Summary
Background
The lack of a well-designed brain tumour registry with standardized pathological diagnoses in underdeveloped countries hinders the ability to compare epidemiologic data across the globe. The National Brain Tumour Registry of China (NBTRC), created in January 2018, is the first multi-hospital-based brain tumour registry in China. Patient data reported to the NBTRC in years 2019–2020 were assessed.
Methods
Tumour pathology was based on the 2016 World Health Organization (WHO) classification of tumours of the central nervous system and ICD-O-3. The anatomical site was coded per the Surveillance, Epidemiology, and End Results (SEER) solid tumour module (version of July 2019). The cases were tabulated by histology and anatomical site. Categorical variables were reported as numbers (percentages). The distribution of tumours by age (0–14, 15–19, 20–39, 40–64, and 65+ years) was analysed.
Findings
There were a total of 25,537 brain tumours, foremost among them meningioma (23.63%), followed by tumours of the pituitary (23.42%), and nerve sheath tumours (9.09%). Glioblastoma, the most common and lethal form of primary brain cancer in adults, represented 8.56% of all cases. Of note, 6.48% of the malignant tumours were located in the brain stem. The percentage of malignant brain tumours decreased with increasing age, 24.08% in adults (40+ years), 30.25% in young adults (20–39 years), 35.27% in adolescents (15–19 years), and 49.83% in children (0–14 years). Among the 2107 paediatric patients, the most common sites were ventricle (17.19%), brainstem (14.03%), pituitary and craniopharyngeal duct (13.4%), and cerebellum (12.3%), a distribution that differed from that of the entire cohort. The histology distribution was also unique in children, with glioblastoma much less incident compared to the whole cohort (3% vs. 8.47%, p < 0.01). 58.80% of all patients chose higher-level neurosurgical hospitals outside of their province of residence. The median in-hospital length of stay (LOS) for the various pathologies ranged from 11 to 19 days.
Interpretation
The histological and anatomical site distribution of brain tumours in the NBTRC was statistically different in the subgroup of children (0–14 years). Patient choice of pursuing trans-provincial treatment was common and the in-hospital LOS was longer compared to that reported in similar European and American patient populations, which merits further attention.
Funding
The National Key Research and Development Program of China (2015BAI12B04, 2013BAI09B03, 2014BAI04B01, and 2021YFF1201104) and Chinese National Natural Science Foundation of China (81971668).
Keywords: Brain tumor, Registry, Distribution, China
Research in context.
Evidence before this study
Comprehensively assessing the distribution of all subtypes of brain tumour in a national registry with a substantial number of patients is crucial for overall evaluation of different disease burden. Without regard to language, we searched for articles published before December 1, 2021, using the keywords “brain tumour registry,” “China,” and “distribution” in PubMed and the China National Knowledge Infrastructure. We discovered that there is wide international heterogeneity in the first research of the histological distribution of brain tumours worldwide published in 2021, which hampers the harmonization of data collection. Throughout the years 2000–2014, less than 5000 cases were recorded without information on the disease's pathology, anatomical site, or age distribution. In another pooled analysis of 17 cancer registries in China during 2003–2013, only 10,391 brain tumour cases were reported. Importantly, no details of tumour histology and site was covered in these reports due to the shortage of brain tumour specific registry with standardized and uniform criteria, particularly tumour pathology and exact anatomical site definition.
Added value of this study
Brain tumour histology, anatomical site distribution, and their link with sex and age have been proven by the first multi-hospital-based brain tumour registry (the NBTRC), which has the largest patient volume across China and employs the most recent tumour pathology and anatomical site criteria. The subgroup of children (0–14 years) in the NBTRC had a significantly distinct histological and anatomical distribution of brain tumours. 58.80% of all patients preferred higher-level neurosurgical care located outside of their home province.
Implications of all the available evidence
The distribution of brain tumour reported in the first nationwide brain tumour registry in China can be comparable to that in Europe and the USA in future. This study contributes to the expanding body of information indicating that patients with brain tumour in the subgroup of children (0–14 years) require increased attention given the differences in pathology and anatomical site distribution. The results emphasize the need for more balanced neurosurgical skills development in China's provinces and more efficient patient management after operation.
Introduction
Primary brain tumours represent more than 100 histologically distinct subtypes and cause disproportionately grim cancer prognoses, especially in adolescents and young adults.1, 2, 3, 4, 5, 6, 7 To date, most large-population epidemiologic data in this patient population are available from European and American registries.5,6,8,9 It is important to assess different disease characteristics and burdens by analyzing the clinical data of brain tumour patients from as many geographically and ethnically diverse global regions as possible. However, in underdeveloped countries, where there aren't standard and uniform patient registries, it is challenging to collect and analyze the data of patients from multiple centers. Furthermore, due to the rapid development of molecular characterization of brain tumour pathology, the World Health Organization (WHO) diagnosis criteria are revised every several years, which renders it difficult to compare newly-classified pathologies with those based on prior criteria. As a result, to date there has been no representative nationwide epidemiologic report summarizing all subtypes of brain tumours in China.
Several important issues related to brain tumour epidemiology in China remain to be fully elucidated. For example, the distribution of brain tumours by age group is currently unknown. Because of the uneven development of neurosurgical skills and the increasing urbanization in China, the role of trans-provincial medical care in the field of brain tumour treatment needs to be understood, particularly with respect to patients from rural areas traveling far distances to seek appropriate subspecialty care. Given the current hierarchical medical system in China, knowing how resources are utilized for people with brain tumours in tertiary neurosurgical hospitals would be very important.10,11
Only a well-designed brain tumour registry containing standardized diagnoses based on the current WHO criteria can provide insight into the histologic distribution of brain tumours and relevant patient features in a region as large as China.12 The National Brain Tumour Registry of China (NBTRC) is the first multi-hospital-based brain tumour registry in China that requires patient data to be collected in a manner that is timely, high-quality, complete, and internally consistent13,14 for the express purpose of improving the management of patients with brain tumours in China and thereby increasing the rigor of brain tumour programs across the country.
In this biennial report (2019–2020) of the NBTRC with 25,239 brain tumour patients from 50 registered hospitals across China, we provide a comprehensive and descriptive distribution of brain tumour cases, including histology and anatomical sites, with additional analysis to understand the relationship between tumour type and patient demographic features including age and sex.
Methods
Data source and study population
The study data were obtained from the NBTRC, the hospital-based real-world brain tumour clinical database supported by the Chinese government.13,14 The NBTRC was approved by the ethics committee of the Beijing Tiantan Hospital, Capital Medical University (Beijing, China; KY 2019-124-02). All participating hospitals obtained central IRB approval. Informed consent was obtained from all patients, and the confidentiality and anonymity of medical information was fully guaranteed. Consent for the adolescent patients’ participation was also obtained from their parents or guardians via written consent. As of December 2021, a total of 50 hospitals with advanced neurosurgical skills from 28 provinces/municipalities in China have participated in the NBTRC.
Patients with primary brain tumours diagnosed in hospitals participating in the NBTRC from January 2019 to December 2020 were included in this study, including inpatients and outpatients who were diagnosed with diffuse intrinsic pontine glioma (DIPG) in brainstem based on MRI images, and inpatients with other brain tumours who were histologically proven via surgical resection or biopsy of tumour. The inpatients were included based on the data from hospital admissions, while the outpatients were based on the patients’ images and strictly diagnosed by neuro-specific tumour board.
Data abstraction
The number of cases represents individual tumours but not patients. A patient was defined as a single case if he or she had multiple outpatient visits or hospitalizations for the same diagnosis of brain tumour, while multiple cases were defined if a patient had multiple brain tumour subtypes and went to outpatient visits or hospitalizations multiple times. In this study, a single patient could contribute to multiple counted tumour cases.
Based on the inclusion criteria above, several steps for strict data abstraction were implemented for all brain tumour patients registered from January 2019 through December 2020. Firstly, after examining all brain tumour cases, patients registered several times with the same diagnosis were merged into a single case. Secondly, DIPG patients duplicated due to being included by outpatient and inpatient centers were merged into a single case. Thirdly, patients with different brain tumour diagnoses were registered as multiple cases. The last step was to extract the age, gender, hospital name and province, home province, tumour diagnosis, NBTRC code, anatomical site, tumour laterality, and hospitalization time from each case, for further data analysis.
Classification by histology
The histology coding library of NBTRC combined the 2016 WHO Classification of Tumours of the Central Nervous System15 and ICD-O-3 Histology Validation List (also in SEER system).16 If there was a discrepancy in the term and code of a case's tumour histology between these two systems, then the 2016 WHO code was used. The version of brain tumour histological codes used by the NBTRC in the years 2019–2020 is shown (Supplementary Table S1), alongside those based on the 2016 WHO criteria and ICD-O-3 list mentioned above.
Anatomic location of tumour sites
The specific laterality and anatomical sites used in this report are based on the categories and site codes defined in the SEER Solid Tumour Module (version July 2019).17 However, NBTRC adopted a more comprehensive code (Supplementary Table S2). In brief, anatomical sites were further subdivided and coded with more granularity. For example, brain stem tumours (classified as C717) were sub-classified in the NBTRC as being located in the midbrain (C717.1), pons (C717.2), or medulla oblongata (C717.3). Multifocal tumours were classified by all anatomical sites in which they were present.
Statistical analysis
The characteristics of primary brain tumours were described by histology, malignancy or not, tumour site, and demographic characteristics. Categorical variables were reported as numbers (percentages), and continuous variables were reported as means (SDs) or medians (with interquartile range [IQR]). The Chi-square test was used to compare proportions. A two-tailed test resulting in p < 0.05 was considered a statistically significant finding. The cumulative number of brain tumour patients in each province was mapped in the study period, with a color gradient corresponding to the number of cases in each province. Patients who sought care outside of the province in which they lived (trans-provincial care) were tabulated in their home province, not the one in which they received care.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc). The spatial map analysis was performed using ArcGIS 10.7 (Esri Inc, Redlands, CA, USA).
Role of the funding source
The funders do not have any role in study design, data collection, data analysis, interpretation, writing of the report.
Results
Overview and distribution of brain tumours by province
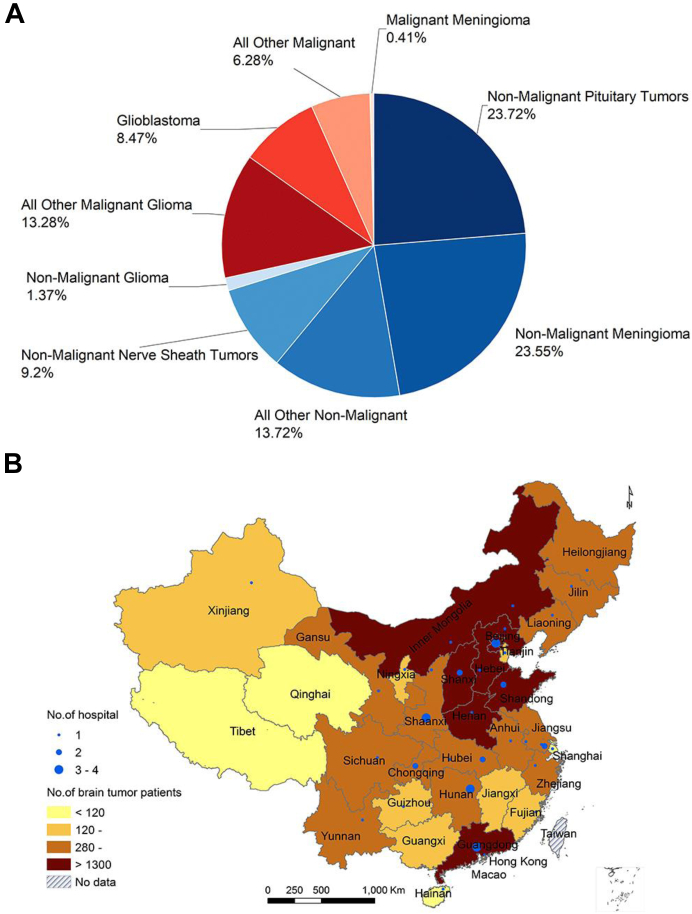
This study included 25,537 brain tumour cases from 25,223 individuals treated at 50 participating hospitals. Of these 25,223 individuals, there were 213 individuals (0.84%) that contributed information to multiple cases (≥2). Malignant tumours accounted for 28.44% of cases and non-malignant tumours accounted for 71.56% of cases (Fig. 1A). The patients were from 50 hospitals located in 33 provinces, and the spatial distribution of brain tumour patients by home province is illustrated (Fig. 1B, and Supplementary Table S3).
Fig. 1.
The distribution of brain tumor patients by A) tumor type and B) province.
Distribution of brain tumours by histology and site
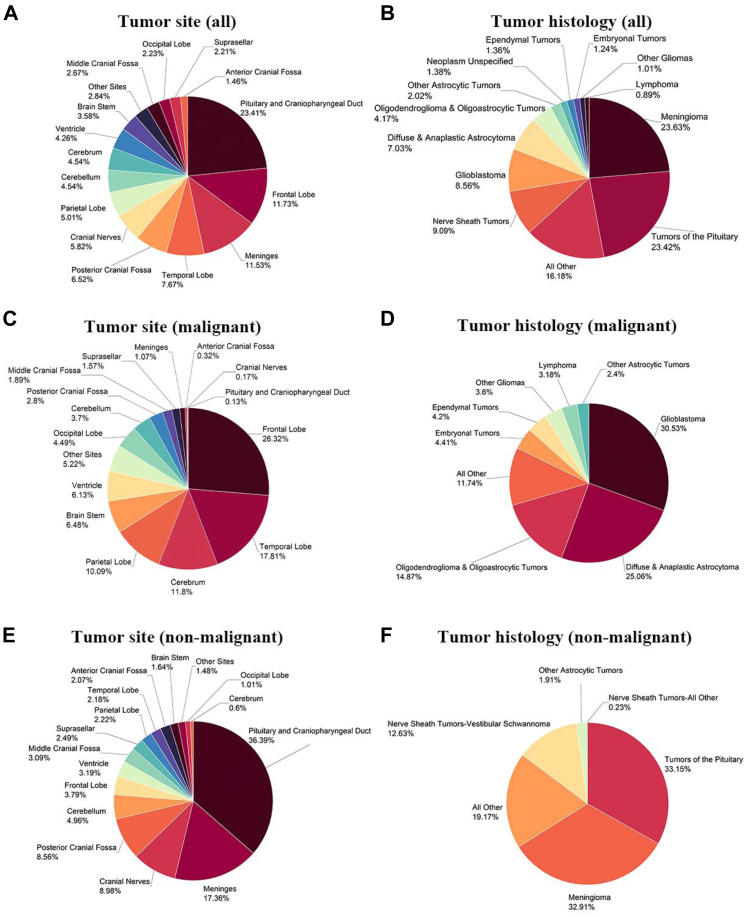
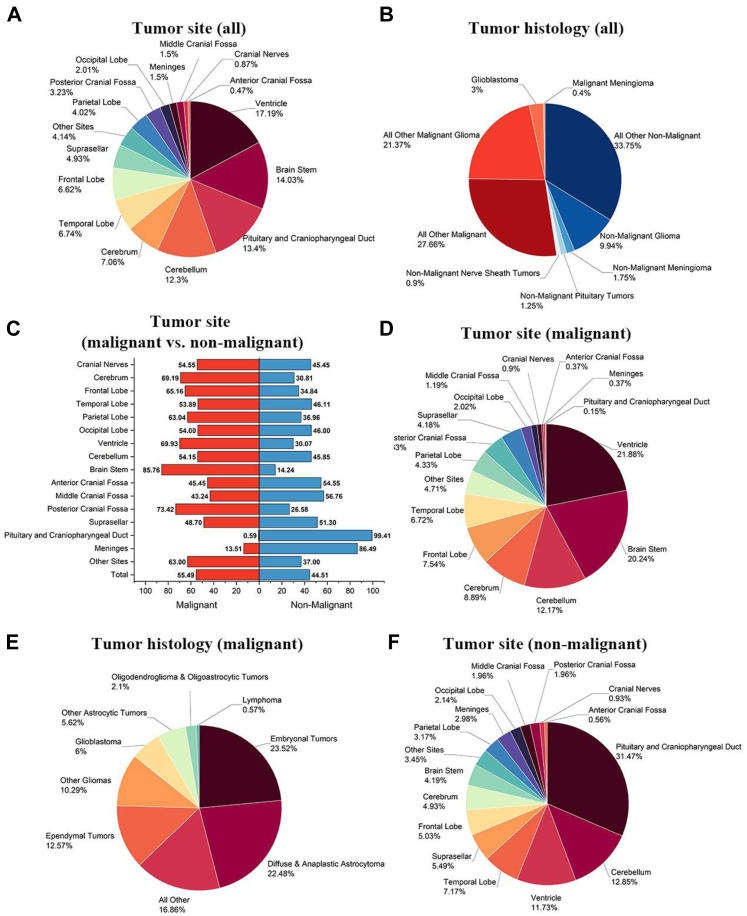
The distribution of all brain tumours by malignancy status, anatomic site, and histology is shown (Fig. 2A–F, Supplementary Tables S4 and S5). The tumours of the pituitary gland and craniopharyngeal duct had the highest incidence (23.41%), followed by frontal lobe (11.73%) and meninges (11.53%) (Fig. 2A). In regard to histology, meningioma was the most common (23.63%), followed by tumours of the pituitary (23.42%) and nerve sheath tumours (9.09%). Glioblastoma, the most common and lethal form of primary brain cancer in adults, constituted 8.56% of all cases (Fig. 2B). For malignant tumours, anatomical location was predominantly in the cerebrum (26.32% frontal lobe, 17.81% temporal lobe, 10.09% parietal lobe, and 4.49% occipital lobe). Of note, 6.48% of malignant brain tumours were located in the brain stem (Fig. 2C). Among malignant cases, glioblastoma was the most common (30.53%), followed by diffuse and anaplastic astrocytoma (25.06%) and oligodendroglioma and oligoastrocytic tumours (14.87%). Primary central nervous system lymphoma (PCNSL), thought to be a rare brain tumour, represented 3.18% of the malignant brain tumour cases (Fig. 2D). The anatomical distribution of non-malignant brain tumours was different; pituitary gland and craniopharyngeal duct were the most common sites (36.39%), followed by meninges (17.36%) and cranial nerves (8.98%) (Fig. 2E). Tumours of the pituitary (33.15%), meningioma (32.91%), and nerve sheath (12.63%) comprised the most common non-malignant brain tumours (Fig. 2F).
Fig. 2.
Distribution of brain tumors reported to NBTRC by type, anatomical site and histology, 2019–2020. A) all brain tumors by anatomical site, B) all brain tumors by histology, C) malignant brain tumors by anatomical site, D) malignant brain tumors by histology, E) non-malignant brain tumors by site, and F) non-malignant brain tumors by histology. Percentages may not add up to 100% due to rounding.
Distribution of malignant and non-malignant tumours based on anatomical site
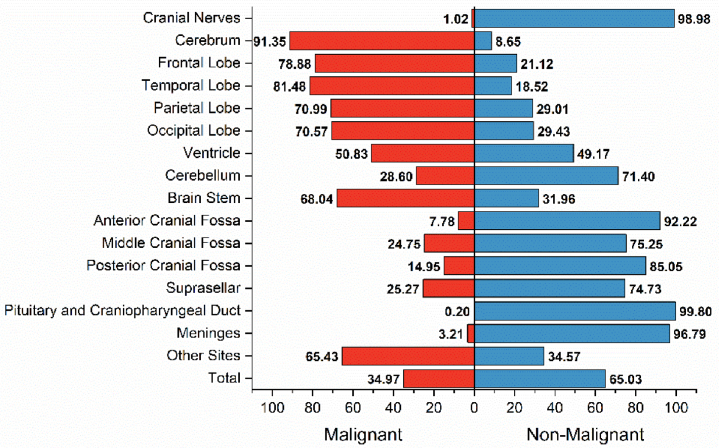
Understanding the anatomical sites where malignant and non-malignant brain tumours are likely to be located is important to construct an accurate differential diagnosis based on neuroimaging findings (e.g., MRI and CT). The incidence of malignant brain tumours by anatomic site was 81.48% (temporal lobe), 78.88% (frontal lobe), 70.99% (parietal lobe), 70.57% (occipital lobe), 68.04% (brainstem), 28.60% (cerebellum), cranial nerves (1.02%), and pituitary gland or craniopharyngeal duct (0.20%) (Fig. 3). For several extracerebellar sites, non-malignant brain tumours were prone to occur in meninges (96.79%), anterior cranial fossa (92.22%), posterior cranial fossa (85.05%), middle cranial fossa (75.25%), and suprasellar (74.73%).
Fig. 3.
The distribution of malignant and non-malignant tumors based on anatomical site.
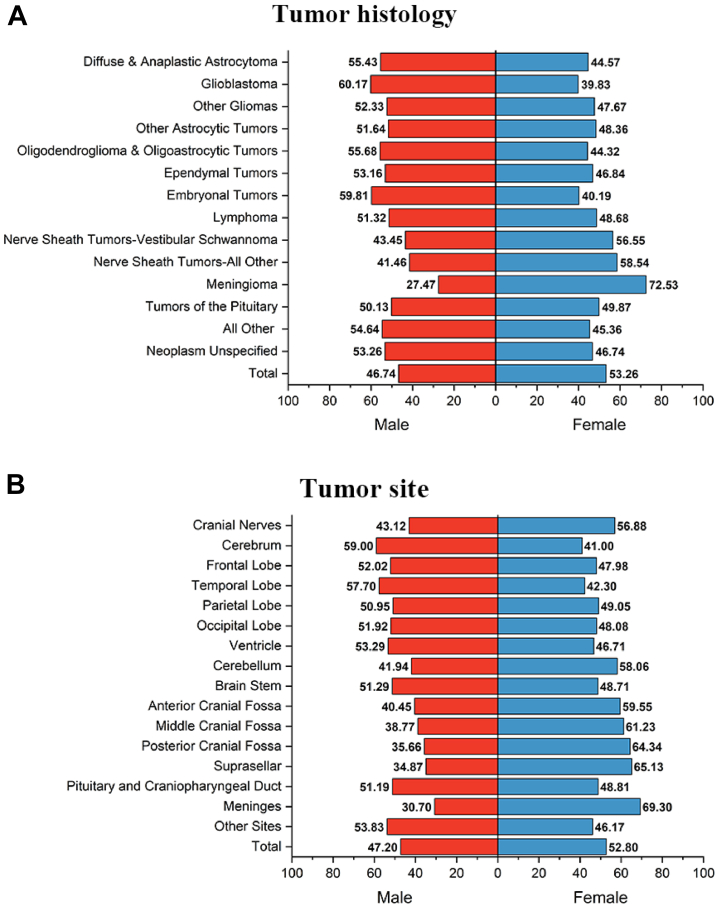
Distribution of brain tumour histology and sites by sex
The distribution of brain tumour histology by patient sex is shown (Fig. 4, and Supplementary Table S5). Overall, there were more female patients in this cohort than male patients (53.26% vs. 46.74%). The proportion of malignant tumours in female and male patients was 22.95% and 34.72%, respectively. The neuroepithelial tumours were more common in males than females (respectively 30.62% vs 20.74%). In contrast, two predominate nonmalignant subtypes, nerve sheath tumours and meningioma, have different trends with more females than males (respectively 56.55% and 72.53% in females). Glioblastoma, the most malignant neuroepithelial tumour, in male patients occupied 60.17%, reflecting the higher risk in males than females (39.83%). PCNSL and tumours of the pituitary were similar in males and females (respectively, 51.32% vs. 48.68%, 50.13% vs. 49.87%).
Fig. 4.
The distribution ofA)brain tumor histology andB)anatomical site by sex.
Distribution of brain tumour histology by age and specific characteristics in children
The proportion of tumour histology (malignant or non-malignant) varied by age subgroup (Table 1). The percentage of malignant brain tumours gradually increased with the decreasing age, 24.08% in adults (age 40+ years), 30.25% in young adults (20–39 years), 35.27% in adolescents (15–19 years), and 49.83% in children (0–14 years). This trend demonstrated that the proportion of malignant brain tumours significantly increased with decreasing patients’ age.
Table 1.
The proportion of tumour histology and malignancy status by age subgroup.
| Age group | Total | Malignant, n (%) | Non-malignant, n (%) | Neoplasm unspecified, n (%) |
|---|---|---|---|---|
| 0–14 Years | 2107 | 1050 (49.83) | 953 (45.23) | 104 (4.94) |
| 15–19 Years | 584 | 206 (35.27) | 359 (61.47) | 19 (3.25) |
| 20–39 Years | 5943 | 1798 (30.25) | 4066 (68.42) | 79 (1.33) |
| 40–64 Years | 14,335 | 3452 (24.08) | 10,760 (75.06) | 123 (0.86) |
| 65+ Years | 2568 | 657 (25.58) | 1883 (73.33) | 28 (1.09) |
| Total | 25,537 | 7163 (28.05) | 18,021 (70.57) | 353 (1.38) |
Therefore, more details were analyzed from the 2107 patients in the pediatric (age 0–14 years) subgroup of patients (Fig. 5). The most common anatomic sites were ventricle (17.19%), brainstem (14.03%), pituitary and craniopharyngeal duct (13.40%) and cerebellum (12.30%), which differed markedly from site distribution among the entire cohort (Fig. 5A). It is worth noting that the histology distribution was also distinct in this age group. Among malignant brain tumour entities, glioblastoma was much less incident in the 0–14 year age subgroup compared to the entire cohort (3% vs. 8.47%, p < 0.01), however, all other malignant or gliomas occupied the most part of malignant histology (Fig. 5B). The most common anatomical sites for malignant brain tumours in this age subgroup were brainstem (85.76%), suprasellar (48.70%), and meninges (13.51%). Of note, the cerebellum and posterior cranial fossa were the two sites where malignant brain tumours were prone to occur (54.15% and 73.42%, Fig. 5C), which is significantly different from the findings in the whole cohort (42.73% and 24.65%). Among the pediatric patients with malignant brain tumour histology, the most common anatomic sites were ventricle (21.88%), brainstem (20.24%), and cerebellum (12.17%), however much less common in frontal, temporal, parietal, and occipital lobes (respectively 7.54%, 6.72%, 4.33% and 2.02%) (Fig. 5D). The most common histological diagnoses were embryonal tumours (23.52%), diffuse and anaplastic astrocytoma (22.48%), and ependymal tumours (12.57%) (Fig. 5E). For the non-malignant brain tumour entities, the most common sites were the pituitary gland or craniopharyngeal duct (31.47%), cerebellum (12.85%), and ventricle (11.73%) (Fig. 5F).
Fig. 5.
The distribution of brain tumors in the subgroup of children aged 0–14 years (N = 2107). A) distribution of tumor anatomical sites; B) distribution of tumor histology including malignant and non-malignant tumors; C) distribution of malignant and non-malignant tumors based on the anatomical sites; D) the tumor sites of malignant tumors; E) the tumor histology of malignant tumors; F) the tumor sites of non-malignant tumors.
Patients’ preference for trans-provincial treatment by tumour histology and anatomic site
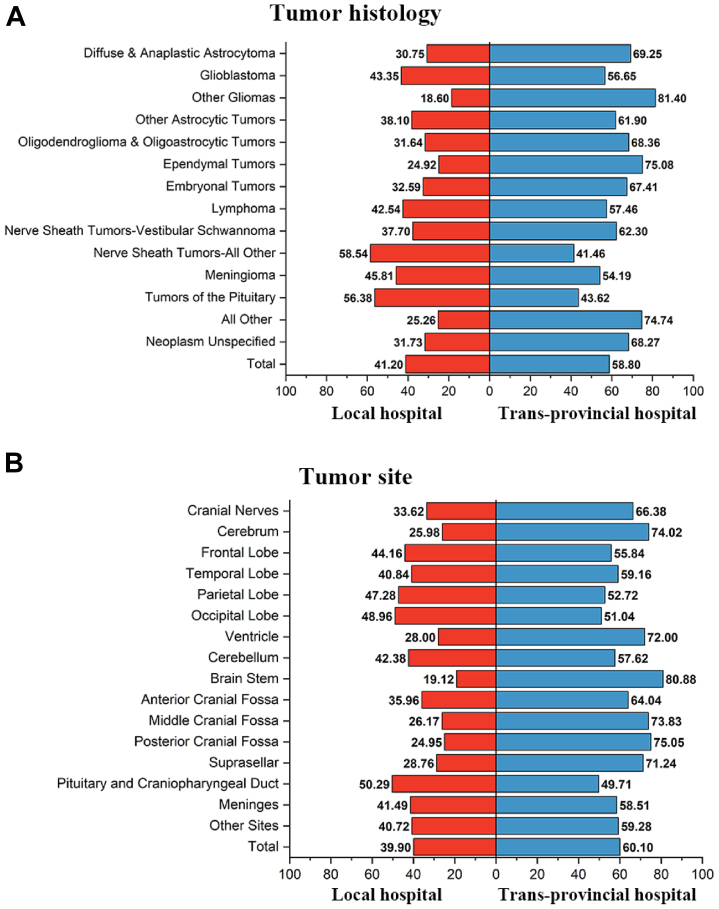
Due to the imbalanced development of neurosurgical competencies across China, 58.80% of the patients chose higher-level neurosurgical hospitals outside of their home province (Fig. 6A). With regard to tumour histology, patients with ependymal tumours, embryonal tumours, and gliomas more often sought trans-provincial treatment (respectively, 75.08%, 67.41%, and 64.24%). For patients with vestibular schwannoma, one of the most challenging nerve sheath tumours, more than 62% of patients sought surgery in neurosurgical hospitals outside of their home province. However, 56.38% of patients with tumours of the pituitary gland received treatment in local hospitals rather than seeking trans-provincial care. With regard to anatomical sites, the deep locations that pose challenging surgical approaches resulted in patients with tumours in these regions seeking trans-provincial care: brainstem (80.88%), posterior cranial fossa (75.05%), middle cranial fossa (73.83%), ventricle (72.00%), and anterior cranial fossa (64.04%) (Fig. 6B).
Fig. 6.
Patients' preference for trans-provincial treatment by tumor histology (A) and anatomical site (B).
In-hospital length of stay by tumour histology and anatomic site
In-hospital length of stay (LOS) can be used as an indicator of disease burden, neurosurgical competency, and hospital efficiency. Patients with pituitary tumours and lymphomas had the shortest median LOS with 11 and 12 days, respectively. The patients with ependymal tumours, embryonal tumours, and nerve sheath tumours-all other experienced a longer median LOS (19, 18, and 19 days, respectively) (Supplementary Table S6). With regard to anatomical site, patients with tumours located in the pituitary gland or craniopharyngeal duct had a median LOS of 12 days, while those with deep periventricular tumours had a median LOS of 18 days (Supplementary Table S7).
Discussion
The distribution of brain tumour histology and anatomical sites in Chinese patients has been reported in this annual report of the NBTRC, which is the first multi-hospital-based brain tumour registry across China. This report can be used as an important reference for policymakers, hospital managers, and physicians (neurosurgeons, radiation oncologists, medical neuro-oncologists, neuropathologists, and neuroradiologists) in China to efficiently allocate relevant resources as well as for international scholars to permit comparisons with similar large registries. The relationship between brain tumour histology and sex or age has also been demonstrated. Specific characteristics and distribution of brain tumours in children aged 0–14 years are similar to those found in CBTRUS and Canadian tumour registry.6,9 More interestingly, several differences were found by comparing the abovementioned data to those reported by CBTRUS, including a smaller percentage of glioblastoma (30.5% vs. 59.2%) but a larger proportion of diffuse and anaplastic astrocytoma (25.1% vs. 13.7%), and a smaller percentage of meningioma (32.9% vs. 55.4%).18 The caveat is that the NBTRC is not a population-based registry, so incidences cannot be derived or compared with those of other countries. The further development of the nationwide NBTRC will permit such comparisons of brain tumour percentages across ethnicities and the conduct of in-depth research into the possible mechanisms underlying any such differences.
The NBTRC has been able to register the largest volume of brain tumour patients with standard and structured information in China owing to sponsorship of the platform by the Chinese government and initial construction by dozens of top-tier neurosurgical hospitals across the country. In a report covering the decade 2003–2013, 17 cancer registries in China reported 10,391 brain tumour cases.3 On the platform of NBTRC, an annual average of 12,768 cases of brain tumour had been registered during 2019–2020, which was roughly 10 times the number of cases reported in the previous decade. More importantly, no details of tumour histology and site was covered in the prior 10-year cancer registry, because the tenth edition of the International Classification of Diseases (ICD-10) was used for classification. Another advancement of the NBTRC database is the inclusion of histological subtypes, which was not as frequently reported in the previous Chinese registry's report.3,19
The complexity of brain tumour histology and site made registration in the entire spectral cancer system challenging. A global study with more than 600,000 brain tumour cases in 60 countries aimed to analyze the histology distribution, however, the proportion of tumours with unspecified histology accounted for 65% in adults and 52% in children.5 The researchers advocated to improve data quality and harmonized data collection worldwide because of wide international variation. Compared to the report,6,19 more accurate information with specified histology and detailed anatomical locations of brain tumour is available on the platform of NBTRC, even with some rare tumours such as diffuse midline glioma.
Using the most scientific coding system of tumour pathology and anatomic sites for registry is important to analyze the brain tumour distribution and understand the patient characteristics. Take meningiomas as an example: they can grow in multiple anatomical locations (e.g., ventricles; anterior, middle, or posterior cranial fossa) besides those pre-specified by the NBTRC code for anatomical sites of the meninges (e.g., convexity, falx cerebri, parasagittal, tentorium, sphenoid wing, cavernous sinus, etc.). This is illustrated by the discrepancy between the percentage of meningiomas (32.91%) and the percentage of tumours located in the meninges (17.36%) (Fig. 2). This is due to the most updated revision of brain tumour pathology diagnosis and anatomic sites based on the 2016 WHO Classification of Tumours of the Central Nervous System and the ICD-O-3 coding. Another advancement of the NBTRC is additional coding of existing site codes by sub-regions of anatomical sites, from the perspective of clinical research. For example, brainstem glioma can have a few categories of glioma in different more specific sites, such as diffuse intrinsic pontine glioma (DIPG) with very poor survival and exogenous medulla glioma with excellent survival after successful operation.20 It is convinced that only a well-designed brain tumour patients’ registry can provide more details on brain tumour patient characteristics as well as the disease burden.
The fact that 58.80% of the registered patients chose trans-provincial treatment outside their residential provinces can be explained by the following reasons. Firstly, the majority of patients can afford treatment in higher-level hospitals for two probable causes: 1) people have become wealthier as a result of the rapid economic development of China in decades11; 2) the proportion of out-of-pocket expenses has declined due to the comprehensive universal health insurance coverage.21,22 Secondly, the extraordinary pace of urbanization and increasingly popular internet provide access to obtain information on brain tumour-relevant strength of specific hospitals (such as neurosurgical ranking overall China).23,24 Thirdly, the development of neurosurgery focusing on brain tumours is uneven across provinces. Hospitals with a long history and more neurosurgeons in big cities may have more advantages in treating difficult brain tumours.25,26 In this report, treatment in more challenging deep-anatomic sites is assumed to be a more difficult surgical approach, resulting in a higher likelihood for patients to seek and receive trans-provincial treatment. Addressing these potential influences on treating brain tumours will require further strengthening of the unified training of neurosurgeons and promotion of the homogeneity of medical quality across provinces.22
The cost-efficiency of brain tumour treatment in China should be improved by this report on a multi-hospital registry. The median LOS for each tumour histology ranged from 11 to 19 days, which was significantly longer than that reported internationally. For example, the average LOS was 4.58 days in 48 consecutive patients with glioblastoma, which is similar in other cohorts.27,28 This important indicator, LOS, reflects how many resources brain tumour patients need to occupy in high-level hospitals with the capability of neurosurgical operations. Because of some potential postoperative complications, nearly half of the patients were discharged to short-term skilled nursing facilities or rehabilitation centers.27 Longer LOS for brain tumour patients in this study may be explained by multiple factors, including the need for (1) improved coordination between various inpatient departments (e.g., neurosurgery, medicine, etc.), (2) national guidelines and high-quality facility for the postoperative management pathways for patients with brain tumours. On the other hand, policy focus requires a shift, likely designing a reasonable hospital reimbursement system according to the in-hospital stage (preoperative, perioperative, or rehabilitation) to guide patients to transfer out of high-level hospitals as soon as possible. In future, the cost-efficiency for brain tumour treatment in China should be strengthened and further analyzed in this well-designed multi-hospital brain tumour registry after policy reform.
There are several limitations in this report. Firstly, lack of differentiation between newly diagnosed and recurrent tumour classification, different subtypes of patients may have different characteristics and disease burdens. The structured item relevant to newly diagnosed vs. recurrent tumours will be implemented in the future. Secondly, a short time span of 2 years was analyzed here. Future comprehensive reports will cover a longer time period (e.g., 5 years) while also permitting the reporting of interim findings from subpopulations within the database. Thirdly, there are currently 54 advanced neurosurgical hospitals that are part of the registry. The representative level of the real situation of brain tumours in China as a whole should be more accurately reflected by recruiting more centers to the registry.
In conclusion, this report is based on the first multi-hospital brain tumour registry in China and contains the largest sample size to date with detailed pathology diagnosis and anatomic sites. The case registration methodology and quality controls enabled the reporting of anatomical distributions of brain tumour subtypes. More than half of patients pursued trans-provincial treatment, and longer in-hospital LOS compared to Europe and the USA suggests room for improvement in coordination between inpatient departments and the formulation of national guidelines for postoperative management of patients in China. Future research relevant to pediatric vs. adult patients, newly-diagnosed vs. recurrent cases, and survival analyses will be conducted in the NBTRC to better characterize and assess the state of brain tumour diagnosis and management in China.
Contributors
DX, DL, TX, NJ, WJ, and LZ designed and managed the NBTRC. DX, TX, and GS did data analyses. DX, DL, TX, CBP, and LZ designed the pictures and wrote the first draft of the manuscript. DX, CY, DL, NJ, WJ, and LZ provided critical comments on the interpretation of all results and participated the revision of the manuscript. XL, DZ, GH, JX, ZH, AW, CM, JL, KS, HJ, NW, GC, JY, HM, ZL, XS, YQ, ZL, XJ, CT, SN, RZ, LC, MG, MW, XJ, GG, ZH, CZ, TZ, CD, LC, PW, JS, XW, JY, YW, NW, RZ, MZ, YH, JG, YL, YP, and BZ, as members of consecutive committee in NBTRC, supervised the data control of every participating neurosurgical center and supervised the overall conduct of this study. All authors had full access to all data in the study and are responsible for reviewing and submitting the manuscript.
Data sharing statement
Data for this study are available upon reasonable request from the principal investigator.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
CP reports grants from American Association for Cancer Research (AACR), grants from The Robert and Janice McNair Foundation, grants from the University of Texas MD Anderson Cancer Centre SPORE Program, grants, personal fees and non-financial support from Novocure Ltd., outside of the submitted work. In addition, CP has a patent using alternating electric fields to increase cell membrane permeability (us PATENT 11103698 B2) issued, and a patent Methods of Normalizing Aberrant Glycolytic Metabolism in Cancer Cells (US patent application US 2021 0199640 A1) with royalties paid and Foreign Senior Advisor of the National Brain Tumor Registry of China (NBTRC). The NBTRC is an initiative sponsored by the China National Clinical Research Center for Neurologic Diseases with the support of the Chinese government. This is an unpaid advisory role in which CP provides guidance on the development of the platform. Deling Li reports a patent issued entitled with novel method for data integration and classification, related to this work and was developed during the NBTRC platform construction. The rest of the authors declare no competing interests.
Acknowledgements
The construction of NBTRC is supported by the following grants including: Chinese National Natural Science Foundation (81971668), the National Key Research and Development Program of China (2015BAI12B04, 2013BAI09B03, 2014BAI04B01, and 2021YFF1201104), and Clinical Scientist Supporting grant of Beijing Tiantan Hospital (YSP201902). China Health Group is the Contract Research Organization for third-party data quality supervision and course management for the NBTRC, guided by Dr. Xuemei Song. United Imaging Healthcare Data Services is the information technique supporting company, which is responsible for constructing the NBTRC website and database, as well as ensuring the security of website and data.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100715.
Appendix A. Supplementary data
References
- 1.Kruchko C., Ostrom Q.T., Gittleman H., Barnholtz-Sloan J.S. The CBTRUS story: providing accurate population-based statistics on brain and other central nervous system tumors for everyone. Neuro Oncol. 2018;20(3):295–298. doi: 10.1093/neuonc/noy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom Q.T., Gittleman H., Truitt G., Boscia A., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng H., Chen W., Zheng R., et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 4.Ostrom Q.T., Cote D.J., Ascha M., Kruchko C., Barnholtz-Sloan J.S. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4(9):1254–1262. doi: 10.1001/jamaoncol.2018.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girardi F., Rous B., Stiller C.A., et al. The histology of brain tumors for 67 331 children and 671 085 adults diagnosed in 60 countries during 2000-2014: a global, population-based study (CONCORD-3) Neuro Oncol. 2021;23(10):1765–1776. doi: 10.1093/neuonc/noab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller K.D., Ostrom Q.T., Kruchko C., et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021;71(5):381–406. doi: 10.3322/caac.21693. [DOI] [PubMed] [Google Scholar]
- 7.Wanis H.A., Moller H., Ashkan K., Davies E.A. The incidence of major subtypes of primary brain tumors in adults in England 1995-2017. Neuro Oncol. 2021;23(8):1371–1382. doi: 10.1093/neuonc/noab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng S., Zouaoui S., Bessaoud F., et al. An epidemiology report for primary central nervous system tumors in adolescents and young adults: a nationwide population-based study in France, 2008-2013. Neuro Oncol. 2020;22(6):851–863. doi: 10.1093/neuonc/noz227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker E.V., Davis F.G., affiliates Cf. Malignant primary brain and other central nervous system tumors diagnosed in Canada from 2009 to 2013. Neuro Oncol. 2019;21(3):360–369. doi: 10.1093/neuonc/noy195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Sun L., Hou J. Hierarchical medical system based on big data and mobile internet: a new strategic choice in health care. JMIR Med Inform. 2017;5(3):e22. doi: 10.2196/medinform.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang G., Wang Y., Zeng Y., et al. Rapid health transition in China, 1990-2010: findings from the global burden of disease study 2010. Lancet. 2013;381(9882):1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnholtz-Sloan J.S. Brain and central nervous system tumor statistics: access to accurate data for all countries is critical! Neuro Oncol. 2019;21(3):291–292. doi: 10.1093/neuonc/noy205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Jia W., Ji N., Li D., Xiao D. Establishment of the National Brain Tumor Registry of China. JCO Glob Oncol. 2020;6:47–48. doi: 10.1200/JGO.19.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L., Jia W., Ji N., et al. Construction of the National Brain Tumor Registry of China for better management and more efficient use of data: a protocol. BMJ Open. 2021;11(1) doi: 10.1136/bmjopen-2020-040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis D.N., Perry A., Reifenberger G., et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 16.ICD-O-3 Coding Materials. https://seer.cancer.gov/icd-o-3/
- 17.July 2019 Revision History for the Solid Tumor Rules. https://seer.cancer.gov/tools/solidtumor/revisions-jul2019.html
- 18.Ostrom Q.T., Price M., Neff C., et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022;24(Suppl 5):v1–v95. doi: 10.1093/neuonc/noac202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W., Zheng R., Baade P.D., et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 20.Leibetseder A., Leitner J., Mair M.J., et al. Prognostic factors in adult brainstem glioma: a tertiary care center analysis and review of the literature. J Neurol. 2022;269(3):1574–1590. doi: 10.1007/s00415-021-10725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao W., Zeng Z., Dang H., et al. Towards universal health coverage: lessons from 10 years of healthcare reform in China. BMJ Glob Health. 2020;5(3) doi: 10.1136/bmjgh-2019-002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yip W.C., Hsiao W.C., Chen W., Hu S., Ma J., Maynard A. Early appraisal of China's huge and complex health-care reforms. Lancet. 2012;379(9818):833–842. doi: 10.1016/S0140-6736(11)61880-1. [DOI] [PubMed] [Google Scholar]
- 23.Cai Z., Chen M., Ye P., Yip P.S.F. Socio-economic determinants of suicide rates in transforming China: a spatial-temporal analysis from 1990 to 2015. Lancet Reg Health West Pac. 2022;19 doi: 10.1016/j.lanwpc.2021.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang T.B., Deng Z.W., Zhi Y.P., Cheng H., Gao Q. The effect of urbanization on population health: evidence from China. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.706982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J.Z., Zhou L.F., Zhou D.B., Tang J., Zhang D. The status quo of neurosurgery in China. Neurosurgery. 2008;62(2):516–520. doi: 10.1227/01.neu.0000316020.28421.18. discussion 20-1. [DOI] [PubMed] [Google Scholar]
- 26.Wu J.S., Zhang J., Zhuang D.X., et al. Current status of cerebral glioma surgery in China. Chin Med J (Engl) 2011;124(17):2569–2577. [PubMed] [Google Scholar]
- 27.Barak T., Vetsa S., Nadar A., et al. Surgical strategies for older patients with glioblastoma. J Neuro Oncol. 2021;155(3):255–264. doi: 10.1007/s11060-021-03862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang O.Y., Clarke R.A., Rivera Perla K.M., Corcoran Ruiz K.M., Toms S.A., Weil R.J. Brain tumor craniotomy outcomes for dual-eligible medicare and medicaid patients: a 10-year nationwide analysis. J Neuro Oncol. 2022;156(2):387–398. doi: 10.1007/s11060-021-03922-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.