Abstract
Atypical anti-glomerular basement membrane (anti-GBM) disease is characterized by linear immunoglobulin G (IgG) deposition along the GBM without circulating IgG anti-GBM antibodies. Compared to classic anti-GBM disease, atypical anti-GBM disease tends to be milder with a more indolent course in certain cases. Moreover, pathologic disease pattern is much more heterogenous in atypical anti-GBM disease than in the classic type, which is uniformly characterized by diffuse crescentic and necrotizing glomerulonephritis. Although there is no single well-established target antigen in atypical anti-GBM disease, the target antigen (within the GBM) and the autoantibody type are hypothesized to be different from the classic type. Some patients have the same antigen as the Goodpasture antigen that are detected only by a highly sensitive technique (biosensor analysis). Some cases of atypical anti-GBM disease have autoantibodies of a different subclass restriction like IgG4, or of monoclonal nature. Antibodies targeting antigen/epitope structure other than the Goodpasture antigen can be detected using modified assays in some cases. Patients with IgA- and IgM-mediated anti-GBM disease are known to have negative circulating antibodies because conventional assays do not detect these classes of antibodies. A significant proportion of cases with atypical anti-GBM disease do not have any identifiable antibodies despite extensive evaluation. Nevertheless, extensive evaluation of atypical autoantibodies using modified assays and sensitive techniques should be attempted, if feasible. This review summarizes the recent literature on atypical anti-GBM disease.
Keywords: anti-GBM disease, atypical, IgA-mediated, IgG4-mediated, linear IgG, monotypic anti-GBM
Anti-GBM disease is an autoimmune disorder with the target antigen present within specific basement membranes, such as GBM and alveolar basement membrane. Anti-GBM disease is rare with an incidence of about 1 per million population per year.1 However, it accounts for approximately 15% of various causes of crescentic glomerulonephritides, and prompt treatment has significantly improved survival in the recent past.1 Presentation is abrupt and severe, with approximately 90% of patients having rapidly progressive glomerulonephritis, with or without lung hemorrhage. Strong linear Ig G deposition along the GBM on direct immunofluorescence (IF) is the pathologic hallmark and basis for the diagnosis of anti-GBM disease. Histologically, it typically shows diffuse crescents and fibrinoid necrosis. Patients with 100% crescents on kidney biopsy who require dialysis and show kidney failure at presentation have a dismal kidney survival rate of 8% at 1 year of follow-up.2 This makes anti-GBM disease one of the most aggressive forms of glomerulonephritides. Serum anti-GBM antibodies are detected in approximately 90% of patients. Anti-GBM disease is observed to be abrupt in onset and recurrence is uncommon. Circulating anti-GBM antibodies are pathogenic3 and directly associated with disease severity. Rare cases of disease recurrence, before and after a kidney transplant, that correlated with circulating anti-GBM antibodies, have been reported.4,5 However, reports describing severe relapse of the disease without the appearance of circulating antibodies in patients with previous positive serology are also known.6 In the last decade, isolated case reports and series7,8 describing similar IF findings as classic anti-GBM disease on kidneys but without other typical features such as aggressive clinical course and/or diffuse crescentic glomerulonephritis and/or circulating anti-GBM antibodies (tested by conventional assays) have increasingly emerged and are termed “atypical anti-GBM disease”9 (Table 1). A subset of patients with atypical anti-GBM disease have linear monotypic Ig deposits on IF without other features of monoclonal gammopathy of renal significance. This review compiles the recent literature on atypical anti-GBM disease to understand the disease pattern and guide patient management.
Table 1.
Comparison of reported literature on polytypic atypical anti-GBM disease in the last decade (2012–2022)
| Author/yr | N | Age (yr) | Peak serum creatinine (mg/dl) | Presenting symptoms/syndrome | Light microscopy | Class/subclass of Ig on immunofluorescence | Serology | Immunosuppressive treatment | Outcome-kidney |
|---|---|---|---|---|---|---|---|---|---|
| Troxell and Houghton9 | 4 | 23–64 | 0.75–7.1 | Macrohematuria: 1 Fever: 1 Hemoptysis: 1 Constitutional: 1 |
Mesangial sclerosis: 1 Focal crescents: 2 Diffuse crescents: 1 IFTA: 4 Interstitial nephritis: 2 |
IgG dominant, subclass available in 1 showed IgG1 dominance | Negative: 1 Weakly positive: 1 Positive: 2 |
PLEX/CYC/steroids: 2 PLEX: 1 Steroids/Methotrexate: 1 |
Improved serum creatinine: 2 Normalized serum creatinine: 1 ESKD: 1 |
| Nasr et al.7 | 10 | 18–85 | 2.4 (median) | Nephrotic syndrome: 5 (50%) Macrohematuria: 30% |
MPGN: 3, MesPGN: 2, EPGN: 4, FSGS: 1, Focal crescent: 4, Diffuse crescent: 0, IFTA: 8, TMA: 6 | IgG1 dominant:2 IgG4 dominant:3 IgG1/IgG4 codominant:1 |
Negative in 9/10 tested | Steroids /CYC:2 Steroids/MMF: 3 Steroids/CYC/PLEX: 1 |
1-year kidney survival: 67% |
| Fernandes et al.12 | 1 | 27 | 5.13 | Hemoptysis, dyspnea | Diffuse necrotizing with focal crescents | IgG dominant (subclass NA) | Negative | Steroids/CYC/PLEX | Improved serum creatinine |
| AlSowailmi et al.13 | 1 | 27 | 3.13 | AKI, nephrotic-range proteinuria | MPGN with focal crescents | IgG dominant (subclass NA) | Weakly positive | Steroids/CYC/PLEX | Improved serum creatinine |
| Liang et al.10 | 19 | 15–61 | 1.8 | Nephrotic syndrome: 37% AKI:63%, Lung involvement: 16% |
MesPGN: 6, MPGN: 2, EPGN: 2, FSGS: 3, Focal crescents: 11, Diffuse crescents: 4, IFTA: 84% | IgG4 dominant: 7 IgG1 dominant: 6 IgG4 and IgG1 codominant: 2 NA: 2 |
Negative | Steroids: 3 Steroids/CYC: 3 Steroids/MMF: 2 Steroids/MMF/tacrolimus: 1 Steroids/Thalidomide: 1 |
ESKD: 32% |
| Singhal et al.14 | 1 | 37 | 0.76 | Hemoptysis and dyspnea | Focal fibrinoid necrosis, mild IFTA | IgG4 dominant | Weakly positive | Steroids/PLEX | Chest symptoms normalized |
| Sporinova et al.15 | 1 | 24 | 12.04 | AKI, nephrotic-range proteinuria, hemoptysis and dyspnea | Diffuse crescentic and necrotizing MPGN | IgG4 dominant | Negative | Steroids/CYC/PLEX/RTX | ESKD (chest symptoms improved) |
| Adapa et al.16 | 1 | 46 | 10.8 | Episodic macrohematuria, AKI | Diffuse heterogenous crescents and fibrinoid necrosis | IgG4 dominant | Negative | Steroids/CYC | ESKD |
| Shen et al.8 | 60 | 51.7 15.6 (mean) | 1.61 | AKI: 45%, nephrotic syndrome: 31.7%, hemoptysis: 5%, Macrohematuria: 6.7% | Focal crescents: 20 Diffuse crescents: 5 MPGN: 6, MN: 8, IgAN:12, AAV: 4, FSGS: 3, TMA: 1, TBMN: 1 IFTA: 96.7%, interstitial nephritis: 91.7% |
IgG1: 45.8% IgG2: 35.6% IgG4: 18.6% IgG3: 11.9% |
Negative | Steroids: 53.3% Cytotoxic drugs: 30% PLEX: 6.7% |
1-year kidney survival: 83.3% |
| Zhong et al.17 | 1 | 38 | 1.01 | Occasional hemoptysis, microhematuria, nephrotic-range proteinuria | Cellular crescents and segmental necrosis of glomeruli | IgG (subclass NA) | Negative | Steroids/CYC/PLEX/tacrolimus | Proteinuria reduced, chest symptoms normalized |
| Elshirbeny et al.18 | 1 | 37 | 2.4 | RPGN, nephrotic-range proteinuria, macrohematuria | Diffuse crescentic MPGN with mild IFTA | IgG dominant (subclass NA) | Negative | PLEX/CYC/steroids | Improved serum creatinine |
| Jamboti et al.19 | 1 | 30 | 0.7 | Microscopic hematuria, hemoptysis with dyspnea | Normal | IgG dominant (subclass NA) | Negative | RTX/steroids/PLEX | Normal (+no chest symptoms) |
| Ramesh et al.20 | 1 | 28 | 1.3, 8.3 (during relapse) | Edema, Nephrotic-range proteinuria; RPGN and hemoptysis during relapse | Diffuse crescentic | IgG (subclass IgG4 was negative) | Weakly positive, negative (during relapse) | Steroids/CYC/PLEX | Improved serum creatinine (hemoptysis resolved) |
| Kyriazis et al.11 | 1 | 58 | 5.8 | AKI after immune checkpoint inhibitor (nivolumab) | Focal crescentic and proliferative | IgG dominant (subclass NA) | Negative | Steroids/CYC | ESKD |
| Guo et al.21 | 1 | 38 | 4.35 | RPGN | Diffuse crescentic, IFTA: 30% | IgG dominant | Negative | Steroids/PLEX | Improved serum creatinine |
| Tamura et al.22 | 1 | 43 | 1.44 | Episodic macrohematuria | Focal crescents, endocapillary hypercellularity | IgG1 dominant | Negative by ELISA, positive by IIF | Steroids/CYC/PLEX | Normalized serum creatinine |
| Javaugue et al.23 | 1 | 73 | 4 | AKI, nephrotic-range proteinuria after immune checkpoint inhibitor therapy (pembrolizumab) | Focal crescents, fibrinoid necrosis with endocapillary hypercellularity, moderate IFTA | IgG2 dominant | Negative | Steroids/PLEX/RTX | ESKD |
AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; AKI, acute kidney injury; CYC, cyclophosphamide; EPGN, endocapillary proliferative glomerulonephritis; ESKD, end-stage kidney disease; FSGS, focal segmental glomerulosclerosis; IFTA, interstitial fibrosis tubular atrophy; IgAN, IgA nephropathy; IIF, indirect immunofluorescence; MesPGN, mesangial proliferative glomerulonephritis; MMF, mycophenolate mofetil; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; PLEX, plasma exchange; RPGN, rapidly progressive glomerulonephritis; RTX, rituximab; TBMN, thin basement membrane nephropathy; TMA, thrombotic microangiopathy.
Pathogenesis of Atypical Anti-GBM Disease
Atypical anti-GBM disease comprises 8% to 12% of all anti-GBM disease cases.7,9 As seen in the classic type, atypical anti-GBM disease has been described mostly in Asians and the white population, with a slight predominance in men.7, 8, 9, 10 Although a significant number of patients can have a heterogenous clinical-pathologic presentation, atypical anti-GBM disease is usually a milder and slower disease with less aggressive pathologic features setting it apart from the aggressive pattern of classic anti-GBM disease. Target antigens and autoantibodies seem to differ from those in classic anti-GBM disease in most cases.
The antigen in classic anti-GBM disease, also called Goodpasture antigen, comprises cryptic epitopes of the noncollagenous 1 (NC1) domain of the α3 subunit of type IV collagen.24 The cryptic epitopes, Ea and Eb, are critical amino acid residues (17–31 and 127–141, respectively) of the α3NC1 monomers. Dissociation of the alpha3 monomer from the hexamer structure results in exposure of cryptic epitopes to host immune system.25 Although genetic association and environmental triggers26 are known to be associated with classic anti-GBM disease, such associations are not yet studied systematically in patients with atypical anti-GBM disease.
Several methods for testing circulating anti-GBM antibodies include indirect immunofluorescence assay, enzyme-linked immunosorbent assay (ELISA), chemiluminescence, radioimmunoassay, multiplex bead test, western blot, and biosensor system. The sensitivity and specificity of commercial immunoassays, which measure the Goodpasture antigen, are 95% to 100% and 91% to 100%, respectively.27 ELISAs are more sensitive than immunofluorescence assay.28,29 Western blots use native GBM containing the NC1 domain of all alpha chains of type IV collagen, unlike ELISA, which uses denatured recombinant antigens comprised the NC1 domain of the α3 chain. The high diagnostic accuracy of commercial assays to detect serum anti-GBM antibodies could be limited to classic anti-GBM disease because most patients in the studies had acute nephritic syndrome and/or lung hemorrhage, preventing generalizability.30 About 2% to 8% of patients with anti-GBM disease have negative serology on rigorous testing. Nasr et al.7 found undetectable circulating anti-GBM antibodies on ELISA, indirect immunofluorescence, and western blot in all patients with atypical anti-GBM disease. Following changes in the antigen or autoantibody composition could result in a negative serology in atypical anti-GBM disease.
Composition of Epitope/Antigen
Conformational epitopes on the alpha3NC1 domain of type IV collagen, other than Ea and Eb, were detected using nonreducing western blot analysis in anti-GBM disease patients with negative serology on ELISA.31 Antibodies against the NC1 domain of other alpha chains like alpha1, alpha4, or alpha5 chains of type IV collagen or to the NC1 domain of alpha345 hexamers32 can cause rare cases of anti-GBM disease. Linear epitopes of the collagenous domain of type IV collagen or antigens other than type IV collagen, such as entactin, also cause anti-GBM disease. Could specific triggers be factors behind the change in the target antigen/epitope within the GBM? For example, immune checkpoint inhibitors ipilimumab and nivolumab are reported to be associated with atypical anti-GBM disease24 characterized by negative circulating anti-GBM antibodies. What makes this entity atypical, unlike the anti-GBM disease developing after anti-CD52 monoclonal antibody (alemtuzumab) use, is not yet known.33 Modifying the existing assays to detect epitopes and antigens beyond the Goodpasture antigen can aid the diagnosis in many such cases of atypical anti-GBM disease.
Affinity of Antibody
Serum anti-GBM antibodies are shown to correlate with clinical and pathologic severity of anti-GBM disease.1,34,35 However, some patients have high-affinity autoantibodies trapped in the kidneys and present with low titer in circulation. This low titer is not detectable in conventional assays, thereby giving false-negative results.36,37 High-affinity antibodies bind to the GBM strongly and dissociate slowly. Therefore, despite negative circulating autoantibodies, such patients have persistent kidney injury. Autoantibodies could also have a low affinity for the substrate in the assay. A biosensor analysis system or antigen-inhibition ELISAs can detect the affinity-binding characteristics of such antibodies, which are missed on conventional commercial assays.38,39
Type of Antibody
Nonspecific polyclonal antibodies in the plasma could alter the detection of anti-GBM antibodies.40 Polytypic antibodies comprising more than 1 subclass of IgG are the norm in classic anti-GBM disease. IgG1 is the most dominant IgG subclass in classic anti-GBM disease, with an equal preponderance of IgG341 and IgG4.42, 43, 44 Although IgG1 and IgG4 were the most common IgG subclasses in atypical anti-GBM disease, dominance of one IgG subclass is common. In addition, IgG2 is reported frequently in atypical anti-GBM disease7, unlike classic anti-GBM disease. Both IgG2 and IgG4 are weak activators of the complement system and anti-GBM antibodies found in normal human serum also belong to IgG2/IgG4 subclass.45 Cases of atypical anti-GBM disease presenting as nephrotic syndrome and having focal segmental glomerulosclerosis (FSGS) on kidney biopsy had IgG2 and IgG4 subclasses, likely explaining their less aggressive course. These cases did not have any other clinico-pathologic characteristics of anti-GBM disease. Given that Ig classes other than IgG are not detected on commercial assays, serology is negative in IgA-mediated and IgM-mediated anti-GBM disease despite having clinical features like that of classic anti-GBM. Moreover, for unknown reasons, some atypical anti-GBM diseases with monotypic IgG deposits do not have detectable circulating antibodies.
Pathology
Deposition of autoantibodies along the GBM leads to complement activation and inflammation followed by rupture of the GBM and fibrinoid necrosis in the endocapillary tuft. Leakage of proinflammatory plasma into the Bowman’s space causes parietal epithelial cell activation and crescent formation, typically diffuse involving >50% of the glomeruli.46 Diffuse crescentic and necrotizing glomerulonephritis with a normal appearance of glomeruli without crescents is the prototypical histopathology in classic anti-GBM disease. Bright (intensity of 2+, on a scale of 0–3+) linear Ig deposition along the GBM on IF and the absence of corresponding electron-dense deposits along the GBM on electron microscopy, are the hallmarks of the classic anti-GBM disease, except in cases of advanced glomerular injury where GBM structure is altered because of several breaks. Atypical anti-GBM disease is characterized by a histopathology picture comprising the hallmarks of classic anti-GBM disease, that is, bright linear Ig deposits along the GBM and absence of electron-dense deposits, but without the typical light microscopy findings of diffuse crescentic and necrotizing glomerulonephritis. Segmental linear staining of tubular basement membrane in atypical anti-GBM disease is often appreciated, similar to classic anti-GBM disease. The light microscopy appearance of atypical anti-GBM is heterogenous, unlike classic anti-GBM disease. Nasr et al.7 noted hypercellularity in the mesangium or endocapillary areas in all biopsies of atypical anti-GBM disease. Endocapillary proliferative glomerulonephritis (GN), mesangial proliferative GN, and membranoproliferative GN patterns were noted in 45%, 30%, and 15% of patients, respectively, of the 20 cases described.7 Similarly, Liang et al.10 noted mesangial proliferative GN in 63%, endocapillary proliferative GN in 10.5%, and membranoproliferative GN in 10.5% of patients, respectively. Whereas focal crescents and/or fibrinoid necrosis were noted in 40% of the cases in the series by Nasr et al.,7 none of them had diffuse crescentic necrotizing GN. Patients with nephrotic syndrome had FSGS concurring with mesangial hypercellularity and global foot process effacement on electron microscopy. Further, nodular glomerulosclerosis with occasional focal crescents and strong linear IgG along the GBM has been reported in 3 patients with history of heavy smoking.47 Tubular atrophy and interstitial fibrosis were noted in almost all (84%, 97%, and 100%),7,8,10 and chronic arterial/arteriolar injury was noted in most (75% and 98.3%) patients with atypical anti-GBM disease.7,8 Interstitial inflammation was frequent too (65% and 91.7%). Global glomerulosclerosis was reported in approximately 80% of patients with atypical anti-GBM disease in a retrospective study,10 reflecting a more chronic process than the monophasic and transitory nature of classic anti-GBM disease. In addition, glomerular thrombotic microangiopathy, characterized by subendothelial zone widening by electron-lucent material on electron microscopy, was noted in 40% of cases.7 However, none of these patients had glomerular or vascular thrombi. There were no systemic findings of microangiopathic hemolytic anemia or thrombocytopenia. Complement C3 staining was present (trace or 1+) in 55%7 and 65%8 of cases, respectively, reflecting complement system activation by the autoantibodies in approximately half of the patients, unlike its universal presence in classic anti-GBM disease. Patients with crescents had complement C3 staining universally, emphasizing the pathogenic role of complement activation in more severe atypical anti-GBM disease.8 Electron-dense deposits corresponding to Ig deposition on IF were universally absent, similar to classic anti-GBM disease.7 More than half (58.3%) of patients in the study from China had concurrent glomerular diseases like IgA nephropathy, FSGS, membranous nephropathy, thrombotic microangiopathy, anti-neutrophil cytoplasmic antibody-associated vasculitis, which likely explains the high prevalence (55.9%) of electron-dense deposits in respective locations in this study.8 Taken together, the subtle clinical features and heterogenous pathologic findings different from that of classic anti-GBM disease, along with the absence of an animal disease model, make one doubt if the linear IgG deposits are the reason behind the glomerular alterations in atypical anti-GBM disease. And does this entity truly belong to the spectrum of anti-GBM disease?48 Further, acquired T-cell sensitivity to the alpha 3 chain of type IV collagen, in the absence of anti-GBM antibodies, has been shown to cause crescentic GN in animals with presumed anti-GBM nephritis.49 This finding, if confirmed, could also be used to argue against the pathogenic role of linear Ig deposits in atypical anti-GBM disease. We believe that an added statement describing important pathologic findings associated with the linear IgG deposits, rather than only stating atypical anti-GBM disease, would best serve to identify this entity until new evidence emerges on the direct role of these deposits in the pathogenesis.
Clinical Course
Classic anti-GBM disease presents abruptly with severe kidney with or without lung involvement. Nephritic syndrome and rapidly progressive glomerulonephritis are typical clinical manifestations of classic anti-GBM disease, characterized by a rapid clinical deterioration of the glomerular filtration rate. Patients present with rapid onset of oliguria or anuria followed by volume overload, often with hemoptysis. Atypical anti-GBM disease is a milder disease with an indolent course. Although kidney dysfunction was common (53%–75%), the median serum creatinine at presentation was mildly elevated (1.61–1.9 mg/dl)7,8,10 in patients with atypical anti-GBM disease. Moreover, severe kidney dysfunction requiring dialysis was infrequent (0%, 8.3%),7,8 unlike >two-thirds of patients with classic anti-GBM disease requiring dialysis at presentation.48 The prevalence of microhematuria was similar in both atypical (63.3%–95%)7,8,10 and classic anti-GBM disease,50 macroscopic hematuria occurred in a variable number (6.7%–20%) of atypical anti-GBM disease cases. Median proteinuria was higher than that in classic anti-GBM disease, with a more frequent prevalence of nephrotic-range proteinuria (42%–53%), likely implying a more chronic process causing glomerulosclerosis and more severe podocyte injury without rapidly shutting down glomerular filtration completely.7,8,10 Nephrotic syndrome was reported in approximately one-third of such patients, and all of them had FSGS as the dominant light microscopy finding.7,8,10 Hypertension was reported in 68% to 75% of the patients with atypical anti-GBM disease,7,10 unlike the scanty prevalence of hypertension in the classic disease. Despite a high prevalence of positive smoking history (53%–58%), lung hemorrhage was either absent7 or present in a few cases (5%–16%),8,10 unlike classic anti-GBM disease having lung involvement in 40% to 60% of patients.1 The reason behind the low prevalence of lung hemorrhage in atypical anti-GBM disease is not understood. In patients with atypical anti-GBM disease who had lung hemorrhage, kidney biopsy showed crescents, and kidney outcome was poor.8 Rarely, atypical anti-GBM disease having pulmonary manifestation and linear IgG staining of GBM without any kidney manifestation is also observed.44 Similarly, cases with negative serology but severe kidney injury are reported because of atypical anti-GBM disease.39 Therefore, although most atypical anti-GBM disease cases have milder diseases, severe kidney dysfunction and lung hemorrhage are also known in some patients. The absence of serum anti-GBM antibodies can delay diagnosis and lead to poor outcomes if a kidney biopsy is not sought early. An algorithm showing the approach to management of suspected atypical anti-GBM disease is shown in Figure 1.
Figure 1.
Algorithm showing the approach to management of suspected atypical anti-GBM disease. FSGS, focal segmental glomerulosclerosis; GBM, glomerular basement membrane; GN, glomerulonephritis; MIDD, monoclonal immunoglobulin deposition disease; MPGN, membranoproliferative glomerulonephritis; TBM, tubular basement membrane.
Treatment Options
Treatment of atypical anti-GBM disease has been heterogenous, including nonimmunosuppressive conservative therapies like renin-angiotensin-aldosterone blockers monotherapy (43.3%), immunosuppressive therapy like steroids, cyclophosphamide, mycophenolate mofetil, tacrolimus, rituximab (50%–70%), and rarely plasmapheresis (6.7%–10%).7,8 About one-quarter of patients progressed to end-stage kidney disease during a median follow-up of 15 to 35.7 months.7,8,10 The 1-year kidney survival (83.3%–85%) and patient survival (93%–95%),7,8 respectively, were better than classic anti-GBM disease (<10%–25%). Although short-term kidney survival appears favorable, kidney dysfunction persists in most cases. Therefore, longer follow-ups of such patients would provide practical data on prognosis.
Various Forms of Atypical Anti-GBM Disease
IgA-Mediated Anti-GBM Disease
Although IgA-mediated anti-GBM disease is not a variant of atypical anti-GBM disease (because the deposited Ig in the GBM is IgA and not IgG), false-negative serology on conventional assays could initially mimic atypical anti-GBM disease. IgA-mediated anti-GBM disease is rare, with only 16 case reports in the literature to date,51, 52, 53 an additional case of IgG and IgA-mediated anti-GBM disease was recently reported.54 Further, Borza et al.55 described an elderly man with monoclonal IgA autoantibody mediated anti-GBM disease, which was associated with plasma cell dyscrasia. Clinical and pathologic manifestations are similar to classic IgG-mediated anti-GBM disease, however, circulating IgA anti-GBM antibodies are not detected on commercial assays similar to atypical IgG-mediated anti-GBM disease. Unlike the favorable 1-year kidney outcomes in treated patients with classic IgG-mediated disease, IgA-mediated anti-GBM disease was observed to have worse short-term outcomes because most patients required maintenance dialysis on follow-up despite treatment.53 Two groups of investigators tried to understand the specificity of the autoantigen in IgA-mediated anti-GBM disease.52,55 The IgA autoantibodies were found to react against cryptic linear epitopes in the collagenous domain of alpha1 and alpha2 chains of type IV collagen. This finding could explain the dominant presence of linear IgA staining along the tubular basement membrane and Bowman’s capsule. Others have reported the antigen target of IgA anti-GBM antibodies to be the NC domain of the alpha5 and alpha6 chain of type IV collagen.56 A delay in the diagnosis or the unmet need to target the disease differently might be factors responsible for the dismal prognosis of this variant of anti-GBM disease.
IgG4-Associated Anti-GBM Disease
IgG4-mediated anti-GBM disease is typically associated with favorable kidney outcomes.57,58 The variance of IgG4 antibodies from other IgG subclasses makes the disease pattern different. Weak classical complement pathway activation and weak binding to IgG Fc receptors on mononuclear by IgG4 makes it less likely to cause aggressive inflammatory injury.58 Anti-GBM disease mediated by IgG4 autoantibodies is known to be associated with milder clinical kidney dysfunction and often lacks demonstrable circulating anti-GBM antibodies by commercial assays. Modified assays that detect quaternary epitopes within alpha345 hexamers detected IgG4 subclass restricted anti-GBM antibodies in a patient with atypical anti-GBM disease.35 Ohlsson et al.57 described 4 cases of IgG4-mediated anti-GBM disease who presented with severe lung hemorrhage. All 4 patients were young women with either negative standard ELISA (for IgG anti-GBM antibodies), weakly positive standard ELISA, or rapidly fluctuating ELISAs that did not correlate with clinical disease severity. Specific ELISA against IgA, IgM, and IgG subclasses was undertaken promptly, which showed IgG4 anti-GBM antibodies that showed increased reactivity in the absence of a denaturing agent, confirming the reactivity of IgG4 toward the intact α345 hexamer. Kidney dysfunction was noted in 2 of the 4 patients with crescentic GN that improved with immunosuppressive therapy. Cui et al.58 described a similar case of IgG4-mediated anti-GBM disease in a young male with intermittent lung hemorrhage and mild kidney involvement in the form of microscopic hematuria and proteinuria. However, standard ELISA for anti-GBM antibodies against the α3 chain was positive (although weakly) in this patient, pointing toward the heterogenous nature of target antigens in IgG4-mediated anti-GBM disease. Although IgG4 antibodies are typically noninflammatory, the pathogenesis of lung hemorrhage and crescentic GN in some of these cases could be related to lectin complement pathway activation by IgG4 or another unknown mechanism(s). Therefore, in patients with lung hemorrhage without severe kidney involvement and doubtful circulating anti-GBM antibodies, testing for IgG4 anti-GBM antibodies by special assays should be considered.
Monotypic Atypical Anti-GBM Disease
Monotypic Ig deposits along the GBM, defined based on light chain restriction on IF of kidney tissue, are increasingly observed to cause a disease pattern similar to atypical anti-GBM disease. Patients typically lack circulating anti-GBM antibodies and have a milder presentation. In the study by Nasr et al.,7 monotypic deposits were defined as one type of immunoglobulin with either kappa or lambda light chain restriction. IgG1 and lambda light chain restriction was noted in two-thirds of cases of atypical anti-GBM disease with monotypic deposits.7 Similar isolated case reports showed monotypic deposits in patients presenting as atypical anti-GBM disease, such as 1 case with IgG3-lambda9 and 3 cases with IgG1-kappa59, 60, 61 restriction, respectively. Monoclonal IgA and IgM autoantibodies such as IgA1-kappa,55 IgA-lambda,7 and IgM-kappa7 are also reported. The patient with IgA1-kappa described by Borza et al.55 had a plasma cell dyscrasia and presented with mild kidney dysfunction having nonspecific light microscopic findings (without diffuse crescents), which remained stable with treatment over 4 to 5 years; however, it rapidly deteriorated to ESKD later. Of the 2 patients with IgM-kappa monotypic deposits along the GBM, 1 had plasma cell dyscrasia as evidenced by serum monoclonal protein (IgG-lambda) and bone marrow showing lambda-restricted plasmacytosis. However, the discrepancy between the type of monoclonal proteins (IgG-lambda in serum/bone marrow and IgM-kappa in kidneys) brings ambiguity in terming the IgM autoantibodies as pathogenic. In the series by Nasr et al.,7 light microscopy findings of the monotypic cases overlapped with those of polytypic cases except that crescent formation (which was universally “focal”) were seen in only 1 patient (10%) with monotypic deposits as compared to 70% of cases with polytypic deposits. The significance of this finding is unclear, because clinical outcomes and patterns did not differ between monotypic and polytypic forms of the disease. Unlike monoclonal gammopathy of renal significance, Randall-type deposits and diffuse bright linear staining of tubular basement membrane are not seen ultrastructurally in monotypic atypical anti-GBM disease.7 In only 1 patient, there was a concordant monoclonal protein in the serum and in the kidneys.7 Serum-free light chains were normal in all patients. Treatment was individualized, similar to the polytypic atypical anti-GBM disease, except that bortezomib and rituximab were more commonly used in patients with the monotypic type. The 1-year kidney and patient survival rates were similar in polytypic and monotypic diseases, respectively.7 Does this entity represent a form of monoclonal gammopathy of renal significance? It remains to be decided as new evidence on its pathogenesis and long-term course evolves. A summary of the differences between the classic and the atypical form of anti-GBM disease is shown in Figure 2.
Figure 2.
Summary of the differences between the classic and the atypical form of anti-GBM disease. GBM, glomerular basement membrane; NC1, noncollagenous 1; RPGN, rapidly progressive glomerulonephritis.
Differential Diagnoses for Linear IgG Deposition Along the GBM
Patients with diabetic nephropathy, monoclonal immunoglobulin deposition disease (MIDD), and fibrillary GN can have linear IgG deposition along the GBM (Figure 3). A passive linear trap of IgG along the GBM because of loss of negative charge from altered GBM structure is known in diabetic kidneys. This usually appears fainter than the IgG staining in anti-GBM disease. A comparison with albumin staining can help differentiate nonspecific passively trapped IgG (which is weaker or equal to albumin staining intensity) in diabetics from anti-GBM disease and MIDD. Moreover, owing to their dominance in the serum, IgG1 is the dominant passively trapped IgG subtype in the glomeruli. Therefore, dominance of IgG2, IgG3, or IgG4 subtypes in glomeruli are unlikely to be passively trapped. Mild linear IgG trapping is described in otherwise healthy old populations. Electron microscopy would help differentiate MIDD and fibrillary GN from anti-GBM disease. Linear IgG deposits along the GBM/ tubular basement membrane in MIDD are due to the physicochemical properties of the Ig chain and not because of autoantibody response to GBM antigen in situ. Whereas established cases of MIDD are associated with punctate powdery electron-dense deposits along the basement membrane, some cases of MIDD may have very subtle or even absent immune deposits on electron microscopy despite strong IF staining62 resembling anti-GBM disease, so called “MIDD by IF only.”47 Fibrillary GN is known to present with pseudolinear IgG deposits along the GBM on IF,63,64 although a smudgy appearance of the immune deposits is characteristic. About a quarter of cases of diffuse crescentic fibrillary GN show linear GBM staining for IgG mimicking anti-GBM nephritis.47 Electron microscopy showing fibrillary electron-dense deposits along the GBM makes the diagnosis of fibrillary GN straightforward. Nonspecific linear Ig deposits should be further analyzed with elution studies of the kidney tissue to confirm the nature of Ig as anti-GBM antibodies if the initial evaluation does not point to a diagnosis.
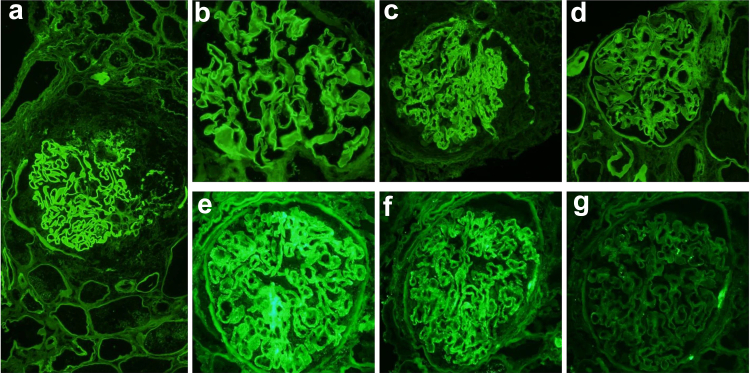
Figure 3.
(a) Classic anti-GBM disease with strong IgG staining along GBM; (b) IgG reactivity along GBM in fibrillary glomerulopathy; (c) linear IgG staining along GBM in an atypical anti-GBM disease; (d) faint linear IgG entrapment along GBM in diabetic nephropathy; _(e–g) IgG linear stain along GBM with kappa restriction. (e) strong IgG staining along GBM; (f): strong kappa staining along GBM; (g) negative lambda stain. (magnification: 200X)
Conclusion
Atypical anti-GBM disease is a variant of the classic anti-GBM disease characterized by linear Ig deposition along the GBM on kidney biopsy and lack of circulating antibodies. Other causes of linear Ig deposition along the GBM must be excluded based on concomitant pathology and clinical background. Kidney dysfunction is usually milder and evolves slower than classic anti-GBM disease; however, presentation as rapidly progressive glomerulonephritis has also been reported. Proteinuria and nephrotic syndrome are more frequent than classic anti-GBM disease. Light microscopy shows heterogenous patterns like mesangial and/or endocapillary proliferative GN, membranoproliferative glomerulonephritis, FSGS, mesangial sclerosis; and glomerular endothelial changes resembling thrombotic microangiopathy are common in atypical anti-GBM disease. Diffuse crescentic and necrotizing GN is uncommon. Differences in the entire target antigen or epitope structure and/or type of antibody can result in negative serology on commercial assays. Modified assays to detect a wide range of antigens or epitopes or antibodies and more sensitive techniques like biosensors can unmask circulating anti-GBM antibodies in some cases and should be attempted. The monotypic subtype of atypical anti-GBM disease needs further study to confirm if it is a form of monoclonal gammopathy of renal significance. Treatment of atypical anti-GBM disease is individualized and typically consists of immunosuppressive agents used to treat classic anti-GBM disease or monotherapy with renin-angiotensin-aldosterone blockers. A prospective multicenter study on a large cohort of patients with atypical anti-GBM disease would be worthwhile to understand its pathogenesis, clinical course, and outcome
Disclosure
KDJ reports consultancy agreements with Secretome, George Clinicals, PMV pharmaceuticals, and Calliditas. KDJ is a founder and co-president of the American Society of Onco-Nephrology (ASON); KDJ reports honoraria from the American Society of Nephrology, and UpToDate.com; reports serving on the editorial boards of CJASN, AJKD, CKJ, Journal of Onconephrology, Kidney International, and NDT; reports serving as Editor-in-Chief of ASN Kidney News and section editor for onconephrology for Nephrology Dialysis Transplantation. Other authors have nothing to disclose.
References
- 1.McAdoo S.P., Pusey C.D. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2017;12:1162–1172. doi: 10.2215/CJN.01380217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy J.B., Turner A.N., Rees A.J., Pusey C.D. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033–1042. doi: 10.7326/0003-4819-134-11-200106050-00009. [DOI] [PubMed] [Google Scholar]
- 3.Lerner R.A., Glassock R.J., Dixon F.J. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126:989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dammacco F., Battaglia S., Gesualdo L., Racanelli V. Goodpasture’s disease: a report of ten cases and a review of the literature. Autoimmun Rev. 2013;12:1101–1108. doi: 10.1016/j.autrev.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Levy J.B., Lachmann R.H., Pusey C.D. Recurrent Goodpasture’s disease. Am J Kidney Dis. 1996;27:573–578. doi: 10.1016/s0272-6386(96)90169-9. [DOI] [PubMed] [Google Scholar]
- 6.Rohm C.L., Acree S., Shrivastava A., Saberi A.A., Lovett L. Antibody-negative relapse of Goodpasture syndrome with pulmonary hemorrhage. Case Rep Med. 2019;2019:2975. doi: 10.1155/2019/2975131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasr S.H., Collins A.B., Alexander M.P., et al. The clinicopathologic characteristics and outcome of atypical anti-glomerular basement membrane nephritis. Kidney Int. 2016;89:897–908. doi: 10.1016/j.kint.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Shen C.R., Jia X.Y., Cui Z., Yu X.J., Zhao M.H. Clinical-pathological features and outcome of atypical anti-glomerular basement membrane disease in a large single cohort. Front Immunol. 2020;11:2035. doi: 10.3389/fimmu.2020.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troxell M.L., Houghton D.C. Atypical anti-glomerular basement membrane disease. Clin Kidney J. 2016;9:211–221. doi: 10.1093/ckj/sfv140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang D., Liang S., Xu F., et al. Clinicopathological features and outcome of antibody-negative anti-glomerular basement membrane disease. J Clin Pathol. 2019;72:31–37. doi: 10.1136/jclinpath-2018-205278. [DOI] [PubMed] [Google Scholar]
- 11.Kyriazis P., Tiwary A., Freeman J., Landry D., Braden G. Atypical anti-glomerular basement membrane glomerulonephritis in a patient with metastatic melanoma treated with mitogen-activated protein kinase and immune checkpoint inhibitors: a case report. J Med Case Rep. 2021;15:186. doi: 10.1186/s13256-021-02766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes R., Freitas S., Cunha P., Alves G., Cotter J. Goodpasture’s syndrome with absence of circulating anti-glomerular basement membrane antibodies: a case report. J Med Case Rep. 2016;10:205. doi: 10.1186/s13256-016-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AlSowailmi B., AlSowailmi G., Aloudah N., Alsaad K.O., Elhassan E., Al Sayyari A.A. Atypical antiglomerular basement membranes disease with nephrotic-range proteinuria, mesangial proliferation, and membranoproliferative glomerulonephritis pattern of injury. Saudi J Kidney Dis Transpl. 2017;28:1397–1403. doi: 10.4103/1319-2442.220868. [DOI] [PubMed] [Google Scholar]
- 14.Singhal P., Ren K.Y.M., Curtis B.M., MacPherson I., Avila-Casado C. Atypical noncrescentic antiglomerular basement membrane disease with concurrent thin basement membrane nephropathy. Kidney Int Rep. 2018;3:991–996. doi: 10.1016/j.ekir.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sporinova B., McRae S.A., Muruve D.A., et al. A case of aggressive atypical anti-GBM disease complicated by CMV pneumonitis. BMC Nephrol. 2019;20:29. doi: 10.1186/s12882-019-1227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adapa S., Konala V.M., Hou J., et al. Seronegative atypical anti-glomerular basement membrane crescentic glomerulonephritis. Ann Transl Med. 2019;7:246. doi: 10.21037/atm.2019.04.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong Z., Tan J., Tang Y., Li Z., Qin W. Goodpasture syndrome manifesting as nephrotic-range proteinuria with anti-glomerular basement membrane antibody seronegativity: a case report. Med (Baltim) 2020;99 doi: 10.1097/MD.0000000000022341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elshirbeny M., Alkadi M.M., Mujeeb I., Fituri O. Atypical anti-glomerular basement membrane disease with diffuse crescentic membranoproliferative glomerulonephritis: case report and review of literature. Qatar Med J. 2020;2020:16. doi: 10.5339/qmj.2020.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamboti J.S., Sinniah R., Dorsogna L., Holmes C. Recurrent, atypical anti-glomerular basement membrane disease. Indian J Nephrol. 2021;31:319–321. doi: 10.4103/ijn.IJN_414_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramesh K., Thoppalan B., Venkatraman R., Gandhi S. Anti-glomerular basement membrane disease: a rare case report of changing phenotype and atypicalities. Saudi J Kidney Dis Transpl. 2021;32:841–850. doi: 10.4103/1319-2442.336781. [DOI] [PubMed] [Google Scholar]
- 21.Guo N., Yin Q., Lei S., He Y., Fu P. Atypical anti-glomerular basement membrane disease with anti-GBM antibody negativity and ANCA positivity: a case report. BMC Nephrol. 2021;22:53. doi: 10.1186/s12882-021-02232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura R., Doi T., Hirashio S., et al. A case report of atypical anti-glomerular basement membrane disease. BMC Nephrol. 2022;23:373. doi: 10.1186/s12882-022-03007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javaugue V., Watson M.J., Fervenza F.C., Nasr S.H. Atypical antiglomerular basement membrane nephritis following immune checkpoint inhibitor. Kidney Int Rep. 2022;7:1913–1916. doi: 10.1016/j.ekir.2022.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedchenko V., Bondar O., Fogo A.B., et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borza D.B., Netzer K.O., Leinonen A., et al. The goodpasture autoantigen. Identification of multiple cryptic epitopes on the NC1 domain of the alpha3(IV) collagen chain. J Biol Chem. 2000;275:6030–6037. doi: 10.1074/jbc.275.8.6030. [DOI] [PubMed] [Google Scholar]
- 26.Hellmark T., Segelmark M. Diagnosis and classification of Goodpasture disease (anti-GBM) J Autoimmun. 2014;48-49:108–112. doi: 10.1016/j.jaut.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 27.O’Sullivan M. Goodpasture’s syndrome: an example of an autoimmune process in the clinical and diagnostic setting. Aust J Med Sci. 2014;35:74–86. [Google Scholar]
- 28.Wilson C.B., Dixon F.J. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973;3:74–89. doi: 10.1038/ki.1973.14. [DOI] [PubMed] [Google Scholar]
- 29.Litwin C.M., Mouritsen C.L., Wilfahrt P.A., Schroder M.C., Hill H.R. Anti-glomerular basement membrane disease: role of enzyme-linked immunosorbent assays in diagnosis. Biochem Mol Med. 1996;59:52–56. doi: 10.1006/bmme.1996.0064. [DOI] [PubMed] [Google Scholar]
- 30.Shiroshita A., Oda Y., Takenouchi S., Hagino N., Kataoka Y. Accuracy of anti-GBM antibodies in diagnosing anti-glomerular basement membrane disease: a systematic review and meta-analysis. Am J Nephrol. 2021;52:531–538. doi: 10.1159/000518362. [DOI] [PubMed] [Google Scholar]
- 31.Jia X.Y., Qu Z., Cui Z., Yang R., Zhao J., Zhao M.H. Circulating anti-glomerular basement membrane autoantibodies against α3(IV)NC1 undetectable by commercially available enzyme-linked immunosorbent assays. Nephrol (Carlton) 2012;17:160–166. doi: 10.1111/j.1440-1797.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 32.Olaru F., Wang X.P., Luo W., et al. Proteolysis breaks tolerance toward intact α345(IV) collagen, eliciting novel anti-glomerular basement membrane autoantibodies specific for α345NC1 hexamers. J Immunol. 2013;190:1424–1432. doi: 10.4049/jimmunol.1202204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clatworthy M.R., Wallin E.F., Jayne D.R. Anti-glomerular basement membrane disease after alemtuzumab. N Engl J Med. 2008;359:768–769. doi: 10.1056/NEJMc0800484. [DOI] [PubMed] [Google Scholar]
- 34.Ang C., Savige J., Dawborn J., et al. Anti-glomerular basement membrane (GBM)-antibody-mediated disease with normal renal function. Nephrol Dial Transplant. 1998;13:935–939. doi: 10.1093/ndt/13.4.935. [DOI] [PubMed] [Google Scholar]
- 35.Yang R., Hellmark T., Zhao J., et al. Levels of epitope-specific autoantibodies correlate with renal damage in anti-GBM disease. Nephrol Dial Transplant. 2009;24:1838–1844. doi: 10.1093/ndt/gfn761. [DOI] [PubMed] [Google Scholar]
- 36.Rutgers A., Meyers K.E., Canziani G., Kalluri R., Lin J., Madaio M.P. High affinity of anti-GBM antibodies from Goodpasture and transplanted Alport patients to alpha3(IV)NC1 collagen. Kidney Int. 2000;58:115–122. doi: 10.1046/j.1523-1755.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 37.Vries T.B., Boerma S., Doornebal J., Dikkeschei B., Stegeman C., Veneman T.F. Goodpasture’s syndrome with negative anti-glomerular basement membrane antibodies. Eur J Case Rep Intern Med. 2017;4 doi: 10.12890/2017_000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segelmark M., Hellmark T., Wieslander J. The prognostic significance in Goodpasture’s disease of specificity, titre and affinity of anti-glomerular-basement-membrane antibodies. Nephron Clin Pract. 2003;94:c59–c68. doi: 10.1159/000072022. [DOI] [PubMed] [Google Scholar]
- 39.Salama A.D., Dougan T., Levy J.B., et al. Goodpasture’s disease in the absence of circulating anti-glomerular basement membrane antibodies as detected by standard techniques. Am J Kidney Dis. 2002;39:1162–1167. doi: 10.1053/ajkd.2002.33385. [DOI] [PubMed] [Google Scholar]
- 40.Henderson S.R., Salama A.D. Diagnostic and management challenges in Goodpasture’s (anti-glomerular basement membrane) disease. Nephrol Dial Transplant. 2018;33:196–202. doi: 10.1093/ndt/gfx057. [DOI] [PubMed] [Google Scholar]
- 41.Qu Z., Cui Z., Liu G., Zhao M.H. The distribution of IgG subclass deposition on renal tissues from patients with anti-glomerular basement membrane disease. BMC Immunol. 2013;14:19. doi: 10.1186/1471-2172-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao J., Yan Y., Cui Z., Yang R., Zhao M.H. The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rHalpha3(IV)NC1 is associated with disease severity. Hum Immunol. 2009;70:425–429. doi: 10.1016/j.humimm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Segelmark M., Butkowski R., Wieslander J. Antigen restriction and IgG subclasses among anti-GBM autoantibodies. Nephrol Dial Transplant. 1990;5:991–996. doi: 10.1093/ndt/5.12.991. [DOI] [PubMed] [Google Scholar]
- 44.Bowman C., Ambrus K., Lockwood C.M. Restriction of human IgG subclass expression in the population of auto-antibodies to glomerular basement membrane. Clin Exp Immunol. 1987;69:341–349. [PMC free article] [PubMed] [Google Scholar]
- 45.Cui Z., Wang H.Y., Zhao M.H. Natural autoantibodies against glomerular basement membrane exist in normal human sera. Kidney Int. 2006;69:894–899. doi: 10.1038/sj.ki.5000135. [DOI] [PubMed] [Google Scholar]
- 46.Jennette J.C. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164–1177. doi: 10.1046/j.1523-1755.2003.00843.x. [DOI] [PubMed] [Google Scholar]
- 47.Batal I., Reyes D.B., Popham S., Bijol V. Nodular glomerulosclerosis with anti-glomerular basement membrane-like glomerulonephritis; a distinct pattern of kidney injury observed in smokers. Clin Kidney J. 2014;7:361–366. doi: 10.1093/ckj/sfu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosales I.A., Colvin R.B. Glomerular disease with idiopathic linear immunoglobulin deposition: a rose by any other name would be atypical. Kidney Int. 2016;89:750–752. doi: 10.1016/j.kint.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 49.Wu J., Hicks J., Borillo J., Glass W.F., 2nd, Lou Y.H. CD4(+) T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J Clin Invest. 2002;109:517–524. doi: 10.1172/JCI13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savage C.O., Pusey C.D., Bowman C., Rees A.J., Lockwood C.M. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980–4. Br Med J (Clin Res Ed) 1986;292:301–304. doi: 10.1136/bmj.292.6516.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bacalja J., Zibar L., Ljubanović D.G. IgA-mediated anti-glomerular basement membrane disease. A case report. Nefrol (Engl Ed) 2018;38:339–341. doi: 10.1016/j.nefro.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Antonelou M., Henderson S.R., Bhangal G., et al. Binding truths: atypical anti glomerular basement membrane disease mediated by IgA anti glomerular basement membrane antibodies targeting the α1 chain of Type IV collagen. Kidney Int Rep. 2019;4:163–167. doi: 10.1016/j.ekir.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moulis G., Huart A., Guitard J., Fortenfant F., Chauveau D. IgA-mediated anti-glomerular basement membrane disease: an uncommon mechanism of Goodpasture’s syndrome. Clin Kidney J. 2012;5:545–548. doi: 10.1093/ckj/sfs087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou G., Lu H., Zhuo L., Zou W., Li W. Anti-glomerular basement membrane disease mediated by IgG and IgA: a case report. Ren Fail. 2021;43:774–778. doi: 10.1080/0886022X.2021.1914658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borza D.B., Chedid M.F., Colon S., Lager D.J., Leung N., Fervenza F.C. Recurrent Goodpasture’s disease secondary to a monoclonal IgA1-kappa antibody autoreactive with the alpha1/alpha2 chains of type IV collagen. Am J Kidney Dis. 2005;45:397–406. doi: 10.1053/j.ajkd.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 56.Ghohestani R.F., Rotunda S.L., Hudson B., et al. Crescentic glomerulonephritis and subepidermal blisters with autoantibodies to alpha5 and alpha6 chains of type IV collagen. Lab Investig. 2003;83:605–611. doi: 10.1097/01.lab.0000067497.86646.4d. [DOI] [PubMed] [Google Scholar]
- 57.Ohlsson S., Herlitz H., Lundberg S., et al. Circulating anti-glomerular basement membrane antibodies with predominance of subclass IgG4 and false-negative immunoassay test results in anti-glomerular basement membrane disease. Am J Kidney Dis. 2014;63:289–293. doi: 10.1053/j.ajkd.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 58.Cui Z., Zhao M.H., Singh A.K., Wang H.Y. Antiglomerular basement membrane disease with normal renal function. Kidney Int. 2007;72:1403–1408. doi: 10.1038/sj.ki.5002525. [DOI] [PubMed] [Google Scholar]
- 59.Coley S.M., Shirazian S., Radhakrishnan J., D’Agati V.D. Monoclonal IgG1κ anti-glomerular basement membrane disease: a case report. Am J Kidney Dis. 2015;65:322–326. doi: 10.1053/j.ajkd.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 60.Olivier M., Watson H., Lee D., Mohanlal V., Madruga M., Carlan S. Monotypic IgG1-kappa atypical anti-glomerular basement membrane nephritis: a case report. Case Rep Nephrol Dial. 2019;9:8–14. doi: 10.1159/000498844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonilla M., Bijol V., Kello N., Jhaveri K.D., Ross D.W. A case of glomerulopathy associated with monoclonal glomerular basement membrane antibody. Kidney Int Rep. 2021;6:1444–1448. doi: 10.1016/j.ekir.2021.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sethi S., Rajkumar S.V., D’Agati V.D. The complexity and heterogeneity of monoclonal immunoglobulin-associated renal diseases. J Am Soc Nephrol. 2018;29:1810–1823. doi: 10.1681/ASN.2017121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sethi S., Adeyi O.A., Rennke H.G. A case of fibrillary glomerulonephritis with linear immunoglobulin G staining of the glomerular capillary walls. Arch Pathol Lab Med. 2001;125:534–536. doi: 10.5858/2001-125-0534-ACOFGW. [DOI] [PubMed] [Google Scholar]
- 64.Thomas J.A., Vasin D., Lin M., Anderson A.E., Alpers C.E. A case of mistaken identity: fibrillary glomerulonephritis masquerading as crescentic anti-glomerular basement membrane disease. Clin Nephrol. 2016;85:114–120. doi: 10.5414/CN108667. [DOI] [PubMed] [Google Scholar]