Abstract
Purpose
To compare the diagnostic value of testicular tissue touch print smear (TPS) conducted on azoospermic patients with results obtained from histopathology and in vitro fertility (IVF) lab findings.
Methods
Microdissection testicular sperm extraction was performed on a group of 148 azoospermic patients and testicular samples obtained intraoperatively. Using TPS, the samples were smeared onto a sterile slide, followed with staining using thionine. The testis tissue bulk samples were also transferred to the IVF lab, and determinations of sperm presence or absence obtained from IVF lab tests were compared with the TPS sample results. Needle testis biopsy was separately performed on a group of 360 azoospermic patients, and results of pathohistology review on the biopsies were further compared with determinations of spermatogenesis stage obtained from TPS for those patients.
Results
When compared with IVF lab results, TPS was found to have 100% (126/126) positive predictive value and 95.5% (25/26) negative predictive value for predicting sperm presence or absence, respectively. Furthermore, TPS was further found to have a 93.6% correlation (337 of 360 biopsies) with results of histological diagnoses performed by needle biopsy. Results from histology and TPS for the detection of sperm presence were concordant in 96.1% (346/360) of biopsies. Diagnosis of SCO by TPS shows the highest correlation with histopathology (98.6%), followed by complete spermatogenesis (97.5%), early maturation arrest (78.9%), and late maturation arrest (27.3%).
Conclusions
The results support the continued use of TPS in testicular tissue analysis for more rapid assessment of spermatogenesis and for detection of spermatozoa in azoospermic subjects.
Keyword: Azoospermia, Spermatogenesis, Germ cells, Testis, Thionine
Introduction
Obtaining sperm from the testis through testicular sperm extraction (TESE), e.g., either with conventional TESE or with microdissection TESE (mTESE), has become an essential technique to help azoospermic patients achieve optimal fertilization via intracytoplasmic sperm injection (ICSI). TESE can be utilized to help either obstructive or non-obstructive azoospermic (NOA) men who seek fertility treatment to father biological children. Although some factors are known to be predictive of whether the TESE procedure will result in successful sperm retrieval [1], it remains difficult to pre-determine spermatogenesis in NOA patients prior to performing TESE. To increase the chances of successful spermatozoa yield, mTESE is employed to provide a rigorous examination of testicular parenchyma for dilated seminiferous tubules. Identifying the presence of viable spermatozoa during surgical sperm retrieval remains a challenge, however, as intraoperative assessment typically requires analyzing meticulously excised tubules from the testis in real time.
Various staining methods and techniques have been proposed to aid in the real-time identification of viable spermatozoa during TESE, including Papanicolaou’s stain, hematoxylin-eosin (H-E), the Diff-Quik staining method, and the wet prep technique [2–4]. Of these methods, the wet prep technique is currently the most popular, being widely used for at least two main reasons: (a) wet prep samples examined under high (40×) objective magnification are useful for observing sperm motility, which is not readily accessible via either of the aforementioned intra-operative staining methods, and (b) the fine details of sperm morphology can be thoroughly examined, thus enabling qualitative analysis. Despite these advantages, one drawback of the wet prep technique is that it usually requires an experienced embryologist to inspect the wet prep slides at high magnification to properly identify active spermatogenesis. Albeit effective, such real-time inspection is considered technique-demanding, labor-intensive, and time-consuming. While individuals with training can typically visually detect the presence of mature sperm, they may not have the ability to fully appreciate the nuances of the testicular histology that can inform the physician about the presence of spermatogenesis. In view of such limitations in the state of the art, we have developed a simple and rapid modified touch imprint method, which we refer to as touch print smear (TPS), for determining the quality and quantity of spermatogenesis. The smears from TPS can be readily and accurately interpreted by reproductive urologists, thus potentially obviating the need for intraoperative assistance from an embryologist. The aim of this study is to report on our experience using TPS, and to compare the diagnostic value of TPS with corresponding reference results obtained from histopathology and IVF lab sperm recovery results.
Materials and methods
Study population
The study was carried out retrospectively by reviewing the medical records of azoospermic patients who presented between Apr 2015 and Dec 2018 at Taipei Veterans General Hospital and who underwent testicular biopsy or mTESE, either for the purpose of differentiating the etiology of azoospermia or for retrieving sperm for use in assisted reproductive techniques by intracytoplasmic sperm injection (ICSI). A majority of cases undergoing mTESE for subsequent ICSI cycles which we enrolled in our study are the ones who had a prior diagnostic mTESE and spermatogenesis has been confirmed. Ethical approval was obtained from Taipei Veterans General Hospital Human Research Ethics Committee (IRB: 2020-01-020 BC#1)
Touch print smear procedure
Retrieved testicular tissue was blotted gently on dry gauze and touched and smeared in whirl manner upon a sterile microscope slide and let air-dried for 5 s. The smeared slide was first immersed in thionine stain solution (0.5%) for about 3 s, and then rinsed with running water to wash away the thionine stain. After a sample was drained and blot dried completely, it was mounted with a small drop of mounting fluid (refractive index closes to 1.52) and covered by a glass coverslip. A single staining procedure typically required less than a minute to complete.
Touch print smear interpretation
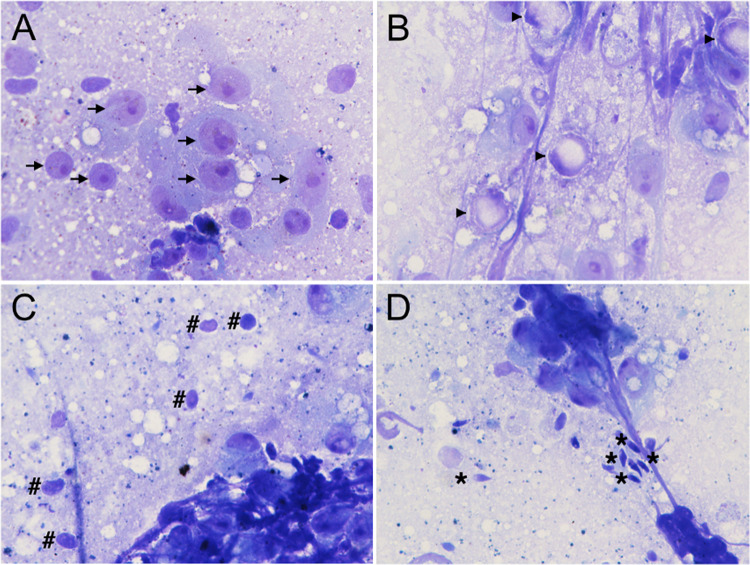
Once the slide was prepared, it was examined under a standard optical microscope at 40×, 100×, or 400× magnification. Two reproductive urologists evaluated the slides and categorized them into various pathologic entities as demonstrating either complete spermatogenesis (CS), early maturation arrest (EMA), late maturation arrest (LMA), or a Sertoli cell-only (SCO) pattern (Fig. 1). Note we chose to employ the term CS to encompass both normal spermatogenesis and hypospermatogenesis, as these conditions may be hard to distinguish solely on the basis of cytology out of a piece of testicular sample.
Fig. 1.
Cytological features of patients diagnosed with A Sertoli cell-only: showing only scattered clusters of Sertoli cell (arrow). B Early maturation arrest: note the absence of spermatid or spermatozoa, while Sertoli cell and primary spermatocyte (arrowhead) continue to be prominent. C Late maturation arrest: spermatids (hashtag) are detected but without spermatozoa. D Complete spermatogenesis: the asterisk indicates mature spermatozoa. (Thionine stain, reduced from × 400)
Initial low-power examination of the focal area with heterogeneity usually provides a predictive indication of which spermatogenesis is more likely to occur. Since the Sertoli cell and the primary spermatocyte are clearly identifiable based on the characteristic criteria, they are typically recognized as landmark cells. Morphologically, the head of the sperm is about the size of nucleolus of the Sertoli cell. The presence of primary spermatocytes is crucial for a diagnosis of presence of developing germ cells. For cases identified as CS, the sperm could then be further identified at higher magnification.
Histopathologic diagnosis of testicular specimens and results from IVF lab
Touch imprints of testicular tissue obtained from a Vim-Silverman 14G biopsy needle (standard 3-piece needle with split cannula) were immersed in Bouin’s solution, followed by paraffin-wax embedding and sections staining with routine hematoxylin and eosin (H-E) for pathohistology review. As for mTESE testis tissue bulk, they were transferred to the IVF lab and further examined by an embryologist to determine the presence or absence of viable sperm within the sample under a stereomicroscope. All the studied specimens were processed for TPS prior to further examination (histopathology review or sperm retrieval in IVF lab).
Results
Results correlation between TPS and IVF
Successful sperm retrieval was found to be highly correlated with the identification of sperm presence using TPS. In particular, of the total 148 patients undergoing mTESE, 126 were identified by TPS as having sperm (i.e., spermatozoa present), and sperm was successfully isolated from the samples of all 126 patients at the IVF lab. As for the remaining 22 patients with no spermatozoa (i.e., spermatozoa absent) as identified by TPS, spermatozoa were successfully isolated at the IVF lab for only 1 patient. Thus, the positive predictive value (PPV) of TPS as an indicator of sperm presence was found to be 100%, whereas its negative predictive value (NPV) was 95.5%. In addition, the sensitivity and specificity were found to be 99.2% and 100%, respectively (Table 1).
Table 1.
Positive and negative predicting values for TPS in relation to sperm recovery by IVF lab
| TPS | IVF lab (sperm +) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| mTESE | ||||
| Spermatozoa (+) | 126 | 126 | 100% | – |
| Spermatozoa (−) | 22 | 1 | – | 95.5% |
| Overall | 148 | 127 | – | – |
mTESE microscopic testicular sperm extraction, IVF in vitro fertilization, PPV positive predictive value, NPV negative predictive value, TPS touch print smear
Further considering the 22 patients with no spermatozoa as identified by TPS, the presence of round spermatid was identified in 11 of those cases, solely primary spermatocyte was only identified in one case, while Sertoli cell-only patterns were identified for the other ten patients. The one out of the 22 patients with round spermatid but no spermatozoa showed on TPS were later found positive for germ cells in IVF lab.
Correlation between TPS and histopathology
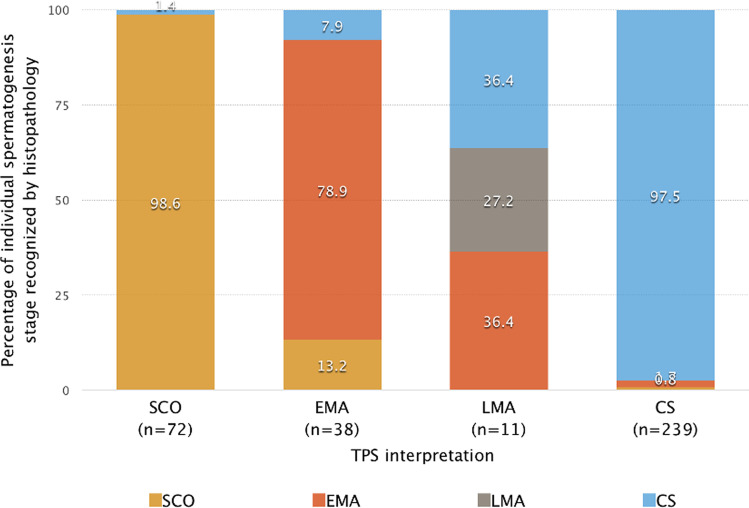
Of the 360 men with azoospermia undergoing needle biopsy, we found a 96.1% correlation (346/360) between histology and TPS results when it came to detecting the presence or absence of sperm. A 93.6% correlation (337 of 360 biopsies) was found between TPS and histological in the classification of spermatogenesis stage. In particular, diagnosis of SCO by TPS was found to have the highest correlation with histopathology (98.6%, 71/72), followed by CS (97.5%, 233/239), EMA (78.9%, 30/38), and LMA (27.3%, 3/11) (Table 2; Fig. 2). Of the eight patients (2.2%) with germ cells identified by histopathology exclusively (i.e., no spermatogenesis by TPS), TPS identified one patient as SCO, three as EMA, and four as LMA. Interestingly, of the 247 subjects classified as complete spermatogenesis (CS) by TPS, histological exam gave a discordant result of no spermatozoa in 6 cases (2.4%). Of those 6 cases, histological exam classified 2 as SCO and 4 as EMA. Sperm could only be identified by histopathology in one patient diagnosed as SCO by TPS.
Table 2.
Concordance between TPS and histopathology diagnosis
| TPS diagnosis | Concordance with histopathology | Presence of sperm by histopathology | Spearman’s correlation coefficient (rs) |
|---|---|---|---|
| SCO (n = 72) | 98.6% (71/72) | 1.4% (1/72) | |
| EMA (n = 38) | 78.9% (30/38) | 7.9% (3/38) | |
| LMA (n = 11) | 27.3% (3/11) | 36.4% (4/11) | |
| CS (n = 239) | 97.5% (233/239) | 97.5% (233/239) | |
| Overall (n = 360) | 93.6 (337/360) | – | 0.936 |
SCO Sertoli cell only, EMA early maturation arrest, LMA late maturation arrest, CS complete spermatogenesis
Fig. 2.
Concordance of TPS and histopathology in relation to individual germ cell types recognized by histopathology in relation to TPS findings
Follow-up procedure for subjects having sperm presence as identified by TPS or histopathology
With further regard to those 8 subjects having sperm presence as identified by histopathology exclusively (i.e., no sperm presence was identified by TPS), four (50%) subsequently underwent mTESE to have their spermatozoa retrieved for further ICSI. Of the 6 needle biopsy subjects having sperm presence as identified by TPS exclusively (i.e., no sperm presence identified by histopathology), three proceeded to further mTESE and 100% (3/3) of them had sperm retrieval for ICSI.
Time and cost saving at the operating room
By using TPS methodology, the saving of time and personnel at mTESE is tremendous. Thirty percent of operating time is shortened in average. Besides, since there is no need to have an embryologist to stand by in the operating room, the cost of the expert personnel is also saved.
Discussion
Given the importance of promptly diagnosing azoospermia, we undertook the current study to assess the feasibility and efficacy of TPS as a standalone method for rapid intra-operative diagnosis. It was found that results obtained from applying TPS to azoospermic men during testicular biopsies demonstrate a strong correlation with those obtained from histopathology and are thus highly predictive of the subsequent chances for successful sperm harvesting in the IVF lab. Furthermore, this simple staining method permits clear visualization of spermatogenic cells at different developmental stages, thus affording an efficient technique for helping reproductive urologists make accurate diagnoses in significantly less time.
Typically, staining techniques are required to visualize cell structures. Such staining must preserve the details of cell architecture and nuclei to allow physicians to distinguish among the different stages of germ cell maturation. Staining utilizes specific chemical reactions to highlight certain parts of the tissue by changing their color, thereby revealing their details and components. In our study, this is achieved by staining with thionine, a cation that intercalates into the DNA GC duplex structure and imparts a strong blue color to the nucleic acid abundant structure [5].
Spermatogenic cells at different stages of development have discrete morphological features that uniquely distinguish them from each other. For example, Sertoli cells tend to be homogenous and exhibit generally prominent nucleoli under staining, while pre-meiotic diploid primary spermatocytes are correspondingly characterized by deeply stained coarse chromatin that is peripherally distributed. Spermatozoa, on the other hand, are marked by their unevenly stained oval-shaped heads, giving them an appearance distinct from the uniformly stained round-headed spermatids. However, spermatozoa may sometimes be difficult to distinguish from late spermatids, which generally have an elongated oval shape. Finally, secondary spermatocytes are observed less frequently than germ cells in other stages of spermatogenesis, presumably due to their shorter appearance span (Fig. 1).
Several characteristic staining methods have been proposed for touch imprint specimens, for the purpose of examining the cellular content of the seminiferous tubules. For example, evaluation with Papanicolaou stains (a method adopted for cervical cancer screening since 1942 [6]) was described in 1987 as a way to interpret real-time intraoperative testicular histology during testicular needle biopsy, while simultaneously providing the capability to distinguish later-stage sperm maturation arrest from spermatozoa [2]. Modified staining methods such as H-E stain and Diff-Quick test have been reported to be much faster to process than Pap stains; however, they still require an estimated 1–2 min for nuclear and cytoplasm staining before sample fixation [3, 4]. In contrast, the thionine-based staining used in our study achieved a much shorter processing time of about 3 s. Another previous study compared wet preparation to touch-print cytology for sperm recovery and found that the latter method resulted in higher PPV, NPV, sensitivity, and specificity in those cases wherein histopathology indicated a low level (2%) of hypospermatogenesis [7]. The findings suggest there may be considerable room for improvement in the wet prep techniques currently used for intraoperative evaluation and diagnosis.
To evaluate the concordance between TPS and histopathology in our study, we first categorized the observed pathologic entities of 360 subjects using TPS, and then compared those categorizations with instances in which spermatozoa was subsequently discovered by histopathology analysis. Of the 72 of 360 cases initially categorized as SCO using TPS, 1.4% (1/72) was later discovered to contain spermatozoa using histopathology; of the 38 EMA cases, 7.9% (3/38) contained spermatozoa; and of the 11 LMA cases, 36.4% (4/11) contained spermatozoa. As for the 247 testicular needle biopsy specimens with sperm presence, 2.4% (6/247) was diagnosed as having sperm presence by TPS only, while cases having sperm presence diagnosed solely by histopathology exam accounted for 3.2% (8/247) of the total. The results suggest that combining TPS with histopathology may significantly increase the diagnostic accuracy of our technique for resolving complete spermatogenesis.
In one particular instance of note, three of the 6 subjects identified only by TPS as showing evidence of spermatozoa (i.e., histopathology found no spermatozoa) went on to undergo successful mTESE-ICSI procedures. This suggests that any diagnosis made solely on the basis histopathology may itself have accuracy limitations in assessing spermatogenesis. Note in this case, the other 3 of the 6 subjects did not undergo further sperm retrieval procedure due to financial and other personal reasons.
The abovementioned cases of discrepancy between TPS and histopathology diagnoses, especially the cases of LMA, may possibly be attributed to the fact that low numbers of spermatids are not clearly distinguishable in every pathological section, and/or to the difficulties of distinguishing spermatid from spermatozoa in those rare cases where only a small amount of specimen could be obtained by a single biopsy. If these are indeed the causes of the discrepancies, then they may be resolved by performing TPS multiple times when prior histopathology results indicate an absence of sperm, as demonstrated by our mTESE and IVF results correlation, which shows considerable promise with 100% of PPV and nearly 100% NPV by using TPS.
Another application of the present staining method may be to the determination of detailed testicular histology in varicocele repair. A meta-analysis clearly demonstrated that testis histopathology is a significant predictor of outcome with respect to NOA patients undergoing varicocelectomy [8]. In particular, NOA patients classified as LMA or HS, rather than EMA or SCO, are more likely to benefit from clinical varicocele repair. Of note, it was found that among all the classified stages of spermatogenesis, EMA had the worst outcome [8]. Therefore, assessment of cells in various stages of spermatogenesis obtained by testis biopsy using TPS can be incorporated into the real-time determination of whether to proceed with varicocelectomy in NOA men. In particular, for those men having unfavorable prognoses based on TPS findings, it would be rendered unnecessary to undergo the procedure to repair the varicocele.
It is noted that the relatively high sperm retrieval rates by mTESE in this study were significantly higher than the rates reported in a previous study evaluating SRR for azoospermia patients [9]. The reason for our higher retrieval rates may be that the medical expenses of obtaining first-attempt mTESE for diagnostic purposes (or “diagnostic mTESE”) are covered under the government-run, single-payer National Health Insurance (NHI) system in Taiwan, thus ensuring that such services are financially accessible to every resident. Accordingly, the majority of cases undergoing mTESE for subsequent ICSI cycles, which we analyzed in our study, were the ones for which sperm or spermatid was already discovered by diagnostic mTESE. In this study, we chose not to include cases undergoing initial “diagnostic mTESE” so as not to confuse our readers. At present, our IVF lab performs ICSI with fresh sperm instead of cryopreserved ones, despite the current state of the art being capable of achieving comparable fertility rates using either fresh or frozen sperm [10]. This is done by the IVF lab presumably to avoid potential failures in recovering post-thawed sperm, which was reported to have occurred in up to 5.8% of testicular cryopreserved samples [11]. For this reason, sperm identified during diagnostic mTESE was not cryopreserved.
To our knowledge, this is the first study done to compare intra-operative staining with IVF results. When mature sperm is present, the results obtained using this method were found to be perfectly correlated with other reference diagnostic techniques. At our institution, we are currently using this method to eliminate the need to have a standby embryologist present in the operating room, thereby substantially reducing the operating time of mTESE procedures by up to 30%.
Based on our results, TPS is a useful diagnostic modality for reproductive practice and can be recommended as a complementary approach to other established modalities, thereby significantly aiding in the determination of spermatogenesis stages. In fact, its applications are not limited to IVF sperm harvesting, but it may also be used as an effective diagnostic tool when performing testicular biopsies. In particular, practitioners in limited-resource settings such as office-based urology clinics may consider adopting TPS as a simple and efficient means to distinguish ductal obstructions from ablative testicular pathology.
Acknowledgements
The authors thank Howard En-Hao Tien and Irene Yea-Lan Chang for their valuable statistical assistance.
Author contribution
Conceptualization: William J Huang. Data curation: IS Huang, WJ Chen, William J Huang. Formal analysis: All authors.
Funding acquisition: William J Huang. Investigation: IS Huang, William J Huang. Title Page (with ALL authors information). Methodology: William J Huang. Project administration: IS Huang, William J Huang. Resources: William J Huang. Software: IS Huang. Supervision: William J Huang. Validation: IS Huang, William J Huang. Visualization: IS Huang, William J Huang. Writing — original draft: All authors. Writing — review and editing: All authors.
Funding
This work was supported by the Taipei Veterans General Hospital (VGH109-C-132) and National Science and Technology Council of Taiwan (109-2314-B-075-073-MY2) to William J. Huang.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flannigan R, Bach PV, Schlegel PN. Microdissection testicular sperm extraction. Transl Androl Urol. 2017;6(4):745–752. doi: 10.21037/tau.2017.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coburn M, Wheeler T, Lipshultz LI, Testicular biopsy. Its use and limitations. Urol Clin North Am. 1987;14(3):551–561. doi: 10.1016/S0094-0143(21)01759-6. [DOI] [PubMed] [Google Scholar]
- 3.Belker AM, Sherins RJ, Dennison-Lagos L. Simple, rapid staining method for immediate intraoperative examination of testicular biopsies. J Androl. 1996;17(4):420–426. [PubMed] [Google Scholar]
- 4.Kim ED, Greer JA, Abrams J, Lipshultz LI. Testicular touch preparation cytology. J Urol. 1996;156(4):1412–1414. doi: 10.1016/S0022-5347(01)65603-9. [DOI] [PubMed] [Google Scholar]
- 5.Reid GD, Whittaker DJ, Day MA, Turton DA, Kayser V, Kelly JM, et al. Femtosecond electron-transfer reactions in mono- and polynucleotides and in DNA. J Am Chem Soc. 2002;124(19):5518–5527. doi: 10.1021/ja0172363. [DOI] [PubMed] [Google Scholar]
- 6.Papanicolaou GN. A new procedure for staining vaginal smears. Science. 1942;95(2469):438–439. doi: 10.1126/science.95.2469.438. [DOI] [PubMed] [Google Scholar]
- 7.Kahraman S, Yakin K, Samli M, Vanlioğlu F, Karlikaya G, Sertyel S, et al. A comparative study of three techniques for the analysis of sperm recovery: touch-print cytology, wet preparation, and testicular histopathology. J Assist Reprod Genet. 2001;18(7):357–363. doi: 10.1023/A:1016602804661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weedin JW, Khera M, Lipshultz LI. Varicocele repair in patients with nonobstructive azoospermia: a meta-analysis. J Urol. 2010;183(6):2309–2315. doi: 10.1016/j.juro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Ramasamy R, Trivedi NN, Reifsnyder JE, Palermo GD, Rosenwaks Z, Schlegel PN. Age does not adversely affect sperm retrieval in men undergoing microdissection testicular sperm extraction. Fertil Steril. 2014;101(3):653–655. doi: 10.1016/j.fertnstert.2013.11.123. [DOI] [PubMed] [Google Scholar]
- 10.Kalsi J, Thum MY, Muneer A, Pryor J, Abdullah H, Minhas S. Analysis of the outcome of intracytoplasmic sperm injection using fresh or frozen sperm. BJU Int. 2011;107(7):1124–1128. doi: 10.1111/j.1464-410X.2010.09545.x. [DOI] [PubMed] [Google Scholar]
- 11.Kathrins M, Abhyankar N, Shoshany O, Liebermann J, Uhler M, Prins G, et al. Post-thaw recovery of rare or very low concentrations of cryopreserved human sperm. Fertil Steril. 2017;107(6):1300–1304. doi: 10.1016/j.fertnstert.2017.04.016. [DOI] [PubMed] [Google Scholar]