Abstract
Purpose
To provide an agreed upon definition of hyper-response for women undergoing ovarian stimulation (OS)?
Methods
A literature search was performed regarding hyper-response to ovarian stimulation for assisted reproductive technology. A scientific committee consisting of 5 experts discussed, amended, and selected the final statements in the questionnaire for the first round of the Delphi consensus. The questionnaire was distributed to 31 experts, 22 of whom responded (with representation selected for global coverage), each anonymous to the others. A priori, it was decided that consensus would be reached when ≥ 66% of the participants agreed and ≤ 3 rounds would be used to obtain this consensus.
Results
17/18 statements reached consensus. The most relevant are summarized here. (I) Definition of a hyper-response: Collection of ≥ 15 oocytes is characterized as a hyper-response (72.7% agreement). OHSS is not relevant for the definition of hyper-response if the number of collected oocytes is above a threshold (≥ 15) (77.3% agreement). The most important factor in defining a hyper-response during stimulation is the number of follicles ≥ 10 mm in mean diameter (86.4% agreement). (II) Risk factors for hyper-response: AMH values (95.5% agreement), AFC (95.5% agreement), patient’s age (77.3% agreement) but not ovarian volume (72.7% agreement). In a patient without previous ovarian stimulation, the most important risk factor for a hyper-response is the antral follicular count (AFC) (68.2% agreement). In a patient without previous ovarian stimulation, when AMH and AFC are discordant, one suggesting a hyper-response and the other not, AFC is the more reliable marker (68.2% agreement). The lowest serum AMH value that would place one at risk for a hyper-response is ≥ 2 ng/ml (14.3 pmol/L) (72.7% agreement). The lowest AFC that would place one at risk for a hyper-response is ≥ 18 (81.8% agreement). Women with polycystic ovarian syndrome (PCOS) as per Rotterdam criteria are at a higher risk of hyper-response than women without PCOS with equivalent follicle counts and gonadotropin doses during ovarian stimulation for IVF (86.4% agreement). No consensus was reached regarding the number of growing follicles ≥ 10 mm that would define a hyper-response.
Conclusion
The definition of hyper-response and its risk factors can be useful for harmonizing research, improving understanding of the subject, and tailoring patient care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02757-4.
Keywords: Hyper-response, Ovarian stimulation, Ovarian hyperstimulation
Introduction
Whether there is an upper limit for the number of oocytes to be collected in an assisted reproductive technology (ART) cycle is controversial due to two breakthroughs. The first is the advent of vitrification. It has greatly increased the effectiveness of gamete and embryo cryopreservation. The chance of a cryopreserved embryo to reach live birth is now equivalent to a fresh transferred embryo [1]. Moreover, the balance tilts in favor of frozen embryo transfers (ET) as ovarian response in the stimulation cycle increases [2]. The increased effectiveness of cryopreservation rendered the cumulative live birth rate (CLBR) per stimulation cycle or even family completion rate per stimulated cycle, the ultimate measures of success; and CLBR linearly increases with the number of oocytes collected [3, 4]. The second is development of multiple strategies to prevent ovarian hyperstimulation syndrome (OHSS) including the GnRH agonist trigger, use of dopamine agonists and luteal GnRH antagonists and the increasing use of freeze-all approach [5]. These preventive measures are not mutually exclusive and the risk of moderate – severe OHSS, when these measures are used, is now low [6–8].
While these two developments significantly relieve our concerns about a hyper-response to ovarian stimulation (OS), there are still questions to be answered about its occurrence, downstream effects on the endometrium, possibly the oocytes and on obstetric outcomes, making it an area of research. As such, a uniform definition of what constitutes a hyper-response would be useful to harmonize research efforts and enable the pooling of results from various studies that use the same inclusion criteria, even if it may not affect routine clinical practice significantly.
A Delphi consensus was conducted with the aim of developing an agreed-upon definition of hyper-response in women undergoing OS for ART, since such a definition is currently absent.
Materials and methods
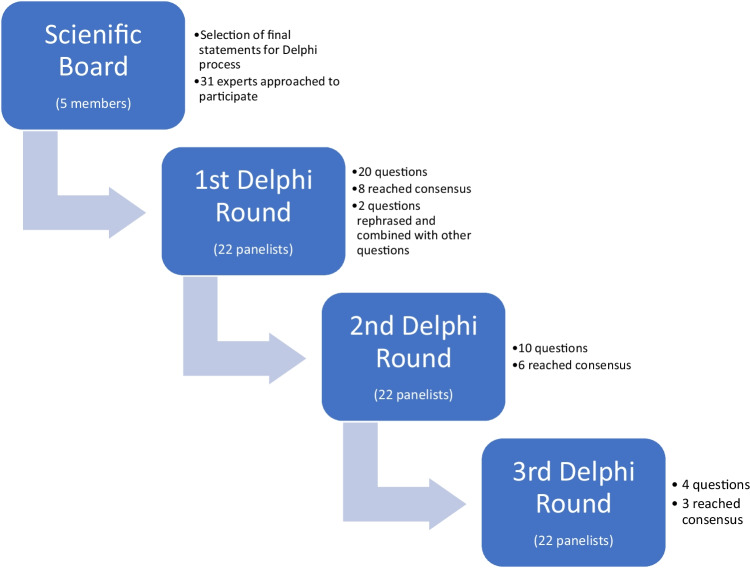
The consensus coordinators (Michael H. Dahan, Baris Ata and Ido Feferkorn) performed a literature search on commonly used definitions for hyper-responders. Based on these definitions, an initial list of defining questions was developed. This list was disseminated to a scientific board consisting of: Michael H. Dahan, Baris Ata, Sandro Esteves, Richard Paulson, and Antonio La Marca. The scientific board discussed, amended, and selected the final statements in the questionnaire for the first round of the Delphi consensus, these are presented in Fig. 1s. The board also discussed and agreed upon the method of disseminating the questionnaire, processing the results, and establishing a threshold for accepting a statement as a consensus. Each member of the scientific board was able to suggest experts who would participate in the Delphi consensus expert panel. The facilitator of the Delphi consensus was Dr. Ido Feferkorn.
Consensus participants
A group of 31 professionals in the field of reproductive medicine from different geographic regions were approached by email and offered to participate as panelists. Each was provided with information regarding the study’s purpose and methods. Twenty-two experts (including the board members) agreed to participate as panelists, and each remained anonymous to the others throughout the process (Table 1s). The geographic representation of the panelists is presented in Table 1.
Table 1.
Geographic representation of the Delphi panelists
| Continent | Country of Origin | Number of panelists |
|---|---|---|
| Europe | Italy | 3 |
| Europe | Spain | 2 |
| Europe | Belgium | 1 |
| Europe | Denmark | 1 |
| Europe | Greece | 1 |
| Europe | Portugal | 1 |
| Europe | United Kingdom | 1 |
| Asia | Turkey | 2 |
| Asia | Israel | 2 |
| Asia | United Arab Emirates | 2 |
| North America | Canada | 2 |
| North America | United States of America | 1 |
| Oceania | Australia | 2 |
| South America | Brazil | 1 |
The consensus process
The consensus process is depicted in Fig. 1. After selecting the final statements for voting, the scientific board determined, a priori, that a consensus would be reached when at least 66% of experts agreed and that up to three rounds would be used to obtain this consensus.
Fig. 1.
The Delphi process
In the first round, an online survey consisting of 20 questions and statements was circulated to the Delphi participants. The survey included five statements on which the experts could agree or disagree, two ranking questions and 13 multiple choice questions in three of which the experts could choose the option “other” and add an opinion in free text.
In the second and third rounds panelists were given the results from the previous round and the survey started with questions in which a consensus was not reached. These questions were revised according to the feedback and the results from the previous round with responses with the least consensus omitted. This part of the survey was followed by a second part where all panelists had the option to discuss a statement that reached a consensus. If two or more panelists would opt to discuss a statement and had the same motivation for this, the statement would be reintroduced in the following stage of the Delphi process. If no strong objection was raised to an achieved consensus, the agreed-upon statement would not be reintroduced in the subsequent round. The panelists were requested to define specific cut-offs for markers of ovarian response that could be used to group women undergoing OS into low and high risk for a hyper-response. The panelists had the option of suggesting their own cut-off or choosing one of the proposed cut-offs.
The questionnaire had the following order: the definition of hyper-response during OS and after the oocyte pick-up (OPU) procedure and the factors that are considered to be associated with a higher risk of a hyper-response.
Results
Overall, 100% (22/22) of experts completed all three rounds of the Delphi. 17/18 statements reached a consensus. The statements are accompanied by the round number in which the consensus was reached in parentheses. The consensus statements are presented in Table 2 and Fig. 2 s.
Table 2.
The consensus statements
| Definitions of a hyper-response |
|---|
| Hyper-response is characterized by the collection of ≥ 15 oocytes |
| A history of a hyper-response or OHSS in a prior cycle is not required to define a hyper-responder |
| OHSS is not relevant for the definition of hyper-response if the number of collected oocytes is above a threshold (≥ 15) |
| Recognition of pending hyper-response during ovarian stimulation |
| The most important factor in defining a hyper-response during the ovarian stimulation is the number of follicles ≥ 10 mm in mean diameter |
| Risk factors for a hyper-response – risk factors prior to OS |
| I consider the serum Anti Mullerian Hormone (AMH) levels when assessing the risk for a possible hyper-response |
| I consider the antral follicular count (AFC) when assessing the risk for a possible hyper-response |
| I consider the patient’s age when assessing the risk for a possible hyper-response |
| I do not consider the ovarian volume when assessing the risk for a possible hyper-response |
| In a patient without a previous ovarian stimulation, the most important risk factor for a hyper-response is the antral follicular count (AFC) |
| In a patient without a previous ovarian stimulation, when AMH and AFC are discordant, with one suggesting a hyper-response and the other not, AFC is the more reliable marker |
| Hyper-response can occur at any age |
| The lowest serum AMH value that would put a woman at risk for a hyper-response is ≥ 2 ng/ml (14.3pMol/L) |
| The lowest AFC that would put a woman at risk for a hyper-response is ≥ 18 |
| A patient with a history of hyper-response in a previous stimulation cycle would be considered at high risk for another hyper-response if there was no significant change in the serum AMH levels and the AFC (± 3 follicles) |
| Women with the polycystic ovarian syndrome (PCOS) as per Rotterdam criteria are at a higher risk of hyper-response than women without PCOS with equivalent follicle counts and gonadotropin doses used to stimulate |
| Risk factors for a hyper-response—Risk factors for hyper-response during ovarian stimulation |
| The lowest number of growing follicles ≥ 14 mm that would characterize a hyper-response to ovarian stimulation is ≥ 15 follicles |
| The lowest peak estradiol level that would indicate a hyper-response to ovarian stimulation is ≥ 3,000pg/ml (11,013pMol/l) |
* No consensus was reached regarding the number of growing follicles ≥ 10 mm that would define a hyper-response
OS – ovarian stimulation
Definition of a hyper-response
Hyper-response is characterized by the collection of ≥ 15 oocytes. (2.nd round, 73% consensus)
A history of a hyper-response or OHSS is not required to define a hyper-responder. (1.st round, 68% consensus)
OHSS is not relevant for the definition of hyper-response if the number of collected oocytes is above a threshold (≥ 15). (2.nd round, 77% consensus)
Recognition of pending hyper-response during ovarian stimulation
-
4.
The most important criterion in defining a hyper-response during ovarian stimulation is the number of follicles ≥ 10 mm in mean diameter. (1.st round, 86% consensus)
-
5.
No consensus was reached regarding the number of growing follicles ≥ 10 mm that would define a hyper-response.
In the first round, 50% chose ≥ 20 follicles, and 36.4% chose ≥ 15 follicles. In the second Delphi round, 59.1% chose ≥ 20 follicles, and 40.9% chose ≥ 15 follicles. In the third round, 63.6% chose ≥ 20 follicles, and 36.4% chose ≥ 15 follicles.
Risk factors for a hyper-response
Risk factors for a hyper-response prior to OS
-
6.
I consider serum Anti Mullerian Hormone (AMH) level when assessing the risk for a possible hyper-response. (1.st round, 96% consensus)
-
7.
I consider antral follicle count (AFC) when assessing the risk for a possible hyper-response. (1.st round, 95.5% consensus)
-
8.
I consider the patient’s age when assessing the risk for a possible hyper-response. (2.nd round, 77% consensus)
-
9.
I do not consider ovarian volume when assessing the risk for a possible hyper-response. (2.nd round, 73% consensus)
-
10.
In a patient without previous ovarian stimulation, the most important risk factor for a hyper-response is AFC. (1.st round, 68% consensus)
-
11.
In a patient without previous ovarian stimulation, when AMH and AFC are discordant, one suggesting a hyper-response and the other not, AFC is the more reliable marker. (1.st round, 68% consensus)
-
12.
Hyper-response can occur at any age. (2.nd round, 73% consensus)
-
13.
The lowest serum AMH value that would place a woman at risk for a hyper-response is ≥ 2 ng/ml (14.3pMol/L). (3.rd round, 73% consensus)
-
14.
The lowest AFC that would place a woman at risk for a hyper-response is ≥ 18. (3.rd round, 82% consensus)
-
15.
A patient with a history of hyper-response in a previous stimulation cycle would be considered at high risk for another hyper-response if there was no significant change in the serum AMH levels and the AFC (± 3 follicles). (1.st round, 77% consensus)
-
16.
Women with polycystic ovarian syndrome (PCOS) as per Rotterdam criteria are at a higher risk of hyper-response than women without PCOS with equivalent follicle counts and gonadotropin doses used to stimulate. (2.nd round, 86% consensus)
Risk factors for hyper-response during ovarian stimulation
-
17.
The lowest number of growing follicles ≥ 14 mm that would characterize a hyper-response to ovarian stimulation is ≥ 15 follicles. (1.st round, 73% consensus)
-
18.
The lowest peak estradiol level that would characterize a hyper-response to ovarian stimulation is ≥ 3,000 pg/ml (11,013pMol/l). (3.rd round, 96% consensus)
Discussion
We suggest that hyper-response to OS should be defined as the collection of > 15 oocytes, regardless of the development or history of OHSS (statements 1 to 3). While the panel agreed that a hyper-response can occur at any age, we still regard age as a factor that determines the risk of a hyper-response (statements 8 and 12). Among the markers of the ovarian reserve, while high AMH and AFC were both recognized to be associated with the risk of hyper-response (statements 6, 7, 13, 14, 15), AFC was given priority over AMH (statements 10 & 11). Ovarian volume was not used in risk assessment (statement 9). While markers of ovarian reserve including AMH, AFC, age, and PCOS status (statement 16) were regarded as primary risk factors, i.e., that exist prior to OS, the number of growing follicles was regarded as the most important secondary risk factor, i.e., a risk factor that became apparent during OS (Statement 5). It is worth emphasizing that the panel differentiated between follicles (antral or growing follicles) and oocytes as not all aspirated follicles are expected to yield oocytes. While the panel could reach a consensus on the lowest cut-offs for AMH, AFC and the number of follicles > 14 mm to define as risk factors for hyper-response (statements 14, 17, 18), a consensus was not reached on the number of follicles > 10 mm, despite recognizing this as the most important risk factor (statements 4 and 5).
The terminology for an excessive response differs in the literature. Some use the term high response/high responder, while others use the loanword from Greek hyper-response/hyper-responder. We selected the prefix “hyper” due to its implication of an excessive or exaggerated response.
While the ESHRE OS guideline considers ≥ 18 oocytes as a high risk for OHSS, it is just one complication of hyper-response [9]. The other is a decrease in LBR following a fresh ET in a hyper-response cycle [3, 10–13]. In a randomized trial, Chen et al. studied the LBR and rates of OHSS in women with PCOS after the transfer of fresh vs. frozen embryos [14]. The mean number of oocytes retrieved was 14 in both groups, and the LBR was significantly lower after a fresh transfer, whereas the OHSS rate was significantly higher. In another randomized trial including ovulatory women, LBR was similar between fresh vs. frozen ET [15]. Of note, the mean number of oocytes collected in this trial was twelve. Moreover, large-scale retrospective studies suggest that live birth rates per fresh ET plateaus at around 15 oocytes [3, 10, 16]. Based on these observations it can be reasonable to suggest that the cut-off value of 15 oocytes might represent a threshold above which there is a possible detrimental effect of hyperstimulation on LBR following a fresh ET, supporting the panel’s opinion.
The consensus statements emphasize the difference between a hyper-response and OHSS. While OHSS most commonly occurs in combination with a hyper-response cycle, the latter represents a separate entity with consequences as reflected with decreased LBR in the above-mentioned trials and other evidence supporting a negative correlation between an excessive ovarian response and the LBR, which becomes perceptible after a certain threshold [2, 14]. These negative outcomes of a hyper-response can occur in the absence of OHSS. Moreover, while several preventive measures allow the prevention of OHSS despite a hyper-response [5] this may not change other downstream effects of the hyper-response.
Disregarding ovarian volume and focusing on high AFC and AMH as risk factors are in agreement with the ASRM committee’s opinion on testing and interpreting measures of ovarian reserve [17]. The ovarian reserve markers AMH and AFC were found to be the most sensitive and specific in predicting ovarian response to stimulation (both excessive and poor response). On the other hand, ovarian volume is less accurate and does not add to the prediction ability when combined with the other ovarian reserve markers [18, 19]. In a meta-analysis comparing AMH and AFC as predictors of excessive response in OS cycles (each study defining excessive response differently), both markers performed equally (sensitivity and specificity for AMH were 82% and 76%, respectively, and 82% and 80% for AFC) [20]. In an individual patient data meta-analysis, AMH and AFC had comparable high accuracy [21]. On the other hand, a few multicenter studies found AMH to be more predictive of an excessive response [22–25]. The multitude of AMH measuring platforms, the analytical variation in AMH measurement [26] and the lack of a common AMH reference preparation [27] may have contributed to the Panel’s preference of AFC over AMH. Moreover, since hyper-response was defined with the number of oocytes collected, and the number of follicles > 10 mm was regarded as the strongest sign of pending hyper-response in an OS cycle, it may be intuitive to correlate these with AFC as assessed at the start of the cycle, rather than AMH level, which is often not measured at the start of OS cycle per se. With that in mind, the panel acknowledges that evidence on the matter is lacking, and it is likely that with a uniform AMH platform this preference might change. Moreso, clinicians are encouraged to practice according to their own experience however the importance of including AFC as a parameter in future studies on hyper-responders is emphasized.
While consensus was reached on AFC being more reliable than AMH in predicting a hyper- response, the minimum number of AFC being 18 as a risk factor for hyper-response, and collection of ≥ 15 oocytes defining a hyper-response; a consensus was not reached regarding the number of developing follicles ≥ 10 mm that would define a hyper-response during OS, which was regarded as “the most important factor in defining a hyper-response during ovarian stimulation”. Logically, the number should have fallen between 18 (the minimum number of AFC as a risk factor) and 15 (the number of oocytes to define a hyper-response, assuming 100% of > 10 mm follicles yielding an oocyte), it seems more than one-third of panelists thought that identifying a minimum of 15 follicles > 10 mm as a risk factor could lead to an overestimation of the risk and perhaps bring about unnecessary preventive measures, whereas a similar proportion of panelists might have thought that 20 follicles > 10 mm would be too high a number, and could have led to an underestimation of the risk of hyper-response. Given the increasing numbers of panelists switching from 15 to 20 during the 2nd and 3rd rounds, probably a number in between, e.g., 17 or 18, might have provided consensus. This lack of consensus may highlight the need for further research, hopefully utilizing the agreed-upon definition of an outcome that is considered a hyper-response (collection of ≥ 15 oocytes).
Young age is related to higher ovarian reserve and is a well-known risk factor for a hyper- response [6, 21, 28]. However, due to the significant overlap in age distribution among women with and without a hyper-response, it is impossible to define a single cut-off [29, 30]. It is also known that even women above the age of 40 years can develop OHSS due to a hyper-response [31–33]
Likewise, it is difficult to define an AMH or AFC cut-off value to predict hyper-response. While there seems to be a rather narrow range of cut-off values for the prediction of poor response [34–36], it is logical that differences on the other end of the spectrum would be wider. Indeed, a wider range of cut-off levels for an excessive response has been described in the literature [20, 37–42]. If one assumes that hyper-response to OS should be avoided, it would be safer to use a lower cut-off value to err on the safe side. The Panel emphasizes that caution should be taken from AMH values ≥ 2 ng/ml. The ASRM guideline on the prevention and treatment of OHSS cites an AFC ≥ 24 to be associated with a higher risk for OHSS [6]. The NICE guidelines cite AFC > 16 as a predictor of hyper-response, while the majority of other studies reported a range of AFC between 14–18 as predictor of hyper-response [20, 39, 43–45] Likely, the Panel chose an AFC of 18 as the cut off in relation to the definition of the collection of > 15 oocytes as hyper-response. With an expected average oocyte retrieval rate of 70–75% of all aspirated follicles, the number of follicles resulting in 15 oocytes would be around 18–20. The suggested cut-off value of 18 follicles for hyper-response again seems to err on the safe side and is consistent with the numbers in the literature. Serum levels of estradiol were found less predictive for OHSS than the number of growing follicles assessed with ultrasound [46, 47]. The association between high estradiol levels and its effect on implantation is a matter of debate, and most studies that support such an association do not define a clear cut-off level after which a fresh transfer is not recommended [48–53]. While the consensus is that a level ≥ 3,000 pg/ml puts a patient at risk for a hyper-response, further study is required to delineate the association between high estradiol levels and implantation rate, pregnancy rate, and neonatal outcome.
There are no studies addressing the importance of a history of a hyper-response to OS and its recurrence or there is no defined period during which women undergoing OS should be considered at higher risk following an initial hyper-response. Since many women return several years after their initial treatment cycle (e.g., for conceiving another child), it is important to define how long this increased risk persists. According to the panel, ovarian reserve markers serve as better indicators of the current risk. However, supposing that no significant change occurred in the ovarian reserve markers, the response to the previous stimulation can better guide the clinician to tailor the treatment for the present stimulation cycle. While a significant change in AFC was defined as a change of more than 3 follicles, the Delphi panel did not discuss what constitutes a significant change in serum AMH levels and this could be a subject of future studies.
Women with PCOS, by definition have high AFC and are likely to hyper-respond to OS. A retrospective cohort study found that while the ovarian response to stimulation was comparable in women with PCOS and polycystic ovarian morphology (and higher than in women with normal ovaries), rates of severe OHSS were higher in the group of women with PCOS (when compared to the group of women with normal ovarian morphology), but not in the group with isolated PCOM [54]. One possible explanation for the increased risk, at least for OHSS, in women with PCOS is the insulin-mediated increased VEGF production in the vascular smooth muscle cells [55] and the low dopaminergic tone in women with PCOS [56] which too can cause an increase in VEGF levels that likely mediate OHSS. Moreover, the observed imbalance in PCOS patients between pro-inflammatory mediators and anti-inflammatory cytokines, which is associated with a systemic low-grade inflammation, might contribute to the excessive response to OS and the occurrence of OHSS, one of the complications of hyper-response [57].
An experiment-based approach defining “hyper-response” and its predictors could be done by identifying women with and without adverse outcomes that would be associated with hyper-response. These could include for exmaple lower LBR per fresh ET and a higher risk of OHSS.The investigator would subsequently compare the detected descriptive parameters to investigate whether a certain cut-off for the number of oocytes could be identified. This would then be followed with a prospective validation of these results. The Delphi process does not replace such studies, it provides expert opinion about this multifaceted issue, which lacks a consensus due to an absence of adequate studies with a holistic assessment of the subject. While there are concerns with the validity of the Delphi methods and the role of expert opinion in evidence-based medicine, there is value for expert judgment in areas where evidence is lacking. We tried to form a diverse and geographically representative panel to reflect different opinions from around the world. While the majority of the invited experts joined the panel, the participation rate from the U.S. was low and the majority of North American participants were Canadians. Furthermore, the lack of any representation from China, India, and Africa also serves as a limitation in the study design. It is possible that in many places 15 oocytes may be the norm or even the goal. However, we believe that the definition of hyper-response does not necessarily mean that it is an unwanted response, rather, that this response is associated with other implications (such as decreased pregnancy rates in a fresh transfer)..The number of participating panelists is also relatively small, although not at an uncommon level for Delphi studies and committee opinions previously published in reproductive medicine. Hopefully, in future studies and when updating this definition. more experts and of a wider geographic representation will accept to take part.
While the choice of an online survey rather than a in-person or web conferences can be considered a limitation of our study it was due to panelists living in different time zones and restrictions secondary to the COVID19 pandemic. However, the opportunity to comment on and discuss items with a consensus after the first two rounds and re-introduction of those items for consensus at the discretion of the experts were provided. Each statement that reached consensus was shown to the panelist, who had the option of objecting to the statement with reasons. The online survey provided anonymity and the blinding of experts to one another, enabling panelists to freely express and change their views. The 100% response rate in all rounds is also a strength of this Delphi consensus.
In conclusion, this Delphi consensus utilized a systematic compilation of experts’ opinions to generate a definition of a hyper-response to OS for ART. We believe that this definition of hyper-response, collection of 15 or more oocytes, and the definitions of a hyper-response during stimulation and the risk factors for a hyper-response (Table 2) can assist in the design of future studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Figure 1s: The initial questionnaire. (PDF 204 kb)
Supplementary file2 Figure 2s: Pie charts – agreement on the consensus statements (DOCX 95 kb)
Acknowledgements
Hera was the goddess of women, marriage, family, and childbirth in ancient Greek mythology and, as such, was felt to make a fitting title for a woman with hyper-response.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
All declarations of interest are outside the submitted work.
• Peter Humaidan reports reception of lecture honoraria from Merck, Gedoeon Richter and IBSA.
• Antonio La Marca reports reception of consulting fees from Merck, Organon, Ferring, Gedeon Richter, Theramex, Beckman Coulter, and Roche.
• Samuel Santos-Ribeiro reports reception of research funding from Roche diagnostics, Organon and Theramex; reception of consulting fees from Organon, MSD and Ferring; honoraria for lectures from Ferring, Besins, MSD/Organon, Theramex and Gedeon Richter; reception of equipment materials or other services from Roche diagnostics and Ferring and is deputy of the SQART SIG in ESHRE.
• Alessandro Conforti reports reception of grants from the University of Naples Federico II; recpetion honoraria from Medea, Event Planet and Merck.
• Baris Ata – reports reception of consulting fees from Merck GmBH – Turkey, reception of payment or honoraria from Abbott, Merck GmBH and Ferring; reception of support for attending meetings or travel from IBSA; is president of the Turkish Society of Reproductive Medicine and is an executive committee member of ESHRE.
• Juan Garcia Velasco – reports reception of payment or honoraria for lectures or educational events from Merck, Ferring, MSD, Organon, Theramex and Gedeon Richter.
• George Lainas—reports reception of payment or honoraria for lectures or educational events from Merck and Ferring, payment for expert testimony from Merck and support for attending meetings from ESHRE. He also participated in data safety monitoring or advisory board of Merck.
• Filippo Maria Ubaldi is the scientific director of GeneraLife and minority shareholder of the company. He is also president of SIFES-MR (the Italian Society of Fertility, Sterility and Reproductive Medicine), and member of the scientific board of Medea. In the last three years, F.M. Ubaldi has received honoraria or consultation fees from Merck, MSD, Ferring, Gedeon Richter, Organon and Ibsa.
• Sesh Sunkara reports reception of payment or honoraria for lectured from, Merck Ferring and MSD.
• Raoul Orvieto reports reception of consulting fees from Merck and Ferring and payment or honoraria for lectures from Merck and Ferring.
• Nikolaos Polyzos reports reception of grants or contracts from Merck Serono, IBSA, Organon, Ferring, Roche, Theramex, Besins Healthcare and Gedeon Richter and reception of consulting fees from Merck Serono, IBSA, Organon, Ferring, Besins Healthcare and Gedeon Richter.
• Ben Mol reports reception of an investigator grant from NHMRC, he reports consultancy at an hourly rate for ObsEva, Merck Merck KGaA and Guerbet and reception of travel support from Merck Merck KGaA.
• Hakan Yarali reports unrestricted grants from Merck, honoraria for lectures from Merck and IBSA, support for attending meetings from Merck, IBSA and Ferring.
• Human Fatemi reports receiving research grants from Merck Serono and Organon, consulting fees from Ferring global, speaker honoraria from Organon, Merck Serono and Ferring and participation in data safety monitoring or advisory board for Ferring.
• Sandro Esteves reports unrestricted research grants from Merck KGaA, reception of consulting fees from Merck, MeD.E.A and event planet, reception of honoraria for lectures from Merck, MeD.E.A and event planet, has a patent on the ART calculator, is an unpaid advisory board member for Nature Reviews and for Urology, Is the Head, Department of Education and Research, Brazilian Society of Urology (São Paulo section; unpaid) and is the Co-chair, Male Infertility Special Interest Group, WHO Infertility Guidelines (unpaid).
Conflicts of interest
• Ariel Weissman reports no conflicts of interest.
• Christophe Blockeel reports no conflicts of interest.
• Seang Lin Tan reports no conflicts of interest.
• Michael Dahan reports no conflicts of interest.
• Bulent Urman reports no conflicts of interest.
• RJ Norman reports no conflict of interest.
• Richard Paulson reports no conflicts of interest.
• Ido Feferkorn reports no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weiss MS, Luo C, Zhang Y, Chen Y, Kissin DM, Satten GA, et al. Fresh vs. frozen embryo transfer: new approach to minimize the limitations of using national surveillance data for clinical research. Fertil Steril [Internet]. Fertil Steril; 2022 [cited 2023 Feb 1]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/36567206/. [DOI] [PMC free article] [PubMed]
- 2.Ata B. Haste makes waste: don’t rush for a fresh embryo transfer in high responders. Human Reproduction [Internet]. Oxford Academic; 2020 [cited 2022 May 3];35:2660–2. Available from: https://academic.oup.com/humrep/article/35/12/2660/5917721. [DOI] [PubMed]
- 3.Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, et al. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ∼15,000 women. Fertil Steril. 2018;110:661–670.e1. doi: 10.1016/j.fertnstert.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 4.Scaravelli G, Levi-Setti PE, Livi C, la Sala G, Ubaldi FM, Greco E, et al. Contribution of cryopreservation to the cumulative live birth rate: a large multicentric cycle-based data analysis from the Italian National Registry. J Assist Reprod Genet [Internet]. J Assist Reprod Genet; 2019 [cited 2022 Sep 6];36:2287–95. Available from: https://pubmed.ncbi.nlm.nih.gov/31463873/. [DOI] [PMC free article] [PubMed]
- 5.Dahan MH, Tannus S, Seyhan A, Tan SL, Ata B. Combined modalities for the prevention of ovarian hyperstimulation syndrome following an excessive response to stimulation. Gynecol Endocrinol [Internet]. Gynecol Endocrinol; 2018 [cited 2022 Sep 6];34:252–5. Available from: https://pubmed.ncbi.nlm.nih.gov/29057693/. [DOI] [PubMed]
- 6.Committee of the American Society for Reproductive Medicine P. Pfeifer S, Butts MSCES, Dumesic D, Fossum G, Gracia MSCEC, et al. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril [Internet] 2016;106:1634–47. doi: 10.1016/j.fertnstert.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Tomás C, Colmorn L, Rasmussen S, Lidegaard Ø, Pinborg A, Andersen AN. Annual incidence of severe ovarian hyperstimulation syndrome. Dan Med J Almindelige Danske Laegeforening. 2021;68:1–8. [PubMed] [Google Scholar]
- 8.Sood A, Goel A, Boda S, Mathur R. Prediction of significant OHSS by ovarian reserve and ovarian response - implications for elective freeze-all strategy. Hum Fertil (Camb) [Internet]. Hum Fertil (Camb); 2022 [cited 2023 Feb 2];25:390–6. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/32835544/. [DOI] [PubMed]
- 9.The ESHRE Guideline Group on Ovarian Stimulation, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI†. Hum Reprod Open [Internet]. Oxford Academic; 2020 [cited 2022 Mar 17];2020:1–13. Available from: https://academic.oup.com/hropen/article/2020/2/hoaa009/5827574.
- 10.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod [Internet]. Hum Reprod; 2011 [cited 2022 Mar 17];26:1768–74. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/21558332/. [DOI] [PubMed]
- 11.Bosdou JK, Venetis CA, Tarlatzis BC, Grimbizis GF, Kolibianakis EM. Higher probability of live-birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: a meta-analysis. Human Reproduction [Internet]. Oxford Academic; 2019 [cited 2022 Mar 17];34:491–505. Available from: https://academic.oup.com/humrep/article/34/3/491/5303709. [DOI] [PubMed]
- 12.Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update [Internet]. Oxford Academic; 2019 [cited 2022 Jun 25];25:2–14. Available from: https://academic.oup.com/humupd/article/25/1/2/5155324. [DOI] [PubMed]
- 13.Boynukalin FK, Turgut NE, Gultomruk M, Ecemis S, Yarkiner Z, Findikli N, et al. Impact of elective frozen vs. fresh embryo transfer strategies on cumulative live birth: Do deleterious effects still exist in normal & hyper responders? PLoS One [Internet]. PLoS One; 2020 [cited 2022 Mar 17];15. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/32589634/. [DOI] [PMC free article] [PubMed]
- 14.Chen Z-J, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. New England Journal of Medicine [Internet]. New England Journal of Medicine (NEJM/MMS); 2016 [cited 2022 Apr 20];375:523–33. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1513873. [DOI] [PubMed]
- 15.Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of Fresh versus Frozen Embryos in Ovulatory Women. New England Journal of Medicine [Internet]. Massachusetts Medical Society; 2018 [cited 2022 Apr 20];378:126–36. Available from: https://www-nejm-org.proxy3.library.mcgill.ca/doi/10.1056/NEJMoa1705334. [DOI] [PubMed]
- 16.Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. 2014;101:967–973. doi: 10.1016/j.fertnstert.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer S, Butts S, Dumesic D, Fossum G, Giudice L, Gracia C, et al. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril [Internet]. Elsevier; 2015 [cited 2022 Mar 28];103:e9–17. Available from: http://www.fertstert.org/article/S0015028214025187/fulltext.
- 18.Lorusso F, Vicino M, Lamanna G, Trerotoli P, Serio G, Depalo R. Performance of different ovarian reserve markers for predicting the numbers of oocytes retrieved and mature oocytes. Maturitas. 2007;56:429–435. doi: 10.1016/j.maturitas.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Jayaprakasan K, Campbell B, Hopkisson J, Clewes J, Johnson I, Raine-Fenning N. Establishing the intercycle variability of three-dimensional ultrasonographic predictors of ovarian reserve. Fertil Steril. 2008;90:2126–2132. doi: 10.1016/j.fertnstert.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJM. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update [Internet]. Oxford Academic; 2011 [cited 2022 Mar 17];17:46–54. Available from: https://academic.oup.com/humupd/article/17/1/46/639734. [DOI] [PubMed]
- 21.Broer SL, Dólleman M, van Disseldorp J, Broeze KA, Opmeer BC, Bossuyt PMM, et al. Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta-analysis. Fertil Steril. 2013;100:420–429.e7. doi: 10.1016/j.fertnstert.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Anckaert E, Smitz J, Schiettecatte J, Klein BM, Arce JC. The value of anti-Müllerian hormone measurement in the long GnRH agonist protocol: association with ovarian response and gonadotrophin-dose adjustments. Hum Reprod [Internet]. Oxford University Press; 2012 [cited 2022 Mar 23];27:1829. Available from: /pmc/articles/PMC3357198/. [DOI] [PMC free article] [PubMed]
- 23.Arce JC, la Marca A, Mirner Klein B, Nyboe Andersen A, Fleming R. Antimüllerian hormone in gonadotropin releasing-hormone antagonist cycles: Prediction of ovarian response and cumulative treatment outcome in good-prognosis patients. Fertil Steril [Internet]. Elsevier Inc.; 2013 [cited 2022 Mar 23];99:1644–53. Available from: http://fertstertforum.com/arcejc-antimullerian-hormone-gnrh-antagonist-ovarian-response/. [DOI] [PubMed]
- 24.Nelson SM, Klein BM, Arce JC. Comparison of antimüllerian hormone levels and antral follicle count as predictor of ovarian response to controlled ovarian stimulation in good-prognosis patients at individual fertility clinics in two multicenter trials. Fertil Steril. 2015;103:923–930.e1. doi: 10.1016/j.fertnstert.2014.12.114. [DOI] [PubMed] [Google Scholar]
- 25.Peluso C, Oliveira R de, Laporta GZ, Christofolini DM, Fonseca FLA, Laganà AS, et al. Are ovarian reserve tests reliable in predicting ovarian response? Results from a prospective, cross-sectional, single-center analysis. Gynecol Endocrinol [Internet]. Gynecol Endocrinol; 2021 [cited 2022 Mar 23];37:358–66. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/32613875/. [DOI] [PubMed]
- 26.Punchoo R, Bhoora S. Variation in the Measurement of Anti-Müllerian Hormone – What Are the Laboratory Issues? Front Endocrinol (Lausanne) [Internet]. Frontiers Media SA; 2021 [cited 2022 Mar 31];12. Available from: /pmc/articles/PMC8446602/. [DOI] [PMC free article] [PubMed]
- 27.Li HWR, Robertson DM, Burns C, Ledger WL. Challenges in Measuring AMH in the Clinical Setting. Front Endocrinol (Lausanne) [Internet]. Frontiers Media S.A.; 2021 [cited 2022 Mar 23];12. Available from: /pmc/articles/PMC8183164/. [DOI] [PMC free article] [PubMed]
- 28.Tarlatzi TB, Venetis CA, Devreker F, Englert Y, Delbaere A. What is the best predictor of severe ovarian hyperstimulation syndrome in IVF? A cohort study. J Assist Reprod Genet [Internet]. Springer; 2017 [cited 2022 Mar 28];34:1341. Available from: /pmc/articles/PMC5633577/. [DOI] [PMC free article] [PubMed]
- 29.Delvigne A. Epidemiology of OHSS. Reprod Biomed Online [Internet]. 2009 [cited 2022 Mar 31];19:8–13. Available from: www.rbmonline.com/Article/3923. [DOI] [PubMed]
- 30.Sousa M, Cunha M, Teixeira da Silva J, Oliveira C, Silva J, Viana P, et al. Ovarian hyperstimulation syndrome: a clinical report on 4894 consecutive ART treatment cycles. Reprod Biol Endocrinol [Internet]. Reprod Biol Endocrinol; 2015 [cited 2022 Mar 31];13. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/26100393/. [DOI] [PMC free article] [PubMed]
- 31.Shibahara H, Shimada K, Morimatsu Y, Kikuchi K, Hirano Y, Suzuki T, et al. Severe ovarian hyperstimulation syndrome in a 42‐year‐old woman with successful pregnancy after intracytoplasmic sperm injection embryo transfer. Reprod Med Biol [Internet]. Wiley-Blackwell; 2005 [cited 2022 Mar 31];4:265. Available from: /pmc/articles/PMC5904648/. [DOI] [PMC free article] [PubMed]
- 32.van der Weiden RMF, Meijers CJH, Hegt VN. Ectopic mesothelial cell proliferation in cervical lymph nodes after severe ovarian hyperstimulation syndrome. Fertil Steril [Internet]. Fertil Steril; 2005 [cited 2022 Mar 31];83:739–41. Available from: https://pubmed.ncbi.nlm.nih.gov/15749507/. [DOI] [PubMed]
- 33.Luke B, Brown MB, Morbeck DE, Hudson SB, Coddington CC, Stern JE. Factors associated with ovarian hyperstimulation syndrome (OHSS) and its effect on assisted reproductive technology (ART) treatment and outcome. Fertil Steril. 2010;94:1399–1404. doi: 10.1016/j.fertnstert.2009.05.092. [DOI] [PubMed] [Google Scholar]
- 34.la Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Human Reproduction [Internet]. Oxford Academic; 2007 [cited 2022 Mar 31];22:766–71. Available from: https://academic.oup.com/humrep/article/22/3/766/2939092. [DOI] [PubMed]
- 35.Ferraretti AP, la Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of ‘poor response’’ to ovarian stimulation for in vitro fertilization: the Bologna criteria’. Human Reproduction [Internet]. Oxford Academic; 2011 [cited 2022 Mar 31];26:1616–24. Available from: https://academic.oup.com/humrep/article/26/7/1616/2913872. [DOI] [PubMed]
- 36.Alviggi C, Andersen CY, Buehler K, Conforti A, de Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril [Internet]. Elsevier; 2016 [cited 2022 Mar 31];105:1452–3. Available from: http://www.fertstert.org/article/S0015028216000893/fulltext. [DOI] [PubMed]
- 37.Nakhuda GS, Chu MC, Wang JG, Sauer M v., Lobo RA. Elevated serum müllerian-inhibiting substance may be a marker for ovarian hyperstimulation syndrome in normal women undergoing in vitro fertilization. Fertil Steril [Internet]. Elsevier; 2006 [cited 2022 Mar 31];85:1541–3. Available from: http://www.fertstert.org/article/S0015028206000963/fulltext. [DOI] [PubMed]
- 38.Lee TH, Liu CH, Huang CC, Wu YL, Shih YT, Ho HN, et al. Serum anti-müllerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Human Reproduction [Internet]. Oxford Academic; 2008 [cited 2022 Mar 31];23:160–7. Available from: https://academic.oup.com/humrep/article/23/1/160/561979. [DOI] [PubMed]
- 39.Ocal P, Sahmay S, Cetin M, Irez T, Guralp O, Cepni I. Serum anti-Müllerian hormone and antral follicle count as predictive markers of OHSS in ART cycles. J Assist Reprod Genet [Internet]. J Assist Reprod Genet; 2011 [cited 2022 Mar 31];28:1197–203. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/21882017/. [DOI] [PMC free article] [PubMed]
- 40.Salmassi A, Mettler L, Hedderich J, Jonat W, Deenadaya A, von Otte S, et al. Cut-Off Levels of Anti-Mullerian Hormone for The Prediction of Ovarian Response, In Vitro Fertilization Outcome and Ovarian Hyperstimulation Syndrome. Int J Fertil Steril [Internet]. Royan Institute; 2015 [cited 2022 Mar 31];9:157. Available from: /pmc/articles/PMC4518483/. [DOI] [PMC free article] [PubMed]
- 41.Vembu R, Reddy NS. Serum AMH Level to Predict the Hyper Response in Women with PCOS and Non-PCOS Undergoing Controlled Ovarian Stimulation in ART. J Hum Reprod Sci [Internet]. J Hum Reprod Sci; 2017 [cited 2022 Mar 31];10:91–4. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/28904496/. [DOI] [PMC free article] [PubMed]
- 42.Sene AA, Ashrafi M, Alaghmand-Fard N, Mohammadi N, Alisaraie MM, Alizadeh A. Anti-Müllerian Hormone Predictive Levels to Determine The Likelihood of Ovarian Hyper-Response in Infertile Women with Polycystic Ovarian Morphology. Int J Fertil Steril [Internet]. Int J Fertil Steril; 2021 [cited 2022 Mar 31];15:115–22. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/33687164/. [DOI] [PMC free article] [PubMed]
- 43.Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Prediction of high ovarian response to controlled ovarian hyperstimulation: anti-Müllerian hormone versus small antral follicle count (2–6 mm). J Assist Reprod Genet [Internet]. J Assist Reprod Genet; 2009 [cited 2022 Mar 24];26:319–25. Available from: https://pubmed.ncbi.nlm.nih.gov/19543966/. [DOI] [PMC free article] [PubMed]
- 44.Fertility problems: assessment and treatment Clinical guideline. 2013 [cited 2022 Apr 1]; Available from: www.nice.org.uk/guidance/cg156.
- 45.Izhar R, Husain S, Tahir MA, Kausar M, Sana T, Ghalib F. Antral follicle count and anti-Müllerian hormone level as predictors of ovarian hyperstimulation syndrome in women with polycystic ovarian syndrome undergoing controlled ovarian stimulation. J Ultrason [Internet]. Polish Ultrasound Society; 2021 [cited 2022 Apr 1];21:e200. Available from: /pmc/articles/PMC8439128/. [DOI] [PMC free article] [PubMed]
- 46.Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2006;85:112–120. doi: 10.1016/j.fertnstert.2005.07.1292. [DOI] [PubMed] [Google Scholar]
- 47.Griesinger G, Verweij PJM, Gates D, Devroey P, Gordon K, Stegmann BJ, et al. Prediction of Ovarian Hyperstimulation Syndrome in Patients Treated with Corifollitropin alfa or rFSH in a GnRH Antagonist Protocol. PLoS One [Internet]. Public Library of Science; 2016 [cited 2022 Apr 4];11:e0149615. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0149615. [DOI] [PMC free article] [PubMed]
- 48.Simón C, Cano F, Valbuena D, Remohí J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod [Internet]. Hum Reprod; 1995 [cited 2022 Apr 4];10:2432–7. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/8530680/. [DOI] [PubMed]
- 49.Simón C, Garcia Velasco JJ, Valbuena D, Peinado JA, Moreno C, Remohí J, et al. Increasing uterine receptivity by decreasing estradiol levels during the preimplantation period in high responders with the use of a follicle-stimulating hormone step-down regimen. Fertil Steril. 1998;70:234–239. doi: 10.1016/S0015-0282(98)00140-X. [DOI] [PubMed] [Google Scholar]
- 50.Pellicer A, Valbuena D, Cano F, Remohi J, Simon C. Lower implantation rates in high responders: evidence for an altered endocrine milieu during the preimplantation period. Fertil Steril. 1996;65:1190–1195. doi: 10.1016/S0015-0282(16)58337-X. [DOI] [PubMed] [Google Scholar]
- 51.Sharara FI, McClamrock HD. High estradiol levels and high oocyte yield are not detrimental to in vitro fertilization outcome. Fertil Steril. 1999;72:401–405. doi: 10.1016/S0015-0282(99)00293-9. [DOI] [PubMed] [Google Scholar]
- 52.Erşahin AA, Acet M, Erşahin SS, Dokuzeylül Güngör N. Frozen embryo transfer prevents the detrimental effect of high estrogen on endometrium receptivity. J Turk Ger Gynecol Assoc [Internet]. J Turk Ger Gynecol Assoc; 2017 [cited 2022 Apr 4];18:38–42. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/28506949/. [DOI] [PMC free article] [PubMed]
- 53.Ng EHY, Yeung WSB, Lau EYL, So WWK, Ho PC. High serum oestradiol concentrations in fresh IVF cycles do not impair implantation and pregnancy rates in subsequent frozen–thawed embryo transfer cycles. Human Reproduction [Internet]. Oxford Academic; 2000 [cited 2022 Apr 4];15:250–5. Available from: https://academic.oup.com/humrep/article/15/2/250/555181. [DOI] [PubMed]
- 54.Li HWR, Lee VCY, Lau EYL, Yeung WSB, Ho PC, Ng EHY. Cumulative live-birth rate in women with polycystic ovary syndrome or isolated polycystic ovaries undergoing in-vitro fertilisation treatment. J Assist Reprod Genet [Internet]. Springer; 2014 [cited 2022 Mar 28];31:205. Available from: /pmc/articles/PMC3933606/. [DOI] [PMC free article] [PubMed]
- 55.Doronzo G, Russo I, Mattiello L, Anfossi G, Bosia A, Trovati M. Insulin activates vascular endothelial growth factor in vascular smooth muscle cells: influence of nitric oxide and of insulin resistance. Eur J Clin Invest [Internet]. John Wiley & Sons, Ltd; 2004 [cited 2022 Apr 20];34:664–73. Available from: https://onlinelibrary-wiley-com.proxy3.library.mcgill.ca/doi/full/10.1111/j.1365-2362.2004.01412.x. [DOI] [PubMed]
- 56.Gómez R, Ferrero H, Delgado-Rosas F, Gaytan M, Morales C, Zimmermann RC, et al. Evidences for the existence of a low dopaminergic tone in polycystic ovarian syndrome: implications for OHSS development and treatment. J Clin Endocrinol Metab [Internet]. J Clin Endocrinol Metab; 2011 [cited 2022 Apr 20];96:2484–92. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/21646367/. [DOI] [PubMed]
- 57.Carvalho LML, dos Reis FM, Candido AL, Nunes FFC, Ferreira CN, Gomes KB. Polycystic Ovary Syndrome as a systemic disease with multiple molecular pathways: a narrative review. Endocr Regul [Internet]. Endocr Regul; 2018 [cited 2022 Apr 30];52:208–21. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/31517612/. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Figure 1s: The initial questionnaire. (PDF 204 kb)
Supplementary file2 Figure 2s: Pie charts – agreement on the consensus statements (DOCX 95 kb)
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.