Abstract
Purpose
To determine whether using progesterone as a trigger of a gonadotropin surge will induce ovulation and a competent corpus luteum.
Methods
Patients were administered 5 or 10 mg of progesterone intramuscularly when the leading follicle reached preovulatory size.
Results
We demonstrate that progesterone injections result in classical ultrasonographic hallmarks of ovulation about 48 h later and the formation of a corpus luteum competent to support pregnancy.
Conclusion
Our results support further exploration of using progesterone to trigger a gonadotropin surge in assisted human reproduction.
Keywords: Progesterone, Trigger, IVF, IUI, Timed intercourse, Ovulation, Pregnancy, surge
Introduction
Ovulation is central to human reproduction. It is well established that ovulation is induced by GnRH binding to its pituitary receptors, thereby causing gonadotropins to surge. However, the identity of the upstream trigger of GnRH until recently remained not entirely settled. The working consensus has been that the upstream trigger is estradiol, since its peak coincides with the ovulatory surge of gonadotropins. In line with this observation, Leyendecker [1], in his foundational work, has demonstrated that in a postmenopausal female, the luteinizing hormone (LH) surge can be observed about 72 h after injection of estradiol benzoate. However, attempts to reproduce this finding in non-menopausal women were unsuccessful for reasons that were not understood.
For the lack of a usable physiological ovulation trigger, assisted reproduction has adopted human chorionic gonadotropin (hCG) and gonadotropin-releasing hormone (GnRH) receptors agonist as surrogates. However, neither of them creates a luteinization profile comparable to what is observed during natural ovulation [2], requiring luteal support, which can be painful and/or expensive, lasting for up to 12 weeks. Also, one of the crucial roles of the trigger is setting the parameters for the resumption of meiosis when most chromosomal errors occur [3]. The significance of this for the success of reproduction cannot be overstated. Since the surrogates cannot reproduce the natural profile of gonadotropins release, this may at least partially account for chromosomal errors in developing embryos.
Therefore, the search for a physiological trigger is ongoing. One of the promising candidates is kisspeptin, a potent second messenger in the GnRH signaling pathway. Kisspeptin is currently in the early stage of clinical trials [2]; its arrival to the market is uncertain.
Recently [4], we re-evaluated Leyendecker’s experiments in light of recent findings in human reproductive physiology and concluded that the gonadotropin surge observed by the Leyendecker group was not caused by peaking estradiol. On the contrary, we demonstrated that it was caused by estradiol falling below the threshold when it could no longer prevent GnRH-independent gonadotropin release from the pituitary [4]. This explained why estradiol was only “effective” in postmenopausal women and could not possibly be a physiological trigger. Ironically, in the same experiments, Leyendecker demonstrated an apparent surge of gonadotropins after progesterone (P4) injection. However, he dismissed progesterone as a potential ovulation trigger for two reasons. First, at the time, it was not known that progesterone begins to rise before LH surge. This will be shown only a decade later [5]. The second stumbling block was progesterone’s well-known ability to block ovulation, which made it an essential component of birth control pills. We reconciled the ability of progesterone to block and induce ovulation by postulating that progesterone is a GnRH agonist (not to be confused with GnRH receptors agonist). When administered close to the end of the follicular phase, progesterone’s binding to its hypothalamic receptors activates the GnRH signaling pathway and induces an LH surge. However, when present continuously at a high level, as in birth control pills, it desensitizes progesterone receptors in the hypothalamus, downregulating the GnRH signaling pathway and preventing an LH surge [4, 6]. This dual effect is similar to that of a pituitary GnRH receptors agonist, i.e., Lupron.
Thus, we proposed that the actual physiological trigger of ovulation is progesterone, which rises in an LH-independent fashion as a follicle begins to lose its integrity, signaling readiness for rupture [4, 6]. Since progesterone is already approved for many indications, including assisted reproduction, has an excellent safety record, and is inexpensive, it may become the ovulation trigger of choice. Here we report two cases of using progesterone to trigger ovulation.
Case reports
Case report 1, evidence of ovulation after progesterone trigger
A 30-year-old female, G2 P1, SAB1, with height 152 cm and weight 164 lb, was referred for infertility treatment following a 6-month period of inability to conceive. The patient’s medical history was significant for polycystic ovarian syndrome (PCOS) and for subclinical hypothyroidism, for which she was taking levothyroxine 100 mcg. The patient had menarche at age 12, an average period duration of 45 days, and an average menses duration of 6 days. Per HSG, the patient’s fallopian tubes were patent, and the uterine cavity was normal. Her AMH was 9.3 ng/ml. Partner’s sperm parameters were normal. Prior to the cycle with progesterone as a trigger, this patient had two unsuccessful intrauterine insemination (IUI) cycles using clomiphene citrate (CC)/letrozole and 10,000 IU of Novarel as a trigger.
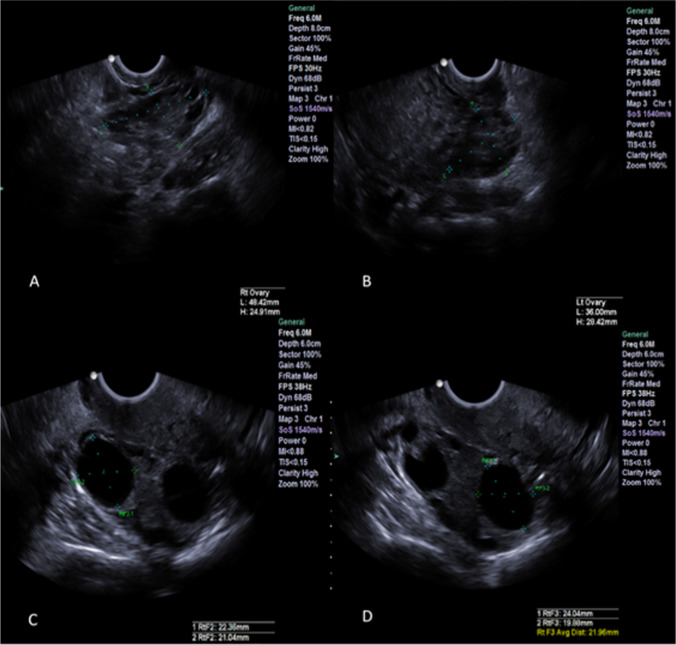
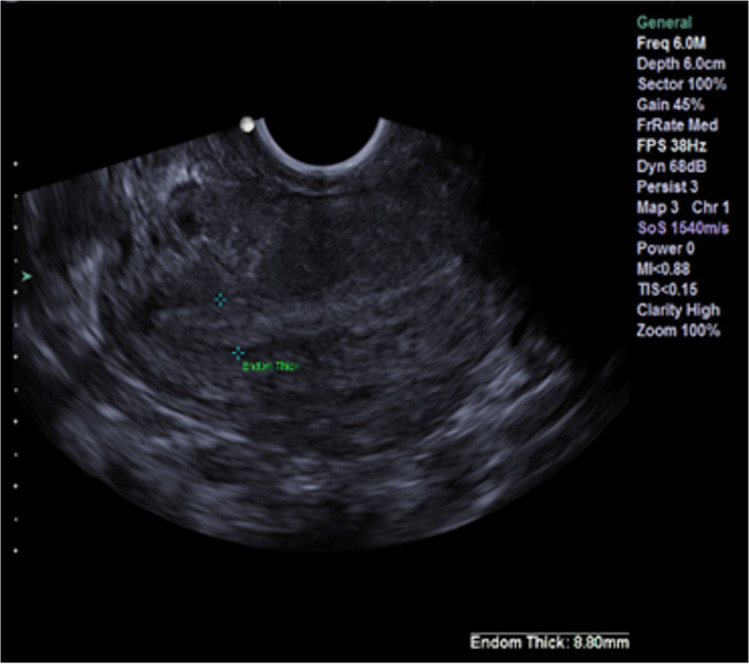
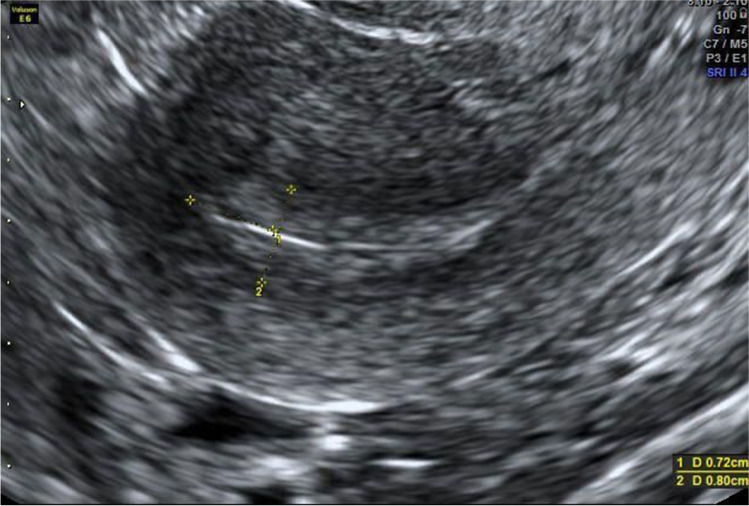
The medication regimen in the third attempt was the same as in the previous two cycles, with the exception of a trigger. On day 12 of the cycle, when the largest follicle was 19 mm, the patient was administered 0.25 mg of cetrotide. On day 14 of the cycle, the patient received a single injection of 5 mg of progesterone in oil. Just before the trigger her P4 and LH were 0.62 ng/ml and 4.26 IU/L, respectively, and ultrasound identified three follicles of 22, 22, and 19 mm (Fig. 1) and a receptive uterine lining (Fig. 2).
Fig. 1.
Case report 1: A–B Left and right ovary, respectively; C–D leading follicles in the right ovary before trigger shot
Fig. 2.
Case report 1: Uterine lining before trigger shot
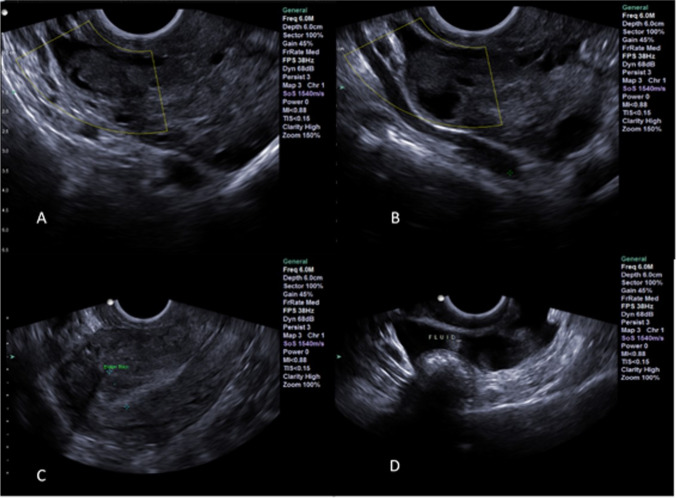
Seventeen hours later, the patient’s serum P4 and LH rose to 8.01 ng/ml and 37.01 IU/L, respectively, indicating a gonadotropin surge has taken place. The ultrasound performed on day 3 following the trigger shot confirmed that all three follicles ruptured (Fig. 3 A,B,C). Additionally, the patient had fluid behind the uterus (Fig. 3D)—a classic hallmark of ovulation. Seven days after the trigger shot, her serum P4 level was 38.39 ng/ml at which time the patient was started on 200-mg progesterone vaginal suppository daily. The patient had her period 2 weeks later indicating that no pregnancy occurred.
Fig. 3.
Case report 1: Three days after progesterone trigger shot. A–B Ruptured follicles (third ruptured follicle not shown); C uterine lining; D fluid behind the uterus
Case report 2, birth after progesterone trigger
A 37-year-old female, G0 P0, with height 163 cm and weight 136 lb, was first seen in the infertility clinic after 2 years of trying to conceive naturally. Her partner was diagnosed with male infertility following an unsuccessful vasectomy reversal. The patient has a regular ovulatory cycle of 29 days of duration.
In the first in vitro fertilization (IVF) cycle, 10 oocytes were retrieved following 12 days of controlled ovarian stimulation with recombinant follicle-stimulating hormone (FSH), and triggering oocytes maturation with 250-μg Ovidrel when the largest follicle reached 19 mm. The intracytoplasmic sperm injection (ICSI) of 6 matured oocytes yielded four zygotes and three blastocysts on day five. However, a fresh transfer of 2 embryos failed to establish pregnancy.
In the second IVF cycle, 8 oocytes were retrieved following 13 days of controlled ovarian stimulation by recombinant FSH with progesterone priming and triggering oocyte final maturation by Ovidrel, once the largest follicle reached 19 mm. The ISCI of 7 mature oocytes yielded 4 zygotes, of which 2 developed to below average quality blastocysts that were cryopreserved by vitrification on day 6.
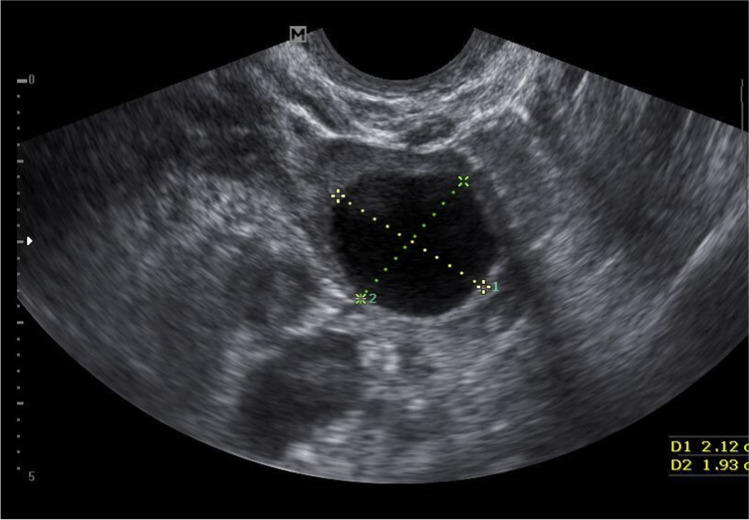
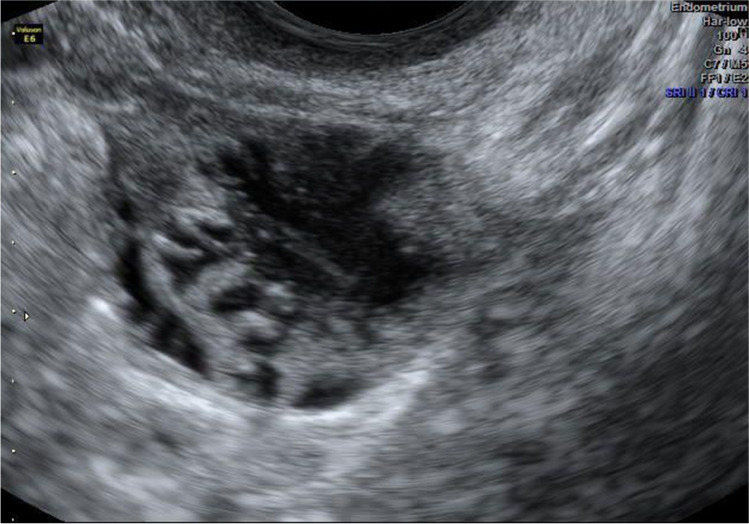
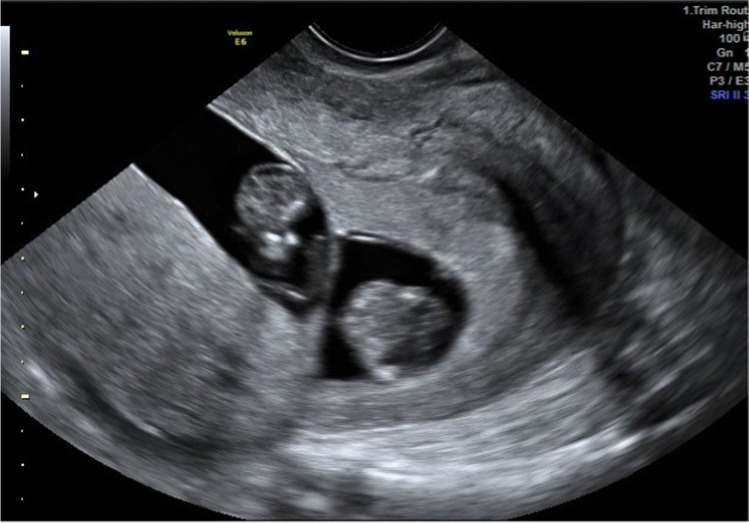
Frozen transfer was performed in a modified natural cycle as the following. On day 10, the patient was started on 100 mg of oral diclofenac/day. On day 12, when her dominant follicle measured 20 mm (Fig. 4), 10 mg of progesterone in oil was administered intramuscularly to trigger a gonadotropin surge. On the day of the progesterone injection, endometrial thickness was measured at 7 mm, and LH and P4 were at 9.3 mIU/ml and 0.3 ng/ml, respectively. Twenty-four hours later, LH and P4 were at 39 mIU/ml and 0.7 ng/ml, respectively. About 48 h after progesterone injection, the uterine ultrasound revealed a receptive uterine lining (Fig. 5) and a ruptured follicle with early evidence of corpus luteum formation measuring 20 mm (Fig. 6). Three days after the trigger, the patient started receiving 100 mg of micronized progesterone intravaginally twice a day. Two thawed average quality day 6 blastocysts were transferred into the uterus on day 7 after the trigger shot. Ten days after embryo transfer, beta hCG was measured at 1822 mIU/ml and at that time progesterone supplementation was stopped. At 6 weeks gestational ultrasound, two fetal sacks were observed. Subsequent ultrasound at 8.3 weeks showed two gestational sacs with heartbeat parameters expected for gestational age. At 10.1 weeks, ultrasound confirmed the vitality of both fetuses, with adequate growth (Fig. 7. Structural ultrasound at 12.3 weeks of gestation was unremarkable for both fetuses. The patient had a C-section at 34.5 weeks gestation, and two children, weighing 4.850 lb and 3.147 lb, with APGAR scores of 9–9 and 7–9, respectively, were delivered.
Fig. 4.
Case report 2: Follicle before the trigger measuring about 20 mm
Fig. 5.
Case report 2: Receptive uterine lining
Fig. 6.
Case report 2: Early corpus luteum measuring about 20 mm
Fig. 7.
Case report 2: Two gestational sacs at 10 weeks
Discussion
In various formulations and dosages, progesterone was previously shown to induce a gonadotropin surge [1, 7–10]. However, to our knowledge, this report is the first demonstration that progesterone triggers ovulation with a subsequent formation of a competent corpus luteum. Based on previously reported data [11], we estimate peak concentration of progesterone in blood 4 h postinjection at 3 ng/ml and 6 ng/ml for cases 1 and 2, respectively. This is well above the triggering threshold of progesterone of about 1 ng/ml found by Hoff and co-authors [5] in the natural cycle. Therefore, we believe that as little as 2 mg of progesterone can trigger ovulation. This amount contrasts sharply with the 100 mg daily injections required for luteal phase support.
One could argue that patients in this report ovulated spontaneously. Although such a possibility can never be ruled out completely, it is improbable for the following reasons. In both cases, at the time of the progesterone injection, its level in circulation was well below its reported triggering threshold [5], and the timeline of the LH rise following progesterone administration followed a virtually identical pattern, reaching 37 and 39 mIU/ml, respectively, at 17–24 h. Moreover, in the first case, the progesterone trigger was administered on day 14. In contrast, the patient’s spontaneous LH surge, given her 45 days cycle, would be expected not earlier than day 25, and no spontaneous LH surges were observed during her previous two IUI cycles. Spontaneous LH surge on day 12 in the second case is even less likely, because this patient has a very regular cycle of 29 days with ovulation on day 18, placing an estimated LH surge not earlier than day 16. Since this patient was receiving diclofenac, known to delay or block ovulation in a natural cycle IVF [12], a premature spontaneous surge of LH on day 12 becomes highly improbable.
Pregnancy in the second case progressed uneventfully until past the luteal-placental shift at 6–8 weeks of gestation following cessation of progesterone supplementation 2 weeks after the trigger. This indicates that the respective corpus luteum was fully competent. The physician’s decision to trigger on day 12 was performed preemptively to prevent a spontaneous LH surge. We speculate that delaying the administration of progesterone as a trigger to around day 16, as in the first case, may make progesterone supplementation for luteal phase support completely unnecessary. At 34.5 weeks, one of the fetuses was judged to be small for gestational age, necessitating a C-section. This outcome is not uncommon for twin pregnancies, including natural conceptions, and is not linked to corpus luteum deficiency. The mechanism of ovulation has been traditionally viewed as a solely hormonal interplay: rising estradiol engages the GnRH signaling pathway that produces a midcycle surge of gonadotropins. However, in practice, not only does estradiol fail to trigger ovulation when injected, but its continued suppression throughout the follicular phase does not affect the timing of ovulation, suggesting that its role in inducing ovulation has been misinterpreted [13]. Furthermore, it was never satisfactorily explained how estradiol, with its large inter-individual variation, can be timed to follicular readiness to rupture: the premature surge would produce a cyst [14] and a delayed release of a postmature oocyte [15]. Unlike estradiol, a progesterone rise can be traced directly to the initial stage of a follicle losing its integrity [4, 6]. According to our theory, binding to its receptors in the hypothalamus, progesterone signals to the GnRH pathway that the follicle is primed to rupture and ovulate [4, 6]. Even though this LH-independent rise in progesterone is small in absolute numbers, increasing from about 0.2 ng/ml to about 1 ng/m in circulation, in relative terms, this is about a 300% surge within 12 h [5]. Thus, according to our theory, disruption of follicular integrity resulting from inflammation and the inability of the ovarian cortex to accommodate further follicular expansion mediates the interplay of the hormones responsible for eliciting ovulation.
As a GnRH agonist, similar to a GnRH receptor agonist, progesterone would be expected to block or induce ovulation, depending on the timing and mode of administration [4, 6]. Indeed, in the second case, progesterone was used to prevent ovulation during controlled ovarian stimulation and trigger ovulation for embryo transfer in the natural cycle.
Our results show that injection of 5 to 10 mg of progesterone close to the end of the follicular phase results in ovulation, formation of a competent corpus luteum, and pregnancy. This indicates that triggering with progesterone may render luteal phase support unnecessary or considerably truncated. A clinical trial would be a logical next step in exploring all benefits and limitations of using progesterone as a physiological trigger of gonadotropins.
Declarations
Conflict of interest
Dr. Diamond and Dr. Dozortsev have pending patent for progesterone repurposing for triggering ovulation.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leyendecker G, Wardlaw S, Nocke W. Experimental studies on the endocrine regulations during the periovulatory phase of the human menstrual cycle. The effects of exogenous 17 -oestradiol and progesterone on the release of pituitary luteinizing and follicle stimulating hormones. Acta Endocrinol (Copenh) 1972;71(1):160–78. doi: 10.1530/acta.0.0710160. [DOI] [PubMed] [Google Scholar]
- 2.Sharma B, Koysombat K, Comninos AN, Dhillo WS, Abbara A. Use of kisspeptin to trigger oocyte maturation during in vitro fertilisation (IVF) treatment. Front Endocrinol (Lausanne) 2022;13:972137. doi: 10.3389/fendo.2022.972137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wartosch L, Schindler K, Schuh M, et al. Origins and mechanisms leading to aneuploidy in human eggs. Prenat Diagn. 2021;41(5):620–630. doi: 10.1002/pd.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dozortsev DI, Diamond MP. Luteinizing hormone-independent rise of progesterone as the physiological trigger of the ovulatory gonadotropins surge in the human. Fertil Steril. 2020;114(2):191–199. doi: 10.1016/j.fertnstert.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Hoff JD, Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57(4):792–796. doi: 10.1210/jcem-57-4-792. [DOI] [PubMed] [Google Scholar]
- 6.Dozortsev D, Pellicer A, Diamond MP. Progesterone is a physiological trigger of ovulatory gonadotropins. Fertil Steril. 2020;113(5):923–924. doi: 10.1016/j.fertnstert.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Rothchild I. Interrelations between progesterone and the ovary, pituitary, and central nervous system in the control of ovulation and the regulation of progesterone secretion. Vitam Horm. 1965;23:210–327. [PubMed] [Google Scholar]
- 8.Odell WD, Swerdloff RS. Progestogen-induced luteinizing and follicle-stimulating hormone surge in postmenopausal women: a simulated ovulatory peak. PNAS. 1968;61(2):529–536. doi: 10.1073/pnas.61.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JH, Yen SS. Induction of midcycle gonadotropin surge by ovarian steroids in women: a critical evaluation. J Clin Endocrinol Metab. 1983;57(4):797–802. doi: 10.1210/jcem-57-4-797. [DOI] [PubMed] [Google Scholar]
- 10.Zalányi S. Progesterone and ovulation. Eur J Obstet Gynecol Reprod Biol. 2001;98(2):152–159. doi: 10.1016/S0301-2115(01)00361-X. [DOI] [PubMed] [Google Scholar]
- 11.Nillius SJ, Johansson ED. Plasma levels of progesterone after vaginal, rectal, or intramuscular administration of progesterone. Am J Obstet Gynecol. 1971;110(4):470–477. doi: 10.1016/0002-9378(71)90686-7. [DOI] [PubMed] [Google Scholar]
- 12.Rijken-Zijlstra TM, Haadsma ML, Hammer C, Burgerhof JG, Pelinck MJ, Simons AH, van Echten-Arends J, Arts JG, Land JA, Groen H, Hoek A. Effectiveness of indomethacin to prevent ovulation in modified natural-cycle IVF: a randomized controlled trial. Reprod Biomed Online. 2013;27(3):297–304. doi: 10.1016/j.rbmo.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Hurst BS, Merriam KS, Elliot M, Matthews ML, Marshburn PB, Usadi RS, Hurst BS. A sustained elevated estradiol is not the trigger for the preovulatory luteinizing hormone surge. Women’s Health Gynecol. 2015;1:1–3. [Google Scholar]
- 14.Dozortsev DI, Pellicer A, Diamond MP. Premature progesterone rise as a trigger of polycystic ovarian syndrome. Fertil Steril. 2020;114(5):943–944. doi: 10.1016/j.fertnstert.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Dozortsev DI, Diamond MP. Two peas from the same pod: vanishing follicles and postmature oocytes. Fertil Steril. 2022;117(1):40–41. doi: 10.1016/j.fertnstert.2021.09.027. [DOI] [PubMed] [Google Scholar]