Abstract
Introduction
The global incidence of sexually transmitted infections (STIs) has been rapidly increasing over the past decade, with more than one million curable STIs being acquired daily. Young women in sub-Saharan Africa have a high prevalence and incidence of both curable STIs and HIV. The use of doxycycline as a prophylaxis to prevent STIs is promising; however, clinical trials, to date, have only been conducted among men who have sex with men (MSM) in high-income settings. We describe the characteristics of participants enrolled in the first trial to determine the efficacy of doxycycline post-exposure prophylaxis (PEP) to reduce STI incidence among women taking daily, oral HIV pre-exposure prophylaxis (PrEP).
Methods
This is an open-label 1:1 randomized clinical trial on the efficacy of doxycycline PEP compared with standard of care (e.g., quarterly STI screening and treatment) to reduce incident bacterial STIs – Neisseria gonorrhoeae, Chlamydia trachomatis, and Treponema pallidum – among Kenyan women aged ≥18 and ≤30 years. All were also taking HIV pre-exposure prophylaxis (PrEP). We describe the baseline characteristics, STI prevalence, and STI risk perception of participants.
Results
Between February 2020 and November 2021, 449 women were enrolled. The median age was 24 years (IQR 21–27), the majority were never married (66.1%), 370 women (82.4%) reported having a primary sex partner, and 33% had sex with new partners in the three months prior to enrolment. Two-thirds (67.5%, 268 women) did not use condoms, 36.7% reported transactional sex, and 43.2% suspected their male partners of having sex with other women. Slightly less than half (45.9%, 206 women) were recently concerned about being exposed to an STI. The prevalence of STIs was 17.9%, with C. trachomatis accounting for the majority of infections. Perceived risk of STIs was not associated with the detection of an STI.
Conclusion
Young cisgender women using HIV PrEP in Kenya and enrolled in a trial of doxycycline postexposure prophylaxis had a high prevalence of curable STIs and represent a target population for an STI prevention intervention.
Keywords: STI, Cisgender young women, HIV, PrEP, Doxycycline post-exposure prophylaxis
Introduction
Global trends reveal a rapid increase in the incidence of sexually transmitted infections (STIs) over the past decade, with more than one million curable STIs acquired daily [1]. In 2020, the World Health Organization (WHO) estimated 374 million new infections of four curable STIs, Chlamydia trachomatis, Neisseria gonorrhoeae, Treponema pallidum, and Trichomonas vaginalis [2, 3]. Young women in sub-Saharan Africa face a high prevalence of curable STIs and HIV [4] and limited data from HIV PrEP trials suggests high incidence rates [5]. STIs can severely affect mortality and morbidity for cisgender women by causing conditions such as tubal infertility, chronic pelvic pain, pelvic inflammatory disease, ectopic pregnancy, post-partum endometriosis, adverse neonatal outcomes, and an increase in susceptibility to HIV [6, 7]. Women are more biologically predisposed to complications from STIs than men [8]. Several studies conducted in sub-Saharan Africa reveal higher STI prevalence among younger women compared to their age-matched male peers and older women [8, 9]. In the region, the cultural, economic, and social marginalization of women contributes to the risk of HIV and STIs [10, 11], in part by rendering the negotiation of preventive measures such as condom use, abstinence, and partner notification ineffective [5, 12].
Taking antibiotics following sexual exposure to prevent bacterial STIs places preventive care in the hands of the user. Interventions that are individually controlled are greatly needed, especially for women, and the use of doxycycline as a post-exposure prophylaxis (PEP) has been proposed as a novel STI prevention strategy [13]. Doxycycline is already standardly used as prophylaxis to prevent infections such as malaria, lyme, and leptospirosis [14, 15]. A recent open-label clinical trial of doxycycline PEP among men who have sex with men (MSM) who were using HIV pre-exposure prophylaxis (PrEP) in France found a 47% relative reduction in bacterial STIs overall and a greater reduction specifically for C. trachomatis (70%) and T. pallidum (73%) [10]. Doxycycline PEP was well-tolerated in that study [18]. Several clinical trials of doxycycline as PEP or PrEP among MSM are ongoing worldwide to test this initial finding.
Studies among young African women have evaluated the association between HIV incidence and perception of HIV risk with disparate results [16–18]. STI risk perception and the prevalence of STIs among women receiving HIV prevention care, which is limited to syndromic management of STIs and daily oral PrEP, have not yet been described. Although women disproportionately bear the burden of adverse sequelae of curable STIs, trials on doxycycline PEP in this population have not yet been completed. The doxycycline postexposure prophylaxis (dPEP) Trial is an open-label, randomized clinical trial evaluating the efficacy of doxycycline PEP for STI prevention (C. trachomatis, N. gonorrhoeae, and T. pallidum) in Kisumu, Kenya, and is the first study to assess the efficacy of doxycycline PEP in cisgender women. In this paper, we describe the baseline characteristics of the dPEP Trial population.
Methods
Ethics statement
Before implementation, ethical approval of the protocol was received from Kenya Medical Research Institute’s Scientific Ethics Review Unit (KEMRI-SERU) and the University of Washington’s Institutional Review Board. Written informed consent to participate in the study was obtained from all participants and legal guardians of the illiterate participants before enrolment. All methods were carried out in accordance with the relevant guidelines and regulations. The trial was registered on 08/08/2019 at ClinicalTrials.gov, number NCT04050540[20].
Study design
This is an open-label 1:1 randomized clinical trial evaluating the efficacy of doxycycline PEP to reduce incident curable, bacterial STIs – N. gonorrhoeae, C. trachomatis, and T. pallidum among Kenyan cisgender young women. Inclusion criteria included willingness and ability to give written informed consent, ≥18 and ≤30 years, female sex assigned at birth, HIV seronegative, and a current prescription for HIV PrEP according to the national guidelines of Kenya. Exclusion criteria included pregnancy, breastfeeding, allergy to tetracyclines, on current medications that may impact doxycycline metabolism or that are contraindicated with doxycycline as per the prescribing information, or recent use of prolonged antibiotics (more than a 14-day course) in the month before enrolment, or active clinically significant medical or psychiatric conditions that would interfere with study participation per the discretion of the study investigator. Use of contraception was not required, and those who became pregnant after enrolment were not disenrolled, but among those assigned to the dPEP group, doxycycline was discontinued and only resumed if no longer pregnant or breastfeeding. Participants were primarily recruited from clinics providing HIV PrEP within Kisumu County. Quarterly follow-up visits were scheduled for each participant for 12 months. The study site is situated within a clinic at the Lumumba sub-County hospital in Kisumu, Kenya.
Demographic and behavioural data were electronically collected (REDCap), including questionnaires on STI and HIV risk perception and potential exposures. Biological sample collection (including serum, endocervical and vaginal swabs) and testing were done by trained study clinicians and laboratory technologists on site. Rapid HIV testing was completed using HIV 4th generation combination test (Abbott Determine) followed by confirmation for any positive results using repeat HIV antibody rapid testing (First Response) and then enzyme linked immunosorbent assay (ELISA) testing per Kenyan National Guidelines. Testing for C. trachomatis and N. gonorrhoeae was done using nucleic acid amplification test (Cepheid GeneXpert or Aptima Combo 2). T. pallidum screening was completed using rapid plasma regain (RPR) test (BD Macro Vue) followed by Treponema pallidum haemagglutinin (TPHA) assay (Fortress Diagnostics Limited) to confirm positive RPR results [20].
This study is an open-label, randomized clinical trial of doxycycline hyclate (200 mg taken up to 72 hours after sex, with no more than 200 mg each day) to reduce bacterial STIs. Participants were randomized 1:1 to doxycycline PEP vs. standard of care using computer-based randomization (Randomize.net). The trial’s plan to enrol 446 participants was determined based on an anticipated 66 women with new STIs (N. gonorrhoeae, C. trachomatis, or early syphilis) occurring in the 12 months of follow-up, corresponding to an annual incidence of 22% in the standard of care arm. The trial was designed to achieve 80% power to detect a 50% reduction in infections in the doxycycline arm compared to standard of care. Full details of the trial protocol can be found in the Supplementary Appendix, available with the full text of this article at https://trialsjournal.biomedcentral.com [21]. All procedures were performed in accordance with relevant guidelines.
We present descriptive statistics, and compared the detection of an STI (N. gonorrhoeae, C. trachomatis, and/or T. pallidum) at baseline between women, who perceived themselves as being at risk of acquiring an STI in the next three months, and women, who did not self-perceive STI risk. Relative risks and 95% confidence intervals were estimated using relative risk regression via modified Poisson regression with robust standard errors. All analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
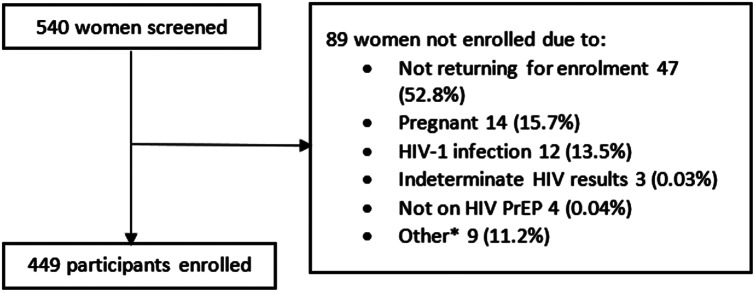
Between February 2020 and November 2021, 540 cisgender women were screened for study eligibility and 449 were enrolled (Fig. 1). The screening to enrolment ratio was 1.2:1. The median age of participants was 24 years (IQR 21–27) (Table 1). Most were never married (66.1%, 297 women). One hundred and thirty-eight women (30.7%) had not given birth at baseline. Over three quarters (76.8%, 345 women) had attended at least secondary schooling or higher, and 62.4% (280 women) reported that they earned their own income.
Fig. 1.
Flow diagram of participant screening and enrolment. (*Other includes age out of study range, breastfeeding, declined study procedures, comorbidities, and not sexually active)
Table 1.
Baseline demographic data, sexual behaviour, contraceptive use, and STI prevalence
| Variable characteristic | N (%) |
|---|---|
| Median age (IQR) years | 24 (21–27) |
| Marital status | |
| Never married | 297 (66.1%) |
| Married (monogamous) | 82 (18.3%) |
| Married (polygamous) | 10 (2.2%) |
| Separated | 52 (11.6%) |
| Divorced | 6 (1.3%) |
| Widowed | 2 (0.4%) |
| Has a primary sex partner | 362 (80.6%) |
| Had a primary sex partner in 3 months before enrolment | 370 (82.4%) |
| Tested for HIV together with primary partner | 254/370 (68.6%) |
| Reported sex with new partners 3 months before enrolment | 149 (33.2%) |
| Parity | |
| None | 137 (30.5%) |
| 1 live birth | 172 (38.3%) |
| 2 live births | 106 (23.6%) |
| 3 or more live births | 34 (7.6%) |
| Highest level of education | |
| No schooling | 1 (0.2%) |
| Primary school, some or complete | 103 (22.9%) |
| Secondary school, not complete | 116 (25.8%) |
| Secondary school, complete | 147 (32.7%) |
| Attended post-secondary school | 82 (18.3%) |
| Participant earns own income | 280 (62.4%) |
| Transactional sex (prior 3 months) | 165 (36.7%) |
| Condom use in most recent vaginal sex act | 129/397* (32.1%) |
| Contraceptive type | |
| Implant | 149 (33.2%) |
| None | 100 (22.3%) |
| DMPA | 98 (21.8%) |
| Condoms | 65 (14.5%) |
| Oral contraceptives | 20 (4.5%) |
| Intrauterine Device | 11 (2.4%) |
| Emergency contraceptives & Condoms | 6 (1.3%) |
*Fifty-two participants did not have vaginal sex in the 3 months prior to enrolment
The majority of women enrolled (82.4%, 370 women) reported having a primary sex partner in the three months prior to enrolment, and one-third (33.2%, 149 women) reported sex with a new partner in the past three months. A total of 268 (67.5%) women reported not using a condom in the most recent vaginal sex act. Over half (56.6%, 254 women) had tested for HIV together with their male partners. Recent transactional sex, sex in exchange for goods, gifts, or money, was reported by 36.7% (165 women), and 16.7% (75 women) reported drinking alcohol before sex in the past month.
More than half of the women were using long-acting reversible contraceptives: implant 149 (33.2%), injectable depot-medroxyprogesterone acetate 98 (21.8%), and intrauterine device 11 (2.4%). One hundred women (22.3%) reported not using any modern contraceptive method. Symptoms commonly associated with doxycycline were reported by some participants prior to randomization, including nausea 38 (8.5%), diarrhoea 19 (4.2%), vomiting 13 (2.9%), and photosensitivity 4 (0.9%).
Overall, 17.9% of women (80/448) had any bacterial STI, including 14.1% (63/448) with C. trachomatis, 5.8% (17/448) with N. gonorrhoeae, and 0.4% (2/449) with T. pallidum, where two participants (0.4%) presented with both N. gonorrhoeae and C. trachomatis (Table 2).
Table 2.
Baseline STI prevalence among cisgender women enrolled in a trial of doxycycline postexposure prophylaxis in Kisumu, Kenya
| STI type | n/N(%) |
|---|---|
| C. trachomatis | 63 /448** (14.1%) |
| N. gonorrhoeae | 17 /448** (3.8%) |
| T. pallidum | 2 /449 (0.4%) |
| STI co-infection | 2/448 (0.4%) |
| Any STI | 80 /448** (17.9%) |
**One participant enrolled without baseline vaginal swab collection
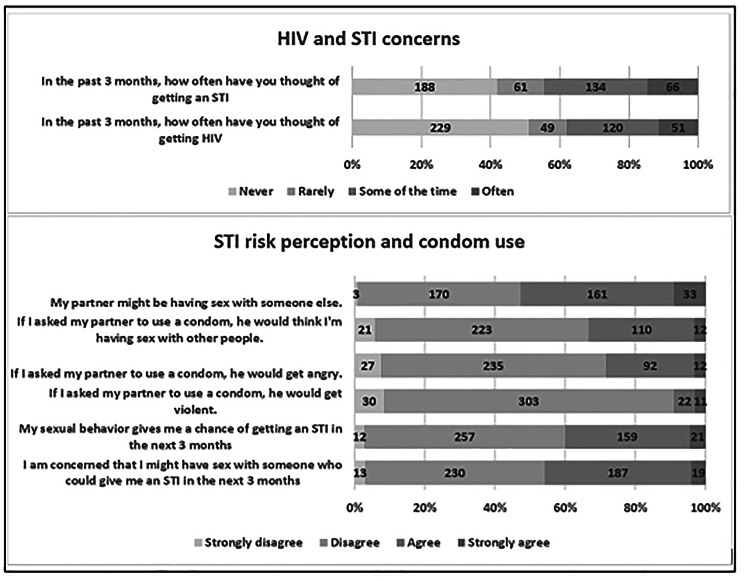
Participants reported being often or sometimes concerned with contracting STIs (44.5%, 200 women) and HIV (38.1%, 171 women) (Fig. 2). Cumulatively, about half of the participants (45.9%, 206 women) either agreed or strongly agreed that they were concerned they might engage in sex with someone who could infect them with an STI in the next three months. One hundred and eighty women (40.1%) perceived that their sexual behaviour could give them a chance of getting an STI in the next three months. About half (52.9%, 194 women) suspected that their male partners might be having sex with someone else. However, there was no association between women’s self-perceived STI risk and STI detection at enrolment, RR 0.90, 95% (CI 0.60–1.36, P = 0.622).
Fig. 2.
Risk concerns and perceptions among the 449 women taking HIV PrEP and enrolled in the dPEP Trial
Discussion
The trial enrolled 449 young women, who were taking HIV PrEP, and half (224) were randomly assigned to take doxycycline PEP. The use of doxycycline as PEP or PrEP for STI prophylaxis is concurrently being evaluated in multiple ongoing trials in the US and Australia among MSM [22]. The potential for long-term complications that result from a bacterial STI, however, is much greater in cisgender women[7, 11, 23]. The dPEP Trial is among the first to focus on the use of doxycycline prophylaxis for the primary prevention of bacterial STI among women.
Social structures and sexual behaviour that necessitate HIV PrEP use often correlate with a high risk of being exposed to a bacterial STI [4, 5]. These baseline data indicate that women who are taking HIV PrEP are at significant risk of contracting curable STIs with an overall prevalence of 17.9% (80), the majority (63) due to C. trachomatis. These data support findings from previous studies which equally indicated a high prevalence of C. trachomatis among women of reproductive age in sub-Saharan Africa [9, 18]. Also consistent with other studies in Kenya, T. palladium had the lowest prevalence, with only two participants testing positive [24]. These baseline data further suggest the need for STI prevention and treatment integrated into PrEP care in sub-Saharan Africa. Furthermore, among the 540 women, primarily recruited from PrEP care, a significant proportion tested positive for HIV at screening (2.2%, 12 women) highlighting the persistent risk of HIV infection and challenges with adherence to HIV PrEP.
A total of 180 (40.1%) of the cohort identified their sexual behaviours as possibly increasing the risk of STIs with more reporting concerns with the behaviours of their male partners (45.9%, 206 women). Overall, slightly less than half of the women, 200 (44.5%) were concerned about contracting STIs in general. This cohort reported low rates of condom use and fear of conflict with negotiated condom use, highlighting the need for structural interventions beyond individual behaviour to reduce the risk of STIs among women [25]. Moreover, the low rates of condom use and high rates of STIs in this cohort indicate that women need a strategy to prevent STIs that they can control by themselves since condoms are typically controlled by their male partners.
Health complications that may stem from STIs among cisgender women include tubal infertility, chronic pelvic pain, pelvic inflammatory disease, ectopic pregnancy, post-partum endometriosis, adverse neonatal outcomes like premature death and premature delivery, and increased risk of HIV acquisition[6, 7, 23, 26]. Despite the high frequency of sexual activity, not all study participants reported prior live births, and of the 137 women (30.5%) without prior delivery, 100 (72.5%) were not using hormonal contraception at enrolment. Multiple factors could explain this observation and may be due to intentional choice for delayed fertility, frequent use of emergency contraception, induced abortions, miscarriage, and/or infertility due to prior STI. Additionally, a significant number of study participants were using contraceptives, and this indicates that women seeking family planning services may benefit from integrating STI prevention, PrEP care, and family planning.
Women of reproductive age in middle and low-income countries are at increased risk of STI-related complications due to limited access to effective prevention strategies, diagnostic testing, or timely treatment [19, 20]. The need for primary prevention of STIs is of global importance with the highest potential for impact among women in low-resource settings. Overall, young women taking HIV PrEP are at risk of curable STIs and have a demonstrated need for women-centered STI prevention programs.
Conclusion
Young women using HIV PrEP in Kenya have a high prevalence of bacterial STIs. Should doxycycline PEP be proven to be efficacious at preventing STIs, there is substantial potential for benefit, especially in high-prevalence settings such as among women taking HIV PrEP.
Acknowledgements
This work has been made possible with the acceptance of the young women in Kisumu who volunteered to participate in the study. We thank the study team members (Lawrence Juma, Linda Aswani, Christine Otieno, Violet Kwach, Elizabeth Koyo, Greshon Rota, Loice Okumu, and Alfred Obiero) for their contributions to data collection. The Kisumu dPEP Kenya Study staff and Benn Kwach offered valued guidance for manuscript development.
Authors’ contributions
KO, LA, and JS led the manuscript development, BR and LRV conducted the data analysis. JMB, EB, and JS designed the trial and wrote the protocol. DD was the lead trial statistician. KO, LA, BR, LRV, RSM, DD, CWS, JO, JMB, EB, and JS participated in manuscript.
Funding statement
This research is supported by US National Institutes of Health (grants R01AI145971, P30AI027757, K23MH124466).
Data Availability
The datasets generated and/or analysed during the current study are not publicly available due to the fact that the study is still ongoing but will be available once study comes to an end in December 2022 on reasonable request from the corresponding author.
Declarations
Competing interests
Dr. McClelland has received honoraria for consulting for Lupin Pharmaceuticals and research funding, paid to the University of Washington, from Hologic Corporation. Dr. Baeten is an employee of Gilead Sciences outside of the submitted work. The author and all other co-authors do not have any declared conflict of interest.
Ethics approval and consent to participate
Ethics approval was received from all pertinent approving bodies in Kenya and the USA: University of Washington’s Institutional Review Board (UW IRB STUDY00007487), Kenya Medical Research Institute Scientific Ethics Review Unit (KEMRI SERU P00122/3915), and Pharmacy and Poisons Board (PPB ECCT 19/11/02/2020(013). Written informed consent to participate in the study was obtained from all participants and legal guardians of the illiterate participants before enrolment. All methods were carried out in accordance with relevant guidelines and regulations of the Kenyan Ministry of Health.
Consent for publication
Not applicable—no identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization WH, World Health Organization. Global health sector strategy on sexually transmitted infections, 2016–2021: Towards ending STIs. WHO/RHR/1609 Geneva WHO; [Internet]. 2016 [cited 2022 Feb 22];20(1):58–63. Available from: https://www.who.int/reproductivehealth/publications/rtis/ghss-stis/en/.
- 2.Korenromp EL, Rowley J, Alonso M, Mello MB, Saman Wijesooriya N, Guy Mahiané S et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes—Estimates for 2016 and progress since 2012. PLoS One. 2019 Feb 1;14(2). [DOI] [PMC free article] [PubMed]
- 3.Unemo M, Lahra MM, Escher M, Eremin S, Cole MJ, Galarza P et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017–18: a retrospective observational study. The Lancet Microbe. 2021 Nov 1;2(11):e627–36. [DOI] [PubMed]
- 4.Amornkul PN, Vandenhoudt H, Nasokho P, Odhiambo F, Mwaengo D, Hightower A et al. HIV prevalence and associated risk factors among individuals aged 13–34 years in rural Western Kenya. PLoS ONE. 2009;4(7). [DOI] [PMC free article] [PubMed]
- 5.Stewart J, Bukusi E, Celum C, Delany-Moretlwe S, Baeten JM. Sexually transmitted infections among African women: an opportunity for combination sexually transmitted infection/HIV prevention. AIDS [Internet]. 2020 Apr 1 [cited 2022 Feb 9];34(5):651–8. https://pubmed.ncbi.nlm.nih.gov/32167988/. [DOI] [PMC free article] [PubMed]
- 6.Stephens AJ, Aubuchon M, Schust DJ. Antichlamydial antibodies, human fertility, and pregnancy wastage. Infect Dis Obstet Gynecol. 2011;2011. [DOI] [PMC free article] [PubMed]
- 7.Steen R, Wi TE, Kamali A, Ndowa F. Control of sexually transmitted infections and prevention of HIV transmission: mending a fractured paradigm. Bull World Health Organ. 2009;87(11):858–65. doi: 10.2471/BLT.08.059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis SC, Mthiyane TN, Baisley K, Mchunu SL, Ferguson JB, Smit T, et al. Prevalence of sexually transmitted infections among young people in South Africa: a nested survey in a health and demographic surveillance site. PLoS Med. 2018;15(2):1–25. doi: 10.1371/journal.pmed.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrone EA, Morrison CS, Chen P-L, Kwok C, Francis SC, Hayes RJ et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. London Sch Hyg Trop Med [Internet]. 360. 10.1371/journal.pmed.1002511. [DOI] [PMC free article] [PubMed]
- 10.Molina J-M, Charreau I, Chidiac C, Pialoux G, Cua E, Delaugerre C et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis [Internet]. 2018;18:308–17. . [DOI] [PubMed]
- 11.Steen R, Chersich M, Gerbase A, Neilsen G, Wendland A, Ndowa F, et al. Periodic presumptive treatment of curable sexually transmitted infections among sex workers: a systematic review. Aids. 2012;26(4):437–45. doi: 10.1097/QAD.0b013e32834ed991. [DOI] [PubMed] [Google Scholar]
- 12.Ong JJ, Baggaley RC, Wi TE, Tucker JD, Fu H, Smith MK, et al. Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure Prophylaxis for the Prevention of HIV infection: a systematic review and Meta-analysis. JAMA Netw Open. 2019;2(12):1–16. doi: 10.1001/jamanetworkopen.2019.17134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celum C, Luetkemeyer AF. Doxycycline for Sexually Transmitted Infection Prevention: Evolving Evidence and Implementation Perspectives. Sex Transm Dis [Internet]. 2021 Sep 1 [cited 2022 Feb 22];48(9):620–1. https://journals.lww.com/stdjournal/Fulltext/2021/09000/Doxycycline_for_Sexually_Transmitted_Infection.2.aspx. [DOI] [PMC free article] [PubMed]
- 14.Tan KR, Magill AJ, Parise ME, Arguin PM. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg. 2011;84(4):517–31. doi: 10.4269/ajtmh.2011.10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chusri S, McNeil EB, Hortiwakul T, Charernmak B, Sritrairatchai S, Santimaleeworagun W, et al. Single dosage of doxycycline for prophylaxis against leptospiral infection and leptospirosis during urban flooding in southern Thailand: a non-randomized controlled trial. J Infect Chemother. 2014 Nov;20(1):709–15. [DOI] [PubMed]
- 16.Santelli JS, Edelstein ZR, Mathur S, Wei Y, Zhang W, Orr MG, et al. Behavioral, biological, and demographic risk and protective factors for new HIV infections among youth in Rakai, Uganda. J Acquir Immune Defic Syndr. 2013;63(3):393–400. doi: 10.1097/QAI.0b013e3182926795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corneli A, Wang M, Agot K, Ahmed K, Lombaard J, Van Damme L. Perception of HIV risk and adherence to a daily, investigational pill for HIV prevention in FEM-PrEP. J Acquir Immune Defic Syndr. 2014;67(5):555–63. doi: 10.1097/QAI.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 18.Price JT, Rosenberg NE, Vansia D, Phanga T, Bhushan NL, Maseko B, et al. Predictors of HIV, HIV Risk Perception, and HIV worry among adolescent girls and Young Women in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2018;77(1):53–63. doi: 10.1097/QAI.0000000000001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rönn MM, Mc Grath-Lone L, Davies B, Wilson JD, Ward H. Evaluation of the performance of nucleic acid amplification tests (NAATs) in detection of chlamydia and gonorrhoea infection in vaginal specimens relative to patient infection status: a systematic review. BMJ Open. 2019;9(1):1–9. doi: 10.1136/bmjopen-2018-022510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakku-Joloba E, Kiragga A, Mbariza J, Kambugu F, Jett-Goheen M, Ratanashi R. Clinical Evaluation of Two Point-Of-Care Lateral Flow Tests for the Diagnosis of Syphilis. Sex Transm Dis [Internet]. 2016;43(10):623–5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5026394/. [DOI] [PMC free article] [PubMed]
- 21.Stewart J, Bukusi E, Sesay FA et al. Doxycycline post-exposure prophylaxis for prevention of sexually transmitted infections among Kenyan women using HIV pre-exposure prophylaxis: study protocol for an open-label randomized trial. Trials. 2022;23:495 (2022). 10.1186/s13063-022-64. [DOI] [PMC free article] [PubMed]
- 22.Grant JS, Stafylis C, Celum C, Grennan T, Haire B, Kaldor J et al. Doxycycline Prophylaxis for Bacterial Sexually Transmitted Infections. Clin Infect Dis [Internet]. 2020 Mar 3 [cited 2022 Mar 11];70(6):1247–53. https://pubmed.ncbi.nlm.nih.gov/31504345/. [DOI] [PMC free article] [PubMed]
- 23.Mwatelah R, McKinnon LR, Baxter C, Abdool Karim Q, Abdool Karim SS. Mechanisms of sexually transmitted infection-induced inflammation in women: implications for HIV risk. J Int AIDS Soc. 2019;22(S6):32–9. doi: 10.1002/jia2.25346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuh T, Micheni M, Selke S, Oluoch L, Kiptinness C, Magaret A et al. Sexually Transmitted Infections Among Kenyan Adolescent Girls and Young Women With Limited Sexual Experience. Front Public Heal [Internet]. 2020 Jul 14 [cited 2022 Feb 9];8. https://www.readcube.com/articles/10.3389%2Ffpubh.2020.00303. [DOI] [PMC free article] [PubMed]
- 25.Madiba S, Ngwenya N. Cultural practices, gender inequality and inconsistent condom use increase vulnerability to HIV infection: narratives from married and cohabiting women in rural communities in Mpumalanga province, South Africa. https://doi.org/101080/1654971620171341597 [Internet]. 2017 [cited 2022 Feb 23];10(sup2). https://www.tandfonline.com/doi/abs/10.1080/16549716.2017.1341597. [DOI] [PMC free article] [PubMed]
- 26.Chesson HW, Mayaud P, Aral SO. Sexually Transmitted Infections: Impact and Cost-Effectiveness of Prevention. Dis Control Priorities, Third Ed (Volume 6) Major Infect Dis [Internet]. 2017 Nov 6 [cited 2022 Feb 9];203–32. https://pubmed.ncbi.nlm.nih.gov/30212101/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to the fact that the study is still ongoing but will be available once study comes to an end in December 2022 on reasonable request from the corresponding author.