Abstract
Considerable research exists on the life history traits, evolutionary history, and environmental factors that shape the population genetic structure of marine organisms, including sharks and rays. Conservation concerns are particularly strong for this group as they are highly susceptible to anthropogenic stressors due to a combination of life history traits including late maturity and low fecundity. Here, we provide a review and synthesis of the global phylogeography of sharks and rays. We examined existing data for 40 species of sharks belonging to 17 genera and 19 species of rays belonging to 11 genera. Median joining haplotype networks were constructed for each species for the mtDNA cytochrome C oxidase subunit I (COI), and an Analysis of Molecular Variance (AMOVA) was conducted to understand patterns of genetic diversity and structure across the three major ocean basins—the Indian, Atlantic and Pacific Oceans. Haplotype networks showed very shallow coalescence in most species, a finding previously reported for marine teleosts. Star topologies were predominant among sharks while complex mutational topologies predominated among rays, a finding we attribute to extremely limited dispersal in the early life history of rays. Population structuring varied amongst species groups, apparently due to differences in life history traits including reproductive philopatry, site fidelity, pelagic habitat, migratory habits, and dispersal ability. In comparison to reef-associated and demersal species, pelagic and semi pelagic species showed lower levels of structure between and within ocean basins. As expected, there is variation between taxa and groups, but there are also some broad patterns that can guide management and conservation strategies.
Keywords: Conservation genetics, Elasmobranchs, Genetic diversity, Mitochondrial DNA, Population structure, Sharks, Rays, Phylogeography, Life history
Introduction
Many marine organisms are characterized by very large distribution ranges, a finding often attributed to the lack of physical barriers. Allen (2008) estimated that the average range of a teleost (bony) reef fish in the Indo–Pacific is 9 million km2, roughly the size of China, compared to 350,000 km2 for a typical freshwater fish range (Albert & Carvalho, 2011). The spatial scales of population structure and dispersal in marine ecosystems are also much larger than in terrestrial and freshwater environments (Avise, 2000). Long-distance colonisation and range expansion, both facilitated and constrained by oceanographic and geographic processes, have shaped the distribution and genetic architecture of marine fishes (Hellberg, 2009).
The patterns of genetic variation within species are linked to geographical processes that give rise to sub-divided populations, as indicated by quantifiable factors such as genetic connectivity and demography. The study of population connectivity is especially pertinent to management practices for commercial exploitation and conservation (Allendorf, Luikart & Aitken, 2013). Genetic diversity is also an important axis for species assessment in a conservation context, especially for wide-ranging marine species like sharks (Domingues, Hilsdorf & Gadig, 2018).
Sharks and rays play a crucial role in the sea by maintaining coastal and oceanic ecosystem structure and function. Large sharks function as top predators while smaller sharks are mesopredators and prey of larger sharks and other oceanic predators (Dulvy et al., 2008). Unlike teleosts, most elasmobranchs (sharks, rays, and skates) show late sexual maturity, long gestation period, low fecundity, slow growth rate, high level of maternal investment and long-life spans (Dulvy et al., 2014). These extreme life histories result in elasmobranchs being among the slowest reproducing vertebrates in the ocean and make their populations extremely vulnerable to anthropogenic pressures such as overfishing, habitat modification, pollution and climate change (Heist, 2008; Snelson, Burgess & Roman, 2008). The primary cause of declining shark and ray populations is overfishing, as harvest rates exceed their capacity to replenish, and their life history traits render them vulnerable to rapid declines (Bräutigam et al., 2015; Dulvy et al., 2021; Pacoureau et al., 2021). More than half of this fishing mortality is due to bycatch (Hall, Alverson & Metuzals, 2000; Gupta et al., 2020; Dulvy et al., 2021). The expansion of the shark and ray fishing industry is an outcome of declining commercial fish populations (teleosts) and/or stringent restrictions on their capture (Bräutigam et al., 2015).
Sharks are found in coastal, demersal, and pelagic habitats that are largely limited to continental shelves, although there are a few completely oceanic species like Carcharhinus longimanus (oceanic whitetip shark). Several species in the family Sphyrnidae (hammerheads), Carcharhinus falciformis (silky shark), Galeocerdo cuvier (tiger shark), and Carcharodon carcharias (white shark) migrate between coastal and oceanic waters (Ferretti et al., 2010). Rays are mostly marine except for a few species in the family Dasyatidae capable of living in low salinity habitats, and members of Potamotrygonidae completely adapted to a life cycle in freshwater (Last et al., 2016). Like sharks, they occupy a variety of niches with pelagic rays capable of undertaking long migrations (Last et al., 2016). However, population subdivisions may be more common in rays because of limited dispersal and greater susceptibility to geographical impediments (Heist, 2005; Heupel, Carlson & Simpfendorfer, 2007). Sharks that inhabit coastal waters aggregate for mating and parturition at specific discrete locations which provide protection for juveniles (Heupel, Carlson & Simpfendorfer, 2007). The extent of population subdivision and genetic divergence between populations in different geographic regions is directly influenced by such segregation and philopatry (Hueter et al., 2005) and the dispersal ability of individuals.
For example, philopatry to natal sites in blacktip reef sharks (Carcharhinus melanopterus) is a major contributor to genetic structuring within the Indo-Pacific and between islands in French Polynesia, by reducing dispersal (Mourier & Planes, 2013; Vignaud et al., 2014). Bull shark juveniles from nurseries in the Gulf of Mexico and Atlantic showed significant genetic variation in the mitochondrial control region (mtDNA-CR) but were homogenous with nuclear microsatellites indicating male biased dispersal (Laurrabaquio et al., 2019). Similarly, population structure (using mtNADH sequences) among juvenile bull sharks from 13 nurseries located in rivers around Northern Australia also indicated a strong influence of female reproductive philopatry (Tillett et al., 2012). These observed genetic differences support philopatry and indicate a strong role in shaping population separations in bull sharks (Tillett et al., 2012; Laurrabaquio et al., 2019). Site fidelity and long-term residency also resulted in fine-scale genetic structuring within reef manta rays (Mobula alfredi) in New Caledonia (Lassauce et al., 2022).
Understanding elasmobranch biology and life history is therefore important for evolving species-specific management plans. Their governance poses a challenge as many species of sharks and rays migrate across national boundaries and international waters, and there is little knowledge/information about the migratory habits of transboundary species in international waters (Bräutigam et al., 2015; Kizhakudan et al., 2015). Presently, conservation measures for sharks and rays are influenced by political boundaries, oceanic expanses, and centres of high demand (Bräutigam et al., 2015). Conservation efforts are under-resourced due to lack of adequate funds, technical capacity and political will to efficiently monitor, control and manage elasmobranch fisheries/trade (Bräutigam et al., 2015).
Hence, we examined patterns of phylogeography and population structure within and across multiple families of sharks (Carcharhinidae, Cetorhinidae, Hemiscyllium, Odontaspididae, Stegostomatidae, Alopiidae, Rhincodontidae, Sphyrnidae and Lamnidae) and rays (Dasyatidae, Mobulidae, Myliobatidae and Gymnuridae) in relation to their habitat and life history. We explored these patterns by (a) compiling data from a variety of published and unpublished sources, (b) constructing haplotype networks and examining network topology, (c) estimating nucleotide and haplotype diversity, and (d) assessing population genetic structure using AMOVA. In a few species, we report data from a single study, but for many species, our meta-analysis combines data from multiple sources, both published and unpublished, and provides insights from comparisons across genera, and between sharks and rays.
Methods
A literature review was carried out on the phylogeography of shark and ray species to check for availability of sequence data. The accession numbers provided in publications were used to identify sequences from GenBank (Benson et al., 2013), a National Centre for Biological Sciences (National Center for Biotechnology Information, 1988) and US National Institute of Health (NIH) sequence database, as well as the geographical locations of specimens. Additional unpublished sequences were downloaded from GenBank and included in the study. We then narrowed the species list to those with sufficient sample sizes (>10). Cytochrome C oxidase subunit I (COI) was selected for this analysis because it was the most common sequence across species. Other markers may provide greater resolution for the detection of intraspecific (population level) divergences but it is a pragmatic choice based on data availability. Our final set of target species included 40 shark species from 17 genera (Table 1) and 19 ray species from 11 genera (Table 2). All mitochondrial DNA sequences (mtDNA) were obtained/downloaded from GenBank (Tables S1 and S2). The sample collection locations provided in the published research article and/or on GenBank were used for population analysis.
Table 1. Life history attributes and distribution of shark species assessed in the present study.
| Species name | Common name | Distribution | Habitat | Body size a (cm) | IUCN category | Traits b |
|---|---|---|---|---|---|---|
| Alopias pelagicus | Pelagic thresher shark | Indo–Pacific | Oceanic and pelagic | 259–376 | Endangered | No evidence of philopatry; ovoviviparous |
| Alopias superciliosus | Bigeye thresher shark | Global | Coastal, oceanic and pelagic | 470–484 | Vulnerable | No evidence of philopatry; ovoviviparous |
| Alopias vulpinus | Common thresher shark | Global | Coastal, oceanic and pelagic | 575–635 | Vulnerable | No evidence of philopatry; ovoviviparous |
| Carcharhinus altimus | Bignose shark | Global | Benthopelagic | 300 | Near threatened | Viviparous |
| Carcharhinus amblyrhynchoides | Graceful shark | Indo–west Pacific | Coastal and pelagic | 178–190 | Near threatened | Viviparous |
| Carcharhinus amboinensis | Pigeye shark | Global | Coastal, oceanic and demersal | 280 | Data deficient | Residency, regional philopatry; viviparous |
| Carcharhinus brevipinna | Spinner shark | Global | Coastal and pelagic | 280–283 | Near threatened | Regional philopatry, site fidelity; viviparous |
| Carcharhinus dussumieri | Whitecheek shark | Indo–west Pacific | Coastal and demersal | 100–110 | Endangered | Viviparous |
| Carcharhinus falciformis | Silky shark | Global | Coastal, oceanic and pelagic | 330–350 | Vulnerable | Site fidelity; viviparous |
| Carcharhinus leucas | Bull shark | Global | Coastal & estuarine and reef-associated | 340–360 | Near threatened | Residency, regional philopatry and site fidelity; viviparous |
| Carcharhinus limbatus | Common blacktip shark | Global | Coastal and reef-associated | 258–297 | Near threatened | Seasonal residency, regional philopatry and site fidelity; viviparous |
| Carcharhinus longimanus | Oceanic whitetip shark | Global | Oceanic and pelagic | 350–395 | Critically endangered | Seasonal residency and site fidelity; viviparous |
| Carcharhinus macloti | Hardnose shark | Indo–west Pacific | Reef-associated and demersal | 100–110 | Near threatened | Viviparous |
| Carcharhinus melanopterus | Blacktip reef shark | Indo–Pacific | Coastal and reef-associated | 160 ≤ 200 | Vulnerable | Residency, site fidelity, natal philopatry; viviparous |
| Carcharhinus plumbeus | Sandbar shark | Global | Coastal and benthopelagic | 200–300 | Near threatened | Seasonal residency, site fidelity; viviparous |
| Carcharhinus sealei | Blackspotted shark | Indo–west Pacific | Reef-associated | 100 | Near threatened | Viviparous |
| Carcharhinus sorrah | Spot-tail shark | Indo–west Pacific | Coastal and reef-associated | 160–180 | Near threatened | Residency; viviparous |
| Carcharodon carcharias | Great white shark | Global | Oceanic and pelagic | 400–600 | Vulnerable | Ovoviviparous (oophagus) |
| Carcharias taurus | Sand tiger | Atlantic, Indo–west Pacific | Coastal, reef-associated and demersal | 320 | Vulnerable | Seasonal residency, site fidelity; ovoviviparous |
| Cetorhinus maximus | Basking shark | Atlantic, Pacific and Artic | Coastal, oceanic and pelagic | 1,200–1,220 | Endangered | Ovoviviparous (oviphagy) |
| Chiloscyllium griseum | Grey bamboo shark | Indo–west Pacific | Reef-associated and demersal | 77 | Vulnerable | Oviparous |
| Chiloscyllium indicum | Slender bamboo shark | Indo–west Pacific | Coastal and demersal | 65 | Vulnerable | Oviparous |
| Chiloscyllium punctatum | Brown banded bamboo shark | Indo–west Pacific | Reef-associated and demersal | 104 | Near threatened | Oviparous |
| Galeocerdo cuvier | Tiger shark | Global | Coastal, oceanic and semi pelagic | 400 ≥ 550 | Near threatened | Residency, site fidelity; ovoviviparous |
| Isurus oxyrinchus | Short mako shark | Global | Coastal, oceanic and pelagic | 200–400 | Endangered | Site fidelity; ovoviviparous |
| Isurus paucus | Longfin mako | Global | Oceanic, pelagic | 417–430 | Endangered | Site fidelity; ovoviviparous (oviphagy) |
| Negaprion acutidens | Sicklefin lemon shark | Indo–Pacific | Coastal and demersal | 310–380 | Vulnerable | Residency, site fidelity; viviparous |
| Negaprion brevirostris | Lemon shark | Atlantic, East Pacific | Coastal, reef-associated and demersal | 250–300 | Near threatened | Residency, site fidelity, natal philopatry; viviparous |
| Prionace glauca | Blue shark | Global | Oceanic and pelagic | 383–385 | Near threatened | Viviparous |
| Rhincodon typus | Whale shark | Global | Coastal, oceanic and semi pelagic | 1,600–2,100 | Endangered | Site fidelity; ovoviviparous |
| Rhizoprionodon oligolinx | Grey sharpnose shark | Indo–west Pacific | Coastal, reef-associated | 70 | Least concern | Viviparous |
| Rhizoprionodon acutus | Milk shark | Indo–west Pacific | Benthopelagic | 178–180 | Vulnerable | Viviparous |
| Scoliodon laticaudus | Spadenose shark | Indo–west Pacific | Coastal and demersal | 74–75 | Vulnerable | Viviparous |
| Sphyrna lewini | Scalloped hammerhead shark | Global | Semi oceanic and semi pelagic | 370–420 | Critically endangered | Seasonal residency, regional philopatry and site fidelity; viviparous |
| Sphyrna mokarran | Great hammerhead shark | Global | Semi oceanic and semi pelagic | 550–610 | Critically endangered | Site affinity; viviparous |
| Sphyrna zygaena | Smooth hammerhead shark | Global | Semi oceanic and semi pelagic | 370–400 | Vulnerable | Regional philopatry; viviparous |
| Stegostoma fasciatum | Zebra shark | Not mapped | Coastal and demersal | 235–246 | Endangered | Seasonal residency and site fidelity; oviparous |
| Triaenodon obesus | Whitetip reef shark | Indo–Pacific | Coastal and reef-associated | 170–213 | Vulnerable | Residency; viviparous |
| Lamna ditropis | Salmon shark | North Pacific | Coastal, oceanic and pelagic | 221–305 | Least concern | Residency, seasonal residency, site fidelity, regional philopatry; ovoviviparous (oophagus) |
| Lamna nasus | Porbeagle | North Atlantic and Southern hemisphere | Oceanic, coastal and pelagic | 350–365 | Vulnerable | Ovoviviparous (oophagus) |
Notes.
Body size refers to the maximum total length of the adult shark. Total length is the length measured from the tip of the snout to the tip of the longer lobe of the caudal fin.
Traits refer to the type of philopatry and reproduction shown by the particular shark species.
Table 2. Life history attributes and distribution of ray species assessed in the present study.
| Species name | Common name | Distribution | Habitat | Disc width a (cm) | IUCN category | Traits b |
|---|---|---|---|---|---|---|
| Aetobatus narinari | Spotted eagle ray | Atlantic Ocean | Oceanic, coastal and benthopelagic | 330 | Near threatened | Site fidelity; ovoviviparous |
| Aetobatus ocellatus | Ocellated eagle ray | Indo–west Pacific | Oceanic, coastal and benthopelagic | 300 | Vulnerable | Ovoviviparous |
| Brevitrygon imbricata | Bengal whipray | Northern Indian Ocean | Coastal and demersal | 23 | Data deficient | Ovoviviparous |
| Brevitrygon walga | Scaly whipray/ Dwarf whipray | Western Indian Ocean | Coastal and demersal | 32 | Near threatened | Ovoviviparous |
| Gymnura micrura | Smooth butterfly ray | Atlantic Ocean | Coastal and demersal | 110 | Data deficient | Ovoviviparous |
| Gymnura poecilura | Longtail butterfly ray | Indo–Pacific | Coastal and demersal | 104 | Near threatened | Ovoviviparity |
| Himantura leoparda | Leopard whipray | Indo–west Pacific | Coastal and demersal | 140 | Vulnerable | Ovoviviparous |
| Himantura uarnak | Honeycomb stingray/ Reticulate whipray | Indo–west Pacific Lessepsian migrant found in the Mediterranean Sea. |
Coastal and intertidal | 160 | Vulnerable | Site affinity; ovoviviparous |
| Maculabatis gerrardi | Sharpnose stingray | Indo–west Pacific | Coastal and demersal | 116 | Endangered | Ovoviviparous |
| Mobula birostris | Giant oceanic manta ray | Indo-Pacific | Oceanic, coastal and demersal | 670–900 | Vulnerable | Site fidelity, seasonal residency; ovoviviparous |
| Mobula kuhlii | Shortfin devil ray | Indo–west Pacific | Coastal, oceanic and pelagic | 135 | Endangered | Ovoviviparous |
| Mobula mobular | Devil Fish | Global | Oceanic, coastal and pelagic | 520 | Endangered | Ovoviviparous |
| Mobula tarapacana | Sicklefin devil ray | Global | Coastal, oceanic and pelagic | 370 | Endangered | Ovoviviparous |
| Mobula thurstoni | Bentfin devil ray | Global | Coastal and pelagic | 189 | Endangered | Ovoviviparous |
| Neotrygon indica | Indian ocean blue spotted maskray | Western Indian Ocean | Coastal and reef-associated | 31.4 | Newly described species | |
| Neotrygon kuhlii | Bluespotted maskray | Southwest Pacific Ocean | Reef-associated and demersal | 30 | Data deficient | Site affinity, regional philopatry: ovoviviparous |
| Pateobatis jenkinsii | Jenskin’s whipray | Indo–Pacific | Coastal and demersal | 150 | Vulnerable | Ovoviviparous |
| Pteroplatytrygon violacea | Pelagic stingray | Global | Oceanic and pelagic | 96 | Least concern | Ovoviviparous |
| Taeniura lymma | Blue spotted ribbon ray | Indo–west Pacific | Coastal, reef-associated and benthic | 30–35 | Near threatened | Ovoviviparous |
Notes.
Disc Width refers to the maximum measured wing-span of the ray species.
Traits refer to the type of philopatry and reproduction shown by the particular ray species.
Alignment of sequences
The sequences were downloaded and aligned using ‘Clustal W’ in MEGA 5.05 (MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms; Kumar et al., 2018). The length of shark sequences ranged from 648 bp to 687 bp and the sequence length for rays ranged from 651 bp to 693 bp. Stop codons, if present in the aligned DNA segment, were deleted and sequences re-aligned before constructing the haplotype networks.
Data analysis
DNA Sequence Polymorphism 6.12.03 (DnaSP 6; Rozas et al., 2017) was used to generate the haplotype data file and calculate haplotype and nucleotide diversities (Nei, 1987; Nei & Miller, 1990) with corresponding standard deviations. The sample sites were categorised as Western, Eastern and Central Indian Ocean within the Indian Ocean group; Northern & Southern Atlantic within the Atlantic Ocean group; and Northern & Southern Pacific within the Pacific Ocean group. A Median Joining Haplotype Network (Bandelt, Forster & Röhl, 1999) was constructed using PopART 1.7 (Population Analysis with Reticulate Trees; Leigh & Bryant, 2015) for sharks (Table 1) and rays (Table 2). In population genetics, haplotype networks are used to understand biogeography and genealogical relationships at intraspecific levels (Leigh & Bryant, 2015). Median joining networks can handle large data sets (Posada & Crandall, 2001) and combines the features of minimum spanning trees (Kruskal’s algorithm) and Farris’s maximum-parsimony heuristic algorithm (Bandelt, Forster & Röhl, 1999).
All the observed haplotype networks were classified into seven types of topologies. Based on Jenkins, Castilho & Stevens (2018), we classified networks as star (a single dominant haplotype with many haplotypes related to it), complex mutational (a few haplotypes differing by one or two mutations and some by a very large number) and complex star (many dominant haplotypes and high frequency connections). When species from different geographic ranges did not share any haplotypes and differed by several mutations, we termed it as ‘simple exclusive’ (referred to as reciprocally monophyletic by some authors). We classified networks as ‘single’ when species from different geographic regions were represented by a single haplotype. The term ‘simple linear’ was used to refer to networks having three or more haplotypes arranged linearly with no branches. Networks that did not fall into any of the above mentioned six categories and had two or more haplotypes arranged in no particular pattern were categorized as ‘simple’.
Statistical analyses were carried out using PopART. To understand the extent of geographic structuring of evolutionary lineages, nested/hierarchical AMOVA (Excoffier, Smouse & Quattro, 1992) was conducted after defining the groups. The groups defined were Indian Ocean, Pacific Ocean, and Atlantic Ocean with subgroups as defined above. Fixation indices (ΦST, ΦSC, ΦCT) and percentage variation among groups, among populations and within populations were reported from AMOVA. Differentiation among all populations i.e., all subgroups is represented by overall ΦST values, while population structuring within individual ocean basins is represented by ΦSC; population structuring across ocean basins is given by ΦCT values. Genetic structuring of populations between Indo–Pacific and Atlantic species was also carried out, wherein the samples from Indian and Pacific Oceans were combined (Indo–Pacific) and samples from Atlantic Ocean formed the second group. The extent of genetic structuring among shark and ray species from different regions was analysed using the haplotype networks, AMOVA, and diversity values.
Kruskal–Wallis (Siegel & Castellan, 1988) tests were carried out to statistically test if the haplotype (h) and nucleotide ( π) diversity values differed among different families of sharks and rays. The test was also carried out to determine if the diversity values differed significantly between species based on their habitat. A Mann–Whitney U test (Siegel & Castellan, 1988) was used to determine whether diversity values differed significantly between sharks and rays. Fisher’s exact test was used to assess the effect of structuring across habitats for both sharks and rays. The habitats occupied by species of both sharks and rays were classified into three broad categories–pelagic (includes semi pelagic species as well), demersal (includes benthopelagic species) and reef-associated (includes those that are bottom dwelling among coral reefs, i.e., reef-associated and demersal).
Results
Before presenting results, we reiterate that data was drawn primarily from published studies (for 11 shark and three ray species) but also include unpublished data (for 28 shark and 17 ray species; Tables S1 and S2). We regard the published data as highly reliable. The unpublished data was given a higher level of scrutiny, including contribution source, chronology, and volume of sequence data. In cases where unpublished data corroborated published data, we considered the results to be supported. In instances where observed divergent lineages were entirely based on unpublished data, we regarded the results as provisional. In particular, Gymnura micrura had highly divergent lineages and the results should be treated as provisional. The careful use of GenBank data, while provisional, is unlikely to introduce a systemic bias that would alter the interpretation of results.
SHARKS: patterns of genetic structure and diversity
Network topology
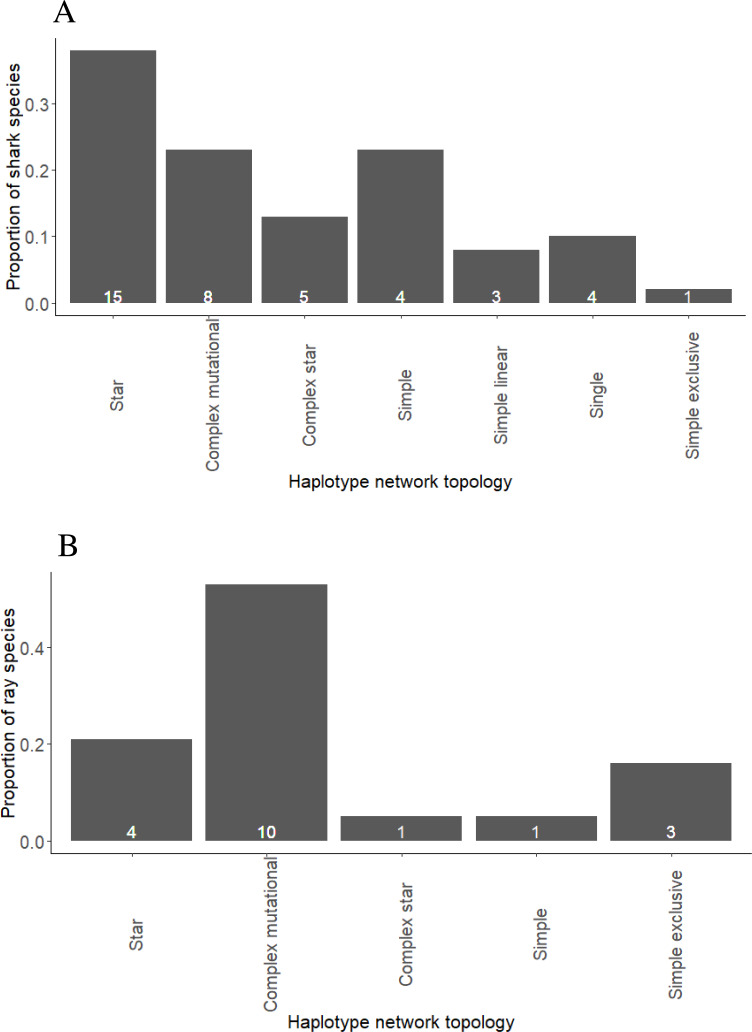
Star networks were predominant among shark species, including eight pelagic, two reef-associated, two demersal and reef-associated, two demersal and one semi-pelagic species (Fig. 1A, Fig. S1 and Table S3). Complex mutational networks were observed in three reef-associated, one demersal & reef-associated, two semi-pelagic, one pelagic and one benthopelagic species. Many species exhibiting complex mutational networks also had a dominant (presumably) ancestral haplotype at the centre of a star topology. Complex star topologies were observed in four species inhabiting pelagic waters and one reef-associated species. The coastal-demersal Ca. dussumieri had two small clusters of haplotypes, corresponding to the Western Indian Ocean and Eastern Indian/South Pacific, separated by a long branch with 33 mutations (Fig. S1). Two globally distributed pelagic species (P. glauca and Sp. zygaena) and, one demersal (Ch. Griseum) and one Atlantic species (N. brevirostris) were represented by a single haplotype (Fig. S1 and Table S3).
Figure 1. Proportion of shark (A) and ray (B) species exhibiting different haplotype network topologies.
The total number of shark species in the present study is forty and number of ray species is nineteen.
Haplotype and nucleotide diversity
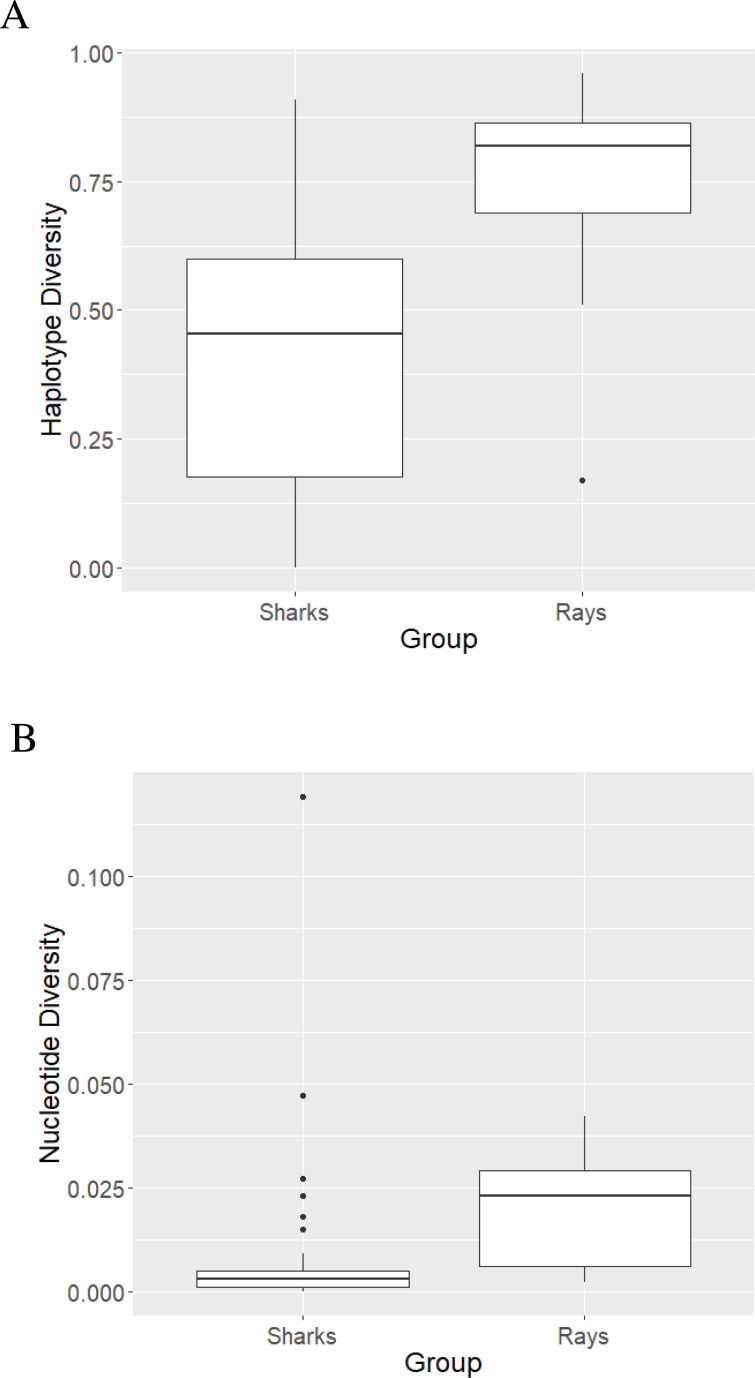
The mean haplotype diversity value of sharks was 0.422 ±0.260 (Fig. 2A) with the highest diversity (h = 0.91 ± 0.047) observed in L. ditropis, a coastal-oceanic-pelagic species. The lowest (non-zero) diversity (h = 0.004 ± 0.066) was observed in Carcharias carcharias (Table S3). Within genus Carcharhinus, the haplotype diversity value was lowest for a coastal-demersal species Ca. amboinensis (h = 0.108 ± 0.049) and highest for Ca. plumbeus, a coastal-benthopelagic species (h = 0.671 ± 0.083). Haplotype diversity was high (h ≥ 0.50) for eighteen species, of which twelve were coastal. Haplotype diversity was low (h ≤ 0.50) for twenty-two species, with the majority (12) inhabiting oceanic/semi-oceanic waters (Table S3). There was no difference in h values between the nine families of sharks (Kruskal–Wallis χ2 = 738, p = 0.49).
Figure 2. Haplotype (A) and nucleotide (B) diversity values of sharks and rays represented as boxplots.
The highest nucleotide diversity was observed in the coastal, reef associated Rhiz. oligolinx (π = 0.119 ± 0.00787) while the coastal-oceanic Rhin. typus had the lowest (non-zero) diversity (0.0002 ± 0.0001; Table S3). The mean nucleotide diversity value for sharks was 0.0086 ± 0.02 (Fig. 2B). Within genus Carcharhinus, coastal, reef associated Ca. dussumieri had the highest nucleotide diversity (π = 0.027 ± 0.0039) while oceanic/pelagic Ca. longimanus had the lowest (π = 0.0003 ± 0.0001). The differences in nucleotide diversity for the remaining eight species within the genus Carcharhinus were minimal. High nucleotide diversity values (π ≥ 0.005) were observed in twelve species with the most common habitat being pelagic waters (five). Twenty-eight species had low nucleotide diversity values (π ≥ 0.005) with the most common habitat being pelagic (10) followed by reef-associated (six) and demersal (five). There was no difference in π values between the nine families of sharks (Kruskal–Wallis χ2 = 6.55, p = 0.59). There was no difference in diversity values between species occupying different habitats—h (χ2 = 4.04, p = 0.40) and π (χ2 = 2.78, p = 0.59).
Genetic structure
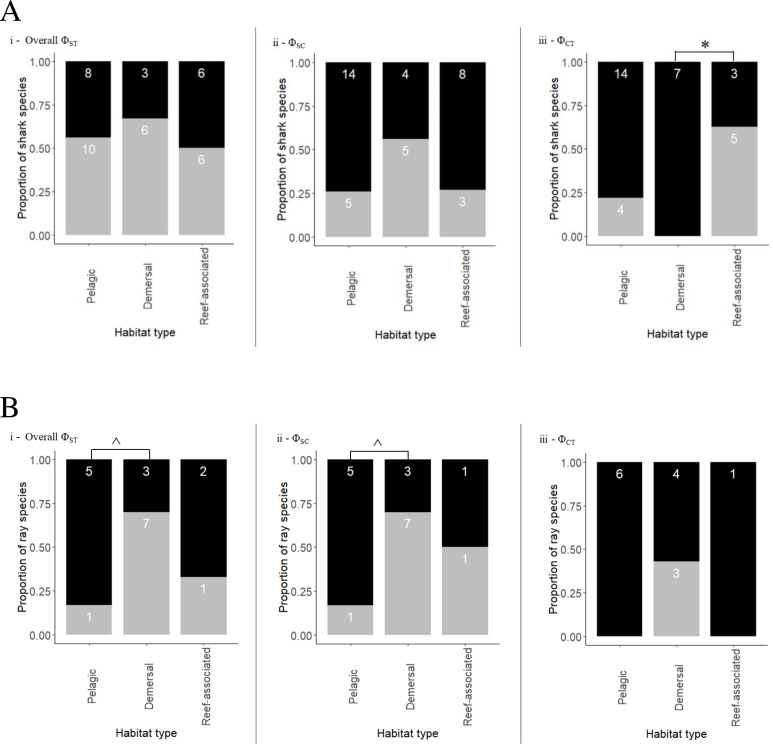
The coastal-demersal Ca. dussumieri had the highest overall ΦST value (0.99, p < 0.001; Table 3). Eleven of 22 species (50%) belonging to the family Carcharhinidae and four pelagic species of family Lamnidae (80%) had significant overall ΦST values. Most species that exhibited structuring within Carcharhinidae were reef-associated and/or demersal. Isurus oxyrinchus had the lowest ΦST value (0.067, p = 0.018) indicating weak but significant structuring. Very weak structuring was observed in Ca. longimanus, I. oxyrhincus and G. cuvier, all inhabiting pelagic and semi pelagic waters. Four pelagic and one semi pelagic species with Indo–Pacific/global distribution showed negative or ΦST = 0 values, indicating a lack of structure among oceans. Overall, there was no significant difference between habitats in ΦST (Fisher’s exact test, p = 0.796) (Fig. 3A; See Table 3 for a summary).
Table 3. AMOVA analysis carried out using cytochrome C oxidase subunit I for sharks.
The numerals in bold indicate significant structuring, p values are given in brackets and n is the sample size.
| Species name | Overall ΦST | Global comparison (nested AMOVA) | Indo–Pacific vs Atlantic | ||||
|---|---|---|---|---|---|---|---|
| ΦST | ΦSC | ΦCT | ΦST | ΦSC | ΦCT | ||
| A. pelagicus (n = 146) | 0.211** | 0.308** | 0.218* (0.002) | 0.116** | Indo–Pacific distribution | ||
| A. superciliosus (n = 104) | −0.07 (0.882) | −0.066 (0.755) | −0.149 (0.949) | 0.072 (0.228) | −0.12 (0.735) | −0.078 (0.814) | −0.038 (0.793) |
| A. vulpinus (n = 44) | 0.513** (0.001) | 0.613** | 0.374 (0.365) | 0.382* (0.025) | 0.56** (0.001) | 0.59* (0.019) | −0.086 (0.267) |
| Ca. altimus (n = 29) | 0.714** | 0.735** | 0.787** | −0.245 (0.602) | 0.759** | 0.75* (0.003) | 0.038 (0.297) |
| Ca. amblyrhynchoides (n = 32) | 0.024 (0.296) | −0.105 (0.875) | 0.303 (0.144) | 0.152 (0.728) | Indo–west Pacific distribution | ||
| Ca. amboinensis (n = 72) | 0.018 (0.601) | −0.086 (0.904) | 0.065 (0.246) | 0.162 (0.888) | Indo–Pacific distribution | ||
| Ca. brevipinna (n = 132) | 0.052 (0.183) | 0.036 (0.423) | 0.124 (0.06) | 0.106 (0.807) | 0.0018 (0.537) | 0.11 (0.222) | −0.13 (0.527) |
| Ca. dussumieri (n = 24) | 0.99** | 0.99** | 0.99** | −0.388 (0.665) | Indo–Pacific distribution | ||
| Ca. falciformis (n = 116) | 0.206 (0.081) | −0.054 (0.483) | 0.247* (0.007) | ||||
| Ca. leucas (n = 66) | 0.37 (0.017) | 0.423* (0.003) | 0.398 (0.046) | 0.041 (0.279) | 0.369* (0.015) | 0.46* (0.029) | −0.17 (0.477) |
| Ca. limbatus (n = 78) | 0.244* (0.008) | 0.238* (0.02) | 0.007 (0.22) | 0.067** | 0.22* (0.046) | 0.35* (0.016) | −0.201 (0.915) |
| Ca. longimanus (n = 30) | 0.091* (0.058) | 0.163 (0.15) | −0.289 (0.497) | 0.351 (0.096) | 0.306* (0.032) | −0.175 (0.17) | 0.409** |
| Ca. macloti (n = 12) | 0.633** | 0.915** | 0.191** | 0.894** | IP | IP | IP |
| Ca. melanopterus (n = 54) | 0.408* (0.021) | 0.735** | 0.151 (0.291) | 0.692* | Indo–Pacific distribution | ||
| Ca. plumbeus (n = 48) | 0.493* | 0.601** | 0.645** | −0.122 (0.087) | 0.582** | 0.649** | −0.109 (0.791) |
| Ca. sealei (n = 18) | −0.013 (0.292) | All sequences from Indian Ocean | |||||
| Ca. sorrah (n = 124) | 0.012 (0.521) | 0.06 (0.345) | 0.007 (0.601) | 0.067** | Indo–Pacific distribution | ||
| Ca. taurus | 0.997** | 0.998** | 0.994** | 0.667 (0.105) | 0.998** | 0.993** | 0.756* (0.05) |
| Carcharodon carcharias (n = 18) | 0.379* (0.041) | 0.442* (0.026) | 0.355 (0.361) | 0.134 (0.187) | 0.342 (0.084) | 0.501* (0.027) | −0.319 (0.879) |
| Ce. maximus (n = 56) | −0.0104 (0.246) | 0.0005 (0.2250) | −0.065 (0.329) | 0.066 (0.389) | −0.013 (0.3) | −0.022 (0.31) | 0.009 (0.478) |
| Ch. griseum (n = 12) | All sequences from central Indian Ocean | ||||||
| Ch. indicum (n = 14) | All sequences from eastern Indian Ocean | ||||||
| Ch. punctatum (n = 20) | 0.206* (0.019) | All sequences from eastern Indian and south Pacific Ocean | |||||
| G. cuvier (n = 228) | 0.068** | 0.833** | 0.174* (0.006) | 0.798* (0.057) | 0.88** | 0.13* (0.009) | 0.86** |
| I. oxyrinchus (n = 140) | 0.067* | 0.0707* (0.037) | 0.112* (0.016) | −0.047 (0.698) | 0.085* | 0.076* | 0.0104 |
| I. paucus (n = 46) | 0.401** | 0.519** | 0.157 (0.163) | 0.429 (0.123) | 0.562** | 0.107 (0.213) | 0.509** |
| L. ditropis (n = 15) | North Pacific distribution | ||||||
| L. nasus (n = 81) | 0.455** | 0.427** | 0.646** | −0.621 (0.731) | All sequences from Atlantic and Pacific Ocean | ||
| N. acutidens (n = 31) | 0.568** | 0.718** | 0.714** | 0.016 (0.325) | Indo–Pacific distribution | ||
| N. brevirostris (n = 11) | 0 | Atlantic distribution | |||||
| P. glauca (n = 534) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhin. typus (n = 48) | −0.001 (0.935) | −0.055 (0.993) | −0.05 (0.532) | 0.0022 (0.482) | All sequences from Indo–Pacific | ||
| Rhiz. acutus (n = 78) | 0.678** | 0.818** | 0.744** | 0.286 (0.263) | Indo–west Pacific distribution | ||
| Rhiz. oligolinx (n = 15) | 0.026 (0.37) | All sequences from Atlantic Ocean | |||||
| S. laticaudus (n = 27) | −0.0105 (0.622) | All sequences from Indian Ocean | |||||
| Sp. mokarran (n = 59) | 0.084 (0.129) | 0.075 (0.153) | 0.361 (0.0156) | −0.447 (0.728) | 0.095 (0.139) | 0.24 (0.202) | −0.188 (0.704) |
| Sp. lewini (n = 323) | 0.726** | 0.80** | 0.76** | 0.207 (0.344) | 0.734** | 0.81** | −0.375 (0.678) |
| Sp. zygaena (n = 91) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| St. fasciatum (n = 26) | 0.306* (0.013) | 0.28* (0.071) | 0.466* (0.017) | −0.35 (0.495) | Indo–Pacific distribution | ||
| T. obesus (n = 53) | 0.467** | 0.654** | 0.843* (0.05) | −1.2 (0.767) | All sequences from Indo–Pacific | ||
Notes.
p < 0.05.
p < 0.001.
Figure 3. Proportion of shark (A) and ray (B) species exhibiting population structuring/no structuring in relation to their habitats across different Φ statistics (grey bars represent significant structuring; black bars represent no structure).
(i) Population structuring among all populations (overall ΦST), (ii) population structuring within ocean basins (ΦSC), (iii) population structuring across ocean basins (ΦCT). Semi pelagic species have been grouped with pelagic species and benthopelagic with demersal species. Demersal & reef-associated species have been grouped with reef-associated species. Asterisk (*) indicates the p-value (p = 0.026) of test of proportions. Caret (∧) indicates the p-value (p = 0.13) for the test of proportions.
Nested AMOVA results show significant structuring of population samples among oceans (ΦCT) in five reef-associated, one semi pelagic and three pelagic species (Fig. 3A; Table 3). Among these, four reef-associated, one semi-pelagic and two pelagic species had distribution ranges in the Indo–Pacific, indicating that structuring was primarily between the Indian and Pacific Ocean. In contrast to the findings above for ΦST, ΦCT was significant (Fisher’s exact test p = 0.018) for species occupying different habitats. Pairwise comparison revealed that the proportion of structured populations was significantly lower in demersal species than reef-associated species (p = 0.026).
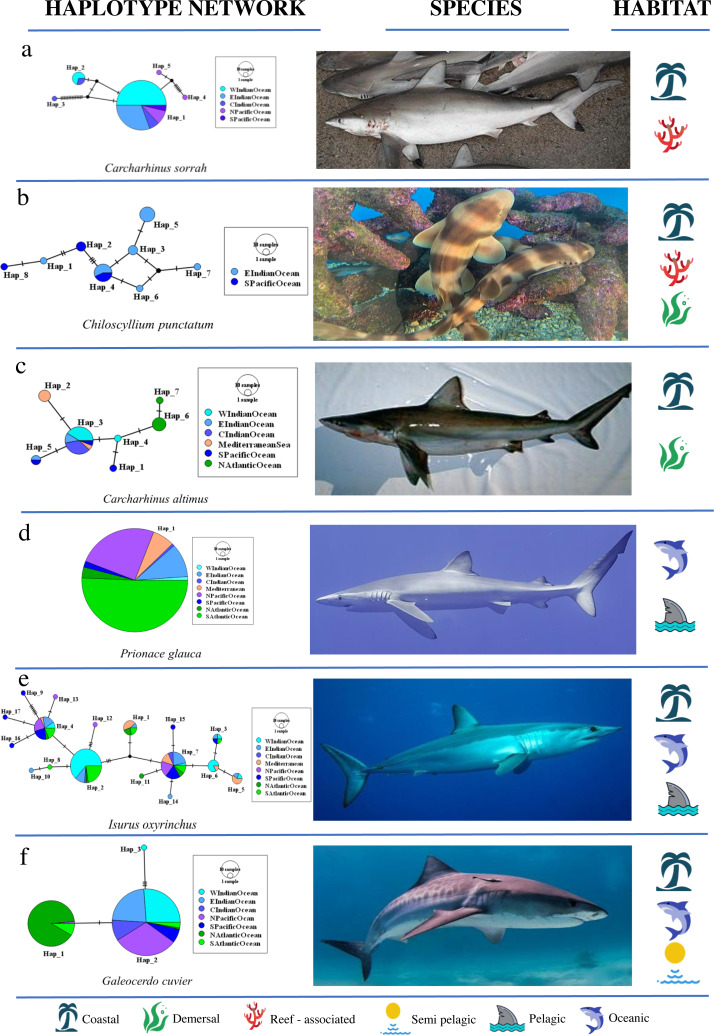
Ca. macloti a reef-associated & demersal species had the highest ΦCT value (0.894, p < 0.001) while the lowest was observed in two reef-associated species (Ca. sorrah and Ca. limbatus). Pelagic and semi-pelagic species in five families had non-significant ΦCT values, thereby lacking structuring across the three (or two Indian & Pacific) ocean basins. A few reef-associated, demersal and three benthopelagic sharks also lacked structuring (Fig. 3A). Differentiation among population samples within ocean (ΦSC) was significant for 13 species. These are five reef-associated and/or demersal, three benthopelagic, three pelagic and two semi-pelagic species (Fig. 3A; Table 3). Carcharias taurus had the highest ΦSC value (0.994, p < 0.001; reef-associated & demersal) and the lowest was for I. oxyrinchus (0.112, p = 0.016; oceanic & semi pelagic). There was no significant difference in the proportion of structured populations within ocean basins (Fisher’s exact test, p = 0.37) across the three habitats. In comparisons of Indo–Pacific vs Atlantic groupings, three of 17 globally distributed species (Ca. longimanus, G. cuvier and I. paucus) showed structuring. A. superciliosus, Ca. brevipinna, Ca. amblyrhynchoides, Ca. amboinensis, P. glauca, Ce. maximus, Rhin. typus, Sp. mokarran and Sp. zygaena lacked detectable structuring at all levels (Table 3). A few haplotypes of Ca. brevipinna are separated by more mutations from the central haplotype compared to Ca. amboinensis, in the same region (Indo-Pacific). This could be due to differences in the number of sequences used covering a broader range for Ca. brevipinna (132 sequences), while Ca. amboinensis was represented by 72 sequences. See Fig. 4A for an overview of the network topologies and life history characteristics of five shark species.
Figure 4. Haplotype network structures of six shark species representing different habitats.
All images have been taken from Wikimedia Commons. Picture credits: (A) Carcharhinus sorrah, Tassapon Krajangdara, CC BY 3.0; (B) Chiloscyllium punctatum, Coughdrop12, CC BY-SA 4.0; (C) Carcharhinus altimus, NOAA; (D) Prionace glauca, Diego Delso, CC BY-SA 4.0; (E) Isurus oxyrinchus, Patrick Doll, CC BY-SA 3.0; (F) Galeocerdo cuvier, Albert Kok, CC BY-SA 3.0. The icons representing habitat type are available at https://icons8.com.
RAYS: patterns of genetic structure and diversity
Network topology
Complex mutational topologies were observed in 10 out of 19 ray species (Fig. 1B, and Fig. S2 and Table S4), including one pelagic, two benthopelagic, two reef-associated & demersal, four demersal, one reef-associated species. Long branches between two clusters of haplotypes were observed in three demersal species while complex star topology was observed in one pelagic species. Star shaped haplotype networks were observed in only four species of rays (Fig. S2, Table S4) inhabiting pelagic (three) and benthopelagic (one) habitats.
Haplotype and nucleotide diversity
The mean haplotype diversity value was 0.74 ± 0.217 (Fig. 2A). B. walga, a coastal-benthic species, had the highest haplotype diversity (h = 0.963 ± 0.028) among rays while the pelagic Mob. tarapacana had the lowest (h = 0.17 ± 0.021; Table S4). All the species belonging to family Dasyatidae had high h values (0.67 to 0.91) while the diversity values for family Mobulidae ranged from 0.17 to 0.85. Haplotype diversity was high (h ≥ 0.50) for eighteen species representing all habitats, while only one pelagic species—Mob. tarpacana had a low value (h = 0.17 ± 0.102). The mean nucleotide diversity value for rays was 0.0184 ± 0.0134 (Fig. 2B). Nucleotide diversity was highest in the coastal-demersal G. poecilura (π = 0.042 ± 0.0087) while Mob. birostris and Mob. tarapacana had the lowest value (π = 0.002; Table S4). In rays, all the nucleotide diversity values were lower than 0.005 (Table S4). There were no differences in the diversity values between ten families of rays, h (Kruskal–Wallis χ2 = 12.19, p = 0.25) and π (χ2 = 15.86, p = 0.069). However, demersal habitats were significantly higher in h and π relative to pelagic habitats (for h, p = 0.03 and for π, p = 0.002). The mean of both the diversity values was significantly higher for rays than for sharks—h (Mann Whitney U test, p < 0.0000096) and π (Mann Whitney U test, p < 0.00016).
Genetic structure
Gymnura micrura (demersal) showed very high structuring (ΦST = 0.989, p < 0.001) between North and South Atlantic samples with no sharing of haplotypes (simple exclusive topology; Table 3). A. narinari showed weak but significant structuring between the North and South Atlantic Ocean. The Western and Central Indian Ocean samples of N. indica (reef-associated) differed significantly with no haplotype sharing (ΦST = 0.357, p < 0.001). Himantura leoparda and G. poecilura showed highly significant population structure (ΦST = 0.918, p < 0.001 and 0.989, p < 0.001 respectively). In addition to these cases, three globally distributed species and four Indo–Pacific species had significant overall ΦSTvalues. No significant structuring was observed in ten species, including T. lymma, B. imbricata, N. kuhlii, Pa. jenkinsii, Pt. violacea, Mob. kuhlii, Mob. tarapacana, Mob. thurstoni, Mob. birostris, and A. ocellatus (Fisher’s exact test, p = 0.13). Of these, five are pelagic, two are reef-associated & demersal, one is benthopelagic and two are demersal species (Fig. 3B). (See Table 4 for a summary).
Table 4. AMOVA analysis carried out using cytochrome C oxidase subunit I for rays.
The numerals in bold indicate significant structuring, p values are given in brackets and n is the sample size.
| Species name | Simple Φ ST | Global comparison (nested AMOVA) | Indo–Pacific vs Atlantic | ||||
|---|---|---|---|---|---|---|---|
| ΦST | ΦSC | ΦCT | ΦST | ΦSC | ΦCT | ||
| A. narinari (n = 29) | 0.021* (0.007) | Atlantic Ocean distribution | |||||
| A. ocellatus (n = 18) | -0.028 (0.522) | −0.12 (0.704) | 0.117 (0.218) | 0.27 (0.674) | Indo–Pacific distribution | ||
| B. imbricata (n = 23) | 0.011 (0.317) | All sequences from western or central Indian Ocean | |||||
| B. walga (n = 20) | 0.567* (0.002) | 0.688** | 0.714** | 0.092 (0.327) | Indo–Pacific distribution | ||
| G. micrura (n = 15) | 0.989** | All sequences from north or south Atlantic | |||||
| G. poecilura (n = 39) | 0.339** (0.001) | 0.438** (0.001) | 0.618* (0.026) | 0.618 (0.247) | Indo–Pacific distribution | ||
| H. leoparda (n = 23) | 0.918** | 0.974** | 0.626* (0.025) | 0.931** | Indo–Pacific distribution | ||
| H. uarnak (n = 49) | 0.636** | 0.915** (0.001) | 0.913** | 0.019 (0.359) | Indo–Mediterranean distribution | ||
| Mac. gerrardi (n = 37) | 0.318** (0.001) | 0.595* (0.015) | 0.384* (0.004) | 0.344** | Indo–Pacific distribution | ||
| Mob. birostris (n = 16) | 0.05 (0.364) | −1.09 (0.543) | 0.165 (0.116) | −1.502 (0.751) | Indo–Pacific distribution | ||
| Mob. kuhlii (n = 18) | −0.278 (0.998) | −0.462 (0.96) | −0.457 (0.999) | −0.0032 (0.826) | Indo–Pacific distribution | ||
| Mob. mobular (n = 15) | 0.1587* (0.044) | 0.228* (0.052) | 0.165* (0.013) | 0.774 (0.145) | 0.277 (0.135) | 0.208** | 0.087 (0.213) |
| Mob. tarapacana (n = 23) | −0.062 (0.659) | −0.134 (0.905) | −0.0086 (0.534) | −0.124 (0.682) | −1.057 (0.642) | −0.0267 (0.432) | −1.0043 (0.772) |
| Mob. thurstoni (n = 32) | 0.099 (0.116) | 0.201 (0.085) | −0.1014 (0.409) | 0.274 (0.28) | 0.341 (0.059) | 0.053 (0.328) | 0.304 (0.301) |
| N. indica (n = 15) | 0.357** | All sequences from western or central Indian Ocean | |||||
| N. kuhlii (n = 59) | 0 | Southwest Pacific Ocean distribution | |||||
| P. jenkinsii (n = 22) | 0.074 (0.272) | All sequences from central or eastern Indian Ocean | |||||
| P. violacea (n = 25) | −0.812 (0.782) | −0.233 (0.628) | −0.291 (0.887) | 0.045 (0.683) | −0.204 (0.666) | −0.332 (0.859) | 0.096 (0.31)S |
| T. lymma (n = 20) | −0.046 (0.671) | Indo–Pacific distribution | |||||
Notes.
p < 0.05.
p < 0.001.
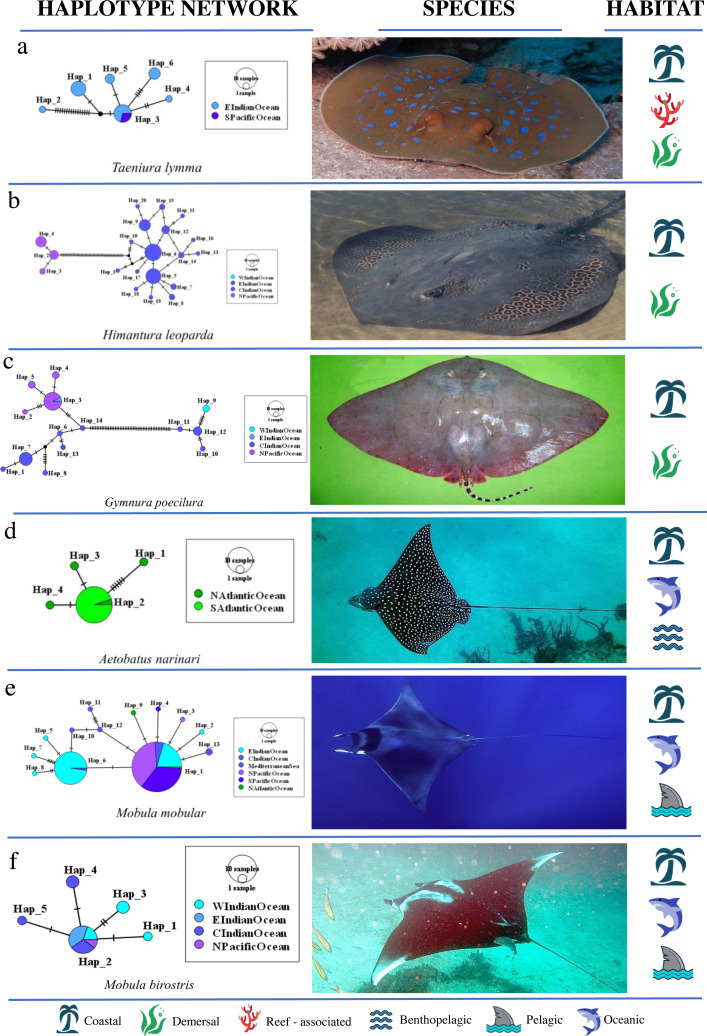
A nested AMOVA revealed that none of the four globally distributed species showed structuring across the three major ocean basins. Two of nine Indo–Pacific species (H. leoparda and Mac. gerrardi) showed structuring (Table 4). Structuring at the level of ocean basins was only detected in benthopelagic and demersal rays (Fig. 3B). There was no significant difference in the proportion of structured population samples among oceans (Fisher’s exact test, p = 0.25). Variation among populations within an ocean basin (ΦSC) was significant for nine species of rays–six demersal, one benthopelagic and two pelagic (Fig. 3B). The highest variation within oceans was observed in G. micrura (ΦSC = 0.989, p = 0.001, demersal) followed by an intertidal species–H. uarnak (ΦSC = 0.913, p < 0.001; Lessepsian migrant), found in Indian Ocean and Mediterranean Sea (Table 4). There was no significant difference in the proportion of structured and non-structured population samples within ocean basins (Fisher’s exact test, p = 0.13). None of the four globally distributed ray species showed Indo–Pacific vs Atlantic structuring. Seven species of rays having distribution ranges in either all three or two ocean basins lacked structuring at all levels–five pelagic (four belong to genus Mobula), one reef-associated (T. lymma) and one demersal (M. birostris) species. See Fig. 5 for an overview of the network topologies and life history characteristics of five ray species.
Figure 5. Haplotype network structures of six ray species representing different habitats.
All images have been taken from Wikimedia Commons. Picture credits: (A) Taeniura lymma, Jon Hanson CC BY-SA 2.0; (B) Himantura leoparda, Julie Lawson ; (C) Gymnura poecilura, Hamid Badar CC BY 3.0; (D) Aetobatus narinari, Nicholas Lindell Reynolds CC BY-SA 4.0; (E) Mobula mobular, Julien Renoult ; (F) Mobula birostris, Jon Hanson CC BY-SA 2.0. The icons representing habitat type are available at https://icons8.com/ (Icons8 LLC., 2023).
Discussion
Genetic structure in sharks and rays
There are at least three tiers of population structure in marine fishes. The low-dispersal damselfishes and anemonefishes (family Pomacentridae) can have population structure at the scale of individual bays and archipelagos (e.g., Dohna et al., 2015; Tenggardjaja, Bowen & Bernardi, 2016). This is often attributed, at least in part, to a greatly attenuated pelagic larval duration (PLD), but larval duration can only provide part of the answer. In a survey of 35 reef-associated species across the Hawaiian Archipelago, Selkoe et al. (2014) could attribute only 50% of the variance in population structure to PLD. The second tier is coastal fishes with broad ranges in the Indo–Pacific and Atlantic. These species typically have population structures at the scale of biogeographic provinces such as the Caribbean vs Brazil (Rocha et al., 2002), Red Sea vs Western Indian Ocean (Coleman et al., 2016), or Hawaiian Archipelago vs West Pacific (Leray et al., 2010). The third tier is pelagic wanderers or those that have very long PLD, including tunas (family Scombridae, Pecoraro et al., 2018; Puncher et al., 2018; Rodríguez-Ezpeleta et al., 2019) and billfishes (family Istiophoridae, Graves & McDowell, 2015). These species show population structure at the scale of ocean basins. Some moray eels (family Muraenidae), with PLDs exceeding 100 days, show no population structure across the entire Indo–Pacific basin (Reece, Bowen & Larson, 2011). Deepwater species, those below 200 m depth, seem to fall into this category as well (Andrews et al., 2020; McDowell & Brightman, 2018).
Based on our analysis of existing data sets, sharks and rays (lacking a PLD) fall primarily in the second tier (biogeographic provinces) and third tier (ocean basins) because a majority of the genetic structuring was observed within or across ocean basins. Examples of the second tier include pelagic sand tiger shark (Carcharias taurus; Ahonen, Harcourt & Stow, 2009), and blacktip reef shark (Ca. melanopterus; Vignaud et al., 2014) where structuring was observed within the Atlantic and Pacific oceans respectively. The genetic structure within the Atlantic basin (Northwest Atlantic and Brazil samples) for sand tiger sharks has been attributed to the warm equatorial currents acting as a barrier along with high breeding site fidelity (Dicken et al., 2007; Ahonen, Harcourt & Stow, 2009; Bansemer & Bennett, 2009), while for blacktip reef sharks, philopatry and deep oceanic waters influence genetic structuring (Vignaud et al., 2014). Shortfin mako shark (I. oxyrhynchus; Heist, Musick & Graves, 1996) and zebra shark (St. fasciatum; Dudgeon, Broderick & Ovenden, 2009) exhibited structuring at the level of biogeographic provinces. The porbeagle shark (L. nasus) has an anti-tropical distribution (absent in tropical waters) because it prefers regions where the mean water temperature is 7–18 °C (González et al., 2021). In the present study, South Atlantic and South Pacific samples comprise a southern lineage, and samples from the Mediterranean Sea and North Atlantic are united in a northern lineage. Isurus oxyrinchus, a highly migratory species also shows anti-tropical structuring. It is known to exhibit extended periods of residency and has an affinity to coastal waters and therefore does not frequently undertake trans-equatorial migrations (Corrigan et al., 2018). Apart from the ones mentioned above, bignose shark (Ca. altimus), sandbar shark (Ca. plumbeus), lemon shark (N. acutidens), milk shark (Rhiz. acutus), scalloped hammerhead shark (S. lewini) and whitetip reef shark (T. obesus) showed structuring within basins. Among rays, the spotted eagle ray (A. narinari), scaly whipray (B. walga), reticulate whipray (H. uarnak), blue spotted stingray (N. indica), giant devil ray (Mob. mobular), longtail butterfly ray (G. poecilura) and smooth butterfly ray (G. micrura) showed within basin structuring.
In the third tier, several oceanic and pelagic sharks exhibit population structure between the Atlantic and Indo–Pacific basins. These include the whale shark (Rhin. typus) (ΦST = 0.107; Castro et al., 2007), longfin mako (I. paucus; present study) and oceanic whitetip shark (Ca. longimanus; present study). This is a common pattern in pelagic teleost fishes where there is genetic structuring between the Atlantic and Indo–Pacific with little to no genetic structuring within ocean basins (Graves & McDowell, 2015; Bowen et al., 2016). At least two pelagic sharks show low-to-no genetic structure worldwide: basking shark (Ce. maximus; Hoelzel et al., 2006), and blue shark (P. glauca; Veríssimo et al., 2017). Other pelagic sharks with more limited distribution–bigeye thresher shark (A. superciliosus), great hammerhead shark (Sp. mokarran), smooth hammerhead shark (Sp. zygaena) and spinner shark (Ca. brevipinna)–showed no structuring (panmictic population) across their entire range. Silky shark (Ca. falciformis; pelagic) and spot-tail shark (Ca. sorrah) showed structured lineages only between three and two (Indo–Pacific) basins respectively.
In rays, such clear structuring was not observed wherein the species showed genetic separations only between ocean basins. This difference can be attributed to the habitat preference of target species, with the majority of the ray species being demersal and exhibiting genetic structuring within ocean basins. While pelagic rays lacked genetic structuring at all levels, two demersal species (H. leoparda and Mac. gerrardi) had structuring both within and between ocean basins. Pelagic stingray (P. violacea), and five pelagic species—Mob. kuhlii, Mob. tarapcana, Mob. thurstoni, Mob. birostris and A. ocellatus showed no structuring, possibly indicating greater gene flow between and within ocean basins.
The mean haplotype and nucleotide diversity (h and π) of rays were significantly higher than that of sharks. While sharks did not show any significant differences in diversity among species occupying different habitats, rays showed significantly higher diversity in species occupying demersal (rather than pelagic) habitats. This is in keeping with the observation that pelagic organisms are more dispersive and have geographically larger populations. However, neither sharks nor rays showed significant differences in diversity values among families.
Drivers of geographical genetic structure
Habitat and depth preference clearly shapes the geographical genetic structure of sharks and rays (Hirschfeld et al., 2021; Canfield, Galván-Magaña & Bowen, 2022). Among sharks, genetic structure between ocean basins was observed predominantly in the shallow reef-associated species, but four pelagic (one of which is semi pelagic) sharks also showed this type of structuring. In both sharks and rays, within basin structuring was observed primarily in benthopelagic and reef-associated and/or demersal species, although exceptions did exist where pelagic species also showed structuring. In rays, structure of lineages was observed primarily in demersal and benthopelagic species (categorised as demersal in Fig. 3B). A few reef-associated and pelagic sharks and rays also showed within ocean basin structuring. There was a significant difference in the proportion of structured species between demersal and reef-associated sharks across ocean basins but not within basins.
While one semi-pelagic and three pelagic sharks showed genetic structure at the scale of Indo–Pacific vs Atlantic, none of the ray species exhibited this structuring. However, this comparison has limited utility as most rays had distributions limited to the Indo–Pacific (8), with one species (H. uarnak) found in Indian Ocean and Mediterranean Sea (Table 2). H. uarnak, with a natural distributional range in the Indo–Pacific, is the largest Lessepsian elasmobranch species reported from the Mediterranean Sea (Golani, 1998; Ali et al., 2010; Amor et al., 2016). This species showed within basin structuring. A significant difference in the proportion of structured species at the ocean basin level (ΦCT), was observed between demersal and reef-associated shark species but not in rays. The test of proportions for structuring between demersal and pelagic rays for overall ΦST and ΦSC was not significant when all three habitats were considered. However, there appeared to be a stronger trend when reef-associated species were excluded from the analysis - ΦST and ΦSC (Fisher’s exact test, p = 0.13). Therefore, the inability to observe significant differences between species of these two habitats in the present study may be due to small sample sizes.
The dispersal of shark and ray species is entirely mediated by the active movement of juveniles and adults, unlike teleosts whose dispersal depends on planktonic larval stage as well as oceanic circulation (Taguchi et al., 2015). As expected, large-bodied oceanic sharks tend to have a lower population structure (Hirschfeld et al., 2021). Adult mediated population connectivity (AMPC) may result in different population structuring because the ability to overcome physical-biological barriers will be different across ontogenic stages (Frisk, Jordaan & Miller, 2014). Greater genetic connectivity may be observed in AMPC when compared to the classical larval-mediated geneflow as genetic exchange occurs over large distances—100s to 1,000s km (Frisk et al., 2010; Frisk, Jordaan & Miller, 2014). In winter skates (Leucoraja ocellata), adult migration strongly influenced connectivity and was responsible for increased abundance of the species along George’s Bank (Frisk et al., 2010). Apart from causing an increase in the abundance of species during a particular season, adult migrations also result in open populations where emigration and immigration play important roles in maintaining connectivity among locations (Frisk et al., 2010).
Just like in any landscape, physical barriers in the marine environment affect the movement of individuals. The three major ocean basins are separated by the Isthmus of Panama, Old World Barrier and the Sunda Shelf Barrier (also referred to as Indo–Pacific barrier). Ocean basins also have mid-oceanic barriers like the East Pacific Barrier, Indian Ocean Barrier and Mid-Atlantic Barrier. Thermal barriers (equatorial warm-water barrier and Aghulas-Benguela), ocean currents, hyaline barriers, straits and depth also hinder the mobility of marine organisms (Toonen et al., 2016; Hirschfeld et al., 2021; Canfield, Galván-Magaña & Bowen, 2022). These physical and environmental barriers pose different constraints on species with varying life histories and would therefore influence the genetic structuring of sharks and rays differently. Apart from the limitations imposed by geophysical barriers, wide-ranging pelagic species may exhibit population structuring due to philopatry.
Philopatric behaviour has been documented in a variety of marine taxa including at least 31 sharks (Chapman et al., 2015). Every species has unique migrational tendencies and reproductive strategies which guide their movement; therefore, it is not possible to find a general pattern of stock structure or gene flow that would apply to all species, even those occupying similar habitats (Heist, 2008). For example, tiger sharks (G. cuvier) have a regional population structure even though they undertake trans-oceanic migrations (Ferreira et al., 2015). This population genetic structure, detected in the maternally-inherited mtDNA, is attributed to female site fidelity (philopatry) to reproductive areas (Bernard et al., 2016), resulting in more structured populations (Chapman et al., 2004). Tiger sharks are monandrous and polyandry has not been detected in this species (Pirog et al., 2020). On the other hand, population structuring in pelagic thresher sharks and silky sharks may be shaped by oceanic currents and geography (Cardeñosa, Hyde & Caballero, 2014; Clarke et al., 2015; Domingues et al., 2018; Kraft et al., 2020). Juvenile sharks have been observed to remain in their natal sites for a long time before moving to habitats used by older juveniles and then to those used by adults (Springer, 1967). Hueter et al. (2005) reported that the traits such as residency, site fidelity and philopatry, either in combination or alone, influence population structuring at finer geographic scales among coastal shark species. Therefore, behavioural patterns (like philopatry) that inhibit reproductive mixing can also result in isolated adjacent populations in the absence of geophysical barriers (Chapman et al., 2015) in addition to environmental features restricting movement (Dudgeon et al., 2012).
Topology of haplotype networks
Life history traits which influence the geographic structuring of evolutionary lineages could also affect the topology of a network. A star topology typically has a single widely-distributed haplotype that is positioned at the centre of the network (Jenkins, Castilho & Stevens, 2018). This central haplotype is thought to be the ancestral haplotype with the additional haplotypes linked to it differing by a single or few mutational steps (Jenkins, Castilho & Stevens, 2018). This was the predominant topology in which sharks occupy pelagic/semi pelagic habitat (eight) of which five were found in both coastal and oceanic waters. Seven reef-associated and/or demersal species also exhibited star topologies. However, four pelagic, one demersal and one reef-associated shark species with star topology did not exhibit genetic structuring. In rays, this topology was observed in three pelagic and one benthopelagic species found in both coastal and oceanic waters and all three pelagic species lacked genetic structuring. The benthopelagic species, A. narinari, exhibited weak but significant structuring possibly as a result of site affinity (Flowers et al., 2016). Star-like networks can indicate high connectivity, recent coalescence to a common ancestor, or population expansion. In the present study, star networks predominate among highly mobile species that lacked structuring.
In strong contrast to sharks (predominantly star topology), the majority of rays showed complex mutational topology, where several mutations separate the central and peripheral haplotypes. Eight ray species exhibiting this topology were either demersal and/or were found around coral reefs in coastal waters, including four with genetic structure. This topology was also observed in one oceanic-pelagic and one benthopelagic species, both found in coastal and oceanic habitats and both lacked structuring. The benthopelagic species, A. ocellatus, possibly lacked structuring because studies so far have not reported site affinity in this species. In sharks, complex mutational topologies were found in four reef-associated, two semi pelagic, one pelagic and one benthopelagic species all of which had structured populations. Therefore, shark species with complex mutational topology were structured and all of them except Rhiz. acutus exhibited philopatry such as seasonal residency, site fidelity, or natal philopatry.
Another difference between sharks and rays was the number of species that showed simple exclusive network topology. Three coastal-demersal rays (B. walga, H. leoparda, G. micrura) and one shark (Ca. dussumieri) showed this topology and all of them showed genetic structuring. Complex star topology was also observed in some pelagic sharks (four) and ray species (one) and one reef-associated shark. In this topology, there are multiple connections and high-frequency haplotypes (Jenkins, Castilho & Stevens, 2018). Networks with a single haplotype were observed in three sharks but not in rays. The tendency towards star mtDNA networks in sharks, and complex networks in demersal rays, may indicate a fundamental difference in phylogeographic patterns. Complex networks are common in terrestrial and freshwater organisms that inhabit highly structured habitats such as rivers and streams (e.g., Schönhuth et al., 2018). Complex networks are seldom observed in marine fishes but are a recurring pattern in marine invertebrates that lack a pelagic larval stage (Bowen et al., 2020).
Shallow coalescence
Marine teleosts tend to show very shallow coalescence in haplotype networks, indicating a shared common ancestor on a timescale much shorter than the age of the species (Grant & Bowen, 1998). Furthermore, pelagic teleosts tend to have shallower coalescence than coastal fishes (Graves, 1998). The causes for this phenomenon have been debated in the literature for over 20 years (e.g., Copus et al., 2022). Here we extend these conclusions to sharks and rays, which have nearly uniformly shallow coalescence in haplotype networks (Figs. S1 & S2).
What could cause shallow mtDNA coalescences in marine teleosts, sharks and rays, relative to freshwater and terrestrial organisms? Certainly, part of the answer for sharks and rays is the low mutation rate relative to other vertebrates, initially proposed by Martin, Naylor & Palumbi (1992) and confirmed with comparisons across the Isthmus of Panama (Duncan et al., 2006; Keeney & Heist, 2006; Schultz et al., 2008). A second explanation is the vast medium of the ocean with few barriers and high biological connectivity. This is a condition shared by marine teleosts and elasmobranchs and separates their environmental regime from those of freshwater and terrestrial biota.
A third explanation for shallow coalescence, postulated for marine teleosts, is derived from r/K selection theory (MacArthur & Wilson, 1967). Marine teleost fishes are almost universally identified with an extreme version of the r-selected strategy, with high fecundity and no parental care. Thousands or millions of eggs are produced, but few survive to reproduce. This would result in a small effective population size (Ne; Wright, 1931) relative to the census size of reproducing adults. Sweepstakes reproduction, wherein a small number of females produce most of the next generation by fortuitously placing progeny in optimal conditions for survival, would further reduce Ne (Hedgecock & Pudovkin, 2011). The r-selected strategy, combined with sweepstakes reproduction, could explain the shallow mtDNA coalescence in marine teleosts, but not in sharks and rays. Here, sharks and rays provide a unique insight into the genetic architecture of marine organisms. They are decidedly closer to the K-selected strategy, producing fewer progenies after a long gestation. Progeny are much further along in development, mostly arriving as miniature adults that can swim at birth. We conclude that since the r-selected teleosts and the K-selected sharks and rays both have shallow coalescence, the reproductive strategy may not drive this shared trait. The alternate explanation of high connectivity should be given greater weight and could be tested with genomic kinship analyses.
Conservation implications
A comparison of structuring at the family level shows that two of three species within Alopiidae exhibit genetic structuring within ocean basins, followed by Hemiscyllium (1 of 3) and Carcharhinidae (5 of 22). Overall ΦSTwas significant for all species belonging to 3 families–Odontaspididae, Stegostomatidae and Lamnidae. This was followed by Carcharhinidae (11 of 22), Alopiidae and Hemiscyllium (1 of 3) indicating structuring at some level. Structuring between ocean basins was observed in two of three species within Alopiidae, one species of Hemiscyllium and six species of Carcharhinidae. In rays, only Myliobatidae (1 of 2) and Dasyatidae (2 of 10) showed genetic structuring within ocean basins. However, on comparing overall ΦST, significant values were observed in species from all families–Dasyatidae (5 of 10), Myliobatidae (1 of 2), Mobulidae (1 of 5) and Gymnuridae (2 of 2). Two species belonging to Dasyatidae and one belonging to Myliobatidae showed structuring across ocean basins. Hence one conclusion from our review is that management units based on political boundaries may be too small to be effective, which points to the need for transboundary collaboration (see Shiffman & Hammerschlag, 2016).
Resource managers need to understand the pattern and degree of population subdivision to prevent over-exploitation and loss of genetic diversity. The lesson from comparative phylogeography of sharks is that multiple population units with unique genetic signatures exist in most species, except in some of the large oceanic migrants. The corresponding lesson for pelagic rays is that whole ocean basins may be the scale of population units. Demersal rays may require management on a much smaller scale, based on the implications of complex haplotype networks. When population partitions exist, they are usually concordant with biogeographic boundaries such as those between ocean basins. Of course, while there will be exceptions to these trends, these can provide broad directions for management as well as point to species that urgently need genetic studies.
In at least four cases, we detected genetic separations that approach or meet the criterion for evolutionary significant units (ESUs; Moritz, 1994). The smooth butterfly ray (Gymnura micrura) shared no haplotypes between North and South Atlantic samples (ΦST = 0.989, p < 0.001). The reef-associated Indian-Ocean blue spotted maskray (Neotrygon indica, described in 2018 by Pavan-Kumar et al., 2018) shared no haplotypes between the Western and Central Indian Ocean (ΦST = 0.357, p < 0.001). Likewise, the leopard whipray (Himantura leoparda) and longtailed butterfly ray (Gymnura poecilura) showed highly significant population structure (ΦST = 0.918, p < 0.001 and 0.989, p = 0.001 respectively). It is not surprising that our survey revealed evidence for undescribed species; however, species misidentification cannot be ruled out, and where possible sequences were verified by carrying out a BLAST search on NCBI to compare and confirm the species identification. In a survey of 284 globally distributed fish species (both teleost and elasmobranch), at least 35 showed genetic evidence of cryptic evolutionary diversity (Gaither et al., 2016). In these cases, additional studies are strongly mandated to investigate the likelihood of cryptic evolutionary lineages at or below the species level. Taxonomic assignments would result in higher conservation priorities.
The conservation outlook for elasmobranchs is dire. Over the last decade, fishing has moved into deeper regions of the world’s oceans (Morato et al., 2006) and several elasmobranchs found in deep waters have been exploited (Kyne & Simpfendorfer, 2010). Many elasmobranchs that are under immense pressure from fishing activities show low levels of genetic diversity while continued overfishing can result in changes in population subdivision and loss of genetic variation (Allendorf, Luikart & Aitken, 2013; Domingues, Hilsdorf & Gadig, 2018). Globally, 1,199 species of sharks and rays have been assessed for the IUCN Red List, including a minimum of 391 (32.6%) species assigned to three threatened categories–Critically Endangered, Endangered and Vulnerable (IUCN Red List Assessment, https://www.iucnredlist.org/, Dulvy et al., 2021). A total of 299 species (24.9%) are classified as Data Deficient and 44.1% are categorised as Least Concern indicating that a majority of them need proper assessment and conservation effort (IUCN SSC Shark Specialist Group, 2019). In addition, most of these species are classified based on abundance and geographic range size, which may not necessarily be important determinants of extinction risk (Payne et al., 2011; Harnik, Simpson & Payne, 2012). In the cases considered here, large range sizes and geographic scope of populations provide some buffer from depletion and extirpation. Abundance, on the other hand, is a more serious concern for sharks and rays, especially if demographic trends lead to the erosion of genetic diversity, the necessary building blocks to adapt to a changing world.
Genetic diversity has largely been overlooked in conservation policy and fisheries management plans (Domingues, Hilsdorf & Gadig, 2018). Only about 10% of the 2014 IUCN listed shark and ray species have been studied for genetic diversity and structuring (Domingues, Hilsdorf & Gadig, 2018). Commonly caught by-catch species like pelagic sting ray (P. violacea) and the Critically Endangered daggernose shark (Isogomphodon oxyrhynchus) with narrow distribution have not yet been evaluated for discrete populations (Domingues, Hilsdorf & Gadig, 2018). It is therefore important to understand the nature of population subdivision and the type of structuring especially in those that are commercially exploited with narrow distributional ranges. This knowledge can aid in establishing policies and improving conservation plans that prevent overexploitation and aim to preserve natural genetic diversity.
Conclusions
Our metadata analysis provides insights into how populations of sharks and rays are structured globally. It was evident that populations of sharks and rays primarily show genetic structuring across biogeographic provinces and ocean basins and, like marine teleosts, exhibit shallow coalescence in haplotype networks. No clear pattern of population subdivision could be observed for species occupying similar habitats because the reasons for structuring are complex and multifaceted. Apart from biogeographic barriers, philopatry also plays an important role in population connectivity and structure. This study was able to identify certain trends in structuring with populations of reef-associated shark species showing a higher proportion of genetic structuring across ocean basins when compared to demersal species. For rays, although non-significant, the results suggested that within basin genetic structuring could be higher for demersal species when compared to pelagic species. Network topologies of sharks were predominantly star-shaped while for rays (mostly demersal) they were complex mutational, indicating that the latter has more structured populations. Therefore, special recognition needs to be given to demersal rays which require management at a smaller scale. Since most of the shark and ray species in this study are migratory and showed genetic subdivisions among population samples, it is important that these ‘population units’ are assessed and managed individually. Conservation efforts need to move beyond political boundaries and require transboundary collaborations spanning neighbouring countries for the effective management of elasmobranchs.
Limitations of the study
The present study has used COI sequences given their availability for a large number of sharks and rays. Other mtDNA markers like the control region could reveal a different or more nuanced view of the observed population structure patterns. The present study also did not include skates (order Rajiformes) which are an important group of egg-laying elasmobranchs. Skates could potentially show tier 1 or 2 genetic structuring at regional and local levels (Misawa et al., 2019; however, further study is needed to compare sharks, rays and skates, the three most speciose groups of elasmobranchs.
Supplemental Information
Acknowledgments
We thank Muralidharan Manoharakrishnan for comments and insights that improved the manuscript. SK conducted a part of this work during her Master’s dissertation at the TERI School of Advanced Studies. BWB thanks RJ Toonen and members of the ToBo Lab at Hawai’i Institute of Marine Biology for useful discussion and comment. We thank Fausto Tinti and two anonymous reviewers for insightful comments that improved the manuscript. We thank Shruthi Kottillil for proofreading the final manuscript and giving her inputs.
Funding Statement
The authors received no funding for this work. The open access funding for this paper has been provided by the Chan Zuckerberg Initiative. Brian W. Bowen is supported by the National Geographic Society Pristine Seas Initiative and the University of Hawai’i Sea Grant College Program. Kartik Shanker is supported by the DBT-IISc Partnership Programme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Chetan Rao used to be an employee with Dakshin Foundation and this project was undertaken during his time with Dakshin Foundation. He is no longer an employee at Dakshin Foundation. Kartik Shanker is a founder trustee of Dakshin Foundation. The authors declare there are no competing interests.
Author Contributions
Sudha Kottillil conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Chetan Rao conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Brian W. Bowen analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Kartik Shanker conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
References
- Ahonen, Harcourt & Stow (2009).Ahonen H, Harcourt RG, Stow AJ. Nuclear and mitochondrial DNA reveals isolation of imperilled grey nurse shark populations (Carcharias taurus) Molecular Ecology. 2009;18(21):4409–4421. doi: 10.1111/j.1365-294X.2009.04377.x. [DOI] [PubMed] [Google Scholar]
- Albert & Carvalho (2011).Albert JS, Carvalho TP. Neogene assembly of modern faunas. In: Albert JS, Reis RE, editors. Historical biogeography of neotropical freshwater fishes. University of California Press; California: 2011. pp. 119–136. [Google Scholar]
- Ali et al. (2010).Ali M, Saad A, Ben Amor MM, Capapé C. First records of the honeycomb stingray, Himantura uarnak (Forskål, 1775), off the Syrian coast (eastern Mediterranean) (Chondrichthyes: Dasyatidae) Zoology in the Middle East. 2010;49(1):104–106. [Google Scholar]
- Allen (2008).Allen GR. Conservation hotspots of biodiversity and endemism for Indo–Pacific coral reef fishes. Aquatic Conservation Marine and Freshwater Ecosystems. 2008;18(5):541–556. doi: 10.1002/aqc.880. [DOI] [Google Scholar]
- Allendorf, Luikart & Aitken (2013).Allendorf FW, Luikart G, Aitken SN. Conservation and the genetics of populations. 2nd ed Wiley-Blackwell; West Sussex: 2013. [Google Scholar]
- Amor et al. (2016).Amor BMM, Diatta Y, Diop M, Ben Salem M, Capapé C. Confirmed occurrence in the Mediterranean Sea of milk shark Rhizoprionodon acutus (Chondrichthyes: Carcharhinidae) and first record off the Tunisian coast. Cahiers de Biologie Marine. 2016;57:145–149. doi: 10.21411/CBM.A.9BC69C09. [DOI] [Google Scholar]
- Andrews et al. (2020).Andrews KR, Copus JM, Wilcox C, Williams AJ, Newman SJ, Wakefield CB, Bowen BW. Range-wide population structure of three deepwater Eteline snappers across the Indo–Pacific basin. Journal of Heredity. 2020;111(5):471–485. doi: 10.1093/jhered/esaa029. [DOI] [PubMed] [Google Scholar]
- Avise (2000).Avise JC. Phylogeography: The history and formation of species. Cambridge Massachusetts: Harvard University Press; 2000. [Google Scholar]
- Bandelt, Forster & Röhl (1999).Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16(1):37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Bansemer & Bennett (2009).Bansemer CS, Bennett MB. Reproductive periodicity, localized movements and behavioural segregation of pregnant Carcharias taurus at Wolf Rock, southeast Queensland, Australia. Marine Ecology Progress Series. 2009;374:215–227. doi: 10.3354/meps07741. [DOI] [Google Scholar]
- Benson et al. (2013).Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Research. 2013;41(Database issue):D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard et al. (2016).Bernard AM, Feldheim KA, Heithaus MR, Wintner SP, Wetherbee BM, Shivji MS. Global population genetic dynamics of a highly migratory, apex predator shark. Molecular Ecology. 2016;25(21):5312–5329. doi: 10.1111/mec.13845. [DOI] [PubMed] [Google Scholar]
- Bowen et al. (2020).Bowen BW, Forsman ZH, Whitney JL, Faucci A, Hoban M, Canfield SJ, Johnston EC, Coleman RR, Copus JM, Vicente J, Toonen RJ. Species radiations in the sea: What the flock? Journal of Heredity. 2020;111(1):70–83. doi: 10.1093/jhered/esz075. [DOI] [PubMed] [Google Scholar]
- Bowen et al. (2016).Bowen BW, Gaither MR, Di Battista JD, Iacchei M, Andrews KR, Grant WS, Toonen RJ, Briggs JC. Comparative phylogeography of the ocean planet. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(29):7962–7969. doi: 10.1073/pnas.1602404113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam et al. (2015).Bräutigam A, Callow M, Campbell IR, Camhi MD, Cornish AS, Dulvy NK, Fordham SV, Fowler SL, Hood AR, McClennen C, Reuter EL, Sant G, Simpfendorfer CA, Welch DJ. Global priorities for conserving sharks and rays: a 2015–2025 strategy. International Union for Conservation of Nature; Gland: 2015. [Google Scholar]
- Canfield, Galván-Magaña & Bowen (2022).Canfield SJ, Galván-Magaña F, Bowen BW. Little sharks in a big world: Mitochondrial DNA reveals small-scale population structure in the demersal California Horn Shark (Heterodontus francisci) Journal of Heredity. 2022;113:298–310. doi: 10.1093/jhered/esac008. [DOI] [PubMed] [Google Scholar]
- Cardeñosa, Hyde & Caballero (2014).Cardeñosa D, Hyde J, Caballero S. Genetic diversity and population structure of the pelagic thresher shark (Alopias pelagicus) in the Pacific Ocean: evidence for two evolutionarily significant units. PLOS ONE. 2014;9(10):e110193. doi: 10.1371/journal.pone.0110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro et al. (2007).Castro ALF, Stewart BS, Wilson SG, Hueter RE, Meekan MG, Motta PJ, Bowen BW, Karl SA. Population genetic structure of Earth’s largest fish, the whale shark (Rhincodon typus) Molecular Ecology. 2007;16(24):5183–5192. doi: 10.1111/j.1365-294X.2007.03597.x. [DOI] [PubMed] [Google Scholar]
- Chapman et al. (2015).Chapman DD, Feldheim KA, Papastamatiou YP, Hueter RE. There and back again: a review of residency and return migrations in sharks, with implications for population structure and management. Annual Review of Marine Science. 2015;7:547–570. doi: 10.1146/annurev-marine-010814-015730. [DOI] [PubMed] [Google Scholar]
- Chapman et al. (2004).Chapman DD, Prodohl PA, Gelsleichter J, Manire CA, Shivji MS. Predominance of genetic monogamy by females in a hammerhead shark, Sphyrna tiburo: implications for shark conservation. Molecular Ecology. 2004;13(7):1965–1974. doi: 10.1111/j.1365-294X.2004.02178.x. [DOI] [PubMed] [Google Scholar]
- Clarke et al. (2015).Clarke CR, Karl SA, Horn RL, Bernard AM, Lea JS, Hazin FH, Prodöhl PA, Shivji MS. Global mitochondrial DNA phylogeography and population structure of the silky shark, Carcharhinus falciformis. Marine Biology. 2015;162(5):945–955. doi: 10.1007/s00227-015-2636-6. [DOI] [Google Scholar]
- Coleman et al. (2016).Coleman RR, Eble JA, Di Battista JD, Rocha LA, Randall JE, Berumen ML, Bowen BW. Regal phylogeography: range-wide survey of the marine angelfish Pygoplites diacanthus reveals evolutionary partitions between the Red Sea, Indian Ocean, and Pacific Ocean. Molecular Phylogenetics and Evolution. 2016;100:243–253. doi: 10.1016/j.ympev.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Copus et al. (2022).Copus JM, Walsh CAJ, Hoban ML, Lee AM, Pyle RL, Kosaki RK, Toonen RJ, Bowen BW. Phylogeography of mesophotic coral ecosystems: squirrelfish and soldierfish (Holocentriformes: Holocentridae) Diversity. 2022;14:691. doi: 10.3390/d14080691. [DOI] [Google Scholar]
- Corrigan et al. (2018).Corrigan S, Lowther AD, Beheregaray LB, Bruce BD, Cliff G, Duffy CA, Foulis A, Francis MP, Goldsworthy SD, Hyde JR, Jabado RW, Kacev D, Marshall L, Mucientes GR, Naylor GJP, Pepperell JG, Queiroz N, White WT, Wintner SP, Rogers PJ. Population connectivity of the highly migratory shortfin mako (Isurus oxyrinchus Rafinesque 1810) and implications for management in the Southern Hemisphere. Frontiers in Ecology and Evolution. 2018;6:187. doi: 10.3389/fevo.2018.00187. [DOI] [Google Scholar]
- Dicken et al. (2007).Dicken ML, Booth AJ, Smale MJ, Cliff G. Spatial and seasonal distribution patterns of juvenile and adult raggedtooth sharks (Carcharias taurus) tagged off the east coast of South Africa. Marine and Freshwater Research. 2007;58(1):127–134. doi: 10.1071/MF06018. [DOI] [Google Scholar]
- Dohna et al. (2015).Dohna TA, Timm J, Hamid L, Kochzius M. Limited connectivity and a phylogeographic break characterize populations of the pink anemonefish. Amphiprion perideraion, in the Indo-Malay Archipelago: Inferences from a mitochondrial and microsatellite loci. Ecology and Evolution. 2015;5(8):717–1733. doi: 10.1002/ece3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues, Hilsdorf & Gadig (2018).Domingues RR, Hilsdorf AWS, Gadig OBF. The importance of considering genetic diversity in shark and ray conservation policies. Conservation Genetics. 2018;19(3):501–525. doi: 10.1007/s105922-017-1038-3. [DOI] [Google Scholar]
- Domingues et al. (2018).Domingues RR, Hilsdorf AW, Shivji MM, Hazin FV, Gadig OB. Effects of the Pleistocene on the mitochondrial population genetic structure and demographic history of the silky shark (Carcharhinus falciformis) in the western Atlantic Ocean. Reviews in Fish Biology and Fisheries. 2018;28(1):213–227. doi: 10.1007/s11160-017-9504-z. [DOI] [Google Scholar]
- Dudgeon et al. (2012).Dudgeon CL, Blower DC, Broderick D, Giles JL, Holmes BJ, Kashiwagi T, Krück NC, Morgan JA, Tillett BJ, Ovenden JR. A review of the application of molecular genetics for fisheries management and conservation of sharks and rays. Journal of Fish Biology. 2012;80(5):1789–1843. doi: 10.1111/j.1095-8649.2012.03265.x. [DOI] [PubMed] [Google Scholar]
- Dudgeon, Broderick & Ovenden (2009).Dudgeon CL, Broderick D, Ovenden JR. IUCN classification zones concord with, but underestimate, the population genetic structure of the zebra shark Stegostoma fasciatum in the Indo-West Pacific. Molecular Ecology. 2009;18(2):248–261. doi: 10.1111/j.1365-294X.2008.04025.x. [DOI] [PubMed] [Google Scholar]
- Dulvy et al. (2008).Dulvy NK, Baum JK, Clarke S, Compagno LJ, Cortés E, Domingo A, Fordham S, Fowler S, Francis MP, Gibson C, Martínez J. You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquatic Conservation: Marine and Freshwater Ecosystems. 2008;18(5):459–482. doi: 10.1002/aqc.975. [DOI] [Google Scholar]
- Dulvy et al. (2014).Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, Carlson JK, Davidson LNK, Fordham SV, Francis MP, Pollock CM, Simpfendorfer CA, Burgess GH, Carpenter KE, Compagno LJ, Ebert DA, Gibson C, Heupel MR, Livingstone SR, Sanciangco JC, Stevens JD, Valenti S, White WT. Extinction risk and conservation of the world’s sharks and rays. eLife. 2014;3:e00590. doi: 10.7554/eLife.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulvy et al. (2021).Dulvy NK, Pacoureau N, Rigby CL, Pollom RA, Jabado RW, Ebert DA, Finucci B, Pollock CM, Cheok J, Derrick DH, Herman KB, Sherman SC, VanderWright WJ, Lawson JM, Walls RHL, Carlson JK, Charvet P, Bineesh KK, Fernando D, Ralph GM, Matsushiba JH, Hilton-Taylor C, Fordham SV, Simpfendorfer CA. Overfishing drives over one-third of all sharks and rays towards a global extinction crisis. Current Biology. 2021;31(21):4773–4785. doi: 10.1016/j.cub.2021.08.062. [DOI] [PubMed] [Google Scholar]
- Duncan et al. (2006).Duncan KM, Martin AP, Bowen BW, De Couet HG. Global phylogeography of the scalloped hammerhead shark (Sphyrna lewini) Molecular Ecology. 2006;15(8):2239–2251. doi: 10.1111/j.1365-294X.2006.02933.x. [DOI] [PubMed] [Google Scholar]
- Excoffier, Smouse & Quattro (1992).Excoffier L, Smouse PE, Quattro J. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131(2):6479–6491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira et al. (2015).Ferreira LC, Thums M, Meeuwig JJ, Vianna GM, Stevens J, McAuley R, Meekan MG. Crossing latitudes—long-distance tracking of an apex predator. PLOS ONE. 2015;10(2):e0116916. doi: 10.1371/journal.pone.0116916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti et al. (2010).Ferretti F, Worm B, Britten GL, Heithaus MR, Lotze HK. Patterns and ecosystem consequences of shark declines in the ocean. Ecology Letters. 2010;13(8):1055–1071. doi: 10.1111/j.1461-0248.2010.01489.x. [DOI] [PubMed] [Google Scholar]
- Flowers et al. (2016).Flowers KI, Ajemian MJ, Bassos-Hull K, Feldheim KA, Hueter RE, Papastamatiou YP, Chapman DD. A review of batoid philopatry, with implications for future research and population management. Marine Ecology Progress Series. 2016;562:251–261. doi: 10.3354/meps11963. [DOI] [Google Scholar]
- Frisk, Jordaan & Miller (2014).Frisk MG, Jordaan A, Miller TJ. Moving beyond the current paradigm in marine population connectivity: are adults the missing link? Fish and Fisheries. 2014;15(2):242–254. doi: 10.1111/faf.12014. [DOI] [Google Scholar]
- Frisk et al. (2010).Frisk MG, Martell SJD, Miller TJ, Sosebee K. Exploring the population dynamics of winter skate (Leucoraja ocellata) in the Georges Bank region using a statistical catch-at-age model incorporating length, migration, and recruitment process errors. Canadian Journal of Fisheries and Aquatic Sciences. 2010;67(5):774–792. doi: 10.1139/F10-008. [DOI] [Google Scholar]
- Gaither et al. (2016).Gaither MR, Bowen BW, Rocha LA, Briggs JC. Fishes that rule the world: Circumglobal distributions revisited. Fish and Fisheries. 2016;17(3):664–679. doi: 10.1111/faf.12136. [DOI] [Google Scholar]
- Golani (1998).Golani D. Impact of Red Sea fish migrants through the Suez Canal on the aquatic environment of the Eastern Mediterranean. Bulletin Series Yale School of Forestry and Environmental Studies. 1998;103:375–387. [Google Scholar]
- González et al. (2021).González MT, Sepúlveda FA, Zárate PM, Baeza JA. Regional population genetics and global phylogeography of the endangered highly migratory shark Lamna nasus: implications for fishery management and conservation. Aquatic Conservation: Marine and Freshwater Ecosystems. 2021;31(3):620–634. doi: 10.1002/aqc.3455. [DOI] [Google Scholar]
- Grant & Bowen (1998).Grant WS, Bowen BW. Shallow population histories in deep evolutionary lineages of marine fishes: insights from sardines and anchovies and lessons for conservation. Journal of Heredity. 1998;89(5):415–426. doi: 10.1093/jhered/89.5.415. [DOI] [Google Scholar]
- Graves (1998).Graves JE. Molecular insights into the population structures of cosmopolitan marine fishes. Journal of Heredity. 1998;89(5):427–437. doi: 10.1093/jhered/89.5.427. [DOI] [Google Scholar]
- Graves & McDowell (2015).Graves JE, McDowell JR. Population structure of istiophorid billfishes. Fisheries Research. 2015;166:21–28. doi: 10.1016/j.fishres.2014.08.016. [DOI] [Google Scholar]
- Gupta et al. (2020).Gupta T, Booth H, Arlidge W, Rao C, Manoharakrishnan M, Namboothri N, Shanker K, Milner-Gulland EJ. Mitigation of elasmobranch bycatch in trawlers: a case study in Indian fisheries. Frontiers in Marine Science. 2020;7:571. doi: 10.3389/fmars.2020.00571. [DOI] [Google Scholar]
- Hall, Alverson & Metuzals (2000).Hall MA, Alverson DL, Metuzals KI. By-catch: problems and solutions. Marine Pollution Bulletin. 2000;41(1–6):204–219. doi: 10.1016/S0025-326X(00)00111-9. [DOI] [Google Scholar]
- Harnik, Simpson & Payne (2012).Harnik PG, Simpson C, Payne JL. Long-term differences in extinction risk among the seven forms of rarity. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1749):4969–4976. doi: 10.1098/rspb.2012.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock & Pudovkin (2011).Hedgecock D, Pudovkin AI. Sweepstakes reproductive success in highly fecund marine fish and shellfish: a review and commentary. Bulletin of Marine Science. 2011;87(4):971–1002. doi: 10.5343/bms.2010.1051. [DOI] [Google Scholar]
- Heist (2005).Heist EJ. Genetics: stock identification. In: Musick JA, Bonfil R, editors. FAO fisheries technical paper. Vol. 474. FAO; Rome: 2005. pp. 62–75. [Google Scholar]
- Heist (2008).Heist EJ. Molecular markers and genetic population structure of pelagic sharks. In: Camhi MD, Pikitch E, Babcock EA, editors. Sharks of the open ocean: biology, fisheries & conservation. Blackwell Publishing Ltd; Oxford: 2008. pp. 323–333. [Google Scholar]
- Heist, Musick & Graves (1996).Heist EJ, Musick JA, Graves JE. Genetic population structure of the shortfin mako (Isurus oxyrinchus) inferred from restriction fragment length polymorphism analysis of mitochondrial DNA. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53(3):583–588. doi: 10.1139/cjfas-53-3-583. [DOI] [Google Scholar]
- Hellberg (2009).Hellberg ME. Gene flow and isolation among populations of marine animals. Annual Review of Ecology, Evolution, and Systematics. 2009;40:291–310. doi: 10.1146/annurev.ecolsys.110308.120223. [DOI] [Google Scholar]
- Heupel, Carlson & Simpfendorfer (2007).Heupel MR, Carlson JK, Simpfendorfer CA. Shark nursery areas: concepts, definition, characterization and assumptions. Marine Ecology Progress Series. 2007;337:287–297. [Google Scholar]
- Hirschfeld et al. (2021).Hirschfeld M, Dudgeon C, Sheaves M, Barnett A. Barriers in a sea of elasmobranchs: from fishing for populations to testing hypotheses in population genetics. Global Ecology and Biogeography. 2021;30:2147–2163. doi: 10.1111/geb.13379. [DOI] [Google Scholar]
- Hoelzel et al. (2006).Hoelzel AR, Shivji MS, Magnussen J, Francis MP. Low worldwide genetic diversity in the basking shark (Cetorhinus maximus) Biology Letters. 2006;2(4):639–642. doi: 10.1098/rsbl.2006.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueter et al. (2005).Hueter RE, Heupel MR, Heist EJ, Keeney DB. Evidence of philopatry in sharks and implications for the management of shark fisheries. Journal of Northwest Atlantic Fishery Science. 2005;35:239–247. doi: 10.2960/J.v35.m493. [DOI] [Google Scholar]
- Icons8 LLC. (2023).Icons8 LLC 2023 https://icons8.com/ (accessed March 2021)
- IUCN SSC Shark Specialist Group (2019).IUCN SSC Shark Specialist Group 2019 Red List Update. https://www.iucnssg.org/2019-iucn-rl-update.html. 2019 (accessed March 2021)
- Jenkins, Castilho & Stevens (2018).Jenkins TL, Castilho R, Stevens JR. Meta-analysis of northeast Atlantic marine taxa shows contrasting phylogeographic patterns following post-LGM expansions. PeerJ. 2018;28(6):5684. doi: 10.7717/peerj.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney & Heist (2006).Keeney DB, Heist EJ. Worldwide phylogeography of the blacktip shark (Carcharhinus limbatus) inferred from mitochondrial DNA reveals isolation of western Atlantic populations coupled with recent Pacific dispersal. Molecular Ecology. 2006;15(12):3669–3679. doi: 10.1111/j.1365-294X.2006.03036. [DOI] [PubMed] [Google Scholar]
- Kizhakudan et al. (2015).Kizhakudan SJ, Zacharia PU, Thomas S, Vivekanandan E, Muktha M. CMFRI marine fisheries policy series2: guidance on national plan of action for sharks in India. http://eprints.cmfri.org.in/10403/ CMFRI Marine Fisheries Policy Series. 2015;2:1–102. [Google Scholar]
- Kraft et al. (2020).Kraft DW, Conklin E, Barba E, Hutchinson M, Toonen RJ, Forsman ZH, Bowen BW. Genomics versus mtDNA for resolving stock structure in the silky shark (Carcharhinus falciformis) PeerJ. 2020;8:e10186. doi: 10.7717/peerj.10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. (2018).Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyne & Simpfendorfer (2010).Kyne PM, Simpfendorfer CA. Deepwater chondrichthyans. In: Carrier JC, Musick JA, Heithaus MR, editors. Sharks and their relatives II: biodiversity, adaptive physiology, and conservation. CRC Press; Boca Raton: 2010. pp. 37–114. [Google Scholar]
- Lassauce et al. (2022).Lassauce H, Dudgeon CL, Armstrong AJ, Wantiez L, Carroll EL. Evidence of fine-scale genetic structure for reef manta rays Mobula alfredi in New Caledonia. Endangered Species Research. 2022;47:249–264. doi: 10.3354/esr01178. [DOI] [Google Scholar]
- Last et al. (2016).Last PR, de Carvalho MR, Naylor GJP, Séret B, Stehmann MFW, White WT. Introduction. In: Last PR, White WT, de Carvalho MR, Séret B, Stehmann MFW, Naylor JP, editors. Rays of the world. CSIRO Publishing; Ithaca: 2016. pp. 1–9. [Google Scholar]
- Laurrabaquio et al. (2019).Laurrabaquio ANS, Islas-Villanueva V, Adams DH, Uribe-Alcocer M, Alvarado-Bremer JR, Díaz-Jaimes P. Genetic evidence for regional philopatry of the Bull Shark (Carcharhinus leucas), to nursery areas in estuaries of the Gulf of Mexico and western North Atlantic ocean. Fisheries Research. 2019;209:67–74. doi: 10.1016/j.fishres.2018.09.013. [DOI] [Google Scholar]
- Leigh & Bryant (2015).Leigh JW, Bryant D. PopART: full-feature software for haplotype network construction. Methods in Ecology and Evolution. 2015;6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- Leray et al. (2010).Leray M, Beldade R, Holbrook SJ, Schmitt RJ, Planes S, Bernardi G. Allopatric divergence and speciation in coral reef fish: the three-spot Dascyllus, Dascyllus trimaculatus, species complex. Evolution. 2010;64(5):1218–1230. doi: 10.1111/j.1558-5646.2009.00917.x. [DOI] [PubMed] [Google Scholar]
- MacArthur & Wilson (1967).MacArthur R, Wilson EO. The theory of Island biogeography. Princeton University Press; Princeton: 1967. [Google Scholar]
- Martin, Naylor & Palumbi (1992).Martin AP, Naylor GJ, Palumbi SR. Rates of mitochondrial DNA evolution in sharks are slow compared with mammals. Nature. 1992;357(6374):153–155. doi: 10.1038/357153a0. [DOI] [PubMed] [Google Scholar]
- McDowell & Brightman (2018).McDowell JR, Brightman HL. High level of genetic connectivity in a deep-water reef fish, textitCaulolatilus microps. Journal of Fish Biology. 2018;93(5):766–777. doi: 10.1111/jfb.13779. [DOI] [PubMed] [Google Scholar]
- Misawa et al. (2019).Misawa R, Narimatsu Y, Endo H, Kai Y. Population structure of the ocellate spot skate (Okamejei kenojei) inferred from variations in mitochondrial DNA (mtDNA) sequences and from morphological characters of regional populations. Fishery Bulletin. 2019;117(1–2):24–43. [Google Scholar]
- Morato et al. (2006).Morato T, Watson R, Pitcher TJ, Pauly D. Fishing down the deep. Fish and Fisheries. 2006;7(1):24–34. doi: 10.1111/j.1467-2979.2006.00205.x. [DOI] [Google Scholar]
- Moritz (1994).Moritz C. Defining ‘evolutionarily significant units’ for conservation. Trends in Ecology & Evolution. 1994;9(10):373–375. doi: 10.1016/0169-5347(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Mourier & Planes (2013).Mourier J, Planes S. Direct genetic evidence for reproductive philopatry and associated fine-scale migrations in female blacktip reef sharks (Carcharhinus melanopterus) in French Polynesia. Molecular Ecology. 2013;22(1):201–214. doi: 10.1111/mec.12103. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information (1988).National Center for Biotechnology Information (NCBI) Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 1988. [12 July 2021]. https://www.ncbi.nlm.nih.gov/ https://www.ncbi.nlm.nih.gov/
- Nei (1987).Nei M. Molecular evolutionary genetics. Columbia University Press; New York: 1987. [Google Scholar]
- Nei & Miller (1990).Nei M, Miller JC. A simple method for estimating average number of nucleotide substitutions within and between populations from restriction data. Genetics. 1990;125(4):873–879. doi: 10.1093/genetics/125.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacoureau et al. (2021).Pacoureau N, Rigby CL, Kyne PM, Sherley RB, Winker H, Carlson JK, Fordham SV, Barreto R, Fernando D, Francis MP, Jabado RW, Herman KB, Liu KM, Marshall AD, Pollom RA, Romanov EV, Simpfendorfer CA, Kindsvater HK, Dulvy NK. Half a century of global decline in oceanic sharks and rays. Nature. 2021;589:67–571. doi: 10.1038/s41586-020-03173-9. [DOI] [PubMed] [Google Scholar]
- Pavan-Kumar et al. (2018).Pavan-Kumar A, Kumar R, Pitale P, Shen KN, Borsa P. Neotrygon indica sp. nov. the Indian Ocean blue-spotted maskray (Myliobatoidei, Dasyatidae) Comptes Rendus Biologies. 2018;341(2):120–130. doi: 10.1016/j.crvi.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Payne et al. (2011).Payne JL, Truebe S, Nützel A, Chang ET. Local and global abundance associated with extinction risk in late Paleozoic and early Mesozoic gastropods. Paleobiology. 2011;37(4):616–632. doi: 10.1666/10037.1. [DOI] [Google Scholar]
- Pecoraro et al. (2018).Pecoraro C, Babbucci M, Franch R, Rico C, Papetti C, Chassot E, Bodin N, Cariani A, Bergelloni L, Tinti F. The population genomics of yellowfin tuna (Thunnus albacares) at global geographic scale challenges current stock delineation. Scientific Reports. 2018;8(1):1–10. doi: 10.1038/s41598-018-32331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirog et al. (2020).Pirog A, Magalon H, Poirout T, Jaquemet S. New insights into the reproductive biology of the tiger shark Galeocerdo cuvier and no detection of polyandry in Reunion Island, western Indian Ocean. Marine and Freshwater Research. 2020;71(10):1301–1312. [Google Scholar]
- Posada & Crandall (2001).Posada D, Crandall KA. Intraspecific gene genealogies: trees grafting into networks. Trends in Ecology & Evolution. 2001;16(1):37–45. doi: 10.1016/S0169-5347(00)02026-7. [DOI] [PubMed] [Google Scholar]
- Puncher et al. (2018).Puncher NG, Cariani A, Maes GE, Van Houdt J, Herten K, Cannas R, Rodriguez-Ezpeleta N, Albaina A, Estonba A, Lutcavage M, Hanke A, Rooker J, Franks SJ, Quattro MJ, Basilone G, Fraile I, Laconcha U, Goñi N, Kimoto A, Macías DA, Alemany F, Deguara S, Zgozi WS, Garibaldi F, Oray KI, Karakulak FS, Abid N, Santos NM, Addis P, Arrizabalaga H, Tinti F. Spatial dynamics and mixing of bluefin tuna in the Atlantic Ocean and Mediterranean Sea revealed using next generation sequencing. Molecular Ecology Resources. 2018;18:1–51. doi: 10.1111/1755-0998.12764. [DOI] [PubMed] [Google Scholar]
- Reece, Bowen & Larson (2011).Reece JS, Bowen BW, Larson A. Long larval duration in moray eels (Muraenidae) ensures ocean-wide connectivity despite differences in adult niche breadth. Marine Ecology Progress Series. 2011;437:269–277. [Google Scholar]
- Rocha et al. (2002).Rocha LA, Bass AL, Robertson DR, Bowen BW. Adult habitat preferences, larval dispersal, and the comparative phylogeography of three Atlantic surgeonfishes (Teleostei: Acanthuridae) Molecular Ecology. 2002;11(2):243–252. doi: 10.1046/j.0962-1083.2001.01431.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ezpeleta et al. (2019).Rodríguez-Ezpeleta N, Díaz-Arce N, Walter III JF, Richardson DE, Rooker JR, Nøttestad L, Hanke AR, Franks JS, Deguara S, Lauretta MV, Addis P. Determining natal origin for improved management of Atlantic bluefin tuna. Frontiers in Ecology and the Environment. 2019;17(8):439–444. doi: 10.1002/fee.2090. [DOI] [Google Scholar]
- Rozas et al. (2017).Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular Biology and Evolution. 2017;34(12):3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- Schönhuth et al. (2018).Schönhuth S, Gagne RB, Alda F, Neely DA, Mayden RL, Blum MJ. Phylogeography of the widespread creek chub Semotilus atromaculatus (Cypriniformes: Leuciscidae) Journal of Fish Biology. 2018;93(5):778–791. doi: 10.1111/jfb.13778. [DOI] [PubMed] [Google Scholar]
- Schultz et al. (2008).Schultz JK, Feldheim KA, Gruber SH, Ashley MV, McGovern TM, Bowen BW. Global phylogeography and seascape genetics of the lemon sharks (genus Negaprion) Molecular Ecology. 2008;17(24):5336–5348. doi: 10.1111/j.1365-294X.2008.04000.x. [DOI] [PubMed] [Google Scholar]
- Selkoe et al. (2014).Selkoe KA, Gaggiotti OE, Laboratory ToBo, Bowen BW, Toonen RJ. Emergent patterns of population genetic structure for a coral reef community. Molecular Ecology. 2014;23(12):3064–3079. doi: 10.1111/mec.12804. [DOI] [PubMed] [Google Scholar]
- Shiffman & Hammerschlag (2016).Shiffman DS, Hammerschlag N. Shark conservation and management policy: a review and primer for non-specialists. Animal Conservation. 2016;19(5):401–412. doi: 10.1111/acv.12265. [DOI] [Google Scholar]
- Siegel & Castellan (1988).Siegel S, Castellan NJ. Nonparametric statistics for the behavioural sciences. 2nd edn McGraw-Hill Book Company; New York: 1988. pp. 144–151. [Google Scholar]
- Snelson, Burgess & Roman (2008).Snelson Jr FF, Burgess GH, Roman BL. The reproductive biology of pelagic elasmobranchs. In: Camhi MD, Pikitch E, Babcock EA, editors. Sharks of the open ocean: biology, fisheries & conservation. Blackwell Publishing Ltd; Oxford: 2008. pp. 140–145. [Google Scholar]
- Springer (1967).Springer S. Social organization of shark population. In: Gilbert PW, Mathewson RF, Rall DP, editors. Sharks, skates and rays. John Hopkins Press; Baltimore: 1967. pp. 149–174. [Google Scholar]
- Taguchi et al. (2015).Taguchi M, King JR, Wetklo M, Withler RE, Yokawa K. Population genetic structure and demographic history of Pacific blue sharks (Prionace glauca) inferred from mitochondrial DNA analysis. Marine and Freshwater Research. 2015;66(3):267–275. doi: 10.1071/MF14075. [DOI] [Google Scholar]
- Tenggardjaja, Bowen & Bernardi (2016).Tenggardjaja KA, Bowen BW, Bernardi G. Reef fish dispersal in the Hawaiian Archipelago: comparative phylogeography of three endemic damselfishes. Journal of Marine Biology. 2016;165(1) doi: 10.1155/2016/3251814. Article ID 3251814. [DOI] [Google Scholar]
- Tillett et al. (2012).Tillett BJ, Meekan MG, Field IC, Thorburn DC, Ovenden JR. Evidence for reproductive philopatry in the bull shark Carcharhinus leucas. Journal of Fish Biology. 2012;80(6):2140–2158. doi: 10.1111/j.1095-8649.2012.03228.x. [DOI] [PubMed] [Google Scholar]
- Toonen et al. (2016).Toonen RJ, Bowen BW, Iacchei M, Briggs JC. Biogeography, marine. In: Kliman RM, editor. Encyclopedia of evolutionary biology. Academic Press; Oxford: 2016. pp. 166–178. [Google Scholar]
- Veríssimo et al. (2017).Veríssimo A, Sampaio Í, McDowell JR, Alexandrino P, Mucientes G, Queiroz N, da Silva C, Jones CS, Noble LR. World without borders—genetic population structure of a highly migratory marine predator, the blue shark (Prionace glauca) Ecology and Evolution. 2017;7(13):4768–4781. doi: 10.1002/ece3.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaud et al. (2014).Vignaud TM, Mourier J, Maynard JA, Leblois R, Spaet JL, Clua E, Neglia V, Planes S. Blacktip reef sharks, Carcharhinus melanopterus, have high genetic structure and varying demographic histories in their Indo-Pacific range. Molecular Ecology. 2014;23(21):5193–5207. doi: 10.1111/mec.12936. [DOI] [PubMed] [Google Scholar]
- Wright (1931).Wright S. Evolution in Mendelian populations. Genetics. 1931;16(2):97–159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The cytochrome c oxidase subunit I (COI) sequences of shark species are available at NCBI GenBank (Tables S1 and S2).