Abstract
Haemodialysis is life sustaining but expensive, provides limited removal of uraemic solutes, is associated with poor patient quality of life and has a large carbon footprint. Innovative dialysis technologies such as portable, wearable and implantable artificial kidney systems are being developed with the aim of addressing these issues and improving patient care. An important challenge for these technologies is the need for continuous regeneration of a small volume of dialysate. Dialysate recycling systems based on sorbents have great potential for such regeneration. Novel dialysis membranes composed of polymeric or inorganic materials are being developed to improve the removal of a broad range of uraemic toxins, with low levels of membrane fouling compared with currently available synthetic membranes. To achieve more complete therapy and provide important biological functions, these novel membranes could be combined with bioartificial kidneys, which consist of artificial membranes combined with kidney cells. Implementation of these systems will require robust cell sourcing; cell culture facilities annexed to dialysis centres; large-scale, low-cost production; and quality control measures. These challenges are not trivial, and global initiatives involving all relevant stakeholders, including academics, industrialists, medical professionals and patients with kidney disease, are required to achieve important technological breakthroughs.
Subject terms: Haemodialysis, Biomedical engineering
Portable, wearable and implantable artificial kidney systems require compact and efficient dialysate regeneration systems and novel membranes for improved toxin removal and long-term patency. Here, the authors discuss efforts to overcome these challenges and future perspectives for achieving miniaturized dialysis.
Key points
Haemodialysis is expensive and is associated with high patient mortality and poor quality of life; portable, wearable and implantable artificial kidney systems are being developed to improve patient care.
An important challenge for designing portable or wearable artificial kidney systems is the continuous regeneration of a small volume of dialysate; recycling systems based on sorbents have great potential for dialysate regeneration.
Novel dialysis membranes composed of polymeric or inorganic materials are being developed to improve the removal of uraemic toxins, with low levels of membrane fouling.
Bioartificial kidney systems can provide important biological functions and thereby potentially improve patient outcomes; however, their implementation has manufacturing, feasibility and logistics challenges.
Important technological breakthroughs can be achieved via global initiatives involving relevant stakeholders including academics, industrialists, medical professionals and patients.
Introduction
Kidney failure is an increasing public health care problem, with ~4.7 million patients worldwide receiving kidney replacement therapy (KRT) in 2021 (refs. 1–3). The growing number of patients is the result of several factors, including an increased number of people at risk, for example, owing to hypertension4 and/or diabetes mellitus5, an ageing population and events such as the COVID-19 pandemic6. Kidney transplantation is the only curative form of KRT but is often not available or patients are not eligible. Hence, many patients with kidney failure rely on haemodialysis or peritoneal dialysis.
Despite its life-sustaining nature, dialysis results in a considerable burden for patients and is associated with a low quality of life7–9 and high morbidity and mortality10. Haemodialysis is expensive, invasive and offers low patient mobility and autonomy compared with peritoneal dialysis; however, the efficacy of peritoneal dialysis is lower than that of haemodialysis and technique failure is often unavoidable after a few years11. Home dialysis, both home haemodialysis and peritoneal dialysis, offers patients more flexibility, mobility and autonomy than in-centre dialysis and may improve their well-being12,13. In addition, home haemodialysis enables the use of intensive treatment protocols (e.g. 6x2h or 6x8h per week) that are associated with improvements in survival and uraemic symptoms14–16 compared with less intensive protocols (3x4h per week)17, but are difficult to attain with conventional in-centre haemodialysis. Home haemodialysis is also cost-effective when compared with conventional in-centre haemodialysis18, although reimbursement policies vary among countries.
The COVID-19 pandemic and resulting requirement for self-isolation as well as the advent of small, easy-to-use haemodialysis machines that do not require modification of the home to provide a purified water source have led to a resurgence in interest in home haemodialysis. Several devices, including the Physidia S3 (ref. 19), Quanta SC20 and NxStage21, are in clinical use and the DIMI22 device is undergoing clinical testing. Various initiatives to improve and/or miniaturize home haemodialysis devices are currently underway23, including the development of dialysis membranes to enable longer and more effective blood purification, systems to mimic selective ion transport in the nephron24 and sorbents and membranes for dialysate regeneration25. Such regeneration reduces the amount of water that is required for each treatment session and could thereby reduce the ecological impact of dialysis26.
Despite these important advances, few portable artificial kidney (PAK) and wearable artificial kidney (WAK) devices are being developed25. Most of the current devices are fairly bulky (10–30 kg including the dialysate) and further miniaturization and/or the development of implantable devices will require several challenges to be overcome, such as the development of more compact and efficient dialysate regeneration systems, improved toxin removal and long-term maintenance of membrane patency. In this Review, we discuss the current efforts aimed at overcoming these challenges as well as future perspectives for achieving miniaturized dialysis.
Dialysate regeneration
A single 4-h haemodialysis session generally uses 120–150 l of dialysate. The miniaturization of dialysis machines to create an efficient portable or even wearable device will require the development of strategies to enable continuous regeneration of a small volume of dialysate in a closed-loop system (Fig. 1).
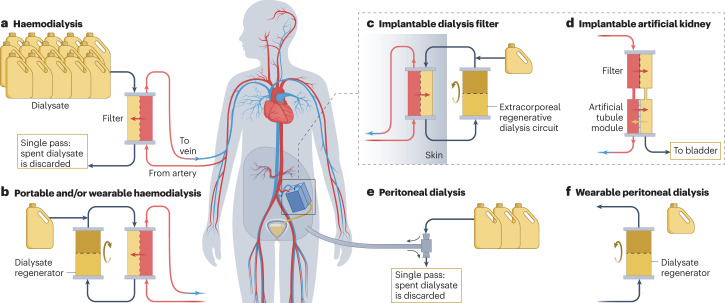
Fig. 1. Dialysis technologies.
a, Single-pass haemodialysis is the most common modality of kidney replacement therapy, but requires very large volumes of dialysate, which limits the portability of the system. b, Portable and/or wearable haemodialysis devices use dialysate regeneration systems based on chemical sorbents, urease, electro-oxidation, photo-oxidation or combinations of these approaches. c, Haemodialysis can also be performed using an implantable dialysis filter (typical Si-wafer based) with an external regenerative dialysate circuit. d, Fully implantable artificial kidneys are also being developed. These systems use a silicon-wafer filter as an artificial glomerulus in combination with an artificial tubule module (which might be a bioreactor or a fully technological approach) that has a urine outlet to the bladder. e, Single-pass peritoneal dialysis also uses large volumes of dialysate (image shows typical tidal peritoneal dialysis). f, Peritoneal dialysis can also be miniaturized using dialysate regeneration systems. This approach is suitable for continuous flow peritoneal dialysis.
Removal of organic solutes and ions
Dialysate regeneration systems commonly use cation exchangers (e.g. zirconium phosphate27 or polystyrene-based resins28) to exchange potassium for other cations (primarily Na+ or H+). Dialysate electrolyte concentrations are kept within an acceptable range through various strategies, such as dilution with sodium-free dialysate (to prevent Na+ release to the patient) and the use of basic anion exchangers that neutralize H+ and release bicarbonate or hydroxide ions (OH−). Ca2+ and Mg2+ depletion is prevented by replenishment from a reservoir or by preloading the cation exchanger with Ca2+ and Mg2+ (ref. 28). Phosphate is removed by anion exchangers such as (hydrous) zirconium oxide27 or polystyrene-based resins with immobilized metal ions such as lanthanum (La3+) or iron ions (Fe2+ or Fe3+)29 that exchange phosphate for a base. This approach may correct metabolic acidosis, which is a common complication of kidney failure owing to impaired excretion of non-volatile acids. Removal of organic uraemic solutes is mainly accomplished by physisorption to activated carbon with a surface area commonly within the range 500–1500 m2/g30. Activated carbon has been reported to adsorb 81% of the organic uraemic solutes that are identified in spent dialysate, including protein-bound solutes31.
Removal of urea
Urea removal is one of the main challenges for dialysate regeneration32. During conventional single-pass haemodialysis, urea is easily removed from the blood to the dialysate compartment via diffusion. By contrast, removal of urea from closed loop dialysate circuits in WAK systems is difficult32. The affinity of activated carbon for urea is fairly low (0.1–0.2 mmol/g at uraemic concentrations32) and urea is fairly unreactive (uncharged at physiological pH and neither very nucleophilic nor electrophilic). Moreover, the daily molar production of urea — the primary nitrogenous waste product — is higher than that of other waste solutes (240–470 mmol per day, depending on protein intake33). Several urea removal methods are available, including enzymatic hydrolysis, electrochemical decomposition and adsorption.
Enzymatic hydrolysis
Urease-catalysed hydrolysis of urea into ammonium and bicarbonate is very efficient. In theory, <1 g of active urease is sufficient for complete urea removal from the dialysate during a 4-h dialysis session in a patient with uraemia32. In practice, urease needs to be immobilized to a solid support and sterilized before use; therefore, ~30–50 g of immobilized urease is typically needed for a dialysis session32. Unfortunately, urea hydrolysis results in the production of ammonium, which is much more toxic than urea. A urea removal strategy based on urease should therefore be complemented by a strategy to remove ammonium.
The first system for dialysis regeneration was the Recirculating Dialysis (REDY) sorbent system, which uses zirconium phosphate to bind ammonium34. However, zirconium phosphate also completely removes calcium, magnesium and potassium ions from the dialysate. These ions need to be re-infused from a separate reservoir, which enables personalization of calcium, magnesium and potassium concentrations in the dialysate but increases the size and weight of the device. The adsorbed cations are exchanged for hydrogen and sodium ions. The released protons (partially) react with bicarbonate generated during urea hydrolysis to form water and carbon dioxide, which can be effectively removed from the dialysate circuit via a degasser. However, sodium release is a concern as higher dialysate sodium concentrations are associated with hypertension and weight gain between dialysis sessions35. To prevent a rise in dialysate sodium concentration, a sodium-free dialysate reservoir could be used to dilute the released sodium, but this approach would be at the expense of miniaturization32. Alternative methods for ammonium capture that minimize Ca2+ and Mg2+ removal and Na+ and H+ release should be explored, such as use of a gas-permeable hydrophobic coating for zirconium phosphate to enable binding of ammonia (NH3)32,36.
Electrochemical decomposition
Electrochemical decomposition of urea into compounds that can be outgassed from the dialysate can be achieved using a compact, lightweight and durable device with reusable electrodes32. During direct oxidation, urea is converted at the anode into N2 and CO2. During indirect oxidation, oxidizing chlorine species such as hypochlorite (OCl−) are generated at the anode and subsequently convert urea into N2 and CO2. To date, no material has been found that is selective for urea oxidation over chloride oxidation at neutral pH; therefore, indirect oxidation of urea occurs under these conditions. The direct oxidation of urea at neutral pH requires large overpotentials37, which is the additional voltage required for the reaction to occur that exceeds the theoretical value. With a higher overpotential, additional reactions are also more likely to occur. For example, reactive chlorine species such as chloramines and chlorine can be formed owing to the abundant presence of readily oxidizable Cl− ions in the dialysate. Other unwanted by-products include nitrite, nitrate, ammonium and cyanate32.
One approach to urea electro-oxidation that substantially reduced chlorine by-product formation involved optimizing the electrode distance and current density using a graphite electrode system38,39. This approach resulted in a urea removal rate of 16 mmol/h. However, glucose degradation products were still formed, compromising the biocompatibility of the system40. The application of an aquaporin-based biomimetic membrane that is permeable for urea but blocks other solutes has also been proposed41. This membrane adds an additional loop to the dialysate circuit that enables urea to reach the electrodes but blocks glucose molecules. The mass transfer coefficient for urea through the membrane was reportedly 3 orders of magnitude larger than that of glucose. Furthermore, a urea removal rate of 0.32 mg cm−2 h−1 was achieved, suggesting that an electrode area of 0.2 m2 would be required for the removal of 15 g urea per day41.
Light assisted photo-electrocatalytic oxidation of urea has been investigated using titanium dioxide (TiO2) as both the photo-active material and the electrocatalytic material for urea oxidation at neutral and alkaline pH41–43. At neutral pH, a urea removal rate of 2.7 μg cm−2 h−1 was achieved. This finding suggests that a total electrocatalytic electrode area of 0.23 m2 would be sufficient for targeted removal of 15 g of urea per day44. This device is reported to be selective for urea at neutral pH based on comparison of the Faradaic current (six electrons are necessary to remove 1 urea molecule) and the amount of removed urea. However, selectivity of direct urea oxidation over indirect oxidation via chlorine by-products generated at the electrode has not yet been proven. The study also reported an increase in reactive chlorine species to almost 1.0 mg/l in the first hour. This increase equilibrates to ~0.6 mg/l until the end of the experiment after 4 h. As the measured chlorine species also react with urea, whether urea removal was via direct oxidation at the electrode surface or indirect oxidation via the formation of active chlorine species is not clear44.
To date, selective and direct urea oxidation rather than chloride oxidation has only been achieved in an alkaline environment with mostly nickel (Ni)-based catalysts37,42,43. Anodic oxidation of urea over these catalysts is energetically favourable compared with the oxygen evolution reaction when generating hydrogen at the cathode (0.37 V versus 1.23 V). Ni-based catalysts are already used for wastewater treatment and hydrogen storage and generation technologies. Advances in the removal of urea via electrolysis and photo-electrocatalytic oxidation have also enabled the development of direct urea fuel cells37,42,43. Although early studies using NiOOH catalysts reported that urea is mostly converted into N2 and CO2, data published in the past few years suggest that urea is mostly converted into nitrite (up to ~80%) as well as small amounts of cyanate and other by-products45–47, which are highly toxic. Both the selectivity towards N2 over nitrite and the requirement for a high alkaline solution would have to be solved to enable an Ni-based catalytic system to be used for dialysate regeneration. The addition of Cu atoms or polymer coatings to the Ni catalyst has been shown to increase the amount of N2 versus nitrite that is produced during urea electrolysis45,46.
Adsorption
Urea sorption relies on the formation of covalent or coordination bonds with urea acting as the nucleophile (chemisorption) or hydrogen bond formation and dipole interactions (physisorption). To remove daily urea production during a 2–8-h dialysis session with a reasonable amount of sorbent (<0.5 kg), both high binding capacity and fast sorption kinetics are required. In general, chemisorption is an exothermic process in which strong, practically irreversible covalent bonds are formed. However, the kinetics of urea chemisorption are relatively slow. Urea physisorption is a faster process than chemisorption, but the resulting bonds are weaker and sorbent-bound urea is in equilibrium with urea dissolved in the dialysate, resulting in a decrease in the amount of urea bound per time unit when the dialysate urea concentration decreases during a dialysis session. As mentioned above, most types of activated carbon have relatively low urea-binding capacity (UBC), approximately 0.2 mmol/g at an equilibrium urea concentration of 20 mM, which can be enhanced by cooling and increasing the number of functional oxide groups48–53. Silicon dioxides (silica) and zeolites seem to be attractive urea sorbents with reported UBCs of 0.2–8 mmol/g at an equilibrium urea concentration of 20 mM48,49,53; however, aluminium leaching from zeolites is a potential hazard.
Two-dimensional metal carbide nanosheets known as MXenes bind urea via hydrogen bonds and dipole interactions54. The UBC of MXenes of titanium carbide (Ti3C2Tx with Tx representing terminal surface groups O−, OH− and F−) was relatively low at 37 °C (0.12 mmol/g). A computational chemistry study showed that MXenes of Cd2C and Mn2C may be most appropriate for urea sorption55. Chitosan, a linear polysaccharide composed of randomly distributed β-1,4-linked d-glucosamine and N-acetyl-d-glucosamine units, is capable of binding urea via hydrogen bonds, albeit with fairly low binding capacity (<0.2 mmol/g)56. Complexation of chitosan with metal ions (e.g. Cu2+) could increase urea binding (up to 4.4 mmol/g at a urea concentration of 30 mM) via coordinate bonds between urea and metal ions57–59.
Several studies have investigated the use of molecularly imprinted polymers (MIPs) for urea adsorption. Urea is used as a template during the synthesis of the polymer and recognition of urea by the polymer is based on hydrogen bonding. These studies have reported high UBCs of the MIPs; a UBC of 6.3 mmol/g was reported for a MIP based on methacrylic acid as the functional monomer (unfortunately [urea] was not reported)57 and a UBC of 0.4 mmol/g (at [urea] 16.7 mM) was reported using ethylene-co-vinyl alcohol as a functional monomer60,61. Chitosan has also been used to design urea-imprinted polymers but the resulting UBC was very low (0.16 ± 0.003 mmol/g)62.
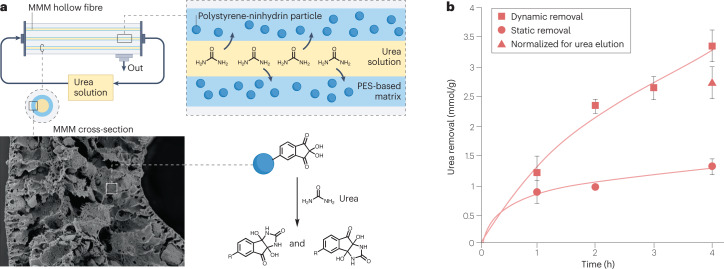
Our studies using methacrylic acid, acrylic acid, sorbitol di-methacrylate and allyl urea as functional monomers resulted in low UBCs of the MIPs (<0.1 mmol/g) (K.G., unpublished work). However, when urea was dissolved in an organic solvent, we found that the UBCs of the MIPs were close to the theoretical maximums based on the number of imprinting sites. We obtained high UBCs (up to 3.2 mmol/g) with macromolecular compounds using phenyl glyoxaldehyde and ninhydrin as functional units that contain adjacent highly electrophilic (hydrated) carbonyl groups that covalently bind the nucleophilic sites of urea (nitrogen atoms) by formation of an energy-favourable 5-membered ring. However, the urea sorption kinetics were relatively slow (~0.6 mmol/g in 8 h at 37 °C)63,64. To improve the kinetics and total urea removal, we incorporated polystyrene ninhydrin particles in a polyethersulfone (PES)/polyvinylpyrrolidone (PVP)-based mixed matrix membrane (MMM)65 (Fig. 2). At 70oC, the UBC of this MMM was higher and the binding kinetics were faster than that of the particles alone. These differences were probably due to increased accessibility of the sorbent and low particle aggregation within the MMM. We found that urea removal by the MMM was due not only to chemisorption (owing to interaction with the Nin functional groups, which comprised the highest percentage of urea binding) but also to physisorption (probably owing to urea–urea hydrogen bonding)65. Further studies are required to optimize the sorbent and MMM to achieve high urea removal at 37oC.
Fig. 2. Removal of urea using a mixed matrix membrane.
a, Urea solution is pumped through a mixed matrix membrane (MMM) hollow fibre device consisting of polystyrene ninhydrin particles within a polyethersulfone (PES)-based polymer matrix. Ninhydrin contains highly electrophilic carbonyl groups that covalently bind to the nitrogen atoms of urea and thereby remove it from dialysate solution. b, Urea removal by the MMM at 70°C under static (stirring) and dynamic (filtration and recirculation) conditions. Urea removal is estimated per grams of particles incorporated into the membrane matrix. Adapted from ref. 65, CC BY 4.0 (https://creativecommons.org/licenses/ by/4.0/).
In our opinion, dialysate recycling systems based on sorbents have great potential for implementation in PAK and WAK systems. However, the potential ecological benefit of such systems related to low dialysate water consumption needs to be weighed against the ecological footprint of sorbent production and recycling26. Notably, ninhydrin sorbents can be regenerated using strong acid (K.G., unpublished work). This technique is probably too complex and/or hazardous for the home setting so should be performed at a central location after return of the used sorbent cartridges, which would entail a logistical challenge.
Membranes for artificial kidneys
Current haemodialysis membranes can provide high removal of small water-soluble uraemic solutes and toxins (molecular weight <500 Da, e.g. urea and creatinine), but fail to provide sufficient removal of middle and large solutes and toxins (molecular weight >500 Da, e.g. β2-microglobulin and parathyroid hormone) and of protein-bound uraemic toxins (PBUTs, e.g. indoxyl sulfate or p-cresol sulfate). To achieve more effective removal of uraemic solutes, the membranes should mimic kidney glomerular filtration66 and have high molecular weight cut-offs close to, but lower than that of albumin (~66 kDa)67. During conventional haemodialysis, the removal of uraemic toxins takes place in an intermittent manner, typically for 4 h, three times per week. Longer and/or continuous KRT with PAK or WAK devices would more closely mimic the continuous nature of kidney filtration and likely improve patient outcomes68–70. However, longer operational times pose additional challenges for membrane dialysers, including long-term haemocompatibility and minimization of blood clotting.
Polymeric membranes
Most currently available synthetic dialysis membranes are composed of hydrophobic polymers such as PES or polysulfone blended with hydrophilic additives, such as PVP to improve biocompatibility. However, these additives could be eluted from the membrane during sterilization or long-term use71–73. To solve this problem, various methods of grafting and/or coating of hydrophilic additives to the membrane have been proposed. One such approach involved modifying polyvinylidene fluoride membranes by coating them with polyvinyl alcohol and chitosan to improve biocompatibility74.
Researchers have also grafted argatroban, a direct thrombin inhibitor, onto the surface of a polysulfone membrane, to reduce thrombosis75. Similar antithrombogenic results were obtained by immobilizing argatroban and mPEG-NH2 onto a PES membrane surface76. We have developed membranes based on blends of PES and SlipSkin, which have very good haemocompatibility (e.g. low cell adhesion and low complement system activation) and provide high removal of uraemic toxins77,78. SlipSkin is a random copolymer consisting of hydrophilic N-vinylpyrrolidone and hydrophobic N-butylmethacrylate) moieties that does not elute from the membrane within 24 h of filtration78.
Silicon-based membranes
Despite their broad implementation, polymeric membranes have limitations that could decrease their potential for further miniaturization79. They often have long tortuosity, which limits permeability, and relatively high hydraulic resistance requiring application of rather large blood pumps. Their pore-size distribution is also relatively broad, which limits filtration selectivity. Silicon-based nanoporous membranes (SNMs) can have very uniform nanopores (<1% deviation between pore sizes) within the size range required for haemodialysis and haemofiltration (5–10 nm)79–81. However, the porosity of these membranes is low (≤1%). To improve permeability, SNMs have been fabricated with arrays of nanoslits79–81 (10 nm wide and 4.5 µm long). However, these nanoslits have to be spaced 100 nm to 2 μm apart owing to photolithographic limitations; hence, the membrane porosity remains relatively low. Nanoporous membranes have also been developed from silicon nitride (SiN) with a porosity of up to 40%82. However, their pore sizes (40–80 nm) are far too large for haemodialysis applications. Importantly, owing to the poor haemocompatibility of silicon, a hydrophilic coating is crucial to prevent protein deposition, platelet aggregation and thrombosis. Polyethylene glycol (PEG) brushes are often applied to improve haemocompatibility but these degrade over time81. Hence, for application in an implantable artificial kidney, much longer lasting alternatives are needed.
The Kidney Project researchers utilize a SNM in their bioartificial kidney (BAK)83. Studies of this SNM have demonstrated that 0.17 m2 of membrane surface (10× less than that of current dialysers) would be required for 3× 8-h dialysis at a standard targeted Kt/V of 2.0 per week84–86. In 2022, a small-scale, arteriovenous, coated silicon nanopore membrane haemodialyser was successfully implanted in pigs87. After showing patency of the modules for up to 32 days, the researchers performed three haemodialysis sessions in 7 days, achieving blood flow via the arterial venous pressure differential only. Creatinine and urea clearance were comparable with traditional fibre-based dialysers but at 1/20th of the blood flow rate, which could facilitate function via natural blood pressure and potentially eliminate the need for a blood pump. An attractive feature of silicon-wafer-based filters is that electronics, sensors and micro-actuators (microelectromechanical systems) can potentially be directly integrated onto the SNM, enabling further miniaturization. For example, just 5 × 5 mm2 of silicon chip surface can hold a complete multiparameter medical monitor, including microprocessor, memory and wireless communication88.
Mixed matrix membranes
PBUTs are poorly removed by haemodialysis because their free fraction in plasma is rather low. To improve PBUT removal from plasma, we proposed the application of MMMs that combine filtration and adsorption89. These MMMs consist of a haemocompatible inner porous layer based on PES/PVP90 that is in direct contact with blood plasma, and an outer layer composed of activated carbon dispersed within a PES/PVP matrix in contact with the dialysate. The inner porous layer has hydrophilic and hydrophobic polymer patches, particularly on the membrane surface, to ensure low cell adhesion and improve haemocompatibility. Toxins that are removed by diffusion and/or convection through the inner membrane layer are adsorbed to the activated carbon particles. This approach leads to a high toxin concentration gradient across the membrane, which stimulates further dissociation of protein-bound toxins from the proteins, resulting in an increase in the free fraction of toxins in plasma. In ex vivo studies, the MMMs achieved higher PBUT removal than the membranes of standard dialysers89,91. Moreover, the high adsorptive properties of the membrane could enable the application of lower amounts of dialysate than conventional haemodialysis membranes. MMMs can also act as an adsorptive barrier to protect the patient against bacterial pyrogens from the dialysate92. These advantages could be important assets for PAK and WAK systems where a low amount of dialysate is needed for prolonged application. As discussed above, we have also developed an MMM for urea removal by adsorption65.
Outside-in filtration
Standard haemodialysis uses the ‘inside-out filtration’ mode in which the blood flows in the lumen of the hollow fibre and the dialysate flows in the inter-fibre space (IFS). Thrombi can be deposited and blood clots can form at the inlet of the fibre, blocking the blood flow through the entire fibre and consequently lowering blood clearance and filter life93. In the ‘outside-in filtration’ (OIF) mode, the blood flows in the IFS while the dialysate flows in the intraluminal space. Thus, thrombi that are deposited in the IFS will have minimal effect on membrane function because blood can flow around and bypass them without any marked reduction in membrane surface area93. Commercial dialysers that were designed for standard inside-out haemofiltration can operate for more than 100 h when applied in the OIF mode, without a significant increase in extracorporeal blood volume94. This advantage of the OIF mode could be beneficial for PAK and WAK systems in which a prolonged blood filtration time with low membrane fouling and no membrane clogging is needed. We have developed a MMM for OIF mode95 with an outer membrane layer of PES/PVP and an inner layer of PES/PVP/activated carbon. This OIF MMM had superior toxin removal from human plasma in vitro compared with commercial membranes and to an MMM designed for inside-out filtration. These promising results warrant further investigation of these membranes under clinically relevant experimental conditions.
Activated wafer electro-deionization
The membrane concepts described above are aimed at mimicking glomerular function. To use them in a WAK, the system should be equipped with a closed loop dialysate regeneration system as described above. Alternatively, a technology that reabsorbs water, ions, glucose and amino acids from the filtrate could be used to mimic tubular reabsorption. Activated wafer electro-deionization (AWEDI) based on a combination of ion exchange resins, ion exchange membranes and an externally applied voltage has been used to accomplish selective ion rebsorption24. The filtrate is flushed through a column or wafer that is packed with ion exchange beads. The walls of the column consist of an anion exchange membrane on one side and a cation exchange membrane on the other. When an external voltage is applied, cations move from the dilute stream through the cation exchange membrane and anions move through the anion exchange membrane towards a concentrated stream surrounding the resin column. The type of ion that is exchanged is determined by the resin that is used. Selective reabsorption of Na+, K+, Mg2+ and Ca2+ has been demonstrated using the AWEDI system24. The researchers equipped their artificial kidney system with a nanofilter to retain glucose. However, this approach hampers optimal clearance of uraemic toxins with a molecular weight >180 Da.
Another challenge for the AWEDI system is improving the efficiency of ion transport and water splitting24. The ion transport efficiency is limited by the power density, which is reported to be 25–35 mA/cm2 for a typical electro-deionization system. Ion transport is mostly driven by water splitting, which generates oxygen and hydrogen gas bubbles and a local acidic and alkaline region around the electrodes. Furthermore, different wafers can have large variability in ion selectivity (up to 42%) depending on their age, bead size and the concentration and pH of the feed solution24.
Bioartificial kidney systems
The BAK is aimed at mimicking proximal tubule function by employing “living membranes” comprising renal proximal tubule cells with transport, metabolic and endocrine activity, cultured on artificial membranes. The first BAK applied a commercial haemofilter connected in series with a renal assist device96. The renal assist device consisted of hollow fibre membranes with a luminal renal epithelial cell monolayer (108–109 cells per device). Studies in patients with acute kidney injury suggested that the device could improve patient survival, mainly as a result of reducing their pro-inflammatory status97. However, these studies identified several challenges related to cell source availability, storage, distribution and reconstruction at point-of-care facilities. To circumvent these issues, the researchers developed a bioartificial renal epithelial cell system (BRECS), consisting of niobium-coated carbon disks in a perfused housing that were loaded with renal epithelial cells derived from adult progenitor cells98. BRECS can be cryopreserved, cryostored, shipped and thawed at the end-use location.
To produce an extracorporeal wearable BRECS, the BAK design was adapted to support a continuous flow peritoneal dialysis regime. The wearable BRECS was tested in anephric sheep for 7 days99,100. Cell viability and activity were maintained in the system with extracorporeal peritoneal fluid circulation. This study raises the possibility of providing a prolonged and continuous use wearable BRECS without the need for an anticoagulated blood circuit.
In the past decade, we have developed a small-scale BAK consisting of conditionally immortalized proximal tubule epithelial cells (ciPTECs) on functionalized hollow fibres101,102 (Fig. 3). This BAK can be connected in-series with a conventional dialysis filter to enable easier integration and compatibility with existing dialysis equipment and duration, making it a more feasible option for widespread use in clinical settings. This approach enables glomerular filtration, tubular secretion and reabsorption to be concomitantly reproduced, providing a more comprehensive treatment for patients. We demonstrated active PBUT secretion by the ciPTECs in vitro through the concerted action of basolateral influx and apical efflux transporters expressed by these cells as well as reabsorption and subsequent activation of vitamin D103–105. In addition, after an inflammatory insult (exposure to lipopolysaccharide and IFN-γ), we found increased, polarized secretion of pro-inflammatory cytokines into the extra luminal (filtrate) space compared with the intraluminal (blood) space, indicating the immunological safety of the system103. We also showed that ciPTECs do not exhibit tumorigenic and/or oncogenic potential in vitro or in vivo106. Current challenges for this system are upscaling the BAK to a clinically relevant size and developing a production process that would make the device affordable for maintenance dialysis.
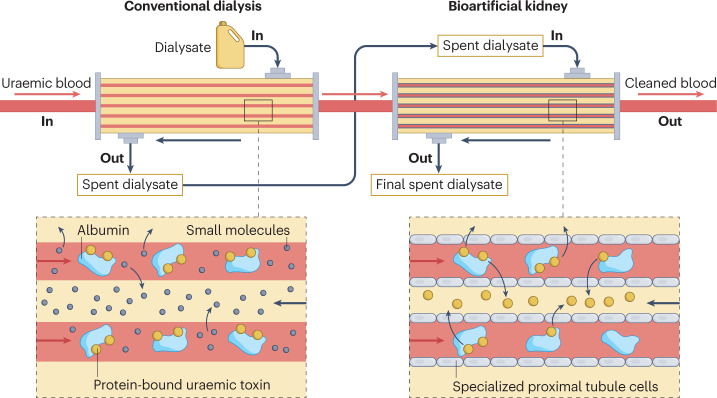
Fig. 3. A bioartificial kidney.
Schematic representation of a bioartificial kidney integrated with a conventional dialysis filter in a series configuration. First, the patient’s blood flows through a dialysis filter, which removes small molecules and medium-sized molecules (up to 45 kDa) and excess fluid. The blood then enters the bioartificial kidney, which consists of immortalized proximal tubule cells cultured on polymeric hollow fibre membranes. These cells take up protein-bound uraemic toxins from the blood after release of the molecules from the plasma protein (predominantly albumin) to the free solute, owing to the higher affinity of the solutes for the basolateral influx transporters, which can then be secreted into the dialysate via the actions of apical efflux transporters. The blood is then returned to the patient.
The Kidney Project researchers developed a prototype implantable BAK by connecting their biomimetic SNM haemofilter with sub-10-nm-wide slit pores in series with a bioreactor unit with porcine renal cells (LL-CPK1) cultured on an SNM107,108. The system is powered by blood pressure and the first operational feasibility study in a healthy pig model showed promising results without the use of anticoagulants or immune suppressants108.
To support the development of BAKs, experimental studies can be complemented by mathematical models that provide a critical assessment of factors that are relevant for BAK implementation. For example, we developed a 3D mathematical model of our ciPTEC-based BAK and used this model to simulate the transport and reaction mechanisms that are associated with the removal of PBUT109. Another study developed a model for cell-transport-aided dialysis that incorporates the effect of wall-shear stress on the cell monolayer and estimates its influence on toxin transport. The researchers concluded that hollow fibres with a wavy design would outperform those with a flat surface110; however, experimental studies should be performed to calibrate and validate these results.
PAK and WAK devices
Several PAKs and WAKs are currently under development. Modified versions of the REDY system are being used in sorbent-based miniaturized haemodialysis and peritoneal dialysis devices. The best known is the WAK (~5 kg) developed by Gura and colleagues, which was evaluated in three small clinical trials applying shorter (4–8 h) and longer (24 h) haemodialysis sessions27,111,112. The system provided effective ultrafiltration and urea, creatinine and phosphorus clearances of 17 ± 10, 16 ± 8 and 15 ± 9 ml/min, respectively, over 24 h. However, adverse events such as excessive carbon dioxide bubbles in the dialysate and clotting of the extracorporeal circuit prompted early termination of the 24-h haemodialysis session trial27. The researchers showed that clinically relevant (diffusive) middle molecule clearance could only be achieved by near-continuous operation of the WAK.
For effective clearance of middle molecules with intermittent use of the WAK, the addition of convection may be required, which is challenging in a closed-loop system with a small dialysate volume. To accomplish such convection, ‘push-pull’ techniques may be used based on alternating transmembrane pressure and half-cycle differences in dialysate volume (created by moving a small volume of liquid back and forth across the dialyser within seconds)113. The compact Physidia home dialysis system actively uses ‘push-pull’ technology for ‘self-convective haemodialysis’19. This technology could be utilized in wearable systems using microvalves, which can be integrated into standard silicon and microelectronics technology and used to open and close fluidic channels and to pump fluid114,115.
The automated WAK (AWAK) is a small (<2 kg) peritoneal dialysis device that uses modified REDY technology for continuous (24 h) regeneration of dialysate. In 2022, this WAK was evaluated in a first-in-human clinical trial in 14 patients who were receiving peritoneal dialysis116. Treatment with the automated WAK for >10.5 h per day for 3 days resulted in significant decreases in the median serum concentrations of urea, creatinine and phosphate, from 20.8 to 14.9 mM (P = 0.001), 976 to 668 μM (P = 0.001) and 1.7 to 1.5 mM (P = 0.03), respectively, and a weekly peritoneal Kt/V urea of >1.7. No serious adverse events were reported, although 73% of the patients experienced abdominal discomfort, which resolved after dialysate drainage or bowel movement. Four other PAK devices (>10 kg) are approaching first-in-human clinical trials: a home haemodialysis system from Mozart Medical, formerly Medtronic, which uses sorbent-based regeneration of dialysate23; NeoKidney, which uses modified REDY technology for dialysate regeneration117; Diality, which uses a novel sorbent dialysis system118; and Qidny, which uses an organic polymer hydrogel for urea removal119.
Conclusions and outlook
Current haemodialysis therapy removes a limited range of uraemic toxins, is discontinuous and requires large volumes of dialysate, which limits portability and patient autonomy. The application of PAK or WAK systems requires the development of strategies to enable continuous regeneration of a small volume of dialysate. Dialysate recycling systems based on sorbents have great potential, but their benefits need to be weighed against the ecological footprint of sorbent production and recycling. Novel dialysis membranes composed of polymeric or inorganic materials could potentially provide improved removal of a broad range of uraemic toxins via filtration and/or adsorption, with low levels of membrane fouling compared with currently available synthetic dialysis membranes.
To achieve more complete therapy, such as effective removal of PBUTs, and to provide important biological functions, such as production of erythropoietin and vitamin D, the new artificial kidney systems could be combined with BAKs. However, such an approach requires several manufacturing, feasibility and logistics challenges to be resolved, including stable and robust cell sourcing, cell culture facilities annexed to dialysis centres, large-scale low-cost production and quality control measures. Transition of therapy from the dialysis centre to the home could be facilitated by equipping (bio)artificial systems with smart, unobtrusive miniaturized sensing technologies to enable the provision of individualized treatments to improve patient outcomes120. For example, body composition (e.g. fluid load, lean mass and fat), respiratory rate and haemoconcentration can be estimated by bioimpedance spectroscopy121, whereas optical122, ion-selective123 and electrical conductivity sensors124 can be used to monitor the composition of blood and dialysate.
We strongly believe that to achieve important technological breakthroughs that improve haemodialysis, innovators in academia and industry and other stakeholders including doctors, nurses and patients with kidney disease, need to work together within global initiatives. An important example of such a collaboration is the technology roadmap for innovative approaches to KRT that was published by the Kidney Health Initiative in 2018 (ref. 125). This roadmap has already been adopted by many innovators, including the American Association of Kidney Patients, the European Kidney Patient Federation and the European Kidney Health Alliance, who have developed an initiative — The Decade of the Kidney — that actively involves patient associations in steering innovations for kidney diseases126–128. Regulatory agencies such as the FDA, the EMA and EU-notified bodies as well as standards issuing organizations also have a large role in promoting innovation and expediting access to new technologies to improve the survival and quality of life of patients with kidney failure.
Acknowledgements
D.L. Ramada acknowledges the financial support of the Top Sector Life Sciences & Health (Health~Holland), NODIAL project 21OP + 035). D. Stamatialis, S.M. Mihaila, R. Masereeuw, K. Gerritsen and N. Noor acknowledge the financial support of the Strategic alliance of the University of Twente, University of Utrecht, and University Medical Center Utrecht. D. Stamatialis and R. Masereeuw acknowledge the financial support of the “European Uremic Toxin working group” (EUTox) of the “European Society for Artificial Organs” (ESAO) endorsed by the “European Renal Association-European Dialysis Transplantation Association” (ERA-EDTA). K. Gerritsen, J. de Vries, R. Masereeuw and F. Wieringa acknowledge the financial support of the European Commission (KIDNEW, HORIZON-EIC-2022 Pathfinder program, grant agreement no. 101099092). The authors thank J.A.W. Jong (Neokidney BV) for critically reviewing the manuscript and Dr. A. Verschueren (IMEC) for creating the initial concept for Fig. 1.
Author contributions
All authors researched data for the article, contributed substantially to discussion of the content, wrote the article and reviewed or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Nephrology thanks Ira Kurtz and the other, anonymous, reviewers for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Renal Data System - USA, Annual Data Report https://usrds-adr.niddk.nih.gov/2022 (2022).
- 2.Saran R, et al. US Renal Data System 2019 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2020;75:A6–A7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Fresenius Annual Report 2021. https://www.fresenius.com/sites/default/files/2022-03/Fresenius_Annual_Report_2021.pdf (2021).
- 4.Zhou B, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magliano DJ, et al. Trends in incidence of total or type 2 diabetes: systematic review. Br. Med. J. 2019 doi: 10.1136/bmj.l5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geetha D, et al. Impact of the COVID-19 pandemic on the kidney community: lessons learned and future directions. Nat. Rev. Nephrol. 2022;18:724–737. doi: 10.1038/s41581-022-00618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal SK, et al. Self‐assessed physical and mental function of haemodialysis patients. Nephrol. Dial. Transplant. 2001;16:1387–1394. doi: 10.1093/ndt/16.7.1387. [DOI] [PubMed] [Google Scholar]
- 8.Zazzeroni L, et al. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and meta-analysis. Kidney Blood Press. Res. 2017;42:717–727. doi: 10.1159/000484115. [DOI] [PubMed] [Google Scholar]
- 9.van Sandwijk MS, et al. Fatigue, anxiety, depression and quality of life in kidney transplant recipients, haemodialysis patients, patients with a haematological malignancy and healthy controls. Nephrol. Dial. Transplant. 2019;34:833–838. doi: 10.1093/ndt/gfy103. [DOI] [PubMed] [Google Scholar]
- 10.ERA-EDTA. ERA-EDTA Registry — Annual Report 2019 (2019).
- 11.Yang F, Liao M, Wang P, Yang Z, Liu Y. The cost-effectiveness of kidney replacement therapy modalities: a systematic review of full economic evaluations. Appl. Health Econ. Health Policy. 2021;19:163–180. doi: 10.1007/s40258-020-00614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonenkamp AA, et al. Health-related quality of life in home dialysis patients compared to in-center hemodialysis patients: a systematic review and meta-analysis. Kidney Med. 2020;2:139–154. doi: 10.1016/j.xkme.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filipčič T, Bogataj Š, Pajek J, Pajek M. Physical activity and quality of life in hemodialysis patients and healthy controls: a cross-sectional study. Int. J. Environ. Res. Public. Health. 2021;18:1–10. doi: 10.3390/ijerph18041978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathew A, et al. Mortality and hospitalizations in intensive dialysis: a systematic review and meta-analysis. Can. J. Kidney Health Dis. 2018;5:2054358117749531. doi: 10.1177/2054358117749531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kjellstrand CM, et al. Short daily haemodialysis: survival in 415 patients treated for 1006 patient-years. Nephrol. Dial. Transplant. 2008;23:3283–3289. doi: 10.1093/ndt/gfn210. [DOI] [PubMed] [Google Scholar]
- 16.Kurella M, Suri RS, Chertow GM. Frequent hemodialysis and psychosocial function. Semin. Dial. 2005;18:132–136. doi: 10.1111/j.1525-139X.2005.18216.x. [DOI] [PubMed] [Google Scholar]
- 17.Bonenkamp AA, et al. Home haemodialysis in the Netherlands: state of the art. Neth. J. Med. 2018;76:144–157. [PubMed] [Google Scholar]
- 18.Walker RC, Howard K, Morton RL. Home hemodialysis: a comprehensive review of patient-centered and economic considerations. Clinicoecon. Outcomes Res. 2017;9:149–161. doi: 10.2147/CEOR.S69340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fessi H, et al. Safety and efficacy of short daily hemodialysis with physidia S3 system: clinical performance assessment during the training period. J. Clin. Med. 2022;11:2123. doi: 10.3390/jcm11082123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komenda PVJ, et al. Hemodialysis with the quanta SC+: efficacy and safety of a self-care hemodialysis machine. Kidney Med. 2020;2:724–731.e721. doi: 10.1016/j.xkme.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glickman JD, Teitelbaum I, Golper TA. Prescribing home hemodialysis. Adv. Chronic Kidney Dis. 2021;28:157–163. doi: 10.1053/j.ackd.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Di Liberato L, et al. SP482A New portable device for home haemodialysis. Nephrol. Dial. Transplant. 2019 doi: 10.1093/NDT/GFZ103.SP482. [DOI] [Google Scholar]
- 23.van Gelder MK, et al. From portable dialysis to a bioengineered kidney. Expert. Rev. Med. Devices. 2018;15:323–336. doi: 10.1080/17434440.2018.1462697. [DOI] [PubMed] [Google Scholar]
- 24.Hestekin CN, et al. Simulating nephron ion transport function using activated wafer electrodeionization. Commun. Mater. 2020;1:20. doi: 10.1038/s43246-020-0016-3. [DOI] [Google Scholar]
- 25.Groth T, et al. Wearable and implantable artificial kidney devices for end-stage kidney disease treatment — current status and review. Artif. Organs. 2022 doi: 10.1111/aor.14396. [DOI] [PubMed] [Google Scholar]
- 26.Vanholder R, et al. The European Green Deal and nephrology: a call for action by the European Kidney Health Alliance. Nephrol. Dial. Transplant. 2022 doi: 10.1093/ndt/gfac160. [DOI] [PubMed] [Google Scholar]
- 27.Gura V, et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight. 2016;1:e86397. doi: 10.1172/jci.insight.86397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wester M, et al. A regenerable potassium and phosphate sorbent system to enhance dialysis efficacy and device portability: a study in awake goats. Nephrol. Dial. Transplant. 2017;32:951–959. doi: 10.1093/ndt/gfw108. [DOI] [PubMed] [Google Scholar]
- 29.Wallenas, A., Meinander, N., Malmborg, C., Landholm, S. & Bengtsson, H. Cartridge and apparatus for performing adsorption dialysis. Patent WO2016190794A3 (2016).
- 30.Yin CY, Aroua MK, Daud WMAW. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007;52:403–415. doi: 10.1016/j.seppur.2006.06.009. [DOI] [Google Scholar]
- 31.Lee S, et al. Removal of uremic solutes from dialysate by activated carbon. Clin. J. Am. Soc. Nephrol. 2022;17:1168–1175. doi: 10.2215/CJN.01610222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Gelder MK, et al. Urea removal strategies for dialysate regeneration in a wearable artificial kidney. Biomaterials. 2020;234:119735. doi: 10.1016/j.biomaterials.2019.119735. [DOI] [PubMed] [Google Scholar]
- 33.Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin. J. Am. Soc. Nephrol. 2015;10:1444–1458. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blumenkrantz MJ, et al. Applications of the Redy® sorbent system to hemodialysis and peritoneal dialysis. Artif. Organs. 1979;3:230–236. doi: 10.1111/j.1525-1594.1979.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 35.Hecking M, et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin. J. Am. Soc. Nephrol. 2012;7:92–100. doi: 10.2215/CJN.05440611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soto-Herranz M, Sánchez-Báscones M, Antolín-Rodríguez JM, Martín-Ramos P. Evaluation of different capture solutions for ammonia recovery in suspended gas permeable membrane systems. Membranes. 2022;12:572. doi: 10.3390/membranes12060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye K, Wang G, Cao D, Wang G. Recent advances in the electro-oxidation of urea for direct urea fuel cell and urea electrolysis. Top. Curr. Chem. 2018;376:42. doi: 10.1007/s41061-018-0219-y. [DOI] [PubMed] [Google Scholar]
- 38.Wester M, et al. Removal of urea in a wearable dialysis device: a reappraisal of electro-oxidation. Artif. Organs. 2014;38:998–1006. doi: 10.1111/aor.12309. [DOI] [PubMed] [Google Scholar]
- 39.Wester M, et al. Removal of urea by electro-oxidation in a miniature dialysis device: a study in awake goats. Am. J. Physiol. Renal Physiol. 2018;315:F1385–F1397. doi: 10.1152/ajprenal.00094.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Gelder MK, et al. Safety of electrooxidation for urea removal in a wearable artificial kidney is compromised by formation of glucose degradation products. Artif. Organs. 2021;45:1422–1428. doi: 10.1111/aor.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao G, et al. Dialysate regeneration with urea selective membrane coupled to photoelectrochemical oxidation system. Adv. Mater. Interfaces. 2022;9:2102308. doi: 10.1002/admi.202102308. [DOI] [Google Scholar]
- 42.Dector D, et al. Harvesting energy from real human urine in a photo-microfluidic fuel cell using TiO2–Ni anode electrode. Int. J. Hydrog. Energy. 2021;46:26163–26173. doi: 10.1016/j.ijhydene.2021.02.148. [DOI] [Google Scholar]
- 43.Zhu B, Liang Z, Zou R. Designing advanced catalysts for energy conversion based on urea oxidation reaction. Small. 2020;16:1906133. doi: 10.1002/smll.201906133. [DOI] [PubMed] [Google Scholar]
- 44.Shao G, Zang Y, Hinds BJ. TiO2 nanowires based system for urea photodecomposition and dialysate regeneration. ACS Appl. Nano Mater. 2019;2:6116–6123. doi: 10.1021/acsanm.9b00709. [DOI] [Google Scholar]
- 45.Li J, et al. Deciphering and suppressing over-oxidized nitrogen in nickel-catalyzed urea electrolysis. Angew. Chem. Int. Ed. 2021;60:26656–26662. doi: 10.1002/anie.202107886. [DOI] [PubMed] [Google Scholar]
- 46.Tatarchuk SW, Medvedev JJ, Li F, Tobolovskaya Y, Klinkova A. Nickel-catalyzed urea electrolysis: from nitrite and cyanate as major products to nitrogen evolution. Angew. Chem. Int. Ed. 2022;61:e202209839. doi: 10.1002/anie.202209839. [DOI] [PubMed] [Google Scholar]
- 47.Rebiai L, et al. Photoelectrocatalytic conversion of urea under solar illumination using Ni decorated Ti-Fe2O3 electrodes. Electrochim. Acta. 2023;438:141516. doi: 10.1016/j.electacta.2022.141516. [DOI] [Google Scholar]
- 48.Cheah WK, Sim YL, Yeoh FY. Amine-functionalized mesoporous silica for urea adsorption. Mater. Chem. Phys. 2016;175:151–157. doi: 10.1016/j.matchemphys.2016.03.007. [DOI] [Google Scholar]
- 49.Cheng YC, et al. Clearance of low molecular-weight uremic toxins p-cresol, creatinine, and urea from simulated serum by adsorption. J. Mol. Liq. 2018;252:203–210. doi: 10.1016/j.molliq.2017.12.084. [DOI] [Google Scholar]
- 50.Giordano C, et al. Cold carbon apparatus for hemodialysis. J. Dial. 1976;1:165–179. doi: 10.3109/08860227609039143. [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, et al. Development of a cold dialysate regeneration system for home hemodialysis. Blood Purif. 2009;28:84–92. doi: 10.1159/000218088. [DOI] [PubMed] [Google Scholar]
- 52.Ooi CH, et al. Conversion and characterization of activated carbon fiber derived from palm empty fruit bunch waste and its kinetic study on urea adsorption. J. Environ. Manag. 2017;197:199–205. doi: 10.1016/j.jenvman.2017.03.083. [DOI] [PubMed] [Google Scholar]
- 53.Wernert V, Schäf O, Ghobarkar H, Denoyel R. Adsorption properties of zeolites for artificial kidney applications. Microporous Mesoporous Mater. 2005;83:101–113. doi: 10.1016/j.micromeso.2005.03.018. [DOI] [Google Scholar]
- 54.Meng F, et al. MXene sorbents for removal of urea from dialysate: a step toward the wearable artificial kidney. ACS Nano. 2018;12:10518–10528. doi: 10.1021/acsnano.8b06494. [DOI] [PubMed] [Google Scholar]
- 55.Zandi P, et al. Shedding light on miniaturized dialysis using MXene 2D materials: a computational chemistry approach. ACS Omega. 2021;6:6312–6325. doi: 10.1021/acsomega.0c06118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou YG, Yang YD, Guo XM, Chen GR. Effect of molecular weight and degree of deacetylation of chitosan on urea adsorption properties of copper chitosan. J. Appl. Polym. Sci. 2003;89:1520–1523. doi: 10.1002/app.12235. [DOI] [Google Scholar]
- 57.Alizadeh T. Preparation of molecularly imprinted polymer containing selective cavities for urea molecule and its application for urea extraction. Anal. Chim. Acta. 2010;669:94–101. doi: 10.1016/j.aca.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Chen X, Shao Z, Zhou P. Preparation and characterization of chitosan/Cu(II) affinity membrane for urea adsorption. J. Appl. Polym. Sci. 2003;90:1108–1112. doi: 10.1002/app.12841. [DOI] [Google Scholar]
- 59.Xue C, Wilson LD. Kinetic study on urea uptake with chitosan based sorbent materials. Carbohydr. Polym. 2016;135:180–186. doi: 10.1016/j.carbpol.2015.08.090. [DOI] [PubMed] [Google Scholar]
- 60.Huang CY, et al. Urinalysis with molecularly imprinted poly(ethylene-co-vinyl alcohol) potentiostat sensors. Biosens. Bioelectron. 2009;24:2611–2617. doi: 10.1016/j.bios.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 61.Lee MH, et al. Synthesis of magnetic molecularly imprinted poly(ethylene-co-vinyl alcohol) nanoparticles and their uses in the extraction and sensing of target molecules in urine. ACS Appl. Mater. Interfaces. 2010;2:1729–1736. doi: 10.1021/am100227r. [DOI] [PubMed] [Google Scholar]
- 62.Cheng Y, et al. Preparation of urea-imprinted cross-linked chitosan and its adsorption behavior. Nanotechnology. 2014;47:1063–1078. [Google Scholar]
- 63.Jong JAW, et al. A ninhydrin-type urea sorbent for the development of a wearable artificial kidney. Macromol. Biosci. 2020;20:1900396. doi: 10.1002/mabi.201900396. [DOI] [PubMed] [Google Scholar]
- 64.Jong JAW, et al. Phenylglyoxaldehyde-functionalized polymeric sorbents for urea removal from aqueous solutions. ACS Appl. Polym. Mater. 2020;2:515–527. doi: 10.1021/acsapm.9b00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geremia I, et al. New mixed matrix membrane for the removal of urea from dialysate solution. Sep. Purif. Technol. 2021;277:119408. doi: 10.1016/j.seppur.2021.119408. [DOI] [Google Scholar]
- 66.Storr M, Ward RA. Membrane innovation: closer to native kidneys. Nephrol. Dial. Transplant. 2018;33:iii22–iii27. doi: 10.1093/ndt/gfy228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geremia I, Stamatialis D. Innovations in dialysis membranes for improved kidney replacement therapy. Nat. Rev. Nephrol. 2020;16:550–551. doi: 10.1038/s41581-020-0293-6. [DOI] [PubMed] [Google Scholar]
- 68.Cornelis T, et al. Protein-bound uraemic toxins, dicarbonyl stress and advanced glycation end products in conventional and extended haemodialysis and haemodiafiltration. Nephrol. Dial. Transplant. 2015;30:1395–1402. doi: 10.1093/ndt/gfv038. [DOI] [PubMed] [Google Scholar]
- 69.Dam M, Weijs PJM, van Ittersum FJ, van Jaarsveld BC. Physical performance in patients treated with nocturnal hemodialysis — a systematic review of the evidence. BMC Nephrol. 2019;20:317. doi: 10.1186/s12882-019-1518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham-Brown MP, Churchward DR, Smith AC, Baines RJ, Burton JO. A 4-month programme of in-centre nocturnal haemodialysis was associated with improvements in patient outcomes. Clin. Kidney J. 2015;8:789–795. doi: 10.1093/ckj/sfv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zawada AM, et al. Polyvinylpyrrolidone in hemodialysis membranes: impact on platelet loss during hemodialysis. Hemodial. Int. 2021;25:498–506. doi: 10.1111/hdi.12939. [DOI] [PubMed] [Google Scholar]
- 72.Woiterski C, Jäger S, Dröschel S. Comparative study on elution of polyvinylpyrrolidone on dialyzers using ultraviolet analysis and iodine method. ASAIO J. 2023;69:225–230. doi: 10.1097/MAT.0000000000001751. [DOI] [PubMed] [Google Scholar]
- 73.Namekawa K, Matsuda M, Fukuda M, Kaneko A, Sakai K. Poly(N-vinyl-2-pyrrolidone) elution from polysulfone dialysis membranes by varying solvent and wall shear stress. J. Artif. Organs. 2012;15:185–192. doi: 10.1007/s10047-012-0629-5. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Q, Lu X, Yang S, Zhang Q, Zhao L. Preparation of anticoagulant polyvinylidene fluoride hollow fiber hemodialysis membranes. Biomed. Tech. 2017;62:57–65. doi: 10.1515/bmt-2015-0149. [DOI] [PubMed] [Google Scholar]
- 75.Fu X, Ning JP. Synthesis and biocompatibility of an argatroban-modified polysulfone membrane that directly inhibits thrombosis. J. Mater. Sci. Mater. Med. 2018;29:66. doi: 10.1007/s10856-018-6054-4. [DOI] [PubMed] [Google Scholar]
- 76.Dai Y, Dai S, Xie X, Ning J. Immobilizing argatroban and mPEG-NH2 on a polyethersulfone membrane surface to prepare an effective nonthrombogenic biointerface. J. Biomater. Sci. Polym. Ed. 2019;30:608–628. doi: 10.1080/09205063.2019.1595891. [DOI] [PubMed] [Google Scholar]
- 77.ter Beek O, et al. New membranes based on polyethersulfone — SlipSkin™ polymer blends with low fouling and high blood compatibility. Sep. Purif. Technol. 2019;225:60–73. doi: 10.1016/j.seppur.2019.05.049. [DOI] [Google Scholar]
- 78.ter Beek OEM, Pavlenko D, Stamatialis D. Hollow fiber membranes for long-term hemodialysis based on polyethersulfone-SlipSkin™ polymer blends. J. Membr. Sci. 2020 doi: 10.1016/j.memsci.2020.118068. [DOI] [Google Scholar]
- 79.Ghosh A, et al. Effective clearance of uremic toxins using functionalised silicon nanoporous membranes. Biomed. Microdev. 2021;23:4. doi: 10.1007/s10544-020-00539-8. [DOI] [PubMed] [Google Scholar]
- 80.Fissell WH, et al. High-performance silicon nanopore hemofiltration membranes. J. Memb. Sci. 2009;326:58–63. doi: 10.1016/j.memsci.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kensinger C, et al. First implantation of silicon nanopore membrane hemofilters. ASAIO J. 2016;62:491–495. doi: 10.1097/MAT.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DesOrmeaux JPS, et al. Nanoporous silicon nitride membranes fabricated from porous nanocrystalline silicon templates. Nanoscale. 2014;6:10798–10805. doi: 10.1039/C4NR03070B. [DOI] [PubMed] [Google Scholar]
- 83.Tang Y-S, Tsai Y-C, Chen T-W, Li S-Y. Artificial kidney engineering: the development of dialysis membranes for blood purification. Membranes. 2022;12:177. doi: 10.3390/membranes12020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fissell WH, Roy S, Davenport A. Achieving more frequent and longer dialysis for the majority: wearable dialysis and implantable artificial kidney devices. Kidney Int. 2013;84:256–264. doi: 10.1038/ki.2012.466. [DOI] [PubMed] [Google Scholar]
- 85.Kim S, et al. Diffusive silicon nanopore membranes for hemodialysis applications. PLoS One. 2016;11:e0159526. doi: 10.1371/journal.pone.0159526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L, Marchant RE, Dubnisheva A, Roy S, Fissell WH. Anti-biofouling sulfobetaine polymer thin films on silicon and silicon nanopore membranes. J. Biomater. Sci. Polym. Ed. 2011;22:91–106. doi: 10.1163/092050609X12578498982998. [DOI] [PubMed] [Google Scholar]
- 87.Moyer, J. et al. Endovascular nephrectomy in swine for evaluation of implantable devices for renal replacement therapy. Abstract: SA-PO018 ASN 2022 (American Society of Nephrology, 2022).
- 88.Song S, et al. A 769 μW battery-powered single-chip SoC with BLE for multi-modal vital sign monitoring health patches. IEEE Trans. Biomed. Circuits Syst. 2019;13:1506–1517. doi: 10.1109/TBCAS.2019.2945114. [DOI] [PubMed] [Google Scholar]
- 89.Tijink MSL, et al. Mixed matrix hollow fiber membranes for removal of protein-bound toxins from human plasma. Biomaterials. 2013;34:7819–7828. doi: 10.1016/j.biomaterials.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Geremia I, et al. Ex vivo evaluation of the blood compatibility of mixed matrix haemodialysis membranes. Acta Biomater. 2020;111:118–128. doi: 10.1016/j.actbio.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 91.Kim D, Stamatialis D. High flux mixed matrix membrane with low albumin leakage for blood plasma detoxification. J. Membr. Sci. 2020;609:118187. doi: 10.1016/j.memsci.2020.118187. [DOI] [Google Scholar]
- 92.Geremia I, Bansal R, Stamatialis D. In vitro assessment of mixed matrix hemodialysis membrane for achieving endotoxin-free dialysate combined with high removal of uremic toxins from human plasma. Acta Biomater. 2019;90:100–111. doi: 10.1016/j.actbio.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 93.Catapano G, Wodetzki A, Baurmeister U. Blood flow outside regularly spaced hollow fibers: the future concept of membrane devices. Int. J. Artif. Organs. 1992;15:327–330. doi: 10.1177/039139889201500602. [DOI] [PubMed] [Google Scholar]
- 94.Dukhin SS, et al. Outside-in hemofiltration for prolonged operation without clogging. J. Memb. Sci. 2014;464:173–178. doi: 10.1016/j.memsci.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.ter Beek OEM, et al. In vitro study of dual layer mixed matrix hollow fiber membranes for outside-in filtration of human blood plasma. Acta Biomater. 2021;123:244–253. doi: 10.1016/j.actbio.2020.12.063. [DOI] [PubMed] [Google Scholar]
- 96.Humes HD, et al. Metabolic replacement of kidney function in uremic animals with a bioartificial kidney containing human cells. Am. J. Kidney Dis. 2002;39:1078–1087. doi: 10.1053/ajkd.2002.32792. [DOI] [PubMed] [Google Scholar]
- 97.Humes HD, et al. Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int. 2004;66:1578–1588. doi: 10.1111/j.1523-1755.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 98.Buffington DA, et al. Bioartificial renal epithelial cell system (BRECS): a compact, cryopreservable extracorporeal renal replacement device. Cell Med. 2012;4:33–43. doi: 10.3727/215517912X653328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnston KA, et al. Development of a wearable bioartificial kidney using the bioartificial renal epithelial cell system (BRECS) J. Tissue Eng. Regen. Med. 2017;11:3048–3055. doi: 10.1002/term.2206. [DOI] [PubMed] [Google Scholar]
- 100.Pino CJ, Westover AJ, Buffington DA, Humes HD. Bioengineered renal cell therapy device for clinical translation. ASAIO J. 2017;63:305–315. doi: 10.1097/MAT.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schophuizen CM, et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch. 2013;465:1701–1714. doi: 10.1007/s00424-013-1307-z. [DOI] [PubMed] [Google Scholar]
- 102.Nieskens TT, et al. A human renal proximal tubule cell line with stable organic anion transporter 1 and 3 expression predictive for antiviral-induced toxicity. AAPS J. 2016;18:465–475. doi: 10.1208/s12248-016-9871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chevtchik NV, et al. A bioartificial kidney device with polarized secretion of immune modulators. J. Tissue Eng. Regen. Med. 2018;12:1670–1678. doi: 10.1002/term.2694. [DOI] [PubMed] [Google Scholar]
- 104.Chevtchik NV, et al. Upscaling of a living membrane for bioartificial kidney device. Eur. J. Pharmacol. 2016;790:28–35. doi: 10.1016/j.ejphar.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 105.Mihajlovic M, et al. Role of vitamin D in maintaining renal epithelial barrier function in uremic conditions. Int. J. Mol. Sci. 2017;18:2531. doi: 10.3390/ijms18122531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mihajlovic M, et al. Safety evaluation of conditionally immortalized cells for renal replacement therapy. Oncotarget. 2019;10:5332–5348. doi: 10.18632/oncotarget.27152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fissell WH, Roy S. The implantable artificial kidney. Semin. Dial. 2009;22:665–670. doi: 10.1111/j.1525-139X.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 108.Caressa Chen et al. Demonstrating preclinical proof of concept of an implantable bioartificial kidney (iBAK). Abstract PO0513 ASN 2022 (2022).
- 109.Refoyo R, Skouras ED, Chevtchik NV, Stamatialis D, Burganos VN. Transport and reaction phenomena in multilayer membranes functioning as bioartificial kidney devices. J. Membr. Sci. 2018;565:61–71. doi: 10.1016/j.memsci.2018.08.007. [DOI] [Google Scholar]
- 110.Viguerie A, Swapnasrita S, Veneziani A, Carlier A. A multi-domain shear-stress dependent diffusive model of cell transport-aided dialysis: analysis and simulation. Math. Biosci. Eng. 2021;18:8188–8200. doi: 10.3934/mbe.2021406. [DOI] [PubMed] [Google Scholar]
- 111.Davenport A, et al. A wearable haemodialysis device for patients with end-stage renal failure: a pilot study. Lancet. 2007;370:2005–2010. doi: 10.1016/S0140-6736(07)61864-9. [DOI] [PubMed] [Google Scholar]
- 112.Castro AC, et al. Wearable artificial kidney and wearable ultrafiltration device vascular access — future directions. Clin. Kidney J. 2019;12:300–307. doi: 10.1093/ckj/sfy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee K. Engineering perspective on the evolution of push/pull-based dialysis treatments. Expert. Rev. Med. Dev. 2013;10:611–620. doi: 10.1586/17434440.2013.827504. [DOI] [PubMed] [Google Scholar]
- 114.Anjewierden D, Liddiard GA, Gale BK. An electrostatic microvalve for pneumatic control of microfluidic systems. J. Micromech. Microeng. 2012;22:025019. doi: 10.1088/0960-1317/22/2/025019. [DOI] [Google Scholar]
- 115.Atik AC, Özkan MD, Özgür E, Külah H, Yıldırım E. Modeling and fabrication of electrostatically actuated diaphragms for on-chip valving of MEMS-compatible microfluidic systems. J. Micromech. Microeng. 2020;30:115001. doi: 10.1088/1361-6439/aba16f. [DOI] [Google Scholar]
- 116.Htay H, et al. Preliminary safety study of the automated wearable artificial kidney (AWAK) in peritoneal dialysis patients. Perit. Dial. Int. 2022;42:394–402. doi: 10.1177/08968608211019232. [DOI] [PubMed] [Google Scholar]
- 117.Bluechel, C. G., Koh, Y. N., Tan, C. S., Chen, K. & Zhuang, K. D. Animal trial of sorbent cartridge for portable artificial kidney (PAK). Abstract PO0962 ASN 2022 (2022).
- 118.Borillo, B. B., Chen, T. T., Khawar, O. & Poppe, C. Validation of automated sodium control in a novel dialysis system. In ASN Kidney Weekhttps://www.asn-online.org/education/kidneyweek/2021/program-abstract.aspx?controlId=3604706 (ASN, 2021).
- 119.Ahmadi, M., Sud, R. & Graansma, C. Sorbent for use in renal therapy. US Patent Application 2022/0161233, https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/20220161233 (2022).
- 120.Wieringa FP, Kooman JP. Smart sensors for real-time monitoring of patients on dialysis. Nat. Rev. Nephrol. 2020;16:554–555. doi: 10.1038/s41581-020-0287-4. [DOI] [PubMed] [Google Scholar]
- 121.Lindeboom L, et al. On the potential of wearable bioimpedance for longitudinal fluid monitoring in end-stage kidney disease. Nephrol. Dial. Transplant. 2021;37:2048–2054. doi: 10.1093/ndt/gfab025. [DOI] [PubMed] [Google Scholar]
- 122.Paats J, et al. Optical method and biochemical source for the assessment of the middle-molecule uremic toxin β2-microglobulin in spent dialysate. Toxins. 2021;13:255. doi: 10.3390/toxins13040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dam VAT, Zevenbergen MAG, van Schaijk R. Flexible ion sensors for bodily fluids. Proc. Eng. 2016;168:93–96. doi: 10.1016/j.proeng.2016.11.155. [DOI] [Google Scholar]
- 124.Brom-Verheijden GJAM, Goedbloed MH, Zevenbergen MAG. A microfabricated 4-electrode conductivity sensor with enhanced range. Proceedings. 2018;2:797. [Google Scholar]
- 125.KHI. Kidney Health Initiative — kidney replacement therapy roadmap. https://khi.asn-online.org/roadmap/ (2018).
- 126.Vanholder R, Conway PT, Gallego D, Scheres E, Wieringa F. The European Kidney Health Alliance (EKHA) and the Decade of the KidneyTM. Nephrol. Dial. Transplant. 2022 doi: 10.1093/ndt/gfac211. [DOI] [PubMed] [Google Scholar]
- 127.Wieringa FP, Sheldon M. The Kidney Health Initiative innovation roadmap for renal replacement therapies: building the yellow brick road, while updating the map. Artif. Organs. 2020;44:111–122. doi: 10.1111/aor.13621. [DOI] [PubMed] [Google Scholar]
- 128.Wieringa FP, Sheldon MI, Hidalgo-Simon A. Regulatory approaches to stimulate innovative renal replacement therapies. Nat. Rev. Nephrol. 2020;16:546–547. doi: 10.1038/s41581-020-0275-8. [DOI] [PubMed] [Google Scholar]