Abstract
Objectives:
Electrical stimulation mapping (ESM) is the clinical standard for functional localization with subdural electrodes (SDE). As stereo-EEG (SEEG) has emerged as an alternative option, we compared functional responses, after-discharges (ADs) and unwanted ESM-induced seizures (EIS) between the two electrode types.
Methods:
Incidence and current thresholds for functional responses (sensory, motor, speech/language), ADs and EIS were compared between SDE and SEEG using mixed models incorporating relevant covariates.
Results:
We identified 67 SEEG ESM and 106 SDE ESM patients (7207 and 4980 stimulated contacts respectively). We found similar incidence of language and motor responses between electrode types, however, more SEEG patients reported sensory responses. ADs and EIS occurred less commonly with SEEG than SDE. Current thresholds for language, face motor, upper extremity (UE) motor, and EIS significantly decreased with age. However, they were not affected by electrode type, premedication, or dominant hemispheric stimulation. AD thresholds were higher with SEEG than with SDE. For SEEG ESM, language thresholds remained below AD thresholds up to 26 years-of-age whereas this relationship was inverse for SDE. Also, face and UE motor thresholds fell below AD thresholds at earlier ages for SEEG than SDE. AD and EIS thresholds were not affected by premedication.
Significance:
SEEG and SDE have clinically relevant differences for functional brain mapping with electrical stimulation. While evaluation of language and motor regions is comparable between SEEG and SDE, SEEG offers higher likelihood of identifying sensory areas. A lower incidence of ADs and EIS, and a favorable relationship between functional and AD thresholds suggest superior safety and neurophysiologic validity for SEEG ESM than SDE ESM.
Keywords: Functional brain mapping, Intracranial EEG, Drug-resistant epilepsy
INTRODUCTION
Before epilepsy surgery, intracranial electroencephalographic evaluation is often performed to identify the seizure-onset zone/s (SOZ) and functional networks to individualize the best neurosurgical approach. Delineation of functional networks, particularly those underlying motor and speech/language functions, is typically achieved by electrical stimulations of the stereotactic depth electrodes (SEEG) or subdural electrodes (SDE), so-called electrical stimulation mapping (ESM). Despite ESM being a standard procedure for SDE, there is a lack of consensus on its utility with SEEG1–6. In addition, previous evidence suggests some limitations of SDE ESM including a high incidence of after-discharges (ADs) and ESM-induced seizures (EIS), and its unfavorable relationship between functional and AD thresholds particularly in children7–10. On the other hand, although there is preliminary evidence supporting SEEG ESM for functional mapping11–13, and higher gray matter sampling compared to SDE14, there is a concern that poorly contiguous cortical surface coverage by SEEG may limit its ability to localize the extent of functional areas.
This study compared ESM with SEEG and SDE for incidence and current thresholds of speech/language and sensorimotor responses, ADs, and EIS. We hypothesized that the incidence of functional responses would be comparable for SEEG and SDE based on similar gray matter sampling14, 15. We further hypothesized that the incidence of ADs and unwanted EIS would be lower with SEEG ESM than SDE ESM and that the relationship between functional and AD thresholds would be different for the two electrode types, because SEEG lacks the shunting of stimulation currents by cerebrospinal fluid (CSF) and produces a more focal perturbation of the neuronal assemblies16–18.
METHODS
Patient Sample
Using the epilepsy surgery database at the Cincinnati Children’s Hospital, patients who underwent SDE or SEEG monitoring between January 2007 and December 2020 and completed ESM were identified. The study was approved by the institutional review board (IRB 2017-4025).
Intracranial EEG Recordings
The configuration of intracranial electrodes was individualized by reviewing the noninvasive presurgical data in a multidisciplinary conference. Our practice changed from exclusively using SDE to predominantly using SEEG in 2016.
With SDE, intracranial EEG was recorded using Bio-Logic Ceegraph XL-II amplifiers (Bio-Logic Systems Inc., Mundelein, IL, USA) at 0.1-400 Hz before April 2008 (17 patients), and subsequently using Stellate eAmp (Stellate Systems Inc., Montreal, Canada) at 0.1-2000 Hz in all other SDE patients. SDE were 4.75 mm platinum/ iridium discs embedded in silicone elastomer having 2.5 mm exposed contacts and a center-to-center distance of 1 cm (Integra Neurosciences, Plainsboro, NJ). A distant 2-contact subdural strip facing the dura served as the reference and ground.
With SEEG, intracranial EEG was recorded using Natus Quantum/Quantum Plus amplifiers at 0.1-2048 Hz. SEEG electrodes had a diameter of 0.86 mm with 2.41 mm platinum contacts and 1 cm center-to-center contact spacing. Two electrode contacts distant from the presumed seizure onset zone and with low artifacts were used as the reference and ground.
ESM
The decision to perform ESM and selection of electrode contacts for stimulation were individualized for each patient based on the proximity of the presumed SOZ and eloquent cortex as assessed from the noninvasive data. Simultaneous video and intracranial EEG were recorded during the ESM sessions. We performed bipolar ESM with both SEEG and SDE using 50 Hz, 250-350 μs, biphasic square wave pulses, beginning with 1-2 mA current and a stepwise increment of 0.5-2 mA unless the functional response, evolving ADs, or EIS was encountered. The train duration was 2-3 seconds for sensorimotor ESM and 5 seconds for language ESM. The maximum current strength differed between the two electrode types. For the initial 80 patients who underwent SDE ESM, we used OCS2 Ojemann stimulator (Integra Life Sciences, Plainsboro, NJ) with a maximum current of 10 mA, and for subsequent 26 SDE patients Natus stimulator (Natus Medical Inc., Middleton, WI) was used with a maximum current of 15 mA. SEEG ESM was also performed using Natus stimulator but the maximum current was limited to 8 mA. This reflects a change in our clinical practice with time and not deliberate choices for individual patients. Also, the maximum current strength for SEEG is based on the accepted safety limits for charge density (<30 μC/cm2/phase) and lack of current shunting into CSF16, 19. SEEG electrodes were typically stimulated from the deepest to the most superficial contact, because of the concern that stimulation of the cortex could possibly render the white matter contact(s) refractory to stimulation due to orthodromic conduction of the stimulation impulse. Additional details of our ESM protocol have been published previously7, 8, 11, 12.
Data Extraction
The following data were extracted for each patient: (a) demographics: age, sex, handedness, dominant hemisphere; (b) ESM: premedication, stimulated hemisphere, functional responses, ADs, EIS, and their current thresholds. Intracranial EEG recordings during ESM were independently reviewed for the study purposes, blinded to the clinical report. Hemispheric language dominance was derived from fMRI whenever available, or the left hemisphere was regarded as dominant unless the patient was left-handed and had a left hemispheric developmental pathology20.
Outcomes
We analyzed incidence and current thresholds for functional responses, ADs, and EIS. Language responses were assessed with an overt picture naming task21. Because it is often challenging to differentiate between cognitive and motor components of language interference in pediatric patients, we scored paraphasic errors (semantic or phonemic), aphasia, anomia, dysarthria, or speech interruptions as positive language responses as per usual clinical practice22. We have used the term language responses in this manuscript for both speech and language responses. Motor responses consisting of involuntary movement of facial, upper, or lower extremity (UE/LE) muscles, were visually evaluated by the bedside team, and sensory responses were based on patient reports. Reproducibility of all functional responses was verified.
ADs were defined as rhythmic discharges (spikes, poly-spikes, sharp waves, or spike-wave complexes) clearly distinct from the pre-stimulation electrographic activity and occuring immediately following electrical stimulation7, 23. EIS was defined as a train of ADs that evolved in distribution, morphology, or frequency, and was accompanied by clinical changes. Because seizure elicitation can sometimes be a desired endpoint with SEEG ESM, we included only unwanted EIS in this study. Current thresholds were defined as minimum stimulation currents (mA) that resulted in aforementioned functional responses, ADs (lasting ≥3 seconds), or EIS.
Statistical Analysis
Both patient-level and electrode contact level variables were compared between SEEG and SDE using t-test for independent samples (continuous variables) and Fisher’s exact test (categorical variables). Incidence of functional responses, ADs, and EIS were analyzed as a function of the electrode type (SDE/SEEG), age, stimulation of the dominant hemisphere, premedication, and current strength, with generalized linear mixed models. Similarly, current thresholds for functional responses, ADs, and EIS were analyzed as a function of the electrode type (SDE/SEEG), age, stimulation of the dominant hemisphere, and premedication, with linear mixed models. Because the maximum current used for SDE ESM was higher than that used for SEEG ESM, it was scaled group-wise to obtain z-scores which were used for the aforementioned mixed models. These mixed models respect the hierarchical structure of the data and incorporate nesting of electrode contacts under the patient-level random effects. Similar mixed models were also fitted separately for SDE and SEEG subgroups. Statistical analyses were performed with R 4.1 with “lme4” and “lmerTest” libraries used for fitting mixed models and testing their statistical significance respectively24.
RESULTS
ESM was performed in 106 patients with SDE aged 0.9-25.5 years (4980 stimulated electrode contacts) and 67 patients with SEEG aged 1.9-32.0 years (7207 stimulated electrode contacts). The cohorts were comparable in terms of relevant clinical variables (Table 1), however, premedication was used more frequently before SDE ESM (37%) compared to SEEG ESM (19%; OR 0.42; p=0.017).
Table 1.
Summary of patient-level and electrode contact-level analyses
| SEEG | SDE | Odds Ratio (95% Confidence Interval) | p-value | |
|---|---|---|---|---|
| Patient-level Analyses | ||||
| Number of patients | 67 | 106 | ||
| Mean age (years) | 12.2 | 11.6 | SMD 0.11, −0.42 to 0.19 | 0.477 |
| Left hemispheric dominant (%) | 82.1 | 87.6 | 1.54, 0.58 to 4.05 | 0.372 |
| Left hemispheric stimulation (%) | 62.7 | 61.3 | 0.94, 0.48 to 1.86 | 0.874 |
| Dominant hemispheric stimulation (%) | 50.7 | 54.6 | 0.85, 0.44 to 1.67 | 0.637 |
| Premedication (%) | 19.4 | 36.8 | 0.42, 0.18 to 0.89 | 0.017* |
| Language response (%) | 43.3 | 42.5 | 1.03, 0.53 to 2.00 | 0.999 |
| Motor response (%) | 68.7 | 72.6 | 0.83, 0.40 to 1.71 | 0.608 |
| Sensory response (%) | 58.2 | 1.0 | 141.5, 22.0 to 5752.0 | <0.001* |
| After discharges (%) | 80.6 | 84.0 | 0.79, 0.33 to 1.93 | 0.681 |
| ESM-induced seizure (%) | 28.4 | 40.6 | 0.58, 0.28 to 1.17 | 0.108 |
| Electrode Contact-level Analyses | ||||
| Number of stimulations | 7207 | 4980 | ||
| Language responses (%) | 4.8 | 5.6 | 0.85, 0.72 to 1.00 | 0.049 |
| Motor responses (%) | 5.3 | 9.7 | 0.52, 0.45 to 0.59 | <0.001* |
| Sensory responses (%) | 4.8 | 0.3 | 19.03, 10.96 to 36.17 | <0.001* |
| After-discharges (%) | 12.2 | 29.3 | 0.34, 0.31 to 0.37 | <0.001* |
| ESM-induced seizure (%) | 0.4 | 1.7 | 0.24, 0.15 to 0.37 | <0.001* |
Notes: Odds ratio>1 represents higher proportion/% in SEEG compared to SDE.
p<0.05
Abbreviations: ESM electrical stimulation mapping, SDE subdural electrodes, SEEG stereo-electroencephalography, SMD Standardized Mean Difference (Hedge’s ‘g’)
The proportions of patients with language (43% vs. 43%) and motor responses (69% vs. 73%) were comparable between SEEG and SDE groups, however, significantly more SEEG patients (58%) reported sensory responses than with SDE (1.0%; OR 141.6, p <0.001). On analyzing at electrode contact level, the incidence of language responses was comparable (SDE 5.6%; SEEG 4.8%, Figure 1), however, motor responses were less common with SEEG (5.3%) than with SDE stimulations (9.7%; OR 0.52, p <0.001), whereas sensory responses were predominantly elicited with SEEG stimulations (4.8%) than SDE (0.3%, OR 19.03, p<0.001).
Figure 1. Incidence of functional responses.
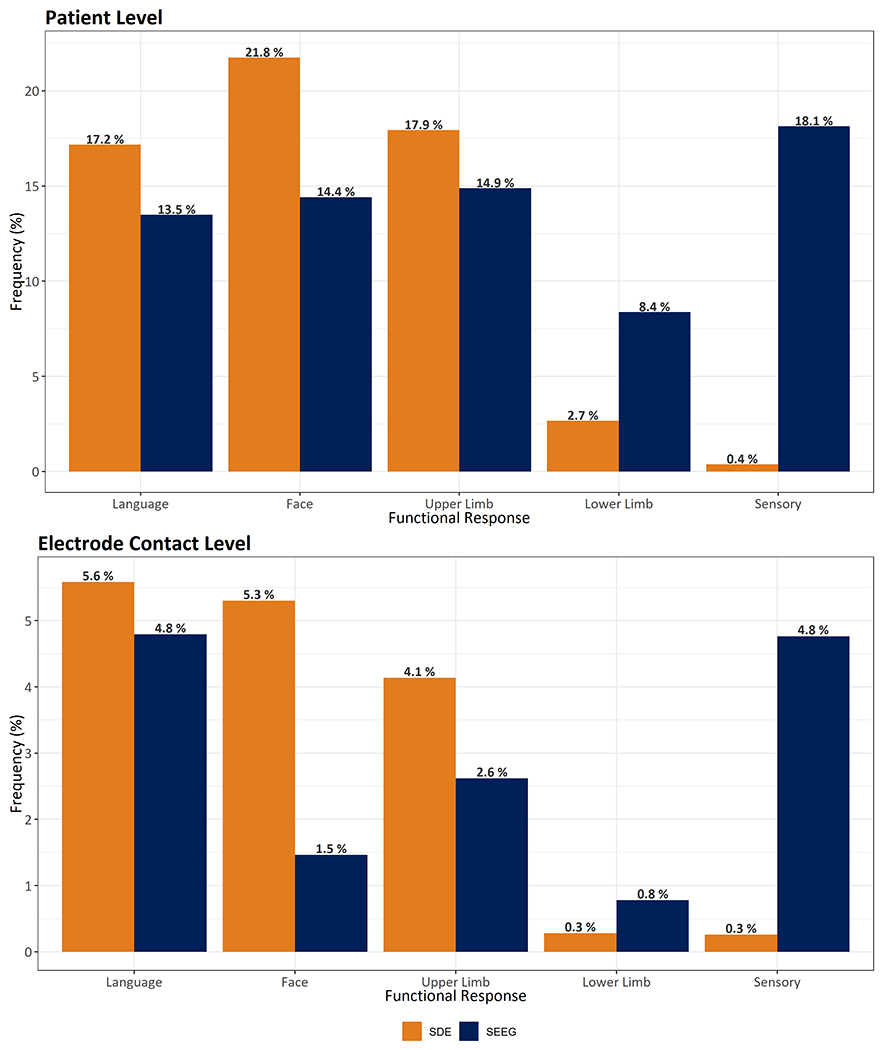
The proportion of patients (top panel) and stimulated contacts (bottom panel) with different functional responses are shown for SEEG and SDE electrical stimulation.
Abbreviations: SDE subdural electrodes, SEEG stereo-electroencephalography
Again, the proportions of patients with ADs and EIS were not significantly different between SEEG and SDE. However, at the electrode contact levels, ADs were seen less frequently with SEEG stimulations (12.2%) than SDE (29.3%; OR 0.34, p<0.001), and the incidence of unwanted EIS was also approximately 4-fold less common with SEEG ESM (0.4%) than SDE ESM (1.7%; OR 0.24, p<0.001).
Incidence of Functional Responses
Language:
Mixed models did not show a significant effect of the electrode type or the use of pre-medication on the occurrence of language responses during ESM (Table 2). The incidence of language responses on ESM increased with age (OR 1.13, p=0.002), stimulation of language dominant hemisphere (OR 25.1, p<0.001), and current strength (OR 1.89, p<0.001).
Table 2.
Incidence of responses as a function of electrode type, age, premedication, dominant hemisphere stimulation, and scaled current analyzed using generalized linear mixed models
| Response | Variable | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|---|
| Language | SEEG (vs SDE) | 0.56 | 0.25 to 1.22 | 0.142 |
| Age | 1.13 | 1.04 to 1.21 | 0.002* | |
| Premedication | 0.70 | 0.39 to 1.26 | 0.234 | |
| Dominant hemispheric stimulation | 25.10 | 11.63 to 54.10 | <0.001* | |
| Scaled current | 1.89 | 1.70 to 2.11 | <0.001* | |
| Face | SEEG (vs SDE) | 0.24 | 0.13 to 0.44 | <0.001* |
| Age | 1.14 | 1.08 to 1.21 | <0.001* | |
| Premedication | 0.87 | 0.45 to 1.67 | 0.681 | |
| Scaled current | 0.95 | 0.83 to 1.09 | 0.462 | |
| Upper extremity | SEEG vs SDE | 0.67 | 0.30 to 1.50 | 0.329 |
| Age | 1.03 | 0.96 to 1.11 | 0.422 | |
| Premedication | 0.89 | 0.38 to 2.12 | 0.799 | |
| Scaled current | 0.74 | 0.64 to 0.84 | <0.001* | |
| Lower extremity | SEEG (vs SDE) | 4.79 | 1.39 to 16.58 | 0.013* |
| Age | 0.94 | 0.85 to 1.05 | 0.279 | |
| Premedication | 0.64 | 0.16 to 2.57 | 0.530 | |
| Scaled current | 0.75 | 0.58 to 0.96 | 0.025* | |
| Sensory | SEEG (vs SDE) | 255.14 | 28.81 to 2259.82 | <0.001* |
| Age | 1.13 | 0.99 to 1.30 | 0.067 | |
| Premedication | 1.08 | 0.19 to 6.26 | 0.933 | |
| Scaled current | 0.69 | 0.58 to 0.82 | <0.001* | |
| After-discharges | SEEG (vs SDE) | 0.23 | 0.12 to 0.45 | <0.001* |
| Age | 0.99 | 0.93 to 1.05 | 0.689 | |
| Premedication | 0.35 | 0.20 to 0.63 | <0.001* | |
| Dominant hemispheric stimulation | 1.29 | 0.87 to 1.92 | 0.200 | |
| Scaled current | 1.46 | 1.36 to 1.57 | <0.001* | |
| ESM-induced seizures | SEEG (vs SDE) | 0.19 | 0.09 to 0.38 | <0.001 |
| Age | 1.07 | 1.00 to 1.14 | 0.054 | |
| Premedication | 0.33 | 0.15 to 0.71 | 0.005* | |
| Dominant hemispheric stimulation | 0.58 | 0.31 to 1.11 | 0.098 | |
| Scaled current | 1.43 | 1.14 to 1.78 | 0.002* |
Notes: Odds ratio>1 represents higher proportion/% in SEEG compared to SDE. Current strengths were scaled separately for SEEG and SDE.
p<0.05.
Abbreviations: ESM electrical stimulation mapping, SDE subdural electrodes, SEEG stereo-electroencephalography
Motor:
Face motor responses were less frequent with SEEG ESM (OR 0.24, p<0.001), whereas LE responses were predominantly seen with SEEG ESM (OR 4.79, p=0.013), compared to SDE ESM (Table 2). Electrode type did not significantly impact the incidence of UE responses. Increasing age significantly increased the odds of eliciting face motor responses (OR 1.14, p<0.001), but not UE and LE responses. Additionally, increase in stimulation current paradoxically decreased the odds of eliciting UE (OR 0.74, p<0.001) and LE (OR 0.75, p=0.025) responses, but not face motor responses. Pre-medication did not have a significant impact on the incidence of motor responses.
Sensory:
Mixed models again confirmed significantly higher likelihood of eliciting sensory responses with SEEG ESM (OR 255.14, p<0.001) compared to SDE ESM (Table 2). Increasing current strengths decreased the odds of sensory responses (OR 0.69, p<0.001), but age or premedication did not have a significant effect.
Incidence of ADs and EIS
The incidence of both ADs (OR 0.23, p<0.001) and EIS (OR 0.19, p<0.001) was significantly lower with SEEG ESM compared to SDE ESM, and with the use of premedication (ADs: OR 0.35, p<0.001; EIS: OR 0.33, p=0.005) in mixed models incorporating other variables (Table 2). Also, increasing current strengths increased the likelihood of eliciting ADs (OR 1.46, p<0.001) and EIS (OR 1.43, p=0.002).
Thresholds for Functional Responses
Current thresholds for language (slope=−0.04, p=0.008), face motor (slope=−0.06, p<0.001), and UE motor (slope=−0.04, p=0.006) responses showed a significant linear decrease with age, whereas the effect of age was not significant for LE motor and sensory response thresholds (Table 3). Although stimulation of the dominant hemisphere was associated with a negative slope in the model for language thresholds, it did not attain statistical significance. There was no significant effect of the electrode type (SEEG/SDE), premedication, or dominant hemisphere stimulation on any of the functional thresholds.
Table 3.
Current thresholds for functional responses, after-discharges and ESM-induced seizures as functions of electrode type, age, premedication, and dominant hemispheric stimulation analyzed with linear mixed models
| Response | Variable | Slope | 95% Confidence Interval | p-value |
|---|---|---|---|---|
| Language | SEEG (vs SDE) | −0.29 | −0.67 to 0.09 | 0.145 |
| Age | −0.04 | −0.08 to −0.004 | 0.008* | |
| Premedication | 0.09 | −0.23 to 0.41 | 0.587 | |
| Dominant hemispheric stimulation | −0.43 | −0.93 to 0.07 | 0.095 | |
| Face | SEEG (vs SDE) | 0.03 | −0.35 to 0.41 | 0.882 |
| Age | −0.06 | −0.10 to −0.03 | <0.001* | |
| Premedication | 0.12 | −0.24 to 0.48 | 0.526 | |
| Dominant hemispheric stimulation | 0.22 | −0.10 to 0.53 | 0.176 | |
| Upper extremity | SEEG (vs SDE) | −0.09 | −0.37 to 0.18 | 0.509 |
| Age | −0.04 | −0.07 to −0.01 | 0.006* | |
| Premedication | 0.05 | −0.25 to 0.36 | 0.726 | |
| Dominant hemispheric stimulation | 0.01 | −0.25 to 0.27 | 0.927 | |
| Lower extremity | SEEG (vs SDE) | −0.43 | −1.41 to 0.56 | 0.405 |
| Age | −0.06 | −0.13 to 0.01 | 0.142 | |
| Premedication | 0.24 | −0.77 to 1.24 | 0.650 | |
| Dominant hemispheric stimulation | −0.44 | −1.26 to 0.38 | 0.309 | |
| Sensory | SEEG (vs SDE) | 0.12 | −1.48 to 1.72 | 0.883 |
| Age | 0.004 | −0.05 to 0.05 | 0.873 | |
| Premedication | 0.05 | −0.64 to 0.73 | 0.898 | |
| Dominant hemispheric stimulation | −0.05 | −0.34 to 0.24 | 0.731 | |
| After-discharge | SEEG (vs SDE) | 0.24 | 0.01 to 0.46 | 0.045* |
| Age | −0.02 | −0.04 to −0.002 | 0.009* | |
| Premedication | 0.08 | −0.16 to 0.31 | 0.521 | |
| Dominant hemispheric stimulation | 0.11 | −0.07 to 0.28 | 0.243 | |
| ESM-induced seizure | SEEG (vs SDE) | 0.17 | −0.28 to 0.61 | 0.463 |
| Age | −0.06 | −0.11 to −0.02 | 0.006* | |
| Premedication | 0.14 | −0.39 to 0.67 | 0.605 | |
| Dominant hemispheric stimulation | 0.21 | −0.21 to 0.63 | 0.333 |
Notes: Current strengths were scaled separately for SEEG and SDE.
p<0.05.
Abbreviations: ESM electrical stimulation mapping, SDE subdural electrodes, SEEG stereo-electroencephalography
The random effects showed higher inter-patient but lower intra-patient heterogeneity for both language and motor thresholds as functions of age (Figure 2), justifying the use of mixed models.
Figure 2. Contribution of random effect of each patient in the relationship between language (A) or motor (B) thresholds and age.
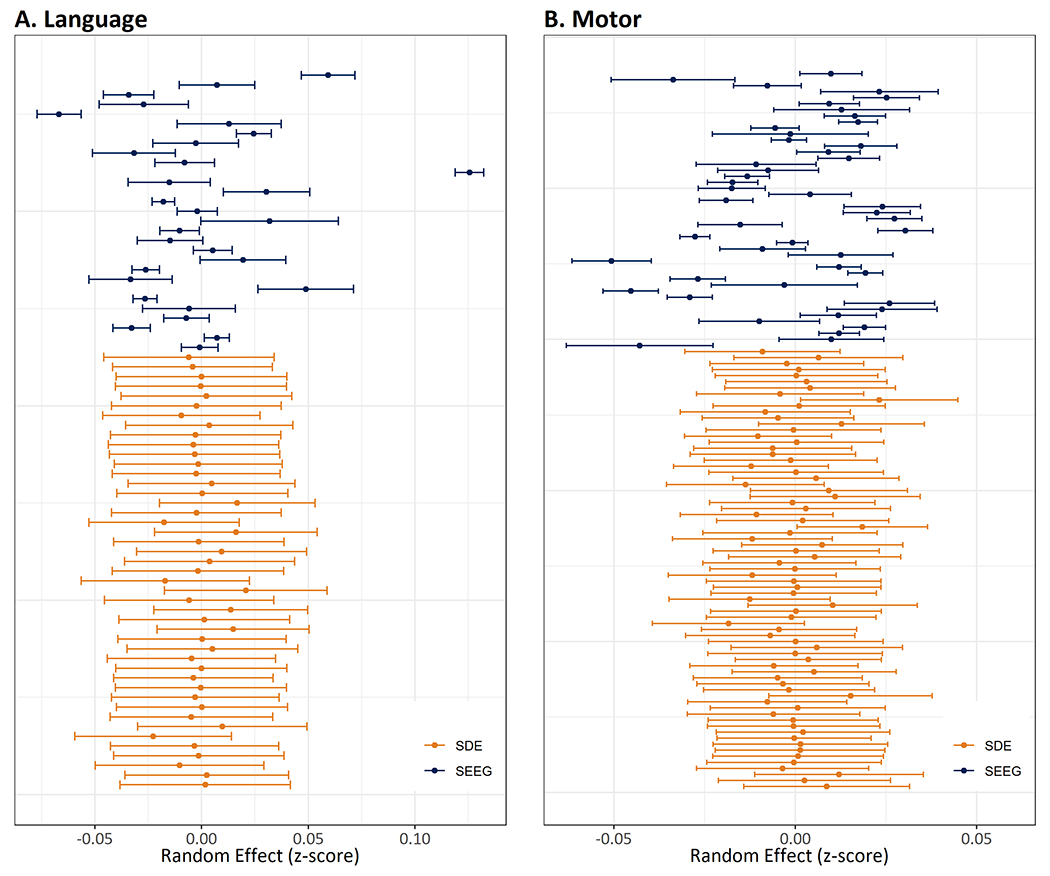
Each horizontal line shows the magnitude of random effect (z-score) for an individual patient. Note that SEEG shows high between-subject heterogeneity (non-overlapping error bars) but low within-subject heterogeneity (tighter error bars), whereas SDE has low between-subject heterogeneity but high within-subject heterogeneity. Higher heterogeneity seen in motor thresholds (z-scores on x-axis) is probably partly due to inclusion of disparate motor responses (face, upper extremity, and lower extremity) in this analysis.
Abbreviations: SDE subdural electrodes, SEEG stereo-electroencephalography
Thresholds for ADs and EIS
Both AD (slope=−0.02, p=0.009) and EIS (slope=−0.06, p=0.006) thresholds showed a significant linear decline with age (Table 3). While the electrode type did not significantly impact EIS thresholds, SEEG ESM was associated with higher AD thresholds (slope=0.24, p=0.045). Premedication and stimulation of dominant hemisphere did not significant impact AD and EIS thresholds.
Thresholds for SEEG and SDE subgroups
When the SDE subgroup was separately analyzed, language (slope=−0.04, p=0.021), face motor (slope=−0.07, p=0.006), UE motor (slope=−0.06, p=0.009), and AD (slope=−0.02, p=0.023) thresholds significantly decrease with age (Table S1). LE and EIS thresholds also decreased with age, but the relationship was not statistically significant. In addition, language thresholds (slope 0.71, p=0.012) increased with the use of premedication. However, neither premedication nor stimulation of the dominant hemisphere had any significant impact on other thresholds.
In the SEEG subgroup, language (slope=−0.02, p=0.007), face motor (slope=−0.06, p=0.027), and EIS (slope=−0.10, p=0.050) thresholds significantly decreased with age (Table S1). UE motor, LE motor, and AD thresholds also decreased with age but the relationship failed to achieve statistical significance. Premedication and dominant hemisphere stimulation did not influence SEEG ESM thresholds.
For SDE ESM, language thresholds were above AD thresholds throughout the age range included in the study, whereas an inverse relationship was seen for SEEG ESM (Figure 3), such that SEEG language thresholds remain below the AD thresholds up to 26 years-of-age. Furthermore, for SDE ESM, face motor and UE motor thresholds were above AD thresholds in younger children and fell below the AD thresholds at 11.2 and 7.4 years-of-age respectively (Figure 3). To compare, SEEG UE motor thresholds remained below AD thresholds throughout the age range, and face motor thresholds fell below AD thresholds by 4.8 years-of-age.
Figure 3. Language, motor, and AD thresholds as functional of age.
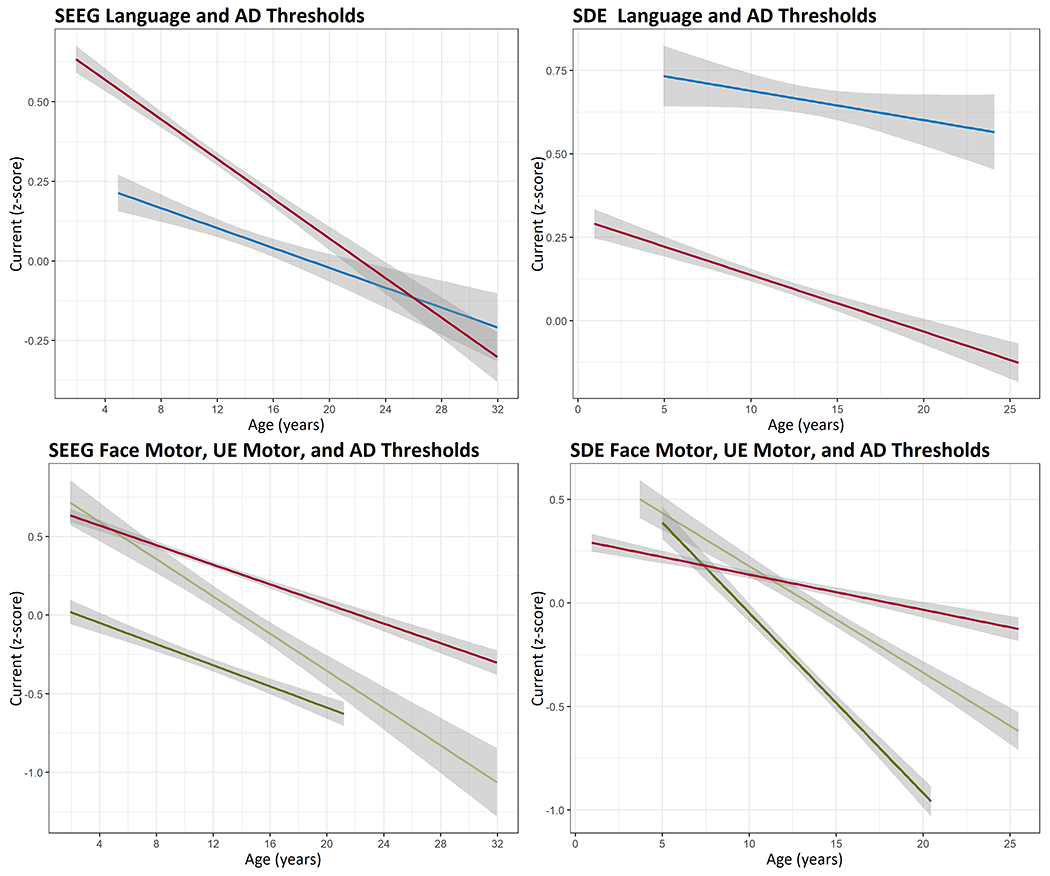
Language (blue) thresholds remain below AD (red) thresholds for SEEG ESM for most of the age range whereas the relationship is inverse for SDE ESM. UE motor (dark green) thresholds remain below AD thresholds (red) throughout the age range for SEEG ESM, whereas they fall below AD thresholds (red) at 7.4 years-of-age for SDE ESM. Face motor thresholds (light green) also fall below AD thresholds (red) at an earlier age for SEEG ESM compared to SDE ESM. Although the data was analyzed with mixed models, ordinary least square lines are shown for convenience. For SEEG graph showing language thresholds, the number of data points for age>24 years is small (wide confidence interval band) and should be interpreted with caution.
Abbreviations: AD after-discharges, ESM electrical stimulation mapping, SDE subdural electrodes, SEEG stereo-electroencephalography
DISCUSSION
We report one of the most extensive analyses of functional ESM including 173 children. This large dataset facilitates statistically valid inferences and highlights important comparisons between SEEG ESM and SDE ESM.
Impact of the Electrode Type on ESM
Language:
Our analyses did not show a significant impact of the electrode type (SEEG/SDE) on the incidence or the thresholds for language responses (Tables 1–3). Also, SEEG ESM thresholds for language responses were below those for ADs across the included age range up to 26 years, whereas this relationship is inverse for SDE ESM (Figure 3). Given that AD and EIS thresholds are closely correlated7, we posit that language ESM is safer with SEEG than SDE. This is because language responses in pediatric patients are typically elicited at currents approaching or exceeding AD thresholds for SDE ESM but not for SEEG ESM (Figure 3).
When comparing SEEG and SDE, it is important to be cognizant of the essential differences in their cortical sampling. SEEG offers access to sulcal folds and cortex on medial and basal cerebral surfaces inaccessible to SDE, whereas SDE samples gyral crowns with apparent contiguity. However, a recent study has shown that the overall average recording volume of gray matter is similar between SEEG and SDE14. In fact, SEEG sampled an average of 20% more gray matter and had 77% more of its contacts in gray matter within sulci. Because cerebral cortex is extensively folded, SEEG provides discrete local coverage across a wider region of the brain, whereas SDE covers relatively contiguous areas on the cortical surface with lower density. Therefore, it is conceivable that functional responses elicited with SEEG ESM, especially in the absence of ADs, are better localized and have superior neurophysiologic validity compared to SDE ESM.
We found that SEEG language (and to a lesser extent motor) thresholds have higher inter-patient but lower intra-patient heterogeneity compared to SDE thresholds (Figure 2). This interesting observation lends support to our use of mixed models. The neurophysiologic mechanism of this observation requires further research, however, we speculate that while SDE are approximately perpendicular to dendrites of pyramidal cells, SEEG electrodes have different trajectories such that their contacts have different geometrical relationship to pyramidal axons and dendrites. For the same reason, SEEG ESM may result in stimulation of cells other than pyramidal neurons, contributing to inter-patient heterogeneity in functional thresholds.
Motor:
SEEG ESM showed a more favorable relationship between AD and motor thresholds than SDE ESM. For SDE ESM, AD thresholds were below the motor thresholds until 7.4 years for UE and until 11.2 years for face motor response, whereas SEEG ESM showed AD thresholds higher than UE thresholds throughout the included age range and above face motor thresholds in children over 4.8 years-of-age (Figure 3).
The proportions of patients with motor responses did not differ between the electrode types. However, at electrode contact level, a more nuanced picture is noted, with SEEG ESM resulting in higher odds of LE responses (OR 4.79), lower odds of face motor responses (OR 0.24), and no difference in UE motor responses compared to SDE ESM (Table 1). We believe that the LE representation on the medial cerebral surface is preferentially accessible to SEEG compared to SDE. In contrast, we hypothesize that UE function is distributed over a sizeable extent of the precentral gyrus and is therefore equally accessible to both electrode types. The lower odds of face motor responses with SEEG ESM are challenging to explain. Although we do not completely understand propagation of stimulation current within brain tissue and its cellular and network level excitatory and inhibitory effects25, 26, evidence suggests that SDE ESM may result in a wider extent of cortical stimulation than SEEG ESM. SDE ESM is associated with current shunting through the CSF and increased current density towards the perimeter of the electrode16, 27. Hence, we speculate that SEEG activation is more localized, recruits smaller neuronal pools, and results in lower amplitude face motor responses which may be subtle and less observable. In contrast, cortical motor representations of extremities have larger motor units typically resulting in more obvious motor responses. Also, it is possible that negative face motor responses are triggered at lower current intensities at some of the same sites where higher intensities trigger positive responses given the proximity of negative motor area in the dorsal inferior frontal gyrus and facial representation in the inferior precentral gyrus9, 28.
Sensory:
Sensory responses were significantly more common with SEEG stimulations than SDE (Table 1). Also, the thresholds for sensory responses were substantially lower with SEEG ESM (Table 3). Sensory representation is predominantly localized to the apposed lips of the Rolandic sulcus29. We believe that SEEG has better access to this intra-sulcal primary somatosensory cortex, as well as secondary somatosensory cortex in the parietal operculum and the sensory representation in posterior insula30, 31.
ADs and EIS:
A lower incidence of ADs and unwanted EIS was seen with SEEG ESM compared to SDE ESM. Also, the thresholds (scaled for the electrode type) for eliciting ADs and EIS were higher for SEEG ESM than SDE ESM (Tables 1–3). ADs and EIS can compromise the validity and safety of ESM. When a functional response is elicited simultaneously with ADs, it is unclear if the observed response is attributable to stimulation or to ADs and whether it localizes to the stimulated cortex or to remote cortical areas with preferential connectivity9.
Effect of age
We found an overall trend of increasing incidence and lower current thresholds with age for functional responses in both SEEG and SDE groups, similar to previous studies7, 8, 11, 12, 32. Specifically: (1) incidence of language responses increased while thresholds decreased with age, (2) increasing age significantly increased the odds of eliciting face motor responses, but not UE and LE responses. Also, thresholds for face motor and UE motor responses significantly decreased with age. (3) AD and EIS thresholds, but not the incidence, decreased with age. When the SDE group was separately analyzed, language, face motor, UE motor, and AD thresholds significantly decreased with age (Table S1). Similarly, in the SEEG subgroup, language, face motor, and EIS thresholds significantly decreased with age (Table S1).
We hypothesize higher cortical excitability (lower thresholds) in older patients may be potentially explained by right-wards shift of the curve between the current intensity and pulse duration in non-myelinated neurons compared to myelinated neurons, such that non-myelinated neurons require higher rheobase currents for excitation33. The interaction among the electrode type, current thresholds, and age is further insightful. While SDE language thresholds were above AD thresholds throughout the included age range, SEEG language thresholds remain below the AD thresholds up to 26 years-of-age (Figure 3). Also, SDE face and UE motor thresholds were above AD thresholds in younger children and decrease below the AD thresholds at 11.2 and 7.4 years-of-age respectively, whereas SEEG UE motor thresholds remained below AD thresholds throughout the age range, and face motor thresholds decreased below AD thresholds by 4.8 years-of-age (Figure 3). Because the incidence and thresholds for ADs and EIS are closely correlated7, SEEG ESM evoking functional responses without crossing the AD thresholds, offers better safety and neurophysiologic validity compared to SDE ESM.
Conundrum of current strength
With an increase in current strength, while the incidence of language responses, ADs and EIS increased, a decrease in the odds of eliciting UE motor, LE motor, and sensory responses were observed (Table 2). We speculate that these findings may be explained by differential excitability of functional cortical regions. ESM results in a complex summation of excitation and inhibition both locally and in remotely connected networks25, 34, as has been shown for sensorimotor cortices35. Transcranial magnetic stimulation studies have shown differential excitation and inhibition over short and long distances as a function of current strength36. Also, in certain cortical regions, ESM has been shown to generate an early inhibitory effect that may be overcome by subsequent excitation, depending on current strength, frequency, and the pre-stimulation firing rate of the neuronal populations37. Hence, it is possible that sensorimotor cortices may be susceptible to strength-dependent responses, however, this finding warrants future evaluation.
Pre-medication
Premedication was used more frequently before SDE ESM (37%) than before SEEG ESM (19%), which reflects a variation in practice and underscores the need for standardized ESM protocols. Overall, the incidence of language, motor, and sensory responses was not affected by premedication, nor were the thresholds for functional (language, motor, and sensory) responses, ADs, and EIS. Only with SDE, premedication was associated with decreased incidence of ADs and EIS, and increased language thresholds. This is consistent with our previous study where fosphenytoin premedication before SDE ESM reduced the risk of EIS but increased temporal language thresholds38. Thus, our findings suggest little justification for the use of premedication with SEEG ESM, however, premedication may be considered before SDE ESM.
Limitations
Our study is one of the largest comparisons of SEEG and SDE ESM with fairly uniform protocols in the respective subgroups, however, there are some limitations. We used visual naming for language mapping, a practice based on the observation that dysnomia is seen in nearly all aphasias, although probably during an auditory discourse rather than for naming objects in the visual environment22. There may be value in performing ESM using multiple language tasks, probably depending on the cortical region being tested, however, the optimal protocol for such testing, particularly in children, remains undefined39. Also, we relied on visual observation for ascertaining motor responses and did not use electromyography (EMG). This is because using EMG is challenging in many children, and because most ESM sessions result in stimulation of several muscles, which would have required placing a prohibitive number of EMG electrodes. Hence, subtle motor responses may have been undetected. For SEEG ESM, we did not distinguish contacts in gray and white matter, because previous studies found no differences in the incidence or thresholds between gray and white matter stimulation11, 12. Finally, we could not analyze duration of epilepsy as a covariate due to variability in the documentation. In addition, some inadvertent bias may also be present from being a single-center retrospective study.
Conclusion and Implications for Clinical Practice
We found that SEEG ESM is similar to SDE ESM for eliciting language and motor responses. In addition, SEEG ESM offers a unique opportunity to map sensory cortices. SEEG ESM is probably safer than SDE ESM given the lower incidence of ADs and unwanted EIS, and a favorable relationship between AD and functional thresholds with age (Figure 3). Because SEEG ESM evokes functional responses usually below AD thresholds, SEEG ESM may have better concordance between the observed response and the stimulated tissue especially in pediatric patients. In addition, while premedication may be considered for SDE ESM perhaps in some patients with a higher risk of unwanted EIS, routine use of premedication for SEEG ESM is not well-supported.
Although the choice of intracranial modality is often based on institutional experience and practice, our study demonstrates that for presurgical localization of sensorimotor and language cortices, SEEG is comparable to, and in some ways safer than SDE. However, between SEEG and SDE it is not entirely correct to say if one is superior to the other for functional brain mapping, rather, it is more pertinent to realize that they assess different cortical areas. SEEG offers wider coverage with relatively sparse surface sampling, access to deeper cortices including that within sulcal folds, and overall higher gray matter sampling, whereas SDE offers relatively contiguous but lower density coverage on the cortical surface. Also, SEEG may not be feasible in some younger children due to insufficient bone strength to hold the bolts, although alternative approaches have been tried40. This is reflected in our data also where age distribution for SEEG was relatively higher than that for SDE, although the difference was not statistically significant. We think that postoperative functional outcomes will likely be the definitive arbiter for evaluating effectiveness of ESM with SDE and SEEG, however, this was outside the scope of present manuscript focused on safety and neurophysiology, and are planned for future study.
Supplementary Material
KEY POINTS.
ESM with SEEG and SDE is similar for eliciting motor and language responses
Sensory responses are elicited more commonly with SEEG ESM than SDE ESM
ADs and unwanted ESM-induced seizures are less common with SEEG than SDE
Functional and AD thresholds exhibit more favorable age-relationship for SEEG ESM than SDE ESM
ACKNOWLEDGEMENTS
Authors wish to acknowledge support from EEG technologists especially Danielle Givens and Owen America.
Funding Statement.
Research reported in this manuscript was supported by NIH NINDS R01 NS115929 and the Division of Neurology of the Cincinnati Children’s Hospital Medical Center. Funding sources had no role in study design, conduct of the study, writing the manuscript, and the decision to submit for publication.
Conflict of Interest Disclosure.
RA receives research support from NIH NINDS R01 NS115929 and Cincinnati Children’s Research Foundation (Research Innovation Project Grant). None of the other authors have any relevant potential conflicts of interest to report.
Footnotes
Ethical Statement. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Ethics Approval Statement. The study was approved by the Cincinnati Children’s Hospital Medical Center’s Institutional Review Board (IRB #2017-4025).
Data Availability Statement.
Data used in this study including raw EEG files will be made available on reasonable academic requests after negotiation of appropriate agreements with the study institution.
REFERENCES
- 1.Jayakar P, Gaillard WD, Tripathi M, et al. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia 2014;55:507–518. [DOI] [PubMed] [Google Scholar]
- 2.Gavvala J, Zafar M, Sinha SR, Kalamangalam G, Schuele S, American Seeg Consortium sbTACNS. Stereotactic EEG Practices: A Survey of United States Tertiary Referral Epilepsy Centers. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society 2022;39:474–480. [DOI] [PubMed] [Google Scholar]
- 3.Serletis D, Bulacio J, Bingaman W, Najm I, Gonzalez-Martinez J. The stereotactic approach for mapping epileptic networks: a prospective study of 200 patients. Journal of neurosurgery 2014;121:1239–1246. [DOI] [PubMed] [Google Scholar]
- 4.Alomar S, Jones J, Maldonado A, Gonzalez-Martinez J. The Stereo-Electroencephalography Methodology. Neurosurgery clinics of North America 2016;27:83–95. [DOI] [PubMed] [Google Scholar]
- 5.Trebuchon A, Chauvel P. Electrical Stimulation for Seizure Induction and Functional Mapping in Stereoelectroencephalography. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society 2016;33:511–521. [DOI] [PubMed] [Google Scholar]
- 6.Grande KM, Ihnen SKZ, Arya R. Electrical Stimulation Mapping of Brain Function: A Comparison of Subdural Electrodes and Stereo-EEG. Frontiers in human neuroscience 2020;14:611291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aungaroon G, Zea Vera A, Horn PS, et al. After-discharges and seizures during pediatric extra-operative electrical cortical stimulation functional brain mapping: Incidence, thresholds, and determinants. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2017;128:2078–2086. [DOI] [PubMed] [Google Scholar]
- 8.Zea Vera A, Aungaroon G, Horn PS, et al. Language and motor function thresholds during pediatric extra-operative electrical cortical stimulation brain mapping. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2017;128:2087–2093. [DOI] [PubMed] [Google Scholar]
- 9.Lesser RP, Luders H, Klem G, et al. Extraoperative cortical functional localization in patients with epilepsy. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society 1987;4:27–53. [DOI] [PubMed] [Google Scholar]
- 10.Corley JA, Nazari P, Rossi VJ, et al. Cortical stimulation parameters for functional mapping. Seizure : the journal of the British Epilepsy Association 2017;45:36–41. [DOI] [PubMed] [Google Scholar]
- 11.Arya R, Ervin B, Dudley J, et al. Electrical stimulation mapping of language with stereo-EEG. Epilepsy & behavior : E&B 2019;99:106395. [DOI] [PubMed] [Google Scholar]
- 12.Arya R, Ervin B, Holloway T, et al. Electrical stimulation sensorimotor mapping with stereo-EEG. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2020;131:1691–1701. [DOI] [PubMed] [Google Scholar]
- 13.Cuisenier P, Testud B, Minotti L, et al. Relationship between direct cortical stimulation and induced high-frequency activity for language mapping during SEEG recording. Journal of neurosurgery 2020:1–11. [DOI] [PubMed] [Google Scholar]
- 14.Tantawi M, Miao J, Matias C, et al. Gray Matter Sampling Differences Between Subdural Electrodes and Stereoelectroencephalography Electrodes. Frontiers in neurology 2021;12:669406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minkin K, Gabrovski K, Karazapryanov P, Milenova Y, Sirakov S, Dimova P. Theoretical stereoelectroencephalography density on the brain convexity. Epilepsy research 2021;179:106845. [DOI] [PubMed] [Google Scholar]
- 16.Nathan SS, Sinha SR, Gordon B, Lesser RP, Thakor NV. Determination of current density distributions generated by electrical stimulation of the human cerebral cortex. Electroencephalography and clinical neurophysiology 1993;86:183–192. [DOI] [PubMed] [Google Scholar]
- 17.Koessler L, Colnat-Coulbois S, Cecchin T, et al. In-vivo measurements of human brain tissue conductivity using focal electrical current injection through intracerebral multicontact electrodes. Human brain mapping 2017;38:974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kombos T, Suss O. Neurophysiological basis of direct cortical stimulation and applied neuroanatomy of the motor cortex: a review. Neurosurgical focus 2009;27:E3. [DOI] [PubMed] [Google Scholar]
- 19.Cogan SF, Ludwig KA, Welle CG, Takmakov P. Tissue damage thresholds during therapeutic electrical stimulation. Journal of neural engineering 2016;13:021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakai Y, Jeong JW, Brown EC, et al. Three- and four-dimensional mapping of speech and language in patients with epilepsy. Brain : a journal of neurology 2017;140:1351–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of experimental psychology: Human learning and memory 1980;6:174. [DOI] [PubMed] [Google Scholar]
- 22.Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychology review 2007;17:477–489. [DOI] [PubMed] [Google Scholar]
- 23.Blume WT, Jones DC, Pathak P. Properties of after-discharges from cortical electrical stimulation in focal epilepsies. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2004;115:982–989. [DOI] [PubMed] [Google Scholar]
- 24.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823 2014. [Google Scholar]
- 25.Ranck JB. Which Elements Are Excited in Electrical-Stimulation of Mammalian Central Nervous-System - Review. Brain research 1975;98:417–440. [DOI] [PubMed] [Google Scholar]
- 26.Rattay F The basic mechanism for the electrical stimulation of the nervous system. Neuroscience 1999;89:335–346. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Webster JG, Tompkins WJ. Simulated and experimental studies of temperature elevation around electrosurgical dispersive electrodes. IEEE transactions on bio-medical engineering 1984;31:681–692. [DOI] [PubMed] [Google Scholar]
- 28.Mikuni N, Ohara S, Ikeda A, et al. Evidence for a wide distribution of negative motor areas in the perirolandic cortex. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2006;117:33–40. [DOI] [PubMed] [Google Scholar]
- 29.Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Frontiers in human neuroscience 2010;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afif A, Minotti L, Kahane P, Hoffmann D. Anatomofunctional organization of the insular cortex: a study using intracerebral electrical stimulation in epileptic patients. Epilepsia 2010;51:2305–2315. [DOI] [PubMed] [Google Scholar]
- 31.Schluppeck D, Sanchez-Panchuelo RM, Francis ST. Exploring structure and function of sensory cortex with 7T MRI. NeuroImage 2018;164:10–17. [DOI] [PubMed] [Google Scholar]
- 32.Chitoku S, Otsubo H, Harada Y, et al. Extraoperative cortical stimulation of motor function in children. Pediatric neurology 2001;24:344–350. [DOI] [PubMed] [Google Scholar]
- 33.Jayakar P, Alvarez LA, Duchowny MS, Resnick TJ. A safe and effective paradigm to functionally map the cortex in childhood. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society 1992;9:288–293. [DOI] [PubMed] [Google Scholar]
- 34.Tolias AS, Sultan F, Augath M, et al. Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron 2005;48:901–911. [DOI] [PubMed] [Google Scholar]
- 35.Kinnischtzke AK, Simons DJ, Fanselow EE. Motor cortex broadly engages excitatory and inhibitory neurons in somatosensory barrel cortex. Cerebral cortex 2014;24:2237–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? The Journal of physiology 2009;587:725–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollen DA. Responses of single neurons to electrical stimulation of the surface of the visual cortex. Brain Behav Evol 1977;14:67–86. [DOI] [PubMed] [Google Scholar]
- 38.Arya R, Aungaroon G, Zea Vera A, et al. Fosphenytoin pre-medication for pediatric extra-operative electrical stimulation brain mapping. Epilepsy research 2018;140:171–176. [DOI] [PubMed] [Google Scholar]
- 39.Wellmer J, Weber C, Mende M, et al. Multitask electrical stimulation for cortical language mapping: hints for necessity and economic mode of application. Epilepsia 2009;50:2267–2275. [DOI] [PubMed] [Google Scholar]
- 40.Karsonovich T, Alexander A, Graber S, O’Neill BR. Placement of leads for stereotactic electroencephalography without the use of anchor bolts: technical note. Journal of neurosurgery Pediatrics 2020;27:253–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study including raw EEG files will be made available on reasonable academic requests after negotiation of appropriate agreements with the study institution.


