Abstract
Vitamins are essential micronutrients, but the mechanisms of vitamin chemoreception in animals are poorly understood. Here, we provide evidence that vitamin C doubles starvation resistance and induces egg laying in Drosophila melanogaster. Our behavioral analyses of genetically engineered and anatomically ablated flies show that fruit flies sense vitamin C via sweet‐sensing gustatory receptor neurons (GRNs) in the labellum. Using a behavioral screen and in vivo electrophysiological analyses of ionotropic receptors (IRs) and sweet‐sensing gustatory receptors (GRs), we find that two broadly tuned IRs (i.e., IR25a and IR76b) and five GRs (i.e., GR5a, GR61a, GR64b, GR64c, and GR64e) are essential for vitamin C detection. Thus, vitamin C is directly detected by the fly labellum and requires at least two distinct receptor types. Next, we expand our electrophysiological study to test attractive tastants such as sugars, carboxylic acids, and glycerol. Our analysis elucidates the molecular basis of chemoreception in sweet‐sensing GRNs.
Keywords: GR5a, GR61a, GR64 cluster, taste, vitamin C
Subject Categories: Membranes & Trafficking, Neuroscience
Drosophila melanogaster tastes vitamin C using gustatory receptors (GRs) and ionotropic receptors (IRs) in sweet‐sensing gustatory receptor neurons (GRNs).
Introduction
Vitamin C (ascorbic acid), the dietary antioxidant, is abundant in citrus fruits and vegetables. It plays a role in supporting daily activities and good health. It has a direct effect on energy levels, brain function, and cell metabolism (Poljsak & Ionescu, 2009). In addition, it has wound healing effect and the shortage of it causes the deadly disease, scurvy. Vitamin C cannot be stored in the body, so it needs to be supplied with diet. It is assumed that human adults require at least 40 mg of vitamin C every day. Several studies have suggested that it can influence insecticide resistance and oxidative stress in Drosophila melanogaster (Huang et al, 2006; González et al, 2018; Man Anh et al, 2019). Although the advantageous properties of the postingestive mechanism have been highlighted before, how the animal detects vitamins directly has been enigmatic.
Sense of taste plays a key factor in determining nourishing foods and avoiding dangerous toxins. Animals' ability to taste is crucial for recognizing beneficial substances in the environment (Shrestha & Lee, 2023). The taste of various macronutrients and micronutrients is required for the regulation of the feeding behavior of the animal (Freeman & Dahanukar, 2015; Delompré et al, 2019). Drosophila melanogaster offers an excellent choice for the researcher to perform conceivable research for the behavioral and neurophysiological analysis of taste (Moon et al, 2006; Aryal et al, 2022b). The distribution of gustatory organs throughout the body including the labellum, forelegs, pharynx, wings, and female genitalia makes the fruit fly interesting model animals (Vosshall & Stocker, 2007; Rimal & Lee, 2018; Chen & Dahanukar, 2020). The labellum has a special role in contact chemosensation as it contains special types of hair‐like structure called taste sensilla. Each half of the labellum has 31 hair sensilla, which are further classified into the L (long), I (intermediate), and S (short) morphological subtypes according to the length of hair. The taste sensilla are made up of 2–4 chemosensory, 1 mechanosensory, and 3 supporting cells (Liman et al, 2014).
Also, most animals rely on chemosensory mechanisms to evaluate the beneficial and harmful value of their nutrition. Flies can taste macronutrients, such as carbohydrates, lipids, proteins, and micronutrients, such as minerals, in their peripheral sensory systems (Dahanukar et al, 2007; Ahn et al, 2017; Kim et al, 2018; Wu et al, 2021; Aryal et al, 2022a). However, it is unclear how vitamins, which are generally known as micronutrients, are sensed in the chemosensory organs.
Results and Discussion
Beneficial effect of vitamin C
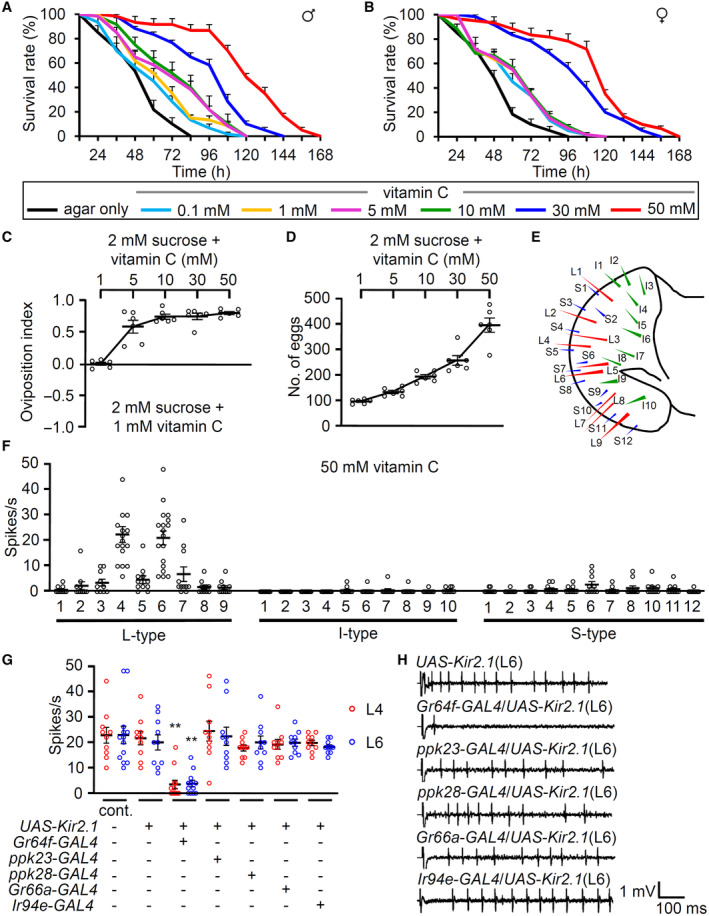
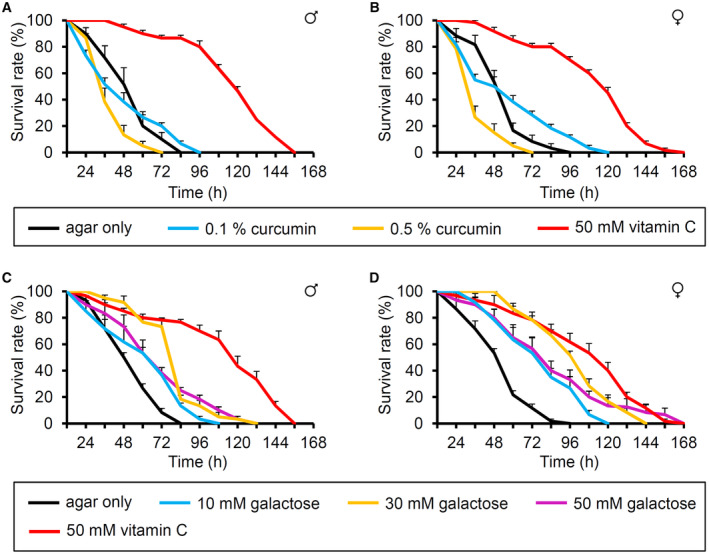
Vitamin C is beneficial and gives pleasant taste to humans (Schiffman & Dackis, 1975; NTP, 1992). We aimed to decipher whether vitamin C has beneficial and attractive effects on fruit flies. On performing a starvation resistance assay, starved wild‐type male and female flies lived significantly longer after feeding on micronutrient vitamin C (0.1–50 mM). This concentration protects cells from free radical damage (Fig 1A and B). We found that 1% agar (without any nutrients) caused lethality in 50% of the flies after 46 h (LT50) in both sexes. However, the LT50 increased to 53.6 ± 5.14 and 56.6 ± 3.78 h in males and females, respectively, in the presence of 0.1 mM vitamin C. This effect increased in a dose‐dependent manner. For example, the LT50 of males in the presence of 30 and 50 mM vitamin C were 99.3 ± 2.55 and 120.9 ± 2.31 h, respectively (Appendix Table S1). This indicates that feeding 50 mM vitamin C can extend the starvation resistance by 2.6 and 2.5 under starved conditions in males and females, respectively. Moreover, we wondered whether this starvation resistance can be regulated by another potent antioxidant, curcumin (Suckow & Suckow, 2006). Previously vitamin C and curcumin have extended the lifespan in a standard food condition (Suckow & Suckow, 2006; Suh et al, 2017). However, the LT50 decreased to 39.3 ± 3.88 and 46.0 ± 5.51 h in males and females, respectively, in the presence of 0.1% curcumin (Fig EV1A and B, and Appendix Table S2). Furthermore, 0.5% curcumin was very toxic without sugars (Fig EV1A and B, and Appendix Table S2). Therefore, the increased starvation resistance may be caused not by a simple antioxidant effect but by others. Vitamin C is a weak sugar acid, related to galactose (Vinson et al, 1992). When 50 mM galactose was fed, the LT50 increased, though not as much as vitamin C (Fig EV1C and D, and Appendix Table S3). This indicates that vitamin C enhances the starvation resistance properties by acting as a possible nutrient source rather than an antioxidant agent to the flies.
Figure 1. Beneficial effect of vitamin C and neuronal response of sweet‐sensing GRNs to vitamin C.
-
A, BTime‐dependent effects of ingesting 0, 0.1, 1, 5, 10, 30, and 50 mM vitamin C without any further nutrition on the survival of wild‐type control flies (w 1118 ) (A) males (B) females, n = 6. Kaplan–Meier survival analyses were performed to compare the survival rates of agar only and fed conditions at different concentrations of vitamin C (Appendix Table S1).
-
CDose‐dependent oviposition choice at indicated concentrations of vitamin C versus 1 mM vitamin C. Sucrose (2 mM) was included on both sides, n = 6.
-
DNumber of eggs laid at the indicated concentrations of vitamin C with 2 mM sucrose, n = 6.
-
ESchematic illustration of taste sensilla on the fly labellum.
-
FElectrophysiology mapping of 31 sensilla with control to 50 mM vitamin C, n = 10–18.
-
GTip recordings from the L4 and L6 sensilla of specific GRN‐ablated flies with 50 mM vitamin C. +/− indicates the presence or absence of the transgene, respectively, n = 10–13.
-
HRepresentative sample traces of (G).
Data information: All error bars represent SEMs. Single‐factor ANOVA coupled with the Scheffe's post hoc test was conducted to compare multiple datasets. Asterisks indicate statistical significance compared with the control. **P < 0.01.
Source data are available online for this figure.
Figure EV1. Effect of curcumin and galactose food on starved flies.
-
A, BTime‐dependent starvation resistance properties of flies to 0.1% (~3 mM), 0.5% curcumin, and 50 mM vitamin C food without the supplement of other nutrition in wild‐type control flies (w 1118 ) (A) males (B) females, n = 6.
-
C, DTime‐dependent starvation resistance properties of flies to 10 mM galactose, 30 mM galactose, 50 mM galactose, and 50 mM vitamin C food without the supplement of other nutrition in wild‐type control flies (w 1118 ) (C) males (D) females, n = 6.
Data information: Error bars represent SEMs. Kaplan–Meier survival analyses were performed to compare the survival rates of agar only and fed conditions with the indicated concentrations of curcumin and galactose (Appendix Tables S2 and S3).
Next, we sought to understand whether flies are attracted to vitamin C. To test the palatability for vitamin C, we allowed females to lay eggs on 1 mM vitamin C + 2 mM sucrose versus higher concentrations of vitamin C + 2 mM sucrose for 18 h using binary oviposition choice assays (Fig 1C). Flies laid more eggs on the side plated with ≥ 5 mM of vitamin C. This indicates that flies can distinguish a higher concentration of vitamin C and are attracted to concentrations higher than 5 mM vitamin C. Females often lay eggs on the high nutritional food source. Therefore, we tested whether females lay more eggs in a dose‐dependent manner (Fig 1D). Food containing 50 mM vitamin C induced a 4‐fold higher number of eggs when compared with 1 mM vitamin C. These results indicate that vitamin C is beneficial for animal fecundity.
Vitamin C is considered a micronutrient to humans, but we found that flies can use it as a beneficial energy source like galactose. Therefore, flies increase survival rates and conduct egg‐laying in a dose‐dependent manner. Furthermore, flies prefer a higher concentration of vitamin C when finding better places for their progenies. The oviposition experiments can be mediated by short‐term effects, such as attractive taste, and long‐term effects, such as internal physiological changes.
Neuronal responses to vitamin C
Vitamin C is water‐soluble and has a pleasant taste. To analyze its mode of action in the fly gustatory system, we tested all the sensilla in the labellum in the presence of vitamin C. Vitamin C induced strong neuronal activity from several L‐type sensilla (L4 and L6 induced highest action potentials, with L5 and L7 inducing relatively milder potentials) but not from S‐ and I‐type sensilla (Fig 1E and F). Moreover, there was a dose‐dependent increase in action potentials on L6 sensilla (Fig EV2A). D. melanogaster has a strong preference for a vitamin‐containing diet including folic acid (vitamin B9) and riboflavin (vitamin B2) (Wu et al, 2021). Thus, we compared the attractive properties of vitamin B and C via electrophysiology. However, neuronal responses were not significantly detected following stimulations with folic acid and riboflavin on L4, L6, and S6 sensilla (Fig EV2B).
Figure EV2. Behavioral and electrophysiological analyses of Vitamin C.
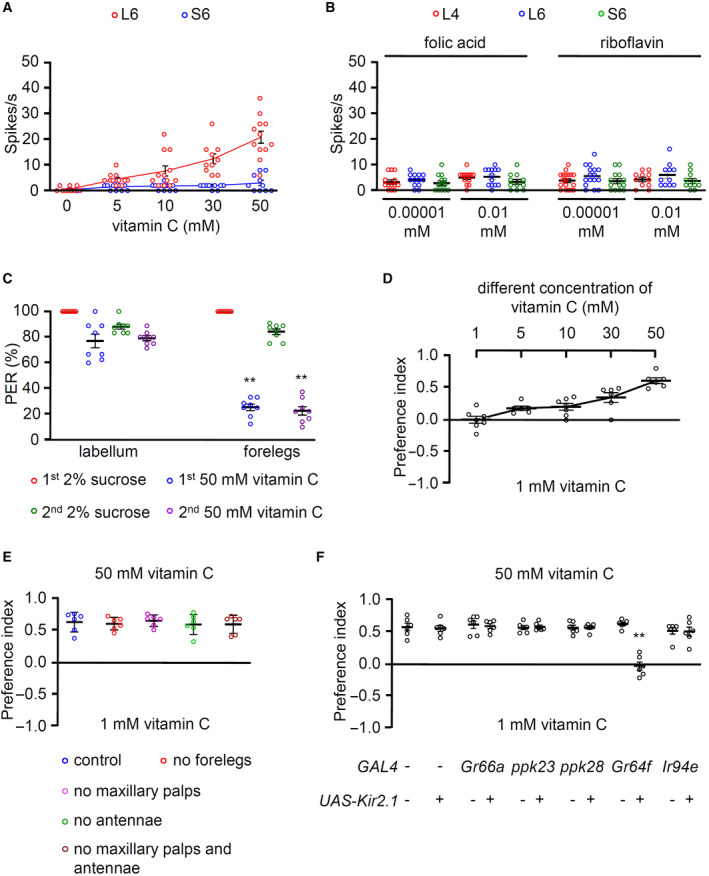
- Tip recording of w 1118 (control) flies at the indicated concentrations of vitamin C from L6 and S6 sensilla, n = 10–12.
- Neuronal firings from L4, L6, and S6 sensilla to folic acid and riboflavin at the indicated concentrations, n = 10–18.
- PER assays were given stimuli to the indicated organs: forelegs or labellum, n = 6–8.
- Dose‐dependent binary food choice assays at different concentrations of vitamin C (1, 5, 10, 30, and 50 mM) versus 1 mM, n = 6–7.
- Binary food choice assays with different organ‐ablated flies in the presence of 50 mM vitamin C versus 1 mM, n = 6.
- Binary food choice assays of specific GRN‐ablated flies and control. +/− indicate the presence or absence of each transgene, respectively, n = 6.
Data information: All error bars represent the SEM. Multiple sets of data were compared using single‐factor ANOVA coupled with the Scheffe's post hoc test. Asterisks indicate statistical significance compared with the control. **P < 0.01.
Next, we tested flies in which neuronal inactivation was selectively induced in specific types of neurons via the expression of UAS‐Kir2.1 under the control of GAL4s (Paradis et al, 2001). Flies have two or four gustatory receptor neurons (GRNs) in each sensillum. Each type of GRN can be categorized based on functional GAL4 expressions (Rimal & Lee, 2018). Gr64f‐GAL4 and Gr66a‐GAL4 are expressed in sweet‐sensing and bitter‐sensing GRNs, respectively (Thorne et al, 2004; Dahanukar et al, 2007). Ir94e‐GAL4 is expressed in one cell per L‐type sensillum without overlapping with other GRNs (Jaeger et al, 2018). Furthermore, ppk23‐GAL4 and ppk28‐GAL4 are expressed in Ca2+‐sensing and water‐sensing GRNs, respectively (Cameron et al, 2010; Lee et al, 2018). The induction of neuronal inhibition under the Gr64f‐GAL4/+ background significantly suppressed the action potentials of L4 and L6 sensilla but not ppk23‐GAL4, ppk28‐GAL4, Gr66a‐GAL4, and Ir94e‐GAL4 (Fig 1G and H). This indicates that vitamin C activates sweet‐sensing GRNs and may induce attractive signals to the brain.
Subsequently, we assessed whether the attraction to vitamin C was mediated via the labellum and legs because flies have multiple sensory organs responsible for sensing nonvolatile chemicals. To verify our findings, we used proboscis extension response (PER) assays and applied the stimulus to the labellum or forelegs. The test solution contained 2% sucrose or 50 mM vitamin C. We found a significant difference in PER between the test solutions when the stimulus was applied to the forelegs but not the labellum (Fig EV2C). Further, we confirmed that flies prefer a higher concentrations of vitamin C using the binary food choice assay (Fig EV2D). The preference for vitamin C was not affected by surgical removals of the forelegs, maxillary palps, or antennae, as well as of both maxillary palps and antennae (Fig EV2E). However, the preference was affected by the genetic ablation of sweet‐sensing GRNs (Fig EV2F). These results indicate that vitamin C is tasted by the sweet‐sensing GRNs in the labellum but not the legs or olfactory organs.
Molecular sensors of vitamin C
Vitamin C is also known as ascorbic acid. The ability to taste attractive carboxylic acids requires ionotropic receptors (IRs) and sweet‐sensing GRs (Stanley et al, 2021; Shrestha & Lee, 2021a). We have previously reported that flies are highly attracted to glycolic, citric, and lactic acid and avoid tartaric and propionic acid (Rimal et al, 2019). Intriguingly, the activation of sweet‐sensing GRNs by lactic acid has two distinct phases, as verified by calcium imaging in the brain (Stanley et al, 2021). IR25a mediates the onset response, while sweet GRs mediate the removal phases, verified as elevation of calcium responses upon removal of lactic acid, which cannot be quantified with tip recordings. Therefore, we tested a repertoire of putative taste receptors (31 candidate Ir and nine candidate Gr loss‐of‐function mutants) via electrophysiology on L4 and L6 sensilla to elucidate the vitamin C receptors (Fig 2A and B). This included the broadly required Ir25a and Ir76b (Zhang et al, 2013; Chen & Amrein, 2017; Ganguly et al, 2017; Lee et al, 2018; Dhakal et al, 2021; Shrestha & Lee, 2021a; Xiao et al, 2022; Aryal et al, 2022a). Ir7a and Ir62a are required for sensing acetic acid and Ca2+, respectively (Lee et al, 2018; Rimal et al, 2019). Ir56d is essential for sensing hexanoic acid and carbonation buffer (Sánchez‐Alcañiz et al, 2018; Dweck et al, 2022). Ir56b is essential for detecting attractive Na+ (Dweck et al, 2022). We detected reduced responses from IR25a and IR76b alone (Fig 2A). The other 29 Ir mutants elicited normal responses to vitamin C. However, we cannot exclude other IRs that have not been tested. Next, we tested 9 Gr mutants (Fig 2B). From these putative sweet‐sensing GRs, we identified that Gr5a, Gr61a, Gr64b, Gr64c, and Gr64e mutants had significantly reduced spike activities to vitamin C (Fig 2B). Then, we performed recovery experiments using its own GAL4 drivers and sweet‐sensing GRNs‐expressing Gr64f‐GAL4 (Fig 2C). When each wild‐type cDNA was expressed in the mutant background, the Ir25a and Ir76b mutants were fully rescued (Fig 2C). This indicates that IR25a and IR76b act in Gr64f‐positive neurons. We also genetically recovered each Gr mutant by expressing each wild‐type cDNA with its specific Gr5a‐GAL4 and Gr61a‐GAL4 or the broadly expressed sweet‐sensing Gr64f‐GAL4 driver for each Gr64 cluster mutant (Fig 2D). These data support the evidence that two coreceptor IRs, IR25a and IR76b, and the five sweet GRs are essential for detecting vitamin C in sweet‐sensing GRNs. As previously reported (Stanley et al, 2021), it would be interesting to test whether two coreceptor IRs are required for the onset response by vitamin C stimulation and whether the other GRs are essential for the removal phase by calcium imaging. Ir25a, Ir76b, and the other Grs are known to be expressed not only in labellum but also in the internal taste organ, pharynx (Chen & Dahanukar, 2017; Sánchez‐Alcañiz et al, 2018). Furthermore, vitamin B preference is mediated by external and internal taste organs (Wu et al, 2021). It would be interesting to test the vitamin C response in the pharynx as well.
Figure 2. Test of IRs and sweet‐sensing GRs to detect vitamin C.
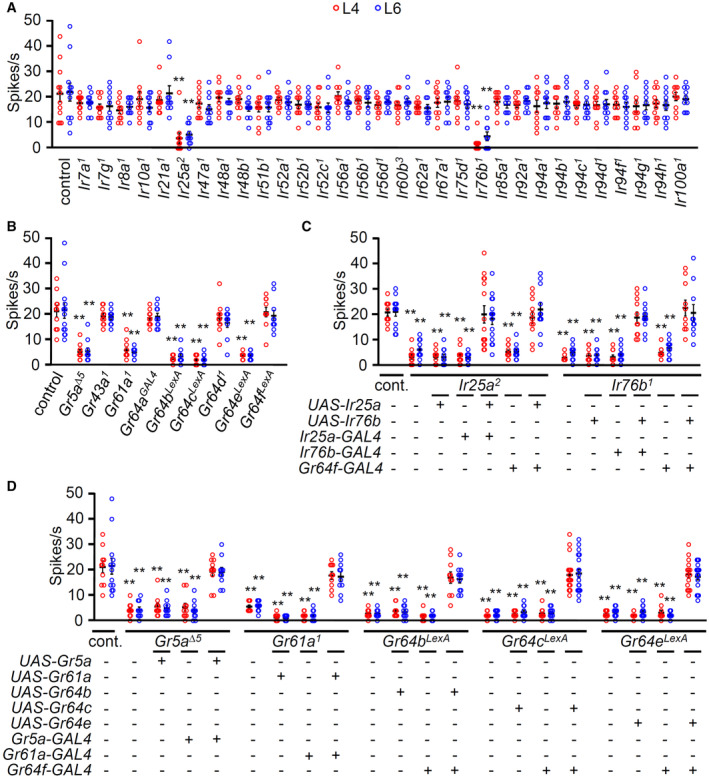
- Tip recording screens performed with 31 Ir mutants and control (w 1118 ) from L4 and L6 sensilla stimulated with 50 mM vitamin C, n = 10–12.
- Tip recording screens performed with sweet Gr mutants from L4 and L6 to 50 mM vitamin C, n = 10–13.
- Genetically recovered flies of Ir25a 2 and Ir76b 1 driven by their own GAL4 or Gr64f‐GAL4 with the wild‐type cDNA transgene. +/− indicate the presence or absence of the transgene, respectively, n = 10–15.
- Genetically recovered flies of Gr5a, Gr61a, Gr64b, Gr64c, and Gr64e mutants driven by their own GAL4 or Gr64f‐GAL4. +/− indicate the presence or absence of the transgene, respectively, n = 10–21.
Data information: All error bars represent the SEM. Multiple sets of data were compared using single‐factor ANOVA coupled with the Scheffe's post hoc test. Asterisks indicate statistical significance compared with the control. **P < 0.01.
Source data are available online for this figure.
GR5a, GR61a, GR64b, GR64c, and GR64e are required in combination with IR25a and IR76b to detect vitamin C
To address how taste receptor activities are translated into behavioral responses, we adopted two different behavioral paradigms. First, we used the well‐established binary food choice assay (Aryal et al, 2022b). Vitamin C physiologically activated sweet‐sensing GRNs (Fig 1G and H); therefore, no sucrose was employed in the assays. This was verified by a preference of 50 mM vitamin C versus 1 mM in wild‐type flies, while Gr64f‐GAL4/UAS‐Kir2.1 flies had no preference (Fig EV2F). We screened the 31 Ir mutants with the same paradigm and found that only Ir25a and Ir76b were required for vitamin C attraction (Fig 3A). Next, we tested the nine sweet Gr mutants. Consistent with the electrophysiology results, Gr5a, Gr61a, Gr64b, Gr64c, and Gr64e mutant flies, but not Gr43a, Gr64a, Gr64d, and Gr64f mutants, were deficient in their attraction to vitamin C (Fig 3B). Rescue experiments revealed the expression of each wild‐type cDNA under the control of its GAL4 or the Gr64f promoter increased the preference index to the level of wild‐type flies (Fig 3C and D). This indicates that IR25a and IR76b function in the sweet‐sensing GRNs with GR5a, GR61a, GR64b, GR64c, and GR64e when being attracted to vitamin C.
Figure 3. Ir25a, Ir76b, Gr5a, Gr61a, Gr64b, Gr64c, and Gr64e are indispensable for the attraction of vitamin C.
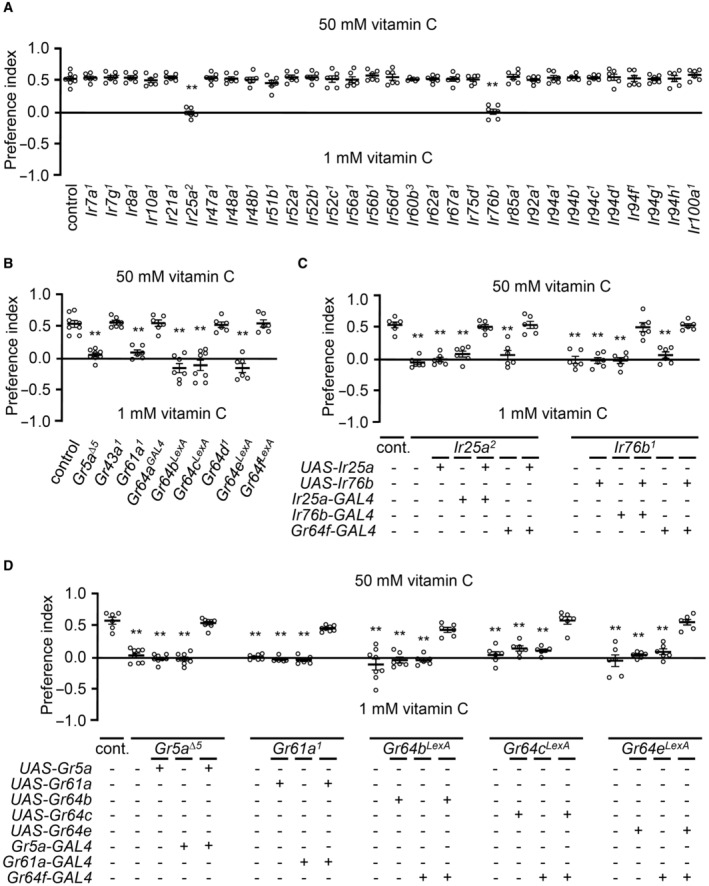
- Binary food choice assay performed with 31 Ir mutants and control (w 1118 ), n = 6.
- Binary food choice assay performed with nine sweet Gr mutants, n = 6–8.
- Rescue of Ir25a 2 and Ir76b 1 mutant deficit in feeding assay. Respective UAS lines were driven by their own GAL4s or Gr64f‐GAL4, n = 6–8.
- Behavioral rescue of Gr5a ∆5 , Gr61a 1 , Gr64b LEXA , Gr64c LEXA , and Gr64e LEXA deficits in the vitamin C attraction via the expression of the respective UAS transgenes under the control of their specific GAL4s or Gr64f‐GAL4, n = 6–8.
Data information: All error bars represent the SEM. Multiple sets of data were compared using single‐factor ANOVA coupled with the Scheffe's post hoc test. Asterisks indicate statistical significance compared with the control. **P < 0.01.
Source data are available online for this figure.
We employed a second behavioral paradigm to further investigate the role of the seven receptors (IR25a, IR76b, GR5a, GR61a, GR64b, GR64c, and GR64e) in the behavioral response to vitamin C. We used the oviposition assay, in which the flies were allowed to freely fly and walk in the arena. It is a complex process to determine how organisms integrate signals and are involved in decision‐like processes and balances conflicting behavioral activities (Joseph et al, 2009). We used this paradigm to replicate the behavior of flies in a natural environment. First, 2 mM sucrose was included in the agarose on both sides to induce more egg‐laying. Second, 1 mM versus 50 mM vitamin C was mixed on each side without any visible dyes. We counted the number of eggs over an 18 h period after the flies were adapted to the arena for 5–6 h. We found that sweet‐GRNs‐inhibited flies (Gr64f‐GAL4/UAS‐Kir2.1), but not others, had defects selecting the egg deposition site (Fig EV3A and B). Furthermore, the seven receptor mutants showed comparable defects to the sweet‐GRNs‐inhibited flies; these defects were completely recovered in the rescue experiments (Fig EV3C–E). Overall, the results of binary food choice feeding assays and oviposition assays were consistent. Flies preferred vitamin C, which is mediated by two broadly expressed IRs and five sweet GRs.
Figure EV3. Oviposition preference assays on vitamin C.
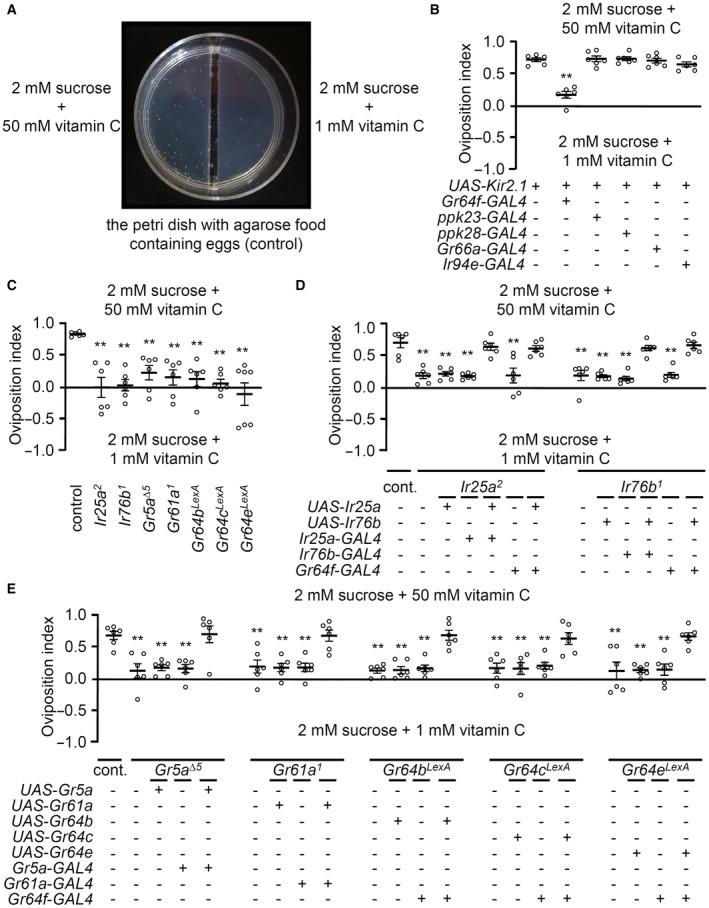
- Photograph of egg‐laying plate to show the differentiating behavior of females in the presence of 50 mM vitamin C. Higher number of eggs were seen on the side of 50 mM vitamin C than 1 mM.
- Oviposition preference assays of specific GRN‐ablated flies and control with the indicated concentrations of vitamin C, n = 6.
- Oviposition preference assays with Ir25a 2 , Ir76b 1 , Gr5a ∆5 , Gr61a 1 , Gr64b LexA , Gr64c LexA , and Gr64e LexA , n = 6–7.
- Oviposition preference assays to rescue Ir25a 2 and Ir76b 1 defects by expression of wild‐type cDNA, Ir25a + and Ir76b +, driven by its own GAL4s and Gr64f‐GAL4, n = 6.
- Rescue of the oviposition defects of Gr5a ∆5 , Gr61a 1 , Gr64b LexA , Gr64c LexA , and Gr64e LexA using GAL4/UAS system with the indicated GAL4s, n = 6. +/− indicate the presence or absence of the transgene, respectively.
Data information: All error bars represent the SEM. Multiple sets of data were compared using single‐factor ANOVA coupled with the Scheffe's post hoc test. Asterisks indicate statistical significance compared with the control. **P < 0.01.
The oviposition behavior for attractive acids is mainly driven by tarsal taste neurons and requires IR25a and IR76b (Chen & Amrein, 2017). However, vitamin C, one of the attractive acids, functioned in the labellum when PER assays were performed (Fig EV2C). Therefore, we tested organ‐ablated flies in oviposition (Fig EV4A–C). First, we found that oviposition preference to carboxylic acids (1 and 10% lactic acid, citric acid, and glycolic acid) is mediated by both forelegs and the labellum but not the antennae (Fig EV4A and B). Second, the flies that were ablated both organs (forelegs and labellum) have an additive effect on average but not significantly different from flies with only one of the organs ablated. Third, these results were consistent when vitamin C was tested (Fig EV4C). Therefore, we conclude that two distinct types of sweet‐sensing GRNs in the labellum and forelegs drive egg‐laying preference behavior.
Figure EV4. Oviposition preference assays on acids.
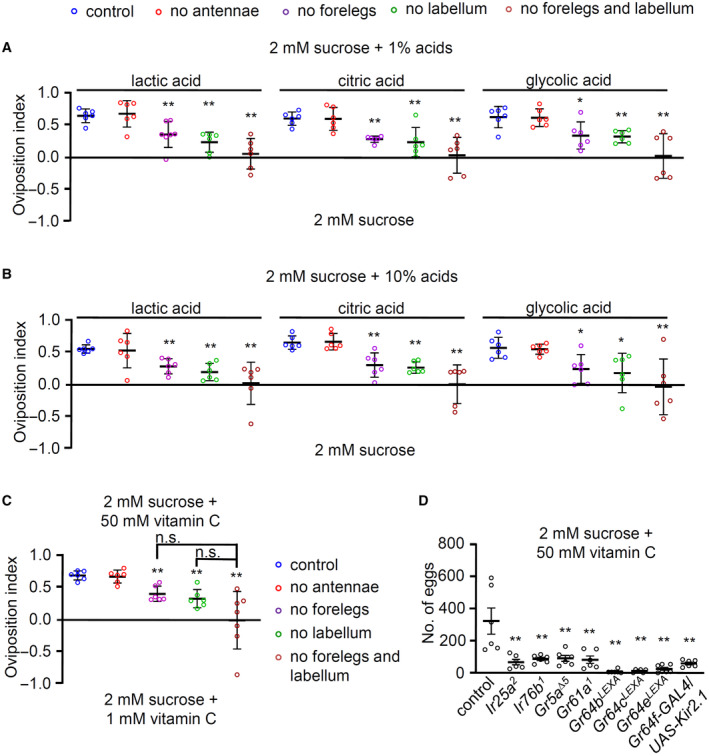
- Oviposition assays of indicated organ‐ablated flies and control at 1% carboxylic acids (lactic acid, citric acid, and glycolic acid), n = 6.
- Oviposition assays of indicated organ‐ablated flies and control at 10% carboxylic acids (lactic acid, citric acid, and glycolic acid), n = 6.
- Oviposition assays of specific organ‐ablated flies and control at 50 mM vitamin C versus 1 mM, n = 6.
- Number of eggs laid at 50 mM vitamin C mixed with 2 mM sucrose by Ir25a 2 , Ir76b 1 , Gr5a ∆5 , Gr61a 1 , Gr64b LexA , Gr64c LexA , Gr64e LexA , and vitamin C insensitive flies (Gr64f‐GAL4/UAS‐Kir2.1), n = 6.
Data information: All error bars represent the SEM. Multiple sets of data were compared using single‐factor ANOVA coupled with the Scheffe's post hoc test. Asterisks indicate statistical significance compared with the control. *P < 0.05, **P < 0.01.
We raised a question as to whether the increase in the number of eggs laid on the vitamin C in a dose‐dependent manner (Fig 1D) can be seen in the mutants. Regulating the number of eggs laid is generally controlled by the physiological effect of the ingested nutrients. Unlike most cases, the seven mutants, as well as sweet‐GRNs‐inhibited flies, laid a significantly lower number of eggs (Fig EV4D). Instead of attributing the reduction of egg numbers to the sensing of vitamin C, however, we expect that vitamin‐insensitive mutants feed on less amount of vitamin C, which results in suppressed egg‐laying.
Though the attraction to vitamin C was significant, flies showed only a slight attraction or stayed neutral to vitamin B2 and B9 in our binary food choice and oviposition assays (Fig EV5A and B). Taken together with behavioral assays and tip recordings with vitamin C, B2, and B9, we conclude that vitamin C can attract flies more than vitamin B2 and B9.
Figure EV5. Binary food choice assay and oviposition preference assay with vitamin B2 and B9.
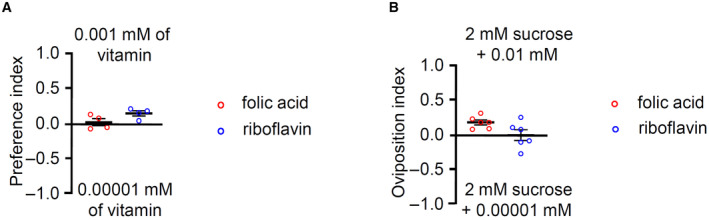
- Binary food choice assays performed with wild‐type flies in the presence of 0.001 mM versus 0.00001 mM riboflavin (vitamin B2) and folic acid (vitamin B9), n = 4.
- Oviposition assays performed with wild‐type flies, n = 6.
Data information: All error bars represent the SEM.
Electrophysiological response profiles for candidate taste receptors to attractive tastants
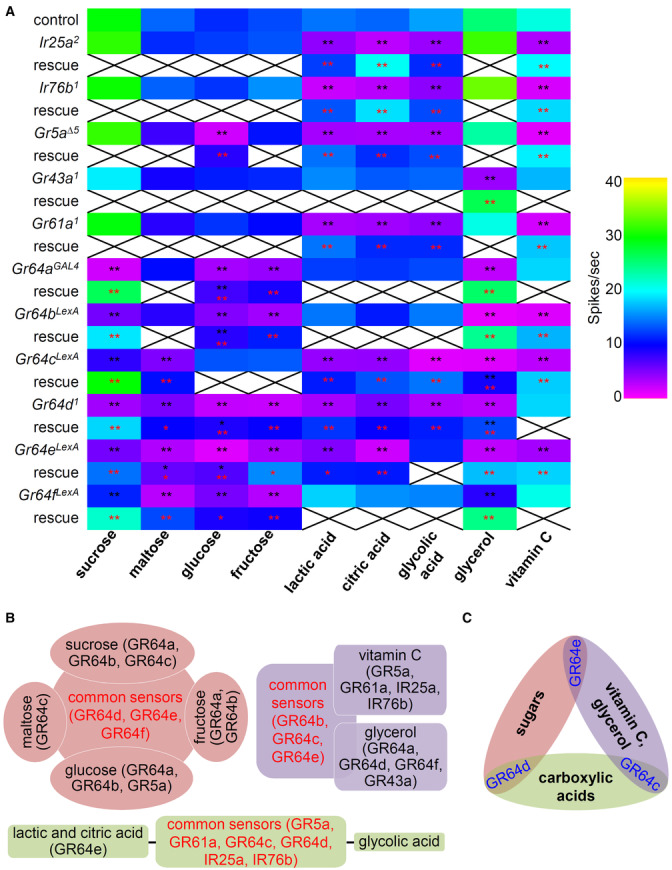
Electrophysiological phenotyping of candidate Ir and Gr mutants, in combination with the use of its genetic rescues, have been conclusive in the identification of ligands and their receptors. Previous studies have sought to characterize these sweet taste receptor candidates by the combinational expression of two Grs in the Gr64 cluster mutants or ectopic expression of one Gr in the empty olfactory neurons using the GAL4/UAS system (Jiao et al, 2007, 2008; Freeman et al, 2014; Kim et al, 2018). The recent discovery of six individual mutants of the Gr64 cluster in addition to the other mutants allowed us to verify attractive tastant responses. Several studies have uncovered the functions of each sweet Gr gene (Jiao et al, 2007, 2008; Freeman et al, 2014; Fujii et al, 2015; Kim et al, 2018; Aryal et al, 2022a). However, some functions are controversial because they have used different mutants that may have affected other cluster genes. Fujii et al (2015) have generated many reagents to delete each of the six Gr64 cluster genes to study the expression and behavioral analysis of each gene. However, these mutants were not evaluated at the level of single sensillum recordings. Therefore, we evaluated each Gr64 mutant (Gr64a GAL4 , Gr64b LexA , Gr64c LexA , Gr64d 1 , Gr64e LexA , Gr64f LexA ) and other potential candidates, such as Gr5a Δ5 , Gr61a 1 , Ir25a 2 , and Ir76b 1 , with the newly generated Gr43a 1 to delete Gr43a using homologous recombination via electrophysiology (Fig 4A and Appendix Fig S1). First, we tested four sugars. Using electrophysiological recordings from the L4 sensilla, we found a significant reduction in spike activity to sucrose in all six Gr64 mutants. The Gr64 gene cluster has a complex transcription unit that has a polycistronic transcription (Dahanukar et al, 2007; Jiao et al, 2007; Slone et al, 2007). Therefore, we also recovered all defects using rescue flies, using wild‐type cDNA (red asterisks). The significance of this study is the following. Firstly, the functions of Gr64b, Gr64c, Gr64d, and Gr64e to sense sucrose were first identified in this study (Fig 4A and Appendix Fig S2A). The role of Gr64a and Gr64f to sense sucrose has been previously identified (Jiao et al, 2007, 2008; Freeman et al, 2014). We also find conflicting evidence regarding the function of Gr5a in sucrose sensation. In previous research, Gr64b and Gr5a have been presented as sucrose sensors via PER assays (Fujii et al, 2015). However, we could not find any defect in Gr5a mutant testing sucrose. Second, we found a normal maltose response using Gr64a GAL4 and Gr64b LexA . However, the other four mutants from Gr64c to Gr64f had a significant reduction in neuronal activity (Fig 4A and Appendix Fig S2B). The mutant defects were fully rescued, except Gr64e. The rescue of Gr64e was significantly increased when compared with the mutant, but it did not reach the level of the control (black asterisk indicates a significant difference versus control). The roles of Gr64c and Gr64d in maltose detection were first identified here. However, we could not observe any reduced action potential from Gr5a and Gr64a mutants as compared to their previous role as maltose sensors (Jiao et al, 2007; Freeman et al, 2014; Fujii et al, 2015). Third, Gr5a with most Gr64 cluster mutants except Gr64c LexA , showed a significant reduction in glucose responses (Fig 4A and Appendix Fig S2C). This aligns with previous findings that Gr5a with Gr64a and Gr64f are glucose sensors (Jiao et al, 2007, 2008). Interestingly, Gr61a 1 was normal in this study, contrary to the previous finding that Gr61a has defects in its electrophysiology (Freeman et al, 2014). There was only a partial rescue of each mutant, including Gr64a, Gr64b, Gr64d, and Gr64e (Fig 4A, black asterisks). Nevertheless, we propose that Gr64b, Gr64d, and Gr64e are required for sensing glucose. Fourth, the internal fructose sensor, Gr43a, was not required for sensing fructose in the labellum. The fructose response profile was quite similar to glucose, except Gr5a. We confirmed the requirement of Gr64a to detect fructose (Freeman et al, 2014). We further identified that Gr64b, Gr64d, Gr64e, and Gr64f were essential for fructose‐induced neuronal spikes (Fig 4A and Appendix Fig S2D). Therefore, we propose that Gr64d, Gr64e, and Gr64f are more commonly required for detecting sugars (sucrose, maltose, glucose, and fructose), whereas Gr64a and Gr64b are relatively specific to sucrose, glucose, and fructose (Fig 4B). Furthermore, Gr64c is specific to sucrose and maltose, whereas Gr5a is only specific to glucose (Fig 4B). Interestingly, Gr43a, Gr61a, Ir25a, and Ir76b were dispensable to detect four sugars. For sugar detection, sweet GR members, but not IRs, are solely required.
Figure 4. Tip recording analysis of sugars, carboxylic acids, glycerol, and vitamin C with 11 potential candidate mutants.
-
AElectrophysiology with the indicated mutants and control (w 1118 ) in the presence of 50 mM sugars (sucrose, maltose, glucose, and fructose), 1% carboxylic acids (lactic acid, citric acid, and glycolic acid), and 50 mM vitamin C from L4 sensilla. The neuronal response to 10% glycerol was recorded from L7 sensilla. The defects of Ir25a, Ir76b, Gr5a, Gr61a, Gr64a, and Gr64f mutants were rescued by their own cDNA expression driven with their own GAL4s, while the rescue of Gr64b, Gr64c, Gr64d, and Gr64e were driven by Gr64f‐GAL4, n = 10–29. Multiple datasets were compared using a single‐factor ANOVA coupled with Scheffe's post hoc test. Black asterisks indicate statistical significance compared with the control. All the rescued flies had significantly increased spike activities; the red asterisks indicate statistical significance compared between the respective mutant and the rescued flies. *P < 0.05, **P < 0.01.
-
BFigurative illustration showing the functional requirement of GRs and IRs to summarize (A).
-
CFigurative illustration characterizing distinct common GR comparing three categories.
Next, we tested the carboxylic acid responses (Stanley et al, 2021; Shrestha & Lee, 2021a). First, we confirmed that Ir25a, Ir76b, Gr5a, and Gr61a were similarly required for sensing lactic, citric, and glycolic acid (Fig 4A, and Appendix Fig S2E–G). The deficits were completely recovered by the wild‐type cDNA expression of each gene. Although the role of Gr64 cluster deletion mutant was identified (Stanley et al, 2021; Shrestha & Lee, 2021a), each Gr64 cluster mutant has not been tested. Therefore, we further identified that Gr64c, Gr64d, and Gr64e were essential for the neuronal responses to lactic acid and citric acid (Fig 4A, and Appendix Fig S2E and F). However, Gr64e was dispensable in detecting glycolic acid (Fig 4A and Appendix Fig S2G). Gr64a, Gr64b, and Gr64f were not required for sensing any carboxylic acids. These results support the previous findings that both GRs and IRs are essential for detecting carboxylic acids as common sensors (Fig 4B). Although we did not reconfirm the separate roles of IRs and sweet GRs as stimulus onset (ON response) and removal phase (OFF response) sensors when the signals were conveyed to the brain (Stanley et al, 2021), we expanded the findings on carboxylic acids by identifying the individual GR64 cluster genes. Therefore, it will be interesting to test that newly identified each GR mutant is responsible for the OFF response by the way of brain calcium imaging. Overall, our results show that carboxylic acids differentially affect ligand‐receptor interactions. Gr5a, Gr61a, Gr64c, Gr64d, Ir25a, and Ir76b were common sensors for the tested carboxylic acids, while Gr64e was specifically required for detecting lactic acid and citric acid (Fig 4B).
Glycerol is the backbone of triglyceride and nutritious. Gr64e is a glycerol sensor (Freeman et al, 2014; Kim et al, 2018). Additionally, Gr64a, Gr64c, and Gr64d are also identified as glycerol sensors in ectopic experiments (Freeman et al, 2014). Gr64a, Gr64b, and Gr64c with Gr5a have behavioral defects that attract glycerol (Fujii et al, 2015). Our electrophysiology revealed that all six Gr64 mutants had significantly reduced spikes (Fig 4A and Appendix Fig S2H). The role of Gr64e and Gr64f were first identified in this study. Furthermore, Gr43a, but not Gr5a, was indispensable in detecting glycerol in the L7 sensilla. The rescued flies of Gr64c and Gr64d only partially recovered their physiological defects (Fig 4A and Appendix Fig S2H). The roles of Ir25a and Ir76b were not observed in the sensation of glycerol. This indicated that only sweet GRs except Gr5a and Gr61a, but not IRs, functioned in glycerol sensation. On comparing the neuronal responses to glycerol and vitamin C, the common sensors were Gr64b, Gr64c, and Gr64e (Fig 4B). Gr5a, Gr61a, Ir25a, and Ir76b were specifically required for sensing vitamin C, while Gr43a, Gr64a, Gr64d, and Gr64f were specific to glycerol.
Overall, we propose that GR64c was a common receptor for detecting carboxylic acid, glycerol, and vitamin C (Fig 4C). GR64e was common for detecting sugars, glycerol, and vitamin C, while GR64d was a sole common receptor for sensing sugars and carboxylic acids (Fig 4C). There are multiple complex arrangements for forming heteromultimeric ion channels; therefore, we proposed a model in which distinct common GRs have category‐dependent contributions to attractive taste depending on different categories (Fig 4C and Appendix Fig S2E, F, G and I). Furthermore, the requirement of IR25a and IR76b in combination with GRs to sense carboxylic acids and vitamin C should be further studied because it remains unclear how this complex physically works or how the intracellular signaling mechanisms of IRs and GRs converge. It would be interesting to study whether the gating or conformation of GRs would change with supplements of acidic compounds like carboxylic acids and vitamin C leading to additional ion flux. These can be enthralling mechanisms to be studied to link the relationship of GRs and IRs in future. Nevertheless, in this study, we showed how flies taste vitamin C.
Materials and Methods
Drosophila strains
In this investigation, the strain w 1118 was employed as a control strain. All flies were kept at a constant temperature of 25°C and 50–60% humidified incubator with a 12‐h light/12‐h dark cycle. Male and female flies were utilized in the studies at random basis. The following lines were obtained from the Bloomington Drosophila Stock Center (https://bdsc.indiana.edu): Ir7g 1 (BL42420), Ir8a 1 (BL41744), Ir10a 1 (BL23842), Ir21a 1 (BL10975), Ir48a 1 (BL26453), Ir48b 1 (BL23473), Ir51b 1 (BL10046), Ir52b 1 (BL25212), Ir52c 1 (BL24580), Ir56b 1 (BL27818), Ir56d 1 (BL81249), Ir62a 1 (BL32713), Ir67a 1 (BL56583), Ir75d 1 (BL24205), Ir85a 1 (BL24590), Ir92a 1 (BL23638), Ir94b 1 (BL23424), Ir94d 1 (BL33132), Ir94f 1 (BL33095), Ir94g 1 (BL25551), Ir100a 1 (BL31853), Ir94e‐GAL4 (BL81246), and UAS‐Kir2.1 (BL6596). The source of following lines was described earlier: Ir7a 1 , Ir47a 1 , Ir52a 1 , Ir56a 1 , Ir60b 3 , Ir94a 1 , Ir94c 1 , Ir94h 1 (Rimal et al, 2019), UAS‐Ir25a (Lee et al, 2018). C. Montell kindly provided strains Ir76b 1 , Ir76b‐GAL4, UAS‐Ir76b (Zhang et al, 2013), UAS‐Gr64a, UAS‐Gr64b (Jiao et al, 2007), UAS‐Gr64c, UAS‐Gr64d, UAS‐Gr64f (Jiao et al, 2008), and UAS‐Gr64e (N/A). Further, J. Carlson generously provided strains Gr5a ∆5 , Gr5a ∆5 /FM7;UAS‐Gr5a/CyO, Gr5a ∆5 /FM7;Gr5a‐GAL4/CyO, Gr61a 1 , Gr61a‐GAL4, and Gr61a 1 ;UAS‐Gr61a (Dahanukar et al, 2007). K. Scott provided strains ppk23‐GAL4 (Thistle et al, 2012) and ppk28‐GAL4 (Cameron et al, 2010). Gr66a‐GAL4, Gr5a‐GAL4, UAS‐Gr43a (Thorne et al, 2004), Gr64a GAL4 , Gr64b LEXA , Gr64c LEXA , Gr64e LEXA , Gr64f LEXA (Fujii et al, 2015), UAS‐Gr64b, UAS‐Gr64c, and UAS‐Gr64e were provided by H. Amrein. Gr64d 1 (Uchizono et al, 2017) was provided by Dr. S.J. Moon. Gr64f‐GAL4 (Dahanukar et al, 2007) were obtained from A. Dahanukar and L. Vosshall provided Ir25a‐GAL4 and Ir25a 2 (Benton et al, 2009) strains. Gr43a‐GAL4 (KDRC2785) was obtained from Korea Drosophila Resource Center.
Chemical reagents
Vitamin C (L‐ascorbic acid; CAS No 50‐81‐7), riboflavin (CAS No 83‐88‐5), folic acid (CAS No 59‐30‐3), tricholine citrate (CAS No 546‐63‐4), sucrose (CAS No 57‐50‐1), fructose (CAS No 57‐48‐7), glucose (CAS No 50‐99‐7), maltose (CAS No 6363‐53‐7), glycerol (CAS No 56‐81‐5), curcumin (CAS No 458‐37‐7), sulforhodamine B (CAS No 3520‐42‐1), glycolic acid (CAS No 79‐14‐1), citric acid (CAS No 77‐92‐9), and lactic acid (CAS No 50‐21‐5) were purchased from Sigma‐Aldrich Co. D(+)‐Galactose (CAS No 59‐23‐4) was purchased from Daejung chemicals & metals Ltd (Korea). Brilliant blue FCF (CAS No 3844‐45‐9, Cat No 027‐12842) was purchased from Wako Pure Chemical Industry Ltd (Japan).
Generation of Gr43a 1 mutant lines
The Gr43a 1 mutation was generated by ends‐out homologous recombination (Gong & Golic, 2003). To create the construct for injections, we amplified two 3‐kb genomic fragments by PCR (NotI and BglII were used) and subcloned the DNA into the pw35 vector. Using the “A” of the “ATG” start codon as +1, the deleted region was +711 to +1,666 (955 bp). The construct was injected into w 1118 embryos by BestGene Inc. We outcrossed the mutant with w 1118 for six generations.
Binary food choice assay
As in a prior study, binary food choice assays were performed (Aryal et al, 2022b). In a humidified chamber, 50–70 flies (3–6 days old; mixed sexes) were starved for 18 h. Next, two distinct food sources containing 1% agarose were prepared. The first food source had 1 mM vitamin C, while the second had higher concentrations of vitamin C, as indicated in the Figures. These foods were combined with either blue (brilliant blue FCF, 0.125 mg/ml) or red (sulforhodamine B, 0.1 mg/ml) food coloring. In a 72‐well microtiter dish (Thermo Fisher Scientific, cat. no. 438733), the two‐food mixtures were placed in alternate wells. Within 30 min of food preparation, approximately 50–70 starved flies were transferred to the plate. The flies were allowed to feed for 90 min at room temperature by incubating the dishes in a dark, humidified container. The tested flies were frozen at −20°C. A stereomicroscope was used to examine the color of their abdomens. Blue (NB), red (NR), and purple (NP) flies were tabulated based on ocular assessment. Based on the dye/tastant combinations, the preference index (PI) was determined using the following formula: (NB − NR)/(NR + NB + NP) or (NR − NB)/(NR + NB + NP). PI values of 1.0 or −1.0 indicated that the flies had a strong preference for one of the two food options. A PI of 0.0 suggested that the flies had no bias.
Tip recording assay
Electrophysiology (i.e., tip recording assay) was performed as previously described (Lee et al, 2009). Flies (4–7‐day‐old males and females) were anesthetized on ice. In the thorax of the flies, a reference glass electrode filled with Ringer's solution was introduced. The electrode was then gradually extended towards the fly's proboscis. Adults that were 5–6 days old were prepared per cycle to avoid any experimental biases. The same method was performed for numerous rounds on different days. The sensilla were stimulated for 5 s in recording pipettes (10–20 mm tip diameter) attached to a preamplifier with a mixture of chemical stimulants in a 30 mM tricholine citrate (TCC) solution (i.e., electrolyte solution). Using a Syntech signal connection interface box and a 100–3,000 Hz band‐pass filter, the recorded signals were collected and amplified by 10×. Action potential recordings were made at a sampling rate of 12 kHz and analyzed with Autospike 3.1 (Syntech). All recordings were performed at 1‐min intervals to ensure appropriate signals. The number of insects tested is shown by the dots in each figure.
Oviposition preference assay
We performed oviposition preference experiments as described earlier (Shrestha & Lee, 2021b). Thirty to 40 flies (mixed sexes) aged 5–7 days were acclimated in 1% agarose for 5–6 h in an egg‐laying chamber (Code No. FEC‐50200, Hansol Tech, Korea). Following the adaption process, the food supply was shifted to a 35 mm diameter petri dish separated into two parts: one with 2 mM sucrose and 1 mM vitamin C, while the other side contained 2 mM sucrose with the indicated concentrations of vitamin C (1–50 mM). This allowed female flies to choose where they want to lay their eggs. The numbers of eggs laid on each portion were counted after 18‐h incubation. Only the assays in which flies laid more than 50 eggs were considered when calculating the oviposition index. The oviposition index was determined as [{(number of eggs on choice A side) − (number of eggs on choice B side)} ÷ (total number of eggs laid)].
Egg count assay
For this assay, 10 females were permitted to lay eggs on media in an egg‐laying chamber overnight. After 18 h, the flies were transferred to new vials and the number of eggs was counted immediately. The indicated concentrations of vitamin C (1, 5, 10, 30, and 50 mM) in 1% agarose and 2 mM sucrose were tested. The numbers of eggs are presented as dots in Fig 1D. The experiment was repeated six times.
Proboscis extension response assay
The proboscis extension response (PER) experiment was performed as described previously (Poudel et al, 2015). Twenty to 25 flies (3–7‐day‐old males and females) were starved for 18–20 h in vials containing 1% agarose. After anesthetizing briefly on ice, the flies were trapped in a 20–200 μl pipette tip. The pipette tip's edge was chopped using a paper cutter blade to reveal the head. The head and proboscis were protruded outside the pipette tip to provide the stimuli to the proboscis. To stimulate the fly's tarsi, the head/proboscis and forelegs were pushed outside of the pipette tip without injuring any body parts. Water was first fed to remove any kind of thirstiness‐based biases. Two percent sucrose alone was used for both the positive control and initial stimulation. The tastant stimuli were presented using Kimwipe paper as the medium. The tarsi or proboscis were exposed to wet wicks containing either 2% sucrose or 50 mM vitamin C. The flies were not included in the experiment if they did not react to the sucrose during the first exposure. The same circumstances as the initial exposures were used for the second exposure. For each test round, > 10 flies were used. The experiment was repeated six times.
Starvation resistance assay
Fly starvation resistance assays were conducted as previously described (Lee et al, 2018). The assays were performed with different concentrations of vitamin C only food (0, 0.1, 1, 5, 10, 30, and 50 mM), curcumin only food (0.1 and 0.5%), and galactose only food (10, 30, and 50 mM) without any further nutrition. Control food was 1% agar alone. Briefly, 3–5‐day‐old 10 male and 10 female flies were allowed to feed on the above‐described food sources. The flies were observed every 12 h and then transferred to new vials containing the same food source. The survivability of flies was assessed up to 156 h, then the LT50 was calculated.
Quantification and statistical analyses
Data were analyzed using GraphPad Prism version 8.0 (RRID: SCR 002798). All trials were repeated on separate days. The number of trials for each experiment is represented by dots in the graphs. The standard error of the mean (SEM) is represented by error bars. Using single‐factor ANOVA and Scheffe's post hoc analysis, multiple datasets were compared. Origin (Origin Lab Corporation, RRID: SCR 002815) was used for all statistical analyses. In the figures, asterisks denote statistical significance (*P < 0.05, **P < 0.01).
Author contributions
Bhanu Shrestha: Conceptualization; formal analysis; validation; investigation; visualization; methodology; writing – original draft. Binod Aryal: Conceptualization; investigation; methodology. Youngseok Lee: Conceptualization; supervision; funding acquisition; writing – original draft; project administration; writing – review and editing.
Disclosure and competing interests statement
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
PDF+
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Acknowledgements
This work was supported by grants to Y. L. from the National Research Foundation of Korea (NRF) funded by the Korea government (MIST) (NRF‐2021R1A2C1007628) and the Korea Environmental Industry and Technology Institute (KEITI) grant funded by the Ministry of Environment of Korea. B.S. and B.A. were supported by the Global Scholarship Program for Foreign Graduate Students at Kookmin University in Korea.
EMBO reports (2023) 24: e56319
Data availability
The datasets produced in this study are available in the following database: Source data—BioStudies S‐BSST991 (https://www.ebi.ac.uk/biostudies/studies/S‐BSST991). Any additional information required to analyze the data reported in this paper is available from the lead contact upon request.
References
- Ahn J‐E, Chen Y, Amrein H (2017) Molecular basis of fatty acid taste in Drosophila . Elife 6: e30115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal B, Dhakal S, Shrestha B, Lee Y (2022a) Molecular and neuronal mechanisms for amino acid taste perception in the Drosophila labellum. Curr Biol 32: 1376–1386 [DOI] [PubMed] [Google Scholar]
- Aryal B, Dhakal S, Shrestha B, Sang J, Pradhan RN, Lee Y (2022b) Protocol for binary food choice assays using Drosophila melanogaster . STAR protoc 3: 101410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez‐Diaz C, Vosshall LB (2009) Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila . Cell 136: 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K (2010) The molecular basis for water taste in Drosophila . Nature 465: 91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Amrein H (2017) Ionotropic receptors mediate Drosophila oviposition preference through sour gustatory receptor neurons. Curr Biol 27: 2741–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y‐CD, Dahanukar A (2017) Molecular and cellular organization of taste neurons in adult Drosophila pharynx. Cell Rep 21: 2978–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y‐CD, Dahanukar A (2020) Recent advances in the genetic basis of taste detection in Drosophila . Cell Mol Life Sci 77: 1087–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Lei Y‐T, Kwon JY, Carlson JR (2007) Two gr genes underlie sugar reception in Drosophila . Neuron 56: 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delompré T, Guichard E, Briand L, Salles C (2019) Taste perception of nutrients found in nutritional supplements: a review. Nutrients 11: 2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal S, Sang J, Aryal B, Lee Y (2021) Ionotropic receptors mediate nitrogenous waste avoidance in Drosophila melanogaster . Commun Biol 4: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck HK, Talross GJ, Luo Y, Ebrahim SA, Carlson JR (2022) Ir56b is an atypical ionotropic receptor that underlies appetitive salt response in Drosophila . Curr Biol 32: 1776–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EG, Dahanukar A (2015) Molecular neurobiology of Drosophila taste. Curr Opin Neurobiol 34: 140–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EG, Wisotsky Z, Dahanukar A (2014) Detection of sweet tastants by a conserved group of insect gustatory receptors. Proc Natl Acad Sci USA 111: 1598–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Yavuz A, Slone J, Jagge C, Song X, Amrein H (2015) Drosophila sugar receptors in sweet taste perception, olfaction, and internal nutrient sensing. Curr Biol 25: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Pang L, Duong V‐K, Lee A, Schoniger H, Varady E, Dahanukar A (2017) A molecular and cellular context‐dependent role for Ir76b in detection of amino acid taste. Cell Rep 18: 737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong WJ, Golic KG (2003) Ends‐out, or replacement, gene targeting in Drosophila . Proc Natl Acad Sci USA 100: 2556–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González E, Cruces MP, Pimentel E, Sánchez P (2018) Evidence that the radioprotector effect of ascorbic acid depends on the radiation dose rate. Environ Toxicol Pharmacol 62: 210–214 [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS (2006) The cells and logic for mammalian sour taste detection. Nature 442: 934–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger AH, Stanley M, Weiss ZF, Musso P‐Y, Chan RC, Zhang H, Feldman‐Kiss D, Gordon MD (2018) A complex peripheral code for salt taste in Drosophila . Elife 7: e37167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C (2007) A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci USA 104: 14110–14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Wang X, Ren Q, Montell C (2008) Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila . Curr Biol 18: 1797–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Devineni AV, King IF, Heberlein U (2009) Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila . Proc Natl Acad Sci USA 106: 11352–11357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim H, Kwon JY, Seo JT, Shin DM, Moon SJ (2018) Drosophila Gr64e mediates fatty acid sensing via the phospholipase C pathway. PLoS Genet 14: e1007229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Moon SJ, Montell C (2009) Multiple gustatory receptors required for the caffeine response in Drosophila . Proc Natl Acad Sci USA 106: 4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Poudel S, Kim Y, Thakur D, Montell C (2018) Calcium taste avoidance in Drosophila . Neuron 97: 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C (2014) Peripheral coding of taste. Neuron 81: 984–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man Anh H, Linh DM, My Dung V, Thi Phuong Thao D (2019) Evaluating dose‐and time‐dependent effects of vitamin c treatment on a parkinson's disease fly model. Parkinsons Dis 2019: 9720546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C (2006) A taste receptor required for the caffeine response in vivo. Curr Biol 16: 1812–1817 [DOI] [PubMed] [Google Scholar]
- National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP) (1992) National toxicology program chemical repository database. Research Triangle Park, NC: U.S. Department of Health and Human Services; [Google Scholar]
- Paradis S, Sweeney ST, Davis GW (2001) Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron 30: 737–749 [DOI] [PubMed] [Google Scholar]
- Poljsak B, Ionescu JG (2009) Pro‐oxidant vs. antioxidant effects of vitamin C. In Handbook of Vitamin C Research: Daily Requirements, Dietary Sources and Adverse Effects, Kucharski H, Zajac J (eds), pp 153–183. New York, NY: Nova Science Publishers, Inc; [Google Scholar]
- Poudel S, Kim Y, Kim YT, Lee Y (2015) Gustatory receptors required for sensing umbelliferone in Drosophila melanogaster . Insect Biochem Mol Biol 66: 110–118 [DOI] [PubMed] [Google Scholar]
- Rimal S, Lee Y (2018) The multidimensional ionotropic receptors of Drosophila melanogaster . Insect Mol Biol 27: 1–7 [DOI] [PubMed] [Google Scholar]
- Rimal S, Sang J, Poudel S, Thakur D, Montell C, Lee Y (2019) Mechanism of acetic acid gustatory repulsion in Drosophila . Cell Rep 26: 1432–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Alcañiz JA, Silbering AF, Croset V, Zappia G, Sivasubramaniam AK, Abuin L, Sahai SY, Münch D, Steck K, Auer TO et al (2018) An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing. Nat Commun 9: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Dackis C (1975) Taste of nutrients: amino acids, vitamins, and fatty acids. Percept Psychophys 17: 140–146 [Google Scholar]
- Shrestha B, Lee Y (2021a) Mechanisms of carboxylic acid attraction in Drosophila melanogaster . Mol Cells 44: 900–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Lee Y (2021b) Mechanisms of DEET gustation in Drosophila . Insect Biochem Mol Biol 131: 103550 [DOI] [PubMed] [Google Scholar]
- Shrestha B, Lee Y (2023) Molecular sensors in the taste system of Drosophila . Genes Genomics 1–15 10.1007/s13258-023-01370-0 [DOI] [PubMed] [Google Scholar]
- Slone J, Daniels J, Amrein H (2007) Sugar receptors in Drosophila . Curr Biol 17: 1809–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M, Ghosh B, Weiss ZF, Christiaanse J, Gordon MD (2021) Mechanisms of lactic acid gustatory attraction in Drosophila . Curr Biol 31: 3525–3537 [DOI] [PubMed] [Google Scholar]
- Suckow BK, Suckow MA (2006) Lifespan extension by the antioxidant curcumin in Drosophila melanogaster . Int J Biomed Sci 2: 402–405 [PMC free article] [PubMed] [Google Scholar]
- Suh HJ, Shin B, Han S‐H, Woo MJ, Hong K‐B (2017) Behavioral changes and survival in Drosophila melanogaster: effects of ascorbic acid, taurine, and caffeine. Biol Pharm Bull 40: 1873–1882 [DOI] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K (2012) Contact chemoreceptors mediate male‐male repulsion and male‐female attraction during Drosophila courtship. Cell 149: 1140–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H (2004) Taste perception and coding in Drosophila . Curr Biol 14: 1065–1079 [DOI] [PubMed] [Google Scholar]
- Uchizono S, Itoh TQ, Kim H, Hamada N, Kwon JY, Tanimura T (2017) Deciphering the genes for taste receptors for fructose in Drosophila . Mol Cells 40: 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson JA, Courey JM, Maro NP (1992) Comparison of two forms of vitamin C on galactose cataracts. Nutr Res 12: 915–922 [Google Scholar]
- Vosshall LB, Stocker RF (2007) Molecular architecture of smell and taste in Drosophila . Annu Rev Neurosci 30: 505–533 [DOI] [PubMed] [Google Scholar]
- Wu Q, Park SJ, Yang M, William WJ (2021) Vitamin preference in Drosophila . Curr Biol 31: R946–R947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Baik LS, Shang X, Carlson JR (2022) Meeting a threat of the Anthropocene: taste avoidance of metal ions by Drosophila . Proc Natl Acad Sci USA 119: e2204238119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Ni J, Montell C (2013) The molecular basis for attractive salt‐taste coding in Drosophila . Science 340: 1334–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File
PDF+
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Data Availability Statement
The datasets produced in this study are available in the following database: Source data—BioStudies S‐BSST991 (https://www.ebi.ac.uk/biostudies/studies/S‐BSST991). Any additional information required to analyze the data reported in this paper is available from the lead contact upon request.


