Abstract
Despite many efforts to improve the transdermal permeability of drugs, most of them are blocked by the skin barrier. Niacinamide (NAC) is a Biopharmaceutics Classification System class I drug with high aqueous solubility and intestinal permeability. Due to the high solubility and intestinal permeability of NAC, the development of new formulations is insufficient as transdermal, injection etc. Thus, this study aimed to develop the novel NAC formulation with improved skin permeability and secured stability. The NAC formulation approach is to first select a solvent that improves skin permeability, and then select a second penetration enhancer to determine the final formulation. All formulations were evaluated for skin permeability using an artificial membrane (Strat-M®). The optimal formulation (non-ionic formulations (NF1) consisted of NAC/Tween®80 = 1:1 wt ratio in dipropylene glycol [DPG]) showed the highest permeability in all formulations in PBS buffer (pH 7.4). The thermal properties of NF1 were altered. Moreover, NF1 maintained a stable drug content, appearance, and pH value for 12 months. In conclusion, DPG had an excellent effect in increasing the NAC permeation, and Tween®80 played a boosting role. Through this study, an innovative NAC formulation was developed, and good results are expected for human transdermal research.
Keywords: Niacinamide, Dipropylene glycol, In vitro permeability test, Artificial membrane
1. Introduction
Disease treatment has been important ever since, and choosing the appropriate route of administration is essential for effective treatment. The oral route of drug administration is the most common, followed by injection (Carillon et al., 2013). There are not only several studies have been conducted on transdermal delivery, but thousands of them (patches and lotions are also available for external use) for years (Nayak et al., 2018, Santos et al., 2018, Sohn and Choi, 2021). However, transdermal drug delivery is difficult because it is not easy for a drug to penetrate the stratum corneum (Som et al., 2012). The stratum corneum is the outermost layer of the skin and serves as a main barrier to drug penetration. The human stratum corneum consists of a layer of dead, keratinized cells with an intercellular matrix consisting mainly of ceramides, cholesterol, and free fatty acids (Zhang et al., 2019b). Therefore, it is important to identify the substances that enhance skin penetration.
The model drug, niacinamide (NAC), is a freely water-soluble vitamin B3. Its beneficial effects include anti-inflammatory, antioxidant, prevention of UV-induced immunosuppression, skin whitening effects (Hakozaki et al., 2002, Iliopoulos et al., 2020, Zhang et al., 2020). Niacinamide (NAC) is a Biopharmaceutics Classification System class I drug with high aqueous solubility and intestinal permeability. For this reason, only tablets and capsules have been developed and have limitations in formulation.
Many researchers have used solvents and permeation enhancers to deliver drugs transdermally. To improve the transdermal permeability of NAC, propylene glycol (PG), dipropylene glycol (DPG), polyethylene glycol 400 and 600 (PEG 400 and PEG 600), dimethyl isosorbide (DMI), t-butyl alcohol (T-BA), Transcutol® P (TC), lemon oil, oleic acid, aloe, linolenic acid, and caprylic/capric triglyceride have been used in previous studies (Iliopoulos et al., 2020, Jafri et al., 2019, Zhang et al., 2019a, Zhang et al., 2019b, Zhang et al., 2020). The above studies developed formulations and applied it to actual artificial skin or animal skin. However, most formulations have low permeability and high standard deviation (SD). An important part of the study is not only formulation development but also accurate evaluation. The evaluation of the in vitro permeation of the products was less accurate. However, a product called the Strat-M® membrane (Sigma-Aldrich Inc., St. Louis, MO, USA) that can replace animal testing has emerged. This has the advantage of not being subject to ethical issues when conducting research. In addition, many research results similar to those of the Strat-M® membrane, animal skin, and human skin are available (Kaur et al., 2018, Touti et al., 2020, Arce et al., 2020). It is simple and easy to experiment on and has the advantage of saving time and research cost compared to animal experiments. Moreover, Strat-M® membrane has a good reproducibility (standard deviation; SD) compared to animal skin and human skin.
Strat-M® membrane was divided into three main layers. The upper layer is composed of a layer similar to the stratum corneum, the middle layer is composed of layer (two porous layers of polyethersulfone) similar to the dermis in the skin and the lower layer is composed of polyolefin non-woven similar to the subcutaneous tissue. Strat-M® membrane is treated with synthetic lipids that resemble human skin (Haq et al., 2018).
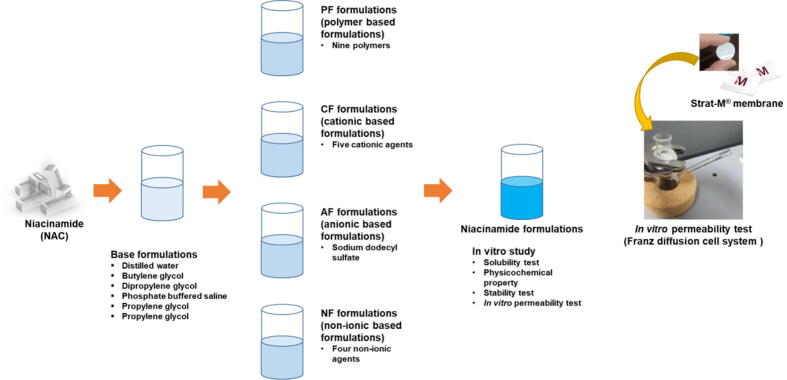
NAC is known to have high water solubility and low pKa (3.35), so that it has good permeability compared to other water-soluble drugs (Park et al., 2019). It was hypothesized that the permeability would be improved by using a polyol-based solvent with high permeability. The difference between this study and the previous studies was that it was developed for external use in this study due to the lack of development of a new formulation due to the high solubility and intestinal permeability of NAC. This study aimed to develop the NAC formulation with improved permeability in the Strat-M® membrane. Moreover, it is to develop the formulation that has both permeability and stability by securing the stability of the liquid phase. Therefore, the following approaches were prepared: First, the base formulations (B) were prepared to select the solvent, including distilled water (DW), phosphate-buffered saline (PBS pH7.4), butylene glycol (BG), and dipropylene glycol (DPG), propylene glycol (PG), and polyethylene glycol 400 (PEG 400). Second, the enhancer was added to the selected solvent to enhance the skin penetration of NAC as non-ionic polymer formulations (PF), cationic formulations (CF), anionic formulations (AF), and non-ionic formulations (NF) were prepared with non-ionic polymer, cationic, anionic, and non-ionic agents, respectively. The third is an in vitro study for each evaluation (including solubility test, physicochemical properties, stability, and in vitro permeability test) (Fig. 1).
Fig. 1.
Process of preparation of niacinamide (NAC) formulations. First, screening for solvents to enhance the permeability of NAC; second, screening to enhance the permeability of NAC with non-ionic polymers and cationic, anionic, and non-ionic agents; and third, an in vitro study for each evaluation.
2. Materials and methods
2.1. Materials
Niacinamide 99.5% (nicotinamide, NAC), hexadecyltrimethylammonium bromide 98.0%, benzalkonium chloride 95.0%, alkyltrimethylammonium bromide 95.0%, dodecylamine 98.0%, cetylpyridinium chloride 98.0%, sodium dodecyl sulfate (SDS) 98.5%, Tween®80 (polysorbate 80), Tween®20 (polysorbate 20), Span®20 (sorbitan laurate), phosphate-buffered saline (PBS pH 7.4) powder, and Strat-M® Membrane (25 mm) were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). TEGOSOFT® CT was obtained from Evonik (Essen, Germany). Butylene glycol 99.0% (BG), dipropylene glycol 97.0% (DPG), and propylene glycol 99.0% (PG) were purchased from Samchun Pure Chemical Co., Ltd. (Pyeongtaek, Korea). Polyethylene glycol 400 was purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan). Polyoxyethylene (1 6 0) and polyoxylpropylene (30) glycol (Kolliphor® P188), polyoxyethylene (1 9 6) and polyoxylpropylene (67) glycol (Kolliphor® P407), Polyethylene glycol 6000 (PEG6000), polyvinyl alcohol-polyethylene glycol graft copolymer (Kollicoat® IR), polyvinylpyrrolidone (Kollidon® K12), macrogol 15 hydroxystearate (Kolliphor® HS 15), polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus®), and D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) were obtained from BASF (Ludwigshafen, Germany). PVP/VA S-630 was obtained from ISP Technologies Inc. (Wayne, NJ, USA).
2.2. Preparation of the NAC formulations
The base formulations (B) were prepared with DW, PBS (pH7.4), BG, DPG, PG, and PEG 400 (Table 1). Briefly, NAC (100 mg) was dissolved in 10 mL of the above solutions using a multichannel stirrer operating at 500 rpm for 60 min at 32 ± 1.0 °C.
Table 1.
Composition of the base formulations (B). The ratios represent percentage weight to volume (w/v).
| B1 | B2 | B3 | B4 | B5 | B6 | |
|---|---|---|---|---|---|---|
| NAC | 1% | |||||
| DW | 10 mL | |||||
| PBS | 10 mL | |||||
| BG | 10 mL | |||||
| DPG | 10 mL | |||||
| PG | 10 mL | |||||
| PEG 400 | 10 mL | |||||
Niacinamide (NAC), distilled water (DW), butylene glycol (BG), dipropylene glycol (DPG), phosphate-buffered saline (PBS, pH 7.4), propylene glycol (PG), propylene glycol 400 (PEG 400).
Non-ionic polymer formulations (PF) were prepared using non-ionic polymers (Table 2). After permeability evaluation of B, DPG was selected as the solvent, and non-ionic polymers were added. Briefly, NAC (100 mg) and non-ionic polymers (P188®, P407®, PEG 6000, IR®, K12®, PVP/VA S630, TPGS, HS-15®, and Soluplus®) were dissolved in DPG (10 mL) and then same process of B formulation.
Table 2.
Composition of the non-ionic polymer formulations (PF). The ratios represent percentage weight to volume (w/v).
| PF1 (B4) | PF2 | PF3 | PF4 | PF5 | PF6 | PF7 | PF8 | PF9 | PF10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| NAC | 1% | |||||||||
| DPG | 10 mL | |||||||||
| P188® | 1% | |||||||||
| P407® | 1% | |||||||||
| PEG 6000 | 1% | |||||||||
| IR® | 1% | |||||||||
| K12® | 1% | |||||||||
| PVA/VA S630 | 1% | |||||||||
| TPGS | 1% | |||||||||
| HS 15® | 1% | |||||||||
| Soluplus® | 1% | |||||||||
Polyoxyethylene (1 6 0) and polyoxypropylene (30) glycol (Kolliphor® P188), polyoxyethylene (1 9 6) and polyoxypropylene (67) glycol (Kolliphor® P407), PEG 6000, polyvinyl alcohol-polyethylene glycol graft copolymer (Kollicoat® IR), polyvinylpyrrolidone (Kollidon® K12), 60:40 linear random copolymer of N-vinyl-2-pyrrolidone and vinyl acetate (PVP/VA S-630), D-α-tocopherol polyethylene glycol 1000 succinate (TPGS), macrogol 15 hydroxystearate (Kolliphor® HS 15), polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus®), Niacinamide (NAC), dipropylene glycol (DPG).
CF, AF, and NF were prepared with cationic, anionic, and non-ionic agents, respectively (Table 3). The CFs were prepared; NAC (100 mg) and cationic agents (hexadecyltrimethylammonium bromide, benzalkonium chloride, alkyltrimethylammonium bromide, dodecylamine, and cetylpyridinium chloride) were dissolved in DPG (10 mL) and then same process of B formulation. The AF was prepared; NAC (100 mg) and SDS were dissolved in DPG (10 mL) and then same process of B formulation. The NFs were prepared by dissolving NAC (100 mg), and non-ionic agents (Tween®80, Tween®20, Span®20, and TEGOSOFT® CT) were dissolved in DPG (10 mL) and then same process of B formulation.
Table 3.
Composition of the CF (cationic formulations), AF (anionic formulations), and NF (non-ionic formulations). The ratios represent percentage weight to volume (w/v).
| CF1 | CF2 | CF3 | CF4 | CF5 | AF | NF1 | NF2 | NF3 | NF4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| NAC | 1% | |||||||||
| DPG | 10 mL | |||||||||
| Hexadecyltrimethylammonium bromide | 1% | |||||||||
| Benzalkonium chloride | 1% | |||||||||
| Alkyltrimethylammonium bromide | 1% | |||||||||
| Dodecylamine | 1% | |||||||||
| Cetylpyridinium chloride | 1% | |||||||||
| SDS | 1% | |||||||||
| Tween®80 | 1% | |||||||||
| Tween®20 | 1% | |||||||||
| Span®20 | 1% | |||||||||
| TEGOSOFT® CT | 1% | |||||||||
Sodium dodecyl sulfate (SDS), polyethylene glycol sorbitan monooleate, polysorbate 80 (Tween®80), polyoxyethylene sorbitan monolaurate (Tween®20), sorbitan laurate (Span®20), TEGOSOFT® CT (caprylic/capric triglycerides), Niacinamide (NAC), dipropylene glycol (DPG).
2.3. UV–vis spectrophotometry
The drug content and permeability (%) of NAC were evaluated using a spectrophotometer (X-ma 1000; Human Co., Korea) at 254 nm (Park et al., 2019). The drug content of each formulation was confirmed using quantitative testing. Each formulation (100 μL) was diluted with 900 μL of PBS (pH7.4) and diluted once more at the same ratio. All samples were measured in triplicate, and the results were derived using a calibration curve (Coefficient of determination [R2] = 0.9992).
2.4. In vitro permeation test
The Strat-M® membrane (polyethersulfone and polyolefin membrane [synthetic lipid posttreatment]; diameter [ϕ] 25 mm and thickness 300 μm) and donor chamber were assembled, and the receptor chamber was filled with 8 mL of PBS (pH 7.4) using a magnetic stirrer (Hajjar et al., 2018, Odrobińska et al., 2020). All samples (NAC concentration of 10.0 mg/mL [1 mL]) was added to the donor chamber. The sample was stirred at a constant speed of 500 rpm for 24 h at 32 ± 1 °C. Samples were withdrawn at 1, 2, 4, 8, and 24 h and then evaluated using a UV–vis spectrophotometer (n = 3).
2.5. Physicochemical properties of the NAC-formulations
The pH values of the NAC formulations were measured using an Ohaus Starter 3100 pH meter (Ohaus, MA, USA) at 20 ± 1 °C (n = 3). All samples were diluted with 1/10 in DW.
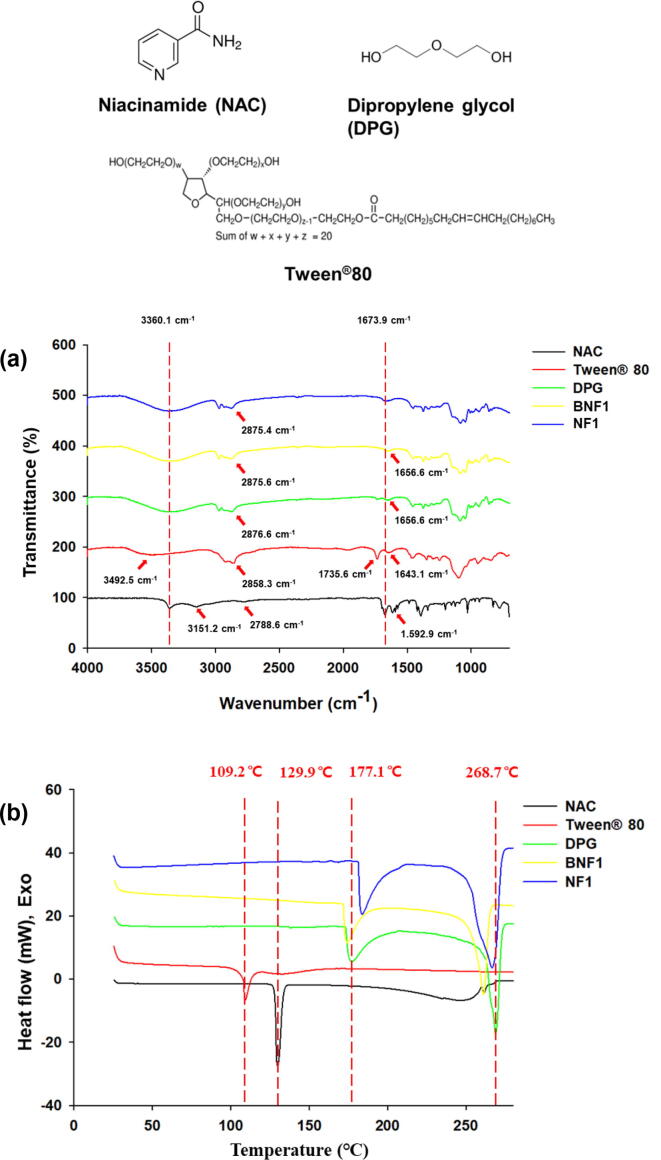
NAC, Tween®80, and DPG, which are components of NF1, the final selected formulation, and blank NF1 were evaluated for chemical structure and thermal characteristics evaluation.
The chemical structures of pure NAC, Tween®80, DPG, blank NF1 (BNF1), and NF1 were confirmed using a Fourier transform infrared (FT-IR) spectrometer (Nicolet 6700, Thermo Scientific, USA). The spectra were recorded in the frequency range of 4,000–500 cm−1 at a resolution of 2 cm−1.
The thermal properties of pure NAC, Tween®80, DPG, blank NF1 (BNF1), and NF1 were evaluated using a differential scanning calorimetry (DSC) 60A instrument (Shimadzu, Japan). These powder samples (2–3 mg) were placed in hermetically sealed aluminum pans and heated from 5 °C to 280 °C at a scanning rate of 10 °C/min under a 40 mL/min nitrogen purge.
2.6. Stability
The NAC formulations were subjected to a stability test including appearance, drug content, and pH value for 12 months. The NAC formulations were stored as a solution in capped glass vials, and their stability was evaluated under laboratory environmental conditions (temperature 20–25 °C, relative humidity 50%–60%). In the case of appearance, color change or the presence of sediment was observed. The pH values of the stability samples were measured, and the drug content was measured in stability samples diluted with PBS (pH7.4) and then by using a UV–vis spectrophotometer (n = 3).
2.7. Statistical analysis
Statistical analysis was performed using Student’s t-test on SigmaPlot (ver. 12.5; SYSTAT, Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation (SD). In all analyses, p < 0.005 (***), p < 0.01 (**), and p < 0.05 (*) indicated statistical significance.
3. Results
3.1. Characterization of the NAC formulations
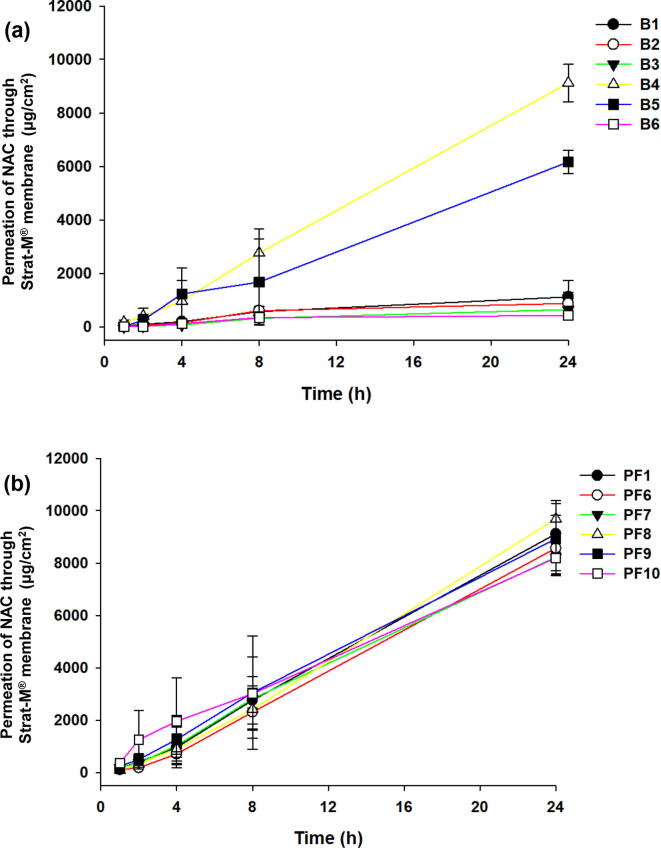
Niacinamide (NAC) is a Biopharmaceutics Classification System class I drug with high aqueous solubility and intestinal permeability. NAC is a water-soluble drug, with no solubility problem. Therefore, this study aimed to improve the transdermal permeability of NAC for the diversification of formulations. The first approach is a solvent that can enhance the permeability of the NAC. To confirm the permeability of NAC, DW was used as a control, and PBS (pH 7.4), BG, DPG, PG, and PEG 400 solutions were compared. B (1–6) formulations were prepared with a clear solution, and all of the NAC were fully dissolved. Through the permeation test of B (1–6) formulations, B4 formulation was selected the DPG as the solvent with highest permeability, among them (Fig. 2a). Thus, PF (1–10) formulations were developed by adding non-ionic polymers to B4 formulation. Except for PF (4–5), all PF were prepared in a clear solution state. The prepared PF4 (PEG 6000) and PF5 (IR®) showed a turbid solution, because PEG 6000 and IR® were considered to have low solubility in DPG. Moreover, PF (2–3) formulations were precipitated 1 h after preparation. These results suggest that the solubility of P188® and P407® in DPG solution decreased when the temperature was lowered to room temperature after stirring at 32 ± 1 °C during preparation. Precipitation was also confirmed during the preparation of blank formulations of PF (2–5) formulations. CF, AF, and NF were developed by adding various cations, anion, and non-ionic agents to PF1 (B4) formulation. All CF, AF, and NF formulations were clear solutions.
Fig. 2.
Screening of solvents for based formulation (B) and non-ionic polymers for non-ionic polymer formulations (PF). The based formulations (B1–B6) were prepared with DW, PBS (pH7.4), BG, DPG, PG, and PEG 400 (a). Non-ionic polymer formulations (PF1-10) were prepared with polymers (including P188®, P407®, PEG 6000, IR®, K12®, PVP/VA S-630, TPGS, HS 15®, Soluplus®)(b). The permeation study was performed with Strat-M® membrane through the Franz diffusion cell system at 32 ± 1 °C for 24 h. Data are expressed as mean ± standard deviation (sd, n = 3).
The drug contents and pH values of All formulations were represented in Table 4. The drug contents of B (1–6) formulations showed from 9.99 ± 0.42 mg/mL (B1) to 10.12 ± 0.23 mg/mL (B6). There was no change in the drug content in each solvent. The pH values of B (1–6) formulations showed from 6.41 ± 0.07 (B1) to 4.83 ± 0.06 (B6). The pH of B (1–6) was the same as that of the pH of each solution, either blank or B formulations. The drug contents of PF formulations showed from 10.03 ± 0.41 mg/mL (PF1) to 10.22 ± 0.13 mg/mL (PF10). There was no change in the drug content in each formulation. The pH values of the PF formulations showed from 5.83 ± 0.09 (PF1) to 4.58 ± 0.08 (PF10). CF, AF, and NF were developed by adding various cations, anion, and non-ionic agents to PF1 (B4) formulation. All CF, AF, and NF formulations were clear solutions. The drug content of formulations showed from 10.02 ± 0.20 mg/mL (CF1) to 10.01 ± 0.09 mg/mL (NF4). There was no change in the drug content in each formulation. The pH values of samples (CF, AF and NF) showed from 5.91 ± 0.08 (CF1) to 5.96 ± 0.08 (NF4). CF4 (dodecylamine) was originally a basic substance and also affected formulations. The solubility of NAC in different solvents is not an issue as the drug content of NAC formulations contain almost 100%.
Table 4.
Drug contents and pH values of NAC formulations.
| B1 | B2 | B3 | B4 | B5 | B6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug contents | 9.99 ± 0.42 | 10.01 ± 0.22 | 10.03 ± 0.41 | 10.05 ± 0.31 | 10.09 ± 0.19 | 10.12 ± 0.23 | ||||
| pH values | 6.41 ± 0.07 | 7.42 ± 0.05 | 5.89 ± 0.05 | 5.83 ± 0.09 | 6.87 ± 0.10 | 4.83 ± 0.06 | ||||
| PF1 | PF2 | PF3 | PF4 | PF5 | PF6 | PF7 | PF8 | PF9 | PF10 | |
| Drug contents | 10.03 ± 0.41 | – | – | – | – | 9.99 ± 0.10 | 10.06 ± 0.21 | 10.02 ± 0.11 | 9.98 ± 0.23 | 10.22 ± 0.13 |
| pH values | 5.83 ± 0.09 | – | – | – | – | 5.53 ± 0.12 | 5.04 ± 0.10 | 5.46 ± 0.07 | 5.35 ± 0.11 | 4.58 ± 0.08 |
| CF1 | CF2 | CF3 | CF4 | CF5 | AF | NF1 | NF2 | NF3 | NF4 | |
| Drug contents | 10.02 ± 0.20 | 9.98 ± 0.09 | 9.98 ± 0.13 | 9.95 ± 0.32 | 9.98 ± 0.21 | 10.10 ± 0.32 | 10.19 ± 0.21 | 10.02 ± 0.21 | 10.09 ± 0.13 | 10.01 ± 0.09 |
| pH values | 5.91 ± 0.08 | 5.92 ± 0.09 | 5.84 ± 0.11 | 9.87 ± 0.15 | 5.97 ± 0.11 | 5.78 ± 0.15 | 5.98 ± 0.08 | 5.93 ± 0.11 | 6.12 ± 0.15 | 5.96 ± 0.08 |
3.2. Permeation study
An artificial membrane, Strat-M®, was used for the permeation test of NAC formulations. It can be seen that B4 (DPG) permeates NAC significantly compared to other solvents in B formulations (Fig. 2a). For 24 h, the permeations of B (1–6) were 1,123.8 ± 606.4 μg/cm2 (B1), 882.4 ± 406.8 μg/cm2 (B2), 650.3 ± 118.5 μg/cm2 (B3), 9,118.2 ± 696.5 μg/cm2 (B4), 6,171.4 ± 437.3 μg/cm2 (B5), and 429.2 ± 104.2 μg/cm2 (B6). Moreover, the permeations (%) of B (1–6) were 6.8 ± 3.7% (B1), 5.4 ± 2.4% (B2), 3.9 ± 0.7% (B3), 55.9 ± 4.2% (B4), 37.8 ± 2.6% (B5), and 2.6 ± 0.6% (B6) for 24 h. The analysis results of B4 were significantly different (p < 0.005, Student’s t-test) from those of the other formulations.
The PFs (2–5) formulations were excluded due to precipitation, and a permeability test was performed. The PF8 (TPGS) formulation showed slightly higher permeability than the other PF formulations (Fig. 2b). For 24 h, the permeations of the samples were 9,118.2 ± 696.5 μg/cm2 (PF1), 8,573.7 ± 998.0 μg/cm2 (PF6), 8,227.2 ± 522.1 μg/cm2 (PF7), 9,686.0 ± 694.8 μg/cm2 (PF8), 8,929.0 ± 1,340.4 μg/cm2 (PF9), and 8,198.1 ± 671.0 μg/cm2 (PF10). Moreover, the permeations (%) of the samples were 55.9 ± 4.2% (PF1), 52.5 ± 6.1% (PF6), 50.4 ± 3.2% (PF7), 59.4 ± 4.2% (PF8), 54.7 ± 8.2% (PF9), and 50.2 ± 4.1% (PF10) for 24 h. Although PF8 formulation showed the highest permeability result, it showed a slight increase compared to that in PF1 formulation (B4), and no significant difference was observed (p = 0.37). The analysis results of PF8 formulation were similar to those of the other formulations (p > 0.05, Student’s t-test).
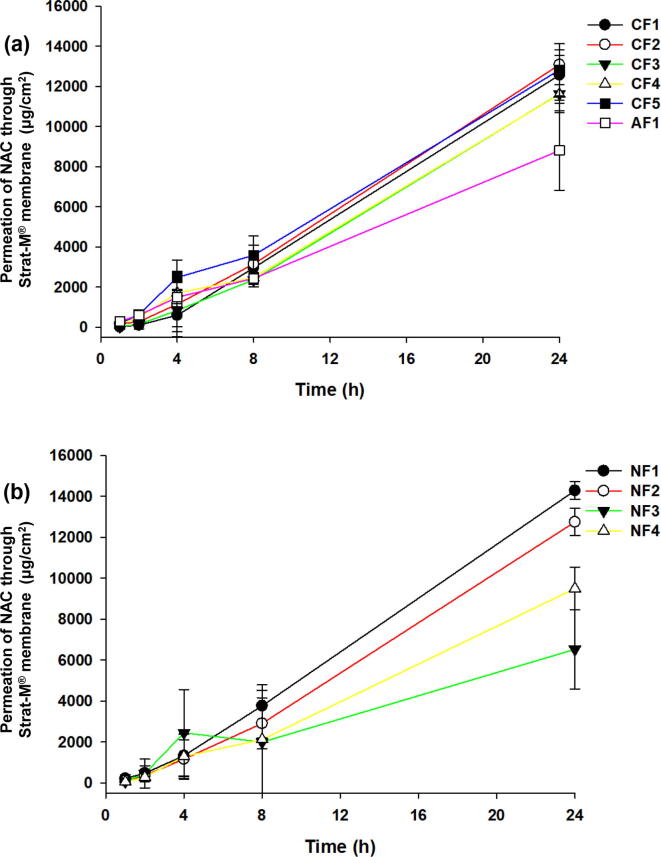
The CF (1–5) formulations showed significantly improved permeability compared to PF1 formulation (B4) (Fig. 3a). For 24 h, the permeations of the samples were 12,560.1 ± 1,255.2 μg/cm2 (CF1), 13,078.4 ± 1,685.9 μg/cm2 (CF2), 11,619.5 ± 459.0 μg/cm2 (CF3), 11,605.0 ± 910.2 μg/cm2 (CF4), and 12,813.4 ± 1,296.9 μg/cm2 (CF5). Moreover, the permeations (%) of the samples were 77.0 ± 7.6% (CF1), 80.2 ± 2.8% (CF2), 71.2 ± 2.8% (CF3), 71.2 ± 5.6% (CF4) and 78.5 ± 7.9% (CF5) for 24 h. These results showed that the cation agents definitely improved the permeability of NAC than non-ionic polymers. Moreover, the permeability of the AF formulation (8,800.8 ± 1,973 μg/cm2, 53.9 ± 12.0%) was lower than that of PF1 formulation (B4). The CF2 formulation (benzalkonium chloride) showed a significant increase compared to that of PF1 formulation, but the analysis results of CF2 formulation were similar to those of other CF formulations, except for the CF3 and AF formulation (p > 0.05, Student’s t-test).
Fig. 3.
Screening of cation and anionic agents for cationic (CF), anionic formulations (AF), and non-ionic agents for non-ionic formulations (NF). The cationic-based formulations (CF1-5) were prepared with cationic agents (including hexadecyltrimethylammonium bromide, benzalkonium chloride, alkyltrimethylammonium bromide, dodecylamine, cetylpyridinium chloride) and an anionic agent such as SDS (a). The NF (1–4) were prepared with non-ionic agents (including Tween®80, Tween®20, Span®20, TEGOSOFT® CT) (b). The permeation study was performed with Strat-M® membrane through the Franz diffusion cell system at 32 ± 1 °C for 24 h. Data are expressed as mean ± standard deviation (sd, n = 3).
The NF (1–2) formulations showed significantly improved permeability compared to that of PF1 formulation (B4)(Fig. 3b). For 24 h, the permeations of the samples were 14,275.2 ± 430.9 μg/cm2 (NF1), 12,743.5 ± 663.6 μg/cm2 (NF2), 6,526.7 ± 1,927.6 μg/cm2 (NF3), and 9,493.9 ± 1,051.2 μg/cm2 (NF4). Moreover, the permeations (%) of the samples were 87.5 ± 2.6% (NF1), 78.2 ± 4.7% (NF2), 40.0 ± 11.8% (NF3), and 58.2 ± 6.4% (NF4) for 24 h. In particular, compared with PF1 formulation, the NF3 formulation (span®20) showed significantly lower NAC transmittance, and the NF4 formulation (TEGOSOFT® CT) also showed lower permeations. The analysis results of NF1 formulation was significantly different (p < 0.05, Student’s t-test) from those of the other NF formulations.
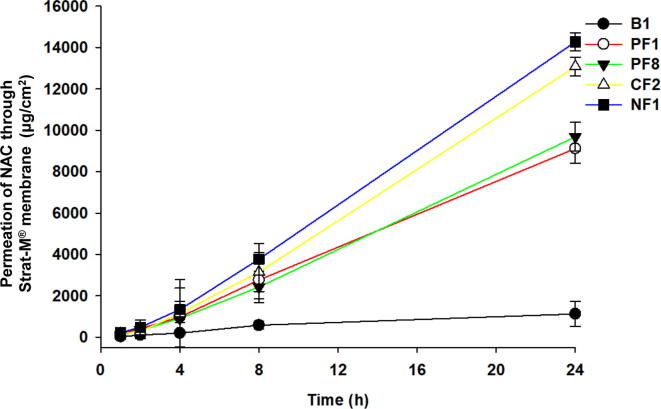
In summary, the permeability results of NAC were as follows: 1,123.8 ± 606.4 μg/cm2 (6.8 ± 3.7%) for the control DW-based formulation (B1), 9,118.2 ± 696.5 μg/cm2 (55.9 ± 4.2%) for the DPG-based formulation (PF1, [B4]), 9,686.0 ± 694.8 μg/cm2 (59.4 ± 4.2%) for the TPGS-DPG-based formulation (PF8), 13,078.4 ± 1,685.9 μg/cm2 (CF2) (80.2 ± 2.8%) for the benzalkonium chloride-DPG-based formulation (CF2), and 14,275.2 ± 430.9 μg/cm2 (87.5 ± 2.6%) for the Tween®80-DPG-based formulation (NF1), indicating that the NF1 formulation had the highest permeability. The NF1 formulation increased the NAC permeability by 12.7-, 1.6-fold, 1.5- and 1.1-fold compared to those of B1, PF1 (B4), PF8, and CF2 formulations, respectively (Fig. 4). The analysis results of NF1 formulation were significantly different (p < 0.005, Student’s t-test) from those of other formulations, except CF2 (p < 0.05, Student’s t-test). The order of flux enhancement on NAC permeation from NAC formulations was NF1 > CF2 > PF1 (B4) > PF8 > B1 (Table 5). Moreover, Flux (μg/h/cm2) and Kp (cm/h*10-3) of all NAC formulations ware presented in Table S2.
Fig. 4.
Comparison of permeation of NAC for each formulation. Comparative analysis of permeation of NAC depending on each formulation (B1 [in DW], PF1 [in DPG], PF8 [ TPGS in DPG], CF2 [benzalkonium chloride in DPG], NF1 [Tween®80 in DPG]). The permeation study was performed with the Strat-M® membrane through the Franz diffusion cell system at 32 ± 1 °C for 24 h. Data are expressed as mean ± standard deviation (sd, n = 3).
Table 5.
Permeability (%) and coefficients of various NAC-formulations through Strat-M®.
| B1 | PF1(B4) | PF8 | CF2 | NF1 | |
|---|---|---|---|---|---|
| Flux (μg/h/cm2) | 48.2 ± 12.2 | 269.2 ± 111.1 | 240.3 ± 56.7 | 306.4 ± 196.9 | 371.1 ± 142.2 |
| Kp (cm/h*10-3) | 4.8 ± 1.2 | 26.8 ± 11.1 | 24.1 ± 5.7 | 30.7 ± 19.7 | 36.4 ± 13.9 |
3.3. FT-IR spectroscopy
The interaction between NAC and the excipients in NF1 formulation was evaluated using an FT-IR scanner (Fig. 5a). The characteristic FT-IR bands of pure NAC represented N—H stretching at 3,360.1 and 3,151.2 cm−1, C—H stretching at 2,788.6 cm−1, C O stretching at 1,673.9 cm−1, and C C stretching at 1,592.9 cm−1 (Trivedi et al., 2015). The characteristic FT-IR bands of Tween®80 represented —OH stretching vibration at 3,492.5 cm−1, C—H stretching at 2,858.3 cm−1, C O stretching at 1,735.6 cm−1, and C C stretching at 1,643.1 cm−1 (Wang et al., 2018). The characteristic FT-IR bands of DPG represented –OH stretching vibration at 3,360.1 cm−1, C—H stretching at 2,875.6 cm−1, C O stretching at 1,735.6 cm−1, and C—C bend of diol at 1,656.6 cm−1. The characteristic FT-IR bands of BNF1 (blank NF1) represented —OH stretching vibration at 3,360.1 cm−1, C—H stretching at 2,875.6 cm−1, and C—C bend of diol at 1,656.6 cm−1 (Khaparde et al., 2017). The characteristic FT-IR bands of NF1 represented —OH stretching vibration at 3,360.1 cm−1, C—H stretching at 2,875.4 cm−1, and C O stretching in NAC at 1,673.9 cm−1. It was impossible to prepare a physical mixture in a liquid phase with NF1 formulation, and it was determined that there is no chemical bond based on the above results.
Fig. 5.
Fourier-transform infrared spectroscopy (FT-IR) and Differential scanning calorimetry (DSC) images. FT-IR spectra of pure NAC, Tween®80, DPG, blank NF1 (BNF1), and NF1(a). DSC images of pure NAC, Tween®80, DPG, blank NF1 (BNF1), and NF1 (b).
3.4. Thermal properties
The thermal properties of pure NAC, Tween®80, DPG, BNF1, and NF1 formulations were evaluated by DSC (Fig. 5b). NAC and Tween®80 displayed one main endothermic peak at 129.9 °C and 109.2 °C, respectively. DPG had two main endothermic peaks at 177.1 °C and 268.7 °C. The BNF1 (blank NF1) formulation had two main endothermic peaks at 174.1 °C and 261.2 °C. NF1 had two main endothermic peaks at 183.8 °C and 266.6 °C. The difference between BNF1 and NF1 was confirmed by thermal property evaluation. As for the main peaks of NF1, the DPG peaks were changed compared to those of BNF1, and it was confirmed that the main peak of NAC disappeared. Based on these results, the thermal change in the NAC of NF1 formulation was confirmed.
3.5. Stability study
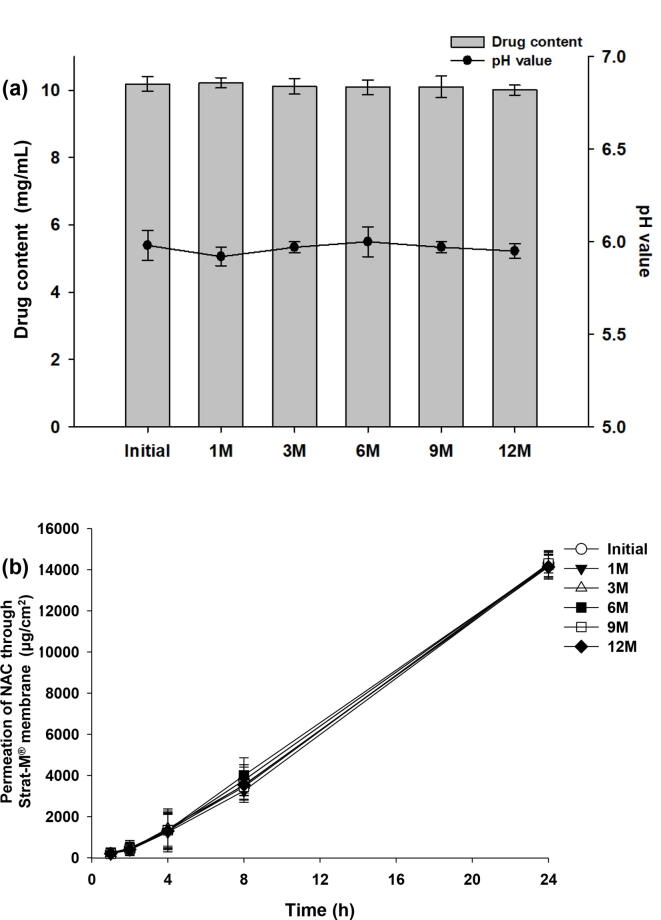
The optimal formulation (NF1) was tested for stability, including appearance, drug content, and pH value, for 12 months. No change in the appearance of NF1 was observed, and a clear solution was maintained for 12 months (Fig. 6). The drug contents of the NF1 showed from 10.19 ± 0.21 mg/mL (initial) to 10.01 ± 0.15 mg/mL (12 M). The pH values of NF1 showed from 5.98 ± 0.08 (initial) to 5.95 ± 0.04 (12 M) (Fig. 6a). The drug contents and pH values of NF1 were maintained for 12 months. Moreover, permeation tests of NF1 showed from 14,275.2 ± 430.9 μg/cm2 (initial) to 14,135.9 ± 598.1 μg/cm2 (12 M) at 24 h (Fig. 6b). As a result, it was determined that the stability of NF1 formulation was well maintained for 12 months.
Fig. 6.
Stability test. The optimal formulation (NF1) was evaluated for its stability over time. The following stability tests were performed: drug content, pH value (a), and in vitro transdermal permeability (b) over 12 months.
4. Discussion
Since NAC is highly soluble in water, most clear formulations could be developed. The pH of the formulation was found to be affected by each solvent and additive. Among the polymers, PEG6000 and IR did not dissolve due to low solubility in DPG. In addition, P188® and P407® were initially dissolved in DPG, but it was confirmed that they were immediately precipitated when stored at room temperature.
An artificial membrane, Strat-M®, was used for the permeation test of NAC formulations. It can be seen that B4 (DPG) permeates NAC significantly compared to other solvents in B formulations (Fig. 2a). It was confirmed that DPG had a stronger viscosity than DW, but improved the permeation of NAC. Except for DPG and PG, other solutions showed lower permeability than DW. Both B1 (DW) and B2 (PBS) were expected to be difficult to permeate due to the characteristics of Strat-M® membrane. B6 (PGE400) was thought to take longer permeation time because of its higher viscosity. B3 (BG) was considered to have a viscosity similar to other polyols, but low permeability. DPG not only improves the permeability of NAC, but also makes it less irritating to the skin. Undiluted DPG showed mild irritation when 500 mg was applied to rabbit skin for 24 h (Glycol and Glycol, 1985). In previous studies, the finite-dose human skin permeation study of ibuprofen with DPG formulation was similar to that of PG, but higher than that of tripropylene glycol and PEG 300-based formulations (Patel et al., 2021). The finite-dose porcine skin permeation study of NAC with the Transcutol®P formulation was higher than that of the PG- and DPG-based formulations (Iliopoulos et al., 2020). Several studies have shown that the permeation of DPG was lower than that of PG, but the results were reversed in our study (Fasano et al., 2011, Haque et al., 2017, Iliopoulos et al., 2020). Since the p-values of the NAC-DPG and PG formulations were also low, we proceeded according to the results of this study.
The PF8 (TPGS) formulation showed slightly higher permeability than the other PF formulations (Fig. 2b). It was found that depending on the type of non-ionic polymer, it could be a factor inhibiting the permeability of NAC. In previous studies, raloxifene-loaded TPGS transferosome films showed improved permeation compared to that in raw raloxifene film in rat skin (Alhakamy et al., 2019). Moreover, TPGS improved the permeability of several drugs (ibuprofen, cyclosporin A, quercetin, etc.) in animal skin (Pham and Cho, 2017). The CF (1–5) formulations showed significantly improved permeability compared to PF1 formulation (B4) (Fig. 3a). These results showed that the cation agents definitely improved the permeability of NAC than non-ionic polymers. Moreover, the permeability of the AF formulation (8,800.8 ± 1,973 μg/cm2, 53.9 ± 12.0%) was lower than that of PF1 formulation (B4). The NF (1–2) formulations showed significantly improved permeability compared to that of PF1 formulation (B4) (Fig. 3b). Studies on enhancing the permeability of NAC using Tween®20 and Tween®80 have not yet been conducted. The reason the permeability is lower than that of PF1 formulation in all formulations is considered to be due to the increase in viscosity or adsorption by additives.
In previous studies, the finite-dose (5 μL) porcine skin permeation study of NAC with Transcutol®P formulation (approximately 50.8 μg/cm2) was higher than that of PG (approximately 22 μg/cm2) and DPG (approximately 10 μg/cm2) (Iliopoulos et al., 2020). The NAC-Transcutol®P formulation showed a permeation of 32% of the initial NAC concentration. The finite-dose (50 μL) of porcine skin permeation of NAC with t-butyl alcohol (approximately 474 μg/cm2, 18%) and dimethyl isosorbide formulation (approximately 116 μg/cm2, 4.4%) was higher than that of Transcutol®P (approximately 39.5 μg/cm2, 1.5%) and PG (approximately 46 μg/cm2, 2.3%) (Zhang et al., 2019b). The finite-dose (1 μL) mammalian skin permeation study of NAC with Transcutol®P/t-butyl alcohol (9:1) and PG/t-butyl alcohol (9:1) formulation (approximately 178 and 150 μg/cm2, respectively) was higher than that of the other formulations (Zhang et al., 2019a). The NAC-Transcutol®P/t-butyl alcohol (9:1) and PG/t-butyl alcohol (9:1) formulations showed a permeation of 79.5% and 71.3% of the initial concentration of NAC, respectively. Our results reveal that NF1 showed a skin permeation of 87.5% of the initial concentration of NAC, which is superior to that in previous studies.
The interaction between NAC and the excipients in NF1 formulation was evaluated using an FT-IR scanner (Fig. 5a). It was impossible to prepare a physical mixture in a liquid phase with NF1 formulation, and it was determined that there is no chemical bond based on the above results. The thermal properties of pure NAC, Tween®80, DPG, BNF1, and NF1 formulations were evaluated by DSC (Fig. 5b). As for the main peaks of NF1, the DPG peaks were changed compared to those of BNF1, and it was confirmed that the main peak of NAC disappeared. Based on these results, the thermal change in the NAC of NF1 formulation was confirmed.
The final formulation, NF1, was a liquid formulation and proved to be a stable formulation without changes in appearance, drug content, pH and transdermal permeability for 12 months in Fig. 6.
5. Conclusion
A Novel transdermal formulation of NAC was successfully developed. DPG was selected as a solvent to improve the permeability of NAC, and NAC formulations were prepared for various non-ionic polymers and cationic, anionic, and non-ionic agents as permeation enhancers. The permeability of all NAC formulations in the artificial membrane (Strat-M®) was evaluated. The optimal NAC formulation (NF1) consisted of NAC and Tween®80 in a 1:1 wt ratio in DPG solution (10 mL). The NF1 formulation improved NAC permeability by 12.7-fold compared to that of B1 (consisting of NAC in DW) in PBS (pH 7.4) through the Strat-M® membrane for 24 h. This is a result of the significantly improved permeability compared with that of previous studies. Moreover, the stability of NF1 formulation was maintained for 12 months. Since the Strat-M® membrane is recognized as an alternative test for animal experiments, it is considered to be accurate for evaluation. As a result, it is expected that good results will be obtained in human experiments.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT)(No. NRF-2020R1G1A1100266 and No. NRF-2021R1F1A1059684).
This work was supported by a research program funded by Chodang University in 2022.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Editage (www.editage.co.kr) for the English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2023.05.018.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Alhakamy N.A., Fahmy U.A., Ahmed O.A. Vitamin E TPGS based transferosomes augmented TAT as a promising delivery system for improved transdermal delivery of raloxifene. Plos one. 2019;14:e0226639. doi: 10.1371/journal.pone.0226639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce F., Jr, Asano N., See G.L., Itakura S., Todo H., Sugibayashi K. Usefulness of artificial membrane, Strat-M®, in the assessment of drug permeation from complex vehicles in finite dose conditions. Pharmaceutics. 2020;12:173. doi: 10.3390/pharmaceutics12020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carillon J., Rouanet J.-M., Cristol J.-P., Brion R. Superoxide dismutase administration, a potential therapy against oxidative stress related diseases: several routes of supplementation and proposal of an original mechanism of action. Pharm. Res. 2013;30(11):2718–2728. doi: 10.1007/s11095-013-1113-5. [DOI] [PubMed] [Google Scholar]
- Fasano W.J., ten Berge W.F., Banton M.I., Heneweer M., Moore N.P. Dermal penetration of propylene glycols: Measured absorption across human abdominal skin in vitro and comparison with a QSAR model. Toxicol. Vitro. 2011;25(8):1664–1670. doi: 10.1016/j.tiv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Glycol B., Glycol E. 8 Final report on the safety assessment of butylene glycol, hexylene glycol, ethoxydiglycol, and dipropylene glycol. Int. J. Toxicol. 1985;4:223–248. [Google Scholar]
- Hajjar B., Zier K.-I., Khalid N., Azarmi S., Löbenberg R. Evaluation of a microemulsion-based gel formulation for topical drug delivery of diclofenac sodium. Int. J. Pharm. Investig. 2018;48(3):351–362. [Google Scholar]
- Hakozaki T., Minwalla L., Zhuang J., Chhoa M., Matsubara A., Miyamoto K., Greatens A., Hillebrand G.G., Bissett D.L., Boissy R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002;147(1):20–31. doi: 10.1046/j.1365-2133.2002.04834.x. [DOI] [PubMed] [Google Scholar]
- Haq A., Goodyear B., Ameen D., Joshi V., Michniak-Kohn B. Strat-M® synthetic membrane: permeability comparison to human cadaver skin. Int. J. Pharm. 2018;547(1-2):432–437. doi: 10.1016/j.ijpharm.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Haque T., Rahman K.M., Thurston D.E., Hadgraft J., Lane M.E. Topical delivery of anthramycin I. Influence of neat solvents. Eur. J. Pharm. Sci. 2017;104:188–195. doi: 10.1016/j.ejps.2017.03.043. [DOI] [PubMed] [Google Scholar]
- Iliopoulos F., Sil B.C., Monjur Al Hossain A.S.M., Moore D.J., Lucas R.A., Lane M.E. Topical delivery of niacinamide: Influence of neat solvents. Int. J. Pharm. 2020;579:119137. doi: 10.1016/j.ijpharm.2020.119137. [DOI] [PubMed] [Google Scholar]
- Jafri I., Shoaib M.H., Yousuf R.I., Ali F.R. Effect of permeation enhancers on in vitro release and transdermal delivery of lamotrigine from Eudragit® RS100 polymer matrix-type drug in adhesive patches. Prog. Biomater. 2019;8(2):91–100. doi: 10.1007/s40204-019-0114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur L., Singh K., Paul S., Singh S., Singh S., Jain S.K. A mechanistic study to determine the structural similarities between artificial membrane Strat-M™ and biological membranes and its application to carry out skin permeation study of amphotericin B nanoformulations. AAPS Pharmscitech. 2018;19(4):1606–1624. doi: 10.1208/s12249-018-0959-6. [DOI] [PubMed] [Google Scholar]
- Khaparde A., Vijayalakshmi M., Tetala K.K. Development of a metal/chelate polyhydroxyethylmethacrylate monolith capillary for selective depletion of immunoglobulin G from human plasma for proteomics. J. Chromatogr. A. 2017;1517:117–125. doi: 10.1016/j.chroma.2017.08.047. [DOI] [PubMed] [Google Scholar]
- Nayak A.K., Ahmad S.A., Beg S., Ara T.J., Hasnain M.S. Drug delivery: present, past, and future of medicine, applications of nanocomposite materials in drug delivery. Elsevier. 2018:255–282. [Google Scholar]
- Odrobińska J., Skonieczna M., Neugebauer D. PEG graft polymer carriers of antioxidants: in vitro evaluation for transdermal delivery. Pharmaceutics. 2020;12:1178. doi: 10.3390/pharmaceutics12121178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Lee H., Lim G.-S., Kim N., Kim D., Kim Y.-C. Enhanced transdermal drug delivery by sonophoresis and simultaneous application of sonophoresis and iontophoresis. AAPS PharmSciTech. 2019;20:1–7. doi: 10.1208/s12249-019-1309-z. [DOI] [PubMed] [Google Scholar]
- Patel A., Iliopoulos F., Caspers P.J., Puppels G.J., Lane M.E. In vitro–in vivo correlation in dermal delivery: the role of excipients. Pharmaceutics. 2021;13:542. doi: 10.3390/pharmaceutics13040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham C.V., Cho C.-W. Application of d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) in transdermal and topical drug delivery systems (TDDS) J. Pharm. Investig. 2017;47(2):111–121. [Google Scholar]
- Santos L.F., Correia I.J., Silva A.S., Mano J.F. Biomaterials for drug delivery patches. Eur. J. Pharm. Sci. 2018;118:49–66. doi: 10.1016/j.ejps.2018.03.020. [DOI] [PubMed] [Google Scholar]
- Sohn J.S., Choi J.-S. Development and evaluation of pseudoephedrine hydrochloride abuse-deterrent formulations using thermal modified rice starch. Int. J. Biol. Macromol. 2021;182:1248–1258. doi: 10.1016/j.ijbiomac.2021.05.055. [DOI] [PubMed] [Google Scholar]
- Som I., Bhatia K., Yasir M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012;4:2. doi: 10.4103/0975-7406.92724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touti R., Noun M., Guimberteau F., Lecomte S., Faure C. What is the fate of multi-lamellar liposomes of controlled size, charge and elasticity in artificial and animal skin? Eur. J. Pharm. Biopharm. 2020;151:18–31. doi: 10.1016/j.ejpb.2020.03.017. [DOI] [PubMed] [Google Scholar]
- Trivedi M.K., Branton A., Trivedi D., Nayak G., Bairwa K., Jana S. Spectroscopic characterization of disulfiram and nicotinic acid after biofield treatment. J. Anal. Bioanal. Tech. 2015;6:1000265. [Google Scholar]
- Wang J., Pan T., Zhang J., Xu X., Yin Q., Han J., Wei M. Hybrid films with excellent oxygen and water vapor barrier properties as efficient anticorrosive coatings. RSC Adv. 2018;8(38):21651–21657. doi: 10.1039/c8ra03819h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kung C.-P., Sil B.C., Lane M.E., Hadgraft J., Heinrich M., Sinko B. Topical delivery of niacinamide: influence of binary and ternary solvent systems. Pharmaceutics. 2019;11:668. doi: 10.3390/pharmaceutics11120668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lane M.E., Hadgraft J., Heinrich M., Chen T., Lian G., Sinko B. A comparison of the in vitro permeation of niacinamide in mammalian skin and in the Parallel Artificial Membrane Permeation Assay (PAMPA) model. Int. J. Pharm. 2019;556:142–149. doi: 10.1016/j.ijpharm.2018.11.065. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lane M.E., Moore D.J. An investigation of the influence of PEG 400 and PEG-6-Caprylic/Capric glycerides on dermal delivery of niacinamide. Polymers. 2020;12:2907. doi: 10.3390/polym12122907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.