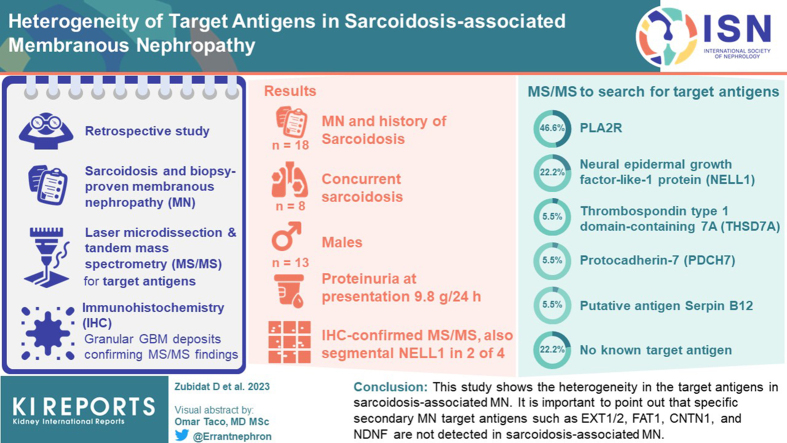
Abstract
Introduction
Membranous nephropathy (MN) is the most common glomerular disease associated with sarcoidosis. The target antigen M-type phospholipase A2 receptor 1 (PLA2R) has been identified in a subset of sarcoidosis-associated MN. The target antigen is not known in the remaining sarcoidosis-associated MN.
Methods
Data of patients with history of sarcoidosis and biopsy-proven MN were retrieved and analyzed. Mass spectrometry (MS/MS) was performed on all kidney biopsies of sarcoidosis-associated MN to detect the target antigens. Immunohistochemistry (IHC) studies were performed to confirm and localize the target antigens along the glomerular basement membrane (GBM).
Results
Eighteen patients with history of sarcoidosis and biopsy-proven MN were identified, of whom 3 were known to be PLA2R-negative, and in the remaining patients the target antigen was unknown. Thirteen (72%) patients were males; the median age at MN diagnosis was 54.5 years. The median proteinuria at presentation was proteinuria 9.8 g/24 h. Eight patients (44.4%) had concurrent sarcoidosis. Using MS/MS, we detected PLA2R and neural epidermal growth factor-like-1 protein (NELL1) in 7 (46.6%) and 4 (22.2%) patients, respectively. In addition, 1 case each (5.5%) was positive for thrombospondin type 1 domain-containing 7A (THSD7A), protocadherin-7 (PCDH7), and putative antigen Serpin B12. No known target antigen was detected in the remaining 4 patients (22.2%).
Conclusion
Patients with sarcoidosis and MN exhibit heterogeneous target antigens. We identified, along with PLA2R, the presence of previously unreported antigens, including NELL1, PCDH7, and THSD7A. The incidence of the target antigens in sarcoidosis appears to mirror the overall incidence of target antigens in MN. MN in sarcoidosis may be the result of a heightened immune response and is not associated with a single target antigen.
Keywords: mass spectrometry, membranous nephropathy, NELL1, PLA2R, sarcoidosis
Graphical abstract
Kidney involvement in sarcoidosis occurs in 10% to 20% of affected patients.1,2 It typically results in nephrocalcinosis or interstitial nephritis secondary to granulomatous infiltration.1,3, 4, 5, 6 Glomerular involvement in sarcoidosis has been reported in 10% of cases, and when present, the most common histopathological pattern of injury is MN.7,8 MN results from antigen-antibody complexes along the subepithelial region of the GBM. There are only 2 small studies on the target antigens present in sarcoidosis-associated MN. In these studies, the target antigen M-type PLA2R was detected in 55% (5 out of 9 patients) and 75% (3 out of 4 patients) of sarcoidosis-associated MN, respectively.8, 9, 10, 11 In the remaining cases, the target antigen is not known. In recent years, novel target antigens have been identified in MN.12 These include exostosin 1/2, NELL1, semaphorin 3B, PCDH7, neural cell adhesion molecule 1, contactin-1, protocadherin FAT1, and neuron-derived neurotrophic factor.13, 14, 15, 16, 17, 18, 19 The role of these target antigens in sarcoidosis-associated MN is not known. In this study, we aimed to determine whether any of the newly identified MN antigens were present in sarcoidosis-associated MN.
Methods
Patient Selection
A total of 18 patients with sarcoidosis-associated MN were identified for the study. We retrospectively identified 15 cases (cases 4–18) with an established diagnosis of sarcoidosis and biopsy-proven MN. The diagnosis of MN was confirmed by light microscopy, immunofluorescence (IF), and electron microscopy in all cases. Kidney biopsies were received at Mayo Clinic between January 1, 2010 and December 31, 2020, in the Renal Pathology Laboratory (Department of Laboratory Medicine and Pathology). The study was approved by the Mayo Clinic Institutional Review Board. In addition, 3 cases (cases 1–3) of PLA2R-negative sarcoidosis-associated MN were received from the Department of Pathology and Cell Biology, Columbia University. Clinical, demographic, and laboratory data at the time of MN diagnosis were obtained from medical charts. Medical records with clinical data were requested for biopsy cases received from outside institutions. Patients were diagnosed with sarcoidosis according to the clinical or biopsy findings (either the presence of noncaseating granulomas, which comprised of epithelioid histocytes with multinucleated forms, and negative staining for fungal microorganisms or acid-fast bacilli on a lung or lymph node biopsy specimen or diagnostic radiological signs of pulmonary nodules or mediastinal lymphadenopathy). The estimated glomerular filtration rate was calculated using the CKD-EPI creatinine equation 2021. Sarcoidosis and MN were considered concurrent when the diagnosis of MN was within a year of the diagnosis of sarcoidosis.
Detection of Target Antigens in Sarcoidosis-Associated MN Using Laser Microdissection and Tandem (MS/MS)
Sample cases were processed beginning with 10-micron thick formalin-fixed paraffin-embedded sections that were cut on a special polyethylene naphtalate membrane laser microdissection slide then deparaffinized in xylene. The glomeruli were microdissected using a Zeiss Palm Microbeam microscope to accumulate approximately 250,000 to 550,000 μm2 per case into 100 mM Tris pH 8.2/0.005% zwittergent 3 to 16. The collected formalin-fixed paraffin-embedded fragments were heated at 98 °C for 60 minutes for protein extraction, then reduced with tris carboxyethyl phosphine, alkylated with iodoacetamide followed by digestion with trypsin/LysC mix (Promega) at 37 °C overnight. The digest mixture was acidified with dilute trifluoroacetic acid and loaded onto a C18 trap column (EXP Halo 2.7um, Optimize Technologies). The peptides were separated with a 100 μm × 40 cm C18 column (PepSep 1.5 μm) running 0.1% formic acid/acetonitrile buffers on a Thermo Ultimate 3000 RSLCnano HPLC system coupled to a Thermo Scientific Exploris480 Orbitrap Mass Spectrometer (Thermo Fisher Scientific, Bremen, Germany) for nano-flow liquid chromatography electrospray tandem MS/MS (nanoLC-ESI-MS/MS) analysis. The raw data files were processed in MaxQuant using Andromeda to search against the Swissprot human database (release 2021_03) and the identified peptides and proteins were filtered at 1% false discovery rate. The MaxQuant reported spectral counts (MS/MS counts) and iBAQ values19 were used for comparisons to controls.
Confirmation of New Antigens by IHC
The methods have been previously described.14,16 Briefly, IHC staining was performed at the Pathology Research Core (Mayo Clinic, Rochester, MN) using the Leica Bond RX stainer (Leica). Formalin-fixed paraffin-embedded tissues were sectioned at 5 microns and IHC staining was performed on-line. The NELL-1 primary antibody (Rabbit Polyclonal, Sigma #HPA051535, diluted to 1:100) and the PCDH7 primary antibody (Mouse Monoclonal, Clone OT12G6, Abcam, diluted to 1:400) were diluted in background reducing diluent (Dako) and incubated for 30 minutes. The detection system used was polymer refine detection system (Leica). This system includes the hydrogen peroxidase block, post primary and polymer reagent, 3,3′-Diaminobenzidine (DAB), and hematoxylin. Immunostaining visualization was achieved by incubating the slides for 10 minutes in DAB and DAB buffer (1:19 mixture) from the bond polymer refine detection system. Slides were then rinsed between steps with 1× bond wash buffer (Leica). The slides were counterstained for 5 minutes using Schmidt hematoxylin and molecular biology grade water (1:1 mixture), followed by several rinses in 1× bond wash buffer and distilled water, this is not the hematoxylin provided with the refine kit. Once the immunochemistry process was completed, the slides were removed from the stainer and rinsed in tap water for 5 minutes. Slides were dehydrated in increasing concentrations of ethyl alcohol and cleared in 3 changes of xylene before permanent coverslipping in xylene-based medium. Established IF microscopy methodology in the Renal Pathology Laboratory, Mayo Clinic was used for the detection of PLA2R and THSD7A.
Statistical Analysis
Descriptive statistics were performed using JMP Pro software version 10.0.2 (SAS Institute Inc., Cary, NC). Continuous variables were skewed and are reported as median with an interquartile range of Q1 to Q3 (25th, 75th). Further, categorical variables were presented as frequency (%).
Results
Clinical and Laboratory Characteristics
We identified 18 cases with an established diagnosis of sarcoidosis and a biopsy-proven MN. Patients were mostly males 13 (72.2%), the median age at MN diagnosis was 54.5 years (47.2, 70.5), with median serum creatinine at 1.7 mg/dl (1.14, 3.31), median estimated glomerular filtration rate of 46.5 ml/min (20.0, 75.5), median proteinuria 9.5 g/24 h (5.0, 14.0) and median serum albumin at 2.6 g/dl (1.9, 3.2). Sarcoidosis diagnosis was confirmed in 8 (44%) patients by either a lymph node biopsy (in 4 patients) or pulmonary biopsy (4 patients) with findings showing noncaseating granulomas, composed of epithelioid histocytes with multinucleated appearance and negative staining for fungal microorganisms and acid-fast bacilli in 5 (27.7%) patients. Out of the 18 patients, 8 (44.4%) developed concurrent MN with either active sarcoidosis or within 1 year of the diagnosis of sarcoidosis. In all patients, other diseases associated with MN, including autoimmune diseases (e.g., lupus erythematosus), infections (e.g., hepatitis B or C infection), drugs (e.g., nonsteroidal anti-inflammatory drugs), or malignancy, were not present.
Kidney Biopsy Findings
All cases showed characteristic findings of MN with thickened GBM on light microscopy, granular IgG staining along the capillary walls on IF, and subepithelial electron-dense deposits on electron microscopy. A median of 9.5 (6.0, 18.0) glomeruli were present, of which 1.0 (0.0, 1.25) were globally sclerosed. Proliferative features were not present in any case. There was minimal (<10%) interstitial fibrosis and tubular atrophy in 6 biopsies (33.3%), whereas mild (10%–25%) and moderate (26%–50%) tubular atrophy and interstitial fibrosis were present in 9 (50.0%) and 3 (16.6%) biopsies, respectively. None of the samples had severe (>50%) interstitial fibrosis and tubular atrophy. Noncaseating granulomatous interstitial nephritis was present in 2 kidney biopsies. IF showed staining for IgG (1 − 3+/3) and C3 (1 − 3+/3) in all biopsies. Two cases showed staining for C1q (1 − 3+). IgG subtypes were not performed. On electron microscopy, 2 (11.1%) cases had stage I, 12 cases (66.6%) had stage II, 3 (16.6%) as stage III, and 1 (5.5%) had stage III to IV (Churg and Ehrenreich20). Details on clinical and pathologic findings are presented in Table 1.
Table 1.
Clinical, pathology, and MS findings
| Case number | Age | Gender | Sarcoidosis Dx in relation with MN | Urinary protein g/24h | Serum creatinine mg/dl | Serum albumin g/dl | IFTA | Immunofluorescence | EM | MS/MS |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 72 | Male | Concurrent | 1.8 | 5.5 | 3.2 | 20% | IgG 3+, IgM trace, IgA trace, C3 2+ | Stage II | PCDH7 |
| #2 | 56 | Female | Concurrent | 9.1 | 1.32 | 2.6 | 20% | Segmental IgG 2+, C3 trace | Stage II | NELL1 |
| #3 | 50 | Male | Prior | 14.8 | 1.22 | 1.9 | 10% | IgG 3+, C3 1+ | Stage II | NELL1 |
| #4 | 42 | Male | Prior | 14 | 1.1 | 2.1 | 25% | IgG 3+, IgA 1+, C1q 3+, C3 2+ | Stage II | PLA2R |
| #5 | 28 | Male | Concurrent | 13 | 1.05 | 1.5 | 5% | IgG 3+, C3 3+ | Stage I | PLA2R |
| #6 | 33 | Male | Concurrent | 18 | 0.8 | 1.9 | 5% | IgG 3+, IgA 1+, C1q 1+, C3 2+ | Stage II | PLA2R |
| #7 | 53 | Female | Prior | UA 3+ | 1.8 | NA | 50% | IgG 1+ | Stage III–IV | Unknown |
| #8 | 65 | Male | Prior | 3.7 | 3.3 | 2.6 | 50% | IgG 3+, C3 trace | Stage III | NELL1 |
| #9 | 55 | Female | Prior | 15 | 2.7 | 2.3 | 30% | No cortex | Stage II | PLA2R |
| #10 | 50 | Male | Prior | 4.8 | 3.5 | 3.3 | 10% | IgG 2+, IgA 1+, C3 2+ | Stage III | Unknown |
| #11 | 45 | Male | Concurrent | 14 | 3.3 | 2.9 | 10% | IgG 3+, IgM 1+, C3 1+ | Stage II | Unknown |
| #12 | 54 | Male | Prior | 10 | 0.96 | 1 | <5% | IgG 3+, C3 3+ | Stage I | PLA2R |
| #13 | 70 | Male | Prior | 7 | 1.2 | 3.5 | <5% | IgG 3+, IgA 1+, C3 2+ | Stage II | THSD7A |
| #14 | 65 | Male | Concurrent | 5 | 1.6 | NA | 10% | IgG 2+, IgM 1+, C3 2+ | Stage II | PLA2R |
| #15 | 75 | Female | Prior | 5.2 | 1.16 | 3 | <5% | IgG 3+, C3 3+ | Stage II | NELL1 |
| #16 | 72 | Female | Prior | NA | 4.9 | 2.8 | 10% | No cortex | Stage II | Unknown |
| #17 | 48 | Male | Concurrent | NA | 1.8 | 3.6 | 10% | IgG 1+, IgA 1+, IgM 1+ | Stage III | PLA2R |
| #18 | 78 | Male | Concurrent | NA | 1.8 | NA | <5% | IgG 2+, C3 trace | Stage II | SerpinB12 |
Dx, diagnosis; EM, electron microscopy; IFTA, interstitial fibrosis and tubular atrophy; MN, membranous nephropathy; MS, mass spectrometry; NELL1, neural epidermal growth factor-like-1 protein; PCDH7, protocadherin-7; PLA2R, phospholipase A2 receptor; THSD7A, thrombospondin type 1 domain-containing 7A.
Identification of Target Antigens by MS/MS
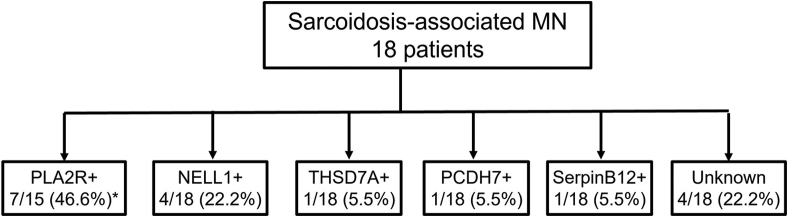
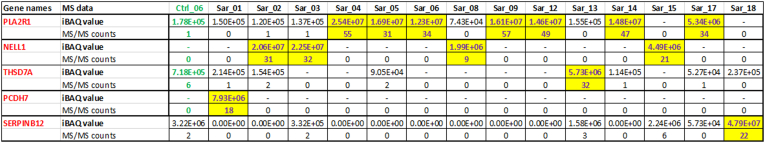
We performed laser microdissection and MS/MS studies on all 18 cases. PLA2R was detected in 7 cases (38.8%) with average total spectral counts of 43.8 (±10.7). NELL1 was identified in 4 cases (22.2%) with total spectral count of 23.5 (±10.7). THSD7A and PCDH7 were detected in 1 case (5.5%) each, with total spectral counts of 32 and 18, respectively. In 1 case, we detected a Serpin B12 that appears to be a unique protein. In the remaining 4 cases (22.2%), no known MN antigens were detected (Figure 1). The MS/MS findings are shown in Figure 2.
Figure 1.
Antigens detected in sarcoidosis-associated. MN, membranous nephropathy; NELL1, neural epidermal growth factor-like-1 protein; PCDH7, protocadherin-7; PLA2R, phospholipase A2 receptor; THSD7A, thrombospondin type 1 domain-containing 7A.
Figure 2.
Proteomic Identification of target antigens in sarcoidosis-associated MN. Target antigens were detected in 14 of the 18 cases. The target antigens are listed in the first column. Each sarcoidosis case is listed as Sar_01, Sar_2, through to Sar_18. No target antigen was detected in 4 cases Sarc_7, _10, _11, _16 are not shown. Each antigen has 2 rows showing the MS/MS data — the top row showing the iBAQ values and the bottom row showing the total spectral counts. The protein iBAQ values are calculated by summing the precursor ion intensities and dividing them by the number of theoretically observable trypsin-generated peptides. MN, membranous nephropathy; NELL1, neural epidermal growth factor-like-1 protein; PCDH7, protocadherin-7; PLA2R, phospholipase A2 receptor; THSD7A, thrombospondin type 1 domain-containing 7A.
IHC and Immunofluorescence Studies
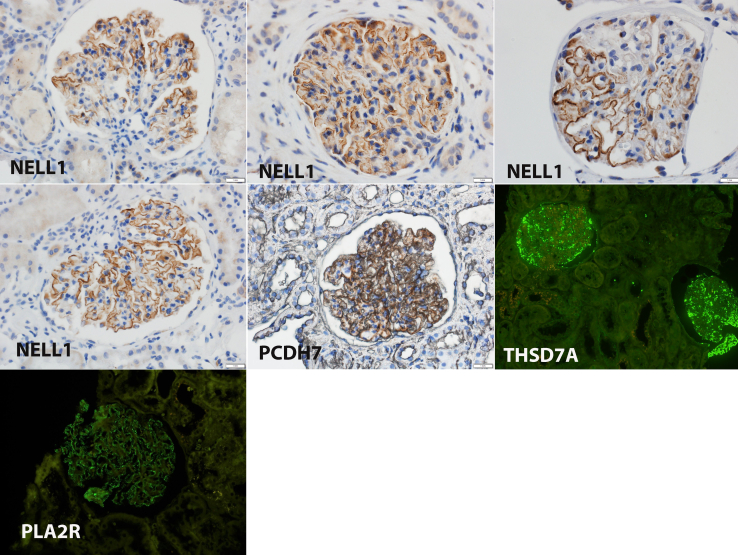
To confirm the findings of MS/MS, we performed IHC for the specific proteins in all cases. All 4 cases of NELL1-associated MN were positive for NELL1 staining along the GBM. Segmental NELL1 staining was noted in 2 of the 4 NELL1 positive cases. The single case of PCDH7-associated MN also showed granular GBM staining. All 7 cases of PLA2R-associated MN and the single case of THSD7A-associated MN were positive for PLA2R and THSD7A on IF studies. IHC and IF findings of all cases of NELL1-associated, PCDH7-associated, THSD7A-associated and a representative case of PLA2R-associated MN are shown in Figure 3.
Figure 3.
Immunohistochemistry showing positive granular staining along the GBM for NELL1 in 4 cases and PCDH7 in 1 case. Immunofluorescence microscopy shows positive staining for THSD7A in 1 case and PLA2R in a representative case. NELL1, neural epidermal growth factor-like-1 protein; PCDH7, protocadherin-7; PLA2R, phospholipase A2 receptor; THSD7A, thrombospondin type 1 domain-containing 7A.
Clinical Follow-Up
Of the 18 patients, 6 (33.3%) were treated with steroids for sarcoidosis, and 5 (27.7%) patients the MN was treated with steroids during the disease. Patient #3 did not receive immunosuppressant treatment and had spontaneous remission. In addition to receiving steroids, patient #4 was treated with mycophenolate mofetil, cyclosporine, and rituximab. Interestingly, 4 of 18 patients were on prednisone at the time of the MN occurrence. Serum creatinine at 1-year follow-up was available in 9 (50%) patients with a median of 1.4 (0.97, 2.04). Serum albumin was available in 6 (33.3%) patients at 1-year follow-up with a median of 3.95 (2.9, 4.4).
Discussion
MN is an autoimmune disease resulting in the accumulation of antigen-antibody complexes in the subepithelial region of the GBM. In a majority (70%) of patients with MN, no underlying disease association is found, and MN is referred to as primary MN. The target antigens PLA2R, NELL1, THSD7A, and PCDH7 have been identified in most (80%–90%) of these patients, with PLA2R being the most common target antigen. In contrast, a specific disease may be associated with MN. In these cases, MN is referred to as secondary MN. In the past, no target antigens were identified in this group. However, recently, it has been shown that target antigens, protocadherin FAT1, are detected in hematopoietic stem cell transplant-associated MN, exostosin 1/2 and neural cell adhesion molecule 1 are detected in autoimmune disease -associated MN, such as lupus MN, neuron-derived neurotrophic factor is detected in infection-(syphilis) associated MN,21 contactin-1 is detected in demyelinating polyneuropathy, and most recently proprotein convertase subtilisin/Kexin type 6 is detected in nonsteroidal anti-inflammatory drugs-associated MN. Therefore, a specific target antigen can be detected in most of the diseases associated with MN.
Sarcoidosis is also associated with MN and is often included in the secondary group of diseases associated with MN. The target antigen PLA2R has been detected in 55% to 75% of the patients of sarcoidosis-associated MN, although the number of patients in these series was small.8 In the remaining patients, the target antigen is unknown. We have successfully used laser microdissection and MS studies to identify novel target antigens in MN.12 In this study, we used MS studies in patients with MN with either concurrent or a prior history of sarcoidosis to either detect a novel target antigen or detect any new antigens that have been discovered in the last few years. In a series of 18 cases of MN with either concurrent or prior history of sarcoidosis, we detected target antigens PLA2R (46.6%), NELL1 (22.2%), THSD7A (5.5%), and PCDH7 (5.5%). We also detected target antigen proprotein convertase subtilisin/Kexin type 6 in 1 case, but the patient had a history of nonsteroidal anti-inflammatory drugs use, and we did not include this patient in our series. Proprotein convertase subtilisin/Kexin type 6 has been recently shown to be the target antigen in nonsteroidal anti-inflammatory drugs-associated MN (KI, in press). Other than sarcoidosis, none of the patients had any other known secondary disease associated with MN.
Of the antigens we detected, only PLA2R has been previously described in sarcoidosis-associated MN.8 To the best of our knowledge, none of the other antigens we detected have been previously reported in sarcoidosis-associated MN. We also take the opportunity to present a putative antigen Serpin B12 in 1 case of sarcoidosis-associated MN, which was unique, present in high spectral counts, and was absent in other MN and control cases. Further studies are required including IHC to localize the protein and western blot studies to detect circulating antibodies to Serpin B12 before confirming Serpin B12 as a likely target antigen.
Concurrent Versus Prior History of Sarcoidosis
Nine patients of MN were reported in the setting of sarcoidosis with no other secondary cause. Of these, only 5 (45.5%) had active sarcoidosis (sarcoidosis diagnosed within 6 months of MN diagnosis).8 In other studies of sarcoidosis-associated MN where 3 out 4 cases (75%) were positive for PLA2R, there is no mention of whether the sarcoidosis was concurrent or the patients had a prior history of sarcoidosis.9 In our study, we found concurrent sarcoidosis in 44.4% of the patients, which is similar to the previous study.8 Therefore, greater than 50% of the patients lack the temporal causality between sarcoidosis and MN.
Target Antigens
Taken together, our studies suggest that target antigens detected in sarcoidosis-associated MN, either concurrent or with a prior history of sarcoidosis, reflect the overall incidence of target antigens in MN. Therefore, PLA2R was the most common antigen in sarcoidosis-associated MN, followed by NELL1, PCDH7, and THSD7A, respectively, thereby reflecting the overall incidence of the target antigens in primary MN. This contrasts with target antigens in other diseases associated with MN in which a distinctive target antigen has been identified, such protocadherin FAT1 in hematopoietic stem cell transplant-associated MN, exostosin 1/2 in autoimmune disease-associated MN, contactin-1 in demyelinating polyneuropathy, and neuron-derived neurotrophic factor in syphilis-associated MN.
Suggested Mechanism
Sarcoidosis is an inflammatory disorder with multisystem involvement resulting from dysregulated antigenic response to unknown environmental exposures in a genetically susceptible person.22 It primarily affects the pulmonary system and, to a lesser extent, other extrapulmonary organs such as the kidneys. It is possible that the inflammation results from immune-dysregulation involving CD4 type-1 helper T lymphocytes and antigen-presenting cells.22 We propose that the inflammation, in turn, in susceptible individuals results in increased expression of proteins, including PLA2R and NELL1, that may result in MN. In other words, a heightened immune response may be responsible for both antigen expression and resulting antibody response. Perhaps this is the likely reason that no single target antigen can account for all cases of sarcoidosis-associated MN, and the pattern of antigen expression follows that of the overall pattern of antigens seen in primary MN.
There are limitations to our study in that we used archival tissue for identifying the target antigens. Frozen tissue and serum were not available. The study is a retrospective study, and clinical data, laboratory findings, detailed treatment, and follow-up are not available in some cases.
To summarize, our study shows a heterogeneity in the target antigens in sarcoidosis-associated MN. The target antigens detected in sarcoidosis-associated MN reflects the overall incidence of target antigens in MN. It is important to point out that specific secondary MN target antigens such as exostosin 1/2, protocadherin FAT1, contactin-1, and neuron-derived neurotrophic factor are not detected in sarcoidosis-associated MN. Finally, the significance of the causal relationship and the clinical implication of the target antigens in sarcoidosis-associated MN needs to be explored in future studies.
Disclosure
All the authors declared no competing interests.
Acknowledgment
This work was supported in part by funding to SS from the Department of Laboratory Medicine and Pathology at the Mayo Clinic (INNOV award). We acknowledge the assistance of the Mayo Clinic Proteomics Core, a shared resource of the Mayo Clinic Cancer Center (NCI P30 CA15083). We would like to thank LouAnn Gross and Vivian Negron in the Pathology Research Core for their help with histology and immunostaining.
Author Contributions
SS and FCF designed the study. SS wrote the manuscript, interpreted the kidney biopsy, clinical, and IHC data. DZ and LN helped write the first draft and collect the clinical data. SN helped in collection of cases and in critical evaluation of the kidney biopsies. SK provided cases and clinical data from Columbia University. BM performed mass spectrometry studies. The manuscript was drafted and written by SS, with input as appropriate from all investigators.
References
- 1.Berliner A.R., Haas M., Choi M.J. Sarcoidosis: the nephrologist’s perspective. Am J Kidney Dis. 2006;48:856–870. doi: 10.1053/j.ajkd.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Bergner R., Löffler C. Renal sarcoidosis: approach to diagnosis and management. Curr Opin Pulm Med. 2018;24:513–520. doi: 10.1097/MCP.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 3.Bijol V., Mendez G.P., Nose V., Rennke H.G. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol. 2006;14:57–63. doi: 10.1177/106689690601400110. [DOI] [PubMed] [Google Scholar]
- 4.Hannedouche T., Grateau G., Noël L.H., et al. Renal granulomatous sarcoidosis: report of six cases. Nephrol Dial Transplant. 1990;5:18–24. doi: 10.1093/ndt/5.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Calatroni M., Moroni G., Reggiani F., Ponticelli C. Renal sarcoidosis. J Nephrol. 2023;36:5–15. doi: 10.1007/s40620-022-01369-y. [DOI] [PubMed] [Google Scholar]
- 6.Mahévas M., Lescure F.X., Boffa J.J., et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Med (Baltim) 2009;88:98–106. doi: 10.1097/MD.0b013e31819de50f. [DOI] [PubMed] [Google Scholar]
- 7.Taylor R.G., Fisher C., Hoffbrand B.I. Sarcoidosis and membranous glomerulonephritis: a significant association. Br Med J (Clin Res Ed) 1982;284:1297–1298. doi: 10.1136/bmj.284.6325.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stehlé T., Joly D., Vanhille P., et al. Clinicopathological study of glomerular diseases associated with sarcoidosis: a multicenter study. Orphanet J Rare Dis. 2013;8:65. doi: 10.1186/1750-1172-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen C.P., Messias N.C., Silva F.G., Messias E., Walker P.D. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 10.Stehlé T., Audard V., Ronco P., Debiec H. Phospholipase A2 receptor and sarcoidosis-associated membranous nephropathy. Nephrol Dial Transplant. 2015;30:1047–1050. doi: 10.1093/ndt/gfv080. [DOI] [PubMed] [Google Scholar]
- 11.Svobodova B., Honsova E., Ronco P., Tesar V., Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant. 2012;28:1839–1844. doi: 10.1093/ndt/gfs439. [DOI] [PubMed] [Google Scholar]
- 12.Sethi S. New ‘antigens’ in membranous nephropathy. J Am Soc Nephrol. 2020;32:268–278. doi: 10.1681/ASN.2020071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S., Madden B.J., Debiec H., et al. Exostosin 1/exostosin 2–associated membranous nephropathy. J Am Soc Nephrol. 2019;30:1123–1136. doi: 10.1681/ASN.2018080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi S., Debiec H., Madden B., et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163–174. doi: 10.1016/j.kint.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Sethi S., Debiec H., Madden B., et al. Semaphorin 3B–associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 2020;98:1253–1265. doi: 10.1016/j.kint.2020.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Sethi S., Madden B., Debiec H., et al. Protocadherin 7-associated membranous nephropathy. J Am Soc Nephrol. 2021;32:1249–1261. doi: 10.1681/ASN.2020081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caza T.N., Hassen S.I., Kuperman M., et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 2021;100:171–181. doi: 10.1016/j.kint.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Quintrec M., Teisseyre M., Bec N., et al. Contactin-1 is a novel target antigen in membranous nephropathy associated with chronic inflammatory demyelinating polyneuropathy. Kidney Int. 2021;100:1240–1249. doi: 10.1016/j.kint.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Sethi S., Madden B., Casal Moura M., et al. Hematopoietic stem cell transplant-membranous nephropathy is associated with protocadherin FAT1. J Am Soc Nephrol. 2022;33:1033–1044. doi: 10.1681/ASN.2021111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Churg J., Ehrenreich T. Membranous nephropathy. Perspect Nephrol Hypertens. 1973;1:443–448. [PubMed] [Google Scholar]
- 21.Sethi S., Madden B., Casal Moura M., et al. Membranous nephropathy in syphilis is associated with neuron-derived neurotrophic factor. J Am Soc Nephrol. 2023;34:374–384. doi: 10.1681/ASN.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drent M., Crouser E.D., Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med. 2021;385:1018–1032. doi: 10.1056/NEJMra2101555. [DOI] [PubMed] [Google Scholar]