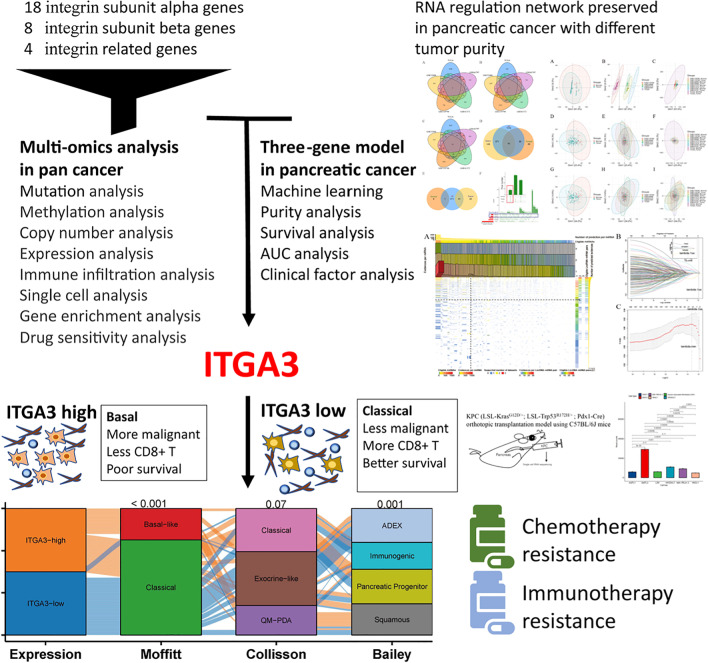
Abstract
Contribution of integrin superfamily genes to treatment resistance remains uncertain. Genome patterns of thirty integrin superfamily genes were analyzed of using bulk and single-cell RNA sequencing, mutation, copy number, methylation, clinical information, immune cell infiltration, and drug sensitivity data. To select the integrins that are most strongly associated with treatment resistance in pancreatic cancer, a purity-independent RNA regulation network including integrins were constructed using machine learning. The integrin superfamily genes exhibit extensive dysregulated expression, genome alterations, epigenetic modifications, immune cell infiltration, and drug sensitivity, as evidenced by multi-omics data. However, their heterogeneity varies among different cancers. After constructing a three-gene (TMEM80, EIF4EBP1, and ITGA3) purity-independent Cox regression model using machine learning, ITGA3 was identified as a critical integrin subunit gene in pancreatic cancer. ITGA3 is involved in the molecular transformation from the classical to the basal subtype in pancreatic cancer. Elevated ITGA3 expression correlated with a malignant phenotype characterized by higher PD-L1 expression and reduced CD8+ T cell infiltration, resulting in unfavorable outcomes in patients receiving either chemotherapy or immunotherapy. Our findings suggest that ITGA3 is an important integrin in pancreatic cancer, contributing to chemotherapy resistance and immune checkpoint blockade therapy resistance.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10142-023-01122-z.
Keywords: Pancreatic cancer, Chemotherapy resistance, Immunotherapy resistance, Machine learning, Integrin, ITGA3
Introduction
Pancreatic cancer is a highly aggressive and lethal tumor known for its resistance to treatment with a 5-year survival rate of approximately 10%. Currently, pancreatic cancer is the seventh leading cause of cancer-related deaths worldwide. However, by 2030, it has been projected to be the second leading cause of cancer-related deaths in the USA (Badheeb et al. 2022; Cai et al. 2021). In China, both the incidence and mortality rates of pancreatic cancer have recently been on the rise (Chen et al. 2016). Radical resection is the most important approach for achieving long-term survival; however, patients in China are often diagnosed at an advanced stage and experience a short period of disease-free survival after surgery (Cai et al. 2021).
Resistance to chemotherapy, radiation therapy, and targeted therapies is a major challenge in pancreatic cancer treatment. Moreover, patients are naturally resistant to immunotherapy (Wu et al. 2022), with only 10% of patients classified as immune hot patients (Cao et al. 2021). Understanding the genomic factors contributing to treatment failure is crucial in pancreatic cancer research and can aid in determining personalized therapies based on individual genome alterations. Pancreatic cancer is characterized by a high frequency of genomic alterations, with common driver gene mutations including KRAS, TP53, SMAD4, and CDKN2A. However, DNA molecular subtyping could not reflect the complexity of pancreatic cancer (Waddell et al. 2015). Several subtyping schemas have been proposed, including Moffitt’s two subtypes (Moffitt et al. 2015), Collisson’s three subtypes (Collisson et al. 2011), Bailey’s four subtypes (Bailey et al. 2016), and Puleo’s five subtypes (Puleo et al. 2018). However, these classification approaches are complex and can be negatively affected by tumor purity or sampling location, possibly hindering accurate classification (Cancer Genome Atlas Research Network. Electronic address and Cancer Genome Atlas Research 2017; Collisson et al. 2019; Waddell et al. 2015). Therefore, these classifiers may not always be clinically implemented. Thus, it is important to identify a marker that is both purity-independent and easy to use for classifying molecular subtypes and guiding treatment decisions.
In the era of single-cell sequencing, the significance of the integrin superfamily genes in complex cellular communication within the tumor microenvironment has been rediscovered. Integrin superfamily genes play an important role in cancer (Miyamoto et al. 2022; Wang et al. 2022), influencing various cancer-related processes including tumor initiation, microenvironment modifications, cell migration, tumor–stroma interactions, fibroblast activation, colonization, immune dysfunction, and drug resistance (Cooper and Giancotti 2019; Kanchanawong and Calderwood 2023; Margadant and Sonnenberg 2010; Mu et al. 2002; Nan et al. 2022; Sheta et al. 2021; Slack et al. 2022). However, the association between genome alteration of integrins and treatment resistance remains unclear. In this article, we conducted a pan-cancer multi-omics analysis of integrin superfamily genes and identified the most critical integrin in pancreatic cancer by constructing a purity-independent RNA regulation network (Yan et al. 2021).
Materials and methods
Patient cohorts
Integrin subunit genes and integrin superfamily genes containing a series of 18 alpha subunit genes (ITGA1, ITGA10, ITGA11, ITGA2, ITGA2B, ITGA3, ITGA4, ITGA5, ITGA6, ITGA7, ITGA8, ITGA9, ITGAD, ITGAE, ITGAL, ITGAM, ITGAV, and ITGAX), 8 beta subunit genes (ITGB1, ITGB2, ITGB3, ITGB4, ITGB5, ITGB6, ITGB7, and ITGB8), and 4 related genes (ITGB1BP1, ITGB3BP, ITGB1BP2, and ITGBL1) were included in the analysis. To analyze the multi-omics of integrins, we downloaded and analyzed source data from The Cancer Genome Atlas (TCGA) database (J Liu et al. 2018a), including expression, mutation, copy number alterations, methylation, clinical information, and immune cell infiltration. For pan-cancer comparison of gene expression, processed transcripts per million data were used by combining TCGA and Genotype-Tissue Expression (GTEx) data (Consortium et al. 2015), which were downloaded from UCSC Xena (Goldman et al. 2017).
For the purity-independent network construction, patient cohorts with both pancreatic cancer and normal adjacent samples were included. Samples other than those identified as outliers during quality control checking were included for regulation prediction, obtained from TCGA datasets and four pathological pancreatic adenocarcinomas datasets: GSE32688, (Donahue et al. 2012), GSE41372, (Frampton et al. 2014), GSE119794, (Lin et al. 2019), and GSE43797 (Park et al. 2014). Tables 1 and 2 present the clinical and quality control information. All five existing studies had provided expression data for mRNAs, long noncoding RNAs (lncRNAs), and microRNAs (miRNAs) in paired pancreatic cancer and normal tissues. Samples with partial or whole neuroendocrine carcinoma were excluded from the analysis (Nagtegaal et al. 2020). After analyzing the prediction results, we only considered the RNA regulatory relationships observed in the cancer tissues obtained from TCGA datasets, GSE32688, and GSE119794 with sufficient cancer samples for constructing the purity-independent network. We excluded GSE41372 and GSE43797 because of insufficient cancer samples.
Table 1.
Patient’s baseline characteristics used to construct the purity-independent RNA network
| TCGA | GSE32688 | GSE41372 | GSE119794 | GSE43797 | |
|---|---|---|---|---|---|
| Country | Global | USA | Italy | China | Korea |
| Age | |||||
| ≥ 65 | 98 (56.3%) | 16 (64%) | NA | 4 (40.0%) | 6(100.0%) |
| < 65 | 76 (43.7%) | 9 (36%) | NA | 6 (60.0%) | 0(0.0%) |
| Sex | |||||
| Male | 97 (55.7%) | 12 (48%) | 6 (66.7%) | 6 (60.0%) | 5 (83.3%) |
| Female | 77 (44.3%) | 13 (52%) | 3 (33.3%) | 4 (40.0%) | 1 (16.7%) |
| Tumor location | |||||
| Head + Neck | 147 (84.5%) | 24 (96%) | NA | NA | 4 (66.7%) |
| Body + Tail | 26 (14.9%) | 1 (4%) | NA | NA | 2 (33.3%) |
| Multiple | 1 (0.6%) | 0 (0.0%) | NA | NA | 0 (0.0%) |
| Pathological nodal stage | |||||
| N+ | 129 (75.0%) | 17 (68%) | 9 (100.0%) | 4 (40.0%) | 1 (16.7%) |
| N0 | 43 (25.0%) | 8 (32%) | 0 (0.0%) | 6 (60.0%) | 5 (83.3%) |
| Pathological stage | |||||
| IV | 5 (2.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (33.3%) |
| I–III | 168 (97.1%) | 25 (100.0%) | 9 (100.0%) | 10 (100.0%) | 4 (66.7%) |
| Resection margin | |||||
| Positive | 55 (34.6%) | 4 (16%) | 5 (55.6%) | NA | NA |
| Negative | 104 (65.4%) | 21 (84%) | 4 (45.4%) | NA | NA |
| Differentiation | |||||
| Poorly differentiated | 53 (31.0%) | 11 (44%) | 5 (55.6%) | 0 (0.0%) | NA |
| Well or moderately differentiated | 118 (69.0%) | 14(56%) | 4 (45.4%) | 10 (100.0%) | NA |
| Lymphovascular invasion | |||||
| Positive | NA | 18 (72%) | 9 (100.0%) | NA | NA |
| Negative | NA | 5 (20%) | 0 (0.0%) | NA | NA |
| Perineural invasion | |||||
| Positive | NA | 21 (84%) | 8 (88.9%) | NA | NA |
| Negative | NA | 2 (8%) | 1 (11.1%) | NA | NA |
TCGA The Cancer Genome Atlas, NA not available
Table 2.
Number of samples and platforms used in purity-independent regulatory network prediction
| TCGA | GSE32688 | GSE41372 | GSE119794 | GSE43797 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Data type | mRNA | miRNA | mRNA | miRNA | mRNA | miRNA | mRNA | miRNA | mRNA | miRNA |
| Subset names | PAAD | PAAD | GSE32676 | GSE32678 | GSE41368 | GSE41369 | GSE119794 | GSE119794 | GSE43795 | GSE43796 |
| Number of tumor samples | 168 | 169 | 25 | 25 | 6 | 9 | 10 | 10 | 6 | 6 |
| Number of tumor samples included | 165 | 165 | 24 | 24 | 6 | 6 | 10 | 10 | 6 | 6 |
| Number of control samples | 4 | 4 | 7 | 7 | 6 | 9 | 10 | 10 | 5 | 5 |
| Number of control samples included | 3 | 3 | 7 | 7 | 6 | 6 | 10 | 10 | 5 | 5 |
| Lower limit of tumor purity | 30.0% | 30.0% | 30.0% | 30.0% | 60.0% | 60.0% | 50.0% | 50.0% | 70.0% | 70.0% |
| Platform number | GPL11154 | GPL11154 | GPL570 | GPL7723 | GPL6244 | GPL16142 | GPL11154 | GPL11154 | GPL10558 | GPL15159 |
TCGA The Cancer Genome Atlas, mRNA messenger RNA, miRNA microRNA, PAAD pancreatic ductal adenocarcinoma, GPL Gene Expression Omnibus Platform
The patient cohorts of the five mRNA datasets, TCGA pancreatic ductal adenocarcinoma (PAAD), E-MTAB-6134 from ArrayExpress (Athar et al. 2019; Puleo et al. 2018), International Cancer Genome Consortium (ICGC)-PACA-AU (Bailey et al. 2016; Perez-Mancera et al. 2012) and ICGC-PACA-CA from ICGC (https://daco.icgc.org/), and GSE21501 and GSE71729 from Gene Expression Omnibus were used to develop the prognostic model. E-MTAB-6134 was used for external validation, and the immunotherapy cohort (Parikh et al. 2021) was used to determine the prognostic values of the genes to guide the immunotherapy treatment. We used data that met the following criteria: availability of mRNA expression profiles of primary pancreatic cancer samples (excluding neuroendocrine carcinoma samples), patients with sufficient clinical data, and patients with at least 30 days of follow-up and survival status information. Table 3 presents the clinical information of the datasets used to construct and validate the purity-independent model. The median overall survival (OS) of the construction group was 540 days, with 1-, 2-, 3-, and 5-year survival rates of 68.7%, 33.7%, 22.9%, and 10.1%, respectively. The median OS of the validation group was 795 days, with 1-, 2-, 3-, and 5-year survival rates of 78.3%, 52.0%, 38.9%, and 27.5%, respectively.
Table 3.
Clinical information of the datasets included in purity-independent three-gene model construction and validation
| Variables (n, %) | Construction | Validation | ||||
|---|---|---|---|---|---|---|
| TCGA | ICGC-PACA-CA | ICGC-PACA-AU | GSE71729 | GSE21501 | E-MTAB-6134 | |
| Number of patients | 160 | 65 | 90 | 123 | 102 | 288 |
| Sex | ||||||
| Male | 88 (55.0) | 33 (50.8) | 52 (57.8) | NA | NA | 166 (57.6) |
| Female | 72 (45.0) | 32 (49.2) | 38 (42.2) | NA | NA | 122 (42.4) |
| Pathological tumor stage | ||||||
| T1 | 6 (3.8) | NA | 2 (2.2) | NA | 2 (2.0) | 12 (4.2) |
| T2 | 15 (9.4) | NA | 14 (15.6) | NA | 16 (16.3) | 39 (13.5) |
| T3 | 135 (84.9) | NA | 72 (80.0) | NA | 79 (80.6) | 237 (82.3) |
| T4 | 3 (1.9) | NA | 2 (2.2) | NA | 1 (1.0) | 0 (0.0) |
| Pathological nodal stage | ||||||
| N0 | 43 (26.9) | NA | 17 (18.9) | NA | 28 (27.7) | 72 (25.0) |
| N+ | 117 (73.1) | NA | 73 (81.1) | NA | 73 (72.3) | 216 (75.0) |
| Differentiation | ||||||
| Well or moderately differentiated | 109 (69.0) | 38 (58.5) | 58 (64.4) | NA | NA | 240 (83.3) |
| Poorly differentiated | 49 (31.0) | 27 (41.5) | 32 (35.6) | NA | NA | 48 (16.7) |
| Pathological stage | ||||||
| I–IIa | 39 (24.5) | 10 (15.6) | 17 (18.9) | NA | 26 (26.8) | 72 (25.0) |
| IIb–IV | 120 (75.5) | 54 (84.4) | 73 (81.1) | NA | 71 (73.2) | 216 (75.0) |
| Resection margin | ||||||
| R0 | 93 (63.3) | NA | NA | NA | NA | 235 (82.7) |
| R1–2 | 54 (36.7) | NA | NA | NA | NA | 49 (17.3) |
TCGA The Cancer Genome Atlas, ICGC International Cancer Genome Consortium, NA missing information
Pancreatic cancer patient cohorts of single-cell RNA sequencing (RNA-seq) studies by Peng et al. (M Li et al. 2022a; Peng et al. 2019) and a single-nucleus RNA-seq study by Hwang et al. (2022) were used together with annotated data from original articles.
Cell cultures and RNA-seq
Primary cultures of human pancreatic cancer-associated fibroblasts (CAFs) were obtained from human pancreatic cancer tissues via pancreatectomy. The tissue was cut and incubated with dispase at 4 °C to separate the fibroblasts from other cells. Furthermore, we obtained other cell lines, including HPDE6C7, PANC1, BxPC3, AsPC1, and MIA-PACA-2, from the American Type Culture Collection (Manassas, VA, USA). RNA-seq was performed by the Annoroad Gene Company (Beijing, China) using the Illumina HiSeq X-ten system. For more detailed information, please refer to the Methods section of our previous work (Du et al. 2022).
Mouse model and single-cell sequencing
We purchased four C57BL/6J mice (12-week-old males) from GemPharmatech Co., Ltd. (Nanjing, China) and housed them under standard pathogen-free conditions. The mice were anesthetized by administering 5% isoflurane in the air, and each mouse received 2% isoflurane via a face mask. Cultured KPC cells (5 × 105; LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx1-Cre) were injected into the tail of pancreas, and they were returned to their cages after recovery. The mice were euthanized after 4 weeks under anesthesia, and the tumors were removed for single-cell sequencing that was performed by the Annoroad Gene Company using NovaSeq 6000.
Preparation of single-cell samples
Mouse pancreatic tissue pieces were washed in phosphate-buffered saline (PBS) and digested in a mixture of collagenase (2 mg/mL), calcium chloride (8.5 mM), and DNA enzyme (50 μg/mL) at 4 °C for 30 min to 1 h. Subsequently, the suspension was filtered using a 70-μm strainer to remove undigested cell clumps, and the resulting liquid was centrifuged at 500 g for 7 min to obtain cell pellets. Dead cells were removed using the Dead Cell Removal Kit (Miltenyi Biotec), and the cells were counted using a LUNA counter. The supernatant was carefully discarded while retaining the precipitate after centrifugation. The cell pellets were resuspended in 1 mL of 1X PBS containing 0.04% bovine serum albumin and washed twice with PBS. The cell concentration was determined using automatic cytometry, and the sample volume was calculated based on the optimal cell sampling concentration provided by 10× Genomics official website and the target capture number. Once we obtained the desired cell suspension, the samples were immediately cooled on ice for subsequent gel beads-in-emulsion (GEM) preparation and reverse transcription.
Library construction and single-cell sequencing
During the library preparation process, gel beads and cells containing a poly-T primer sequence linked with a cell barcode and a unique molecular identifier were encapsulated in oil drops, forming GEM. The cells were lysed within the GEM, and the released mRNA was subjected to reverse transcription, leading to the formation of full-length cDNA sequences. The oil drops were then crushed and purified, and the cDNA library was subjected to polymerase chain reaction amplification, with sequencing primers being connected to complete the library construction. Following library construction, the library concentration was determined using the Qubit® 3.0 Fluorometer (Life Technologies, Carlsbad, CA, USA), whereas the distribution of library product fragments was determined using the Agilent 2100 High Sensitivity DNA Assay Kit (Agilent Technologies, Santa Clara, CA, USA). The library product fragments were required to be > 10 nmol/L. The resulting libraries were sequenced on an Illumina NovaSeq 6000 platform using a 2 × 150-cycle paired-end run.
Analysis and reanalysis of single-cell sequencing
We used the Cell Ranger software (10× Genomics) to perform unique molecular identifier and barcode counting for the mice data. The data were filtered and aligned to the GRCm38 (mm10) reference genome to generate the feature file, barcode file, and matrix. Subsequent analyses of the single-cell data were conducted using Seurat v4.3.0 (Hao et al. 2021) following the official guide (https://www.10xgenomics.com/). Low-quality barcodes were removed using the following criteria: low unique molecular identifiers (< 200); low number of genes expressed (< 200); extremely high unique molecular identifiers (> 30,000); extremely high genes expressed (> 10,000); and high proportion of mitochondrial gene expression (> 10%). The default settings of the Harmony workflow were used for dimensionality reduction, clustering, and removal of sample batch effect (Korsunsky et al. 2019). Rough annotation information was obtained using SingleR (Aran et al. 2019), which used annotations from an embedded mouse dataset (“MouseRNAseqData” function). Auto-annotated mice cell types were summarized into epithelial (acinar, endocrine, tuft, normal ductal, and malignant ductal cell types), immune (T, B, natural killer [NK], and myeloid cell types), and stromal (fibroblast, muscle, endothelial, and nerve cell types) compartments based on the labeled annotations from published data (Cui Zhou et al. 2022; Hwang et al. 2022; Peng et al. 2019). Doublets, erythrocytes, adipocytes, and unknown cell types were removed after manual checking. Processed human single-cell data were reanalyzed by following the same protocol using Seurat v4.3.0 but were annotated cells with original cell annotation.
Multi-omics analyses
RNA-seq data were normalized using trimmed mean of M-values (TMM) and variance modeling at the observational level (VOOM) transformation. Original processed microarray data were used, which were normalized and corrected for batch effects. Low-expressed probes were filtered out, and probes were annotated using Bioconductor and Genecode version 36. Names of miRNA accessions were converted to new ones based on MIRbase version 21. Probes with higher expression values were retained if more than one probe was mapped to the same RNA name. Z-score transformation was performed for each gene to construct (TCGA, ICGC-PACA-CA, ICGC-PACA-AU, GSE71729, and GSE21501) and validate (E-MTAB-6134) the universal risk model. ComBat function in the sva package was used to adjust the batch effect during dataset construction (Leek et al. 2012).
The mutation data were collected from TCGA. Gene set cancer analysis (GSCA) (C J Liu et al. 2023a) includes information regarding missense mutations, nonsense mutation, frame shift insertions, splice sites, frame shift deletions, in-frame deletions, and in-frame insertions as deleterious mutations for analysis. cBioPortal (Cerami et al. 2012; Gao et al. 2013) contains information regarding nonsilent mutations, heterozygous copy number variations, and structural variations as genomic alterations. Copy number data were processed using GISTICS 2.0 (Mermel et al. 2011) and classified into five statuses based on the standards of cBioPortal: no alteration, heterozygous amplification, heterozygous deletions, homozygous amplification, and homozygous deletions. DNA methylation data were compared using β values.
Over-representation analysis (ORA) (Khatri and Draghici 2005), Gene set variation analysis (GSVA) (Hanzelmann et al. 2013), and gene set enrichment analysis (GSEA) (Subramanian et al. 2005) were used to identify the significantly enriched terms from the Gene Ontology (GO) (Gene Ontology 2021), Kyoto Encyclopedia of Genes and Genomes (KEGG) (Gillespie et al. 2022), and Reactome (Gillespie et al. 2022) datasets.
For pan-cancer and PAAD analyses, we used open-access online tools using TCGA datasets such as Gene Expression Profiling Interactive Analysis 2 (Tang et al. 2019), TISIDB (Ru et al. 2019), GSCA (C J Liu et al. 2023b), GSCALite (C J Liu et al. 2018b), Comprehensive Analysis on Multi-Omics of Immunotherapy in Pan-cancer (CAMOIP), cBioPortal (Cerami et al. 2012; Gao et al. 2013), Immune Cell Abundance Identifier (ImmuCellAI) (Miao et al. 2020), Tumor Immune Estimation Resource 2.0 (TIMER 2.0) (Li et al. 2020), and TIMER (Li et al. 2016; Li et al. 2017). The purity analysis was performed using Estimate (Yoshihara et al. 2013).
Quality control and batch correction before RNA regulatory network construction
Before analysis, the ComBat function in the sva package was used to adjust for batch effects (Leek et al. 2012). Samples outside the ellipses in the principal component analysis (PCA) plots were considered outliers, set at four standard deviations of the mean (99.9937% of values lie within one). Additional PCAs were conducted iteratively until no outliers were identified, and samples within the ellipses were selected for further network construction. PCA results were compared based on sample types (tumor vs. normal), studies (TCGA, GSE32688, GSE41372, GSE119794, and GSE43797), and sample types in individual studies. Based on these three factors, mRNA profiles before adjusting for batch effects are plotted in Supplementary Fig. 1A–C, those after adjusting for batch effects are in Supplementary Fig. 1D–F, and those after removing all outliers are in Supplementary Fig. 1G–I. Similarly, miRNA profiles before adjusting for batch effects are plotted in Supplementary Fig. 2A–C, those after adjusting for batch effects are in Supplementary Fig. 2D–F, and those after removing all outliers are in Supplementary Fig. 2G–I. We excluded two tumor samples and one normal sample from TCGA and one patient from GSE32688 after conducting PCA. Finally, 165 tumor samples from TCGA, 24 from GSE32688, 6 from GSE41372, 10 from GSE119794, and 6 from GSE43797 were retained for RNA regulation network prediction. Moreover, 3 control samples from TCGA, 7 from GSE32688, 6 from GSE41372, 10 from GSE119794, and 5 from GSE43797 were selected for analyses (Table 2). Although TCGA–PAAD dataset had the highest number of tumor samples, its purity limit was lower (≥ 30%) than that of the other datasets (Table 3). The expression profiles of the patients who did not meet the study standards were considered outliers in the pre-analysis quality control step and were hence excluded.
Purity-independent network construction
A purity-independent gene network was constructed using experimentally verified RNA regulatory pairs available in StarBase (Li et al. 2014), which implemented the competing endogenous RNA (ceRNA) mechanism among mRNAs, lncRNAs, and miRNAs. Theoretically, we expected the conservation of the actual RNA regulations at different tumor purities. Unverified relationships were eliminated to maintain network reliability. To ensure the relationship between the RNA regulatory pairs that have shared miRNAs, the expression profiles were subjected to correlation analyses and a hypergeometric test in the R package GDCRNAtools (Li et al. 2018). Only one RNA regulation pair with lncRNA–mRNA correlation P < 0.05, correlation coefficient > 0, and hypergeometric test P < 0.05 was considered to be preserved. The resulting co-expression networks were visualized using Cytoscape (Shannon et al. 2003). RNA relationships were calculated for tumor samples (Supplementary Fig. 3A), normal samples (Supplementary Fig. 3B), and all samples (Supplementary Fig. 3C). Significant differences were observed in the prediction results not only among the datasets but also among the sample types included. Only 10 common lncRNA–mRNA pairs were recognized in at least three tumor datasets and three control datasets (Supplementary Fig. 3D). No common predictive pairs were found if the criteria were set to at least four tumor datasets and four control datasets (Supplementary Fig. 3E).
Selection of prognosis-related genes and evaluation of the risk model
We observed differences in ceRNA regulation between cancerous and healthy tissues (Supplementary Fig. 3D and Supplementary Fig. 3E), which were in agreement with the results of prior studies (Li et al. 2018; Loganathan and Doss C 2023). Furthermore, we observed that the total number (red) of predicted ceRNA regulatory pairs significantly decreased with sample number (Supplementary Fig. 3F). Therefore, the prediction results from the tumor samples from GSE41372 and GSE43797 with only less than 10 tumor samples were removed. The prediction results from the tumor samples among the remaining three datasets (TCGA, GSE32688, and GSE119794) were observed. Finally, 201 lncRNA–mRNA pairs were considered to be preserved pairs for constructing the prognostic model (Supplementary Fig. 3F). The combined datasets (TCGA, ICGC-PACA-CA, ICGC-PACA-AU, GSE71729, and GSE21501) were used for constructing the model, and E-MTAB-6134 was used for external validation. To select the most useful prognostic mRNA models, least absolute shrinkage and selection operator (LASSO) (Gui and Li 2005; Tibshirani 1996; Tibshirani 1997) and Cox regression analyses were performed using the “glmnet” package in R (Friedman et al. 2010) for model construction, and the model with a lambda value of lambda.1se was used. Parameters (λ) in the LASSO model were selected using three-fold cross-validation. Blasso, a variant of LASSO, was used to determine the frequency of the variables being selected among different seeds for steady selection of the prognostic genes (Zeng et al. 2020).
Survival analyses
We evaluated the survival data using Kaplan–Meier survival analyses (log-rank method). The optimal cut-off values were determined using the “survminer” package, which divided the patients into high-risk and low-risk groups based on the association between the survival time and risk mode in the cohorts (Kassambara et al. 2017). We conducted Cox regression model analyses as previously described, and P < 0.05 was considered statistically significant for all analyses. We used time-dependent area under the curve (AUC) and decision curve analyses to represent the advantages of the prognostic model.
Statistical analyses
Quantitative data are presented as the median ± interquartile range. Numeric data were analyzed using the Student’s t test and Wilcoxon’s rank-sum tests, whereas classified variables were analyzed using the chi-square and Fisher’s exact tests. Significance of enrichment results was determined using a one-sided Fisher’s exact test and corrected for multiple hypotheses using a Benjamini–Hochberg false discovery rate (FDR). Tests with a FDR or P value < 0.05 were considered significant. All statistical tests were performed in R v4.2.1 (http://www.R-project.org/). Correlation analyses were conducted as mentioned in the figures.
Results
Pan-cancer view of integrin superfamily genes
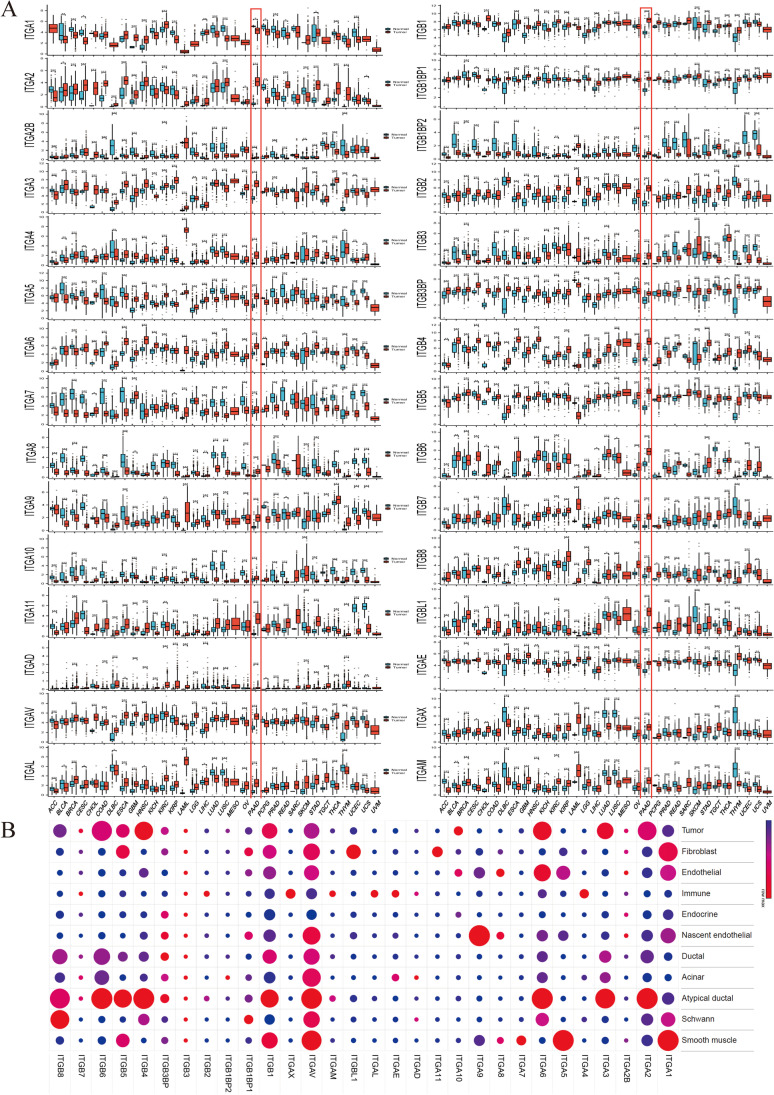
Thirty integrin superfamily genes were comprehensively analyzed to investigate their pan-cancer expression patterns, revealing the heterogeneous expression of most integrins between tumor and normal tissue across human cancer types (Fig. 1A). As determined using the Wilcoxon’s test, the expression of all integrins in pancreatic cancer, except that of ITGA1, increased in cancer tissues (Fig. 1A). Notably, differential expression analysis protocols may generate different results even when using the same datasets, as demonstrated by GEPIA2, wherein ITGA2B, ITGA7, ITGA8, ITGA10, ITGAD, and ITGB1BP2 did not exhibit significant difference (Supplementary Fig. 4A) (Du et al. 2022). Dysregulation of integrins is a characteristic feature of cancer, but the expression of specific genes varies with cancer and cell types. Single-nucleus RNA-seq analysis of untreated pancreatic cancer revealed heterogeneous distribution of integrins among cells. ITGA1, ITGA5, ITGA7, ITGA8, ITGA9, ITGA11, and ITGBL1 were found to be higher in stromal cells (fibroblasts, endothelial cells, nascent endothelial cells, and Schwann cells), while ITGA4, ITGAE, ITGAL, ITGAM, ITGAX, and ITGB2 were found to be higher in immune cells. ITGA2, ITGA3, ITGA10, ITGB4, and ITGB6 were more highly expressed in epithelial cells (tumor, atypical ductal, acinar, ductal, or endocrine cells), and ITGA2, ITGA3, and ITGB4 were highly expressed in malignant cells (tumor or atypical ductal cells) (Fig. 1B).
Fig. 1.
Expression landscape of integrin superfamily genes in multiple cancer types using bulk RNA sequencing data and pancreatic cancer using the single-nucleus RNA sequencing dataset of Hwang et al. A Boxplots depicting integrin expression in normal and tumor tissue samples from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) datasets (n = 20876), with significance assessed using the Wilcoxon sum test. TCGA-pancreatic ductal adenocarcinoma (PAAD) project is highlighted in red. Boxes represent the interquartile range, horizontal lines indicate the median, and whiskers represent 1.5 times the interquartile range. Outliers are represented by individual points. B Dot plot illustrating integrin superfamily gene expression across multiple cell types from the single-cell RNA sequencing dataset of treatment naïve PAAD of Hwang et al (n = 18). Each circle’s size corresponds to the percentage of cells exhibiting gene expression. Gene expression refers to the average expression values of genes rescaled by all cell types. Statistical significance is denoted as follows: *P < 0.05, **P < 0.01, and ***P < 0.001. Cancer project abbreviations are available on the website: https://gdc.cancer.gov/resources-tcga-users/tcga-code-tables/tcga-study-abbreviations
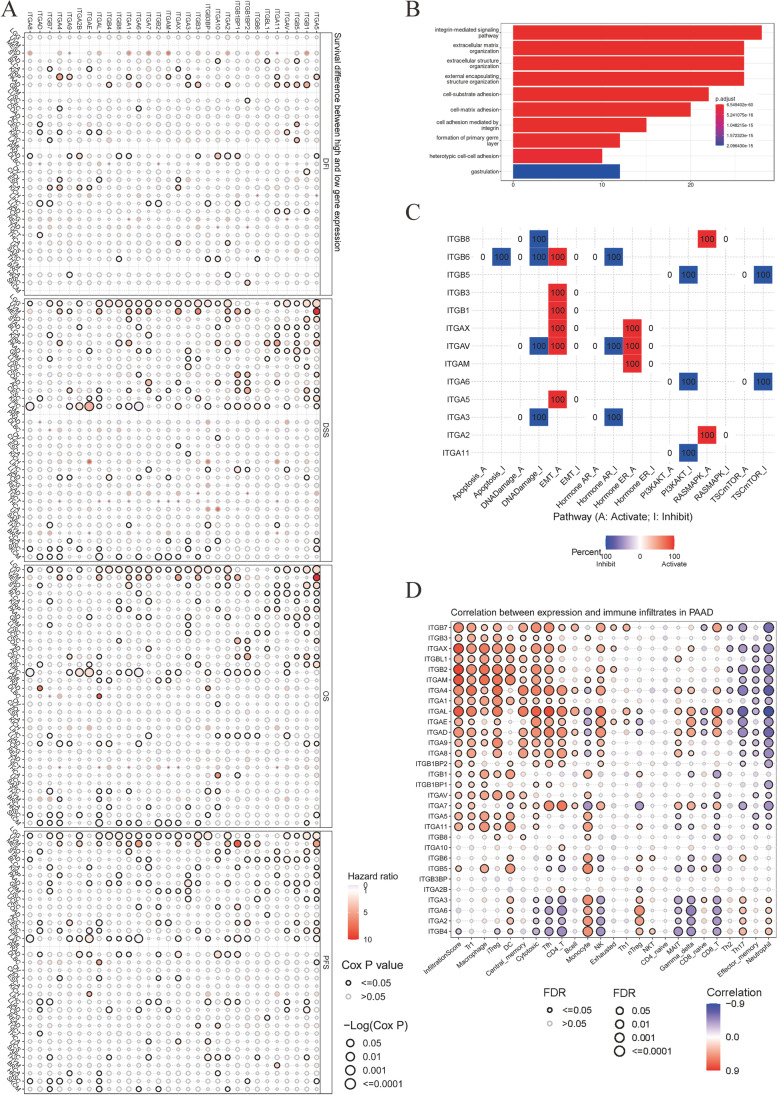
Figure 2A demonstrates the relationship between integrin gene expression and survival outcomes. Several integrins were found to be associated with all four clinical outcomes, namely OS, progression-free survival (PFS), disease-free interval (DFI), and disease-specific survival (DSS). Protective integrins included ITGB7 in bladder urothelial cancer (BLCA), ITGA7 in liver hepatocellular carcinoma (LIHC), and ITGAD and ITGAE in cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC). Conversely, hazardous integrins such as ITGA11 and ITGA5 were found in kidney renal papillary cell carcinoma (KIRP); ITGB1BP1 in LIHC; ITGB1 in lung adenocarcinoma (LUAD); ITGA2, ITGA3, ITGA6, ITGAV, ITGB1, and ITGB5 in PAAD; ITGB3BP in sarcoma; and ITGB5 in stomach adenocarcinoma (STAD). We evaluated the enrichment results of integrin superfamily genes in pancreatic cancer using GO-BP (Fig. 2B) and conducted cancer pathway analyses (Fig. 2C). Genes in the integrin superfamily were associated with the extracellular matrix, which mostly activates epithelial-mesenchymal transition (EMT, 5/13, Fig. 2C). Elevated expression of ITGA7, ITGAD, ITGAE, ITGAL, ITGA4, and ITGB7 positively correlated with a higher degree of CD8+ T cell infiltration into tumor sites, whereas the expression of ITGB4, ITGA2, ITGA6, ITGA3, ITGB5, ITGB6, ITGB8, ITGA11, ITGA5, ITGAV, and ITGB1 were negatively correlated with CD8+ T cell infiltration in pancreatic cancer (Fig. 2D).
Fig. 2.
Association between survival and prognosis and the enrichment pathways of integrin superfamily genes. A Cox proportional hazard regression modeling showing the association between overall survival (OS, n = 11505), progression-free survival (PFS, n = 11505), disease-specific survival (DSS, n = 10947), and disease-free interval (DFI, n = 5790) with integrin superfamily gene expression. B Bar plot displaying the top 10 Gene Ontology terms for biological processes associated with all integrins (n = 30). C Grid diagram illustrating the cancer pathways of the integrin superfamily genes. Red and blue shades represent the activated and inhibited pathways, respectively (n = 123). D Bubble plot illustrating the Spearman’s correlation between integrin superfamily gene expression and immune infiltration in in pancreatic ductal adenocarcinoma (PAAD, n = 183), adjusted using false discovery rate (FDR). Higher color intensity reflects a stronger correlation, while bubble size represents FDR significance. Bubbles with black borders have FDRs < 0.05. Abbreviations for the cancer project are available at https://gdc.cancer.gov/resources-tcga-users/tcga-code-tables/tcga-study-abbreviations. Abbreviations and definitions for infiltrated immune cells were provided by the Immune Cell Abundance Identifier (ImmuCellAI)
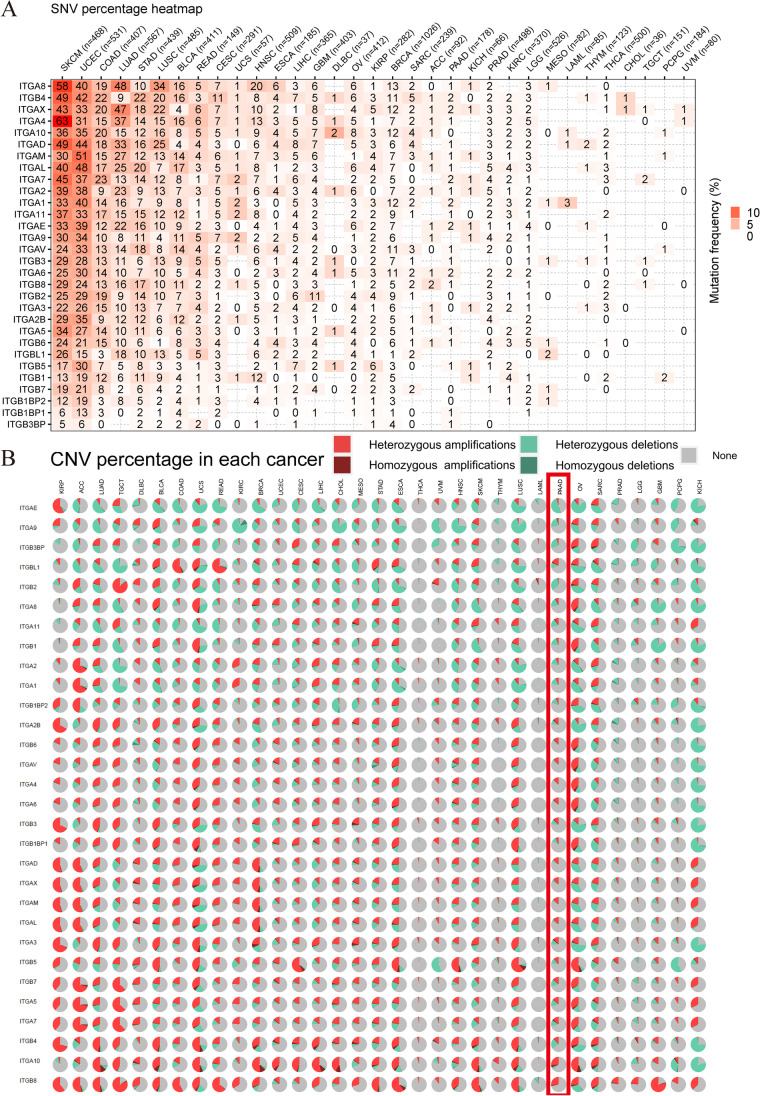
Genomic and epigenetic modifications such as DNA methylation can result in dysregulated gene expression, which can affect clinical outcomes in pancreatic cancer (X Li et al. 2022b). Figure 3A illustrates a pan-cancer overview of the deleterious mutations analyzed using GSCA. The patients with skin cutaneous melanoma (SKCM), uterine corpus endometrioid carcinoma (UCEC), colon adenocarcinoma (COAD), LUAD, STAD, lung squamous cell carcinoma (LUSC), BLCA, CESC, head and neck squamous cell carcinoma (HNSC), glioblastoma multiforme (GBM), and breast invasive carcinoma (BRCA) exhibited deleterious mutation rates of ≥ 10% among all cancers, whereas pancreatic cancer had a low rate. Copy number variants are believed to be an important source of transcriptomic dysregulation (Mielczarek et al. 2022). However, the cBioPortal oncoplot demonstrated that integrin superfamily genes have relatively low alteration rates (somatic mutations and homozygous alterations) ranging from 0 to 5% in pancreatic cancer (Supplementary Fig. 4B). Nevertheless, ITGA10 was identified as one of the most frequently altered integrin genes in patients with pancreatic cancer. When considering both homozygous and heterozygous alterations (homozygous deletions and homozygous amplifications), the number and types of alterations in integrin superfamily genes varied across cancers and most integrin superfamily genes were still not altered (Fig. 3B). Pan-cancer DNA methylation analysis revealed that ITGB3 was the most common hypomethylated integrin across cancer types, whereas ITGA8 was the most common hypermethylated integrin (Fig. 4A). We also observed a relationship between methylation and cell infiltration (Fig. 4B). ITGB6, ITGA10, ITGA3, ITGA6, ITGB3, and ITGA10 were positively correlated with infiltration scores, whereas ITGA5, ITGA9, ITGAM, ITGA7, ITGB1, ITGAE, ITGB2, ITGB7, ITGA4, ITGA8, ITGAL, ITGA11, and ITGAV were negatively correlated. Higher methylation of ITGA3, ITGA6, ITGB3, ITGB4, ITGB8, ITGBL1, and ITGB5 were associated with higher infiltration of CD8+ T cells into tumor sites, while ITGA9, ITGAM, ITGA7, ITGAE, ITGB2, ITGA4, ITGA8, and ITGAL had a negative impact in pancreatic cancer. TP53 is the second most commonly mutated gene in pancreatic cancer. The role of TP53 mutation in methylation regulation has been extensively studied (Sadzeviciene et al. 2022; Xiao et al. 2022; Zhou et al. 2023). In this study, we compared methylation levels between patients with and without TP53 mutation. Our findings indicated that methylation levels not only differ between pancreatic cancer and normal tissues but also differ among patients with or without TP53 mutation. In particular, ITGAV, ITGB4, ITGB5, ITGA2, ITGA3, ITGAX, ITGAE, ITGAL, ITGB6, and ITGBL1 exhibited lower methylation levels, whereas ITGA4, ITGA8, ITGA11, ITGAL, ITGAM, and ITGB2 showed higher methylation levels (Fig. 4C). Methylation levels inversely correlated with the majority of integrin expression, suggesting the possibility of methylation-dependent regulation of integrin mRNA expression (Supplementary Fig. 4C).
Fig. 3.
Mutation and alteration landscapes of integrin superfamily genes across multiple cancer types. A Deleterious mutations in integrin superfamily genes. The numbers within the boxes represent the number of deleterious gene mutations in different cancer types. An increase in color intensity indicates an increase in the percentage of deleterious mutations (n = 10234). B Pie charts showing the proportions of copy number alterations across cancer types (n = 11461). The red rectangle highlights information related to copy number variations in pancreatic ductal adenocarcinoma (PAAD, n = 184). CNV copy number variation, SNV single-nucleotide variation. The abbreviations for the cancer projects are available on the website: https://gdc.cancer.gov/resources-tcga-users/tcga-code-tables/tcga-study-abbreviations
Fig. 4.
DNA methylation of integrins in human cancers. A Bubble plot comparing methylation levels (β values) of integrin superfamily genes in normal and tumor tissues across multiple cancer types from The Cancer Genome Atlas (TCGA). Cancer types with < 10 normal samples were excluded (n = 6560). Bubble color represents methylation level differences between tumor and normal samples, ranging from blue to red. P values were estimated using the two-tailed unpaired t test and adjusted using the false discovery rate (FDR). Bubbles with black borders indicate FDRs < 0.05. Bubble size indicates FDR significance, while color intensity increases with correlation. B Spearman’s correlation between DNA methylation levels (β values) and cell infiltration degree for each cancer type from TCGA, evaluated using ImmuCellAI (n = 183). P values were adjusted using FDR. Color intensity increases with correlation. Bubbles with black borders represent FDRs < 0.05. Bubble size indicates FDR significance. C Boxplots showing the distribution of DNA methylation levels (β values) of integrins among normal pancreatic tissues, TP53 wild-type, and TP53 mutant pancreatic cancer tissues (n = 192). P values were calculated using the two-tailed unpaired t-test. Boxes indicate the interquartile range, horizontal lines represent the median, and whiskers indicate values 1.5 times the interquartile range. Abbreviations for cancer projects are available at https://gdc.cancer.gov/resources-tcga-users/tcga-code-tables/tcga-study-abbreviations. Abbreviations and definitions for infiltrated cells were provided by Immune Cell Abundance Identifier (ImmuCellAI). Methy.diff methylation difference, ns no significance
In Supplementary Fig. 5, we predicted 30 chemotherapy drugs associated with integrin expression using the Drug Sensitivity in Cancer (GDSC) and the Cancer Therapeutics Response Portal (CTRP) datasets. Analyzing the relationship between drug sensitivity and integrin expression, we found that increased expression of ITGB5, ITGA2, ITGA3, ITGAV, ITGB8, ITGBL1, and ITGA6 was positively correlated with resistance to 5-fluorouracil, methotrexate, trametinib (GDSC datasets, Supplementary Fig. 5a), lapatinib, bendamustine, brivanib, and ouabain (CTRP datasets, Supplementary Fig. 5B). Conversely, increased expression of ITGA4, ITGAL, ITGB7, ITGA9, ITGB1BP2, and ITGB3BP was correlated with enhanced drug sensitivity to the aforementioned drugs.
From these analyses, we observed that integrins play a critical role in cancer omics, with pancreatic cancer exhibiting the highest number of prognostic integrins. Our focus lies in identifying an integrin with promising molecular and clinical applications in patients with pancreatic cancer. However, tumor purity poses a challenge to the analyses and selection process, given the high stromal content of pancreatic cancer. Therefore, we aimed to address this issue by developing a purity-independent prognostic model that incorporates integrins for clinical applications.
Purity-independent RNA regulation network uncovered the significance of ITGA3 in pancreatic cancer
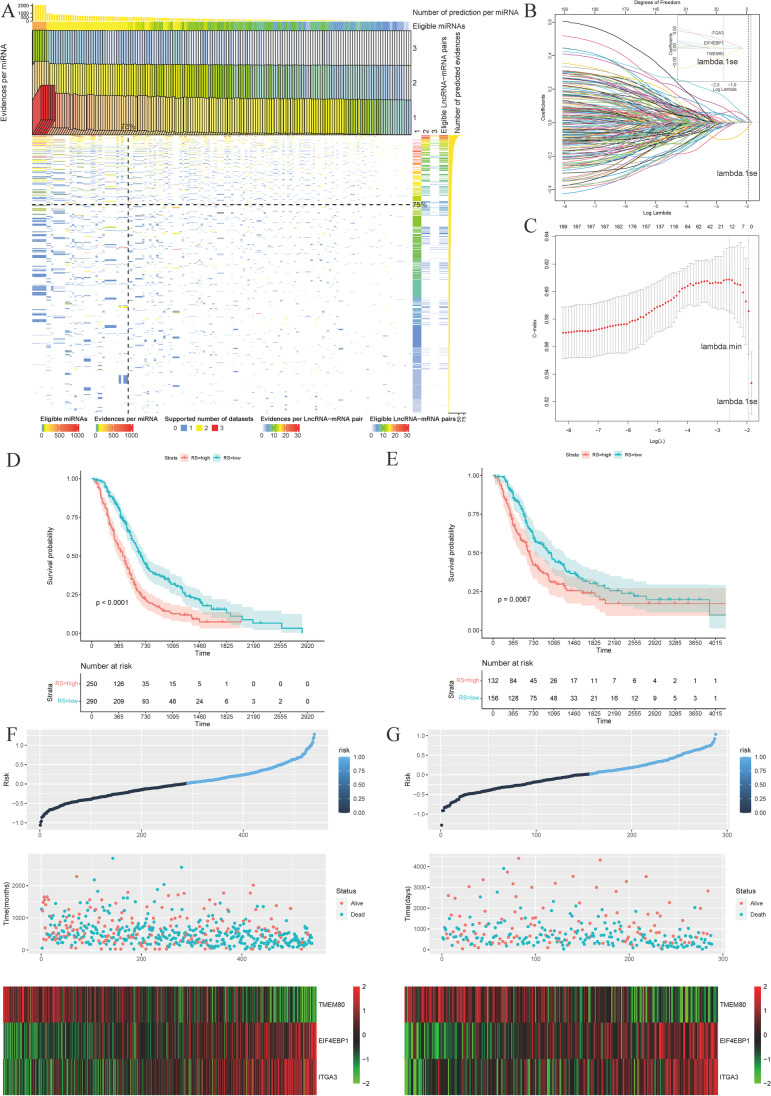
Integrins are instrumental in the management of noncoding RNAs (Tan et al. 2018; Xu et al. 2022; Zheng et al. 2019). Therefore, we used experimentally confirmed RNA regulatory relationships to computationally predict these relationships under different levels of purity to establish a purity-independent prognostic model. The detailed protocol for constructing the purity-independent RNA regulatory network has been described in the “Materials and methods” section. Figure 5A presents a heatmap illustrating the total evidence and number of evidence for each lncRNA–mRNA or miRNA pair using prediction results from TCGA datasets, GSE32688 and GSE119794. We observed that the top 75% of the RNA regulatory relationships accounted for the majority of the regulatory pairs, suggesting that the shared prediction results are more credible.
Fig. 5.
Construction and validation of the purity-independent regulatory network. A Heatmap displaying eligible lncRNA–miRNA–mRNA pairs supported by GDCRNAtools. Only experiments confirming RNA relationships were included. RNA relationships with consistent evidence support across datasets with varying tumor purity levels were considered preserved in pancreatic cancer (n = 3). B Model coefficients (top axis) versus the logarithm of lambda (bottom axis) for LASSO analysis of the construction group (n = 540). The coefficients of three genes were non-zero at lambda.1se, which represents the lambda value generating the most regularized model with cross-validated errors within one standard error of the minimum. C Three-fold cross-validation C-indexes versus the logarithm of lambda (bottom axis) and the number of non-zero coefficients (top axis) in the construction group (n = 540). D Kaplan–Meier curve illustrating the high- and low-risk groups in the construction model using the three-gene model (n = 540, log-rank test, P < 0.0001). E Kaplan–Meier curve illustrating the high- and low-risk groups in the validation model using the three-gene model (n = 288, log-rank test, P = 0.0067). F Risk score (RS) panel of the construction model (n = 540). G Risk score (RS) panel of the validation model (n = 288)
To establish a purity-independent network, we utilized 201 mRNA–lncRNA pairs exclusively from calculation results obtained from three high-quality datasets using pancreatic cancer samples exclusively. After excluding genes absent in the survival cohorts, we obtained 194 qualified genes present in all prognostic datasets. These genes were used to establish the optimal prognosis model through LASSO regression analysis. The genes suitable for the Cox model were selected from the candidate genes using the LASSO algorithm. We performed data subsampling and determined tuning parameters based on the C-index, estimated via three-fold cross-validation. The lambda coefficients and C-indexes were plotted for different lambda values (Fig. 5 B and C). Finally, we selected lambda.1se (0.143) for further model selection and chose three genes—TMEM80, EIF4EBP1, and ITGA3—to construct the multiple Cox risk regression model for predicting OS (Fig. 5 B and C).
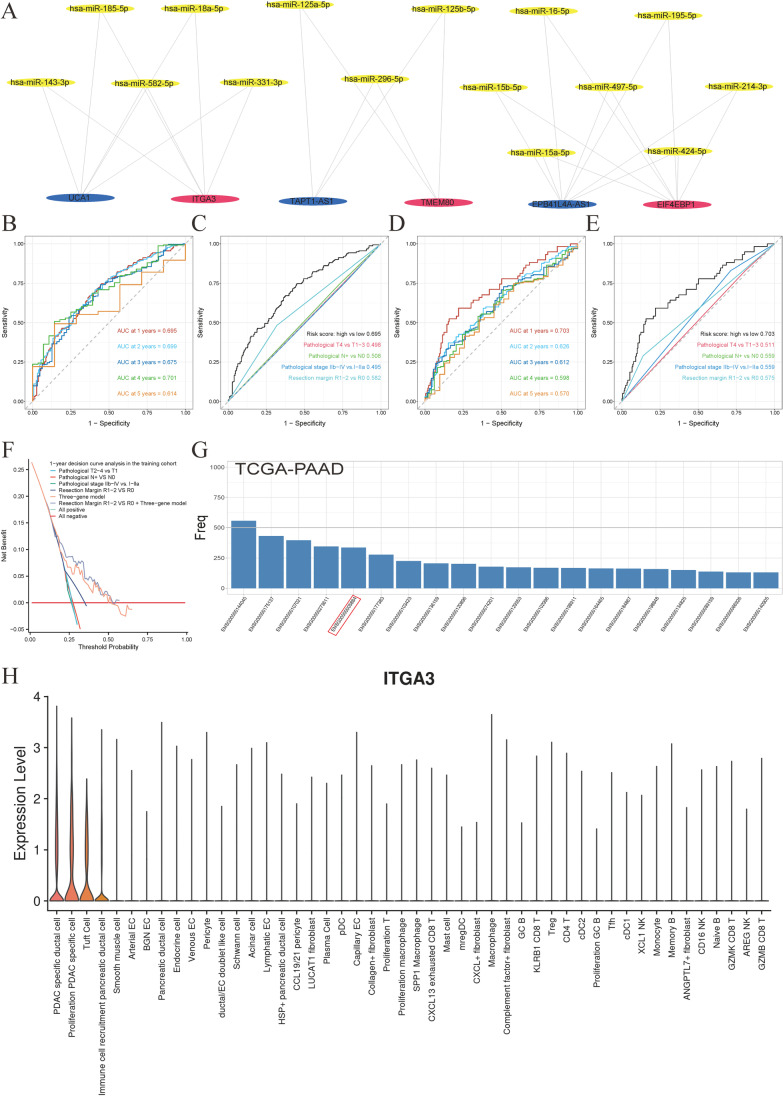
The optimal cut-off value for the risk score, which distinguishes the high-risk group from the low-risk group, was determined to be 0.02. In the construction group (Fig. 5 D and F) and the validation groups (E-MTAB-6134, Fig. 5 E and G), this value showed significance with P < 0.0001 and P = 0.0067, respectively. Higher scores in the model were associated with poor prognoses. The generalization of the model among each cohort was visualized at P < 0.05 (Supplementary Fig. 6A–E). The final mRNA-miRNA-lncRNA network genes, including three prognosis-related mRNAs, 15 miRNAs, and three lncRNAs, are shown in Fig. 6A. Time-dependent receiver operating characteristic (ROC) curves illustrated the 1-, 2-, 3-,4- and 5-year AUC values (0.695, 0.699, 0.675, 0.701, and 0.614, respectively) for the construction cohort (Fig. 6B). Given the high annual death rates in pancreatic cancer, we compared the 1-year AUC values of the three-gene model to the clinical model for 1-year survival in the construction group. The three-gene model proved to be a superior model for determining patients’ prognosis compared to commonly used clinical information markers, namely pathological tumor stage (pT), pathological nodal stage (pN), pathological stage, and resection margin (Fig. 6C). Figure 6D displays the 1-, 2-, 3-, 4-, and 5-year AUC values (0.703, 0.626, 0.612, 0.598, and 0.570) of the three-gene model for the validation group (E-MTAB-6134). The 1-year time-dependent AUC plots reveal that the three-gene model also emerged as the best clinical marker for determining patients’ prognosis in the validation groups (Fig. 6E). Decision curve analyses showed that the use of the three-gene predictive model was beneficial for patients in the construction group at 12 months after pancreatectomy (Fig. 6F). Furthermore, the three-gene model and the combined model (three-gene model high vs. low plus resection margin R1-2 vs. R0) showed a higher net benefit across all thresholds compared to other models for predicting OS of patients from the validation group at 12 months (Supplementary Fig. 6F).
Fig. 6.
Prognosis-related purity-independent three-gene network visualization. A Purity-independent regulatory network constructed using 3 prognosis-related mRNAs, 15 miRNAs, and 3 lncRNAs. B Time-dependent receiver operating characteristic (ROC) curves of the construction group (n = 540) for overall survival prediction using the three-gene model. C One-year time-dependent ROC curves for overall survival, showing higher area under the curve (AUC) values for the three-gene model compared to other clinic al factors in the validation groups (n = 288). D Time-dependent ROC curves of the validation group (n = 288) for overall survival prediction using the three-gene model. E One-year time-dependent ROC curves for overall survival, demonstrating higher AUC values for the three-gene model compared to other clinical factors in the validation groups (n = 288). F Decision curve analysis of the three-gene model, clinical models, and the combined model at 12 months post-pancreatectomy in the construction group (n = 540). G Frequency count of variables selected using the LASSO algorithm, repeated 1000 times in The Cancer Genome Atlas (TCGA) using BLASSO (n = 160). H Violin plot of ITGA3 expression constructed using the single-cell pancreatic cancer dataset of Peng et al. Cell types are arranged from left to right based on decreasing ITGA3 expression (n = 35)
To identify a reliable prognostic marker from the three-gene purity-independent model for further investigations, we employed the Blasso algorithm (Zeng et al. 2020). This algorithm involves frequency-based screening of the stability of prognostic variables based on LASSO. We presented the top 20 variables selected in the TCGA–PAAD cohort with 1000 repetitions. We observed that ITGA3 (ENSG00000005884) was the only gene among the top 20 variables in the selection results of the three-gene purity-independent model (Fig. 6G). ITGA3 was chosen for further investigation because of its stability in the selection process (Fig. 6G) and its significant role in pancreatic cancer, as highlighted by various studies (Liu et al. 2021; Rashid et al. 2020; Wenric et al. 2022). Single-cell analyses of ITGA3 using the dataset provided by Peng et al. (2019) demonstrated its high expression in malignant cells (Fig. 6H), indicating its potential biological implications.
Implications of ITGA3 in genomics and association with treatment resistance
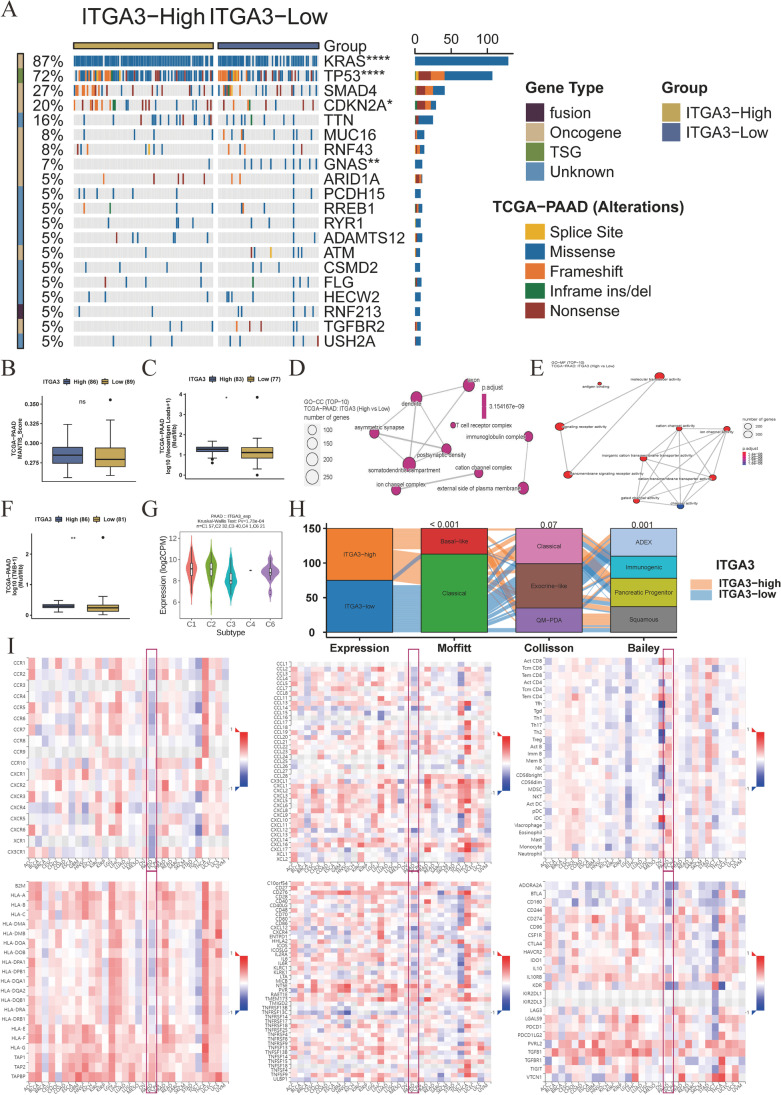
The mutation landscape differs between the high-ITGA3 and low-ITGA3 groups. The high-ITGA3 group exhibited higher rates of driver gene alterations, including KRAS, TP53, and CDKN2A. GNAS mutations, commonly found in intraductal papillary mucinous neoplasms, were more frequent in the low-ITGA3 group (Fig. 7A). We assessed the ability of ITGA3 participating in regulating the immune response. Although, there were no differences in Microsatellite Analysis for Normal-Tumor InStability (MANTIS) scores (regarding microsatellite instability, Fig. 7B) (Kautto et al. 2017). The high-ITGA3 group exhibited higher neoantigen loads (P < 0.05, Fig. 7C), cell components (CC) in T cell receptor complex and immunoglobulin complex (Fig. 7D), molecular function (MF) in antigen binding (Fig. 7E), and tumor mutational burden (P < 0.01, Fig. 7F) in high-ITGA3 samples. ITGA3 expression also differed among immune subtypes (P = 1.73 × 10−4, Fig. 7H). Most patients with the Moffit-basal subtype (Moffitt et al. 2015) exhibited high ITGA3 expression (P < 0.001). Patients with the Collisson quasi mesenchymal (QM-PDA) (Collisson et al. 2011) and Bailey squamous (Bailey et al. 2016) subtypes also exhibited high ITGA3 expression because of the overlapping classification of molecular subtypes (Fig. 7H). The correlation plots between ITGA3 and receptors, chemokines, tumor-infiltrating lymphocytes, major histocompatibility complex (MHC) molecules, immunostimulators, and immune inhibitors are shown in Fig. 7I. We observed that ITGA3 was positively correlated with classical and nonclassical MHC-I molecules and negatively correlated with MHC-II molecules and immune cell receptors.
Fig. 7.
Association of ITGA3 with genomic and molecular characteristics of pancreatic cancer. A Mutation landscape of the ITGA3-high and ITGA3-low expression groups. The Fisher’s exact test was used to determine statistical differences between groups (n = 175). B Microsatellite Analysis for Normal-Tumor InStability (MANTIS) scores in the ITGA3-high and ITGA3-low groups (n = 175). C Neoantigen loads in the ITGA3-high and ITGA3-low groups (n = 160). D Gene Ontology (GO) analysis of cell component (CC) in the ITGA3-high and ITGA3-low groups (n = 179). E GO analysis of molecular function (MF) in the ITGA3-high and ITGA3-low groups. Adjusted P values were calculated using the one-sided Fisher’s exact test and corrected for multiple hypotheses using the Benjamini–Hochberg false discovery rate (FDR) of 5% (n = 179). F Tumor mutation burden (TMB) in the ITGA3-high and ITGA3-low groups (n = 167). G Differential expression of ITGA3 among immune subtypes (n = 151). H High expression of ITGA3 in common molecular subtypes, including Moffit basal subtypes, Collisson quasimesenchymal subtypes (QM-PDA), and Bailey squamous subtypes. Differences between groups were assessed using the chi-square test (n = 150). I Spearman’s correlation analysis between ITGA3 and receptors, chemokines, tumor-infiltrating lymphocytes, MHC molecules, immune stimulators, and inhibitors in pan-cancer analysis. Blue squares indicate negative correlations, while red squares indicate positive correlations; darker colors indicate stronger correlations (n = 179). B, C, F, and G: Data represented as boxplots, and differences in immune-related scores or immune cells were compared using the Mann–Whitney U test. Boxes represent interquartile ranges, horizontal lines within each box represent the median, and whiskers represent values 1.5 times the interquartile range. Black dots indicate outliers. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001
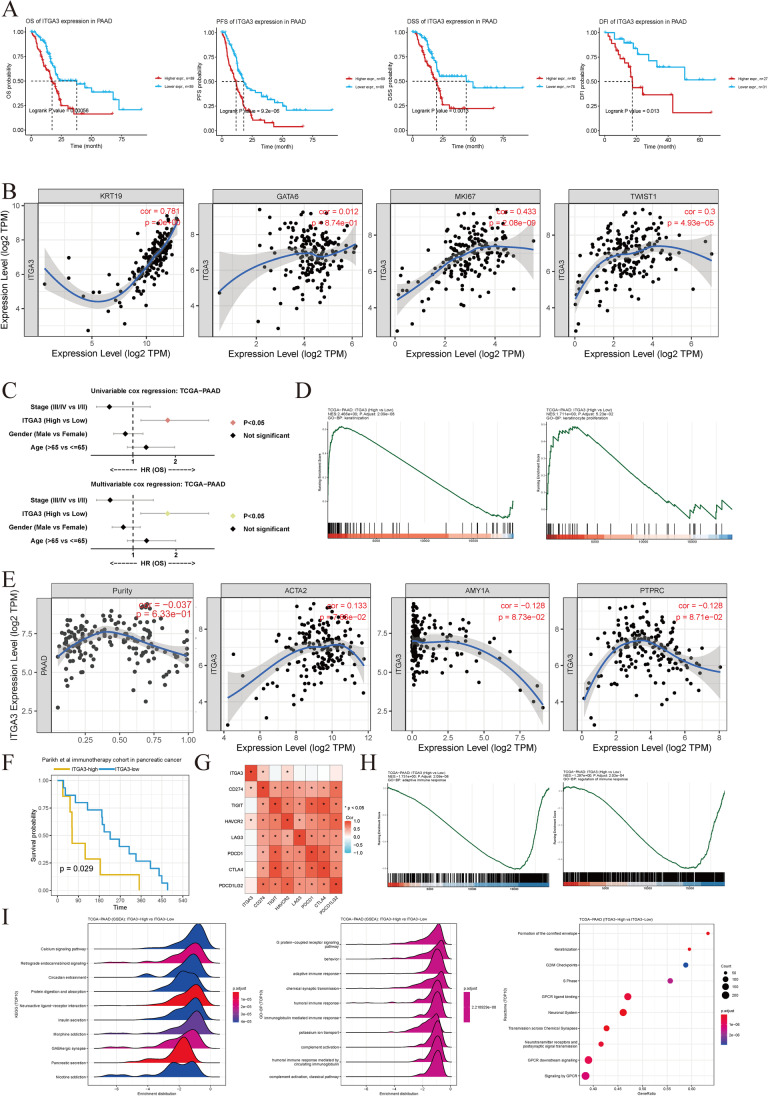
Figure 8A demonstrates that ITGA3 is a risk factor for OS, PFS, DSS, and DFI. The expression of ITGA3 was strongly correlated with KRT19 (a marker for malignant ductal cells, correlation coefficient 0.781, P < 0.001), MKI67 (a marker for cell proliferation, correlation coefficient 0.433, P < 0.001), and TWIST (a marker for EMT, correlation coefficient 0.3, P < 0.001). Patients with proliferative cancer cells and EMT features exhibited ITGA3 expression independent of GATA6 (a marker for the basal-classical subtype, with a correlation P > 0.05). ITGA3 was a better prognostic marker than clinical factors (stage, gender, and age) in both univariate and multivariate Cox regression models (Fig. 8C) in TCGA dataset plotted using CAMOIP. Using our own processing protocols, we observed that ITGA3 (hazard ratio = 1.32, in multivariate analysis) was as an independent hazardous marker along with surgical margin (hazard ratio = 1.86, in multivariate analysis; Supplementary Fig. 7A) in TCGA–PAAD. GSEA results reveal that the keratinization and keratinocyte proliferation pathways differed between high-ITGA3 and low-ITGA3 groups, which explain the malignant phenotype in the high-ITGA3-high pancreatic cancer cohort with or without chemotherapy (Fig. 8D). No significant association between ITGA3 expression and tumor purity was found (Fig. 8E). Furthermore, the other two genes in the risk model did not exhibit a high correlation with tumor purity (Supplementary Fig. 7B). ITGA3 was not related to other compartment markers such as ACTA2 (a muscle and fibroblast marker), AMY1A (an acinar cell marker), or PTPRC (CD45, an immune cell marker), further supporting the idea that ITGA3 is not severely affected by environmental cells (Fig. 8E). Higher ITGA3 expression was correlated with poor prognosis in the Parikh et al. immunotherapy cohort, which includes pancreatic and colorectal cancer (Fig. 8F). ITGA3 was positively correlated with immune exhaustion markers CD274 (PD-L1, P < 0.05) and HAVCR2 (Tim-3, P < 0.05). Regarding the GSEA of immune pathways, downregulation of immune response, especially in adaptive immune response, was found to be more common in high-ITGA3 patients (Fig. 8H). The top 10 KEGG pathways using GSEA, GO-BP terms using GSEA, and Reactome terms using ORA reveal enrichment of various cancer and immune suppression pathways in high-ITGA3 pancreatic cancer samples (P < 0.05, Fig. 8I).
Fig. 8.
Association of ITGA3 expression with chemotherapy and immunotherapy resistance. A ITGA3 is a risk factor for overall survival (OS, n = 178), progression-free survival (PFS, n = 178), disease-specific survival (DSS, n = 158), and disease-free interval (DFI, n = 58) (P values determined using log-rank tests). B Correlation of ITGA3 with KRT19 (malignant ductal cell marker, Spearman’s correlation coefficient: 0.781, P < 0.001), MKI67 (cell proliferation marker, Spearman’s correlation coefficient: 0.433, P < 0.001), and TWIST (EMT marker, Spearman’s correlation coefficient: 0.3, P < 0.001). ITGA3 expression is independent of GATA6 expression (pancreatic cancer basal-classical subtype marker, Spearman correlation, P > 0.05). n = 179 C ITGA3 is a superior univariable and multivariable hazard factor compared to clinical markers (stage, age, and sex) based on CAMOIP. D Gene set enrichment analysis (GSEA) of Gene Ontology (GO) terms for biological processes in the ITGA3-high and ITGA3-low expression groups. Adjusted P values were calculated using the one-sided Fisher’s exact test and corrected for multiple hypotheses using the Benjamini–Hochberg false discovery rate (FDR) of 5% (n = 179). E Spearman’s correlation analysis of ITGA3 expression and cell component markers (ACTA2, AMY1A, and PTPRC), indicating the purity independence of ITGA3 expression in pancreatic cancer (P > 0.05, n = 179). F ITGA3 was associated with immunotherapy resistance for OS (Parikh et al., 2021, n = 23). P values determined using log-rank tests (P < 0.05). G Positive correlation between ITGA3 expression and the immunotherapy genes CD274 (PD-L1) and HAVCR2 (Spearman’s correlation, P < 0.05). n = 179 H GSEA of GO terms for biological processes indicating the suppression of adaptive immunity in patients with high ITGA3 expression. n = 179 I GSEA of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and GO terms for biological processes, as well as Reactome analysis in patients with high ITGA3 expression (n = 179). Adjusted P values were calculated using the one-sided Fisher's exact test and corrected for multiple hypotheses using FDR
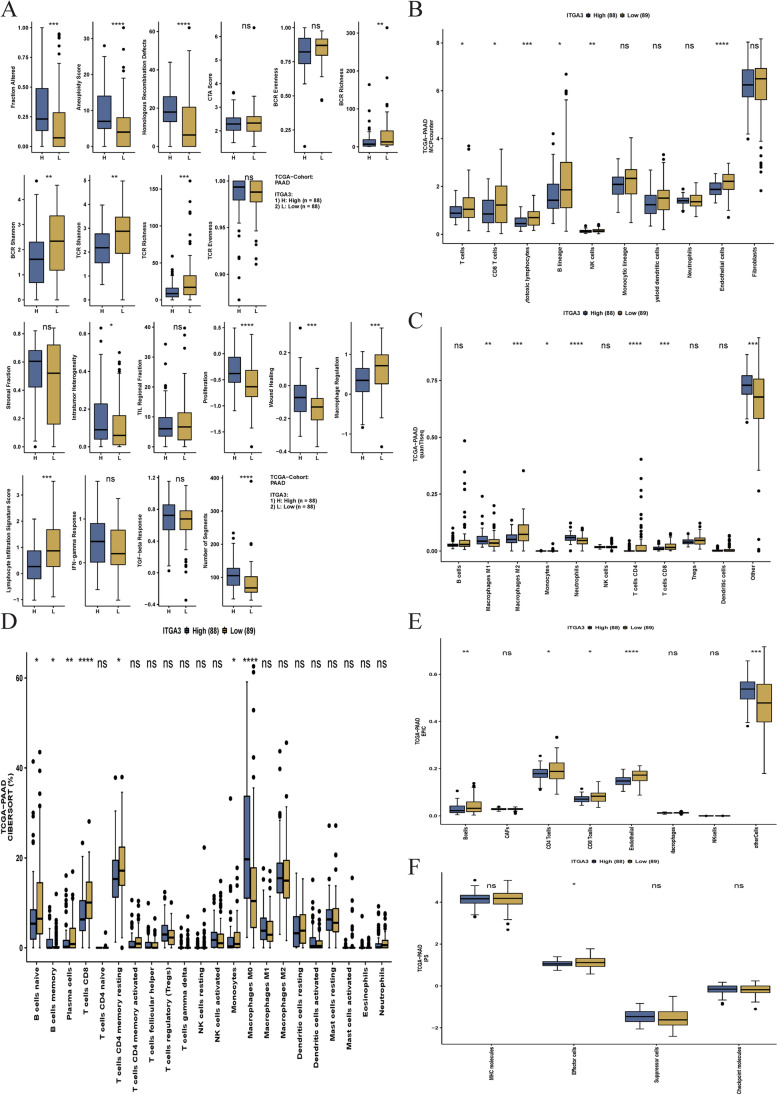
GSVA results show that high-ITGA3 patients exhibited a higher altered fraction, aneuploidy score, homologous recombination defects, proliferation, intratumor heterogeneity, wound healing, and number of segments. However, patients with high ITGA3 expression exhibited lower lymphocyte infiltration, TCR and BCR Shannon, TCR and BCR richness, macrophage regulation, and lymphocyte infiltration signature scores (Fig. 9A). We plotted current benchmark deconvolution methods in Fig. 9B–F and observed contradictory results because of different algorithms (MCPcounter, Fig. 9B; quanTIseq, Fig. 9C; CIBERSORT, Fig. 9D; EPIC, Fig. 9E; IPS, Fig. 9F). Interestingly, we observed that CD8+ T cells were consistently downregulated across the methods in patients with high ITGA3 expression, suggesting that high ITGA3 expression is associated with an immune suppressive environment and a poorer response to immunotherapy.
Fig. 9.
Effect of ITGA3 expression on immune response pathways and cell composition in pancreatic cancer tissue. A Gene set variation analysis (GSVA) comparing immune response pathways in ITGA3-high and ITGA3-low groups (n = 176). B–E Deconvolution results of cell components using bulk RNA sequencing with The Cancer Genome Atlas-pancreatic ductal adenocarcinoma (TCGA–PAAD) dataset (n = 177). Cell composition analyzed using MCPcounter (B), quanTIseq (C), CIBERSORT (D), EPIC (E), and IPS (F) in samples with high and low ITGA3 expression. Boxplots illustrate the data, and the Mann–Whitney U test was used to compare immune-related scores or immune cells. Boxes represent the interquartile range, horizontal lines represent the median, and whiskers indicate values 1.5 times the interquartile range. Outliers are depicted as black dots. Note: ns no significance; *P < 0.05, **P < 0.01, and ***P < 0.001
Validation of ITGA3 expression in vivo and in vitro
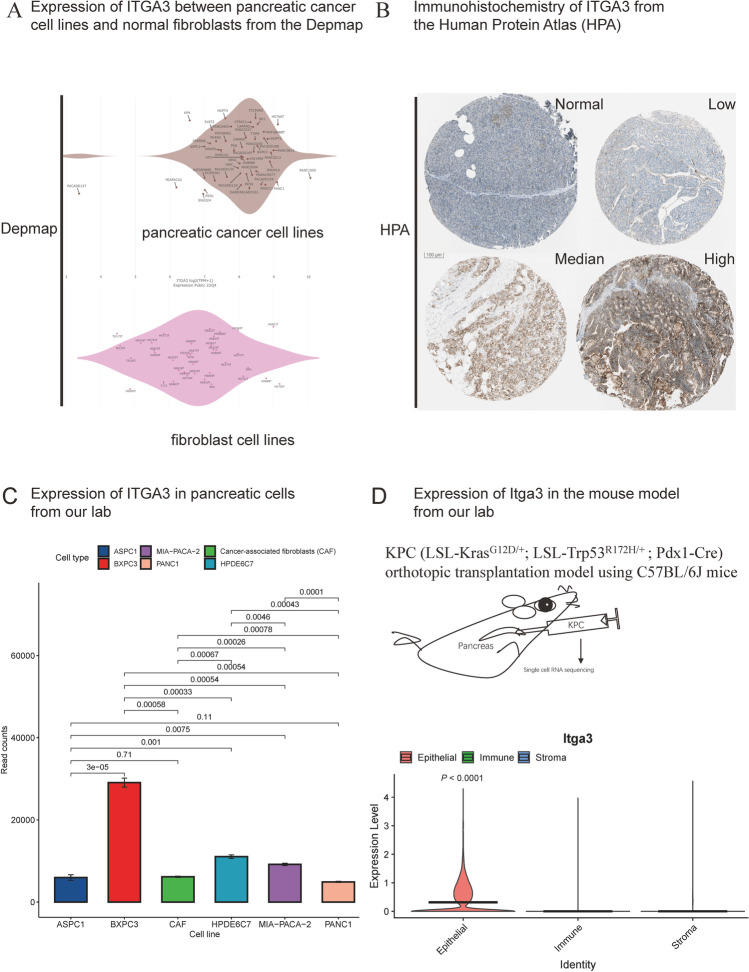
In the present study, we analyzed ITGA3 in patient genomics data and validated its expression in cell lines and immunohistochemistry imaging, which are widely used by laboratory researchers and pathologists. ITGA3 expression was found to vary among pancreatic cancer cell lines from different molecular subtypes (Fig. 10A) but was relatively higher than in pan-organ normal fibroblasts based on data from the Cancer Dependency Map Project (DepMap) (Yu et al. 2019). Figure 10B depicted ITGA3 expression in normal pancreatic samples as well as low-, median-, and high-ITGA3 pancreatic cancer samples using the Human Protein Atlas (HPA, https://www.proteinatlas.org/). While non-epithelial cells showed low but relatively constant ITGA3 expression, the greatest expression variances were observed in cancer cells (Fig. 10B). We also generated RNA-seq data from KRAS wild-type (BXPC3) and mutant (PANC1, MIA-PACA-2, and ASPC1) cancer cell lines, as well as the HPDE6C7 (an immortalized normal ductal cell line) and an immortalized primary CAF cell line (Fig. 10C, each cell line sequenced in triplicate). Comparing with single-cell (Fig. 1B) and single-nucleus (Fig. 6H) RNA-sequencing results, we observed notable ITGA3 expression in nonneoplastic cells in vitro (Fig. 10C).
Fig. 10.
Validation of ITGA3 expression in pancreatic cancer. A Expression of ITGA3 in pancreatic cancer cell lines and normal fibroblasts from the Cancer Dependency Map Project (DepMap, n = 88). B Immunohistochemistry of ITGA3 in pancreatic tissue and cancer samples from the Human Protein Atlas (HPA, n = 4). C ITGA3 expression in pancreatic cells (PANC-1, BxPC3, HPDE6C7, ASPC1, MIA-PACA-2, and cancer-associated fibroblasts [CAFs]) in our laboratory; each sequencing analysis was performed three times. Gene expression differences among cell lines were compared using the t-test, n = 3. D Validation of Itga3 expression using single-cell RNA sequencing in a mouse model developed in our laboratory. The violin plot illustrates significantly higher expression of Itga3 in epithelial cells than in immune cells (P < 0.0001) and stroma cells (P < 0.0001). Gene expression differences between epithelial cells and other cells were compared using the Mann–Whitney U test, n = 4
The Moffit basal subtype cell line BXPC3 displayed the highest ITGA3 expression, supporting our inference (all P < 0.001). However, PANC1 exhibited a lower ITGA3 mRNA expression than the normal epithelial cell line HPDE6C7 (P = 4.3 × 10−4) and CAFs (P = 7.8 × 10−4). To address this seemingly contradictory result, we established an orthotopic transplantation model by injecting 5 × 105 cultured KPC cells into four C57BL/6J mice to investigate Itga3 expression in vivo. Subsequently, we performed single-cell sequencing of pancreatic cancer tissues 28 days after orthotopic transplantation. We categorized the cell types in mice into epithelial, immune, and stromal compartments. Violin plots illustrating the expression of Itga3 (mouse gene version, Fig. 10D) using the Wilcoxon test revealed higher median expression in epithelial cells (including malignant cells and a few normal ductal, endocrine, and acinar cells) than in immune cells (mainly T, B, NK, and myeloid cells, P < 0.0001) and stromal cells (fibroblasts and endothelial cells, P < 0.0001). Our animal model results and human results are consistent with each other. We found that normal cells also express ITGA3; however, single-cell sequencing results in both humans and mice demonstrated that ITGA3 is primarily expressed in the epithelial compartment in vivo. Only a small number of epithelial cells exhibit weaker ITGA3 expression compared to immune and stromal compartments (Fig. 10D).
Discussion
Herein, the integrin superfamily genes were comprehensively analyzed. Using a purity-independent gene regulatory network, we demonstrated the importance of ITGA3 in pancreatic cancer as a prognostic marker for molecular transformation into the basal subtype and for patients who receive either chemotherapy or immunotherapy.
Studies have focused on the involvement of integrins in tumor stemness, proliferation, metastasis, and other malignant phenotypes (Cooper and Giancotti 2019; Dustin 2019; Weiss et al. 2022). Integrative analysis of a pan-cancer TCGA cohort, mostly comprising patients who received chemotherapy, revealed that the prognostic significance of distinct integrins varies across tumor types. The integrin/FAK cascade controls YAP phosphorylation and nuclear localization, resulting in the activation of the YAP/TAZ signaling pathway, which controls stem cell expansion in normal and cancer tissues (Cooper and Giancotti 2019; Hu et al. 2017). The integrins α3β1 and α6β1 on the tumor surface mediate the transendothelial migration of circulating tumor cells.
The significance of integrins in immunotherapy has been reported (Cooper and Giancotti 2019; Ruan et al. 2020; Shaim et al. 2021). The integrins on immune cells play critical roles in adhesion, homing, and trafficking and can be used to specifically target a particular cell, thereby increasing or inhibiting the immune response (Dustin 2019; Ran et al. 2022). For example, in benign diseases, intestinal antigen-presenting cells can promote regulatory T cell activity via ITGB8, resulting in immune tolerance in the intestine to alleviate inflammatory bowel disease (Akagbosu et al. 2022). Furthermore, integrin αV/β8 on the surface of pancreatic cells induces DNA methylation and downregulates the expression of glucose metabolism- and oxidative phosphorylation-related genes in M1-like macrophages, but not in M2-like macrophages, resulting in immune suppression via direct cell–cell interaction. Blocking of integrin αV/β8 can suppress tumor-induced DNA methylation of Nqo-1 and reprogramming of macrophage metabolism (Zhang et al. 2021). In tumor therapy, T cell cytotoxicity is enhanced by targeting the myeloid cell surface marker CD11b (ITGAM), resulting in the repolarization of macrophages, improvements in the responses of dendritic cells, and a decrease in myeloid-derived suppressor cells in pancreatic cancer (Bagati et al. 2021). We expanded the application of the integrin ITGA3, a basal subtype transforming gene associated with lesser degree of infiltration of CD8+ T cells and higher expression of PD-L1, to both chemotherapy and immunotherapy resistance. These lines of evidence confirm that using integrins to modulate immune responses may be a potential therapeutic target for treating pancreatic cancer.
Another aspect of the present study was the development of a purity-independent prognostic model, identification of the significance of ITGA3 as a single gene marker. Purity Independent Subtyping of Tumors (PurIST) and some studies have comprehensively explained risk stratification and treatment response from every aspect (Rashid et al. 2020; Shi et al. 2022; Tang et al. 2022). Compared to other models, our model possesses the advantage of being both simple and independent of tumor purity. Notably, the benchmark study PurIST also included ITGA3 in the 14-gene purity independent model to determine the basal subtype in pancreatic cancer (Rashid et al. 2020). Sample purity is an important issue hampering the identification of molecular subtypes in cancer. The application of spatial transcriptomics, bulk RNA sequencing, and bulk proteomics as well as other novel omics technologies for clinical sample analysis is currently affected by sample location, with different tumor heterogeneity and purity (Cao et al. 2021; Cui Zhou et al. 2022; Hwang et al. 2022). To construct the purity-independent regulatory model in pancreatic cancer, we adopted the ceRNA mechanism, which forms a link between the functions of protein-coding mRNAs and noncoding RNAs (such as lncRNAs, circRNAs, and miRNAs, including NamiRNAs) (Bailey et al. 2016; Liang et al. 2019; Yan et al. 2021) and has been considered a post-transcriptional regulation mechanism that is widely involved in the pathological processes of various cancers, including breast, colon, lung, gastric, and pancreatic cancers (Abdollahzadeh et al. 2019; X Liu et al. 2023b; Qi et al. 2015; Shuwen et al. 2018; Yan et al. 2021). By preserving the correlation between RNA interaction pairs under different tumor purities, we constructed the three-gene purity-independent network. For cancer research, it is important to select a feasible single-gene model (Ortega et al. 2022). Although the 14-gene model from the PurIST study and GATA6 as a single-gene model have been selected as benchmark classifiers for pancreatic cancer, we recommend ITGA3 as a promising supplementary marker for determining the immunotherapy or chemotherapy refractory status of patients with pancreatic cancer, regardless of the sampling location. We observed that HPDE6C7 cells and CAFs in pancreatic cancer also express ITGA3 to a certain extent in vitro. Similar results were observed in DepMap-based cell line analysis. Nonmalignant cells expressing ITGA3 contribute to steady ITGA3 expression, suggesting that ITGA3 serves as a marker for variations among molecular subtypes rather than for variations among tumor purities. However, we also observed a discrepancy in the expression of ITGA3 between laboratory cell lines and clinical samples, that the low expression of ITGA3 in some pancreatic cancer cells compared to non-malignant cells in vitro. However, using single-cell RNA-seq in humans and mice, and single-nucleus RNA-seq in humans, we observed that average ITGA3 expression was significantly higher in epithelial cells than that in immune and stroma cells. This situation dominates the majority in vivo. As cell lines represent only a fraction of the heterogeneous cancer cells found in vivo, and their gene expression in vitro may not align with in vivo observations. This highlights the issue of inadequate representativeness when relying solely on tumor cell lines for research purposes. Collaboration between clinicians and researchers are needed to bridge the gap between laboratory and clinical results (Sailer et al. 2023). The use of single-cell RNA sequencing has greatly expanded our understanding of precision therapy; the results of single-cell analysis can be combined to select the most efficient therapeutic targets (Yang et al. 2023; Yu et al. 2023). For example, a treatment regimen aimed at targeting the tumor stem cell marker ITGA4 may result in undesired effects owing to the high expression of ITGA4 in immune cells other than tumor cells, as illustrated in Fig. 1A (Ruan et al. 2020). In contrast, ITGA3 is highly localized to the tumor epithelium within tumor tissues, with weak expression in surrounding cells.
A limitation of the present study is that we analyzed public databases because we could not collect freely accessible data from single-cell and bulk sequencing data (Cui Zhou et al. 2022). To address this, we employed a combination of bulk, single-cell, and single-nucleus sequencing datasets, laboratory-collected cell lines, and mouse models to support the reliability of our conclusions. Another limitation is the inability to develop a highly generalized model from a single dataset with a small sample without combining the datasets, possibly introducing bias in the combined datasets used for model construction, which may introduce bias. However, we independently assessed the prognostic values of the three-gene model in each database. Finally, although we identified the advantages of ITGA3 in predicting chemotherapy and immunotherapy resistance, additional prospective clinical trials are warranted for further investigation (Dean et al. 2022; Principe et al. 2021; Slack et al. 2022; Zeltz et al. 2020).
Conclusions
Comprehensive multi-omics analyses showed dysregulation of integrin superfamily genes in pancreatic cancer and other cancers. Among all integrins, ITGA3 emerged as a promising single-gene biomarker with clinical relevance, specifically associated with resistance to chemotherapy and immunotherapy in pancreatic cancer owing to its purity-independent characteristics.
Supplementary information
Acknowledgements
We appreciate the research conditions provided by the Peking Union Medical College.
Inclusion of identifiable human data
No potentially identifiable human images or data are presented in this study.
Abbreviations
- TCGA
The Cancer Genome Atlas
- ICGC
International Cancer Genome Consortium
- GPL
Gene Expression Omnibus Platform
- TMM
Trimmed mean of M-values
- VOOM
Variance modeling at the observational level
- LASSO
Least absolute shrinkage and selection operator
- GISTIC
Genomic Identification of Significant Targets in Cancer
- ORA
Over-representation analysis
- GSVA
Gene set variation analysis
- GSEA
Gene set enrichment analysis
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- PCA
Principal component analysis
- lncRNA
Long non-coding RNA
- miRNA
MicroRNA
- mRNA
Messenger RNA
- StarBase
Splicing and Transcriptome Analysis Resource Database
- CAF
Cancer-associated fibroblast
- ATCC
American Type Culture Collection
- GRCm38
Genome Reference Consortium Mouse Build 38
- ceRNA
Competitive endogenous RNA
- AUC
Area under the curve
- Methyl.diff
Methylation difference
- NA
Missing
- ns
No significance
- CNV
Copy number variation
- SNV
Single-nucleotide variation
- FDR
False discovery rate
- ROC
Receiver operating characteristic
- MF
Molecular function
- RS
Risk score
- CC
Cell component
- BP
Biological process
- OS
Overall survival
- PFS
Progression-free survival
- DSS
Disease-specific survival
- DFI
Disease-free interval
- EMT
Epithelial-mesenchymal transition
- MHC
Major histocompatibility complex
- LAML
Acute myeloid leukemia
- ACC
Adrenocortical carcinoma
- BLCA
Bladder urothelial carcinoma
- LGG
Brain lower grade glioma
- BRCA
Breast invasive carcinoma
- CESC
Cervical squamous cell carcinoma and endocervical adenocarcinoma
- CHOL
Cholangiocarcinoma
- COAD
Colon adenocarcinoma
- ESCA
Esophageal carcinoma
- GBM
Glioblastoma multiforme
- HNSC
Head and neck squamous cell carcinoma
- KICH
Kidney chromophobe
- KIRC
Kidney renal clear cell carcinoma
- KIRP
Kidney renal papillary cell carcinoma
- LIHC
Liver hepatocellular carcinoma
- LUAD
Lung adenocarcinoma
- LUSC
Lung squamous cell carcinoma
- DLBC
Lymphoid neoplasm diffuse large B-cell lymphoma
- MESO
Mesothelioma
- OV
Ovarian serous cystadenocarcinoma
- PAAD
Pancreatic adenocarcinoma
- PCPG
Pheochromocytoma and paraganglioma
- PRAD
Prostate adenocarcinoma
- READ
Rectum adenocarcinoma
- SARC
Sarcoma
- SKCM
Skin cutaneous melanoma
- STAD
Stomach adenocarcinoma
- TGCT
Testicular germ cell tumors
- THYM
Thymoma
- THCA
Thyroid carcinoma
- UCS
Uterine carcinosarcoma
- UCEC
Uterine corpus endometrial carcinoma
- UVM
Uveal melanoma
Authors’ contributions
XHZ, YXD, MYL, and CFW contributed to study conception and manuscript writing; XHZ, YXD, and MYL contributed to data collection and analysis. XHZ, YXD, MYL, and CFW contributed to manuscript revision. The manuscript has been read and approved by all authors.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 81972314) and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (Grant No. 2022-I2M-1-010).
Data availability
Raw RNA sequencing data for BxPC3, PANC1, and HPDE6C7 cell lines have been deposited in the Gene Expression Omnibus database under the accession number GSE185069. For raw RNA sequencing data of MIA-CAPA-2, ASPC1, the cancer-associated fibroblast line, and single-cell RNA sequencing data of transplanted tumors, please contact the corresponding author. The open-access data can be downloaded from the original articles as described.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (Grant No. NCC2022C-928). The need for informed consent for using open access databases was waived. Animal experiments were approved by the Animal Committee of the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (Grant No. NCC2022A521).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s10142-024-01373-4
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/11/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s10142-024-01373-4
References
- Abdollahzadeh R, Daraei A, Mansoori Y, Sepahvand M, Amoli MM, Tavakkoly-Bazzaz J. Competing endogenous RNA (ceRNA) cross talk and language in ceRNA regulatory networks: A new look at hallmarks of breast cancer. J Cell Physiol. 2019;234(7):10080–10100. doi: 10.1002/jcp.27941. [DOI] [PubMed] [Google Scholar]
- Akagbosu B, Tayyebi Z, Shibu G, Paucar Iza YA, Deep D, Parisotto YF, Fisher L, Pasolli HA, Thevin V, Elmentaite R, Knott M, Hemmers S, Jahn L, Friedrich C, Verter J, Wang ZM, van den Brink M, Gasteiger G, Grunewald TGP, Marie JC, Leslie C, Rudensky AY, Brown CC. Novel antigen-presenting cell imparts T (reg)-dependent tolerance to gut microbiota. Nature. 2022;610(7933):752–760. doi: 10.1038/s41586-022-05309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, Chak S, Naikawadi RP, Wolters PJ, Abate AR, Butte AJ, Bhattacharya M. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20(2):163–172. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athar A, Fullgrabe A, George N, Iqbal H, Huerta L, Ali A, Snow C, Fonseca NA, Petryszak R, Papatheodorou I, Sarkans U, Brazma A. ArrayExpress update - from bulk to single-cell expression data. Nucleic Acids Res. 2019;47(D1):D711–D715. doi: 10.1093/nar/gky964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badheeb M, Abdelrahim A, Esmail A, Umoru G, Abboud K, Al-Najjar E, Rasheed G, Alkhulaifawi M, Abudayyeh A, Abdelrahim M. Pancreatic tumorigenesis: precursors, genetic risk factors and screening. Curr Oncol. 2022;29(11):8693–8719. doi: 10.3390/curroncol29110686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagati A, Kumar S, Jiang P, Pyrdol J, Zou AE, Godicelj A, Mathewson ND, Cartwright ANR, Cejas P, Brown M, Giobbie-Hurder A, Dillon D, Agudo J, Mittendorf EA, Liu XS, Wucherpfennig KW. Integrin alphavbeta6-TGFbeta-SOX4 pathway drives immune evasion in triple-negative breast cancer. Cancer Cell. 2021;39(1):54–67 e59. doi: 10.1016/j.ccell.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, Zhang T, Dai M, Zhao Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1–11. doi: 10.1016/j.canlet.2021.06.027. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Electronic address a a d h e, & Cancer Genome Atlas Research N Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185–203. doi: 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Huang C, Cui Zhou D, Hu Y, Lih TM, Savage SR, Krug K, Clark DJ, Schnaubelt M, Chen L, da Veiga LF, Eguez RV, Yang W, Pan J, Wen B, Dou Y, Jiang W, Liao Y, Shi Z, Terekhanova NV, Cao S, Lu RJ, Li Y, Liu R, Zhu H, Ronning P, Wu Y, Wyczalkowski MA, Easwaran H, Danilova L, Mer AS, Yoo S, Wang JM, Liu W, Haibe-Kains B, Thiagarajan M, Jewell SD, Hostetter G, Newton CJ, Li QK, Roehrl MH, Fenyo D, Wang P, Nesvizhskii AI, Mani DR, Omenn GS, Boja ES, Mesri M, Robles AI, Rodriguez H, Bathe OF, Chan DW, Hruban RH, Ding L, Zhang B, Zhang H, Clinical Proteomic Tumor Analysis C Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell. 2021;184(19):5031. doi: 10.1016/j.cell.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer JClin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16(4):207–220. doi: 10.1038/s41575-019-0109-y. [DOI] [PubMed] [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, Gray JW. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium G. Ardlie KG, Deluca DS, Segrè AV, Sullivan TJ, Young TR, Gelfand ET, Trowbridge CA, Maller JB, Tukiainen T. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J, Giancotti FG. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell. 2019;35(3):347–367. doi: 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Zhou D, Jayasinghe RG, Chen S, Herndon JM, Iglesia MD, Navale P, Wendl MC, Caravan W, Sato K, Storrs E, Mo CK, Liu J, Southard-Smith AN, Wu Y, Naser Al Deen N, Baer JM, Fulton RS, Wyczalkowski MA, Liu R, Fronick CC, Fulton LA, Shinkle A, Thammavong L, Zhu H, Sun H, Wang LB, Li Y, Zuo C, McMichael JF, Davies SR, Appelbaum EL, Robbins KJ, Chasnoff SE, Yang X, Reeb AN, Oh C, Serasanambati M, Lal P, Varghese R, Mashl JR, Ponce J, Terekhanova NV, Yao L, Wang F, Chen L, Schnaubelt M, Lu RJ, Schwarz JK, Puram SV, Kim AH, Song SK, Shoghi KI, Lau KS, Ju T, Chen K, Chatterjee D, Hawkins WG, Zhang H, Achilefu S, Chheda MG, Oh ST, Gillanders WE, Chen F, DeNardo DG, Fields RC, Ding L. Spatially restricted drivers and transitional cell populations cooperate with the microenvironment in untreated and chemo-resistant pancreatic cancer. Nat Genet. 2022;54(9):1390–1405. doi: 10.1038/s41588-022-01157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A, Gill S, McGregor M, Broadbridge V, Jarvelainen HA, Price T. Dual alphaV-integrin and neuropilin-1 targeting peptide CEND-1 plus nab-paclitaxel and gemcitabine for the treatment of metastatic pancreatic ductal adenocarcinoma: a first-in-human, open-label, multicentre, phase 1 study. Lancet Gastroenterol Hepatol. 2022;7(10):943–951. doi: 10.1016/S2468-1253(22)00167-4. [DOI] [PubMed] [Google Scholar]
- Donahue TR, Tran LM, Hill R, Li Y, Kovochich A, Calvopina JH, Patel SG, Wu N, Hindoyan A, Farrell JJ, Li X, Dawson DW, Wu H. Integrative survival-based molecular profiling of human pancreatic cancer. Clin Cancer Res. 2012;18(5):1352–1363. doi: 10.1158/1078-0432.CCR-11-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Gu Z, Li Z, Yuan Z, Zhao Y, Zheng X, Bo X, Chen H, Wang C. Dynamic interplay between structural variations and 3Dgenome organization in pancreatic cancer. Adv Sci (Weinh) 2022;9(18):e2200818. doi: 10.1002/advs.202200818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. Integrins and Their Role in Immune Cell Adhesion. Cell. 2019;177(3):499–501. doi: 10.1016/j.cell.2019.03.038. [DOI] [PubMed] [Google Scholar]
- Frampton AE, Castellano L, Colombo T, Giovannetti E, Krell J, Jacob J, Pellegrino L, Roca-Alonso L, Funel N, Gall TM, De Giorgio A, Pinho FG, Fulci V, Britton DJ, Ahmad R, Habib NA, Coombes RC, Harding V, Knosel T, Stebbing J, Jiao LR. MicroRNAs cooperatively inhibit a network of tumor suppressor genes to promote pancreatic tumor growth and progression. Gastroenterology. 2014;146(1):268–277 e218. doi: 10.1053/j.gastro.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. JStat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene OC. The gene ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49(D1):D325–D334. doi: 10.1093/nar/gkaa1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M, Jassal B, Stephan R, Milacic M, Rothfels K, Senff-Ribeiro A, Griss J, Sevilla C, Matthews L, Gong C, Deng C, Varusai T, Ragueneau E, Haider Y, May B, Shamovsky V, Weiser J, Brunson T, Sanati N, Beckman L, Shao X, Fabregat A, Sidiropoulos K, Murillo J, Viteri G, Cook J, Shorser S, Bader G, Demir E, Sander C, Haw R, Wu G, Stein L, Hermjakob H, D'Eustachio P. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022;50(D1):D687–D692. doi: 10.1093/nar/gkab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M, Craft B, Zhu JC, Haussler D. The UCSCXena system for cancer genomics data visualization and interpretation. Cancer Res. 2017;77(13):2584–2584. doi: 10.1158/1538-7445.Am2017-2584. [DOI] [Google Scholar]
- Gui J, Li H. Penalized Cox regression analysis in the high-dimensional and low-sample size settings, with applications to microarray gene expression data. Bioinformatics. 2005;21(13):3001–3008. doi: 10.1093/bioinformatics/bti422. [DOI] [PubMed] [Google Scholar]
- Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Hao S, Andersen-Nissen E, Mauck WM, 3rd, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, Hoffman P, Stoeckius M, Papalexi E, Mimitou EP, Jain J, Srivastava A, Stuart T, Fleming LM, Yeung B, Rogers AJ, McElrath JM, Blish CA, Gottardo R, Smibert P, Satija R. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–3587 e3529. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JK, Du W, Shelton SJ, Oldham MC, DiPersio CM, Klein OD. An FAK-YAP-mTORsignaling axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell. 2017;21(1):91–106 e106. doi: 10.1016/j.stem.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WL, Jagadeesh KA, Guo JA, Hoffman HI, Yadollahpour P, Reeves JW, Mohan R, Drokhlyansky E, Van Wittenberghe N, Ashenberg O, Farhi SL, Schapiro D, Divakar P, Miller E, Zollinger DR, Eng G, Schenkel JM, Su J, Shiau C, Yu P, Freed-Pastor WA, Abbondanza D, Mehta A, Gould J, Lambden C, Porter CBM, Tsankov A, Dionne D, Waldman J, Cuoco MS, Nguyen L, Delorey T, Phillips D, Barth JL, Kem M, Rodrigues C, Ciprani D, Roldan J, Zelga P, Jorgji V, Chen JH, Ely Z, Zhao D, Fuhrman K, Fropf R, Beechem JM, Loeffler JS, Ryan DP, Weekes CD, Ferrone CR, Qadan M, Aryee MJ, Jain RK, Neuberg DS, Wo JY, Hong TS, Xavier R, Aguirre AJ, Rozenblatt-Rosen O, Mino-Kenudson M, Castillo CF, Liss AS, Ting DT, Jacks T, Regev A. Single-nucleus and spatial transcriptome profiling of pancreatic cancer identifies multicellular dynamics associated with neoadjuvant treatment. Nat Genet. 2022;54(8):1178–1191. doi: 10.1038/s41588-022-01134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanawong P, Calderwood DA. Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions. Nat Rev Mol Cell Biol. 2023;24(2):142–161. doi: 10.1038/s41580-022-00531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A, Kosinski M, Biecek P, Fabian S. Package ‘survminer’. Drawing Survival Curves using ‘ggplot2’.(R package version 0.3. 1.) 2017. [Google Scholar]
- Kautto EA, Bonneville R, Miya J, Yu L, Krook MA, Reeser JW, Roychowdhury S. Performance evaluation for rapid detection of pan-cancer microsatellite instability with MANTIS. Oncotarget. 2017;8(5):7452–7463. doi: 10.18632/oncotarget.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P, Draghici S. Ontological analysis of gene expression data: current tools, limitations, and open problems. Bioinformatics. 2005;21(18):3587–3595. doi: 10.1093/bioinformatics/bti565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods. 2019;16(12):1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28(6):882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, Signoretti S, Liu JS, Liu XS. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174. doi: 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang X, Ang KS, Ling J, Sethi R, Lee NYS, Ginhoux F, Chen J. DISCO: a database of Deeply Integrated human Single-Cell Omics data. Nucleic Acids Res. 2022;50(D1):D596–D602. doi: 10.1093/nar/gkab1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Qu H, Wang S, Wei J, Zhang L, Ma R, Lu J, Zhu J, Zhong WD, Jia Z. GDCRNATools: an R/Bioconductor package for integrative analysis of lncRNA, miRNA and mRNA data in GDC. Bioinformatics. 2018;34(14):2515–2517. doi: 10.1093/bioinformatics/bty124. [DOI] [PubMed] [Google Scholar]
- Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: AWeb server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–W514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang X, Lin X, Cai L, Wang Y, Chang Z. Classification and prognosis analysis of pancreatic cancer based on DNAmethylation profile and clinical information. Genes (Basel) 2022;13(10):1913. doi: 10.3390/genes13101913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Xu P, Zou Q, Luo H, Yu W. An epigenetic perspective on tumorigenesis: Loss of cell identity, enhancer switching, and NamiRNA network. Semin Cancer Biol. 2019;57:1–9. doi: 10.1016/j.semcancer.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu YJ, Liang X, Ji M, Ying HM, Wang XY, Sun X, Shao CH, Zhan LX, Zhang Y. Network-based integration of mRNA and miRNA profiles reveals new target genes involved in pancreatic cancer. Mol Carcinog. 2019;58(2):206–218. doi: 10.1002/mc.22920. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Hu FF, Xia MX, Han L, Zhang Q, Guo AY. GSCALite: a web server for gene set cancer analysis. Bioinformatics. 2018;34(21):3771–3772. doi: 10.1093/bioinformatics/bty411. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Hu FF, Xie GY, Miao YR, Li XW, Zeng Y, Guo AY. GSCA: an integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief Bioinform. 2023;24(1):bbac558. doi: 10.1093/bib/bbac558. [DOI] [PubMed] [Google Scholar]
- Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee AV, Omberg L, Wolf DM, Shriver CD, Thorsson V, Cancer Genome Atlas Research N. Hu H. An integrated TCGAPan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173(2):400–416 e411. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhang Y, Yang J, Zhan H, Zhou Z, Jiang Y, Shi X, Fan X, Zhang J, Luo W, Fung KA, Xu C, Bronze MS, Houchen CW, Li M. Zinc-dependent regulation of ZEB1 and YAP1 coactivation promotes epithelial-mesenchymal transition plasticity and metastasis in pancreatic cancer. Gastroenterology. 2021;160(5):1771–1783 e1771. doi: 10.1053/j.gastro.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Y, Zhou G, Zhou J, Tian Z, Xu J. circGRAMD1B contributes to migration, invasion and epithelial-mesenchymal transition of lung adenocarcinoma cells via modulating the expression of SOX4. Funct Integr Genomics. 2023;23(1):75. doi: 10.1007/s10142-023-00972-x. [DOI] [PubMed] [Google Scholar]
- Loganathan T, Doss CGP. Non-coding RNAs in human health and disease: potential function as biomarkers and therapeutic targets. Funct Integr Genomics. 2023;23(1):33. doi: 10.1007/s10142-022-00947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11(2):97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12(4):R41. doi: 10.1186/gb-2011-12-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YR, Zhang Q, Lei Q, Luo M, Xie GY, Wang H, Guo AY. ImmuCellAI: AUnique method for comprehensive T-cell subsets abundance prediction and its application in cancer immunotherapy. Adv Sci (Weinh) 2020;7(7):1902880. doi: 10.1002/advs.201902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielczarek M, Frąszczak M, Zielak-Steciwko AE, Nowak B, Hofman B, Pierścińska J, Kruszyński W, Szyda J. An effect of large-scale deletions and duplications on transcript expression. Funct Integr Genomics. 2022;23(1):19. doi: 10.1007/s10142-022-00946-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Nagano Y, Miyazaki M, Nagamura Y, Sasaki K, Kawamura T, Yanagihara K, Imai T, Ohki R, Yashiro M, Tanaka M, Sakai R, Yamaguchi H. Integrin alpha5 mediates cancer cell-fibroblast adhesion and peritoneal dissemination of diffuse-type gastric carcinoma. Cancer Lett. 2022;526:335–345. doi: 10.1016/j.canlet.2021.11.008. [DOI] [PubMed] [Google Scholar]
- Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, Smyla JK, Anderson JM, Kim HJ, Bentrem DJ, Talamonti MS, Iacobuzio-Donahue CA, Hollingsworth MA, Yeh JJ. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. JCell Biol. 2002;157(3):493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA, Board WHOC, o TE. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan P, Dong X, Bai X, Lu H, Liu F, Sun Y, Zhao X. Tumor-stroma TGF-beta1-THBS2 feedback circuit drives pancreatic ductal adenocarcinoma progression via integrin alpha(v)beta(3)/CD36-mediated activation of the MAPK pathway. Cancer Lett. 2022;528:59–75. doi: 10.1016/j.canlet.2021.12.025. [DOI] [PubMed] [Google Scholar]
- Ortega MA, Pekarek L, Fraile-Martinez O, Garcia-Montero C, Saez MA, Asúnsolo A, Alvarez-Mon MA, Monserrat J, Ruiz-Llorente L, García-Honduvilla N, Albillos A, Buján J, Alvarez-Mon M, Guijarro LG. Implication of ERBB2 as a Predictive Tool for Survival in Patients with Pancreatic Cancer in Histological Studies. Curr Oncol. 2022;29(4):2442–2453. doi: 10.3390/curroncol29040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, Thel H, Hoyos D, Mehta A, Arshad S, Lieb DJ, Drapek LC, Blaszkowsky LS, Giantonio BJ, Weekes CD, Zhu AX, Goyal L, Nipp RD, Dubois JS, Van Seventer EE, Foreman BE, Matlack LE, Ly L, Meurer JA, Hacohen N, Ryan DP, Yeap BY, Corcoran RB, Greenbaum BD, Ting DT, Hong TS. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer. 2021;2(11):1124–1135. doi: 10.1038/s43018-021-00269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Kim M, Hwang D, Park M, Kim WK, Kim SK, Shin J, Park ES, Kang CM, Paik YK, Kim H. Characterization of gene expression and activated signaling pathways in solid-pseudopapillary neoplasm of pancreas. Mod Pathol. 2014;27(4):580–593. doi: 10.1038/modpathol.2013.154. [DOI] [PubMed] [Google Scholar]
- Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, Liu L, Huang D, Jiang J, Cui GS, Yang Y, Wang W, Guo D, Dai M, Guo J, Zhang T, Liao Q, Liu Y, Zhao YL, Han DL, Zhao Y, Yang YG, Wu W. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29(9):725–738. doi: 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Mancera PA, Rust AG, Van Der Weyden L, Kristiansen G, Li A, Sarver AL, Silverstein KA, Grützmann R, Aust D, Rümmele P, Knösel T. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012;486(7402):266–270. doi: 10.1038/nature11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Principe DR, Korc M, Kamath SD, Munshi HG, Rana A. Trials and tribulations of pancreatic cancer immunotherapy. Cancer Lett. 2021;504:1–14. doi: 10.1016/j.canlet.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, Quertinmont E, Svrcek M, Elarouci N, Iovanna J, Franchimont D, Verset L, Galdon MG, Deviere J, de Reynies A, Laurent-Puig P, Van Laethem JL, Bachet JB, Marechal R. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology. 2018;155(6):1999–2013 e1993. doi: 10.1053/j.gastro.2018.08.033. [DOI] [PubMed] [Google Scholar]
- Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52(10):710–718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- Ran GH, Lin YQ, Tian L, Zhang T, Yan DM, Yu JH, Deng YC. Natural killer cell homing and trafficking in tissues and tumors: from biology to application. Signal Transduct Target Ther. 2022;7(1):205. doi: 10.1038/s41392-022-01058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid NU, Peng XL, Jin C, Moffitt RA, Volmar KE, Belt BA, Panni RZ, Nywening TM, Herrera SG, Moore KJ, Hennessey SG, Morrison AB, Kawalerski R, Nayyar A, Chang AE, Schmidt B, Kim HJ, Linehan DC, Yeh JJ. Purity independent subtyping of tumors (PurIST), aclinically robust, single-sample classifier for tumor subtyping in pancreatic cancer. Clin Cancer Res. 2020;26(1):82–92. doi: 10.1158/1078-0432.CCR-19-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, Chan NW, Zhang J. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35(20):4200–4202. doi: 10.1093/bioinformatics/btz210. [DOI] [PubMed] [Google Scholar]
- Ruan S, Lin M, Zhu Y, Lum L, Thakur A, Jin R, Shao W, Zhang Y, Hu Y, Huang S, Hurt EM, Chang AE, Wicha MS, Li Q. Integrin beta4-targeted cancer immunotherapies inhibit tumor growth and decrease metastasis. Cancer Res. 2020;80(4):771–783. doi: 10.1158/0008-5472.CAN-19-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadzeviciene I, Snipaitiene K, Scesnaite-Jerdiakova A, Daniunaite K, Sabaliauskaite R, Laurinaviciene A, Drobniene M, Ostapenko V, Jarmalaite S (2022) Analysis of intrinsic breast cancer subtypes: the clinical utility of epigenetic biomarkers and TP53 mutation status in triple-negative cases. Int J Mol Sci 23(23). 10.3390/ijms232315429 [DOI] [PMC free article] [PubMed]
- Sailer V, von Amsberg G, Duensing S, Kirfel J, Lieb V, Metzger E, Offermann A, Pantel K, Schuele R, Taubert H, Wach S, Perner S, Werner S, Aigner A. Experimental in vitro, ex vivo and in vivo models in prostate cancer research. Nat Rev Urol. 2023;20(3):158–178. doi: 10.1038/s41585-022-00677-z. [DOI] [PubMed] [Google Scholar]
- Shaim H, Shanley M, Basar R, Daher M, Gumin J, Zamler DB, Uprety N, Wang F, Huang Y, Gabrusiewicz K, Miao Q, Dou J, Alsuliman A, Kerbauy LN, Acharya S, Mohanty V, Mendt M, Li S, Lu J et al (2021) Targeting the alphav integrin/TGF-beta axis improves natural killer cell function against glioblastoma stem cells. J Clin Invest 131(14). 10.1172/JCI142116 [DOI] [PMC free article] [PubMed]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheta M, Hassan G, Afify SM, Monzur S, Kumon K, Abu Quora HA, Farahat M, Zahra MH, Fu X, Seno A, Seno M. Chronic exposure to FGF2 converts iPSCs into cancer stem cells with an enhanced integrin/focal adhesion/PI3K/AKT axis. Cancer Lett. 2021;521:142–154. doi: 10.1016/j.canlet.2021.08.026. [DOI] [PubMed] [Google Scholar]
- Shi W, Li C, Wartmann T, Kahlert C, Du R, Perrakis A, Brunner T, Croner RS, Kahlert UD (2022) Sensory ion channel candidates inform on the clinical course of pancreatic cancer and present potential targets for repurposing of FDA-approved agents. J Pers Med 12(3). 10.3390/jpm12030478 [DOI] [PMC free article] [PubMed]
- Shuwen H, Qing Z, Yan Z, Xi Y. Competitive endogenous RNA in colorectal cancer: a systematic review. Gene. 2018;645:157–162. doi: 10.1016/j.gene.2017.12.036. [DOI] [PubMed] [Google Scholar]
- Slack RJ, Macdonald SJF, Roper JA, Jenkins RG, Hatley RJD. Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov. 2022;21(1):60–78. doi: 10.1038/s41573-021-00284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci US A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Banerjee P, Liu X, Yu J, Gibbons DL, Wu P, Scott KL, Diao L, Zheng X, Wang J, Jalali A, Suraokar M, Fujimoto J, Behrens C, Liu X, Liu CG, Creighton CJ, Wistuba II, Kurie JM. The epithelial-to-mesenchymal transition activator ZEB1 initiates a prometastatic competing endogenous RNA network. JClin Invest. 2018;128(4):1267–1282. doi: 10.1172/JCI97225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Wu Z, Rong Z, Xu J, Wang W, Zhang B, Yu X, Shi S. Ferroptosis-related lncRNA pairs to predict the clinical outcome and molecular characteristics of pancreatic ductal adenocarcinoma. Brief Bioinform. 2022;23(1):bbab388. doi: 10.1093/bib/bbab388. [DOI] [PubMed] [Google Scholar]
- Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–W560. doi: 10.1093/nar/gkz430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc B Methodol. 1996;58(1):267–288. doi: 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385–395. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wren JD, Ding Y, Chen J, Mittal N, Xu C, Li X, Zeng C, Wang M, Shi J, Zhang YH, Han SJ, Zhang XA. EWI2 promotes endolysosome-mediated turnover of growth factor receptors and integrins to suppress lung cancer. Cancer Lett. 2022;536:215641. doi: 10.1016/j.canlet.2022.215641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lauffenburger D, Friedl P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat Rev Cancer. 2022;22(3):157–173. doi: 10.1038/s41568-021-00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenric S, Davison JM, Wang YE, Mayhew GM, Beebe K, Kang HP, Milburn MV, Chung V, Bekaii-Saab T, Perou CM. Abstract A002: purity independent subtyping of tumor (PurIST): real-world data validation of a pancreatic ductal adenocarcinoma (PDAC) gene expression classifier and its prognostic implications. Cancer Res. 2022;82(22):A002–A002. doi: 10.1158/1538-7445.Panca22-a002. [DOI] [Google Scholar]
- Wu L, Lu Y, Chen L, Yue X, Cen C, Zheng C, Han P. The effects of radiotherapy on pancreatic ductal adenocarcinoma in patients with liver metastases. Curr Oncol. 2022;29(10):7912–7924. doi: 10.3390/curroncol29100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Liang X, Yan Z, Chen J, Zhu Y, Xie Y, Li Y, Li X, Gao Q, Feng F, Fu G, Gao Y. ADNA-methylation-driven genes based prognostic signature reveals immune microenvironment in pancreatic cancer. Front Immunol. 2022;13:803962. doi: 10.3389/fimmu.2022.803962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Li Y, Li P, Sun Y, Lv S, Wang Y, He X, Xu J, Xu Z, Li L, Li Y. Soft substrates promote direct chemical reprogramming of fibroblasts into neurons. Acta Biomater. 2022;152:255–272. doi: 10.1016/j.actbio.2022.08.049. [DOI] [PubMed] [Google Scholar]
- Yan L-r, Ding H-x, Shen S-x, Lu X-d, Yuan Y, Xu Q. Pepsinogen C expression–related lncRNA/circRNA/mRNA profile and its co-mediated ceRNA network in gastric cancer. Funct Integr Genomics. 2021;21(5):605–618. doi: 10.1007/s10142-021-00803-x. [DOI] [PubMed] [Google Scholar]
- Yang F, Yu Y, Zhou H, Zhou Y. Prognostic subtypes of thyroid cancer was constructed based on single cell and bulk-RNA sequencing data and verified its authenticity. Funct Integr Genomics. 2023;23(2):89. doi: 10.1007/s10142-023-01027-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, Trevino V, Shen H, Laird PW, Levine DA, Carter SL, Getz G, Stemke-Hale K, Mills GB, Verhaak RG. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4(1):2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Chen B, Aran D, Charalel J, Yau C, Wolf DM, van 't Veer LJ, Butte AJ, Goldstein T, & Sirota M. Comprehensive transcriptomic analysis of cell lines as models of primary tumors across 22 tumor types. Nat Commun. 2019;10(1):3574. doi: 10.1038/s41467-019-11415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Zhou Y, Li Y, Dong Z. Integration of clinical and spatial data to explore lipid metabolism-related genes for predicting prognosis and immune microenvironment in gliomas. Funct Integr Genomics. 2023;23(2):82. doi: 10.1007/s10142-023-01010-6. [DOI] [PubMed] [Google Scholar]
- Zeltz C, Primac I, Erusappan P, Alam J, Noel A, Gullberg D. Cancer-associated fibroblasts in desmoplastic tumors: emerging role of integrins. Semin Cancer Biol. 2020;62:166–181. doi: 10.1016/j.semcancer.2019.08.004. [DOI] [PubMed] [Google Scholar]
- Zeng D, Ye Z, Wu J, Zhou R, Fan X, Wang G, Huang Y, Wu J, Sun H, Wang M, Bin J, Liao Y, Li N, Shi M, Liao W. Macrophage correlates with immunophenotype and predicts anti-PD-L1 response of urothelial cancer [Research Paper] Theranostics. 2020;10(15):7002–7014. doi: 10.7150/thno.46176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Pan X, Fujiwara K, Jurcak N, Muth S, Zhou J, Xiao Q, Li A, Che X, Li Z, Zheng L. Pancreatic cancer cells render tumor-associated macrophages metabolically reprogrammed by a GARP and DNA methylation-mediated mechanism. Signal Transduct Target Ther. 2021;6(1):366. doi: 10.1038/s41392-021-00769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL, Lv JW, Huang XD, Liu RQ, Chen F, He XJ, Kou J, Zhang J, Wen X, Li YQ, Ma J, Liu N, Sun Y. Long Noncoding RNAFAM225APromotes Nasopharyngeal Carcinoma Tumorigenesis and Metastasis by Acting as ceRNA to Sponge miR-590-3p/miR-1275 and Upregulate ITGB3. Cancer Res. 2019;79(18):4612–4626. doi: 10.1158/0008-5472.CAN-19-0799. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Jin H, Xu J. A gene signature driven by abnormally methylated DEGs was developed for TP53 wild-type ovarian cancer samples by integrative omics analysis of DNA methylation and gene expression data. Ann Transl Med. 2023;11(1):20. doi: 10.21037/atm-22-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw RNA sequencing data for BxPC3, PANC1, and HPDE6C7 cell lines have been deposited in the Gene Expression Omnibus database under the accession number GSE185069. For raw RNA sequencing data of MIA-CAPA-2, ASPC1, the cancer-associated fibroblast line, and single-cell RNA sequencing data of transplanted tumors, please contact the corresponding author. The open-access data can be downloaded from the original articles as described.