Abstract
A small fraction of recipients who receive polyethylene-glycol (PEG)-containing COVID-19 mRNA-LNP vaccines (Comirnaty and Spikevax) develop hypersensitivity reactions (HSRs) or anaphylaxis. A causal role of anti-PEG antibodies (Abs) has been proposed, but not yet been proven in humans. We used ELISA for serial measurements of SARS-CoV-2 neutralizing Ab (anti-S) and anti-PEG IgG/IgM Ab levels before and after the first and subsequent booster vaccinations with mRNA-LNP vaccines in a total of 291 blood donors. The HSRs in 15 subjects were graded and correlated with anti-PEG IgG/IgM, just as the anti-S and anti-PEG Ab levels with each other. The impacts of gender, allergy, mastocytosis and use of cosmetics were also analyzed. Serial testing of two or more plasma samples showed substantial individual variation of anti-S Ab levels after repeated vaccinations, just as the levels of anti-PEG IgG and IgM, which were over baseline in 98–99 % of unvaccinated individuals. About 3-4 % of subjects in the strongly left-skewed distribution had 15–45-fold higher values than the median, referred to as anti-PEG Ab supercarriers. Both vaccines caused significant rises of anti-PEG IgG/IgM with >10-fold rises in about ∼10 % of Comirnaty, and all Spikevax recipients. The anti-PEG IgG and/or IgM levels in the 15 vaccine reactors (3 anaphylaxis) were significantly higher compared to nonreactors. Serial testing of plasma showed significant correlation between the booster injection-induced rises of anti-S and anti-PEG IgGs, suggesting coupled anti-S and anti-PEG immunogenicity. Conclusions: The small percentage of people who have extreme levels of anti-PEG Ab in their blood may be at increased risk for HSRs/anaphylaxis to PEGylated vaccines and other PEGylated injectables. This risk might be further increased by the anti-PEG immunogenicity of these vaccines. Screening for anti-PEG Ab “supercarriers” may help predicting reactors and thus preventing these adverse phenomena.
Keywords: Covid-19, SARS-CoV-2, mRNA, Allergy, Anaphylaxis, Neutralizing antibodies, Spike protein, Hypersensitivity reactions, Complement, ELISA, Anti-PEG IgG, IgM
1. Introduction
The efficacy of mRNA-lipid nanoparticle (LNP)-based Covid-19 vaccines (Comirnaty and Spikevax) in reducing death or severe illness from SARS-CoV-2 infections is well recognized. However, as with all vaccinations, these vaccines may also have side effects in some people [1]. One of them is an allergic reaction, also known as a hypersensitivity reaction (HSR), whose most severe manifestation is anaphylaxis. Such anaphylactic reactions are rare and mostly controllable with epinephrine and other means of first aid. However, there are still life-threatening anaphylaxis cases, which is a major problem for severely allergic people [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]. Most recently, the manufacturer of Comirnaty lists these reactions prominently among the vaccine's adverse effects [19].
Since the mRNA-LNP vaccines include polyethylene glycol (PEG) as an excipient, allergy to PEG has been an obvious explanation for the anaphylaxis. However, it has been found that the overwhelming majority of these reactions are not IgE-mediated classic type I allergies against PEG [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [17], [20], leaving the underlying cause unclear. Among the studies addressing this puzzle, the possible causal role of anti-PEG antibodies (Abs) was raised by many authors [6], [13], [14], [16], [17], [20], [21], [22], but conclusive experimental or clinical evidence has not been presented to date.
Most recently, anti-PEG Abs have been reported in studies reporting their rise in Spikevax-vaccinated people [23], [24] and in rats immunized with PEGylated LNPs [25]. These data attest to the inducibility of these Abs by mRNA-LNP vaccines, while other studies highlight their specific binding to PEG on the surface of LNPs or liposomes, causing complement-mediated structural damage [26], [27] or accelerated blood clearance (ABC) [25], [28], [29], with concomitant loss of vaccine efficacy [30], [31]. Elevated levels of anti-PEG Abs that can contribute to HSRs were previously shown in clinical trials with the PEGylated protein, urate oxidase [30], [31] the PEGylated RNA aptamer, Pegnivacogin [32], [33] and most recently with PEGylated-granulocyte colony-stimulating factor (PEG-G-CSF) [34]. In animals, we have described lethal anaphylaxis caused by PEGylated liposomes in anti-PEG immunized pigs [35].
Based on these data, we hypothesized that a contributing factor to the occasional anaphylactic reactivity of mRNA-LNP vaccines could be high levels of anti-PEG IgG and/or IgM Abs. Accordingly, we measured the plasma levels of these Abs in people reporting HSRs after vaccinations with mRNA-LNP vaccines, as well as in nonreactor recipients of Comirnaty and Spikevax, before and after one or multiple vaccinations. We also compared the postvaccination anti-PEG Ab levels with the pre-vaccination values in both vaccine groups to explore anti-PEG immunogenicity, and correlated the SARS-CoV-2 neutralizing and anti-PEG Ab levels to explore possible joint immunogenicity.
2. Materials and methods
2.1. Study population
The volunteers for blood sample donations were between 8 months and 85 years of age from both sexes and were vaccinated once or several times with combinations of mRNA (Comirnaty or Spikevax) and/or other vaccines, including Astra Zeneca, Jansen, Sputnik, and Sinopharm. A total of 291 volunteers could be grouped, according to their immune status, to 1) “non-allergic”, 2) “allergic” and 3) “mastocytosis” subjects. The participant number and distinguishing features of these groups are specified in Table 1 .
Table 1.
Numbers of study participant grouped by their immune status and sample collection time relative to vaccination.
| Sample collection relative to vaccination |
|||||||
|---|---|---|---|---|---|---|---|
| Allergy status |
No vaccination |
pre + post |
Only post |
All | |||
| M | F | M | F | M | F | ||
| Non-allergic | 29 | 37 | 8 | 8 | 66 | 84 | 232 |
| Allergic | 3 | 16 | 1 | 5 | 8 | 15 | 48 |
| Mastocytosis* | 0 | 11 | 0 | 0 | 0 | 0 | 11 |
| total | 32 | 64 | 9 | 13 | 74 | 99 | 291 |
| All | 96 | 22 | 173 | ||||
Abbreviations: M, male; F, female; “No vaccination”, donors who were not vaccinated any COVID-19 vaccine vaccine; “Pre + post”, people gave blood both before and after vaccination with an mRNA-LNP vaccine; “Only post”, people who gave blood only after vaccination with an any COVID-19 vaccine.
Yet another grouping of blood donors considered the timing of blood withdrawal relative to their vaccination. Notably, 1) people who gave blood without Covid-19 vaccination (for exploring their anti-S antibody level as a sign of infection), 2) people who gave their blood both before and after vaccination with an any COVID-19 vaccine; and 3) those, who undertook the measurements only after their vaccination.
It should be noted that not all subjects were analyzed for all parameters, and some special conditions, e.g., steroid of cytostatic treatment, excluded people from certain assays despite being non-allergic and free from Covid-19.
In the column “Allergy status”, “Non-allergic” people did not reveal any major allergy in their life. The “allergic” group included people who displayed transient or persistent allergy to, among others, dust mite, mold, cat hair, foods, numerous pollen types, chemicals, drugs, or cosmetics. The “mastocytosis” group included only women who were diagnosed with the disease and had variable degrees of systemic and cutaneous symptoms (e.g., fatigue, watery stool, osteoporosis, bone and joint pain, hypotension, bradycardia, hyperventilation, asphyxia, coma, generalized eruption of erythematous macules, papules and plaques with variable amounts of brown pigment) with or without elevated blood tryptase. In particular, 1 participant had cutaneous symptoms only in the past, 2 had weak, and 1 strong cutaneous symptoms along with elevated blood tryptase levels. Three subjects had systemic symptoms occasionally with (2 subjects) or without (1 subject) weak cutaneous symptoms, and another 4 had severe systemic and cutaneous manifestations at the same time.
All participants or parents of minors filled out a consent form and questionnaire asking about their age, medical and vaccination history, cosmetic use, and symptoms of HSRs, if they had experienced them after Covid-19 vaccination. The study was approved by the Scientific and Research Ethics Committee of the Hungarian Medical Research Council (52685-6/2022/EÜIG) and, for Miskolc University, BORS-02/2021.
2.2. Materials
The Covid-19 vaccines were administered to the study participants at official vaccination sites. The ELISA kits for measuring SARS-CoV-2 neutralization antibody (TE 1076) were from TECOMedical AG, Sissach, Switzerland. Dulbecco’s phosphate-buffered saline (PBS) without Ca++/Mg++, 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS), bovine serum albumin, Tween-20 and peroxidase-labeled goat polyclonal anti-human IgG and IgM were from Sigma Chemical Co. (St. Louis, MO, USA).
2.3. Blood samples
Blood was collected into EDTA vacutainers, and the plasma was separated by centrifugation and then stored in aliquots at −80 °C until the various assays were performed. We also obtained samples from vaccine reactors from other health care facilities, transported on dry ice.
2.4. Antibody tests
Plasma levels of SARS-CoV-2 neutralization Ab, specific against the receptor binding region of viral spike protein (anti-S) [36], [37], were measured with an ELISA kit provided by TECOmedical AG. (Sissach, Switzerland, Catalog No. TE 1076). Serial measurements of blood anti-PEG IgM and IgG levels were performed with ELISA as described earlier [35], [38], [39].
Briefly, Polysorp (Nunc) plates were coated with DSPE-PEG-2000 overnight at 4 °C, followed by blocking of the wells with PBS/0.05 % Tween-20 (or, for IgG, 0.05 % CHAPS) + 2 % bovine serum albumin (BSA) at 37 °C for 1.5 h. Before blocking, wells were washed three times with wash buffer containing PBS/0.05 % Tween-20 (or 0.05 % CHAPS) for 1 min. Plasma samples, as well as humanized recombinant anti-PEG IgG, or IgM (Institute of Biomedical Sciences, Academia Sinica), used as reference standards, were diluted in the wash buffer supplemented with 1 % BSA, and incubated in the wells for 1.5 h at 37 °C, with slow shaking. Wells were washed five times with wash buffer for 1 min. After staining with HRP-conjugated anti-human IgM (Sigma) or IgG (Sigma) for 1 h, wells were washed again five times with wash buffer as mentioned. The Abs were stained by incubation for 15 min with substrate solution (Neogen) containing 3,3′,5,5′-tetramethylbenzidine (TMB) and hydrogen peroxide. The reaction was stopped with 2 N H2SO4, and A450 was read with a Fluostar Omega 96-well plate reader (BMG Labtech, Ortenberg, Germany).
2.5. Statistical analysis
The applied approaches, specified in the figure legends, included descriptive statistics, 2-tailed t-test on logarithmic transformed data, checking the normality of distribution by Shapiro-Wilk test, one-way ANOVA followed by Dunnett test, Wilcoxon signed rank test of normalized data and correlation analysis to test linear relationship between two variables.
3. Results
3.1. Plasma anti-PEG IgG and IgM levels before vaccination: impact of age, sex, cosmetic use, mastocytosis, and seasonal and chronic allergies
We found detectable levels of anti-PEG IgG in 98 %, and anti-PEG IgM in 99 % of unvaccinated blood donors, whose highly left-skewed distributions are shown in Fig. 1 A and B, respectively. The data showed log-normal distribution with considerable, 5–6 orders of magnitude span between the lowest and highest values in case of both Ab classes, with 4–5 substantially high values (15–45-fold higher than the respective median) in both groups. We refer to this 3–4 % of people as “anti-PEG Ab supercarriers”.
Fig. 1.
Pre-vaccination anti-PEG antibody concentrations in a mixed population of blood donors. Anti-PEG-IgG (A, green) and anti-PEG-IgM (B, red) values were sorted in growing order. The bottom bars (which look like lines) are absolute Ab concentrations. The inserted probability distribution histograms are made from the log of absolute Ab levels, grouped into bins on the abscissa [40]. The normality of log-transformed data was established by the Shapiro-Wilk test. The anti-PEG Ab levels were determined as described in the Methods, and the descriptive statistics of these data are shown in Table 2.
Table 2 lists the main statistical parameters derived from the anti-PEG Ab levels in men and women, revealing significantly higher median of original and mean of logarithmic transformed data in women compared to men.
Table 2.
Descriptive statistics of pre-vaccination anti-PEG-IgG and IgM levels and distributions in humans.
|
Anti-PEG IgG [ng(eq)/mL] |
Anti-PEG IgM [ng(eq)/mL] |
|||||||
|---|---|---|---|---|---|---|---|---|
| All | Male | Female | F/M# | All | Male | Female | F/M | |
| N of subjects | 116& | 40 | 76 | 1.90 | 118& | 41 | 77 | 1.88 |
| N below detection limit# | 2 | 1 | 1 | 1.00 | 1 | 0 | 1 | NA |
| 25 % Percentile | 10,584 | 8,281 | 14,929 | 1.80 | 38 | 23 | 46 | 2.00 |
| Median | 30,681 | 23,108 | 37,198 | 1.61 | 105 | 60 | 132* | 2.20 |
| Log-normal mean (μ) | 35,893 | 30,903 | 38,815 | 1.26 | 109 | 73 | 136** | 1.86 |
| SD of log-normal mean (σ) | 4 | 4 | 4 | 1.00 | 4 | 4 | 4 | 1.00 |
| Lower 99 % Cl of μ | 25,119 | 16,106 | 25,003 | 1.55 | 78 | 42 | 89 | 2.12 |
| Upper 99 % Cl of μ* | 51,404 | 59,293 | 60,395 | 1.02 | 153 | 129 | 207 | 1.60 |
| 75 % Percentile | 87,165 | 76,176 | 88,111 | 1.16 | 297 | 209 | 397 | 1.90 |
| Maximum | 1,362,175 | 1,048,553 | 1,362,175 | 1.30 | 2,796 | 1,829 | 2,796 | 1.53 |
| Maximum/Median | 44 | 45 | 37 | 0.81 | 27 | 30 | 21 | 0.69 |
| 95 % Percentile | 674,368 | 665,283 | 612,709 | 0.99 | 1,344 | 706 | 1,657 | 2.35 |
| 95 % Percentile/Median | 22.0 | 28,8 | 16,5 | 0.57 | 12.8 | 11.8 | 12.6 | 10.8 |
N, number; CI, confidence interval; #, F/M, female/male ratios; &The difference in male and female number of subjects is due to 2 erroneous IgG determinations; #, Samples below the detection limit were excluded from normalized data analysis; *, significant difference between female and male at p = 0,044 (Mann Whitney test); ** p = 0,023 (unpaired t test on logarithmic transformed data after their normal distribution was checked by the Shapiro-Wilk test).
Table 2 also shows that the pre-vaccination level anti-PEG IgM was significantly higher in women compared to men. Questioning about cosmetic use revealed that 1/3 of men were frequent cosmetic users versus >3/4 of women (Fig. 2 A), and the plasma level of anti-PEG IgM was significantly higher in frequent cosmetic users compared to rare users (Fig. 2C). Thus, the higher anti-PEG IgM in women can be explained, at least in part, by frequent use of cosmetics. Our data also suggested higher anti-PEG IgG levels in those donors who suffered from persistent allergies due to dust mites, mold, cat hair, foods, numerous pollen type, chemicals, drugs, or cosmetics (data not shown).
Fig. 2.
Use of cosmetics by women and men and blood anti-PEG antibody levels in the two genders. A) Ratio of frequent cosmetic users vs. no users or rare users in men and women. B) Blood levels of anti-PEG-IgG (B) and anti-PEG-IgM (C) in frequent vs. no/rare cosmetic users before COVID-19 vaccination. The anti-PEG Ab levels were determined as described in the Methods. *P < 0.05 by 2-tailed t-test of logarithmic transformed data.
Correlating the anti-PEG IgG or IgM levels with age showed no significant correlation (data not shown), and surprisingly, we found relatively high anti-PEG IgG (36 µg(eq)/mL) and IgM (401 ng(eq)/mL) even in a baby less than one year old. Eight children in the 6–11 year-old range also displayed high values (median of anti-PEG IgG and IgM were 27 µg(eq)/mL and 56 ng(eq)/mL, respectively). We have also analyzed the relative changes of anti-PEG-IgG and IgM levels in the blood of 6 people in whom at least 2 measurements were available at different time intervals before vaccination (different symbols in Fig. 3 A). Expressing the relative changes of the 2nd sample to the first as a ratio showed substantial variation of anti-PEG IgM over time, while the anti-PEG IgG remained relatively constant in the same individual regardless of time and baseline anti-PEG IgG value (Fig. 3A). This suggests that screening of people for anti-PEG IgG levels provides a better long-term estimate of their anti-PEG Ab status, while the level of anti-PEG IgM may rather reflect the actual level of immunization. Plotting all pre- and postimmunization anti-PEG IgG to IgM levels against each other in the same individuals also showed substantial individual variation without statistical correlation (Fig. 3B), implying different mechanisms of these anti-PEG Abs' formation. Notably, 3 of 4 extreme values on the anti-PEG IgG and IgM axes were from people with allergies or mastocytosis, raising the possibility of increased propensity towards abnormal isotype switching in them. Fig. 3C and D compares the pre-vaccination anti-PEG IgG (C) and IgM (D) levels in healthy, mastocytic, allergic, and persistent allergic people (to dust mite, dog hair and mold), suggesting significant elevation of only anti-PEG Ig in the persistent allergy group (Fig. 3C).
Fig. 3.
Anti-PEG antibody levels in people before Covid-19 vaccinations. A) Changes over time in 6 subjects who gave blood at least 2 times before vaccination against Covid-19. The second/first anti-PEG IgG and IgM ratios were plotted against the time elapsed time between the 1st and 2nd blood withdrawal. The different symbols represent different individuals, and the filling color of symbols distinguishes IgG and IgM (as shown by the keys). The non-significant linear fits on the relative changes of anti-PEG IgG and IgM indicate no significant impact of time on inter-individual changes. Moreover, the smaller variation of the relative changes of anti-PEG IgG compared to IgM in most individuals suggest higher intra-individual stability of blood anti-PEG IgG levels compared to anti-PEG IgM. B) Plotting of plasma anti-PEG-IgG against anti-PEG IgM in healthy blood donors before vaccination (black dots), as well as those with allergy (red squares) and mastocytosis (green triangles), shows no linear correlation between these Ig classes. R2 of regression line is 0.0213 (p >0.1). The blue-framed extreme values on both axes are mainly from subjects with allergy or mastocytosis. C) Anti-PEG IgG and (D) anti-PEG IgM levels in the groups indicated on the X axis on panel D. Antibody levels were determined as described in the Methods.
3.2. Plasma anti-PEG IgG and IgM levels after vaccination with Comirnaty, Spikevax and other vaccines
Fig. 4A and B show the individual and median anti-PEG IgG and IgM levels after vaccination with mRNA vaccines as well as other, PEG-free Covid-19 vaccines (Sinopharm, Sputnik V and Astra Zeneca), used to evaluate the effect of PEG upon antibody production. The pre-vaccination baseline, also shown in Fig. 1, displayed substantial individual variation for both Ab classes, relative to which highly significant rises after the first and 2nd booster vaccinations were found only for Spikevax (Fig. 3A and B). For Comirnaty, we found significant post-vaccination increase only in anti-PEG IgM after the second jab (Fig. 4B). However, the considerable individual variation of anti-PEG Ab levels over a broad range both before and after vaccination and the irrelevance of vaccination numbers when the question is the presence or absence of PEG immunogenicity led us to introduce another type of analysis, namely relating the maximal postvaccination values to the pre-vaccination baselines for each person regardless of injection number. This approach yielded highly significant rises in both anti-PEG IgG and IgM for Comirnaty also (Fig. 3C). As for other vaccines, the small number of preimmunization data did not yield statistically analyzable pre- and post-vaccination pairs, but the pooled data -as mentioned for Fig. 4A and B-, did not suggest the rise of anti-PEG Abs.
Fig. 4.
Pre- and postvaccination anti-PEG IgG and IgM antibodies of non-allergic participants. Anti-PEG IgG (A, green rectangles) and anti-PEG IgM (B, red circles) levels in people vaccinated with different Covid-19 vaccines shown on the X axis, wherein the numbers (1, 2 and 3) represent the order of vaccinations. C) Relative rises of maximal postvaccination anti-PEG IgG and IgM levels relative to the respective preimmunization value in people undergoing serial (at least 2) immunizations with Comirnaty. Both absolute (A and B) and relative values (C) are presented on a log scale. Of note, the time elapsed between the pre- and postvaccination measurements differs for each point. *, P < 0.05, ***, P < 0.001, ****, P < 0.0001 by one-way ANOVA of logarithmic transformed data followed by Dunnett test (A and B) and Wilcoxon signed rank test of normalized data (C).
3.3. Hypersensitivity reactions to Covid-19 mRNA-LNP vaccines and their association with anti-PEG antibodies
Table 3 categorizes all adverse events described by the 195 Comirnaty or Spikevax recipients of this study. Based on the definitions in the Table's legend we have grouped the study participants according to reaction type, clinical grades, and Brighton levels [41]. 72 % of vaccinees were reaction free (Grade 0), 20 % experienced common vaccine side effects (Grade 1), and 8 % reported Grade 2 or 3 HSR symptoms. There were more women among the reactors than men, and the 3 anaphylaxis cases were all women.
Table 3.
Incidence of adverse reactions to mRNA/LNP Covid-19 vaccines.
| Clinically-based grading* | Symptom types | Brighton Levels** | Female | Male | All*** |
|---|---|---|---|---|---|
| Grade 0 | none | NA | 74 | 67 | 141 (72 %) |
| Grade 1 (Mild) | usual vaccine reactions | 28 | 11 | 39 (20 %) | |
| Grade 2 (Moderate) | hypersensitivity reactions | ≥2 | 7 | 5 | 12 (6 %) |
| Grade 3 (Severe) | 1 | 3 | 0 | 3 (2 %) | |
| Total | 112 | 83 | 195 (100 %) | ||
*Grade 0 is no adverse effect, Grade 1 “usual vaccine symptoms” include fever, arm pain, local redness, weakness, headache, chills, pruritus, depression, abdominal pain, indigestion, bloating, arthralgia, muscle pain, or fatigue. Grade 2 reactions represent allergic symptoms which pass spontaneously with or without epinephrine, antihistamines, or other pharmaceutical interventions. Grade 3 defines anaphylaxis or life-threatening reactions requiring emergency care (resuscitation and/or hospitalization).
**Level of diagnostic certainty for anaphylaxis, as defined in the Brighton Collaboration's anaphylaxis case definition guidelines [41]. According to this system level 1 “true anaphylaxis” is distinguished from other manifestations of allergy by its “diagnostic certainty”, defined by a complex matrix of major and minor criteria. Our Grade 3 reactions roughly correspond to Brighton level 1, i.e., true anaphylaxis, wherein at least a major hemodynamic/circulatory or cardiopulmonary symptom (heart/back/limb pain, hypo- or hypertension, (angio)edema, swelling of the lips, tongue, or face) and a major skin alteration (i.e., flushing, rash, erythema) are concurrently present. Brighton levels ≥ 2 (Grade 2) are defined as all moderate (Grade 2) HSRs that involve non-life-threatening symptoms.
***The % values represent incidence in the 195 participants of this study involving both healthy and allergic people who were recalled for blood donation because of his/her HSR.
The symptoms of 15 allergy responders included in this study are listed in Table 4 , along with the gender, age range, the vaccine type and number, and the severity of HSRs.
Table 4.
Hypersensitivity reactions in recipients of mRNA-containing Covid-19 vaccines.*
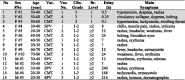 |
*Immediate and delayed reactions combined. Shaded area: cases diagnosed as anaphylaxis by the referring vaccination center. Abbreviations: F, female; M, male; Vac: vaccine; CMT, Comirnaty; SPV, Spikevax; Vac. No., jab number triggering the reaction; Clin., clinical; Br., Brighton; Delay (h), hours between the HSR and vaccination; question mark (?), unknown.
The above information in Table 4 reveals substantial individual variation of symptoms affecting mainly the circulatory, cardio-pulmonary, the nervous system, and the skin Fig. 4.
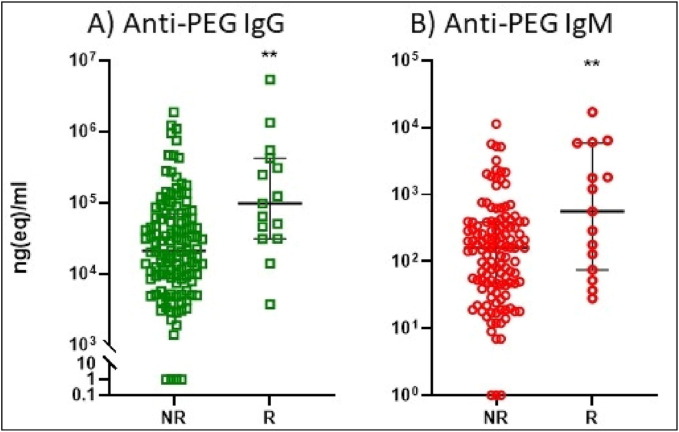
Fig. 5 hows the anti-PEG IgG (A) and IgM (B) levels in reactor people displaying HSRs (R) compared to non-reactor (NR) subjects. Both Ab levels were significantly higher in the R group compared to the NR, which suggests increased propensity of R people for anti-PEG Ab responses. However, because the blood withdrawals were not coincidental with the HSRs, temporal linkage between anti-PEG Ab rises and HSRs could not be assessed from these data.
Fig. 5.
Anti-PEG IgG (A), IgM (B) in reactor people displaying HSRs (R) compared to non-reactor (NR) subjects. The symptoms observed in the NR (no adverse effects and Grade 1) and R (Grade 2 and 3) patients are detailed in Table 4. The time span between Ab determinations and vaccinations was 4 months. **P < 0.01 by t-test of logarithmic transformed data.
3.4. Correlation between the anti-PEG IgG and SARS-CoV-2 neutralization antibody (anti-S IgG) levels after booster vaccinations with Comirnaty: Coupled memory immune response against the S-protein and PEG
Serial measurements of anti-SARS-CoV-2 neutralizing, S protein-binding Abs (anti-S) in the plasma from 190 blood donors (n = 362 tests) before and after vaccinations multiple times (in the 1–9 range) showed several versions of anti-S responses, including small and large increases followed by steeper or weaker declines, constant low or high levels, or strong or weak initial or late responses (Supplement Table S1). This individual variation of antiviral immune response is in keeping with the variable and relatively short duration of immunity provided by the current mRNA/LNP vaccines, necessitating booster injections.
Plotting the anti-S levels versus the anti-PEG IgG and IgM after the first immunizations and first booster injections in different subjects within 100 days after the first vaccination with Comirnaty (without any sign of Covid-19 infection) (Fig. 6 A-D) showed significant correlation only for IgG after the booster injection (Fig. 6B). This observation, referred to as coupled memory immune response against the S-protein and PEG, is consistent with the claim of PEG immunogenicity of mRNA/LNP vaccines and may have major clinical implications but needs more studies to confirm and understand.
Fig. 6.
Anti-S Ab levels as a function of anti-PEG Abs within 100 days after the first or the second vaccination with Comirnaty. Donors with any sign of Covid-19 infection were excluded. Each points represent the highest Anti-S value obtained in serial plasma samples in each individual. The goodness of linear fitting is represented by R2 values on the figures. P < 0.05 implies that the slope is significantly non-zero after linear regression.
4. Discussion
4.1. Statistics on severe hypersensitivity reactions and anaphylaxis to mRNA/LNP vaccines
There is consensus in the literature [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [20] that the prevalence of anaphylaxis caused by mRNA-LNP vaccinations is slightly increased relative to that of flu vaccines (∼1.3 anaphylaxis/million [42]. Notably, the estimated prevalence of anaphylaxis to Comirnaty and Spikevax is in the 3/million [4], [18], [43] to 443/million range [3] (Table 5 ), and its incidence is in decline as awareness of the risk grows and precautions are heightened [18].
Table 5.
Different statistics on the incidence and prevalence of anaphylaxis to mRNA-LNP vaccines.
| Vaccine | Incidence (n*) | Total N | Ana/million | Ana % | References |
|---|---|---|---|---|---|
| Comirnaty | 47 | 9,943,247 | 5 | 0.00047 | [5], [57] |
| Spikevax | 19 | 7,581,429 | 3 | 0.00025 | [4], [43] |
| Comirnaty | 46 | 14,475,979 | 3 | 0.00032 | [18] |
| Comirnaty | 3730 | 242,000,000 | 15 | 0.00154 | [39] |
| Spikevax | 1455 | 154,000,000 | 9 | 0.00094 | |
| Comirnaty + Spikevax | 1 | 4000 | 250 | 0.02500 | [8] |
| Comirnaty | 7 | 25,929 | 270 | 0.02700 | [9] |
| Spikevax | 9 | 38,971 | 231 | 0.02309 | |
| Comirnaty | 14 | 31,635 | 443 | 0.04425 | [3] |
| Spikevax | 3 | 7260 | 413 | 0.04132 | |
| Comirnaty** | 3 | 429 | 6993 | 0.69900 | [11] |
n*, number of reported anaphylaxis or “severe” HSR cases after “N” total number of immunizations; **, only highly allergic people were included in the statistic and the Ana/million figure represents a projection to 1 million vaccinations assuming identical incidence. The median of Ana/million in the normal population, i.e., 123, was calculated from the entries in the Ana/million column (except 6993).
Nevertheless, the ∼1.8 billion mRNA-LNP injections given worldwide until Dec. 31, 2022 (1,461,838,752 Comirnaty and 374,447,652 Spikevax) [44] places even the lowest estimate of the sheer number of anaphylaxis cases in the multiple thousand range and calculating with the median of estimated anaphylaxis rate (123 cases/million) results in 223,200 cases of anaphylaxis. It should not be forgotten either that anaphylaxis is only the tip of the iceberg, the worst scenario in a broad spectrum of HSR symptoms.
Table 5 also shows that the incidence of vaccine-induced anaphylaxis in people with severe allergies is ∼0.7 % [11] (bolded), which is ∼57-fold higher than the median of reported rates (i.e., 0.0123 %) in the normal population.
In keeping with these numbers, despite the exclusion from vaccination of people with a history of a severe allergic reaction to any vaccine component, anaphylaxis and/or allergic reactions to Comirnaty became front runners in the manufacturer's most recent list of adverse effects [19].
4.2. Prevalence of plasma anti-PEG IgG and IgM levels before vaccination
Our detection of anti-PEG IgG and IgM positivity in 98–99 % of blood donors is higher than the 10–76 % prevalence range for these Abs described in older studies [23], [32], [33], [45], [46], [47], [48], [49]. The variation may partly be due to the differences in cohort size, inclusion criteria, time points for blood collection, and methodical differences in assaying [50]. Our 541 tests in 291 blood donors represent the largest exploration of anti-PEG IgG and IgM levels in humans to date, measuring both the pre-vaccination (pre-existing) levels and the immunogenicity of PEGylated Covid-19 vaccines in a mixed human population “enriched” with allergic people and patients with mastocytosis. The sampling time was random in the 2 to 626 days interval after the 1st vaccination, thus enabling the detection of immune responses over a broad time window.
As for the methodological differences, unlike all other above-referred studies, we used 2 K-PEGylated lipid antigen in the sandwich ELISA, which is also present in the vaccine. It should be noted in this regard that natural anti-PEG Abs are polyclonal; they recognize different parts of PEG with different affinities and avidities [38]. Thus, depending on the PEGylated immunogen that induced high levels of anti-PEG Abs in different individuals (which may show geographical, regional and local differences), the spectrum of these Abs can show substantial individual variation and, hence, different results with different assays using unique combination of antigens and assay conditions. For these reasons, it is perhaps not surprising that the anti-PEG ELISA results show substantial inter-laboratory variation.
The presence of baseline Ab levels in most participants is most easily rationalized by a low-level immunization via daily exposure to PEGylated immunogens that is present in many oral, topical, and parenteral pharmaceutical products, hygiene and cosmetic items, and processed food and beverages [51]. Immunization by cosmetic products via the skin of mice was shown recently by Ibrahim et al [52]. It is also important to mention that Abs against PEG and polysorbate-80 (PS-80), which is a branched PEG derivative, can mutually cross-react [38], [53], [54], and PS-80 is also widespread as an ingredient in drugs, vaccines, vitamins, food products, and beverages [51], [55]. Thus, the anti-PEG ELISA does not necessarily distinguish between anti-PEG and anti-polysorbate Abs.
The highly left-skewed distribution of pre-vaccination anti-PEG Abs in our study resembles the distribution of these Abs in patients treated with the PEGylated RNA aptamer, Pegnivacogin [32]. On the other hand, our data may be the first to document this distribution of natural anti-PEG Abs in the preimmunization blood samples. The finding of an increased prevalence of anti-PEG Abs in females compared to males is likely a consequence of increased cosmetics use by women and is in line with many studies reporting such gender difference [23], [32], [33], [45], [46], [47], [48].
4.3. Plasma anti-PEG IgG and IgM levels after vaccinations
Our finding that Spikevax induces a highly significant increase in anti-PEG Abs is consistent with the recent data of Carreno et al. [24] and Ju et al. [23], with both groups coming to the same conclusion. Regarding our finding that Comirnaty also causes a similar, although less pronounced effect, which is seen only upon pairwise comparison, is also consistent with the latter two studies although both concluded that there was no such effect in the case of Comirnaty [23], [24]. However, a closer look at their data reveals very similar increases in anti-PEG IgG and IgM after vaccination with Comirnaty as we have seen in a pairwise comparison (Fig. 4C). Notably, in the study of Carreno et al. [24], Fig. 2 shows a 50–300 % rise of prime/baseline anti-PEG IgG in 4/10 subjects expressed as log AUC, and Fig. S1 in the study of Ju et al. [23], which shows 13/17, 4/9 and 10/15 endpoint dilution value pairs in cohorts 1, 2 and 3, respectively, where the post-booster values visibly exceed the pre-vaccination values by >20 % up to several-fold on a log scale.
Most recently, Bavli et al reported highly significant increases in mean anti-PEG IgG from 7.8 to 17.5 μg/mL three weeks after immunization of 79 people with Comirnaty, while no rise of anti-PEG IgM or IgE were found [50]. Consistent with our observations in occasional super-responders, 2 of the 79 individuals experienced large (>50 μg/mL) increases in anti-PEG IgG [50].
In animals, Wang et al showed that Comirnaty as well as other simulations of approved PEGylated LNP drugs caused significant time- and dose-dependent generation of anti-PEG IgM and IgG after immunization in rats [25]. Furthermore, these authors found that these injections caused not only isotype switching but also immune memory, inasmuch as the response times and intensities were enhanced after the second injection [25]. Such “full-scale” immunogenicity of PEG is in keeping with our observation on significant linear correlation between anti-PEG IgG and anti-S IgG Ab formations in people obtaining the booster dose of Comirnaty (Fig. 6B). Although the possibility of a positive feedback loop between anti-PEG and anti-S immunogenicity has not been formally explored, complement activation by Comirnaty by at least 4 different mechanisms (i.e., antibody binding, liberation of mRNA and positively charged lipids and S-protein liberation [17], [39]) could feed a vicious cycle among anaphylatoxin liberation, HSRs and anti-PEG Ab formation [56], thereby explaining the occasional spike of anaphylaxis in anti-PEG super-carriers [30], [31], [32], [33].
5. Limitations of the study
HSR/anaphylaxis to mRNA-LNP vaccines is a very rare, unpredictable acute disease. It is therefore not possible to recruit reactors as a group for prospective studies. To study the phenomenon, we can use model systems, such as the pig model described earlier [39] or collect information about past reactions and recruit the reactors for retrospective studies. Here we pursued the latter approach and analyzed the blood of reactors (as well as nonreactors) within an individually variable, up to ∼ 3-months’ time window after vaccination. Therefore, the anti-PEG Ab levels we measured informed about the propensity of the individuals to produce such antibodies, rather than measuring their actual level at the time of the reactions. Another limitation is the heterogenicity of study population. To our best knowledge, the only condition that has been known to have significant impact on the rate of HSR/anaphylaxis is allergy [11], which is also a frequent symptom of mastocytosis. Thus, we classified these people in separate groups and made no exclusion criteria for the participants. Nevertheless, because the study was “enriched” with atopic, hypersensitive people, it is not a classical epidemiological surveillance of vaccine adverse effects, and the reaction prevalence data do not apply for the general population.
6. Outlook
We found in this study significantly increased levels of anti-PEG Abs in people who displayed HSRs to PEGylated vaccines compared to non-reactors, which finding, by itself, does not prove a causal relationship between anti-PEG Abs and HSRs. However, taken together with the unambiguous evidence for at least a contributing role of anti-PEG Abs to anaphylaxis and efficacy loss in clinical and experiential studies with PEGylated NPs bearing similarities to mRNA-LNP [28], [29], [30], [31], [32], [33], [35], the warning by Ganson et al.: “we advise testing for pre-existing anti-PEG antibodies during clinical trials of new PEGylated therapeutic agents” [32] seems to be valid for mRNA-LNP vaccinations too, particularly in light of the potential need for frequent booster injections with these Covid vaccines and the intense development of other mRNA-LNP injectables. The continual exposure to PEGylated food and consumer products in our everyday life and the high frequency of atopic disease in humans are additional good reasons for increased attention to the “bystander damage” caused by anti-PEG Abs.
Funding
The financial support by the European Union Horizon 2020 projects 825828 (Expert) and 952520 (Biosafety) are acknowledged. This project was supported by a grant from the National Research, Development, and Innovation Office (NKFIH) of Hungary (2020-1.1.6-JÖVŐ-2021-00013). JS thanks the logistic support by the Applied Materials and Nanotechnology, Center of Excellence, Miskolc University, Miskolc, Hungary.
Author contribution
GTK and JS conceived and designed the study, GTK, Zs.P., Cs.Zs. O, A.N., and K.A.G. recruited the participants, T.M., P.B., R.F., collected the data, GTK, JS, T.R, B.M analyzed and interpreted the data; J.Sz. wrote the manuscript, GTK, J.Sz., T.R, B.M. discussed the data and critically reviewed and revised the manuscript; J.S., T.R. and B.M. provided grant support for the measurements. All authors approved the final version of the manuscript for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Marieluise Wippermann (TECOMedical, Sissach, Switzerland) for providing the SARS-CoV-2 neutralization kits. Thanks are due to Dr. Judit Varkonyi for recruiting patients with mastocytosis, Ms. Anum Bashir and Mr. Zoltan Szebeni for proofreading the ms.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.06.009.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Fraiman J., Erviti J., Jones M., Greenland S., Whelan P., Kaplan R.M., et al. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine. 2022;40:5798–5805. doi: 10.1016/j.vaccine.2022.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: Current evidence and suggested approach. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren C.M., Snow T.T., Lee A.S., Shah M.M., Heider A., Blomkalns A., et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. Am J Transplant. 2021;21:1326–1331. doi: 10.1111/ajt.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021 doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kounis N.G., Koniari I., de Gregorio C., Velissaris D., Petalas K., Brinia A., et al. Allergic reactions to current available COVID-19 vaccinations. Pathophysiol, Causality, Therap Considerations Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim X.R., Leung B.P., Ng C.Y.L., Tan J.W.L., Chan G.Y.L., Loh C.M., et al. Pseudo-anaphylactic reactions to Pfizer BNT162b2 vaccine: Report of 3 cases of anaphylaxis post Pfizer BNT162b2 vaccination. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krantz M.S., Bruusgaard-Mouritsen M.A., Koo G., Phillips E.J., Stone C.A., Jr., Garvey L.H. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: Don't give up on the second dose! Allergy. 2021;76:2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B., et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nittner-Marszalska M., Rosiek-Biegus M., Kopec A., Pawlowicz R., Kosinska M., Lata A., et al. Pfizer-BioNTech COVID-19 vaccine tolerance in allergic versus non-allergic individuals. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9060553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shavit R., Maoz-Segal R., Iancovici-Kidon M., Offengenden I., Haj Yahia S., Machnes Maayan D., et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gringeri M., Mosini G., Battini V., Cammarata G., Guarnieri G., Carnovale C., et al. Preliminary evidence on the safety profile of BNT162b2 (Comirnaty): new insights from data analysis in EudraVigilance and adverse reaction reports from an Italian health facility. Hum Vaccin Immunother. 2021;17:2969–2971. doi: 10.1080/21645515.2021.1917236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risma K.A., Edwards K.M., Hummell D.S., Little F.F., Norton A.E., Stallings A., et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021 doi: 10.1016/j.jaci.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McSweeney M.D., Mohan M., Commins S.P., Lai S.K. Anaphylaxis to Pfizer/BioNTech mRNA COVID-19 vaccine in a patient with clinically confirmed PEG allergy. Front Allergy. 2021;2 doi: 10.3389/falgy.2021.715844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfson A.R., Robinson L.B., Li L., McMahon A.E., Cogan A.S., Fu X., et al. First-dose mRNA COVID-19 vaccine allergic reactions: Limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9:3308–3320. doi: 10.1016/j.jaip.2021.06.010. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luxi N., Giovanazzi A., Arcolaci A., Bonadonna P., Crivellaro M.A., Cutroneo P.M., et al. Allergic reactions to COVID-19 vaccines: Risk factors, frequency, mechanisms and management. BioDrugs. 2022;36:443–458. doi: 10.1007/s40259-022-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szebeni J., Storm G., Ljubimova J.Y., Castells M., Phillips E.J., Turjeman K., et al. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat Nanotechnol. 2022;17:337–346. doi: 10.1038/s41565-022-01071-x. [DOI] [PubMed] [Google Scholar]
- 18.Anis E., Preis S.A., Cedar N., Tal Y., Hershkowitz I., Hershko A.Y. Reporting of allergic reactions during Pfizer-BioNTech BNTT162B2 vaccination in Israel. J Allergy Clin Immunol Pract. 2022 doi: 10.1016/j.jaip.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfizer Responds to Research Claims. Press release on January 27, 2023 - 08:00pm. https://wwwpfizercom/news/announcements/pfizer-responds-research-claims; 2023.
- 20.Garvey L.H., Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anesthesia. 2020 doi: 10.1016/j.bja.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrieze J.d. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science. 2020 [Google Scholar]
- 22.Sellaturay P., Nasser S., Islam S., Gurugama P., Ewan P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51:861–863. doi: 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju Y., Lee W.S., Pilkington E.H., Kelly H.G., Li S., Selva K.J., et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano. 2022 doi: 10.1021/acsnano.2c04543. [DOI] [PubMed] [Google Scholar]
- 24.Carreno J.M., Singh G., Tcheou J., Srivastava K., Gleason C., Muramatsu H., et al. mRNA-1273 but not BNT162b2 induces antibodies against polyethylene glycol (PEG) contained in mRNA-based vaccine formulations. Vaccine. 2022 doi: 10.1016/j.vaccine.2022.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Wang Y, Yuan C, Xu X, Zhou W, Huang Y, et al. Polyethylene glycol (PEG)-associated immune responses triggered by clinically relevant lipid nanoparticles. 2023: 2022.11.24.516986. [DOI] [PMC free article] [PubMed]
- 26.Chen E., Chen B.M., Su Y.C., Chang Y.C., Cheng T.L., Barenholz Y., et al. Premature drug release from polyethylene glycol (PEG)-coated liposomal doxorubicin via formation of the membrane attack complex. ACS Nano. 2020 doi: 10.1021/acsnano.9b07218. [DOI] [PubMed] [Google Scholar]
- 27.Estape Senti M., de Jongh C.A., Dijkxhoorn K., Verhoef J.J.F., Szebeni J., Storm G., et al. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J Control Release. 2021;341:475–486. doi: 10.1016/j.jconrel.2021.11.042. [DOI] [PubMed] [Google Scholar]
- 28.Ishida T., Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int J Pharm. 2008;354:56–62. doi: 10.1016/j.ijpharm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Abu Lila A.S., Szebeni J., Ishida T. In: Immune effects of biopharmaceuticals and nanomedicines. Bawa R., Szebeni J., Webster T.J., Audette G.F., editors. Pan Stanford Publishing Pte. Ltd.; Singapore: 2018. Accelerated blood clearance phenomenon and complement activation-related pseudoallergy: Two sides of the same coin; pp. 771–800. [Google Scholar]
- 30.Hershfield M.S., Ganson N.J., Kelly S.J., Scarlett E.L., Jaggers D.A., Sundy J.S. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther. 2014;16 doi: 10.1186/ar4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calabrese L.H., Kavanaugh A., Yeo A.E., Lipsky P.E. Frequency, distribution and immunologic nature of infusion reactions in subjects receiving pegloticase for chronic refractory gout. Arthritis Res Ther. 2017;19 doi: 10.1186/s13075-017-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganson N.J., Povsic T.J., Sullenger B.A., Alexander J.H., Zelenkofske S.L., Sailstad J.M., et al. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol. 2016;137:1610–1613. doi: 10.1016/j.jaci.2015.10.034. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Povsic T.J., Lawrence M.G., Lincoff A.M., Mehran R., Rusconi C.P., Zelenkofske S.L., et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J Allergy Clin Immunol. 2016;138:1712–1715. doi: 10.1016/j.jaci.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 34.Okada N., Taro S., Ando H., Nakamura S., Goda M., Abe M., et al. Clinical impact of antipolyethylene glycol (PEG) antibody in hematological patients administered PEGylated-granulocyte colony-stimulating factor. Clin Pharmacol Drug Dev. 2023 doi: 10.1002/cpdd.1225. [DOI] [PubMed] [Google Scholar]
- 35.Kozma G.T., Meszaros T., Vashegyi I., Fulop T., Orfi E., Dezsi L., et al. Pseudo-anaphylaxis to polyethylene glycol (PEG)-coated liposomes: Roles of anti-PEG IgM and complement activation in a porcine model of human infusion reactions. ACS Nano. 2019;13:9315–9324. doi: 10.1021/acsnano.9b03942. [DOI] [PubMed] [Google Scholar]
- 36.Neumann F., Rose R., Rompke J., Grobe O., Lorentz T., Fickenscher H., et al. Development of SARS-CoV-2 specific IgG and virus-neutralizing antibodies after infection with variants of concern or vaccination. Vaccines (Basel) 2021 doi: 10.3390/vaccines9070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose R., Neumann F., Grobe O., Lorentz T., Fickenscher H., Krumbholz A. Humoral immune response after different SARS-CoV-2 vaccination regimens. BMC Med. 2022;20 doi: 10.1186/s12916-021-02231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozma G.T., Shimizu T., Ishida T., Szebeni J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. 2020;154–155:163–175. doi: 10.1016/j.addr.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Dezsi L., Meszaros T., Kozma G., HV M., Olah CZ, Szabo M., et al. A naturally hypersensitive porcine model may help understand the mechanism of COVID-19 mRNA vaccine-induced rare (pseudo) allergic reactions: complement activation as a possible contributing factor. Geroscience. 2022;44:597–618. doi: 10.1007/s11357-021-00495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roederer M., Moore W., Treister A., Hardy R.R., Herzenberg L.A. Probability binning comparison: a metric for quantitating multivariate distribution differences. Cytometry. 2001;45:47–55. doi: 10.1002/1097-0320(20010901)45:1<47::aid-cyto1143>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 41.Ruggeberg J.U., Gold M.S., Bayas J.M., Blum M.D., Bonhoeffer J., Friedlander S., et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 42.Bardenheier B.H., Duderstadt S.K., Engler R.J., McNeil M.M. Adverse events following pandemic influenza A (H1N1) 2009 monovalent and seasonal influenza vaccinations during the 2009–2010 season in the active component U.S. military and civilians aged 17–44years reported to the Vaccine Adverse Event Reporting System. Vaccine. 2016;34:4406–4414. doi: 10.1016/j.vaccine.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Our-World-In-Data Internet link. Covid-vaccine-doses-by-manufacturer. https://ourworldindataorg/grapher/covid-vaccine-doses-by-manufacturer; 2023.
- 45.Armstrong JK. The occurrence, induction, specificity and potential effect of antibodies against poly(ethylene glycol). In: Veronese FM, editor. PEGylated protein drugs: Basic science and clinical applications. Birkhäuser Verlag/Switzerland; 2009. p. 147-68.
- 46.Garay R.P., El-Gewely R., Armstrong J.K., Garratty G., Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 2012;9:1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- 47.Chen B.M., Su Y.C., Chang C.J., Burnouf P.A., Chuang K.H., Chen C.H., et al. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal Chem. 2016;88:10661–10666. doi: 10.1021/acs.analchem.6b03109. [DOI] [PubMed] [Google Scholar]
- 48.Lubich C., Allacher P., de la Rosa M., Bauer A., Prenninger T., Horling F.M., et al. The mystery of antibodies against polyethylene glycol (PEG) - what do we know? Pharm Res. 2016;33:2239–2249. doi: 10.1007/s11095-016-1961-x. [DOI] [PubMed] [Google Scholar]
- 49.Chen B.M., Cheng T.L., Roffler S.R. Polyethylene glycol immunogenicity: Theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano. 2021;15:14022–14048. doi: 10.1021/acsnano.1c05922. [DOI] [PubMed] [Google Scholar]
- 50.Bavli Y., Chen B.M., Gross G., Hershko A., Turjeman K., Roffler S., et al. Anti-PEG antibodies before and after a first dose of Comirnaty(R) (mRNA-LNP-based SARS-CoV-2 vaccine) J Control Release. 2023;354:316–322. doi: 10.1016/j.jconrel.2022.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The allergist. Medications containing PEG/Polysorbate. The allergist; 2021 https://theallergist.wordpress.com/2021/03/02/medications-containing-peg-polysorbate/.
- 52.Ibrahim M., Shimizu T., Ando H., Ishima Y., Elgarhy O.H., Sarhan H.A., et al. Investigation of anti-PEG antibody response to PEG-containing cosmetic products in mice. J Control Release. 2023;354:260–267. doi: 10.1016/j.jconrel.2023.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Nin-Valencia A., Fiandor A., Lluch M., Quirce S., Caballero T., Heredia Revuelto R., et al. Safe administration of SARS-CoV-2 vaccine after desensitization to a biologic containing polysorbate 80 in a patient with polyethylene glycol-induced severe anaphylaxis and sensitization to polysorbate 80. J Investig Allergol Clin Immunol. 2022;0 doi: 10.18176/jiaci.0830. [DOI] [PubMed] [Google Scholar]
- 54.Li Y., Duan J., Xia H., Li Y., Shu B., Duan W. Macromolecules in polysorbate 80 for injection: an important cause of anaphylactoid reactions. BMC Pharmacol Toxicol. 2022;23 doi: 10.1186/s40360-022-00591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drugs.com. Polysorbate-80. https://wwwdrugscom/inactive/polysorbate-80-372html#ref1; 2022.
- 56.Gabizon A., Szebeni J. Complement activation: A potential threat on the safety of poly(ethylene glycol)-coated nanomedicines. ACS Nano. 2020;14:7682–7688. doi: 10.1021/acsnano.0c03648. [DOI] [PubMed] [Google Scholar]
- 57.FDA Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.