Graphical abstract
Keywords: COVID-19, Aptasensor, Paper-based, Biosensor
Abstract
Corona Virus Disease 2019 (COVID-19) as the infectious disease caused the pandemic disease around the world through infection by SARS-CoV-2 virus. The common diagnosis approach is Quantitative RT-PCR (qRT-PCR) which is time consuming and labor intensive. In the present study a novel colorimetric aptasensor was developed based on intrinsic catalytic activity of chitosan film embedded with ZnO/CNT (ChF/ZnO/CNT) on 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. The main nanocomposite platform was constructed and functionalized with specific COVID-19 aptamer. The construction subjected with TMB substrate and H2O2 in the presence of different concentration of COVID-19 virus. Separation of aptamer after binding with virus particles declined the nanozyme activity. Upon addition of virus concentration, the peroxidase like activity of developed platform and colorimetric signals of oxidized TMB decreased gradually. Under optimal conditions the nanozyme could detect the virus in the linear range of 1–500 pg mL and LOD of 0.05 pg mL. Also, a paper-based platform was used for set up the strategy on applicable device. The paper-based strategy showed a linear range between 50 and 500 pg mL with LOD of 8 pg mL. The applied paper based colorimetric strategy showed reliable results for sensitive and selective detection of COVID-19 virus with the cost-effective approach.
1. Introduction
The COVID-19 as the infectious respiratory illness was spread around the world in recent years with the mortality rate about 5% [1]. More than its mortality rate, the high transmission ability of SARS-CoV-2 virus is the main reason for the pandemic outcome. The most common symptoms of this illness include fever, fatigue, cough, myalgia and headache. These symptoms are very similar to the infection by other viruses such as influenza A and B which make it difficult to distinguish SARS-CoV-2 virus from other viruses. This problem has been raised with development of new serotypes with moderate symptoms recently.
The worldwide gold standard method across the world is quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR). Despite of qRT-PCR detection system priorities, it has some drawbacks including time consuming, high cost and strict requirements for laboratory settings and trained personnel. Several electrochemical based methods have been developed for detection of COVID-19 [2], [3]. The proposed assays showed lower detection limits (LODs) at micromolar per liter levels. Some detection methods has been developed based on lateral flow immunoassay (LFA) which the detection strategy are based on antibody-antigen interaction [4]. For fabrication of these systems the recombinant antibodies with high binding affinity have been produced by the immune system in response to an antigen and deposited on lateral flow surface. Despite specificity of antibodies their production is complicated and expensive process. So, there is an urgent need for development of cost effective and sensitive method with the same or improved performance. Several colorimetric approaches have been reported based on peroxidase like activity of nanostructures to catalyze the TMB substrate oxidation by H2O2 which result to blue color appearance [5], [6], [7].
Aptamers are specific molecular detection probe which composed of short sequence of oligonucleotides and showed comparable results with antibodies in biosensors performance [8], [9]. Among different optical assays for development of biosensors, fluorescence based strategies illustrated the higher sensitivity in different applied strategies for detection of variety of bioanalytes [10], [11]. But they are not applicable for utilization in conventional and rapid test detection kits and need instruments and equipment. Colorimetric methods are most reliable assays among optical approaches and their detection results through naked eye introduce them as the applicable strategy in biosensing of bioanalytes [12], [13]. Several aptasensor based strategies has been conducted for detection of SARS-CoV-2 virus including fluorescence [14], [15], electrochemical [16], surface enhanced Raman spectroscopy (SERS) [17].
In the present study, we developed a novel platform composed of chitosan film (ChF), ZnO nanoparticles (ZnO NPs) and carbon nanotubes (CNTs) for quantitative detection of the COVID-19. The synthesized hybrid (ChF/ZnO/CNT) showed superior performance in biomimetic catalytic activity which was applicable in construction of colorimetric aptasensor. Intrinsic affinity of aptamer to CNT part of platform resulted to deposition of aptamer on ChF/ZnO/CNT nanohybrid and also enhanced the oxidized TMB color in the presence of H2O2. Addition of COVID-19 virus in reaction resulted to removal of aptamer and decreased the appeared blue color and solution absorbance (Scheme 1 ). The platform was deposited on the specific µpad along with corresponding aptamer and target virus. Developed biosensing platform showed efficient performance in both mode of colorimetric and paper-based approaches with low sensitivity. Moreover, developed strategy is the low-cost, fast and facile with high sensitivity toward detection of SARS-CoV-2 virus.
Scheme 1.
Schematic representation of proposed platform for detection of COVID-19.
2. Materials and methods
2.1. Materials
The applied solution through the experiments were prepared by double distilled and deionized water. CNTs with the length of 1–25 µm and 10–40 nm diameters were obtained from the Research Institute of Iran’s Petroleum Industry. Chitosan with the low molecular weight was purchased from Sigma-Aldrich, Munich, Germany. Other applied chemicals including ammonium hydroxide (NH4OH), zinc sulfate heptahydrate (ZnSO4·7H2O, 99%), acetic acid (CH3COOH, 99%), polyethylene glycol (PEG), nitric acid (HNO3, 70%), sodium hydroxide (NaOH, 98%), hydrochloric acid (HCl, 37%) H2O2 and TMB were obtained from Merck (Germany). Specific COVID-19 aptamer including 41 bp (GTAGGGTTTGGCTCCGGGCCTGGCGTCGGTCGTCTCTCGCC) was synthesized by Generay Biotech Co. (Shanghai) according to previous study [18].
2.2. Synthesis of ChF/ZnO/CNT platform
For synthesis of ZnO NPs, zinc sulfate heptahydrate (ZnSO4・7H2O) was used as a precursor and sol–gel method was applied in this experiment. In the beaker containing 10 mL distilled water, 5 g polyethylene glycol (PEG) was dissolved and following 2.875 g, ZnSO4·7H2O added to solution. Titration with 0.1 M NH4OH was performed until colloidal sediment precipitated. After centrifugation at 9000 rpm, it was washed twice with water. Finally, precipitant calcinated in air condition at 500 °C and 2 h to obtain the ZnO NPs. For synthesis of ChF/ZnO/CNT, chitosan solution (0.2%) was prepared through solving in 0.1 M acetic acid and stirred for 3 h for complete dissolution. Then ZnO NPs and CNT were dispersed in chitosan solution with the molar ratio of 3:1:100 for CNT:ZnO:chitosan respectively. Finally, the obtained mixture stirred for 6 h and sonicated subsequently. Synthesized colloidal solution dried at the room temperature and stored for further analysis and experiments.
2.3. Apparatuses
The spectroscopic analysis was performed through UV– Visible spectrophotometer (Shimadzu, double beam, Japan) along with another single beam spectrophotometer (Jenway, UK) for optimization process of applied strategy. Homogenization and dispersion of hybrid platform was performed with high energy sonicator (Hielscher ultrasonic processor 200 W, 50 kHz, UTR 200, Germany). Field emission scanning electron microscopy (FESEM energy-dispersive) was used for determination of morphology and size of synthesized nanostructure. The surface morphology was also characterized using atomic force microscopy (AFM, model Full plus), in tapping mode. The X-ray diffraction pattern and crystalline nature of nanostructures was analyzed with Philips analytical diffractometer. The Fourier transform infrared analysis was performed by FTIR spectroscopy (Shimadzu 8400, Japan).
2.4. Catalytic activity of synthesized nanohybrid
The acetate buffer was used as the solution substrate for determination of peroxidase like activity of ChF/ZnO/CNT nanohybrid. The optimization was performed to determine the efficient performance of biosensor in different conditions. Various concentration of TMB and H 2O2 were added to ChF/ZnO/CNT nanohybrid solution to determine the optimum concentration. Also, other parameters including aptamer concentration, incubation time and ChF/ZnO/CNT Concentration were optimized through UV–Vis spectroscopy.
2.5. Colorimetric detection of COVID-19
The effect of COVID-19 concentration on catalytic performance of proposed method was determined in the optimized concentration of other parameters. Briefly, the concentration of virus was prepared with dilution of Sinopharm vaccine as the source of inactivated virus in PBS buffer (pH = 7). Subsequently, the same amount of ingredients added to a test tube solution containing 50 µL of ChF/ZnO/CNT nanohybrid solution, 25 µL of TMB, 200 µL of acetate buffer solution and different concentration of COVID-19 virus. Afterwards, the 25 µL of H2O2 was added to solution and incubated for 15 min. Finally, the blue color was appeared and absorbance of solution was measured through UV–Vis spectrometry.
2.6. Paper based detection approach
For preparation of paper-based platform, filter paper (Whatman, No.1) was cut according to designed pattern by laser engraver machine. The process was performed through burning the paper to obtain the ideal pattern. Obtained paper platforms were soaked in distilled water for 2 h to discard the burning impurities. Subsequently, for paper platform preparation, the spot zones were coated with the nanohybrids and following the optimum concentration of aptamer and TMB substrate were added on paper surface. After being dried, different concentration of COVID-19 virus was poured on the paper. Finally, H2O2 was added to center entrance zone. While the H2O2 reached to around spot zones resulted to appearance of blue color due to the enzymatic activity of proposed biosensor. The intensity of sample colors was observable with naked eye and determined quantitively through Image J software.
3. Results and discussion
3.1. Principle of mechanism
Colorimetric detection of COVID-19 would be superior detection approach compared to other applied method due to its application in rapid test detection kits. The applied ternary nanocomposite provided a sufficient substrate by integration of CNT in platform structure for attachment of applied COVID-19 aptamer through van der Waals and π–π stacking interaction with CNT component. Due to the poor dispersibility of CNT, it is necessary to combine appropriate dispersants. Chitosan with excellent film-forming ability and good adhesion is one of the most popular dispersants. Also, Chitosan has been used to overcome the insolubility caused by attraction between CNTs, which prevents their stable dispersion in aqueous solution. The ZnO nanoparticles could accelerate the catalytic activity of peroxidase with the substrate 3,3,5,5-tetramethylbenzidine (TMB). Also, integration of ZnO NPs in different heterostructures have been reported for improving of TMB oxidation in previous studies [19], [20]. Regarding to intrinsic catalytic activity of applied components, the fabrication of ChF/ZnO/CNT resulted in catalytic activity and oxidation on TMB substrate. Addition of TMB and H2O2 led to appearance of blue color which demonstrate the peroxidase like activity of fabricated nanocomposite. The immobilized aptamer on nanocomposite induced higher peroxidase activity compared to bare nanocomposite platform. Addition of virus samples resulted to separation of aptamer from nanocomposite due to its more affinity to the virus target. Detachment of aptamer reduced the peroxidase like activity and decreasing the color and absorbance of oxidized TMB molecules. So, the detection platform was based on specific interaction between virus concentration and specific aptamer.
3.2. Characterization of proposed platform
The synthesized ChF/ZnO/CNT hybrid morphology and nanostructure was investigated by field emission scanning electron microscope (FESEM). Fig. 1 a demonstrated the spherical shaped of synthesized ZnO NPs with the average size of 20 nm. The nanostructure hybrid image showed the deposited CNTs on the surface of ChF and ZnO NPs which dispersed uniformly on CNTs (Fig. 1b). The electrostatic interaction between the positively charged of chitosan and negatively charged CNTs resulted to coating of ChF surface by CNTs. The atomic force microscopy (AFM) was also used to obtain the topographic image of ChF/ZnO/CNT. As illustrated in Fig. 1c, high roughness was observed on the sample which confirmed presence of ZnO deposited CNTs on the surface of chitosan substrate. FTIR analysis of prepared sample was performed to determine the functionalized groups on the surface. As illustrated in Fig. 1d, the characteristic stretching bands of ZnO NPs appeared at 430, 500 cm−1 in spectrum a, with distinguished band at 3555 cm−1 which indicate surface hydroxyl group of ZnO NPs [21]. The vibration bands of C = O and C-O groups in the CNT related bands was observed at 1715 and 1172 cm−1 bands in spectrum b. Another weak band was observed at 3415 cm−1 which was attributed to O–H stretching band of carboxylic acid on the CNTs surface [22]. In the hybrid spectrum c, diverse bands were observed that attributed to the components of nanostructure. Also, the bands at 3433, 2888, 1728, 1560, 1413, 1343 and 1061 cm−1 were belonged to the chitosan film. To determine the crystalline structure of hybrid, XRD analysis was performed. The Fig. 2 e shows the obtained results while the spectra (a) has diffraction peaks of 31.62, 34.27°, 36.07°, 47.42°, 56.47°, 62.72°, 66.32°, 67.82°, 68.97°, 72.57° and 76.77° related to planes of ZnO crystal and demonstrates the wurtzite structure spherical phase of ZnO NPs (JCPDS card NO. 36–1415) [23]. The diffraction peak in CNTs related spectrum (b) at 26.13°is because of the reflection of (0 0 2) planes of graphite (JSPDS = 96–101-1061). Another diffraction peak at 43.94° is also related to the diffraction of C (1 0 0) planes of graphite (JCPDS = 96–100-1061). Finally, as depicted in spectrum (c), the diffraction peaks of ChF, ZnO and CNTs are illustrated with the same characteristics in synthesized ChF/ZnO/CNT hybrid.
Fig. 1.
Characterization of proposed platform, a) FESEM analysis of synthesized ZnO NPs b) FESEM image of ChF/ZnO/CNT nanohybrid, c) AFM image of ChF/ZnO/CNT nanohybrid, d) FTIR spectra of ZnO, CNT and ChF/ZnO/CNT nanohybrid, e) XRD analysis of ZnO, CNT and ChF/ZnO/CNT nanohybrid, f) UV–Vis spectra of ChF/ZnO/CNT nanohybrid before (a) and after immobilization of COVID-19 aptamer.
Fig. 2.
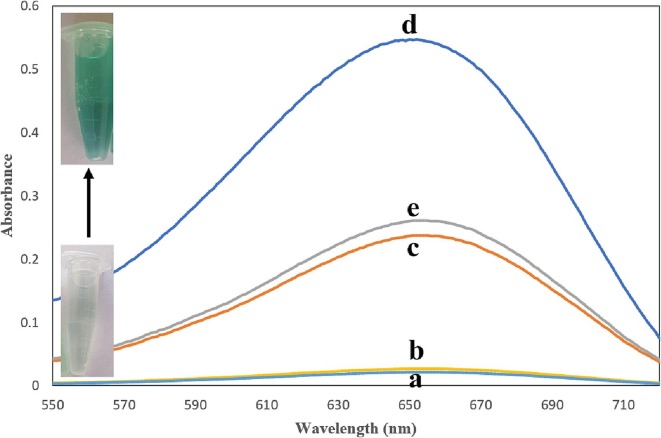
Feasibility of performed experiment in the presence of TMB + H2O2 + COVID-19 aptamer (a), TMB + H2O2 + COVID-19 virus (b), ChF/ZnO/CNT + TMB + H2O2 (c), ChF/ZnO/CNT + TMB + H2O2 + COVID-19 aptamer (d) and ChF/ZnO/CNT + TMB + H2O2 + COVID-19 aptamer + COVID-19 virus (e).
3.3. Immobilization of aptamer on the applied nanohybrid
The binding of DNA aptamer to the nanocomposite was confirmed with UV–Vis analysis. The obtained spectra (Fig. 1f) for ChF/ZnO/CNT hybrids (a) and aptamer functionalized nano-hybrids (b) showed overlapped in absorbance at 260 nm and this absorbance was higher in functionalized nanocomposite due to presence of aptamer in the structure. The previous studies clearly illustrated the efficient and considerable interaction of DNA oligonucleotides with carbon-based structures such as CNTs and GOs. The aptamer binding to nanocomposite attributed the intrinsic affinity of applied CNT in the nanocomposite structure. To evaluate the catalytic performance of aptamer conjugated nanostructure, obtained assembly catalytic activity evaluated in the presence of TMB and H2O2. Improvement of catalytic performance was observed after incubation with aptamer oligonucleotides which illustrated the role of aptamer in electron transfer from TMB substrate to H2O2.
3.4. Peroxidase like activity of constructed nanohybrid
The conducted experiment showed peroxidase like activity of synthesized ChF/ZnO/CNT through color change from colorless to green color and maximum UV–Vis absorption (λ max) at 650 nm (Fig. 2). Determination of peroxidase activity of TMB substrate and H2O2 in the presence of COVID-19 aptamer and COVID-19 virus showed no chromogenic response (Fig. 2a,b). These results indicated that the COVID-19 aptamer and COVID-19 virus ingredients could not have catalytic activity alone. Integration of applied nanostructures on the chitosan film as the organic compound resulted in enhancement of catalytic activity of proposed platform (Fig. 2c). Immobilization of aptamer on the surface of ChF/ZnO/CNT enhanced more the catalytic activity of biosensor which attributed to π-π stacking and electrostatic interaction of oligonucleotides with ChF/ZnO/CNT assembly (Fig. 2d). Addition of COVID-19 virus resulted to separation of aptamer from the surface of nanocomposite and reduced the absorbance of UV–Vis spectrum (Fig. 2e). The peroxidase like activity of ZnO, CNT and chitosan constitutes in several nanocomposite structures has been reported in previous studies [24], [25], [26]. Integration of these nanostructures brought significant enzyme activity as expected on TMB substrate in the presence of H2O2.
3.5. Optimization of assay condition
For optimization of assay condition, first the various concentration of TMB and H2O2 were added to determine the best condition for the catalytic activity of TMB. Different concentrations of TMB (2, 4, 8,10, 15, 20 mM) were used in the presence of 10 mM H2O2 and the 1 mg/mL of synthesized platform. The obtained results showed the optimum point for TMB concentration at the 15 mM (Fig. 3 a). Following, the series of H2O2 concentration (2, 4, 8,10, 15, 20 mM) were checked in the presence of 15 mM TMB and 20 mM showed the highest absorbance and performance for further experiments (Fig. 3b). The various concentrations of ChF/ZnO/CNT nanocomposite were also used in the range between 0.01 and 3 mg/mL and the maximum absorbance was determined at the 1 mg/mL and higher concentration showed no further increase in absorbance (Fig. 3c). Moreover, the effect of different concentrations of COVID-19 aptamer were determined in the range of 0.05–1 µM. Increase of aptamer concentration enhanced the absorbance at 650 nm gradually and reached the plateau after 0.5 µM and it was selected as the optimum concentration (Fig. 3d). Peroxidase like activity of proposed platform was also determined at every 5 min incubation time from 5 to 30 min. The maximum absorbance value was obtained after 15 min incubation and then decreased at next incubation times. Thus, 15 min was chosen as the ideal incubation time (Fig. 3e).
Fig. 3.
Optimization of different parameters including TMB concentration (a), H2O2 concentration (b), ChF/ZnO/CNT Concentration (c), aptamer concentration (d), incubation time (e).
3.6. Colorimetric detection of COVID-19 virus
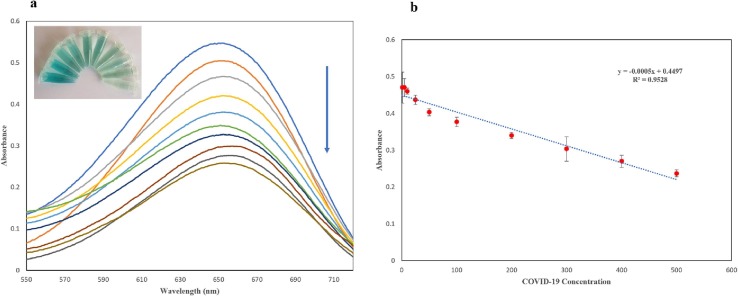
A Sinopharm vaccine was used as the source of inactivated SARS-CoV-2 and was diluted to a series of concentrations in PBS buffer (pH = 7.5). The different dilutions of COVID-19 virus sample were added to the prepared detection reaction with the optimized concentration of nanocomposite, aptamer, H2O2 and TMB. After incubation at the obtained optimum incubation time (15 min), the visual detection showed blue color appearance which decreased after addition of more concentration of virus samples (Fig. 4 ). Also, the absorbance spectra of the samples were recorded and showed gradual decrease which was dependent in addition of virus sample concentration (Fig. 4a). The linear relationship was observed according to standard calibration curve which demonstrated a linear range between 1 and 500 pg mL (1 × 10-12, 5 × 10-12, 1 × 10-11, 2.5 × 10-11, 5 × 10-11, 1 × 10-10, 2 × 10-10, 3 × 10-10 4 × 10-10, 5 × 10-10 g mL) with a linear relationship of Y = -0.0005X + 0.4497, R2 = 0.9446 where Y is the absorbance and X is the concentration of COVID-19 virus (Fig. 4b). The limits of detection (LOD) was calculated based on following formula: LOD = 3σ/s, where σ is the standard deviation of baseline noise and s is the analytical sensitivity of the calibration curve [27]. So, the LOD was calculated at 0.05 pg mL. This novel detection platform presented some advantages including quick response, simple and cost-effective approach toward virus detection. Also, all the experiment condition was performed at room temperature and peroxidase like activity showed reliable response which was reproducible in triplicate test.
Fig. 4.
Catalytic activity of proposed biosensor in the presence of COVID-19 virus concentration through UV–Vis spectroscopy (a), The obtained calibration curve for virus detection (b) and naked-eye color change of TMB oxidation by addition of virus concentration.
3.7. Paper based detection of COVID-19 virus
Paper based detection strategy as the low cost and rapid detection approach is highly considered in recent years. For determination of proposed approach performance on paper-based test a circle shaped paper device was used as depicted in Fig. 5 a. The µpad consists of a central zone for sample inlet that connects to separate detection zones around it. This pattern was designed for conduction of H2O2 toward detection zones. The negative sample without nanohybrids could not exhibit color change after addition of H2O2 while presence and biomimetic activity of nanohybrid in the presence of aptamer showed intense blue color (Upper spot zones). After spotting of all components, different concentrations of COVID-19 virus were also added in each spot zone and showed gradual decrease in color which was detectable with naked eye. The detachment process of aptamer and subsequent color change was performed on the paper through weakening of electrostatic interaction between CNT and aptamer in the presence of COVID-19 virus as the target. So, according to obtained results the assay strategy showed similar results compared to detection in solution test. A smart phone was used for picture capturing. The obtained picture was analyzed with Image J software and showed decreasing trend of grey intensity by addition of COVID-19 concentration between 50 and 500 pg mL with LOD of 8 pg mL (Fig. 5b).
Fig. 5.
Paper-based detection platform for determinization of COVID-19 virus (a), and the calibration curve for grey intensity of each sample (b).
3.8. Selectivity of experiment
The selectivity of the proposed biosensor was evaluated in the presence of possible concomitant microorganisms found in environment and body fluid as the pathogen and non-pathogen strains (500 µmol L−1), including Bacillus subtilis and staphylococcus aureus gram positive bacteria and Pseudomonas aeruginosa and Escherichia coli strains. The same concentration of above microorganisms was studied to evaluate the maximum levels of each interference. It was possible to observe that after the addition of the interferents, the obtained absorbance values decreased significantly (Fig. 6 ). This shows that the selectivity of the ChF/ZnO/CNT based aptasensor for the detection of COVID-19 virus has not been affected in the presence of the evaluated interfering analytes.
Fig. 6.
Selectivity of proposed strategy in the presence of different microorganisms.
4. Conclusion
The novel and colorimetric method has been developed for detection of COVID-19 virus. In the present strategy ChF/ZnO/CNT was introduced for the first time as the nanocomposite with peroxidase like activity which also could play a role as the detection platform. Intrinsic affinity of oligonucleotides toward CNTs facilitated immobilization of aptamer and COVID-19 virus addition decreased the appeared blue color and absorbance of oxidized TMB as the chromogenic substrate. This strategy was also performed on paper-based platform to demonstrate the applicability of method with naked eye detection. Analytical performance of ChF/ZnO/CNT nanohybrid were evaluated and found to be linear in the range of 1 pg mL to 500 pg mL in colorimetric mode and 50 to 500 pg mL in paper-based platform. Proposed biosensor showed low LOD in colorimetric and paper-based detection modes and could be as the alternative cheap and portable device for COVID-19 detection. Feasibility of proposed biosensor for detection of whole particle of virus would be another advantage of this method which can be applicable in detection kits. Developed biosensor bring down the cost of COVID-19 detection assays and make them affordable and reliable for onsite detection and screening of virus particles.
CRediT authorship contribution statement
Mostafa Vafabakhsh: Investigation. Mehdi Dadmehr: Project administration, Supervision, Writing – review & editing. Sakineh Kazemi Noureini: Supervision. Zarrin Es'haghi: Supervision, Validation. Mitra Malekkiani: Formal analysis, Investigation. Morteza Hosseini: Validation, Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eissa S., Zourob M. Development of a low-cost cotton-tipped electrochemical immunosensor for the detection of SARS-CoV-2. Anal. Chem. 2020;93:1826–1833. doi: 10.1021/acs.analchem.0c04719. [DOI] [PubMed] [Google Scholar]
- 3.Yu M., Zhang X., Zhang X., ul ain Zahra Q., Huang Z., Chen Y., Song C., Song M., Jiang H., Luo Z. An electrochemical aptasensor with N protein binding aptamer-complementary oligonucleotide as probe for ultra-sensitive detection of COVID-19. Biosens. Bioelectron. 2022;213 doi: 10.1016/j.bios.2022.114436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant B.D., Anderson C.E., Williford J.R., Alonzo L.F., Glukhova V.A., Boyle D.S., Weigl B.H., Nichols K.P. SARS-CoV-2 coronavirus nucleocapsid antigen-detecting half-strip lateral flow assay toward the development of point of care tests using commercially available reagents. Anal. Chem. 2020;92:11305–11309. doi: 10.1021/acs.analchem.0c01975. [DOI] [PubMed] [Google Scholar]
- 5.Li H., Song P., Wu T., Zhao H., Liu Q., Zhu X. In situ decorating of montmorillonite with ZnMn2O4 nanoparticles with enhanced oxidase-like activity and its application in constructing GSH colorimetric platform. Appl. Clay Sci. 2022;229 [Google Scholar]
- 6.Zhu X., Li H., Wu T., Zhao H., Wu K., Xu W., Qin F., Chen W., Zheng J., Liu Q. In situ decorating the surface and interlayer of montmorillonite with Co0. 5Ni0. 5Fe2O4 nanoparticles: A sustainable, biocompatible colorimetric platform for H2O2 and acetylcholine. Nano Res. 2022;15:9319–9326. [Google Scholar]
- 7.Zhu X., Li H., Zhang D., Chen W., Fu M., Nie S., Gao Y., Liu Q. Novel “on–off” colorimetric sensor for glutathione based on peroxidase activity of Montmorillonite-loaded TiO2 functionalized by porphyrin precisely controlled by visible light. ACS Sustain. Chem. Eng. 2019;7:18105–18113. [Google Scholar]
- 8.Dadmehr M., Shahi S.C., Malekkiani M., Korouzhdehi B., Tavassoli A. A stem-loop like aptasensor for sensitive detection of aflatoxin based on graphene oxide/AuNPs nanocomposite platform. Food Chem. 2023;402 doi: 10.1016/j.foodchem.2022.134212. [DOI] [PubMed] [Google Scholar]
- 9.Sabet F.S., Hosseini M., Khabbaz H., Dadmehr M., Ganjali M.R. FRET-based aptamer biosensor for selective and sensitive detection of aflatoxin B1 in peanut and rice. Food Chem. 2017;220:527–532. doi: 10.1016/j.foodchem.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Dadmehr M., Mortezaei M., Korouzhdehi B. Dual mode fluorometric and colorimetric detection of matrix metalloproteinase MMP-9 as a cancer biomarker based on AuNPs@ gelatin/AuNCs nanocomposite. Biosens. Bioelectron. 2023;220 doi: 10.1016/j.bios.2022.114889. [DOI] [PubMed] [Google Scholar]
- 11.Dadmehr M., Karimi M.A., Korouzhdehi B. A signal-on fluorescence based biosensing platform for highly sensitive detection of DNA methyltransferase enzyme activity and inhibition. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020;228 doi: 10.1016/j.saa.2019.117731. [DOI] [PubMed] [Google Scholar]
- 12.Mortezaei M., Dadmehr M., Korouzhdehi B., Hakimi M., Ramshini H. Colorimetric and label free detection of gelatinase positive bacteria and gelatinase activity based on aggregation and dissolution of gold nanoparticles. J. Microbiol. Methods. 2021;191 doi: 10.1016/j.mimet.2021.106349. [DOI] [PubMed] [Google Scholar]
- 13.Shahi S.C., Dadmehr M., Korouzhdehi B., Tavassoli A. A novel colorimetric biosensor for sensitive detection of aflatoxin mediated by bacterial enzymatic reaction in saffron samples. Nanotechnology. 2021;32 doi: 10.1088/1361-6528/ac23f7. [DOI] [PubMed] [Google Scholar]
- 14.Liu R., He L., Hu Y., Luo Z., Zhang J. A serological aptamer-assisted proximity ligation assay for COVID-19 diagnosis and seeking neutralizing aptamers. Chem. Sci. 2020;11:12157–12164. doi: 10.1039/d0sc03920a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z., Zhang C., He S., Xu D. An ultrasensitive fluorescence aptasensor for SARS-CoV-2 antigen based on hyperbranched rolling circle amplification. Talanta. 2023;255 doi: 10.1016/j.talanta.2022.124221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu N., Liu R., Zhang J. CRISPR-Cas12a-mediated label-free electrochemical aptamer-based sensor for SARS-CoV-2 antigen detection. Bioelectrochemistry. 2022;146 doi: 10.1016/j.bioelechem.2022.108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian C., Zhao L., Qi G., Zhu J., Zhang S. One-pot and rapid detection of SARS-CoV-2 viral particles in environment using SERS aptasensor based on a locking amplifier. Sens. Actuators B. 2022;371 doi: 10.1016/j.snb.2022.132445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z., Li J., Gu J., Amini R., Stacey H.D., Ang J.C., White D., Filipe C.D., Mossman K., Miller M.S. A Universal DNA Aptamer that Recognizes Spike Proteins of Diverse SARS-CoV-2 Variants of Concern, Chemistry–A. European Journal. 2022;28:e202200078. doi: 10.1002/chem.202200078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christus A.A.B., Ravikumar A., Panneerselvam P., Radhakrishnan K. A novel Hg (II) sensor based on Fe3O4@ ZnO nanocomposite as peroxidase mimics. Appl. Surf. Sci. 2018;449:669–676. [Google Scholar]
- 20.Wang X., Zhao M., Song Y., Liu Q., Zhang Y., Zhuang Y., Chen S. Synthesis of ZnFe2O4/ZnO heterostructures decorated three-dimensional graphene foam as peroxidase mimetics for colorimetric assay of hydroquinone. Sens. Actuators B. 2019;283:130–137. [Google Scholar]
- 21.Malekkiani M., Heshmati Jannat Magham A., Ravari F., Dadmehr M. Facile fabrication of ternary MWCNTs/ZnO/Chitosan nanocomposite for enhanced photocatalytic degradation of methylene blue and antibacterial activity. Sci. Rep. 2022;12:5927. doi: 10.1038/s41598-022-09571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallakpour S., Dinari M., Behranvand V. Anionic clay intercalated by multi-walled carbon nanotubes as an efficient 3D nanofiller for the preparation of high-performance l-alanine amino acid containing poly (amide-imide) nanocomposites. J. Mater. Sci. 2014;49:7004–7013. [Google Scholar]
- 23.Preethi S., Abarna K., Nithyasri M., Kishore P., Deepika K., Ranjithkumar R., Bhuvaneshwari V., Bharathi D. Synthesis and characterization of chitosan/zinc oxide nanocomposite for antibacterial activity onto cotton fabrics and dye degradation applications. Int. J. Biol. Macromol. 2020;164:2779–2787. doi: 10.1016/j.ijbiomac.2020.08.047. [DOI] [PubMed] [Google Scholar]
- 24.Ragavan K., Ahmed S.R., Weng X., Neethirajan S. Chitosan as a peroxidase mimic: Paper based sensor for the detection of hydrogen peroxide. Sens. Actuators B. 2018;272:8–13. [Google Scholar]
- 25.Tripathi R.M., Ahn D., Kim Y.M., Chung S.J. Enzyme mimetic activity of ZnO-Pd nanosheets synthesized via a green route. Molecules. 2020;25:2585. doi: 10.3390/molecules25112585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L., Zhou X., Wan G., Shi S., Wang G. NiFe2O4/CNTs fabricated by atomic layer deposition as highly stable peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Mater. Res. Bull. 2022;147 [Google Scholar]
- 27.Long G.L., Winefordner J.D. Limit of detection. A closer look at the IUPAC definition. Anal. Chem. 1983;55:712A–724A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.