Abstract
Transcatheter tricuspid valve edge-to-edge repair (T-TEER) has emerged as an option for treating patients with tricuspid regurgitation. Few studies have explored intraprocedural maneuvers to optimize leaflet-grasping T-TEER in order to improve technical success. This case series of 3 patients describes maneuvers that facilitated T-TEER in patients with large coaptation gaps or short leaflet lengths. (Level of Difficulty: Advanced.)
Key Words: transcatheter, transesophageal echocardiography, tricuspid regurgitation, tricuspid valve
Central Illustration
Treatment options for severe tricuspid regurgitation (TR) have been limited given a lack of evidence-based medical therapy and the high mortality rate of isolated tricuspid valve (TV) surgery.1 Transcatheter TV repair has emerged as a viable option that is safe and effective in reducing TR while alleviating symptoms and improving quality of life,2,3 with the potential of improving survival.4 The most common transcatheter device currently used in Europe and in U.S. trials is transcatheter TV edge-to-edge repair (T-TEER).5 The main anatomical predictor of poor technical success is the coaptation gap (CG) that is measured on transesophageal echocardiography (TEE).6, 7, 8 Few studies have explored maneuvers to reduce the CG during T-TEER.9 This case series describes maneuvers other than table tilt that facilitate T-TEER in patients with excessive CGs or short leaflet lengths. The patients presented in this case series had persistent severe, symptomatic TR after evaluation by a multidisciplinary team and medication optimization.
Learning Objectives
-
•
To describe maneuvers that might facilitate transcatheter tricuspid valve repair by reducing the CG between the tricuspid leaflets during T-TEER.
-
•
To understand procedural T-TEER scenarios in which these maneuvers might prove beneficial.
Case Presentation 1
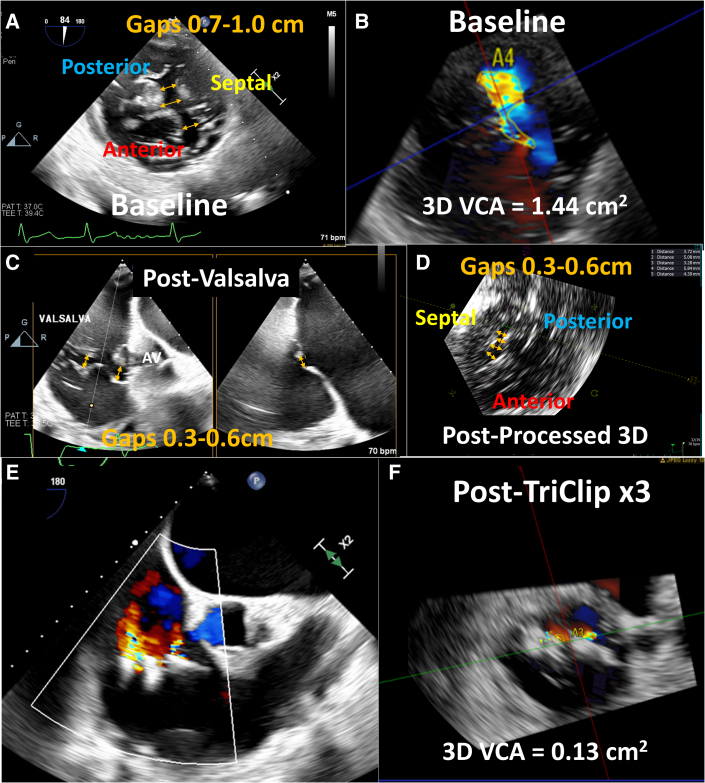
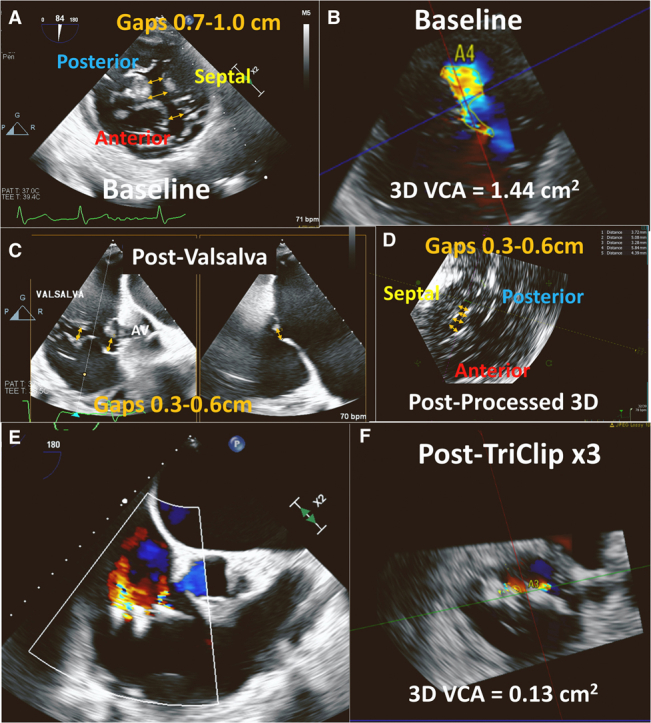
The patient was an 84-year-old man with symptomatic severe TR. His medical history was significant for remote atrial septal defect repair, recent MitraClip NTR (Abbott Vascular) placement with residual mild to moderate mitral regurgitation (MR), and permanent atrial fibrillation (AF). He was enrolled in the previously reported TRILUMINATE (Trial to Evaluate Treatment With Abbott Transcatheter Clip Repair System in Patients With Moderate or Greater Tricuspid Regurgitation; NCT03227757) early feasibility trial for the TriClip device (Abbott Vascular).3 Preprocedural TEE revealed normal left ventricular (LV) function, moderately reduced right ventricular (RV) function, a severely enlarged RV, and leaflet tethering consistent with ventricular functional TR. The central CG was along the entire septal commissure (4.1 cm anteroposterior length), with septolateral CGs of 0.7 to 1.0 cm (Figure 1A, Video 1). The 3-dimensional vena contracta area (VCA) was 1.44 cm2, with regurgitant volume of 61 mL (using a TR velocity-time integral of 42.4 cm) (Figure 1B), consistent with torrential TR. Simultaneous and independent leaflet grasping was unsuccessful. A ventilator-assisted or intraoperative Valsalva maneuver was initiated by increasing ventilatory gas flow and squeezing the breathing circuit bag temporarily with the adjustable pressure-limiting valve fully closed, increasing intrathoracic pressure to approximately 30 mm Hg. During the ventilator-assisted Valsalva maneuver, the CGs reduced to 0.3 to 0.6 cm2 (Figures 1C and 1D, Videos 2 and 3) and the initial tricuspid clip could be placed during the 15 to 20 seconds of the maneuver. Following initial clip placement, 2 subsequent clips could be placed, without the need for a ventilator-assisted Valsalva maneuver. Three tricuspid clips resulted (Figure 1E) in a final reduction of TR to a 3-dimensional VCA of 0.13 cm2 and regurgitant volume of 11 mL (Figure 1F), consistent with mild TR.
Figure 1.
Case 1: Use of Ventilator-Assisted Valsalva Maneuver
(A) Transesophageal echocardiogram with transgastric short-axis view of the tricuspid valve showing preprocedural septolateral gaps of 0.7 to 1.0 cm (yellow arrows) (Video 1). Color Doppler 3-dimensional (3D) vena contracta area (VCA) was 1.44 cm2(B), consistent with torrential tricuspid regurgitation. Transgastric short-axis view with biplane of the tricuspid valve during transcatheter tricuspid edge-to-edge repair (T-TEER) after ventilator-assisted Valsalva maneuver, showing a reduction of the coaptation gaps to 0.3 to 0.6 cm (C) (Video 2), which can be also seen on postprocessed 3D transgastric short-axis view of the tricuspid valve (D) (Video 3). After a total of 3 T-TEER clips were placed (E), the 3D VCA was reduced to 0.13 cm2(F), consistent with mild TR. AV = aortic valve.
Case Presentation 2
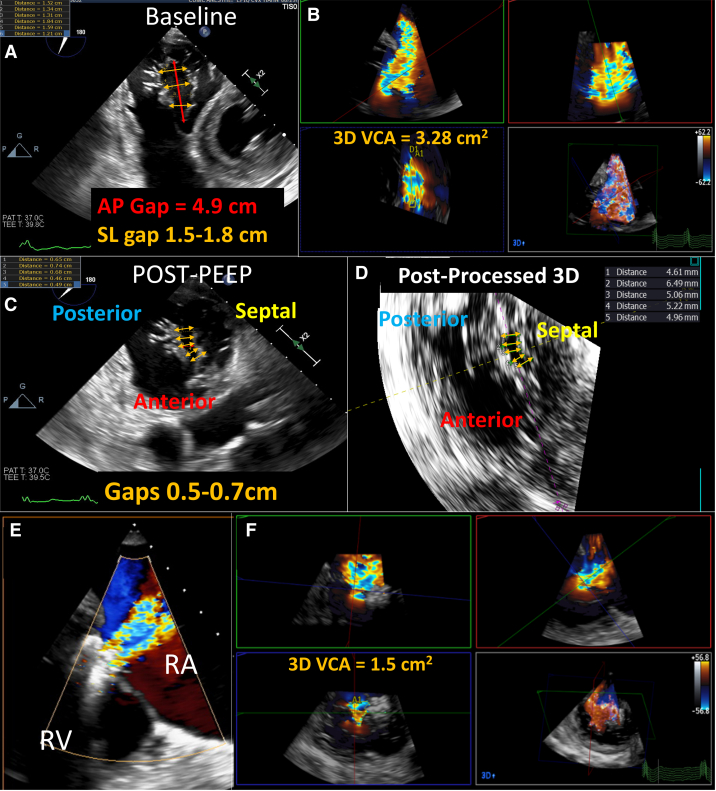
The patient was an 86-year-old woman with symptomatic severe TR despite optimal medical therapy. Her medical history included permanent AF and hypertension. She was accepted for compassionate use of the PASCAL device (Edwards Lifesciences). Preprocedural TEE showed normal LV and RV function, a severely enlarged right atrium and RV, and torrential mixed atrial and ventricular functional TR. The anteroposterior CG was 4.92 cm, with a septolateral CG of 1.5 to 1.8 cm (Figure 2A, Video 4) and 3-dimensional VCA of 3.28 cm2 (Figure 2B). T-TEER with either simultaneous or independent leaflet grasping was unsuccessful. With a positive end-expiratory pressure (PEEP) of 20 mm Hg to reduce venous return in combination with external pressure applied to the patient’s sternum to physically narrow the lateral-to-septal dimension, the CG was reduced to 0.5 to 0.7 cm (Figures 2C and 2D, Video 5). A T-TEER device was placed across the anteroseptal leaflets, and a second T-TEER device could be placed across the posteroseptal leaflet without need for further maneuvers (Figure 2E), with reduction of TR by more than 50% (Figure 2F).
Figure 2.
Case 2: Use of PEEP and Chest Pressure
(A) Baseline preprocedural transesophageal echocardiographic transgastric short-axis view of the tricuspid valve (Video 4) shows an anteroposterior (AP) gap of 4.9 cm (red arrow), with septolateral (SL) gaps between 1.5 and 1.8 cm (yellow arrows). (B) Color Doppler 3D VCA was 3.28 cm2, consistent with torrential tricuspid regurgitation. (C,D) During T-TEER, transgastric short-axis view and postprocessed 3D short-axis view of the tricuspid valve after the use of positive end-expiratory pressure (PEEP) and chest pressure show a reduction of the coaptation gaps to 0.5 to 0.7 cm (yellow arrows) (Video 5). After successful T-TEER with 2 devices (E), the 3D VCA was reduced to 1.5 cm2(F), a >50% reduction from baseline. RA = right atrium; RV = right ventricle; other abbreviations as in Figure 1.
Case Presentation 3
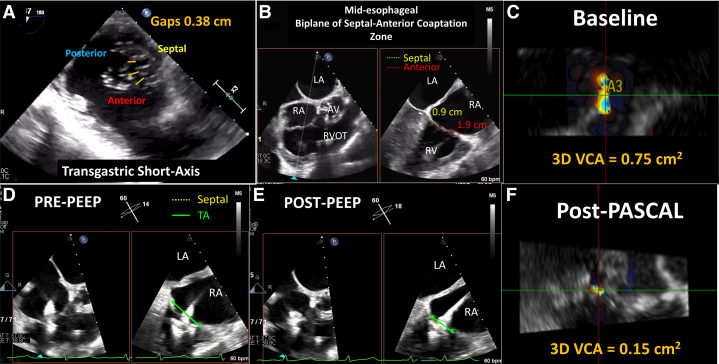
The patient was a 91-year-old woman with symptomatic severe TR. Her medical history included moderate aortic stenosis, severe MR status post recent MitraClip implantation with residual moderate to severe MR, chronic AF, and sinus node dysfunction status post permanent pacemaker (PPM) implantation, complicated by PPM-related TR. She underwent PPM lead extraction and wireless pacemaker implantation, but right-sided heart failure symptoms persisted despite optimal medical therapy. She was admitted for compassionate use of the T-TEER device. Preprocedural TEE revealed normal LV and RV function, with moderately increased RV size and biatrial dilation. There was severe atrial functional TR with a septolateral CG of 0.38 cm with dense chordae (Figure 3A) and a short septal leaflet (Figure 3B). Three-dimensional VCA was 0.75 cm2, with regurgitant volume of 75 mL (using a TR velocity-time integral of 100 cm) (Figure 3C). Attempts at simultaneous grasping were unsuccessful, so independent grasping was performed. After anterior leaflet grasp (Figure 3D, Video 6), attempts to maneuver closer to the septal leaflet were ineffectual, and PEEP of 15 cm H2O was instituted, with subsequent narrowing of the tricuspid annulus (Figure 3E, Video 7), and the septal leaflet was grasped. A single T-TEER device was required for a 2-grade TR reduction from severe to mild, with a final 3-dimensional VCA of 0.15 cm2 (Figure 3F).
Figure 3.
Case 3: Use of PEEP
(A) Baseline transgastric short-axis view shows a septolateral gap of 0.38 cm with dense chordae. (B) Tricuspid valve leaflets close at the annular plane (midesophageal RV inflow view with biplane) with a relatively short septal leaflet. (C) Color Doppler 3D VCA was 0.75 cm2, consistent with severe tricuspid regurgitation (TR). (D) During T-TEER, after the anterior leaflet was grasped, the device could not be maneuvered close enough to the septum for an adequate grasp of the septal leaflet (midesophageal RV inflow view with biplane) (Video 6). (E) PEEP was used to reduce the tricuspid annular (TA) diameter (green arrows) (Video 7). (F) After single-device T-TEER, the 3D VCA was reduced to 0.15 cm2, consistent with mild TR. Abbreviations as in Figures 1 and 2.
Discussion
Various anatomical criteria have been proposed to determine the suitability of patients for different categories of transcatheter devices.5 Worldwide, T-TEER is the most common approach given its early commercial approval in Europe and proceduralists’ familiarity. However, several anatomical criteria limit the procedural success of these devices: large CGs, noncentral regurgitant orifices, increased tethering height, and greater leaflet complexity with dense chordae.5 Atrial functional TR can result in suboptimal anatomy, as the leaflets are not long enough to cover the markedly dilated annular area and thus may be particularly difficult for TEER devices. Given the growing use of T-TEER, site-reported techniques for facilitating procedural success become integral, with greater reduction in TR associated with better outcomes.10
In this case series, ventilatory maneuvers and chest compression were used in 2 different scenarios: excessive CGs and inadequate leaflet lengths (Table 1). Intraprocedural maneuvers such as the reverse Trendelenburg position, the Valsalva maneuver, and PEEP reduce systemic venous return, which decreases tricuspid annular size and CG, effectively increasing the grasping lengths of the leaflets. Preprocedural aggressive diuresis also reduces right heart size and CGs and may obviate the need for additional intraprocedural maneuvers. Which procedure to use may depend on the proceduralist’s and anesthesiologist’s comfort with each maneuver. The reverse Trendelenburg position is safe but requires changing the settings of the catheterization table to permit this change in angle. The Valsalva maneuver is not commonly performed and may require experience to achieve a consistent intrathoracic pressure using manual methods. We have obtained the most consistent results with PEEP. Finally, in case 2, the effect of chest compression alone was not captured by imaging, as PEEP and chest compression were performed in close temporal proximity, but chest compression to physically reduce the tricuspid annular dimension and thus leaflet gap size has led to inconsistent gap reduction in our experience, given the variability of cardiac position and rotation within the chest.
Table 1.
Echocardiographic and invasive patient characteristics
| Patient 1: Valsalva | Patient 2: PEEP + Chest Compression |
Patient 3: PEEP | |
|---|---|---|---|
| RV basal: mid-dimension (ratio) | 6.18 cm/4.48 cm (1.38) | 4.75 cm/4.57 cm (1.03) | 5.27 cm/3.64 cm (1.45) |
| TA 4-chamber dimension | 6.39 cm | 4.87 cm | 3.62 cm |
| Tethering height | 1.43 cm | 1.23 cm | 0 cm |
| LVEF | 59% | 67% | 63% |
| Estimated PASP | 31 mm Hg | 31 mm Hg | 36 mm Hg |
| RV function | Moderately reduced | Mildly reduced | Normal |
| RV FWS pre–T-TEER | -12.5% | -16.6% | -21% |
| RV FWS post–T-TEER | -11.0% | -16.4% | -22.9% |
| Maximal S-L gap pre-maneuver | 1.03 cm | 1.84 cm | 0.38 cm |
| Maximal S-L gap post-maneuver | 0.58 cm | 0.74 cm | 0 cm |
| TR severity/3D VCA pre-TEER | Torrential/1.44 cm2 | Torrential/3.28 cm2 | Severe/0.75 cm2 |
| TR severity/3D VCA post-TEER | Mild/0.13 cm2 | Severe/1.5 cm2 | Mild/0.15 cm2 |
3D VCA = 3-dimensional vena contracta area; FWS = free wall longitudinal strain; LVEF = left ventricular ejection fraction; PASP = pulmonary artery systolic pressure; RV = right ventricular; S-L = septal-lateral; TA = tricuspid annulus; TEER = transcatheter tricuspid edge-to-edge repair; TR = tricuspid regurgitation.
Importantly, the Valsalva maneuver, increased PEEP, and chest compression can result in lung barotrauma and chest trauma and can have detrimental hemodynamic effects by reducing venous return making it imperative to perform these maneuvers with continuous hemodynamic monitoring. To minimize the adverse hemodynamic effect and complications, the duration of these maneuvers is intentionally brief (15-20 seconds at most), and any delay to assess hemodynamic effect (either invasively or noninvasively) is not required, as the gap reduction itself is the proof of adequate hemodynamic effect.
Conclusions
Maneuvers for reducing the CG and improving grasping leaflet lengths can help increase procedural success rates for T-TEER and clinical outcomes. A ventilator-assisted Valsalva maneuver and PEEP should be added to other maneuvers (ie, reverse Trendelenburg position) to improve technical outcomes in challenging cases.
Funding Support and Author Disclosures
Dr Hahn has received speaker fees from Abbott Structural, Baylis Medical, Edwards Lifesciences, and Philips Healthcare; has institutional consulting contracts for which she receives no direct compensation with Abbott Structural, Boston Scientific, Edwards Lifesciences, Medtronic, and Novartis; and is chief scientific officer for the echocardiography core laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored TV trials, for which she receives no direct industry compensation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Case 1: Intraprocedural Transgastric Short-Axis Biplane View of the Tricuspid Valve at Baseline
Case 1: Intraprocedural Transgastric Short-Axis Biplane View of the Tricuspid Valve After Ventilator-Assisted Valsalva Maneuver
Case 1: Three-Dimensional Multiplanar Reconstruction of the Tricuspid Valve After Valsalva Maneuver
Case 2: Intraprocedural Transgastric Short-Axis View of the Tricuspid Valve at Baseline
Case 2: Intraprocedural Transgastric Short-Axis View of the Tricuspid Valve With Biplane During Positive End-Expiratory Pressure and Chest Compression
Case 3: Intraprocedural Midesophageal Right Ventricular Inflow Biplane View of the Tricuspid Valve at Baseline
Case 3: Intraprocedural Midesophageal Right Ventricular Inflow Biplane View of the Tricuspid Valve After Initiation of Positive End-Expiratory Pressure
References
- 1.Dreyfus J., Flagiello M., Bazire B., et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J. 2020;41:4304–4317. doi: 10.1093/eurheartj/ehaa643. [DOI] [PubMed] [Google Scholar]
- 2.Kodali S., Hahn R.T., Eleid M.F., et al. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol. 2021;77:345–356. doi: 10.1016/j.jacc.2020.11.047. [DOI] [PubMed] [Google Scholar]
- 3.Nickenig G., Weber M., Lurz P., et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–2011. doi: 10.1016/S0140-6736(19)32600-5. [DOI] [PubMed] [Google Scholar]
- 4.Taramasso M., Benfari G., van der Bijl P., et al. Transcatheter versus medical treatment of patients with symptomatic severe tricuspid regurgitation. J Am Coll Cardiol. 2019;74:2998–3008. doi: 10.1016/j.jacc.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Praz F., Muraru D., Kreidel F., et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention. 2021;17:791–808. doi: 10.4244/EIJ-D-21-00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besler C., Orban M., Rommel K.-P., et al. Predictors of procedural and clinical outcomes in patients with symptomatic tricuspid regurgitation undergoing transcatheter edge-to-edge repair. J Am Coll Cardiol Intv. 2018;11:1119–1128. doi: 10.1016/j.jcin.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Mehr M., Taramasso M., Besler C., et al. 1-Year outcomes after edge-to-edge valve repair for symptomatic tricuspid regurgitation: results from the TriValve Registry. J Am Coll Cardiol Intv. 2019;12:1451–1461. doi: 10.1016/j.jcin.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Ruf T.F., Hahn R.T., Kreidel F., et al. Short-term clinical outcomes of transcatheter tricuspid valve repair with the third-generation MitraClip XTR system. J Am Coll Cardiol Intv. 2021;14:1231–1240. doi: 10.1016/j.jcin.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Wild M.G., Weckbach L., Braun D., et al. Mind the gap: facilitating tricuspid transcatheter edge-to-edge repair procedures by standardized table tilt. J Am Coll Cardiol Intv. 2022;15:1004–1006. doi: 10.1016/j.jcin.2022.02.046. [DOI] [PubMed] [Google Scholar]
- 10.Sannino A., Ilardi F., Hahn R.T., et al. Clinical and echocardiographic outcomes of transcatheter tricuspid valve interventions: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.919395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case 1: Intraprocedural Transgastric Short-Axis Biplane View of the Tricuspid Valve at Baseline
Case 1: Intraprocedural Transgastric Short-Axis Biplane View of the Tricuspid Valve After Ventilator-Assisted Valsalva Maneuver
Case 1: Three-Dimensional Multiplanar Reconstruction of the Tricuspid Valve After Valsalva Maneuver
Case 2: Intraprocedural Transgastric Short-Axis View of the Tricuspid Valve at Baseline
Case 2: Intraprocedural Transgastric Short-Axis View of the Tricuspid Valve With Biplane During Positive End-Expiratory Pressure and Chest Compression
Case 3: Intraprocedural Midesophageal Right Ventricular Inflow Biplane View of the Tricuspid Valve at Baseline
Case 3: Intraprocedural Midesophageal Right Ventricular Inflow Biplane View of the Tricuspid Valve After Initiation of Positive End-Expiratory Pressure