Abstract
Mycobacterial membrane protein Large 3 (MmpL3), an inner membrane protein, plays a crucial role in the transport of mycolic acids that are essential for the viability of M. tuberculosis and has been a promising therapeutic target for new anti-TB agents. Herein, we report the discovery of pyridine-2-methylamine antitubercular compounds using a structure-based drug design strategy. Compound 62 stands out as the most potent compound with high activity against M.tb strain H37Rv (MIC = 0.016 μg/mL) as well as the clinically isolated strains of MDR/XDR-TB (MIC = 0.0039-0.0625 μg/mL), low Vero cell toxicity (IC50 ⩾ 16 μg/mL), and moderate liver microsomal stability (CLint = 28 μL/min/mg). Furthermore, the resistant mutant of S288T due to single nucleotide polymorphism in mmpL3 was resistant to pyridine-2-methylamine 62, demonstrating compound 62 is likely target to MmpL3.
Keywords: MDR and XDR tuberculosis, MmpL3, structure-based drug design, pyridine-2-methylamine
Graphical Abstract

1. Introduction
Tuberculosis (TB) remains a serious global health security threat and is the second leading cause of death from a single infectious agent after COVID-19. According to the 2022 World Health Organization (WHO) report, approximately a quarter of the world population has been infected with Mycobacterium tuberculosis (Mtb) but is not yet sick and persists in a dormant or latent state.[1]. In addition, people infected with HIV are 18 times more likely to develop TB, and those with other immune-compromising disorders are also at higher risk of developing TB. The emergence of drug-resistant TB is the greatest obstacle to the “End TB Strategy”. Only three antitubercular drugs, namely bedaquiline, delamanid, and pretomanid, have been approved to treat drug-resistant TB over five decades (Figure 1). Bedaquiline inhibits mycobacterial ATP synthase, thereby interfering with the energy metabolism of Mtb [2, 3]. Both delamanid and pretomanid belong to the class of nitroimidazoles, which affect the synthesis of bacterial cell walls by inhibiting the biosynthesis of mycolic acids [4-6]. The combination of bedaquiline-pretomanid-linezolid can significantly shorten the treatment cycle of drug-resistant TB patients to 6 months [7]. However, bedaquiline resistance has emerged in high TB burden countries [8]. The development of antitubercular drugs with novel chemical structures and distinct mechanisms of action is still urgently needed.
Figure 1.

Structure of bedaquiline (1), delamanid (2), pretomanid (3) and representative MmpL3 inhibitors SQ109 (4), ICA38 (5), 6, AU1235 (7).
Mycobacterial membrane protein Large 3 (MmpL3) is a membrane protein encoded by the mycobacterial genome and belongs to the resistance, nodulation, and cell division (RND) protein family [9]. MmpL3 is the only essential MmpL gene among the 13 MmpL genes of Mtb and is responsible for the transportation of trehalose-monomycolates (TMM) from the cytoplasm to the cell envelope. Transportation relies on proton-motive force (PMF) as the energy source [10, 11]. Chemical or genetic silencing of MmpL3 affects the passage of TMM through the inner membrane. Consequently, the TMM accumulates in the cytoplasm which affects the formation of the bacterial cell wall and Mtb viability [12].
MmpL3 inhibitors with different scaffolds have been reported including 1,2-ethylenediamines (SQ109, 4), indole-2-carboxamides (ICA38, 5 and 6), adamantyl ureas (AU1235, 7), dis- or tri-substituted pyrroles and pyrazoles, spiro piperidines, and tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamides [13-18]. SQ109 is the most advanced MmpL3 inhibitor currently in phase IIb/III. A recent clinical trial showed that SQ109 could improve sputum clearance in patients with drug-resistant TB, similar to that of bedaquiline and delamanid [19]. However, the resistant mutant of SQ109 could not be directly isolated in vitro or in vivo [20]. Other inhibitors are still in the early stage of research due to inadequate water solubility, unsatisfactory ADME-Tox properties, etc. Therefore, it is an urgent need to develop MmpL3 inhibitors with novel scaffolds [21, 22].
Most of the anti-TB drugs are discovered using a phenotype/cell-based screening strategy, as the screened compounds generally have better permeability against the unique mycobacterial cell wall [23-26]. Since the publication of the complete Mtb genome sequence in 1998, multiple potential TB drug targets have been identified and validated[27]. Herein, we present our design and synthesis of pyridine-2-methylamine derivatives as MmpL3 inhibitors using a structure-guided approach. The antitubercular activity of designed compounds was initially evaluated using the Mtb H37Rv strain. Cytotoxicity, metabolic stability, and mechanism of action studies for selected compounds were carried out.
2. Results and discussion
2.1. Rational drug design
In an analysis of the crystal structures of Mycobacterium smegmatis (Msmg) MmpL3 and its inhibitors [28], we found that SQ109, ICA38, and AU1235 share similar binding modes. As shown in Figure 1 and Figure 2A, the nitrogen atom forms a crucial hydrogen bond with D645, thereby blocking two pairs of Asp-Tyr hydrogen bonds (Y256–D646; Y257–D645) and interfering with the proton transportation. In addition, there are two important hydrophobic regions S1 (residues F260, S293, and F649), S2 (residues I249, I297, V638, G641, L642, and L686) within the binding pocket. We used a structure-guided strategy to design MmpL3 inhibitors with a novel scaffold (Figure 2B): 1) retain the nitrogen atom to form the essential hydrogen bond with D645; 2) utilize the aliphatic heterocycles and aromatic rings to occupy the S1 and S2 pockets, respectively; 3) select 5- or 6-membered aromatic heterocycles as the linker. Subsequent molecular docking studies showed that compounds containing pyridine, pyrimidine, or oxazole moiety could nicely bind to the active pocket of MmpL3 (Supporting Information Figure S1). In addition, the six-membered aromatic heterocycles could readily form π-π stacking with Y646 residue compared to the five-membered aromatic heterocycles Thus, we chose the six-membered aromatic heterocycles for the linker region.
Figure 2.
(A) Superposition of the structures of the MmpL3 inhibitors (SQ109, purple, PDB: 6AJG; AU1235, light blue, PDB: 6AJH; and ICA38, pink, PDB: 6AJJ) binding pockets (B) Structural-based design of MmpL3 inhibitors.
2.2. Chemistry
Compounds 11–68 were synthesized through a four-step route as shown in Scheme 1. Briefly, commercially available methyl 4-bromopicolinate 7 underwent a Suzuki coupling reaction with 4-isopropylbenzeneboronic acid in the presence of Pd(PPh3)4 and Na2CO3 in a refluxing mixture of toluene and water to give compound 8. Aldehyde 9 was obtained from ester 8 through a sequential reduction and oxidation with NaBH4 and Dess-Martin periodinane respectively. Compounds 20–21 were obtained via reductive amination reactions with N-4,4-dimethylpiperidine or N-4,4-dimethyl-1,4-azasilyl. Similarly, compounds 11–19, 22–39, and 45–68 were successfully synthesized following the same synthetic route. The ester 8 was hydrolyzed to carboxylic acid 10 under basic conditions, which was subjected to amide coupling to afford compounds 40–44.
Scheme 1. Synthesis of compounds 11–68a.
aReagents and conditions: (a) 4-isopropylbenzeneboronic acid, Pd(PPh3)4, Na2CO3, PhMe: H2O = 2:1, 100 °C, N2, overnight; (b) NaBH4, MeOH, rt, overnight; (c) Dess-Martin periodinane, CH2Cl2, rt, 2 h; (d) LiOH, MeOH: THF: H2O = 2 : 1: 1, 50 °C, 3 h; (e) amine, NaBH(AcO)3, CH2Cl2, rt, overnight; (f) amines, EDCI, HOBt, DIPEA, CH2Cl2, rt, 6 h.
2.3. Structure-Activity Relationships.
Antitubercular activity evaluation for compounds 11–30
All final compounds were initially evaluated for their antitubercular activity against the Mtb strain H37Rv using a standard microplate Alamar Blue assay (MABA) [29, 30]. As shown in Table 1, compounds 11-30 exhibited antitubercular activity of varying potency with MIC values ranging from 0.5 to 64 μg/mL. For the substituent at the R1 position, the antitubercular activity of 4,4-dimethyl-1,4-azasilyl was significantly better than that of 4,4-dimethylpiperidinyl (16 vs 17; 18 vs 19; 20 vs 21; 24 vs 25; 28 vs 29). Similarly, compared to the hydrogen atom, the isopropyl group at the R2 could better occupy the S2 hydrophobic pocket 11 vs 12; 14 vs 16; 15 vs 17; 18 vs 20; 19 vs 21; 22 vs 24; 23 vs 25; 29 vs 30). Compounds with pyridine-2-methylamine-6-aryl (13), pyridine-2-aryl-4-methylamine (17), and pyrimidine-4-methylamine-6-aryl (27) showed moderate antitubercular activity (MIC = 4-8 μg/mL). To our delight, pyridine-2-methylamine-4-aryl (21), pyridine-3-methylamine-5-aryl (25), and oxazole-2-aryl-4-methylamine (30) showed good activity (MIC = 0.5-1 μg/mL).
Table 1.
Antitubercular Activity of Compounds 11–30 against Mtb Strain H37Rv.

| |||||
|---|---|---|---|---|---|
| Comp. | R1 | Ar | R2 | MICa (μg/mL) | cLogPb |
| 11 |

|

|
H | 32 | 4.81 |
| 12 |

|

|
isopropyl | 8 | 6.24 |
| 13 |

|

|
isopropyl | 8 | 7.80 |
| 14 |

|

|
H | >64 | 4.81 |
| 15 |

|

|
H | 64 | 6.37 |
| 16 |

|

|
isopropyl | 16 | 6.24 |
| 17 |

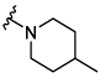
|

|
isopropyl | 4 | 7.80 |
| 18 |

|

|
H | 64 | 4.60 |
| 19 |

|

|
H | 8 | 6.16 |
| 20 |

|

|
isopropyl | 2 | 6.03 |
| 21 |

|

|
isopropyl | 0.5 | 7.59 |
| 22 |

|

|
H | 32 | 4.60 |
| 23 |

|

|
H | 32 | 6.16 |
| 24 |

|

|
isopropyl | 8 | 6.03 |
| 25 |

|

|
isopropyl | 1 | 7.59 |
| 26 |

|

|
isopropyl | 4 | 5.28 |
| 27 |

|

|
isopropyl | 8 | 6.84 |
| 28 |

|

|
H | > 64 | 3.98 |
| 29 |

|

|
H | 32 | 5.55 |
| 30 |

|

|
isopropyl | 1 | 6.98 |
The lowest concentration of compounds leading to at least 90% inhibition of bacterial growth signal by MABA. MIC values are reported as an average of three individual measurements.
cLogP was calculated using ChemBioDraw Ultra 20.0.
A molecular docking study showed that pyridine-2-methylamine 21 nicely binds to the active pocket of MmpL3 (docking score = −10.934) (Figure 3A). The nitrogen atom of N-4,4-dimethyl-1,4-azasilyl formed a hydrogen bond with D645 (2.62 Å), and the pyridine-2-methylamine maintained the π-π stacking with Y646. We noticed that there were still non-interacting regions within the S1 and S2 pockets. Therefore, we next tried to introduce additional hydrophobic groups at the para-position of benzyl and expand the hydrophobic ring of N-4,4-dimethyl-1,4-azasilyl.
Figure 3.
(A) Predicted 3D binding mode of compound 21 and MmpL3 (generated from PDB code 6AJJ); (B) Further structural optimization.
Antitubercular activity evaluation for pyridine-2-methylamines 31–66
The N-heterocycle (R1) could form a crucial hydrogen bond with the carboxyl of D645 and hydrophobic interaction with residues (F260, S293, and F649) in the hydrophobic pocket S1. N-heterocyclyls (31–39) maintained the activity (31–36 and 38; MIC = 1-2 μg/mL) compared to compound 21 (Table 2). The N-8-azaspiro[4.5] decyl (37) had a 4-fold increase in activity (MIC = 0.125 μg/mL), likely because the bulker N-8-azaspiro[4.5] decyl group could insert deeper into the S1 hydrophobic pocket. Replacing methylene amines with amides resulted in a significant decrease in activity compared to the corresponding pyridine-2-amines (31 vs 40, 33 vs 41, 32 vs 43, and 35 vs 44), likely due to the loss of hydrogen bond with the carboxyl of D645.
Table 2.
Antitubercular Activity of Compounds 31–44 against Mtb Strain H37Rv

| |||
|---|---|---|---|
| Comp. | R1 | MICa (μg/mL) |
cLogPb |
| 31 |

|
1 | 7.03 |
| 32 |

|
1 | 5.99 |
| 33 |

|
1 | 5.92 |
| 34 |

|
1 | 6.48 |
| 35 |

|
2 | 5.51 |
| 36 |

|
2 | 5.30 |
| 37 |

|
0.125 | 6.42 |
| 38 |

|
1 | 3.30 |
| 39 |

|
8 | 3.53 |
| 40 |

|
> 64 | 7.47 |
| 41 |

|
8 | 6.36 |
| 42 |

|
8 | 5.80 |
| 43 |

|
> 64 | 6.43 |
| 44 |

|
32 | 4.53 |
The lowest concentration of compounds leading to at least 90% inhibition of bacterial growth signal by MABA. MIC values are reported as an average of three individual measurements.
cLogP was calculated using ChemBioDraw Ultra 20.0.
Next, various aromatic groups at the R2 were examined (Table 3). N- or O-containing aromatic rings (45–48) decreased antitubercular activity (MIC = 32–64 μg/mL). The electron-donating groups (EDGs; isobutyl, tert-butyl, and cyclohexyl, 52–54) maintained activity compared to 35, while the electron-withdrawing groups (EWGs; -NO2, -Br, and -CF3, 49-51) resulted in 2 to 32-fold decrease in activity. The bulkier naphthyl and 4-biphenyl (55-57) also maintained the antitubercular activity (MIC = 2–4 μg/mL). Replacement of N-4-methylpiperidinyl (52-55) with N-4,4-dimethylazasilyl (58-61) resulted in a 4-fold increase in activity. The combination of dominant groups (N-8-azaspiro[4,5] decyl and 4-biphenyl) at R1 and R2 gave the most potent compounds 62 and 63 (MIC = 0.0156 μg/mL). Substitution at the para-position of 4-biphenyl with chloro or methyl led to a decrease in activity (64 and 65). 3-Pyridyl (66), 2,4-difluoropyridyl (67), and furyl (68) decreased the activity by 2 to 32-fold compared to compound 62.
Table 3.
Antitubercular Activity of Compounds 45–68 Against Mtb Strain H37Rv

| ||||
|---|---|---|---|---|
| Comp. | R1 | R2 | MICa (μg/mL) | cLogPb |
| 45 |

|

|
32 | 3.97 |
| 46 |

|

|
64 | 2.69 |
| 47 |

|

|
64 | 3.19 |
| 48 |

|

|
32 | 3.26 |
| 49 |
|

|
64 | 3.86 |
| 50 |

|

|
4 | 4.96 |
| 51 |

|

|
4–8 | 4.99 |
| 52 |

|
|
1 | 6.04 |
| 53 |

|
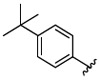
|
2 | 5.91 |
| 54 |

|

|
2 | 6.70 |
| 55 |

|

|
4 | 5.25 |
| 56 |

|

|
2 | 5.97 |
| 57 |

|

|
2–4 | 5.25 |
| 58 |

|
|
0.5 | 8.12 |
| 59 |

|

|
0.5 | 8.78 |
| 60 |

|

|
0.5 | 7.99 |
| 61 |

|

|
0.125 | 8.05 |
| 62 |

|

|
0.0156 | 6.88 |
| 63 |

|

|
0.0156 | 7.44 |
| 64 |

|

|
0.125 | 7.59 |
| 65 |

|

|
0.125–0.25 | 7.38 |
| 66 |

|

|
0.25 | 5.38 |
| 67 |

|

|
0.5 | 5.78 |
| 68 |

|

|
0.0313 | 6.06 |
The lowest concentration of compounds leading to at least 90% inhibition of bacterial growth signal by MABA. MIC values are reported as an average of three individual measurements.
cLogP was calculated using ChemBioDraw Ultra 20.0.
We plotted the correlation between the MIC and cLogP values of pyridine-2-methylamines. As shown in Figure 4, the antitubercular activity is positively correlated with the cLogP values. When the values of cLogP are greater than 6.8, the MIC values were less than 1 μg/mL. The compounds with higher lipophilicity exhibit more potent activity, indicating that higher lipophilicity is beneficial to membrane permeability. Compounds 62 and 68 attracted our attention due to their relatively low cLogP values and potent antitubercular activity.
Figure 4.
Correlation plot of the MIC (Mtb, H37Rv) with cLogP for the pyridine-2-methylamines
2.4. Toxicity to mammalian cells
Compounds 21, 25, 30-34, 38, 52, and 58-65 with potent antitubercular activity were selected to further evaluate their cytotoxicity profiles against Vero cells derived from African green monkey kidneys [31]. As shown in Table 4, all tested compounds showed low cytotoxicity in Vero cells with IC50 values of no less than 16 μg/mL.
Table 4.
Vero Toxicity of Selected Compounds 21, 25, 30-34, 38, 52, and 58-65.
| Comp. | Vero cells IC50 (μg/mL) |
MIC H37Rv (μg/mL) |
Comp. | Vero cells IC50 (μg/mL) |
MIC H37Rv (μg/mL) |
|---|---|---|---|---|---|
| 21 | ⩾ 32 | 0.5 | 58 | ⩾ 32 | 0.5 |
| 25 | ⩾ 32 | 1 | 59 | ⩾ 32 | 0.5 |
| 30 | ⩾ 32 | 1 | 60 | 16 | 0.5 |
| 31 | ⩾ 32 | 1 | 61 | ⩾ 32 | 0.125 |
| 32 | 16 | 1 | 62 | 16 | 0.0156 |
| 33 | 16 | 1 | 63 | ⩾ 32 | 0.0156 |
| 34 | 16 | 1 | 64 | ⩾ 32 | 0.125 |
| 38 | ⩾ 32 | 1 | 65 | ⩾ 32 | 0.125–0.25 |
| 52 | ⩾ 32 | 1 |
The lowest concentration of compounds leading to at least 90% inhibition of bacterial growth signal by MABA. MIC values are reported as an average of three individual measurements.
2.5. In vitro microsomal stability and hERG inhibition
To evaluate the metabolic stability of pyridine-2-methylamine compounds, we selected the compounds with potent antitubercular activity and obvious structural differences to assess their microsomal stability (Table 5). Compounds 37 and 68 were extremely unstable in human liver microsomes, with only 1.5% and 0.2% remaining after 60 min incubation in the presence of NADPH. It was speculated that the methyl of the isopropyl and 2-H-furyl shown in compounds 37 and 68 could be readily oxidized to the ─CH2OH (subsequently ─COOH) and epoxide respectively [32]. Replacing the isopropyl (37) and furyl (68) with the phenyl group (62) significantly improved the liver microsomal stability with 43.1% remaining after 60 min incubation in the presence of NADPH, and the half-life was 49.5 min.
Table 5.
Human Liver Microsomal Stability of Selected Compounds 37, 62, and 68.
| Comp. | T1/2a, (min) | CLint(mic)b, (μL/min/mg) |
Remainingc, (T = 60 min) |
Remainingd, (NCF = 60 min) |
|---|---|---|---|---|
| 37 | 11.3 | 122.9 | 1.5% | stable |
| 62 | 49.5 | 28.0 | 43.1% | stable |
| 68 | 1.8 | 758.2 | 0.2% | stable |
| Testosterone | 12.9 | 107.1 | 3.9% | stable |
| Diclofenac | 7.7 | 180.4 | 0.4% | stable |
| Propafenone | 6.0 | 230.4 | 0.1% | stable |
T 1/2 is half-life.
CLint(mic) is the intrinsic clearance; bCLint(mic) = 0.693/half-life/mg of microsome protein per mL.
Substrate concentrations were determined in incubations with NADPH after 60 min and normalized to concentrations at time zero.
Stability was determined without the NADPH cofactor.
Compound 62 was next evaluated for hERG inhibition using a manual patch-clamp electrophysiology assay. As shown in Table 6, compound 62 inhibited the hERG K+ channel by 52.7% at the initial test concentration of 10 μM, while the inhibition rate of cisapride was 97.2% at 300 nM.
Table 6.
Inhibition rate of compound 62 on hERG K+ channel at 10 μM.
| Compound | hERG K+ inhibition rate a | Concentration |
|---|---|---|
| 62 | 52.7% | 10 μM |
| cisapride | 97.2% | 300 nM |
an average of three individual measurements
2.6. Antitubercular activity against drug-susceptible/resistant strains of Mtb
The representative compound 62 was evaluated for its activities against susceptible-TB (V4207), MDR-TB (V2475 and KZN494), and XDR-TB (R506 and TF274) strains of clinical isolates [33]. First-line antitubercular drugs (Isoniazid and Rifampin) and second-line antitubercular drugs (Levofloxacin, Ofloxacin, and Kanamycin) were as positive controls (Table 7). Compound 62 exhibited high activity not only against susceptible Mtb strains but also against all clinical isolates of MDR and XDR-TB strains tested, suggesting that they will not exhibit cross-resistance with currently used medications.
Table 7.
Antitubercular Activity of Compound 62 Against Susceptible, MDR, and XDR Strains of Mtb
| Strain/phenotype | Mtb MIC (μg/mL) | ||||
|---|---|---|---|---|---|
| V4207/ DSa |
V2475/ MDRb |
KZN494/ MDRb |
R506/ XDRc |
TF274/ XDRc |
|
| 62 | 0.0039 | 0.0625 | 0.0156–0.0313 | 0.0313 | 0.0039 |
| Isoniazid | 0.04 | 16 | 16 | 8 | 8 |
| Rifampin | 0.0625 | 4 | 128 | >128 | >128 |
| Levofloxacin | 0.125 | NT | NT | 2 | 1 |
| Ofloxacin | 0.5 | 0.5 | 0.5 | 4 | 8 |
| Kanamycin | 2 | 2 | 2 | >128 | >128 |
Drug susceptible strain of Mtb.
Multidrug resistant strain of Mtb.
Extensively drug resistant strain of Mtb.
The lowest concentration of the drug leads to at least a 90% reduction of bacterial growth signal by microplate Alamar Blue assay (MABA); reported MIC values are an average of three individual measurements.
2.7. Molecular docking
To investigate the binding mode of pyridine-2-methylamine derivatives and MmpL3, we performed molecular docking studies for compound 62. The crystal structure of the MmpL3-ICA38 complex (PDB:6AJJ) was used [28]. The docking calculation was carried out using Schrödinger's Glide-XP module, and Pymol 2.5 was used for visualization. As shown in Figure 5, compound 62 successfully binds to the active pocket of MmpL3, and the docking score (−11.474) is higher than that of compound 21 (−10.934). The N-8-azaspiro[4.5]decyl inserts deeper into the hydrophobic region S1 (residues F260, S293, and F649) than the N-4,4-dimethylsilyl, which likely enhances the hydrophobic interaction. The nitrogen atom of compound 62 forms a hydrogen bond (3.1 Å) with D645, which blocks the hydrogen bonding interaction of the Asp-Tyr pair, thus disrupting the proton motive force for substrate translocation. The bulky biphenyl fits the hydrophobic region at S2 (residues I249, I297, V638, G641, L642, and L686) better than the p-isopropylphenyl. The pyridine skeleton forms the π-π stacking interaction with the phenyl ring of Y646. The superposition of compound 62 and indole-2-carboxamide ICA38 showed that their binding modes are consistent (Figure 5B), suggesting that the mechanism of action of 62 was likely the same as that of ICA38.
Figure 5.
(A) Proposed binding mode of compound 62 with MmpL3 (generated from PDB code 6AJJ). The atoms of 62 are colored as follows: carbon, green; nitrogen, blue. The cartoon shows the protein and key residues are colored as follows: carbon, grey; oxygen, red; nitrogen, blue. Yellow dotted lines indicate H-bonds, and the orange dotted lines represent π-π stacking interactions. (B) Superposed docking poses of 62 and ICA38. The atoms of ICA38 are colored as follows: carbon, pink; oxygen, red; nitrogen, blue.
2.8. Mechanism of Action
Drug-resistant strains provide services for the study of the mechanism of action with similar chemical structures or binding modes [34, 35]. Indole-2-carboxamide 6 is a well-characterized MmpL3 inhibitor (Figure 1). [31] Whole genome deep sequencing of the compound 6-resistant mutants identified a single nucleotide polymorphism (S288T) in the mmpL3 gene responsible for the resistance phenotype (IAR2). The mutated residue S288 in Mtb corresponded to the S293 in Msng, which was located in the S1 region of the MmpL3 active pocket. We evaluated the antitubercular activity of compound 62 against the mutant Mtb strain IAR2 and calculated the ratio of MIC (IAR2)/MIC(WT). As shown in Table 8, the growth of the mutant strain treated with compound 62 was significantly inhibited compared to the WT strain (MIC (IAR2)/MIC(H37Rv) = 64), while INH remained susceptible to resistant strain IAR2. These results suggest that pyridine-2-methylamine 62 likely shares a common binding pocket with the indole-2-carboxamides.
Table 8.
Antitubercular activity of pyridine-2-methylamine 62 and indole-2-carboxamide 6 against mutant of Mtb IAR2 and H37Rv
| Comp. | MIC IAR2 (μg/mL) | MIC H37Rv (μg/mL) | MIC (IAR2)/MIC (WT) |
|---|---|---|---|
| 62 | 1 | 0.0156 | 64 |
| 6 [31] | 0.0125 | 0.0039 | 32 |
| INH | 0.04 | 0.04 | 1 |
2. Conclusions
In summary, a novel series of pyridine-2-methylamine derivatives were designed, synthesized, and evaluated as potential antitubercular agents against Mtb strains. Firstly, the general structure was obtained through the binding mode analysis of MmpL3 with SQ109, ICA38, and AU1235. The pyridine-2-methylamine skeleton was identified as a suitable module. Extensive SAR studies focused on the hydrophobic regions S1 and S2 identified a lead compound 62, which showed high potency against Mtb strain H37Rv (MIC = 0.016 μg/mL) as well as the clinically isolated strains of MDR and XDR-TB (MIC = 0.0039-0.0625 μg/mL). Compound 62 exhibited low Vero cell toxicity and moderate metabolic stability profiles in human liver microsomes. Lastly, the mechanism of action exploration revealed that mutation of conserved residue (S288T) within the S1 region of the MmpL3-indocarboxymide binding pocket significantly affected the antitubercular activity of 62, suggesting that pyridine-2-methylamine series of compounds likely target MmpL3. Overall, pyridine-2-methylamine derivatives represent promising antitubercular agents with high efficacy and low toxicity and warrant further explorations.
3. Experimental section
4.1. General synthetic methods.
Starting materials, reagents, and solvents were purchased from commercial suppliers and used without further purification unless otherwise stated. Anhydrous CH2Cl2 was obtained by distillation over calcium hydride. All nonaqueous reactions were run under a nitrogen atmosphere with the exclusion of moisture from reagents. The progress of reactions was monitored by liquid chromatography mass spectrometry and thin-layer chromatography on SiO2. Silica gel for column chromatography was of 200–300 mesh particle size, and an EtOAc/petroleum ether mixture or gradient was used unless stated otherwise. High-resolution mass spectra (HRMS) were performed using a Bruker ESI-TOF high-resolution mass spectrometer. 1H NMR spectra were recorded at a spectrometer frequency of 400 MHz, and 13C NMR spectra were recorded at 101 MHz. Chemical shifts were reported in δ (ppm) using the δ 0 signal of tetramethylsilane and δ 7.26 signal of CDCl3 as internal standards. Purities of final compounds were established by analytical HPLC (>95%), which was carried out on a Waters HPLC system using Eclipse XDB-C18 column with detection at 254 and 285 nm on 2998 PDA, flow rate = 1.0 mL/min, the gradient of 30-95% acetonitrile aqueous solution over 30 min.
4.2. General method for preparation of compounds 8–39 and 45–68.
Methyl 4-(4-isopropylphenyl)picolinate (8).
To a solution of methyl 4-bromopicolinate (3 mmol) in toluene (10 mL) and water (5 mL) at room temperature were added 4-isopropylbenzeneboronic acid (1 mmol), Na2CO3 (8 mmol) and Pd(PPh3)4 (0.15 mmol). The mixture was stirred at 100 °C for 3 h under a nitrogen atmosphere, then it cooled to room temperature. The reaction mixture was then extracted with EtOAc. The combined organic layer was washed with brine, and dried over anhydrous Na2SO4, purified by silica gel chromatography (petroleum ether/EtOAc, 4:1, v/v) to afford 8. Yield 85%, white solid. 1H NMR (400 MHz, CDCl3) δ 8.76 (d, J = 5.0 Hz, 1H), 8.38 (s, 1H), 7.69 (dd, J = 5.0, 1.7 Hz, 1H), 7.64 (d, J = 8.2 Hz, 2H), 7.38 (d, J = 8.1 Hz, 2H), 4.04 (s, 3H), 3.04 – 2.94 (m, 1H), 1.30 (d, J = 6.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 150.8, 150.2, 149.6, 148.4, 134.5, 127.4, 127.0, 124.4, 122.9, 53.0, 34.0, 23.9.
4-(4-Isopropylphenyl)picolinaldehyde (9).
To a solution of ester 8 (2 mmol) in methanol at room temperature was added NaBH4 (4 mmol). The mixture was stirred at room temperature until the disappearance of the starting material (usually 2-3 h). The reaction mixture was then extracted with EtOAc. The combined organic layer was washed with brine and dried over anhydrous Na2SO4, concentrated in vacuo to afford crude alcohol intermediate. Then, to a solution of crude alcohol intermediate in CH2Cl2 was added Dess-Martin Periodinane (4 mmol) at room temperature. The mixture was stirred at room temperature for 3-5 h. The reaction mixture was slowly quenched with water at 0 °C and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo; the following purification by silica gel chromatography (petroleum ether/EtOAc, 5:1, v/v) led to the aldehyde intermediate 9. Yield 47%, white solid.1H NMR (400 MHz, CDCl3) δ 10.14 (s, 1H), 8.79 (d, J = 5.0 Hz, 1H), 8.18 (s, 1H), 7.72 (d, J = 5.0 Hz, 1H), 7.63 (d, J = 8.1 Hz, 2H), 7.37 (d, J = 8.1 Hz, 2H), 2.98 (m, 1H), 1.29 (d, J = 6.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 193.5, 153.3, 150.9, 150.6, 149.6, 134.3, 127.4, 127.0, 125.3, 119.2, 115.3, 33.9, 23.9.
1-((4-(4-Isopropylphenyl)pyridin-2-yl)methyl)-4,4-dimethyl-1,4-azasilinane (21).
To a solution of aldehyde intermediate 9 (1 mmol) in CH2Cl2 at room temperature was added 4,4-dimethyl-1,4-azasilinane hydrochloride (1.1 mmol). After stirring for 30 min, the sodium triacetoxyborohydride (2 mmol) at 0 °C was added, and the reaction mixture was stirred at room temperature for 3-5 h. The reaction mixture was slowly quenched with water at 0 °C and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo; the following purification by silica gel chromatography (petroleum ether/EtOAc, 1:1, v/v) led to the desired compound 21. Yield 56%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.68 (d, J = 5.4 Hz, 1H), 8.49 (s, 1H), 7.79 – 7.73 (m, 2H), 7.67 – 7.62 (m, 1H), 7.41 – 7.35 (m, 2H), 4.57 (s, 2H), 3.80 – 3.22 (m, 4H), 3.05 – 2.89 (m, 1H), 1.45 – 1.09 (m, 10H), 0.20 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 151.3, 151.1, 149.7, 149.2, 133.6, 127.5, 127.4, 124.9, 121.9, 59.9, 52.6, 33.9, 23.8, 10.4, −3.8.
2-((4,4-Dimethylpiperidin-1-yl)methyl)-6-phenylpyridine (11).
Compound 11 was prepared according to the method described for the preparation of compound 21 except using methyl 6-bromopicolinate, phenylboronic acid, and 4,4-dimethylpiperidine instead of methyl 4-bromopicolinate, 4-isopropylbenzeneboronic acid, and 4,4-dimethyl-1,4-azasilinane hydrochloride, respectively. Yield 47%, yellow oil. 1H NMR (400 MHz, CD3OD) δ 8.17 – 8.00 (m, 4H), 7.60 – 7.49 (m, 4H), 4.64 (s, 2H), 3.72 – 3.33 (m, 4H), 1.90 – 1.65 (m, 4H), 1.13 (s, 6H). HR-MS (ESI): m/z calcd for C19H25N2 (M + H)+: 281.2012; found: 281.2028.
2-((4,4-Dimethylpiperidin-1-yl)methyl)-6-(4-isopropylphenyl)pyridine (12).
Compound 12 was prepared according to the method described for the preparation of compound 21 except using methyl 6-bromopicolinate and 4,4-dimethylpiperidine instead of methyl 4-bromopicolinate and 4,4-dimethyl-1,4-azasilinane hydrochloride, respectively. Yield 58%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.01 – 7.86 (m, 4H), 7.83 – 7.75 (m, 1H), 7.41 – 7.35 (m, 2H), 4.63 (s, 2H), 3.66 – 3.55 (m, 2H), 3.29 – 3.13 (m, 2H), 3.09 – 2.92 (m, 1H), 2.29 – 2.10 (m, 2H), 1.63 – 1.51 (m, 2H), 1.31 (d, J = 6.9 Hz, 6H), 1.08 (s, 3H), 1.03 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.2, 150.5, 149.2, 138.5, 136.0, 127.0, 126.8, 124.9, 120.7, 61.0, 49.3, 35.3, 33.9, 30.7, 27.6, 23.9. HR-MS (ESI): m/z calcd for C22H31N2 (M + H)+: 323.2482; found: 323.2470.
1-((6-(4-Isopropylphenyl)pyridin-2-yl)methyl)-4,4-dimethyl-1,4-azasilinane (13).
Compound 13 was prepared according to the method described for the preparation of compound 21 except using methyl 6-bromopicolinate instead of methyl 4-bromopicolinate. Yield 62%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.01 – 7.87 (m, 4H), 7.80 – 7.73 (m, 1H), 7.41 – 7.32 (m, 2H), 4.67 (s, 2H), 3.97 – 3.80 (m, 2H), 3.52 – 3.39 (m, 2H), 3.05 – 2.93 (m, 1H), 1.62 – 1.49 (m, 2H), 1.29 (d, J = 6.9 Hz, 6H), 1.14 – 1.03 (m, 2H), 0.19 (d, J = 10.8 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 157.0, 150.5, 149.4, 138.6, 135.9, 127.0, 126.8, 124.7, 120.6, 60.2, 52.8, 33.9, 23.9, 10.4, −3.3, −4.0. HR-MS (ESI): m/z calcd for C21H31N2Si (M + H)+: 339.2251; found: 339.2246.
4-((4,4-Dimethylpiperidin-1-yl)methyl)-2-phenylpyridine (14).
Compound 14 was prepared according to the method described for the preparation of compound 21 except using 2-bromopyridine-4-carboxylate, phenylboronic acid, and 4,4-dimethylpiperidine instead of methyl 4-bromopicolinate, 4-isopropylbenzeneboronic acid, and 4,4-dimethyl-1,4-azasilinane hydrochloride, respectively. Yield 56%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.78 (d, J = 4.8 Hz, 1H), 8.33 (s, 1H), 8.12 (d, J = 7.4 Hz, 2H), 7.67 – 7.58 (m, 1H), 7.54 – 7.40 (m, 3H), 4.20 (s, 2H), 3.43 – 3.32 (m, 2H), 2.92 – 2.78 (m, 2H), 2.33 – 2.19 (m, 2H), 1.57 – 1.44 (m, 2H), 1.08 (s, 3H), 1.03 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.5, 150.5, 138.0, 129.6, 128.9, 127.1, 123.8, 122.5, 77.2, 59.4, 49.3, 34.8, 30.7, 27.7, 23.5. HR-MS (ESI): m/z calcd for C19H25N2 (M + H)+: 281.2012; found: 281.2015.
4,4-Dimethyl-1-((2-phenylpyridin-4-yl)methyl)-1,4-azasilinane (15).
Compound 15 was prepared according to the method described for the preparation of compound 21 except using methyl 2-bromopyridine-4-carboxylate and phenylboronic acid instead of methyl 4-bromopicolinate and 4-isopropylbenzeneboronic acid, respectively. Yield 56%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.92 – 8.79 (m, 1H), 8.60 (s, 1H), 8.21 (d, J = 7.4 Hz, 2H), 7.81 – 7.65 (m, 1H), 7.61 – 7.44 (m, 3H), 4.26 (s, 2H), 3.75 – 3.60 (m, 2H), 3.15 – 3.00 (m, 2H), 1.77 – 1.57 (m, 2H), 1.06 – 0.88 (m, 2H), 0.23 (s, 3H), 0.21 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.4, 150.4, 129.4, 128.9, 127.1, 123.3, 121.9, 59.3, 52.5, 10.9, −3.7. HR-MS (ESI): m/z calcd for C18H25N2Si (M + H)+: 297.1782; found: 297.1775.
4-((4,4-Dimethylpiperidin-1-yl)methyl)-2-(4-isopropylphenyl)pyridine (16).
Compound 16 was prepared according to the method described for the preparation of compound 21 except using methyl 2-bromopyridine-4-carboxylate and 4,4-dimethylpiperidine instead of methyl 4-bromopicolinate and 4,4-dimethyl-1,4-azasilinane hydrochloride, respectively. Yield 59%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.77 (s, 1H), 8.47 – 8.30 (m, 1H), 8.07 (d, J = 7.5, 2H), 7.68 – 7.56 (m, 1H), 7.44 – 7.36 (m, 2H), 4.19 (s, 2H), 3.45 – 3.28 (m, 2H), 3.06 – 2.93 (m, 1H), 2.93 – 2.74 (m, 2H), 2.35 – 2.17 (m, 2H), 1.61 – 1.44 (m, 2H), 1.28 (d, J = 6.9 Hz, 6H), 1.08 (s, 3H), 1.02 (s, 3H). HR-MS (ESI): m/z calcd for C22H31N2 (M + H)+: 323.2482; found: 323.2457.
1-((2-(4-Isopropylphenyl)pyridin-4-yl)methyl)-4,4-dimethyl-1,4-azasilinane (17).
Compound 17 was prepared according to the method described for the preparation of compound 21 except using methyl 2-bromopyridine-4-carboxylate instead of methyl 4-bromopicolinate. Yield 53%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.79 – 8.72 (m, 1H), 8.23 (s, 1H), 8.09 – 7.99 (m, 2H), 7.56 (s, 1H), 7.41 – 7.31 (m, 2H), 4.17 (s, 2H), 3.75 – 3.60 (m, 2H), 3.03 – 2.95 (m, 2H), 1.69 – 1.61 (m, 3H), 1.35 – 1.23 (m, 6H), 0.94 (m, 2H), 0.23 (s, 3H), 0.18 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.6, 150.7, 150.5, 138.3, 135.6, 127.1, 127.0, 123.2, 122.0, 77.2, 58.6, 52.4, 33.9, 23.8, 10.1, −3.5, −4.2. HR-MS (ESI): m/z calcd for C21H31N2Si (M + H)+: 339.2251; found: 339.2235.
2-((4,4-Dimethylpiperidin-1-yl)methyl)-4-phenylpyridine (18).
Compound 18 was prepared according to the method described for the preparation of compound 21 except using phenylboronic acid and 4,4-dimethylpiperidine instead of 4-isopropylbenzeneboronic acid and 4,4-dimethyl-1,4-azasilinane hydrochloride, respectively. Yield 63%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.62 (d, J = 5.3 Hz, 1H), 8.47 (s, 1H), 7.80 (d, J = 7.0 Hz, 2H), 7.62 (d, J = 6.7 Hz, 1H), 7.55 – 7.43 (m, 3H), 4.38 (s, 2H), 3.35 – 3.01 (m, 4H), 1.92 – 1.41 (m, 4H), 1.04 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 150.0, 149.9, 136.7, 129.6, 129.3, 127.2, 124.9, 122.1, 61.4, 49.0, 35.1, 27.7. HR-MS (ESI): m/z calcd for C19H25N2 (M + H)+: 281.2012; found: 281.2007.
4,4-Dimethyl-1-((4-phenylpyridin-2-yl)methyl)-1,4-azasilinane (19).
Compound 19 was prepared according to the method described for the preparation of compound 21 except using phenylboronic acid instead of 4-isopropylbenzeneboronic acid. Yield 55%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.62 (d, J = 5.2 Hz, 1H), 8.38 (s, 1H), 7.84 – 7.76 (m, 2H), 7.62 – 7.56 (m, 1H), 7.54 – 7.48 (m, 2H), 7.48 – 7.44 (m, 1H), 4.36 (s, 2H), 3.57 – 3.20 (m, 4H), 1.39 – 1.08 (m, 4H), 0.19 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 150.7, 150.1, 129.9, 129.5, 127.4, 124.8, 122.1, 60.7, 52.4, 10.3, −3.6. HR-MS (ESI): m/z calcd for C18H25N2Si (M + H)+: 297.1782; found: 297.1787.
2-((4,4-Dimethylpiperidin-1-yl)methyl)-4-(4-isopropylphenyl)pyridine (20).
Compound 21 was prepared according to the method described for the preparation of compound 21 except using 4,4-dimethylpiperidine instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 49%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.60 (d, J = 5.2 Hz, 1H), 8.38 (s, 1H), 7.75 – 7.69 (m, 2H), 7.60 – 7.55 (m, 1H), 7.38 – 7.32 (m, 2H), 4.40 (s, 2H), 3.32 – 3.08 (m, 4H), 3.02 – 2.88 (m, 1H), 2.22 – 1.48 (m, 4H), 1.26 (d, J = 6.9 Hz, 6H), 1.02 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 150.9, 150.2, 149.8, 149.7, 134.0, 127.4, 127.2, 124.6, 121.8, 61.0, 48.9, 35.1, 33.9, 27.7, 23.8. HR-MS (ESI): m/z calcd for C22H31N2 (M + H)+: 323.2482; found: 323.2462.
3-((4,4-Dimethylpiperidin-1-yl)methyl)-5-phenylpyridine (22).
Compound 22 was prepared according to the method described for the preparation of compound 21 except using methyl 5-bromopyridine-3-carboxylate, phenylboronic acid, and 4,4-dimethylpiperidine instead of methyl 4-bromopicolinate, 4-isopropylbenzeneboronic acid, and 4,4-dimethyl-1,4-azasilinane hydrochloride, respectively. Yield 56%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.82 (s, 1H), 8.53 (s, 1H), 8.25 (s, 1H), 7.68 – 7.63 (m, 2H), 7.51 – 7.46 (m, 2H), 7.43 – 7.38 (m, 1H), 3.84 (s, 2H), 2.81 – 2.59 (m, 4H), 1.67 – 1.46 (m, 4H), 0.98 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 149.4, 148.1, 137.0, 136.6, 136.5, 129.1, 128.3, 127.2, 59.0, 49.5, 36.8, 28.1. HR-MS (ESI): m/z calcd for C19H25N2 (M + H)+: 281.2012; found: 281.2002.
4,4-Dimethyl-1-((5-phenylpyridin-3-yl)methyl)-1,4-azasilinane (23).
Compound 23 was prepared according to the method described for the preparation of compound 21 except using methyl 5-bromopyridine-3-carboxylate and phenylboronic acid instead of methyl 4-bromopicolinate and 4-isopropylbenzeneboronic acid, respectively. Yield 51%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.83 (s, 1H), 8.54 (s, 1H), 8.40 (s, 1H), 7.69 – 7.64 (m, 2H), 7.49 – 7.44 (m, 2H), 7.42 – 7.36 (m, 1H), 3.99 (s, 2H), 3.21 – 2.94 (m, 4H), 1.18 – 0.98 (m, 4H), 0.12 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 149.3, 148.4, 136.8, 136.8, 136.6, 129.1, 128.4, 127.2, 58.1, 52.3, 11.6, −3.5. HR-MS (ESI): m/z calcd for C18H25N2Si (M + H)+: 297.1782; found: 297.1782.
3-((4,4-Dimethylpiperidin-1-yl)methyl)-5-(4-isopropylphenyl)pyridine (24).
Compound 24 was prepared according to the method described for the preparation of compound 21 except using methyl 5-bromopyridine-3-carboxylate and 4,4-dimethylpiperidine instead of methyl 4-bromopicolinate and 4,4-dimethyl-1,4-azasilinane hydrochloride, respectively. Yield 60%, yellow oil. NMR (400 MHz, CDCl3) δ 8.91 (s, 1H), 8.81 (s, 1H), 8.58 (s, 1H), 7.66 (d, J = 8.0 Hz, 2H), 7.35 (d, J = 8.1 Hz, 2H), 4.25 (s, 2H), 3.41 – 3.29 (m, 2H), 2.98 – 2.92 (m, 1H), 2.91 – 2.79 (m, 2H), 2.27 – 2.17 (m, 2H), 2.04 – 1.93 (m, 2H), 1.27 (d, J = 6.9 Hz, 6H), 1.06 (s, 3H), 1.02 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 149.6, 149.3, 137.5, 137.1, 133.6, 127.4, 127.2, 57.8, 49.0, 35.1, 33.8, 27.8, 23.9. HR-MS (ESI): m/z calcd for C22H31N2 (M + H)+: 323.2482; found: 323.2496.
1-((5-(4-Isopropylphenyl)pyridin-3-yl)methyl)-4,4-dimethyl-1,4-azasilinane (25).
Compound 25 was prepared according to the method described for the preparation of compound 21 except using methyl 5-bromopyridine-3-carboxylate instead of methyl 4-bromopicolinate. Yield 61%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.92 (s, 1H), 8.85 (s, 1H), 8.61 (s, 1H), 7.70 – 7.65 (m, 2H), 7.39 – 7.34 (m, 2H), 4.28 (s, 2H), 3.71 – 3.59 (m, 2H), 3.12 – 3.01 (m, 2H), 2.98 – 2.92 (m, 1H), 1.98 (s, 2H), 1.30 – 1.24 (m, 6H), 1.01 – 0.92 (m, 2H), 0.20 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 189.6, 166.4, 156.3, 152.4, 148.8, 133.0, 128.5, 124.8, 117.2, 105.2, 88.3, 73.8, 34.7, 31.4, 31.1, 26.5, 26.4, 15.6. HR-MS (ESI): m/z calcd for C21H31N2Si (M + H)+: 339.2251; found: 339.2269.
4-((4,4-Dimethylpiperidin-1-yl)methyl)-6-(4-isopropylphenyl)pyrimidine (26).
Compound 26 was prepared according to the method described for the preparation of compound 21 except using methyl 6-bromopyrimidine-4-carboxylate and 4,4-dimethylpiperidine instead of methyl 4-bromopicolinate and 4,4-dimethyl-1,4-azasilinane hydrochloride, respectively. Yield 52%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.18 (s, 1H), 8.08 – 7.92 (m, 3H), 7.38 (d, J = 7.9 Hz, 2H), 3.82 (s, 2H), 3.01 – 2.94 (m, 1H), 2.82 – 2.50 (m, 4H), 1.70 – 1.36 (m, 4H), 1.29 (d, J = 6.9 Hz, 6H), 0.98 (s, 6H). HR-MS (ESI): m/z calcd for C21H30N3 (M + H)+: 324.2434; found: 324.2453.
4-((4,4-Dimethyl-1,4-azasilinan-1-yl)methyl)-6-(4-isopropylphenyl)pyrimidine (27).
Compound 27 was prepared according to the method described for the preparation of compound 21 except using methyl 6-bromopyrimidine-4-carboxylate instead of methyl 4-bromopicolinate. Yield 56%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.06 (s, 1H), 7.97 (d, J = 8.1 Hz, 2H), 7.84 (s, 1H), 7.29 (d, J = 8.1 Hz, 2H), 3.64 (s, 2H), 2.96 – 2.85 (m, 1H), 2.76 – 2.67 (m, 4H), 1.21 (d, J = 6.9 Hz, 6H), 0.79 – 0.71 (m, 4H), 0.00 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 169.0, 164.1, 158.5, 152.1, 134.5, 127.2, 127.0, 114.9, 63.6, 53.1, 34.1, 23.8, 13.8, −3.0. HR-MS (ESI): m/z calcd for C20H30N3Si (M + H)+: 340.2204; found: 340.2213.
4-((4,4-Dimethylpiperidin-1-yl)methyl)-2-phenyloxazole (28).
Compound 28 was prepared according to the method described for the preparation of compound 21 except using methyl 2-bromo-1,3-oxazole-4-carboxylate, phenylboronic acid, and 4,4-dimethylpiperidine instead of methyl 4-bromopicolinate, 4-isopropylbenzeneboronic acid, and 4,4-dimethyl-1,4-azasilinane hydrochloride, respectively. Yield 53%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H), 8.00 (d, J = 4.9 Hz, 2H), 7.51 – 7.44 (m, 3H), 4.15 (s, 2H), 3.43 – 2.96 (m, 4H), 2.31 – 1.42 (m, 4H), 1.01 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 162.2, 141.1, 131.0, 131.0, 128.9, 126.6, 126.5, 51.3, 48.5, 35.3, 27.7. HR-MS (ESI): m/z calcd for C17H23N2O (M + H)+: 271.1805; found: 271.1817.
4-((4,4-Dimethyl-1,4-azasilinan-1-yl)methyl)-2-phenyloxazole (29).
Compound 29 was prepared according to the method described for the preparation of compound 21 except using methyl 2-bromo-1,3-oxazole-4-carboxylate and phenylboronic acid instead of methyl 4-bromopicolinate and 4-isopropylbenzeneboronic acid, respectively. Yield 59%, yellow oil. 1H NMR (400 MHz, CDC13) δ 8.25 (s, 1H), 8.04 – 7.96 (m, 2H), 7.51 – 7.43 (m, 3H), 4.18 (s, 2H), 3.70 – 3.08 (m, 4H), 1.54 – 0.92 (m, 4H), 0.16 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 162.1, 141.1, 131.2, 131.0, 128.9, 126.6, 126.5, 52.2, 51.4, 10.7, −3.9. HR-MS (ESI): m/z calcd for C16H23N2OSi (M + H)+: 287.1574; found: 287.1585.
4-((4,4-Dimethyl-1,4-azasilinan-1-yl)methyl)-2-(4-isopropylphenyl)oxazole (30).
Compound 30 was prepared according to the method described for the preparation of compound 21 except using methyl 2-bromo-1,3-oxazole-4-carboxylate instead of methyl 4-bromopicolinate. Yield 49%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.09 (s, 1H), 7.91 (d, J = 7.5 Hz, 2H), 7.29 (d, J = 7.6 Hz, 2H), 4.08 (s, 2H), 3.38 – 3.20 (m, 4H), 2.97 – 2.88 (m, 1H), 1.24 (d, J = 6.6 Hz, 6H), 1.20 – 1.12 (m, 4H), 0.12 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 162.2, 152.1, 140.0, 131.2, 126.9, 126.5, 124.4, 52.2, 51.9, 34.1, 23.7, 11.2, −3.7. HR-MS (ESI): m/z calcd for C19H29N2OSi (M + H)+: 329.2044; found: 329.2047.
N-((4-(4-Isopropylphenyl)pyridin-2-yl)methyl)adamantan-2-amine (31).
Compound 31 was prepared according to the method described for the preparation of compound 21 except using 2-amantadine instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 61%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.54 (d, J = 5.2 Hz, 1H), 7.74 (s, 1H), 7.66 – 7.56 (m, 2H), 7.50 – 7.43 (m, 1H), 7.39 – 7.31 (m, 2H), 4.21 (s, 2H), 3.10 (s, 1H), 3.03 – 2.87 (m, 1H), 2.25 – 2.11 (m, 4H), 1.93 – 1.82 (m, 4H), 1.76 – 1.68 (m, 4H), 1.63 – 1.54 (m, 2H), 1.28 (d, J = 6.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 156.4, 150.5, 149.1, 134.8, 127.3, 127.0, 120.7, 62.1, 50.7, 37.5, 37.2, 33.9, 31.0, 30.8, 27.3, 27.1, 23.8. HR-MS (ESI): m/z calcd for C25H33N2 (M + H)+: 361.2638; found: 361.2664.
N-((4-(4-Isopropylphenyl)pyridin-2-yl)methyl)adamantan-1-amine (32).
Compound 32 was prepared according to the method described for the preparation of compound 21 except using 1-amantadine instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 55%, yellow oil. 1H NMR (400 MHz, CDC13) δ 8.59 – 8.48 (m, 1H), 7.69 (s, 1H), 7.64 – 7.54 (m, 2H), 7.53 – 7.41 (m, 1H), 7.39 – 7.32 (m, 2H), 4.28 (s, 2H), 3.02 – 2.92 (m, 1H), 2.21 – 2.09 (m, 3H), 2.03 – 1.91 (m, 6H), 1.67 (s, 6H), 1.28 (d, J = 6.9 Hz, 6H). 13C NMR (101 MHz, CDC13) δ 150.7, 150.2, 148.8, 127.3, 127.0, 120.4, 43.9, 39.9, 35.7, 33.9, 29.2, 23.8. HR-MS (ESI): m/z calcd for C25H33N2 (M + H)+: 361.2638; found: 361.2651.
N-((4-(4-Isopropylphenyl)pyridin-2-yl)methyl)cycloheptanamine (33).
Compound 33 was prepared according to the method described for the preparation of compound 21 except using cycloheptylamine instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 56%, yellow oil. 1H NMR (400 MHz, CDC13) δ 8.56 (d, J = 5.2 Hz, 1H), 7.84 (s, 1H), 7.63 (d, J = 8.1 Hz, 2H), 7.45 (d, J = 4.8 Hz, 1H), 7.33 (d, J = 8.0 Hz, 2H), 4.23 (s, 2H), 3.07 – 3.00 (m, 1H), 2.98 – 2.91 (m, 1H), 2.19 – 2.07 (m, 2H), 1.82 – 1.68 (m, 4H), 1.57 – 1.49 (m, 4H), 1.45 – 1.36 (m, 2H), 1.27 (d, J = 6.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 154.3, 150.5, 149.6, 149.3, 134.6, 127.3, 127.0, 121.2, 120.9, 58.0, 49.8, 33.8, 30.3, 26.6, 25.6, 23.8. HR-MS (ESI): m/z calcd for C22H31N2 (M + H)+: 323.2482; found: 323.2504.
N-((4-(4-Isopropylphenyl)pyridin-2-yl)methyl)cyclooctanamine (34).
Compound 34 was prepared according to the method described for the preparation of compound 21 except using cyclooctylamine instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 63%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.56 (d, J = 5.2 Hz, 1H), 7.88 (s, 1H), 7.64 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 4.8 Hz, 1H), 7.33 (d, J = 8.0 Hz, 2H), 4.25 (s, 2H), 3.21 – 3.08 (m, 1H), 3.01 – 2.88 (m, 1H), 2.15 – 2.00 (m, 2H), 1.90 – 1.72 (m, 4H), 1.63 – 1.38 (m, 8H), 1.27 (d, J = 6.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 154.7, 150.5, 149.6, 149.3, 134.7, 127.2, 127.0, 121.1, 120.8, 58.9, 50.0, 33.9, 32.4, 27.8, 24.0, 23.8. HR-MS (ESI): m/z calcd for C22H31N2 (M + H)+: 323.2482; found: 323.2495.
4-(4-Isopropylphenyl)-2-((4-methylpiperidin-1-yl)methyl)pyridine (35).
Compound 35 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 54%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.58 (d, J = 5.2 Hz, 1H), 7.77 (s, 1H), 7.62 (d, J = 8.1 Hz, 2H), 7.44 (s, 1H), 7.35 (d, J = 8.0 Hz, 2H), 3.85 (s, 2H), 3.05 (s, 1H), 3.02 – 2.92 (m, 1H), 2.38 – 2.13 (m, 2H), 1.75 – 1.61 (m, 2H), 1.53 – 1.37 (m, 2H), 1.29 (d, J = 6.9 Hz, 6H), 0.95 (d, J = 4.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 150.1, 149.4, 148.9, 135.4 (d, J = 23.6 Hz), 127.2, 127.0, 121.5, 120.4, 120.1, 64.1, 54.0, 33.9, 33.3, 30.2, 23.8, 21.6. HR-MS (ESI): m/z calcd for C21H29N2 (M + H)+: 309.2325 found: 309.2347.
6-((4-(4-Isopropylphenyl)pyridin-2-yl)methyl)-6-azaspiro[2.5]octane (36).
Compound 36 was prepared according to the method described for the preparation of compound 21 except using 6-azaspiro[2.5]octane instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 51%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.58 (d, J = 4.5 Hz, 1H), 7.78 (s, 1H), 7.63 (d, J = 7.8 Hz, 2H), 7.45 – 7.38 (m, 1H), 7.35 (d, J = 7.7 Hz, 2H), 3.84 (s, 2H), 3.04 – 2.92 (m, 1H), 2.79 – 2.58 (m, 4H), 1.76 – 1.34 (m, 4H), 1.30 (d, J = 6.9 Hz, 6H), 0.35 – 0.22 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 150.1, 149.5, 148.8, 135.6, 127.2, 127.0, 121.5, 120.0, 64.4, 53.3, 34.5, 33.9, 23.9, 17.1, 11.4. HR-MS (ESI): m/z calcd for C22H29N2 (M + H)+: 321.2325; found: 321.2350.
8-((4-(4-Isopropylphenyl)pyridin-2-yl)methyl)-8-azaspiro[4.5]decane (37).
Compound 37 was prepared according to the method described for the preparation of compound 21 except using 8-azaspiro[4.5]decane instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 60%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.58 (d, J = 4.9 Hz, 1H), 7.85 (s, 1H), 7.64 (d, J = 7.8 Hz, 2H), 7.47 – 7.41 (m, 1H), 7.35 (d, J = 7.7 Hz, 2H), 3.88 (s, 2H), 3.07 – 2.91 (m, 1H), 2.83 – 2.53 (m, 4H), 1.70 – 1.55 (m, 8H), 1.48 – 1.39 (m, 4H), 1.29 (d, J = 6.8 Hz, 6H). HR-MS (ESI): m/z calcd for C24H33N2 (M + H)+: 349.2638; found: 349.2637.
9-((4-(4-Isopropylphenyl)pyridin-2-yl)methyl)-1,5-dioxa-9-azaspiro[5.5] undecane (38).
Compound 38 was prepared according to the method described for the preparation of compound 21 except using l,5-dioxa-9-azaspiro[5.5]undecane instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 53%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.58 (d, J = 5.0 Hz, 1H), 7.81 (s, 1H), 7.63 (d, J = 7.7 Hz, 2H), 7.48 – 7.40 (m, 1H), 7.35 (d, J = 7.6 Hz, 2H), 3.96 – 3.84 (m, 6H), 3.05 – 2.90 (m, 1H), 2.83 – 2.63 (m, 4H), 2.14 – 2.00 (m, 4H), 1.77 – 1.66 (m, 2H), 1.29 (d, J = 6.8 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 150.2, 149.6, 148.9, 135.3, 130.1, 128.7, 127.2, 127.0, 121.6, 120.3, 95.6, 63.5, 59.3, 49.9, 49.4, 33.9, 32.0, 25.5, 23.9. HR-MS (ESI): m/z calcd for C22H31N2 (M + H)+: 323.2380; found: 323.2376.
8-((4-(4-Isopropylphenyl)pyridin-2-yl)methyl)-1,4-dioxa-8-azaspiro[4.5]decane (39).
Compound 39 was prepared according to the method described for the preparation of compound 21 except using 1,4-dioxa-8-azaspiro[4.5]decane instead of 4,4-dimethyl-1,4-azasilinane hydrochloride. Yield 55%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.59 (d, J = 5.0 Hz, 1H), 7.77 (s, 1H), 7.62 (d, J = 7.7 Hz, 2H), 7.43 (s, 1H), 7.35 (d, J = 7.7 Hz, 2H), 3.95 (s, 4H), 3.86 (s, 2H), 3.06 – 2.91 (m, 1H), 2.88 – 2.65 (m, 4H), 1.96 – 1.75 (m, 4H), 1.29 (d, J = 6.8 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 150.2, 149.5, 149.1, 135.4, 127.2, 127.0, 121.4, 106.5, 64.3, 63.5, 51.4, 34.2, 33.9, 23.9. HR-MS (ESI): m/z calcd for C22H28N2O2 (M + H)+: 353.2224; found: 353.2242.
8-(2-((4-Methylpiperidin-1-yl)methyl)pyridin-4-yl)quinoline (45).
Compound 45 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 8-quinolinylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 56%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.98 – 8.91 (m, 1H), 8.68 (d, J = 4.9 Hz, 1H), 8.24 (d, J = 8.2 Hz, 1H), 7.96 (s, 1H), 7.94 – 7.89 (m, 1H), 7.88 – 7.83 (m, 1H), 7.70 – 7.63 (m, 2H), 7.49 – 7.44 (m, 1H), 4.01 (s, 2H), 3.27 – 3.13 (m, 2H), 2.57 – 2.39 (m, 2H), 1.78 – 1.66 (m, 2H), 1.65 – 1.44 (m, 3H), 0.96 (d, J = 5.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 150.5, 148.8, 148.1, 145.4, 137.5, 136.5, 130.6, 129.0, 128.7, 126.4, 124.9, 121.4, 63.5, 53.4, 32.7, 29.9, 21.4. HR-MS (ESI): m/z calcd for C21H24N3 (M + H)+: 318.1965; found: 318.1977.
2-((4-Methylpiperidin-1-yl)methyl)-4,4'-bipyridine (46).
Compound 46 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 4-pyridinylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 50%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.92 (s, 1H), 8.67 (d, J = 12.7 Hz, 2H), 8.00 (d, J = 7.2 Hz, 1H), 7.90 – 7.74 (m, 1H), 7.52 – 7.36 (m, 2H), 3.82 (s, 2H), 3.14 – 2.82 (m, 2H), 2.39 – 2.09 (m, 2H), 1.74 – 1.59 (m, 2H), 1.51 – 1.32 (m, 3H), 0.96 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 150.1, 149.8, 134.5, 123.8, 121.6, 120.9, 120.1, 64.4, 54.0, 33.6, 30.3, 21.7. HR-MS (ESI): m/z calcd for C17H22N3 (M + H)+: 268.1808; found: 268.1827.
6-Methyl-2'-((4-methylpiperidin-l-yl)methyl)-3,4'-bipyridine (47).
Compound 47 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 2-picoline-5-boronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 59%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.81 (s, 1H), 8.63 (d, J = 4.7 Hz, 1H), 8.07 – 7.90 (m, 2H), 7.53 – 7.41 (m, 1H), 7.31 – 7.21 (m, 1H), 3.96 (s, 2H), 3.30 – 3.00 (m, 2H), 2.63 (s, 3H), 2.55 – 2.32 (m, 2H), 1.79 – 1.66 (m, 2H), 1.65 – 1.44 (m, 3H), 0.98 (d, J = 5.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 159.4, 155.8, 149.8, 147.3, 146.2, 135.0, 130.5, 123.6, 120.4, 63.5, 53.6, 32.7, 29.9, 24.3, 21.3. HR-MS (ESI): m/z calcd for C18H24N3 (M + H)+: 282.1965; found: 282.1957.
4-(Furan-3-yl)-2-((4-methylpiperidin-1-yl)methyl)pyridine (48).
Compound 48 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 3-furanboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 53%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.57 – 8.47 (m, 1H), 8.13 – 7.95 (m, 2H), 7.52 (s, 1H), 7.38 – 7.31 (m, 1H), 6.84 (s, 1H), 4.01, (s, 2H), 3.26 – 3.14 (m, 2H), 2.63 – 2.43 (m, 2H), 1.78 – 1.44 (m, 5H), 0.98 (d, J = 6.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 154.3, 149.7, 144.4, 141.0, 128.8, 123.9, 121.8, 119.7, 108.3, 63.1, 53.3, 32.1, 29.6, 21.2. HR-MS (ESI): m/z calcd for C16H21N2O (M + H)+: 257.1648; found: 257.1630.
2-((4-Methylpiperidin-1-yl)methyl)-4-(4-nitrophenyl)pyridine (49).
Compound 49 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and (4-nitrophenyl)boronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 53%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.60 (m, 2H), 8.40 – 8.24 (m, 1H), 8.02 (m, 2H), 7.83 – 7.59 (m, 1H), 7.57 – 7.38 (m, 1H), 3.90 (s, 2H), 3.28 – 2.90 (m, 2H), 2.52 – 2.20 (m, 2H), 1.80 – 1.60 (m, 2H), 1.60 – 1.34 (m, 3H), 0.97 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 150.0, 148.8, 146.6, 139.8, 133.2, 130.3, 123.7, 122.1, 122.0, 120.4, 64.0, 53.8, 33.1, 30.1, 21.5. HR-MS (ESI): m/z calcd for C18H22N3O2 (M + H)+: 312.1707; found: 312.1696.
4-(4-Bromophenyl)-2-((4-methylpiperidin-1-yl)methyl)pyridine (50).
Compound 50 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 4-bromophenylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 49%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.66 (s, 1H), 7.79 – 7.46 (m, 6H), 4.01 (s, 2H), 3.47 – 3.07 (m, 2H), 2.71 – 2.35 (m, 2H), 1.8 8– 1.50 (m, 5H), 1.00 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 149.6, 132.4, 132.0, 131.9, 128.7, 128.6, 128.6, 127.7, 127.5, 63.7, 53.8, 32.9, 30.0, 21.4. HR-MS (ESI): m/z calcd for C18H22BrN2 (M + H)+: 345.0961; found: 345.0959.
2-((4-Methylpiperidin-1-yl)methyl)-4-(4-(trifluoromethyl)phenyl)pyridine (51).
Compound 51 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 4-trifluoromethylphenylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 63%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.69 (s, 1H), 8.03 – 7.47 (m, 6H), 3.97 (s, 2H), 3.32 – 2.98 (m, 2H), 2.57 – 2.24 (m, 2H), 1.90 – 1.47 (m, 5H), 0.98 (s, 3H). HR-MS (ESI): m/z calcd for C19H22F3N2 (M + H)+: 335.1730; found: 335.1722.
4-(4-Isobutylphenyl)-2-((4-methylpiperidin-1-yl)methyl)pyridine (52).
Compound 52 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 4-isobutylphenylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 52%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.58 (d, J = 4.7 Hz, 1H), 8.11 (s, 1H), 7.66 (d, J = 7.5 Hz, 2H), 7.56 – 7.46 (m, 1H), 7.30 – 7.24 (m, 2H), 4.10 (s, 2H), 3.32 – 3.16 (m, 2H), 2.74 – 2.56 (m, 2H), 2.53 (d, J = 7.0 Hz, 2H), 1.96 – 1.87 (m, 1H), 1.84 – 1.66 (m, 4H), 1.63 – 1.49 (m, 1H), 0.99 (d, J = 6.2 Hz, 3H), 0.93 (d, J = 6.5 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 149.7, 149.4, 143.4, 134.5, 130.0, 126.8, 123.2, 121.0, 62.8, 53.1, 45.1, 31.8, 30.1, 29.5, 22.3, 21.1. HR-MS (ESI): m/z calcd for C22H31N2 (M + H)+: 323.2482; found: 323.2482.
4-(4-(Tert-butyl)phenyl)-2-((4-methylpiperidin-1-yl)methyl)pyridine (53).
Compound 53 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 4-tert-butylphenylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 60%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.59 (d, J = 5.1 Hz, 1H), 8.03 (s, 1H), 7.68 (d, J = 8.0 Hz, 2H), 7.52 (d, J = 8.2 Hz, 2H), 7.50 – 7.45 (m, 1H), 4.03 (s, 2H), 3.13 – 3.28 (m, 2H), 2.62 – 2.42 (m, 2H), 1.79 – 1.59 (m, 4H), 1.58 – 1.45 (m, 1H), 1.36 (s, 9H), 0.98 (d, J = 6.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 152.6, 149.6, 149.2, 134.5, 126.8, 126.1, 122.8, 120.8, 63.1, 53.2, 34.7, 32.3, 31.2, 29.7, 21.2, HR-MS (ESI): m/z calcd for C22H31N2 (M + H)+: 323.2482; found: 323.2463.
4-(4-Cyclohexylphenyl)-2-((4-methylpiperidin-1-yl)methyl)pyridine (54).
Compound 54 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 4-cyclohexylbenzeneboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 56%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.57 (s, 1H), 8.16 – 7.95 (m, 1H), 7.76 – 7.61 (m, 2H), 7.57 – 7.43 (m, 1H), 7.39 – 7.29 (m, 2H), 4.06 (s, 2H), 3.32 – 3.13 (m, 2H), 2.74 – 2.36 (m, 3H), 1.98 – 1.82 (m, 4H), 1.82 – 1.62 (m, 5H), 1.58 – 1.25 (m, 6H), 0.98 (d, J = 5.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.9, 149.6, 127.7, 127.0, 122.9, 120.9, 63.0, 53.1, 44.3, 34.3, 32.1, 29.6, 26.8, 26.0, 21.2. HR-MS (ESI): m/z calcd for C24H33N2 (M + H)+: 349.2638; found: 349.2621.
2-((4-Methylpiperidin-1-yl)methyl)-4-(naphthalen-1-yl)pyridine (55).
Compound 55 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 1-naphthylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 51%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.68 (d, J = 4.8 Hz, 1H), 7.96 – 7.89 (m, 2H), 7.87 – 7.83 (m, 1H), 7.76 (s, 1H), 7.59 – 7.44 (m, 4H), 7.43 – 7.37 (m, 1H), 4.00 (s, 2H), 3.28 – 3.13 (m, 2H), 2.58 – 2.38 (m, 2H), 1.77 – 1.66 (m, 2H), 1.63 – 1.40 (m, 3H), 0.95 (d, J = 5.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 155.3, 149.5, 149.1, 136.9, 133.7, 130.6, 129.0, 128.5, 127.2, 126.7, 126.1, 125.9, 125.4, 125.0, 124.3, 63.3, 53.4, 32.7, 29.8, 21.4. HR-MS (ESI): m/z calcd for C22H25N2 (M + H)+: 317.2012; found: 317.2032.
4-([1,1'-Biphenyl]-4-yl)-2-((4-methylpiperidin-1-yl)methyl)pyridine (56).
Compound 56 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and 4-biphenylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 62%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.62 (s, 1H), 8.18 – 8.03 (m, 1H), 7.86 – 7.78 (m, 2H), 7.77 – 7.70 (m, 2H), 7.68 – 7.61 (m, 2H), 7.56 – 7.51 (m, 1H), 7.49 – 7.41 (m, 2H), 7.42 – 7.35 (m, 1H), 4.04 (s, 2H), 3.28 – 3.14 (m, 2H), 2.64 – 2.40 (m, 2H), 1.79 – 1.46 (m, 5H), 0.98 (d, J = 5.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 154.7, 149.7, 148.8, 142.1, 140.1, 136.3, 128.9, 127.8, 127.7, 127.5, 127.0, 122.9, 120.8, 63.2, 53.3, 32.3, 29.7, 21.3. HR-MS (ESI): m/z calcd for C24H27N2 (M + H)+: 343.2169; found: 343.2162.
2-((4-Methylpiperidin-1-yl)methyl)-4-(naphthalen-2-yl)pyridine (57).
Compound 57 was prepared according to the method described for the preparation of compound 21 except using 4-methylpiperidine and naphthalen-2-ylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 55%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.63 (d, J = 4.9 Hz, 1H), 8.19 (s, 1H), 8.11 – 8.01 (m, 1H), 7.99 – 7.91 (m, 2H), 7.91 – 7.84 (m, 1H), 7.83 – 7.78 (m, 1H), 7.63 – 7.49 (m, 3H), 3.96 (s, 2H), 3.23 – 3.04 (m, 2H), 2.49 – 2.33 (m, 2H), 1.78 – 1.63 (m, 2H), 1.63 – 1.40 (m, 3H), 0.96 (d, J = 5.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 156.2, 149.7, 149.1, 135.0, 133.5, 133.4, 128.9, 128.5, 127.7, 126.8, 126.6, 124.6, 122.6, 120.9, 63.8, 53.6, 32.8, 30.0, 21.4. HR-MS (ESI): m/z calcd for C22H25N2 (M + H)+: 317.2012; found: 317.2002.
1-((4-(4-Isobutylphenyl)pyridin-2-yl)methyl)-4,4-dimethyl-1,4-azasilinane (58).
Compound 58 was prepared according to the method described for the preparation of compound 21 except using 4-isobutylphenylboronic acid instead of 4-isopropylbenzeneboronic acid. Yield 49%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 4.1 Hz, 1H), 7.75 (s, 1H), 7.52 (d, J = 7.5 Hz, 2H), 7.33 (s, 1H), 7.17 (d, J = 6.9 Hz, 2H), 3.82 (s, 2H), 2.95 – 2.77 (m, 4H), 2.44 (d, J = 7.0 Hz, 2H), 1.88 – 1.74 (m, 1H), 0.88 – 0.75 (m, 10H), 0.00 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 149.4, 148.9, 143.0, 135.2, 129.8, 126.8, 121.4, 120.2, 52.6, 45.1, 30.2, 29.7, 22.3, 12.8, −3.2. HR-MS (ESI): m/z calcd for C22H33N2Si (M + H)+: 353.2408; found: 353.2386.
1-((4-(4-Cyclohexylphenyl)pyridin-2-yl)methyl)-4,4-dimethyl-1,4-azasilinane (59).
Compound 59 was prepared according to the method described for the preparation of compound 21 except using 4-cyclohexylbenzeneboronic acid instead of 4-isopropylbenzeneboronic acid. Yield 62%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.46 (d, J = 4.4 Hz, 1H), 7.83 (s, 1H), 7.53 (d, J = 7.6 Hz, 2H), 7.41 – 7.30 (m, 1H), 7.26 – 7.19 (m, 2H), 3.88 (s, 2H), 3.04 – 2.82 (m, 4H), 2.53 – 2.36 (m, 1H), 1.84 – 1.71 (m, 4H), 1.40 – 1.24 (m, 4H), 1.23 – 1.08 (m, 2H), 0.97 – 0.80 (m, 4H), 0.00 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 149.5, 149.1, 135.1, 127.6, 127.0, 122.1, 120.5, 62.8, 52.5, 44.3, 34.3, 29.7, 26.8, 26.1, 12.3, −3.3. HR-MS (ESI): m/z calcd for C24H35N2Si (M + H)+: 379.2564; found: 379.2572.
1-((4-(4-(Tert-butyl)phenyl)pyridin-2-yl)methyl)-4,4-dimethyl-1,4-azasilinane (60).
Compound 60 was prepared according to the method described for the preparation of compound 21 except using 4-tert-butylphenylboronic acid instead of 4-isopropylbenzeneboronic acid. Yield 55%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 4.9 Hz, 1H), 7.81 (s, 1H), 7.55 (d, J = 7.6 Hz, 2H), 7.42 (d, J = 7.6 Hz, 2H), 7.37 – 7.30 (m, 1H), 3.86 (s, 2H), 3.00 – 2.79 (m, 4H), 1.26 (s, 9H), 0.95 – 0.80 (m, 4H), 0.00 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 152.4, 149.5, 148.9, 134.9, 126.8, 126.1, 121.7, 120.3, 63.1, 52.6, 34.7, 31.2, 29.7, 12.6, −3.3. HR-MS (ESI): m/z calcd for C22H33N2Si (M + H)+: 353.2408; found: 353.2404.
1-((4-([1,1'-Biphenyl]-4-yl)pyridin-2-yl)methyl)-4,4-dimethyl-1,4-azasilinane (61).
Compound 61 was prepared according to the method described for the preparation of compound 21 except using 4-biphenylboronic acid instead of 4-isopropylbenzeneboronic acid. Yield 59%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.50 (d, J = 4.4 Hz, 1H), 7.83 (s, 1H), 7.68 (d, J = 7.7 Hz, 2H), 7.62 (d, J = 7.7 Hz, 2H), 7.54 (d, J = 7.4 Hz, 2H), 7.37 (t, J = 6.3 Hz, 3H), 7.28 (t, J = 7.1 Hz, 1H), 3.83 (s, 2H), 2.96 – 2.76 (m, 4H), 0.92 – 0.76 (m, 4H), 0.00 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 149.6, 148.5, 142.0, 140.2, 136.8, 128.9, 127.8, 127.7, 127.5, 127.0, 121.5, 120.2, 63.3, 52.7, 29.6, 12.8, −3.2. HR-MS (ESI): m/z calcd for C24H29N2Si (M + H)+: 373.2095; found: 373.2097.
8-((4-([1,1'-Biphenyl]-4-yl)pyridin-2-yl)methyl)-8-azaspiro[4.5]decane (62).
Compound 62 was prepared according to the method described for the preparation of compound 21 except using 8-azaspiro[4.5]decane and 4-biphenylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 51%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.69 – 8.57 (m, 1H), 8.08 – 7.91 (m, 1H), 7.84 – 7.77 (m, 2H), 7.77 – 7.70 (m, 2H), 7.70 – 7.60 (m, 2H), 7.53 – 7.44 (m, 3H), 7.42 – 7.34 (m, 1H), 3.94 (s, 2H), 2.87 – 2.58 (m, 4H), 1.72 – 1.54 (m, 8H), 1.49 – 1.40 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 149.7, 148.6, 145.2, 143.6, 142.0, 140.2, 136.6, 128.9, 127.8, 127.7, 127.5, 127.1, 120.4, 63.8, 51.3, 40.2, 36.3, 24.3, HR-MS (ESI): m/z calcd for C27H31N2 (M + H)+: 383.2482; found: 383.2508.
3-((4-([1,1'-Biphenyl]-4-yl)pyridin-2-yl)methyl)-3-azaspiro[5.5]undecane (63).
Compound 63 was prepared according to the method described for the preparation of compound 21 except using 8-azaspiro[5.5]undecane and 4-biphenylboronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 56%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.64 – 8.59 (m, 1H), 8.28 – 8.09 (m, 1H), 7.88 – 7.81 (m, 2H), 7.75 – 7.72 (m, 2H), 7.66 – 7.63 (m, 2H), 7.57 – 7.53 (m, 1H), 7.50 – 7.45 (m, 2H), 7.41 – 7.36 (m, 1H), 4.10 (s, 2H), 3.05 – 2.78 (m, 4H), 1.82 – 1.71 (m, 4H), 1.44 – 1.38 (m, 10H). HR-MS (ESI): m/z calcd for C28H33N2 (M + H)+: 397.2638; found: 397.2661.
8-((4-(4'-Chloro-[1,1'-biphenyl]-4-yl)pyridin-2-yl)methyl)-8-azaspiro[4.5]decane (64).
Compound 64 was prepared according to the method described for the preparation of compound 21 except using 8-azaspiro[4.5]decane and (4'-chloro-[1,1'-biphenyl]-4-yl)boronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 50%, yellow oil. 1H NMR (400 MHz, CDC13) δ 8.78 – 8.61 (m, 1H), 7.81 – 7.69 (m, 5H), 7.63 (s, 1H), 7.61 – 7.55 (m, 2H), 7.49 – 7.42 (m, 2H), 4.02 (s, 2H), 3.38 – 3.13 (m, 2H), 2.68 – 2.40, (m, 2H), 2.33 – 2.09 (m, 2H), 1.65 – 1.43 (m, 10H). HR-MS (ESI): m/z calcd for C27H30CIN2 (M + H)+: 417.2092; found: 417.2111.
8-((4-(4'-Methyl-[1,1'-biphenyl]-4-yl)pyridin-2-yl)methyl)-8-azaspiro[4.5]decane (65).
Compound 65 was prepared according to the method described for the preparation of compound 21 except using 8-azaspiro[4.5]decane and [4-(4-methylphenyl)phenyl]boronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 61%, yellow oil. 1H NMR (400 MHz, CDC13) δ 8.65 (d, J = 5.4 Hz, 1H), 7.82 – 7.64 (m, 6H), 7.59 – 7.51 (m, 2H), 7.33 – 7.27 (m, 2H), 4.03 (s, 2H), 3.42 – 3.08 (m, 2H), 2.77 – 2.49 (m, 2H), 2.46 – 2.40 (m, 3H), 2.29 – 2.02 (m, 2H), 1.66 – 1.45 (m, 10H). HR-MS (ESI): m/z calcd for C28H33N2 (M + H)+: 397.2638; found: 397.2662.
8-((4-(4-(Pyridin-3-yl)phenyl)pyridin-2-yl)methyl)-8-azaspiro[4.5]decane (66).
Compound 66 was prepared according to the method described for the preparation of compound 21 except using 8-azaspiro[4.5]decane and (4-(pyridin-3-yl)phenyl)boronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 51%, yellow oil. 1H NMR (400 MHz, CD3OD ) δ 8.88 (s, 1H), 8.70 (s, 1H), 8.57 (s, 1H), 8.17 (s, 1H), 8.03 – 7.78 (m, 6H), 7.57 (s, 1H), 4.29 (s, 2H), 3.21 – 2.87 (m, 4H), 1.86 – 1.45 (m, 12H).
8-((4-(4-(2,6-Difluoropyridin-3-yl)phenyl)pyridin-2-yl)methyl)-8-azaspiro[4.5]decane (67).
Compound 67 was prepared according to the method described for the preparation of compound 21 except using 8-azaspiro[4.5]decane and (4-(2,6-difluoropyridin-3-yl)phenyl)boronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 57%, yellow oil. 1H NMR (400 MHz, CDC13) δ 8.66 (s, 1H), 8.04 (d, J = 8.2 Hz, 1H), 7.88 – 7.63 (m, 6H), 6.98 (d, J = 6.9 Hz, 1H), 3.92 (s, 2H), 2.84 – 2.57 (m, 4H), 1.56 – 1.07 (m, 12H).
8-((4-(4-(Furan-3-yl)phenyl)pyridin-2-yl)methyl)-8-azaspiro[4.5]decane (68).
Compound 68 was prepared according to the method described for the preparation of compound 21 except using 8-azaspiro[4.5]decane and (4-(furan-3-yl)phenyl)boronic acid instead of 4,4-dimethyl-1,4-azasilinane hydrochloride and 4-isopropylbenzeneboronic acid, respectively. Yield 61%, yellow solid. 1H NMR (400 MHz, CD3OD ) δ 8.61 (d, J = 4.3 Hz, 1H), 8.01 (s, 1H), 7.86 (s, 1H), 7.81 (s, 1H), 7.72 (d, J = 7.6 Hz, 2H), 7.60 (s, 1H), 4.05 (s, 2H), 2.99 – 2.72 (m, 4H), 1.77 – 1.39 (m, 12H).
4.3. General method for preparation of compounds 40–44.
N-(Adamantan-2-yl)-4-(4-isopropylphenyl)picolinamide (40).
To a solution of the carboxylic acid, 10 (1 mmol) in anhydrous dichloromethane (6 mL) at room temperature were added HOBt (1.2 mmol) and EDC·HCl (1.2 mmol). After stirring for 10 min, the appropriate 2-amantadine (1.2 mmol) and DIPEA (2.0 mmol) were added, and the reaction mixture was stirred at room temperature for 4-5 h. The reaction mixture was quenched with water and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo; the following purification by silica gel chromatography (petroleum ether/EtOAc, 1:1, v/v) led to the desired compound 40. Yield 68%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.69 – 8.52 (m, 2H), 8.45 (s, 1H), 7.72 – 7.59 (m, 3H), 7.36 (d, J = 7.5 Hz, 2H), 4.35 – 4.24 (m, 1H), 3.06 – 2.89 (m, 1H), 2.07 – 1.67 (m, 14H), 1.29 (d, J = 6.6 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 163.4, 150.8, 150.5, 149.6, 148.4, 134.9, 127.3, 127.0, 123.3, 119.7, 53.3, 37.6, 37.2, 33.9, 32.1, 32.0, 27.3, 27.2, 23.8. HR-MS (ESI): m/z calcd for C25H31N2O (M + H)+: 375.2436; found: 375.2431.
N-Cycloheptyl-4-(4-isopropylphenyl)picolinamide (41).
Compound 41 was prepared according to the method described for the preparation of compound 40 except using cycloheptylamine instead of 2-amantadine. Yield 71%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.55 (d, J = 5.0 Hz, 1H), 8.45 (s, 1H), 8.08 (d, J = 8.1 Hz, 1H), 7.66 (d, J = 7.8 Hz, 2H), 7.61 (d, J = 5.0 Hz, 1H), 7.36 (d, J = 7.8 Hz, 2H), 4.26 – 4.13 (m, 1H), 3.04 – 2.92 (m, 1H), 2.12 – 1.98 (m, 2H), 1.73 – 1.52 (m, 10H), 1.29 (d, J = 6.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 163.1, 150.7, 150.5, 149.6, 148.4, 134.9, 127.3, 127.0, 123.3, 119.8, 50.3, 35.0, 33.9, 28.1, 24.1, 23.8. HR-MS (ESI): m/z calcd for C22H28N2ONa (M + Na)+: 359.2099; found: 359.2094.
N-Cyclohexyl-4-(4-isopropylphenyl)picolinamide (42).
Compound 42 was prepared according to the method described for the preparation of compound 40 except using cyclohexylamine instead of 2-amantadine. Yield 75%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.55 (d, J = 5.0 Hz, 1H), 8.45 (s, 1H), 8.02 (d, J = 8.0 Hz, 1H), 7.66 (d, J = 7.8 Hz, 2H), 7.61 (d, J = 4.9 Hz, 1H), 7.36 (d, J = 7.9 Hz, 2H), 4.07 – 3.91 (m, 1H), 3.05 – 2.91 (m, 1H), 2.10 – 1.98 (m, 2H), 1.83 – 1.73 (m, 2H), 1.53 – 1.21 (m, 12H). 13C NMR (101 MHz, CDCl3) δ 163.4, 150.7, 150.5, 149.6, 148.4, 134.9, 127.3, 127.0, 123.3, 119.8, 48.1, 33.9, 33.1, 25.6, 24.9, 23.8. HR-MS (ESI): m/z calcd for C21H26N2ONa (M + Na)+: 345.1943; found: 345.1937.
N-(Adamantan-1-yl)-4-(4-isopropylphenyl)picolinamide (43).
Compound 43 was prepared according to the method described for the preparation of compound 40 except using 1-amantadine instead of 2-amantadine. Yield 66%, yellow oil. 1HNMR (400 MHz, CDCl3) δ 8.52 (d, J = 5.0 Hz, 1H), 8.43 (s, 1H), 7.95 (s, 1H), 7.65 (d, J = 7.9 Hz, 2H), 7.59 (d, J = 4.9 Hz, 1H), 7.35 (d, J = 7.9 Hz, 2H), 3.06 – 2.90 (m, 1H), 2.20 – 2.12 (m, 9H), 1.78 – 1.68 (m, 6H), 1.29 (d, J = 6.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 163.2, 151.4, 150.4, 149.6, 148.1, 134.9, 127.3, 126.9, 123.2, 119.3, 51.6, 41.5, 36.4, 33.9, 29.5, 23.8. HR-MS (ESI): m/z calcd for C25H30N2ONa (M + Na)+: 397.2256; found: 397.2250.
(4-(4-Isopropylphenyl)pyridin-2-yl)(4-methylpiperidin-1-yl)methanone (44).
Compound 44 was prepared according to the method described for the preparation of compound 40 except using 4-methylpiperidine instead of 2-amantadine. Yield 71%, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.60 (d, J = 5.1 Hz, 1H), 7.79 (s, 1H), 7.61 (d, J = 7.9 Hz, 2H), 7.58 – 7.48 (m, 1H), 7.35 (d, J = 7.9 Hz, 2H), 4.84 – 4.65 (m, 1H), 3.93 – 3.81 (m, 1H), 3.13 – 3.02 (m, 1H), 3.01 – 2.92 (m, 1H), 2.89 – 2.73 (m, 1H), 1.87 – 1.74 (m, 1H), 1.71 – 1.55 (m, 2H), 1.35 – 1.19 (m, 8H), 0.97 (d, J = 6.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 167.8, 155.2, 150.4, 149.3, 148.9, 134.9, 127.3, 126.9, 121.7, 120.8, 47.6, 42.7, 34.7, 33.9, 33.7, 31.1, 23.8, 21.7. HR-MS (ESI): m/z calcd for C21H27N2O (M + H)+: 323.2123; found: 323.2118.
4.4. Minimum Inhibitory Concentration (MIC).
Mtb H37Rv was cultured to mid log phase in 7H9 broth complemented with 10% OADC, 0.05% Tween 80, and 0.1% cholesterol. MIC determination was done as reported previously [29, 30].
4.5. Cytotoxicity assay.
Cytotoxicity of selected compounds, expressed as IC50 was determined using Vero cells and the MABA assay. The details of the specific experiments refer to previous reports [31].
4.6. Molecular Docking.
Selected protein crystal complexes were repaired, hydrogenated, decrystallized, and energy optimized using the Protein Preparation module of Meastro, and to define binding pockets. Small molecules were then optimized for 3D conformation and energy minimization using the LigPre module. Finally, the small molecules were flexibly docked into the binding pocket using the XP docking module of Glide. Top poses were used for visualization analysis by pymol 2.5.
Supplementary Material
Highlights.
Utilizing a structure-directed approach, a series of pyridine-2-methylamine compounds were designed and synthesized.
Comprehensive structure-activity relationship exploration resulted in the identification of lead compound 62, which possess potent antitubercular activity against the clinical isolates of MDR and XDR-TB (MIC = 0.004–0.063 μg/mL).
Compound 62 showed low Vero cytotoxicity and moderate metabolic stability.
Target verification demonstrated compound 62 is likely to target MmpL3.
Acknowledgments
This work has been supported by the National Natural Science Foundation of China (21778019 and 22107031). This project has also been funded in part with Federal funds from the National Institutes of Health and National Institute of Allergy and Infectious Diseases, Department of Health and Human Services, under grant All 55602.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].WHO Global Tuberculosis Report 2022, World Health Organization, (2022). [Google Scholar]
- [2].Lakshmanan M, Xavier AS, Bedaquiline-The first ATP synthase inhibitor against multi drug resistant tuberculosis, J. Young Pharm, 5 (2013) 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fiorillo M, Lamb R, Tanowitz HB, Cappello AR, Martinez-Outschoorn UE, Sotgia F, P Lisanti M, Bedaquiline, an FDA-approved antibiotic, inhibits mitochondrial function and potently blocks the proliferative expansion of stem-like cancer cells (CSCs), Aging (Albany NY), 8(2016)1593–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Szumowski JD, Lynch JB, Profile of delamanid for the treatment of multidrug-resistant tuberculosis, Drug Des. Dev. Ther, 9 (2015) 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sasabe H, Shimokawa Y, Shibata M, Hashizume K, Hamasako Y, Ohzone Y, Kashiyama E, Umehara K, Antitubercular agent delamanid and metabolites as substrates and inhibitors of ABC and solute carrier transporters, Antimicrob. Agents Chemother, 60 (2016) 3497–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lenaerts AJ, Gruppo V, Marietta KS, Johnson CM, Driscoll DK, Tompkins NM, Rose JD, Reynolds RC, Orme IM, Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models, Antimicrob. Agents Chemother, 49 (2005) 2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, Treatment of highly drug-resistant pulmonary tuberculosis, N. Engl. J. Med, 382 (2020) 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chesov E, Chesov D, Maurer FP, Andres S, Utpatel C, Barilar I, Donica A, Reimann M, Niemann S, Lange C, Emergence of bedaquiline resistance in a high tuberculosis burden country, Euro. Respir. J, 59 (2022) 2100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Viljoen A, Dubois V, Girard-Misguich F, Blaise M, Herrmann JL, Kremer L, The diverse family of MmpL transporters in mycobacteria: from regulation to antimicrobial developments, Mol. Microbiol, 104 (2017) 889–904. [DOI] [PubMed] [Google Scholar]
- [10].Kalscheuer R, Weinrick B, Veeraraghavan EL, Besra GS, Jacobs WR Jr, Trehalose-recycling ABC transporter LpqY-SugA-SugB-SugC is essential for virulence of Mycobacterium tuberculosis, Proc. Natl. Acad. Sci, 107 (2010) 21761–21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Su C-C, Klenotic PA, Bolla JR, Purdy GE, Robinson CV, Yu EW, MmpL3 is a lipid transporter that binds trehalose monomycolate and phosphatidylethanolamine, Proc. Natl. Acad. Sci, 116(2019) 11241–11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Viljoen A, Herrmann J-L, Onajole OK, Stec J, Kozikowski AP, Kremer L, Controlling extra-and intramacrophagic Mycobacterium abscessus by targeting mycolic acid transport, Front. Cell. Infect. Microbiol, 7 (2017) 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scherman MS, North EJ, Jones V, Hess TN, Grzegorzewicz AE, Kasagami T, Kim I-H, Merzlikin O, Lenaerts AJ, Lee RE, Screening a library of 1600 adamantyl ureas for anti-Mycobacterium tuberculosis activity in vitro and for better physical chemical properties for bioavailability, Bioorg. Med. Chem, 20 (2012) 3255–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stec J, Onajole OK, Lun S, Guo H, Merenbloom B, Vistoli G, Bishai WR, Kozikowski AP, Indole-2-carboxamide-based MmpL3 inhibitors show exceptional antitubercular activity in an animal model of tuberculosis infection, J. Med. Chem, 59 (2016) 6232–6247. [DOI] [PubMed] [Google Scholar]
- [15].Jia L, Tomaszewski JE, Hanrahan C, Coward L, Noker P, Gorman G, Nikonenko B, Protopopova M, Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug, Brit. J. Pharmacol, 144 (2005) 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Umare MD, Khedekar PB, Chikhale RV, Mycobacterial membrane protein large 3 (MmpL3) inhibitors: a promising approach to combat tuberculosis, ChemMedChem, 16 (2021) 3136–3148. [DOI] [PubMed] [Google Scholar]
- [17].Shao M, McNeil M, Cook GM, Lu X, MmpL3 inhibitors as antituberculosis drugs, Eur. J. Med. Chem, 200 (2020) 112390. [DOI] [PubMed] [Google Scholar]
- [18].Zhao H, Gao Y, Li W, Sheng L, Cui K, Wang B, Fu L, Gao M, Lin Z, Zou X, Design, Synthesis, and Biological Evaluation of Pyrrole-2-carboxamide Derivatives as Mycobacterial Membrane Protein Large 3 Inhibitors for Treating Drug-Resistant Tuberculosis, J. Med. Chem, 65 (2022) 10534–10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gil Z, Martinez-Sotillo N, Pinto-Martinez A, Mejias F, Martinez JC, Galindo I, Oldfield E, Benaim G, SQ109 inhibits proliferation of Leishmania donovani by disruption of intracellular Ca2+ homeostasis, collapsing the mitochondrial electrochemical potential (ΔΨm) and affecting acidocalcisomes, Parasitol. Res, 119 (2020) 649–657. [DOI] [PubMed] [Google Scholar]
- [20].Tahlan K, Wilson R, Kastrinsky DB, Arora K, Nair V, Fischer E, Barnes SW, Walker JR, Alland D, Barry III CE, SQ109 targets MmpL3, a membrane transporter of trehalose monomycolate involved in mycolic acid donation to the cell wall core of Mycobacterium tuberculosis, Antimicrob. Agents Chemother, 56 (2012) 1797–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brown JR, North EJ, Hurdle JG, Morisseau C, Scarborough JS, Sun D, Korduláková J, Scherman MS, Jones V, Grzegorzewicz A, The structure–activity relationship of urea derivatives as anti-tuberculosis agents, Bioorganic & medicinal chemistry, 19 (2011) 5585–5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hammock BD, Wagner K, Inceoglu B, The soluble epoxide hydrolase as a pharmaceutical target for pain management, Pain manag., 1 (2011) 383–386. [DOI] [PubMed] [Google Scholar]
- [23].Manjunatha UH , Smith PW, Perspective: Challenges and opportunities in TB drug discovery from phenotypic screening, Bioorg. Med. Chem, 23 (2015) 5087–5097. [DOI] [PubMed] [Google Scholar]
- [24].Aminov R, History of antimicrobial drug discovery: Major classes and health impact, Biochem. pharmacol, 133 (2017) 4–19. [DOI] [PubMed] [Google Scholar]
- [25].Machado D, Girardini M, Viveiros M, Pieroni M, Challenging the drug-likeness dogma for new drug discovery in tuberculosis, Front, microbiol, 9 (2018) 1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wagner BK, The resurgence of phenotypic screening in drug discovery and development, Expert Opin. DrugDis, 11 (2016) 121–125. [DOI] [PubMed] [Google Scholar]
- [27].Borsari C, Ferrari S, Venturelli A, Costi MP, Target-based approaches for the discovery of new antimycobacterial drugs, Drug Discov. Today, 22 (2017) 576–584. [DOI] [PubMed] [Google Scholar]
- [28].Zhang B, Li J, Yang X, Wu L, Zhang J, Yang Y, Zhao Y, Zhang L, Yang X, Yang X, Crystal structures of membrane transporter MmpL3, an anti-TB drug target, Cell, 176 (2019) 636–648. e613. [DOI] [PubMed] [Google Scholar]
- [29].Collins L, Franzblau SG, Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium, Antimicrob. Agents Chemother, 41 (1997) 1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Onajole OK, Pieroni M, Tipparaju SK, Lun S, Stec J, Chen G, Gunosewoyo H, Guo H, Ammerman NC, Bishai WR, Preliminary structure–activity relationships and biological evaluation of novel antitubercular indolecarboxamide derivatives against drug-susceptible and drug-resistant Mycobacterium tuberculosis strains, J. Med. Chem, 56 (2013) 4093–4103. [DOI] [PubMed] [Google Scholar]
- [31].Lun S, Guo H, Onajole OK, Pieroni M, Gunosewoyo H, Chen G, Tipparaju SK, Ammerman NC, Kozikowski AP, Bishai WR, Indoleamides are active against drug-resistant Mycobacterium tuberculosis, Nat. Commun, 4 (2013) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jaladanki CK, Gahlawat A, Rathod G, Sandhu H, Jahan K, Bharatam PV, Mechanistic studies on the drug metabolism and toxicity originating from cytochromes P450, Drug Metab. Rev, 52 (2020) 366–394. [DOI] [PubMed] [Google Scholar]
- [33].Ioerger TR, Koo S, No E-G, Chen X, Larsen MH, Jacobs WR Jr, Pillay M, Sturm AW, Sacchettini JC, Genome analysis of multi-and extensively-drug-resistant tuberculosis from KwaZulu-Natal, South Africa, PloS one, 4 (2009) e7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chan PF, Germe T, Bax BD, Huang J, Thalji RK, Bacqué E, Checchia A, Chen D, Cui H, Ding X, Thiophene antibacterials that allosterically stabilize DNA-cleavage complexes with DNA gyrase, Proc. Nati. Acad. Sci, 114 (2017) E4492–E4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Imai Y, Hauk G, Quigley J, Liang L, Son S, Ghiglieri M, Gates MF, Morrissette M, Shahsavari N, Niles S, Evybactin is a DNA gyrase inhibitor that selectively kills Mycobacterium tuberculosis, Nat. Chem. Biolog, 18 (2022) 1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







