Abstract
Background:
Polygenic risk scores (PRS) are associated with atherosclerotic cardiovascular disease (ASCVD) events. We studied incident ASCVD among individuals with absent coronary artery calcium (CAC = 0), to investigate the association of PRS with incident ASCVD among such individuals.
Methods:
Data was used from Multi-Ethnic Study of Atherosclerosis (MESA), a prospective cohort study of participants free of clinical CVD at baseline. PRS were developed based on a literature-derived list of single-nucleotide polymorphisms (SNPs) weighted by effect size. The coronary heart disease (CHD) PRS contained 180 SNPs, and the stroke PRS had 32 SNPs. These SNPs were combined to compute an ASCVD PRS. The PRS were calculated among 3132 participants with CAC = 0. Multivariable-adjusted Cox proportional hazards models evaluated the association between each PRS (top 20% vs bottom 50%) and ASCVD.
Results:
The study population included 3132 individuals with CAC = 0 [mean (SD) age 58 (9) years; 63% female, 33% White, 31% Black, 12% Chinese-American, 24% Hispanic]. Over a median follow-up of 16 years, there were 108 incident CHD events and 93 stroke events. ASCVD event rates were generally <7.5 per 1000-person years for all ASCVD events regardless of PRS risk stratum. The ASCVD PRS was significantly associated with incident ASCVD: (HR; 95% CI) (1.63; 1.11, 2.39). The CHD PRS was not associated with any ASCVD outcome, whereas the stroke PRS was significantly associated with ASCVD (1.84; 1.27, 2.68), CHD (1.79; 1.05, 3.06), and stroke (1.96; 1.19, 3.23). The stroke PRS results were significant among women and non-Whites.
Conclusions:
Among individuals with CAC = 0, the ASCVD PRS was associated with incident ASCVD events. This appears to be driven by genetic variants related to stroke but not CHD, and particularly among women and non-Whites. ASCVD event rates remained below the threshold recommended for consideration for initiation of statin therapy even in the high PRS groups.
Keywords: Polygenic risk score, Coronary artery calcium, Atherosclerotic cardiovascular disease, Coronary heart disease
Introduction
The clinical approach to prevention of atherosclerotic cardiovascular disease (CVD;ASCVD) involves identifying high risk individuals who may benefit from pharmacotherapy.1 The Pooled Cohort Equations (PCE) form the recommended first step for estimating absolute ASCVD risk.2,3 Coronary artery calcium (CAC) offers superior risk discrimination and risk reclassification compared to other CVD risk markers and is considered the strongest negative risk marker.4–6 CAC = 0 is associated with low absolute ASCVD event rates.5 However, CVD risk factors remain associated with incident ASCVD events among individuals with CAC = 0.7–11 It is unclear whether this residual risk is due to genetic factors independent of traditional CVD risk factors.
Coronary heart disease (CHD) risk scores have been developed using single-nucleotide polymorphisms (SNPs) identified from genome-wide association studies (GWAS).12 A prior study found that individuals in the highest decile of a polygenic risk score (PRS) had an almost 4-fold higher likelihood of prevalent CHD compared with lower risk individuals.13 Among individuals who underwent coronary angiography, high PRS was independently associated with higher risk of all-cause mortality.14 A CHD PRS was also associated with incident myocardial infarction and mortality, particularly among men between the ages of 40 and 51 years.15
Less is known about the association of PRS (especially those combining both the CHD and stroke PRS) with ASCVD outcomes among those with CAC = 0. As imaging of atherosclerosis represents a “risk integrator” combining risk of ASCVD from both traditional and non-traditional CVD risk factors, it is possible that the utility of PRS may be low in those with CAC =0. In this study, we evaluated the association of genetic variants with incident ASCVD events among those with absent CAC at baseline. We leveraged the extensive information on CVD risk factors and genetic information and long-term follow-up for incident ASCVD events that are available in Multi-Ethnic Study of Atherosclerosis (MESA.).
Methods
Study design and population
Details of the MESA design have been reported elsewhere.16 Briefly, MESA is a prospective cohort study of 6814 U.S adults aged 45 to 84 years of White, Black, Hispanic, or Chinese American race/ethnicity. Participants were enrolled from 6 U.S. field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota) between 2000 and 2002. All participants were required to be free of clinical CVD at the time of enrollment. Institutional review boards at each site approved the study, and all participants provided written informed consent.
Inclusion/exclusion criteria
MESA participants with CAC = 0 at baseline and available information on PRS were included. Participants with missing follow-up event data were excluded in analyses of incident ASCVD events (n = 13).
Assessment of polygenic risk scores
SNP genotype data were acquired on the Affymetrix 6.0 SNP array using stored samples. SNPs were imputed using the 1000 Genomes cosmopolitan phase 3 version 5 reference haplotypes. Closely related individuals were excluded by randomly removing one of each pair of individuals with pi-hat genetic relatedness that was >0.2 in MESA. Principal components used to control for population stratification were generated from the underlying SNP genotypes using the EIGENSOFT package.17,18
The CHD SNPs were identified from the Coronary Artery Disease Genome Wide Replication and Meta-analysis plus the Coronary Artery Disease Genetics (CARDIOGRAMplusC4D) consortium GWAS analysis comprised of White individuals.19–23 The linkage-disequilibrium SNP-reweighting approach encoded in the LDpred software package24 was used to generate the best performing PRS.25 A total of 180 SNPs were used in the CHD PRSs.
The stroke SNPs were identified in the large scale study by Malik et al. which tested ~8 million SNPs and combined 29 studies including 67,612 cases and 454,450 controls of multiple ancestries and stroke subtypes.26 Genome-wide genotypes were imputed to 1000 Genomes Project (1000G) phase 1v3 or UK10K/HRC and ancestry-specific meta-analyses and subsequent fixed-effects transancestral meta-analyses and MANTRA transancestral meta-analyses were conducted. The study identified 32 loci associated with stroke and stroke subtypes. The same 32 SNPs were used in the stroke PRS.
A PRS was computed for each individual by summing the product of the allele weighting and the allele dosage across the selected SNPs. The CHD and stroke SNPs were then combined to compute an ASCVD PRS. There were no overlapping SNPs between the CHD and stroke SNPs. For each MESA participant, 3 sets of PRSs were derived: a CHD PRS, a stroke PRS, and an ASCVD PRS. The PRS for each individual was categorized a priori as being in the top 20% versus the bottom 50%. These categories best fit the distribution of data in our study in order to preserve power for prospective analyses of incident ASCVD outcomes.
Ascertainment of incident outcome
The outcomes for this analysis were CHD (including myocardial infarction, resuscitated cardiac arrest, definite angina, probable angina if followed by revascularization, and CHD death), stroke (including fatal and non-fatal stroke events), and ASCVD events (a composite of CHD and stroke outcomes). Two physicians from the MESA study events committee independently adjudicated all medical records and death certificates for endpoint classification and assignment of incidence dates. The reviewers were blinded to CAC score and used prespecified criteria.16 The median follow-up time for incident outcomes was approximately 16 years.
Assessment of CAC
CAC was assessed at the baseline examination using either an electron-beam CT scanner (Chicago, Los Angeles, and New York centers) or a multidetector CT system (Baltimore, Forsyth County, and St. Paul centers). Each participant was scanned twice and all images were interpreted at a central reading center (the Lundquist Institute at Harbor–University of California Los Angeles Medical Center, Torrance).27 A CAC score was calculated for each scan, and the mean score of the two scans was used in all analyses. Intraobserver and interobserver agreement were excellent (kappa statistics, 0.93 and 0.90, respectively).28
Assessment of covariates
Information pertaining to demographics, medical history, medication use, and cigarette smoking was collected using validated questionnaires at Visit 1. MESA participants at visit 2 were asked about family history of premature CHD defined as occurrence in any first-degree relative (mother, father, siblings, or child) of CHD or a heart attack occurring before the age of 55 years in men and 65 years in women, respectively. Anthropometric measurements were performed according to predefined protocols. Systolic and diastolic blood pressure (SBP and DBP, respectively) were measured three times using an automated sphygmomanometer (Dinamap, Critikon, Tampa, FL), and the mean of the last two measurements was used in these analyses. Hypertension was defined according to the JNC VI criteria as blood pressure ≥ 140/ 90 mmHg. A central laboratory (University of Vermont, Burlington, VT, USA) measured concentrations of total and high-density lipoprotein cholesterol (HDL-C), and plasma glucose, after a 12-h fast. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation.29 Diabetes mellitus was defined as a fasting glucose of ≥7 mmol/L (126 mg/dL) or use of hypoglycemic medication (oral agents and/or insulin). ASCVD risk was estimated using the Pooled Cohort Equations (PCE) and categorized as <7.5%, 7.5%–<15%, and 15%–<20%.30
Statistical analysis
Characteristics were tabulated for participants with CAC = 0 at baseline. Values for participants in the top 20% of the ASCVD PRS distribution were compared to values for participants in the bottom 50%. Continuous variables were reported using the mean (standard deviation) or median (interquartile range) and compared using the t-test or Kruskal–Wallis test as appropriate. Categorical variables were summarized as the count (percentage) and compared using the chi-square test.
Unadjusted incidence rates of ASCVD, CHD, and stroke were reported as the number of events per 1000 person-years among those in the top 20% versus those in the bottom 50% of the ASCVD PRS distribution. Rates were compared using log-rank testing. Incidence results were first calculated in the overall study population with CAC = 0, and then were further analyzed by sex (men versus women) and race/ethnicity (Whites versus non-Whites).
After confirming the proportionality assumption, multivariable Cox proportional hazards models were used to study the association between ASCVD, CHD, and stroke PRSs (top 20% versus bottom 50%) and risk of incident ASCVD, CHD, and stroke events among those with CAC = 0. Three sequentially adjusted models were adjusted for as follows: Model 1 was adjusted for age, sex, race, and principal components 1 to 5; Model 2 was additionally adjusted for education and PCE; Model 3 was further adjusted for family history of premature CHD. To further explore the subsequent positive results, sensitivity analyses were performed in sex and race subgroups. Multiplicative interaction testing was performed between each PRS score (top 20% versus bottom 50%) and sex and race with cardiovascular outcomes.
A p-value <0.05 was considered statistically significant. All analyses were conducted using SAS 9.4.
Results
Baseline characteristics
The study population consisted of 3132 individuals with CAC = 0 (mean (SD) age 58 (9) years; 63% female; 33% White, 31% Black, 12% Chinese-American, and 24% Hispanic individuals). ASCVD PRS categories were: 1566 individuals in the bottom 50%; 940 in the middle 30%; and 626 in the top 20%. There were no statistically significant differences in baseline demographics, CVD risk factors, or 10-year ASCVD risks when comparing individuals in the top 20% of the ASCVD PRS distribution versus individuals in the bottom 50%, with the exception of a higher prevalence of family history of premature CHD (24.9% vs 19.0%; p = 0.002) and slightly higher LDL-C (118 mg/dL vs 115 mg/dL; p = 0.04) (Table 1).
Table 1.
Baseline characteristics of the study population stratified by atherosclerotic cardiovascular disease polygenic risk score.
| Bottom 50% ASCVD PRS (N = 1566) | Middle 30% ASCVD PRS (N = 940) | Top 20% ASCVD PRS (N = 626) | p-value comparing top 20% and bottom 50% ASCVD PRS | |
|---|---|---|---|---|
| Age | 58.2(9.3) | 57.9(9.0) | 57.6(9.1) | 0.15 |
| Sex | 0.79 | |||
| Women | 979 (62.5%) | 593(63.1%) | 401(64.1%) | |
| Men | 587(37.4%) | 347(36.9%) | 225(35.9%) | |
| Race/Ethnicity | 0.65 | |||
| White | 571(36.5%) | 289(30.7%) | 225(35.9%) | |
| Chinese-American | 203(13.0%) | 107(11.4%) | 72(11.5%) | |
| Black | 408(26.1%) | 292(31.1%) | 177(28.3%) | |
| Hispanic American | 384(24.5%) | 252(26.8%) | 152(24.3%) | |
| Education | 0.78 | |||
| Less than college | 530(33.8%) | 342(36.4%) | 208(33.2%) | |
| College or above | 1036(66.2%) | 598(63.6%) | 418(66.8%) | |
| Cigarette smoking status | 0.85 | |||
| Never | 864(55.2%) | 554(58.9%) | 338(54.0%) | |
| Former | 486(31.0%) | 274(29.2%) | 202(32.3%) | |
| Current | 216(13.8%) | 112(11.9%) | 86(13.7%) | |
| Diabetes mellitus | 152(9.7%) | 81(8.6%) | 52(8.3%) | 0.31 |
| Hypertension | 518(33.1%) | 336(35.7%) | 217(34.7%) | 0.48 |
| Systolic blood pressure, mm Hg | 121.5(20.1) | 122.8(20.5) | 122.2(21.1) | 0.48 |
| Aspirin use at baseline | 377(24.1%) | 267(28.4%) | 171(27.3%) | 0.12 |
| Statin use at baseline | 132(8.4%) | 97(10.3%) | 68(10.9%) | 0.07 |
| Family history of premature CHD | 298(19.0%) | 198(21.1%) | 156(24.9%) | 0.002 |
| LDL-C, mg/dL | 115.3 (30.4) | 116.5(31.2) | 118.3(30.5) | 0.04 |
| HDL-C, mg/dL | 52.6 (15.1) | 52.1(14.7) | 52.3(14.8) | 0.70 |
| Triglycerides, mg/dL | 128.0(92.9) | 128.3(79.3) | 131.0 (80.6) | 0.45 |
| 10-year ASCVD risk, % | 0.23 | |||
| <7.5% | 958(69.1%) | 589(69.5%) | 406(73.0%) | |
| 7.5%–<15% | 331(23.8%) | 195(23.0%) | 115(20.7%) | |
| 15%–<20% | 97(7.0%) | 63(7.4%) | 35(6.3%) |
Continuous variables are summarized as mean (standard deviation) or median (interquartile range)* as appropriate. Categorical variables are summarized as count (percentage). Abbreviations: PRS (polygenic risk score); CHD (coronary heart disease); HDL-C (high-density lipoprotein cholesterol); LDL-C (low-density lipoprotein cholesterol); ASCVD (atherosclerotic cardiovascular disease).
Distribution of PRS
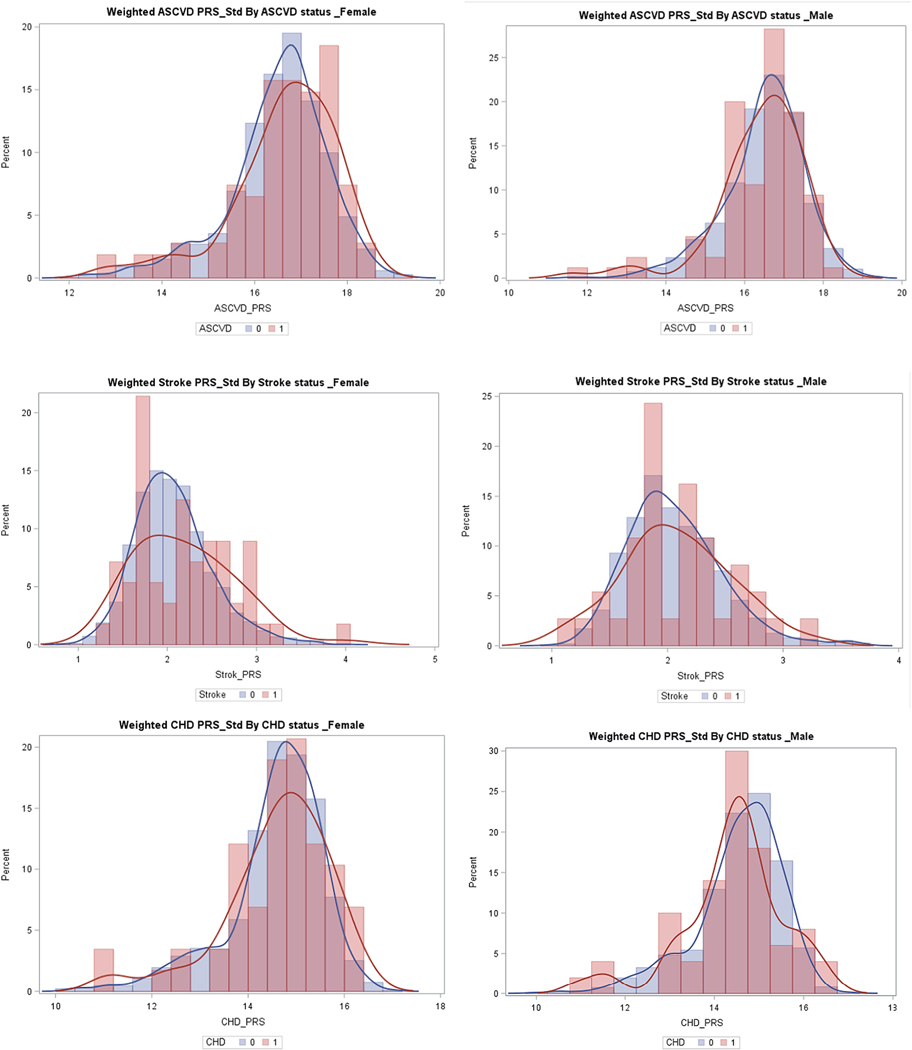
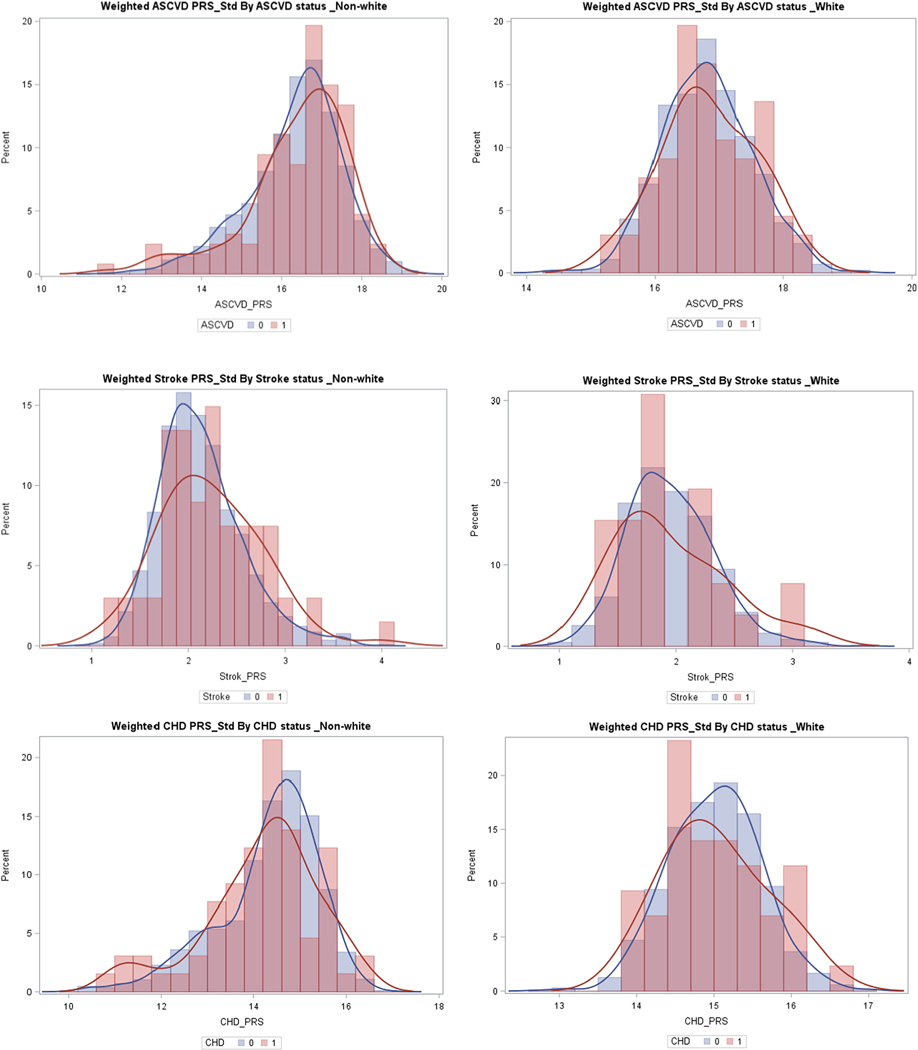
The distribution of PRSs by sex and race/ethnicity and development of incident cardiovascular outcomes is shown in Figs. 1 and 2. The distribution of PRSs was slightly more right skewed in those who developed ASCVD events. Also, there was a higher proportion of the participants who developed future ASCVD events at the higher end of the PRS distribution. This was most pronounced for the stroke PRS in men, women, and non-Whites, and for the ASCVD PRS among women.
Fig. 1.
Polygenic risk score distribution by sex and case/control status. Abbreviations: PRS (polygenic risk score); ASCVD (atherosclerotic cardiovascular disease), CHD (coronary heart disease); 1 = cases, 0 = controls.
Fig. 2.
Polygenic risk score distribution by race/ethnicity and case/control status. Abbreviations: PRS (polygenic risk score); ASCVD (atherosclerotic cardiovascular disease), CHD (coronary heart disease); 1 = cases, 0 = controls.
Polygenic risk scores and incident CVD outcomes
Over a median follow-up of 16 years, there were 193 incident ASCVD outcomes, including 108 CHD and 93 stroke events. Unadjusted incident event rates were generally <5 per 1000 person-years among those in the bottom 50% of the ASCVD PRS distribution, with the exception of ASCVD event rates for men (5.28). ASCVD event rates were >5 per 1000 person-years for those with PRS values in the top 20%. ASCVD event rates were higher for the top 20% versus the bottom 50% of the ASCVD distribution in the overall study population with CAC = 0 (5.73 vs 4.01; p = 0.03) and among women (5.67 vs 3.24; p = 0.01) and non-Whites (5.85 vs 3.81; p = 0.04). While there were no statistically significant differences in individual CHD or stroke event rates individuals in the top 20% versus the bottom 50% of the ASCVD PRSs, they were both increased and their combined frequencies led to statistically higher ASCVD event rates in the top 20% PRS group (Table 2). Cardiovascular disease event rates stratified by the CHD and stroke PRS are displayed in Supplementary Tables 1a and 1b.
Table 2.
Unadjusted incidence rates of atherosclerotic cardiovascular disease, coronary heart disease and stroke (per 1000 person-years) stratified by atherosclerotic cardiovascular disease polygenic risk score overall and among sex and racial/ethnic subgroups.
| Bottom 50% ASCVD PRS | Top 20% ASCVD PRS | p-value | |
|---|---|---|---|
| Overall | N = 1566 | N = 626 | |
| Atherosclerotic Cardiovascular Disease | 4.01 | 5.73 | 0.03 |
| Coronary Heart Disease | 2.29 | 3.21 | 0.14 |
| Stroke | 1.89 | 2.65 | 0.18 |
| Men | N = 587 | N = 225 | |
| Atherosclerotic Cardiovascular Disease | 5.28 | 5.82 | 0.71 |
| Coronary Heart Disease | 3.17 | 3.37 | 0.87 |
| Stroke | 2.11 | 2.45 | 0.73 |
| Women | N = 979 | N = 401 | |
| Atherosclerotic Cardiovascular Disease | 3.24 | 5.67 | 0.01 |
| Coronary Heart Disease | 1.76 | 3.09 | 0.06 |
| Stroke | 1.77 | 2.75 | 0.15 |
| Whites | N = 571 | N = 225 | |
| Atherosclerotic Cardiovascular Disease | 4.34 | 5.52 | 0.39 |
| Coronary Heart Disease | 2.79 | 3.98 | 0.19 |
| Stroke | 1.69 | 1.83 | 0.84 |
| Non-Whites | N = 995 | N = 401 | |
| Atherosclerotic Cardiovascular Disease | 3.81 | 5.85 | 0.04 |
| Coronary Heart Disease | 2.03 | 2.59 | 0.43 |
| Stroke | 2.02 | 3.29 | 0.09 |
Abbreviations: PRS (polygenic risk score); ASCVD (atherosclerotic cardiovascular disease). Bold items are statistically significant.
Multivariable-adjusted analyses are presented in Table 3. In general, the results were consistent across all 3 models. There was a significant association between the ASCVD PRS and incident ASCVD events in the overall study population: Model 3, Hazard Ratio; 95% Confidence Interval (1.63; 1.11, 2.39). There was no statistically significant association between the CHD PRS and any CVD outcome. The stroke PRS was significantly associated with higher risks of all outcomes – ASCVD: (1.84; 1.27, 2.68), CHD (1.79; 1.05, 3.06), and stroke: (1.96; 1.19, 3.23).
Table 3.
Hazard ratios (95% confidence interval) for the association of polygenic risk scores and incident atherosclerotic cardiovascular disease, coronary heart disease and stroke overall and stratified by sex and race/ethnicity.
| ASCVD | CHD | Stroke | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Overall | |||||||||
| ASCVD PRS | 1.77 (1.20,2.60) | 1.70 (1.16,2.49) | 1.63 (1.11,2.39) | 1.61 (0.98,2.66) | 1.54 (0.94,2.53) | 1.45 (0.88,2.38) | 1.77 (0.99,3.17) | 1.72 (0.96,3.06) | 1.71 (0.95,3.06) |
| CHD PRS | 0.99 (0.66,1.51) | 0.98 (0.65,1.49) | 0.94 (0.62,1.43) | 0.92 (0.54,1.55) | 0.91 (0.54,1.53) | 0.87 (0.51,1.47) | 1.05 (0.55,2.00) | 1.06 (0.55,2.02) | 1.04 (0.54,1.98) |
| Stroke PRS | 1.84 (1.27,2.68) | 1.88 (1.29,2.73) | 1.84 (1.27,2.68) | 1.87 (1.10,3.18) | 1.86 (1.09,3.18) | 1.79 (1.05,3.06) | 1.91 (1.16,3.15) | 1.97 (1.20,3.24) | 1.96 (1.19,3.23) |
| Men | |||||||||
| ASCVD PRS | 1.29 (0.68,2.47) | 1.32 (0.69,2.50) | 1.29 (0.68,2.45) | 1.08 (0.48,2.43) | 1.13 (0.51,2.54) | 1.11 (0.50,2.49) | 1.77 (0.62,5.04) | 1.76 (0.63,4.90) | 1.74 (0.62,4.89) |
| CHD PRS | 0.77 (0.40,1.47) | 0.77 (0.40,1.48) | 0.77 (0.40,1.46) | 0.66 (0.30,1.47) | 0.68 (0.31,1.52) | 0.68 (0.30,1.50) | 0.98 (0.32,2.99) | 1.05 (0.35,3.10) | 1.09 (0.36,3.29) |
| Stroke PRS | 0.82 (0.43,1.54) | 0.76 (0.40,1.45) | 0.76 (0.40,1.44) | 0.57 (0.22,1.46) | 0.54 (0.21,1.38) | 0.53 (0.21,1.36) | 1.30 (0.56,2.99) | 1.25 (0.54,2.91) | 1.25 (0.54,2.91) |
| Women | |||||||||
| ASCVD PRS | 2.04 (1.24,3.35) | 1.96 (1.20,3.21) | 1.83 (1.11,3.02) | 2.01 (1.04,3.07) | 1.90 (0.99,3.64) | 1.68 (0.87,3.25) | 1.77 (0.86,3.61) | 1.70 (0.84,3.47) | 1.70 (0.83,3.49) |
| CHD PRS | 1.23 (0.72,2.11) | 1.22 (0.71,2.08) | 1.13 (0.66,1.94) | 1.22 (0.60,2.47) | 1.18 (0.58,2.39) | 1.08 (0.53,2.21) | 1.13 (0.52,2.44) | 1.13 (0.52,2.43) | 1.08 (0.50,2.35) |
| Stroke PRS | 2.90 (1.81,4.65) | 3.10 (1.93,4.98) | 3.03 (1.88,4.87) | 3.54 (1.77,7.07) | 3.60 (1.80,7.20) | 3.42 (1.71,6.83) | 2.50 (1.35,4.64) | 2.79 (1.49,5.23) | 2.78 (1.48,5.19) |
| Whites | |||||||||
| ASCVD PRS | 1.36 (0.76,2.43) | 1.34 (0.74,2.40) | 1.23 (0.68,2.21) | 1.61 (0.81,3.21) | 1.58 (0.79,3.14) | 1.41 (0.70,2.83) | 1.05 (0.37,2.96) | 1.03 (0.37,2.91) | 0.97 (0.35,2.75) |
| CHD PRS | 0.91 (0.49,1.68) | 0.90 (0.49,1.67) | 0.85 (0.46,1.57) | 1.12 (0.53,2.35) | 1.11 (0.53,2.34) | 0.98 (0.46,2.08) | 0.73 (0.27,1.97) | 0.73 (0.27,1.97) | 0.73 (0.27,1.99) |
| Stroke PRS | 1.57 (0.84,2.91) | 1.53 (0.82,2.84) | 1.49 (0.80,2.78) | 2.23 (1.02,2.91) | 2.15 (0.98,4.72) | 2.06 (0.94,4.54) | 1.17 (0.47,2.92) | 1.16 (0.46,2.89) | 1.14 (0.46,2.87) |
| Non-Whites | |||||||||
| ASCVD PRS | 2.12 (1.28,3.51) | 2.11 (1.29,3.46) | 2.09 (1.27,3.43) | 1.09 (0.53,2.21) | 1.12 (0.57,2.23) | 1.10 (0.55,2.18) | 3.64 (1.80,7.34) | 3.50 (1.75,6.99) | 3.69 (1.83,7.42) |
| CHD PRS | 1.31 (0.77,2.24) | 1.24 (0.73,2.11) | 1.23 (0.73,2.10) | 0.87 (0.41,1.85) | 0.82 (0.41,1.85) | 0.82 (0.39,1.74) | 1.85 (0.88,3.91) | 1.81 (0.87,3.75) | 1.89 (0.90,3.94) |
| Stroke PRS | 1.90 (1.21,2.96) | 2.00 (1.28,3.11) | 1.97 (1.26,3.07) | 1.51 (0.78,2.93) | 1.56 (0.81,3.01) | 1.52 (0.79,2.92) | 2.27 (1.25,4.11) | 2.40 (1.33,4.33) | 2.39 (1.32,4.32) |
Abbreviations: PRS (polygenic risk score); ASCVD (atherosclerotic cardiovascular disease), CHD (coronary heart disease); PC (principal component).
Model 1: age, sex, race/ethnicity, PC1-PC5.
Model 2: age, sex, race/ethnicity, principal components 1–5, education, Pooled Cohort Equations.
Model 3: age, sex, race/ethnicity, principal components 1–5, education, Pooled Cohort Equations, family history of premature coronary heart disease. Sx was not adjusted for in sex-stratified analyses.
Bolded items are significant.
There was no statistically significant association between any PRS and CVD outcome among men. In women, the ASCVD PRS was associated with higher risk of ASCVD, while the stroke PRS was associated with all CVD outcomes (Table 3). There was a significant interaction between the stroke PRS and sex in the association with incident ASCVD and CHD (p = 0.003 and 0.009 respectively) (Supplementary Table 2).
There was no statistically significant association between any PRS and CVD outcomes among Whites. In non-Whites, there was a significant association between the ASCVD PRS and risk of ASCVD: (2.09; 1.27, 3.43) and stroke: (3.69; 1.83, 7.42). The CHD PRS was not statistically associated with cardiovascular events, whereas the stroke PRS was associated with higher risks of ASCVD (1.97; 1.26, 3.07) and stroke (2.39; 1.32, 3.42) (Table 3). There was no significant interaction between any PRS and race/ethnicity in the association with incident CVD outcomes (Supplementary Table 2).
Discussion
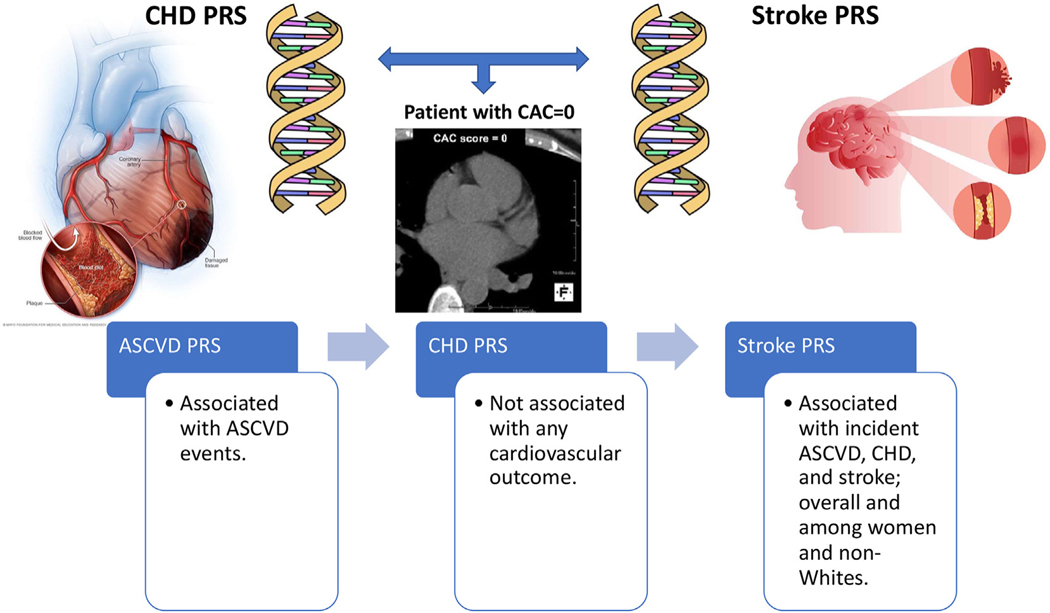
In this study of middle-aged individuals with CAC = 0 at baseline, the incidence rates of ASCVD events were low regardless of the PRS groups. Nevertheless, within this group the ASCVD PRS was associated with incident ASCVD, which appeared to be driven by genetic variants related to stroke but not CHD, and only among women and nonWhites (Fig. 3). The PRS association was independent of traditional CVD risk factors that are represented by the pooled cohort equations and family history of premature CHD.
Fig. 3.
Graphical summary of the association of polygenic risk scores with atherosclerotic cardiovascular disease outcomes. Abbreviations: PRS (polygenic risk score); ASCVD (atherosclerotic cardiovascular disease), CHD (coronary heart disease).
We found that demographics and cardiovascular risk factors were similar for participants in the top 20% and the bottom 50% of the PRS distribution. Exceptions were a higher prevalence of family history of premature CHD and slightly higher LDL-C levels in the top 20% group. Among individuals without CAC, a family history of premature CHD may indicate presence of inherited factors that predispose to incident ASCVD. Similarly, higher LDL-C levels may also be related to genetic variants that correlate with both higher serum LDL-C levels and ASCVD.13 However, the ASCVD PRS was significantly associated with subsequent events independent of the PCE and family history of premature CHD (Model 3).
The interplay of CAC and PRS might be particularly instructive, because PRSs may reflect lifetime ASCVD risks. Presumably, individuals with CAC = 0 may have protective genetic variants. Nevertheless, the top 20% of the ASCVD PRS distribution was associated with a higher risk of ASCVD events, which appeared to be driven by the stroke PRS but not the CHD PRS. We therefore posit that this observed association between stroke PRS with ASCVD may represent a pathway to CAD and stroke independent of mechanisms that lead to CAC.31 The stroke PRS association could be due to a preponderance of stroke events that occur in those with CAC = 0,11,32 or possibly a different pathophysiology of ASCVD in this group, driven more by stroke risk factors such as atrial fibrillation and hypertension rather than atherosclerotic factors, such as dyslipidemia. These results are hypothesis generating and require additional studies to elucidate potential mechanisms predisposing to CHD and stroke events among those with CAC = 0. Nevertheless, our study identifies the stroke PRS as a potential novel risk factor for ASCVD among those with CAC = 0.
Interestingly, the stroke PRS was associated with ASCVD risk among women but not men. This may be particularly true for CHD given a significant statistical interaction between thee stroke PRS and sex in the association with incident CHD but not stroke. We hypothesize that CHD events among women with CAC = 0 may be uniquely driven by stroke-related disease pathways as aforementioned. Similarly, the stroke PRS was associated with incident ASCVD events among non-Whites, possibly because it was derived from a multiethnic population unlike the CHD PRS which was derived exclusively from White populations. These results may be especially informative given that ASCVD risk among women and non-Whites is not well understood resulting in inadequate treatment.33
Although the ASCVD and stroke PRS were associated with ASCVD events, the ASCVD event rates were generally lower than the currently recommended threshold to initiate statin therapy. Therefore, at this time the PRS may not be useful to identify high-risk individuals with CAC = 0 since ASCVD event rates remained fairly modest in those with CAC = 0 regardless of the PRS risk stratum (<7.5 per 1000-person years). As the decision to initiate statin therapy is based on global ASCVD risk estimation, the current results suggest that the PRS may have limited clinical utility to guide statin treatment among those with CAC = 0 even over 16-years follow-up. Among young adults, a PRS added to clinical risk factors resulted in a significant but small improvement in model discrimination for CAC.34 Mosely et al. found that PRS was associated with incident CHD events but did not improve reclassification, discrimination, or calibration of CHD over conventional risk predictors.25 A recent study in an East Asian cohort found that the addition of a PRS to clinical risk factors yielded a very modest yet significant improvement in discrimination (1%) and reclassification (3.5%) of CAD risk.35
On the other hand, a higher risk from PRS could guide further discussion around adherence to lifestyle therapy between a patient and a clinician. A recent study found that adherence to a healthy lifestyle (using the Heart Association’s Life’s Simple 7 recommendations) was associated with a lower lifetime risk of CHD, especially in those with high genetic risk.36 Higher genetic risk may also identify individuals who would benefit from earlier CAC screening.31,37
The present results should be interpreted in the context of important limitations. The sample size was possibly underpowered to detect statistically significant associations between PRSs and incident ASCVD among those with CAC = 0 especially in subgroup analyses. This study used less SNPs (restricted to those at genome-wide significance) compared to that by Khera et al.13 The CHD PRS used in this analysis was derived from White populations, though there was no significant association with incident ASCVD in Whites and non-Whites. As discussed above, no conclusions can be made regarding disease mechanisms linking stroke genetic variants and ASCVD events in our study alone. Residual confounding cannot totally be excluded given the observational design of our study. Lastly, these findings may not necessarily be extrapolated to other study populations. Therefore, these results warrant replication in other cohorts before more definitive conclusions can be drawn regarding pathophysiological mechanisms and clinical utility. If so replicated, this may lead to a more granular precision medicine approach to ASCVD.
In summary, among individuals with CAC = 0, the ASCVD PRS is associated with incident ASCVD events. This appears to be driven by genetic variants related to stroke but not CHD, with the effect predominantly among women and non-Whites. However, ASCVD event rates remained below the threshold recommended for consideration for statin therapy even in the high PRS groups, suggesting limited utility of the PRS to guide statin therapy in those with CAC = 0.
Supplementary Material
Acknowledgements
MESA and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020 D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N920 20D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N9 2020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420. Funding for SHARe genotyping was provided by NHLBI Contract N02-HL-64278. Genotyping was performed at Affymetrix (Santa Clara, California, USA) and the Broad Institute of Harvard and MIT (Boston, Massachusetts, USA) using the Affymetrix Genome-Wide Human SNP Array 6.0. Supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR001881, NHLBI grants R01-HL151855-01 and R01HL146860, Sanford Health Polygenic Risk Score Development contract 2018-1690, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center. Infrastructure for the CHARGE Consortium is supported in part by the National Heart, Lung, and Blood Institute (NHLBI) grant R01HL105756. Also supported in part by the NHLBI South Bay Heart Watch – PREDICT – The role of PRoteomics, gEnetics, and Directed Imaging using CT grant R01 HL128801.
Disclosures
Dr. Virani: Research support: Department of Veterans Affairs, National Institutes of Health, World Heart Federation, Tahir and Jooma Family.
Honorarium: American College of Cardiology (Associate Editor for Innovations, acc.org).
Dr. Greenland: Grants from the National Institutes of Health and the American Heart Association.
Dr. Ballantyne: Grant/Research Support- All significant. (All paid to institution, not individual): Abbott Diagnostic, Akcea, Amgen, Arrow-head, Esperion, Ionis, Novartis, Regeneron, Roche Diagnostic, NIH, AHA, ADA.
Consultant- Abbott Diagnostics, Althera, Amarin*, Amgen, Arrowhead, Astra Zeneca, Denka Seiken*, Esperion, Genentech, Gilead, Illumina, Matinas BioPharma Inc., Merck, New Amsterdam*, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic, Sanofi-Synthelabo.
Dr. Blumenthal: Grants from the National Institutes of Health.
Dr. Budoff: Grant 5R01HL146666 from the National Institutes of Health.
Dr. Rotter: Grants from the National Institutes of Health.
Dr. Guo: Grants from the National Institutes of Health.
Dr. Taylor: Grants from the National Institutes of Health.
Abbreviations:
- ASCVD
Atherosclerotic Cardiovascular Disease
- CAC
Coronary Artery Calcium
- CHD
Coronary Heart Disease
- CVD
Cardiovascular Disease
- PCE
Pooled Cohort Equations
- PRS
Polygenic Risk Score
- SNP
Single Nucleotide Polymorphism
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pcad.2022.08.003.
References
- 1.Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2018. 10.1016/j.jacc.2018.11.005. published online Nov. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey A, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of Blood Cholesterol. Circulation 2018;139:e1082-e1143. CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation 2019;140:e596–e646. CIR0000000 000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 5.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the multi-ethnic study of atherosclerosis (MESA). Circulation 2016;133:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeboah J, Polonsky TS, Young R, et al. Utility of non-traditional risk markers in individuals ineligible for statin therapy according to the 2013 ACC/AHA cholesterol guidelines. Circulation 2015;132:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2013;61:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okwuosa TM, Greenland P, Burke GL, et al. Prediction of coronary artery calcium progression in individuals with low Framingham Risk Score: the Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging 2012;5:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen R, Budoff M, McClelland RL, et al. Significance of a positive family history for coronary heart disease in patients with a zero coronary artery calcium score (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2014;114:1210–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi PH, Blaha MJ, Budoff MJ, et al. The 10-year prognostic value of zero and minimal CAC. JACC Cardiovasc Imaging 2017;10:957–958. [DOI] [PubMed] [Google Scholar]
- 11.Al Rifai M, Blaha MJ, Nambi V, et al. Determinants of incident atherosclerotic cardiovascular disease events among those with absent coronary artery calcium: multi-ethnic study of atherosclerosis. Circulation 2022. 10.1161/CIRCULATIONAHA.121.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet 2018;19:581–590. [DOI] [PubMed] [Google Scholar]
- 13.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018;50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin MG, Kember RL, Judy R, et al. Genomic risk stratification predicts all-cause mortality after cardiac catheterization. Circ Genomic Precis Med 2018;11, e002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manikpurage HD, Eslami A, Perrot N, et al. Polygenic risk score for coronary artery disease improves the prediction of early-onset myocardial infarction and mortality in men. Circ Genomic Precis Med 2021;14, e003452. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 17.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet 2006;2, e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 2012;28:3326–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikpay M, Goel A, Won H-H, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schunkert H, König IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 2011;43:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kral BG, Mathias RA, Suktitipat B, et al. A common variant in the CDKN2B gene on chromosome 9p21 protects against coronary artery disease in Americans of African ancestry. J Hum Genet 2011;56:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res 2018;122:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts R. Genetics in the prevention and management of coronary artery disease. Curr Opin Cardiol 2018;33:257–268. [DOI] [PubMed] [Google Scholar]
- 24.Vilhjálmsson BJ, Yang J, Finucane HK, et al. Modeling linkage disequilibrium increases accuracy of polygenic risk scores. Am J Hum Genet 2015;97:576–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosley JD, Gupta DK, Tan J, et al. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA 2020;323: 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik R, Chauhan G, Traylor M, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 2018;50:524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 28.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/ American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severance LM, Carter H, Contijoch FJ, McVeigh ER. Targeted coronary artery calcium screening in high-risk younger individuals using consumer genetic screening results. JACC Cardiovasc Imaging 2021;14:1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta A, Pandey A, Ayers CR, et al. Predictive value of coronary artery calcium score categories for coronary events versus strokes: impact of sex and race: MESA and DHS. Circ Cardiovasc Imaging 2020;13, e010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev 2015;11:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells QS, Bagheri M, Aday AW, et al. Polygenic risk score to identify subclinical coronary heart disease risk in Young adults. Circ Genomic Precis Med 2021;14, e003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu X, Liu Z, Cui Q, et al. A polygenic risk score improves risk stratification of coronary artery disease: a large-scale prospective Chinese cohort study. Eur Heart J 2022: ehac093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasbani NR, Ligthart S, Brown MR, et al. American Heart Association’s Life’s simple 7: lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation 2022. 10.1161/CIRCULATIONAHA.121.053730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Severance LM, Contijoch FJ, Carter H, et al. Using a genetic risk score to calculate the optimal age for an individual to undergo coronary artery calcium screening. J Cardiovasc Comput Tomogr 2019;13:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.