Abstract
Background/Aim
Patients with cirrhosis have poor outcomes once decompensation occurs; however, we lack adequate predictors of decompensation. To use a national claim database to compare the predictive accuracy of seven models for decompensation and hospitalization in patients with compensated cirrhosis.
Methods
We defined decompensation as ascites, hepatic encephalopathy, hepato-renal syndrome, and variceal bleeding. Patients without decompensation at the time of cirrhosis diagnosis were enrolled from 2001 to 2015. Patients with hepatitis B and/or C were grouped as viral cirrhosis. We compared the predictive accuracy of models with the AUC (area under the curve) and c-statistic. The cumulative incidence of decompensation and incidence risk ratios of hospitalization were calculated with the Fine–Gray competing risk and negative binomial models, respectively.
Results
A total of 3722 unique patients were enrolled with a mean follow-up time of 524 days. The mean age was 59 (standard deviation 12), and the majority were male (55%) and white (65%). Fifty-three percent of patients had non-viral cirrhosis. Sixteen and 20 percent of patients with non-viral and viral cirrhosis, respectively, developed decompensation (P = 0.589). The FIB-4 model had the highest 3-year AUC (0.73) and overall c-statistic (0.692) in patients with non-viral cirrhosis. The ALBI-FIB-4 model had the best 1-year (AUC = 0.741), 3-year (AUC = 0.754), and overall predictive accuracy (c-statistic = 0.681) in patients with viral cirrhosis. The MELD score had the best predictive power for hospitalization in both non-viral and viral patients.
Conclusions
FIB-4-based models provide more accurate prediction for decompensation, and the MELD model has the best predictive ability of hospitalization.
Keywords: Alcohol, Ascites, Decompensation, Hepatic encephalopathy, Nonalcoholic fatty liver disease (NAFLD)
Introduction
The prognosis of patients with cirrhosis is mainly determined by the control of underlying liver disease and the development of decompensation (such as ascites and hepatic encephalopathy) [1]. Without decompensation, patients with cirrhosis can have a favorable prognosis and preserved quality of life while decompensation carries a substantial symptom burden, increased healthcare utilization, and mortality [2]. Clinicians can provide prognostic information for patients with decompensated cirrhosis using the Child–Turcotte–Pugh (CTP) score and model for end-stage liver disease (MELD). However, these indices poorly discriminate survival when the patient is compensated and give little information on the risk of decompensation [3]. Accurate risk stratification tools for patients with compensated disease remain an unmet need.
Emerging evidence suggests that prognosis can be informed by noninvasive scoring systems developed to discriminate the presence of advanced fibrosis in patients with chronic liver disease. Recently, the albumin–bilirubin (ALBI) and fibrosis-4 (FIB-4) scores have been combined in an international validation study and accurately identified patients at risk for decompensation [4]. However, this study has notable limitations including a small sample size, failure to account for the competing risk of death before decompensation, and inclusion of few patients with nonalcoholic fatty liver disease (NAFLD), which is increasingly prevalent in Western populations with cirrhosis. Finally, an additional measure to validate the clinical utility of a risk score for decompensation is its association with the patient’s future burden of hospitalizations [5–7].
We aimed to investigate the short- and long-term prognostic accuracy of several noninvasive models of liver disease severity for predicting decompensation and burden of hospitalization in a large, nationally representative cohort of commercially insured patients with compensated cirrhosis.
Patients and Methods
Data Sources
Patient data were obtained from the Clinformatics Data Mart (Optum), which contains comprehensive health claims submitted for payment including medical service, pharmacy, laboratory, facility, hospitalization, procedures, and de-identified demographics of patient population across the USA. The Optum system has collected healthcare data from more than 100 million customers and 80 percent of hospitals in the USA (https://www.optum.com/content/dam/optum/resources/productSheets/Clinformatics_for_Data_Mart.pdf). The Optum database had been used extensively for clinical research by multiple independent research groups [8].
Patients
Patients with two claims of International Classification of Disease (ICD) 9th edition of cirrhosis from 2001 to 2015 were extracted from the main Optum database (ICD-9 571.2, 571.5, 571.6, suppl. table 1). The first medical claim of cirrhosis was defined as the index date of enrolled patients. We excluded patients with decompensation events before or within 3 months of the cirrhosis diagnosis; this included claims for ascites, hepatic encephalopathy, hepato-renal syndrome, or variceal bleeding, or two pharmacy refill records for loop diuretics, spironolactone, rifaximin, or lactulose. We allowed this 3-month window for the clinicians and patients to complete laboratory, endoscopic, and imaging studies to decide if patients were compensated when the cirrhosis was diagnosed. We excluded patients without a medical encounter within 6 months before the index date or patients who did not have full laboratory records (albumin, bilirubin, creatinine, aspartate aminotransferase [AST], alanine aminotransferase [ALT], international normalized ratio [INR] of prothrombin time [PT], platelet, sodium) within 3 months of the cirrhosis diagnosis. The comparison between patients with and without full laboratory records (excluded by the algorithm) was provided in suppl. table 2. Patients who were diagnosed with hepatocellular carcinoma or had liver transplantation before the index date were removed. Patients who did not have subsequent medical claims of cirrhosis were excluded because of loss of follow-up (Fig. 1). Patients with interruption of insurance within 6 months before the event were removed from the comparison of cumulative incidence of decompensation and predictive accuracy of seven models.
Fig. 1.
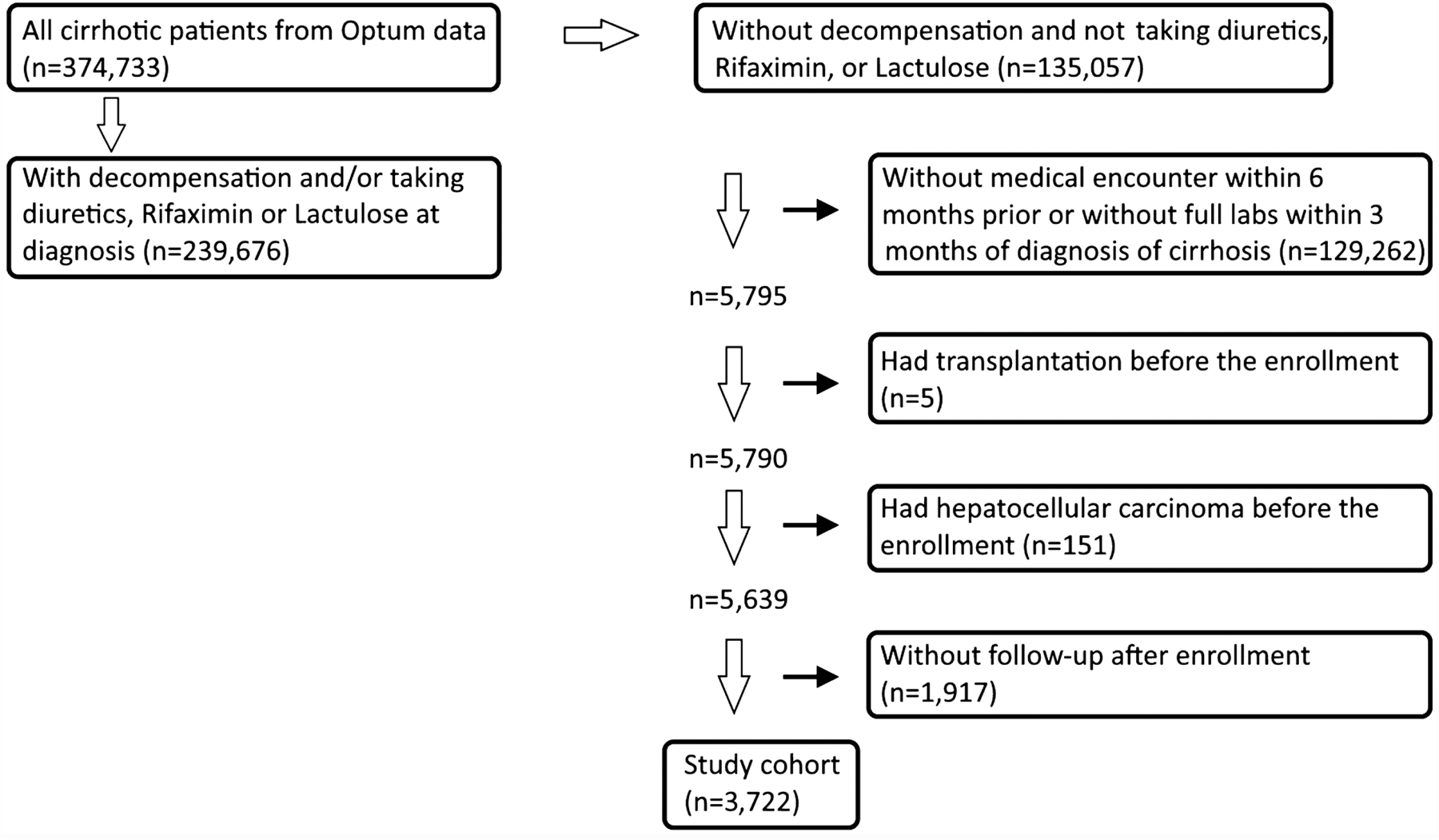
Patient selection algorithm of Optum data bank. A total of 3722 patients with compensated cirrhosis were enrolled
Diagnosis and Measures
Patient demographics were extracted from the enrollment records. The race was grouped as white and others. Chronic medical diseases including alcoholic liver disease, alpha-1-antitrypsin deficiency, autoimmune hepatitis, diabetes mellitus, hemochromatosis, hepatitis B, hepatitis C, morbid obesity, primary biliary cholangitis, primary sclerosing cholangitis, and Wilson’s disease were obtained by using ICD codes of claims. Patients without other specific liver disease codes including viral hepatitis, alcoholic liver disease, alcohol use, cholestatic liver disease, etc., were recorded as NAFLD (suppl. table 3) [9]. Laboratory results that were closest to the index date were reported and used to calculate the seven noninvasive scoring systems including the ALBI, ALBI-FIB-4, aspartate aminotransferase (AST)-to-platelet ratio index (APRI), CTP, FIB-4, Lok index, and MELD (suppl. table 4). Enrolled patients with hepatitis B and/or hepatitis C were grouped as patients with viral cirrhosis. Hospitalization data were only available between 2004 and 2017; therefore, patients diagnosed with cirrhosis before 2004 were not included in the hospitalization analysis.
Outcomes
The primary outcome of this study was the decompensation of cirrhosis, defined as the development of ascites, hepatic encephalopathy, hepato-renal syndrome, or variceal bleeding after enrollment until the end of 2017. Liver transplantation and death before the development of decompensation were considered competing events. We conducted a secondary analysis to determine which scoring system predicted days of all-cause hospitalization per year during the follow-up period.
Statistical Analysis
The chi-squared or Fisher’s exact test was used to analyze categorical variables, and the Mann–Whitney rank-sum test was used for continuous variables. The distribution of decompensation (event) was investigated with the Fine–Gray competing risk regression model, which also generated the sub-distribution hazard ratio to evaluate the overall increased risk of decompensation by 1-unit increment of the scoring system [10]. Predictive accuracy of the seven noninvasive models at 1 year and 3 years were compared by using the time-dependent area under a curve (AUC). The overall performance of models was evaluated with Uno’s c-statistics [11]. Bootstrapping with 200 unrestricted random samples was used for the AUC and Uno’s c-statistics. The incidence risk ratio was calculated by using the negative binomial model. For all tests, a P value less than 0.05 is considered statistically significant. All statistical analyses were conducted with SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient Characteristics
The baseline demographics of study patients stratified by viral etiologies are shown in Table 1. Upon the time of enrollment, there were 1978 and 1744 patients with non-viral and viral cirrhosis, respectively. When compared to non-viral etiologies, patients with viral cirrhosis were younger and had a higher proportion of males and a lower proportion of white patients. Patients with viral cirrhosis had significantly lower albumin and platelet, but higher bilirubin, AST, and ALT. Patients with viral cirrhosis had more advanced fibrosis based on higher ALBI, ALBI-FIB-4, APRI, and FIB-4 scores. In addition, patients with viral cirrhosis had significantly higher rates of variceal bleeding as the first complication of cirrhosis and significantly higher incidence of hepato-renal syndrome, variceal bleeding, and transplantation during the follow-up period.
Table 1.
Baseline characteristics of enrolled patients
| Non-viral (n = 1978) | Viral (n = 1744) | P | |
|---|---|---|---|
| Demographics | |||
| Age (years; mean/median, IQR) | 60/61 (17) | 57/57 (13) | < 0.001 |
| Male (%) | 967 (49) | 1076 (62) | < 0.001 |
| White (%) | 1248 (63) | 1004 (58) | < 0.001 |
| Etiology of liver disease | |||
| Alcoholic liver disease (%) | 561 (28) | 424 (24) | 0.005 |
| Alpha-1-antitrypsin deficiency (%) | 16 (0.8) | 5 (0.3) | 0.046 |
| Autoimmune hepatitis (%) | 118 (6) | 54 (3) | < 0.001 |
| Hemochromatosis (%) | 66 (3) | 45 (3) | 0.176 |
| Hepatitis B (%) | 0 | 321 (18) | N/A |
| Hepatitis C (%) | 0 | 1639 (94) | N/A |
| NAFLD (%) | 760 (38) | 0 | N/A |
| Primary biliary cholangitis (%) | 422 (21) | 133 (8) | < 0.001 |
| Primary sclerosing cholangitis (%) | 109 (6) | 46 (3) | < 0.001 |
| Wilson’s disease (%) | 10 (0.5) | 3 (0.2) | 0.1 |
| Comorbidity | |||
| Diabetes mellitus (%) | 1011 (51) | 715 (41) | < 0.001 |
| Morbid obesity (%) | 314 (16) | 153 (9) | < 0.001 |
| Biochemistries (mean/median, IQR) | |||
| Albumin (g/dL) | 3.9/4 (0.7) | 3.9/3.9 (0.7) | 0.004 |
| Bilirubin (mg/dL) | 1.4/0.7 (0.7) | 1.3/0.8 (0.8) | 0.008 |
| Creatinine (mg/dL) | 0.9/0.8 (0.3) | 0.9/0.9 (0.3) | 0.1 |
| Aspartate aminotransferase (U/L) | 59/39 (38) | 89/61 (69) | < 0.001 |
| Alanine aminotransferase (U/L) | 52/32 (35) | 87/58 (68) | < 0.001 |
| INR of prothrombin time | 1.2/1.1 (0.2) | 1.1/1.1 (0.2) | 0.423 |
| Platelet (109/L) | 193/179 (131) | 139/139 (114) | < 0.001 |
| Sodium (mmol/L) | 139/139 (4) | 139/139 (4) | 0.527 |
| Medications during enrollment | |||
| Beta-blockers (%) | 382 (19) | 341 (20) | 0.853 |
| Hepatitis B medications (%) | 79 (5) | N/A | |
| Interferon (%) | 42 (2) | N/A | |
| Direct antiviral agents (%) | 4 (0.2) | N/A | |
| Cirrhosis staging systems (mean/median, IQR) | |||
| ALBI | − 2.6/− 2.7 (0.8) | − 2.5/− 2.6 (0.8) | 0.003 |
| ALBI-FIB-4 | − 2.8/− 3.1 (1.5) | − 2.6/− 2.9 (1.7) | < 0.001 |
| APRI | 1/0.6 (0.9) | 2/1.2 (1.8) | < 0.001 |
| CTP | 5.6/5 (1) | 5.5/5 (1) | 0.568 |
| FIB-4 | 3.6/2.6 (3.2) | 4.7/3.4 (4) | < 0.001 |
| Lok index | 0.6/0.6 (0.6) | 0.6/0.6 (0.4) | 0.096 |
| MELD | 9.6/7.8 (4.5) | 9/7.9 (3.5) | 0.281 |
| Outcomes | |||
| First decompensation | 0.013 | ||
| Ascites | 146 | 153 | |
| Hepatic encephalopathy | 122 | 107 | |
| Hepato-renal syndrome | 4 | 11 | |
| Variceal bleeding | 41 | 70 | |
| Decompensations during follow-up | |||
| Ascites (%) | 194 (10) | 207 (12) | 0.043 |
| Hepatic encephalopathy (%) | 164 (8) | 175 (10) | 0.065 |
| Hepato-renal syndrome (%) | 12 (0.6) | 26 (1) | 0.007 |
| Variceal bleeding (%) | 48 (2) | 80 (5) | < 0.001 |
INR, international normalized ratio; IQR, interquartile range; NAFLD, nonalcoholic fatty liver disease. Beta-blockers include Carvedilol, Nadolol, and Propranolol. Hepatitis B medications include Adefovir, Entecavir, Lamivudine, Telbivudine, and Tenofovir. Direct antiviral agents include Boceprevir, Daclatasvir, Harvoni, Simeprevir, and Telaprevir
Events of Decompensation Stratified by Etiologies
For all the enrolled patients, the average follow-up period was 524 days. (The mean was 218 with a standard deviation of 654 days.) The decompensation events at 1 year and 3 years are presented in Table 2. There were 165 (8%) non-viral and 167 (10%) viral cirrhosis patients who had decompensation within 1 year; and 273 (14%) non-viral and 293 (17%) viral cirrhosis patients had decompensation within 3 years. Overall, 312 and 337 patients with non-viral and viral cirrhosis, respectively, had decompensation during the follow-up period; and competing events were noted in 106 non-viral (91 patients died and 15 patients had liver transplantation) and 67 viral cirrhotic patients (41 patients died and 26 patients had liver transplantation). Among patients who had liver transplantation before decompensation, 22 patients had hepatocellular carcinoma (HCC) and 2 patients had cholangiocarcinoma. For both groups of patients, ascites was the most common presentation of decompensation in the early follow-up period, followed by hepatic encephalopathy (Table 2).
Table 2.
Events of decompensation at different time points stratified by the etiology of cirrhosis
| All patients (n = 3722) |
Non-viral (n = 1978) |
Viral (n = 1744) |
||||
|---|---|---|---|---|---|---|
| 1-year | 3-year | 1-year | 3-year | 1-year | 3-year | |
| Ascites | 175 | 329 | 86 | 159 | 89 | 170 |
| Hepatic encephalopathy | 162 | 292 | 84 | 143 | 78 | 149 |
| Decompensation | 332 | 566 | 165 | 273 | 167 | 293 |
| Transplantation | 26 | 57 | 9 | 19 | 17 | 38 |
| Died | 142 | 324 | 92 | 192 | 50 | 132 |
| Number at risk | 1578 | 478 | 767 | 213 | 811 | 265 |
Comparison of Cumulative Incidences of Decompensation Between Patients with Non‑viral and Viral Cirrhosis
Patients with interruption of insurance within 6 months before the event were removed (5 patients with non-vial cirrhosis and 14 patients with viral cirrhosis). The cumulative incidences of decompensation were similar between patients with non-viral and viral cirrhosis (P = 0.589, Fig. 2)
Fig. 2.
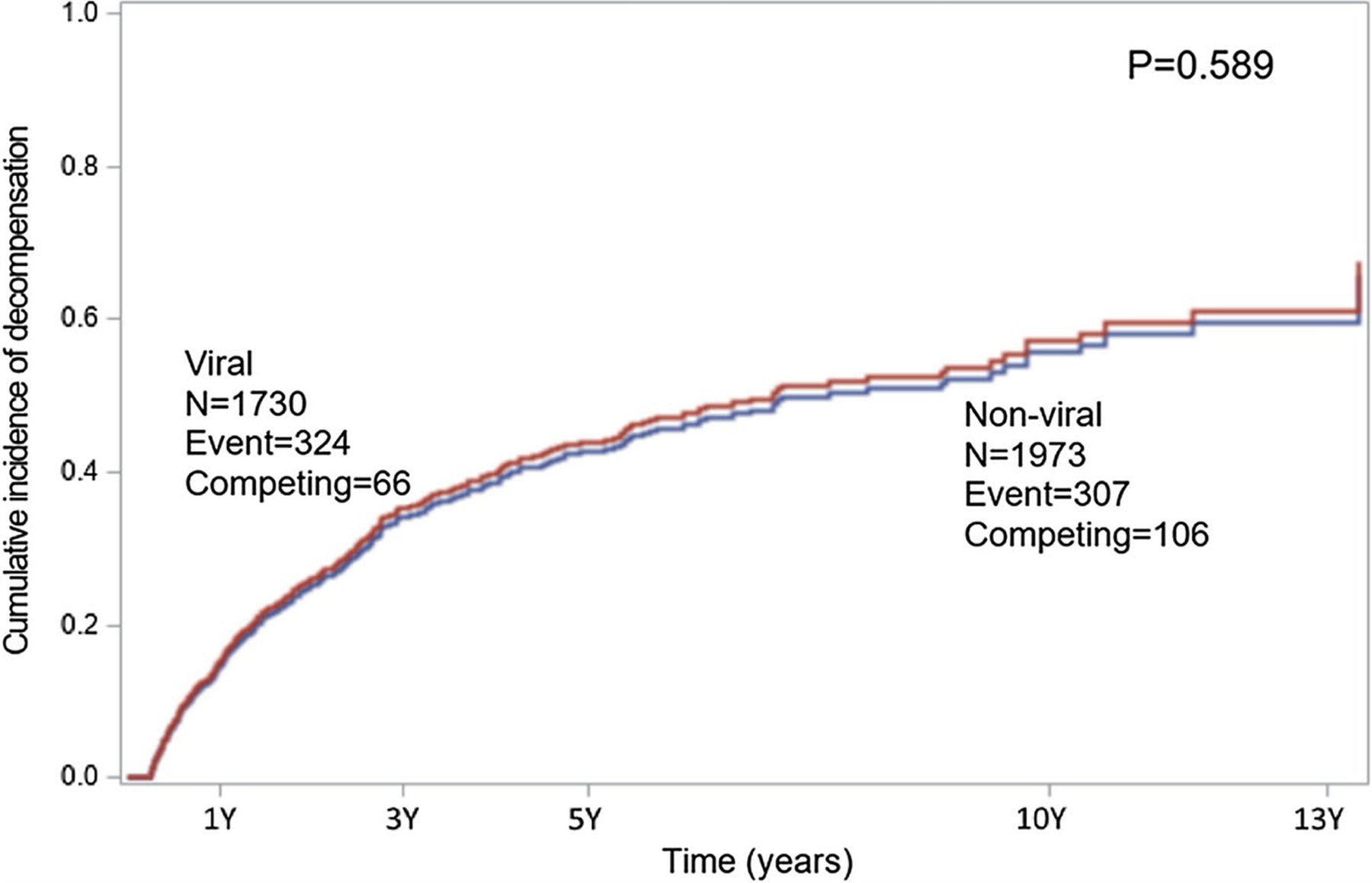
Distribution of cumulative incidence of decompensation stratified by non-viral and viral cirrhosis with Fine–Gray competing factor regression model. Patients with non-viral and viral cirrhosis had similar cumulative incidence of decompensation (P = 0.589)
Predictive Accuracy of Seven Noninvasive Models for Decompensation
For patients with non-viral cirrhosis, the ALBI-FIB-4 had the best predictive power among seven models for ascites, hepatic encephalopathy, and decompensation with all presentations at 1 year (Table 3). The FIB-4 had the highest AUCs for ascites, hepatic encephalopathy, and decompensation with all presentations prediction at 3 years. The performance for predicting overall decompensation was evaluated by the Uno’s c-statistic; the FIB-4 had the best c-statistic (0.692), which was significantly better than the c-statistics of other noninvasive models. All seven models had a sub-distribution hazard ratio (HR) larger than 1 with statistical significance in the Fine–Gray model (all P values < 0.001).
Table 3.
Predictive accuracy of noninvasive models for decompensation and hospital stay in patients with non-viral cirrhosis
| Non-viral cirrhosis (n = 1973) | AUC for the risk of ascites (95% CI) |
AUC for the risk of hepatic encephalopathy (95% CI) |
AUC for the risk of any decompensation (95% CI) |
Hazard ratio of decompensation (95% CI, P) | Concordance index (P)g | Hospital days IRR (95% CI, P)h | |||
|---|---|---|---|---|---|---|---|---|---|
| 1-yeara | 3-yearb | 1-yearc | 3-yeard | 1-yeare | 3-yearf | ||||
| ALBI | 0.657 (0.652–0.661) | 0.627 (0.623–0.632) | 0.785 (0.781–0.789) | 0.703 (0.697–0.709) | 0.708 (0.704–0.712) | 0.667 (0.661–0.673) | 1.88 (1.61–2.16, < 0.01) | 0.658 (< 0.01) | 1.21 (0.99–1.5, 0.07) |
| ALBI-FIB-4 | 0.68 (0.674–0.686) | 0.662 (0.657–0.668) | 0.806 (0.802–0.811) | 0.741 (0.736–0.746) | 0.734 (0.73–0.738) | 0.706 (0.701–0.711) | 1.22 (1.13–1.32, < 0.01) | 0.682 (< 0.01) | 1.04 (0.93–1.16, 0.51) |
| APRI | 0.628 (0.622–0.634) | 0.624 (0.62–0.629) | 0.706 (0.701–0.711) | 0.672 (0.667–0.677) | 0.672 (0.668–0.676) | 0.658 (0.652–0.663) | 1.12 (1.07–1.16, < 0.01) | 0.62 (< 0.01) | 0.98 (0.93–1.04, 0.58) |
| CTP | 0.613 (0.608–0.618) | 0.586 (0.581–0.59) | 0.739 (0.735–0.743) | 0.647 (0.642–0.652) | 0.661 (0.657–0.665) | 0.62 (0.616–0.623) | 1.29 (1.19–1.4, < 0.01) | 0.612 (< 0.01) | 1.35 (1.17–1.54, < 0.01) |
| FIB-4 | 0.673 (0.668–0.677) | 0.687 (0.682–0.692) | 0.763 (0.759–0.767) | 0.754 (0.75–0.759) | 0.717 (0.714–0.719) | 0.73 (0.725–0.736) | 1.03 (1.02–1.05, < 0.01) | 0.692 (< 0.01) | 0.98 (0.96–1.01, 0.23) |
| Lok index | 0.652(0.647–0.656) | 0.658 (0.653–0.663) | 0.79 (0.785–0.794) | 0.741 (0.735–0.746) | 0.721 (0.718–0.725) | 0.716 (0.711–0.721) | 7.81 (5.25–11.6, < 0.01) | 0.668 (< 0.01) | 1.46 (1.0–2.14, 0.05) |
| MELD | 0.634 (0.628–0.64) | 0.642 (0.637–0.648) | 0.748 (0.743–0.753) | 0.68 (0.674–0.685) | 0.69 (0.688–0.693) | 0.664 (0.66–0.67) | 1.05 (1.03–1.07, < 0.01) | 0.629 (< 0.01) | 1.09 (1.06–1.12, < 0.01) |
AUC area under the curve, CI confidence interval, IRR incidence risk ratio
AUC of ALBI-FIB-4 is significantly larger than AUCs of ALBI, APRI, CTP, Lok index, and MELD. There is no significant difference between ALBI-FIB-4 and FIB-4
AUC of FIB-4 is significantly larger than AUCs of other noninvasive models
AUC of ALBI-FIB-4 is significantly larger than AUCs of other noninvasive models
AUC of FIB-4 is significantly larger than AUCs of ALBI, APRI, CTP, FIB-4, Lok index, and MELD. There is no significant difference between ALBI-FIB-4 and FIB-4
AUC of ALBI-FIB-4 is significantly larger than AUCs of other noninvasive models
AUC of FIB-4 is significantly larger than AUCs of other noninvasive models
Concordance index of FIB-4 is significantly larger than concordance indexes of other noninvasive models
Akaike information criterion (AIC) of MELD (11,472) is smaller than the AIC of CTP (11,490); the MELD has the best predictive ability of hospitalization
The comparison of seven models for patients with viral cirrhosis is shown in Table 4. For the prediction for ascites at 1 year and 3 years and the prediction for hepatic encephalopathy at 1 year and 3 years, the ALBI-FIB-4 had the best predictive accuracy with AUCs between 0.694 and 0.792. For the prediction for all presentations of decompensation, the ALBI-FIB-4 had the best prognostic ability at 1 year and 3 years with AUCs between 0.741 and 0.754 and also provided the highest Uno’s c-statistic (0.681) for overall decompensation during follow-up time, which were significantly larger than the AUCs and c-statistics of other noninvasive models. All sub-distribution HRs were larger than 1, which predicted significantly increased risk of decompensation in the Fine–Gray model (P values between 0.011 and < 0.001).
Table 4.
Predictive accuracy of noninvasive models for decompensation and hospital stay in patients with viral cirrhosis
| Viral cirrhosis (n = 1730) | AUC for the risk of ascites (95% CI) |
AUC for the risk of hepatic encephalopathy (95% CI) |
AUC for the risk of any decompensation (95% CI) |
Hazard ratio of decompensation (95% CI, P) | Concordance index (P)g | Hospital days IRR (95% CI, P)h | |||
|---|---|---|---|---|---|---|---|---|---|
| 1-yeara | 3-yearb | 1-yearc | 3-yeard | 1-yeare | 3-yearf | ||||
| ALBI | 0.686 (0.682–0.69) | 0.717 (0.713–0.721) | 0.776 (0.771–0.78) | 0.787 (0.782–0.792) | 0.728 (0.725–0.731) | 0.739 (0.734–0.744) | 2.35 (1.98–2.78, < 0.01) | 0.672 (< 0.01) | 1.46 (1.15–1.85, < 0.01) |
| ALBI-FIB-4 | 0.694 (0.689–0.699) | 0.724 (0.719–0.728) | 0.78 (0.775–0.784) | 0.792 (0.788–0.796) | 0.741 (0.737–0.745) | 0.754 (0.751–0.757) | 1.29 (1.16–1.44, < 0.01) | 0.681 (< 0.01) | 1.09 (0.98–1.22, 0.11) |
| APRI | 0.617 (0.612–0.621) | 0.624 (0.618–0.63) | 0.669 (0.665–0.673) | 0.658 (0.652–0.663) | 0.646 (0.642–0.651) | 0.649 (0.644–0.653) | 1.04 (1.01–1.07, 0.011) | 0.614 (< 0.01) | 0.99 (0.95–1.03, 0.539) |
| CTP | 0.658 (0.653–0.663) | 0.666 (0.661–0.671) | 0.732 (0.728–0.736) | 0.733 (0.728–0.737) | 0.687 (0.683–0.691) | 0.686 (0.682–0.69) | 1.49 (1.36–1.64, < 0.01) | 0.628 (< 0.01) | 1.55 (1.3–1.85, < 0.01) |
| FIB-4 | 0.675 (0.67–0.68) | 0.692 (0.688–0.696) | 0.731 (0.725–0.738) | 0.735 (0.73–0.741) | 0.704 (0.701–0.709) | 0.719 (0.715–0.724) | 1.04 (1.02–1.07, < 0.01) | 0.66 (< 0.01) | 0.99 (0.97–1.02, 0.636) |
| Lok index | 0.684 (0.679–0.689) | 0.709 (0.704–0.713) | 0.741 (0.737–0.744) | 0.759 (0.754–0.764) | 0.71 (0.706–0.714) | 0.722 (0.718–0.726) | 9.4 (5.84–14.97, < 0.01) | 0.663 (< 0.01) | 1.17 (0.73–1.88, 0.511) |
| MELD | 0.632 (0.626–0.638) | 0.632 (0.627–0.638) | 0.698 (0.693–0.702) | 0.683 (0.678–0.688) | 0.656 (0.653–0.66) | 0.638 (0.634–0.642) | 1.08 (1.05–1.11, < 0.01) | 0.611 (< 0.01) | 1.13 (1.08–1.17, < 0.01) |
AUC area under the curve, CI confidence interval, IRR incidence risk ratio
AUC of ALBI-FIB-4 is significantly larger than AUCs of APRI, CTP, FIB-4, and MELD. There is no significant difference between ALBI, ALBI-FIB-4, and Lok index
AUC of ALBI-FIB-4 is significantly larger than AUCs of APRI, CTP, FIB-4, Lok index, and MELD. There is no significant difference between ALBI and ALBI-FIB-4
AUC of ALBI-FIB-4 is significantly larger than AUCs of APRI, CTP, FIB-4, Lok index, and MELD. There is no significant difference between ALBI and ALBI-FIB-4
AUC of ALBI-FIB-4 is significantly larger than AUCs of APRI, CTP, FIB-4, Lok index, and MELD. There is no significant difference between ALBI and ALBI-FIB-4
AUC of ALBI-FIB-4 is significantly larger than AUCs of other noninvasive models
AUC of ALBI-FIB-4 is significantly larger than AUCs of other noninvasive models
Concordance index of ALBI-FIB-4 is significantly larger than AUCs of other noninvasive models
Akaike information criterion (AIC) of MELD (8109) is smaller than the AICs of ALBI (8141) and CTP (8121); the MELD has the best predictive ability of hospitalization
Prediction for All‑Cause Hospital Stay After Cirrhosis Diagnosis
For patients with non-viral cirrhosis (1776 patients with admission record, mean days of hospitalization after enrollment were 12 [interquartile range = 10]), the CTP and MELD had significantly predictive accuracy for all-cause hospital stay after cirrhosis diagnosis (both P < 0.05, Table 3). The MELD had an incidence rate ratio (IRR) of 1.09 (95% CI 1.06–1.12, P < 0.05) with the lower Akaike information criterion (AIC) compared to the CTP.
For patients with viral cirrhosis (1489 patients with admission record, mean days of hospitalization after enrollment were 10 [interquartile range = 8]), the ALBI, CTP, and MELD had significant predictive ability for all-cause hospitalization (all P < 0.05, Table 4). The MELD had the lowest AIC with an IRR of 1.13 (95% CI 1.08–1.17, P < 0.05).
Discussion
Patients with compensated cirrhosis have a median survival of 10 or more years; in contrast, once decompensation develops, the median survival of patients with cirrhosis significantly reduces to 2 to 4 years [12, 13]. Recently, Guha et al. validated the use of combined noninvasive indices (ALBI and FIB-4) for the prediction of decompensation in 379 patients with largely viral or alcohol-related compensated cirrhosis. Our data extend this research in several ways. First, we examined a cohort severalfold larger (> 3700 patients with compensated cirrhosis). Second, we compared seven models for predicting decompensation accounting for the competing risk factors (death and transplantation before decompensation). Third, we distinguished predictions based on disease etiology highlighting estimates for persons with non-viral cirrhosis. Fourth, we evaluated predictions for all-cause hospitalization.
Choosing Models Based on the Outcome of Interest: First Decompensation
In our large cohort, the ALBI-FIB-4 and FIB-4 displayed significantly better predictive ability for decompensation and the MELD had the best predictive power for all-cause hospitalization. The FIB-4 has been extensively validated as a feasible tool to diagnose cirrhosis and shown to have consistent prognostic power to predict clinical outcomes for patients with different underlying liver diseases [14, 15]. The ALBI-FIB-4 and FIB-4 not only predicted decompensation at 1 year and 3 years, but the ALBI-FIB4 and FIB-4 consistently had a better predictive ability for long-term decompensation compared to others. These indices may be able to discern which patients with cirrhosis will remain compensated for more than 3 years [2]. The ALBI was designed initially to evaluate the severity of fibrosis in HCC patients and had been validated externally for patients with chronic liver disease, cirrhosis, and acute-on-chronic liver failure [16–18]. Low albumin is a known associated factor of ascites formation, which reasonably provides the advantage of ALBI for the prediction for ascites. However, the best AUCs of these seven models for ascites prediction at different time points were only 0.72, which could be related to the fact that the ascites formation is associated not only with portal hypertension but also with the influence of cardiac and renal dysfunctions. Of note, the CTP score inevitably lost some accuracy because patients in the final cohort had no ascites or hepatic encephalopathy; its predicting power might perform better for unselect cirrhotic patients.
Risk of Hospitalization
Cirrhosis is associated with longer hospital stay and a high readmission rate of patients admitted for non-liver diseases [7]. With the progression to decompensation, patients would have more frequent hospital visits and admission for liver and non-liver causes. We found that higher ALBI, CTP, and MELD scores were significantly associated with increased length of all-cause hospital stay per follow-up year in patients with viral cirrhosis; for patients with non-viral cirrhosis, higher CTP and MELD scores were significantly associated with longer hospital stay per year. Regardless of the etiologies of cirrhosis, the MELD had the most accurate prognostication (smallest AIC). Interestingly, the dominant predictive power of FIB-4-based models for decompensation was not found for predicting hospitalizations. The renal dysfunction included in the MELD could be the surrogate of progression of portal hypertension and end-organ hypoperfusion, which is considered the cornerstone of the prognostic power of MELD [19–21].
Impact of Etiology on Prediction
There were more than 1500 patients for both non-viral and viral groups in our study, which assures that our findings have generalizability for predicting patients with different underlying liver diseases from various geographical areas. Patients with viral cirrhosis were significantly younger, with higher liver enzymes and worse liver function markers (albumin, bilirubin, and platelet), and had more advanced fibrosis/cirrhosis based on the higher ALBI, ALBI-FIB-4, APRI, and FIB-4. Ninety-four percent of the patients had hepatitis C in the viral cirrhosis group; this finding is consistent with the known disease course of patients with hepatitis C, which causes chronic hepatitis and cirrhosis in approximately 50 to 85 and 20 to 30 percent of cases, respectively [22]. The numbers of non-viral and viral cirrhosis patients suffering from decompensation at 1 year and 3 years were similar, which was also displayed in the cumulative incidence of decompensation. Notably, fewer patients from the non-viral cirrhosis group had liver transplantation compared to the viral cirrhosis group, which could result from the higher prevalence of obesity, alcohol use, and other comorbidities.
Contextual Factors
One of the major strengths of this study is the Optum data, which have been utilized by independent research groups for various clinical topics [8, 23]. The Optum data include all medical claims of privately insured patients from all 50 states of the USA. The large patient cohort, diversity of basic demographics, and underlying liver disease support the conduct of studies focusing on specific complications of cirrhosis (ascites and hepatic encephalopathy) and overall decompensation stratified by non-viral and viral cirrhosis [24]. Additionally, with complete pharmacy records within the Optum, we can exclude all patients receiving loop diuretics, spironolactone, rifaximin, and lactulose at enrollment to better identify patients without decompensation as the base of our study cohort.
This study has a few limitations. First, this study is conducted with a commercial insurance database, which is usually paid by the employer in the USA. When patients’ cirrhosis or general medical condition deteriorates to a degree that patients could not keep their job, they may lose insurance coverage and the follow-up in this database. This feature prevents us from doing an accurate analysis of overall mortality. Second, most patients diagnosed with compensated cirrhosis were excluded from this study due to the lack of a full laboratory panel, which is an inevitable weakness of retrospective studies using an administrative dataset. Third, the diagnosis code of decompensation might not be updated timely if clinicians continued to provide care under the same ICD code of cirrhosis after complications developed. Fourth, the diagnosis criteria of cirrhosis and decompensation could not be completely standardized because of the nature of this insurance database study. Fifth, because of the method of NAFLD diagnosis applied in the study, some patients with cryptogenic cirrhosis would be coded as NAFLD. Last, hepatitis B and C viral statuses were not included in the analysis. Also, the management and outcome of hepatitis C patients have been significantly changed by the success of direct antiviral agents recently. The predictive accuracy of these seven noninvasive models for hepatitis C patients with cirrhosis after sustained viral response requires further study.
Conclusion
In conclusion, noninvasive models composed of routinely available laboratory tests can serve as a tool to predict the development of decompensation in patients with compensated cirrhosis. Among the seven current models, the FIB-4 and ALBI-FIB-4 had the best prognostic power to serve patients with non-viral and viral cirrhosis, respectively; however, due to the mediocre prediction of overall decompensation (AUCs less than 0.7), a modified model based on FIB-4 or identification of cutoff values of FIB-4-based models to predict decompensation is warranted in the future. On the other hand, the MELD showed the best predictive accuracy for the all-cause hospital stay. Altogether, our findings showed that the currently used noninvasive models could provide short- and long-term guidance of the clinical course of cirrhosis, which could help the healthcare system to select candidates for clinical trials and offer more individualized care plans for patients with compensated cirrhosis.
Supplementary Material
Funding
Elliot Tapper receives funding from the National Institutes of Health through the NIDDK (1K23DK117055–01A1).
Abbreviations
- ALBI
Albumin–bilirubin
- APRI
Aspartate aminotransferase-to-platelet ratio index
- CI
Confidence interval
- CTP
Child–Turcotte–Pugh
- FIB-4
Fibrosis-4
- HCC
Hepatocellular carcinoma
- HR
Hazard ratio
- MELD
Model for end-stage liver disease
Footnotes
Supplementary Information The online version of this article (https://doi.org/10.1007/s10620-020-06763-9) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards
Conflict of interest Neehar Parikh serves as a consultant for Bristol Myers-Squibb, Exact Sciences, Eli Lilly, Freenome, has served on advisory boards of Genentech, Easai, Bayer, Exelexis, Wako/Fujifilm, and has received research funding from Bayer, Target Pharmasolutions, Exact Sciences, and Glycotest. Elliot Tapper has served as a consultant to Novartis, Kaleido, Axcella, and Allergan, has served on advisory boards for Mallinckrodt, Rebiotix, and Bausch Health, and has received unrestricted research grants from Gilead and Valeant. The remaining authors indicate no potential conflicts of interest.
References
- 1.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G, Morabito A, D’Amico M, et al. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol Int. 2018;12:34–43. [DOI] [PubMed] [Google Scholar]
- 3.Tapper EB, Zhang P, Garg R, et al. Body composition predicts mortality and decompensation in compensated cirrhosis patients: A prospective cohort study. JHEP Rep. 2020;2:100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guha IN, Harris R, Berhane S, et al. Validation of a model for identification of patients with compensated cirrhosis at high risk of decompensation. Clin Gastroenterol Hepatol. 2019;17:e1. [DOI] [PubMed] [Google Scholar]
- 5.Hung TH, Hsieh MH, Lay CJ, et al. Increased occurrence of native septic arthritis in adult cirrhotic patients: a population-based three-year follow-up study in Taiwan. Prz Gastroenterol. 2014;9:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung TH, Hsieh YH, Tseng KC, et al. The risk for bacterial endocarditis in cirrhotic patients: a population-based 3-year follow-up study. Int J Infect Dis. 2013;17:e391. [DOI] [PubMed] [Google Scholar]
- 7.Parikh NS, Kamel H, Navi BB, et al. Liver fibrosis indices and outcomes after primary intracerebral hemorrhage. Stroke 2020:STROKEAHA119028161. [DOI] [PMC free article] [PubMed]
- 8.Mittal VS, Wu B, Song J, et al. Healthcare resource utilization and costs among nonvalvular atrial fibrillation patients initiating rivaroxaban or warfarin in skilled nursing facilities: a retrospective cohort study. Curr Med Res Opin. 2019;20:1. [DOI] [PubMed] [Google Scholar]
- 9.Allen AM, Therneau TM, Larson JJ, et al. Nonalcoholic fatty liver disease incidence and impact on metabotlic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36:4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uno H, Cai T, Pencina MJ, et al. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planas R, Montoliu S, Ballesté B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol 2006;4:1385–1394. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180–1193. [DOI] [PubMed] [Google Scholar]
- 14.Chen RC, Cai YJ, Wu JM, et al. Usefulness of albumin-bilirubin grade for evaluation of long-term prognosis for hepatitis B-related cirrhosis. J Viral Hepat. 2017;24:238–245. [DOI] [PubMed] [Google Scholar]
- 15.Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen B, Lin S. Albumin-bilirubin (ALBI) score at admission predicts possible outcomes in patients with acute-on-chronic liver failure. Medicine (Baltimore). 2017;96:e7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Zhang Z, Yan X, et al. Albumin-Bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig Liver Dis. 2019;51:1172–1178. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh YC, Lee KC, Wang YW, et al. Correlation and prognostic accuracy between noninvasive liver fibrosis markers and portal pressure in cirrhosis: role of ALBI score. PLoS One. 2018;13:e0208903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piano S, Romano A, Di Pascoli M, et al. Why and how to measure renal function in patients with liver disease. Liver Int. 2017;37:116–122. [DOI] [PubMed] [Google Scholar]
- 20.Piano S, Brocca A, Angeli P. Renal function in cirrhosis: a critical review of available tools. Semin Liver Dis. 2018;38:230–241. [DOI] [PubMed] [Google Scholar]
- 21.Fede G, D’Amico G, Arvaniti V, et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol. 2012;56:810–818. [DOI] [PubMed] [Google Scholar]
- 22.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 23.Alberts M, Chen YW, Lin JH, et al. Risks of Stroke and Mortality in Atrial Fibrillation Patients Treated With Rivaroxaban and Warfarin. Stroke 2019:STROKEAHA119025554. [DOI] [PMC free article] [PubMed]
- 24.Tapper EB, Hao S, Lin M, et al. The quality and outcomes of care provided to patients with cirrhosis by advanced practice providers. Hepatology. 2019;71:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


