Abstract
Objective: This meta-analysis aimed to determine the efficacy of curcumin in preventing myocardial ischemia/reperfusion (I/R) injury in animal models.
Methods: Studies published from inception to January 2023 were systematically searched in databases including PubMed, Web of Science, Embase, China’s National Knowledge Infrastructure (CNKI), Wan-Fang database, and VIP database (VIP). The SYRCLE’s RoB tool was used to determine methodological quality. Sensitivity analysis and subgroup analysis were performed when there was high heterogeneity. Publication bias was assessed using a funnel plot.
Results: Thirty-seven studies involving 771 animals were included in this meta-analysis with methodology quality scores ranging from 4 to 7. The results indicated that curcumin treatment significantly improved myocardial infarction size standard mean difference (SMD) = −5.65; 95% confidence interval (CI): 6.94, −4.36; p < 0.01; I2 = 90%). The sensitivity analysis for infarct size showed that the results were stable and reliable. However, the funnel plot was asymmetric. The subgroup analysis included species, animal model, dose, administration, and duration. The results showed that the subgroup dose was statistically significant between subgroups. In addition, curcumin treatment improved cardiac function, myocardial injury enzymes, and oxidative stress levels in animal models of myocardial I/R injury. The funnel plot revealed that there is publication bias for creatine kinase and lactate dehydrogenase. Finally, we performed a meta-analysis of inflammatory cytokines and apoptosis index. The results showed that curcumin treatment downregulated serum inflammatory cytokine levels and myocardial apoptosis index.
Conclusion: This meta-analysis suggests that curcumin has excellent potential for the treatment of myocardial I/R injury in animal models. However, this conclusion needs to be further discussed and verified in large animal models and human clinical trials.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022383901.
Keywords: curcumin, myocardial ischemia/reperfusion injury, myocardial infarction, preclinical evidence, meta-analysis
1 Introduction
Acute coronary syndrome (ACS) is estimated to affect more than 7 million people annually, with a significant increase in the number of young people receiving this diagnosis (Gulati et al., 2020; Bhatt et al., 2022). The pathological basis of ACS is the rupture or invasion of coronary atherosclerotic plaque and the secondary complete or incomplete occlusive thrombosis, which fails coronary blood flow to meet the demands of myocardial metabolism, leading to sharp and temporary myocardial ischemia and hypoxia. Reperfusion therapy is the most important and effective treatment strategy known for acute myocardial infarction (AMI) (O'Gara et al., 2013). Reperfusion therapy improves myocardial ischemia and hypoxia, but it also progressively aggravates the myocardial injury and leads to myocardial cell death, which is described as myocardial ischemia/reperfusion (I/R) injury (Pagliaro et al., 2011). Oxidative stress, inflammation, and intracellular Ca2+ overload occur in a time-dependent manner during myocardial I/R injury, ultimately leading to cardiomyocyte death (Hausenloy and Yellon, 2013). Therefore, reducing cardiomyocyte death is an important therapeutic principle for myocardial I/R injury (Heusch, 2020).
The inherent health benefits and hypotonicity of natural products have attracted growing interest worldwide, leading to a remarkable increase in the use of nutraceuticals and dietary supplements. Turmeric, a curry spice originating in India, has attracted increasing attention in recent years for its medicinal properties. Curcumin, an extract of turmeric, is a lipophilic polyphenol with antioxidant, anti-inflammatory, and anti-fibrotic properties (Wang et al., 2012; Kotha and Luthria, 2019). Curcumin was proven to be well tolerated at high oral doses (12 g/d) and was generally considered to be safe (Lao et al., 2006; Gupta et al., 2013; Prasad et al., 2014).
Recent studies have shown that curcumin protects cardiomyocytes from myocardial I/R injury through multiple and diverse mechanisms (Wang et al., 2018a; Mokhtari-Zaer et al., 2018; Wu et al., 2021a; Pawar et al., 2022). Curcumin has been shown to improve cardiac function after myocardial I/R injury by reducing extracellular matrix degradation and inhibiting collagen synthesis via the TGFβ/Smad signaling pathway (Wang et al., 2012). In addition, curcumin attenuates oxidative damage and inhibits cardiomyocyte apoptosis by activating the JAK2/STAT3 signaling pathway, thereby ameliorating myocardial I/R injury (Liu et al., 2017a). Although the molecular mechanisms of curcumin’s cardioprotective effect in myocardial I/R injury have been partially elucidated, they are still insufficient. Therefore, there is still a long way to go before curcumin be used clinically. Furthermore, it is difficult to transfer finding of animal researches into human clinical trials. The first and most important recommendation of Rainer Spanagel’s list of 10 recommendations for improving reproducibility and translation is to perform a preclinical meta-analysis (Spanagel, 2022). Therefore, this preclinical meta-analysis aimed to determine whether curcumin has a cardioprotective role in animal models of myocardial I/R injury.
2 Methods
This meta-analysis has been registered in PROSPERO (ID: CRD42022383901). This study was conducted in Covidence from literature selection to data extraction.
2.1 Search strategy
Six databases, including Web of Science, PubMed, Embase, China’s National Knowledge Infrastructure (CNKI), the Wan-Fang database, and the VIP database (VIP), were searched systematically between database inception and January 2023 without language restrictions. The keywords mainly used were “myocardial infarction”, “myocardial ischemia”, “myocardial I/R″, “myocardial I/R injury”, “myocardial ischemia-reperfusion injury”, “myocardial ischemia-reperfusion”, and “curcumin”.
2.2 Study selection
The included studies met the following criteria: (Bhatt et al., 2022): myocardial I/R injury experimental models: left anterior descending (LAD) ligation, injecting isoprenaline (ISO) intravenously, or endovascular embolization; (Gulati et al., 2020); Treatment: curcumin was the only intervention with a control group receiving placebo fluid or no treatment at all; and (O'Gara et al., 2013) Data: detailed data of the primary or secondary outcomes in the articles.
Exclusion Criteria were as follows: (Bhatt et al., 2022): no detailed data was provided, (Gulati et al., 2020), no animal model, (O'Gara et al., 2013), without a control group, (Pagliaro et al., 2011), Curcumin is not the only intervention, (Hausenloy and Yellon, 2013), in vitro studies, (Heusch, 2020), article types: review, conference abstract, case reports, meta-analysis and clinical trials.
2.3 Data extraction
Two independent reviewers screened relevant articles for titles and abstracts, viewed full texts, and then extracted data from identified studies by Covidnce in the same standard form. The search results were then checked by the third reviewer. Any disagreements therein were resolved with discussion and adjudication by group discussion.
Information extracted includes as follows: (Bhatt et al., 2022): Author information: the first author, publication year, and country; (Gulati et al., 2020); Animal information: species, sex, and sample size; (O'Gara et al., 2013); Animal model: anesthetic and model methods; (Pagliaro et al., 2011); Drug administration: method, dosage, and duration of administration; (Hausenloy and Yellon, 2013); Outcome record: the mean and standard deviation of the primary outcomes. Primary outcomes include myocardial infarction size (IS). Secondary outcomes include left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), left ventricular developed pressure (LVDP), maximum 1st derivative of developed pressure (dP/dt max), serum creatine kinase (CK), serum creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and apoptosis index. Primary and secondary outcomes were extracted in detail. When different doses of the curcumin were used, data in the highest dose group was extracted. When there are multiple time points after myocardial ischemia, only the last time point is recorded.
2.4 Quality assessment
The quality assessment of the included studies was independently evaluated by two authors using the SYRCLE’s RoB tool (Hooijmans et al., 2014).
2.5 Statistical analysis
The summary statistics were considered by standard mean difference (SMD) and 95% confidence interval (CI). The Cochran Q test and the I2 statistics were used for the study heterogeneity assessment. Different effect models were used according to the heterogeneity. The random effect model was used when I2>50%, on the contrary, the fixed effect model was used. All statistics were analyzed by R (Version 4.2.2). Subgroup analysis and sensitivity analysis were conducted to find the source of heterogeneity. Publication bias was evaluated, when more than 10 studies were included, by funnel plot. p < 0.05 indicated that the difference was statistically significant.
3 Results
3.1 Study selection
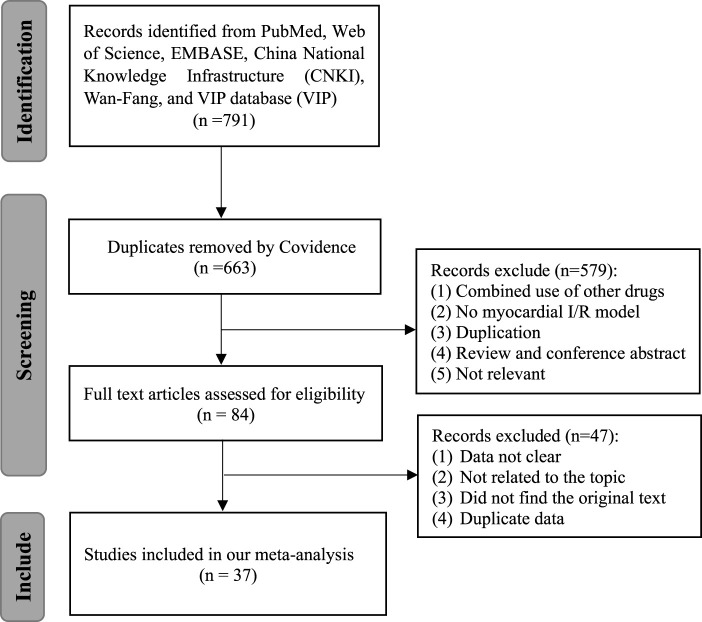
Seven hundred and ninety-one articles were involved after the primary retrieval. One hundred and twenty-eight duplicate articles were excluded by Covidence. After carefully reading the abstracts and titles, 579 articles were excluded. Forty-seven studies were excluded after full-text articles were assessed due to articles with unclear data, irrelevant to our topic, the original text being unavailable, and duplicate data. Finally, a total of 37 studies involving 755 animals were included in the present meta-analysis (Nirmala and Puvanakrishnan, 1996; Manikandan et al., 2004; Cheng et al., 2005; Ansari et al., 2007; Tanwar et al., 2010; Zhao et al., 2010; Wu et al., 2011; Duan et al., 2012; Izem-Meziane et al., 2012; Jeong et al., 2012; Kim et al., 2012; Yang et al., 2012; Wang et al., 2013; Yang et al., 2013; Su et al., 2014; Wang et al., 2014; Yao and Jiang, 2014; Xiao et al., 2016a; Geng et al., 2016; Gu et al., 2016; Sun et al., 2016; Liu et al., 2017b; Liu et al., 2017c; Wang et al., 2018b; Chen et al., 2018; Liu et al., 2018; Boarescu et al., 2019a; Li et al., 2019a; Boarescu et al., 2019b; Javanmard et al., 2019; Rahnavard et al., 2019; Li et al., 2020a; Ding et al., 2020; Jo et al., 2020; Wu et al., 2021b; Yan et al., 2021; Zhao et al., 2022). The PRISMA flow diagram is shown in Figure 1.
FIGURE 1.
Flow diagram of database searches and study selection.
3.2 Characteristics of included studies
In terms of species, 21 studies used Sprague-Dawley rats, 10 studies used Wistar rats, and 6 studies used C57BL/6 mice. In terms of gender, only 3 studies used female animals and all the others were male. The methods used to establish animal models of myocardial I/R injury included LAD ligation in 26 studies, ISO injection in 10 studies, and coronary microembolization in 1studies.
Curcumin was administered by multiple routines including oral in 20 studies, intraperitoneal in 7 studies, intravenous in 4 studies, and perfused in 6 studies. Additionally, the duration of curcumin treatment varied from 1 min to 12 weeks. The publication year of the included studies varied between 1996 and 2022. Twenty-four of 37 studies were conducted in China, 4 studies in India, 3 studies in Korea, 2 studies each in Iran or Romania, and 1 study each in France or the United States. All the basic information about the included studies in this meta-analysis is shown in Table 1.
TABLE 1.
Baseline characteristics of included studies.
| Author | Year | Country | Species | N (CCM/Ctrl) | Anesthetic | Model method | Methods to administration (dose) | Duration | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Ansari et al., | 2007 | India | male Wistar rats | 8/8 | — | ISO | Oral (200 mg/kg/d) | 20 days | 1.CK, 2.LDH, 3.CAT. |
| Boarescu et al., | 2019 | Romania | male Wistar rats | 7/7 | — | ISO | Oral (200 mg/kg/d) | 2 weeks | 1.CK, 2.CK-MB, 3.LDH, 4.MDA, 5.IL-1β, 6.IL-6, 7.TNF-α |
| Boarescu et al., | 2019 | Romania | female Wistar rats | 7/7 | — | ISO | Oral (200 mg/kg/d) | 2 weeks | 1.CK, 2.CK-MB, 3.MDA, 4.IL-1β, 5.IL-6, 6.TNF-α |
| Chen et al., | 2018 | China | male SD rats | 20/20 | Pentobarbital sodium | Ischemia 30 min then reflow 6 h | Intravenous (20 mg/kg) | 1 min | 1.LVDP |
| 2.+dP/dt max | |||||||||
| 3.-dP/dt max, 4.CK, 5.LDH. | |||||||||
| Cheng et al., | 2005 | China | male SD rats | 10/10 | Pentobarbital sodium (0.9 mg/kg) | Ischemia 60 min then reflow 30 min | Intravenous (40 mg/kg) | 5 min | 1. Infarction size |
| Ding et al., | 2020 | China | male SD rats | 10/10 | Phenobarbital | Ischemia 30 min then reflow 60 min | Oral (400 mg/kg/d) | 12 weeks | 1.CK, 2.CK-MB, 3.LDH, 4.SOD. |
| Duan et al., | 2012 | China | male SD rats | 8/8 | Pentobarbital sodium (30 mg/kg) | Ischemia 60 min then reflow 60 min | Perfused (1uM) | 10 min | 1.Infarction size, 2.LVDP |
| 3.+dP/dt max | |||||||||
| Geng et al., | 2016 | China | male C57BL/6 J | 8/8 | 5.0% isoflurane | LAD ligation | Oral (50 mg/kg) | 1 week | 1.Infarction size |
| Gu et al., | 2016 | China | male C57BL/6 mice | 10/10 | Pentobarbital sodium (0.5 mg/kg) | Ischemia 30 min then reflow 4 h | Intraperitoneal (100 mg/kg) | 30 min | 1.Infarction size |
| 2.TNF-α | |||||||||
| Izem-Meziane et al., | 2012 | France | male Wistar rats | 5/5 | — | ISO | Intraperitoneal (60 mg/kg/d) | 2 days | 1.CAT, 2.Apoptosis Index |
| Javanmard et al., | 2019 | Iran | male Wistar rats | 6/6 | — | ISO | Oral (80 mg/kg/d) | 12 days | 1.Infarction size, 2.CK, 3.LDH, 4.MDA, 5.SOD |
| 6.Apoptosis Index | |||||||||
| Jeong et al., | 2012 | Korea | male SD rats | 7/7 | Pentobarbital (60 mg/kg) | Ischemia 30 min then reflow 2 h | Intraperitoneal (100 mg/kg/d) | 20 min | 1.Infarction size |
| Jo et al., | 2020 | Korea | male SD rats | 8/8 | Alfaxalone (50 mg/kg) and xylazine (5 mg/kg) | Ischemia 30 min then reflow 4 days | Oral (50 mg/kg/d) | 5 days | 1.Infarction size, 2.LVEF, 3.LVFS, 4.CK-MB, 5.LDH. |
| Kim et al., | 2012 | Korea | male Wistar rats | 3/4 | Ketamine (50 mg/kg) and xylazine (5 mg/kg) | Ischemia 30 min then reflow 14 days | Oral (300 mg/kg/d) | 2 weeks | 1.Infarction size, 2.LVEF, 3.LVFS, 4.+dP/dt max |
| 5.-dP/dt max | |||||||||
| Li et al., | 2019 | China | male C57BL/6 J | 32/32 | sodium pentobarbital (50 mg/kg) | LAD ligation | Oral (100 mg/kg/d) | 4 weeks | 1. Infarction size, 2. LVEF |
| 3. LVFS. | |||||||||
| Li et al., | 2020 | China | male SD rats | 10/10 | Isoflurane | Ischemia 30 min then reflow 2 h | Intraperitoneal (20 mg/kg) | 4 h | 1.LVDP, 2.+dP/dt max, 3.-dP/dt max, 4.CK, 5.LDH, 6.MDA, 7.SOD, 8.Apoptosis Index |
| Liu et al., | 2017 | China | male SD rats | 10/10 | Ketamine (90 mg/kg) and xylazine (10 mg/kg) | Ischemia 60 min then reflow 3 h | Oral (30 mg/kg/d) | 20 days | 1.Infarction size, 2.LVDP, 3.+dP/dt max, 4.-dP/dt max, 5.MDA. |
| Liu et al., | 2017 | China | male SD rats | 10/10 | chloral hydrate (350 mg/kg) | Ischemia 30 min then reflow 30 min | Perfused (0.5 mg/kg) | 30 min | 1.Infarction size |
| Liu et al., | 2018 | China | male SD rats | 10/10 | pentobarbital (30–40 mg/kg) | Endovascular embolization by microspheres | Oral (150 mg/kg/d) | 5 days | 1.LVEF, 2.LVFS, 3.IL-1β, 4.TNF-α, 5.Apoptosis Index |
| Manikandan et al., | 2004 | India | female Wistar rats | 6/6 | — | ISO | Oral (15 mg/kg/d) | 30 min | 1.CAT. |
| Nirmala et al., | 1996 | India | female Wistar rats | 6/6 | — | ISO | Oral (200 mg/kg/d) | 4 days | 1.CK, 2.LDH, 3.CAT. |
| Rahnavard et al., | 2019 | Iran | male Wistar rats | 6/6 | — | ISO | Oral (50 mg/kg/d) | 9 days | 1.Infarction size, 2.CK, 3.LDH, 4.MDA, 5.SOD. |
| Su et al., | 2014 | China | male SD rats | 10/10 | NA | Ischemia 30 min then reflow 2 h | Oral (200 mg/kg/d) | 4 weeks | 1.CK-MB. |
| Sun et al., | 2016 | China | male SD rats | 10/10 | Chloral hydrate | Ischemia 45 min then reflow 2 h | Intraperitoneal (50 mg/kg) | 45 min | 1.+dP/dt max |
| 2.-dP/dt max, 3.CK, 4.LDH. | |||||||||
| Tanwar et al., | 2010 | India | male Wistar rats | 10/10 | — | ISO | Oral (400 mg/kg/d) | 2 weeks | 1.CAT |
| Wang et al., | 2013 | China | male SD rats | 8/8 | Pentobarbital (30 mg/kg) | Ischemia 30 min then reflow 150 min | Perfused (1uM) | 60 min | 1.Infarction size, 2.LVDP, 3.+dP/dt max |
| Wang et al., | 2014 | United States | male SD rats | 8/8 | ketamine (90 mg/kg) and zylaxine (10 mg/kg) | Ischemia 30 min then reflow 3 h | Oral (150 mg/kg/d) | 5 days | 1.Infarction size |
| 2.IL-6 | |||||||||
| 3.TNF-a | |||||||||
| Wang et al., | 2018 | China | male SD rats | 6/6 | sodium pentobarbital (50 mg/kg) | Ischemia 45 min then reflow 2 h | Perfused (1uM) | 120 min | 1.Infarction size |
| 2.LDH | |||||||||
| 3.Apoptosis Index | |||||||||
| Wu et al., | 2011 | China | male SD rats | 10/10 | Sodium pentobarbital (0.9 mg/kg) | Ischemia 60 min then reflow 30 min | Oral (40 mg/kg) | 30 min | 1.Infarction size, 2.LDH, 3.MDA, 4.SOD. |
| Wu et al., | 2021 | China | male SD rats | 10/10 | 10% chloral hydrate (3.5 mL/kg) | Ischemia 30 min then reflow 3 h | Intraperitoneal (100 mg/kg/d) | 30 min | 1.Infarction size, 2.CK-MB, 3.LDH, 4.MDA, 5.SOD. |
| Xiao et al., | 2016 | China | male C57BL/6 J mice | 12/12 | 2% isoflurane | LAD ligation | Oral (100 mg/kg) | 5 weeks | 1.Infarction size |
| Yan et al., | 2021 | China | male C57BL/6 J mice | 33/33 | ketamine (50 mg/kg) and pentobarbital sodium (50 mg/kg) | LAD ligation | Intraperitoneal (100 mg/kg/d) | 6 weeks | 1.Infarction size, 2.LVEF, 3.LVFS, 4.IL-1β, 5.IL-6, 6.TNF-α |
| Yang et al., | 2012 | China | male SD rats | 8/8 | Pentobarbital (30 mg/kg) | Ischemia 45 min then reflow 60 min | Perfused (1uM) | 5 min | 1.Infarction size, 2.LVDP |
| 3.+dP/dt max | |||||||||
| Yang et al., | 2013 | China | male SD rats | 8/8 | sodium pentobarbital (50 mg/kg) | Ischemia 45 min then reflow 60 min | Perfused (1uM) | 60 min | 1.Infarction size, 2.Apoptosis Index |
| Yao et al., | 2014 | China | male SD rats | 12/12 | Sodium pentobarbital (0.9 mg/kg) | Ischemia 30 min then reflow 6 h | Intravenous (20 mg/kg) | 1 min | 1.LVDP |
| 2.+dP/dt max | |||||||||
| 3.-dP/dt max, 4.CK, 5.LDH, 6.MDA, 7.SOD. | |||||||||
| Zhao et al., | 2010 | China | male SD rats | 20/20 | Urethane (0.1 mL/kg) | Ischemia 30 min then reflow 2 h | Intravenous (20 mg/kg) | 30 min | 1.CK, 2.LDH. |
| Zhao et al., | 2022 | China | male C57BL/6 J mice | 5/5 | 1.5%–2% isoflurane | LAD ligation | Oral (100 mg/kg/d) | 4 weeks | 1.Infarction size, 2.LVEF, 3.LVFS. |
Note: SD rats, Sprague-Dawley rats; ISO, isoproterenol; LAD, left anterior descending branch; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVDP, left ventricular diastolic pressure; dP/dT max, maximum 1st derivative of developed pressure; CK, creatine kinase; CK-MB, creatine kinase isoenzyme; LDH, lactic dehydrogenase; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; IL, interleukin; TNF, tumor necrosis factor.
The studies included in this meta-analysis scored between 4 and 7 on the quality assessment. The details of the methodological quality of the included literature are shown in Table 2. The brief molecular mechanism by which curcumin protects cardiomyocytes from myocardial I/R injury is summarized in Table 3.
TABLE 2.
Quality assessment of included studies by the SYRCLE’s RoB tool.
| Study | Published year | A | B | C | D | E | F | G | H | I | J | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ansari et al., | 2007 | √ | √ | √ | √ | √ | 5 | |||||
| Boarescu et al., | 2019 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Boarescu et al., | 2019 | √ | √ | √ | √ | √ | 5 | |||||
| Chen et al., | 2018 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Cheng et al., | 2005 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Ding et al., | 2020 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Duan et al., | 2012 | √ | √ | √ | √ | √ | 5 | |||||
| Geng et al., | 2016 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Gu et al., | 2016 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Izem-Meziane et al., | 2012 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Javanmard et al., | 2019 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Jeong et al., | 2012 | √ | √ | √ | √ | √ | 5 | |||||
| Jo et al., | 2020 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Kim et al., | 2012 | √ | √ | √ | √ | 4 | ||||||
| Li et al., | 2020 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Li et al., | 2019 | √ | √ | √ | √ | √ | 5 | |||||
| Liu et al., | 2017 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Liu et al., | 2017 | √ | √ | √ | √ | √ | 5 | |||||
| Liu et al., | 2018 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Manikandan et al., | 2004 | √ | √ | √ | √ | √ | 5 | |||||
| Nirmala et al., | 1996 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Rahnavard et al., | 2019 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Su et al., | 2014 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Sun et al., | 2016 | √ | √ | √ | √ | 4 | ||||||
| Tanwar et al., | 2010 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Wang et al., | 2013 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Wang et al., | 2014 | √ | √ | √ | √ | √ | 5 | |||||
| Wang et al., | 2018 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Wu et al., | 2011 | √ | √ | √ | √ | √ | 5 | |||||
| Wu et al., | 2021 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Xiao et al., | 2016 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Yan et al., | 2021 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Yang et al., | 2012 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Yang et al., | 2013 | √ | √ | √ | √ | √ | 5 | |||||
| Yao et al., | 2014 | √ | √ | √ | √ | √ | √ | 6 | ||||
| Zhao et al., | 2010 | √ | √ | √ | √ | √ | 5 | |||||
| Zhao et al., | 2022 | √ | √ | √ | √ | √ | √ | √ | 7 |
Note: A, sequence generation; B, baseline characteristics; C, allocation concealment; D, random housing; E, blinding investigators; F, random outcome assessment; G, blinding outcome assessor; H, incomplete outcome data; I, selective outcome reporting; J, other sources of bias.
TABLE 3.
The molecular and cellular mechanisms underlying the cardioprotective effect of Curcumin treatment in myocardial ischemia/reperfusion injury.
| Author | Published year | Proposed mechanisms |
|---|---|---|
| Ansari et al., | 2007 | Enhance the antioxidant capability |
| Boarescu et al., | 2019 | Enhance antioxidative and anti-inflammatory effects |
| Boarescu et al., | 2019 | Limit the increase in inflammatory cytokine levels and MMP2/9 expression |
| Chen et al., | 2018 | Increase HO-1 activity and expression |
| Cheng et al., | 2005 | Reduce lipid peroxide and increase production of endogenous antioxidative substances |
| Ding et al., | 2020 | Reduce myocardial enzyme release and enhance antioxidant capacity |
| Duan et al., | 2012 | Activate of the JAK2/STAT3 signaling pathway |
| Geng et al., | 2016 | Upregulate miR-7a/b expression and downregulate of SP1 expression |
| Gu et al., | 2016 | Inhibit TLR4/NF-kappaB signaling pathway |
| Izem-Meziane et al., | 2012 | Prevent mitochondrial damaging and mitochondrial permeability transition pore (mPTP) opening |
| Javanmard et al., | 2019 | NA |
| Jeong et al., | 2012 | Activate RISK/GSK-3β and inhibit p38 and JNK. |
| Jo et al., | 2020 | NA |
| Kim et al., | 2012 | Selective inhibit of TLR2 |
| Li et al., | 2019 | Immunomodulatory effect |
| Li et al., | 2020 | NA |
| Liu et al., | 2017 | Stimulate JAK2/STAT3 signal pathway, decrease oxidative damage, and inhibit myocardium apoptosis |
| Manikandan et al., | 2004 | Inhibit xanthine dehydrogenase/xanthine oxidase (XD/XO) conversion and resultant superoxide anion production |
| Nirmala et al., | 1996 | Increase activity of cathepsin D in mitochondrial, lysosomal, and microsomal fractions |
| Rahnavard et al., | 2019 | Reduce oxidative status by reducing SOD and MDA contents |
| Su et al., | 2014 | NA |
| Sun et al., | 2016 | Suppresses ROS-mediating mitochondrial apoptosis |
| Tanwar et al., | 2010 | Stabilize cytoskeleton structure and increase Hsp27 expression |
| Wang et al., | 2013 | Activate Notch signaling pathway |
| Wang et al., | 2014 | Downregulate expression of EGR-1 and inhibit inflammation-mediated processes |
| Wang et al., | 2018 | Activate SIRT3 |
| Wu et al., | 2011 | NA |
| Wu et al., | 2021 | Downregulate the expression of Bax and upregulate the expression of Bcl2, p-mTOR and p-AKT |
| Yan et al., | 2021 | Suppress inflammation by modulating macrophage polarization via the AMPK pathway |
| Yang et al., | 2012 | Activate anti-apoptotic signaling pathway |
| Yang et al., | 2013 | Activate of SIRT1 signaling and the alleviate of mitochondrial oxidative damage |
| Yao et al., | 2014 | Anti-oxidative injury and upregulate activity and expression of HO-1 protein |
| Zhao et al., | 2010 | NA |
| Zhao et al., | 2022 | Inhibit of macrophage-fibroblast crosstalk and activate of IL18-TGFβ1-p-SMAD2/3 signaling pathway in cardiac fibroblasts |
3.3 Outcome measures
3.3.1 Myocardial infarct size
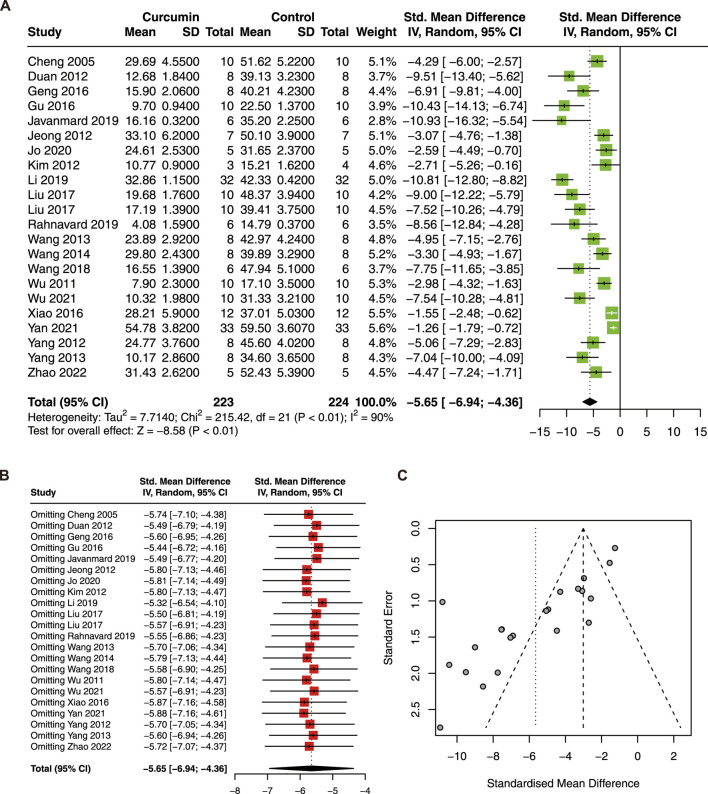
Twenty-two studies with 447 animals were included in this meta-analysis to evaluate the effect of curcumin on IS (Cheng et al., 2005; Duan et al., 2012; Jeong et al., 2012; Kim et al., 2012; Wang et al., 2013; Geng et al., 2016; Gu et al., 2016; Liu et al., 2017b; Liu et al., 2017c; Li et al., 2019a; Javanmard et al., 2019; Rahnavard et al., 2019; Jo et al., 2020). The result showed that curcumin treatment significantly reduced IS (SMD = −5.65; 95% CI: −6.94, −4.36; p < 0.01; I2 = 90%) in the animal model of myocardial I/R injury (Figure 2A).
FIGURE 2.
(A) Forest plot showing cardioprotective effects of curcumin on myocardial infarction size (IS) in myocardial ischemia/reperfusion injury animal model; (B) sensitive analysis of IS; (C) funnel plot of IS.
Due to high heterogeneity, sensitivity analysis, funnel plot, and subgroup analysis were conducted. The result of the sensitivity analysis revealed that omitting a single study did not significantly change the pooled estimate of IS (Figure 2B). However, the funnel plot of IS was asymmetric (Figure 2C). To explore the source of heterogeneity, species, animal models, dose, administration, and duration were included in the subgroup analysis. The results showed that the subgroup dose was statistically significant between subgroups (Table 4). Furthermore, significant decreases in heterogeneity were found in the ISO model and in the administration of perfusion.
TABLE 4.
Subgroup analysis of pooled estimates of infarct size.
| Subgroup | No. of studies | SMD | 95% CI | P value between subgroups | heterogeneity within subgroups | |
|---|---|---|---|---|---|---|
| I2(%) | P value | |||||
| Species | 0.81 | |||||
| SD rats | 13 | -5.34 | -6.60, -4.08 | 72 | < 0.01 | |
| Wistar rats | 3 | -6.98 | -11.96, -1.99 | 81 | < 0.01 | |
| C57BL/6 mice | 6 | -5.73 | -9.14, -2.32 | 96 | < 0.01 | |
| Model | 0.08 | |||||
| I/R | 15 | -5.48 | -6.77, -4.19 | 74 | < 0.01 | |
| MI | 5 | -4.90 | -8.46, -1.33 | 96 | < 0.01 | |
| ISO | 2 | -9.48 | -12.83; -6.13 | 0 | 0.50 | |
| Dose | <0.01 | |||||
| Perfused | 6 | -6.50 | -7.86, -5.14 | 26 | 0.24 | |
| ≤50mg/kg | 6 | -5.32 | -7.49, -3.14 | 77 | <0.01 | |
| 50-100mg/kg | 8 | -5.94 | -8.84, -3.04 | 95 | <0.01 | |
| >100mg/kg | 2 | -3.13 | -4.50, -1.75 | 90 | <0.01 | |
| Drug Delivery | 0.26 | |||||
| Perfused | 6 | -6.50 | -7.86, -5.14 | 26 | 0.24 | |
| Intraperitoneal | 4 | -5.30 | -9.33, -1.28 | 93 | < 0.01 | |
| Oral | 11 | -5.46 | -7.51, -3.42 | 90 | < 0.01 | |
| Intravenous | 1 | -4.29 | -6.00, -2.57 | / | / | |
| Duration | 0.37 | |||||
| <1day | 11 | -5.96 | -7.40, -4.52 | 74 | < 0.01 | |
| 1day-1week | 3 | -4.02 | -6.35, -1.69 | 68 | 0.05 | |
| >1week | 8 | -5.84 | -8.73, -2.95 | 94 | < 0.01 | |
Note: SMD, standardized mean difference; SD rats, Sprague-Dawley rats; I/R, ischemia/reperfusion; MI, myocardial infarction; ISO, isoproterenol.
3.3.2 Cardiac function
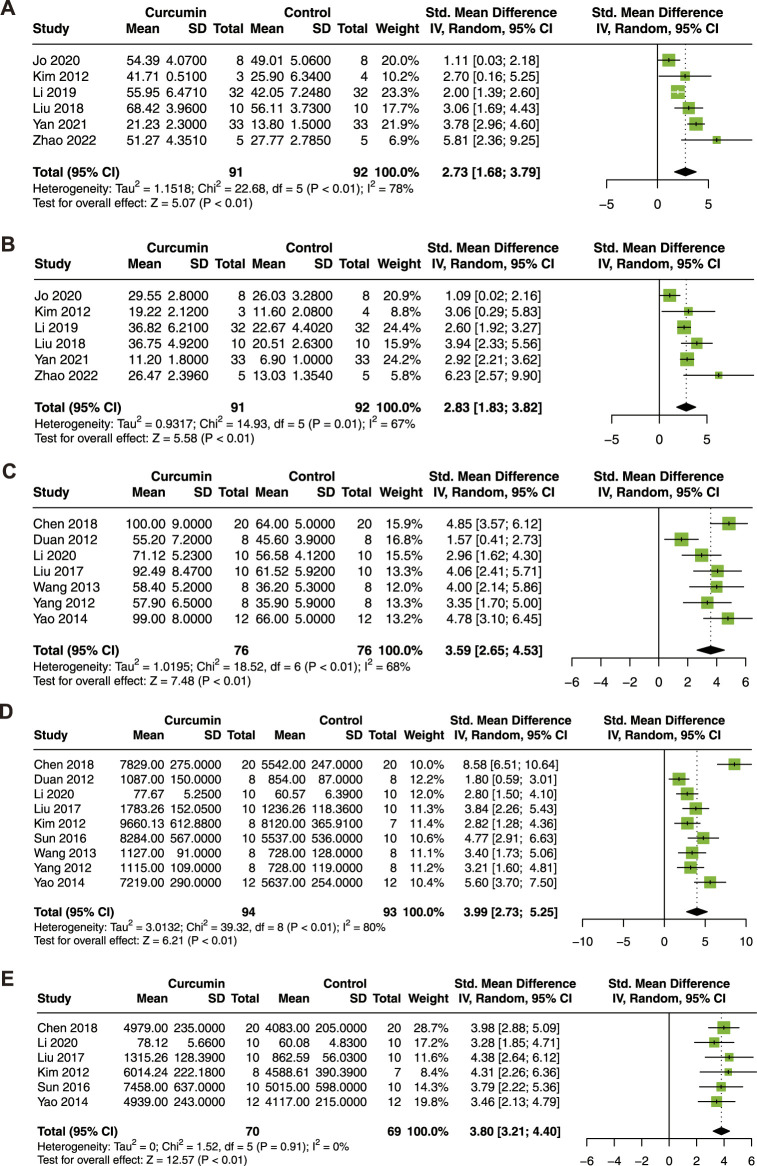
To determine the association between curcumin treatment and cardiac function in the animal model of myocardial I/R injury, LVEF, LVFS, LVDP, +dP/dt max, and -dP/dt max were analyzed. Six studies with 183 animals for LVEF and LVFS (Kim et al., 2012; Liu et al., 2018; Li et al., 2019a; Jo et al., 2020; Yan et al., 2021; Zhao et al., 2022), 7 studies with 152 animals for LVDP (Duan et al., 2012; Yang et al., 2012; Wang et al., 2013; Yao and Jiang, 2014; Liu et al., 2017b; Chen et al., 2018; Li et al., 2020a), 9 studies with 187 animals for + dP/dt max (Duan et al., 2012; Kim et al., 2012; Yang et al., 2012; Wang et al., 2013; Yao and Jiang, 2014; Sun et al., 2016; Liu et al., 2017b; Chen et al., 2018; Li et al., 2020a), and 6 studies with 139 animals for -dP/dt max (Kim et al., 2012; Yao and Jiang, 2014; Sun et al., 2016; Liu et al., 2017b; Chen et al., 2018; Li et al., 2020a) were included in this meta-analysis.
As shown in Figure 3, curcumin treatment exhibited a significant improvement in LVEF (SMD = 2.73; 95% CI: 1.68, 3.79; p < 0.01; I2 = 78%; Figure 3A), LVFS (SMD = 2.83; 95% CI: 1.83, 3.82; p = 0.01; I2 = 67%; Figure 3B), LVDP (SMD = 3.59; 95% CI: 2.65, 4.53; p < 0.01; I2 = 68%; Figure 3C), +dP/dt max (SMD = 3.99; 95% CI: 2.73, 5.25; p < 0.01; I2 = 80%; Figure 3D), and -dP/dt max (SMD = 3.80; 95% CI: 3.21, 4.40; p < 0.01; I2 = 0%; Figure 3E). In conclusion, curcumin treatment significantly improved cardiac function in animal models of myocardial I/R injury.
FIGURE 3.
Forest plot showing cardioprotective effects of curcumin on LVEF (A), LVFS (B), LVDP (C), +dP/dt max (D), and -dP/dt max (E) in myocardial ischemia/reperfusion injury animal model. LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVDP, left ventricular diastolic pressure; dP/dt max, maximum 1st derivative of developed pressure.
3.3.3 Myocardial enzyme
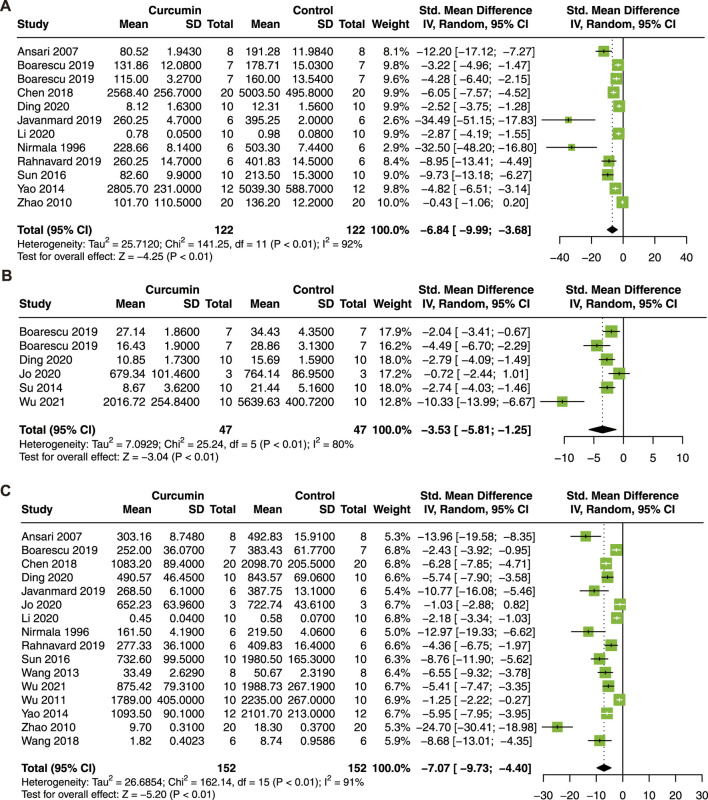
Then, serum biomarkers of myocardial injury, including CK, CK-MB, and LDH, were analyzed. Twelve studies with 244 animals for CK (Nirmala and Puvanakrishnan, 1996; Ansari et al., 2007; Zhao et al., 2010; Yao and Jiang, 2014; Sun et al., 2016; Chen et al., 2018; Boarescu et al., 2019a; Boarescu et al., 2019b; Javanmard et al., 2019; Rahnavard et al., 2019; Li et al., 2020a; Ding et al., 2020), 6 studies with 94 animals for CK-MB (Su et al., 2014; Boarescu et al., 2019a; Boarescu et al., 2019b; Ding et al., 2020; Jo et al., 2020; Wu et al., 2021b), and 16 studies with 304 animals for LDH (Nirmala and Puvanakrishnan, 1996; Ansari et al., 2007; Zhao et al., 2010; Wu et al., 2011; Wang et al., 2013; Yao and Jiang, 2014; Sun et al., 2016; Wang et al., 2018b; Chen et al., 2018; Boarescu et al., 2019a; Javanmard et al., 2019; Rahnavard et al., 2019; Li et al., 2020a; Ding et al., 2020; Jo et al., 2020; Wu et al., 2021b) were included in this meta-analysis.
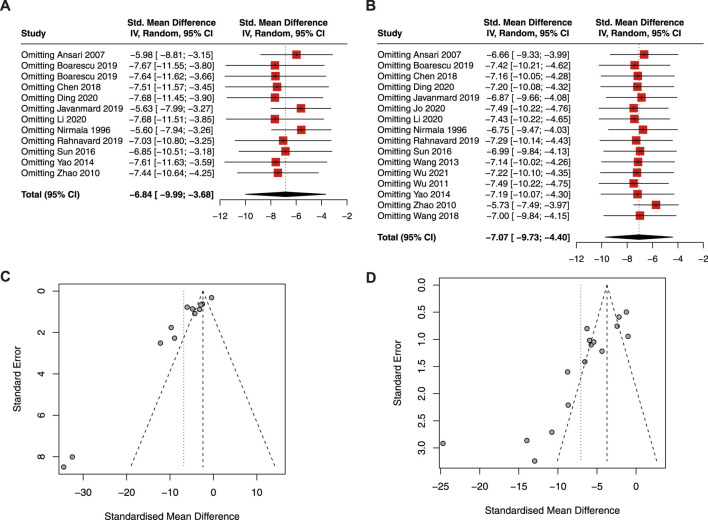
As a result, curcumin treatment presented a significant effect on the reduction of serum CK (SMD = −6.84; 95% CI: −9.99, −3.68; p < 0.01; I2 = 92%; Figure 4A), CK-MB (SMD = −3.53; 95% CI: −5.81, −1.25; p < 0.01; I2 = 80%; Figure 4B), and LDH (SMD = −7.07; 95% CI: −9.73, −4.40; p < 0.01; I2 = 91%; Figure 4C) in animals with myocardial I/R injury.
FIGURE 4.
Forest plot showing cardioprotective effects of curcumin on CK (A), CK-MB (B), and LDH (C) in myocardial ischemia/reperfusion injury animal model. CK, creatine kinase; CK-MB, creatine kinase isoenzyme; LDH, lactic dehydrogenase.
The result of the sensitivity analysis revealed that the pooled estimate of CK (Figure 5A) and LDH (Figure 5B) was stable and reliable. However, the funnel plots of CK (Figure 5C) and LDH (Figure 5D) were asymmetric, indicating publication bias.
FIGURE 5.
Sensitive analysis of CK (A) and LDH (B); funnel plot of CK (C) and LDH (D). CK, creatine kinase; LDH, lactic dehydrogenase.
3.3.4 Oxidative stress levels
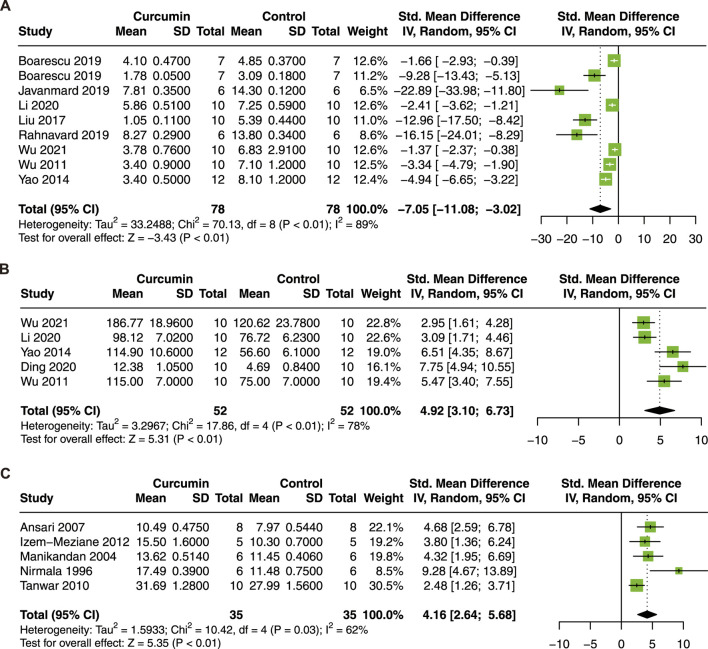
MDA is a crucial indicator of tissue oxidative stress and is a degradation product of lipid peroxidation (Idris et al., 2001). CAT level is known to decrease after myocardial I/R injury (Jin et al., 2017). In addition, SOD is an important antioxidant enzyme. To investigate the association between curcumin treatment and oxidative stress levels, MDA, SOD, and CAT were analyzed. Nine studies with 156 animals for serum MDA (Wu et al., 2011; Yao and Jiang, 2014; Liu et al., 2017b; Boarescu et al., 2019a; Boarescu et al., 2019b; Javanmard et al., 2019; Rahnavard et al., 2019; Li et al., 2020a; Wu et al., 2021b), 5 studies with 104 animals for serum SOD (Wu et al., 2011; Yao and Jiang, 2014; Li et al., 2020a; Ding et al., 2020; Wu et al., 2021b), and 5 studies with 70 animals for CAT in heart tissue (Nirmala and Puvanakrishnan, 1996; Manikandan et al., 2004; Ansari et al., 2007; Tanwar et al., 2010; Izem-Meziane et al., 2012) were included in this meta-analysis.
Curcumin treatment significantly decrease MDA (SMD = −7.05; 95% CI: −11.08, −3.02; p < 0.01; I2 = 89%; Figure 6A), increase SOD (SMD = 4.92; 95% CI: 3.10, 6.73; p < 0.01; I2 = 78%; Figure 6B) and CAT (SMD = 4.16, 95% CI: 2.64, 5.69; p = 0.03; I2 = 62%; Figure 6C) in animal models of myocardial I/R injury.
FIGURE 6.
Forest plot showing cardioprotective effects of curcumin on MDA (A), SOD (B), and CAT (C) in myocardial ischemia/reperfusion injury animal model. MDA malondialdehyde, SOD superoxide dismutase, CAT catalase.
3.3.5 Inflammation cytokine and apoptosis index
It has been reported that curcumin improves myocardial I/R injury through immunomodulatory effects (Li et al., 2019a). Therefore, we took inflammatory cytokines and myocardial apoptosis index into consideration in this meta-analysis. Four studies with 114 animals for IL-1β (Liu et al., 2018; Boarescu et al., 2019a; Boarescu et al., 2019b; Yan et al., 2021), 4 studies with 110 animals for IL-6 (Wang et al., 2014; Boarescu et al., 2019a; Boarescu et al., 2019b; Yan et al., 2021), and 5 studies with 130 animals for TNF-α (Wang et al., 2014; Liu et al., 2018; Boarescu et al., 2019a; Boarescu et al., 2019b; Yan et al., 2021), and 6 studies with 90 animals for apoptosis index (Izem-Meziane et al., 2012; Yang et al., 2013; Wang et al., 2018b; Liu et al., 2018; Javanmard et al., 2019; Li et al., 2020a) were included in the meta-analysis.
The results demonstrated that curcumin treatment decreased the serum level of IL-1β (SMD = −5.01; 95% CI: −5.81, −4.22; p < 0.01; I2 = 0%; Figure 7A), IL-6 (SMD = −6.67; 95% CI: −12.36, −0.98; p = 0.02; I2 = 96%; Figure 7B), TNF-α (SMD = −5.62; 95% CI: −8.50, −2.74; p < 0.01; I2 = 76%; Figure 7C), and the apoptosis index (SMD = −8.00; 95% CI: −14.18, −1.82; p = 0.01; I2 = 84%; Figure 7D). Our results suggest that curcumin has significant anti-inflammatory and anti-apoptosis effects in myocardial I/R injury.
FIGURE 7.
Forest plot showing cardioprotective effects of curcumin on IL-1β (A), IL-6 (B), TNF-α (C), and apoptosis index (D) in myocardial ischemia/reperfusion injury animal model. IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
4 Discussion
4.1 Summary of evidence
A total of 38 studies were included in this meta-analysis, and the results suggested that curcumin significantly reduced the myocardial IS, improved cardiac function parameters, downregulated serum myocardial enzyme, improved antioxidant ability, decreased serum inflammatory cytokines, and myocardial apoptosis index. The subgroup analysis of IS revealed that dose of curcumin may significantly influence its therapeutic effect. In the 50–100 mg/kg group, 8 studies using 100 mg/kg curcumin and 1 study using 80 mg/kg curcumin showed favorable therapeutic effects despite high heterogeneity (94%), suggesting that 100 mg/kg may be the most well-documented effective dose for the treatment of myocardial I/R injury.
In conclusion, our results demonstrated a significant cardioprotective effect of curcumin at multiple levels in animal models of myocardial I/R injury. This result provided a solid theoretical basis for subsequent large animal studies and human clinical trials.
4.2 Molecular mechanisms
The basic pathological processes of myocardial I/R injury include in inflammation, oxidation stress, cardiomyocyte apoptosis, etc (Schirone et al., 2022). Curcumin has been reported to exert anti-inflammatory, anti-apoptosis, antioxidant, and anti-fibrosis properties (Li et al., 2020b). To better understand the of cardioprotective effect of curcumin in myocardial I/R injury, we summarized its molecular mechanism.
Curcumin reduces myocardial I/R injury through multiple molecular mechanisms, including reducing NF-κB activation (Yeh et al., 2005a; Yeh et al., 2005b), activating the JAK2/STAT3 (Jeong et al., 2012; Liu et al., 2017b) and activating the PI3K/AKT/mTOR signaling pathway (Wu et al., 2021a). In rabbit myocardial I/R injury model, curcumin treatment can significantly inhibit NF-κB activity in myocardial cells, resulting in the downregulation of pro-inflammatory genes such as IL-6, TNF-α, and MCP1, and activation of MMPs (Yeh et al., 2005a; Yeh et al., 2005b). Curcumin activated JAK2/STAT3 pathway, reduced oxidative damage, and inhibited myocardium apoptosis, which improved the myocardial IS in turn (Jeong et al., 2012; Liu et al., 2017b). Further study has implicated pro-surviving pathway of PI3K/Akt/mTOR and MAPK was activated by curcumin, resulting in reduction of myocardial cell apoptosis, and inhibition of inflammation and oxidative stress (Wu et al., 2021a).
Sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD)+-dependent histone deacetylase, regulates cell stress, energy metabolism, and cell apoptosis through the deacetylation of specific substrate proteins such as NF-κB, FOXO1, TP53, and AMPK, and thus plays a cardiovascular protective role (Yang et al., 2013; Xiao et al., 2016b; Li et al., 2019b; Ren et al., 2020; Aziz et al., 2022). The upregulation and activation of SIRT1 by curcumin pretreatment have been reported to attenuate the mitochondrial oxidative damage, myocardial enzyme levels, and myocardial apoptosis induced by myocardial I/R injury (Yang et al., 2013). Myocardial cells that die after myocardial I/R injury lose their cell membrane integrity, and a measurable infarct area occurs (Liu et al., 2017c). Curcumin maintains mitochondrial membrane stability and reduces mitochondrial oxidative damage by activating SIRT1 (Yang et al., 2013).
Myocardial fibroblasts and macrophages play vital roles in cardiac repair and remodeling after myocardial infarction and affects the prognosis of patients (Prabhu and Frangogiannis, 2016; Wang et al., 2023). Pro-inflammatory cytokines (such as IL-1β and TNF-α) were thought to promote cardiac fibrosis by inducing the expression of TGF-β in fibrogenic macrophages. The expression of IL-18, a pro-inflammatory cytokine, were increased in MI, resulting in upregulation of TGF-β, inflammatory activation, and activation of SMAD2/3 which eventually activated cardiac fibrosis. Curcumin pretreatment inhibited the pro-fibrotic TGFβ1-SMAD2/3 signaling pathway and then improve cardiac fibrosis and cardiac function after MI (Zhao et al., 2022). In addition, TGFβ1-SMAD2/3 signaling can improve cardiac repair after myocardial infarction by inducing myocardial fibroblasts to express CTHRC1 and then selectively activate WNT5A signialing pathway (Wang et al., 2023).
Oxidative stress is involved in the entire process of myocardial I/R injury (Mokhtari-Zaer et al., 2018). Excessive production of reactive oxygen species (ROS) is generally toxic to cells, causing damage to all components of the cell, such as DNA, proteins, and lipids (Bagheri et al., 2016). Therefore, reducing oxidative stress is a strategy to deal with myocardial I/R injury. Curcumin not only protects cardiomyocyte from hypoxia/reoxygenation (H/R) damage by inhibiting the MAPK signaling pathway and Notch pathway to reduce ROS levels (Wei et al., 2019; Zhu et al., 2019), but also alleviates hyperglycemic-induced H9c2 damage by activating Nrf2/HO-1 signaling pathway to reduce ROS production (Wu et al., 2022).
4.3 Implications
Animal researches are limited by design variations and methodological flaws. In addition, publication bias also limits the clinical translation of animal studies because positive experimental results are easier to publish (van der Worp et al., 2010). Studies have shown that preclinical animal meta-analysis help the translation of research results from animal findings to clinical trials (Sena et al., 2014). Moreover, the rigor and reproducibility of preclinical animal studies varied greatly. Thus, it is of great importance and necessity to improve the rigor and reproducibility of preclinical research on cardioprotection (Bøtker et al., 2018; Lecour et al., 2021). The consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR) develops a multicenter, randomized, controlled, clinical trial-like infrastructure to address these problems (Jones et al., 2015). We are looking forward to finding more high-quality preclinical studies under CAESAR.
The results of this study showed that curcumin significantly reduces myocardial IS, improves cardiac function, downregulates myocardial enzyme levels, inhibits oxidative stress, decreased serum inflammatory cytokines, and myocardial apoptosis index to play a cardioprotective role in animal models of myocardial I/R injury. However, the results of this study only used the target animal model and did not include animal models with multiple coexisting diseases, which may differ from the pathology of patients in the actual clinical setting. Although high heterogeneity exists, our results still suggest that curcumin is effective in myocardial I/R injury, and this study can provide a solid theoretical basis for further clinical trials.
4.4 Limitations
Firstly, only small animals were used in the included studies. There is a lack of evidence in larger animals. According to the criteria of IMproving Preclinical Assessment of Cardioprotective Therapies (IMPACT) (Lecour et al., 2021), large animal models were the final step of validation to be considered before clinical tests. Therefore, our results might be limited by the lack of large animal models. In addition, most studies used only male animals due to a higher model success rate. Secondly, the heterogeneity in IS, LVDP, CF, CK, CK-MB, and LDH was relatively high. Besides, significant publication bias was found for CK and LDH. Therefore, there is a possibility that the efficacy of curcumin may be overestimated. Finally, all included studies were carried out in target animal models, which were inconsistent with complex cardiovascular comorbidities (such as diabetic hypertension and hyperlipidemia) of patients with myocardial I/R injury in a real-world clinical setting (Andreadou et al., 2021). These factors will greatly limit the extrapolation of our results to clinical translation.
5 Conclusion
This meta-analysis suggests that curcumin has excellent potential for the treatment of myocardial I/R injury in animal models. However, this conclusion needs to be further discussed and verified in large animal models and human clinical trials.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 81903663), and the Hunan Provincial Natural Science Foundation of China (No. 2020JJ5944).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
Y-FZ and Q-HG drafted the manuscript. Y-FZ and W-JZ designed the study. W-JZ, NY, and YL revised the manuscript. X-YW, S-YC, and SD were responsible for the collection of data. Y-FZ and J-JL were responsible for data analysis. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Andreadou I., Daiber A., Baxter G. F., Brizzi M. F., Di Lisa F., Kaludercic N., et al. (2021). Influence of cardiometabolic comorbidities on myocardial function, infarction, and cardioprotection: Role of cardiac redox signaling. Free Radic. Biol. Med. 166, 33–52. 10.1016/j.freeradbiomed.2021.02.012 [DOI] [PubMed] [Google Scholar]
- Ansari M. N., Bhandari U., Pillai K. K. (2007). Protective role of curcumin in myocardial oxidative damage induced by isoproterenol in rats. Hum. Exp. Toxicol. 26 (12), 933–938. 10.1177/0960327107085835 [DOI] [PubMed] [Google Scholar]
- Aziz S. G., Pourheydar B., Chodari L., Hamidifar F. (2022). Effect of exercise and curcumin on cardiomyocyte molecular mediators associated with oxidative stress and autophagy in aged male rats. Microvasc. Res. 143, 104380. 10.1016/j.mvr.2022.104380 [DOI] [PubMed] [Google Scholar]
- Bagheri F., Khori V., Alizadeh A. M., Khalighfard S., Khodayari S., Khodayari H. (2016). Reactive oxygen species-mediated cardiac-reperfusion injury: Mechanisms and therapies. Life Sci. 165, 43–55. 10.1016/j.lfs.2016.09.013 [DOI] [PubMed] [Google Scholar]
- Bhatt D. L., Lopes R. D., Harrington R. A. (2022). Diagnosis and treatment of acute coronary syndromes: A review. Jama 327 (7), 662–675. 10.1001/jama.2022.0358 [DOI] [PubMed] [Google Scholar]
- Boarescu P.-M., Boarescu I., Bocsan I. C., Gheban D., Bulboaca A. E., Nicula C., et al. (2019). Antioxidant and anti-inflammatory effects of curcumin nanoparticles on drug-induced acute myocardial infarction in diabetic rats. Antioxidants 8 (10), 504. 10.3390/antiox8100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarescu P.-M., Chirila I., Bulboaca A. E., Bocsan I. C., Pop R. M., Gheban D., et al. (2019). Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxidative Med. Cell. Longev. 2019, 7847142. 10.1155/2019/7847142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøtker H. E., Hausenloy D., Andreadou I., Antonucci S., Boengler K., Davidson S. M., et al. (2018). Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res. Cardiol. 113 (5), 39. 10.1007/s00395-018-0696-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Lin Z., Gu Y., Yan S., Zhang X. (2018). The protective effect of curcumin on rats with myocardial ischemia-reperfusion injury. J. Clin. Exp. Med. 17 (22), 2374–2378. [Google Scholar]
- Cheng H., Liu W., Ai X. (2005). Protective effect of curcumin on myocardial ischemia reperfusion injury in rats. J. Chin. Med. Mater. 10, 61–63. 10.13863/j.issn1001-4454.2005.10.025 [DOI] [PubMed] [Google Scholar]
- Ding Y., Li J., Zheng N. (2020). The protective effect of curcumin on myocardial tissues in ischemia/reperfusion rats. J. Guangxi Med. Univ. 37 (09), 1598–1604. 10.16190/j.cnki.45-1211/r.2020.09.003 [DOI] [Google Scholar]
- Duan W., Yang Y., Jin Z., He R., Zhang J., Geng X., et al. (2012). Protective effect of Jak2/Stat3 signaling pathway in curcumin posttreatment against myocardial ischemia/reperfusion injury in rats. Chin. Heart J. 24 (03), 298–302. 10.13191/j.chj.2012.03.24.duanwx.002 [DOI] [Google Scholar]
- Geng H. H., Li R., Su Y. M., Xiao J., Pan M., Cai X. X., et al. (2016). Curcumin protects cardiac myocyte against hypoxia-induced apoptosis through upregulating mir-7a/B expression. Biomed. Pharmacother. 81, 258–264. 10.1016/j.biopha.2016.04.020 [DOI] [PubMed] [Google Scholar]
- Gu H., Wu M., Guo X. (2016). Protecitve effect of curcumin onf myocardial ischemia-reperfusion injury in mice as well as its relationship to toll-like receptor 4/nuclear factor kappa-light-chain-enhance of activated B cell signaling pathway. Chin. J. Biol. 29 (09), 932–935. 10.13200/j.cnki.cjb.001449 [DOI] [Google Scholar]
- Gulati R., Behfar A., Narula J., Kanwar A., Lerman A., Cooper L., et al. (2020). Acute myocardial infarction in young individuals. Mayo Clin. Proc. 95 (1), 136–156. 10.1016/j.mayocp.2019.05.001 [DOI] [PubMed] [Google Scholar]
- Gupta S. C., Patchva S., Aggarwal B. B. (2013). Therapeutic roles of curcumin: Lessons learned from clinical trials. Aaps J. 15 (1), 195–218. 10.1208/s12248-012-9432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy D. J., Yellon D. M. (2013). Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Invest. 123 (1), 92–100. 10.1172/jci62874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch G. (2020). Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 17 (12), 773–789. 10.1038/s41569-020-0403-y [DOI] [PubMed] [Google Scholar]
- Hooijmans C. R., Rovers M. M., de Vries R. B. M., Leenaars M., Ritskes-Hoitinga M., Langendam M. W. (2014). Syrcle’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14 (1), 43. 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris I., Gray S., Donnelly R. (2001). Protein kinase C activation: Isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia 44 (6), 659–673. 10.1007/s001250051675 [DOI] [PubMed] [Google Scholar]
- Izem-Meziane M., Djerdjouri B., Rimbaud S., Caffin F., Fortin D., Garnier A., et al. (2012). Catecholamine-induced cardiac mitochondrial dysfunction and mptp opening: Protective effect of curcumin. Am. J. Of Physiology-Heart Circulatory Physiology 302 (3), H665–H674. 10.1152/ajpheart.00467.2011 [DOI] [PubMed] [Google Scholar]
- Javanmard M. Z., Rahnavard M., Soraya H., Karimipour M. (2019). Curcumin improves the efficacy of bmscs in myocardial ischemia injury in rat. Iran. Red Crescent Med. J. 21 (9). 10.5812/IRCMJ.86592 [DOI] [Google Scholar]
- Jeong C. W., Yoo K. Y., Lee S. H., Jeong H. J., Lee C. S., Kim S. J. (2012). Curcumin protects against regional myocardial ischemia/reperfusion injury through activation of risk/gsk-3β and inhibition of P38 mapk and jnk. J. Cardiovasc. Pharmacol. Ther. 17 (4), 387–394. 10.1177/1074248412438102 [DOI] [PubMed] [Google Scholar]
- Jin J. K., Blackwood E. A., Azizi K., Thuerauf D. J., Fahem A. G., Hofmann C., et al. (2017). Atf6 decreases myocardial ischemia/reperfusion damage and links Er stress and oxidative stress signaling pathways in the heart. Circ. Res. 120 (5), 862–875. 10.1161/circresaha.116.310266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo W., Min B. S., Yang H.-Y., Park N.-H., Kang K.-K., Lee S., et al. (2020). Sappanone a prevents left ventricular dysfunction in a rat myocardial ischemia reperfusion injury model. Int. J. Mol. Sci. 21 (18), 6935. 10.3390/ijms21186935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. P., Tang X. L., Guo Y., Steenbergen C., Lefer D. J., Kukreja R. C., et al. (2015). The nhlbi-sponsored consortium for preclinical assessment of cardioprotective therapies (caesar): A new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ. Res. 116 (4), 572–586. 10.1161/circresaha.116.305462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Kwon J. S., Cho Y. K., Jeong M. H., Cho J. G., Park J. C., et al. (2012). Curcumin reduces the cardiac ischemia-reperfusion injury: Involvement of the toll-like receptor 2 in cardiomyocytes. J. Nutr. Biochem. 23 (11), 1514–1523. 10.1016/j.jnutbio.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Kotha R. R., Luthria D. L. (2019). Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 24 (16), 2930. 10.3390/molecules24162930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao C. D., Mtt R., Normolle D., Heath D. D., Murray S. I., Bailey J. M., et al. (2006). Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 6, 10. 10.1186/1472-6882-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecour S., Andreadou I., Bøtker H. E., Davidson S. M., Heusch G., Ruiz-Meana M., et al. (2021). Improving preclinical assessment of cardioprotective therapies (impact) criteria: Guidelines of the Eu-cardioprotection cost action. Basic Res. Cardiol. 116 (1), 52. 10.1007/s00395-021-00893-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Li Y., Wu D. (2020). Preparation of curcumin nanoemulsion and its protective effect on myocardial ischemia-reperfusion in rats. Chin. J. Of Comp. Med. 30 (5), 97–103. 10.3969/j.issn.1671-7856.2020.05.015 [DOI] [Google Scholar]
- Li G., Hao Y., Li S., Lu H., Zhou F. (2019). Therapeutic effects of curcumin on mouse ventricular remodelling. Farmacia 67 (6), 1041–1047. 10.31925/farmacia.2019.6.15 [DOI] [Google Scholar]
- Li H., Sureda A., Devkota H. P., Pittalà V., Barreca D., Silva A. S., et al. (2020). Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 38, 107343. 10.1016/j.biotechadv.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Li K., Zhai M., Jiang L., Song F., Zhang B., Li J., et al. (2019). Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the Sirt1 pathway. Oxid. Med. Cell Longev. 2019, 6746907. 10.1155/2019/6746907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang C., Qiao Z., Xu Y. (2017). Protective effect of curcumin against myocardium injury in ischemia reperfusion rats. Pharm. Biol. 55 (1), 1144–1148. 10.1080/13880209.2016.1214741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang C., Qiao Z., Xu Y. (2017). Protective effect of curcumin against myocardium injury in ischemia reperfusion rats. Pharm. Biol. 55 (1), 1144–1148. 10.1080/13880209.2016.1214741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Chen H., You Q. S., Ye Q., Wang F., Wang S., et al. (2017). Curcumin attenuates myocardial ischemia-reperfusion injury. Oncotarget 8 (67), 112051–112059. 10.18632/oncotarget.23002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu Y., Huang X., Zhang J., Yang L. (2018). Protective effects and mechanism of curcumin on myocardial injury induced by coronary microembolization. J. Cell Biochem. 120 (4), 5695–5703. 10.1002/jcb.27854 [DOI] [PubMed] [Google Scholar]
- Manikandan P., Sumitra M., Aishwarya S., Manohar B. M., Lokanadam B., Puvanakrishnan R. (2004). Curcumin modulates free radical quenching in myocardial ischaemia in rats. Int. J. Biochem. Cell Biol. 36 (10), 1967–1980. 10.1016/j.biocel.2004.01.030 [DOI] [PubMed] [Google Scholar]
- Mokhtari-Zaer A., Marefati N., Atkin S. L., Butler A. E., Sahebkar A. (2018). The protective role of curcumin in myocardial ischemia-reperfusion injury. J. Cell Physiol. 234 (1), 214–222. 10.1002/jcp.26848 [DOI] [PubMed] [Google Scholar]
- Nirmala C., Puvanakrishnan R. (1996). Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 159 (2), 85–93. 10.1007/BF00420910 [DOI] [PubMed] [Google Scholar]
- O'Gara P. T., Kushner F. G., Ascheim D. D., Casey D. E., Jr., Chung M. K., de Lemos J. A., et al. (2013). 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American college of cardiology foundation/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 61(4), e78-e140. 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Pagliaro P., Moro F., Tullio F., Perrelli M. G., Penna C. (2011). Cardioprotective pathways during reperfusion: Focus on redox signaling and other modalities of cell signaling. Antioxid. Redox Signal 14 (5), 833–850. 10.1089/ars.2010.3245 [DOI] [PubMed] [Google Scholar]
- Pawar H. D., Mahajan U. B., Nakhate K. T., Agrawal Y. O., Patil C. R., Meeran M. F. N., et al. (2022). Curcumin protects diabetic mice against isoproterenol-induced myocardial infarction by modulating Cb2 cannabinoid receptors. Life (Basel) 12 (5), 624. 10.3390/life12050624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu S. D., Frangogiannis N. G. (2016). The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circ. Res. 119 (1), 91–112. 10.1161/circresaha.116.303577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Gupta S. C., Tyagi A. K., Aggarwal B. B. (2014). Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 32 (6), 1053–1064. 10.1016/j.biotechadv.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Rahnavard M., Hassanpour M., Ahmadi M., Heidarzadeh M., Amini H., Javanmard M. Z., et al. (2019). Curcumin ameliorated myocardial infarction by inhibition of cardiotoxicity in the rat model. J. Cell. Biochem. 120 (7), 11965–11972. 10.1002/jcb.28480 [DOI] [PubMed] [Google Scholar]
- Ren B. C., Zhang Y. F., Liu S. S., Cheng X. J., Yang X., Cui X. G., et al. (2020). Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via sirt1-foxo1 and pi3k-akt signalling pathways. J. Cell. Mol. Med. 24 (21), 12355–12367. 10.1111/jcmm.15725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirone L., Forte M., D'Ambrosio L., Valenti V., Vecchio D., Schiavon S., et al. (2022). An overview of the molecular mechanisms associated with myocardial ischemic injury: State of the art and translational perspectives. Cells 11 (7), 1165. 10.3390/cells11071165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena E. S., Currie G. L., McCann S. K., Macleod M. R., Howells D. W. (2014). Systematic reviews and meta-analysis of preclinical studies: Why perform them and how to appraise them critically. J. Cereb. Blood Flow. Metab. 34 (5), 737–742. 10.1038/jcbfm.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. (2022). Ten points to improve reproducibility and translation of animal research. Front. Behav. Neurosci. 16, 869511. 10.3389/fnbeh.2022.869511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., Liao W., Liu J. (2014). Experimental study on reducing myocardial ischemia/reperfusion injury by curcumin. Contemp. Med. 20 (33), 12–13. [Google Scholar]
- Sun B., Li G., Rui R., Li H., Wang H. (2016). Curcumin attenuates myocardial ischemia-reperfusion injury by suppressing mitochondrial apoptosis. J. Clin. Cardiol. 32 (06), 619–623. [Google Scholar]
- Tanwar V., Sachdeva J., Golechha M., Kumari S., Arya D. S. (2010). Curcumin protects rat myocardium against isoproterenol-induced ischemic injury: Attenuation of ventricular dysfunction through increased expression of Hsp27 alongwith strengthening antioxidant defense system. J. Cardiovasc. Pharmacol. 55 (4), 377–384. 10.1097/FJC.0b013e3181d3da01 [DOI] [PubMed] [Google Scholar]
- van der Worp H. B., Howells D. W., Sena E. S., Porritt M. J., Rewell S., O'Collins V., et al. (2010). Can animal models of disease reliably inform human studies? PLoS Med. 7 (3), e1000245. 10.1371/journal.pmed.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhang Y., Ye T., Zhang R., Zhang L., Shi D., et al. (2023). Cthrc1 deficiency aggravates wound healing and promotes cardiac rupture after myocardial infarction via non-canonical Wnt5a signaling pathway. Int. J. Biol. Sci. 19 (4), 1299–1315. 10.7150/ijbs.79260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Duan W., Yang Y., Li Y., Liang Z., Xiaozhen J., et al. (2013). Effects of curcumin post-treatment against myocardial ischemia and reperfusion by activation of the Notch signaling pathway. Shandong Med. J. 53 (06), 4–8. [Google Scholar]
- Wang N. P., Pang X. F., Zhang L. H., Tootle S., Harmouche S., Zhao Z. Q. (2014). Attenuation of inflammatory response and reduction in infarct size by postconditioning are associated with downregulation of early growth response 1 during reperfusion in rat heart. Shock 41 (4), 346–354. 10.1097/SHK.0000000000000112 [DOI] [PubMed] [Google Scholar]
- Wang N. P., Wang Z. F., Tootle S., Philip T., Zhao Z. Q. (2012). Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br. J. Pharmacol. 167 (7), 1550–1562. 10.1111/j.1476-5381.2012.02109.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Zhang J. Y., Zhang M., Zhai M. G., Di S. Y., Han Q. H., et al. (2018). Curcumin attenuates Ir-induced myocardial injury by activating Sirt3. Eur. Rev. Med. Pharmacol. Sci. 22 (4), 1150–1160. 10.26355/eurrev_201802_14404 [DOI] [PubMed] [Google Scholar]
- Wang R., Zhang J. Y., Zhang M., Zhai M. G., Di S. Y., Han Q. H., et al. (2018). Curcumin attenuates Ir-induced myocardial injury by activating Sirt3. Eur. Rev. Med. Pharmacol. Sci. 22 (4), 1150–1160. 10.26355/eurrev_201802_14404 [DOI] [PubMed] [Google Scholar]
- Wei W., Peng J., Li J. (2019). Curcumin attenuates hypoxia/reoxygenation-induced myocardial injury. Mol. Med. Rep. 20 (6), 4821–4830. 10.3892/mmr.2019.10742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Whye D., Glazewski L., Choe L., Kerr D., Lee K. H., et al. (2011). Proteomic assessment of a cell model of spinal muscular atrophy. Chongqing Med. 40 (01), 25–26+9. 10.1186/1471-2202-12-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. J., Zhang K., Ma J. J., Wang L., Zhuang Y. (2021). Mechanism of curcumin against myocardial ischaemia-reperfusion injury based on the P13k/akt/mtor signalling pathway. Eur. Rev. Med. Pharmacol. Sci. 25 (17), 5490–5499. 10.26355/eurrev_202109_26658 [DOI] [PubMed] [Google Scholar]
- Wu H. J., Zhang K., Ma J. J., Wang L., Zhuang Y. (2021). Mechanism of curcumin against myocardial ischaemia-reperfusion injury based on the P13k/akt/mtor signalling pathway. Eur. Rev. Med. Pharmacol. Sci. 25 (17), 5490–5499. 10.26355/eurrev_202109_26658 [DOI] [PubMed] [Google Scholar]
- Wu X., Zhou X., Lai S., Liu J., Qi J. (2022). Curcumin activates Nrf2/Ho-1 signaling to relieve diabetic cardiomyopathy injury by reducing ros in vitro and in vivo . Faseb J. 36 (9), e22505. 10.1096/fj.202200543RRR [DOI] [PubMed] [Google Scholar]
- Xiao J., Sheng X., Zhang X., Guo M., Ji X. (2016). Curcumin protects against myocardial infarction-induced cardiac fibrosis via Sirt1 activation in vivo and in vitro . Drug Des. Dev. Ther. 10, 1267–1277. 10.2147/DDDT.S104925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Sheng X., Zhang X., Guo M., Ji X. (2016). Curcumin protects against myocardial infarction-induced cardiac fibrosis via Sirt1 activation in vivo and in vitro . Drug Des. Devel Ther. 10, 1267–1277. 10.2147/dddt.S104925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Zhou M., Zheng X., Xing Y., Dong J., Yan M., et al. (2021). Anti-inflammatory effect of curcumin on the mouse model of myocardial infarction through regulating macrophage polarization. Mediat. Inflamm. 2021, 9976912. 10.1155/2021/9976912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Duan W., Lin Y., Yi W., Liang Z., Yan J., et al. (2013). Sirt1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic. Biol. Med. 65, 667–679. 10.1016/j.freeradbiomed.2013.07.007 [DOI] [PubMed] [Google Scholar]
- Yang Y., Wang N., Liang Z., Li Y., Duan W., Jin Z., et al. (2012). Protective effect of curcumin pre-treatment against myocardial ischemia reperfusion injury in isolated perfusion rat hearts. J. Cardiovasc. Surgery(Electronic Ed. 1 (01), 26–30. [Google Scholar]
- Yao B., Jiang W. (2014). Protective effects of curcumin post-treatment on myocardial ischemia/reperfusion injury via heme oxygenase-1. Chin. J. Arteriosclerosis 22 (07), 685–689. [Google Scholar]
- Yeh C. H., Chen T. P., Wu Y. C., Lin Y. M., Jing Lin P. (2005). Inhibition of nfkappab activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J. Surg. Res. 125 (1), 109–116. 10.1016/j.jss.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Yeh C. H., Lin Y. M., Wu Y. C., Lin P. J. (2005). Inhibition of nf-kappa B activation can attenuate ischemia/reperfusion-induced contractility impairment via decreasing cardiomyocytic proinflammatory gene up-regulation and matrix metalloproteinase expression. J. Cardiovasc Pharmacol. 45 (4), 301–309. 10.1097/01.fjc.0000155385.41479.b3 [DOI] [PubMed] [Google Scholar]
- Zhao J., Chen Y., Chen Q., Hong T., Zhong Z., He J., et al. (2022). Curcumin ameliorates cardiac fibrosis by regulating macrophage-fibroblast crosstalk via Il18-P-Smad2/3 signaling pathway inhibition. Front. Pharmacol. 12, 784041. 10.3389/fphar.2021.784041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Chang C., Xin S., Tian L., Zhu Y. (2010). Effect of curcumin on myocardial ischemia reperfusion in rats. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 8 (08), 956. [Google Scholar]
- Zhu P., Yang M., He H., Kuang Z., Liang M., Lin A., et al. (2019). Curcumin attenuates hypoxia/reoxygenation-induced cardiomyocyte injury by downregulating Notch signaling. Mol. Med. Rep. 20 (2), 1541–1550. 10.3892/mmr.2019.10371 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.