Abstract
A previously well 34-year-old man presents with electrical storm after performing headstands. A step-by-step review of clinical information and case development is presented with discussion. Ultimately, 2 rare diagnoses are discovered and their potential contribution to a cascade of complications resulting in ventricular arrhythmia are discussed. (Level of Difficulty: Intermediate.)
Key Words: congenital heart disease, pheochromocytoma, ventricular tachycardia
Central Illustration
Patient Presentation
A 34-year-old man presents for assessment of new-onset chest pain and palpitations that developed while performing a headstand. He reports no prior medical history. The patient denies any history of dyspnea, syncope, or presyncope. He does not take any medications, and denies smoking and recreational drug use. Triage vitals are as follows: 153/74 mm Hg (right arm), 72 beats/min, 18 breaths/min, oxygen saturation 100% on room air, and 36.5° Celsius. He appears fit and in no acute distress, with normal peripheral and carotid pulses that are equal bilaterally. Thorough neurological, cardiac, respiratory, and abdominal examination is normal. A 12-lead electrocardiogram (ECG) demonstrates normal sinus rhythm with diffuse upsloping ST depression (Figure 1). Laboratory investigations reveal the following: white blood count 14.8 × 109/L with 82% neutrophils, hemoglobin 153 g/L, and platelet count 318 × 109/L. Serum chemistry reveals the following: creatinine 100 μmol/L, sodium 138 mmol/L, and potassium 3.1 mmol/L. Initial troponin is 458 ng/L on a high-sensitivity assay. A repeat 12-lead ECG (Figure 2) demonstrates ongoing sinus rhythm with 1 mm of ST-segment elevation in aVR and new hyperacute T waves in the anterior leads. A 2-view chest x-ray is normal with no mediastinal widening or any airspace disease.
Learning Objectives
-
•
To review the approach to management and workup of undifferentiated electrical storm to improve resuscitation outcomes.
-
•
To outline the cardiac manifestations associated with pheochromocytoma to recognize the potential complications.
-
•
To discuss the physiological implications of anomalous aortic origin of a coronary artery to guide a management approach.
Figure 1.
Electrocardiogram Demonstrating Diffuse Ischemia
Electrocardiogram demonstrating sinus rhythm with ST-segment elevation in aVR and V1 and diffuse upsloping ST-segment depressions.
Figure 2.
Evolution of Electrocardiographic Changes
Electrocardiogram demonstrating sinus rhythm with ST-segment elevation in aVR and peaked T waves in the anterior leads.
Question 1: What is the initial differential diagnosis and following steps?
Chest pain and palpitations are common patient complaints with a broad differential. Initial investigations often focus on cardiac and respiratory etiologies. Despite the young age and lack of cardiovascular risk factors, acute coronary and aortic syndromes remain high on the initial differential diagnosis, particularly considering the ECG changes. The elevated blood pressure raises the index of suspicion for aortic dissection. The reported palpitations necessitate ongoing telemetry and consideration for an arrhythmia as the etiology of the patient’s presentation. The initial presentation is consistent with an acute coronary syndrome and compels the physician to arrange for emergent coronary angiography. A computed tomography (CT) aortogram would be prudent given that the clinical examination is insufficiently sensitive to rule out aortic dissection. Alternatively, a D-dimer may be considered because of its high sensitivity in both pulmonary embolism and aortic dissection. If these entities are definitively ruled out, there remains a wide differential that falls under the term myocardial infarction with nonobstructive coronary arteries.
Patient Presentation
A CT aortogram and CT pulmonary angiogram are carried out and initially reported as normal. The patient is transferred to the cardiac care unit for ongoing monitoring and management. Shortly after arrival, the patient complains of acute epigastric pain and nausea and develops a wide-complex tachycardia on the monitor. The patient remains alert but endorses palpitations. A 6-mg dose of adenosine is administered with no effect, followed by a second dose of 12 mg that precedes acceleration of the arrhythmia. A 12-lead ECG demonstrates a wide-complex tachycardia with 1 capture beat (Figure 3) before loss of consciousness, no palpable pulse, and development of ventricular fibrillation on the monitor. The patient is electrically defibrillated with successful return of spontaneous circulation and makes a rapid and full neurological recovery and sinus rhythm on the monitor. However, over the subsequent hour the patient requires 4 further electrical defibrillations for ventricular arrhythmias (VAs), despite intravascular treatment with 300 mg amiodarone, 100 mg lidocaine, and 50 μg esmolol.
Figure 3.
Ventricular Tachycardia
Electrocardiogram demonstrating a wide-complex tachycardia with a capture beat consistent with ventricular tachycardia.
Question 2: What is your updated differential diagnosis and next steps in the management?
The recurrent episodes of VAs fit the definition of an electrical storm and provide an etiology for the reported palpitations and ischemia on initial investigations. The ECG in Figure 3 confirms that the arrhythmia has a ventricular origin due to the capture beat. The management of VAs initially follows advanced cardiac life support guidelines with the use of electrical defibrillation, antiarrhythmic agents, and treatment of the “5 Hs and Ts” where appropriate—in this case the hypokalemia may be contributing to the VA and has not yet been treated. Escalation of therapy may include sedation with intubation and ventilation to reduce sympathetic drive. In this case, the underlying etiology of the VA remains unclear; however, an acute coronary syndrome event remains on the differential and thus coronary angiography is indicated. Other important considerations include an arrhythmogenic or structural cardiomyopathy, or an underlying metabolic etiology. One should also consider ingestion of toxic and potentially proarrhythmic agents by the patient.
Patient Presentation
The patient remains in sinus rhythm with no further episodes of VAs. The hypokalemia is treated with 40 mEq of intravenous KCl. Hypertension remains refractory with an arterial line tracing of 210/120 mm Hg despite escalating doses of nitroglycerin infusion up to 200 μg/min. An emergent coronary angiogram is arranged (Video 1) and demonstrates an anomalous right coronary artery (ARCA) arising from the left coronary sinus. There is no atherosclerosis and left ventricular function is preserved on ventriculogram. Medical management is continued with further escalation of antihypertensives, including ongoing nitroglycerin infusion, beta blockers, and angiotensin-converting enzyme inhibitors. Further investigations include a transthoracic echocardiogram, cardiac magnetic resonance imaging scan, and CT coronary angiogram (Video 2), which demonstrates a slit-like orifice of the ARCA with an interarterial course. The diagnosis of ischemia secondary to a malignant course of an ARCA is established, and thus noninvasive functional testing for ischemia was not indicated, and a cardiovascular surgeon is consulted for surgical correction. As part of a workup for secondary causes of hypertension, an abdominal ultrasound demonstrates an incidental mass in the right adrenal region measuring 5 × 4 × 4 cm3. A 24-hour urine specimen is sent to the lab for metanephrines and catecholamines.
Question 3: What are the cardiac implications of an anomalous aortic origin of a coronary artery?
Anomalous aortic origin of a coronary artery (AAOCA) is rare, but increasingly recognized.1
AAOCA may predispose an individual to sudden cardiac death with multiple proposed mechanisms.1 The anomalous artery often takes an acute angle near its ostium, placing it at risk of kinking, particularly when the aorta expands as occurs with exercise. Similarly, increased flow through the aorta or pulmonary artery may compress the artery, particularly if it runs an interarterial or intramural course,2 which may be exacerbated during exercise or hypertension. The 2018 American Heart Association/American College of Cardiology guidelines for the management of adult congenital heart disease recommend surgery for AAOCA in patients with typical angina symptoms, evidence of stress-induced ischemia, or if they have high-risk anatomy such as an intramural course or specific orifice anomalies (Class 1, Level of Evidence: C).3 Surgical options include bypass grafting, reimplantation of the coronary artery, augmentation of the sinus from which the artery arises, or unroofing of the intramural segment.2 Pharmacological options include treatment with a beta-blocker or angiotensin-converting enzyme inhibitor therapy. Sport restriction for individuals with AAOCA is recommended.3
Question 4: What are the cardiac manifestations and management of a pheochromocytoma and how do you work up a pheochromocytoma in a patient who has had a cardiac arrest?
The refractory hypertension and reported incidental adrenal mass on ultrasound strongly suggest a pheochromocytoma as a contributing factor to the patient's presentation. Pheochromocytomas are catecholamine-secreting tumors that arise from the adrenal medulla. Clinical manifestations of pheochromocytoma include hypertension and the classic triad of headaches, sweating, and tachycardia.4 Patients may develop left ventricular hypertrophy secondary to hypertension, dilated cardiomyopathy, stress-mediated cardiomyopathy, and thickening of coronary artery media leading to ischemia, myocarditis, and tachyarrhythmias.5 Pheochromocytomas may be associated with familial pathologies including Multiple Endocrine Neoplasia 2A and 2B syndromes. Screening investigations for a pheochromocytoma include a 24-hour urine fractionated metanephrine and catecholamine test, or a plasma fractionated metanephrine following 30 minutes of rest. Notably, plasma and urine catecholamine levels rise markedly with cardiac arrest and should be evaluated days to weeks after a cardiac arrest. Surgical resection of the pheochromocytoma following optimal medical preparation with combined alpha- and beta-adrenergic blockade is recommended. If a hypertensive crisis occurs, patients may be treated with nitroprusside or phentolamine.
Patient Presentation
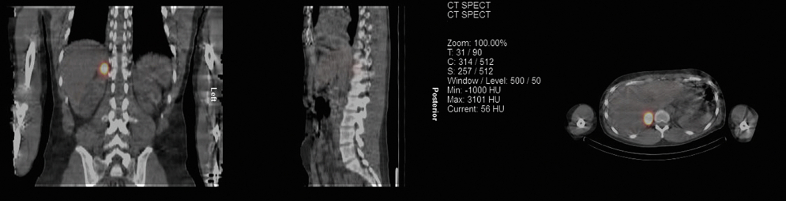
On post admission day 4, the patient is brought to the operating room for surgical correction of the ARCA with stand-by alpha-blockade. On induction with 2 mg midazolam, 100 μg fentanyl, 100 mg propofol, and 100 μg rocuronium, the patient's blood pressure paradoxically increases from 130/64 mm Hg to 198/104 mm Hg. This necessitates treatment with a further 300 mg propofol, 250 μg fentanyl, and eventually 50 mg phentolamine to correct the blood pressure. The surgery continues as planned with no further hemodynamic instability or complications. The surgeon initially dissects the right coronary artery (RCA) from the pulmonary artery and aorta. The RCA is then ligated at the ostium and subsequently spatulated before anastomosis to the right coronary sinus. The patient has a positive postoperative recovery and is extubated on postoperative day 1. Workup for a pheochromocytoma is shown in Table 1. An abdominal CT demonstrates a 5.7-cm right adrenal mass with evidence of hemorrhage (Figure 4). A total body metaiodobenzylguanidine scan demonstrates clear evidence of isolated metaiodobenzylguanidine accumulation in the right adrenal region, consistent with pheochromocytoma (Figure 5). Forty-nine days after RCA reimplantation, the patient undergoes a laparoscopic right adrenalectomy with no complications after optimal medical preparation with combined alpha- and beta-adrenergic blockade. Histopathological assessment shows a well-circumscribed mass surrounded by a thin fibrous capsule (Figure 6). Tumor cells are visualized with morphology of adrenal medullary cells, with foci of hemorrhage. Immunohistochemical assessment demonstrates tumor cells strongly and diffusely positive for the neuroendocrine marker chromogranin (Figure 7), confirming the neuroendocrine nature of the tumor. All features are in keeping with a pheochromocytoma. The patient is reassessed 4 months after initial presentation and has made a full recovery to his baseline physical status.
Table 1.
Results of Laboratory Screening Investigations
| 24-h urine normetanephrines | 4720 nmol/d (ref <300 nmol/d) |
| Normetanephrine/creatinine ratio | 470 (ref <34) |
| 24-h urine metanephrines | 2,508 nmol/d (ref <270 nmol/d) |
| Metanephrine/creatinine ratio | 250 (ref <31) |
| 24-h urine 3-Methoxytyramine | 1,026 nmol/d (ref <380 nmol/d) |
| Urine 3-Methoxytyramine/creatinine ratio | 102.6 (ref <45) |
| Chromogranin A levela | 766 μg/L (ref <89 μg/L) |
Drawn 40 days after initial presentation, others were drawn 4 days after initial presentation.
Figure 4.

Right Adrenal Mass
Computed tomography scan revealing a 5.7-cm right adrenal mass with evidence of hemorrhage (arrow).
Figure 5.
Metaiodobenzylguanidine Accumulation in the Right Adrenal Region
A total body metaiodobenzylguanidine scan demonstrating evidence of accumulation in the right adrenal region.
Figure 6.
Histopathological Assessment of the Pheochromocytoma
Histopathological assessment showing a well-circumscribed mass surrounded by a thin fibrous capsule (red arrow) (Hematoxylin and eosin staining magnification is x4).
Figure 7.

Immunohistochemical Assessment of the Pheochromocytoma
Immunohistochemical assessment demonstrating tumor cells strongly positive for the neuroendocrine marker chromogranin (Magnification is x20).
Question 5: Is it possible to tie together these 2 rare diagnoses with the initial presentation?
Palpation of a catecholamine-releasing mass has been reported to trigger the signs and symptoms of a pheochromocytoma—perhaps in this case the headstand led to a cascade of complications starting with unintended manipulation of the adrenal mass, causing malignant hypertension that obstructed the interarterial coronary artery resulting in ischemia and the electrical storm.
Conclusions
We present a case of synchronous pheochromocytoma and AAOCA presenting with electrical storm. Surgical correction of the AAOCA through reimplantation to the right sinus of Valsalva, and laparoscopic right adrenalectomy were carried out with no major long-term complications. A precise mechanism connecting the pheochromocytoma, AAOCA, and presentation with electrical storm remains speculative; however, it is plausible that the headstand was a trigger leading to a cascade of complications starting with unintended manipulation of the adrenal mass, causing malignant hypertension that obstructed the interarterial coronary artery resulting in ischemia and the electrical storm.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Anomalous Origin of the Right Coronary Artery
Coronary angiogram demonstrating an anomalous origin of the right coronary artery from the left coronary sinus.
Interarterial Course of RCA
CT coronary angiogram demonstrating interarterial course of the RCA between the aorta and pulmonary artery.
References
- 1.Cheezum M.K., Liberthson R.R., Shah N.R., et al. Anomalous aortic origin of a coronary artery from the inappropriate sinus of Valsalva. J Am Coll Cardiol. 2017;69(12):1592–1608. doi: 10.1016/j.jacc.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Peñalver J.M., Mosca R.S., Weitz D., Phoon C.K. Anomalous aortic origin of coronary arteries from the opposite sinus: a critical appraisal of risk. BMC Cardiovasc Disord. 2012;12:83. doi: 10.1186/1471-2261-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):e81–e192. doi: 10.1016/j.jacc.2018.08.1029. [DOI] [PubMed] [Google Scholar]
- 4.Young W. Clinical presentation and diagnosis of pheochromocytoma. In: Post TW, ed. UpToDate, Waltham, MA. Accessed July 28, 2021. https://www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma
- 5.Zipes D.P., Libby P., Bonow R.O., Mann D.L., Tomaselli G.F., editors. founding editor and online editor Eugene Braunwald. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Elsevier/Saunders; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anomalous Origin of the Right Coronary Artery
Coronary angiogram demonstrating an anomalous origin of the right coronary artery from the left coronary sinus.
Interarterial Course of RCA
CT coronary angiogram demonstrating interarterial course of the RCA between the aorta and pulmonary artery.