Abstract
Providing quality cardiovascular disease (CVD) care in low resource setting requires understanding of priority and effective interventions. This study aimed to identify and prioritize evidence-based quality improvement strategies for CVD care in India using a modified two-round Delphi process in which, we asked 46 experts (clinicians, researchers, program implementers and policy makers) to rate 25 proven CVD care strategies grouped into: (1) patient support, (2) information communication technology (ICT) for health, (3) group problem solving, (4) training, and (5) multicomponent strategy on a scale of 1 (highest/best)—5 (lowest/worst) on priority, relative advantage, and feasibility. Subsequently, we convened an expert consensus panel of 32 members to deliberate and achieve consensus regarding the prioritized set of strategies for CVD care. The Delphi study found that group problem solving strategies achieved the best score for priority (1.80) but fared poorly on feasibility (2.88). Compared to others, multicomponent strategies were rated favorably across all domains (priority = 1.84, relative advantage = 1.94, and feasibility = 2.40). The ICT for health strategies achieved the worst scores for priority = 2.01, relative advantage = 2.31, and feasibility = 2.85. Training and patient support strategies scored moderately across all domains. The expert panel narrowed the selection of a multicomponent strategy consisting of (1) electronic health records with clinical decision-support system, (2) non-physician health worker facilitated care, (3) patient education materials, (4) text-message based reminders for healthy lifestyle, and (5) audit and feedback report for providers. Future research will evaluate the real-world feasibility and effectiveness of the multicomponent strategy in patients with CVD in a low- and middle-income country setting.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43477-023-00087-2.
Keywords: Delphi study, Cardiovascular disease care, Quality improvement, India
Introduction
In 2019, cardiovascular diseases (CVD) caused around 18.5 million deaths, with over half a billion prevalent cases globally (Roth et al., 2019). Notably, 80% of premature CVD deaths (< 70 years) now occur in low- and middle-income countries (LMICs) (Khetan et al., 2019). Indians particularly develop CVD at a much younger age and have one of the highest age-standardized CVD death rates compared to western countries thus leading to significant productivity loss, poor economic growth, and social challenges (India State-Level Disease Burden Initiative, 2018; Ke et al., 2018; Yusuf et al., 2001). Factors contributing to high CVD burden in India are complex including patient-level factors such as high propensity to cardiometabolic risk factors, unhealthy diets, physical inactivity, tobacco use, and low disease awareness; clinician-level factors such as inadequate screening, diagnosis, treatment, and monitoring of CVD; and system- and policy-level factors such as suboptimal availability, access, and quality of care, and weak social health protection schemes for outpatient CVD care (Gupta et al., 2018; Mensah et al., 2019). There are multiple levels at which CVD can be prevented—primordial prevention that focusses on population-based policy interventions (e.g., tobacco taxation, tax on sugar sweetened beverages) to promote healthy lifestyles, primary prevention to prevent or delay the onset of CVD in individuals with elevated risk factors (e.g., screening of hypertension and diabetes), and secondary prevention to prevent the occurrence/recurrence of heart attacks or strokes (after disease onset).
Several effective, evidence-based treatment for CVD prevention exist, including aspirin, blood pressure lowering drugs, and cholesterol lowering drugs. However, uptake and implementation of proven treatment for CVD are deficient, leading to a 55%-point gap in the expected efficacy of CVD secondary prevention resulting in highly reduced real-world effectiveness (Perel et al., 2015). This gap is ostensibly due to factors related to suboptimal prescription (e.g., provider-level inertia, time constraints, large patient volume) and adherence rates (e.g., patient-level: low health literacy, cost). In the Indian context, barriers to CVD care are further exacerbated by the economic burden on patients and their family from treatment (Mohanan et al., 2019), poor understanding of the need for lifelong medication particularly for diseases like CVD that are frequently free of symptoms, and poor access and supply of evidence-based treatments to a vast majority of the population in need of these treatments. Bridging these implementation gaps require multidisciplinary collaborations between policymakers, academia, implementation partners, trained health workforce, and public–private partnerships (Collaborators et al., 2021; Rehman et al., 2020). Further, contextual adaptation and implementation of successful/standardized CVD care models are important due to significant variations in CVD care quality across states and socio-demographic groups within India (Geldsetzer et al., 2018). Despite global recognition of CVD as a direct and indirect threat to economic development and identification of many implementation gaps in clinical care quality, research to close “know-do” gaps remains scarce in India (Collaborators et al., 2021; Singh et al., 2021).
Among the proven strategies for CVD care that may potentially close these “know-do” gaps within LMIC context (fragmented healthcare system) are task-sharing through involvement of non-physician health workers, and clinical decision support tools for providers to enhance responsiveness to timely treatment modification (Singh et al., 2021; Xavier et al., 2016). Further, prompts and reminders for the clinical team, and storing serial data in accessible software programs (i.e., electronic health records) may stimulate better continuity, coordinated and comprehensive CVD care delivery. Prior reports indicate that several other strategies exist to improve outcomes in patients with CVD, but not all of them can be implemented in resource constrained settings such as India (Singh et al., 2021). Hence, we need to identify and prioritize CVD prevention strategies within local LMIC context, so the strategies can be effectively and consistently implemented across diverse healthcare settings. Further, a prioritized set of quality improvement strategies for CVD care could assist policy makers to inform resource investment decisions considering relative advantage, feasibility, and the potential public health impact of interventions across socio-demographic groups. This study aimed to identify and prioritize evidence-based quality improvement strategies for CVD care in India using a modified Delphi process by integrating the perspectives of clinicians, program implementers and policy makers.
Methods
Overview: Modified Delphi Technique
To identify which interventions and implementation strategies to prevent and control CVD in India are considered priorities, we performed a modified Delphi process between September 2020 and November 2020 that consisted of a systematic two-round web-based Delphi questionnaire with a panel of leading experts representing diverse geographic regions in India, followed by a virtual face-to-face consensus meeting. The Delphi method is an iterative consensus-based process that uses serial surveys or rounds to gather information from an expert panel, thereby gives anonymity and an equal opportunity for voice and participation to all participants (Fink et al., 1984). The Delphi process is widely used to attain consensus regarding quality indicators (Evangelidis et al., 2017) (Linstone, 1975), development of consensus statements for CVD diagnosis/treatment (Verhestraeten et al., 2020), identification of gaps in CVD guidelines (Panchal et al., 2018) and implementation strategies (Powell et al., 2015). In this Delphi study, the main outcome of interest was development of consensus on a prioritized set of CVD care strategies that, if implemented fully, have the greatest impact to reduce CVD burden.
The study was approved by the Public Health Foundation of India’s Institutional Ethics Committee (Reference no. TRC-IEC-382/18). Agreement to take part in the Delphi process implied voluntary consent to participate in this study.
Selection of Expert Panelists
Individuals were defined as “experts” if they held a senior position as a professor in cardiology, community medicine, or a related medical field at a major research university or were a director, administrator, program implementer, health services researcher, or advisor to a governmental or nongovernmental organization. The formation of experts group was guided by a list of investigators who previously participated in CVD research in India (Huffman et al., 2018; Lamy et al., 2016; Prabhakaran et al., 2018, 2020; Singh et al., 2019; Thom et al., 2014) or served as faculty in the hypertension and CVD management training programs conducted by the Public Health Foundation of India (Sharma et al., 2019). Ideal panel sizes for the Delphi method have been cited as 20–50 participants to allow for group dynamics to be established (Linstone, 1975).
In October 2020, first an email invitation to participate in the two-round Delphi survey was sent to 84 experts. To improve survey response and participation in the Delphi process, reminders to participate were sent out about 1 and 2 weeks after each round’s survey invitation. Participation and completion of the survey was voluntary and without compensation.
In November 2020 expert panel group for the final round of face-to-face meeting was constituted. Although an in-person consensus meeting was originally planned, however, due to the COVID-19 pandemic related restrictions we conducted a virtual expert panel meeting in November end 2020. The inclusion criteria for the final round of expert panel were experience of designing or implementing CVD quality improvement strategies or specialist expertise and authority or influence in the science of CVD care delivery in low resource settings. For their identification, a “snowball” strategy was used based on the personal contacts of the study steering committee who, in turn proposed new relevant candidates in their professional settings. Following this process, 40 professionals were invited. Of those 32 experts agreed to take part in the final expert consensus meeting. Figure 1 summarizes the Delphi process.
Fig. 1.
Delphi process diagram. QI quality improvements, CVD cardiovascular disease. Likert scale 1–5, 1 = highest priority/feasibility or best strategy, 5 = lowest priority/feasibility or worst strategy
Questionnaire Development
The quality improvement strategies included in the Delphi survey questionnaire was derived from a previously published scoping review (Singh et al., 2021). Briefly, the goal of the scoping review was to map the global evidence on the intervention strategies to enhance outcomes in patients with CVD. The review included randomized trials and quasi-experimental studies that assessed the effectiveness of patient-, provider- or health system level strategies among patients with CVD and were published between January 1, 2009, and October 25, 2019. The search was limited to studies published since January 2009 to capture recent advancements in health technology and implementation strategies that would make contextualization and adaptation of quality improvement interventions more relevant and applicable to current healthcare settings. Observational studies and quasi-experimental studies without comparator arm, narrative reviews and editorials were excluded. Further, to guide data extraction and synthesis the scoping review used a previously published “Health Care Provider Performance Review” (HCPPR) (Rowe et al., 2018) framework, which systematically reviewed the effectiveness of strategies to improve health care provider performance in LMICs. The HCPPR framework allowed a comprehensive mapping of the CVD care strategies. In summary, the scoping review included 456 studies from 45 countries involving 150,148 patients that assessed 186 unique interventions to improve outcomes in patients with CVD.
From the 186 interventions assessed in the scoping review, we narrowed the selection of 25 strategies considering the transferability of successful CVD care delivery models to a LMIC setting. The decision criteria used to select the initial set of 25 strategies from the scoping review included: (1) proven effectiveness of the strategies on either reducing the risk of CVD events, improving risk factor control, or increasing adherence to CVD medications, (2) demonstration of implementation feasibility, (3) adaptability to local context in terms of resources required and economic costs (or cost-effectiveness) if data were available. In addition, through an informal process single proven strategies were combined to construct five different sets of multicomponent strategies.
Next, the strategies were grouped into five categories using the HCPPR framework: (1) patient support strategies (n = 9) (e.g., patient-counseling; nurse-, pharmacist- or community health worker facilitated care), (2) Information communication, and technology (ICT) for health strategies (n = 7) (e.g., text reminders, use of digital apps, telehealth services for CVD care), (3) group problem solving (n = 1) (e.g., goal setting for better self-care), (4) training strategies targeted at patients as well as providers (n = 3) (e.g., yoga and exercise training for patients, physician’s training on motivational interviewing technique), and (5) multicomponent strategies (n = 5) (e.g., patient education + telephonic support by nurse + clinical decision support system for providers + electronic health records). See supplementary file for a complete list of strategies included in the Delphi study.
Data Collection Process
The Delphi survey tool consisting of 25 strategies and basic demographic characteristics of experts was designed and pre-tested in September 2020. Refinements to the questionnaire were discussed within the core group of study investigators. The online two-round Delphi survey was carried out between October 2020 and November 2020, followed by a virtual consensus meeting held in November end 2020. The two-round Delphi survey was digitalized using the Research Electronic Data Capture (REDCap) (Harris et al., 2009). Participant responses without personal identifiers were maintained in the REDCap database to ensure anonymity. Before beginning to answer the Delphi survey, the experts had to answer a series of questions about their medical specialty, years of professional practice, and number and characteristics of CVD patients treated.
Delphi round 1 was an “informed” assessment in which respondents were informed/provided an adapted list of 25 proven interventions identified through an informal process as described above based on the evidence gathered from a published scoping review. Experts in round 1 were asked to rank each strategy on three main attributes: (i) priority (on a Likert scale of 1–5, where 1 indicates high priority and 5 indicates low priority), (ii) relative advantage (versus usual care) using a 5-point Likert-type scale, from 1 = Best (“very important/critical”) to 5 = worst (“not at all important”), and (iii) four feasibility criteria (each on a scale of 1–5, where 1 indicates high feasibility and 5 indicates low feasibility): (i) Reach defined as ability to reach the target population, (ii) Technical complexity defined as the level of medical technologies or expertise needed to implement an intervention, (iii) capital intensity defined as the amount of capital resources required for an intervention, and (iv) cultural acceptability defined as appropriateness of an intervention regarding social norms or religious beliefs in the respondent’s geographic region.
Round 2 was an “uninformed” assessment (meaning no prior information on the interventions or strategies were provided to the experts) carried out to identify the most important strategies and tactics to achieve the priorities for CVD care (beyond the strategies already listed in Round 1). Specifically, respondents were asked to provide a list of up to five innovative or novel strategies that would facilitate CVD prevention and control in India.
Finally, the data gathered from the two-round Delphi survey were synthesized and presented at a virtual expert panel meeting to develop consensus on the prioritized set of strategies for CVD care. The expert panel meeting included formal presentations on the two-round Delphi survey results, break-out sessions to have in-depth discussion around priority, and feasibility of implementing each of the selected strategies. The break-out session moderators reported back to the expert panel on the major concerns, agreements, and disagreements on individual strategies. Lastly, through an open consultation/voting, we arrived at a consensus on the design of the intervention strategy for CVD care considering priority, relative advantage, and feasibility.
Statistical Analysis
Quantitative Analysis
Data collected in Delphi Round 1 were analyzed using Microsoft Office Excel 2013, and STATA (version 16.0 SE; StataCorp, TX, USA). Participants’ demographic characteristics were summarized using mean (standard deviation, SD) for age, and numbers (percentages) for categorical variables (sex, education, clinical experience, hospital type). Further, mean scores (SD) for single strategy, as well as aggregate mean score (SD) for the five strategy groups (i.e., patient support, ICT for health, training, group problem solving and multicomponent strategies) were calculated separately for priority, relative advantage, and the four feasibility criteria (i.e., reach, technical complexity, capital intensity, cultural acceptability, and appropriateness). Next, we plotted the summary scores for the five strategy groups to compare the mean scores across the three domains: priority, relative advantage, and feasibility. In addition, the summary results for each strategy group were plotted on a two-quadrant graph represented by high/low priority (y-axis) and high/low feasibility (x-axis). The size of the bubble represented the relative advantage of the five strategy groups.
Further, to analyze the expert group opinion regarding each strategy and for the interpretative purposes of the Likert-type scale questions, the presentation of the answers/scores was systematized by grouping the range of possible values between 1 and 5 into 3 levels: 1–2 (agreements), 3 (neutral), 4–5 (disagreements). A consensus was defined as one reached in disagreement or agreement when at least 75% of the panelists had given scores of 1–2 (consensus in agreement) or 4–5 (consensus in disagreement), respectively. This level of agreement has been considered appropriate in previous published Delphi studies. (Cases Amenos et al., 2016; Diamond et al., 2014; Panchal et al., 2018; Woodcock et al., 2020). The data were analyzed as whole comparing all strategies and comparing the answers within each strategy group. For subsequent round, strategies that were deemed as important by less than 75% of the panelists were considered as non-consensus and removed. Between the two-round Delphi survey and expert panel meeting, data were summarized by two independent researchers (K.S. and A.J.) who were not a part of the panel.
Qualitative Analysis
A qualitative synthesis of data collected in Delphi Round 2 was performed to identify common themes using the qualitative framework method involving data familiarization, framework identification, indexing, charting, and mapping and interpretation (Hackett & Strickland, 2019). An iterative thematic analysis (Braun & Clarke, 2006) approach was followed to identify the themes by two researchers (A.J., K.S.), which was developed by interrogating data categories through comparison between and within expert responses. Finally, we enumerated how many times each of the strategy/theme was mentioned by the respondents in Delphi round 2.
During the virtual face-to-face expert panel meeting, consensus was recorded using electronic survey forms. Data analysis was performed in November–December 2020.
Results
Of the 84 experts approached, 46 responded to the online two-round Delphi survey (response rate = 54.7%). Mean (standard deviation) age of respondents was 51.5 (11.2) years, 95% male, and mean (SD) number of years practicing CVD care was 20.5 (11.6) years (see Table 1).
Table 1.
Characteristics of study participants (Experts, n = 46)
| Participant’s characteristics | n | (%) |
|---|---|---|
| Age, mean (SD) | 51.5 (11.2) | – |
| Sex, Male | 40 | (95%) |
| Highest qualifications | ||
| Doctorate in Medicine (Cardiology) | 32 | (71%) |
| MD, Medicine | 10 | (21%) |
| PhD | 4 | (7%) |
| Years practicing cardiology, mean (SD) | 20.5 | (11.6%) |
| Private hospital | 30 | (71%) |
SD Standard deviation; PhD Doctorate in Philosophy
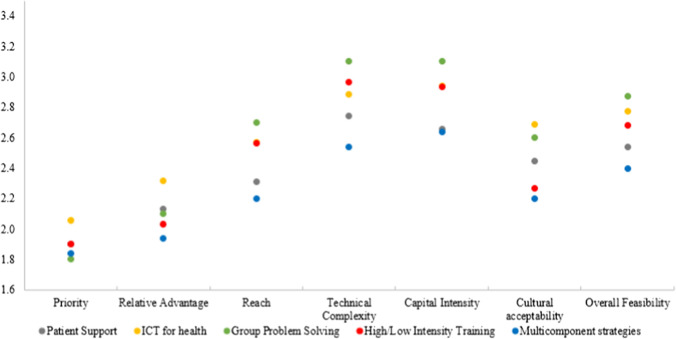
The mean scores (SD) for individual strategies are provided in the supplementary file (eTable 1). All strategy groups scored relatively well for priority (range: 1.80–2.06) and relative advantage (range: 1.94–2.31) but scored poorly for feasibility criteria: technical complexity (range: 2.54–3.10) and capital intensity (range: 2.64–3.10) (see Fig. 2). Group problem solving achieved the best score for priority (1.80) but was rated poorly on feasibility criteria (2.88). Multicomponent strategies rated well across all domains (priority = 1.84, relative advantage = 1.94, feasibility = 2.40). ICT for health strategy achieved the worst scores across all domains (priority = 2.01, relative advantage = 2.31, feasibility = 2.85).
Fig. 2.
Mean scores of strategy groups for priority, relative advantage, and feasibility criteria. (1 = Best; 5 = Worst). *Mean scores for five quality improvement strategy groups: (1) patient support, (2) information communication and technology (ICT) for health, (3) group problem solving, (4) high/low intensity training, and (5) multicomponent strategies. These strategies were rated by experts across three domains on a scale of 1–5 (1 = best, 5 = worst): priority, relative advantage (compared to usual care scenario), and feasibility criteria (reach, technical complexity, capital intensity and cultural acceptability)
Priority
Mean scores for priority ranged from 1.80 (group problem solving strategies) to 2.06 (ICT for health strategies). At the individual strategy level, strategies with the best scores (1.60) were: (1) patient support strategy of: In-clinic patient educational video + patient report card + text messages on self-care management for CVD, and (2) training strategy of physician’s training on motivational interviewing. The ICT for health intervention: mHealth based intervention with automated emails sent to care partners received the worst score of 2.40.
Relative Advantage
Mean scores for relative advantage ranged from 1.94 (multicomponent strategies) to 2.31 (ICT for health strategies). At the individual strategy level, multicomponent strategies of: (1) face-to-face patient counselling by health worker + patient education and (2) self-care manual + telephone support by trained nurse, and training strategy of: physician’s training on motivational interviewing fared the best with an average score of 1.70. The worst mean score (2.60) was given to the ICT for health strategy using automated emails sent to care partners.
Feasibility
Mean scores for feasibility criteria ranged from 2.40 (multicomponent strategies) to 2.88 (group problem solving strategies). At the individual strategy level, 3 (of 7) ICT for health strategies received poor mean scores of > 3 for both technical complexity and capital intensity. Likewise, 1 patient support strategy (of 9), the single group problem solving strategy, and one training strategy (of 3) received mean scores of > 3.
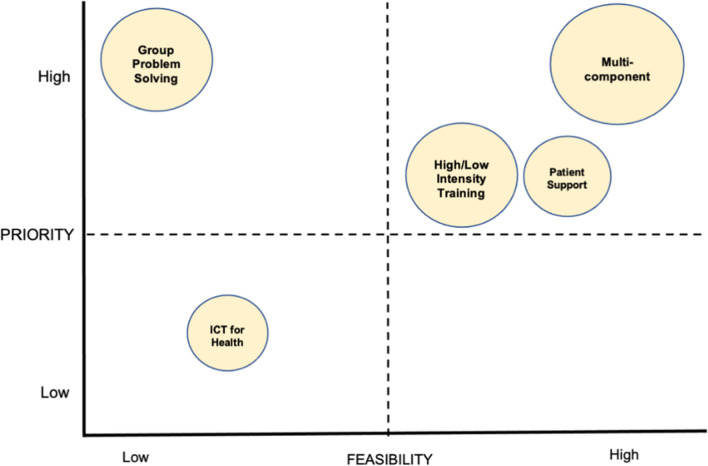
While most multicomponent, training, and patient support strategies scored well for both feasibility and priority, ICT for health strategies scored poorly for both (see Fig. 3). Group problem solving strategies scored well for priority, but poorly for feasibility. ICT for health strategies scored poorly on relative advantage whereas multicomponent strategies scored the best. The expert (dis)agreement rates for the individual strategies are shown in eTable 2, which are consistent with the average priority or feasibility scores as in eTable 1. Several strategies (n = 11) such as non-physician health workers led lifestyle counselling, patient education materials, text-message based reminders, electronic health records, group problem solving, training providers on motivational interviewing and multicomponent interventions received consensus (agreement rate of > 75%) in Delphi round 1.
Fig. 3.
Strategy groups represented on a graph based on high and low rankings for feasibility and priority. *Mean scores for relative advantage are represented by the size of the bubble, i.e., high, medium, low
Delphi round 2 process yielded 142 strategies, which were organized into 29 categories or themes. The most common themes that emerged from Delphi Round 2 to improve CVD care quality in India were: standardized health information systems to maintain and track regular patient follow-ups, mHealth tools-based alerts to improve medication adherence, telehealth services, and consideration of using polypill for secondary prevention of CVD (see Table 2). Strategies recommended in Round 2 that were not part of Round 1 included efforts to minimize multiple drug prescriptions (polypharmacy), and use of polypills to improve medication adherence, and integration of mental health workers alongside CVD care teams. In addition, several policy-level strategies were recommended such as mass media campaigns to improve awareness around CVD and risk factors; cross-subsidies for medicines, fruits, and vegetables; increasing tobacco taxation; strengthening primary care centers; cardiovascular health education in school children; educating policymakers about the value of population level CVD prevention strategies; mass targeted screening programs for CVD, and involvement of health professional in policymaking.
Table 2.
Number of mentions of novel implementation strategies to prevent or control CVD in India
| No | Strategies | Number | Percent |
|---|---|---|---|
| 1 | Trained NPHW (nurse, pharmacist) for chronic care of CVD in primary care setting | 22 | 15.5 |
| 2 | Patient and Caregiver education/counselling using printed materials/booklet | 17 | 12.0 |
| 3 | Mobile app for medication reminders and alters when medications are missed/non-compliance | 14 | 9.9 |
| 4 | Systems in place to track, monitor, and maintain regular follow-up care | 13 | 9.2 |
| 5 | Physician education | 9 | 6.3 |
| 6 | Lifestyle modification prompted by trained NPHW | 7 | 4.9 |
| 7 | SMS, Mails, or other electronic alerts for medication adherence | 7 | 4.9 |
| 8 | Utilizing multimedia channels, posters in clinics/hospitals | 7 | 4.9 |
| 9 | Patient education videos | 5 | 3.5 |
| 10 | Telephone follow-ups with patients | 5 | 3.5 |
| 11 | EHR-DSS focused on secondary prevention of CVD | 4 | 2.8 |
| 12 | Pharmacotherapy for CVD (minimize over prescription/polypharmacy) | 4 | 2.8 |
| 13 | Yoga-based interventions | 4 | 2.8 |
| 14 | Peer support groups | 3 | 2.1 |
| 15 | Strengthening primary care for CVD management | 3 | 2.1 |
| 16 | Audit and feedback systems for providers and hospitals | 2 | 1.4 |
| 17 | Goal setting and monthly review of clinical team performance | 2 | 1.4 |
| 18 | Heart health policy involving doctors | 2 | 1.4 |
| 19 | Tobacco taxation | 2 | 1.4 |
| 20 | Admission and discharge checklists to manage acute CVD patients | 1 | 0.7 |
| 21 | CVD risk and disease symptoms awareness camps | 1 | 0.7 |
| 22 | CVD risk factor screening programs | 1 | 0.7 |
| 23 | Establishing lifestyle counselling centers in the community | 1 | 0.7 |
| 24 | Patient centered care (involving patients and caregivers in decision making) | 1 | 0.7 |
| 25 | Patient group meetings facilitated by community health workers | 1 | 0.7 |
| 26 | Polypill for secondary prevention of CVD | 1 | 0.7 |
| 27 | Community engagement programs to prevent sudden cardiac death | 1 | 0.7 |
| 28 | Provision of medicines, healthy fruits, and vegetables at subsidized price | 1 | 0.7 |
| 29 | Provision of telehealth in remote areas for secondary prevention of CVD using hub and spoke model | 1 | 0.7 |
NPHW Non-physician health worker, CVD Cardiovascular disease; EHR-DSS Electronic health records decision support software
Expert Panel Results
A total of 32 panelists participated in the face-to-face virtual consensus meeting. Of these panelists, 14 were specialist clinicians, 6 primary care providers, 5 non-physician health workers, 4 health services researchers or program implementers, 3 health administrators or policy makers. During this meeting, the top 3–4 ranked strategies from each of the strategy groups were discussed within the overall group and condensed into a final shortlist of a multicomponent strategy with 100% agreement achieved for various components. The multicomponent strategy comprised of: (1) clinical decision supported electronic health records to standardize CVD care across diverse care settings, (2) cardiovascular care coordinator facilitated care for patients with CVD, (3) patient education materials containing reinforcement tool (patient diary) to support lifestyle modification and visual assessment tool for medication adherence (VITA), (4) text-message based reminders for the next clinic appointment or laboratory testing and for self-care, and (5) audit and feedback reports for providers. A prospective randomized trial will evaluate how contextual clinic- and patient-level factors influence the real-world implementation feasibility and clinical effectiveness of such a multicomponent strategy across a mix of public and private healthcare facilities in India.
Discussion
We conducted this modified Delphi consensus study involving expert clinicians, researchers, implementers, and policy makers, to collaboratively design the intervention strategy for CVD care in Indian context. This unique two-round Delphi process, followed by an expert consensus meeting improves the clinical and socio-political relevance of the intervention strategy by integrating diverse stakeholder’s perspectives. Given the paucity of implementation research in LMICs, research to establish priority and feasible interventions for CVD care using expert consensus process may lead to better adoption, acceptability, and maintenance. This Delphi study showed that multicomponent, provider training, and patient support strategies were rated most favorably by experts than group problem solving strategies in terms of priority and feasibility. In general, the mean scores for all strategy groups were internally consistent i.e., all strategies were rated relatively poorly for technical complexity and capital intensity and relatively well for priority, which reinforces the urgent need for new, simpler strategies that require fewer resources and have lower technical complexity.
ICT for health strategies were rated the least favorably. Among ICT for health measures, strategies such as telemedicine and mobile apps were rated poorly on technical complexity and capital intensity, while strategies involving text messages, phone-support, and electronic health records were rated slightly better. Lower priority and feasibility ratings of ICT for health measures may indicate perceived barriers among providers due to cost and technical expertise required for the set-up, implementation, and sustainment. Training strategies involving yoga and aerobic exercise were rated poorly on technical complexity. This may be partially explained by the low availability of trained/skilled workforce to deliver these strategies and lower awareness or knowledge about training programs and their direct benefits for secondary prevention of CVD. Therefore, more studies on the feasibility and effectiveness of training strategies are needed in low resource settings. The group problem solving strategy of goal setting and the adaptation of the Stanford Chronic Disease Self-Management Program (Iyngkaran et al., 2016; Liddy et al., 2016) was rated well for priority (1.80), but poorly on technical complexity (3.10) and capital intensity (3.10). This discordance likely indicates a higher perceived value for such strategy, but uncertainty around its implementation. Low priming of experts regarding the adaptations of internationally successful chronic disease management programs could have influenced these results.
Prior research from LMICs have shown positive impact of patient support strategies involving non-physician health workers on improving adherence to medications and hypertension treatment and control rates (Anand et al., 2019; Lee et al., 2016). In this study, multicomponent strategies targeting multiple levels scored well on priority and relative advantage from the provider’s perspective, which indicates that combining multiple modes of care delivery strategies is perceived more valuable than a single mode. Remarkably, these strategies also all scored relatively well for capital intensity and feasibility criteria (2.64). However, Rowe et al.,’s 2019 review indicates that even a single strategy could be as effective as multicomponent strategies (Rowe et al., 2018), which demands further exploration of individual components more granularly, as well as from the perspectives of those who receive care such as patients and caregivers.
The cardiovascular polypill for CVD prevention was one of the “new” strategies identified in Round 2. Despite the demonstrated benefits of a polypill-based strategy (Roshandel et al., 2019; Yusuf et al., 2021), it has been found to be less popular among clinicians due to challenges in dose adjustment (Roy et al., 2017; Webster et al., 2020). We also observed some overlap between the strategies recommended in Round 2 with those already listed in Round 1, implying that the respondent’s answers may have been influenced by the prior exposure to the extensive list of strategies provided in Round 1.
Strengths and Limitations of the Study
This study has several strengths. First, to the best of our knowledge, this study may be the first of its kind from India that attempts to identify and prioritize strategies to prevent and control CVD. Second, the modified Delphi study was part of a multistep formative research that included a scoping review and qualitative interviews with diverse stakeholders based on an established implementation research framework, followed by an expert consensus meeting. Third, a wide range of evidence-based interventions and implementation strategies were considered, ranging from patient counselling, lifestyle changes, and training of providers to the use of mobile apps for improving implementation of evidence-based interventions to improve outcomes in patients with CVD. Fourth, the Delphi survey involved expert cardiovascular clinicians and researchers from diverse geographic locations in India with a mean practice experience of 20 years, which represents a critical group delivering CVD care in India. Fifth, use of the modified Delphi technique offered several advantages, preserving the anonymity of experts and allowing unrestricted expression of opinions, and thus helping to reduce the influence of dominant personalities and the effect of expert status on results. Lastly, Round 2 allowed coverage of full range of quality improvement strategies, rather than restricting to the initial list of 25 strategies proposed initially. However, the long list (over 140 responses, categorized into 29 themes) of strategies that emerged offers insight into the complexity of real-world implementation as an endeavor in CVD quality improvement research.
The study has limitations. First, by design, our findings are influenced by expert views of clinicians, researchers, administrators, program implementers, and policy makers who were predominately male, located in urban private health centers, which might limit the generalizability of the results to other care providers in rural settings. The low heterogeneity among respondents may be due at least in part to purposive sampling, and future research can consider a more diverse group of providers, as well as end users through meaningful involvement of patients, and caregivers. Second, the pooled nature of strategies might have made it difficult to assess which specific components were being evaluated. However, the presentation of these strategies was based on previous research that grouped strategies for improving quality of care (Rowe et al., 2018; Singh et al., 2021), which was necessary to ensure consistency throughout this modified Delphi study. Third, although several policy-level strategies were suggested in Round 2 of the survey, these were outside the immediate scope of this study, but remains an area for future research. Lastly, the generalizability and adaptability of each strategy selected in this study must be reviewed and examined before application to account for differences in practice settings and local health system contexts.
Implications of Study Findings
To reduce the growing public health impact of CVD, focused efforts are urgently required to address the knowledge gaps in priority and feasible interventions that will drive innovation in implementation science. As part of this goal, we leveraged the knowledge of experts to identify prioritized set of evidence-based interventions, which will be further evaluated for its real-world effectiveness and feasibility in a prospective randomized trial in India. The effectiveness of several interventions to control CVD including lifestyle modification and treatment such as aspirin, statin and blood pressure lowering drugs is undisputed. However, some variation in effectiveness across countries is likely, because of supply-side differences in reach, available trained health workforce and intrinsic health system factors (e.g., provider incentives, social health protection schemes) or because of demand-side differences in patient volume, adherence, and maintenance. This study addresses important gaps regarding supply-side aspects by collecting stakeholders’ views through a modified Delphi consensus process to prioritize a set of proven strategies for CVD care in LMIC context. Indeed, implementation studies of multicomponent strategies hold much promise to optimize limited healthcare resource available and to lower supply-side costs, but data are extremely scarce, particularly from LMICs. Further, a better understanding of existing infrastructure, resources, and health workforce competencies, can help shape how multicomponent CVD care models will be delivered, by whom, and where. Also, each country’s health care financing varies considerably, and policy makers would want to know what up-front, fixed, and variable resource investments (economics costs) are required with the implementation of quality improvement strategies; what return on investment is possible and over what time horizon. These are all important considerations and future research should address these knowledge gaps to inform integration of prioritized set of CVD care strategies into routine healthcare systems.
Recommendations
In this study, we identified several strategies for which the data about clinical effectiveness, and feasibility were most convergent. Further, interventions that were, on aggregate, considered effective, important, and feasible were given highest priority by the experts. We recommend pursuing the following supply- and demand-side interventions (in no specific order): trained non-physician health worker led CVD care to achieve multiple risk factor control among people at high risk of CVD, physician training on motivational interviewing technique and patient education on the entire continuum of CVD prevention and care through trained nonclinical staff (community health workers, pharmacist, nurse). Lifestyle modification to manage CVD are also highly effective but implementing them is challenging, which may reflect our limited understanding of the processes, personnel, financing, and infrastructure needed to deliver and sustain lifestyle modification strategies in various LMIC contexts. Yet lifestyle interventions may offer the greatest long-term possibility to prevent and control CVD and warrant further investigation regarding best practices to prompt/sustain lifestyle modification.
Conclusions
Given the disproportionately high burden of CVD in LMICs and limited health care resources, prioritizing effective, low-cost, and feasible quality improvement strategies for CVD care is crucial. Experts who participated in this Delphi study ranked a multicomponent strategy (consisting of decision supported electronic health records, non-physician health worker facilitated care, patient education tools, text messages for healthy lifestyle and audit and feedback reports for providers) as the best considering priority, relative advantage, and feasibility for CVD care in India. This multicomponent strategy will be evaluated for the real-world feasibility and effectiveness in a prospective randomized trial in India.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the experts who participated in the qualitative interviews, Delphi survey, and expert consultation meeting.
Author Contributions
KS, MDH, NT, and DP: conceived the study. KS, MDH, and NSV: developed the methods and the study tools. KS, AJ, and RR: had primary responsibility for the analysis and initial draft of the manuscript. All authors contributed substantially to the analysis, interpretation of the results, and completion of the manuscript. All authors approved the final manuscript.
Funding
This study is supported by the Fogarty International Centre, National Institutes of Health (NIH), United States (Grant award: 1K43TW011164). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability
Corresponding author has access to all study data. Data will be made available to the external researchers upon request.
Declarations
Conflict of interest
In the last 3 years, MDH has received support from the World Heart Federation through unrestricted education grants from Boehringer Ingelheim, Novartis, and Bupa and from the American Heart Association, Verily, and AstraZeneca for work unrelated to this research. MDH has also received salary support from the American Medical Association for his role as an associate editor for JAMA Cardiology. MDH has planned patents for combination therapy for the treatment of heart failure. The George Institute for Global Health has a patent, license, and has received investment funding with the intent to commercialize fixed-dose combination therapy through its social enterprise business, George Medicines. All other co-authors declare no conflict of interests.
Ethical Approval and Consent to Participate
This study is approved by the Institutional Ethics Committee—Public Health Foundation of India. Reference no. TRC-IEC-382/18. Participants provided consent prior to participating in the Delphi survey.
Consent for Publication
All authors reviewed and approved the final manuscript for submission to the Global Heart.
References
- Anand TN, Joseph LM, Geetha AV, Prabhakaran D, Jeemon P. Task sharing with non-physician health-care workers for management of blood pressure in low-income and middle-income countries: a systematic review and meta-analysis. The Lancet Global Health. 2019;7(6):e761–e771. doi: 10.1016/S2214-109X(19)30077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3(2):77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- Cases Amenos A, Pedro-Botet Montoya J, Pascual Fuster V, Barrios Alonso V, Pinto Sala X, Ascaso Gimilio JF, Millan Nunez-Cortes J, Serrano Cumplido A. Delphi consensus on the diagnosis and management of dyslipidaemia in chronic kidney disease patients: A post hoc analysis of the DIANA study. Nefrologia. 2016;36(6):679–686. doi: 10.1016/j.nefro.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Collaborators. Bradley SM, Adusumalli S, Amin AP, Borden WB, Das SR, Downey WE, Ebinger JE, Gelbman J, Gluckman TJ, Goyal A, Gupta D, Khot UN, Levy AE, Mutharasan RK, Rush P, Strauss CE, Shreenivas S, Ho PM. The cardiovascular quality improvement and care innovation consortium: Inception of a multicenter collaborative to improve cardiovascular care. Circulation: Cardiovascular Quality and Outcomes. 2021;14(1):6753. doi: 10.1161/CIRCOUTCOMES.120.006753. [DOI] [PubMed] [Google Scholar]
- Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. Journal of Clinical Epidemiology. 2014;67(4):401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Evangelidis N, Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, Crowe S, Harris T, Van Biesen W, Winkelmayer WC, Sautenet B, O'Donoghue D, Tam-Tham H, Youssouf S, Mandayam S, Ju A, Hawley C, Pollock C, Harris DC, Johnson DW, Rifkin DE, Tentori F, Agar J, Polkinghorne KR, Gallagher M, Kerr PG, McDonald SP, Howard K, Howell M, Craig JC, Standardized Outcomes in Nephrology–Hemodialysis (SONG-HD) Initiative Developing a set of core outcomes for trials in hemodialysis: An International Delphi Survey. American Journal of Kidney Diseases. 2017;70(4):464–475. doi: 10.1053/j.ajkd.2016.11.029. [DOI] [PubMed] [Google Scholar]
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. American Journal of Public Health. 1984;74(9):979–983. doi: 10.2105/ajph.74.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldsetzer P, Manne-Goehler J, Theilmann M, Davies JI, Awasthi A, Danaei G, Gaziano TA, Vollmer S, Jaacks LM, Barnighausen T, Atun R. Geographic and sociodemographic variation of cardiovascular disease risk in India: A cross-sectional study of 797,540 adults. PLoS Medicine. 2018;15(6):e1002581. doi: 10.1371/journal.pmed.1002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Khedar RS, Gaur K, Xavier D. Low quality cardiovascular care is important coronary risk factor in India. Indian Heart Journal. 2018;70(Suppl 3):S419–S430. doi: 10.1016/j.ihj.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett A, Strickland K. Using the framework approach to analyse qualitative data: A worked example. Nurse Researcher. 2019;26(2):8–13. doi: 10.7748/nr.2018.e1580. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman MD, Mohanan PP, Devarajan R, Baldridge AS, Kondal D, Zhao L, Ali M, Krishnan MN, Natesan S, Gopinath R, Viswanathan S, Stigi J, Joseph J, Chozhakkat S, Lloyd-Jones DM, Prabhakaran D, Acute Coronary Syndrome Quality Improvement in Kerala (ACS QUIK) Investigators Effect of a quality improvement intervention on clinical outcomes in patients in india with acute myocardial infarction: The ACS QUIK randomized clinical trial. JAMA. 2018;319(6):567–578. doi: 10.1001/jama.2017.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- India State-Level Disease Burden Initiative CVDC The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990–2016. Lancet Global Health. 2018;6(12):e1339–e1351. doi: 10.1016/S2214-109X(18)30407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyngkaran P, Toukhsati SR, Harris M, Connors C, Kangaharan N, Ilton M, Nagel T, Moser DK, Battersby M. Self managing heart failure in remote Australia—translating concepts into clinical practice. Current Cardiology Reviews. 2016;12(4):270–284. doi: 10.2174/1573403x12666160703183001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke C, Gupta R, Xavier D, Prabhakaran D, Mathur P, Kalkonde YV, Kolpak P, Suraweera W, Jha P, Million Death Study Collaborators Divergent trends in ischaemic heart disease and stroke mortality in India from 2000 to 2015: A nationally representative mortality study. Lancet Global Health. 2018;6(8):e914–e923. doi: 10.1016/S2214-109X(18)30242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetan A, Zullo M, Rani A, Gupta R, Purushothaman R, Bajaj NS, Agarwal S, Madan Mohan SK, Josephson R. Effect of a community health worker-based approach to integrated cardiovascular risk factor control in India: A cluster randomized controlled trial. Global Heart. 2019;14(4):355–365. doi: 10.1016/j.gheart.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Straka Z, Piegas LS, Avezum A, Akar AR, Lanas Zanetti F, Jain AR, Noiseux N, Padmanabhan C, Bahamondes JC, Novick RJ, Tao L, Olavegogeascoechea PA, Airan B, Sulling TA, Whitlock RP, Ou Y, Gao P, Pettit S, Yusuf S. Five-year outcomes after off-pump or on-pump coronary-artery bypass grafting. New England Journal of Medicine. 2016;375(24):2359–2368. doi: 10.1056/NEJMoa1601564. [DOI] [PubMed] [Google Scholar]
- Lee ES, Vedanthan R, Jeemon P, Kamano JH, Kudesia P, Rajan V, Engelgau M, Moran AE. Quality improvement for cardiovascular disease care in low- and middle-income countries: A systematic review. PLoS One. 2016;11(6):e0157036. doi: 10.1371/journal.pone.0157036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddy C, Johnston S, Nash K, Irving H, Davidson R. Implementation and evolution of a regional chronic disease self-management program. Canadian Journal of Public Health. 2016;107(2):e194–e201. doi: 10.17269/cjph.107.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstone HA, T. M. The Delphi method: Techniques and applications. Addison-Wesley; 1975. [Google Scholar]
- Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. Journal of the American College of Cardiology. 2019;74(20):2529–2532. doi: 10.1016/j.jacc.2019.10.009. [DOI] [PubMed] [Google Scholar]
- Mohanan PP, Huffman MD, Baldridge AS, Devarajan R, Kondal D, Zhao L, Ali M, Joseph J, Eapen K, Krishnan MN, Menon J, Thomas M, Lloyd-Jones DM, Harikrishnan S, Prabhakaran D, ACS QUIK Investigators Microeconomic costs, insurance, and catastrophic health spending among patients with acute myocardial infarction in india: Substudy of a randomized clinical trial. JAMA Network Open. 2019;2(5):e193831. doi: 10.1001/jamanetworkopen.2019.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal AR, Cash RE, Crowe RP, Coute R, Way D, Aufderheide T, Merchant RM. Delphi analysis of science gaps in the 2015 American Heart Association Cardiac Arrest Guidelines. Journal of the American Heart Association. 2018 doi: 10.1161/JAHA.118.008571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perel P, Avezum A, Huffman M, Pais P, Rodgers A, Vedanthan R, Wood D, Yusuf S. Reducing premature cardiovascular morbidity and mortality in people with atherosclerotic vascular disease: The world heart federation roadmap for secondary prevention of cardiovascular disease. Global Heart. 2015;10(2):99–110. doi: 10.1016/j.gheart.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, Proctor EK, Kirchner JE. A refined compilation of implementation strategies: Results from the expert recommendations for implementing change (ERIC) project. Implementation Science. 2015;10:21. doi: 10.1186/s13012-015-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran D, Chandrasekaran AM, Singh K, Mohan B, Chattopadhyay K, Chadha DS, Negi PC, Bhat P, Sadananda KS, Ajay VS, Singh K, Praveen PA, Devarajan R, Kondal D, Soni D, Mallinson P, Manchanda SC, Madan K, Hughes AD, Chathurvedi N, Roberts I, Ebrahim S, Reddy KS, Tandon N, Pocock S, Roy A, Kinra S, Yoga-CaRe Trial I. Yoga-based cardiac rehabilitation after acute myocardial infarction: A randomized trial. Journal of the American College of Cardiology. 2020;75(13):1551–1561. doi: 10.1016/j.jacc.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran D, Jha D, Prieto-Merino D, Roy A, Singh K, Ajay VS, Jindal D, Gupta P, Kondal D, Goenka S, Jacob PD, Singh R, Prakash Kumar BG, Perel P, Tandon N, Patel V. Effectiveness of an mHealth-based electronic decision support system for integrated management of chronic conditions in primary care: The mWellcare cluster-randomized controlled trial. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.118.038192. [DOI] [PubMed] [Google Scholar]
- Rehman H, Kalra A, Kochar A, Uberoi AS, Bhatt DL, Samad Z, Virani SS. Secondary prevention of cardiovascular diseases in India: Findings from registries and large cohorts. Indian Heart J. 2020;72(5):337–344. doi: 10.1016/j.ihj.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshandel G, Khoshnia M, Poustchi H, Hemming K, Kamangar F, Gharavi A, Ostovaneh MR, Nateghi A, Majed M, Navabakhsh B, Merat S, Pourshams A, Nalini M, Malekzadeh F, Sadeghi M, Mohammadifard N, Sarrafzadegan N, Naemi-Tabiei M, Fazel A, Brennan P, Etemadi A, Boffetta P, Thomas N, Marshall T, Cheng KK, Malekzadeh R. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): A pragmatic, cluster-randomised trial. Lancet. 2019;394(10199):672–683. doi: 10.1016/S0140-6736(19)31791-X. [DOI] [PubMed] [Google Scholar]
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernandez-Sola J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundstrom J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V, GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 Study. Journal of the American College of Cardiology. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AK, Rowe SY, Peters DH, Holloway KA, Chalker J, Ross-Degnan D. Effectiveness of strategies to improve health-care provider practices in low-income and middle-income countries: A systematic review. Lancet Global Health. 2018;6(11):e1163–e1175. doi: 10.1016/S2214-109X(18)30398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Naik N, Srinath Reddy K. Strengths and limitations of using the polypill in cardiovascular prevention. Current Cardiology Reports. 2017;19(5):45. doi: 10.1007/s11886-017-0853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Jose AP, Pandey N, Vats S, Bagre V, Kumar H, Nair SC, Kumar P, Bhalla S, Padmanabhan S, Poulter N, Prabhakaran D, Roy A. A collaborative model for capacity building of primary care physicians in the management of Hypertension in India. Journal of Human Hypertension. 2019;33(8):562–565. doi: 10.1038/s41371-019-0213-z. [DOI] [PubMed] [Google Scholar]
- Singh K, Bawa VS, Venkateshmurthy NS, Gandral M, Sharma S, Lodhi S, Wafford QE, Patel SA, Tandon N, Narayan KMV, Prabhakaran D, Huffman MD. Assessment of studies of quality improvement strategies to enhance outcomes in patients with cardiovascular disease. JAMA Network Open. 2021;4(6):e2113375. doi: 10.1001/jamanetworkopen.2021.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Devarajan R, Mohanan PP, Baldridge AS, Kondal D, Victorson DE, Karmali KN, Zhao L, Lloyd-Jones DM, Prabhakaran D, Goenka S, Huffman MD, Investigators AQ. Implementation and acceptability of a heart attack quality improvement intervention in India: A mixed methods analysis of the ACS QUIK trial. Implementation Science. 2019;14(1):12. doi: 10.1186/s13012-019-0857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom S, Field J, Poulter N, Patel A, Prabhakaran D, Stanton A, Grobbee DE, Bots ML, Reddy KS, Cidambi R, Rodgers A. Use of a Multidrug Pill In Reducing cardiovascular Events (UMPIRE): rationale and design of a randomised controlled trial of a cardiovascular preventive polypill-based strategy in India and Europe. European Journal of Preventive Cardiology. 2014;21(2):252–261. doi: 10.1177/2047487312463278. [DOI] [PubMed] [Google Scholar]
- Verhestraeten C, Weijers G, Debleu D, Ciarka A, Goethals M, Droogmans S, Maris M. Diagnosis, treatment, and follow-up of heart failure patients by general practitioners: A Delphi consensus statement. PLoS One. 2020;15(12):e024485. doi: 10.1371/journal.pone.0244485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R, Murphy A, Bygrave H, Ansbro E, Grobbee DE, Perel P. Implementing fixed dose combination medications for the prevention and control of cardiovascular diseases. Global Heart. 2020;15(1):57. doi: 10.5334/gh.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock T, Adeleke Y, Goeschel C, Pronovost P, Dixon-Woods M. A modified Delphi study to identify the features of high quality measurement plans for healthcare improvement projects. BMC Medical Research Methodology. 2020;20(1):8. doi: 10.1186/s12874-019-0886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier D, Gupta R, Kamath D, Sigamani A, Devereaux PJ, George N, Joshi R, Pogue J, Pais P, Yusuf S. Community health worker-based intervention for adherence to drugs and lifestyle change after acute coronary syndrome: A multicentre, open, randomised controlled trialT. The Lancet Diabetes & Endocrinology. 2016;4(3):244–253. doi: 10.1016/S2213-8587(15)00480-5. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Joseph P, Dans A, Gao P, Teo K, Xavier D, Lopez-Jaramillo P, Yusoff K, Santoso A, Gamra H, Talukder S, Christou C, Girish P, Yeates K, Xavier F, Dagenais G, Rocha C, McCready T, Tyrwhitt J, Bosch J, Pais P, International Polycap Study 3 Investigators Polypill with or without aspirin in persons without cardiovascular disease. New England Journal of Medicine. 2021;384(3):216–228. doi: 10.1056/NEJMoa2028220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Corresponding author has access to all study data. Data will be made available to the external researchers upon request.