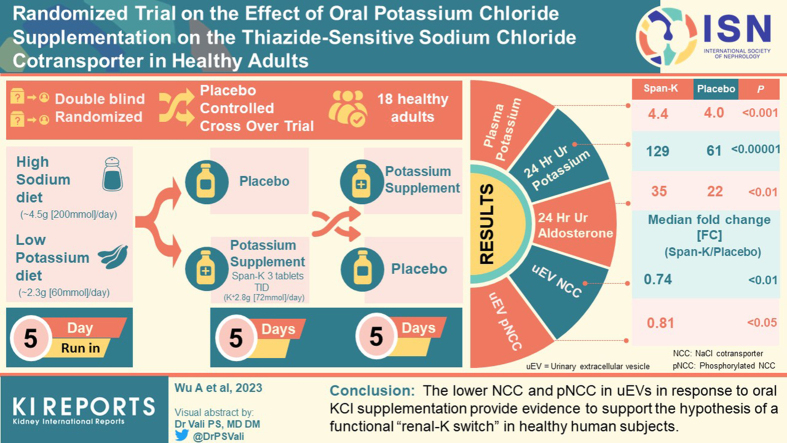
Abstract
Introduction
The putative “renal-K switch” mechanism links dietary potassium intake with sodium retention and involves activation of the sodium chloride (NaCl) cotransporter (NCC) in the distal convoluted tubule in response to low potassium intake, and suppression in response to high potassium intake. This study examined NCC abundance and phosphorylation (phosphorylated NCC [pNCC]) in urinary extracellular vesicles (uEVs) isolated from healthy adults on a high sodium diet to determine tubular responses to alteration in potassium chloride (KCl) intake.
Methods
Healthy adults maintained on a high sodium (∼4.5 g [200 mmol]/d) low potassium (∼2.3 g [60 mmol]/d) diet underwent a 5-day run-in period followed by a crossover study, with 5-day supplementary KCl (active phase, Span-K 3 tablets (potassium 24 mmol) thrice daily) or 5-day placebo administrated in random order and separated by 2-day washout. Ambulatory blood pressure (BP) and biochemistries were assessed, and uEVs were analyzed by western blotting.
Results
Among the 18 participants who met analysis criteria, supplementary KCl administration (vs. placebo) was associated with markedly higher levels of plasma potassium and 24-hour urine excretion of potassium, chloride, and aldosterone. KCl supplementation was associated with lower uEV levels of NCC (median fold change (KCl/Placebo) = 0.74 [0.30–1.69], P < 0.01) and pNCC (fold change (KCl/Placebo) = 0.81 [0.19–1.75], P < 0.05). Plasma potassium inversely correlated with uEV NCC (R2 = 0.11, P = 0.05).
Conclusions
The lower NCC and pNCC in uEVs in response to oral KCl supplementation provide evidence to support the hypothesis of a functional “renal-K switch” in healthy human subjects.
Keywords: high sodium diet, potassium, randomized crossover trial, sodium chloride cotransporter, urinary extracellular vesicles
Graphical abstract
See Commentary on Page 1131
Restriction of dietary sodium (Na+, commonly consumed as NaCl [table salt]) can reduce the risk of heart disease, stroke, or death in people with hypertension.1 Reduction in habitual NaCl intake has been recommended in guidelines not only for people with hypertension but also for the general population.2,3 Although Na+ restriction is well recognized to be an effective component of hypertension prevention and management, it is difficult to achieve in practice because of the high content of Na+ in current patterns of dietary intake globally.4
High Na+ intake may be accompanied by a potassium (K+) intake lower than currently recommended,5, 6, 7, 8, 9, 10, 11 which may be an alternative avenue for intervention. A longitudinal study on the long-term effects of dietary Na+, K+, and the K+/Na+ ratio on BP in female adolescents found that higher K+ intake and the K+/Na+ ratio were inversely associated with BP throughout adolescence.12 Similarly, in the large observational INTERSALT study, urinary K+-to-Na+ ratio had a strong inverse association with BP, and K+ excretion was negatively correlated with BP.13 Intervention with the Dietary Approaches to Stop Hypertension diet demonstrated a significant decrease in BP in healthy adults with low Na+ intake compared with higher levels of intake, but this diet program also encouraged people to enrich their diet with K+ through eating more fruit and vegetables.14 A recent population-wide salt-substitution initiative (replacing regular salt with K+-enriched substitutes) in Peru reported an average reduction of 1.29 mm Hg in systolic BP and 0.76 mm Hg in diastolic BP, and a 51% reduction in hypertension incidence in normotensive people.15 In addition, a recent large randomized control trial demonstrated that, compared with regular salt, a salt substitute (consisting of 75% NaCl and 25% KCl by mass) lowered the rates of stroke, major cardiovascular events and death in populations who had a history of stroke or hypertension, and participants demonstrated a mean reduction of 3.34 mm Hg in systolic BP.16 These observations indicate that the impact of dietary K+ may be higher than that of dietary Na+ in influencing body fluid volume and BP maintenance. The exact mechanisms underlying this K+-driven BP-lowering phenomenon have however been unclear, and this knowledge is vital for designing novel BP interventions.
The “renal-K switch” is a mechanism that proposes that a low K+ intake may act as a trigger which links K+ and Na+-dependent BP.17 Changes in the extracellular fluid K+ concentration under different dietary K+ intakes control the activity of the thiazide-sensitive NCC in the distal convoluted tubule.18 This determines the amount of Na+ delivered downstream to the epithelial sodium channel to regulate distal Na+ reabsorption and K+ excretion. The switch mechanism, built predominantly on data from animal studies, ‘turns on’ NCC in response to low dietary K+ intake and turns NCC off in response to a higher K+ intake, and centers around the chloride (Cl−)-sensitive With-No-Lysine kinases (WNKs)-STE20/SPS1-related proline/alanine-rich kinase (SPAK) network.19 Unfortunately, difficulties in obtaining human kidney tissue have hampered studies of the “renal-K switch” model in the clinical setting.
uEVs are a noninvasive source of potential molecular biomarkers to mirror molecular processes as well as physiological and pathologic conditions in the kidney and other urinary tract tissues.20, 21, 22 In a large scale unbiased analysis of uEV proteins, NCC in uEV tracks the abundance of the parent protein in the kidney.23
To assess the relevance of the “renal-K switch” mechanism in healthy adults, we conducted a double-blind, randomized and placebo-controlled crossover trial. Participants received 1 week of high Na+ high K+ diet and 1 week of a high Na+ low K+ diet. uEVs were isolated from the subjects before and after each dietary phase and the abundances of NCC and phosphorylated NCC (active form, pNCC) were analyzed to determine their responses to alterations in K+ intake.
Methods
Study Design and Ethics
This trial was designed to ascertain the effects of altering oral K+ intake (via supplementation) on NCC in uEVs in healthy adult participants on a high Na+ low K+ diet (Clinical Trials Repository ID: CT-2018-CTN-03504-1 v1). The flow diagram for this trial is shown in Supplementary Figure S1. The trial was conducted at the Endocrine Hypertension Research Centre in Brisbane, Australia. The trial was started in September 2019, and was scheduled to be completed in June 2020, but completion was delayed until July 2021 because of the COVID-19 pandemic and associated regulatory requirements in Queensland, Australia. Ethical approval was granted for this study by the Metro South Human Research Ethics Committee (HREC/18/QPAH/103).
Recruitment
Participants were recruited via advertisements on notice boards and within routine electronic media channels at the Princess Alexandra Hospital and the Translational Research Institute. Respondents to the advertisement were given a questionnaire to assess their eligibility of participation.
Eligibility
Inclusion and exclusion criteria are described in Supplementary Methods.24
Description of Dietary Intervention
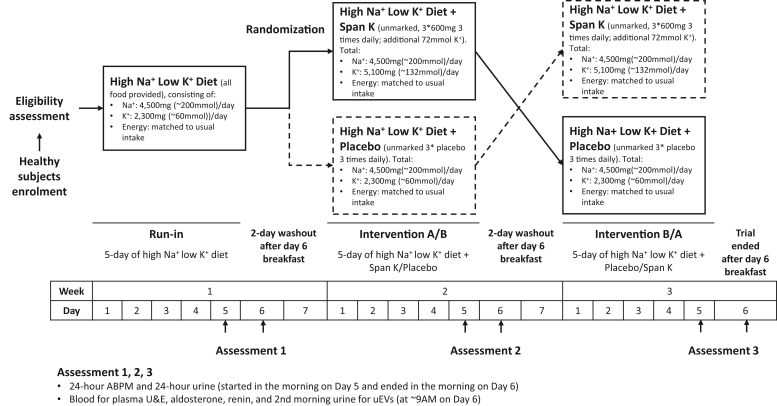
The dietary intervention ran across a total of 20 days, with 3 distinct phases (a preintervention run-in and 2 intervention phases) each from Sunday (day 1) to Friday (day 5) morning as described in Figure 1 and Supplementary Methods.25
Figure 1.
Flowchart of dietary intervention methods. Intervention A, 5-day of high Na+ low K+ diet supplemented with Span- K; Intervention B, 5-day of high Na+ low K+ diet supplemented with placebo; ABPM, ambulatory blood pressure monitoring; K+, potassium; Na+, sodium; uEVs, urinary extracellular vesicles.
Randomization and Masking
Unmarked Span-K tablets and unmarked placebo capsules were used. The allocation sequence of supplements was computer-generated, independent of the investigators, by the clinical trials unit at Princess Alexandra Hospital. Access to the allocation sequence was restricted to the clinical trials pharmacists, and the sequence was concealed until all clinical and laboratory measurements were completed.
Sample Collection
On day 6 of each phase, blood was collected at 8 AM for biochemistry. Midstream second morning urine was also collected at approximately 8 AM for uEV isolation and spot urine creatinine measurement. After initial sample collection at screening, 24-hour ambulatory BP monitoring and 24-hour urine collection commenced on day 5 and were completed in the morning on day 6 after the study diet breakfast in the run-in phase or after the study diet breakfast plus supplements in the intervention phases. Measurements are described in Supplementary Methods.25, 26
uEVs Isolation and Characterization
Collected second morning spot urine samples were immediately treated with cOmplete EDTA-free protease inhibitor cocktail (Roche, Switzerland) and phosphatase inhibitor (Sigma-Aldrich, St. Louis, MO) before aliquoting and freezing at −80 oC. uEVs were isolated using progressive ultracentrifugation techniques with dithiothreitol treatment as previously described.27 uEVs were characterized by size distribution and presence of EV marker proteins. Size distribution was measured by nanoparticle-tracking analysis (as described in Supplementary Methods) using a pool sample of uEVs from participants. The presence of marker proteins of EV (ALIX, TSG101 or CD9) were measured by western blotting among all participants.
Western Blotting
SDS-PAGE used precast 4% to15% TGX midi gels and each gel lane was loaded with uEV isolate containing 10 μg of total protein. Blot transferring was performed using the Bio-Rad turbo transfer system, and obtained blots were then blocked in 5% bovine serum albumin (A3858, Sigma-Aldrich) followed by overnight 4 °C incubation with the antibodies listed in Supplementary Table S1.28 Exposure was performed in configuring signal accumulation mode by Bio-Rad ChemiDoc XRS+ Imager on configure signal accumulation mode with Image Lab software. Images that did not exceed saturation were exported for analyses.
Statistical Analyses
Calculations were processed with R (version 4.0.0)29. Before comparison and correlation assessment, relative protein abundance was determined by dividing the protein band intensity analyzed with ImageJ software by the sum abundance of 3 EV markers (ALIX+TSG101+CD9). Paired Wilcoxon tests were performed to compare the differences of biochemical features and dietary compliance (meal plan and supplements). Paired t tests were performed to compare the changes in dietary content (energy, Na+, and K+) and the log10-transformed relative protein abundance during the trial. Pearson’s correlation was assessed between log10-transformed relative protein abundance and biochemical parameters (including ambulatory BPs; plasma levels of K+, Na+, Cl−, bicarbonate, aldosterone, renin, aldosterone-to-renin ratio; and timed urine levels of K+, Na+, Cl− and aldosterone) during the intervention phases. A P < 0.05 was considered significant. Data are presented as mean [range], unless stated otherwise.
Results
Recruitment and Participants’ Characteristics
A total of 36 healthy volunteers were assessed for eligibility in this study from September 2019 to July 2021, and 6 declined to participate. The remaining 30 subjects provided written consent. Two participants withdrew from the study after their first intervention phase as follows: 1 participant withdrew because of bowel issues during the second intervention phase, and the other withdrew before commencing the second intervention phase because of a family emergency. A total of 28 participants (93%, 20 females and 8 males; Table 1 and Supplementary Spreadsheet S1) completed the trial. Among the female participants, 13 (65%) were premenopausal (including 1 using a Mirena intrauterine device) and 7 (35%) were postmenopausal. At enrolment, all the 28 participants were normotensive (119 [101–141]/77 [67–97] mm Hg) and normokalemic (4.3 [3.7–4.9] mmol/l). Compared with male participants, female participants were older (47.5 [22.5–62.3] vs. 26.3 [23.6–47.6] years, P < 0.01), and had lower levels of estimated glomerular filtration rate (88 [70–120] vs. 113 [110–123] ml/min per 1.73 m2, P < 0.01) and creatinine clearance (101 [62–147] vs. 144 [120–172] ml/min, P < 0.001). This may be due to the fact that the female participants were older than the males. During the trial, ambulatory BP monitoring and biochemistry were assessed in all 28 participants, urine samples for uEVs were collected from 28 participants, but urine samples collected from 3 participants (subject #19, 20, and 22) were inadequate (<20 ml) for uEV isolation.
Table 1.
Participants’ features at enrolment
| Variables | Normal range in adults | Overall (N = 28) | Female (n = 20, 71.4%) | Male (n = 8, 28.6%) | Wilcoxon test P (female vs. male) |
|---|---|---|---|---|---|
| Age, yr | 38.1 [22.5–62.3] | 47.5 [22.5–62.3] | 26.3 [23.6–47.6] | 0.0032 | |
| Body mass index, kg/m2 | 18.5–24.9 | 25.3 [20.4–36.6] | 25.5 [20.4–36.6] | 24.2 [22.2–32.9] | 0.71 |
| Premenopausal female, n (%) | 13 (46.4) | 13 (65) | - | - | |
| Sitting systolic blood pressure, mm Hg | <140 | 119 [101–141] | 116 [101–139] | 122 [113–141] | 0.089 |
| Sitting diastolic blood pressure, mm Hg | <90 | 77 [67–97] | 77 [67–87] | 76 [68–97] | 0.74 |
| Plasma aldosterone, pmol/la | ambulatory 30–800; supine 0– 400 | 270 [43–919] | 267 [43–919] | 301 [109–902] | 0.82 |
| Plasma renin concentration, mU/la | ambulatory 3–40; supine 2–29 | 17.4 [4.2–75.2] | 15.4 [4.2–40.4] | 18.7 [8.7–75.2] | 0.18 |
| Aldosterone/renin, pmol/mUb | <55 | 19 [4–91] | 20 [4–91] | 13 [6–33] | 0.31 |
| Plasma Na+, mmol/l | 135–145 | 138 [134–142] | 138 [134–142] | 139 [135–140] | 0.66 |
| Plasma K+, mmol/l | 3.5–5.2 | 4.3 [3.7–4.9] | 4.4 [3.7–4.9] | 4.3 [4.0–4.6] | 0.76 |
| Plasma Cl−, mmol/l | 95–110 | 104 [100–109] | 104 [100–109] | 104 [101–106] | 0.38 |
| Plasma HCO3-, mmol/l | 22–32 | 26 [22–30] | 26 [22–30] | 26 [23–28] | 0.76 |
| Anion gap, mmol/l | 4–13 | 9.0 [3.0–12.0] | 9.0 [3.0–12.0] | 9.5 [6.0–11.0] | 0.64 |
| Plasma glucose, mmol/l | 3.0–7.8 (fasting 3.0–6.0) | 5.0 [4.4–6.6] | 4.9 [4.4–6.6] | 5.0 [4.4–5.5] | 0.92 |
| eGFR, ml/min per 1.73 m2 | >60 | 103 [70–123] | 88 [70–120] | 113 [110–123] | 0.0068 |
| ClCr, ml/min | male 97–137; female 88–128 | 121 [62–172] | 101 [62–147] | 144 [120–172] | 0.00051 |
Cl−, chloride; ClCr, creatinine clearance estimated using the Cockcroft-Gault equation; eGFR, estimated glomerular filtration rate using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation; HCO3-, bicarbonate; K+, potassium; Na+, sodium.
Data present in median [range].
Plasma aldosterone and renin concentration were measured in 26 participants.
Plasma aldosterone/renin ratio was measured in 27 participants.
Dietary Compliance
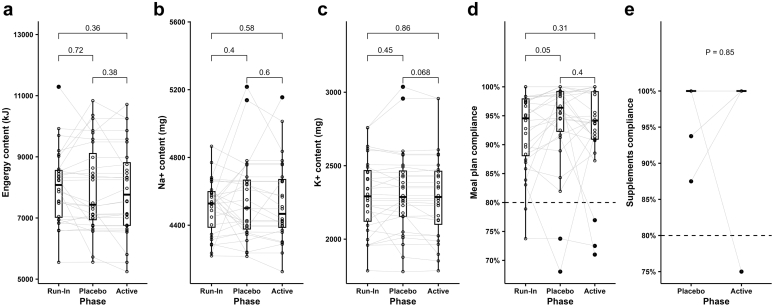
Daily meal plan content and dietary compliance (to meal plan and supplements) based on the participants’ self-reported data are depicted in Figure 2. Among the 28 participants, daily meal plan content of Na+ (run-in: 4512 mg, active: 4467 mg, placebo: 4501 mg) and K+ (run-in: 2292 mg, active: 2287 mg, placebo: 2287 mg) were deliberately intended not to change throughout the trial and met the requirements of a high Na+ (4500 mg) low K+ (2300 mg) diet (Figure 2a–c). Median energy content in the run-in phase was slightly higher than in the intervention phases. This is likely because the volume of food was reduced slightly after run-in because some participants initially gave feedback that there was too much food and that they were not able to consume it all. The overall self-reported meal plan compliance and supplements compliance among the 28 participants were high with the majority reporting over 80% meal plan and supplement compliance in both intervention phases (Figure 2d–e). However, 4 participants reported compliance that was <80% (Supplementary Spreadsheet S2). These included subject #13 who reported low meal plan compliance (68%) in the placebo phase, subject #16 who reported low meal plan compliance (∼70%) through the trial, subject #23 who reported both low meal plan (77%) and supplement (75%) compliance in the active phase and subject #27 who reported low meal plan compliance (71%) in the active phase.
Figure 2.
Meal plan contents and self-reported compliance among 28 participants. (a) Energy content, (b) sodium content, (c) potassium content, (d) participants’ self-reported meal plan compliance, (e) participants’ self-reported supplements (Span-K or placebo) compliance. Energy content, average daily energy content; Na+ content, average daily sodium content; K+ content, average daily potassium content; Meal plan compliance, self-reported average across the components of compliance for all; Supplements compliance, self-reported compliance with supplements (Span-K in the active phase or placebo in the placebo phase). The gray lines in (a)–(e) connect the values in each phase from the same participants. In (e), because all participants except for 3 had 100% compliance in both placebo and active phases and are all encompassed in one gray line. P values for meal contents of energy, sodium and potassium were based on paired t test; P values for compliance of meal plan and supplements were based on paired Wilcoxon test. K+, potassium; Na+, sodium.
Urinary Response
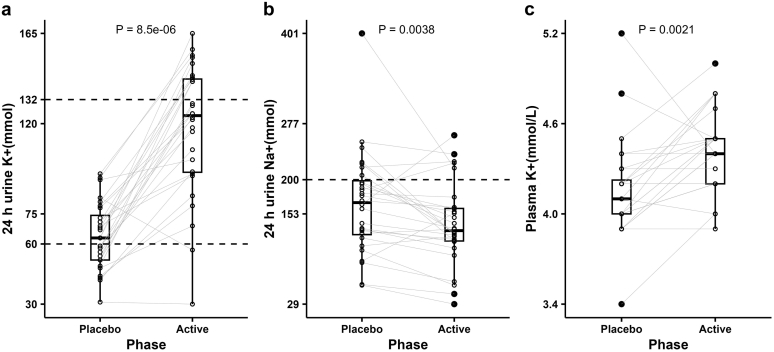
Among the 28 participants, supplementary KCl administration (vs. placebo) in the active phase was associated with higher median levels of urinary K+ (124 [30–165] vs. 63 [31–95] mmol/24h) and plasma K+ (4.4 [3.9–5.0] vs. 4.1 [3.4–5.2] mmol/l, P < 0.01) (Figure 3a and c). However, in several participants (including 3 who reported low dietary compliance), urinary K+ in the active phase did not response to supplemental KCl administration and urinary Na+ did not match the prescribed amount (Figure 3a and b), suggesting that the actual meal plan or supplement intake did not align with self-reported compliance in some of the participants. In subject #16, the low reported meal plan compliance was associated with low urinary Na+ (active: 29 and placebo: 55 mmol/24 h); the similarity of urinary K+ between active (30 mmol/24 h) and placebo (31 mmol/24 h) phases despite the self-reported 100% compliance rates for supplement (placebo or Span-K) intake indicated that the self-reported supplements compliance did not align with actual intake. In subject #23, the low reported meal plan and supplements compliance in the active phases resulted in lower urinary K+ level (57 mmol/24 h) compared with placebo (83 mmol/24 h). In subject #27, the low meal plan compliance in the active phase led to less urinary Na+ excretion in both active (43 mmol/24 h) and placebo (56 mmol/24 h) phases. In subject #11, urinary K+ excretion did not change during the active phase (69 mmol/24 h) compared with the run-in phase (72 mmol/24 h) and was only slightly higher than placebo (48 mmol/24 h), even though supplements compliance was 100% through the trial. In subject #21, urinary Na+ was inexplicably very high (401 mmol/24 h) in the placebo phase, which was double that in the active phases. Therefore, we excluded the data from these 5 participants who did not meet urinary Na+ and K+ response requirements in analyses of biochemical features and BP.
Figure 3.
Urine and plasma responses during interventions among 28 participants. (a) 24-h urinary potassium, (b) 24-h urinary sodium, (c) plasma potassium level. The nonaxial horizontal dashed lines in (a) denote the target values of 24-h urinary potassium in the placebo (60 mmol) and active (132 mmol) phases; the nonaxial horizontal dashed line in (b) denotes the target value of 24-h urinary sodium (200 mmol) during interventions; the gray lines in (a) and (b) connect the values in each phase from the same participants. P values were based on paired Wilcoxon test. K+, potassium; Na+, sodium.
uEV Characterization
Among the 28 participants, urine samples collected from 3 participants (subject #19, #20, and #22) were insufficient (<25 ml) for uEV isolation; therefore, uEV analysis was performed in a total of 25 (18 females and 7 males) participants. The diameter of the particles of uEV pool ranged overall from 150 to 487 nm, with mode particle size of 155 nm (Supplementary Figure S2). Detection of at least 2 EV-enriched proteins (ALIX, TSG101, or CD9) in each sample was considered an indicator of successful uEV isolation. uEVs were successfully isolated from 24 participants (excluding subject #28) in the run-in phase, 25 participants in the active and 23 (excluding subject #14 and #27) in the placebo phase (Supplementary Table S2). Of the 23 (17 females and 6 males) included in biochemical analyses, 20 provided sufficient urine for uEV isolation, but in 1 subject, uEVs were not successfully isolated from placebo phase urine (subject #14), and in another (subject #18) NCC and pT60-NCC were not detected in placebo phase uEVs (Supplementary Figure S3 and Supplementary Table S2). Therefore, the remaining 18 participants (14 females and 4 males) were included in subsequent analyses.
Changes in Biochemistry and BP
Among the 18 participants (14 females and 4 males), supplementary KCl administration compared with placebo was associated with higher levels of plasma K+ (4.4 [3.9–5.0] vs. 4.0 [3.9–4.5], P < 0.001) and 24-hour urinary excretion of K+ (129 [96–164] vs. 61 [42–92], P < 0.00001), Cl− (200 [139–309] vs. 157 [96–268], P < 0.001), and aldosterone (35 [13–83] vs. 22 [6–63], P < 0.01) (Table 2). There were no significant differences in plasma levels of aldosterone, renin, aldosterone-to-renin ratio, Na+, Cl−, and bicarbonate; and estimated glomerular filtration rate, creatinine clearance, ambulatory BPs, and 24-hour urine volume and Na+ were not significantly different between interventions (Table 2). Compared with males (Table 2), supplemental KCl administration in female participants led to greater increases in plasma K+ (0.5 [−0.1 to 0.8] vs. 0.2 [0–0.5] mmol/l) whereas changes in urine K+ excretion remained similar (69 [45–102] vs. 62 [10–92] mmol/24 h), even though the meal plan contents of K+ were slightly lower for females than for males during interventions, and no significant difference was detected in self-reported dietary compliance between male and female participants.
Table 2.
Meal plan contents and changes in biochemical features among 18 participants during interventions
| Variables | Overall (N = 18) | Female (n = 14, 77.8%) | Male (n = 4, 22.2%) | P (female vs. male) |
|---|---|---|---|---|
| Meal energy, kJ/d | ||||
| Placebo | 7332 [5557–10251] | 7198 [5557–8633] | 9053 [6641–10251] | 0.13 |
| Active | 7320 [5246–10251] | 7058 [5246–8751] | 9053 [6641–10251] | 0.10 |
| P (active vs. placebo) | 0.52 | 0.52 | 1.00 | |
| Meal Na+, mg/d | ||||
| Placebo | 4427 [4216–4781] | 4427 [4216–4781] | 4458 [4393–4763] | 0.63 |
| Active | 4427 [4126–4781] | 4427 [4126–4781] | 4458 [4393–4763] | 0.63 |
| P (active vs. placebo) | 0.74 | 0.74 | 1.00 | |
| Meal K+, mg/d | ||||
| Placebo | 2241 [1779–2957] | 2166 [1779–2492] | 2464 [2297–2957] | 0.008 |
| Active | 2236 [1785–2957] | 2146 [1785–2459] | 2464 [2297–2957] | 0.008 |
| P (active vs. placebo) | 0.19 | 0.19 | 1.00 | |
| Meal plan compliance, % | ||||
| Placebo | 97 [68–100] | 95 [68–100] | 100 [95–100] | 0.07 |
| Active | 95 [87–100] | 93 [87–100] | 99 [95–100] | 0.11 |
| P (active vs. placebo) | 0.66 | 0.57 | 1.00 | |
| Supplements compliance, % | ||||
| Placebo | 100 [88–100] | 100 [88–100] | 100 [100–100] | 0.37 |
| Active | 100 [100–100] | 100 [100–100] | 100 [100–100] | NaN |
| P (active vs. placebo) | 0.17 | 0.17 | NaN | |
| Plasma aldosterone, pmol/l | ||||
| Placebo | 230 [78–1080] | 468 [78–1080] | 218 [215–239] | 0.72 |
| Active | 360 [88–1410] | 563 [88–1410] | 218 [157–234] | 0.08 |
| P (active vs. placebo) | 0.47 | 0.33 | 0.62 | |
| Plasma renin mass, mU/l | ||||
| Placebo | 18.7 [5.2–45.6] | 19.3 [5.2–45.6] | 16.1 [14.2–32.7] | 0.96 |
| Active | 18.1 [11.4–97.4] | 18.2 [13.3–41.9] | 16.8 [11.4–97.4] | 0.65 |
| P (active vs. placebo) | 0.46 | 0.67 | 0.62 | |
| Aldosterone/renin, pmol/mU | ||||
| Placebo | 19 [4–42] | 22 [4–42] | 14 [7–17] | 0.20 |
| Active | 18 [2–85] | 24 [5–85] | 13 [2–14] | 1.00 |
| P (active vs. placebo) | 0.29 | 0.13 | 0.27 | |
| Plasma Na+, mmol/l | ||||
| Placebo | 138 [133–143] | 138 [133–143] | 138 [135–138] | 0.45 |
| Active | 139 [135–142] | 139 [135–142] | 139 [137–139] | 0.91 |
| P (active vs. placebo) | 0.31 | 0.76 | 0.27 | |
| Plasma K+, mmol/l | ||||
| Placebo | 4.0 [3.9–4.5] | 4.1 [3.9–4.5] | 4.0 [3.9–4.2] | 0.66 |
| Active | 4.4 [3.9–5.0] | 4.4 [4.0–5.0] | 4.2 [3.9–4.7] | 0.18 |
| P (active vs. placebo) | 0.00086 | 0.0032 | 0.17 | |
| Plasma Cl−, mmol/l | ||||
| Placebo | 105 [101–112] | 106 [102–112] | 103 [101–105] | 0.05 |
| Active | 105 [102–111] | 107 [102–111] | 104 [103–105] | 0.16 |
| P (active vs. placebo) | 0.19 | 0.42 | 0.42 | |
| Plasma HCO3-, mmol/l | ||||
| Placebo | 25 [23–29] | 24 [23–29] | 26 [26–27] | 0.12 |
| Active | 26 [23–28] | 26 [23–28] | 27 [24–27] | 0.74 |
| P (active vs. placebo) | 0.44 | 0.31 | 1.0 | |
| eGFR, ml/min per 1.73 m2 | ||||
| Placebo | 95 [67–115] | 83 [67–115] | 102 [101–106] | 0.23 |
| Active | 93 [62–117] | 82 [62–117] | 105 [98–113] | 0.08 |
| P (active vs. placebo) | 0.30 | 0.17 | 0.62 | |
| ClCr, ml/min | ||||
| Placebo | 107 [64–150] | 103 [64–150] | 126 [109–135] | 0.08 |
| Active | 108 [60–158] | 107 [60–158] | 132 [106–151] | 0.10 |
| P (active vs. placebo) | 0.83 | 0.36 | 0.62 | |
| Ambulatory SBP, mm Hg | ||||
| Placebo | 122 [104–149] | 121 [104–149] | 130 [110–139] | 0.56 |
| Active | 124 [106–149] | 122 [106–149] | 132 [112–136] | 0.34 |
| P (active vs. placebo) | 0.69 | 0.65 | 1.0 | |
| Ambulatory DBP, mm Hg | ||||
| Placebo | 74 [66–91] | 74 [69–91] | 79 [66–87] | 0.39 |
| Active | 75 [66–86] | 74 [67–86] | 80 [66–85] | 0.49 |
| P (active vs. placebo) | 0.80 | 0.86 | 1.0 | |
| Urine volume, ml/24 h | ||||
| Placebo | 2188 [900–3950] | 2188 [900–3950] | 2626 [1130–3778] | 0.88 |
| Active | 2221 [1027–4099] | 2390 [1027–3776] | 1878 [1523–4099] | 0.80 |
| P (active vs. placebo) | 0.67 | 0.81 | 0.88 | |
| Urine creatinine, mmol/24 h | ||||
| Placebo | 11.5 [8.7–20.0] | 10.6 [8.7–19.1] | 18.0 [14.7–20.0] | 0.20 |
| Active | 11.5 [8.5–19.7] | 10.7 [8.5–18.1] | 16.5 [13.9–19.7] | 0.08 |
| P (active vs. placebo) | 0.97 | 0.83 | 1.0 | |
| Urine Na+, mmol/24 h | ||||
| Placebo | 168 [89–251] | 165 [89–251] | 178 [133–224] | 0.38 |
| Active | 143 [55–260] | 143 [55–236] | 143 [116–260] | 0.72 |
| P (active vs. placebo) | 0.18 | 0.27 | 0.62 | |
| Urine K+, mmol/24 h | ||||
| Placebo | 61 [42–92] | 61 [42–82] | 63 [50–92] | 0.80 |
| Active | 129 [96–164] | 129 [96–164] | 123 [102–144] | 0.44 |
| P (active vs. placebo) | 7.6E-06 | 0.00012 | 0.12 | |
| Urine aldosterone, nmol/24 h | ||||
| Placebo | 22 [6–63] | 17 [6–63] | 33 [19–37] | 0.23 |
| Active | 35 [13–83] | 25 [13–83] | 55 [38–70] | 0.13 |
| P (active vs. placebo) | 0.0013 | 0.011 | 0.12 | |
| Urine Cl−, mmol/24 h | ||||
| Placebo | 157 [96–268] | 157 [96–268] | 151 [108–208] | 0.80 |
| Active | 200 [139–309] | 200 [139–309] | 195 [152–282] | 0.88 |
| P (active vs. placebo) | 0.00014 | 0.00024 | 0.25 |
Cl−, chloride; ClCr, creatinine clearance estimated using the Cockcroft-Gault equation; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate using the CKD-EPI (CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration) equation; HCO3-, bicarbonate; K+, potassium; Meal energy, average daily energy content; Meal K+, average daily potassium content; Meal Na+, average daily sodium content; Meal plan compliance, self-reported average across the components of compliance for all; Na+, sodium; NaN, not a number; P (active vs. placebo), for comparisons in meal energy, Na+ and K+; P (female vs. male), P values based on unpaired Wilcoxon test; P values based on paired t-test, other P values based on paired Wilcoxon test; SBP, systolic blood pressure.
Data present in median [range].
Supplementary KCl Reduced NCC and pNCC in uEVs
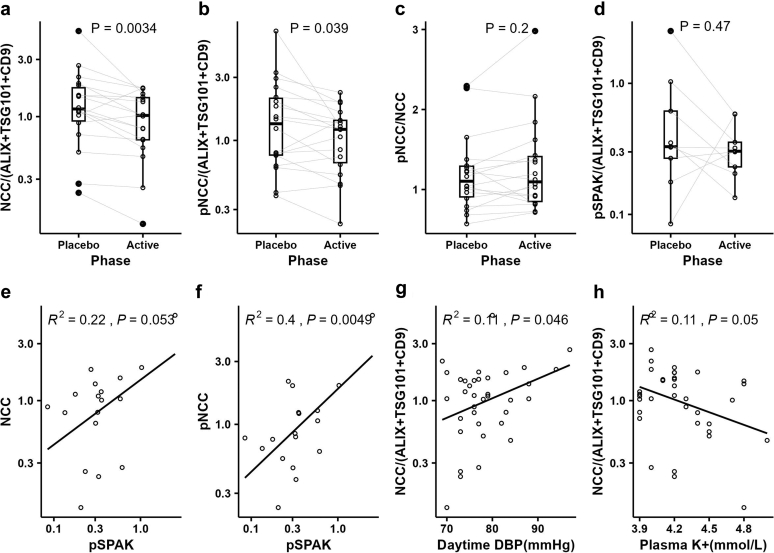
uEV levels of NCC (fold change[active/placebo] = 0.74, P < 0.01) and pT60-NCC (fold change[active/placebo] = 0.81, P < 0.05) were significantly lower in the active phase than in the placebo phase, whereas the ratio of pT60-NCC/NCC (fold change[active/placebo] = 1.09, not significant) remained unchanged (Figure 4a–c). In an attempt to link the reduction in uEV NCC and pNCC in the active phase to the switch mechanism, we assessed phosphorylated STE20/SPS1-related proline/alanine-rich kinase (pSPAK) in uEVs from 11 participants who met the analysis inclusion criteria. pSPAK was detected in 9 participants (Supplementary Table S2). No apparent change in pSPAK was detected (Figure 4d), though pNCC strongly and positively correlated with pSPAK in uEVs (Figure 4f). In the current study, NCC negatively correlated with plasma K+ (R2 = 0.11, P = 0.05) and positively correlated with daytime ambulatory diastolic BP (R2 = 0.11, P < 0.05). (Figure 4g–h)
Figure 4.
Changes in and correlations between uEV proteins. (a)–(c), changes in relative abundances of uEV NCC, pNCC and the ratio of pNCC-to-NCC among 18 participants, (d) change in relative abundance of uEV pSPAK among 9 participants; (e)–(f) correlations of uEV pSPAK with uEV NCC and pNCC during interventions among 9 participants, (g)–(h) NCC correlations with daytime DBP and plasma K+ among 18 participants. The gray lines in (a)–(d) connect the values in each phase from the same participants; P values in (a)–(d) were based on paired t test; Pearson correlation analyses were assessed in (e)–(h). NCC, sodium chloride cotransporter; pNCC, phosphorylated sodium chloride cotransporter; pSPAK, phosphorylated STE20/SPS1-related proline/alanine-rich kinase; uEV, urinary extracellular vesicle.
Sex differences in uEV NCC and pNCC levels are listed in Table 3. NCC reduction remained significant in both females and males, but the significance of pNCC reduction disappeared in both probably because of reduced sample size. After separating females into 2 groups according to menopausal status, NCC reduction remained significant among postmenopausal females. Compared with premenopausal females, supplemental KCl administration among postmenopausal females led to a greater decrease in NCC, with significantly less uEV NCC in the active phase. Compared with males, females had relatively less uEV NCC and pNCC and postmenopausal females showed significantly less uEV NCC across both intervention phases.
Table 3.
Sex difference in uEV NCC and pNCC measured by immunoblotting
| Proteins | Female |
Males (N = 4, 22.2%) | P (female vs. male) | P (premenopausal female vs. male) | P (postmenopausal female vs. male) | |||
|---|---|---|---|---|---|---|---|---|
| All females (N = 14, 77.8%) | Premenopausal (n = 8, 57.1%) | Postmenopausal (n = 6, 42.9%) | P (premenopausal vs. postmenopausal) | |||||
| NCC | ||||||||
| Placebo | 1.12 [0.2, 5.2] | 1.3 [0.7, 5.2] | 0.8 [0.2, 1.8] | 0.094 | 2.0 [1.1, 2.7] | 0.051 | 0.33 | 0.03 |
| Active | 0.9 [0.1, 1.7] | 1.2 [0.6, 1.7] | 0.5 [0.1, 1.4] | 0.035 | 1.4 [0.8, 1.7] | 0.11 | 0.72 | 0.03 |
| P (active vs. placebo) | 0.03 | 0.32 | 0.018 | 0.02 | ||||
| pNCC | ||||||||
| Placebo | 1.0 [0.4, 6.7] | 1.3 [0.4, 6.7] | 0.7 [0.4, 2.5] | 0.43 | 2.5 [0.8, 3.3] | 0.19 | 0.37 | 0.13 |
| Active | 1.1 [0.2, 2.0] | 1.2 [0.5, 1.4] | 0.7 [0.2, 2.0] | 0.35 | 1.6 [0.9, 2.3] | 0.12 | 0.25 | 0.12 |
| P (active vs. placebo) | 0.12 | 0.37 | 0.077 | 0.11 | ||||
NCC, sodium chloride cotransporter; pNCC, phosphorylated sodium chloride cotransporter; uEV, urinary extracellular vesicle.
Discussion
A “renal-K switch” mechanism has been established in rodent models and demonstrates that a low K+ intake acts as a trigger that switches the distal tubule to a state focusing on K+ retention at the expense of sodium reabsorption.17, 18, 19,30 This mechanism involves activation (phosphorylation) of NCC in the distal convoluted tubule in response to low K+ intake and suppression (dephosphorylation) with a high K+ intake. As a first step to establishing a similar mechanism in humans, the current study examined NCC abundance and phosphorylation in uEVs of healthy adults on a high Na+ diet in response to alterations in K+ intake. Our major findings are that there is a fall in uEV NCC and pNCC in response to oral KCl supplementation and a positive correlation between uEV pNCC and pSPAK, providing evidence to support that there is a functional “renal-K switch” in healthy human subjects. Our findings on NCC and pNCC in humans are consistent with those of a previously reported mice study.18 The unchanged pNCC-to-NCC ratio may be because by the time the measurements were complete, the participants were likely already in sodium and potassium balance. It might be next to impossible to pick up small transient effects of NCC dephosphorylation with the KCl supplementation. Overall, the pNCC reduced in response to KCl supplementation, and although the pNCC-to-NCC ratio did not change, the degree of reduction in pNCC was similar to that in total NCC.
Throughout the 3-week trial, the overall self-reported meal plan compliance was high. This was assisted by choosing a meal delivery company which delivers fresh rather than frozen meals, and because of the embedded breaks (2-day washout) of returning to usual diet for 2 days between phases. Having a run-in week was also important because this allowed for suitable amounts and types of food for the individuals to be determined.
Considering that this was a study examining mechanisms rather than being an intention-to-treat interventional study, the analyses included participants whose urinary Na+ and K+ changes were concordant with the prescribed meals and KCl or placebo supplements. Among the included 18 participants, 5 days of supplementary KCl (72 mmol per day, compared with 5 days of placebo) administration was associated with higher levels of plasma K+ and urinary K+, Cl−, and aldosterone, but it did not cause significant rises in plasma aldosterone or suppress plasma renin concentration. These may infer that KCl supplementation during a high Na+ diet directly stimulates the zona glomerulosa, causing angiotensin II-independent aldosterone secretion to promote renal excretion of the excess K+ load rather than reabsorb Na+. The greater change of plasma K+ in response to KCl supplementation in females compared with males may be due to low NCC in females at baseline, but more male participants are required to confirm this.
Increased dietary K+ can cause a natriuretic response.31,32 In the current study, supplementary KCl administration did not cause significant natriuresis, but an unexpected antinatriuresis. The phenomenon of K+-induced natriuresis has been linked to the reduction in NCC activity with high K+ intake and greater delivery of Na+ to downstream segments. However, it may be that the reduction in NCC in the current study was not sufficient to cause natriuresis, and concurrent inhibition of Na+ transport in the proximal tubule and loop of Henle is required.33, 34, 35 Furthermore, in mice there is a biphasic response to high K+ loading which is characterized by an initial natriuresis and kaliuresis over an initial 3-hour period, followed by a sustained kaliuresis. These functional changes were accompanied by a rapid and sustained dephosphorylation of NCC and a late up-regulation of proteolytically activated epithelial sodium channel.36 Therefore, inactivation of NCC in the acute setting may be counterbalanced in the long run by a compensatory response by the epithelial sodium channel that prevents chronic renal Na+ loss. This biphasic response may also exist in humans too, but during our trial we only collected urine from day 5 to 6 of each phase and therefore, any initial natriuresis after the KCl supplementation may have been missed. Although unexpected, the observed antinatriuresis may be due to raised aldosterone secretion. In the current study, plasma aldosterone increased (albeit not statistically significantly) from 230 to 360 pmol/l in the active phase, and the urine aldosterone excretion increased significantly from 22 to 35 nmol/day in the active phase. Urinary aldosterone quantifies only part of aldosterone secretion, but it is a reliable index of aldosterone secretion in the absence of severe renal dysfunction. The raised urinary aldosterone in the active phase indicates an increase in aldosterone secretion, which, by acting on the aldosterone-sensitive distal nephron, may help to explain the overall reduced Na+ excretion. Assessing the effects of thiazide after each intervention to quantify the functional shift in NCC activity triggered by KCl supplementation would have provided further insights.
BP-lowering effects of dietary K+ have been observed in multiple experimental and clinical studies, and various observational and interventional studies have shown an inverse correlation of K+ intake with BP in normotensive adults.37,38 Here, no significant changes in 24-hour ambulatory BP monitoring were observed between the 2 interventions, which is similar to some other studies that have not detected BP-lowering effects of K+ in healthy or normotensive individuals.39,40 The lack of a detectable effect on BP here may relate to the short duration (5 days) of the intervention, the small number of included participants (study was not powered to study effects on BP) and/or the lack of significant natriuresis.41
A limitation of the current study is that the placebo (which was delivered in capsules) was distinguishable in appearance from the Span-K tablets (which were too large to be inserted into capsules), leading to the potential for partial unblinding of participants. However, blinding of the research team was maintained because randomization occurred at the pharmacy dispense point. Another limitation is that the current study included a relatively small number of participants and was not powered to detect changes in BP. Furthermore, the results of the current study were obtained mainly from women (>70%), so a study to confirm the mechanism in healthy men is needed. In addition, the median age of women in this study was twice that of men. Sex-related and age-related nephron mass differences may be responsible for the sex- and menopause-related differences in uEV NCC and pNCC.42
In conclusion, the abundance and phosphorylation of NCC in the uEVs of healthy adults on a high Na+ diet was reduced in response to supplementary K+ intake. The reduced NCC supports the validity of the “renal-K switch” in humans and provides further support that dietary K+ interventions are a viable option to lower BP.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work is supported by a grant from the Leducq Foundation (17CVD05, Potassium in Hypertension Network). AW was supported by a scholarship from the Commonwealth Government of Australia. RAF was funded by the Novo Nordisk Foundation (NNF21OC0067647, NNF17OC0029724)
Footnotes
Supplementary Methods (PDF)
Supplementary Table S1. List of antibodies in use (PDF).
Supplementary Table S2. List of participants included in analyses (PDF).
Supplementary Figure S1. The CONSORT flowchart of the double-blind, randomized and placebo-controlled crossover trial (PDF).
Supplementary Figure S2. Nanoparticle-tracking analysis of uEV pool (PDF).
Supplementary Figure S3 (A-L). Immunoblots of analyzed protein in all participants (PDF).
Supplementary Spreadsheet S1. Details of participants’ features at enrolment (XLSX).
Supplementary Spreadsheet S2. Details of dietary intervention and randomisation (XLSX).
Supplementary Reference (PDF).
Supplementary Material
Supplementary Table S1. List of antibodies in use (PDF).
Supplementary Table S2. List of participants included in analyses (PDF).
Supplementary Figure S1. The CONSORT flowchart of the double-blind, randomized and placebo-controlled crossover trial (PDF).
Supplementary Figure S2. Nanoparticle-tracking analysis of uEV pool (PDF).
Supplementary Figure S3 (A-L). Immunoblots of analyzed protein in all participants (PDF).
Supplementary Spreadsheet S1. Details of participants’ features at enrolment (XLSX).
Supplementary Spreadsheet S2. Details of dietary intervention and randomisation (XLSX).
Supplementary Reference (PDF).
References
- 1.Mente A., O’Donnell M., Rangarajan S., et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388:465–475. doi: 10.1016/S0140-6736(16)30467-6. [DOI] [PubMed] [Google Scholar]
- 2.Rodgers A., Chow C.K., Jackson R.T., et al. Guideline for the diagnosis and management of hypertension in adults - 2016. Med J Aust. 2017;206:141. doi: 10.5694/mja16.01057. [DOI] [PubMed] [Google Scholar]
- 3.Nutrient reference values for Australian and New Zealand including Recommonded dietary intakes. National Health and Medical Research Council. https://www.nhmrc.gov.au/sites/default/files/images/nutrient-refererence-dietary-intakes.pdf
- 4.Mozaffarian D., Fahimi S., Singh G.M., et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 5.Bolton K.A., Trieu K., Woodward M., et al. Dietary intake and sources of potassium in a cross-sectional study of Australian adults. Nutrients. 2019;11:2996. doi: 10.3390/nu11122996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.What we eat in America:2017–2020. US Department of Agriculture Agriculture Research Service. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/
- 7.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Turck D., Bresson J.L., Burlingame B., et al. Dietary reference values for potassium. EFSA J. 2016;14 doi: 10.2903/j.efsa.2016.4592. [DOI] [Google Scholar]
- 8.Okuda N., Okayama A., Miura K., et al. Food sources of dietary potassium in the adult Japanese population: the international study of macro-/micronutrients and blood pressure (INTERMAP) Nutrients. 2020;12:787. doi: 10.3390/nu12030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du S., Wang H., Zhang B., Popkin B.M. Dietary potassium intake remains low and sodium intake remains high, and most sodium is derived from home food preparation for Chinese adults, 1991–2015 trends. J Nutr. 2020;150:1230–1239. doi: 10.1093/jn/nxz332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donfrancesco C., Lo Noce C., Russo O., et al. Trend in potassium intake and Na/K ratio in the Italian adult population between the 2008 and 2018 CUORE project surveys. Nutr Metab Cardiovasc Dis. 2021;31:814–826. doi: 10.1016/j.numecd.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Guideline: potassium intake for adults and children. WHO Guidelines Approved by the Guidelines Review Committee. https://www.who.int/publications/i/item/9789241504829
- 12.Buendia J.R., Bradlee M.L., Daniels S.R., Singer M.R., Moore L.L. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatr. 2015;169:560–568. doi: 10.1001/jamapediatrics.2015.0411. [DOI] [PubMed] [Google Scholar]
- 13.Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ard J.D., Coffman C.J., Lin P.H., Svetkey L.P. One-year follow-up study of blood pressure and dietary patterns in dietary approaches to stop hypertension (DASH)-sodium participants. Am J Hypertens. 2004;17:1156–1162. doi: 10.1016/j.amjhyper.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Bernabe-Ortiz A., Sal Y.R.V.G., Ponce-Lucero V., et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374–378. doi: 10.1038/s41591-020-0754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neal B., Wu Y., Feng X., et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067–1077. doi: 10.1056/NEJMoa2105675. [DOI] [PubMed] [Google Scholar]
- 17.Ellison D.H., Terker A.S., Gamba G. Potassium and its discontents: new insight, new treatments. J Am Soc Nephrol. 2016;27:981–989. doi: 10.1681/ASN.2015070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terker A.S., Zhang C., McCormick J.A., et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoorn E.J., Gritter M., Cuevas C.A., Fenton R.A. Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol Rev. 2020;100:321–356. doi: 10.1152/physrev.00044.2018. [DOI] [PubMed] [Google Scholar]
- 20.Huebner A.R., Somparn P., Benjachat T., et al. Exosomes in urine biomarker discovery. Adv Exp Med Biol. 2015;845:43–58. doi: 10.1007/978-94-017-9523-4_5. [DOI] [PubMed] [Google Scholar]
- 21.Spanu S., van Roeyen C.R., Denecke B., Floege J., Mühlfeld A.S. Urinary exosomes: a novel means to non-invasively assess changes in renal gene and protein expression. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barros E.R., Carvajal C.A. Urinary exosomes and their cargo: potential biomarkers for mineralocorticoid arterial hypertension? Front Endocrinol (Lausanne) 2017;8:230. doi: 10.3389/fendo.2017.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q., Poulsen S.B., Murali S.K., et al. Large-scale proteomic assessment of urinary extracellular vesicles highlights their reliability in reflecting protein changes in the kidney. J Am Soc Nephrol. 2021;32:2195–2209. doi: 10.1681/asn.2020071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wielgosz A., Robinson C., Mao Y., Jiang Y., Campbell N.R., Muthuri S. The impact of using different methods to assess completeness of 24-Hour urine collection on estimating dietary sodium. J Clin Hypertens. 2016;18:581–584. doi: 10.1111/jch.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thuzar M., Young K., Ahmed A.H., Ward G., Wolley M.J., Guo Z. Diagnosis of primary aldosteronism by seated saline suppression test-variability between immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab. 2020;105:e477-483. doi: 10.1210/clinem/dgz150. [DOI] [PubMed] [Google Scholar]
- 26.Guo Z., Poglitsch M., McWhinney B.C., Ungerer J.P., Ahmed A.H., Gordon R.D. Measurement of equilibrium angiotensin II in the diagnosis of primary aldosteronism. Clin Chem. 2020;66:483–492. doi: 10.1093/clinchem/hvaa001. [DOI] [PubMed] [Google Scholar]
- 27.Wolley M.J., Wu A., Xu S., Gordon R.D., Fenton R.A., Stowasser M. In primary aldosteronism, mineralocorticoids influence exosomal sodium-chloride cotransporter abundance. J Am Soc Nephrol. 2017;28:56–63. doi: 10.1681/Asn.2015111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen N.B., Hofmeister M.V., Rosenbaek L.L., Nielsen J., Fenton R.A. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int. 2010;78:160–169. doi: 10.1038/ki.2010.130. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
- 30.Ellison D.H., Welling P. Insights into salt handling and blood pressure. N Engl J Med. 2021;385:1981–1993. doi: 10.1056/NEJMra2030212. [DOI] [PubMed] [Google Scholar]
- 31.van Buren M., Rabelink T.J., van Rijn H.J., Koomans H.A. Effects of acute NaCl, KCl and KHCO3 loads on renal electrolyte excretion in humans. Clin Sci (Lond) 1992;83:567–574. doi: 10.1042/cs0830567. [DOI] [PubMed] [Google Scholar]
- 32.van der Lubbe N., Moes A.D., Rosenbaek L.L., et al. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl- cotransporter. Am J Physiol Ren Physiol. 2013;305:F1177–F1188. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- 33.Hunter R.W., Craigie E., Homer N.Z., Mullins J.J., Bailey M.A. Acute inhibition of NCC does not activate distal electrogenic Na+ reabsorption or kaliuresis. Am J Physiol Ren Physiol. 2014;306:F457–F467. doi: 10.1152/ajprenal.00339.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein A.M. A mathematical model of the rat kidney: K(+)-induced natriuresis. Am J Physiol Ren Physiol. 2017;312:F925–F950. doi: 10.1152/ajprenal.00536.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L., Xu S., Guo X., et al. Regulation of renal Na transporters in response to dietary K. Am J Physiol Ren Physiol. 2018;315:F1032–F1041. doi: 10.1152/ajprenal.00117.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorensen M.V., Grossmann S., Roesinger M., et al. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 37.Braschi A., Naismith D.J. The effect of a dietary supplement of potassium chloride or potassium citrate on blood pressure in predominantly normotensive volunteers. Br J Nutr. 2008;99:1284–1292. doi: 10.1017/S0007114507864853. [DOI] [PubMed] [Google Scholar]
- 38.Naismith D.J., Braschi A. The effect of low-dose potassium supplementation on blood pressure in apparently healthy volunteers. Br J Nutr. 2003;90:53–60. doi: 10.1079/bjn2003861. [DOI] [PubMed] [Google Scholar]
- 39.Aburto N.J., Hanson S., Gutierrez H., Hooper L., Elliott P., Cappuccio F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013;346:f1378. doi: 10.1136/bmj.f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthesen S.K., Larsen T., Vase H., Lauridsen T.G., Pedersen E.B. Effect of potassium supplementation on renal tubular function, ambulatory blood pressure and pulse wave velocity in healthy humans. Scand J Clin Lab Investig. 2012;72:78–86. doi: 10.3109/00365513.2011.635216. [DOI] [PubMed] [Google Scholar]
- 41.Terker A.S., Zhang C., McCormick J.A., Lazelle R.A., Zhang C., Meermeier N.P. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blijdorp C.J., Hartjes T.A., Wei K.Y., et al. Nephron mass determines the excretion rate of urinary extracellular vesicles. J Extracell Vesicles. 2022;11 doi: 10.1002/jev2.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.