Abstract
Objective
To assess the value of intrauterine PRP to improve IVF outcome in women with previous implantation failure.
Methods
Screening of Pubmed, Web of Science, and other databases from inception to August 2022 using the keywords related to “platelet-rich plasma” OR “PRP” AND “IVF” “implantation failure.” Twenty-nine studies (3308 participants) were included in our analysis, 13 were RCTs, 6 were prospective cohorts, 4 were prospective single arm, and 6 were retrospective analyses. Extracted data included settings of the study, study type, sample size, participants’ characteristics, route, volume, timing of PRP administration, and outcome parameters.
Results
Implantation rate was reported in 6 RCTs (886 participants) and 4 non-RCTs (732 participants). The odds ratio (OR) effect estimate was 2.62 and 2.06, with 95% CI of 1.83, 3.76, and 1.03–4.11, respectively. Endometrial thickness was compared in 4 RCTs (307 participants) and 9 non-RCTs (675 participants), which showed a mean difference of 0.93 and 1.16, with 0.59–1.27 and 0.68–1.65 95% CI, respectively.
Conclusion
PRP administration improves implantation, clinical pregnancy, chemical pregnancy, ongoing pregnancy, live birth rates, and endometrial thickness in women with previous implantation failure.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02781-4.
Keywords: Platelet-rich plasma, PRP, Autologous platelet-rich plasma, Implantation failure, Thin endometrium
Introduction
IVF failure is mostly related to implantation failure. Successful implantation requires a precisely synchronized development of both endometrium and blastocyst [1]. Optimized endometrial development requires cellular, vascular, and immunological modifications [2].
These changes include the replacement of the endometrial stromal cells by the decidual cells. The latter is characterized by the development of apical projections (pinopodes), glandular growth, and the development of microvilli on the endometrial luminal epithelial surface [3]. These cellular changes are associated with modifications of adhesion molecules, cytokines, growth factors, and loss of inhibitory mediators, resulting in vascular invasion and endometrial immune cell infiltration [4].
Various interventions have been tried to improve implantation, especially for those with repeated implantation failure (RIF). These interventions include endometrial scratch injury [5], hysteroscopic correction of cavity pathology [6], improving endometrial thickness in women with thin endometrium [7, 8], intrauterine administration of autologous peripheral blood mononuclear cells [9], human chorionic gonadotropin [10], granulocyte colony-stimulating factor [11], growth hormone [12], intravenous Atosiban [13], and the use of immunomodulators [14]. However, even with these new treatment approaches, many patients still suffer from RIF. Therefore, there is a need for an alternative treatment with more success in patients with a history of treatment failure.
Platelet-rich plasma (PRP), also known as autologous conditioned plasma, is a concentrate of platelet-rich blood prepared through centrifugation of fresh whole blood to remove red and white blood cells. The resultant precipitate is rich in growth factors and cytokines (e.g., VEGF, TGFβ, and PDGF) released from activated platelets α-granules [15]. PRP has regenerative and anti-inflammatory characteristics and has been used in various medical fields, such as ophthalmology and orthopedics [16].
It was first applied to improve refractory endometrium by Chang in 2015 [17]. Since then, it has been studied in the treatment of female infertility in women with RIF, thin endometrium, premature ovarian failure, and Asherman syndrome. The results of these studies revealed conflicting findings, especially in those with implantation failure and thin endometrium [9]. This systematic review and meta-analysis aimed to assess the value of intrauterine PRP to improve IVF outcomes in women with previous implantation failure.
Material and methods
A prospectively prepared protocol that follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for meta-analysis was registered at PROSPERO. The registration number was CRD42022327811.
Eligibility criteria, information sources, and search strategy
Two authors (AM, AE) searched Pubmed, Web of Science, Scopus, and the Cochrane Central Register of Controlled Trials electronic databases from inception to August 2022 using the keywords “platelet-rich plasma” OR “PRP” OR “autologous platelet-rich plasma”) AND “IVF” “ICSI” “implantation failure” OR “thin endometrium” and their MeSH terms. Abstracts of conferences, Google Scholar, and reference and citation lists of the subject-related studies were checked for any additional studies. Contacting the authors was done if any clarifications or additional data were needed through emails. Details about the search strategy are provided in Supplementary Table 1.
Study selection
All available studies—with no language limitations—involved PRP administration around the time of embryo transfer in IVF/ICSI cycles. The types of studies included randomized controlled trials (RCTs) mainly. A separate analysis for cohort or single-arm studies, whether prospective or retrospective, was done. Studies compared PRP to no intervention, placebo, or granulocyte colony-stimulating factor (GCSF) were included. All routes of administration, whether intrauterine or subendometrial, were also included. Excluded studies included in vitro (cell culture) studies, animal studies, case reports, and studies with an inadequate methodology or unclear outcomes (and cannot be clarified by author correspondence).
Data extraction
Two authors (AM and NS) examined the search results titles and abstracts according to the predetermined eligibility criteria and then evaluated the full articles of the related studies. Any disagreement between the 2 authors regarding inclusion was discussed with other co-authors. Data from selected articles were extracted independently by 2 authors (AM and NS), and disagreement was dealt with in the same way as inclusion. Extracted data included settings of the study, sample size, participants’ inclusion and exclusion criteria, intervention characteristics, outcome parameters, registration, and funding data. The authors were contacted to clarify any vague or missed data.
Assessment of risk of bias
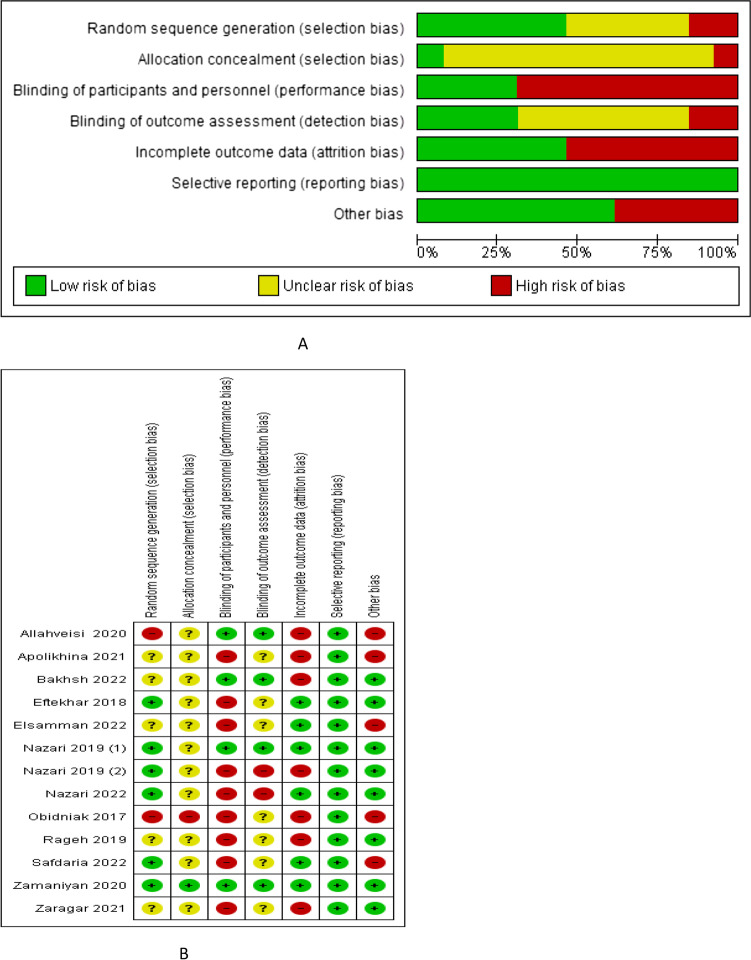
Quality assessment of the included RCTs was done following the Cochrane Handbook of Systematic Reviews recommendations by two investigators (AM and MF), and disagreements were discussed further with other investigators. All studies were assessed for random sequence generation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective reporting, and other bias were done.
Quality assessment of non-RCTs was done using the Newcastle–Ottawa scale (NOS). This “star system” is based on three main perspectives: the selection of the study groups (exposed and non-exposed); the comparability of the groups (cohorts or cases and controls) on the basis of the design or analysis; the ascertainment of exposure or outcome (length and adequacy of follow-up). Absent and unclear data were checked by contacting the corresponding author or other coauthors.
The GRADE system was used to assess the quality of evidence. GRADE included the risk of bias in the included studies due to inconsistency, indirectness, imprecision, and publication bias. Serious concerns in each item decrease the evidence by 1 level, while very serious ones decrease the evidence by 2 levels.
The levels were high, moderate, low, or very low if we were very confident, moderately confident, have limited confidence, or very limited confidence that the true effect is close to the effect estimate, respectively.
Data synthesis
The odd ratio and the corresponding 95% CI were calculated for all dichotomous data, and the mean difference with the corresponding 95% CI was calculated for continuous data. The effect size was obtained using the random effect model by the Mantel–Hansel method.
The heterogeneity of studies included was evaluated by I2 statistic and Cochran’s Q test. Heterogeneity was considered significant at a p-value of < 0.05 in the Q-test or I2 > 40%. A separate analysis was done for RCTs and non-RCTs, and subgroup analysis of the studies according to inclusion criteria of participants (previous implantation failure or thin endometrium), the volume of transferred PRP (ranged from 0.5 to 40 ml), and types of transferred embryos (fresh or frozen). All statistical analysis was performed with the Review Manager (RevMan) version 5.4.1 (The Nordic Cochrane Centre, Cochrane Collaboration 2020; Copenhagen, Denmark).
Results
Study selection
Our search yielded 2398 studies through databases (533 from PubMed, 239 from Embase, 717 from Scopus, 384 from Web of Science, 523 from clinical trials, and 2 through other sources), 985 of them were screened after the removal of duplicates, 50 screened for full text, and 29 studies were included in quantitative and qualitative synthesis (Fig. 1).
Fig. 1.
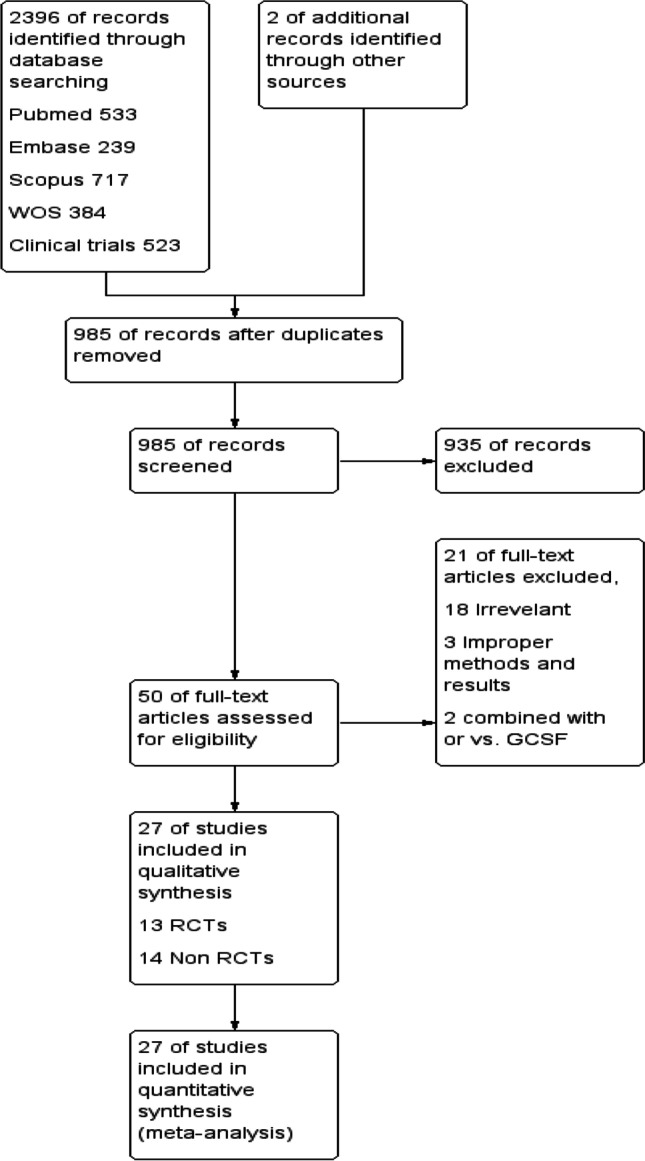
PRISMA flow chart
Study characteristics
Tables S2 and S3 summarized the main characteristics of the included RCTs and non-RCTs. Twenty-seven studies were included in our analysis: 13 were RCTs [18–30], 5 was prospective cohort [17, 31–35], 4 were prospective single-arm [35–38], and 5 were retrospective analysis [39–44]. All the studies were single centers except Kusumi [35], which was conducted in 7 fertility clinics. Eleven studies were conducted in Iran, 3 in Russia [22, 29, 34], 3 in China [17, 36, 39], 2 in India [38, 45], 2 in Japan [35, 41], and 1 study was conducted in each of the following countries: Bahrain [28], Canada [40], Egypt [20], South Korea [37], Turkey [42], and UK [32]. In 12 studies, the participants had recurrent implantation failure, in 5 studies had implantation failure, and in 10 studies, had thin endometrium. Frozen embryo transfer was done in 22 studies, fresh embryo transfer cycles were investigated in 2 studies [28, 34], while in 3 studies the cycles included had both fresh and frozen embryo transfer [20, 27, 38]. All the studies evaluated intrauterine injection except Apolikhina [21], which evaluated subendometrial injection and Nourshin [32], which evaluated both intrauterine and subendometrial PRP injections. PRP was compared to no intervention or placebo in all 27 studies. PRP volume injected was 0.3–0.4 ml in 1 study [45], 0.5–1 ml in 22 studies, 1.5 ml in 1 study [27], 2 ml in 1 study [22], 5–7 ml in 1 study [34], and 35–40 ml in 1 study [29]. The timing of PRP injection was 48 h before ET in 12 studies, between cycle days 6 and 14 in 12 studies, unspecified time in 2 studies, and when the endometrial thickness was below 7 mm in 1 study.
PRP preparation in the included studies was achieved through a two-step process. Venous blood was added to acid citrate and centrifugated for about 10 min to separate the red and white blood cells, then centrifugated again to reach 4–5 times platelet concentration.
Risk of bias of included studies
Quality assessment of the included RCTs was done following the Cochrane Handbook of Systematic Reviews; recommendation is shown in Fig. 2. Quality assessment of the included non-RCTs was done using Newcastle–Ottawa Scale, is summarized in Table 1. GRADE quality of evidence for each outcome criteria is summarized in Table 2.
Fig. 2.
Risk of bias A graph and B summary
Table 1.
Quality assessment of the included non RCTs using Newcastle-Ottawa Scale
| [Study] | Selection | Comparability | Outcome/exposure |
|---|---|---|---|
| Noushin 2021 | *** | * | *** |
| Chang 2019 | *** | * | *** |
| Dzhincharadze 2021 | *** | * | *** |
| Tehraninejad 2020 | *** | * | *** |
| Kim 2019 | *** | --- | *** |
| Kusumi 2020 | *** | --- | *** |
| Dogra 2022 | *** | --- | *** |
| Wang 2018 | ** | --- | * |
| Zadehmodarres 2017 | ** | ---- | * |
| Madhavan 2018 | *** | * | ** |
| Xu 2022 | *** | * | ** |
| Coksuer 2019 | *** | *** | |
| Enatsu 2021 | *** | * | ** |
| Russell 2022 | *** | * | ** |
Table 2.
GRADE quality of evidence
| Outcome | No studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Quality | |
|---|---|---|---|---|---|---|---|---|
| Sample size | Wide CI | |||||||
| Implantation rate | 6 | N | S | N | 886 | N | N | Moderate |
| Clinical pregnancy rate | 11 | S | S | N | 1289 | N | N | Low |
| Chemical pregnancy rate | 7 | N | N | N | 726 | N | N | High |
| Ongoing pregnancy rate | 5 | N | S | N | 488 | N | N | Low |
| Live birth rate | 4 | S | S | N | 523 | S | N | Very low |
| Endometrial thickness | 4 | S | N | N | 307 | N | N | Low |
CI, confidence interval; N, not serious; S, serious
Synthesis of results
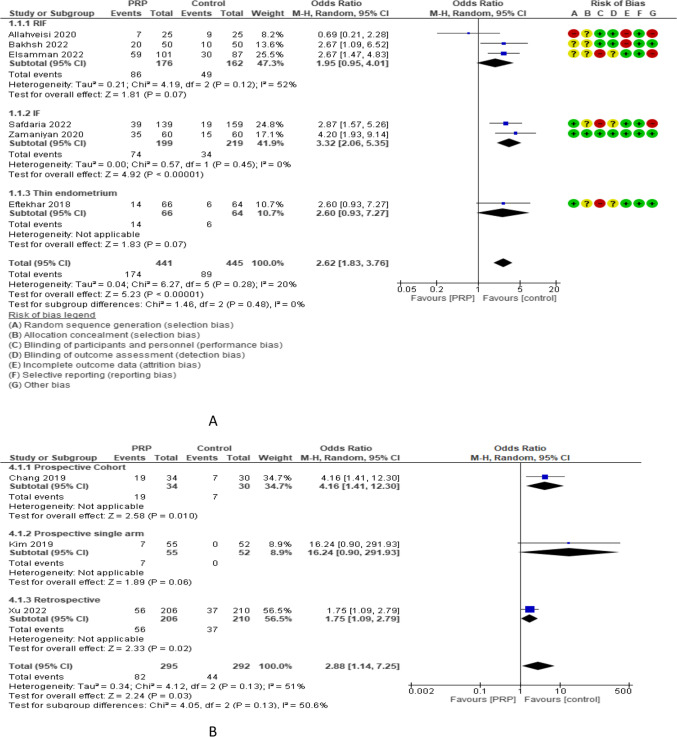
Implantation rate (IR) was reported in 6 RCTs with 886 participants. The odd ratio effect estimate was 2.62 with a 95% CI of [1.83, 3.76]. Subgroup analysis reported IR in 3 studies (338 women) with repeated implantation failure and revealed an overall estimated OR of 1.95 and 95% CI of 0.95–4.01. IR was reported in 2 and 1 studies (418 and 130 women) with previous implantation failure and thin endometrium and revealed overall estimated OR of 3.32 and 2.60 with 95% CI of 2.06–5.35 and 0.93–7.27, respectively. IR was reported in 5 and 1 studies (698 and 188 women) with frozen and both frozen and fresh embryo transfer and revealed overall estimated OR of 2.54 and 2.67 with 95% CI of 1.57–4.11 and 1.47–4.83, respectively. IR was evaluated in 3 non-RCTs with 587 participants and revealed an overall estimated OR of 2.88 with a 95% CI of 1.14–7.25 [1 study (64 women) was prospective cohort and had 4.16 OR and 1.41–12.30 95% CI, 1 study (107 women) was prospective single arm and had 16.24 OR and 0.9–291.93 95% CI, and 1 study (416 women) was retrospective and had 1.75 OR and 1.09–2.79 95% CI (Fig. 3).
Fig. 3.
Implantation rate in A RCTs and B non-RCTs
Clinical pregnancy rate (CPR) was reported in 11 RCTs with 1289 participants. The odd ratio effect estimate was 2.46 with a 95% CI of [1.08, 5.63]. Subgroup analysis reported CPR in 5 studies (416 women) with repeated implantation failure and revealed an overall estimated OR of 2.54 and 95% CI of 1.61–4.02. CPR was reported in 4 and 2 studies (730 and 143 women) with previous implantation failure and thin endometrium and revealed overall estimated OR of 1.88 and 5.02 with 95% CI of 0.35–10.02 and 1.13–22.29, respectively. CPR was reported in 9 and 2 studies (1113 and 176 women) with frozen and both frozen and fresh embryo transfer and revealed overall estimated OR of 2.37 and 2.95 with 95% CI of 0.91–6.20 and 1.35–6.43, respectively. Subgroup analysis of CPR according to the volume of PRP injected revealed that 0.5–1 ml (9 studies, 1119 women), 1.5 ml (1 study, 80 women), and 2 ml (1 study, 90 women) had a CPR OR of 2.31, 3.35, and 3.53 and 95% CI of 0.89–5.96, 0.63–17.74, and 1.44–8.67, respectively. CPR was evaluated in 10 non-RCTs with 1452 participants and revealed an overall estimated OR of 2.39 with a 95% CI of 1.47–3.90 [4 studies (412 women) were prospective cohort and have 2.01 OR and 0.85–4.76 95% CI, 1 study (40 women) was prospective single arm and have 18.38 OR and 0.96–352.57 95% CI, and 5 studies (1000 women) were retrospective and have 2.47 OR and 1.29–4.72 95% CI (Fig. 4).
Fig. 4.
Clinical pregnancy rate in A RCTs and B non-RCTs
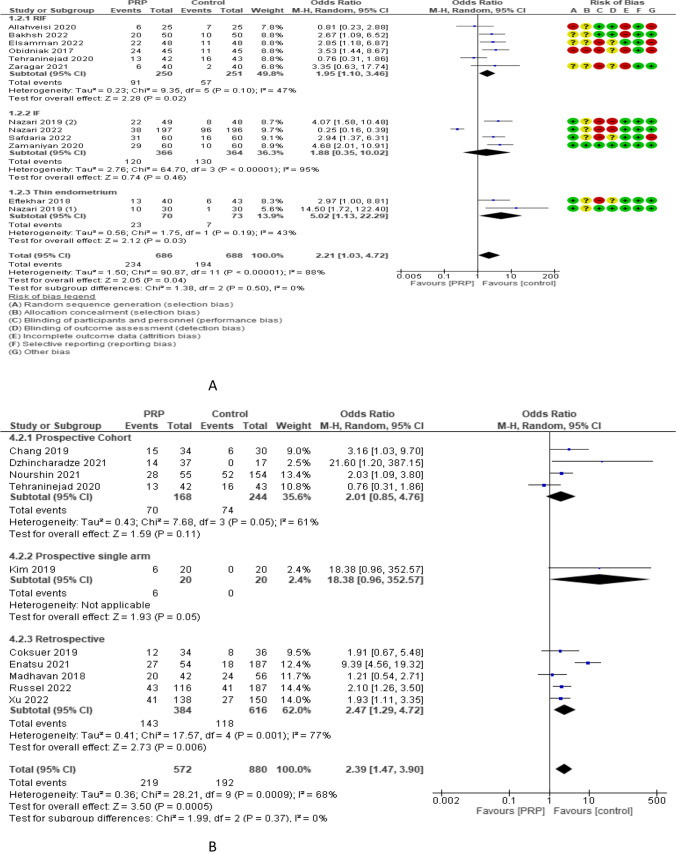
Chemical pregnancy rate was reported in 7 RCTs with 726 participants. The odd ratio effect estimate was 2.92 with a 95% CI of [2.09, 4.08]. Subgroup analysis reported chemical pregnancy rate in 2 studies (246 women) with repeated implantation failure and revealed an overall estimated OR of 3.10 and 95% CI of 1.58–6.10. Chemical pregnancy rate was reported in 3 and 2 studies (337 and 143 women) with previous implantation failure and thin endometrium and revealed overall estimated OR of 2.65 and 4.05 with 95% CI of 1.66–4.25 and 1.08–15.22, respectively. Chemical pregnancy rate was reported in 5.1 and 1 studies (480,150 and 96 women) with frozen, fresh, and both frozen and fresh embryo transfer and revealed overall estimated OR of 2.83, 4.33, and 2.17 with 95% CI of 1.87–4.27, 1.97–9.51, and 0.95–4.96 respectively. The chemical pregnancy rate was evaluated in 6 non-RCTs with 1196 participants and revealed an overall estimated OR of 1.49 with a 95% CI of 0.86–2.58 [2 studies (294 women) was prospective cohort and has 1.58 OR and 0.63–3.94 95% CI and 4 studies (902 women) were retrospective and has 1.40 OR and 0.65–3.02 95% CI (Fig. 5).
Fig. 5.
Chemical pregnancy rate in A RCTs and B non-RCTs
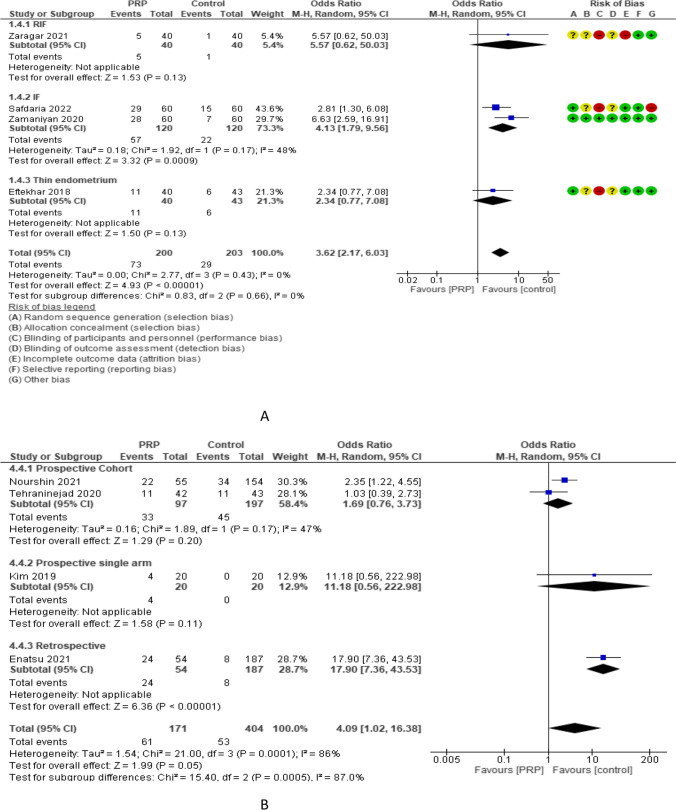
Ongoing pregnancy rate (OPR) was reported in 5 RCTs with 488 participants. The odd ratio effect estimate was 2.78, with a 95% CI of [1.43, 5.41]. Subgroup analysis reported OPR in 2 studies (165 women) with repeated implantation failure and revealed an overall estimated OR of 1.79 and 95% CI of 0.37–8.53. OPR was reported in 2 and 1 studies (240 and 83 women) with previous implantation failure and thin endometrium and revealed overall estimated OR of 4.13 and 2.34 with 95% CI of 1.79–9.56 and 0.77–7.08, respectively. OPR was reported in 4 and 1 studies (408 and 80 women) with frozen and both frozen and fresh embryo transfer and revealed overall estimated OR of 2.62 and 5.57 with 95% CI of 1.26–5.47 and 0.62–50.03, respectively. Subgroup analysis of OPR according to the volume of PRP injected revealed that 0.5–1 ml (4 studies, 408 women) and 1.5 ml (1 study, 80 women) had an OPR OR of 2.62 and 5.57 and 95% CI of 1.26–5.47 and 0.62–50.03, respectively. OPR was evaluated in 4 non-RCTs with 575 participants and revealed an overall estimated OR of 4.09 with a 95% CI of 1.02–16.38 [2 studies (294 women) were prospective cohort and had 1.69 OR and 0.76–3.73 95% CI, 1 study (40 women) was prospective single arm and had 11.18 OR and 0.56–222.98 95% CI, and 1 study (241 women) was retrospective and had 17.90 OR and 7.36–43.53 95% CI (Fig. 6).
Fig. 6.
Ongoing pregnancy rate in A RCTs and B non-RCTs
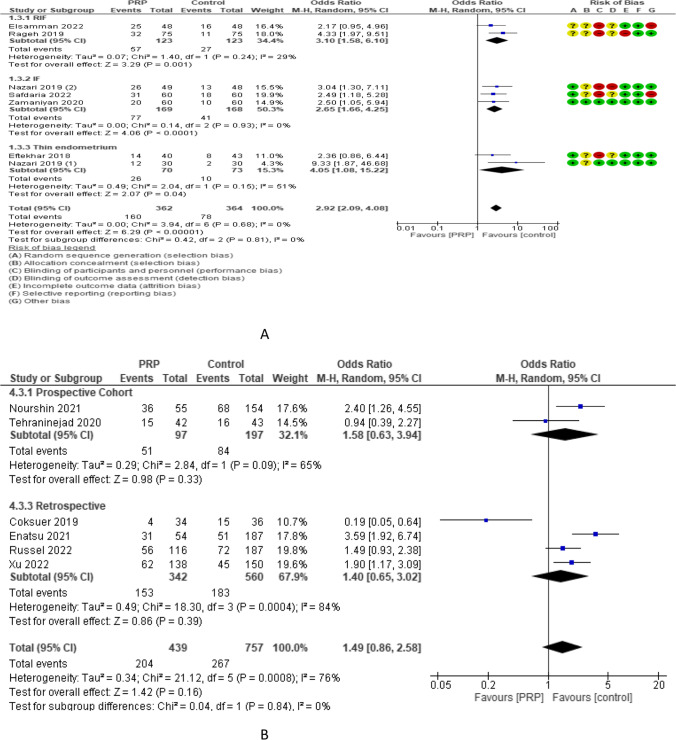
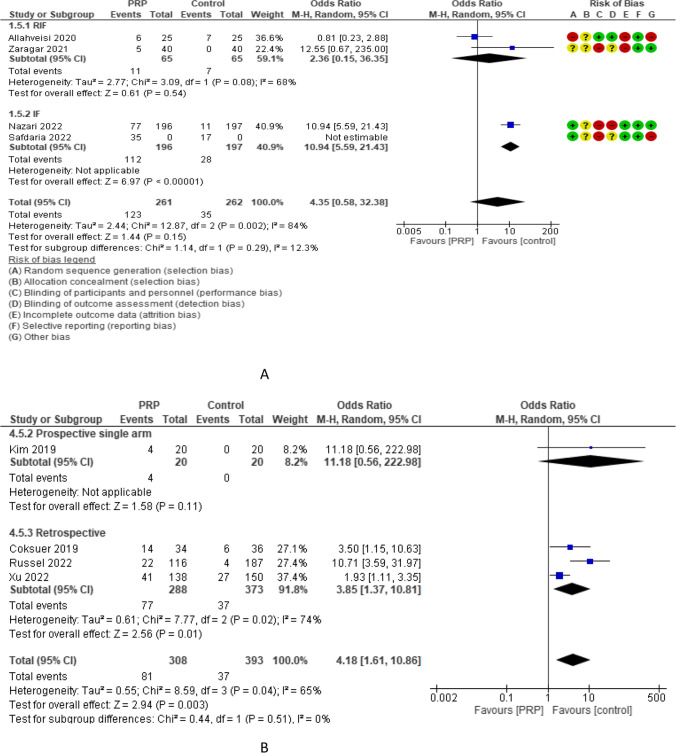
Live birth rate (LBR) was reported in 4 RCTs with 523 participants. The odd ratio effect estimate was 4.35 with a 95% CI of [0.58, 32.38]. LBR was reported in 2 studies (130 women) with repeated implantation failure and 2 studies (393 women) with previous implantation failure and revealed overall estimated OR of 2.36 and 10.94 with 95% CI of 0.15–36.35 and 5.59–21.43, respectively. LBR was reported in 3 studies (563 women) with frozen embryo transfer and 1 study (80 women) with both frozen and fresh embryo transfer and revealed overall estimated OR of 3.47 and 12.55 with 95% CI of 0.93–12.91 and 0.67–235.00, respectively. Subgroup analysis of LBR according to the volume of PRP injected revealed that 0.5–1 ml (3 studies, 563 women) and 1.5 ml (1 study, 80 women) had an LBR OR of 3.47 and 12.55, and 95% CI of 0.93–12.91 and 0.67–235.00, respectively. LBR was evaluated in 4 non-RCTs with 701 participants and revealed an overall estimated OR of 4.18 with a 95% CI of 1.61–10.86 [1 study (40 women) was prospective single arm and had 11.18 OR and 0.56–222.98 95% CI and 3 studies (661 women) were retrospective and had 3.85 OR and 1.37–10.81 95% CI (Fig. 7).
Fig. 7.
Livebirth rate in A RCTs and B non-RCTs
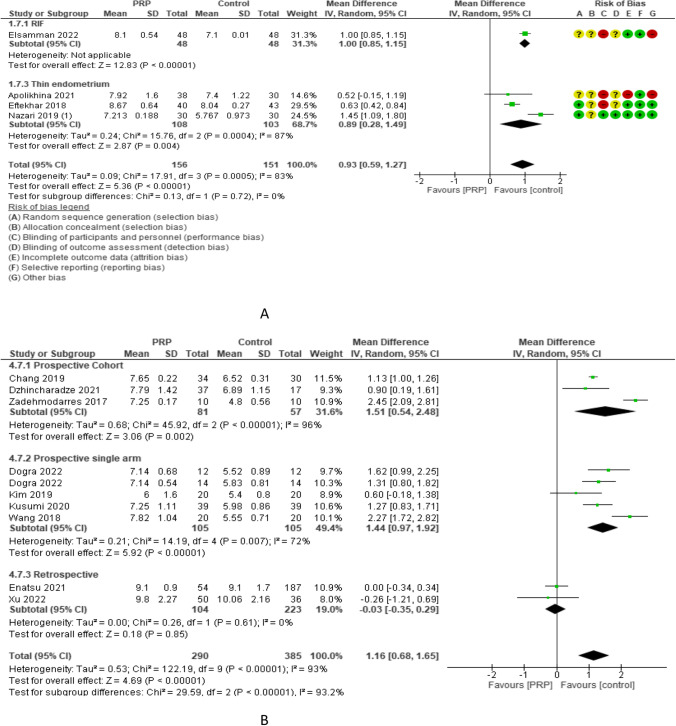
Endometrial thickness was compared in 4 RCTs with 307 participants and showed a mean difference of 0.93 with 0.59–1.27 95% CI between PRP and control cycles. Endometrial thickness was reported in 1 [23] and 3 studies [21, 43, 46] (96 and 211 women) with repeated implantation failure and thin endometrium and revealed an overall estimated mean difference of 1.00 and 0.89 with 95% CI of 0.85–1.15 and 0.28–1.49, respectively. It was also reported in 3 and 1 studies (211 and 96 women) with frozen and both frozen and fresh embryo transfer and revealed an overall estimated mean difference of 0.89 and 1.00 with 95% CI of 0.28–1.49 and 0.85–1.15, respectively. Subgroup analysis of LBR according to the volume of PRP injected revealed that 0.5–1 ml (3 studies, 239 women) and 35–40 ml (1 study, 68 women) had an endometrial thickness mean difference of 1.00 and 0.52 and 95% CI of 0.63–1.38 and − 0.15 to 1.19, respectively. Endometrial thickness was evaluated in 9 non-RCTs with 675 participants and revealed an overall estimated mean difference of 1.16 mm with a 95% CI of 0.68–1.65 mm [3 studies (138 women) in were prospective cohort and had 1.51 mm mean difference and 0.54–2.48 mm 95% CI, 4 studies (210 women) were prospective single-arm and had 1.44 mm mean difference and 0.97–1.92 mm 95% CI, and 2 studies (327 women) were retrospective and had − 0.03 mean difference and − 0.35 to 0.29 mm 95% CI (Fig. 8).
Fig. 8.
Endometrial thickness in A RCTs and B non-RCTs
Two prospective cohort studies by Apolikhina et al. [21] and Noushin et al. [32] evaluated the effects of subendometrial injection of PRP. Apolikhina’s study involved 68 women with a history of cycle cancellation resulting from refractory thin endometrium not responding to the standard treatment. Thirty-eight women were treated by physical electropulse therapy with abdominal and vaginal placement of electrodes on “BTL-4000 Premium G’” unit from cycle days 5–7 for 10 days and then received subendometrial injection of 35–40 ml of autologous PRP during the cycle next to physical therapy. The injection was done by endoscopic needle through hysteroscopy. They were compared to 30 women who were treated only by physical therapy. Endometrial growth was significantly higher in the PRP group compared to controls (7.92 ± 1.6 vs 7.4 ± 1.22, p-value < 0.001, obtained by author contact). They concluded that PRP injection is effective in women with refractory “thin” endometrium and decreased uterine artery hemodynamics.
Noushin et al. study [32] included women with recurrent implantation failure undergoing frozen embryo transfer. They compared 55 women subjected to ultrasonographic guided transvaginal subendometrial injection of PRP during the luteal phase of the cycle prior to the embryo transfer cycle under ultrasound guidance and 109 women subjected to intrauterine PRP injection done during the embryo transfer cycle at an approximate endometrial thickness of 7 mm to 154 women who underwent standard cycle without intervention. Women in the 2 intervention groups received additional subcutaneous injections of 300 mg of GCSF daily for 3 days. They found that ongoing/livebirth rates were higher in the intervention groups compared to the control group [22/55 (40%), 45/109 (41.3%), and 34/154 (22.1%), respectively; p = 0.004]. They reported a similarly higher clinical pregnancy rate [28/55 (51%), 57/109 (52.3%) vs 52/154 (33.8%), respectively; p = 0.006]. They concluded that PRP improves the outcome of frozen embryo transfer cycles with no difference between subendometrial injection and intrauterine infusion.
Discussion
Main findings
This meta-analysis found a beneficial effect of PRP administration on implantation, clinical pregnancy, chemical pregnancy, ongoing live birth rates, and endometrial thickness. These effects on IVF outcomes were constant with changing types of participants, types of embryos transferred, and volume of PRP injected in both RCTs and non-RCTs. However, the quality of evidence of these findings was very low regarding live birth rate (only 4 studies with 523 participants), low in endometrial thickness (4 studies with 307 participants), and clinical pregnancy rate (high risk of bias with the inconsistency of results), moderate in implantation rate and high in chemical pregnancy rate.
The possible mechanisms of the beneficial effects of PRP include synchronization of immunological interactions between the endometrial and embryo development during the implantation window. PRP decreases inflammatory cytokines such as IL-6 and 8 and increases IL-1 β, which is crucial in successful implantation [44].
However, the exact mechanism is still not clear, and these beneficial effects may result from mechanical endometrial injury caused by intrauterine catheter simulating endometrial injury effects, as in most of the included studies, the control group had no intervention.
It is to be noted that the CI is passing through 1 in LBR, endometrial thickness, and chemical pregnancy rate.
Strengths and limitations
Our meta-analysis is the first comprehensive one focusing on the effects of PRP in women with previous implantation failure. It included all the available studies reached by extensive searching of all available databases and the gray literature, trial registration sites, and a reference list of all related studies. A separate analysis for RCTs and non-RCTs was done. Adequate subgroup analysis according to participants’ characteristics, route, and volume of PRP injected for all the available outcomes.
This meta-analysis is not without limitations. Only 11 RCTs were included. Most of them had a high risk of bias, especially in blinding and allocation concealment. The most important outcome (live birth rate) was reported in 4 studies only. Most of the included studies did not describe accurate details about PRP preparation. The exact cause of previous implantation failure was not clarified in most studies except those that described thin endometrium. The studies describing thin endometrium failed to describe the exact method of measuring the endometrial thickness and the presence of any intra- or inter-observer variability. The exact timing and number of PRP administrations were not clear in most of the studies. These led to marked heterogeneity. We tried to compensate for that by using the random effect method for comparison.
Comparison with existing literature
There are many systematic reviews evaluating the effects of PRP in orthopedic, ophthalmic, and dermatological fields. Few ones were done in gynecology. Some evaluated its role in Asherman syndrome and others evaluated it in premature ovarian failure. Only 1 systematic review studied its role in implantation.
Maleki-Hajiagha and colleagues [9] evaluated the effect of PRP on the outcome of embryo transfer in IVF/ICSI. They included 7 studies with 625 women (311 cases vs. 314 controls). They reported higher clinical pregnancy (7 studies, RR: 1.79, 95% CI: 1.37, 2.32; P < 0.001, I2 = 16%), chemical pregnancy (3 studies, RR 1.79, 95% CI: 1.29, 2.50; P < 0.001, I2 = 0%), and implantation rates (n = 3, RR: 1.97, 95% CI: 1.40, 2.79; P < 0.001, I2 = 0%). They also reported more increase in endometrial thickness in women who received PRP compared to the control group (SMD: 1.79, 95% CI: 1.13, 2.44; P < 0.001, I2 = 64%). They concluded that PRP could be used as an accessory strategy in women with RIF and thin endometrium. However, this review included only 3 RCTs and 4 cohort studies. So, the subgroup analysis was defective.
Conclusions
This systematic review showed an increase in all outcomes of IVF cycles, namely implantation, clinical pregnancy, chemical pregnancy, ongoing pregnancy, and live birth rates. It also reported a significant increase in endometrial thickness in women with refractory thin endometrium. However, the quality of evidence was generally low, as the number of well-designed RCTs was inadequate to provide strong evidence, and there was marked heterogeneity among the included studies. More RCTs with adequate blinding, low risk of bias, with precise inclusion criteria considering the possible causes of implantation failure and other markers of endometrial receptivity besides the endometrial thickness should be conducted to provide the needed evidence.
Supplementary information
Supplementary Tables
Authors contribution
AMM search, assessment, writing, revision, and approval of manuscript; AE data extraction, writing, revision, and approval of manuscript; NK search, data analysis, writing, revision, and approval of manuscript; SIM data extraction, writing, revision, and approval of manuscript; MAF data analysis, writing, revision, and approval of manuscript; NE data extraction, writing, revision, and approval of manuscript; AK data extraction, writing, revision, and approval of manuscript; WSR data analysis, writing, revision, and approval of manuscript; AIO data extraction, writing, revision, and approval of manuscript; AAM data extraction, writing, revision, and approval of manuscript; RMF data extraction, writing, revision, and approval of manuscript; HS data extraction, writing, revision, and approval of manuscript; EKS data extraction, writing, revision, and approval of manuscript; NS search, assessment of risk of bias, writing, revision, and approval of manuscript; YL data extraction, writing, revision, and approval of manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Declarations
Ethics approval
Registration number CRD42022327811.
Conflict of interest
The authors declare no competing interests.
Footnotes
Synopsis
PRP improved implantation, clinical pregnancy, chemical pregnancy, ongoing, live birth rates, and endometrial thickness in women with implantation failure.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maged AM, Ogila AI, Mohsen RA, et al. Endometrial scratch injury in infertile women seeking conception through natural or intrauterine insemination cycles: a systematic review and meta-analysis. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2021. 10.1002/ijgo.14030. [DOI] [PubMed]
- 2.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Revel A. Defective endometrial receptivity. Fertil Steril. 2012;97(5):1028–1032. doi: 10.1016/j.fertnstert.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Aplin JD. The cell biological basis of human implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5):757–764. doi: 10.1053/beog.2000.0116. [DOI] [PubMed] [Google Scholar]
- 5.Maged AM, Rashwan H, AbdelAziz S, et al. Randomized controlled trial of the effect of endometrial injury on implantation and clinical pregnancy rates during the first ICSI cycle. Int J Gynaecol Obstet Off organ Int Fed Gynaecol Obstet. 2018;140(2):211–216. doi: 10.1002/ijgo.12355. [DOI] [PubMed] [Google Scholar]
- 6.Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21(12):3036–3043. doi: 10.1093/humrep/del305. [DOI] [PubMed] [Google Scholar]
- 7.Lebovitz O, Orvieto R. Treating patients with “thin” endometrium - an ongoing challenge. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2014;30(6):409–414. doi: 10.3109/09513590.2014.906571. [DOI] [PubMed] [Google Scholar]
- 8.Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane database Syst Rev. 2016;6:CD002118. doi: 10.1002/14651858.CD002118.pub5. [DOI] [PubMed] [Google Scholar]
- 9.Maleki-Hajiagha A, Razavi M, Rouholamin S, Rezaeinejad M, Maroufizadeh S, Sepidarkish M. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: a systematic review and meta-analysis. J Reprod Immunol. 2020;137:103078. doi: 10.1016/j.jri.2019.103078. [DOI] [PubMed] [Google Scholar]
- 10.Bienert M, Habib P, Buck V, Classen-Linke I, Skoblo R, Rösing B. Intrauterine hCG application increases expression of endothelial cell-cell adhesion molecules in human. Arch Gynecol Obstet. 2021;304(6):1587–1597. doi: 10.1007/s00404-021-06031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalem Z, Namli Kalem M, Bakirarar B, Kent E, Makrigiannakis A, Gurgan T. Intrauterine G-CSF administration in recurrent implantation failure (RIF): an Rct. Sci Rep. 2020;10(1):5139. doi: 10.1038/s41598-020-61955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Cao Z, Yu X, Hu W. Effect of growth hormone on thin endometrium via intrauterine infusion. Ann Transl Med. 2021;9(16):1325. doi: 10.21037/atm-21-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang CL, Li QY, Chen FL, et al. A randomized double blind comparison of atosiban in patients with recurrent implantation failure undergoing IVF treatment. Reprod Biol Endocrinol. 2022;20(1):124. doi: 10.1186/s12958-022-00999-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mekinian A, Cohen J, Alijotas-Reig J, et al. Unexplained recurrent miscarriage and recurrent implantation failure: is there a place for immunomodulation? Am J Reprod Immunol. 2016;76(1):8–28. doi: 10.1111/aji.12493. [DOI] [PubMed] [Google Scholar]
- 15.Baba K, Yamazaki Y, Sone Y, et al. An in vitro long-term study of cryopreserved umbilical cord blood-derived platelet-rich plasma containing growth factors-PDGF-BB, TGF-β, and VEGF. J cranio-maxillo-facial Surg Off Publ Eur Assoc Cranio-Maxillo-Facial Surg. 2019;47(4):668–675. doi: 10.1016/j.jcms.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Makrigiannakis A, Makrygiannakis F, Vrekoussis T. Approaches to improve endometrial receptivity in case of repeated implantation failures. Front Cell Dev Biol. 2021;9:613277. doi: 10.3389/fcell.2021.613277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang Y, Li J, Chen Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8(1):1286–1290. [PMC free article] [PubMed] [Google Scholar]
- 18.Nazari L, Salehpour S, Hosseini S, Sheibani S, Hosseinirad H. The effects of autologous platelet-rich plasma on pregnancy outcomes in repeated implantation failure patients undergoing frozen embryo transfer: a randomized controlled trial. Reprod Sci. 2022;29(3):993–1000. doi: 10.1007/s43032-021-00669-1. [DOI] [PubMed] [Google Scholar]
- 19.Nazari L, Salehpour S, Hosseini MS, Hashemi MP. The effects of autologous platelet-rich plasma in repeated implantation failure: a randomized controlled trial. Hum Fertil. 2020;23(3):209–213. doi: 10.1080/14647273.2019.1569268. [DOI] [PubMed] [Google Scholar]
- 20.Rageh K, Barakat A, Ahmed K, Ahmed A. PRP in recurrent implantation failure, hope or hype? A prospective randomized controlled study. Evid Based Women’s Heal J. 2020;10(1):46–53. doi: 10.21608/ebwhj.2019.17936.1039. [DOI] [Google Scholar]
- 21.Apolikhina IA, Efendieva ZN, Fedorova TA, et al. Autologous platelet rich plasma in complex therapy of women with refractory ‘“thin”’ endometrium. Akusherstvo i Ginekologiya/ Obst Gynecol. 2021;2021(4):112–119. doi: 10.18565/aig.2021.4.112-119. [DOI] [Google Scholar]
- 22.Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Azargashb E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: a double-blind RCT. Int J Reprod Biomed. 2019;17(6):445–450. doi: 10.18502/ijrm.v17i6.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Samman A, Wafa Y, Al-Omda F, Ali M. Autologous intrauterine platelet rich plasma instillation in repeated implantation failure in assisted reproductive techniques. Al-Azhar Int Med J. 2022;3(2):100–106. doi: 10.21608/aimj.2022.92034.1557. [DOI] [Google Scholar]
- 24.Allahveisi A, Seyedoshohadaei F, Rezaei M, Bazrafshan N, Rahimi K. The effect of platelet-rich plasma on the achievement of pregnancy during frozen embryo transfer in women with a history of failed implantation. Heliyon. 2020;6(3):e03577. doi: 10.1016/j.heliyon.2020.e03577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obidniak D, Gzgzyan A, Feoktistov A, Niauri D. Randomized controlled trial evaluating efficacy of autologous platelet -rich plasma therapy for patients with recurrent implantation failure. Fertil Steril. 2017;108(3):e370. doi: 10.1016/j.fertnstert.2017.07.1080. [DOI] [Google Scholar]
- 26.Zamaniyan M, Peyvandi S, Heidaryan Gorji H, et al. Effect of platelet-rich plasma on pregnancy outcomes in infertile women with recurrent implantation failure: a randomized controlled trial. Gynecol Endocrinol. 2021;37(2):141–145. doi: 10.1080/09513590.2020.1756247. [DOI] [PubMed] [Google Scholar]
- 27.Eftekhar M, Neghab N, Naghshineh E, Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan J Obstet Gynecol. 2018;57(6):810–813. doi: 10.1016/j.tjog.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Bakhsh AS, Maleki N, Sadeghi MR, et al. Effects of autologous platelet-rich plasma in women with repeated implantation failure undergoing assisted reproduction. J Bras Reprod Assist. 2022;26(1):84–87. doi: 10.5935/1518-0557.20210046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safdarian L, Aleyasin A, Aghahoseini M, et al. Efficacy of the intrauterine infusion of platelet-rich plasma on pregnancy outcomes in patients with repeated implantation failure: a randomized control trial. Int J Women’s Heal Reprod Sci. 2022;10(1):38–44. doi: 10.15296/ijwhr.2022.08. [DOI] [Google Scholar]
- 30.Zargar M, Pazhouhanfar R, Najafian M, Choghakabodi PM. Effects of intrauterine autologous platelet-rich plasma infusions on outcomes in women with repetitive in vitro fertilization failures: a prospective randomized study. Clin Exp Obstet Gynecol. 2021;48(1):180–185. doi: 10.31083/j.ceog.2021.01.2131. [DOI] [Google Scholar]
- 31.Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. J Bras Reprod Assist. 2017;21(1):54–56. doi: 10.5935/1518-0557.20170013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noushin MA, Ashraf M, Thunga C, et al. A comparative evaluation of subendometrial and intrauterine platelet-rich plasma treatment for women with recurrent implantation failure. F S Sci. 2021;2(3):295–302. doi: 10.1016/j.xfss.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Tehraninejad ES, Kashani NG, Hosseini A, Tarafdari A. Autologous platelet-rich plasma infusion does not improve pregnancy outcomes in frozen embryo transfer cycles in women with history of repeated implantation failure without thin endometrium. J Obstet Gynaecol Res. 2021;47(1):147–151. doi: 10.1111/jog.14445. [DOI] [PubMed] [Google Scholar]
- 34.Dzhincharadze LG, Abubakirov AN, Mishieva NG, Bakuridze EM, Bystrykh OA. Effectiveness of intrauterine administration of autologous platelet-rich plasma to prepare “thin” endometrium for the defrosted embryo transfer program. Akusherstvo i Ginekol (Russian Fed). 2021;2021(2):90–95. doi: 10.18565/aig.2021.2.90-95. [DOI] [Google Scholar]
- 35.Kusumi M, Ihana T, Kurosawa T, Ohashi Y, Tsutsumi O. Intrauterine administration of platelet-rich plasma improves embryo implantation by increasing the endometrial thickness in women with repeated implantation failure: a single-arm self-controlled trial. Reprod Med Biol. 2020;19(4):350–356. doi: 10.1002/rmb2.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Liu L, Mou S, et al. Investigation of platelet-rich plasma in increasing proliferation and migration of endometrial mesenchymal stem cells and improving pregnancy outcome of patients with thin endometrium. J Cell Biochem. 2019;120(5):7403–7411. doi: 10.1002/jcb.28014. [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Shin JE, Koo HS, Kwon H, Choi DH, Kim JH. Effect of autologous platelet-rich plasma treatment on refractory thin endometrium during the frozen embryo transfer cycle: a pilot study. Front Endocrinol (Lausanne). 2019;10:1–9. doi: 10.3389/fendo.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dogra Y, Singh N, Vanamail P. Autologous platelet-rich plasma optimizes endometrial thickness and pregnancy outcomes in women with refractory thin endometrium of varied aetiology during fresh and frozen-thawed embryo transfer cycles. J Bras Reprod Assist. 2022;26(1):13–21. doi: 10.5935/1518-0557.20210037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Hao C, Fang J, Liu X, Xue P, Miao R. Intrauterine perfusion of autologous platelet-rich plasma before frozen-thawed embryo transfer improves the clinical pregnancy rate of women with recurrent implantation failure. Front Med. 2022;9:1–5. doi: 10.3389/fmed.2022.850002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell SJ, Kwok YSS, Nguyen TTTN, Librach C. Autologous platelet-rich plasma improves the endometrial thickness and live birth rate in patients with recurrent implantation failure and thin endometrium. J Assist Reprod Genet. 2022;39(6):1305–1312. doi: 10.1007/s10815-022-02505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enatsu Y, Enatsu N, Kishi K, et al. Clinical outcome of intrauterine infusion of platelet-rich plasma in patients with recurrent implantation failure. Reprod Med Biol. 2021;2021:2–7. doi: 10.1002/rmb2.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coksuer H, Akdemir Y, Ulas BM. Improved in vitro fertilization success and pregnancy outcome with autologous platelet-rich plasma treatment in unexplained infertility patients that had repeated implantation failure history. Gynecol Endocrinol. 2019;35(9):815–818. doi: 10.1080/09513590.2019.1597344. [DOI] [PubMed] [Google Scholar]
- 43.Nazari L, Salehpour S, Hosseini S, Hashemi T, Borumandnia N, Azizi E. Effect of autologous platelet-rich plasma for treatment of recurrent pregnancy loss: a randomized controlled trial. Obstet Gynecol Sci. 2022;65(3):266–272. doi: 10.5468/ogs.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med. 2009;27(1):62–79. doi: 10.1055/s-0028-1108011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madhavan A, Naidu P, Rani K, Kaur J, Mahajan N. Intrauterine autologous platelet-rich plasma therapy to improve implantation rates in patients undergoing frozen embryo transfer: a pilot study. Onco Fertil J. 2018;1(2):81. doi: 10.4103/tofj.tofj_11_18. [DOI] [Google Scholar]
- 46.Eftekhar M, Neghab N, Naghshineh E, Khani P. Corrigendum to “can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial” [Taiwan J Obstet Gynecol 57 (2018) 810-813] Taiwan J Obstet Gynecol. 2021;60(5):973. doi: 10.1016/j.tjog.2021.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables