Abstract
Study Purpose:
Lower urinary tract symptoms (LUTS) can occur in chronic pain populations at high rates and drastically affect quality of life. Hypnosis is a nonpharmacological treatment used in chronic pain known to have beneficial implications to health outside of pain reduction. This study evaluated the potential for hypnosis to reduce LUTS in a sample of individuals with chronic pain, if baseline LUTS severity affected outcomes, and specific LUTS that may respond to hypnosis.
Methods:
Sixty-four adults with chronic pain and LUTS at a level of detectable symptom change (American Urological Association Symptom Index, AUASI ≥ 3) participated in an 8-week group hypnosis protocol. Participants completed validated assessments of LUTS, pain, and overall functioning before, after, 3- and 6-months posttreatment. Linear mixed effects models assessed improvement in LUTS over time while accounting for known factors associated with outcome (e.g., age, gender). The interaction of baseline symptom severity and treatment assessed the potential effect of baseline symptoms on change scores.
Results:
Participants experienced significant and meaningful improvements in LUTS following group hypnosis (p = 0.006). There was a significant interaction between baseline symptom severity and treatment (p < 0.001), such that those with severe symptoms experienced the most pronounced gains over time (e.g., an 8.8 point reduction). Gains increased over time for those with moderate and severe symptoms. Changes in LUT symptoms occurred independently of pain relief.
Conclusions:
This pilot study suggests hypnosis has the potential to drastically improve LUTS in individuals with chronic pain, even when pain reduction does not occur. Results provide initial evidence for the treatment potential of hypnosis in urologic pain (and possibly non-pain/benign)populations, with randomized trials needed for definitive outcomes.
Keywords: chronic pain, complementary therapies, hypnosis, lower urinary tract symptoms, psychotherapy
1 |. INTRODUCTION
Lower urinary tract symptoms (LUTS) include urinary urgency, frequency, intermittency, hesitancy, weak stream, among other storage and voiding issues. LUTS increase with age and are expected to affect 42 million individuals in the United States by 2025.1 In a 2022 large community-based survey of adult women in the United States evaluating bladder health, over half of respondents reported LUTS and 52% being at least somewhat bothered by these symptoms.2 The impact of LUTS on quality of life for individuals with moderate to severe symptoms is substantial. LUTS interfere with partnerships, intimacy engagement, ability to work, and overall social functioning.3 Moreover, LUTS are accompanied by a significant degree of embarrassment and shame.3 In international epidemiological studies, the physical health impact of severe LUTS has been compared to that of having a heart attack or stroke.4 A 2018 review reported individuals with LUTS (overactive bladder) have 1.4–2-fold increase in annual healthcare expenses, with comorbidities being important drivers of costs.5
LUTS can co-occur with chronic pain conditions at high rates, particularly those involving widespread pain such as fibromyalgia, and other pain conditions such as irritable bowel syndrome and chronic pelvic pain. A recent study found a prevalence rate of 93% regarding LUTS in individuals with fibromyalgia.6 Together, the presence of both LUTS and fibromyalgia is associated with significantly poorer quality of life than living with fibromyalgia alone.6 Not surprisingly, the presence of LUTS in addition to chronic pain can further deteriorate a person’s functioning and quality of life.
Researchers have called for a multimodal approach to LUTS that stresses the importance of psychological intervention.7 Nonetheless, little work has assessed the potential for psychosocial interventions to alleviate LUTS burden or improve quality of life. One potential intervention is clinical hypnosis. Clinical hypnosis is a psychosocial treatment in which a provider guides a patient into a relaxed and focused state of attention, and then suggests changes in sensations, thoughts, emotions, or behaviors8 that can be incredibly powerful in coping with pain.9 Individuals subsequently learn to apply self-hypnosis as a “skill” through home practice to further enhance symptom coping. Few studies have examined hypnosis as a tool to manage LUTS, with existing studies limited by low sample sizes and limited follow-up.10 However, a recent study demonstrated that hypnosis is feasible and acceptable in urologic populations.11 Previous investigations also indicated that when compared to behavioral interventions alone, clinical hypnosis is superior in reducing overall overactive bladder symptoms.12 Thus, hypnosis may have a unique effect on LUTS.
This study aimed to evaluate the secondary impact of clinical hypnosis on LUTS in a sample of individuals with chronic pain conditions. We hypothesized that individuals with pain and co-morbid LUTS would have reduced urinary symptoms following hypnosis treatment. We then sought to explore how any potential treatment benefits may differ by baseline symptom severity. Lastly, we sought to assess whether posttreatment changes in LUTS may occur outside of pain relief in these individuals.
2 |. MATERIALS AND METHOD
The study methods are described elsewhere13 and are also reviewed below. This project was a planned secondary analysis of a pilot study evaluating group hypnosis applied to chronic pain conditions. The study was a single-arm, single-site pilot clinical trial (NCT#03384953) approved by the institutional review board in accordance with the declaration of Helsinki. A pre-test–posttest design evaluated the overall effect of group hypnosis on patient-reported outcomes across time before treatment, after, and at 3- and 6-months following treatment.
Individuals were invited to “opt into” research following referral to an 8-week group hypnosis treatment14 offered in a tertiary integrative medicine clinic at a large academic medical center. Participants were recruited within the integrative medicine clinic, at other outpatient sites across the medical center, online, and via ClinicalTrials.gov if self-referring to treatment. Potential participants were screened either at the point of care by referring providers or by phone using a structured screening form before service engagement. Screening followed an IRB-approved form14 containing 13 questions evaluating appropriateness for research participation. Questions covered factors such as pain, cognitive limitations, suicidality, mental health, hospitalization history, and current opioid use. Eligible participants were contacted by a member of the research team to gain consent and arrange appointments. The clinic provided services regardless of whether patients participated in the study or not (see Figure 1).
FIGURE 1.
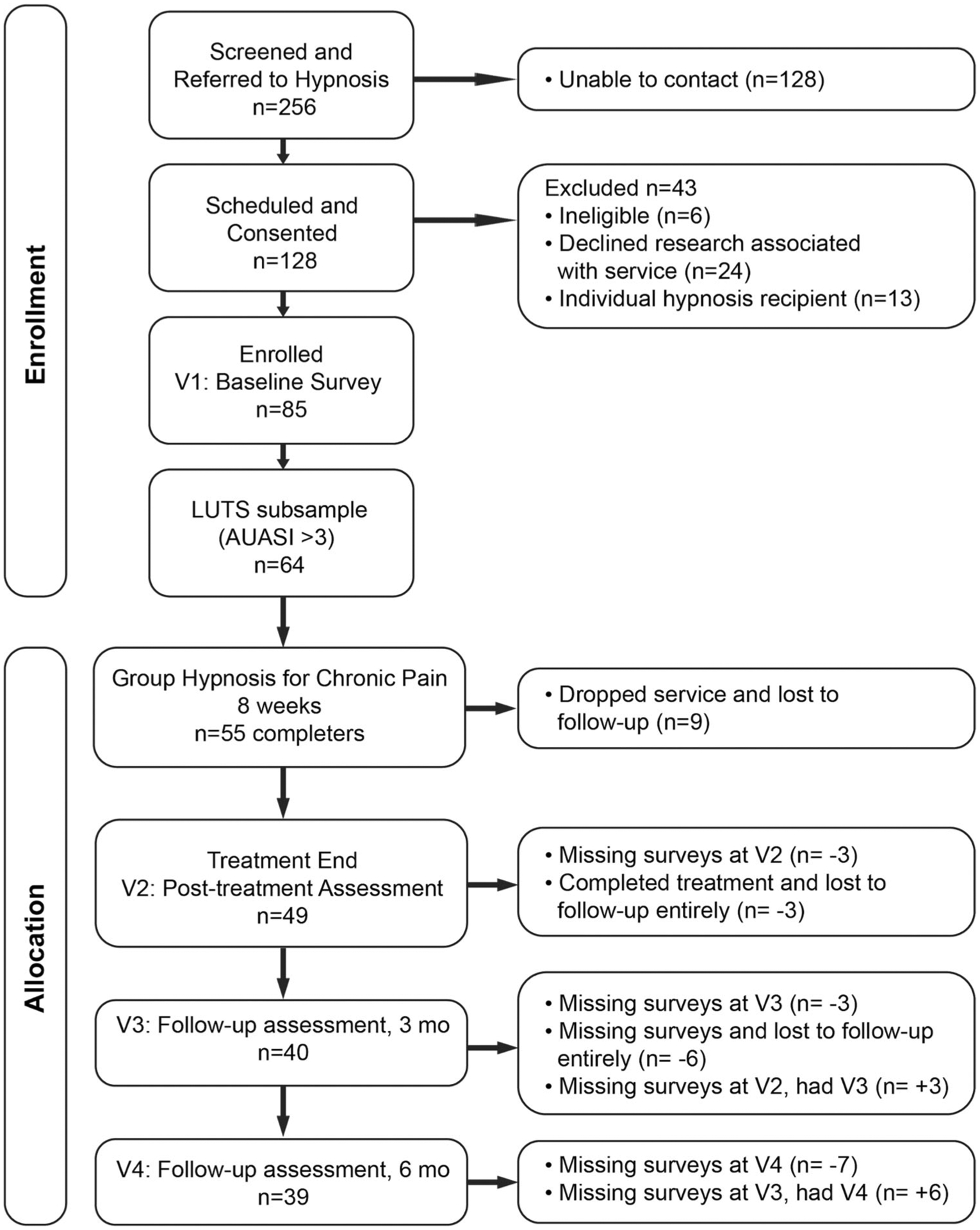
Study flow. LUTS, lower urinary tract symptoms.
Inclusion criteria for the study were broad in an attempt to reflect patients seen in routine clinical practice. Individuals eligible for study participation were English-speaking adults with chronic pain as defined by pain persisting for 6 months or longer at a level of ≥4/10 on a Numeric Rating Scale.15 Participants reporting cognitive limitations, history of audio/visual hallucinations, psychiatric hospitalizations for reasons other than suicidality/homicidal ideation or posttraumatic stress, severe emotional distress, or taking large amounts of opiates (e.g. ≥ 120 mg morphine equivalent dose daily) were not eligible for research participation. The presence of these factors has the potential to interfere with the ability to reliably complete assessment measures, experience a hypnotic induction, or tolerate group interaction. In some cases, alternative referrals were made to best suit patient needs, such as to individual psychotherapy.
Consenting participants agreed to complete a short battery of validated self-reported assessments at four separate timepoints over eight months. These assessments were offered online and on paper and stored in a secure electronic research database.16 Participants completed assessments the week before service initiation, and within approximately seven days of each follow-up timepoint.
2.1 |. Baseline variables and outcomes of interest
For this sub-analysis specifically, the primary outcome of interest was the American Urologic Association Symptom Index.17 Participants also completed assessments evaluating current pain symptoms such as the Brief Pain Inventory (BPI)18 and Patient-Reported Outcome Measurement Information System19 (PROMIS) pain interference scales. The Michigan Body Map (MBM)20 assessed pain extent and Pain Catastrophizing Scale (PCS)21 measured pain-related distress. The Harvard Group Scale of Hypnotic Susceptibility22 (HGSHS) assessed hypnotizability levels posttreatment. Lastly, the PROMIS Global Health—2a23 assessed overall perception of physical health. These measures are detailed below to provide sample characteristics.
2.2 |. Intervention description
Participants completed an 8-session structured hypnosis protocol.14 Each group session lasted 90 minutes. Sessions contained brief psychoeducation, dedicated time to process and troubleshoot home practice experiences, and a group hypnotic induction containing varied suggestions each week. Participants received weekly recordings to practice between appointments. Suggestions spanned important life domains affected by pain, such as increased comfort, reduced pain, better sleep, greater energy levels, enhancing relaxation, addressing negative thoughts, and obtaining distance from discomfort. Within the standardized protocol, treatment allowed for flexibility to individualize experiences and adapt to a person’s circumstances. This occurred via facilitators eliciting comforting imagery from each patient to integrate into inductions and offering varied and nuanced experiences within suggestions to enhance inductions.13 As pain sources and patterns varied, group discussion included exploration of applications of hypnosis practice to individual circumstances, such as managing pain flares or situations that can exacerbate discomfort. Examples of individual applications may include a person with painful urgency applying hypnosis before or during voiding, or an individual with widespread pain using hypnosis in the morning before moving to reduce muscle stiffness. For a detailed description of the intervention and suggestions given, please see McKernan et al.13
2.3 |. Statistical analysis
Linear mixed effects (LME) models were used to estimate and test all hypotheses. All models included a random intercept for each participant to account for repeated measurements. To investigate the hypothesis that hypnosis reduced LUTS, AUASI (American Urological Association Symptom Index) scores were modeled as a function of time (coded categorically: pretreatment, posttreatment, 3 months, 6 months), which allowed the capture of nonlinear changes in the AUASI scores over time. To investigate whether baseline LUTS moderated the treatment effect, an interaction between time by baseline AUASI score was added to the model. Baseline LUTS symptoms were categorized before modeling the interaction by using the following predetermined AUASI severity criteria:17 scores ≤ 7 as mild, 8–19 as moderate, and >19 as severe. The models controlled for participants’ age, gender, past hypnosis experience, and pain intensity. Post hoc, we also explored the effect that pain catastrophizing had on any observed outcomes. To determine which components of the AUASI score contributed to treatment effects on the total score, eight exploratory analyses using the same LME model structure were performed including each symptom as the outcome variable. Multiple comparisons were adjusted for across these eight models using the Benjamini–Hochberg procedure.24 Hypothesis tests were performed for all models using approximate χ2 statistics with Type 2 sum of squares, so that main effects are interpreted without their higher order interactions. This study was a registered clinical trial powered to detect differences in pain intensity as measured by the Brief Pain Inventory.18 The current analyses were not a part of the primary aims of the study design, so a power analysis was not performed for the AUASI.
3 |. RESULTS
3.1 |. Sample characteristics
Participants with a chronic pain diagnosis were recruited for eight sessions of group hypnosis between June 2017–September 2019. A total of 11 group cohorts were run, ranging from 5 to 10 patients, Mode = 8. Of the 85 individuals who completed the program, 64 (75%) reported LUTS at a level of detectable change and were included in the sample for analysis (>3, Figure 1).25 Of those enrolled, 86% completed treatment (n = 55), defined as completing at least four of the eight hypnosis sessions. Participants attended approximately six sessions on average (mean [SD] = 5.70 [1.87]).
Baseline characteristics of the subsample (Table 1) indicate 70% were female (n = 45) and had a median age of 51.5 years (interquartile range [IQR]: 42–61), 86% had some college education or higher (n = 55), and a majority were married, and retired or unable to work. Less than one-third of participants had previous experience with hypnosis (n = 18). The median pain intensity score (BPI) was 5.3 (IQR: 4.3–6.9). Overall, the sample reported moderate LUTS (mean AUASI = 12.9) at baseline, with 26.6% reporting mild (n = 17), 57.8% moderate (n = 37), and 15.6% severe LUTS (n = 10, Table 1). Participants endorsed high levels of diffuse pain, averaging approximately 14 sites of chronic pain across the body (MBM). Participants had moderate to severe pain interference (mean PROMIS-PI-6a = 23.9 ± 5.1), and a lower perception of physical health (mean PROMIS-GH-2a = 5.4 ± 1.6) than the general population due to their symptoms. The mean pain catastrophizing score (mean PCS = 23.3 ± 11.7) trended slightly above the mean for the measure (20), although did not reach the threshold of clinical concern (≥30).26 On average, the sample had moderate hypnotic susceptibility (mean HGSHS = 7.4).
TABLE 1.
Baseline characteristics of participants (N = 64)a
| N | Overall (proportion) | |
|---|---|---|
| Gender: Female | 64 | 45 (0.70) |
| Race/Ethnicity | 64 | |
| African American/Black | 2 (0.03) | |
| Asian/Pacific Islander | 2 (0.03) | |
| Hispanic/Latino | 3 (0.05) | |
| White, non-Hispanic | 55 (0.86) | |
| Other | 2 (0.03) | |
| Education level | 64 | |
| High school diploma or equivalent | 3 (0.05) | |
| Vocational/Technical school | 2 (0.03) | |
| Some college | 19 (0.30) | |
| Bachelor’s degree | 18 (0.28) | |
| Master’s degree | 13 (0.20) | |
| Doctorate or Professional degree | 5 (0.08) | |
| Other | 4 (0.06) | |
| Marital status | 63 | |
| Single, never married | 9 (0.14) | |
| Married or in a domestic partnership | 36 (0.57) | |
| Divorced | 14 (0.22) | |
| Widowed | 4 (0.06) | |
| Employment status | 64 | |
| Employed full-time | 18 (0.28) | |
| Employed part-time | 7 (0.11) | |
| Self-employed | 1 (0.02) | |
| Unemployed | 3 (0.05) | |
| Retired | 13 (0.20) | |
| Unable to work | 22 (0.34) | |
| Had past experience with hypnosis | 63 | 18 (0.29) |
| Lower Urinary Tract Symptoms (AUASI15) | 64 | |
| Mild (0–7) | 17 (0.27) | |
| Moderate (8–19) | 37 (0.58) | |
| Severe (>19) | 10 (0.16) | |
| N | Median (IQR), Mean ± SD | |
| Age | 64 | 51.5 (42–61), 51.7 ± 13.6 |
| Pain intensity (BPI)16 | 64 | 5.3 (4.3–6.9), 5.6 ± 1.5 |
| Pain catastrophizing (PCS)19 | 59 | 23.0 (15.0–33.7), 23.3 ± 11.7 |
| Hypnotizability (HGSHS)20 | 44 | 7.5 (5.0–10.0), 7.4 ± 2.9 |
| Pain extent (MBM)18 | 64 | 8.5 (4.0–17.0), 13.8 ± 14.4 |
| Lower urinary tract symptoms (AUASI)15 | 64 | 11.5 (7.0–17.0), 12.9 ±6.8 |
| N | Median (IQR), Mean ± SD | |
| Global Health (PROMIS-GH-2a)21 | 64 | 6.0 (4.0–6.0), 5.4 ± 1.6 |
| Pain Interference (PROMIS-PI-6a)17 | 63 | 24.0 (21.0–28.0), 23.9 ± 5.1 |
Abbreviations: AUASI, American Urological Association Symptom Index; HGSHS, Harvard Group Scale of Hypnotic Susceptibility; IQR, interquartile range; MBM, Michigan Body Map; PCS, Pain Catastrophizing Scale; PROMIS, Patient-Reported Outcome Measurement Information System.
N reported is the number with non-missing values.
LME models assessed the effect of hypnosis on AUASI score after treatment. Across the whole sample, model results showed that average AUASI decreased by 2.1 points at posttreatment compared to pretreatment, by 3.9 points at 3 months, and by 4.2 points at 6 months (Table 2). The second model included an interaction effect between baseline symptom severity level and time point. This revealed a significant interaction (χ2 = 35.0, df = 6, p < 0.001), suggesting that the pattern of change over time differed by baseline symptom severity. Participants with higher baseline severity had higher AUASI across all time points (χ2 = 87.7, df = 2, p < 0.001). Initial changes in AUASI at posttreatment did not significantly differ by severity level when compared to pretreatment (Table 3). However, differences emerged at both 3- (Moderate: CI = [−6.97, −0.18], p = 0.039; Severe: CI = [−13.37, −3.12], p = 0.002) and 6-(Moderate: CI = [−12.21, −5.21], p < 0.001; Severe: CI = [−16.77, −6.90], p < 0.001) month follow-up timepoints for the moderate and severe groups (Figure 2). Reductions in AUASI were most pronounced in the severe group. Specifically, the moderate group showed an average decrease of 4.2 points at 3 months and an average decrease of 5.6 points at 6 months, while the severe group showed an average decrease of 8.9 points at 3 months and a decrease of 8.8 points at 6 months compared to pretreatment. There was not evidence for decreases in AUASI in the low severity group. When later exploring the effect of pain catastrophizing on outcomes as a covariate, model results remained unchanged. In this model, baseline pain catastrophizing levels (χ2 = 13.4 df = 1, p < 0.0001) and age (χ2 = 7.5 df = 1, p = 0.006) were associated with higher AUASI scores.
TABLE 2.
Linear mixed effects model of overall sample (without interaction effect) predicting AUASI
| Predictors | Estimates | CI | P |
|---|---|---|---|
| (Intercept) | 4.36 | −1.53 to 10.24 | 0.147 |
| Age | 0.07 | −0.01 to 0.14 | 0.105 |
| Gender (reference: Male) | |||
| Female | −0.91 | −3.17 to 1.36 | 0.433 |
| Previous experience with hypnosis hypnosis (eference: No) | |||
| Yes | −0.35 | −2.75 to 2.04 | 0.773 |
| Pain intensity (BPI) | 0.11 | −0.35 to 0.57 | 0.639 |
| Time (reference: Pre) | |||
| Post | −2.13 | −3.64 to −0.62 | 0.006 |
| 3 months | −3.89 | −5.53 to −2.26 | <0.001 |
| 6 months | −4.19 | −5.85 to −2.54 | <0.001 |
| AUASI baseline category (reference:Mild) | |||
| Moderate | 4.95 | 2.59–7.31 | <0.001 |
| Severe | 15.74 | 12.39–19.09 | <0.001 |
Abbreviation: AUASI, American Urological Association symptom index; BPI, Brief Pain Inventory; CI, confidence interval.
TABLE 3.
Linear mixed effects model with interaction between time and baseline LUTS severity predicting AUASI
| Predictors | Estimates | CI | P | |
|---|---|---|---|---|
| (Intercept) | 2.29 | −3.61 to 8.18 | 0.447 | |
| Age | 0.06 | −0.02 to 0.14 | 0.117 | |
| Gender (reference: Male) | ||||
| Female | −0.91 | −3.16 to 1.34 | 0.427 | |
| Previous experience with hypnosis (reference: No) | ||||
| Yes | −0.5 | −2.88 – 1.88 | 0.68 | |
| Pain intensity (BPI) | 0.15 | −0.29 to 0.59 | 0.502 | |
| Time (reference: Pre) | ||||
| Post | −0.73 | −3.21 to 1.75 | 0.564 | |
| 3 months | −0.64 | −3.55 to 2.28 | 0.668 | |
| 6 months | 3.07 | −0.01 to 6.16 | 0.051 | |
| AUASI baseline category (reference: Mild) | ||||
| Moderatea | 7.42 | 4.61–10.24 | <0.001 | |
| Severea | 19.32 | 15.50–23.14 | <0.001 | |
| Time × AUASI baseline category | ||||
| Post × AUASI moderate | −1.66 | −4.63 to 1.31 | 0.273 | |
| 3 months × AUASI moderate | −3.57 | −6.97 to −0.18 | 0.039 | |
| 6 months × AUASI moderate | −8.71 | −12.21 to −5.21 | <0.001 | |
| Post × AUASI severe | −2.49 | −7.12 to 2.13 | 0.29 | |
| 3 months × AUASI severe | −8.24 | −13.37 to −3.12 | 0.002 | |
| 6 months × AUASI severe | −11.83 | −16.77 to −6.90 | <0.001 | |
Abbreviation: AUASI, American Urological Association symptom index; BPI, Brief Pain Inventory; CI, confidence interval; LUTS, lower urinary tract symptoms.
FIGURE 2.
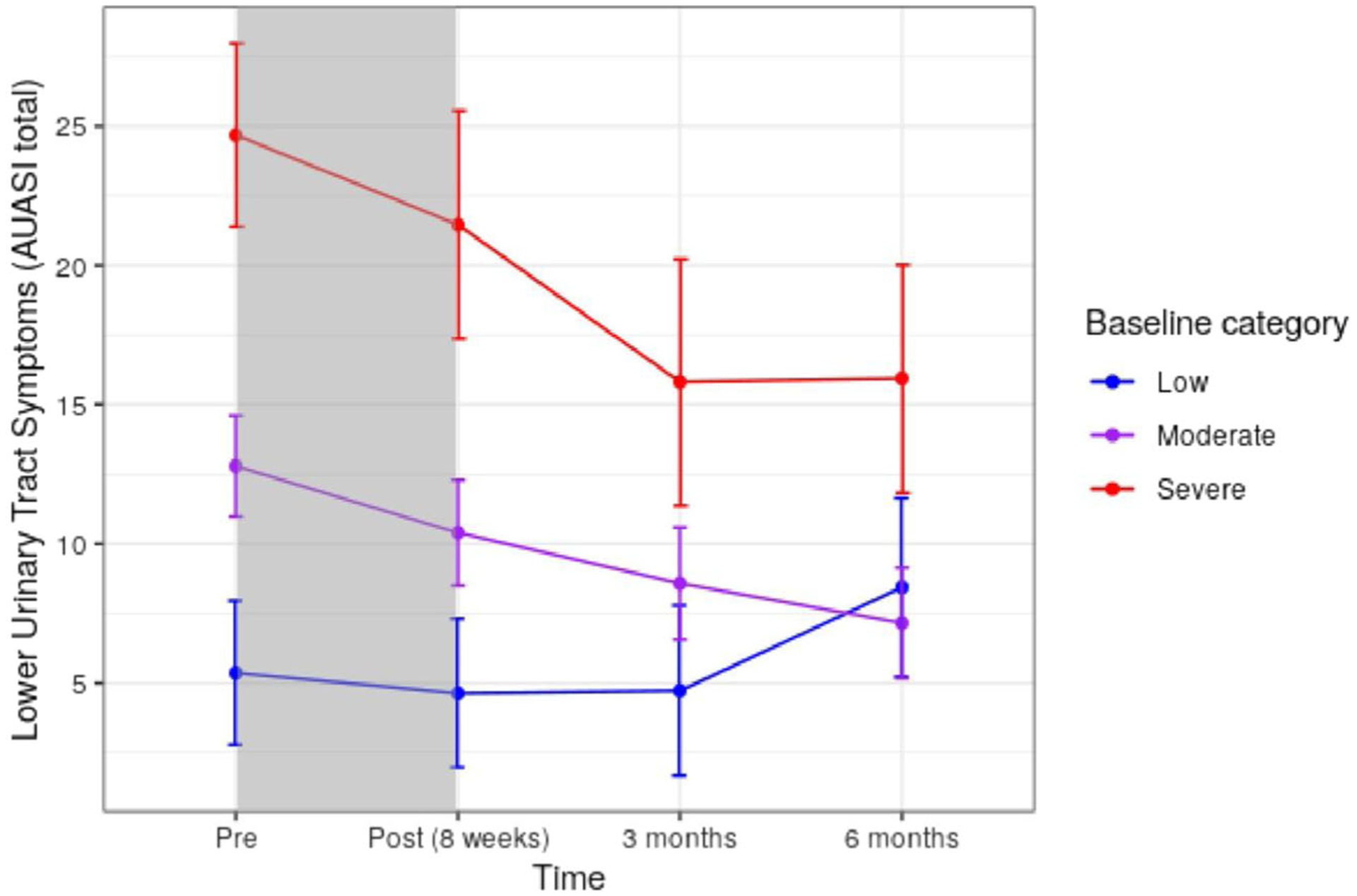
Predicted changes in LUTS over time by baseline symptom severity. LUTS categories defined by AUASI criteria,15 total score of mild ≤7 (n = 17), moderate 8–19 (n = 37), severe ≥20 (n = 10). Shaded area indicates treatment phase. Predictions estimate values for a participant who is female, with no hypnosis experience, of average age, and average pain intensity. AUASI, American Urological Association symptom index; LUTS, lower urinary tract symptoms.
The main effects mixed effects model was fit using each of the AUASI subscales as the outcome to understand what features were driving changes in the total score over time. These exploratory models showed that after controlling for age, gender, and past hypnosis, there were differences in frequency, intermittency, urgency, and weak stream over time (all adjusted p < 0.01). Baseline covariates were not predictive of symptom scores. Model results are presented in Table 4.
TABLE 4.
P values predicting changes in specific LUTS across all timepointsa
| Variable | Incomplete emptying | Frequency | Intermittency | Urgency | Weak stream | Straining | Nocturia | QOL |
|---|---|---|---|---|---|---|---|---|
| Age | 0.57 | 0.97 | 0.57 | 0.53 | 0.53 | 0.53 | 0.53 | 0.53 |
| Gender | 0.79 | 0.79 | 0.79 | 0.23 | 0.23 | 0.65 | 0.98 | 0.79 |
| Past Hypnosis | 0.81 | 0.81 | 0.81 | 0.49 | 0.49 | 0.81 | 0.81 | 0.81 |
| BPI | 0.88 | 0.88 | 0.87 | 0.88 | 0.88 | 0.52 | 0.52 | 0.52 |
| Time | 0.10 | <0.01 | <0.01 | <0.01 | <0.01 | 0.10 | 0.25 | 0.14 |
Abbreviations: BPI, Brief Pain Inventory; LUTS, lower urinary tract symptoms; QOL, quality of life.
P values adjusted for false discovery rates in accordance with Benjamini and Hochberg (1995).
4 |. DISCUSSION
This pilot study demonstrated that individuals with chronic pain and concurrent LUTS who participated in group clinical hypnosis reported significant improvement in urinary symptoms, with clinically meaningful gains maintained over a six-month period. Treatment gains exceeded the threshold of clinical significance for LUTS. Notably, reduction in LUTS posttreatment occurred outside of any potential pain reduction, prior hypnosis experience, and in controlling for the potential effect of age or gender on symptoms. Treatment gains significantly differed by baseline symptom severity, where the most substantial gains occurred in individuals with moderate and severe baseline LUTS. Regarding patterns of change, for these individuals, further improvement occurred at 3-month follow-up. Symptom improvement stabilized at 6 months for persons with severe LUTS, and slightly improved further for individuals with moderate LUTS. Those with mild symptoms did not have significant gains over time. An exploratory analysis of symptom-level changes indicated that urologic symptoms most improved following hypnosis include frequency, intermittency, urgency, and weak stream.
The relevance of these reported treatment gains to patients’ lives warrants consideration. The threshold of clinically meaningful change in treatments for LUTS (adopted as a threshold for any treatment approved by the FDA) was established at ≥ 3.1 points long ago.25 However, additional studies have noted that baseline symptom severity may affect the meaning of a 3-point change to a person with mild versus severe symptoms. Subsequent investigations considering symptom severity indicate changes of 1–1.9 points (mild), 2–3 points (moderate), and 6–6.1 points (severe)25,27 correspond with reports of meaningful symptom improvement to patients. This study suggests that meaningful change in symptoms can occur following hypnosis. Total gains reported by groups (e.g., −5.6 for moderate severity, −8.8 for severe at 6 months, see Table 3) parallel reports of moderate and “much better” perceived symptom improvement in large-scale studies.25
The potential of hypnosis to reduce LUTS independent of pain relief is important for two reasons. First, this study provides preliminary evidence that with hypnosis, individuals can make significant changes to a cluster of urinary symptoms that can negatively affect individuals with chronic pain. Some individuals with chronic pain may not experience pain reduction with treatment. For many, positive change can be viewed in terms of how a given treatment may affect a person’s overall functioning, including managing secondary symptoms that may make pain harder to live with. This study’s indication that hypnosis can have secondary benefits to participants outside of pain relief (LUTS) also coincides with previous research that hypnosis can produce significant long-term benefits to sleep, mood, and energy levels after hypnosis, whether pain reduction occurs.10
Second, given the symptom reductions observed in our sample (e.g., close to 9 points for at 6 months), study findings suggest that hypnosis may be appropriate more broadly for individuals struggling with dysfunctional voiding. Applications of hypnosis to individuals with overactive bladder (OAB), idiopathic retention, or nocturia symptoms may be of particular interest. In related conditions, an initial pilot trial of hypnosis for OAB,13 and recent larger study with long-term follow-up indicated that women with urgency urinary incontinence can benefit substantially from hypnosis, with benefits non-inferior to that of medications at 12 months.10 The authors noted some difference in the rate of change between groups, where those with medication responded at faster rates initially, with equal gains at 6 and 12 months. Our study results suggest that gains may continue to evolve posttreatment for some patients. Throughout treatment, patients are instructed to practice self-hypnosis or recordings outside of sessions for continued benefit. Combined with our initial results, this may suggest that hypnosis requires additional practice and time (e.g., >2 months) to actualize its full benefits.
There is increasing recognition of the influence of psychological factors in how individuals experience, react to, and cope with LUTS.3 While psychological interventions for LUTS are relatively new to urology,4,8 hypnosis may be one of many available nonpharmacological treatment strategies that can improve LUTS self-management.
This study builds upon existing research by demonstrating the additional secondary benefit of hypnosis for LUTS in persons with chronic pain. Study strengths include evaluating the intervention in a representative sample of patients with diverse and complex clinical presentations and longstanding symptoms13 akin to those seen in routine clinical practice. The study also had an extended follow-up period to assess for potential maintenance of gains. The sample size exceeded that of most studies of hypnosis and LUTS, where case reports and case series predominate existing literature.12 The protocol involved a manualized, evidence-based treatment with sessions occurring in person. In gastrointestinal disorders, scripted session-by-session approaches have demonstrated significant impact even in those with severe refractory symptoms.28 Other hypnosis modes of delivery include online, app-based, telephone-delivered, or self-guided intervention. New evidence supports that hypnosis in these flexible formats is both feasible and may have comparable outcomes. Future studies must assess the optimal dose of in-person sessions required to obtain meaningful and durable symptom change.
Any conclusions drawn by study findings are limited by our single-arm, pre-test–posttest design. Due to this pilot trial studying a convenience sample of an ongoing clinical service, randomization or structuring a control group was not possible. Although we had a high rate of research enrollment, only half of individuals referred to the service made contact for scheduling. This could be due to logistical factors such as insurance, availability, or scheduling barriers, as the group required an 8-week commitment at pre-set times. It is unclear how information provided during referrals or participant attitudes toward hypnosis may have impacted service engagement. Our sample was predominately white and educated, which may limit generalizability. We did not collect data on medication intake, other urologic intervention during the study period, duration of LUTS symptoms, nor exclude conditions that may affect findings such as neurogenic bladder. Although we are encouraged by study findings standing after controlling for known factors associated with treatment outcome, additional unmeasured confounding factors such as hormonal changes, other benign urologic conditions, bladder medicines, and anxiety may also affect study findings. Group psychotherapy treatments can have high attrition rates (e.g., 20%). An initial analysis of individuals who completed or dropped treatment revealed no significant differences in baseline demographic or clinical variables.14 While the intervention had a high completion rate (86%), we lost an additional 6% to follow-up at posttreatment and had variable follow-up from some participants who remained in the study (i.e., partial data). This variable survey completion may be in part due to inadequate follow-up communication methods of calling and emailing participants, as participants increasingly respond to text messaging. We assessed LUTS at only three timepoints, where symptoms can fluctuate. This could have affected assessment of severity and null findings regarding our mild LUTS group, which may reflect a regression toward the mean. We did not reliably capture practice effects, which could have provided insight into patients’ continued symptom improvements posttreatment. Future studies may consider stratifying by symptom severity, and assessing baseline LUTS at multiple timepoints to accurately ascertain symptom clusters. Replication of findings within a larger sample of individuals with severe LUTS is also suggested. Studies designed to capture daily practice, account for potential confounders such as concurrent treatment, evaluate the potential role of hormones, and assess mediating or moderating factors associated with outcomes (e.g., practice effects, anxiety) may help overcome these limitations.
5 |. CONCLUSION
Hypnosis training may be an intervention option for treating co-occurring LUTS and chronic pain to complement other treatments. Previous studies note hypnosis can be a helpful nonpharmacological treatment option for LUTS.12,13 With concurrent chronic pain, our pilot study suggests hypnosis can improve LUTS even when pain reduction does not occur. Although our study design limits any definitive conclusions, at a minimum, this study supports the need for additional investigation and randomized trials.
ACKNOWLEDGMENTS
This manuscript was prepared with support from the Vanderbilt University Medical Center CTSA award No. UL1TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Funding information
CTSA UL1TR002243
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This work is derived from a registered clinical trial (NCT#03384953) of an institutionally approved study with consenting participants (IRB#170652).
DATA AVAILABILITY STATEMENT
Data may be made available upon written request with appropriate data use agreements consistent with institutional policy. All authors have had access to, contributed to, read, reviewed, and agreed upon the content of this submission.
REFERENCES
- 1.Litman HJ, McKinlay JB. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int. 2007;100(4):820-825. doi: 10.1111/j.1464-410X.2007.07018.x [DOI] [PubMed] [Google Scholar]
- 2.Smith AL, Chen J, Wyman JF, et al. Survey of lower urinary tract symptoms in United States women using the new Lower urinary tract dysfunction research Network-Symptom Index 29 (LURN-SI-29) and a national research registry. Neurourol Urodyn. 2022;41(2):650-661. doi: 10.1002/nau.24870 [DOI] [PubMed] [Google Scholar]
- 3.Kinsey D, Pretorius S, Glover L, Alexander T. The psychological impact of overactive bladder: a systematic review. J Health Psychol. 2016;21(1):69-81. doi: 10.1177/1359105314522084 [DOI] [PubMed] [Google Scholar]
- 4.Robertson C, Link CL, Onel E, et al. The impact of lower urinary tract symptoms and comorbidities on quality of life: the BACH and UREPIK studies. BJU Int. 2007;99(2):347-354. doi: 10.1111/j.1464-410X.2007.06609.x [DOI] [PubMed] [Google Scholar]
- 5.Powell LC, Szabo SM, Walker D, Gooch K. The economic burden of overactive bladder in the United States: a systematic literature review. Neurourol Urodyn. 2018;37(4):1241-1249. doi: 10.1002/nau.23477 [DOI] [PubMed] [Google Scholar]
- 6.de Araújo MP, Faria AC, Takano CC, et al. Urodynamic study and quality of life in patients with fibromyalgia and lower urinary tract symptoms. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(8):1103-1107. doi: 10.1007/s00192-008-0577-z [DOI] [PubMed] [Google Scholar]
- 7.Bavendam TG, Norton JM, Kirkali Z, et al. Advancing a comprehensive approach to the study of lower urinary tract symptoms. J Urol. 2016;196(5):1342-1349. doi: 10.1016/j.juro.2016.05.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kihlstrom JF. Hypnosis. Annu Rev Psychol. 1985;36(1): 385-418. doi: 10.1146/annurev.ps.36.020185.002125 [DOI] [PubMed] [Google Scholar]
- 9.Jensen MP, Patterson DR. Hypnotic approaches for chronic pain management: clinical implications of recent research findings. Am Psychol. 2014;69(2):167-177. doi: 10.1037/a0035644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komesu YM, Schrader RM, Rogers RG, Sapien RE, Mayer AR, Ketai LH. Hypnotherapy or medications: a randomized noninferiority trial in urgency urinary incontinent women. Am J Obstet Gynecol. 2020;222(2):159.e1-159.e16. doi: 10.1016/j.ajog.2019.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano AJ, Schnur JB, Harvie HS, Newman DK, Montgomery GH, Arya LA. Pilot randomized controlled trial of a hypnosis intervention for women with bladder pain syndrome. Neurourol Urodyn. 2021;40(8):1945-1954. doi: 10.1002/nau.24771 [DOI] [PubMed] [Google Scholar]
- 12.Komesu YM, Sapien RE, Rogers RG, Ketai LH. Hypnotherapy for treatment of overactive bladder: a randomized controlled trial pilot study. Female Pelvic Med Reconstr Surg. 2011;17(6): 308-313. doi: 10.1097/SPV.0b013e31823a08d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKernan LC, Finn MTM, Crofford LJ, Kelly AG, Patterson DR, Jensen MP. Delivery of a group hypnosis protocol for managing chronic pain in outpatient integrative medicine. Int J Clin Exp Hypn. 2022;70:227-250. doi: 10.1080/00207144.2022.2096455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKernan LC, Finn MTM, Patterson DR, Williams RM, Jensen MP. Clinical hypnosis for chronic pain in outpatient integrative Medicine: an implementation and training model. J Altern Complement Med. 2020;26(2):107-112. doi: 10.1089/acm.2019.0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158. doi: 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry MJ, Fowler FJ Jr., O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148(5):1549-1557. Discussion 1564. doi: 10.1016/s0022-5347(17)36966-5 [DOI] [PubMed] [Google Scholar]
- 18.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309-318. [DOI] [PubMed] [Google Scholar]
- 19.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150(1):173-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brummett C, Hassett A, Brummett K, Clauw D, Williams D. The Michigan Body Map and its use in assessing the American College of Rheumatology survey criteria for fibromyalgia. Arthritis Rheumat. 2011;62:744. [Google Scholar]
- 21.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589-605. [DOI] [PubMed] [Google Scholar]
- 22.Shor RE, Orne EC. Norms on the Harvard group scale of hypnotic susceptibility, form A. Int J Clin Exp Hypn. 1963;11: 39-47. doi: 10.1080/00207146308409226 [DOI] [PubMed] [Google Scholar]
- 23.Hays RD, Schalet BD, Spritzer KL, Cella D. Two-item PROMIS® global physical and mental health scales. J Patient Rep Outcomes. 2017;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodol). 1995;57(1):289-300. [Google Scholar]
- 25.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association Symptom Index and the Benign Prostatic Hyperplasia Impact Index is perceptible to patients. J Urol. 1995;154(5):1770-1774. doi: 10.1016/S0022-5347(01)66780-6 [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MJL, Bishop SR, Pivik J The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4): 524-532. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 27.Fuller TW, Ristau BT, Tepe SM, Benoit RM. Characterizing clinically meaningful changes in lower urinary tract symptoms using the American Urological Association Symptom Index. Urology. 2018;115:139-143. doi: 10.1016/j.urology.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 28.Palsson OS, van Tilburg M Hypnosis and guided imagery treatment for gastrointestinal disorders: experience with scripted protocols developed at the university of north carolina. Am J Clin Hypn. 2015;58(1):5-21. doi: 10.1080/00029157.2015.1012705 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be made available upon written request with appropriate data use agreements consistent with institutional policy. All authors have had access to, contributed to, read, reviewed, and agreed upon the content of this submission.


