Abstract
Purpose
One of the causes of infertility is circadian rhythm disorders. This study aimed to investigate Clock 3111 T/C and Period3 VNTR (variable number tandem repeat) gene polymorphisms and these gene proteins, some biochemical parameters, and circadian rhythm hormones in infertile women.
Methods
Thirty-five infertile women and thirty-one healthy fertile women were included. Blood samples were taken in the mid-luteal phase. DNAs obtained from peripheral blood were analyzed using polymerase chain reaction-restriction fragment length polymorphism methods. Follicle-stimulating hormone, LH (luteinizing hormone), estradiol, prolactin, free triiodothyronine, fT4 (free thyroxine), thyroid-stimulating hormone, testosterone, cortisol, progesterone, prolactin, ferritin, vitamin B12, and folate levels in serum samples were determined by the electrochemiluminescence immunoassay method. Melatonin, Clock, and Period3 protein levels were determined with ELISA kits.
Results
There was a significant difference in the frequency of Period3 DD (Per34/4) genotype between the groups. The Clock protein level of the infertile group was higher than the fertile group. Clock protein levels of the fertile group were positively correlated with estradiol levels and negatively correlated with LH, prolactin, and fT4 levels. PER3 protein levels of the infertile group were negatively correlated with LH levels. Melatonin levels of the fertile group were positively correlated with progesterone levels and negatively correlated with cortisol levels. Melatonin levels of the infertile group were positively correlated with LH levels and negatively correlated with cortisol levels.
Conclusion
Per34/4 genotype may be an independent risk factor in infertile women. Different correlation results found in fertile and infertile women can form the basis for future studies.
Keywords: Clock 3111 T/C gene polymorphism, Period3 VNTR gene polymorphism, Melatonin, Infertility, Circadian rhythm
Introduction
Infertility is a growing health problem in industrialized countries. A better understanding of infertility at the molecular level may be significant for the development of new treatments for this disease. One of the causes of infertility is circadian rhythm disorders [1]. It is well known that the circadian rhythm is of great importance for human physiology and that sleep, a significant component of this rhythm, is mainly related to female fertility. Circadian rhythm disorders such as short sleep duration are associated with infertility and recurrent pregnancy losses [2].
Circadian rhythms are endogenously produced cycles with a period of approximately 24 h that include physiological variables such as hormone levels, sleep, body temperature, blood pressure, and metabolism. Human circadian clocks are controlled by a set of functionally linked clock genes, and variations in circadian rhythms can be associated with clock gene polymorphisms [3]. The molecular clock, a group of proteins that work in concert to create a transcriptional-translational feedback loop, controls the circadian rhythm. A significant transcription factor of this feedback loop is the Clock protein. The Clock 3111 T/C single-nucleotide polymorphism (SNP) is a genetic variation of the Clock gene [4]. Mutations or polymorphisms in the Clock gene may be associated with circadian rhythm disorders [5]. It has also been suggested that the variable number tandem repeat (VNTR) in Period3 (PER3) is associated with the circadian rhythm sleep disorder [3]. In addition, circadian rhythm gene disorders are likely to be important in the development of cancer and various other diseases [6].
The most accurate indicator of internal time in low ambient light conditions is the circadian cycle of endogenous melatonin. The endogenous circadian component of the sleepiness rhythm and the endogenous melatonin rhythm are closely related [7]. On the other hand, melatonin and its agonists have chronobiotic effects. So they can recalibrate the circadian system. Melatonin and its agonists help treat circadian rhythm disorders [8]. The use of melatonin as a chronobiotic drug in the treatment of synchronization and circadian disorders in humans and the success of these treatments underline the important role of melatonin in the synchronization of the circadian system [9].
The rhythmic release of melatonin begins during puberty. Rhythmic release continues throughout the life of the fertile woman by regulating ovarian functions. In addition, melatonin improves egg quality and the success of in vitro fertilization treatment [10]. Additionally, melatonin supplementation (dose dependent) has an effect on theca cell steroidogenesis [11].
Circadian rhythm disorders are one of the causes of infertility. Circadian rhythm disorders are associated with Clock 3111 T/C SNP and PER3 VNTR gene polymorphisms. Clock 3111 T/C SNP and PER3 VNTR gene polymorphisms were included in the study because of their association with circadian rhythm disorders. In addition, melatonin can reorganize the circadian system. This study was aimed to investigate the effects of Clock 3111 T/C SNP and PER3 VNTR gene polymorphisms and protein levels, and melatonin levels on infertility in women.
Materials and methods
Ethical approval statement
The study was initiated with the approval of the Ethics Committee of Health Sciences University Samsun Training and Research Hospital Clinical Research Ethics Committee dated 05/01/2020 and numbered 2020/5/5. Each volunteer participating in the study was informed about the study and the consent form was read and signed and included in the study.
Study design
The type of study was a single-center and case–control study. Thirty-five women, who were followed up with the diagnosis of infertility at Health Sciences University Samsun Training and Research Hospital, Department of Medical Genetics, were included in the infertile group. Individuals in this group were determined according to specific criteria. These criteria are as follows: not getting pregnant despite having regular sexual intercourse for at least 1 year, between the ages of 18–40, having regular menstrual cycles, having both tubes open in Hystero-Salpingo X-ray, having both ovaries intact, and having no other health problems other than infertility diagnosis. In addition, blood samples were taken from individuals in this group before undergoing any infertility treatment.
Thirty-one women aged 18–40 years, without any health problems, and with at least one live birth were included in the control group.
Blood samples of the control and infertile groups were taken in the mid-luteal phase (on the 3rd day of the menstrual cycle) in the morning fasting condition. Blood samples were placed in tubes with and without anticoagulant. DNA isolation was performed for genotyping by following the kit protocol and salt precipitation method with blood samples taken into tubes with anticoagulant. Blood samples taken into tubes without anticoagulant were kept in the laboratory for 20 min and coagulated. It was then centrifuged at 1550 × g at + 4 °C for 10 min, and serum samples were collected. Serum samples were placed in Eppendorf and kept in the freezer at – 20 °C until the day of analysis. Clock and PER3 protein and melatonin levels were measured from serum samples. In addition, iron, unsaturated iron binding capacity (UIBC), ferritin, vitamin B12, folate, estradiol (E2), follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone, progesterone, prolactin, free T3 (fT3), free T4 (fT4) thyroid-stimulating hormone (TSH), and cortisol levels were also measured.
Genotyping
Clock 3111 T/C SNP gene
Venous blood samples (~ 7 mL) were collected in tubes with ethylene di amine tetra acetic acid. Genomic DNA was extracted from leukocytes using a QIAamp DNA Blood Kit (Qiagen, Valencia, CA, USA). The Clock 3111 T/C gene polymorphism (rs1801260) was genotyped using polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) analysis. PCR–RFLP analysis of Clock 3111 T/C gen polymorphism is given in Table 1. Ten microliters of successfully amplified PCR product was digested with the restriction enzyme Bsp1286I (New England Biolabs, Beverly, MA) at 37 °C for 5 h, and the resulting fragments were visualized on a 2% agarose gel under ultraviolet light after ethidium bromide staining. The Bsp1286I enzyme cleaves the PCR product (221 bp) in two fragments (126 and 95 bp) in the presence of the C allele [12].
Table 1.
Investigation of polymorphisms of Per3 (VNTR) and Clock 3111 T/C by PCR and RFLP methods
| Gene polymorphism | PCR condition (temperature, °C) | Primers | Method |
|---|---|---|---|
| Per3 (VNTR) | PD: 95 °C. 5 min, D: 94 °C 45 s | F: 5‟-GGA ACC CAT TGC CCG AGC-3” | PCR |
| A: 60 °C 45 s, 72 °C 45 s (35 cycle) | R: 5‟-GGT GAG GAT TGC ATT AGG TC-3” | ||
| E: 72 °C 5 min | |||
| Clock 3111 T/C | PD: 95 °C 5 min, 94 °C 45 s | F: 5‟-GCT CCA ATG TAC GGG GTA AAC-3” | RFLP |
| A: 60 °C 45 s, 72 °C 45 s (35 cycle) | R: 5‟-GGG GAC TGA CTT GAG TGA CCT-3” | ||
| E: 72 °C 5 min |
Sec second, min minute, F forward, R reverse, PD pre-denaturation, D denaturation, A annealing, E extension
PER3 VNTR gen
PCR primer pairs (forward primer: 5’-TGTCTTTTCATGTGCCCTTACTT-3’; reverse primer: 5’-TGTCTGGCATTGGAGTTTGA-3’) were synthesized and purified by Sangon Inc. (Shanghai, China). Each 10 μL PCR reaction mixture contained 150 ng genomic DNA, 5 pM of each primer, and 2 × PCR mix buffer (Tiangen Biotech, Beijing, China). PCR methods of Per3 (VNTR) gen polymorphism is given in Table 1. The amplified products were analyzed by 2.5% agarose gel electrophoresis. A 401-bp fragment was amplified of the PER3 VNTR five repeat alleles. A 347-bp fragment was amplified from the PER3 VNTR four repeat alleles. Two bands of different sizes indicated heterozygote individuals. Genotype results were verified by direct sequencing [13].
Analysis of Clock and PER3 proteins, and melatonin level
The serum Clock protein level was determined using the Human Clock Homolog enzyme-linked immunosorbent assay (ELISA) kit (Bioassay Technology Laboratory (BT-Lab), Cat. No E7330Hu, Jiaxing, Zhejiang Province China). Serum PER3 protein level was determined using the Human Period Protein 3 ELISA kit (BT-Lab, Cat. No E7346Hu, Jiaxing, Zhejiang Province China). Serum melatonin level was determined using the Human Melatonin ELISA assay kit (BT-Lab, Jiaxing, Cat. No E1013Hu Jiaxing, Zhejiang Province China). The ELISA test was performed according to the method reported by the manufacturer. ELISA tests were performed according to the methods reported by the manufacturer. These analyzes were performed on the Tecan Infinite F50 instrument (Fornax Technologies, Breitscheid, Germany).
Other variables
Iron, UIBC, ferritin, vitamin B12, folate, E2, FSH, LH, testosterone, progesterone, prolactin, fT3, fT4, and TSH levels were analyzed in Health Sciences University Samsun Training and Research Hospital Biochemistry laboratory. These analyses were performed on a Roche Cobas 8000 autoanalyzer (Roche Diagnostics, Mannheim, Germany) according to the electrochemiluminescence immunoassay method using commercial kits (FSH; Lot No: 51926400, LH; Lot No: 48094900, E2; Lot No: 53910800, Prolactin; Lot No: 52654800, fT3; Lot No: 5743800, fT4; Lot No: 57294100, TSH; Lot No: 57176500, Testosterone; Lot No: 52096100, Cortisol; Lot No: 52794900, Progesterone; Lot No: 5299700, Prolactin; Lot No: 52654800, Ferritin; Lot No: 56820600, vitamin B12; Lot No: 56721412, Folate; Lot No: 52690940).
Statistical methods
Data analysis was performed using the Statistical Package for the Social Sciences 22 for Windows (IBM, New York, USA). Continuous variables were expressed as mean and standard deviation values. Categorical variables were expressed as percent frequency. p < 0.05 was considered significant. The normal distribution of the data was evaluated with Shapiro–Wilk normality tests. Continuous variables of normally distributed groups were compared with the independent sample t-test, which is a parametric test. Pearson correlation analysis was performed to see the relationship between protein levels and other parameters analyzed. Categorical variable data of PER3 VNTR and Clock 3111 T/C gene polymorphisms were compared with the chi-square analysis test.
Results
Descriptive information
The mean age of the control group included in the study was 30.79 ± 3.80, while the mean age of the infertile group was 26.54 ± 2.38 years. The mean and standard deviation values of sex hormones, circadian rhythm hormones, circadian rhythm proteins, and some biochemical parameters of the groups are presented in Table 2.
Table 2.
Mean and standard deviation values of sex hormones, circadian rhythm hormones, circadian rhythm proteins, and some biochemical parameters of the groups
| Parameters | Control (n = 31) | Infertile (n = 35) | p |
|---|---|---|---|
| Sex hormones | |||
| Estradiol (pg/mL) | 162.57 ± 104.03 | 63.90 ± 24.71 | < 0.001 |
| FSH (mIU/mL) | 12.48 ± 4.39 | 3.83 ± 1.79 | < 0.01 |
| LH (mIU/mL) | 48.98 ± 16.32 | 5.71 ± 2.40 | < 0.001 |
| Progesterone (ng/mL) | 3.56 ± 2.06 | 5.60 ± 3.47 | > 0.05 |
| Testosterone (ng/mL) | 17.90 ± 11.26 | 28.75 ± 14.32 | > 0.05 |
| Prolactin (ng/mL) | 13.29 ± 4.27 | 15.30 ± 3.67 | > 0.05 |
| Circadian rhythm hormones | |||
| Melatonin (ng/L) | 42.45 ± 4.31 | 34.25 ± 4.87 | > 0.05 |
| Cortisol (μg/dL) | 11.69 ± 6.07 | 10.27 ± 4.28 | > 0.05 |
| TSH (mIU/mL) | 2.49 ± 0.81 | 2.93 ± 0.58 | > 0.05 |
| fT3(pg/mL) | 3.21 ± 0.59 | 3.61 ± 0.59 | > 0.05 |
| fT4 (ng/mL) | 1.54 ± 0.22 | 1.64 ± 0.23 | > 0.05 |
| Circadian rhythm proteins | |||
| Period 3 (ng/L) | 840.35 ± 99.88 | 647.43 ± 106.37 | > 0.05 |
| Clock (ng/L) | 46.29 ± 7.31 | 184.44 ± 18.04 | < 0.001 |
| Biochemical parameters | |||
| Iron (mg/dL) | 86.38 ± 4.47 | 43.83 ± 4.50 | < 0.01 |
| UIBC (μg/dL) | 265.83 ± 9.10 | 291.46 ± 19.32 | > 0.05 |
| Ferritin (μg/L) | 32.55 ± 5.00 | 24.21 ± 3.92 | > 0.05 |
| Vitamin B12 (ng/L) | 253.54 ± 17.91 | 356.58 ± 35.27 | > 0.05 |
| Folate (mg/L) | 7.59 ± 0.93 | 12.52 ± 1.15 | < 0.01 |
Sex hormones
E2, FSH, and LH hormone levels of the infertile group were found to be significantly lower than the E2, FSH, and LH hormone levels of the control group (Table 2).
Circadian rhythm hormones
There was no significant difference between the infertile group and the control group in terms of melatonin and other circadian rhythm hormone levels (Table 2).
Clock and PER3 proteins
While there was no significant difference in the PER3 protein levels of the infertile group and the control group, the Clock protein levels of the infertile group were found to be significantly higher than the Clock protein levels of the control group (Table 2).
Biochemical parameters
The iron levels of the infertile group were significantly lower than the control group, and the folate levels of the infertile group were significantly higher than the control group (Table 2).
Correlation analysis
The correlation of Clock and PER3 proteins and melatonin levels with other examined parameters was analyzed (Table 3). Table 3, which includes the parameters that gave significant results according to the correlation analysis, was made.
Table 3.
Correlation of Clock and PER3 proteins and melatonin levels with other parameters
| Control (n = 31) | |||||||
| E2 | LH | Cortisol | Progesterone | Prolactin | fT4 | ||
| Melatonin | Cc | 0.194 | − 0.044 | − 0.972 | 0.453 | − 0.175 | 0.039 |
| P | > 0.05 | > 0.05 | < 0.001 | < 0.05 | > 0.05 | > 0.05 | |
| PER3 | Cc | 0.185 | − 0.289 | − 0.015 | 0.439 | − 0.347 | − 0.288 |
| P | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| Clock | Cc | 0.536 | − 0.483 | 0.231 | − 0.262 | − 0.449 | − 0.483 |
| P | < 0.05 | < 0.05 | > 0.05 | > 0.05 | < 0.05 | < 0.05 | |
| Infertile (n = 35) | |||||||
| E2 | LH | Cortisol | Progesterone | Prolactin | fT4 | ||
| Melatonin | Cc | 0.062 | 0.450 | − 0.797 | − 0.130 | − 0.007 | 0.190 |
| P | > 0.05 | < 0.05 | < 0.001 | > 0.05 | > 0.05 | > 0.05 | |
| PER3 | Cc | − 0.115 | − 0.448 | 0.421 | 0.107 | − 0.319 | − 0.152 |
| P | > 0.05 | < 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| Clock | Cc | 0.017 | − 0.253 | 0.363 | − 0.242 | 0.016 | 0.207 |
| P | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
Cc correlation coefficient
There was a positive correlation between Clock protein levels and E2 levels, and a negative correlation between LH, prolactin, and fT4 levels in the control group (p < 0.05). However, there was no significant relationship between Clock protein levels and all analyzed parameters in the infertile group (p > 0.05).
There was no significant correlation between PER3 protein levels and all analyzed parameters in the control group (P > 0.05). However, there was a negative correlation between PER3 protein levels and LH levels in the infertile group (p < 0.05).
There was a negative correlation between melatonin levels and cortisol levels, and a positive correlation between progesterone levels in the control group (p < 0.05). There was a negative correlation between melatonin levels and cortisol levels, and a positive correlation between LH levels in the infertile group (p < 0.05).
Gene polymorphism
Gene polymorphism, genotype, and allele distributions of the groups are given in Table 4. The PER3 VNTR genotypes were typically shown by 2.5% of agarose gel electrophoresis from PCR products. PER3 VNTR was amplified from five repeat alleles. A 347-bp fragment was amplified from the PER3 VNTR four repeat allele. Two bands of different sizes indicated heterozygous individuals (Fig. 1).
Table 4.
Genotype and allele distributions of PER3 and Clock 3111 T/C gene polymorphisms
| Polymorphism | Control (n = 31) | Infertile (n = 35) | p |
|---|---|---|---|
| PER3 VNTR | HWE p = 0.96 | HWE p = 0.06 | |
| Genotype, n (%) | |||
| DD (PER34/4) | 5 (16.13) | 14 (40.00) | < 0.05 |
| ID (PER34/5) | 14 (45.16) | 10 (28.57) | > 0.05 |
| II (PER35/5) | 12 (38.71) | 11 (31.43) | > 0.05 |
| II: ID + DD | 12:19 | 11:24 | > 0.05 |
| Allele, n (%) | |||
| I | 38 (61.29) | 32 (45.71) | > 0.05 |
| D | 24 (38.71) | 38 (54.29) | |
| Clock 3111 T/C | |||
| Genotype, n (%) | |||
| TT | 31 (100.00) | 33 (94.29) | > 0.05 |
| TC | 0 (0) | 2 (5.71) | > 0.05 |
| CC | 0 (0) | 0 | > 0.05 |
| TT: TC + CC | 31:00:00 | 33:02:00 | > 0.05 |
| Allele, n (%) | |||
| T | 62 (100) | 68 (95.00) | > 0.05 |
| C | 0 (0) | 2 (5.00) | |
| PER3 VNTR/Clock 3111 T/C, n (%) | |||
| II/TT | 12 (38.71) | 10 (28.57) | > 0.05 |
| ID/TT | 14 (45.16) | 10 (28.57) | > 0.05 |
| DD/TT | 5 (16.13) | 13 (37.14) | > 0.05 |
| II/TC | 0 (0) | 1 (2.86) | > 0.05 |
| DD/TC | 0 (0) | 1 (2.86) | > 0.05 |
Fig. 1.
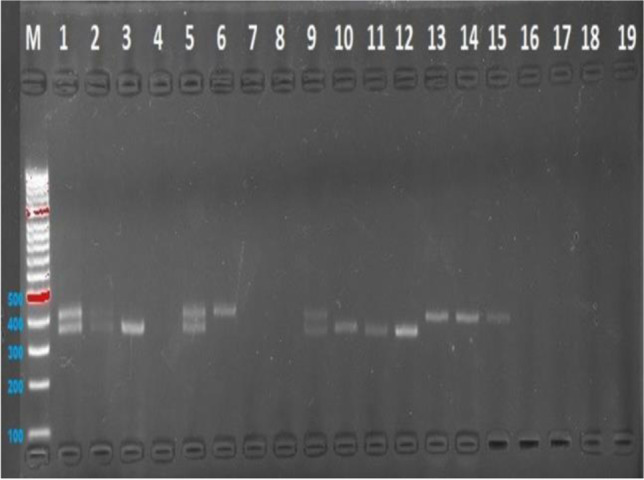
PER3 (VNTR) gen polymorphisms 401-bp fragment
The Clock 3111 T/C genotypes were typically shown by 2% agarose gel electrophoresis from PCR products. The Bsp1286I enzyme cleaves the PCR product (221 bp) into two fragments (126 and 95 bp) in the presence of the C allele (Figs. 2 and 3).
Fig. 2.
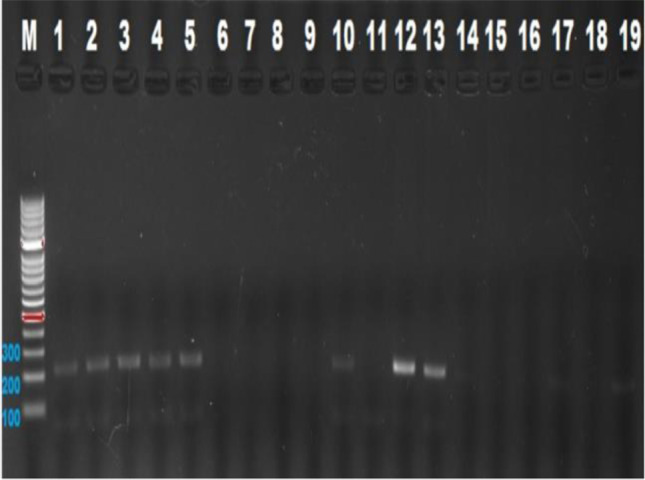
Clock 3111 T/C gen polymorphisms 126-bp fragment
Fig. 3.

Clock 3111 T/C gen polymorphisms 95-bp fragment
PER3 VNTR
The genotype distributions of the control and infertile groups were found to be in accordance with the Hardy–Weinberg equilibrium (HWE). According to the results of the comparison of genotype and allele frequencies of the control and infertile groups, a significant difference was found in the frequency of DD (PER34/4) genotype (Table 4).
Clock 3111 T/C
Since there was no CC homozygous genotype in both groups, concordance analysis of genotype distribution with HWE could not be performed. According to the results of the comparison of genotype and allele frequencies of the control and infertile groups, no statistically significant difference was found between the groups (Table 4).
PER3 VNTR /Clock 3111 T/C
No statistically significant difference was found in terms of PER3 VNTR /Clock 3111 T/C composite genotype frequencies of the control and infertile groups (Table 4).
Discussion
Reproduction is a significant biological function that requires precise synchronization with annual and daily cues to cope with environmental fluctuations. Industrialization and the modern 24/7 human lifestyle have brought harmful changes in their natural habitats and life rhythms in the last century. Shift work, endocrine-disrupting chemicals, and indecent exposure to excessive amounts of artificial light threaten the integrity of biological functions daily and seasonal timing [14]. In addition, the abnormal circadian clock caused by sleep habits and unhealthy diets in modern life has caused a partial decrease in female fertility. Abnormal circadian rhythm contributes to female reproductive system disorders [15].
Physiologically, the female reproductive system is governed in a time-dependent manner by the hypothalamic-pituitary–gonadal axis. The increase in FSH levels in the early phase of the menstrual cycle activates estrogen, which in turn stimulates LH secretion. About 36 to 40 h after LH levels rise mid-cycle, the dominant follicle releases an oocyte, and all hormones return to baseline levels [15]. Both FSH and beta-estradiol levels predict reproductive potential regardless of age [16]. In this study, it was determined that the FSH, LH, and E2 levels of the infertile group were lower than the fertile group in blood samples taken in the mid-luteal phase.
Circadian clock controls 24-h oscillations in physiology, gene expression, and behavior. Clock proteins (Period and Clock) play a significant role in the subnuclear rearrangement of core clock genes to control circadian rhythms [17]. Clock, one of these clock proteins, regulates daily rhythmicity in the binding of BRAHMA, a chromatin remodeler, to deoxyribonucleic acid (DNA) spanning clock-controlled genes to facilitate their rhythmic gene expression cycles [18]. In this study, no significant difference was found between fertile and infertile women’s PER3 protein levels. While there was a negative correlation between PER3 protein levels and LH levels in infertile women, this correlation was not found in fertile women. On the other hand, infertile women had higher Clock protein levels than fertile women. In addition, Clock protein levels were positively correlated with E2 and negatively correlated with LH, prolactin, and fT4 levels in fertile women. However, these correlations were not found in infertile women. Additionally, although some correlations were found, there may be more than one cause, and this study is only one of the first steps in this area.
Circadian release of the hormone melatonin is regulated by the suprachiasmatic nucleus, which feeds back into the nucleus to modulate sleep and the circadian phase. Melatonin has attracted attention as a potential treatment for circadian rhythm sleep disorders due to the sleep and circadian rhythm regulatory role of the suprachiasmatic nucleus [19]. Melatonin has been shown to improve sleep and correct circadian rhythm disturbance. The effectiveness of melatonin in the treatment of circadian rhythm disorders depends on the time of administration of melatonin supplementation. Measuring the endogenous melatonin rhythm is recommended for best results [20]. The effect of melatonin supplementation has been demonstrated in humans, particularly in disorders associated with reduced melatonin rhythms [21]. In addition, melatonin can be considered necessary for both folliculogenesis and spermatogenesis [22]. Moreover, melatonin increases LH receptor immunoreactivity in both theca and granulosa cells [23]. In this study, no difference was found between the melatonin levels of fertile and infertile women. Melatonin levels measured from morning blood samples from fertile and infertile women were also within the reference range. The reference range for serum melatonin in healthy women is 19.0–197 pg/mL at 3:00 am, in near complete darkness, and 3.30–93.2 pg/mL at 8:00 am [24]. On the other hand, melatonin levels were negatively correlated with cortisol in both fertile and infertile women. Additionally, melatonin levels were positively correlated with progesterone levels in fertile women, while melatonin levels were positively correlated with LH levels in infertile women, whereas LH levels are significantly reduced in rats given melatonin supplements [25]. In addition, melatonin supplementation contributes to luteinization for progesterone production during ovulation [26].
The mammalian circadian clock includes feedback (negative) and feed-forward (positive) cycles involving transcription, translation, and post-translational events [27]. The clock genes Clock, Bmal1, Rev-Erbα, Period, and Cryptochrome, which are transcription factors, play a role in these mechanisms [28]. Mutation of these genes disrupts the circadian rhythm, and at the same time, errors occur in the reproductive system, fertility, and pregnancy processes [29]. The main role of PER3 is to regulate sleep/wake timing and sleep homeostasis [30]. A length polymorphism in PER3 is associated with delayed sleep-phase disorder [31]. This length polymorphism is due to VNTR (4 or 5 repeats) in PER3. The shorter 4 alleles are strongly associated with delayed sleep-phase disorder [32]. In this study, the DD (PER34/4) genotype frequency of infertile women was higher than the DD (PER34/4) genotype frequency of fertile women.
The Clock gene is the central regulator of the circadian rhythm. The Clock and Arntl genes represent the central node that creates a positive loop and heterodimerizes and initiates the transcription of other clock genes. In addition, Clock gene polymorphism has a potential relationship with idiopathic recurrent spontaneous abortion [33]. High rates of fetal loss or offspring with an abnormal phenotype have been observed in female mice in which the Clock gene has been deleted. It was also stated that in these gene deletions, the circadian rhythms of the mice were disrupted by behavioral rhythm and locomotor activity experiments [34]. There was a significant relationship between the irregular menstrual cycle and Clock gene polymorphism [12]. In this study, since CC homozygous genotype was not found in both fertile and infertile women, there was no significant difference between the genotype and allele frequencies of the fertile and infertile women.
Conclusion
E2, LH, and FSH hormone levels of infertile women were lower than those of the fertile group. Clock protein levels of infertile women were higher than those of fertile women. Clock protein levels of fertile women were positively correlated with E2 levels and negatively correlated with LH, prolactin, and fT4 levels. However, Clock protein levels of infertile women were not correlated with these parameters. There was no difference between PER3 protein and melatonin levels of fertile and infertile women. While PER3 protein levels of infertile women were negatively correlated with LH levels, no such correlation was found in fertile women. On the other hand, melatonin levels were negatively correlated with cortisol in both fertile and infertile women. In addition, melatonin levels were positively correlated with progesterone levels in fertile women, while melatonin levels were positively correlated with LH levels in infertile women. Iron levels of infertile women were lower than those of fertile women. The folate levels of infertile women were higher than those of fertile women.
The frequency of DD (PER34/4) genotype of infertile women was higher than the frequency of fertile women. There were no significant differences in the frequencies of other genotypes and alleles of PER3 VNTR, and the frequencies of Clock 3111 T/C genotypes and alleles in fertile and infertile women. In addition, no statistically significant difference was found in terms of composite genotype frequencies of PER3 VNTR/Clock 3111 T/C gene polymorphisms.
Data Availability
The data in the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sciarra F, Franceschini E, Campolo F, Gianfrilli D, Pallotti F, Paoli D, Isidori AM, Venneri MA. Disruption of circadian rhythms: a crucial factor in the etiology of infertility. Int J Mol Sci. 2020;21(11):3943. doi: 10.3390/ijms21113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stocker LJ, Cagampang FR, Lu S, Ladyman T, Cheong YC. Is sleep deficit associated with infertility and recurrent pregnancy losses? Results from a prospective cohort study. AOGS. 2021;100(2):302–313. doi: 10.1111/aogs.14008. [DOI] [PubMed] [Google Scholar]
- 3.Osland TM, Bjorvatn BR, Steen VM, Pallesen S. Association study of a variable-number tandem repeat polymorphism in the clock gene PERIOD3 and chronotype in Norwegian university students. Chronobiol Int. 2011;28(9):764–70. doi: 10.3109/07420528.2011.607375. [DOI] [PubMed] [Google Scholar]
- 4.Ozburn AR, Purohit K, Parekh PK., Kaplan GN, Falcon E, Mukherjee S, Cates HM, McClung CA. Functional Implications of the CLOCK 3111T/C single-nucleotide polymorphism. Front Psychiatry 2016;7 10.3389/fpsyt.2016.00067 [DOI] [PMC free article] [PubMed]
- 5.Jones CR, Huang AL, Ptáček LJ, Fu YH. Genetic basis of human circadian rhythm disorders. Exp Neurol. 2013;243:28–33. doi: 10.1016/j.expneurol.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamont EL, James FO, Boivin DB, Cermakian N. From circadian clock gene expression to pathologies. Sleep Med. 2007;8(6):547–556. doi: 10.1016/j.sleep.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 8.Quera Salva MA, Hartley S, Barbot F, Alvarez CJ, Lofaso F, Guilleminault C. Circadian rhythms, melatonin and depression. Curr Pharm Des. 2011;17(15):1459–1470. doi: 10.2174/138161211796197188. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer M, Korf HW, Wicht H. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen Comp Endocrinol. 2018;258:215–221. doi: 10.1016/j.ygcen.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Rai S, Ghosh H. Modulation of human ovarian function by melatonin. Front Biosci (Elite Ed) 2021;13(1):140–157. doi: 10.2741/875. [DOI] [PubMed] [Google Scholar]
- 11.Adriaens I, Jacquet P, Cortvrindt R, Janssen K, Smitz J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology. 2006;228(2–3):333–343. doi: 10.1016/j.tox.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH, Kim Y, Ha J, Shin DW, Shin YC, Oh KS, Woo HY, Lim SW. Association between the CLOCK gene 3111 T > C polymorphism and an irregular menstrual cycle in Korean adolescents. J Psychosom Obstet Gynecol. 2015;36(4):148–154. doi: 10.3109/0167482X.2015.1089229. [DOI] [PubMed] [Google Scholar]
- 13.An H, Zhu Z, Zhou C, Geng P, Xu H, Wang H, Chen R, Qu X, Qian H, Gao Y, Zhao X, Qian Y. Chronotype and a PERIOD3 variable number tandem repeat polymorphism in Han Chinese pilots. Int J Clin Exp Med. 2014;7(10):3770–3776. [PMC free article] [PubMed] [Google Scholar]
- 14.Moralia MA, Quignon C, Simonneaux M, Simonneaux V. Environmental disruption of reproductive rhythms. Front Neuroendocrinol. 2022;66:100990. doi: 10.1016/j.yfrne.2022.100990. [DOI] [PubMed] [Google Scholar]
- 15.Shao S, Zhao H, Lu Z, Lei X, Zhang Y. Circadian Rhythms Within the Female HPG Axis: From Physiology to Etiology. Endocrinol. 2021;162(8):bqab117. doi: 10.1210/endocr/bqab117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makar RS, Toth TL. The evaluation of infertility. Pathology Patterns Reviews. 2002;117:95–103. doi: 10.1309/W8LJ-K377-DHRA-CP0B. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y, Yuan Y, Jimenez M, Soni N, Yadlapalli S. Clock proteins regulate spatiotemporal organization of clock genes to control circadian rhythms. Proc Nat Acad Sci. 2021;118(28):e2019756118. doi: 10.1073/pnas.2019756118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabuloc CA, Kwok RS, Chan EC, Hidalgo S, Cai YD, Chiu JC. CLOCK and TIMELESS regulate rhythmic occupancy of the BRAHMA chromatin-remodeling protein at clock gene promoters. bioRxiv 2022.10.21.513301; 10.1101/2022.10.21.513301 [DOI] [PMC free article] [PubMed]
- 19.Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Skene DJ, Josephine AJ. Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Med. 2007;8(6):651–655. doi: 10.1016/j.sleep.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol. 2018;175:3190–3199. doi: 10.1111/bph.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partonen T. Melatonin-dependent infertility. Med Hypotheses. 1999;52(3):269–270. doi: 10.1054/mehy.1997.0629. [DOI] [PubMed] [Google Scholar]
- 23.Tümentemur G, Altunkaynak BZ, Kaplan S. Is melatonin, leptin or their combination more effective on oxidative stress and folliculogenesis in the obese rats? J Obstet Gynaecol. 2020;40(1):116–127. doi: 10.1080/01443615.2019.1657816. [DOI] [PubMed] [Google Scholar]
- 24.Terzieva DD, Mateva ND, Vladimirova-Kitova LG. Melatonin reference limits at 3:00 AM and 8:00 AM in healthy adults. Clin Lab. 2009;55(9–10):359–361. [PubMed] [Google Scholar]
- 25.A Chuffa LG, Seiva FR, Fávaro WJ, Teixeira GR, Amorim J, Mendes LO, et al. Melatonin reduces LH, 17 beta-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation. Reprod Biol Endocrinol. 2011;9(1):1–9. doi: 10.1186/1477-7827-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taketani T, Tamura H, Takasaki A, Lee L, Kizuka F, Tamura I, Taniguchi K, Maekawa R, Asada H, Shimamura K, Reiter RJ, Sugino N. Protective role of melatonin in progesterone production by human luteal cells. J Pineal Res. 2011;51:207–213. doi: 10.1111/j.1600-079X.2011.00878.x. [DOI] [PubMed] [Google Scholar]
- 27.Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol. 2007;27(8):1694–1705. doi: 10.1161/ATVBAHA.107.144923. [DOI] [PubMed] [Google Scholar]
- 28.Orozco-Solis R, Sassone-Corsi P. Epigenetic control and the circadian clock: linking metabolism to neuronal responses. Neuroscience. 2014;264:76–87. doi: 10.1016/j.neuroscience.2014.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boden MJ, Varcoe TJ, Kennaway DJ. Circadian regulation of reproduction: from gamete to offspring. Prog Biophys Mol Biol. 2013;113(3):387–397. doi: 10.1016/j.pbiomolbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2010;14(3):151–160. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 32.Archer SN, Carpen JD, Gibson M, Lim GH, Johnston JD, Skene DJ, von Schantz M. Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep. 2010;33:695–701. doi: 10.1093/sleep/33.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodžić A, Lavtar P, Ristanović M, Novaković I, Dotlić J, Peterlin B. Genetic variation in the CLOCK gene is associated with idiopathic recurrent spontaneous abortion. PloS One. 2018;13(5):e0196345. doi: 10.1371/journal.pone.0196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14(15):1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in the current study are available from the corresponding author on reasonable request.


