Abstract
Methylsulfonylmethane (MSM), a natural organosulfur compound, is a popular dietary supplement sold both as a single product and as a constituent of multi-ingredient products. It has been postulated that MSM may serve as a donor for methyl groups for various cellular processes; however, studies have yet to demonstrate this. Therefore, the goal of this study was to determine whether or not MSM, supplemented to fully differentiated human HepaRG cells at physiologically-relevant concentrations, can serve as a donor for methyl groups for DNA methylation. For this purpose, methyl groups in the MSM molecule were labeled with deuterium (deuterated) and incorporation of the labeled 5-methylcytosine into the HepaRG cell DNA was evaluated using liquid chromatography/mass spectrometry (LC-MS/MS). We report that MSM supplementation resulted in significant incorporation of deuterated product into DNA in a time- and dose-dependent fashion. These changes were not associated with increased 5-methylcytosine content, did not result in changes of DNA methylation or re-distribution of DNA methylation patterns between the retrotransposons LINE-1 and HERV18, and were not associated with cytotoxicity. In conclusion, short-term supplementation with MSM in vitro demonstrates that MSM can serve as a donor of methyl groups for methylation of DNA, but does not affect the levels of DNA methylation globally and does not lead to redistribution of the DNA methylation patterns within the most abundant repetitive elements. Future studies will be needed to validate these findings in vivo and to investigate whether or not MSM can restore normal DNA methylation patterns within the hypomethylated phenotype.
Keywords: DNA methylation, dietary supplements, LINE-1, methylsulfonylmethane
Introduction
Methylsulfonylmethane (MSM) is a natural organosulfur compound and a widely used dietary supplement (Butawan et al., 2017). A number of experimental studies and clinical trials report benefits of MSM in the treatment of various pathological conditions – from arthritis and other disorders associated with inflammation to improved wound healing and increased energy and metabolism (Herschler, 1982; Herschler, 1984; Herschler, 1985; Herschler, 1986; Kim et al; 2006; Debbie et al., 2011).
As a sulfur-containing compound, MSM has been considered as a potential donor of methyl groups for various cellular process, such as DNA methylation. Given high absorbance rates of MSM in the small intestine and its general safety demonstrated in various experimental systems and in humans (Butawan et al., 2017; Wong et al., 2017; Bloomer et al., 2019; Kutanzi et al., 2020), such a property of MSM could provide great value. Indeed, methylation of DNA is one of the most important cellular processes and a key epigenetic mechanism in regulation of the expression of genetic information (Jones, 2012). Numerous pathological states and diseases, such as cancer and asthma, as well as exposures to various environmental stressors are often associated with the loss of DNA methylation (Baylin and Jones, 2016; Miousse et al., 2017a; Sheikhpour et al., 2021). Furthermore, accumulating evidence indicates that loss of global DNA methylation is not only a consequence of such exposures and disease, but is also one of the major drivers in their initiation and progression (Herceg et al., 2018). Therefore, identification of products that can serve as additional donors of methyl groups that could potentially replenish depleted cellular resources and maintain the stable state of DNA methylation is of particular importance.
Therefore, the first goal of this study was to investigate whether or not MSM could serve as a donor of methyl groups in differentiated human HepaRG hepatic cells at concentrations achievable in plasma and that these concentrations would not demonstrate hepatotoxic potential.
Repetitive elements (RE) comprise a substantial percentage of the genome, with some estimates for this number to be over 60%, with DNA methylation serving as a principal mechanism for regulation of their transcriptional activity (Miousse et al., 2015a; Long et al., 2016; Zhou et al., 2020). Numerous studies demonstrated that changes in global DNA methylation, as a result of various exposures or a disease, often occur within the REs, resulting in their transcriptional and retrotranspositional activity (Miousse et al., 2017b; Prior et al., 2016). Therefore, the second goal of this study was to investigate whether or not supplementation of HepaRG cells with MSM would result in differential methylation among the most abundant REs in the human genome – Long Interspersed Nucleotide Element, 1 (LINE-1) and Long Terminal Repeat (LTR).
Materials and Methods
Reagents
MSM product for experiments (OptiMSM) was provided by Bergstrom Nutrition (Vancouver, WA, USA; Bergstrom SKU: 20020; lot# 1901051). For quantitative studies, deuterated MSM was used (DMSO-d6, Hexadeuterodimethyl sulfoxide; Cat # 492272; deuteration degree min. 99.9%; Sigma-Aldrich, Burlington, MA, USA). Standard for 5-Methyl-d3-cytosine-6-d1 (CAS No.: 1219795-15-9) was purchased from BOC Sciences (Shirley, NY, USA).
Cell culture
HepaRG cells were purchased from Biopredic International (Rennes, France) and were cultured as previously described (McGill et al., 2011). Between passages 17 and 21, the cells were seeded at 100,000 cells/cm2 in Williams’ E medium containing ADD711 supplement (BioPredic). The cells were grown for two weeks (37° C, 21% O2, 5% CO2) with medium changes every two days. At day 14, differentiation medium was introduced – Williams’ E with ADD720 (BioPredic) – for two more weeks to allow for cell differentiation. All MSM treatments were performed within the first two weeks after differentiation.
For toxicological studies, HepaRG cells were treated with the following concentrations of MSM: 0 mM, 3.125 mM, 6.25 mM, 12.5 mM, 25 mM, and 50 mM for 24 h. For quantitative studies, HepaRG cells were treated with either 0 mM, 1 mM, 3.125 mM, or 6.25 mM of MSM – concentrations previously reported in human plasma (Bloomer et al., 2019) for either 24 h or 96 h.
Propidium iodide (PI) fluorescence
HepaRG cells were seeded, grown, and differentiated on 6-well plates. After treating cells with the indicated concentrations of MSM, a 1 mg/mL solution of PI (MilliporeSigma, St. Louis, MO, USA) was added directly into the medium to a final concentration of 10 μM/well. The cells were then incubated at 37° C for 10 min, washed with 1x phosphate-buffered saline (PBS) (pH 7.4) to remove excess PI, and imaged in fresh PBS. Images were taken using a Bio-Rad ZOE Fluorescent Cell Imager system (Bio-Rad, Hercules, CA, USA). Acetaminophen (APAP, 20 mM in 1X PBS served as a positive control).
Lactate dehydrogenase (LDH) release assay
After treatment with the indicated concentrations of MSM, the medium was removed from the cell culture and saved in 2 mL test tubes for subsequent analysis. Then, the cells were washed in 1x PBS (pH 7.4). The cells were then lysed with 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.5) that contained 5 mM Ethylenediaminetetraacetic acid (EDTA), 0.1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), and 1 mg/mL each of the protease inhibitors – aprotinin, leupeptin, and pepstatin A, scraped down with a cell scraper, and transferred to 2 mL test tubes. Next, both lysates and medium were centrifuged (14,000 × g, 5 min, 4° C) in order to pellet cellular debris. LDH release measurement in the supernatants was performed by mixing them in 60 mM potassium phosphate buffer (pH 7.5) containing 0.7 mM pyruvate and 270 μM nicotinamide adenine dinucleotide hydrogen (NADH). NADH absorbance was monitored at 340 nm over time. LDH release was then calculated as % released as follows: LDH units in medium / total LDH units in both medium and lysate × 100%. Acetaminophen (APAP, 20 mM in 1X PBS served as a positive control).
Determination of analytical components of methionine metabolism
Quantitative analysis of cytosine, 5-methylcytosine, and deuterated 5-methylcytosine was performed using liquid chromatography/mass spectrometry (LC-MS/MS) methodology, as previously described (Friso et al., 2022; James et al., 2008; Ewing et al., 2021). Specifically, RNase A (Sigma, St. Louis, MO) was added to genomic DNA (1 μg) to a final concentration of 0.02 mg/mL and DNA was then incubated at 37 °C for 15 min. Next, DNA was denatured by heating at 100 °C for 3 min and rapidly chilled in an ice water bath. One-tenth volume of 0.1 M ammonium acetate (pH 5.3) was added to 2 units of nuclease P1 (Sigma) for every 0.5 A260 unit of DNA and the mixture was then incubated at 45 °C for 2 h. Subsequently, 1/10 volume of 1 M ammonium bicarbonate and 0.002 units of venom phophodiesterase I (both – Sigma) were added and the mixture was incubated at 37 °C for 2 h. Then, 0.5 units of alkaline phosphatase (Sigma) was added to the mixture and incubation continued for an additional hour. The digested nucleotides were stored at −20 °C until LC-MS analysis.
For the chromatographic separation of investigated metabolites – cytosine, 5-methylcytosine, and deuterated 5-methylcytosine – Dionex UHPLC system (UltiMate 3000 series) with Phenomenex Gemini 5 μm, C18, 150×2.0 mm column was used. For the analysis, a mixture of 100% H2O with 25 mM ammonium acetate and 25 mM ammonium hydroxide served as solvent A; 100% acetonitrile served as solvent B. The flow rate was 0.3 mL/min, and the gradient was described as follows: 0–1 min: 95% B; 1–14 min: 95% B to 65% B; 14–16 min: 65% B to 40% B; 16–18 min: 40% B; 18–18.1 min: 40% B to 95% B; and 18.1–23 min: 95% B. The sample injection volume was 5 μL.
For metabolite detection ion trap, triple quadrupole LC-MS system (Thermo LTO series, USA) was set as follows: positive ion mode; mass range was set as m/z 80–500 Da; nitrogen sheath gas flow rate 35; spray voltage +3.5 kV; capillary temperature +275 °C; capillary voltage +28 V; and tube lens −5 V. All data acquisitions were carried out using Xcalibur Software (Thermo, USA).
Nucleic acids extraction
DNA was extracted from flash-frozen HepaRG cells using the DNA extraction kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s protocol. DNA concentrations and integrity were analyzed by the Nanodrop 2000 (Thermo Scientific, Waltham, MA, USA). Only DNA samples with 260/280 ratios between 1.8 and 1.9 and 260/230 ratios above 1.5 were considered for further molecular analyses.
Analysis of repetitive elements DNA methylation
Analysis of RE DNA methylation was performed as previously described (Ewing et al., 2022). First, the consensus sequences of REs were obtained from the Genetic Information Research Institute (GIRI) Database: http://www.girinst.org/ (Bao et al. 2015) and the 5’-UTRs of the select LINE-1 and LTR families were analyzed using NEBcutter® (http://nc2.neb.com/NEBcutter2/). Specific assays for RE elements were developed based on the presence of CpG sites that can be cleaved by the methylation-sensitive restriction enzymes AciI, BstUI, HhaI, HpaII, and SmaI. Then, 1 μg of genomic DNA was digested with restriction enzymes in the following sequence: 1) Sma I (1 U of enzyme at 25°C for 2 h); 2) HpaII, HhaI, and AciI (1 U of each enzyme at 37 °C for 16h); 3). BstUI (0.5 U of enzyme at 60 °C for for 4h). All enzymes were purchased from New England Biolabs, Ipswich, MA, USA. Digested DNA was then analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) using the ViiA 7 System (Applied Biosystems, Forrest City, CA, USA). Undigested samples served as a positive control; samples without primers for DNA amplification served as negative controls. The change in methylation was calculated using the following equation:
Statistical analysis
All data are presented as mean ± standard error of mean(s). Statistically significant differences for each treatment compared to the control (at α=95%) were assessed using Student’s t-test and one-way ANOVA, where applicable. Two-way ANOVA was used for the analysis of the components of methionine metabolism. Statistical analyses were performed using GraphPad Prism 9.4.1 (GraphPad Software Inc., La Jolla, CA, USA).
Results
MSM shows no evidence of toxicity in HepaRG cells
Given the lack of data on MSM toxicity in HepaRG cells, we first aimed to determine the margins of toxicity in our experimental system. There was no morphological evidence of cell death nor any increase in propidium iodide fluorescence at any tested concentration up to 50 mM (Figure 1). The LDH release assay further confirmed these findings, demonstrating that dosing HepaRG cells at MSM concentrations of 0 – 25 mM resulted in no significant increases in LDH release into the media (Figure 2).
Figure 1.
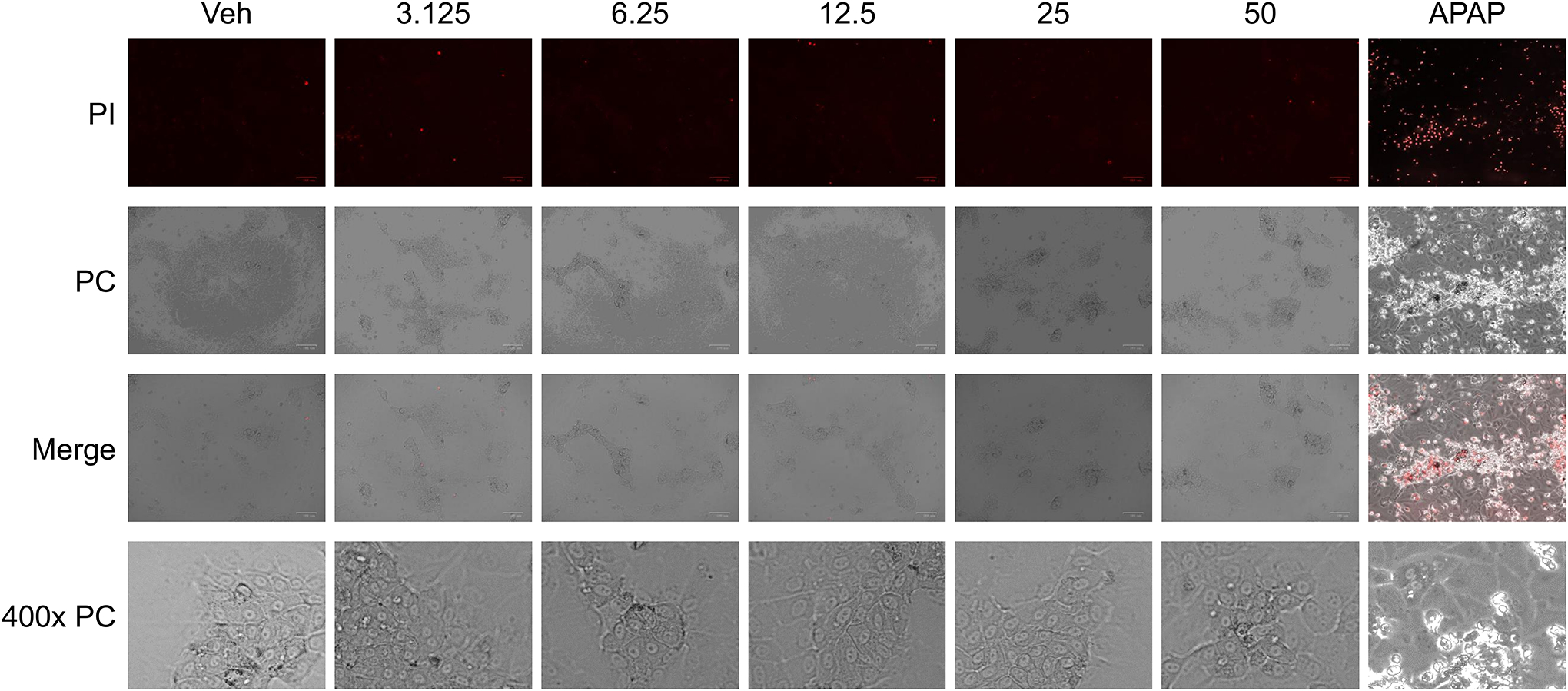
Propidium iodide (PI) staining of human HepaRG cells 24 h after treatment with various concentrations (0 – 50 mM) of MSM and acetaminophen (APAP) at 20 mM concentration (as positive control); mean +/− SEM (n = 3 per group).
Figure 2.
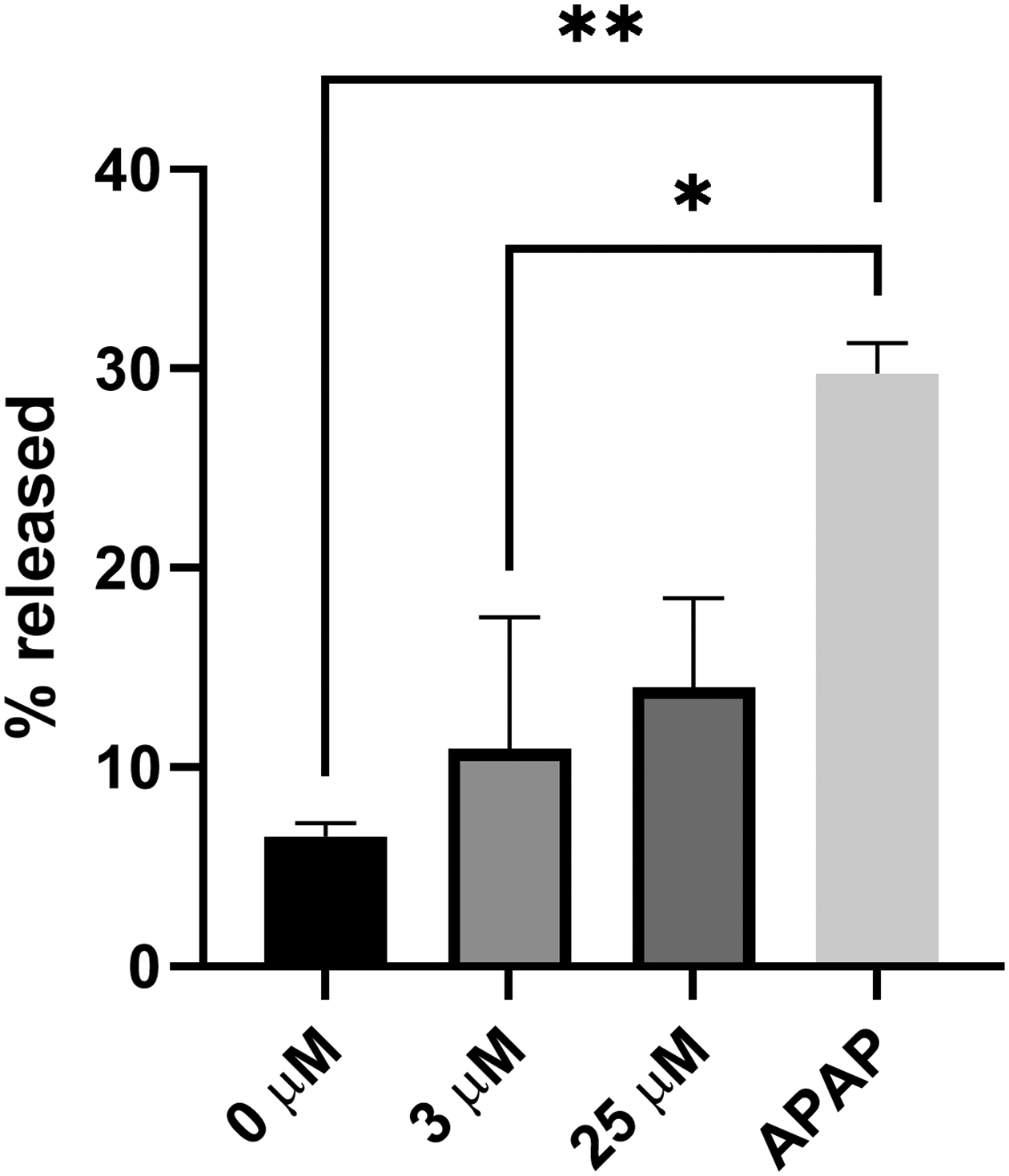
Levels of lactate dehydrogenase (LDH) release 24 h after treatment of human HepaRG cells with various concentrations of MSM and acetaminophen (APAP) at 20 mM concentration (as positive control); mean +/− SEM (n = 3 per group).
MSM as a donor of methyl groups for DNA methylation
Next, we sought to determine whether or not supplementation of HepaRG cells at concentrations that are relevant to those detected in human plasma can serve as a donor of methyl groups. For this purpose, HepaRG cells were dosed with deuterated MSM at three concentrations – 3.125 mM, 6.25 mM, and 10 mM– with the cells being harvested at 24 h and 96 h time-points. Analysis revealed no difference in cytosine levels and subtle, insignificant increases in the levels of 5-methylcytosine observed mostly at 96 h (Figure 3 A–B). At the same time, supplementation of HepaRG cells with MSM resulted in substantial increases in deuterated 5-methylcytosine in a time- and dose-dependent manner (Figure 3C). At the 96 h time-point, these increases were statistically significant (p<0.01) at all three concentrations. Furthermore, there was a trend towards an increase in combined levels of 5-methylcytosine and deuterated 5-methylcytosine; however, this increase did not reach statistical significance (Figure 3D).
Figure 3.
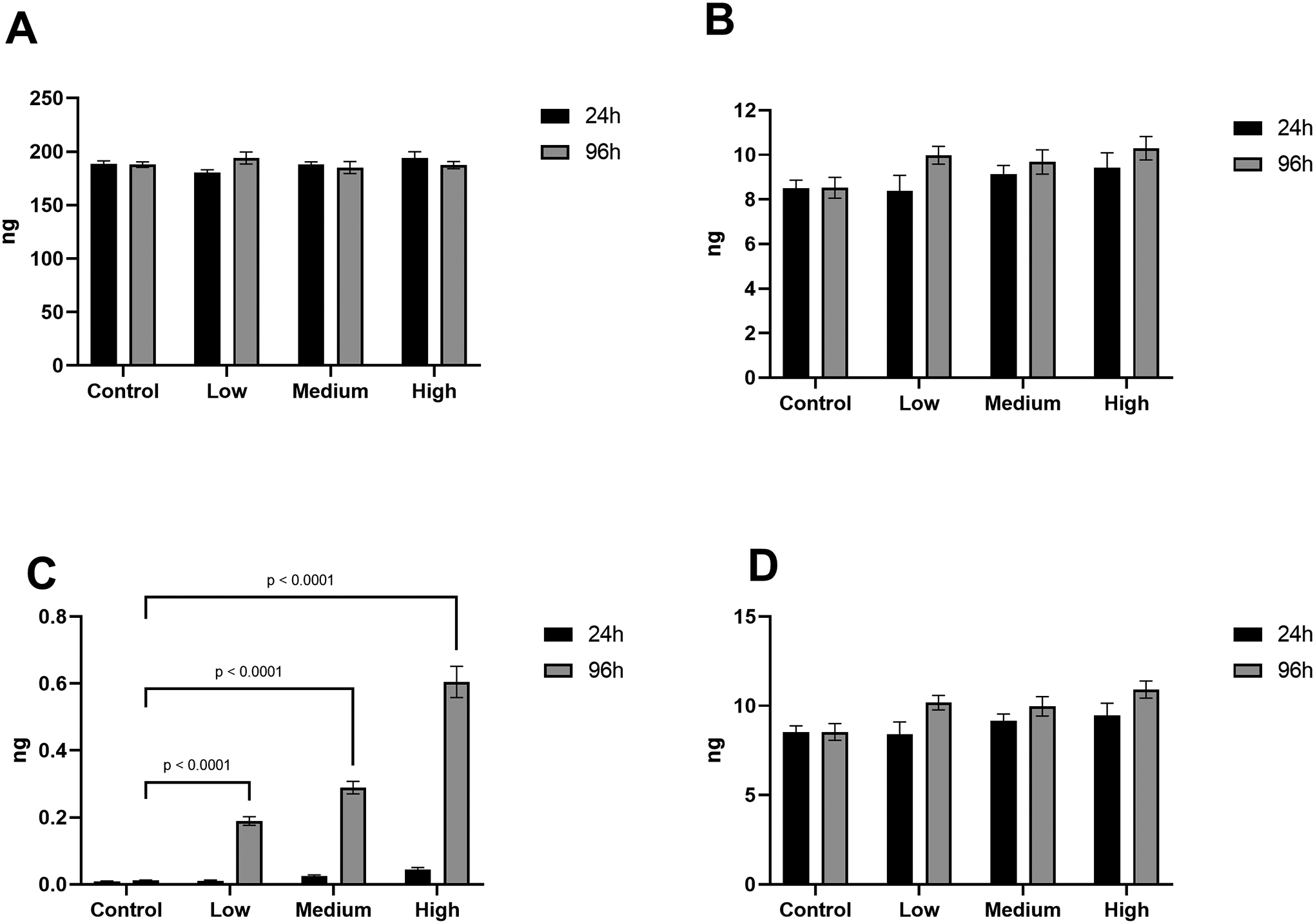
Levels of cytosine (A), 5-methylcytosine (B), deuterated 5-methylcytosine (C), and combined 5-methylcytosine and deuterated 5-methylcytosine (D) as analyzed by LC/MS in DNA of human HepaRG cells after supplementation with MSM; mean +/− SEM (n = 3 per group).
Effects of MSM treatment on DNA methylation of repetitive elements
While only up to 2% of human genomes are comprised of functional genes, REs cover a substantially larger portion, with some studies estimating this number to be over 60% (Miousse et al., 2015). Therefore, we next sought to determine whether or not supplementation of HepaRG cells with MSM would have any effect on DNA methylation of the most abundant and transcriptionally active REs in the human genome – LINE-1 and LTR.
Here, we demonstrate that MSM supplementation does not affect the DNA methylation status of any of the four investigated LINE-1 families (namely, L1P4b, L1MA2, L1PA13, and L1PA2) nor the LTR repeat HERV18 (Figure 4). A nearly significant (p=0.0722) increase in DNA methylation of the L1PA13 LINE-1 element was observed at the 96 h time-point with the MSM concentration of 2.5 mM. These findings suggest that short-term supplementation of MSM does not affect the levels of DNA methylation globally and does not result in redistribution of the DNA methylation patterns within the most abundant REs.
Figure 4.
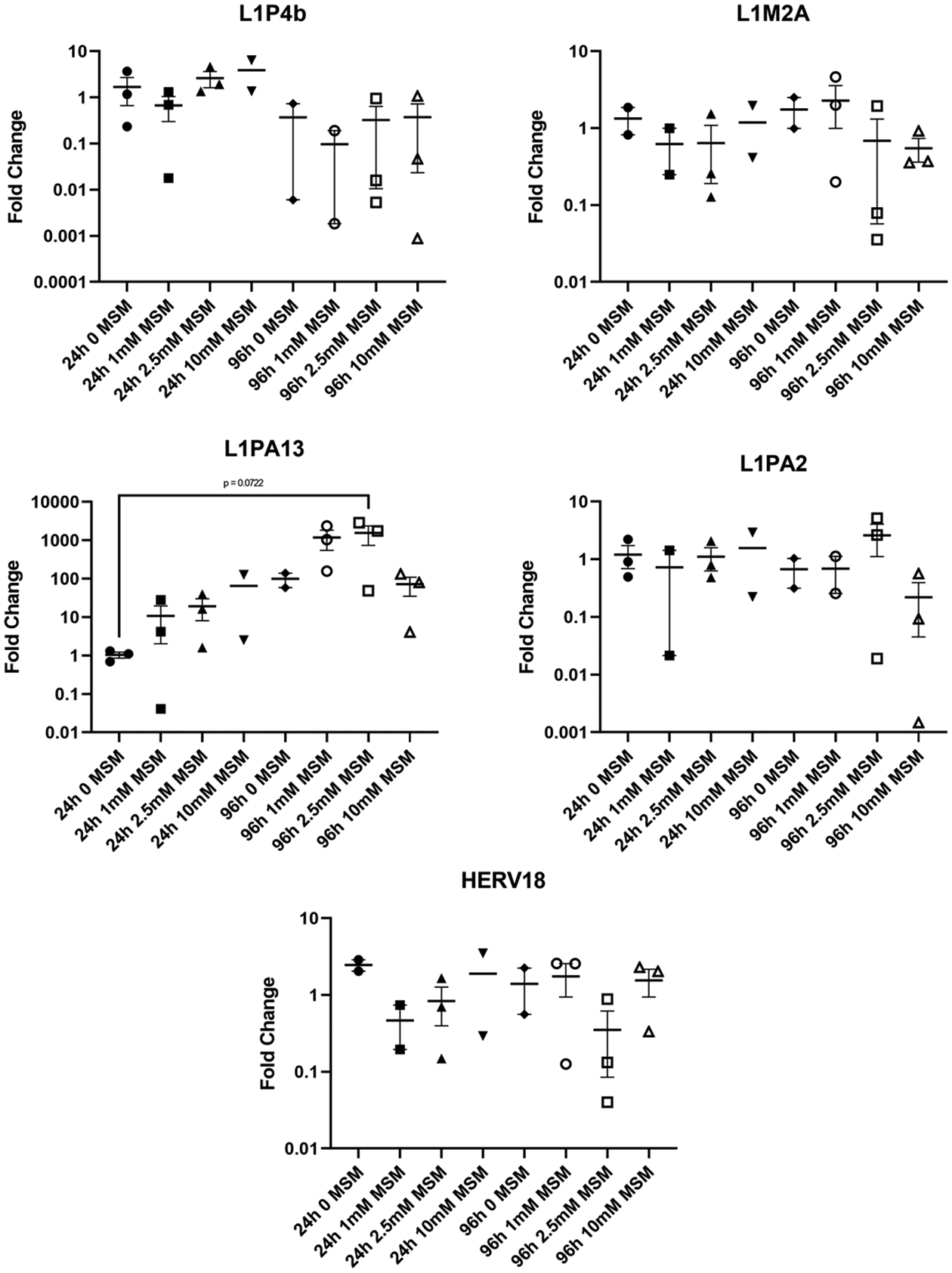
DNA methylation of LINE-1 (L1P4b, L1M2A, L1PA13, and L1PA2) and LTR (HERV18) repetitive elements in human HepaRG cells after supplementation with MSM; mean +/− SEM (n = 3 per group). Data is presented as fold-change from control (0 mM MSM).
Discussion
The aim of this study was to investigate the potential of the natural organosulfur compound MSM to serve as a donor of methyl groups for the purpose of DNA methylation. For this purpose, a culture of fully differentiated, metabolically active human liver cells – HepaRG – was used. These cells hold a number of advantages over other human liver cells historically used in studies. Most importantly, gene expression and morphology in HepaRG cells more closely resemble primary human liver cells and human liver tissue than other, more common liver cell lines like HepG2 (reviewed in Gurley et al., 2022). In addition, use of HepaRG cells eliminates donor variation observed in primary human hepatocytes.
While MSM is generally known to be a safe compound, we know of no data on tolerability of MSM in HepaRG cells. Therefore, first we sought to determine the margins of MSM’s safety in HepaRG cells. Both PI staining and LDH release assays confirmed the results of other studies on hepatocytes’ high tolerance to MSM both in vitro and in vivo (Kutanzi et al., 2020; Kim et al., 2021). Therefore, we next investigated whether or not MSM can provide methyl groups for the purpose of DNA methylation in HepaRG cells. For this purpose, a deuterated MSM product (dimethyl sulfoxide-d6) was used. Subsequently, the deuterium-labeled methyl groups can be then detected as having been incorporated into the DNA of the cell, should the latter be successfully utilized for the process of DNA methylation. Indeed, we observed accumulation of the deuterated 5-methylcytosine in the HepaRG cells DNA as early as 24 h after supplementation. This effect was even more pronounced at the 96 h time-point, which can be explained by the longer exposure of the cells to the labeled product. These findings convincingly demonstrated that MSM can effectively provide methyl groups for intracellular purposes.
While investigation of DNA methylation of individual genes would be daunting and cost-inefficient, analysis of DNA methylation of REs provides an easy and robust assessment of the DNA methylation status. Indeed, the genomic abundance of REs and high frequency of both methylated and unmethylated CpG sites within their sequences allow us to determine the global status of DNA methylation as well as region-specific changes if any are present. For instance, using this approach, we previously demonstrated both DNA hypermethylation (Koturbash et al., 2016; Prior et al., 2016) and DNA hypomethylation (Miousse et al., 2015b; Miousse et al., 2017) as a result of exposure to various environmental stressors and dietary interventions.
Therefore, we then sought to address the methylation status of four LINE-1 elements and one LTR element. These elements belong to retrotransposons – REs known for their ability to propagate through the genome by the “copy-and-paste” mechanism and which are the only retrotransposon elements currently known to be active in humans (Miousse et al., 2015). Undisturbed DNA methylation of these five abundant REs suggests that short-term MSM supplementation, while providing the source for methyl groups for DNA methylation, did not lead to changes in global DNA methylation. Lack of these changes may be explained by the tight control that exists between the synthesis of new methyl groups and the cellular needs for these methyl groups. The cells used in the study are terminally differentiated hepatocytes which are not under an effect of any stressor – the situation when no prerequisite for changes of DNA methylation exists. Another potential explanation is that while being modifiable, DNA methylation itself is quite a stable modification of DNA and, unless challenged by substantial stressors (i.e., treatment with DNA demethylation agents or exposure to high dose of ionizing radiation), 96 hours may be insufficient to detect any significant changes in DNA methylation.
This is an important finding, as the changes in DNA methylation can often lead to development of various pathological states and conditions (Baylin and Jones, 2016). Recently, we have demonstrated that the DNA methylation status of L1PA2 (a LINE-1 element analyzed in our study) was negatively affected by previous environmental exposures and the extent of its DNA methylation was negatively associated with the chromosomal aberrations in peripheral lymphocytes (Ewing et al., 2022). Furthermore, there was no re-distribution of the DNA methylation patterns between the investigated elements, a phenomenon observed when the loss of methylation within certain REs can be compensated by DNA hypermethylation within the other elements that, in total, often result in unaltered global levels of DNA methylation (Prior et al., 2016). We cannot exclude that DNA methylation of other REs could be affected by MSM supplementation. However, given that LINE-1 and LTR elements comprise the vast body of the human REs and that these are the only retrotranspositionally active elements, it would be plausible to consider potential changes in DNA methylation of other REs to be of low-scale and not of high importance.
In conclusion, we demonstrated the ability of MSM, a natural organosulfur compound, to serve as a donor of methyl groups for the purpose of DNA methylation. The used concentrations of MSM were not toxic to metabolically active human hepatocytes and were consistent with MSM concentrations that were detected in human plasma. Furthermore, there were no changes in the global DNA methylation levels – measured as total 5-methylcytosine levels and DNA methylation of individual REs – nor re-distribution of DNA methylation patterns within the investigated REs. Given the high absorbance rates of MSM, this study provides compelling evidence that MSM may serve as a donor of methyl groups to potentially correct or prevent various pathological conditions associated with DNA hypomethylation. Such studies are being pursued in our laboratories and will be reported in subsequent publications.
Acknowledgements
The authors would like to thank Christopher Fettes for his excellent editorial services.
Disclosure of interest
This study, in part, was funded by Bergstrom Nutrition (Vancouver, WA, USA). The funder had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare no other potential conflict of interests.
Funding
This research was funded by Bergstrom Nutrition (Vancouver, WA, USA), Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM109096; Clinical and Translational Science Awards UL1TR000039 and KL2TR000063; and the Arkansas Biosciences Institute.
Biographies
Kirsten Clement is a research associate at the Department of Environmental Health Sciences and the Center for Dietary Supplements Research at the University of Arkansas for Medical Sciences, Fay W. Boozman College of Public Health. Her main interests are devoted to development of in vitro and microphysiological systems for safety and efficacy testing of nutrients and dietary supplements.
Mitchell R. McGill is an Assistant Professor in the Department of Environmental Health Sciences at the University of Arkansas for Medical Sciences, Fay W. Boozman College of Public Health. He is an expert in xenobiotic-induced liver injury with emphasis on both preclinical models and clinical biomarkers. He has authored nearly 100 peer-reviewed publications and a dozen book chapters on the topic of liver injury. He also serves as a drug safety consultant for the pharmaceutical industry and is a practicing board-certified clinical chemist.
Isabelle Racine Miousse is an Assistant Professor in the Department of Biochemistry and Molecular Biology at the University of Arkansas for Medical Sciences. She received her Ph.D. from McGill University in Canada on the metabolism of one-carbon groups. She specializes in epigenetics, with a focus on how environmental and dietary factors linked to cancer affect DNA and histone methylation. Her lab investigates how the supply and metabolism of the methyl donor methionine modulates these responses and alters cancer development.
Sean Young is an Assistant Professor in the Department of Environmental Health Sciences at the University of Arkansas for Medical Sciences, Fay W. Boozman College of Public Health. Sean is a medical geographer, with training in geographic information systems, data science, and data visualization. His interests focus on spatial analysis of human-environment interactions and disease ecology across a wide range of public health topics, including accessibility to cancer screening and treatment, spatio-temporal modeling of emerging infectious diseases, and creating risk indices for prescription opioid misuse, among others.
Stepan Melnyk is a Director of the Core Metabolomics Laboratory at Arkansas Children’s Research Institute. With both an MD and PhD, three decades of his biomedical research career have been dedicated to studying metabolic consequences of oxidative stress, including detection of oxidative damaged biomolecules (3-nitrotyrosine, glutathione), DNA adducts (5-methylcytosine/5-hydroxymethylcytosine, 8-oxo-dG) and universal methylation reaction donor S-Adenosylmethionine (SAM), and development of analytical techniques (HPLC, LC-MS) for those purposes. Stepan has an exceptional list of collaborations on a variety of important biomedical topics (i.e., cancer, autism, congenital cardiac pathology in children, etc.) presented in 120+ peer reviewed journal articles with a high citation index.
Igor Koturbash is an Associate Professor and Chair, Department of Environmental Health Sciences and Co-Director of the Center for Dietary Supplements Research at the University of Arkansas for Medical Sciences, Fay W. Boozman College of Public Health. Igor has a long-lasting interest in diet and dietary supplements and their impact on human health. Therefore, the major focus of his research is safety, efficacy and mechanisms of action of dietary supplements and understanding how diet and dietary supplements can modulate tissue response to cancer therapy. Igor is heavily involved in a number of safety and efficacy studies on various dietary supplements and herbs, including methionine supplementation, green tea extract and cannabidiol (CBD), to name a few. Igor has published 100+ peer-reviewed articles and book chapters and his research has received uninterrupted extramural funding from various sources since the beginning of his independent career.
References
- Amirshahrokhi K, Khalili AR. Methylsulfonylmethane is effective against gastric mucosal injury. Eur J Pharmacol. 2017. Sep 15;811:240–248. doi: 10.1016/j.ejphar.2017.06.034. Epub 2017 Jun 28. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Jones PA. Epigenetic Determinants of Cancer. Cold Spring Harb Perspect Biol. 2016. Sep 1;8(9):a019505. doi: 10.1101/cshperspect.a019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer RJ, Butawan M, Lin L, Ma D, Yates CR. Blood MSM Concentrations Following Escalating Dosages Of Oral MSM In Men And Women. J Nutr Food Sci. 2019, 9:1. [Google Scholar]
- Borzellca JF, Sipes IG, Wallace KB. Dossier in Support of the Generally Recognized as Safe (GRAS) Status of Optimsm (Methylsulfonylmethane; MSM) as a Food Ingredient. Food and Drug Administration; Vero Beach, FL, USA: 2007. [Google Scholar]
- Butawan M, Benjamin RL, Bloomer RJ. Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement. Nutrients. 2017. Mar 16;9(3):290. doi: 10.3390/nu9030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbi EM, Agar G, Fichman G, Ziv YB, Kardosh R, Halperin N, Elbaz A, Beer Y, Debi R. Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: a randomized controlled study. BMC Complement Altern Med. 2011. Jun 27;11:50. doi: 10.1186/1472-6882-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing LE, Skinner CM, Pathak R, Yee EU, Krager K, Gurley PC, Melnyk S, Boerma M, Hauer-Jensen M, Koturbash I. Dietary Methionine Supplementation Exacerbates Gastrointestinal Toxicity in a Mouse Model of Abdominal Irradiation. Int J Radiat Oncol Biol Phys. 2021. Feb 1;109(2):581–593. doi: 10.1016/j.ijrobp.2020.09.042. Epub 2020 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing LE, Pathak R, Landes RD, Skinner CM, Binz R, Young SG, Riklon S, Stahr S, Su J, Boerma M, McElfish PA, Hauer-Jensen M, Koturbash I. Cytogenetic and epigenetic aberrations in peripheral lymphocytes of northwest Arkansas Marshallese. Int J Radiat Biol. 2022. Aug 15:1–12. doi: 10.1080/09553002.2022.2110319. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, Olivieri O, Jacques PF, Rosenberg IH, Corrocher R, Selhub J. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002. Apr 16;99(8):5606–11. doi: 10.1073/pnas.062066299. Epub 2002 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhards E, Gibian H. The metabolism of dimethyl sulfoxide and its metabolic effects in man and animals. Ann N Y Acad Sci. 1967. Mar 15;141(1):65–76. doi: 10.1111/j.1749-6632.1967.tb34867.x. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, McGill MR, Koturbash I. Hepatotoxicity due to herbal dietary supplements: Past, present and the future. Food Chem Toxicol. 2022. Sep 29:113445. doi: 10.1016/j.fct.2022.113445. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herceg Z, Ghantous A, Wild CP, Sklias A, Casati L, Duthie SJ, Fry R, Issa JP, Kellermayer R, Koturbash I, Kondo Y, Lepeule J, Lima SCS, Marsit CJ, Rakyan V, Saffery R, Taylor JA, Teschendorff AE, Ushijima T, Vineis P, Walker CL, Waterland RA, Wiemels J, Ambatipudi S, Degli Esposti D, Hernandez-Vargas H. Roadmap for investigating epigenome deregulation and environmental origins of cancer. Int J Cancer. 2018. Mar 1;142(5):874–882. doi: 10.1002/ijc.31014. Epub 2017 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschler RJ Dietary and Pharmaceutical Uses of Methyl-Sulfonylmethane and Compositions Comprising it. 4514421. U.S. Patent. 1982.
- Herschler RJ Solid Pharmaceutical Compositions Comprising MSM and their Production. 4568547. U.S. Patent. 1984
- Herschler RJ Methylsulfonylmethane in Dietary Products. 4616039. U.S. Patent. 1985.
- Herschler RJ Dietary Products and Uses Comprising Methylsulfonylmethane. 4863748. U.S. Patent. 1986.
- James SJ, Melnyk S, Jernigan S, Hubanks A, Rose S, Gaylor DW. Abnormal transmethylation/transsulfuration metabolism and DNA hypomethylation among parents of children with autism. J Autism Dev Disord. 2008. Nov;38(10):1966–75. doi: 10.1007/s10803-008-0591-5. Epub 2008 May 30. Erratum in: J Autism Dev Disord. 2008 Nov;38(10):1976. Jill James, S [corrected to James, S Jill]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012. May 29;13(7):484–92. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Joung YH, Darvin P, Kang DY, Sp N, Byun HJ, Lee CH, Lee HK, Yang YM. Methylsulfonylmethane Inhibits RANKL-Induced Osteoclastogenesis in BMMs by Suppressing NF-κB and STAT3 Activities. PLoS One. 2016. Jul 22;11(7):e0159891. doi: 10.1371/journal.pone.0159891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel R, El Morsy EM. Hepatoprotective effect of methylsulfonylmethane against carbon tetrachloride-induced acute liver injury in rats. Arch Pharm Res. 2013. Sep;36(9):1140–8. doi: 10.1007/s12272-013-0110-x. Epub 2013 Apr 17. [DOI] [PubMed] [Google Scholar]
- Kim LS, Axelrod LJ, Howard P, Buratovich N, Waters RF. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: a pilot clinical trial. Osteoarthritis Cartilage. 2006. Mar;14(3):286–94. doi: 10.1016/j.joca.2005.10.003. Epub 2005 Nov 23. [DOI] [PubMed] [Google Scholar]
- Kim SH, Smith AJ, Tan J, Shytle RD, Giunta B. MSM ameliorates HIV-1 Tat induced neuronal oxidative stress via rebalance of the glutathione cycle. Am J Transl Res. 2015. Feb 15;7(2):328–38. [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Park JW, Yang YM, Song KD, Cho BW. Effect of methylsulfonylmethane on oxidative stress and CYP3A93 expression in fetal horse liver cells. Anim Biosci. 2021. Feb;34(2):312–319. doi: 10.5713/ajas.20.0061. Epub 2020 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koturbash I, Miousse IR, Sridharan V, Nzabarushimana E, Skinner CM, Melnyk SB, Pavliv O, Hauer-Jensen M, Nelson GA, Boerma M. Radiation-induced changes in DNA methylation of repetitive elements in the mouse heart. Mutat Res. 2016. May;787:43–53. doi: 10.1016/j.mrfmmm.2016.02.009. Epub 2016 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutanzi KR, Ewing LE, Skinner CM, Quick CM, Kennon-McGill S, McGill MR, Walker LA, ElSohly MA, Gurley BJ, Koturbash I. Safety and Molecular-Toxicological Implications of Cannabidiol-Rich Cannabis Extract and Methylsulfonylmethane Co-Administration. Int J Mol Sci. 2020. Oct 21;21(20):7808. doi: 10.3390/ijms21207808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HK, King HW, Patient RK, Odom DT, Klose RJ. Protection of CpG islands from DNA methylation is DNA-encoded and evolutionarily conserved. Nucleic Acids Res. 2016. Aug 19;44(14):6693–706. doi: 10.1093/nar/gkw258. Epub 2016 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson BA, Appleton J, Ames GB. Pharmacokinetics and distribution of [35S]methylsulfonylmethane following oral administration to rats. J Agric Food Chem. 2007. Feb 7;55(3):1033–8. doi: 10.1021/jf0621469. [DOI] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011. Mar;53(3):974–82. doi: 10.1002/hep.24132. Epub 2011 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miousse IR, Chalbot MC, Lumen A, Ferguson A, Kavouras IG, Koturbash I. Response of transposable elements to environmental stressors. Mutat Res Rev Mutat Res. 2015a. Jul–Sep;765:19–39. doi: 10.1016/j.mrrev.2015.05.003. Epub 2015 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miousse IR, Chalbot MC, Pathak R, Lu X, Nzabarushimana E, Krager K, Aykin-Burns N, Hauer-Jensen M, Demokritou P, Kavouras IG, Koturbash I. In Vitro Toxicity and Epigenotoxicity of Different Types of Ambient Particulate Matter. Toxicol Sci. 2015b. Dec;148(2):473–87. doi: 10.1093/toxsci/kfv200. Epub 2015 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miousse IR, Kutanzi KR, Koturbash I. Effects of ionizing radiation on DNA methylation: from experimental biology to clinical applications. Int J Radiat Biol. 2017a. May;93(5):457–469. doi: 10.1080/09553002.2017.1287454. Epub 2017 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miousse IR, Pathak R, Garg S, Skinner CM, Melnyk S, Pavliv O, Hendrickson H, Landes RD, Lumen A, Tackett AJ, Deutz NEP, Hauer-Jensen M, Koturbash I. Short-term dietary methionine supplementation affects one-carbon metabolism and DNA methylation in the mouse gut and leads to altered microbiome profiles, barrier function, gene expression and histomorphology. Genes Nutr. 2017b. Sep 6;12:22. doi: 10.1186/s12263-017-0576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior S, Miousse IR, Nzabarushimana E, Pathak R, Skinner C, Kutanzi KR, Allen AR, Raber J, Tackett AJ, Hauer-Jensen M, Nelson GA, Koturbash I. Densely ionizing radiation affects DNA methylation of selective LINE-1 elements. Environ Res. 2016. Oct;150:470–481. doi: 10.1016/j.envres.2016.06.043. Epub 2016 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhpour M, Maleki M, Ebrahimi Vargoorani M, Amiri V. A review of epigenetic changes in asthma: methylation and acetylation. Clin Epigenetics. 2021. Mar 29;13(1):65. doi: 10.1186/s13148-021-01049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Lima I, Park SY, Chung M, Jung HJ, Kang MC, Gaspar JM, Seo JA, Macedo MP, Park KS, Mantzoros C, Lee SH, Kim YB. Methylsulfonylmethane (MSM), an organosulfur compound, is effective against obesity-induced metabolic disorders in mice. Metabolism. 2016. Oct;65(10):1508–21. doi: 10.1016/j.metabol.2016.07.007. Epub 2016 Jul 20. [DOI] [PubMed] [Google Scholar]
- Wong T, Bloomer RJ, Benjamin RL, Buddington RK. Small Intestinal Absorption of Methylsulfonylmethane (MSM) and Accumulation of the Sulfur Moiety in Selected Tissues of Mice. Nutrients. 2017. Dec 25;10(1):19. doi: 10.3390/nu10010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Liang G, Molloy PL, Jones PA. DNA methylation enables transposable element-driven genome expansion. Proc Natl Acad Sci U S A. 2020. Aug 11;117(32):19359–19366. doi: 10.1073/pnas.1921719117. Epub 2020 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]


