Abstract
Perioperative myocardial infarction is a complication of cardiac surgery, and the cause can be multifactorial. Injury of the left circumflex coronary artery has been described, particularly after mitral valve replacement. We present the case of a 72-year-old woman who underwent mitral valve replacement but developed a lesion in the proximal circumflex coronary artery related to partial mechanical kinking caused by a suture. The therapeutic options are surgical or percutaneous. In this patient, the percutaneous strategy was successful.
Learning objective
• Percutaneous coronary intervention is an option in cases involving kinking of the left circumflex coronary artery after mitral valve replacement.
• If unable to cross the lesion with a workhorse guide wire, one alternative is to use wires with good support properties and avoid very high tip loads to reduce the risk of perforation.
In patients at high risk of bleeding, use of a drug-eluting stent and short-duration dual antiplatelet therapy is recommended.
Keywords: Perioperative myocardial infarction, Left circumflex coronary artery, High bleeding risk
Introduction
Perioperative myocardial infarction is a complication of cardiac surgery, and the cause can be multifactorial and may include thrombosis, ligation, tortuosity, or anatomical alteration of the underlying tissues. Injury of the left circumflex coronary (LCx) artery may occur occasionally after mitral valve replacement. We present the case of a patient who developed myocardial infarction after mitral valve replacement. The patient was treated with percutaneous management, which produced adequate clinical and angiographic results.
Case report
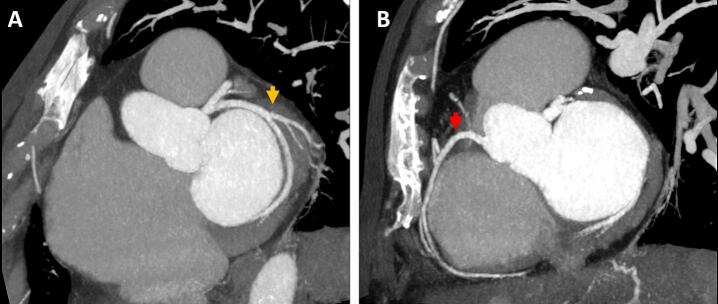
A 72-year-old woman with a past medical history of rheumatic heart disease and mitral valve stenosis underwent percutaneous mitral balloon commissurotomy 40 years before attending our clinic. She was referred to our cardiology outpatient department for elective mitral valve replacement with an STS score of 5.1 % predicted mortality, 19.04 % risk for morbidity, and EuroSCORE II of 1.99 % predicted mortality. Transthoracic echocardiography revealed severe mitral stenosis and tricuspid regurgitation without regional wall motion abnormalities. Coronary computed tomography angiography (CCTA) showed no coronary lesions (Fig. 1A and B).
Fig. 1.
Coronary computed tomography angiography and electrocardiogram before cardiac surgery. (A) Normal left circumflex coronary artery (yellow arrow) and (B) right coronary artery (red arrow).
The patient underwent mitral valve replacement using a St. Jude Epic 27 mm prosthesis (St. Jude Medical. Minneapolis, MN, USA). ETHIBOND EXCEL Polyester Suture (2-0) (Ethicon, Inc. Raritan, NJ, USA) was used to attach the protheses to the mitral ring using the parachute technique, and tricuspid valve annuloplasty with a 28 mm ring and left atrial reduction were performed. During surgery, aortic clamping and myocardial anterograde protection using 1000 mL of Custodiol (Custodiol HTK Organ Preservation Solution. Durham, NC, USA) were performed. During cardiopulmonary bypass (CPB), the patient's mean arterial pressure was 70–80 mmHg. After aortic declamping, she exhibited complete atrioventricular block, and a ventricular epicardial pacemaker was placed.
CPB weaning began with volume administration. A transesophageal echocardiogram revealed poor biventricular mobility and decreased left ventricular ejection fraction of 35 % without paravalvular leaks. Dobutamine was started at a dose of 4 μg/kg/min and later increased gradually to 9 μg/kg/min. Norepinephrine was started at a dose of 0.05 μg/kg/min and was increased to a maximum of 0.2 μg/kg/min. However, these treatments did not increase the mean arterial pressure to >60 mmHg, and vasopressin was added at a dose of 0.02 U/min and increased to 0.06 U/min.
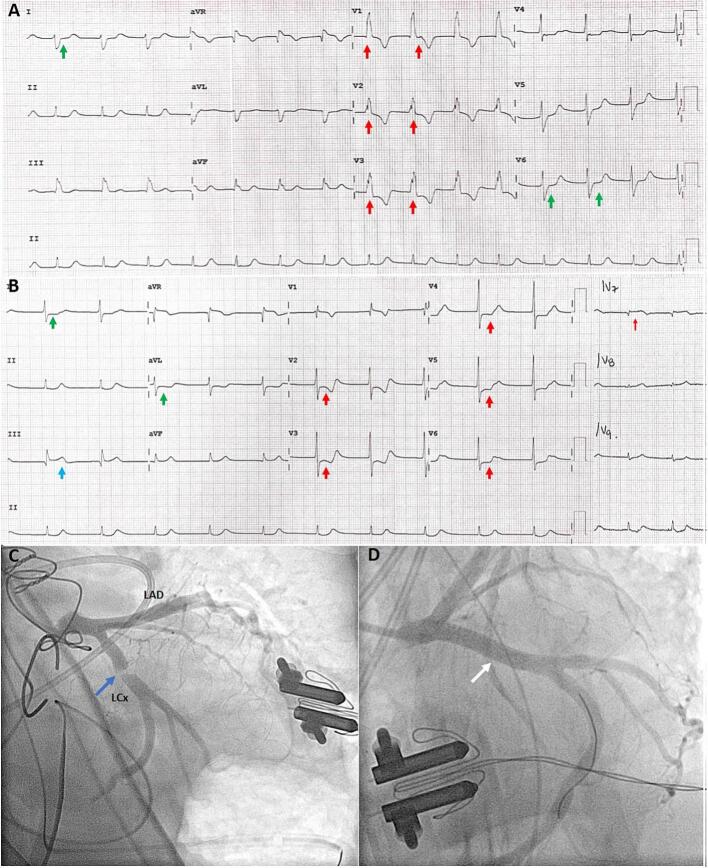
Given the patient's ventricular dysfunction, the coronary ostia were checked by the surgeon and found to be permeable. The patient's mean arterial pressure was maintained above 65 mmHg with adequate acid–base and electrolyte status, and CPB was suspended. She was transferred to the cardiovascular critical care unit. On arrival, the initial electrocardiogram (ECG) showed right bundle branch block (Fig. 2A).
Fig. 2.
Presurgical and postsurgical electrocardiogram and coronary angiography including percutaneous intervention. (A) Electrocardiogram before surgery. Right bundle branch block (QRS duration 80 ms), notched R wave pattern in V1–V3 (red arrows), and S wave >40 ms in leads I and V6 (green arrows). (B) Electrocardiogram after cardiac surgery. ST segment depression in V2–V6 (red arrows), I, and aVL (green arrows), and ST segment elevation III (light blue arrow). ST segment elevation in V7 (red arrow). (C) Lesion in the proximal left circumflex coronary artery (LCx) (blue arrow), left anterior descending artery (LAD) without lesions. (D) Stent placement and final result (white arrow).
The hemodynamic profile showed cardiac output of 1.5 L/min, cardiac index of 1.1 L/min/m2, systemic vascular resistance of 5100 dyn/s/cm−5, pulmonary capillary wedge pressure of 10 mmHg, and central venous pressure of 5 mmHg. Levosimendan infusion was initiated but did not improve the patient's cardiac output. A transthoracic echocardiogram showed biventricular dysfunction and a left ventricular outflow tract velocity time integral of 7 cm. The high-sensitivity troponin I level was 4474 pg/mL, and the 3-hour peak was 6220 pg/mL. The creatine phosphokinase (CK) concentration was 850 U/L, CK-MB concentration 115 ng/mL, hemoglobin concentration 8.7 g/dL, and platelet count 65 × 103/μL. A second ECG showed a posterior myocardial infarction (Fig. 2B).
Emergency coronary angiography revealed a subocclusive lesion in the proximal LCx artery probably because of partial mechanical kinking caused by a suture, which compromised flow. Both surgical (EuroSCORE II 20.3 %, STS 21.1 %) and bleeding (ARC-HBR 5 major criteria, PRECISE DAPT 55) high risk were documented. Given the absence of total occlusion of the LCx artery, percutaneous coronary intervention (PCI) was decided as the initial strategy.
To achieve lesion crossing, a Sion Blue workhorse guide wire (Asahi Intecc USA, Inc. Orange County, CA, USA) was used. Balloon angioplasty was performed with luminal gain and conserved flow. A 4.5 × 22 mm Resolute Onyx drug-eluting stent (Medtronic, Inc. Minneapolis, MN, USA) was deployed in the ostial-proximal segment, and a final flow grade of thrombolysis in myocardial infarction III was attained (Fig. 2C and D; Video 1).
Discussion
Injury to the LCx artery during mitral valve surgery is a life-threatening complication that can be caused either by direct injury by the suture or traction of surrounding tissue. The incidence of this injury is 0.5 to 1.8 % [1], and the risk is closely related to the proximity of the LCx artery to the mitral annulus, especially near the anterior commissure. The risk of injury to the LCx artery is higher in patients with left-dominant coronary circulation [2]. The LCx artery is prone to injury because of its variable anatomy, as seen in this patient, whose injury was probably caused by a poorly placed suture close to the posterior mitral annulus.
In more stable patients, for whom the diagnosis is more elusive, computed tomography coronary angiography (CTCA) may be useful for identifying the mechanism and ruling out other differential diagnoses. The role of CTCA in our patient was limited to diagnosing the clinical instability caused by cardiogenic shock. Because sufficient evidence was obtained from the ECG, a bedside echocardiogram was obtained to diagnose the perioperative myocardial infarction, and the patient was sent immediately to the cardiac catheterization laboratory for diagnosis.
Treatment depends on the degree of obstruction and the mechanism of injury to the artery. In cases of total occlusion, the surgical treatment includes repositioning of the annuloplasty ring or valve and/or coronary artery bypass grafting. PCI is an option in cases of artery kinking causing partial occlusion and a risk of rupture during LCx dilatation. Similar success rates have been reported with both therapeutic strategies (87 % vs. 81 %) [3], [4]. In this patient, we were able to cross the lesion easily, possibly because the suture did not exert much traction on the adjacent tissues. In other possible scenarios, we would use a guide extension catheter (e.g. GuideLiner [Teleflex. Morrisville, NC, USA], or Guidezilla [Boston Scientific. Marlborough, MA, USA]) in combination with a microcatheter with a small distal size (e.g. Corsair distal size 1.3 Fr or Caravel distal size 1.4 Fr [both from Asahi Intecc USA, Inc. Orange County, CA, USA]) and a hydrophilic wire (in this order: PILOT 50 [Abbott Laboratories. Chicago, IL, USA], Sion Black [Asahi Intecc USA, Inc. Orange County, CA, USA], Fielder FC or Fielder XT [Asahi Intecc USA, Inc. Orange County, CA, USA] and Choice PT Extra Support [Boston Scientific. Marlborough, MA, USA]) to cross the lesion, increase the support properties, and avoid very high tip loads, which should reduce the risk of perforation.
The decision to use PCI was based on the patient's high surgical risk and the presence of LCx artery kinking with anterograde flow, given that this type of stenosis was secondary to external compression and was not related to intraluminal material. Because the patient had a high bleeding risk, a drug-eluting stent approved for dual antiplatelet therapy was prescribed for 1 month; this stent was also chosen for its rapid healing and wide diameter range. Given the suspicion about the mechanism of injury and the presence of anterograde flow identified by angiography, intravascular imaging was not considered necessary, but it may have a role in other cases by accurately identifying the coronary lesion wall properties and helping to elucidate the mechanism of injury. The patient had a good postoperative course, was discharged from the cardiovascular critical care unit 2 days after PCI, and was placed on dual antiplatelet and anticoagulation therapy for 1 month.
The following is the supplementary data related to this article.
Percutaneous coronary intervention.
Funding sources
No funding.
Patient consent statement
Written informed consent for patient information and images to be published were provided by the patient or a legally authorized representative.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Aybek T., Risteski P., Miskovic A., Simon A., Dogan S., Abdel-Rahman U., Moritz A. Seven years’ experience with suture annuloplasty for mitral valve repair. J Thorac Cardiovasc Surg. 2006;131:99–106. doi: 10.1016/j.jtcvs.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R., Chun P.K., Parker J., McAllister H.A., Jr. Suture obliteration of the circumflex coronary artery in three patients undergoing mitral valve operation. J Thorac Cardiovasc Surg. 1982;84:773–778. [PubMed] [Google Scholar]
- 3.Hiltrop N., Bennett J., Desmet W. Circumflex coronary artery injury after mitral valve surgery: a report of four cases and comprehensive review of the literature. Catheter Cardiovasc Interv. 2016;89:78–92. doi: 10.1002/ccd.26449. [DOI] [PubMed] [Google Scholar]
- 4.Bargagna M., Trumello C., Sala A., Blasio A., Castiglioni A., Alfieri O., De Bonis M. Left circumflex artery injury after mitral valve surgery: an algorithm management proposal. Ann Thorac Surg. 2021;111:899–904. doi: 10.1016/j.athoracsur.2020.05.160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percutaneous coronary intervention.