Abstract
Purpose
The aim of this study was to determine the relationship between morphological parameters and the incidence of de novo chromosomal abnormalities.
Methods
This was a retrospective cohort study of 652 patients who underwent 921 cycles with 3238 blastocysts biopsied. The embryo grades were evaluated according to Gardner and Schoolcraft’s system. The incidence of euploidy, whole chromosomal aneuploidy (W-aneuploidy), segmental chromosomal aneuploidy (S-aneuploidy), and mosaicism in trophectoderm (TE) cell biopsies was analyzed.
Results
The euploidy decreased significantly with maternal age and was positively correlated biopsy day and morphological parameters. The W-aneuploidy increased significantly with maternal age and was negatively correlated biopsy day and morphological parameters. Parental age, TE biopsy day, and morphological parameters were not associated with S-aneuploidy and mosaicism, except that TE grade C blastocysts had significantly higher mosaicism than TE grade A blastocysts. Subanalysis in different female age groups showed that euploidy and W-aneuploidy had a significant correlation with TE biopsy day among women aged ≤ 30 y and 31–35 y, with expansion degree among women aged ≥ 36 y, with ICM grade among women aged ≥ 31 y, and with TE grade among all female age ranges.
Conclusion
Female age, embryo developmental speed and blastocyst morphological parameters are associated with euploidy and whole chromosomal aneuploidy. The predictive value of these factors varies across female age groups. Parental age, embryo developmental speed, expansion degree, and ICM grade are not associated with the incidence of segmental aneuploidy or mosaicism, but TE grade seemingly has a weak correlation with segmental aneuploidy and mosaicism in embryos.
Keywords: Embryonic developmental competence, Blastocyst morphology, Whole chromosomal aneuploidy, Segmental chromosomal aneuploidy, Mosaicism, PGT-M
Introduction
Blastocyst culture and transfer have been increasingly used in assisted reproductive technology (ART). This approach has several theoretical advantages, such as the opportunity to potentially select the most viable embryo(s) for transfer, better temporal synchronization between the embryo and endometrium at the time of embryo transfer, higher implantation and live-birth rates than those with cleavage-stage embryo transfer, and potential decreases in multiple pregnancy rates and the risks of maternal and fetal morbidity by single blastocyst transfer[1–3].
Embryo quality is traditionally evaluated based on morphological characteristics. The blastocyst morphology grade system introduced by Gardner and Schoolcraft is the most accepted and widely used to select blastocysts in IVF. The system is based on the degree of blastocyst expansion and the morphology of the inner cell mass (ICM) and trophectoderm (TE) cells [4, 5]. Some studies have reported that blastocyst morphology parameters are significantly associated with the implantation rate, clinical pregnancy rate, ongoing pregnancy rate, and live birth rate[6–10]. Additionally, the blastocyst development rate affects clinical outcomes, evidenced by the fact that day-5 blastocysts have significantly higher implantation, clinical pregnancy, and live birth rates than day-6 blastocysts[11, 12].
Chromosomal abnormalities in preimplantation embryos are common[13]. In many cases, the presence of aneuploidy is proposed to be the underlying cause of embryonic arrest, implantation failure, and spontaneous pregnancy loss in natural pregnancy and ART. Approximately 50–70% of all miscarriages are due to aneuploidy, and eliminating aneuploid embryo transfer could significantly reduce the rate of miscarriage after IVF[14]. Selection of single euploid blastocysts for transfer is more likely to result in implantation and development into a healthy pregnancy, avoiding multiple pregnancies and related complications[15, 16]. Previous studies reporting cytogenetic data from oocytes and embryos focused primarily on the incidence of whole chromosome abnormalities. However, segmental aneuploidy, losses or gains of chromosomal fragments, also occur at appreciable frequencies in preimplantation embryos[17, 18]. The frequency of de novo segmental aneuploidies in blastocysts detected by TE biopsies is approximately 5–12%[19–21]. Mosaicism, a mixture of cells with different karyotypes, is another abnormality found in the preimplantation embryo[22, 23]. Although the correlation of blastocyst morphology parameters with ploidy and clinical outcomes has been previously reported, to our best knowledge, no work has been reported to identify the relationships of blastocyst development, expansion, ICM grade, and TE grade with the incidence of whole chromosomal aneuploidies, segmental chromosomal aneuploidies, and mosaicism detected in TE biopsies at the same time.
Preimplantation genetic testing for aneuploidy (PGT-A) is an embryo selection technique used for women of advanced maternal age, those with recurrent pregnancy loss, and patients with recurrent IVF failure or an abnormal chromosome karyogram[24]. The application of PGT to monogenic disorders (PGT-M) represents several important advances, including parallel analysis of mutation-linked markers and prevention and detection of DNA contamination, which helps avoid misdiagnosis and improve accuracy[22]. Monogenic disorders are caused by pathogenic variations in a single gene, which theoretically would not affect the frequency of aneuploidy in embryos in most cases.
To further investigate the association between blastocyst chromosomal abnormalities and morphological characteristics, we conducted a retrospective cohort study with a large sample size in a single center to analyze the correlations of maternal and paternal age, developmental competence of embryos (biopsy days), blastocyst expansion degree, ICM grade, and TE grade with euploidy and the incidences of whole chromosomal aneuploidy, segmental chromosomal aneuploidy, and mosaicism from TE biopsy at the blastocyst stage.
Materials and methods
Patients and study design
The data were collected from May 2015 to July 2022 at the Center for Reproductive Medicine. We enrolled 652 couples carrying monogenic diseases who sought fertility treatment for PGT-M in this retrospective cohort study. The average female age was 31.8 years (range 22–43 years). A total of 921 cycles were performed. Patients with chromosome translocation or Y-chromosome microdeletion were excluded. Cycles, in which no blastocysts were obtained, or blastocyst grading was not recorded, were also excluded.
Clinical protocol
Assisted reproductive methodologies, including controlled ovarian hyperstimulation, oocyte maturation trigger, and oocyte retrieval, were carried out according to standard protocols used at the Center for Reproductive Medicine. Briefly, controlled ovarian stimulation was applied using the GnRH antagonist protocol, oocyte maturation was triggered through hCG administration, and ultrasound-guided ovum retrieval was performed approximately 36 h after hCG injection. Mature oocytes at the MII stage were fertilized by intracytoplasmic sperm injection (ICSI) on the day of egg retrieval, and embryos were cultured for 5–7 days in vitro.
Blastocyst morphological classification and trophectoderm biopsies
Morphological assessments were conducted on the day of TE biopsy (day 5, day 6, or day 7). Blastocysts were graded according to three separate quality scores: the degree of expansion (1–6), the grade of ICM (A, B, C), and the grade of TE (A, B, C) according to Gardner and Schoolcraft’s system[4].
All blastocysts for PGT were artificially hatched on day 3 after fertilization. TE biopsy was performed by laser-assisted methodologies on embryos with expansion grades 4 or above on days 5, 6, or 7. Four to six TE cells were gently aspirated with a biopsy pipette from the trophectoderm of each blastocyst. The biopsied TE cells were washed in sterile phosphate-buffered saline (PBS) solution and then kept in a 0.2-mL polymerase chain reaction (PCR) tube with 5 μl of lysis buffer. After TE biopsy, the blastocysts were cryopreserved by vitrification.
Preimplantation genetic testing and definition
The whole genome of the biopsied TE cells was amplified with a multiple annealing and looping-based amplification cycle (MALBAC) Single-Cell WGA Kit (Yikon Genomics Inc., China) and analyzed using next-generation sequencing (NGS) as described previously[25]. Blastocysts analyzed by TE cell biopsy were classified as euploid, aneuploid or mosaic. All aneuploidies were further classified as whole-chromosome aneuploidy (W-aneuploidy) or segmental-chromosome aneuploidy (S-aneuploidy). In the data analysis, blastocysts with uniform single or multiple whole-chromosome aneuploidies were classified as W-aneuploid blastocysts; blastocysts with one or more segmental aneuploidies were classified as S-aneuploid blastocysts, and blastocysts with at least one mosaic chromosome abnormality were classified as mosaicism blastocysts.
Statistical analysis
All data analyses were performed using IBM SPSS Statistics software version 23.0. The continued data are presented as the mean and standard deviation (SD). The categorical variables are presented as counts (percentages) and were analyzed by a chi-squared test. In the multivariate logistic regression analysis, female age, male age, biopsy day, and blastocyst morphological parameters (expansion degree, ICM grade, and TE grade) were included, and the data are reported as odds ratios (ORs) and 95% confidence intervals (95% CIs). P < 0.05 was considered statistically significant.
Results
Baseline characteristics and description
A general overview of the study population and embryos is presented in Table 1.
Table 1.
Characteristics of the study population and embryos
| Characteristic | Description |
|---|---|
| Number of couples, n | 652 |
| Number of cycles, n | 921 |
| Mean female age, (years, ± SD) | 31.8 ± 4.0 |
| Mean male age, (years, ± SD) | 33.2 ± 4.7 |
| Number of retrieved oocytes, n | 13979 |
| Mature oocytes, n (%) | 10969 (78.5%) |
| Number of cleavage embryos, n (%) | 8102 (73.9%) |
| Blastocysts obtained, n (%) | 3238 (40.0%) |
| Day of biopsy, n (%) | |
| Day 5 | 710 (21.9%) |
| Day 6 | 2462 (76.0%) |
| Day 7 | 66 (2.1%) |
| Blastocyst expansion, n (%) | |
| 4 | 83 (2.6%) |
| 5 | 2523 (77.9%) |
| 6 | 632 (19.5%) |
| ICM grade, n (%) | |
| A | 446 (13.8%) |
| B | 2604 (80.4%) |
| C | 188 (5.8%) |
| TE grade, n (%) | |
| A | 424 (13.1%) |
| B | 1960 (60.5%) |
| C | 854 (26.4%) |
| Blastocysts successfully analyzed (n, %) | 3149 (97.3%) |
Continuous variables are expressed as median ± standard deviation (SD) and categorical values are presented as number (percentages). ICM, inner cell mass, TE, trophectoderm
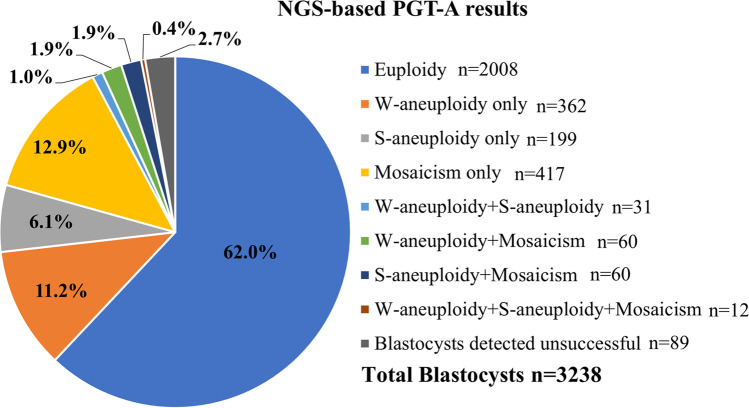
A total of 921 cycles, including 3238 blastocysts from 652 patients, were included in this study. The average age of the female patients was 31.8 ± 4.0 years (range 22–43 years), and the average age of the male patients was 33.2 ± 4.7 years (range 22–56 years). The total number of retrieved oocytes was 13,979, including 10,969 (78.5%) mature (metaphase II, MII) oocytes. The number of cleavage-stage embryos was 8102 (73.9%). A total of 3238 (40.0%) blastocysts were obtained, including 710 (21.9%) blastocysts on day 5, 2462 (76.0%) blastocysts on day 6, and 66 (2.1%) blastocysts on day 7. Among these blastocysts, more than 97% (3149/3238) of NGS-based TE biopsies were successfully analyzed. Figure 1 shows the results of genetic testing for aneuploidies. In all tested blastocysts, 62.0% (2008/3238) of blastocysts were euploid. The percentage of blastocysts with at least one whole aneuploidy was 14.4% (465/3238), and that with at least one segmental aneuploidy was 9.3% (302/3238); the percentage of blastocysts carrying mosaicism was 17.0% (549/3238). Eighty-nine blastocysts were tested unsuccessful (Fig. 1). Among the successfully analyzed TE biopsies, The percentage of blastocysts with euploidy, whole aneuploidy, segmental aneuploidy, and mosaicism was 63.8% (2008/3149), 14.8% (465/3149), 9.6% (302/3149), and 17.4% (549/3149) respectively.
Fig. 1.
Summary of NGS-based PGT-A results in all biopsied blastocysts
Main analysis
The number of euploid blastocysts, W-aneuploid blastocysts, S-aneuploid blastocysts, and mosaicism blastocysts are summarized in terms of biopsy day, degree of expansion, ICM grade, and TE grade in Table 2. Analysis by chi-squared test showed that the embryo development speed (biopsy day), expansion degree, and ICM grade were significantly associated with euploidy and W-aneuploidy but not with the incidence of S-aneuploidy or mosaicism in blastocysts. Among the blastocysts, the rates of euploidy were significantly higher, and the rates of W-aneuploidy were significantly lower on biopsy day 5, at expansion degree 6, and at a higher ICM grade. Among the TE grades (A, B, C), there were significant differences in euploidy, W-aneuploidy, S-aneuploidy, and mosaicism. TE grade C tended to result in a lower proportion of euploidy and higher proportions of W-aneuploidy, S-aneuploidy, and mosaicism (Table 2).
Table 2.
Incidence of euploidy and chromosomal abnormalities at different TE biopsy days and blastocyst morphological parameters
| Variables | Euploid blastocysts n (%) | P value | W-aneuploid blastocysts n (%) | P value | S-aneuploid blastocysts n (%) | P value | Mosaic blastocysts n (%) | P value |
|---|---|---|---|---|---|---|---|---|
| Biopsy day | ||||||||
| Day 5 (n = 684) | 487 (71.2%) | < 0.001 | 62 (9.1%) | < 0.001 | 57 (8.3%) | 0.380 | 107 (15.6%) | 0.265 |
| Day 6 (n = 2401) | 1486 (61.9%) | 390 (16.2%) | 240 (10.0%) | 428 (17.8%) | ||||
| Day 7 (n = 64) | 35 (54.7%) | 13 (20.3%) | 5 (7.8%) | 14 (21.9%) | ||||
| Expansion degree | ||||||||
| 4 (n = 82) | 43 (52.4%) | 0.001 | 17 (20.7%) | 0.011 | 9 (11.0%) | 0.065 | 16 (19.5%) | 0.718 |
| 5 (n = 2449) | 1536 (62.7%) | 378 (15.4%) | 249 (10.2%) | 431 (17.6%) | ||||
| 6 (n = 618) | 429 (69.4%) | 70 (11.3%) | 44 (7.1%) | 102 (16.5%) | ||||
| ICM grade | ||||||||
| A (n = 437) | 319 (73.0%) | < 0.001 | 36 (8.2%) | < 0.001 | 36 (8.2%) | 0.571 | 65 (14.9%) | 0.207 |
| B (n = 2530) | 1583 (62.6%) | 390 (15.4%) | 249 (9.8%) | 447 (17.7%) | ||||
| C (n = 182) | 106 (58.2%) | 39 (21.4%) | 17 (9.3%) | 37 (20.3%) | ||||
| TE grade | ||||||||
| A (n = 414) | 303 (73.2%) | < 0.001 | 37 (8.9%) | < 0.001 | 34 (8.2%) | 0.024 | 56 (13.5%) | 0.009 |
| B (n = 1908) | 1269 (66.5%) | 242 (12.7%) | 169 (8.9%) | 325 (17.0%) | ||||
| C (n = 827) | 436 (52.7%) | 186 (22.5%) | 99 (12.0%) | 168 (20.3%) | ||||
Values are presented as numbers (percentages), W-aneuploidy, whole chromosomal aneuploidy, S-aneuploidy, segmental chromosomal aneuploidy, ICM, inner cell mass, TE, trophectoderm
Logistic regression analysis was conducted to evaluate the contributions of female age, male age, biopsy day, degree of expansion, ICM grade, and TE grade as predictors of chromosome abnormalities. Male age had no significant correlation with euploidy or chromosomal abnormalities in blastocysts. The proportion of euploid blastocysts decreased and W-aneuploid blastocysts increased significantly with advancing maternal age (P < 0.001, OR 0.94, 95% CI 0.92–0.97; P < 0.001, OR 1.14, 95% CI 1.10–1.19, respectively). The proportion of euploid blastocysts was significantly lower among blastocysts biopsied on day 6 or 7 than among those biopsied on day 5 (P < 0.001, OR 0.69, 95% CI 0.56–0.84; P = 0.032, OR 0.55, 95% CI0.32–0.95, respectively). ICM grade C blastocysts had significantly lower euploidy (P = 0.016, OR 0.63, 95% CI 0.43–0.92) and significantly higher W-aneuploidy (P = 0.001, OR 2.37, 95% CI 1.41–4.01) than ICM grade A blastocysts. TE grade B and TE grade C blastocysts had significantly lower euploidy than TE grade A blastocysts (P = 0.038, OR 0.77, 95% CI 0.60–0.99; P < 0.001, OR 0.47, 95% CI 0.36–0.63, respectively), and TE grade C blastocysts had significantly higher W-aneuploidy than TE grade A blastocysts (P < 0.001, OR 2.42, 95% CI 1.60–3.64). However, female age, day of TE biopsy, degree of expansion, ICM grade, and TE grade were not associated with the proportions of S-aneuploidy and mosaicism in blastocysts, except that TE grade C blastocysts had significantly higher mosaicism than TE grade A blastocysts (P = 0.013, OR 1.57, 95% CI 1.10–2.23) (Table 3).
Table 3.
Logistic regression analysis to evaluate the contributions of female age, male age, biopsy day, and morphological parameters as predictors of chromosomal abnormalities in biopsied blastocysts
| Variables | Euploid blastocysts | W-aneuploid blastocysts | S-aneuploid blastocysts | Mosaic blastocysts | ||||
|---|---|---|---|---|---|---|---|---|
| P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | |
| Female age | < 0.001 | 0.94 (0.92–0.97) | < 0.001 | 1.14 (1.10–1.19) | 0.191 | 1.03 (0.98–1.09) | 0.697 | 0.99 (0.96–1.03) |
| Male age | 0.198 | 0.99 (0.96–1.01) | 0.316 | 0.99 (0.96–1.05) | 0.224 | 0.98 (0.94–1.02) | 0.451 | 1.01 (0.98–1.04) |
| Biopsy day | ||||||||
| Day 5 | 0.001 | Ref | 0.001 | Ref | 0.308 | Ref | 0.431 | Ref |
| Day 6 | < 0.001 | 0.69 (0.56–0.84) | < 0.001 | 1.73 (1.28–2.34) | 0.146 | 1.27 (0.92–1.74) | 0.280 | 1.15 (0.90–1.47) |
| Day 7 | 0.032 | 0.55 (0.32–0.95) | 0.058 | 1.97 (0.98–3.97) | 0.960 | 0.98(0.37–2.57) | 0.297 | 1.41 (0.74–2.68) |
| Expansion degree | ||||||||
| 4 | 0.001 | Ref | 0.016 | Ref | 0.061 | Ref | 0.716 | Ref |
| 5 | 0.310 | 1.27 (0.80–1.99) | 0.750 | 0.91 (0.52–1.61) | 0.962 | 1.02 (0.50–2.07) | 0.823 | 0.94 (0.54–1.64) |
| 6 | 0.017 | 1.80 (1.11–2.92) | 0.111 | 0.60 (0.33–1.12) | 0.312 | 0.67 (0.31–1.45) | 0.587 | 0.85 (0.47–1.54) |
| ICM grade | ||||||||
| A | 0.051 | Ref | 0.005 | Ref | 0.940 | Ref | 0.543 | Ref |
| B | 0.100 | 0.81 (0.64–1.04) | 0.073 | 1.43 (0.97–2.10) | 0.759 | 1.06 (0.72–1.58) | 0.763 | 1.05 (0.77–1.42) |
| C | 0.016 | 0.63 (0.43–0.92) | 0.001 | 2.37 (1.41–4.01) | 0.756 | 1.11 (0.59–2.07) | 0.294 | 1.28 (0.81–2.04) |
| TE grade | ||||||||
| A | < 0.001 | Ref | < 0.001 | Ref | 0.129 | Ref | 0.038 | Ref |
| B | 0.038 | 0.77(0.60–0.99) | 0.155 | 1.32 (0.90–1.95) | 0.684 | 1.09 (0.73–1.63) | 0.120 | 1.29 (0.94–1.78) |
| C | < 0.001 | 0.47 (0.36–0.63) | < 0.001 | 2.42 (1.60–3.64) | 0.119 | 1.42 (0.91–2.21) | 0.013 | 1.57 (1.10–2.23) |
OR, odds ratio, CI, confidence interval, W-aneuploidy, whole chromosomal aneuploidy, S-aneuploidy, segmental chromosomal aneuploidy, ICM, inner cell mass, TE, trophectoderm
Subanalysis
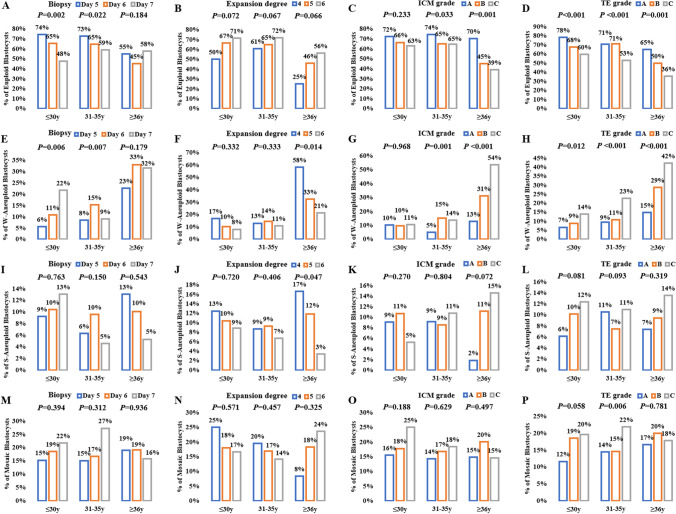
For further subanalysis, data were divided into three groups according to female age (≤ 30 y, 31–35 y, ≥ 36 y), and the results are summarized in Fig. 2. The correlation of embryo development speed and morphologic parameters with euploidy and chromosomal abnormalities appeared to vary by female age. The day of TE biopsy had significant effects on euploidy and W-aneuploidy in the groups of women aged ≤ 30 y and 31–35 y, while expansion degree did not affect euploidy or W-aneuploidy in these age groups. In the group of women aged ≥ 36 y, W-aneuploidy increased significantly in blastocysts with expansion degree 4 compared to those with expansion degrees 5 and 6. The ICM grade was significantly positively correlated with euploidy and significantly negatively correlated with W-aneuploidy in the groups of women aged 31–35 y and ≥ 36 y but not in the group of women aged ≤ 30 y. TE grade was significantly positively correlated with euploidy and significantly negatively correlated with W-aneuploidy in all female age groups. However, S-aneuploidy and mosaicism had no correlation with the biopsy day, expansion degree, ICM grade, or TE grade in any age group, except that the expansion degree was significantly negatively correlated with S-aneuploidy in women aged ≥ 36 y (P = 0.047) and the mosaicism rate was significantly higher in TE grade C than in TE grades A and B among women aged 31–35 y (P = 0.006), though this may be due to the small sample sizes in some subgroups (Fig. 2).
Fig. 2.
The chromosomal abnormalities at different biopsy days, expansion degrees, ICM grades, and TE grades for different female ages. (A ~ D) Incidence of euploidies (E ~ H) Incidence of W-aneuploidies. (I ~ L) Incidence of S-aneuploidies. (M ~ P) Incidence of mosaicism
Discussion
In this study, we demonstrated that male age had no significant correlation with euploidy or chromosomal abnormalities in blastocysts. We found that female age, biopsy day, blastocoel expansion degree, ICM grade, and TE grade were associated with euploidy and W-aneuploidy, and these factors were not associated with S-aneuploidy or mosaicism except that TE grade seemingly has a weak correlation with segmental aneuploidy and mosaicism, in blastocysts detected by TE cells. The contribution of these factors to euploidy and W-aneuploidy varies by female age.
The subjects included in this study were patients who underwent treatment for PGT-M in a single reproductive center. This has several significant strengths. First, the therapeutic methods and techniques used in the clinic and laboratory are relatively unified. Second, compared to most previous studies[26–30], the subjects in this study had a low mean maternal age (31.8 ± 4.0 years) and a large age range (22–43 years), making them more representative of infertile people. Last, some factors for embryonic chromosomal abnormalities, such as parental chromosome structural rearrangement, recurrent miscarriage, and repeated IVF failure, were excluded. Thus, the data of this study can more directly reflect the relationship between blastocyst morphology and de novo chromosomal abnormalities.
Aneuploidy in embryos is the main reason for implantation failure and abortion in IVF cycles and increases with advancing maternal age[31, 32]. Our data confirm this finding: euploidy was significantly lower and W-aneuploidy was significantly higher among women of the advanced age than among young women, and logistic regression analysis showed that the aneuploidy rate increased by ~ 14% per year of female age, which is consistent with the previous studies[13, 33]. Franasiak et al. reported that the rate of aneuploidy in women aged ≤ 23 years is 40%, and that in women aged 26–30 years is the lowest, at approximately 20%-27%. The incidence of aneuploidy increases gradually from 31 to 43 years of age, reaching a high plateau of approximately 85%[13]. Study by Nair et al. showed the incidence of aneuploidy rate was ~ 28% at maternal age < 30 years which steadily increased to ~ 67% in women above 40 years [33]. However, male age had no effect on the euploidy and chromosome abnormalities of embryos. In laboratories, morphological evaluation has been the main strategy applied to choose the embryos to transfer. Most reports demonstrate that the implantation, clinical pregnancy and live birth rates in the day-5 blastocyst group are significantly higher than those in the day-6 blastocyst group[12, 26, 34] and that morphologic parameters (degree of expansion, ICM grade, and TE grade) are significantly positively correlated with the outcomes after blastocyst transfer[27, 29]. Some studies suggested that the transfer of euploid blastocysts tested by PGT-A can lead to higher rates of clinical pregnancy, live birth, and implantation than those of non-PGT-A tested blastocysts [35, 36]. However, some studies showed that PGT-A could not enhance the ongoing pregnancy rate[37] and live birth rate[38] in young patients, which may be due to the fact that embryos from young patients have low aneuploidy. In regard to chromosomes, the rate of euploidy is significantly higher among blastocysts biopsied on day 5 than among those biopsied on day 6 or day 7[39]. Aneuploid blastocysts have significantly delayed development compared with euploid blastocysts at the start of compaction[40]. The average euploid blastocyst expansion rate is significantly faster than that of aneuploid blastocysts. Some studies suggest that a top-quality ICM and TE, higher expansion grade, and shorter time to the start of blastulation have a higher percentage of euploid blastocysts[30, 39] and a lower incidence of de novo chromosomal abnormalities[41]. The conclusions about the relationship of morphological parameters with euploidy and aneuploidy are consistent with its effect on clinical outcomes, and our results are in accordance with these previous studies at the blastocyst chromosomal level. This further suggests that the combination of female age, embryo developmental speed, and the three morphologic parameters can be used to build algorithms to predict embryonic ploidy and clinical success in ART treatment.
Most studies on embryo aneuploidy often refer to whole chromosomal aneuploidy. However, few studies have focused on the relationship between the incidence of segmental aneuploidy and embryo morphological parameters. A dataset from Escribà et al. showed that there was no relationship between the incidence of segmental aneuploidy and the day of blastocyst biopsy or blastocyst stage, but the rate of segmental aneuploidy was significantly higher among blastocysts with poor ICM and TE quality[42]. Liu et al. analyzed the incidence of de novo chromosome abnormalities related to embryo parameters in the cycles performed for chromosome structural rearrangement and PGT for aneuploidy[41]. The results showed that the incidence of de novo segmental chromosome abnormalities was significantly related to TE grade but not to ICM grade or blastocyst development speed. In our study, the rates of S-aneuploidy increased with TE grade in the groups of women aged ≤ 30 y and ≥ 36 y, although the difference was not significant. The inconsistent results may be due to the different subjects and sample sizes included in the study. The average patient age in our study was younger than that of the abovementioned two studies[41, 42]. The proportion of S-aneuploid blastocysts was lower (9.6%) than that of euploid (63.8%) and W-aneuploid (14.8%) blastocysts. Most of the segmental changes in human blastocysts arise from mitotic errors[17, 43] and affect paternally derived chromosomes, which are probably due to sperm DNA fragmentation during spermatogenesis[19]. There are few studies on the relationship between sperm quality and the development and morphological parameters of blastocysts. Sperm quality, including sperm DNA fragmentation, has a significant correlation with male age[44, 45]. However, paternal age has no impact on fertilization rate, embryo quality, pregnancy, live-birth rates, or miscarriage rate[46, 47] and is not associated with rates of aneuploidy in embryos[19, 40]. The incidence of segmental aneuploidies was not related to female age[43], and sperm DNA fragmentation did not correlate with blastocyst aneuploidy or morphological grading[48, 49]. Therefore, we speculate that male factors have little influence on embryo morphology, which may be affected more by female age and egg quality, so the relationship between S-aneuploidy (mainly paternal origin) and embryo morphology is weak.
Mosaicism has been reported in cleavage- and blastocyst-stage embryo biopsies, and the incidence of mosaicism can vary greatly across laboratories due to differences in NGS protocols and reporting practices[40, 50–53]. In our study, the mosaicism rate among blastocysts was 17.4%. Mosaic embryo transfer could result in viable and genetically normal live births, although it has a significantly lower clinical pregnancy rate and ongoing/live birth rate and a significantly higher miscarriage rate than euploid and non-PGT transfers[54, 55]. A retrospective cohort study by Martín et al. compared the morphokinetic characteristics of 1,511 blastocysts classified as euploid, aneuploid, low-degree mosaic (30%-50%), and high-degree mosaic (50%-70%), and the results showed that embryo morphokinetics were not correlated with the degree of mosaicism or a mosaicism configuration.[40]. Another retrospective cohort study by Chen et al. used multivariate generalized estimating equation analysis to evaluate the correlation of time-lapse-based variables and numeric blastocyst morphological scores with different mosaic levels (≤ 20%, ≤ 50% and ≤ 80%) in 918 biopsied blastocysts and suggested that blastocyst morphokinetic are significantly correlated with the threshold levels of mosaicism[28]. The correlation between blastocyst morphology and the mosaicism level is unclear. In our study, we did not classify blastocysts based on the degree of chromosomal mosaicism. Our results suggested that there was no significant correlation between blastocyst morphology and the incidence of mosaicism, except that TE grade had a significant negative correlation with mosaicism in women aged ≤ 35 years. The reason for the inconsistency is probably that some of the mosaic embryos are false-positive diagnoses or that the mosaicism is the byproduct of a misdiagnosis by PGT-A[40]. Further studies are needed to increase the understanding of embryonic mosaicism and its impact on preimplantation dynamics.
It has been demonstrated that TE karyotype can be an excellent predictor of ICM karyotype[56]. Baatarsuren et al. suggested that the TE could be a better predictive parameter than ICM for live birth rate[26]. In our study, TE grade was closely related to euploidy and W-aneuploidy in all female age groups, while the day of biopsy was related to euploidy and W-aneuploidy in the women aged ≤ 35 years. The expansion degree was related to euploidy and W-aneuploidy in only the group of women aged ≥ 35 years, and ICM grade was related to euploidy and W-aneuploidy in the groups of women aged 31–35 years and ≥ 35 years. The effect of development speed, degree of expansion, and ICM grade on euploidy and W-aneuploidy was variable among the different female age groups, probably because female age is the main factor affecting egg quality, which leads to the differences in developmental potential among embryos. Additionally, TE grade had a weak negative relationship with S-aneuploidy and mosaicism in blastocysts, although most of the differences were not significant. The cells used to detect euploidy by NGS are from the TE biopsy, which is why TE morphology is more closely related to the NGS results. TE aneuploidy is an excellent predictor of ICM aneuploidy, as the majority of ICM results were concordant with the TE biopsies[57, 58]. Our study supports the conclusion that TE morphology is a better predictive parameter for embryo aneuploidy.
Conclusion
In conclusion, our study suggests that female age, biopsy day, and blastocyst morphology (expansion degree, ICM grade, TE grade) are fundamental parameters to predict euploidy and whole chromosome aneuploidy in patients undergoing ART. Blastocysts from younger women biopsied at day 5 and having a higher expansion degree, ICM grade, and TE grade are most likely to be euploid. The predictive roles of these parameters, such as biopsy day, expansion degree, ICM grade, and TE grade, vary among different female age ranges. However, parental age, biopsy day, expansion degree, and ICM grade do not correlate with segmental aneuploidy or mosaicism, and TE grade has a weak correlation with segmental aneuploidy and mosaicism in embryos.
There are some limitations in this study. First, this is a retrospective study that has intrinsic limitations, and data on clinical outcomes after blastocyst transfer are lacking. Second, all the blastocysts included in this study can be used for biopsy for chromosome detection on days 5, 6, or 7, so this study did not include blastocysts with expansion degrees of 1, 2, and 3. Third, uniform single or multiple aneuploidies and the level of mosaicism in noneuploid blastocysts were not distinguished.
Funding
This work was supported by Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16020703) and the National Natural Science Foundation of China (31871447, 82125013, 31871482) and the National Key Research and Development Program (2019YFA0801400).
Data Availability
Data will be made available upon request to the corresponding author.
Declarations
Ethical approval
This study was approved by the Ethics Committee of Peking University Third Hospital. All data was retrieved from standard records collected during clinical care, thus informed consent was not required.
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liying Yan, Email: yanliyingkind@aliyun.com.
Jie Qiao, Email: jie.qiao@263.net.
References
- 1.Li Y, Liu S, Lv Q. Single blastocyst stage versus single cleavage stage embryo transfer following fresh transfer: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;267:11–17. doi: 10.1016/j.ejogrb.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Blastocyst culture and transfer in clinically assisted reproduction: a committee opinion. Fertil Steril. 2018;110(7):1246–52. [DOI] [PubMed]
- 3.Thompson SM, et al. Blastocyst expansion score and trophectoderm morphology strongly predict successful clinical pregnancy and live birth following elective single embryo blastocyst transfer (eSET): a national study. J Assist Reprod Genet. 2013;30(12):1577–1581. doi: 10.1007/s10815-013-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner DK, et al. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 5.Subira J, et al. Grade of the inner cell mass, but not trophectoderm, predicts live birth in fresh blastocyst single transfers. Hum Fertil (Camb) 2016;19(4):254–261. doi: 10.1080/14647273.2016.1223357. [DOI] [PubMed] [Google Scholar]
- 6.Hill MJ, et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 2013;99(5):1283–1289e1. doi: 10.1016/j.fertnstert.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, et al. Embryo Selection Based on Morphological Parameters in a Single Vitrified-Warmed Blastocyst Transfer Cycle. Reprod Sci. 2021;28(4):1060–1068. doi: 10.1007/s43032-020-00349-6. [DOI] [PubMed] [Google Scholar]
- 8.Bakkensen JB, et al. Association between blastocyst morphology and pregnancy and perinatal outcomes following fresh and cryopreserved embryo transfer. J Assist Reprod Genet. 2019;36(11):2315–2324. doi: 10.1007/s10815-019-01580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nazem TG, et al. The correlation between morphology and implantation of euploid human blastocysts. Reprod Biomed Online. 2019;38(2):169–176. doi: 10.1016/j.rbmo.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Peng X, et al. Effects of euploid blastocyst morphological development on reproductive outcomes. Reprod Biol. 2020;20(4):496–500. doi: 10.1016/j.repbio.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Irani M, et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110(1):95–102e1. doi: 10.1016/j.fertnstert.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, et al. Frozen blastocysts: Assessing the importance of day 5/day 6 blastocysts or blastocyst quality. Exp Ther Med. 2022;23(5):333. doi: 10.3892/etm.2022.11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franasiak JM, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–663e1. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Dahdouh EM, Balayla J, Garcia-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril. 2015;104(6):1503–1512. doi: 10.1016/j.fertnstert.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan-Pyke C, Dokras A. Preimplantation Genetic Screening and Preimplantation Genetic Diagnosis. Obstet Gynecol Clin North Am. 2018;45(1):113–125. doi: 10.1016/j.ogc.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Glujovsky D, et al. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;6:CD002118. doi: 10.1002/14651858.CD002118.pub5. [DOI] [PubMed] [Google Scholar]
- 17.Babariya D, et al. The incidence and origin of segmental aneuploidy in human oocytes and preimplantation embryos. Hum Reprod. 2017;32(12):2549–2560. doi: 10.1093/humrep/dex324. [DOI] [PubMed] [Google Scholar]
- 18.Rabinowitz M, et al. Origins and rates of aneuploidy in human blastomeres. Fertil Steril. 2012;97(2):395–401. doi: 10.1016/j.fertnstert.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Kubicek D, et al. Incidence and origin of meiotic whole and segmental chromosomal aneuploidies detected by karyomapping. Reprod Biomed Online. 2019;38(3):330–339. doi: 10.1016/j.rbmo.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Magli MC, et al. Permanence of de novo segmental aneuploidy in sequential embryo biopsies. Hum Reprod. 2020;35(4):759–769. doi: 10.1093/humrep/deaa025. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S, et al. Prevalence and authenticity of de-novo segmental aneuploidy (>16 Mb) in human blastocysts as detected by next-generation sequencing. Reprod Biomed Online. 2018;37(5):511–520. doi: 10.1016/j.rbmo.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Treff NR, Marin D. The "mosaic" embryo: misconceptions and misinterpretations in preimplantation genetic testing for aneuploidy. Fertil Steril. 2021;116(5):1205–1211. doi: 10.1016/j.fertnstert.2021.06.027. [DOI] [PubMed] [Google Scholar]
- 23.Capalbo A, et al. Detecting mosaicism in trophectoderm biopsies: current challenges and future possibilities. Hum Reprod. 2017;32(3):492–498. doi: 10.1093/humrep/dew250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman KN, et al. Beyond the biopsy: predictors of decision regret and anxiety following preimplantation genetic testing for aneuploidy. Hum Reprod. 2019;34(7):1260–1269. doi: 10.1093/humrep/dez080. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, et al. Validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of blastocysts. Fertil Steril. 2016;105(6):1532–1536. doi: 10.1016/j.fertnstert.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Baatarsuren M, et al. The trophectoderm could be better predictable parameter than inner cellular mass (ICM) for live birth rate and gender imbalance. Reprod Biol. 2022;22(1):100596. doi: 10.1016/j.repbio.2021.100596. [DOI] [PubMed] [Google Scholar]
- 27.Bori L, et al. The higher the score, the better the clinical outcome: retrospective evaluation of automatic embryo grading as a support tool for embryo selection in IVF laboratories. Hum Reprod. 2022;37(6):1148–1160. doi: 10.1093/humrep/deac066. [DOI] [PubMed] [Google Scholar]
- 28.Chen CH, et al. Blastocyst Morphology Based on Uniform Time-Point Assessments is Correlated With Mosaic Levels in Embryos. Front Genet. 2021;12:783826. doi: 10.3389/fgene.2021.783826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shear MA, et al. Blasts from the past: is morphology useful in PGT-A tested and untested frozen embryo transfers? Reprod Biomed Online. 2020;41(6):981–989. doi: 10.1016/j.rbmo.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan Q, et al. Blastocyst score, a blastocyst quality ranking tool, is a predictor of blastocyst ploidy and implantation potential. F S Rep. 2020;1(2):133–141. doi: 10.1016/j.xfre.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fragouli E, et al. The origin and impact of embryonic aneuploidy. Hum Genet. 2013;132(9):1001–1013. doi: 10.1007/s00439-013-1309-0. [DOI] [PubMed] [Google Scholar]
- 32.Fragouli E, Munne S, Wells D. The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies. Hum Reprod Update. 2019;25(1):15–33. doi: 10.1093/humupd/dmy036. [DOI] [PubMed] [Google Scholar]
- 33.Nair J, et al. Preimplantation genetic testing for aneuploidy (PGT-A)-a single-center experience. J Assist Reprod Genet. 2022;39(3):729–738. doi: 10.1007/s10815-022-02413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji H, et al. Effect of Embryo Developmental Stage, Morphological Grading, and Ploidy Status on Live Birth Rate in Frozen Cycles of Single Blastocyst Transfer. Reprod Sci. 2021;28(4):1079–1091. doi: 10.1007/s43032-020-00381-6. [DOI] [PubMed] [Google Scholar]
- 35.Greco E et al. Preimplantation Genetic Testing: Where We Are Today. Int J Mol Sci. 2020;21(12). [DOI] [PMC free article] [PubMed]
- 36.Haddad G, et al. Assessment of aneuploidy formation in human blastocysts resulting from donated eggs and the necessity of the embryos for aneuploidy screening. J Assist Reprod Genet. 2015;32(6):999–1006. doi: 10.1007/s10815-015-0492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munne S, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071–10797. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 38.Ozgur K, et al. Single best euploid versus single best unknown-ploidy blastocyst frozen embryo transfers: a randomized controlled trial. J Assist Reprod Genet. 2019;36(4):629–636. doi: 10.1007/s10815-018-01399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minasi MG, et al. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31(10):2245–2254. doi: 10.1093/humrep/dew183. [DOI] [PubMed] [Google Scholar]
- 40.Martin A, et al. The morphokinetic signature of mosaic embryos: evidence in support of their own genetic identity. Fertil Steril. 2021;116(1):165–173. doi: 10.1016/j.fertnstert.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, et al. The Relationship between Human Embryo Parameters and De Novo Chromosomal Abnormalities in Preimplantation Genetic Testing Cycles. Int J Endocrinol. 2022;2022:9707081. doi: 10.1155/2022/9707081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escriba MJ, Vendrell X, Peinado V. Segmental aneuploidy in human blastocysts: a qualitative and quantitative overview. Reprod Biol Endocrinol. 2019;17(1):76. doi: 10.1186/s12958-019-0515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girardi L, et al. Incidence, Origin, and Predictive Model for the Detection and Clinical Management of Segmental Aneuploidies in Human Embryos. Am J Hum Genet. 2020;106(4):525–534. doi: 10.1016/j.ajhg.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evenson DP, et al. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA(R)) defined sperm DNA and chromatin integrity. Fertil Steril. 2020;114(2):311–320. doi: 10.1016/j.fertnstert.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 45.Gao J, et al. Age-related changes in human conventional semen parameters and sperm chromatin structure assay-defined sperm DNA/chromatin integrity. Reprod Biomed Online. 2021;42(5):973–982. doi: 10.1016/j.rbmo.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Sagi-Dain L, Sagi S, Dirnfeld M. Effect of paternal age on reproductive outcomes in oocyte donation model: a systematic review. Fertil Steril. 2015;104(4):857–865e1. doi: 10.1016/j.fertnstert.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, et al. Effect of Paternal Age on Reproductive Outcomes of In Vitro Fertilization. PLoS One. 2015;10(9):e0135734. doi: 10.1371/journal.pone.0135734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bronet F, et al. Sperm DNA fragmentation index does not correlate with the sperm or embryo aneuploidy rate in recurrent miscarriage or implantation failure patients. Hum Reprod. 2012;27(7):1922–1929. doi: 10.1093/humrep/des148. [DOI] [PubMed] [Google Scholar]
- 49.Gat I, et al. Sperm DNA fragmentation index does not correlate with blastocyst aneuploidy or morphological grading. PLoS One. 2017;12(6):e0179002. doi: 10.1371/journal.pone.0179002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capalbo A, et al. FISH reanalysis of inner cell mass and trophectoderm samples of previously array-CGH screened blastocysts shows high accuracy of diagnosis and no major diagnostic impact of mosaicism at the blastocyst stage. Hum Reprod. 2013;28(8):2298–2307. doi: 10.1093/humrep/det245. [DOI] [PubMed] [Google Scholar]
- 51.Fragouli E, et al. Cytogenetic analysis of human blastocysts with the use of FISH, CGH and aCGH: scientific data and technical evaluation. Hum Reprod. 2011;26(2):480–490. doi: 10.1093/humrep/deq344. [DOI] [PubMed] [Google Scholar]
- 52.Northrop LE, et al. SNP microarray-based 24 chromosome aneuploidy screening demonstrates that cleavage-stage FISH poorly predicts aneuploidy in embryos that develop to morphologically normal blastocysts. Mol Hum Reprod. 2010;16(8):590–600. doi: 10.1093/molehr/gaq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruttanajit T, et al. Detection and quantitation of chromosomal mosaicism in human blastocysts using copy number variation sequencing. Prenat Diagn. 2016;36(2):154–162. doi: 10.1002/pd.4759. [DOI] [PubMed] [Google Scholar]
- 54.Viotti M, et al. Using outcome data from one thousand mosaic embryo transfers to formulate an embryo ranking system for clinical use. Fertil Steril. 2021;115(5):1212–1224. doi: 10.1016/j.fertnstert.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YX et al. The Pregnancy Outcome of Mosaic Embryo Transfer: A Prospective Multicenter Study and Meta-Analysis. Genes (Basel). 2020;11(9). [DOI] [PMC free article] [PubMed]
- 56.Johnson DS, et al. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol Hum Reprod. 2010;16(12):944–949. doi: 10.1093/molehr/gaq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chavli E et al. Chromosomal mosaicism in human blastocysts: a cytogenetic comparison of trophectoderm and inner cell mass after next-generation sequencing. Reprod Biomed Online. 2022. [DOI] [PubMed]
- 58.Huang J, et al. Re-analysis of aneuploidy blastocysts with an inner cell mass and different regional trophectoderm cells. J Assist Reprod Genet. 2017;34(4):487–493. doi: 10.1007/s10815-017-0875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request to the corresponding author.