 years | |||||||
| Young MUD | 28.5 | 18.8 | 38.9 | Ref | |||
| Young Haplo | 23.1 | 18.3 | 28.3 | 0.89 | 0.56 | 1.45 | 0.66 |
| Old Haplo | 29.3 | 24.6 | 34.0 | 1.16 | 0.74 | 1.83 | 0.52 |
Full multivariate models with all adjusted covariates shown in supplemental table 5.
Abbreviations: CI, confidence interval; aGVHD, acute graft-versus-host disease; cGVHD chronic graft-versus-host disease; haplo, haploidentical; HR, hazard ratio; MUD, matched unrelated donor; NRM, non-relapse mortality; Ref: reference.
Figure 1:
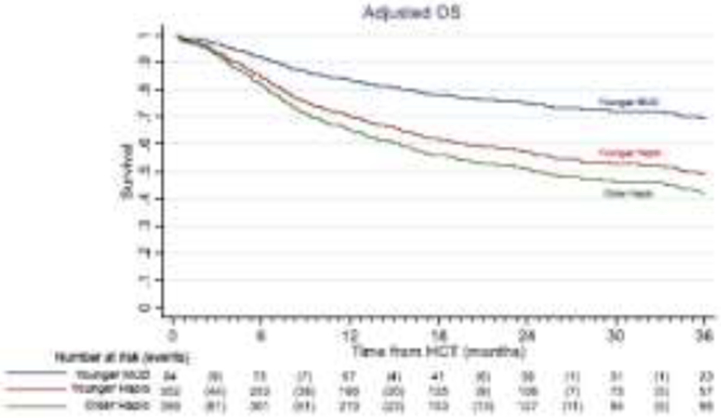
Adjusted overall survival with younger MUD (blue) versus younger haploidentical (maroon) vs an older haploidentical (green) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
Relapse & Non-relapse Mortality
The cumulative incidence of relapse at 3 years was 36.4% (younger MUD) vs 51.3% (younger haploidentical) vs 41.1% (older haploidentical). In the multivariate analysis, no statistically significant differences were observed between the donor groups [Table 2, Figure 2]. The cumulative incidence of NRM at 3 years was 8% (younger MUD) vs 11.0% (younger haploidentical) vs 22.0% (older haploidentical). In multivariate analysis, the younger haploidentical group (HR 3.72, 95% CI 1.39-9.93, p=0.009) and the older haploidentical group (HR 6.91, 95% CI 2.75-17.39, p<0.001) had a significantly higher risk of NRM than in the younger MUD group [Table 2, Figure 3]. Melphalan-containing regimens were associated with a lower risk of relapse but a significantly higher risk of NRM.
Figure 2:

Adjusted relapse after younger MUD (blue) versus younger haploidentical (maroon) vs an older haploidentical (green). The adjusted factors are the variables listed in the multivariable regression analysis.
Figure 3:
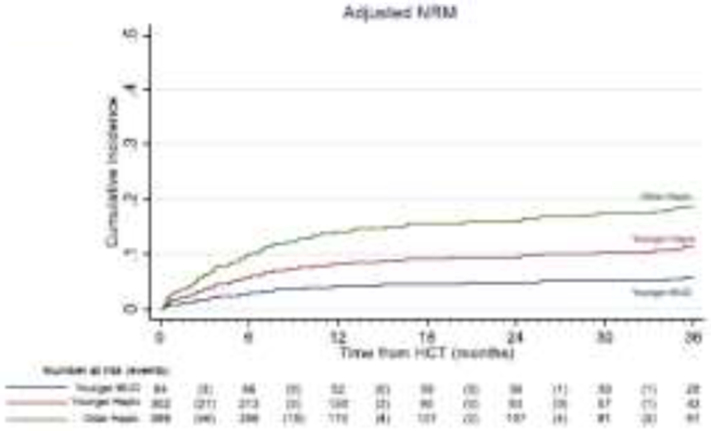
Adjusted non-relapse mortality after younger MUD (blue) versus younger haploidentical (maroon) vs an older haploidentical (green) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
Graft-vs-Host Disease
The cumulative incidence of grade II-IV aGVHD at day 100 was 20.2% (younger MUD) vs 25.8% (younger haploidentical) vs 33.7% (older haploidentical); grade III-IV aGVHD at day 100 was 1.3% vs 7.8% vs 11.0%, respectively. Chronic GVHD at 2 years was 28.5% vs 23.1% vs 29.3%, respectively. In multivariate analysis, the older haploidentical group had a 2.3-fold higher risk of grade II-IV aGVHD (HR 2.29, 95% CI 1.38-3.80, p=0.001) and 2.7-fold higher risk of grade III-IV aGVHD (HR 2.70, 95% CI 1.09-6.71, p=0.03) than the younger MUD group, with no differences in cGVHD [Table 2].
Infection and Causes of Death
There was no difference in the risk of bacterial or viral infections between the groups [supplemental table 3]. The cumulative incidence of fungal infection at 6 months was 7.2% (95% CI 3.5-12.6) in the younger haploidentical vs 10.2% (95% CI 6.4-15.1) in the older haploidentical group, while no fungal infections occurred in the MUD group. Relapse of AML was the most common COD in all groups: 75% (younger MUD) vs 65% (younger haploidentical) vs 55% (older haploidentical). Among non-relapse causes, organ failure accounted for most of the deaths in the MUD group (67% vs 16% vs 20%, respectively), followed by interstitial pneumonitis/ acute respiratory distress syndrome (17% vs 9% vs 6%, respectively). Infections were the leading cause of NRM in both haploidentical groups (36% and 34%, respectively); no infection-related deaths occurred in the MUD group. GVHD accounted for 17% (young MUD) vs 18% (young haploidentical) vs 23% (young haploidentical) of NRM. Graft failure was a rare COD in all groups. Other causes are detailed in table 3 and figure 4.
Table 3:
Causes of death
| Young MUD (donor <35 years) |
Young Haplo (donor <35 years) |
Old Haplo (donor ≥35 years) |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Relapse | 18 | 75 | 82 | 65 | 107 | 55 |
| Infection | 0 | 0 | 16 | 13 | 30 | 15 |
| GVHD | 1 | 4 | 8 | 6 | 20 | 10 |
| Organ failure | 4 | 17 | 7 | 6 | 18 | 9 |
| IPN/ARD | 1 | 4 | 4 | 3 | 5 | 3 |
| Graft failure | 0 | 0 | 1 | 1 | 4 | 2 |
| Secondary malignancy | 0 | 0 | 4 | 3 | 4 | 2 |
| Other | 0 | 0 | 5 | 4 | 7 | 4 |
| Total | 24 | 100 | 127 | 100 | 195 | 100 |
Abbreviations: GVHD, graft-versus-host disease; haplo, haploidentical; MUD, matched unrelated donor; IPN, idiopathic pneumonia syndrome; ARD, acute respiratory distress
Figure 4:

Causes of non-relapse mortality
Pairwise comparisons of younger vs older haploidentical donors
In exploratory pairwise comparisons of the two haploidentical age groups, multivariate analysis showed that the older haploidentical group was associated with a significantly higher risk of NRM (HR 1.88, 95% CI 1.20-2.93, p=0.006), with no difference in relapse (HR 0.82, 95% CI 0.65-1.04, p=0.11) or OS (HR 1.21, 95% CI 0.96-1.52, p=0.11) from the younger haploidentical group. The risk of grade II-IV aGVHD was higher in the older haploidentical group (HR 1.33, 95% CI 1.01-1.78, p=0.046), grade III-IV aGVHD and cGVHD risks were similar [Supplemental Table 4].
Discussion
In this analysis of AML patients who underwent MUD or haploidentical RIC HCT where all patients received PTCy, CNI, and MMF for GVHD prophylaxis, we noted that the haploidentical group - regardless of the donor age categorization (younger or older) had significantly inferior OS and higher NRM as compared to the younger MUD group. A study using propensity score matching and donor age as a continuous variable showed that despite about 54% higher risk of NRM, haploidentical RIC-HCT had similar OS as the MUD RIC-HCT with no difference in relapse.20 Different results seen in our study could be related to varying conditioning intensities and different disease populations studied. While we restricted our analysis to the AML patients who underwent RIC (the largest cohort of patients available for analysis), the previous study included patients with AML, myelodysplastic syndrome, and acute lymphoblastic leukemia who received either RIC or MAC. 20 Another abstract showed superior OS, lower NRM, and lower acute GVHD with a younger MUD (< 35 years old donor) than an older haploidentical donor (≥ 35 years) when PTCy is used for GVHD prophylaxis in both groups but did not compare a younger MUD versus a younger haploidentical donor HCT.21
The earlier study by Gooptu et al3 showed that the haploidentical-PTCy RIC HCT is associated with worse OS than the MUD-PTCy RIC HCT. Our study extends these conclusions and shows that even a younger haploidentical is associated with worse OS than a younger MUD in patients with AML. This has direct clinical implications as the numbers of haploidentical HCTs are increasing rapidly22 and more younger haploidentical donors are becoming available owing to the use of second- or third-degree related donors.14,23 Our study corroborates the finding that older haploidentical donors are associated with worse outcomes. 5,13-16 We found that older haploidentical donors led to an 88% higher risk of NRM with similar risks of aGVHD, cGVHD, and relapse. The older haploidentical group had about 21% higher risk of overall mortality (95% CI 0.96-1.52), but this did not reach statistical significance. Another interesting finding of our study is that melphalan-containing RIC regimens were independently associated with a significantly lower risk of relapse and a higher risk of NRM as compared to other regimens, with no difference in OS.
We restricted the analysis to AML patients who underwent RIC, the largest and more uniform cohort available for analysis, thereby eliminating any confounding attributable to the underlying disease or conditioning intensity. There were too few older MUD HCTs for suitable comparisons. This reflects current clinical practice as a majority (65%) of the MUD HCTs conducted in recent years included donors younger than 30 years old, and only about 15% were ≥40 years old.17 In the past decade, the median recruitment age for the National Marrow Donor Program donors decreased from 35 years to 28 years,17 further enriching the registry with younger donors. Although potentially interesting we were unable to examine whether a younger haploidentical donor should be preferred over an older MUD.
We had insufficient data to address other variables including HLA-DPB1 matching in the MUDs, individual HLA allele mismatches in the haploidentical group,5 gender mismatch (female-to-male), and donor parity—all factors which might further inform the donor selection challenges. Additionally, data on measurable residual disease and molecular classification of AML were not fully available in this dataset.
Conclusion
Among adult AML patients in CR undergoing RIC-HCT with PTCy prophylaxis, our data supports the selection of a younger MUD (age <35 years) as the preferred donor, whenever available, over a younger or haploidentical donor based on a significantly superior OS and lower risks of GVHD and NRM. If possible, older haploidentical donors should be avoided due to the notably high risk of GVHD and NRM. Further study should test whether these findings are applicable to other diseases and to MAC HCT.
Supplementary Material
Highlights.
Younger MUD leads to better OS than either younger or older haploidentical donors in patients with AML undergoing RIC HCT.
Older haploidentical donors are associated with a higher risk of GVHD and NRM than younger haploidentical donors.
Acknowledgment
We thank the CIBMTR staff for providing this dataset and Rima Saliba, PhD for her feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest: No relevant COI to declare.
References:
- 1.Bolanos-Meade J, Reshef R, Fraser R, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. Mar 2019;6(3):e132–e143. doi: 10.1016/S2352-3026(18)30221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holtan SG, Hamadani M, WU J, et al. Post-Transplant Cyclophosphamide, Tacrolimus, and Mycophenolate Mofetil As the New Standard for Graft-Versus-Host Disease (GVHD) Prophylaxis in Reduced Intensity Conditioning: Results from Phase III BMT CTN 1703. American Society Of Hematology Annual Meeting, 2022. 2022; [Google Scholar]
- 3.Gooptu M, Romee R, St Martin A, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood. Jul 22 2021;138(3):273–282. doi: 10.1182/blood.2021011281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guru Murthy GS, Kim S, Hu ZH, et al. Relapse and Disease-Free Survival in Patients With Myelodysplastic Syndrome Undergoing Allogeneic Hematopoietic Cell Transplantation Using Older Matched Sibling Donors vs Younger Matched Unrelated Donors. JAMA Oncol. Mar 1 2022;8(3):404–411. doi: 10.1001/jamaoncol.2021.6846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs EJ, McCurdy SR, Solomon SR, et al. HLA informs risk predictions after haploidentical stem cell transplantation with posttransplantation cyclophosphamide. Blood. Mar 10 2022;139(10):1452–1468. doi: 10.1182/blood.2021013443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw BE, Logan BR, Spellman SR, et al. Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most. Biol Blood Marrow Transplant. May 2018;24(5):1049–1056. doi: 10.1016/j.bbmt.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. Jan 14 2016;127(2):260–7. doi: 10.1182/blood-2015-08-663823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayuk F, Zabelina T, Wortmann F, et al. Donor choice according to age for allo-SCT for AML in complete remission. Bone Marrow Transplant. Aug 2013;48(8):1028–32. doi: 10.1038/bmt.2013.14 [DOI] [PubMed] [Google Scholar]
- 9.Alousi AM, Le-Rademacher J, Saliba RM, et al. Who is the better donor for older hematopoietic transplant recipients: an older-aged sibling or a young, matched unrelated volunteer? Blood. Mar 28 2013;121(13):2567–73. doi: 10.1182/blood-2012-08-453860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroger N, Zabelina T, de Wreede L, et al. Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia. Mar 2013;27(3):604–9. doi: 10.1038/leu.2012.210 [DOI] [PubMed] [Google Scholar]
- 11.Carreras E, Jimenez M, Gomez-Garcia V, et al. Donor age and degree of HLA matching have a major impact on the outcome of unrelated donor haematopoietic cell transplantation for chronic myeloid leukaemia. Bone Marrow Transplant. Jan 2006;37(1):33–40. doi: 10.1038/sj.bmt.1705195 [DOI] [PubMed] [Google Scholar]
- 12.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. Oct 1 2001;98(7):2043–51. doi: 10.1182/blood.v98.7.2043 [DOI] [PubMed] [Google Scholar]
- 13.Mariotti J, Raiola AM, Evangelista A, et al. Impact of donor age and kinship on clinical outcomes after T-cell-replete haploidentical transplantation with PT-Cy. Blood Adv. Aug 25 2020;4(16):3900–3912. doi: 10.1182/bloodadvances.2020001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeZern AE, Franklin C, Tsai HL, et al. Relationship of donor age and relationship to outcomes of haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv. Mar 9 2021;5(5):1360–1368. doi: 10.1182/bloodadvances.2020003922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canaani J, Savani BN, Labopin M, et al. Donor age determines outcome in acute leukemia patients over 40 undergoing haploidentical hematopoietic cell transplantation. American journal of hematology. Feb 2018;93(2):246–253. doi: 10.1002/ajh.24963 [DOI] [PubMed] [Google Scholar]
- 16.Ciurea SO, Shah MV, Saliba RM, et al. Haploidentical Transplantation for Older Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant. Jun 2018;24(6):1232–1236. doi: 10.1016/j.bbmt.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logan BR, Maiers MJ, Sparapani RA, et al. Optimal Donor Selection for Hematopoietic Cell Transplantation Using Bayesian Machine Learning. JCO Clin Cancer Inform. May 2021;5:494–507. doi: 10.1200/CCI.20.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. Jun 1995;15(6):825–8. [PubMed] [Google Scholar]
- 19.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. Mar 2015;21(3):389–401 e1. doi: 10.1016/j.bbmt.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambinder A, Jain T, Tsai HL, Horowitz MM, Jones RJ, Varadhan R. HLA-matching with PTCy: a reanalysis of a CIBMTR dataset with propensity score matching and donor age. Blood Adv. Jul 26 2022;6(14):4335–4346. doi: 10.1182/bloodadvances.2022007741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arcuri LJ, Santos FPdS, Kerbauy L, Kerbauy MN, Ribeiro AF, Hamerschlak N. Impact of Donor Age in the Selection of a Haploidentical or a Matched-Related Donor: A Secondary Analysis of a CIBMTR Database. Blood (2022) 140 (Supplement 1): 4767–4769. [Google Scholar]
- 22.Auletta JJ KJ, Chen M, Shaw BE . Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2021. 2021; [Google Scholar]
- 23.Elmariah H, Kasamon YL, Zahurak M, et al. Haploidentical Bone Marrow Transplantation with Post-Transplant Cyclophosphamide Using Non-First-Degree Related Donors. Biol Blood Marrow Transplant. May 2018;24(5):1099–1102. doi: 10.1016/j.bbmt.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


