Abstract
Purulent bacterial pericarditis is rare and associated with significant short- and long-term morbidity. We report a case of purulent bacterial pericarditis caused by Group A Streptococcus in an immunocompetent young child presenting with a pericardial mass. She was successfully treated with a combined medical and early surgical approach. (Level of Difficulty: Intermediate.)
Key Words: pediatric surgery, pericardial effusion, treatment
Central Illustration
History of Presentation
A 4-year-old girl presented with 1 day of abdominal pain, decreased oral intake, emesis, and subjective fever. She denied recent or current history of sore throat. On initial presentation, she was febrile to 38.2ºC, heart rate was 145 beats/min, respiratory rate was 35 breaths/min, blood pressure was 93/59 mm Hg, and SpO2 was 100% in room air with comfortable work of breathing and a nonfocal physical examination of the oropharynx, heart, lungs, and abdomen.
Learning Objectives
-
•
To maintain a high index of suspicion for purulent pericarditis caused by invasive GAS.
-
•
To understand the importance of a multidisciplinary approach to management in purulent pericarditis, including the role of both surgical and medical management to reduce long-term morbidity.
She was initially admitted in stable condition for supportive management of parainfluenza infection. On hospital day 3, she developed worsening abdominal pain and distension, recurrence of fever, and new respiratory distress requiring bilevel positive airway pressure and intensive care admission. Initial work-up at that time revealed bilateral pleural effusions and pulmonary edema on chest x-ray (Figure 1). Transthoracic echocardiogram (TTE) revealed a large circumferential pericardial effusion with a large pericardial mass located posterior and lateral to the left ventricle (Figure 2).
Figure 1.
CXR Imaging at Presentation and on Hospital Day 3
(A) CXR on the day of presentation. No pleural effusions, no cardiomegaly. (B) CXR on hospital day 3. Bilateral pleural effusions and cardiomegaly noted. CXR = chest x-ray.
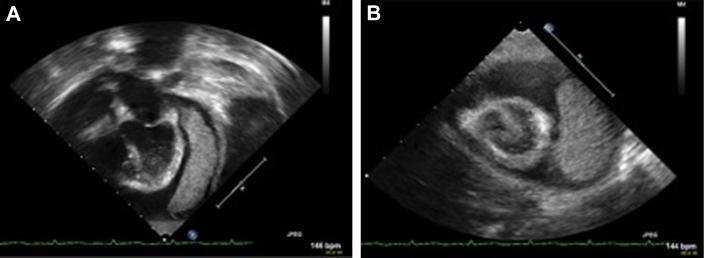
Figure 2.
Initial TTE Imaging
TTE from hospital day 3, before any intervention. (A) Apical 4-chamber view shows a large circumferential pericardial effusion and a large pericardial mass (phlegmon) lateral and posterior to left ventricle (measuring 5.2 cm × 1.8 cm), extending from the region of the AV groove to the apex and appeared to be attached to the epi-pericardium by multiple fibrin strands. (B) Parasternal short-axis view demonstrating the large circumferential pericardial effusion and phlegmon. AV = atrioventricular; TTE = transthoracic echocardiography.
Medical History
There was no significant past medical history and no known cardiovascular risk factors.
Differential Diagnosis
Infectious, hematologic, oncologic, and rheumatologic etiologies of a pericardial mass with acute pericarditis were considered. Infectious organisms included Staphylococcus aureus, Streptococcus pneumoniae, and group A Streptococcus (GAS). Fungal etiologies and Mycobacterium tuberculosis were also considered but thought to be less likely given lack of risk factors. Thrombus was also a consideration. Oncologic processes considered included sarcoma or metastasis from hematologic malignancy such as leukemia. Benign cardiac tumors remained in the differential, including rhabdomyoma, fibroma, myxoma, and teratoma.
Investigations
Initial laboratory testing was notable for elevated white blood cell (WBC) count of 32.0 x 109/L, C-reactive protein (CRP) of 88 mg/L, brain natriuretic peptide (BNP) of 212 pg/mL, and erythrocyte sedimentation rate of 82 mm/h.
Initial TTE revealed a large circumferential pericardial effusion with a large pericardial mass measuring approximately 6 cm (length) x 1.4 cm (width) located posterior and lateral to the left ventricle. This TTE showed right atrial and ventricular free wall collapse with exaggerated respirophasic variation of the mitral and tricuspid valve inflow Dopplers (24% across the mitral and 46% across the tricuspid) with normal biventricular systolic function. Abdominal computed tomography was also performed and was remarkable for wall thickening of terminal ileum and extensive ascites with periportal edema in the liver, without evidence of appendicitis.
Management
The patient underwent pericardiocentesis with removal of 175 mL of cloudy, nonbloody fluid and no drain was left in place. Pericardial fluid studies showed purulent profile with 4,750 WBCs/mL (52% neutrophils, 12% lymphocytes, and 36% macrophages), and Gram stain was positive for Gram-positive cocci in pairs and chains and eventually isolated GAS, or Streptococcus pyogenes, from both aerobic and anaerobic cultures. Pericardial fluid was sent for additional studies including tuberculosis and oncologic pathologies. Fluid adenosine deaminase was elevated 78.2 IU/L but mycobacterial cultures were negative. Fluid flow cytometry was unrevealing for oncologic process.
After pericardiocentesis was performed, she was initiated on empiric IV antibiotics, initially on ceftaroline and then transitioned to penicillin based on culture results.
Repeat echocardiography after pericardiocentesis showed reaccumulation of a large circumferential pericardial effusion and an unchanged pericardial mass (Figure 3). Repeat laboratory investigations showed elevated values for WBC 17.8 × 109/L, CRP >300 mg/L, BNP 3201 pg/mL, and procalcitonin 12.5 ng/mL. All blood cultures, including 2 drawn before antibiotic initiation, remained sterile. Abdominal ultrasound was unrevealing for abscess or any intra-abdominal fluid collection.
Figure 3.
TTE Imaging After Pericardiocentesis
TTE performed after pericardiocentesis with (A) apical 4-chamber, (B) parasternal short-axis, and (C) parasternal long-axis views showing reaccumulation of pericardial effusion and largely unchanged appearance and size of the pericardial mass. Abbreviation as in Figure 2.
Multidisciplinary discussion reached a consensus decision to perform surgical pericardial debridement, drainage, removal of mass, and partial pericardiectomy given the unchanged large size of the mass and potential future risk of restrictive pericarditis if untreated. Surgery was successfully performed 6 days after the pericardiocentesis. A partial sternotomy was performed with opening of the lower quarter of the sternum. A large amount of purulent fluid was drained from the pericardial space. The posterior mass was removed with manual lifting of the cardiac apex. The space was profusely irrigated and a single drain was placed in the pericardial space, which was removed on postoperative day 3 without any complications. Pathology of the pericardial mass showed fibro-purulent exudates with bacterial overgrowth, consistent with bacterial pericarditis with phlegmon formation (Figure 4).
Figure 4.
Gross Specimen and Histopathologic Findings Indicating Phlegmon
(A) Pericardial mass, gross specimen after surgical resection. (B) Histopathology of pericardial mass with a neutrophilic inflammatory infiltrate, fibrin deposition, extravasated red blood cells, granulation tissue, and early fibrosis. Formalin-fixed paraffin-embedded tissue slide stained with hematoxylin and eosin.
Her abdominal pain resolved after initiating antibiotics. The abdominal pain and corresponding wall thickening of terminal ileum was secondary to systemic inflammatory response during the acute phase of illness.
Discussion
Purulent bacterial pericarditis is a rare phenomenon in children, presenting as acute pericarditis with signs of systemic inflammation and symptoms such as chest pain, dyspnea, and fever.1 GAS, or Streptococcus pyogenes, as an etiology of purulent bacterial pericarditis is even more rare with only about 7 reports in the literature2, 3, 4, 5, 6, 7, 8 and none with pericardial mass/phlegmon formation.
There is known risk of constrictive pericarditis after acute pericarditis with higher risk in patients with bacterial pericarditis,9 but there are no data on effects of phlegmon in the pericardial space. Surgical intervention is a consideration in purulent bacterial pericarditis to mitigate risk of constrictive pericarditis and reduce morbidity and mortality.8,10
Of the reported cases of purulent pericarditis in pediatric patients,2, 3, 4, 5, 6, 7, 8 all required pericardial drainage (some with temporary pericardial drain left in place) for both diagnosis and therapeutics. Additionally, all were medically managed with antibiotics of varying lengths (range: 2-8 weeks) and routes (oral/intravenous [IV]/combination). Among those with reported follow-up, no patients had reaccumulation of pericardial effusion or complications such as constrictive pericarditis. One case was complicated by mycotic pseudoaneurysm formation and resultant compression on the pulmonary vasculature,5 which occurred several days after pericardiocentesis. One older study showed that early intervention for purulent pericarditis10 with pericardiectomy should be done if patient has tamponade after initial pericardiocentesis or if fever persists while on appropriate antibiotics.
In this case, the patient had reaccumulation of pericardial effusion despite undergoing pericardiocentesis and had a large pericardial mass that did not decrease in size while on medical management without any evidence of cardiac tamponade during her clinical course. Surgical intervention with removal of pericardial mass and partial pericardiectomy was successfully completed in addition to treatment with IV antibiotics.
Follow-up
After surgical pericardial drainage, phlegmon removal and partial pericardiectomy, only a small pericardial effusion remained. She was discharged on postoperative day 8. She completed a total 4-week course of antibiotics after source control was achieved without any complications.
The patient was followed monthly for 3 months after initial onset of symptoms and remained clinically well without any fevers or respiratory or abdominal symptoms. Her WBC, CRP, and pro-BNP all normalized. One month after completing antibiotics, echocardiogram was normal without any evidence of reaccumulation of fluid or phlegmon and showed normal indices for both systolic and diastolic ventricular function.
Conclusions
To our knowledge, this is the first-reported case of purulent bacterial pericarditis with phlegmon formation due to GAS. Our case provides support for both surgical (pericardial drainage and removal of phlegmon with partial pericardiectomy) and medical management (long-course of IV antibiotics) for this unique presentation. Furthermore, although GAS purulent pericarditis is rare, it is important to have a high clinical suspicion even in the absence of suspected current or prior GAS infection.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Little W.C., Freeman G.L. Pericardial disease [published correction appears in Circulation. 2007;115:e406. Dosage error in article text] Circulation. 2006;113:1622–1632. doi: 10.1161/CIRCULATIONAHA.105.561514. [DOI] [PubMed] [Google Scholar]
- 2.Angoulvant F., Bellanger H., Magnier S., et al. Acute purulent pericarditis in childhood: don't forget β-haemolytic group-A Streptococcus. Intensive Care Med. 2011;37:1709–1710. doi: 10.1007/s00134-011-2259-4. [DOI] [PubMed] [Google Scholar]
- 3.Al-Waili B.R., Zacharias S.K., Aslem E. Group A streptococcal pericarditis in a four-month-old infant: case report. Sultan Qaboos Univ Med J. 2017;17:e241–e243. doi: 10.18295/squmj.2016.17.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pemira S.M., Tolan R.W., Jr. Invasive group A streptococcus infection presenting as purulent pericarditis with multiple splenic abscesses: case report and literature review. Clin Pediatr (Phila) 2012;51:436–441. doi: 10.1177/0009922811430345. [DOI] [PubMed] [Google Scholar]
- 5.Fry E., Urbanczyk J., Price J., et al. Streptococcus pyogenes pericarditis with resultant pulmonary trunk compression secondary to mycotic pseudoaneurysm. Case Rep Cardiol. 2018;2018 doi: 10.1155/2018/3514797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhaduri-McIntosh S., Prasad M., Moltedo J., Vázquez M. Purulent pericarditis caused by group a streptococcus. Tex Heart Inst J. 2006;33:519–522. [PMC free article] [PubMed] [Google Scholar]
- 7.Vigneswaran W.T., Hardie R., Ferguson J.C., Faichney A. Cardiac tamponade due to Lancefield group A beta haemolytic streptococcal pericarditis. Thorax. 1985;40:549–550. doi: 10.1136/thx.40.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megged O., Argaman Z., Kleid D. Purulent pericarditis in children: is pericardiotomy needed? Pediatr Emerg Care. 2011;27:1185–1187. doi: 10.1097/PEC.0b013e31823b44af. [DOI] [PubMed] [Google Scholar]
- 9.Imazio M., Brucato A., Maestroni S., et al. Risk of constrictive pericarditis after acute pericarditis. Circulation. 2011;124:1270–1275. doi: 10.1161/CIRCULATIONAHA.111.018580. [DOI] [PubMed] [Google Scholar]
- 10.Morgan R.J., Stephenson L.W., Woolf P.K., Edie R.N., Edmunds L.H., Jr. Surgical treatment of purulent pericarditis in children. J Thorac Cardiovasc Surg. 1983;85:527–531. [PubMed] [Google Scholar]