Abstract
Background:
Frailty is an increasingly recognized clinical diagnosis associated with high risk of disability and mortality. Frailty in patients after hematopoietic cell transplantation (HCT) is associated with increased non-relapse mortality (NRM) and decreased overall survival (OS). Frailty has not been extensively studied in patients with chronic graft-versus-host disease (cGVHD).
Objectives:
The objectives of this study were to assess the prevalence and clinical correlates of frailty and the association of frailty with NRM and OS in patients enrolled in the Chronic GVHD Consortium. Patients were characterized as frail if they met Fried’s definition: ≥3 of the following criteria at enrollment: unintentional weight loss, exhaustion, slow walking speed, low physical activity, and weakness. Frailty was assessed retrospectively and used surrogate measures for the 5 domains of frailty. Frailty, chronic GVHD organ scores, and patient-reported outcomes were measured at the time of enrollment.
Results:
This study included 399 patients from 9 centers in the United States with 32% characterized as frail and 68% as not frail. The median follow-up time from enrollment was 9 years (interquartile range 7 – 11 years). Frail patients were more likely to be older (p=0.004), have lower Karnofsky performance score (p<0.001), have severe cGVHD (p<0.001), and have GI (p<0.001), liver (p=0.04), or lung cGVHD (p=0.002). In a multivariable analysis, older age, increased cGVHD global severity, and thrombocytopenia were statically associated with frailty when cGVHD organ involvement was excluded. A separate analysis excluding cGVHD severity and including organ involvement showed that lung and liver cGVHD, and older age were associated with frailty. Neither corticosteroid usage at the time of enrollment nor the maximum recorded dose of corticosteroids prior to enrollment were associated with frailty.
Frail patients had higher NRM than not frail patients (p<0.001) with 10-year cumulative incidences of 41% (95% CI 32–49%) vs 22% (95% CI 17–28%), respectively. Reciprocally, frailty was also associated with a significantly lower OS (p<0.001) with 10-year OS of 43% (95% CI 35–53%) in frail patients vs 63% (95% CI 57–69%) in not frail patients. In multivariable analysis that included the individual domains of frailty, weakness, low physical activity, and slow walking speed were associated with survival. Frail patients also had worse scores on various measures of patient reported outcomes including SF-36, Lee Symptom Scale, and the trial outcome of the FACT-BMT index score.
Conclusions:
Frail patients with cGVHD have significantly worse outcomes compared to not frail patients. Clinical features such as older age and lung and liver cGVHD are associated with frailty. Earlier clinical recognition of frailty in cGVHD patients may prompt interventions to counteract frailty that could be beneficial to this population.
Introduction
Frailty is a condition that is increasingly recognized in older populations with around 10% of people >65 classified as frail [1]. Frailty is thought to lead to decreased physiologic reserve for responding to stressors, ultimately leading to increased disability and mortality [2]. Many patients with hematologic malignancies are older and more likely to be frail. Among patients with hematologic malignancies, frailty is associated with worse outcomes [3, 4]. Frailty, measured by Fried’s criteria [5] and by surrogate measures, has also been evaluated in hematopoietic cell transplant (HCT) recipients. Fried previously defined frailty as patients meeting ≥3 of 5 of the following criteria: unintentional weight loss, exhaustion, slow walking speed, low physical activity, and weakness. One study evaluated pre-HCT frailty using Fried’s criteria, although using different measures for the 5 domains of frailty, and reported that frail patients have higher rates of mortality than patients who are not frail[6]. A prospective study showed that the pre-HCT timed get up and go test (TUGT), a measure of functionality, was significantly associated with overall survival (OS), and both frailty, measured using a validated clinical frailty scale[7], and functionality were associated with non-relapse mortality (NRM) [8]. Another prospective German study collected the EORTC QLQ-C30, a questionnaire designed to assess the quality of life in patients with malignancies [9], and TUGT during various time points, both pre and post HCT, and demonstrated that the fatigue score in the EORTC QLQ-C30 and TUGT were associated with OS [10].
A longitudinal evaluation of frailty assessed patients at two time points after HCT with a median interval of 13 years between the time points [11]. This study showed that frailty increased from 5% to 10% and that 19% of patients had worse frailty scores at the later time point. Patients with chronic graft-versus-host (cGVHD) had higher odds of frailty at the second time point [11]. Another retrospective study evaluated frailty in HCT recipients <65 years old who were ≥ 2 years post-HCT. This study reported both resolved cGVHD and active cGVHD were significantly associated with frailty with OR 2.7; 95%CI, 1.1–6.9 and OR 15.0; 95% CI, 6.6–34.3 respectively [12]. These two studies[11, 12] used questions from the bone marrow transplant survivor study (BMTSS) questionnaire[13], a validated tool, as surrogates of the 5 domains of frailty defined by Fried. Although frailty appears to be associated with prognosis and cGVHD, the correlation of frailty and association with major transplant outcomes in patients with cGVHD are unknown. Additionally, many of the prior studies in the HCT population relied solely on subjective measures, such as the clinical frailty scale and the BMTSS questionnaire. In this study, we assess the prevalence of frailty using a combination of objective and patient-reported measures. We also evaluate the correlates of frailty and frailty’s association with NRM and OS in patients enrolled in the Chronic GVHD Consortium.
Methods
Patients
This study received approval through the institutional review board at each institution, and all patients signed informed consent. We identified 399 patients who were enrolled in the Chronic GVHD Consortium between 2007–2012 and had characteristics of frailty measured at enrollment.
End points
Frailty was defined using Fried’s definition: ≥3 of the following 5 criteria at enrollment: unintentional weight loss, exhaustion, slow walking speed, low physical activity, and weakness[5]. Unintentional weight loss was determined by a score of 2 or 3 on the weight loss question on the Lee Symptom Scale (LSS), which is identical to the weight loss question used in the BMTSS questionnaire for prior frailty analyses [11, 12]. Exhaustion was defined by the “loss of energy” question on the LSS [14] with a score ≥2. Slow walking speed was measured by meters walked in 2 minutes based on age and sex, and the cut offs for slow walking speed were based on normative reference values[15]. A timed walk test has been previously used to evaluate frailty and was found to be highly correlated with frailty [16]. Low physical activity was measured by the Human Activity Profile, which has been validated in HCT patients and has been used in other studies to measure frailty [17, 18], with an adjusted activity score ≤53 defining low physical activity. Weakness was measured using grip strength with cutoffs defined by gender and body mass index. Most of these measures were surrogates of Fried’s criteria[5] as data were collected retrospectively. Frailty was defined before starting the analysis. A table to summarize the criteria used in this study compared to Fried’s definition is included. Supplementary Table 1. Pre-frail, defined as patients meeting 1 or 2 criteria, were combined with not frail, defined as patients meeting 0 criteria, as was done in other frailty studies [3, 12]. Chronic GVHD organ scores were recorded at the time of enrollment according to NIH consensus criteria[19]. Subjects were either incident (< 3 months from diagnosis of cGVHD, n=225) or prevalent cases (≥ 3 months from diagnosis of cGVHD, n=174) at enrollment. Case type (incident/prevalent) was not significantly associated with frailty, hence the 2 groups were combined for analyses. We evaluated patient reported outcomes (PROs) using the SF-36 questionnaire [20], FACT-BMT [21], and the LSS [14]. For the SF-36, we calculated the physical and mental component scores (0–100) where 50 is the population mean and higher scores are better with a difference >5 considered clinically meaningful [22]. For the FACT-BMT, we calculated the Trial Outcome Index based on the physical, functional, and bone marrow transplant subscale items (0–96) where higher is better with a difference of >7 considered clinically meaningful [23]. The LSS yields seven domain scores and one summary score (0–100) where higher scores indicate worse symptoms, and an overall difference of 7 is clinically meaningful. For the individual components, a difference of 13 for energy, 15 for eye, 8 for lung, 13 for mouth, 7 for nutrition, 11.5 for psychiatric, and 11 for skin are clinically meaningful [24, 25]. We evaluated the association of corticosteroid use and frailty using the current corticosteroid dose at enrollment and the maximum corticosteroid dose documented since time of cGVHD diagnosis prior to enrollment in mg/kg/day.
Statistical analysis
To examine the associations with frailty, the Chi-square test and Fisher’s exact test were used for categorical factors and the Wilcoxon rank sums test for continuous factors. Stepwise multivariable logistic regression was used to examine factors related univariately to frailty with entry and stay criteria of p=0.10. Because cGVHD severity is calculated using organ involvement, models were fit separately 1) including cGVHD severity but not organ involvement, and 2) including organ scores but not cGVHD severity. Cox proportional hazards models were used in a similar stepwise manner to examine the associations of frailty with overall survival (OS) and non-relapse mortality (NRM); OS was calculated from enrollment to death from any cause with patients censored at last contact if still alive, and NRM was calculated from enrollment to death without relapse, with relapse considered a competing risk.
Analyses were performed using SAS/STAT software, version 9.4 (SAS Institute, Inc, Cary NC).
Results
Baseline Characteristics
The study included 399 patients who were diagnosed with cGVHD, ≥ 18 years of age, from from 9 different centers, from 2007–2012. There were 129 frail patients, 236 pre-frail patients, and 34 not frail patients. Pre-frail and not frail patients were combined (N=270) for the analyses as stated in the methods section. Frail patients were more likely to be >55 at enrollment (51% vs 34%, p=0.004) and have lower Karnofsky performance score (KPS) (70 vs 90, p<0.001), severe cGVHD (42% vs 23%, p<0.001), thrombocytopenia (24% vs 14%, p=0.01), overlap cGVHD (78% vs 66%, p=0.02), and lung (26% vs 14%, p=0.002), gastrointestinal (GI) (28% vs 23%, p<0.001), and liver involvement (11% vs 4.4%, p=0.04), with a suggestive higher hematopoietic cell transplantation-comorbidity index (HCT-CI) (3 vs 2, p=0.06) than not frail patients. There was no difference in sex, diagnosis, disease status, conditioning intensity, donor type, graft source, sex mismatch, prior grade II-IV aGVHD, transplant center, time from HCT to cGVHD, time from HCT to enrollment, case type (incident vs prevalent), or onset type, or with skin, eye, mouth, joints, or genital involvement between frail and not frail patients. The median follow up time was 9 years (interquartile range 7 – 11 years).
Frailty
Among the study population, 32% were characterized as frail and 68% were not-frail. Figure 1A. The most common reported domains of frailty were slow walking speed (99%), exhaustion (92%), and weakness (71%). These were also the most common domains for pre-frail patients with 86.4%, 42.4%, and 21.6% reporting slow walking speed, exhaustion, and weakness respectively. Figure 1B. Slow walking speed and weakness were not highly correlated (ρ=0.14). Multivariable analysis showed that increased cGVHD severity, thrombocytopenia, and older age at enrollment were significantly associated with frailty. Table 2A. When cGVHD global severity was excluded so that we could evaluate individual organ manifestations, older age, lung cGVHD, and liver cGVHD were significantly associated with frailty. Table 2B.
Figure 1.
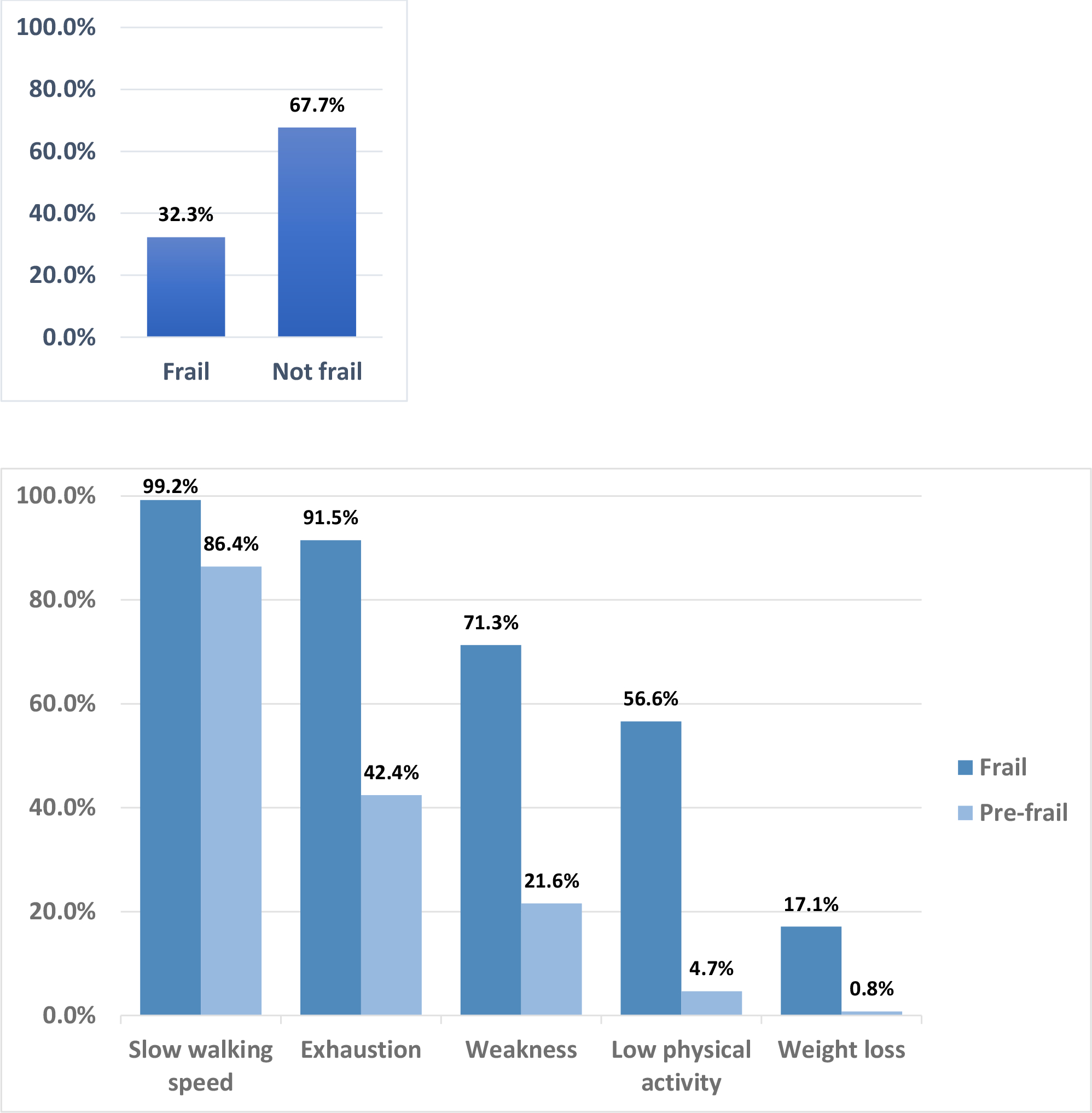
(A) Prevalence of frailty at enrollment, (B) Prevalence of domains of frailty at enrollment
Table 2A:
Multivariable analysis: predictors of frailty (CGVHD severity included, no organs)
| Variable | Class | OR (95% CI) | p-value | Global p-value |
|---|---|---|---|---|
|
| ||||
| CGVHD severity | None/Mild | Reference | <.001 | |
| Moderate | 1.6 (0.8, 3.2) | 0.17 | ||
| Severe | 3.6 (1.7, 7.3) | <.001 | ||
| Thrombocytopenia | Platelets >=100,000/mm3 | Reference | ||
| Platelets <100,000/mm3 | 2.0 (1.1, 3.4) | 0.02 | ||
| Age at enrollment (years) | <40y | Reference | 0.008 | |
| 40–55y | 1.2 (0.6, 2.3) | 0.66 | ||
| >55y | 2.2 (1.1, 4.4) | 0.02 | ||
Abbreviations: CGVHD, chronic graft-versus-host disease
Table 2B:
Multivariable analysis: Predictors of frailty (Organs included, not CGVHD severity)
| Variable | Class | OR (95% CI) | p-value | Global p-value |
|---|---|---|---|---|
|
| ||||
| Thrombocytopenia | Platelets >=100,000/mm3 | Reference | ||
| Platelets <100,000/mm3 | 1.7 (1.0, 3.0) | 0.06 | ||
| Lung | None | Reference | <.001 | |
| Yes | 2.5 (1.5, 4.0) | <.001 | ||
| Liver | None | Reference | ||
| Yes | 1.8 (1.1, 3.1) | 0.02 | ||
| Age at enrollment (years) | <40y | Reference | 0.003 | |
| 40–55y | 1.3 (0.6, 2.5) | 0.52 | ||
| >55y | 2.5 (1.3, 5.0) | 0.008 | ||
Non-relapse mortality
Non-relapse mortality (NRM) was significantly higher for frail patients vs not frail patients (p<0.001), with 10-year NRMs of 41% (95% CI 32%–49%) and 22% (95% CI 17%–;28%), respectively. Figure 2. Multivariable analysis including cGVHD global severity and adjusted for transplant center showed that frailty and higher cGVHD global severity were associated with NRM, but older age was not. Supplementary Table 2. There was no significant interaction between frailty and cGVHD severity in the NRM model (p=0.44). Analysis excluding cGVHD global severity but including the components of frailty and cGVHD organ manifestations showed that slow walking speed, low physical activity, older age, and skin and lung cGVHD were associated with higher NRM. Supplementary Table 3.
Figure 2.
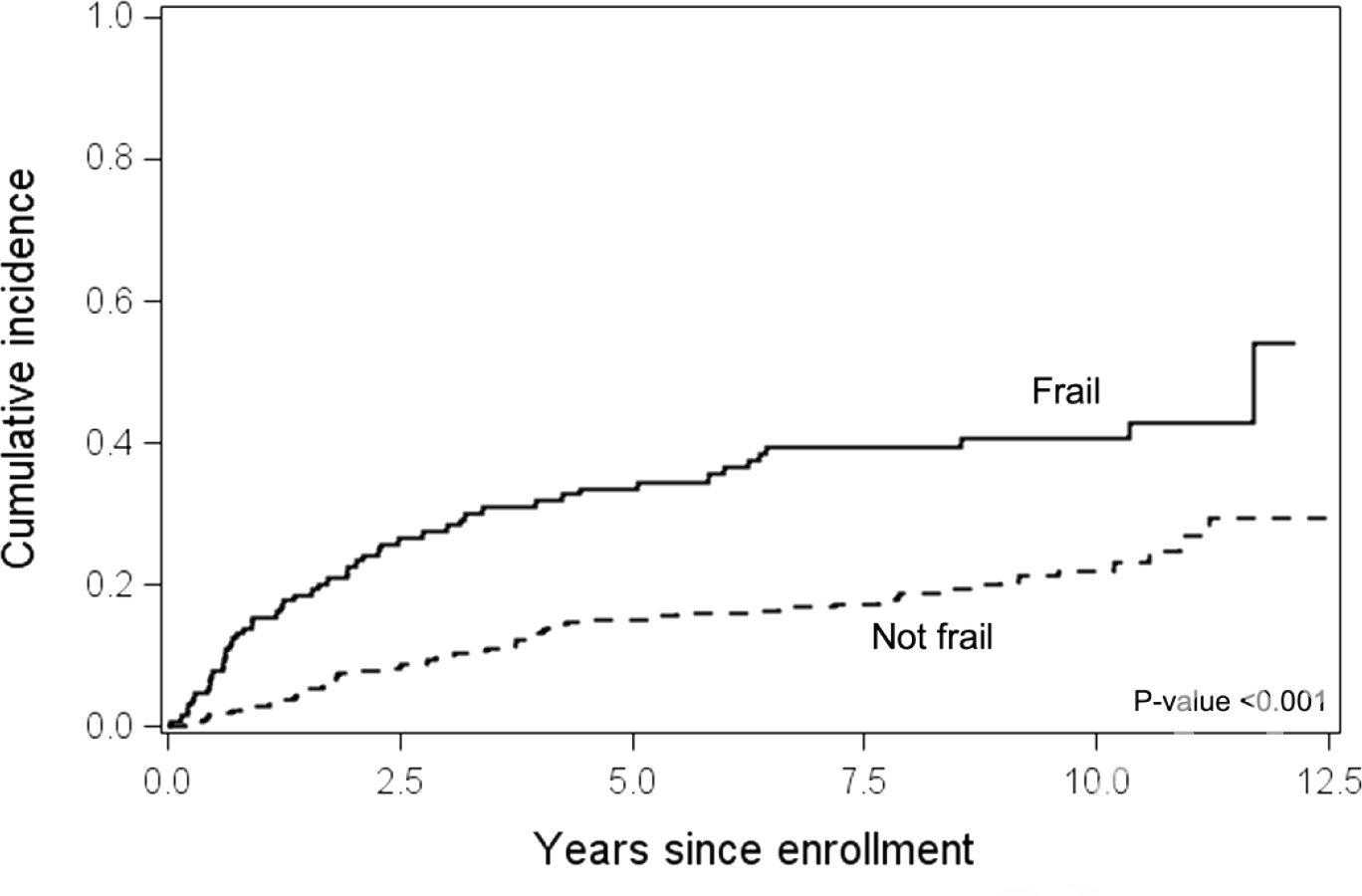
Non-relapse mortality from enrollment
Survival
Ten-year survival was worse in frail (43%, 95% CI 35–53%) compared to not frail (63%, 95% CI 57–69%) patients. Figure 3. In a multivariable analysis including cGVHD global severity and adjusted by transplant center, frailty, HCT-CI, overlap type, and progressive cGVHD onset were associated with overall survival but older age was not. Supplementary Table 4. A separate analysis excluding cGVHD global severity and including frailty components and cGVHD organ involvement showed that weakness, low physical activity, skin, lung, and liver cGVHD, older age and progressive cGVHD onset were associated with worse survival. Supplementary Table 5.
Figure 3.
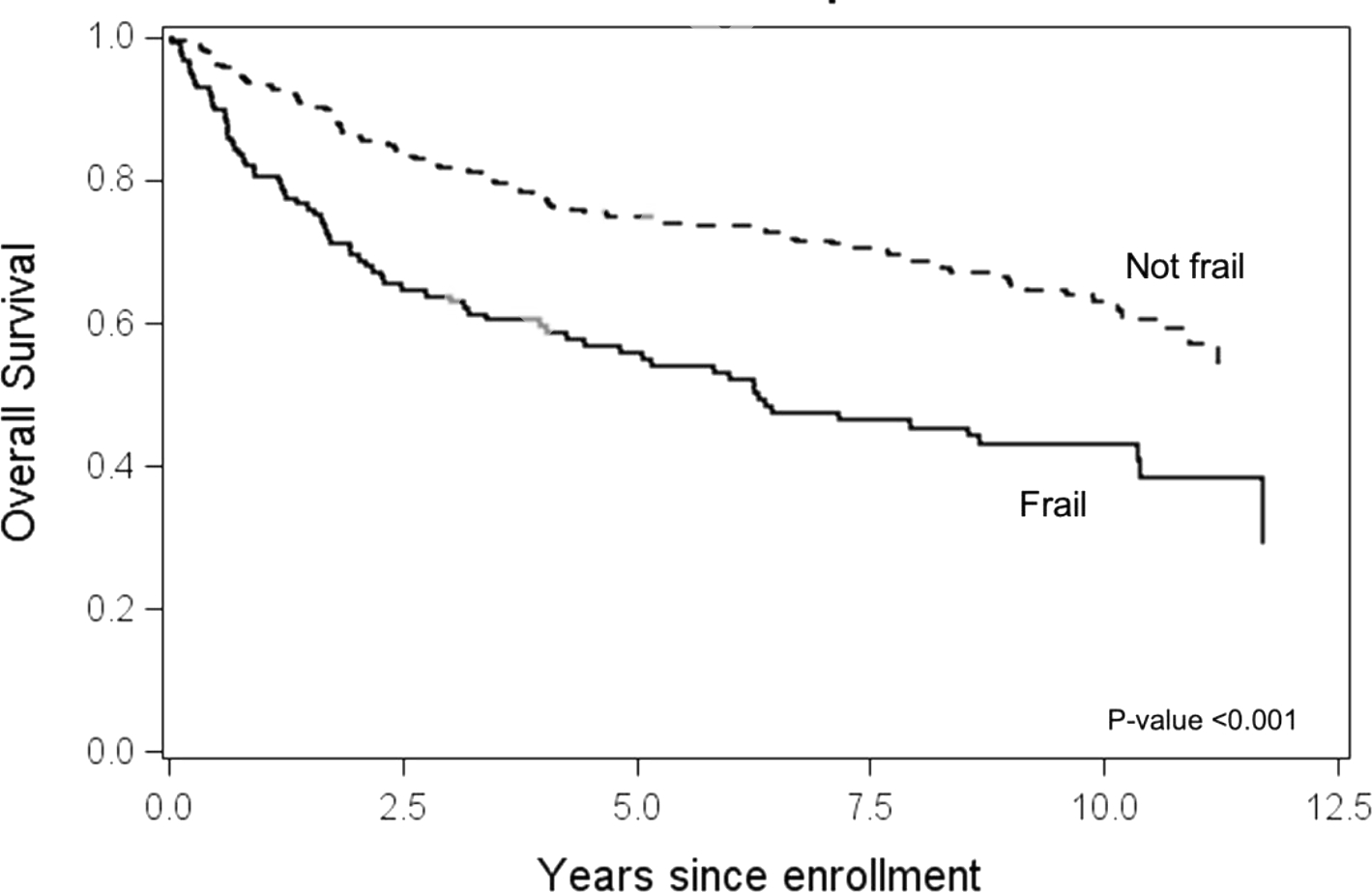
Overall survival from enrollment
Treatment effects
Corticosteroid treatment for cGVHD was not associated with frailty with 72% of frail patients and 66% of not frail patients on corticosteroids at enrollment (p=0.24). The maximum dosage of corticosteroids since diagnosis of cGVHD treatment was also not associated with frailty with 32% of frail patients and 26% of not frail patients having received a dose ≥0.5 mg/kg/day (p=0.22). Maximum corticosteroid dose prior to enrollment for treatment of cGVHD was a median of 0.24 mg/kg/day for frail patients and 0.18 mg/kg/day for not frail patients. The cumulative dose and duration of corticosteroid usage were not available.
Comorbidities
Frail patients had a trend towards higher rates of medical comorbidities prior to transplant, measured using the HCT-CI with a score of 3 (interquartile range 1–5) vs 2 (interquartile range 0–4) p=0.06. Table 1. Multivariable analyses adjusting for HCT-CI showed that frailty was still significantly associated with NRM (HR 2.16, 95% CI 1.47 – 3.19, p<0.001) and OS (HR 1.90, 95% CI 1.38 – 2.63, p<0.001).
Table 1.
Population Characteristics
| Characteristic | Total (n=399) | Not-frail (n=270) | Frail (n=129) | P-value1 |
|---|---|---|---|---|
| Age at enrollment (years) | 0.004 | |||
| 18–39 | 63 (15.8%) | 48 (17.8%) | 15 (11.6%) | |
| 40–55 | 178 (44.6%) | 130 (48.1%) | 48 (37.2%) | |
| >55 | 158 (39.6%) | 92 (34.1%) | 66 (51.2%) | |
| Sex | 0.72 | |||
| Female | 162 (40.6%) | 108 (40.0%) | 54 (41.9%) | |
| Male | 237 (59.4%) | 162 (60.0%) | 75 (58.1%) | |
| Race | 0.57 | |||
| Black or African American | 10 (2.5%) | 5 (1.9%) | 5 (3.9%) | |
| American Indian/Alaskan Native | 2 (0.5%) | 2 (0.7%) | 0 | |
| Asian | 19 (4.8%) | 14 (5.2%) | 5 (3.9%) | |
| Native Hawaiian or Other Pacific Islander | 2 (0.5%) | 2 (0.7%) | 0 | |
| White | 359 (90.0%) | 243 (90.0%) | 116 (89.9%) | |
| Multi-race | 4 (1.0%) | 3 (1.1%) | 1 (0.8%) | |
| Unknown | 3 (0.8%) | 1 (0.4%) | 2 (1.6%) | |
| Hispanic | 10 (2.5%) | 5 (1.9%) | 5 (3.9%) | 0.30 |
| Diagnosis | 0.19 | |||
| AML + MDS | 201 (50.4%) | 145 (53.7%) | 56 (43.4%) | |
| ALL | 40 (10.0%) | 28 (10.4%) | 12 (9.3%) | |
| Lymphoma | 77 (19.3%) | 50 (18.5%) | 27 (20.9%) | |
| Chronic Leukemia | 49 (12.3%) | 27 (10.0%) | 22 (17.1%) | |
| Other | 32 (8.0%) | 20 (7.4%) | 12 (9.3%) | |
| Disease status | 0.28 | |||
| Early | 125 (31.3%) | 90 (33.3%) | 35 (27.1%) | |
| Intermediate | 177 (44.4%) | 120 (44.4%) | 57 (44.2%) | |
| Advanced | 97 (24.3%) | 60 (22.2%) | 37 (28.7%) | |
| Myeloablative conditioning | 211 (53%) | 148 (55.0%) | 63 (48.8%) | 0.25 |
| Donor type | 0.35 | |||
| Matched related | 166 (41.6%) | 116 (43.0%) | 50 (38.8%) | |
| Matched unrelated | 165 (41.4%) | 113 (41.9%) | 52 (40.3%) | |
| Mismatched | 68 (17%) | 41 (15.2%) | 27 (20.9%) | |
| Graft type | 0.99 | |||
| Peripheral blood | 353 (88.5%) | 239 (88.5%) | 114 (88.4%) | |
| Bone marrow | 25 (6.3%) | 17 (6.3%) | 8 (6.2%) | |
| Umbilical cord | 21 (5.3%) | 14 (5.2%) | 7 (5.4%) | |
| Donor-recipient sex mismatch | 0.72 | |||
| Female donor to male recipient | 118 (30.1%) | 81 (30.7%) | 37 (28.9%) | |
| Other | 274 (69.9%) | 183 (69.3%) | 91 (71.1%) | |
| Prior grade 2–4 acute GVHD | 214 (53.6%) | 143 (53.0%) | 71 (55.0%) | 0.70 |
| Site | 0.51 | |||
| Fred Hutchinson Cancer Center | 163 (40.9%) | 115 (42.6%) | 48 (37.2%) | |
| University of Minnesota | 45 (11.3%) | 29 (10.7%) | 16 (12.4%) | |
| Dana-Farber Cancer Institute | 52 (13.0%) | 38 (14.1%) | 14 (10.9) | |
| Stanford University | 55 (13.8) | 39 (14.4%) | 16 (12.4%) | |
| Vanderbilt University | 33 (8.3%) | 19 (7.0%) | 14 (10.9%) | |
| Moffitt Cancer Center | 17 (4.3%) | 11 (4.1%) | 6 (4.7%) | |
| Medical College of Wisconsin | 23 (5.8%) | 14 (5.2%) | 9 (7.0%) | |
| Washington University | 3 (0.8%) | 2 (0.7%) | 1 (0.8%) | |
| Mayo Clinic | 8 (2.0%) | 3 (1.1%) | 5 (3.9%) | |
| Time from HCT to CGVHD, months | 0.52 | |||
| < 7.34 | 198 (49.6%) | 137 (50.7%) | 61 (47.3%) | |
| 7.34 + | 201 (50.4%) | 133 (49.3%) | 68 (52.7%) | |
| Time from HCT to enrollment, months | 0.78 | |||
| < 12.03 | 197 (49.4%) | 132 (48.9%) | 65 (50.4%) | |
| 12.03+ | 202 (50.6%) | 138 (51.1%) | 64 (49.6%) | |
| KPS at enrollment | <0.001 | |||
| Median (IQR) | 80 (70–90) | 90 (80–90) | 70 (60–80) | |
| HCT-CI at enrollment | ||||
| Median (IQR) | 2.0 (1–4) | 2.0 (0–4) | 3 (1–5) | 0.06 |
| CGVHD Characteristics | ||||
| CGVHD severity | <0.001 | |||
| None/mild | 67 (16.8%) | 54 (20.0%) | 13 (10.1%) | |
| Moderate | 215 (53.9%) | 153 (56.7%) | 62 (48.1%) | |
| Severe | 117 (29.3%) | 63 (23.3%) | 54 (41.9%) | |
| Thrombocytopenia (platelets <100,000/mm3) | 69 (17.4%) | 38 (14.1%) | 31 (24.2%) | 0.01 |
| CGVHD type | ||||
| Classic | 121 (30.3%) | 92 (34.1%) | 29 (22.5%) | 0.02 |
| Overlap | 278 (69.7%) | 178 (65.9%) | 100 (77.5%) | |
| Case type | 0.42 | |||
| Prevalent | 174 (43.6%) | 114 (42.2%) | 60 (46.5%) | |
| Incident | 225 (56.4%) | 156 (57.8%) | 69 (53.5%) | |
| Onset type | 0.61 | |||
| De novo | 132 (33.4%) | 91 (34.0%) | 41 (32.3%) | |
| Quiescent | 170 (43.0%) | 111 (41.4%) | 59 (46.5%) | |
| Progressive | 93 (23.5%) | 66 (24.6%) | 27 (21.3%) | |
| Missing | N=4 | N=2 | N=2 | |
| Skin | 0.07 | |||
| Score 1 | 83 (20.8%) | 62 (23.0%) | 21 (16.3%) | |
| Score 2 | 99 (24.8%) | 68 (25.2%) | 31 (24.0%) | |
| Score 3 | 69 (17.3%) | 38 (14.1%) | 31 (24.0%) | |
| Eye | 0.24 | |||
| Score 1 | 129 (32.3%) | 87 (32.2%) | 42 (32.6%) | |
| Score 2 | 60 (15.0%) | 42 (15.6%) | 18 (14.0%) | |
| Score 3 | 14 (3.5%) | 6 (2.2%) | 8 (6.2%) | |
| Mouth | 0.18 | |||
| Score 1 | 179 (44.9%) | 129 (47.8%) | 50 (38.8%) | |
| Score 2 | 48 (12.0%) | 29 (10.7%) | 19 (14.7%) | |
| Score 3 | 5 (1.3%) | 2 (0.7%) | 3 (2.3%) | |
| Lung | 0.002 | |||
| Score 1 | 72 (18.0%) | 39 (14.4%) | 33 (25.6%) | |
| Score 2 | 26 (6.5%) | 15 (5.6%) | 11 (8.5%) | |
| Score 3 | 2 (0.5%) | 0 | 2 (1.6%) | |
| GI | <0.001 | |||
| Score 1 | 97 (24.3%) | 61 (22.6%) | 36 (27.9%) | |
| Score 2 | 23 (5.8%) | 2 (0.7%) | 21 (16.3%) | |
| Score 3 | 1 (0.3%) | 0 | 1 (0.8%) | |
| Liver | 0.04 | |||
| Score 1 | 26 (6.5%) | 12 (4.4%) | 14 (10.9%) | |
| Score 2 | 47 (11.8%) | 31 (11.5%) | 16 (12.5%) | |
| Score 3 | 10 (2.5%) | 5 (1.9%) | 5 (3.9%) | |
| Joints | 0.18 | |||
| Score 1 | 81 (20.3%) | 58 (21.5%) | 23 (17.8%) | |
| Score 2 | 39 (9.8%) | 22 (8.1%) | 17 (13.2%) | |
| Score 3 | 5 (1.3%) | 2 (0.7%) | 3 (2.3%) | |
| Genital | 0.43 | |||
| Score 1 | 17 (4.6%) | 14 (5.6%) | 3 (2.5%) | |
| Score 2 | 11 (3.0%) | 6 (2.4%) | 5 (4.2%) | |
| Score 3 | 5 (1.4%) | 3 (1.2%) | 2 (1.7%) | |
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplant; cGVHD, chronic graft-versus-host disease; KPS, Karnofsky performance score; IQR, interquartile range; HCT-CI, hematopoietic cell transplant-comorbidity index; GI, gastrointestinal
based on the Chi-square test/Fisher’s exact test for categorical variables and the Wilcoxon ranks sums test for continuous variables.
Patient reported outcomes
Frail patients had significantly worse scores on both the physical component (mean 33 vs 43, p<0.001) and mental component (mean 44 vs 50, p<0.001) of the SF-36 compared to not frail patients, and the differences were clinically meaningful. Frail patients also had significantly worse scores for the Trial Outcome Index of the FACT-BMT with a clinically meaningful difference compared to not frail patients (mean 54 vs 70, p<0.001) and statistically worse scores on all LSS components except mouth symptoms (Table 3). The differences in the energy subscale and overall LSS were clinically meaning while the differences in the eye, lung, mouth, nutrition, psychiatric, and skin subscales were not.
Table 3.
Patient Reported Outcomes by Frailty
| Patient-reported outcome score | Total (N=399) | Not frail (n=270) | Frail (n=129) | P-value* |
|---|---|---|---|---|
| SF-36 physical component | ||||
| Mean (STD) | 39.9 (9.6) | 43.1 (8.4) | 33.0 (8.2) | <.001 |
| SF-36 mental component | ||||
| Mean (STD) | 48.2 (10.5) | 50.3 (9.9) | 43.5 (10.3) | <.001 |
| LSS energy | ||||
| Mean (STD) | 32.5 (21.3) | 26.5 (18.8) | 45.0 (20.8) | <.001 |
| LSS eye | ||||
| Mean (STD) | 33.5 (29.8) | 30.8 (27.7) | 39.3 (33.2) | 0.01 |
| LSS lung | ||||
| Mean (STD) | 7.4 (11.4) | 5.5 (9.1) | 11.4 (14.5) | <.001 |
| LSS mouth | ||||
| Mean (STD) | 19.3 (26.9) | 18.7 (25.4) | 20.8 (29.9) | 0.48 |
| LSS nutrition | ||||
| Mean (STD) | 8.4 (11.1) | 6.2 (8.9) | 12.9 (13.6) | <.001 |
| LSS psychological | ||||
| Mean (STD) | 24.4 (20.3) | 21.5 (19.1) | 30.6 (21.2) | <.001 |
| LSS skin | ||||
| Mean (STD) | 22.1 (21.0) | 19.0 (18.4) | 28.5 (24.5) | <.001 |
| LSS overall summary | ||||
| Mean (STD) | 21.1 (12.1) | 18.3 (10.8) | 27.0 (12.6) | <.001 |
| FACT trial outcome index | ||||
| Mean (STD) | 64.7 (15.2) | 70.0 (12.9) | 53.7 (13.8) | <.001 |
Abbreviations: SF-36, short form health survery-36; LSS, Lee Symptom Scale, FACT, functional assessment of cancer therapy
t-test
Discussion
Our study shows that nearly one-third of patients with cGVHD were frail whereas analyses in the general older adult population report a prevalence of frailty around 10%[1]. Among patients who have cGVHD, frailty remains strongly associated with worse OS and higher NRM, although frailty did not disproportionately worsen outcomes of patients with more severe cGVHD given there was no statistical interaction between frailty and cGVHD severity for these outcomes. Frailty was also significantly associated with worse PROs, with frail patients consistently having worse scores on multiple quality of life and symptom domains. One weakness of this study is that we only included patients who were prospectively enrolled, thus excluding some patients with cGVHD. Despite this, we enrolled a relatively large cohort of patients from 9 different centers to have a more comprehensive representation of the cGVHD population. Although this report used objective measures for slow walking speed and weakness, as used in Fried’s criteria, we did not perform the same assessments used in Fried’s original definition. Other retrospective studies in the HCT population have reported an association with frailty with outcomes [6, 8, 11, 12]; however, these analyses also use surrogate measures for the domains of frailty. Frailty should be prospectively studied using standard definitions to fully understand its effects on HCT outcomes.
Prior studies have shown that cGVHD is associated with frailty [12]. Although we hypothesized that long-standing cGVHD and prolonged exposure to corticosteroids would be associated with frailty, there was no difference in the rates of frailty among patients with incident versus prevalent cGVHD, longer duration of cGVHD, or in patients exposed to a higher corticosteroid dose at enrollment or previously. A longitudinal study also using data from the Chronic GVHD Consortium showed the NRM at 5 years after enrollment was 38% and continued to increase with time [26]. Thus, one explanation for the lack of association with duration of cGVHD could be that frail patients with prevalent cGVHD may have died prior to enrollment. The lack of association with corticosteroid treatment was surprising since corticosteroids can lead to loss of muscle mass and weakness, which helps define frailty [27]. However, providers may have limited corticosteroid usage in patients who appeared frail to avoid further weakness and additional side effects. A limitation of this study is that we did not capture total corticosteroid exposure, since the duration of corticosteroid usage greatly impacts its side effects[28].
These data showed that age >55 was associated with frailty. Studies in the general population have shown the association of older age with frailty [2, 29]. A prior study evaluating frailty in HCT patients did not find an association of older age with frailty [11]; however, this study only included patients who were alive and able to answer a questionnaire ≥15 years post-HCT. Given the findings in our study and past studies showing the association of age in frailty, older age should be considered an important risk factor for frailty. We also found that increased cGVHD severity, lung or liver cGVHD, and thrombocytopenia were associated with frailty. Lung cGVHD causes dyspnea, which can impact several markers of frailty such as slow walking speed, exhaustion, and low physical activity. Some forms of liver diseases have been associated with significant fatigue and decreased exercise capacity [30, 31], which may explain why liver cGVHD was associated with frailty. Thrombocytopenia in patients with cGVHD has previously been associated with poor outcomes and increased severity of cGVHD [32]. Being attentive to these features may help with earlier clinical recognition of frailty in the cGVHD population.
The diagnosis of frailty is made from evaluating 5 different domains as previously described. A multivariate analysis was performed to see if certain components of frailty were associated with outcomes and several were associated with higher NRM and worse OS, suggesting the physical functioning domains significantly predict outcomes as has been previously reported [8, 10, 33].
Studies evaluating frailty in older patients describe that frailty alone can lead to an increased risk of poor outcomes, despite patients having either no or mild comorbidities[2, 5]. This study showed similar findings as frail patients had higher NRM and worse OS after adjusting for comorbidities. Physical therapy programs and adequate nutritional support appear to have some benefit on physical function, strength, and maintaining independence [34–36]. Standardized assessments, such as the comprehensive geriatric assessment, can help identify frailty and allow for interventions such as prescribing assistive devices or referring patients to geriatric clinics [37, 38]. The use of these tools has been associated with decreased hospital admissions and emergency room visits [39].
Although frailty is now well described in the medical literature, there have been very few trials evaluating different interventions to reverse or slow the development of frailty. A few randomized cinical trials have evaluated home-based exercise regimens using a combination of strength, balance and aerobic exercises in healthy adults, with the intervention group demonstrating a significant reduction in rates of frailty [40, 41]. This study shows a high prevalence of frailty in the cGVHD population and significantly worse outcomes in frail patients with cGVHD. Upon recognition of frailty, providers can refer patients to physical therapy and provide education about balanced exercise regimens given the data supporting use of these interventions for frailty. Additionally, providers should minimize steroid usage if possible. Ideally, interventions to counteract frailty should be designed and tested in randomized controlled trials.
Supplementary Material
Highlights.
Among patients enrolled in the cGVHD consortium, 32% met criteria for frailty
Frail patients had 10-year NRM of 41% compared to 22% in not frail patients
Frail patients scored significantly worse on SF-36, Lee Symptom Scale, and FACT-BMT
Funding:
S.J.L (CA118953, CA236229), N.R. (T32HL007093)
Footnotes
Conflicts of Interest: The authors do not have any conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collard RM, et al. , Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc, 2012. 60(8): p. 1487–92. [DOI] [PubMed] [Google Scholar]
- 2.Clegg A, et al. , Frailty in elderly people. Lancet, 2013. 381(9868): p. 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel GA and Klepin HD, Frailty and the management of hematologic malignancies. Blood, 2018. 131(5): p. 515–524. [DOI] [PubMed] [Google Scholar]
- 4.Buckstein R, et al. , Patient-related factors independently impact overall survival in patients with myelodysplastic syndromes: an MDS-CAN prospective study. Br J Haematol, 2016. 174(1): p. 88–101. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, et al. , Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci, 2001. 56(3): p. M146–56. [DOI] [PubMed] [Google Scholar]
- 6.Pamukcuoglu M, et al. , Hematopoietic Cell Transplant-Related Toxicities and Mortality in Frail Recipients. Biol Blood Marrow Transplant, 2019. 25(12): p. 2454–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockwood K, et al. , A global clinical measure of fitness and frailty in elderly people. CMAJ, 2005. 173(5): p. 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salas MQ, et al. , Pilot prospective study of Frailty and Functionality in routine clinical assessment in allogeneic hematopoietic cell transplantation. Bone Marrow Transplant, 2021. 56(1): p. 60–69. [DOI] [PubMed] [Google Scholar]
- 9.Aaronson NK, et al. , The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst, 1993. 85(5): p. 365–76. [DOI] [PubMed] [Google Scholar]
- 10.Deschler B, et al. , Geriatric assessment and quality of life in older patients considered for allogeneic hematopoietic cell transplantation: a prospective risk factor and serial assessment analysis. Bone Marrow Transplant, 2018. 53(5): p. 565–575. [DOI] [PubMed] [Google Scholar]
- 11.Arora M, et al. , Longitudinal trajectory of frailty in blood or marrow transplant survivors: Report from the Blood or Marrow Transplant Survivor Study. Cancer, 2021. 127(5): p. 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora M, et al. , Physiologic Frailty in Nonelderly Hematopoietic Cell Transplantation Patients: Results From the Bone Marrow Transplant Survivor Study. JAMA Oncol, 2016. 2(10): p. 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armenian SH, et al. , Health behaviors and cancer screening practices in long-term survivors of hematopoietic cell transplantation (HCT): a report from the BMT Survivor Study. Bone Marrow Transplant, 2012. 47(2): p. 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SW, et al. , Development and validation of a new technique for assessing lumbar spine motion. Spine (Phila Pa 1976), 2002. 27(8): p. E215–20. [DOI] [PubMed] [Google Scholar]
- 15.Bohannon RW, Normative reference values for the two-minute walk test derived by meta-analysis. J Phys Ther Sci, 2017. 29(12): p. 2224–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boxer R, et al. , The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail, 2010. 16(5): p. 208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzberg PY, et al. , Validation of the human activity profile questionnaire in patients after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant, 2010. 16(12): p. 1707–17. [DOI] [PubMed] [Google Scholar]
- 18.Viana JU, et al. , Influence of sarcopenia and functionality indicators on the frailty profile of community-dwelling elderly subjects: a cross-sectional study. Braz J Phys Ther, 2013. 17(4): p. 373–81. [DOI] [PubMed] [Google Scholar]
- 19.Jagasia MH, et al. , National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant, 2015. 21(3): p. 389–401 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE Jr., SF-36 health survey update. Spine (Phila Pa 1976), 2000. 25(24): p. 3130–9. [DOI] [PubMed] [Google Scholar]
- 21.Cella DF, et al. , The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol, 1993. 11(3): p. 570–9. [DOI] [PubMed] [Google Scholar]
- 22.Ogura K, et al. , What Are the Minimum Clinically Important Differences in SF-36 Scores in Patients with Orthopaedic Oncologic Conditions? Clin Orthop Relat Res, 2020. 478(9): p. 2148–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McQuellon RP, et al. , Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant, 1997. 19(4): p. 357–68. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, et al. , Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant, 2002. 8(8): p. 444–52. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, et al. , Correlation of Patient-Reported Outcomes with Clinical Organ Responses: Data from the Belumosudil Chronic Graft-versus-Host Disease Studies. Transplant Cell Ther, 2022. 28(10): p. 700 e1–700 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFilipp Z, et al. , Nonrelapse mortality among patients diagnosed with chronic GVHD: an updated analysis from the Chronic GVHD Consortium. Blood Adv, 2021. 5(20): p. 4278–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clegg A and Hassan-Smith Z, Frailty and the endocrine system. Lancet Diabetes Endocrinol, 2018. 6(9): p. 743–752. [DOI] [PubMed] [Google Scholar]
- 28.Gensler LS, Glucocorticoids: complications to anticipate and prevent. Neurohospitalist, 2013. 3(2): p. 92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Manas L, et al. , Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci, 2013. 68(1): p. 62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carbone M, et al. , Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology, 2013. 144(3): p. 560–569 e7; quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 31.Hollingsworth KG, et al. , Loss of capacity to recover from acidosis in repeat exercise is strongly associated with fatigue in primary biliary cirrhosis. J Hepatol, 2010. 53(1): p. 155–61. [DOI] [PubMed] [Google Scholar]
- 32.Bat T, et al. , Active thrombopoiesis is associated with worse severity and activity of chronic GVHD. Bone Marrow Transplant, 2013. 48(12): p. 1569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muffly LS, et al. , Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica, 2014. 99(8): p. 1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gine-Garriga M, et al. , Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch Phys Med Rehabil, 2014. 95(4): p. 753–769 e3. [DOI] [PubMed] [Google Scholar]
- 35.Apostolo J, et al. , Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database System Rev Implement Rep, 2018. 16(1): p. 140–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dent E, et al. , Management of frailty: opportunities, challenges, and future directions. Lancet, 2019. 394(10206): p. 1376–1386. [DOI] [PubMed] [Google Scholar]
- 37.Preston L, et al. , in What evidence is there for the identification and management of frail older people in the emergency department? A systematic mapping review. 2018: Southampton (UK). [PubMed] [Google Scholar]
- 38.Ambagtsheer RC, et al. , Should we screen for frailty in primary care settings? A fresh perspective on the frailty evidence base: A narrative review. Prev Med, 2019. 119: p. 63–69. [DOI] [PubMed] [Google Scholar]
- 39.Foo CL, et al. , Geriatric assessment and intervention in an emergency department observation unit reduced re-attendance and hospitalisation rates. Australas J Ageing, 2012. 31(1): p. 40–6. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh TJ, et al. , Individualized home-based exercise and nutrition interventions improve frailty in older adults: a randomized controlled trial. Int J Behav Nutr Phys Act, 2019. 16(1): p. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serra-Prat M, et al. , Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing, 2017. 46(3): p. 401–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


