Abstract
A 5-year-old female Beagle Dog was euthanized following ten days of inappetence, lethargy, and pain in the left cervical region that was not responsive to steroids or antibiotics. At necropsy, there were multiple soft dark red to tan nodules throughout all lung lobes, abundant purulent subdural exudate over the right temporal lobe of the brain, and minimally enlarged submandibular and tracheobronchial lymph nodes. Impression smear of the subdural pus and histologic section of the lung and meninges demonstrated small aggregates of rod-shaped to filamentous bacteria often surrounded by Splendori-Hoeppli material. Aerobic culture of the subdural exudate yielded pure growth of Actinomyces bowdenii. To our knowledge, this is the first report of central nervous disease or pneumonia associated with Actinomyces bowdenii.
Keywords: Actinomyces, Bowdenii, Meningitis, Pneumonia
Genus Actinomyces is a ubiquitous group of facultative to obligate anaerobic, gram positive bacteria (Sykes, 2012). Actinomyces spp. are found as commensals in the oral cavity and on other mucosal surfaces in many mammalian species, including humans (Bowden, 1996; Georg et al., 1972). Infections in animals are usually opportunistic and localized, with the classic example being Actinomyces bovis causing “lumpy jaw” in cattle (Mauldin & Peters-Kennedy, 2016). Here, we present a case of systemic Actinomyces bowdenii in a dog.
A 5-year-old female Beagle dog, house dust mite-sensitized and used for atopy investigations by a contract research organization, was examined for inappetence and lethargy of two days’ duration. With the exception of mildly prolonged capillary refill time (CRT), physical exam was normal. Complete blood count (CBC), clinical chemistries, fecal flotation, urinalysis, thoracic radiographs, and abdominal ultrasound were within normal limits. The dog had a history of transient self-resolving inappetence, so it was offered wet food and monitored.
Three days later, the patient developed signs of pain upon cervical spinal manipulation and upon manual opening of its mouth, and was noted to have difficulty masticating. The mandible and maxilla were symmetrical with no appreciable pain when palpated, although there was mild atrophy of the muscles of mastication. Cervical radiographs were normal, a CBC demonstrated mild neutrophilia (10.32 × 103 per µL), and chemistries revealed an increase in alkaline phosphatase (ALP) from baseline (37 IU/L initially, increased to 110 IU/L). The dog received subcutaneous fluids as needed and maropitant citrate (Cerenia®, Zoetis Inc.) 0.89 mg/kg once daily subcutaneously, and dexamethasone 0.2 mg/kg intramuscularly (IM) twice daily was initiated for suspected steroid-responsive meningitis-arteritis (Beagle Pain Syndrome; other primary differentials were cervical intervertebral disc disease, trauma, and early masticatory myositis). Following three days of treatment the dog's clinical signs were unimproved, neutrophilia had progressed and developed a left shift (11.19 × 103 per µL, 35% band neutrophils). Cranial nerve exam, a brief facial palpation, and repeat cervical and thoracic radiographs were normal. Given the development of a left shift and lack of change in clinical signs, differentials of steroid-responsive meningitis-arteritis, intervertebral disc disease, masticatory myositis, and trauma were now deemed less likely, and occult bacterial (less likely: fungal) infection of cranial or cervical soft tissues deemed more likely. Given the dog's status as a research animal, limited diagnostic capacity on site, and potential for advanced interventions to interfere with the dog's use as a research animal, additional diagnostics were not pursued. Dexamethasone dose was decreased to 0.3 mg/kg IM once daily, enrofloxacin 5 mg/kg IM daily was initiated, and when the patient continued to decline over the ensuing 72 h, it was humanely euthanized via intravenous injection of a pentobarbital-based euthanasia solution (nine days after initial presentation).
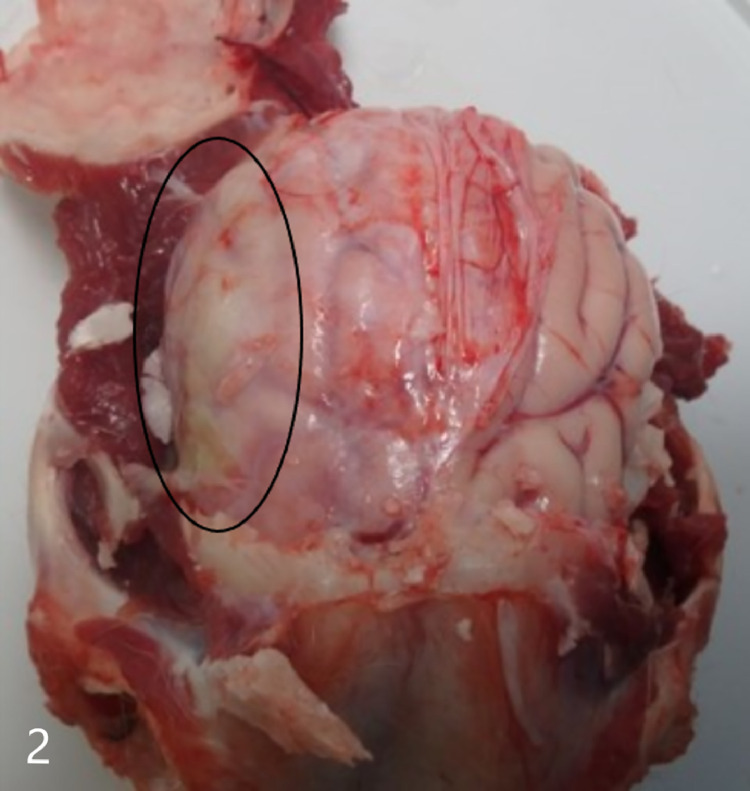
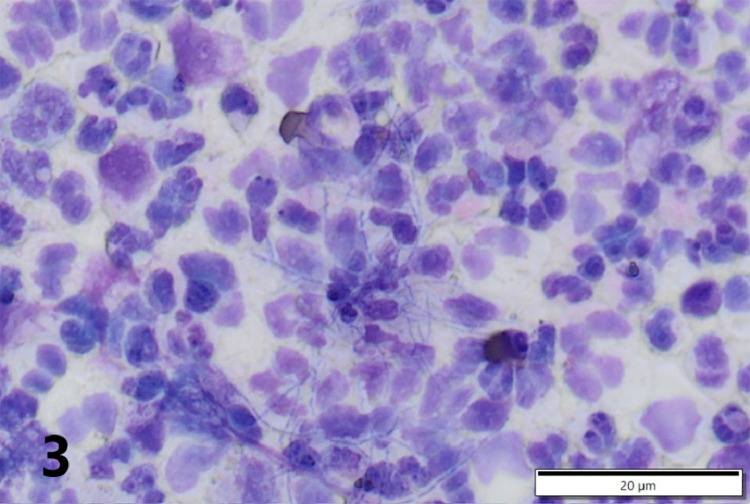
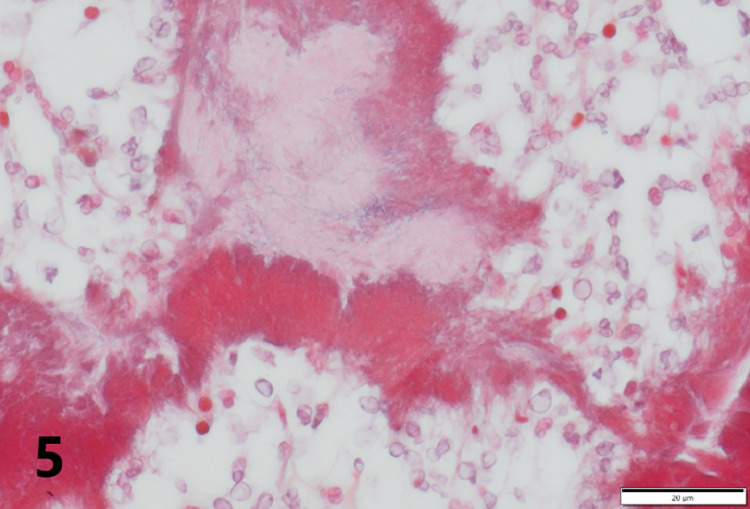
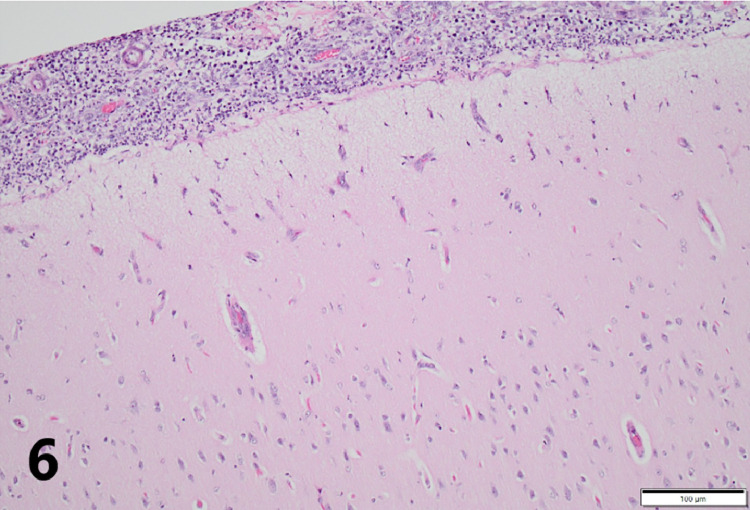
Gross necropsy revealed multiple soft dark red to tan nodules up to 2 cm in diameter scattered throughout all lung lobes (Fig. 1), with the caudal lobes more heavily affected. Tracheobronchial, mesenteric, and submandibular lymph nodes were all slightly enlarged, as were intestinal Peyer's patches. Covering the right temporal lobe was a 4 cm diameter patch of opaque, yellow-green, viscous exudate (Fig. 2), and the surface of the overlying calvarium was discolored brown with prominent blood vessels. An impression smear of the exudate revealed large numbers of intact and degenerate neutrophils surrounding rare aggregates of rod-shaped to filamentous bacteria (Fig. 3). Bacteria were consistently gram positive on Brown-Brenn modified Gram stain, without beading. Histology of the lung revealed that nodules consisted of large numbers of intra-alveolar neutrophils with fewer macrophages at the periphery. Exudate was occasionally centered on aggregates of bacteria with the same morphology as seen on meningeal cytology. Bacteria were often embedded in eosinophilic club-shaped Splendore-Hoeppli material (Fig. 4, Fig. 5). Alveolar septa in affected regions were moderately thickened by infiltrates of neutrophils, histiocytes, lymphocytes, and fibroblasts with small amounts of fibrin and edema. Pleural mesothelium overlying the nodules was hypertrophied. Histology of the brain revealed that the exudate did not infiltrate the underlying parenchyma. The dura was thickened by edema, infiltrating neutrophils and macrophages, and few fibroblasts. There was a copious neutrophilic subdural exudate with rare aggregates of the bacteria seen on cytology, and lesser amounts of exudate overlying the dura (Fig. 6). Given the cytologic appearance and robust neutrophilic response, Actinomyces spp were suspected. A swab of the exudate was submitted to a reference laboratory for aerobic and anaerobic culture. Aerobic culture yielded pure growth of an organism that was speciated by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) as Actinomyces bowdenii.
Fig. 1.
Right lung lobes. There are multiple 1–2 cm tan to dark red nodules scattered throughout all lung lobes.
Fig. 2.
Brain in skull. Overlying the right temporal lobe is a yellow viscous subdural exudate (black circle).
Fig. 3.
A smear of the purulent meningeal exudate reveals small numbers of filamentous rod-type bacteria suggestive of Actinomyces or Nocardia spp.
Fig. 4.
Histology of a pulmonary lesion demonstrates large numbers of neutrophils centered on small colonies of filamentous bacteria (red arrows) associated with small amounts of eosinophilic Splendori-Hoeppli material.
Fig. 5.
Brown-Brenn Gram stain of the pulmonary lesion in Fig. 4 reveals that bacterial colonies consist of gram-positive rods and are surrounded by red-staining Splendori-Hoeppli material.
Fig. 6.
Histology of the brain demonstrates large numbers of subdural neutrophils with few macrophages and fibroblasts. The dura has been separated from the underlying brain by the eduxate. Exudate does not extend into the underlying parenchyma.
Actinomyces spp. are short, slightly curved rods that are most often found in narrow (<1 µm) filaments, which usually but do not always branch. They may easily be mistaken microscopically for aerobic Nocardia spp., and like Nocardia, often produce macroscopic sulfur granules. As microaerophilic facultative to obligate anaerobes, suspected Actinomyces spp. infections should be cultured both anaerobically and aerobically in the presence of carbon dioxide, on blood agar or enriched thioglycolate media. Actinomyces spp. are positive on Gram stain and negative with acid-fast stains. Actinomyces spp. activate macrophages, induce chemotaxis, and bind neutrophils, enhancing phagocytosis and degranulation. They have fimbriae that bind the surface of other bacteria, and this bacterial aggregation inhibits normal neutrophil phagocytosis and metabolism (Sykes, 2012). For this reason, polymicrobial infections are the norm for Actinomyces, and overgrowth of other bacteria may inhibit the successful culturing of the actinomycete. Most Actinomyces isolates are resistant to fluoroquinolones, but can be successfully treated with prolonged courses of penicillins, as well as clindamycin and some other antibiotics (LeCorn et al., 2007; Smith et al., 2005).
Actinomyces spp. are commensals of the oral cavity, pharynx, intestine, and urogenital tract of many species, including humans (Mauldin & Peters-Kennedy, 2016; Thomas-White et al., 2018; Valour et al., 2014). Infections from Actinomyces are usually localized opportunistic infections, with Actinomyces bovis causing lumpy jaw in cattle as a classic example. In lumpy jaw, the bacterium is introduced into the soft tissues of the mouth through open wounds caused by rough forage, grass awns, or sticks or baling wire in hay. This same species may cause poll evil or fistulous withers in horses (Scanlan, 2004). Actinomyces infections usually spread along fascial planes, and rarely spread haematogenously (Sykes, 2012).
Actinomyces infections in dogs are uncommon. These infections are most frequently soft tissue infections (Mauldin & Peters-Kennedy, 2016), though intrathoracic infections occur as mass lesions (Doyle et al., 2009), and there are reports of pneumonia as well (Barnes & Grahn, 2007). Actinomyces viscosus is one of the most common causative agents of actinomycosis in dogs (Donahue & Brightman, 1995; Georg et al., 1972). Actinomyces hordeovulneris is another common causative agent, being associated with the awns produced by Hordeum genus of grasses, which can migrate after penetrating the skin, or respiratory tract if inhaled (Buchanan et al., 1984; Pelle et al., 2000; Sykes, 2012). These grasses are particularly common in California and the western United States, but are also found in Europe. Actinomyces weissii was isolated from one dog with an oral wound and from another with gingivitis (Hijazin et al., 2011). Actinomyces canis is a common component of canine oral plaque, and other Actinomyces spp are frequently identified in canine gingivitis and periodontitis (Davis et al., 2013). Actinomyces coleocanis was isolated from the vagina of a dog (Hoyles et al., 2002), though it is not clear if this was an infection or a commensal organism.
Actinomyces bowdenii was first speciated in 1999, when it was cultured from a submandibular abscess in two dogs, as well as from a subcutaneous pyogranuloma in a dog and from a feline pyothorax (Pascual et al., 2002). Since then, it has also been cultured from the cornea of a diabetic mixed breed dog following a phaecoemulsification surgery (Sherman et al., 2013). Reports of central nervous system (CNS) infections by Actinomyces spp. are rare. Unspeciated actinomycetes were cultured from the cerebrospinal fluid (CSF) of a 1-year-old female German Shepherd dog with a subdural abscess over the dorsal brain stem (Couto et al., 2000), and from a cervical epidural abscess in a 2-year-old female spayed Golden Retriever that survived with aggressive treatment (Song et al., 2015).
The dog in this report did not have any evidence of an injury or foreign body that might have initiated the infection with Actinomyces bowdenii. It is unknown if the organism was part of the dog's native oral flora. It seems most likely that the infection was inhaled and then spread haematogenously to the brain, though an initial CNS infection that then spread haematogenously to shower the lungs cannot be ruled out. Normal thoracic radiographs three days prior to euthanasia suggests exponential growth of bacteria later in the infection course, consistent with the inflammatory infiltrate seen histologically, and is also consistent with the second hypothesis of primary meningeal infection with pulmonary showering. In treating CNS infections, concurrent treatment with steroids in the initial days is often employed to reduce inflammation, but the use of enrofloxacin as an empiric agent would not have been effective against this organism. This paper documents the first case of CNS disease and the first case of pneumonia in a dog caused by Actinomyces bowdenii.
Ethical statement
This paper detailing pneumonia and meningitis caused by Actinomyces bowdenii has not been published in full or in part in any other journal.
The authors declare no conflicts of interest, financial or otherwise.
This manuscript and its associated figures have been reviewed in their entirety by all authors.
The animal described had been previously sensitized to house dust mite antigen and used in non-terminal studies evaluating several different test articles. Both house dust mite sensitization and treatment with test articles were reviewed and approved by the Lovelace Institutional Animal Care and Use Committee. The dog was not on study at the time of its illness, and was treated by multiple veterinarians including a laboratory animal certified veterinarian at the time of its illness.
Kristin Vyhnal, DVM, MS, DACVP.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Barnes L.D., Grahn B.H. Actinomyces endophthalmitis and pneumonia. Canadian Veterinary Journal. 2007;48:1155–1158. [PMC free article] [PubMed] [Google Scholar]
- Bowden G.H.W. In: Medical microbiology. 4th Ed. Baron S, editor. University of Texas Medical Branch at Galveston; Galveston, TX: 1996. Actinomyces, Propionobacterium propionicus, and Streptomyces. ed. Chapter 34. [PubMed] [Google Scholar]
- Buchanan A.M., Scott J.L., Gerenscer M.A., Beaman B.L., Jang S., Biberstein E.L. Actinomyces hordevulneris sp. nov. an agent of canine actinomycosis. International Journal of Systematic Bacteriology. 1984;34:439–443. [Google Scholar]
- Couto S.S., Dickinson P.J., Jang S., Munson L. Pyogranulomatous meningoencephalitis due to Actinomyces sp. in a dog. Veterinary Pathology. 2000;37:650–652. doi: 10.1354/vp.37-6-650. [DOI] [PubMed] [Google Scholar]
- Davis I.J., Wallis C., Deusch O., Colyer A., Milella L., Loman N., et al. A cross-sectional survey of bacterial species in plaque from client-owned dogs with healthy gingiva, gingivitis or mild periodontitis. PloS one. 2013;8:e83158. doi: 10.1371/journal.pone.0083158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue D.E., Brightman A.H.I. Cervicofacial Actinomyces viscosus in a Brazilian fila: A case report and literature review. Journal of the American Animal Hospital Association. 1995;31:501–505. doi: 10.5326/15473317-31-6-501. [DOI] [PubMed] [Google Scholar]
- Doyle J.L., Kuipers von Lande R.G., Worth A.J. Intra-thoracic pyogranulomatous disease in four working dogs. New Zealand Veterinary Journal. 2009;57:346–351. doi: 10.1080/00480169.2009.64721. [DOI] [PubMed] [Google Scholar]
- Georg L.K., Brown J.M., Baker H.J., Cassell G.H. Actinomyces viscosus as an agent of actinomycosis in the dog. American Journal of Veterinary Research. 1972;33:1457–1470. [PubMed] [Google Scholar]
- Hijazin M., Alber J., Lämmler C., Kämpfer P. Actinomyces weissii sp. nov., isolated from dogs. International Journal of Systematic and Evolutionary Microbiology. 2011;62:1755–1760. doi: 10.1099/ijs.0.035626-0. [DOI] [PubMed] [Google Scholar]
- Hoyles L., Falsen E., Foster G., Collins M.D. Actinomyces coleocanis sp. nov., from the vagina of a dog. International Journal of Systematic and Evolutionary Microbiology. 2002;52:1201–1203. doi: 10.1099/00207713-52-4-1201. [DOI] [PubMed] [Google Scholar]
- LeCorn D.W., Vertucci F.J., Rojas M.F., Progulske-Fox A., Belanger M. In vitro activity of amoxicillin, clindamycin, doxycycline, metronidazole, and marbofloxacin against oral Actinomyces. Journal of Endodontics. 2007;33:557–560. doi: 10.1016/j.joen.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Mauldin E.A., Peters-Kennedy J. In: Jubb, Kennedy, and Palmer's pathology of domestic animals. 6th Ed. Maxie MG, editor. Elsevier Inc; St. Louis, MO: 2016. Integumentary system; pp. 637–639. [Google Scholar]
- Pascual C., Foster G., Falsen E., Bergström K., Grecko C., Collins M.D. Actinomyces bowdenii sp. nov., isolated from canine and feline clinical specimens. International Journal of Systematic and Evolutionary Microbiology. 2002;49:1873–1877. doi: 10.1099/00207713-49-4-1873. [DOI] [PubMed] [Google Scholar]
- Pelle G., Makrai L., Fodor L., Dobos-Kovács Actinomycosis of dogs caused by Actinomyces hordeovulneris. Journal of Comparative Pathology. 2000;123:72–76. doi: 10.1053/jcpa.2000.0388. [DOI] [PubMed] [Google Scholar]
- Scanlan C. 2nd Ed. Brown Paw Educational Media; College Station, TX: 2004. Bacterial diseases of domestic animals textbook. [Google Scholar]
- Sherman A., Daniels J.B., Wilkie D.A., Lutz E. Actinomyces bowdenii ulcerative keratitis in a dog. Veterinary Ophthalmology. 2013;16:386–391. doi: 10.1111/vop.12001. [DOI] [PubMed] [Google Scholar]
- Smith A.J., Hall V., Thacker B., Gemmell C.G. Antimicrobial susceptibility testing of Actinomyces species with 12 antimicrobial agents. Journal of Antimicrobial Chemotherapy. 2005;56:407–409. doi: 10.1093/jac/dki206. [DOI] [PubMed] [Google Scholar]
- Song R.B., Vitullo C.A., da Costa R.C., Daniels J.B. Long-term survival in a dog with meningoencephalitis and epidural abscessation due to Actinomyces species. Journal of Diagnostic Investigation. 2015;27:552–557. doi: 10.1177/1040638715586439. [DOI] [PubMed] [Google Scholar]
- Sykes J.E. In: Infectious diseases of the dog and cat. 4th Ed. Greene CE, editor. Elsevier Saunders; St. Louis, MO: 2012. Actinomycosis and nocardiosis; pp. 484–495. [Google Scholar]
- Thomas-White K., Forster S.C., Kumar N., Van Kuiken M., Putonti C., Stares M.D., et al. Culturing of female bladder bacteria reveals an interconnecting urogenital microbiota. Nature Communications. 2018;9:1557. doi: 10.1038/s41467-018-03968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valour F., Sénéchal A., Dupieux C., Karsenty J., Lustig S., Breton P., et al. Actinomycosis: Etiology, clinical features, diagnosis, treatment, and management. Infection and Drug Resistance. 2014;7:183–197. doi: 10.2147/IDR.S39601. [DOI] [PMC free article] [PubMed] [Google Scholar]