Abstract
Salinity is increasingly considered as a major environmental issue, which threatens agricultural production by decreasing yield traits of crops. Seed priming is a useful and cost‐effective technique to alleviate the negative effects of salinity and to enable a fast and uniform germination. In this context, we quantified the effects of priming with gibberellic acid (GP), calcium chloride (CP), and mannitol (MP) on seed germination of three bread wheat cultivars and investigated their response when grown at high salinity conditions (200 mM NaCl). Salt exposure strongly repressed seed imbibition and germination potential and extended germination time, whereas priming enhanced uniformity and seed vigor. Seed preconditioning alleviated the germination disruption caused by salt stress to varying degrees. Priming mitigating effect was agent‐dependent with regard to water status (CP and MP), ionic imbalance (CP), and seed reserve mobilization (GP). Na+ accumulation in seedling tissues significantly impaired carbohydrate and protein mobilization by inhibiting amylase and proteases activities but had lesser effects on primed seeds. CP attenuated ionic imbalance by limiting sodium accumulation. Gibberellic acid was the most effective priming treatment for promoting the germination of wheat seeds under salt stress. Moreover, genotypic differences in wheat response to salinity stress were observed between varieties used in this study. Ardito, the oldest variety, seems to tolerate better salinity in priming‐free conditions; Aubusson resulted the most salt‐sensitive cultivar but showed a high germination recovery under priming conditions; Bologna showed an intermediate behavior.
Keywords: amylases, germination, proteases, salinity, seed priming, wheat
1. INTRODUCTION
Wheat is a major staple crop in the world and the leading source of carbohydrates and plant proteins in the human diet (Fita et al., 2015), hence plays a crucial role in food security. The increase of human population and environmental stresses are big challenges for agriculture in the next years.
Salinity is one of the major abiotic stresses that affect crop production in arid and semi‐arid areas (Hossain et al., 2021; Johnson & Puthur, 2021). In many crop species, seed germination and early seedling growth are the most sensitive stages to salinity stress (Nonogaki, 2006, 2014). Therefore, rapid germination and emergence are critical steps for successful crop establishment (Bewley et al., 2012).
Salinity affects seed germination through osmotic stress, ion‐specific effects and oxidative stress (Debez et al., 2020). Indeed, water uptake during imbibition decreases with increasing external osmotic potential, sodium and chloride ions being toxic on embryo viability (Liu et al., 2018), and high salt accumulation in plant tissues induces an over‐production of ROS leading to an oxidative status imbalance. The toxic effects related to oxidative stress include disruption of the structure of enzymes and other macromolecules, damage to cell organelles and the plasma membrane, disruption of respiration, photosynthesis and protein synthesis (Nxele et al., 2017). Salt stress may also delay the onset, reduce the rate, and increase the dispersion of germination events, leading to reduction in plant growth and final crop yield (Liu et al., 2018). A restriction in mobilization of seed storage reserves in salt‐treated seeds has been reported by (Kazachkova et al., 2016) because many germination‐associated metabolic changes are inhibited or slowed down. However, the adverse effects of salt stress can be alleviated by various approaches, including seed priming. The latter is a pre‐sowing treatment that exposes seeds to a specific solution for a certain period that allows partial hydration without radicle emergence (Farooq et al., 2019). Usually pre‐sowing treatment involves the first two stages of seed germination (imbibition and activation), and it eventually leads to a higher seed germination rate and improves the germination uniformity (Devika et al., 2021). Treated seeds are usually re‐dried before use, but they would exhibit rapid germination when re‐imbibed under normal or stress conditions (Ashraf & Foolad, 2005). When the primed seeds are sown, the imbibition phase and lag phase of water absorption are shortened, and the pre‐germination metabolic processes are stimulated, which makes the seed ready for radicle protrusion (Gerna et al., 2018; Lutts et al., 2016). Besides improving seed germination performance, this cost‐effective method ensures a faster emergence of seedlings, a vigorous growth, and confers tolerance to adverse conditions (Johnson & Puthur, 2021; Marthandan et al., 2020). Priming was suggested to generate a moderate abiotic stress during both soaking and dehydration steps allowing the seeds to cope with environmental stresses during seedling establishment (Lutts et al., 2016).
Seeds generally contain starch, proteins and triacylglycerols, in proportions depending on the plant species, as sources of matter and energy. During germination, these major nutrient reserves are hydrolysed specifically by amylases, proteases and lipases, respectively (Bewley et al., 2012). One of the first signs of metabolic activity in a germinating seed is the activation of enzymes that hydrolyse storage reserves (Chibbar et al., 2004; Ali & Elozeiri, 2017). Hydrolytic enzymes are normally present in the mature grain in a dormant or latent state, becoming activated or synthesized from precursors, only, under humid conditions (El‐Hendawy et al., 2019; Hussain et al., 2022). Gibberellins (GAs) are plant growth regulators and the first to be synthesized in the scutellum of the embryo. GAs diffuse into the aleurone layer and induce the production of hydrolytic enzymes such as α‐amylases active on the starch granules in the endosperm (Rosental et al., 2014; Yahia & Carrillo‐Lopez, 2018).
In wheat, two tissues sustain the embryo axis development, the scutellum and the endosperm. The scutellum is close to the embryo axis and is responsible of sucrose synthesis. The endosperm surrounds the scutellum and consists mainly of dead cells full of starch and other nutrients; its external surface is made of a single layer of living cells called aleurone (Ruan & Fincher, 2017). The scutellum and the aleurone layer provide the endosperm sugar, protein and lipid‐hydrolyzing enzymes near the time of the radicle protrusion (Ali & Elozeiri, 2017; Chibbar et al., 2004). Starch is the most common storage carbohydrate in cereal grains accounting for about 70% of wheat grain on a dry weight basis. It consists of the two glucose polymers amylopectin and amylose, which together form insoluble, semi‐crystalline starch granules (Yahia & Carrillo‐Lopez, 2018). In germinating seeds, the initial degradation of starch granules is associated with an increase in amylolytic activity (Andriotis et al., 2016; López‐Coria et al., 2019). Amylases are a class of enzymes that catalyze the hydrolysis of starch into sugars such as glucose and maltose. α‐amylase, an endoenzyme, preferentially cleaves internal α‐1,4 glycosidic bonds releasing large glucan polymers (Chibbar et al., 2004). Subsequent degradation occurs due to β‐amylases, phosphorylases, maltases, and starch debranching enzymes. β‐amylases, first acts only on the non‐reducing ends of amylose and amylopectin, releasing β‐maltose, which is rapidly converted into natural mixtures of α‐ and β‐isomers by mutarotation (Chibbar et al., 2004). Maltases are specialized α‐glucosidases acting on maltose and other short maltooligosaccharides produced by amylases, converting them to glucose (Marín‐Sanz et al., 2020; Tan et al., 2013). Invertases catalyzes sucrose hydrolysis to glucose and fructose providing monosaccharides to the glycolytic pathway or generating glucose‐mediated signals to regulate plant cell metabolism (Rosental et al., 2014). Carbohydrates stored in the endosperm are the primary source of energy in the developing embryo until it forms leaves and the photosynthetic process takes over to supply the necessary energy and chemical constituents for the developing plantlet (Bewley, 1997; Bewley et al., 2012).
In wheat kernel, approximately 80% of total proteins consists of prolamins, glutenins and gliadins, the so‐called gluten proteins, whereas the remaining 20% is composed of albumins and globulins (Marín‐Sanz et al., 2020; Tan‐Wilson & Wilson, 2012). During seed germination, the storage proteins are hydrolysed into amino acids that remain in the storage tissue or are translocated to the developing axis tissues for the synthesis of various enzymes and structural proteins. Storage proteins mobilization is affected by several environmental factors (Ali & Elozeiri, 2017). The inhibition of storage proteins breakdown could be attributed to the decrease of various members of proteasome and proteinase/peptidase in the germinating seeds under the respective stress conditions (Debez et al., 2012; Tan et al., 2013).
GAs are used as important seed priming agents to mitigate abiotic stresses in different crops. Although the beneficial effects of GAs on germination is relatively well documented, only few reports focused on GAs‐priming‐induced effects on seed reserves mobilization under saline condition (Iqbal & Ashraf, 2013). Mannitol, when applied as osmotic agent in seed osmo‐priming, was found to improve the seed ability to osmotic adjustment (Amooaghaie, 2011; Balaji & Narayanan, 2019; Kumar & Rajalekshmi, 2021). Calcium ion (Ca2+) is also one of the very important ubiquitous intracellular second messenger molecules involved in many signal transduction pathways in plants.
In the present study, we investigated the effects of seed priming under salt excess using seed germination assay of wheat as a model crop. Actually, three varieties were compared. The objectives of this study are (1) to analyze the response of three wheat varieties to salinity during germination (2) to quantify the effects of different priming agents under salt and salt‐free conditions and eventually (3) to define the appropriate combination of priming agent and variety.
2. MATERIAL AND METHODS
2.1. Description of plant material
This research work was carried out in the laboratories of CREA in Vercelli during 2019–2020 winter season. After a preliminary test, three varieties, Ardito, Bologna, and Aubusson, were selected from the collection of Triticum aestivum as representative of wheat breeding in Italy during the 20th century (Laino et al., 2015; Ormoli et al., 2015). Ardito is an ancient bread wheat (bred in 1916) whereas Bologna and Aubusson are modern varieties (released in 2002 and 2003, respectively). According to the Italian bread wheat classification system (Foca et al., 2007); cv Bologna is an improver wheat, with hard seed texture, high protein content and high gluten strength, whereas cv Aubusson is an ordinary breadmaking wheat, with medium seed texture, medium protein content and medium gluten strength. The three cultivars were grown in 2016–2017 season and, after harvest, were kept at 4°C until they were used.
2.2. Experimental design
Wheat grains were surface‐sterilized for 10 min with 2% sodium hypochlorite (NaOCl) and thoroughly washed with distilled water and surface dried. First, two treatments (NPN, no priming and no stress; NPS, no priming and salt stress) were used to determine the effect of salt stress on wheat seed germination. Next, seeds were soaked using either (i) hormonal priming (GP, .1443 mM gibberellic acid) (ii) halo‐priming (CP, 50 mM calcium chloride), or (iii) osmo‐priming (MP, 110 mM mannitol). Priming took place in the dark with continuous gentle stirring for 12 h. The ratio of seeds to solution was 1:5 (w/v) (Chen et al., 2021). After priming, seeds were re‐dried at ambient conditions to reach their original moisture content and then stored at 4°C in a sealed box over silica gel and used for analyses within 1 year. Seed germination was periodically tested to confirm that the advanced germination achieved by the priming treatment was retained.
Petri dishes were covered with double‐layered Whatman filter paper (Whatman 1, GE Healthcare, Little Chalfont, UK) moistened with 10 mL of ultrapure MilliQ water or 10 mL of 200 mM NaCl. Seeds (35 per Petri dish, n = 3 per treatment) were placed to germinate in the dark at 25°C for up to 4 days. The experiment was repeated three times.
Seeds were considered germinated when there was radicle protrusion through the seed coat (Bewley, 1997). The germination percentage was monitored at 8, 16, 24, 32, 48, 56, 72, and 96 h in control and treated seeds. The highest mean daily germination and speed of germination were reached at 24 h; therefore, mobilization activity of stored reserves was studied at that time. Germinating seeds were sampled after 24 h, weighed and stored in liquid nitrogen until used for the analysis of hydrolytic enzyme activities. Seed water content was calculated on an FW basis by the formula: RWC = (FW − DW)/FW × 100.
At the end of experiment duration (96 h), endosperms were removed, dried and weighed. The percentage of mobilized reserves was estimated from the dry weight of remaining endosperms at the end of experiment (tf) as a percentage of the initial weight (t0). At the same time, seedlings were harvested, and their growth determined as radicle and coleoptile length (data not shown), then weighed and stored in liquid nitrogen until use. Sodium accumulation and repartition was determined in radicle, coleoptile and remaining endosperm on dried samples by ICP‐MS.
2.3. Modeling of germination kinetics parameters
In order to test the genotypic variation for salt tolerance, germination analysis in control versus salt conditions was carried out by the “Germinator_curve‐fitting 1.31.xls” Microsoft Excel solver, part of the Germinator software package (Joosen et al., 2010).
Germination performance was assessed by using the following data: maximum germination (Gmax), mean germination time (MGT), time for 50% viable seed germination (T50), germination uniformity (Ub‐a), and area under the germination curve (AUC). AUC is the area between the germination curve and the x‐axis (the germination time). Higher values for AUC correspond to earlier and higher germination rates. The germination uniformity (U) can be set to different ranges of germination; for example, U7525 is the uniformity from 25% to 75% of germinated seeds.
Stress index was calculated as follows: ∆AUC = AUC (water) − AUC (salt).
The coefficient of velocity of germination (CVG), Germination index (GI) and germination rate index (GRI) were calculated as follows, according to Kader (2005):
where N = No. of seeds germinated each day and T = No. of days from seeding corresponding to N.
where n1, n2. … n10 are number of germinated seeds on the first, second and subsequent days until the 10th day and 10, 9, … and 1 are weights given to the number of germinated seeds on the first, second and subsequent days, respectively.
G1 = Germination percentage × 100 at the first day after sowing and G2 = Germination percentage × 100 at the second day after sowing.
2.4. Hydrolytic enzyme activity measurement during germination
For each hydrolytic enzyme extraction, about .5 g of seeds from each treatment were grounded to fine powder in liquid nitrogen in a chilled mortar. The resulting powder was then homogenized with the proper buffer as hereafter described.
2.4.1. Proteolytic activity
Protease activity assay was adapted according to Brouquisse et al. (1998). Extraction of total proteins was performed by homogenization (1:5, w/v) in 50 mM Tris–HCl buffer (pH 7.5), containing 5 mM ß‐mercaptoethanol and .5% (v/v) polyvinylpolypyrrolidone followed by centrifugation at 20,000 g for 30 min. An aliquote of the supernatant was incubated with azocasein (5 mg mL−1 prepared in 100 mM sodium citrate pH 5.5) for 1 h at 37°C. The reaction was stopped by 15% (v/v) trichloracetic acid (TCA). After 10 min on ice, the mixture was centrifuged at 20,000 g for 10 min. One milliliter of 1 M NaOH was then added to the supernatant, and the absorbance was read at 440 nm. The azocasein degrading activity was calculated using the extinction coefficient E1% azocasein in 1 M NaOH = 37 L cm−1 g−1.
2.4.2. Total amylolytic activity
Amylase activity was determined as described by Dure (1960) according to the Bernfeld (1951) procedure, using soluble starch (potato starch, Sigma chemical, USA) as a substrate. The reducing groups released from starch are measured by the reduction of 3,5‐dinitrosalicylic acid (Miller, 1959). Materials were homogenized with 4 (w/v) ice‐cold unbuffered MilliQ water and centrifuged at 14,000 g for 30 min at 4°C. The resulting supernatants were filtered through a single layer of muslin cloth and assayed as a crude extract of enzyme within 1 h after homogenization. The endogenous reducing sugars of the supernatant were measured separately and subtracted from the values obtained in the assay. The standard was run with maltose and the enzyme activity was expressed as μmol maltose min−1 g−1 DW. One unit (U) of amylase is defined as the amount of enzyme that releases 1 μmol of reducing sugar as maltose per minute under the assay condition.
2.4.3. α‐ and β‐Amylase activities
The separation of the two enzymes involved the selective inactivation of one and assaying for the other, based on differences in their physical properties in cereals (Dure, 1960). For α‐amylase assay, the crude enzyme extract (2.4.2) was heated for 5 min at 70°C to inactivate β‐amylase. For β‐amylase assay, 1 mL of the crude enzyme extract was mixed with 1 mL of 1% soluble starch dissolved in citrate buffer at pH 3.4 to discard α‐amylase activity. One unit of enzyme was defined as the amount of enzyme that produced 1 μmol of maltose per minute under the assay conditions.
For the control of inactivation, the supernatant was added to a buffer pH 3.4 and pre‐treated for 5 min at 70°C, in this case, we should not have any activity and then assayed as previously outlined.
2.4.4. Invertase activity
Invertase activity was assayed using sucrose as substrate (Mahadevan & Sridhar, 1982). The enzyme was extracted in cold .1 M phosphate buffer pH 7.0. After centrifugation at 10,000 g for 15 min at 4°C, the supernatant was added to 1.5 mL mixture containing citrate buffer (pH 3.8) and sucrose (200 mM) at 37°C for 30 min. Free hexoses were measured by dinitrosalicylic acid (DNS) method (Bernfeld, 1951), using glucose as standard. Invertase activity was expressed as μmol−1 min−1g−1 DW. One unit of invertase was defined as the amount of enzyme that produced 1 μmol of glucose per minute under the assay conditions.
2.4.5. α‐Glucosidase activity
It was assayed as described by Bergmeyer et al. (1983) using maltose as substrate. The enzyme was extracted in cold .1 M phosphate buffer pH 7.6. After centrifugation at 10,000 g for 15 min at 4°C, an aliquot of supernatant was mixed with 1 mL of assay solution (Na‐acetate .1 M, pH 6.0, .5 M maltose) and incubated at 25°C for 5 min. α‐glucosidase activity, expressed as μmol−1 min−1g−1 DW, was measured by estimating the release of glucose used as standard and measured by DNS method (Bernfeld, 1951). One unit of α‐glucosidase was defined as the amount of enzyme that produced 1 μmol of glucose per minute under the assay conditions.
2.5. Grain reserve content and released products
2.5.1. Starch, soluble carbohydrates, reducing sugars and glucose
Starch was determined as described by McCready et al. (1950). About .5 g of sample seeds were homogenized in hot 80% (v/v) ethanol to remove sugars, centrifuged and the residue was repeatedly washed with hot 80% ethanol until the washing did not give color with anthrone reagent. The residue was then dried over a water bath and subjected to an acidic extraction for starch determination whereas the alcoholic fraction served for the determination of soluble carbohydrates, reducing sugars and glucose contents. For starch determination, an appropriately diluted aliquot from the acidic fraction was mixed with anthrone reagent. The absorbance was measured at 630 nm. The quantity of starch was calculated in terms of glucose equivalent and factor .9 was used to convert the values of glucose to starch (Barry‐Ryan et al., 2020). The quantity of starch was expressed as mg g−1 DW.
Soluble carbohydrates contents were determined by anthrone sulfuric method according to Yemm and Willis (1954). Reducing sugars content was measured by dinitrosalicylic acid method (Bernfeld, 1951). Glucose contents were estimated according to Miller (1959).
2.5.2. Amylose, amylopectin and sucrose contents
Amylose content was estimated using the iodometric method (McCready et al., 1950). Homogenized seeds (about .5 g) were incubated with 1 mL of ethanol and 10 mL of 1 N NaOH at 100°C for 10 min. The volume was made up to 25 mL, followed by centrifugation. The extract (2.5 mL) was added with 20 mL water and three drops of phenolphthalein, and .1 N HCl was added drop‐wise until the pink color disappeared. Lugol reagent (1 mL) was then added, the volume was made up to 50 mL, and the absorbance was read at 590 nm. The amylopectin content was estimated as the difference between starch and amylose.
Sucrose content was determined using the method of Van Handel (1968). The tissue was homogenized with 80% (v/v) ethanol and kept in water bath for 10 min to extract soluble sugars. Aqueous KOH (30% (w/v), .1 mL) was added after cooling and kept in boiling water bath for 10 min. Anthrone reagent was then added after cooling to room temperature and incubated at 38°C for 20 min. The absorbance was recorded at 620 nm, and the amount of sucrose expressed as μg g−1 dry weight was calculated from the standard curve of sucrose (20–250 μg).
2.5.3. Protein and amino acid contents
Protein content was estimated by the Bradford (1976) dye‐binding assay at 595 nm. Bovine serum albumin (BSA) (Sigma) 1 mg/mL was used as standard protein (5–80 μg).
Amino acids were estimated following the method of Moore and Stein (1954). Seeds were grounded in a mortar with 80% ethanol and then boiled for 30 min at 70°C. The homogenate was centrifuged at 8000 g for 20 min at 4°C. The supernatant was tested for amino acid content using the ninhydrin method (Moore & Stein, 1954). The optical density was read at 570 nm using a spectrophotometer. Standard glycine (Sigma) .025–.5 mg mL−1 was used for calibration.
2.6. Sodium accumulation
Dried samples (radicles, coleoptiles, and endosperms collected at 96 h of germination) of about 30 mg were digested in 7 mL of 65% (v/v) HNO3 using a microwave digestion system (Anton Paar MULTIVAWE 3000). The mineralized material was diluted 1:40 (v/v) in Milli‐Q water (to a final volume of 10 mL) and filtered on a .45‐μm PVDF membrane. Mineral content was measured by inductively coupled plasma mass spectrometry (ICP‐MS; Bruker Aurora M90 ICP‐MS).
2.7. Statistical analysis
Significant differences were further analyzed using Duncan's test (at 5% level of significance) to identify differences between treatments for T. aestivum cultivars. All tests were carried out with XLSTAT software version 2014 (Addinsoft, France). To highlight the intraspecific response to salinity in bread wheat cultivars and the efficiency of different priming agents, a principal component analysis (PCA) was performed using XLSTAT software, considering variables centered on their means and normalized with a standard deviation of 1.
3. RESULTS
3.1. Seed germination traits under salt and priming effect
Irrespective of the cultivar, curve fitting and kinetics of germination in un‐primed seeds were clearly affected by salt stress (Figure 1; Table 1). Salt application induced a delay in radicle protrusion concomitant to extended germination time. In fact, as compared with the control, time for 50% viable seed germination, mean germination time, and germination uniformity increased significantly for all varieties (Table 1). Twenty‐four hours after seed sowing, germination percentage reached only 34%, 20% and 39% in stress vs 91%, 88% and 95% in control conditions, in cv Ardito, Bologna and Aubusson, respectively (Figure 1). At the same time, a decline in germination performance was recorded, as revealed by the decrease in maximum germination rate, the area under the curve, the germination rate index and germination index, notably in cv Aubusson (Table 1). Overall, the tested varieties responded differently to salt exposure. Specifically, cv Ardito seemed to be more tolerant in comparison with Bologna and Aubusson. In fact, the germination time (T10) was reduced in Ardito (R = −.43), whereas germination performance (R = +.73) and uniformity (R = +.57) were higher. In absence of priming treatment, cv Aubusson seems the most susceptible to salt stress.
FIGURE 1.

Effect of gibberellins, calcium chloride, and mannitol seed priming on seed germination of the wheat varieties Ardito, Bologna and Aubusson exposed or not to 200 mM NaCl. Data are means of thre replicates of 35 seeds each per treatment from five independent experiments. NPN, no priming, no stress; NPS no priming, salt stress; GP, gibberellins priming, no stress; GPS, gibberellins priming, salt stress; CP, calcium chloride priming, no stress; CPS, calcium chloride priming, salt stress; MP, mannitol priming, no stress; MPS, mannitol priming, salt stress.
TABLE 1.
Relative values of seed germination traits under control and saline conditions in different priming treatment groups.
| Control | Salt‐treated | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | NPN | GP | CP | MP | NPS | GPS | CPS | MPS |
| ARDITO | ||||||||
| Gmax% | 99 ± 1 (ab) | 99 ± 1 (ab) | 96 ± 1 (bc) | 100 ± .1 (a) | 91.4 ± 2 (c) | 99 ± 1 (ab) | 94 ± 2 (bc) | 97 ± 2 (abc) |
| T50 maxG | 18 ± 1 (bc) | 12 ± 4 (bc) | 15 ± 3 (bc) | 14 ± 3 (bc) | 31 ± 2.5 (a) | 17 ± .1 (c) | 21 ± 1 (b) | 19 ± 1 (bc) |
| MGT | 18.2 ± 1 (d) | 13 ± 4 (cd) | 16 ± 2 (cd) | 15 ± 3 (cd) | 32.4 ± 2 (a) | 20 ± .1 (cd) | 24.5 ± 1 (b) | 22 ± 1 (bc) |
| U7525 | 4.8 ± 1 (c) | 3.4 ± 2.5 (c) | 5.5 ± 2 (c) | 5.1 ± 2 (c) | 24.4 ± 1 (a) | 19 ± .4 (b) | 21 ± 3 (ab) | 18.8 ± 2 (b) |
| T10 maxG | 13.6 ± 2(a) | 9.6 ± 5 (abc) | 11 ± 4 (abc) | 10 ± 4 (abc) | 14.3 ± 2 (a) | 5.6 ± .2 (c) | 8 ± .3 (b) | 7 ± .4 (b) |
| AUC 96h | 77 ± .7 (a) | 86 ± 2 (ab) | 76.5 ± 2 (ab) | 80 ± 2 (a) | 58 ± 2 (d) | 73 ± .2 (b) | 67 ± 2.4 (c) | 70 ± .5 (c) |
| SI = ∆AUC | Ns | Ns | Ns | Ns | 19 ± 2 (a) | 13 ± 2 (b) | 9.5 ± 3 (b) | 1.3 ± 2 (b) |
| GRI | 42 ± .5 (bc) | 45.5 ± 1 (a) | 44 ± .4 (cd) | 36 ± 1 (ab) | 31 ± 1 (g) | 42 ± .6 (d) | 40. ± .2 (f) | 38 ± .5 (e) |
| CVG | 13 ± .5 (c) | 12 ± .3 (c) | 13 ± .5 (c) | 16 ± .5 (c) | 19 ± 1.6 (a) | 12 ± .1 (b) | 17 ± .7 (b) | 17 ± 1 (b) |
| GI | 344 ± 4 (ab) | 345 ± 5 (ab) | 347 ± 1 (bc) | 313 ± 8 (a) | 291 ± 6 (e) | 333 ± 5 (bc) | 332 ± 3 (d) | 324 ± 6 (cd) |
| BOLOGNA | ||||||||
| Gmax% | 95 ± 1 (a) | 98 ± 1 (a) | 95 ± 1 (a) | 97 ± .1 (a) | 83 ± 2 (b) | 95 ± 1 (a) | 94 ± 2 (a) | 92 ± 2 (a) |
| T50 maxG | 18 ± 1 (b) | 17 ± 3 (b) | 19 ± 3 (b) | 17 ± 3 (b) | 31 ± 1 (a) | 19 ± 2 (b) | 18 ± 1 (b) | 16 ± 1 (b) |
| MGT | 18 ± 1 (b) | 17 ± 3 (b) | 19 ± 2 (b) | 17 ± 2 (b) | 33 ± 1 (a) | 19 ± 2 (b) | 20 ± 2 (b) | 17 ± 1 (b) |
| U7525 | 4.5 ± 1 (d) | 3.5 ± 2 (d) | 3.5 ± 1 (d) | 3 ± 2 (d) | 17 ± 1 (a) | 4 ± 2 (cd) | 14 ± 3 (ab) | 9 ± 1 (bc) |
| T10 maxG | 14 ± 2 (b) | 14 ± 4 (abc) | 16 ± 4 (b) | 15 ± 4 (ab) | 18 ± 1 (b) | 15 ± 4 (ab) | 8 ± .4 (c) | 9 ± .3 (ab) |
| AUC 96h | 73.5 ± 2 (a) | 76 ± 2 (a) | 75 ± 3 (a) | 76.5 ± 2 (a) | 51 ± .2 (b) | 71 ± 2 (a) | 72 ± 1 (a) | 71 ± 3 (a) |
| SI = ∆AUC | Ns | Ns | Ns | Ns | 22 ± 2 (a) | 5 ± .5 (b) | 3 ± 1.7 (b) | 5.5 ± 2 (b) |
| GRI | 43 ± 1 (b) | 46 ± .5 (a) | 44 ± 1 (ab) | 46 ± 1 (a) | 26 ± .2 (e) | 42 ± 1 (b) | 38 ± 2 (d) | 40 ± 2 (c) |
| CVG | 12 ± .5 (b) | 13 ± .5 (b) | 12 ± .3 (b) | 12 ± 1 (b) | 16 ± 1 (a) | 13 ± 1 (b) | 15.3 ± 2 (a) | 13 ± .2 (b) |
| GI | 330 ± 4 (a) | 341 ± 4 (a) | 331 ± 4 (a) | 339 ± 1 (a) | 262 ± 5 (c) | 327 ± 3 (ab) | 316 ± 6 (b) | 315 ± 9 (b) |
| AUBUSSON | ||||||||
| Gmax% | 100 ± .1 (a) | 100 ± .1 (a) | 98 ± 1.2 (ab) | 100 ± .1 (a) | 79.0 ± 1 (c) | 99 ± 1 (a) | 95 ± 1 (b) | 99 ± 1 (a) |
| T50 maxG | 18 ± 2 (b) | 16 ± 4 (bc) | 16 ± 4 (bc) | 14 ± 3 (bc) | 27 ± 2 (a) | 13 ± 3 (bc) | 15 ± 1 (bc) | 14 ± .3 (c) |
| MGT | 19 ± 1 (b) | 16 ± 4 (bc) | 16 ± 4 (bc) | 15 ± 3 (bc) | 29 ± 1 (a) | 14 ± 3 (bc) | 17 ± 1 (bc) | 15 ± .3 (c) |
| U7525 | 3 ± 1 (c) | 2 ± .5(c) | 1.4 ± .4(c) | 5 ± 1 (bc) | 23 ± .5(a) | 4 ± 2 (bc) | 9 ± 2(b) | 7 ± .4(b) |
| T10 maxG | 16 ± 2 (a) | 14 ± 4 (ab) | 15 ± 4 (ab) | 10 ± 5 (ab) | 12 ± 1(a) | 10 ± 4 (ab) | 8 ± .4(b) | 8 ± .3(b) |
| AUC 96h | 76.5 ± 1 (a) | 81.6 ± 4 (a) | 78 ± 5 (a) | 83 ± .2 (a) | 52 ± 2(b) | 76.2 ± 3 (a) | 74 ± 2(a) | 78 ± .4(a) |
| SI = ∆AUC | Ns | Ns | Ns | Ns | 24.5 ± 2(a) | 5.4 ± .4(b) | 4 ± 2(b) | 5 ± .5(b) |
| GRI | 45 ± .4 (b) | 49 ± .1(a) | 47 ± .5 (ab) | 47 ± .3 (ab) | 27 ± 1(d) | 46 ± 1(b) | 42 ± 2(c) | 45 ± 1(b) |
| CVG | 13 ± .4 (ab) | 12 ± .1 (ab) | 12 ± .3 (ab) | 13 ± 02 (ab) | 13 ± .2 (ab) | 13 ± .4 (ab) | 13 ± 1(a) | 13 ± .4(a) |
| GI | 347 ± 1 (a) | 349 ± .4 (a) | 342 ± 4.5 (a) | 348 ± 1 (a) | 257 ± 5(c) | 344 ± 5(a) | 326 ± 7(b) | 343 ± 4(a) |
Note: Different letters within a line indicate a significant difference at p < .05.
Abbreviations: NPN, no priming, no stress; NPS no priming, salt stress; GP, gibberellins priming, no stress; GPS, gibberellins priming, salt stress; CP, calcium chloride priming, no stress; CPS, calcium chloride priming, salt stress; MP, mannitol priming, no stress; MPS, mannitol priming, salt stress; Gmax%, maximal rate of germination; T50maxG, time for 50% viable seed germination; MGT, mean germination time; U7525, uniformity from 25% to 75% of germinated seeds; T10maxG, time for 10% viable seeds; AUC96h, area under the curve; SI (∆AUC), stress index; GRI, germination rate index; CVG, coefficient of velocity of germination; GI, germination index.
Our data show a beneficial effect of different priming agents on germination, with some differences among varieties: a significant enhancement in GRI was observed, in cv Bologna, upon treatment with hormonal and osmo‐priming, whereas cvs Aubusson and Ardito were positively affected by hormonal treatment (Table 1).
GRI reflects the percentage of germination on each day of the germination period. Therefore, higher GRI values indicate higher and faster germination. Although, the final germination rate was not significantly increased by priming application under salt‐free conditions, hormonal priming accelerated the radicle protrusion. Already, 8 h after the onset of imbibition, germination rate reached 45%, 16%, and 30% in Ardito, Bologna, and Aubusson, respectively, in comparison with un‐primed seeds, which sprouted only after 24 h (Figure 1). Probably, gibberellins seed preconditioning stimulated germination processes through shortening imbibition and activation stages.
Similarly, under saline conditions seed sprouting started at 8 h in gibberellins pre‐treated seeds. But, the germination rate decreased by about 50% compared with the respective GP group. Accordingly, parameters related to germination time decreased significantly after hormonal priming (Table.1). On the other hand, variables reflecting germination potential were increased significantly in CPS and MPS treatments compared with their respective NPS treatments (Table 1). Calcium chloride mitigated the harmful effect of NaCl because the CPS group manifested an early radicle protrusion expressed by a negative correlation of T50 (R = −.36) and MGT (R = −.34) as showed in Pearson's correlation (Table 3).
TABLE 3.
Pearson's correlation matrix.

Note: Heat map of correlations among germination traits under saline conditions comparing the effect of different seed priming in three wheat varieties (Ardito, Bologna, and Aubusson). Variables were centered around their means and normalized with a standard deviation of 1, n = 3. Values in bold represent the statistically significant correlations at .05 (*), .01 (**), and .001 levels (***). Two different colors (red and blue) are used to differentiate positive from negative correlation. Positive correlation interval from (zero to +1): (red gradient). Negative correlation interval from (−1 to zero): (blue gradient). White color correspond to zero.
Abbreviations: Gmax%, maximal rate of germination; T50maxG, time for 50% viable seed germination; MGT, mean germination time; U7525, uniformity from 25% to 75% of germinated seeds; T10maxG, time for 10% viable seed germination; AUC96h, area under the curve; GRI, germination rate index; CVG, coefficient of velocity of germination; GI, germination index; RWC, relative water content; MSR%/mobilized seed reserves percentage; Tot. amy, Total amylolytic activity; α‐amy, α‐amylase activity; β‐amy, β‐amylase activity; invertase, invertase activity; α‐gluc, α‐glucosidase activity; starch, starch content; amylose, amylose content; amylopectin, amylopectin content; sucrose, sucrose content; S.carb, solubles carbohydrates content; Red.sug, reducing sugars content; glucose, glucose content; protease, protease activity; protein, protein content; AA, amino acids content; [Na+] C, sodium concentration in coleoptiles; [Na+] R, sodium concentration in radicles; [Na+] E, sodium concentration in endosperms.
AUC is the area between the germination curve and the x‐axis (the germination time). Under salt exposure, AUC values (Table 1) were enhanced under gibberellins (R = +.36) and mannitol (R = +.33) pre‐treatment, reflecting an earlier and higher germination with respect to unprimed seeds (Table 3).
The stress index (SI) or ∆AUC was significantly higher in the salt‐stressed NPS (no primed and salt stress) treatment compared with GPS (gibberellins priming and salt stress), CPS (calcium chloride priming and salt stress), and MPS (mannitol priming and salt stress) (Table 1).
3.2. Salinity and priming impact on water imbibition and reserve mobilization
All priming agents stimulated water uptake in germinating seeds with a significant increase in water content, which almost doubled compared with NPN treatment (Figure 2a). Salt addition (200 μM NaCl) in the germination media subjected seeds to an osmotic stress limiting water uptake by seed tissues. Consequently, water content in germinating seeds in presence of NaCl showed a drastic decrease with respect to the non‐saline conditions both in primed and unprimed groups (Figure 2a). Water content values were comparable among the primed groups exposed to salinity and NPN treatment. Under salt exposure, pre‐soaking seeds in mannitol or calcium chloride improved water status and increased significantly water content (R = +.48 and R = +.36), respectively, for both agents as demonstrated by Pearson's correlation matrix (Table 3).
FIGURE 2.

Effect of gibberellins, calcium chloride, and mannitol seed priming on relative water content (a) and mobilized seed reserves percentage (b) in the wheat varieties Ardito (white pattern), Bologna (gray pattern) and Aubusson (dark pattern) exposed or not to 200 mM NaCl. Relative water content and mobilized seed reserves percentage were measured 24 and 96 h after the onset of imbibition, respectively. Values are means ± SE of triplicates from five independent experiments (p ≤ .05). Bars with different letters represent values statistically different. Different small letters indicate significance under salt‐free conditions, whereas different capital letters designate significance under salt exposure. NPN, no priming, no stress; NPS no priming, salt stress; GP, gibberellins priming, no stress; GPS, gibberellins priming, salt stress; CP, calcium chloride priming, no stress; CPS, calcium chloride priming, salt stress; MP, mannitol priming, no stress; MPS, mannitol priming, salt stress.
The percentage of mobilized seed reserves calculated at the end of germination experiment provided information about the extent of seed reserves utilized by the embryo for radicle and coleoptile emergence during germination. In unprimed seeds, salinity significantly decreased reserve mobilization in germinating seeds by −43%, −45%, and −38%, in comparison with NPN treatment, in Ardito, Bologna, and Aubusson, respectively (Figure 2b). In non‐saline conditions, gibberellins and, to a lesser extent, mannitol were more effective than calcium chloride as priming agents with regard to improving reserve mobilization (Table 2). This trend was marked in all varieties subjected to hormonal and osmo‐priming, with a more pronounced increase of mobilized seed reserves in seeds pre‐treated by gibberellins (Figure 2b).
TABLE 2.
Pearson's correlation matrix.

Note: Heat map of correlations among germination traits under non‐saline conditions comparing the effect of different seed priming in three wheat varieties (Ardito, Bologna, and Aubusson). Variables were centered around their means and normalized with a standard deviation of 1, n = 3. Values in bold represent the statistically significant correlations at .05 (*), .01 (**), and .001 levels (***). Two different colors (red and blue) are used to differentiate positive from negative correlation. Positive correlation interval from (zero to +1): (red gradient). Negative correlation interval from (−1 to zero): (blue gradient). White color correspond to zero.
Abbreviations: Gmax%, maximal rate of germination; T50maxG, time for 50% viable seed germination; MGT, mean germination time; U7525, uniformity from 25% to 75% of germinated seeds; T10maxG, time for 10% viable seeds; AUC96h, area under the curve; GRI, germination rate index; CVG, coefficient of velocity of germination; GI, germination index; RWC, relative water content; MSR%/mobilized seed reserves percentage; Tot. amy, Total amylolytic activity; α‐amy, α‐amylase activity; β‐amy, β‐amylase activity; invertase, invertase activity; α‐gluc, α‐glucosidase activity; starch, starch content; amylose, amylose content; amylopectin, amylopectin content; sucrose, sucrose content; S.carb, solubles carbohydrates content; Red.sug, reducing sugars content; glucose, glucose content; protease, protease activity; protein, protein content; AA, amino acids content; [Na+] C, sodium concentration in coleoptiles; [Na+] R, sodium concentration in radicles; [Na+] E, sodium concentration in endosperms.
Under salt exposure, gibberellins and calcium chloride were more effective than mannitol in mobilizing reserves when compared with unprimed seeds subjected to the same constraint. This trend was especially true for Ardito and Bologna (Figure 2b). PCA analysis and Pearson's correlation matrix demonstrated a promoting effect of gibberellins on seed reserves mobilization (R = +.72), suggesting a key role in stored reserves hydrolysis through carbohydrates and proteins metabolizing enzymes activation.
3.3. Effect of salt stress on starch breakdown and enzymes involved in polysaccharide hydrolysis
Total amylolytic activity (Figure 3a) and α‐ and β‐amylases activities (Figure 3b; Figure 3c) were significantly enhanced by priming, in particular hormonal priming, resulting in a significant reduction in starch content in endosperms with respect to the NPN group (Figure 3d). This was more pronounced with hormonal priming application, especially in Aubusson, which showed 32% higher hydrolyzed starch compared with NPN treatment in presence of gibberellins (Figure 3d).
FIGURE 3.

Effect of gibberellins, calcium chloride, and mannitol seed priming on total amylase activity (a), α‐amylase activity (b), β‐amylase activity (c), starch content (d), amylose content (e), and amylopectin content (f) measured 24 h after the onset of imbibition in the wheat varieties Ardito (white pattern), Bologna (gray pattern) and Aubusson (dark pattern) exposed or not to 200 mM NaCl. Values are means ± SE of triplicates from five independent experiments (p ≤ .05). Bars with different letters represent values statistically different. Different small letters indicate significance under salt‐free conditions, whereas different capital letters designate significance under salt exposure. NPN, no priming, no stress; NPS no priming, salt stress; GP, gibberellins priming, no stress; GPS, gibberellins priming, salt stress; CP, calcium chloride priming, no stress; CPS, calcium chloride priming, salt stress; MP, mannitol priming, no stress; MPS, mannitol priming, salt stress.
Priming agent‐dependent effect on amylose content was lesser marked than on amylopectin (Figure 3). Only gibberellin pre‐treatment induced a decrease (ca. 14%) in amylose content compared with NPN condition (Figure 3e). On the contrary, a significant decline in amylopectin levels was observed regardless the priming agent (Figure 3f).
In absence of priming, salt exposure reduced significantly total amylolytic activity, α and β‐amylases activities. Salinity impact was more pronounced on α‐amylase (Figure 3b) than on β‐amylase (Figure 3c) with a reduction ranging between −30% and −20%, respectively.
In pre‐treated seeds, gibberellins and calcium chloride seems to be more effective than mannitol in alleviating the deleterious effect of salt on amylolytic activity. Indeed, with respect to NPS treatment, GPS and, to a lesser extent, CPS groups showed an improvement of total amylase activity for all varieties (Figure 3a).
As amylase is considered the main player in starch degradation, the reduction of its activity hampered starch breakdown. Hence, a significant increase (+29%) of starch content was observed in Bologna cultivar in presence of NaCl compared with the NPN treatment (Figure 3d). The amylolytic activity inhibition (Figure 3a–c) results in a slow‐down of starch polymers hydrolysis in soluble carbohydrates (R = −.70).
Interestingly, priming application counteracted the inhibitory effect of salt on starch breakdown. In particular, gibberellins pre‐treatment was the most effective on polysaccharides hydrolysis under salinity constraints with respect to mannitol, followed by calcium chloride (Figure 3d). As demonstrated by Pearson's correlation matrix, the exogenous supply of gibberellins enhanced polysaccharides debranching enzymes activities in particular total amylase (R = +.62) and β‐amylase (R = +.48).
This observation was also valid for mobilized seed reserves percentage in germinating seeds subjected to salt (Figure 2b). Indeed, with respect to NPS treatment, starch content in endosperms undergoes a significant decrease of about 30% and 20%, respectively, in GPS and CPS. Hence, in all varieties, gibberellins were found to enhance not only seed reserve mobilization (Figure 2b) but also amylolytic activity (Figure 3a) and starch hydrolysis when seeds were exposed to salt (Figure 3d).
3.4. Effect of salt on carbohydrate status and enzymes involved in oligosaccharides hydrolysis
Both invertase and α‐glucosidase activities were significantly inhibited in the presence of salt stress under unprimed conditions, cv Aubusson being the most affected compared with the other cultivars. In detail, the NPS treatment of Aubusson induced a significant reduction of α‐glucosidase (Figure 4d) and invertase (Figure 4a) activities compared with NPN (R = −.69 and R = −.68, respectively).
FIGURE 4.
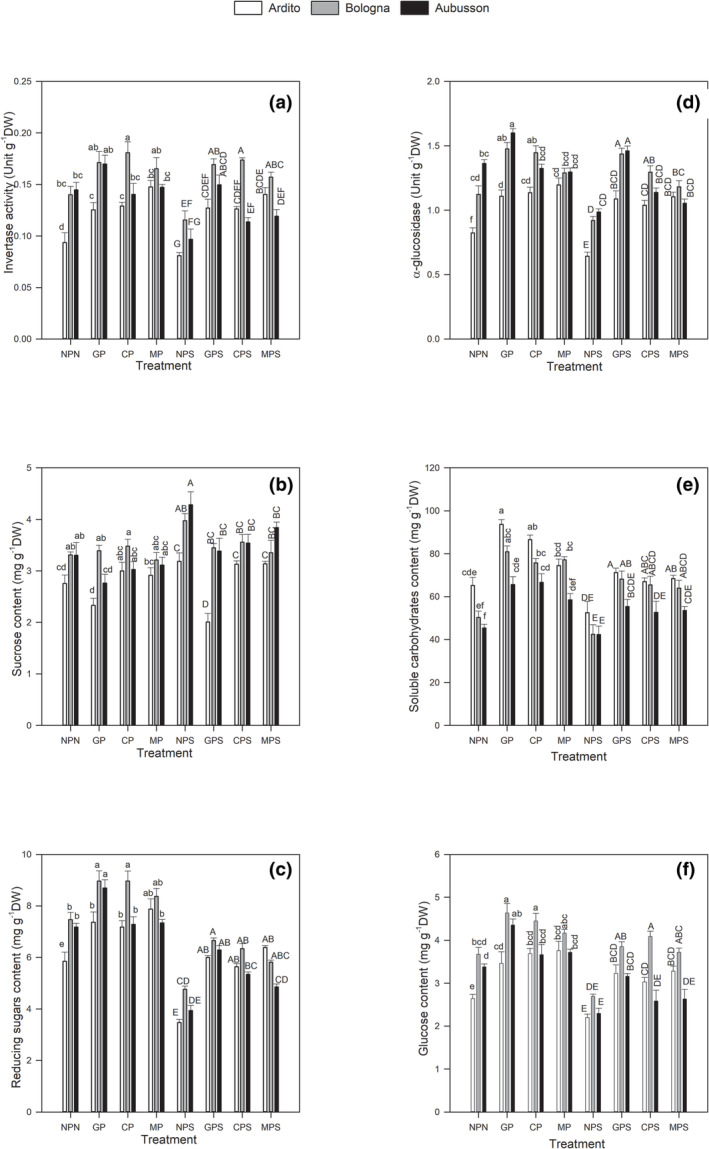
Effect of gibberellins, calcium chloride, and mannitol seed priming on invertase activity (a), sucrose content (b), reducing sugars content (c), α‐glucosidase activity (d), soluble carbohydrates content (e), and glucose content (f) measured 24 h after the onset of imbibition in the wheat varieties Ardito (white pattern), Bologna (gray pattern) and Aubusson (dark pattern) exposed or not to 200 mM NaCl. Values are means ±SE of triplicates from five independent experiments (p ≤ .05). Bars with different letters represent values statistically different. Different small letters indicate significance under salt‐free conditions, whereas different capital letters designate significance under salt exposure. NPN, no priming, no stress; NPS no priming, salt stress; GP, gibberellins priming, no stress; GPS, gibberellins priming, salt stress; CP, calcium chloride priming, no stress; CPS, calcium chloride priming, salt stress; MP, mannitol priming, no stress; MPS, mannitol priming, salt stress.
The inhibition of invertase and α‐glucosidase, two enzymes that play a pivotal role in oligosaccharide mobilization, reduced oligosaccharides, in particular sucrose, hydrolysis (Figure 4b). Total soluble carbohydrates fraction which constitutes both reducing and non‐reducing sugars remains unchanged in Bologna and Aubusson cultivars but decreased significantly (about −20%) in Ardito (Figure 4e). Under salt stress, the content of glucose (Figure 4f) and reducing sugars (Figure 4c), assumed to be the product of invertase and α‐glucosidase enzymes action, showed a drastic decrease with respect to NPN treatment (R = −.64 and R = −.82, respectively).
Under the non‐saline conditions, all priming agents enhanced significantly enzymatic activity of both enzymes except for cv Aubusson, on which only hormonal priming acted positively.
All priming agents also stimulated carbohydrates profile expressed as an increase in soluble carbohydrates (Figure 4e), reducing sugars (Figure 4c) and glucose (Figure 4f) contents especially in germinating seeds pre‐treated with gibberellins. In coherence, also the Pearson's correlation matrix showed a positive correlation between amylase (R = +.76) and α‐glucosidase (R = +.37) activities. The increase of these enzyme activities was followed by a decrease in their respective substrates suggesting probably a contribution of gibberellins on the enhancement of hydrolyzing reserves.
Under salt exposure, Ardito and Bologna did not show a significant inhibition of invertase (Figure 4a) and α‐glucosidase (Figure 4d) in pre‐treated seeds with respect to control conditions.
However, in Aubusson pre‐treated with mannitol and subjected to NaCl, salinity constraint was found to inhibit significantly both enzymes (Figure 4a; Figure 4d) by about −19% in comparison with MP.
The beneficial effect of seed priming under salt constraint alleviated invertase and α‐glucosidase inhibition. The enzymes activity decline was about −30% in NPS group but did not exceed −18% in primed samples when compared with controls (Figure 4a; Figure 4d).
In saline conditions, as invertase activity was inhibited in primed Aubusson (Figure 4a), the substrate (sucrose) showed higher levels than in control conditions (Figure 4b). A significant increase in sucrose contents were recorded in GPS, CPS, and MPS treatments compared with their respective controls. Under salt exposure, soluble carbohydrates (Figure 4e), reducing sugars (Figure 4c) and glucose (Figure 4f) diminished in all varieties but always showed higher levels in primed seeds than in unprimed seeds.
3.5. Effect of salt on proteolytic activity and protein remobilization
In absence of priming, salt affected severely proteolytic activity in all varieties, inhibiting significantly protein hydrolysis (R = −.71) and amino acids release (R = −.75) (Table 3). In comparison with NPN group, NPS treatment showed a reduction of 37%, 55%, and 60% in protease activity in Ardito, Bologna, and Aubusson, respectively (Figure 5a). At the same time a marked decrease in free amino acids release (Figure 5c) was recorded for all varieties in particular Aubusson (−43%). Consequently, an increase in protein content was observed (Figure 5b).
FIGURE 5.

Effect of gibberellins, calcium chloride, and mannitol seed priming on protease activity (a), protein content (b), and amino acids content (c) measured 24 h after the onset of imbibition in the wheat varieties Ardito (white pattern), Bologna (gray pattern) and Aubusson (dark pattern) exposed or not to 200 mM NaCl. Values are means ± SE of triplicates from five independent experiments (p ≤ .05). Bars with different letters represent values statistically different. Different small letters indicate significance under salt‐free conditions, whereas different capital letters designate significance under salt exposure. NPN, no priming, no stress; NPS no priming, salt stress; GP, gibberellins priming, no stress; GPS, gibberellins priming, salt stress; CP, calcium chloride priming, no stress; CPS, calcium chloride priming, salt stress; MP, mannitol priming, no stress; MPS, mannitol priming, salt stress.
Considering all varieties germinated in salt‐free conditions, Ardito got the highest benefits in term of protease activity (Table 2) which was enhanced by about +120%, +48%, and +94% when the cultivar was pre‐treated by gibberellins, calcium chloride and mannitol compared with the NPN (Figure 5a). Considering all priming agents, both under salt (Figure 8) and salt‐free conditions (Figure 7), gibberellins seemed to be the most effective in promoting protein mobilization. Under salt conditions, GP correlated with protease activity (R = +.54), protein hydrolysis (R = −.34) and amino acids release (R = +.50) (Table 3). Under salt exposure, Ardito and Bologna responded better to both hormonal and halopriming, which alleviated significantly the deleterious effect of salinity on proteins mobilization and attenuated enzymatic inhibition (Figure 5a) compared with the unprimed seeds. In detail, with respect to NPS group, gibberellins and, to a less extent calcium chloride, stimulated significantly protease activity (Figure 5a) and amino acids release (Figure 5c) in both varieties.
FIGURE 8.

Principal component analysis of germination traits in the wheat cultivars Ardito, Bologna, and Aubusson, subjected to three different seed priming treatments and germinated in presence of 200 mM NaCl. Circles (•) represent different analysis parameters, squares (■) represent different treatments (NPS, GPS, CPS and MPS), and triangles (▲) represent the three wheat cultivars. Analysis parameters and treatments are projected onto the F1–F2 principal factorial plane that explains 67.91% of the variation in presence of 200 mM NaCl. Abbreviations: Gmax%, maximal rate of germination; T50maxG, time for 50% viable seed germination; MGT, mean germination time; U7525, uniformity from 25% to 75% of germinated seeds; T10maxG, time for 10% viable seed germination; AUC96h:area under the curve; GRI, germination rate index; CVG, coefficient of velocity of germination; GI, germination index; RWC, relative water content; MSR%/mobilized seed reserves percentage; Tot.amy, Total amylolytic activity; α‐amy, α‐amylase activity; β‐amy, β‐amylase activity; invertase, invertase activity; α‐gluc, α‐glucosidase activity; starch, starch content; amylose, amylose content; amylopectin, amylopectin content; sucrose, sucrose content; S.carb, solubles carbohydrates content; Red.sug, reducing sugars content; glucose, glucose content; protease, protease activity; protein, protein content; AA, amino acids content; [Na+] C, sodium concentration in coleoptiles; [Na+] R, sodium concentration in radicles; [Na+] E, sodium concentration in endosperms.
FIGURE 7.

Principal component analysis of germination traits in the wheat cultivars Ardito, Bologna, and Aubusson, subjected to three different seed priming treatments and germinated in salt‐free conditions. Circles (•) represent different analysis parameters, squares (■) represent different treatments (NPN, GP, CP and MP), and triangles (▲) represent the three wheat cultivars. Analysis parameters and treatments are projected onto the F1–F2 principal factorial plane that explains 53.50% of the variation in salt‐free conditions. Abbreviations: Gmax%, maximal rate of germination; T50maxG, time for 50% viable seed germination; MGT, mean germination time; U7525, uniformity from 25% to 75% of germinated seeds; T10maxG, time for 10% viable seed germination; AUC96h: area under the curve; GRI, germination rate index; CVG, coefficient of velocity of germination; GI, germination index; RWC, relative water content; MSR%/mobilized seed reserves percentage; Tot.amy, Total amylolytic activity; α‐amy, α‐amylase activity; β‐amy, β‐amylase activity; invertase, invertase activity; α‐gluc, α‐glucosidase activity; starch, starch content; amylose, amylose content; amylopectin:amylopectin content; sucrose, sucrose content; S.carb, solubles carbohydrates content; Red.sug, reducing sugars content; glucose, glucose content; protease, protease activity; protein, protein content; AA, amino acids content; [Na+] C, sodium concentration in coleoptiles; [Na+] R, sodium concentration in radicles; [Na+] E, sodium concentration in endosperms.
Conversely, in Aubusson, mannitol seems to be more effective on protein hydrolysis than calcium chloride (Figure 5a and Figure 5b). Hence, in this variety, gibberellins, followed by mannitol, acted positively on proteolytic activity (+63% and +42%) (Figure 5a) and amino acids status (+67% and 41%) (Figure 5c) when compared with un‐primed seeds subjected to salt.
3.6. Sodium accumulation and distribution between plumule, radicle, and endosperm
Salt exposure often leads to sodium accumulation in germinating seeds. The repartition of Na+ in seedling tissues is described in Figure 6. Wide differences existed in terms of priming agent specificity and genotypic response. In general, Na+ accumulation decreased from radicles (Figure 6b) and coleoptiles (Figure 6a) to endosperms (Figure 6c). In terms of Na+ concentration, coleoptiles accumulated half of radicles and 25% of endosperm sodium (Figure 6). Under salt exposure, the primed seeds accumulated in all tissues less sodium than unprimed seeds. Hence, all priming agents reduced significantly Na+ concentration in coleoptiles, roots, and endosperms but calcium chloride resulted the most effective agent in limiting sodium accumulation when compared with other priming agents used in this experiment. Significant negative correlations were indeed revealed between CPS and Na+ concentration in coleoptile (R = −.49), radicle (R = −.38), and endosperm (R = −.48) (Table 3). As regards genotypic differences, halopriming, in comparison with NPS treatment, resulted in a decrease of sodium concentration of about −33%, −32%, and −27% in the coleoptyles of Ardito Bologna and Aubusson, respectively (Figure 6a). In radicles (Figure 6b) and under salt conditions, Aubusson accumulated less sodium compared with Ardito and Bologna. Moreover, a significant decrease in Na+ concentration under mannitol (−17%) and calcium chloride (−19%) priming conditions were registered in this variety with respect to NPS treatment. Nevertheless, in Ardito, radicles sodium concentration did not change significantly comparing primed and unprimed conditions.
FIGURE 6.

Effect of gibberellins, calcium chloride, and mannitol seed priming on sodium content in coleoptile (a), radicle (b), and endosperm (c) measured 96 h after the onset of imbibition in the wheat varieties Ardito (white pattern), Bologna (gray pattern) and Aubusson (dark pattern) exposed or not to 200 mM NaCl. Values are means ± SE of triplicates from five independent experiments (p ≤ .05). Bars with different letters represent values statistically different. Different small letters indicate significance under salt‐free conditions, when different capital letters designate significance under salt exposure. NPN, no priming, no stress; NPS no priming, salt stress; GP, gibberellins priming, no stress; GPS, gibberellins priming, salt stress; CP, calcium chloride priming, no stress; CPS, calcium chloride priming, salt stress; MP, mannitol priming, no stress; MPS, mannitol priming, salt stress.
In endosperms (Figure 6c), Na+ concentration was significantly lower under priming conditions compared with NPS. All priming agents reduced significantly sodium accumulation in endosperms but calcium chloride was more effective. In detail, Ardito showed a decrease in sodium concentrations of about −29% and −27%, respectively, in GPS and CPS groups (Figure 6c); Aubusson exhibited a reduction of about −31% and −22% in CPS and MPS, respectively.
3.7. PCA analysis for traits
Under salt free‐conditions, the PCA analysis (Figure 7) revealed variation among the traits for each cultivar, hence genotypic differences in wheat during germination were observed in this study. Ardito, the oldest variety was in the upper side of the score plot and differed from Bologna and Aubusson, which are modern varieties. The first component (F1) and second component (F2) explained 53.5% of the total variance. All priming treatments were on the right side of the score plot but priming‐free treatment was in left side. The variables related to reserve mobilization were closely spaced to priming agents, indicating that their responses during germination were similar. The loading plot of all the measured variables included in the PCA is shown in Figure 7.
Under stress conditions, the use of PCA (Figure 8) provides an indication of the most important traits contributing to salinity tolerance for the studied wheat cvs. The distribution of the genotypes and priming treatments shows that F1 and F2 account for (67.91) % of the total variability in the set of variables (traits) analyzed in each genotype. All priming treatments were on the right side of the score plot and positively correlated with reserves mobilization, relative water content, and germination. In contrast, priming‐free treatment was in left side. Genotypic response varied under optimal and stress conditions. Aubusson, the most salt‐sensitive cultivar was in the upper side of the score plot showing a high germination recovery under priming conditions whereas Bologna with an intermediate behavior was centered.
4. DISCUSSION
Salinity is one of the major environmental constraints restricting cereal productivity and sustainability in arid and semiarid regions (Fita et al., 2015). T. aestivum is a moderately salt‐tolerant crop (Bewley et al., 2012) but is easily inhibited by salt stress, especially during the early developmental stages (germination and seedling). This knowledge is consistent with our data, even if we observed a cultivar‐dependent behavior. Our results revealed two aspects of the inhibitory effect generated by NaCl on germination process, a delay in radicle protrusion (Figure 1) and a reduction in germination performance (Table 1). The delayed and lower seed germination under salt stress is generally attributed to salt‐induced osmotic and ion‐specific effects but also to oxidative stress generated by ROS production (Debez et al., 2020; Hussain et al., 2022; Munns, 2002). The detrimental effect of salinity on cereal crops like wheat (El‐Hendawy et al., 2019; Feghhenabi et al., 2020), barley (Debez et al., 2020), and sorghum (Chen et al., 2021) is widely discussed in the literature and several strategies have been suggested to overcome soil salinity. Therefore, the exploitation of genetic variability of available germplasm constitutes a promising strategy to identify a tolerant genotype that may sustain a reasonable yield on salt affected soils (El‐Hendawy et al., 2019). Seed priming is also a useful and cost‐effective technique to cope with unfavorable conditions and enhance seedling vigor (Lutts et al., 2016).
As expected, an improvement of germinability was observed after seed priming (Figure 1) with respect to unprimed seeds. Interestingly, out of the three wheat varieties analyzed, cv Aubusson, the most susceptible to salt stress, showed a higher germination recovery and the best response to priming. Seed priming has been suggested to be one of the most useful physiological approaches to adapt glycophyte species to saline conditions (Farooq et al., 2019; Ahmad et al., 2021). In this study, although the effects of three priming agents differed, all compounds used promoted the germination of wheat seeds under salt stress to some extent.
Salt exposure during germination results in a damage in hormone balance especially concerning gibberellins (GAs)/abscisic acid (ABA) (Uçarlı, 2020). ABA promotes seed dormancy and inhibits seed germination, whereas GAs release dormancy and stimulate germination. Guangwu and Xuwen (2014) reported that gibberellins promoted seed respiration, lowered ABA level and stimulated IAAs and GAs biosynthesis. Seed priming with gibberellins significantly enhanced germination and seedling parameters in pea and wheat under saline conditions (Rhaman et al., 2021; Tsegay & Andargie, 2018). Likewise, Hussain et al. (2022) reported that the exogenous application of gibberellins mitigated salt and drought stress and enhanced plant growth and development. In the same context, Ibrahim (2016) and Iqbal et al. (2006) reported that seed priming with KCl and CaCl2 alleviates salt stress damage and enhances several germination parameters in rice and maize limiting the absorption of sodium and chloride ions and increasing shoot fresh weight and dry weight.
Seed imbibition and subsequent embryo growth depend on water status with water potential gradients representing the driving force for water flow and finally tissue expansion (Ali & Elozeiri, 2017). Water transport across cell membranes, mediated by aquaporins (Devika et al., 2021) is essential for the initiation of metabolism.
In our study, high salinity induced a substantial decrease of water content in germinating seeds (Figure 2a) especially in NPS but this effect was less severe in pre‐treated seeds. Our results are in accordance with Debez et al. (2020) and Ibrahim (2016), who explained water uptake restriction in dry seeds during imbibition by an increase in external osmotic potential generated by high salinity.
Therefore, internal osmotic adjustment was suggested to be determinant in salinity alleviation and tolerance as reported by Chaffei‐Haouari et al. (2012) in wild and cultivated wheat species differing in osmolyte accumulation when exposed to NaCl. The disruption of water uptake is responsible of germination delay through a decrease in the activity of α‐amylase, and an increase in sodium and chloride concentration (Uçarlı, 2020). A threshold level of hydration is actually required for the synthesis of hydrolytic enzymes, which are responsible for the hydrolysis of the stored compounds in mature seeds (Rosental et al., 2014). Confirming previous studies (Ben Youssef et al., 2021; Debez et al., 2020; Uçarlı, 2020), our findings indicated that salt exposure not only impaired seed imbibition (Figure 2a) but also led to high sodium accumulation in plant tissues.
Interestingly, calcium chloride priming was found to act on ionic balance decreasing significantly Na+ accumulation in coleoptile, radicle and endosperm with respect to NPS group. Ben Youssef et al. (2021) reported similar observations in barley upon use of CaCl2 and KNO3 seed priming, which limited the absorption and accumulation of sodium ions, maintained potassium content and decreased the sodium/potassium ratio in seedling tissues. Tian et al. (2015) reported equivalent finding in wheat seedlings exposed to 100 mM NaCl after calcium application. As the addition of 17.5 mM Ca (NO3)2 mitigated the salt effect and maintained ion homeostasis increasing concentrations of potassium (K+), calcium (Ca2+), magnesium (Mg2+), zinc (Zn2+), iron (Fe2+), and copper (Cu2+). High Na+ resulted in the deficiency of mineral nutrients (Ben Youssef et al., 2021; Tian et al., 2015). The major cause of salt sensitivity in wheat may be attributed to a mineral deficiency, in particular calcium depletion. Considering the crucial role of this element in water and nutrient uptake and also in maintaining the selectivity and integrity of the cell membranes, calcium depletion causes a disorder in these mechanisms (Tian et al., 2015; Yang et al., 2007). In addition, intracellular Ca2+ changes play a role as a signal involved in the regulation of physiological functions in response to salinity (Sen & Puthur, 2020).
However, others investigations on wheat shown that salinity stress increased Ca2+ levels in plants (Yang et al., 2007). An increase of the total calcium content in roots was partly due to plasma membrane NADPH oxidase‐dependent H2O2 generation in the salt‐tolerant wheat cultivar 89,122 exposed to 100 mM NaCl (Yang et al., 2007).
Nxele et al. (2017) ascribed the divergent behavior of salt‐tolerant and salt‐sensitive varieties to a difference in their ability to sequester salt in vacuoles. Other mechanisms are thought to contribute to better salt tolerance, such as the low rate of Na+ and Cl− transport to leaves, and the ability to compartmentalize these ions in vacuoles to prevent their build‐up in cytoplasm or cell walls and thus avoid salt toxicity (Munns, 2002).
The harmful impact of salinity on water and ionic status led to a disruption in reserve mobilization (Figure 2b) and hydrolyzing enzymes functionality (Figures 3 and 5). Besides the lack of sufficient hydration in seed tissues, the toxic effect of excess sodium and chloride ions was reported to impair embryo viability contributing to a disruption in biochemical processes including nucleic and protein metabolism, energy production, and respiration (Hussain et al., 2022). Salt stress was reported to limit the mobilization of starchy endosperm reserves in several species, because of the inhibition of different enzymatic activities (Debez et al., 2020; Feghhenabi et al., 2020). Likewise, Lira et al. (2021) reported that salinity caused the inhibition of lipid mobilization and carbon reserves in two Jatropha curcas genotypes differing in salt tolerance.
In our study, salinity affected carbohydrate and protein breakdown (PCA analysis, Figure 8). Starch mobilization results from simultaneous activities of α‐amylase, β‐amylase, α‐glucosidase, and invertase. Changes in mobilized seed reserves percentage in germinating seeds could be attributed to the starch hydrolysis as wheat is a starchy grain (starch is the main storage carbohydrate in wheat and comprises about 60%–75% of grain weight and 70%–80% of flour).
A positive correlation was observed in NPS group between Na+ accumulation in plant tissues (R = .69 in endosperm) and the failure in polysaccharides debranching activity of starch (R = .67), amylose (R = .37), and amylopectin (R = .66).
Salinity damaged enzymes controlling the mobilization of stored reserves both carbohydrates and proteins. In our study, the strong inhibition of amylase, β‐amylase, invertase, α‐glucosidase, and protease activities was followed by a decrease in final hydrolysis products contents: glucose and amino acids.
The decrease in the α‐amylase activity was suggested as an indicator of germination vulnerability under salt exposure and to be more acute in the salt‐sensitive genotypes than in the salt‐tolerant genotypes (Hussain et al., 2022). Similarly, El‐Hendawy et al. (2019) demonstrated that the reduction in the α‐amylase activity results in a significant limitation in the translocation of sugars, essential for the developing embryo.
Priming is reported to induce the activation and synthesis of enzymes required to breakdown and mobilize stored food reserves during germination (Gerna et al., 2018; Lee & Kim, 2000).
In germinating wheat seeds, water uptake stimulates the embryo to produce phytohormones, mainly gibberellic acid (GA), which diffuse to the aleurone layer and initiate a signaling cascade resulting in the synthesis of α‐amylases and other hydrolytic enzymes (Ali & Elozeiri, 2017).
Seed pre‐treatment with gibberellins was found to activate hydrolyzing metabolism with respect to unprimed conditions. Priming‐induced increase in germination may be associated to a change in plant hormone biosynthesis and signaling which may be impacted directly by gene expression patterns (Lutts et al., 2016). Therefore, a uniform endogenous concentration of GAs in primed seeds may help to synchronize endosperm weakening, embryo cell elongation, and reserve mobilization (Rhaman et al., 2021).
During rice germination, the biosynthesis of gibberellins in embryo epithelium induced α‐amylase in seed endosperm Kaneko et al. (2002). The contents of bioactive gibberellins (GAs) decreased after NaCl exposure. This inhibition was restored by exogenous bioactive GA application as reported by (Liu et al., 2018).
Under salt exposure, our results indicate a higher content of total soluble sugars, reducing sugars, glucose and amino acids in primed seeds with respect to unprimed ones suggesting that salt‐tolerance ability of the pre‐treated seeds was probably related to the accumulation of osmolytes that play a key role in osmotic adjustment and water conductance.
The accumulation of internal solutes at low and moderate salt stress conditions may assist in overcoming osmotic stress in some plants (Johnson & Puthur, 2021; Sen & Puthur, 2020). Our findings are in accordance to Chaffei‐Haouari et al. (2012), showing that the higher proline and total soluble carbohydrates concentrations recorded in salt‐tolerant wheat during germination conferred an enhancement of water status and functionality of enzymes involved in germination process. Thus, hyperaccumulation of soluble sugars under salt stress enabled the cells to improve the tolerance to salinity by increasing the osmotic pressure (Tian et al., 2015).
The present study addressed the relieving of the negative effects of salinity through cost‐effective seed priming in order to improve the production of wheat under saline conditions. Common and low‐cost seed priming agents for osmo‐priming, halopriming and hormonal priming were used in this study. These different agents acted diversely in stress conditions: mannitol and CaCl2 improved water status, CaCl2 mitigated ionic imbalance limiting sodium accumulation in plant tissues, whereas gibberellins stimulated seed reserve mobilization and activated hydrolyzing enzymes of stored reserves. Gibberellic acid was the most effective priming treatment for promoting the germination of wheat seeds under salt stress. Moreover, genotypic differences in wheat response to salinity stress were observed between varieties used in this study. Ardito, the oldest variety, seems to tolerate better salinity in priming‐free conditions; Aubusson resulted the most salt‐sensitive cultivar but showed a high germination recovery under priming conditions; Bologna showed an intermediate behavior.
AUTHOR CONTRIBUTIONS
Souhir Sghayar performed experiments and wrote the original draft of the presented manuscript. Giorgio Lucchini performed ICP‐MS analysis. Alessandro Abruzzese provided technical support. Gian Attilio Sacchi contributed to the study conception and design of experiments. Noemi Negrini and Silvia Morgutti helped in dataset analysis. Walid Zorrig helped in statistical analysis. Patrizia Vaccino supervised the experiments. Ahmed Debez, Nicola Pecchioni, and Chedly Abdelly revised the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This work was conducted in the Research Centre for Cereal and Industrial Crops (CREA‐CI), Vercelli, Italy, in collaboration with the Centre of Biotechnology of Borj‐Cedria (CBBC, Tunisia). A part of the analysis was realized in the Department of Agricultural and Environmental Sciences ‐ Production, Landscape, and Agroenergy (DISAA), University of Milan, Italy. We thank all the technical and administrative staff of CREA‐CI and DISAA for support.
Sghayar, S. , Debez, A. , Lucchini, G. , Abruzzese, A. , Zorrig, W. , Negrini, N. , Morgutti, S. , Abdelly, C. , Sacchi, G. A. , Pecchioni, N. , & Vaccino, P. (2023). Seed priming mitigates high salinity impact on germination of bread wheat ( Triticum aestivum L.) by improving carbohydrate and protein mobilization. Plant Direct, 7(6), e497. 10.1002/pld3.497
Funding information This work was financed by the Italian Ministry of Foreign Affairs and International Cooperation.
DATA AVAILABILITY STATEMENT
All data included in this study are available upon request by contact with the corresponding authors.
REFERENCES
- Ahmad, F. , Kamal, A. , Singh, A. , Ashfaque, F. , Alamri, S. , Siddiqui, M. H. , & Khan, M. I. R. (2021). Seed priming with gibberellic acid induces high salinity tolerance in Pisum sativum through antioxidants, secondary metabolites and up‐regulation of antiporter genes. Plant Biology, 23, 113–121. 10.1111/plb.13187 [DOI] [PubMed] [Google Scholar]
- Ali, A. S. , & Elozeiri, A. A. (2017). Metabolic processes during seed germination. Advances in Seed Biology, 141–166. 10.5772/intechopen.70653 [DOI] [Google Scholar]
- Amooaghaie, R. (2011). The effect of hydro and osmopriming on alfalfa seed germination and antioxidant defenses under salt stress. African Journal of Biotechnology, 10(33), 6269–6275. [Google Scholar]
- Andriotis, V. M. , Saalbach, G. , Waugh, R. , Field, R. A. , & Smith, A. M. (2016). The maltase involved in starch metabolism in barley endosperm is encoded by a single gene. PLoS ONE, 11(3), e0151642. 10.1371/journal.pone.0151642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf, M. , & Foolad, M. R. (2005). Pre‐sowing seed treatment—A shotgun approach to improve germination, plant growth, and crop yield under saline and non‐saline conditions. Advances in Agronomy, 88, 223–271. 10.1016/S0065-2113(05)88006-X [DOI] [Google Scholar]
- Balaji, D. S. , & Narayanan, G. S. (2019). Effect of osmo priming seed treatments on seed quality in certain minor millets. Plant Archieves, 19(1), 508–514. [Google Scholar]
- Barry‐Ryan, C. , Vassalo, M. , & Pojic, M. (2020). The comsumption of Healthy Grains: Products, Health, and Wellness Trends. Innovative Processing Technologies for Healthy Grains, 227‐249. [Google Scholar]
- Ben Youssef, R. , Jelali, N. , Boukari, N. , Albacete, A. , Martinez, C. , Alfocea, F. P. , & Abdelly, C. (2021). The efficiency of different priming agents for improving germination and early seedling growth of local Tunisian barley under salinity stress. Plants, 10(11), 2264. 10.3390/plants10112264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer, H. U. , Bergmeyer, J. , & Gra, M. (1983). Enzymes in methods. Enzymatic analysis. New York: Academic Press. [Google Scholar]
- Bernfeld, P. (1951). Enzymes of starch degradation and synthesis. Advances in Enzymology and Related Subjects of Biochemistry, 12, 379–428. [DOI] [PubMed] [Google Scholar]
- Bewley, J. D. (1997). Seed germination and dormancy. The Plant Cell, 9(7), 1055–1066. 10.1105/tpc.9.7.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley, J. D. , Bradford, K. , & Hilhorst, H. (2012). Seeds: Physiology of development, germination and dormancy (pp. 133‐179). Springer Science & Business Media. [Google Scholar]
- Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry, 72(1‐2), 248‐254. [DOI] [PubMed] [Google Scholar]
- Brouquisse, R. , Gaudillere, J. P. , & Raymond, P. (1998). Induction of a carbon‐starvation‐related proteolysis in whole maize plants submitted to light/dark cycles and to extended darkness. Plant Physiology, 117(4), 1281‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffei‐Haouari, C. , Hajjaji‐Nasraoui, A. , Bouthour, D. , & Gouia, H. (2012). Variations in α‐,β‐amylase and α‐ glucosidase activities in two genotypes of wheat under salinity stress. Plant Breeding and Seed Science, 66, 89–97. [Google Scholar]
- Chen, X. , Zhang, R. , Xing, Y. , Jiang, B. , Li, B. , Xu, X. , & Zhou, Y. (2021). The efficacy of different seed priming agents for promoting sorghum germination under salt stress. PLoS ONE, 16(1), e0245505. 10.1371/journal.pone.0245505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibbar, R. N. , Ganeshan, S. , Båga, M. , & Khandelwal, R. L. (2004). Carbohydrate metabolism. In Encyclopedia of grain science (Vol. 1) (pp. 168–179). Elsevier Academic Press. [Google Scholar]
- Debez, A. , Ben Slimen, I. D. , Bousselmi, S. , Atia, A. , Farhat, N. , El Kahoui, S. , & Abdelly, C. (2020). Comparative analysis of salt impact on sea barley from semi‐arid habitats in Tunisia and cultivated barley with special emphasis on reserve mobilization and stress recovery aptitude. Plant Biosystems‐An International Journal Dealing with all Aspects of Plant Biology, 154(4), 544–552. 10.1080/11263504.2019.1651777 [DOI] [Google Scholar]
- Debez, A. , Braun, H. P. , Pich, A. , Taamalli, W. , Koyro, H. W. , Abdelly, C. , & Huchzermeyer, B. (2012). Proteomic and physiological responses of the halophyte Cakile maritima to moderate salinity at the germinative and vegetative stages. Journal of Proteomics, 75(18), 5667–5694. 10.1016/j.jprot.2012.08.012 [DOI] [PubMed] [Google Scholar]
- Devika, O. S. , Singh, S. , Sarkar, D. , Barnwal, P. , Suman, J. , & Rakshit, A. (2021). Seed priming: A potential supplement in integrated resource management under fragile intensive ecosystems. Frontiers in Sustainable Food Systems, 5, 654001. 10.3389/fsufs.2021.654001 [DOI] [Google Scholar]
- Dure, L. S. (1960). Site of origin and extent of activity of amylases in maize germination. Plant Physiology, 35(6), 925–934. 10.1104/pp.35.6.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Hendawy, S. , Elshafei, A. , Al‐Suhaibani, N. , Alotabi, M. , Hassan, W. , Dewir, Y. H. , & Abdella, K. (2019). Assessment of the salt tolerance of wheat genotypes during the germination stage based on germination ability parameters and associated SSR markers. Journal of Plant Interactions, 14(1), 151–163. 10.1080/17429145.2019.1603406 [DOI] [Google Scholar]
- Farooq, M. , Usman, M. , Nadeem, F. , Rehman, H. , Wahid, A. , Basra, S. M. A. , & Siddique, K. H. M. (2019). Seed priming in field crops: Potential benefits, adoption and challenges. Crop and Pasture Science, 70(9), 731–771. 10.1071/CP18604 [DOI] [Google Scholar]
- Feghhenabi, F. , Hadi, H. , Khodaverdiloo, H. , & Van Genuchten, M. T. (2020). Seed priming alleviated salinity stress during germination and emergence of wheat (Triticum aestivum L.). Agricultural Water Management, 231, 106022. 10.1016/j.agwat.2020.106022 [DOI] [Google Scholar]
- Fita, A. , Rodríguez‐Burruezo, A. , Boscaiu, M. , Prohens, J. , & Vicente, O. (2015). Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Frontiers in Plant Science, 6, 978. 10.3389/fpls.2015.00978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foca, G. , Ulrici, A. , Corbellini, M. , Pagani, M. A. , Lucisano, M. , Franchini, G. C. , & Tassi, L. (2007). Reproducibility of the Italian ISQ method for quality classification of bread wheats: An evaluation by expert assessors. Journal of the Science of Food and Agriculture, 87(5), 839–846. 10.1002/jsfa.2785 [DOI] [Google Scholar]
- Gerna, D. , Roach, T. , Arc, E. , Stöggl, W. , Limonta, M. , Vaccino, P. , & Kranner, I. (2018). Redox poise and metabolite changes in bread wheat seeds are advanced by priming with hot steam. Biochemical Journal, 475(23), 3725–3743. 10.1042/BCJ20180632 [DOI] [PubMed] [Google Scholar]
- Guangwu, Z. , & Xuwen, J. (2014). Roles of gibberellin and auxin in promoting seed germination and seedling vigor in Pinus massoniana. Forest Science, 60(2), 367–373. 10.5849/forsci.12-143 [DOI] [Google Scholar]
- Hossain, A. , Skalicky, M. , Brestic, M. , Maitra, S. , Ashraful Alam, M. , Syed, M. A. , Hossain, J. , Sarkar, S. , Saha, S. , Bhadra, P. , Shankar, T. , Bhatt, R. , Kumar Chaki, A. , el Sabagh, A. , & Islam, T. (2021). Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy, 11(2), 241. 10.3390/agronomy11020241 [DOI] [Google Scholar]
- Hussain, S. , Ali, B. , & Saqib, M. (2022). Seed priming to enhance salt and drought stress tolerance in plants: Advances and prospects. Climate Change and Crop Stress, 441–464. 10.1016/B978-0-12-816091-6.00012-2 [DOI] [Google Scholar]
- Ibrahim, E. A. (2016). Seed priming to alleviate salinity stress in germinating seeds. Journal of Plant Physiology, 192, 38–46. 10.1016/j.jplph.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Iqbal, M. , & Ashraf, M. (2013). Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environmental and Experimental Botany, 86, 76–85. 10.1016/j.envexpbot.2010.06.002 [DOI] [Google Scholar]
- Iqbal, M. , Ashraf, M. , Jamil, A. , & Ur‐Rehman, S. (2006). Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress? Journal of Integrative Plant Biology, 48(2), 181‐189. [Google Scholar]
- Johnson, R. , & Puthur, J. T. (2021). Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiology and Biochemistry, 162, 247–257. 10.1016/j.plaphy.2021.02.034 [DOI] [PubMed] [Google Scholar]
- Joosen, R. V. , Kodde, J. , Willems, L. A. , Ligterink, W. , van der Plas, L. H. , & Hilhorst, H. W. (2010). GERMINATOR: A software package for high‐throughput scoring and curve fitting of Arabidopsis seed germination. The Plant Journal, 62(1), 148–159. 10.1111/j.1365-313X.2009.04116.x [DOI] [PubMed] [Google Scholar]
- Kader, M. A. (2005). A comparison of seed germination calculation formulae and the associated interpretationof resulting data. Journal and Proceeding of the Royal Society of New South Wales, 138, 65‐75. [Google Scholar]
- Kaneko, M. , Itoh, H. , Ueguchi‐Tanaka, M. , Ashikari, M. , & Matsuoka, M. (2002). The α‐amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiology, 128(4), 1264–1270. 10.1104/pp.010785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazachkova, Y. , Khan, A. , Acuña, T. , López‐Díaz, I. , Carrera, E. , Khozin‐Goldberg, I. , Fait, A. , & Barak, S. (2016). Salt induces features of a dormancy‐like state in seeds of Eutrema (Thellungiella) salsugineum, a halophytic relative of Arabidopsis. Frontiers in Plant Science, 7, 1071. 10.3389/fpls.2016.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, V. K. , & Rajalekshmi, R. (2021). Effect of hydro‐, halo‐and osmopriming on seed germination and seedling performance of Psophocarpus tetragonolobus (L.) DC. (winged bean). Journal of Crop Science and Biotechnology, 24(4), 411–428. 10.1007/s12892-021-00090-9 [DOI] [Google Scholar]
- Laino, P. , Limonta, M. , Gerna, D. , & Vaccino, P. (2015). Morpho‐physiolological and qualitative traits of a bread wheat collection spanning a century of breeding in Italy. Biodiversity Data Journal, 3, e4760. 10.3897/BDJ.3.e4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. S. , & Kim, J. H. (2000). Total sugars, $\alpha $‐amylase activity, and germination after priming of normal and aged rice seeds. Korean Journal of Crop Science, 45(2), 108–111. [Google Scholar]
- Lira, E. , Souza, J. , Galdino, L. , Macêdo, C. , Silva, A. , Melo, Y. , Santos, I. , Arriel, N. , Meneses, C. , & Maia, J. (2021). Changes in reserve mobilization caused by salinity could interfere in the initial growth of jatropha curcas. Sustainability, 13(13), 7446. 10.3390/su13137446 [DOI] [Google Scholar]
- Liu, L. , Xia, W. , Li, H. , Zeng, H. , Wei, B. , Han, S. , & Yin, C. (2018). Salinity inhibits rice seed germination by reducing α‐amylase activity via decreased bioactive gibberellin content. Frontiers in Plant Science, 9, 275. 10.3389/fpls.2018.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Coria, M. , Sánchez‐Sánchez, T. , Martínez‐Marcelo, V. H. , Aguilera‐Alvarado, G. P. , Flores‐Barrera, M. , King‐Díaz, B. , & Sánchez‐Nieto, S. (2019). SWEET transporters for the nourishment of embryonic tissues during maize germination. Genes, 10(10), 780. 10.3390/genes10100780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutts, S. , Benincasa, P. , Wojtyla, L. , Kubala, S. , Pace, R. , Lechowska, K. , Quinet, M. , & Garnczarska, M. (2016). Seed priming: new comprehensive approaches for an old empirical technique. In New challenges in seed biology‐basic and translational research driving seed technology (pp. 1–46). InTech. 10.5772/64420 [DOI] [Google Scholar]
- Mahadevan, A. , & Sridhar, R. (1982). Methods in physiological plant pathology (pp. 157‐159). Nature Publishing Group. [Google Scholar]
- Marín‐Sanz, M. , Giménez, M. J. , Barro, F. , & Savin, R. (2020). Prolamin content and grain weight in RNAi silenced wheat lines under different conditions of temperature and nitrogen availability. Frontiers in Plant Science, 11, 314. 10.3389/fpls.2020.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthandan, V. , Geetha, R. , Kumutha, K. , Renganathan, V. G. , Karthikeyan, A. , & Ramalingam, J. (2020). Seed priming: A feasible strategy to enhance drought tolerance in crop plants. International Journal of Molecular Sciences, 21(21), 8258. 10.3390/ijms21218258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCready, R. M. , Guggolz, J. , Silviera, V. , & Owens, H. S. (1950). Determination of starch and amylose in vegetables. Analytical Chemistry, 22(9), 1156–1158. 10.1021/ac60045a016 [DOI] [Google Scholar]
- Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428. 10.1021/ac60147a030 [DOI] [Google Scholar]
- Moore, S. , & Stein, W. H. (1954). A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. The Journal of Biological Chemistry, 211(2), 907–913. 10.1016/S0021-9258(18)71178-2 [DOI] [PubMed] [Google Scholar]
- Munns, R. (2002). Comparative physiology of salt and water stress. Plant, Cell & Environment, 25(2), 239–250. 10.1046/j.0016-8025.2001.00808.x [DOI] [PubMed] [Google Scholar]
- Nonogaki, H. (2006). Seed germination—The biochemical and molecular mechanisms. Breeding Science, 56(2), 93–105. 10.1270/jsbbs.56.93 [DOI] [Google Scholar]
- Nonogaki, H. (2014). Seed dormancy and germination—Emerging mechanisms and new hypotheses. Frontiers in Plant Science, 5, 233. 10.3389/fpls.2014.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nxele, X. , Klein, A. , & Ndimba, B. K. (2017). Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. South African Journal of Botany, 108, 261–266. 10.1016/j.sajb.2016.11.003 [DOI] [Google Scholar]
- Ormoli, L. , Costa, C. , Negri, S. , Perenzin, M. , & Vaccino, P. (2015). Diversity trends in bread wheat in Italy during the 20th century assessed by traditional and multivariate approaches. Scientific Reports, 5(1), 8574. 10.1038/srep08574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaman, M. S. , Imran, S. , Rauf, F. , Khatun, M. , Baskin, C. C. , Murata, Y. , & Hasanuzzaman, M. (2021). Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants, 10(1), 37. 10.3390/plants10010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosental, L. , Nonogaki, H. , & Fait, A. (2014). Activation and regulation of primary metabolism during seed germination. Seed Science Research, 24(1), 1–15. 10.1017/S0960258513000391 [DOI] [Google Scholar]
- Ruan, Y. , & Fincher, G. B. (2017). Carbohydrates. In Encyclopedia of applied plant sciences (Second ed.). Elsevier. 10.1016/B978-0-12-394807-6.00155-6 [DOI] [Google Scholar]
- Sen, A. , & Puthur, J. T. (2020). Influence of different seed priming techniques on oxidative and antioxidative responses during the germination of Oryza sativa varieties. Physiology and Molecular Biology of Plants, 26(3), 551–565. 10.1007/s12298-019-00750-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, L. , Chen, S. , Wang, T. , & Dai, S. (2013). Proteomic insights into seed germination in response to environmental factors. Proteomics, 13(12–13), 1850–1870. 10.1002/pmic.201200394 [DOI] [PubMed] [Google Scholar]
- Tan‐Wilson, A. L. , & Wilson, K. A. (2012). Mobilization of seed protein reserves. Physiologia Plantarum, 145(1), 140–153. 10.1111/j.1399-3054.2011.01535.x [DOI] [PubMed] [Google Scholar]
- Tian, X. , He, M. , Wang, Z. , Zhang, J. , Song, Y. , He, Z. , & Dong, Y. (2015). Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regulation, 77(3), 343–356. 10.1007/s10725-015-0069-3 [DOI] [Google Scholar]
- Tsegay, B. A. , & Andargie, M. (2018). Seed priming with gibberellic acid (GA3) alleviates salinity induced inhibition of germination and seedling growth of Zea mays L., Pisum sativum var. abyssinicum a. Braun and Lathyrus sativus L. Journal of Crop Science and Biotechnology, 21(3), 261–267. 10.1007/s12892-018-0043-0 [DOI] [Google Scholar]
- Uçarlı, C. (2020). Effects of salinity on seed germination and early seedling stage. Abiotic Stress in Plants, 211. 10.5772/intechopen.93647 [DOI] [Google Scholar]
- Van Handel, E. (1968). Direct microdetermination of sucrose. Analytical Biochemistry, 22(2), 280–283. 10.1016/0003-2697(68)90317-5 [DOI] [PubMed] [Google Scholar]
- Yahia, E. M. , & Carrillo‐Lopez, A. (Eds.). (2018). Postharvest physiology and biochemistry of fruits and vegetables. Woodhead publishing. [Google Scholar]
- Yang, Y. , Xu, S. , An, L. , & Chen, N. (2007). NADPH oxidase‐dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. Journal of Plant Physiology, 164(11), 1429–1435. 10.1016/j.jplph.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Yemm, E. W. , & Willis, A. (1954). The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal, 57(3), 508–514. 10.1042/bj0570508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding authors.


