Abstract
Study Design
Systematic Literature Review.
Objective
To propose a systematic imaging algorithm for diagnosing posterior ligamentous complex (PLC) injury in computed tomography (CT) and magnetic resonance imaging (MRI) to improve the reliability of PLC assessment.
Methods
A systematic review was conducted following PRISMA guidelines. The Scopus database was searched from its inception until July 21, 2022, for studies evaluating CT or MRI assessment of the PLC injury following thoracolumbar trauma. The studies extracted key findings, objectives, injury definitions, and radiographic modalities.
Results
Twenty-three studies were included in this systematic review, encompassing 2021 patients. Five studies evaluated the accuracy of MRI in detecting thoracolumbar PLC injury using intraoperative findings as a reference. These studies indicate that black stripe discontinuity due to supraspinous or ligamentum flavum rupture is a more specific criterion of PLC injury than high-signal intensity. Thirteen papers evaluated the accuracy or reliability of CT in detecting thoracolumbar PLC injury using MRI or intraoperative findings as a reference. The overall accuracy rate of CT in detecting PLC injury was 68-90%. Two studies evaluate the accuracy of combined CT findings, showing that ≥2 CT findings are associated with a positive predictive value of 88-91 %. Vertebral translation, facet joint malalignment, spinous process fracture, horizontal laminar fracture, and interspinous widening were independent predictors of PLC injury.
Conclusion
We provided a comprehensive imaging algorithm for diagnosing PLC in CT and MRI based on available literature and our experience. The algorithm will potentially improve the accuracy and reliability of PLC assessment, however it needs multicentre prospective validation.
Keywords: posterior ligamentous complex, thoracolumbar fractures, imaging algorithm, computed tomography, magnetic resonance imaging
Introduction
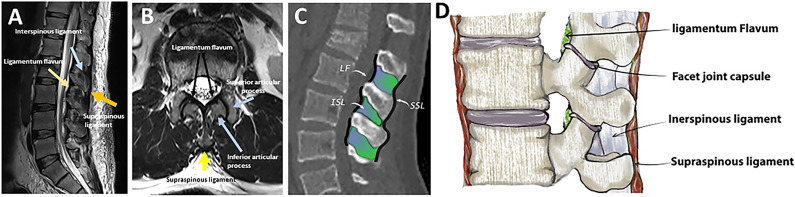
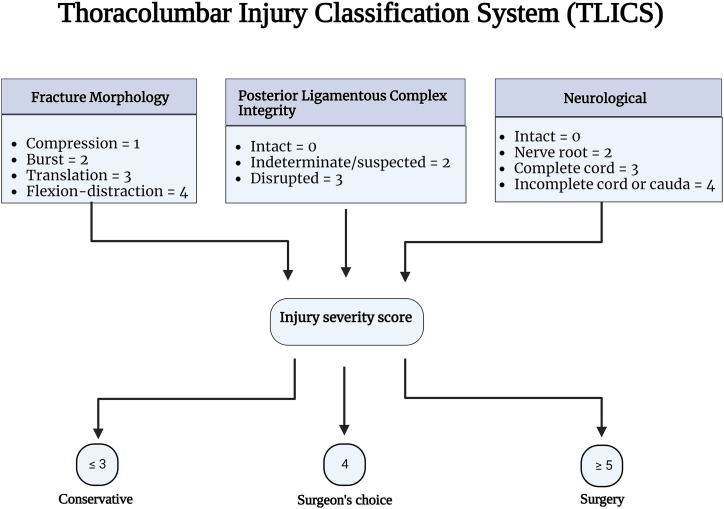
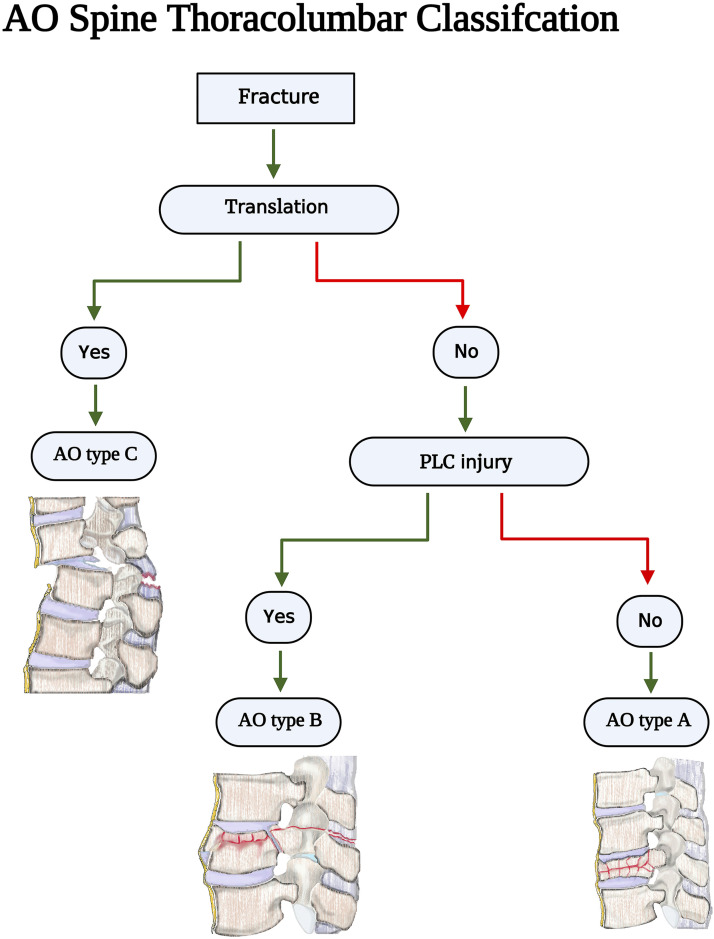
The posterior ligamentous complex (PLC) is a four-structure complex that includes the supraspinous ligament (SSL), interspinous ligament (ISL), ligamentum flavum (LF), and facet joint capsules (FC) ( Figure 1). 1 PLC disruption can cause late-onset kyphosis and chronic back pain and is thus better treated surgically. 2 Thoracolumbar injury classification system (TLICS) and AOSpine classification have recognized PLC integrity as a critical component in fracture classification and treatment algorithms.3,4 PLC integrity is one of three key elements of TLICS, along with fracture morphology and neurological condition, that contribute to injury severity score and decision-making ( Figure 2). 3 Additionally, PLC integrity is the primary criterion for distinguishing AO type B fractures (tension band injuries) from AO type A (compression fractures without evidence of PLC injury) injuries ( Figure 3). 4
Figure 1.
Gross and imaging anatomy of the thoracolumbar posterior ligamentous complex. (A) Midsagittal T2-weighted image shows the supraspinous ligament (yellow arrow), interspinous ligament (blue arrow), and ligamentum flavum (yellow arrow) all appear hypointense (dark); (B) Axial T2-weighted image shows the supraspinous ligament (yellow arrow), ligamentum flavum (two black arrows), and superior and inferior articular facets (two blue arrows); (C) Midsagittal CT images with line drawing show the supraspinous ligament ( black line), interspinous ligament ( green), and ligamentum flavum (black line); (D) Illustration showing the four components of the posterior ligamentous complex. Abbreviations: SSL, Supraspinous ligament; ISL, Interspinous ligament, LF, Ligamentum flavum, FC, facet joint capsule.
Figure 2.
The thoracolumbar injury classification scheme Source- reference. 3
Figure 3.
AOSpine Thoracolumbar classification imaging algorithm. Source - reference 4
There is much debate about the best imaging modality for PLC assessment.1,5 MRI allows direct visualization of PLC structures with high sensitivity and is considered the reference study. 6 Conversely, CT can only detect secondary signs of PLC injury such as vertebral translation or facet diastasis and has a moderate accuracy (50-80 %) and reliability.1,7–10 Important logistical considerations are involved in comparing CT and MRI scans for PLC assessment. 11 Compared to CT, the routine use of MRI in trauma settings was limited by poor availability, higher cost, poor feasibility for polytrauma patients, and considerably longer scanning time. 12 Recognizing these limitations, the current TLFs' classification, such as AOSpine and TLICS, were based on CT.
The PLC assessment has been consistently reported to be the most unreliable component of the AOSpine and TLICS.13,14 The poor reliability in PLC assessment resulted in a bias in the clinical decision-making of TLFs and limited the generalizability of research in this area.15,16 The low reliability of PLC assessment might be attributed to the lack of consensus-based criteria for defining PLC or a standardized method for image interpretation. 12 Poor reliability is caused by a lack of standard definitions for CT/MRI findings, the best imaging planes/sequences, and the pitfalls and pearls of image interpretation. 12 We propose a systematic imaging algorithm to diagnose thoracolumbar PLC injury based on available literature to improve the previously reported poor reliability.
Methods
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. No funding was received for this study, and patient consent was not required since no identifiable health information was collected, and only published studies were incorporated. We conducted a query of the Scopus database from inception until July 21, 2022, using combinations of the words “posterior ligament/ligamentous complex,” “thoracolumbar,” and “injury.” To ensure the inclusion of all available evidence, we also searched the references of available studies for any studies that met inclusion criteria.
Study Eligibility
Studies were included if they described the accuracy or utility of CT or MRI in assessing the PLC injury in the thoracic, lumbar, or thoracolumbar traumatic fractures. Studies were excluded if (1) a full text of the article could not be obtained, (2) the article was written in a language other than English, (3) they were review papers, (4) they were cadaveric or non-human studies. Authors independently screened the abstracts and titles of identified articles. Any articles not meeting exclusion criteria were automatically included for full-text review. At the full-text stage, articles were screened for inclusion based on the predetermined inclusion criteria. A more senior author reconciled discrepancies.
Data Collection Quality Assessment
The authors extracted data from the included studies with a self-designed table. Contents of data selection included objectives, injury definitions, radiographic modalities, and key findings of included studies. Due to significant data heterogeneity, variability in outcome measures and study objectives, and the different imaging modalities utilized, a meta-analysis of the findings was not performed.
Results
Search Results
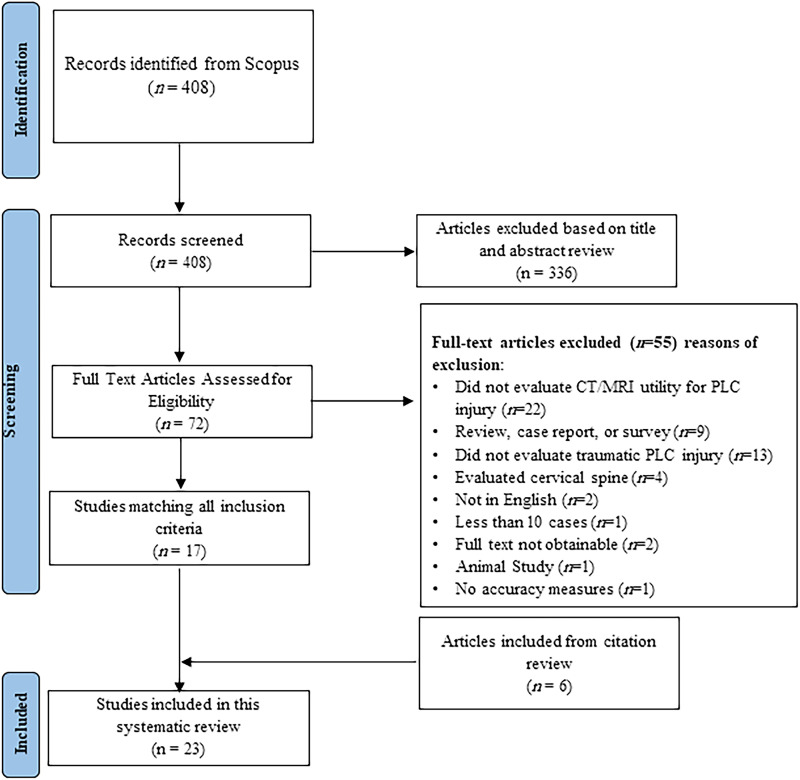
The initial Scopus search identified 408 articles, after which the title and abstract screening identified 72 potentially relevant articles (Figure 4). Twenty-three articles encompassing 2021 patients were included after full text and citation review. Twenty-two studies were excluded because they did not assess the clinical utility of CT or MRI for assessment of the PLC, 13 were excluded because they did not evaluate traumatic PLC injury, four were excluded for analysis of the cervical spine, and nine were excluded because they were surveys, case series, or review articles. Reasons for excluding other articles are further detailed (Figure 4). Four additional studies were identified through a reference review of included studies. The characteristics and key findings of included studies are detailed (Figure 4).
Figure 4.
PRISMA Flow Diagram Describing Article Selection for Inclusion. Abbreviations: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
MRI Accuracy or Reliability in Detection of PLC Injury
Five papers evaluated the accuracy of MRI in detecting thoracolumbar PLC injury using intraoperative findings as a reference (Table 1). When PLC injury was defined as high signal intensity (HSI), two studies reported high sensitivity (91%) and specificity (94%) for detecting PLC injury. 17 However, using HSI, Vaccaro et al reported much lower sensitivity (79-90%) and specificity (53-65%) for different PLC components. 18 Pizones et al defined PLC injury as black stripe discontinuity, reporting the MRI sensitivity and specificity were 100% and 91 %, respectively.19,20 Only three studies have evaluated the interobserver reliability of detection, revealing moderate to perfect agreement (K = .39-.89, Table 1).6,14,21
Table 1.
Studies on the Accuracy or Reliability of Magnetic Resonance Imaging in Diagnosis of Posterior Ligamentous Complex injury.
| Author | Objectives | Levels | Data collection | Number of Patients | Number of reviewers | MRI field strength | Definition of PLC Injury | Key Findings |
|---|---|---|---|---|---|---|---|---|
| Aly et al (2022) | Frequency of change of fracture classification by MRI compared to CT alone | T1-L5 | Retrospective | 244 | 4 | 1.5 T and 3 T MRI | Black stripe discontinuity due to SSL or LF rupture | MRI changes the fracture classification in 10 % of cases. Perfect agreement on MRI evaluation of PLC(k = .89). |
| Mehta et al (2021) | To evaluate the diagnostic accuracy of MRI in PLC injury compared to intraoperative findings | T1-L3 | Prospective | 58 | 2 | Not specified | Black stripe discontinuity due to SSL or LF rupture | MRI sensitivity for intact PLC components ranged from 100% SSL to 66.67% LF. MRI specificity ranged from 100% ISL to 52% thoracolumbar fascia. |
| Lee et al (2015) | To evaluate spine MRI inter-reader and intra-reader reliabilities for the TLICS score | Thoracolumbar | Retrospective | 100 | 1 | 1.5 T and 3 T MRI | Not specified | Inter-reader agreement of MRI was fair to moderate (k = .440 for the first and .389 for the second review) for PLC integrity. Intra-reader agreement was .423-.616. Experience did not influence difference in inter-reader agreement |
| Pizones et al(2013) | To study MRI accuracy for detecting PLC injury | T1-L5 | Prospective | 58 | 3 | Not specified | Black stripe discontinuity due to SSL or LF rupture | MRI sensitivity for injury diagnosis of each isolated PLC component varied between 92.3% ISL and 100% LF 100% SSL. PLC integrity sensitivity and specificity as a whole were 91% and 100%. |
| Vaccaro et al (2009) | To study MRI accuracy for detecting PLC injury compared to intraoperative findings | T1-L3 | Prospective | 42 | 1 | Not specified | Any high signal intensity with or without black stripe discontinuity | MRI sensitivity for the various components of the PLC ranged from 79% left FC to 90% ISL. The specificity ranged from 53% thoracolumbar fascia to 65% LF, the PPV ranged from 70.4% left FC to 78.4% ISL, and the NPV ranged from 50.0% thoracolumbar fascia to 88.2% right FC. |
| Dai et al (2009) | To measure the reliability of MRI in detecting PLC injury. | T11-L1 | Retrospective | 61 | 3 | 1.5-Tesla MRI | Any high signal intensity with or without black stripe discontinuity | The kappa coefficients for ISL or SSL injury ranged .601 to .736, representing substantial to almost perfect agreement. |
| Schweitzner et al (2007) | To determine the interrater reliability of indicators of PLC integrity on MRI and CT | T3-L3 | Retrospective | 13 | 13 | 16-slice CT 1.5-Tesla MRI |
1) facet joint diastasis on CT; (2) high signal intensity in the region of PLC components on sagittal T2W FAT SAT MRI; and (3) discontinuity of black strip on sagittal T1W MRI | Absolute interrater percent agreement on facet diastasis and posterior edema-like signal in the region of PLC components on sagittal T2-weighted MRI was similar (agreement 70.5%).Facet joint diastasis on CT was the most reliable indicator of PLC disruption (κ = .395). |
| Haba et al (2003) | To assess the diagnostic accuracy of MRI in detection of PLC injury compared to intraoperative findings | T2-L5 | Retrospective | 35 | 3 | .5-1.5-tesla MRI | Any high signal intensity with or without black stripe discontinuity | The diagnostic accuracy of MR imaging in detecting injury of the SSL and ISL was 90.5 and 94.3%, respectively. T1W MRI alone was more specific injury than T2W MRI alone. The overall mean κ coefficient for MR imaging findings of PLC injury was .803,and was greater for ISL (κ = .915) compromise than SSL (κ = .69) compromise. |
| Lee et al (2000) | To assess the reliability of MRI for PLC injury in thoracolumbar spinal fractures. | Thoracolumbar | Prospective | 34 | 1 | Not specified | A wide interspinous gap compared with the adjacent interspinous spaces on palpation or plain radiography (more than a 20% increase | SSL injury was suspected on MRI in 27 patients, ISL in 30 patients, and LF in 9 patients. There were 28 SSL injuries, 29 ISL injuries, and 7 LF injuries in operative findings. There was a significant relation between MRI interpretation and operative findings. |
| Emery et al (1989) | To review the accuracy MRI findings of patients with PLC injury compared to CT alone | Thoracolumbar | Retrospective | 37 | NR | 1.0 T or 1.5 Tesla MRI | Any high signal intensity with or without black stripe discontinuity | MRI detected ligament damage in 17/19 (89%) cases of PLC injury. However, the only misdiagnosed thoracolumbar PLC injury was the only MRI performed without T2 sequences and performed at an outside hospital |
Abbreviations: MRI, magnetic resonance imaging; SSL, supraspinous ligament; PLC, posterior ligamentous complex; CT, computed tomography; LF, ligamentum flavum; FC, facet joint capsules; ISL, interspinous ligament.
CT Accuracy or Reliability in Detection of PLC Injury
Thirteen papers evaluated the accuracy or reliability of CT in detecting thoracolumbar PLC injury using MRI or intraoperative findings as a reference (Table 2). The overall accuracy rate of CT in detecting PLC injury was 68-90%.12,22–24 Only two papers provided detailed accuracy measures for individual and combined CT findings in detecting PLC injury.12,23 The findings from these two studies showed that facet joint malalignment, spinous process fracture, interspinous widening, and horizontal laminar fractures were independently associated with PLC injury in MRI. The accuracy of combined CT findings shows that ≥ 2 CT findings yielded a higher PPV for PLC injury than ≥1 CT findings ( 90% vs 66 % and 82% vs67%).12,23 Only three studies have evaluated the interobserver reliability of CT findings, revealing moderate to excellent agreement (K = .30-.91, Table 1).10,12,14
Table 2.
Studies on the Accuracy or Reliability of Computed Tomography in Diagnosis of Posterior Ligamentous Complex Injury.
| Author | Data collection | Number of reviewers | Number of Patients | Objectives | Imaging Evaluated | Definition of PLC Injury | Key Findings |
|---|---|---|---|---|---|---|---|
| Aly et al (2022) | Retrospective | 3 | 41 | To determine the impact of MRI on fracture classification for low lumbar fractures compared to CT alone. | 64-slice multidetector CT scanner and 1.5-Tesla or 3-Tesla MRI | PLC injury in MRI was defined by black stripe discontinuity and in CT by ≥ 1 of the following presence of: vertebral body translation, facet joint malalignment, horizontal laminar or spinous process fracture, and interspinous widening | CT was highly accurate (95%) for diagnosis of PLC injury in lower lumbar fractures. Addition of MRI after CT did not change the AO classification or TLISS, compared to CT alone |
| Aly et al (2021) | Retrospective | 2 | 271 | To determine diagnostic value of morphological features of horizontal laminar fracture and vertical laminar fracture for diagnosis of PLC injury. | 64-slice multidetector CT scanner and 1.5-Tesla or 3-Tesla MRI | Black stripe discontinuity due to SSL or LF rupture | Bilateral HLFs, laminar and pedicle fractures, and displaced HLFs, but not any VLF subtypes, were independently associated with PLC injury, aiding CT-diagnosis of PLC injury |
| Durmaz et al (2021) | Prospective | 3 | 180 | To investigate the role of MRI in decision making for thoracic and lumbar fractures | 3-Tesla MRI scanner | Black stripe discontinuity | There was a weak correlation between Xray + CT and post-MRI classification for AO type B fractures, and MRI classified AO type B fractures significantly more accurately than Xray + CT MRI findings were significantly correlated to PLC disruption during intraoperative ligament assessment MRI changed treatment plan in favor of surgery in 33.9% of patients where CT/X-ray suggested non-operative treatment |
| Aly et al (2021) | Retrospective | 2 | 263 | To determine the diagnostic accuracy of combined CT findings for detecting PLC injury in MRI | CT images were obtained using a 64-slice multidetector CT | Black stripe discontinuity on MRI due to SSL or LF rupture | Facet joint malalignment, spinous process fracture, horizontal laminar fracture, and interspinous widening were independently associated with PLC injury Two or more CT findings yielded a positive predictive value of 91% for PLC injury, while a single finding is associated with 34% PPV for PLC injury |
| Ganjeifar et al (2019) | Retrospective | 98 | To evaluate the diagnostic value of CT scan in predicting PLC injuries | MRI and CT type not specified | Not specified | A significant relationship exists between facet joint widening, increased interspinous process distance, and spinous process avulsion fracture with PLC disruption. Diagnostic results of CT were similar to MRI |
|
| Khurana et al (2018) | Retrospective | 3 | 105 | To determine whether secondary CT findings can predict PLC injury | 128-slice CT scanner and 1.5-Tesla or 3.0-Tesla MRI | Any high signal intensity with or without black stripe discontinuity | At least one positive CT finding was found to yield average sensitivity of 82%, while two or more positive findings yielded average specificity of 88%. Interobserver reliability was poorest for interspinous widening. OR of PLC injury was 3.8 to 5.6 with one CT findings vs no positive CT findings, and 13.6 to 25.1 with two or more positive CT findings |
| Jiang et al (2018) | Retrospective | 2 | 88 | To examine the accuracy of some radiographic parameters and interspinous widening in CT to detect PLC injury in MRI | CT with single-detector helical protocol with a 3-mm-overlapping axial slice thickness and image reformatting in the sagittal and coronal planes | Any high signal intensity with or without black stripe discontinuity | On CT, PLC injury was associated interspinous distance ratio, and interspinous distance minus. |
| Rajasekaran et al (2016) | Retrospective | 2 | 60 | To examine the accuracy of interspinous widening in CT to detect PLC injury in MRI | 1.5 Tesla MRI | hyperintense signal changes in the PLC complex | An increase in interspinous distance by 2mm, on its own, was associated with a sensitivity of 60 % and specificity of 57 %. When considering presence of both factors of LK greater than 25° and interspinous distance greater than 2.5 mm the specificity was 97 %. |
| Barcelos et al (2016) | Retrospective | 3 | 43 | To evaluate the reliability of CT findings in the diagnosis of PLC injury | CT with single-detector helical protocol with a 3-mm- axial slice thickness and image reformatting in the sagittal and coronal planes. | facet joint diastasis, sagittal translation, increased interspinous distance, horizontal translation, and rotation of the vertebra | The intraobserver reliability for the PLC injury parameters ranged from .518 to 1.000, except for increased interspinous distance. Interobserver reliability ranged from .303 to .688 |
| Winklhofer et al (2013) | Retrospective | 3 | 100 | To evaluate the influence of additional MRI compared with CT alone for the classification of thoracolumbar traumatic fractures | CT with .6 mm slice thickness and 1.5-Tesla MRI | On CT, PLC injury was defined when one or more of the following: facet joints diastasis, avulsion fracture of the superior or inferior aspect of contiguous spinous processes, vertebral translation, or an interspinous spacing greater than that of the level above or below On MRI, Any high signal intensity with or without black stripe discontinuity |
With CT alone the integrity of the PLC was defined as intact in 80%, suspect in 2%, and injured in 18% of the 100 patients. With CT and MRI together the PLC was assessed as intact in 55 out of 55%, suspect in 3%, and injured in 42% patients. |
| Pizones e tal (2012) | Prospective | 2 | 33 | Impact of MRI on decision making For thoracolumbar traumatic fracture diagnosis and treatment |
CT not specificized, 1.5-Tesla MRI | Black stripe discontinuity | MRI offers additional information compared to other diagnostic tools: it modified our diagnosis in 40% of our patients, classification fracture pattern in 24% of our fractures and therapeutic management in 16% of our patients. |
| Lefrink et al (2002) | Retrospective | NR | 160 | To determine the diagnostic accuracy of CT findings for detecting PLC injury compared to intraoperative findings | Not specificized | Intraoperative verification of ligamentous injury | Thirty percent of Type B fractures are misdiagnosed when plain X-rays and CT scans with 2D reconstructions are used as the only preoperative diagnostic tools |
| Peterslige et al (1994) | Retrospective | 2 | 21 | frequency of diagnosis of PLC injury In MRI compared to CT alone | 1.0 and 1.5 T MRI | Any high signal intensity with or without discontinuity of SSL | CT predictors of ligamentous injury were present in only 33% of cases with SSL rupture in MRI |
Abbreviations: MRI, magnetic resonance imaging; SSL, supraspinous ligament; PLC, posterior ligamentous complex; CT, computed tomography; LF, ligamentum flavum; FC, facet joint capsules; ISL, interspinous ligament.
Discussion
The integrity of PLC has been cited as a critical element in the TLFs' classification and treatment algorithm by AOSpine classification, but no standard approach for PLC evaluation in CT or MRI has been provided.3,4 As a result, the reliability of PLC assessments and TLFs' classifications were compromised.13,14 Herein, we propose an algorithm for CT/MRI evaluation of a thoracolumbar PLC injury that incorporates a systematic literature review's results and authors' clinical expertise. The systematic review of the literature centered on the diagnostic accuracy and reliability of CT and MRI in PLC injury. Following is a summary of the significant CT and MRI findings for diagnosing PLC injury.
MRI Protocol and Imaging Considerations
The importance of using the optimal MRI sequence parameters cannot be overemphasized. 25 However, these parameters vary greatly depending on the MR imaging system’s field strength, coil design, and gradient strength; a customized approach is needed for each MRI device. 25 The spine trauma protocol includes axial and sagittal T2-WI and T1-WI, sagittal short tau inversion-recovery (STIR), with a slice thickness of 3 mm for sagittal and 4 mm for axial, using a matrix size of 240x320.7,26 T2-weighted fat-suppression sequences such as STIR or fat-saturated T2-weighted sequences are needed to distinguish T2 hyperintensity associated with PLC injuries from adjacent fat. 27 Compared to the fat-saturated T2-weighted sequence, the STIR sequence provides more uniform fat signal suppression and is less affected by the presence of metallic implants; however, it takes slightly longer to acquire and frequently produces a grainier image. 27 There is no evidence that a 3 Tesla (T) MRI scanner is more accurate than a 1.5 Tesla scanner in detecting PLC injury. One study revealed comparable inter-intrareader reliability for 1.5 and 3T. 12 A focused MRI protocol of three levels above and below known TLF has shown a comparable sensitivity to a whole-spine MRI in detecting clinically significant injuries while reducing scanning time. 28 For MRI to be highly sensitive for PLC injuries, it should be performed within 72 hours of injury. After this point, MRI’s sensitivity decrease due to resorption of the T2 signal hyperintensity produced by edema or hemorrhage, which provides an excellent contrast medium to the low signal intensity ligaments. 25
CT Protocol and Imaging Considerations
The CT's perceived image quality and the diagnostic performance depend on the choice of imaging parameters and the post-processing, in particular the reconstruction algorithm use the reformatting parameters. 29 The following parameters are recommended for a 64-slice MDCT scanner in a helical mode: 120 kVp, MA 150-600 smart MA, and pitch and speed (mm/rot) 1.375:1/55.00. The slice thickness for axial images of soft tissue and the bone algorithm was 5 mm and .625 mm, respectively, and 3 mm or less for reformatted images.12,30
MRI Findings for Each Component of PLC
Imaging Anatomy of the Posterior Ligamentous Complex
A low signal intensity (dark) on all MRI sequences is typical for PLC components (due to their collagen content), except for the LF, which has an intermediate signal on T1 and T2 sequences (due to its high elastin content, Figures 1A and B). 27 Table 3. Summarizes the MRI findings of each PLC component as well as their accuracy.
Table 3.
Magnetic Resonance Imaging Findings of Thoracolumbar Posterior Ligamentous Complex Injury.
| MRI finding | Best /additional sequence | Positive predictive value a | Negative predictive value a | Pearls/Pitfalls | |
|---|---|---|---|---|---|
| Supraspinous ligament | Black stripe discontinuity | Sagittal STIR/T2, Axial T2 | 100 | 96 | Classified as intact or disrupted: thinned but continuous ligament classified as intact |
| Interspinous ligament | Hyperintensity | Sagittal STIR/T2 | 100 | 92 | Edema vs disruption: ill-defined hyperintense signal vs sharp horizontal line crossing through the ligament |
| Ligamentum flavum | Black stripe discontinuity | Sagittal STIR/T2, Axial T2 | 100 | 100 | Horizontal vs vertical tear: Localized sharp vertical line vs diffusely macerated ligament |
| Facet capsule | Facet effusion (partial) Malalignment (complete) |
Sagittal STIR (effusion) Axial T1/sagittal T1 (malalignment) | 57 | 100 | — |
Abbreviations: MRI, Magnetic resonance imaging; STIR, Short tau inversion recovery.
aThe accuracy measures were obtained from the study by Pizones et al 19 the only paper that uses dichotomous criteria for each ligament.
Supraspinous Ligament
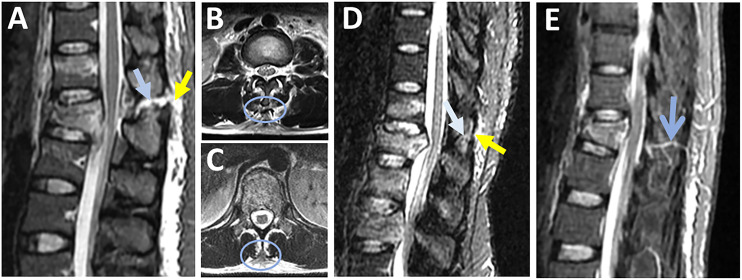
The MRI identification of SSL injury has the highest accuracy for detecting SSL injury intraoperatively (PPV,100% and NPV. 96%, Table 3). 19 According to Pizones et al, PLC disruption due to distraction injury follows an orderly rupture progressing from posterior to anterior; therefore, SSL rupture is the inflection point that marks PLC's incompetence. 31 SSL rupture can be observed as discontinuity of the black stripe, which is best seen in sagittal STIR/T2 images and corroborated by the absence of SSL-related signal intensity in axial T2 images. (Figures 5A and B) 26 Occasionally, axial T2 may show SSL is avulsed from the tip of the spinous process. (Figure 5C) SSL and LF injuries are typically graded as “intact” or “disrupted”; hence a thinned but continuous SSL or LF should be reported as “intact” (Figure 5D). 32 Detecting a ruptured SSL in the mid-thoracic area may be more challenging due to anatomical variations in the SSL/ISL complex.6,26,33 Vascular marking due to a blood vessel crossing the black stripe should not be mistaken as black stripe discontinuity (Figure 5E).
Figure 5.
Magnetic resonance imaging findings in supraspinous ligament injury. (A) Sagittal STIR images are the best sequence to detect the discontinuous black stripe due to supraspinous ligament rupture (yellow arrow) associated with high signal intensity due to interspinous ligament rupture (blue arrow); (B & C) Axial T2-weighted images show supraspinous ligament stripped from the spinous process tip (blue ring, B) or the absence of the signal intensity corresponding to the supraspinal ligament (blue ring, C ): both indicating supraspinous ligament rupture; (D) Sagittal T2-STIR image shows thinned, but continuous black stripe due to supraspinous ligament partial injury (yellow arrow) and high signal intensity due to interspinous ligament edema (white arrow); (E) vascular marking due to a crossing blood vessel (blue arrow) should not be mistaken for black stripe discontinuity. Abbreviations: STIR: short tau inversion-recovery.
Ligamentum Flavum
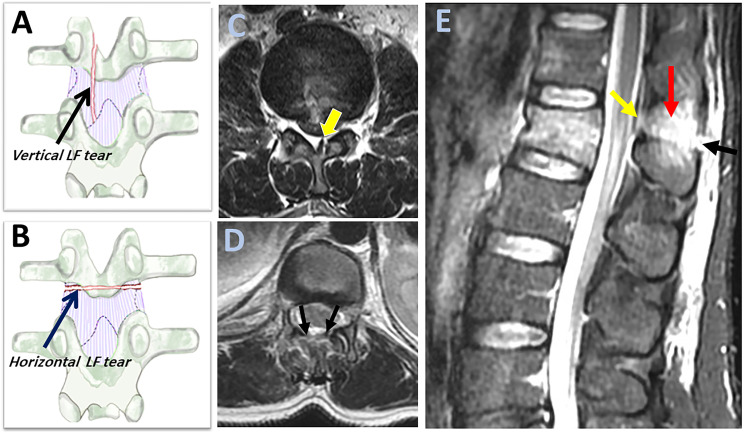
LF rupture can be observed as "black stripe discontinuity," which is best seen in parasagittal T2 or STIR images. It is critical to distinguish between a horizontal LF tear (which indicates incompetent PLC) and a vertical LF tear, considered stable (Figures 6A and B). 34 On axial T2 images, horizontal LF tears are visible as diffusely macerated ligaments, whereas vertical LF tears appear as unilateral focal (Figures 6B and C). Furthermore, horizontal LF tears appear on several sagittal STIR slices, whereas vertical tears are barely found in a single slice. (Figure 6E) According to the orderly sequence of PLC rupture by Pizones et al, a horizontal LF caused by the distraction mechanism is typically accompanied by SSL/ ISL rupture and represents the advanced stage of PLC incompetence. 31 Contrarily, a vertical LF tear is typically caused by a vertical laminar fracture due to axial compression and is not associated with other ligamentous injuries. 34
Figure 6.
Magnetic resonance imaging findings of ligamnetum flavum injury. (A-B) An illustration of the coronal view of a vertebra with the dotted lines indicates the silhouettes of the laminae and inferior articular processes behind the ligament; showing a vertical laminar fracture with an underlying vertical tear in ligamentum flavum ( black arrow, A) and a horizontal laminar fracture with an underlying horizontal tear in ligamentum flavum (blue arrow, B); (C-D) Axial T2 image can help to distinguish between horizontal and vertical ligamentum flavum it tear as it shows unilateral focal ligamentum flavum tears for vertical tears (green arrow, C) and a diffusely macerated tears for horizontal tear ( two black arrows, D); (E) Sagittal STIR image shows black stripe discontinuity due to horizontal ligament flavum tear (green arrow) and supraspinous ligament rupture (black arrow) and high signal intensity due to interspinous ligament rupture (red arrow). Horizontal ligamentum flavum tear is more easily visualized in sagittal STIR in multiple slices than the vertical ligament tear. Abbreviations: STIR: short tau inversion-recovery.
Interspinous Ligament
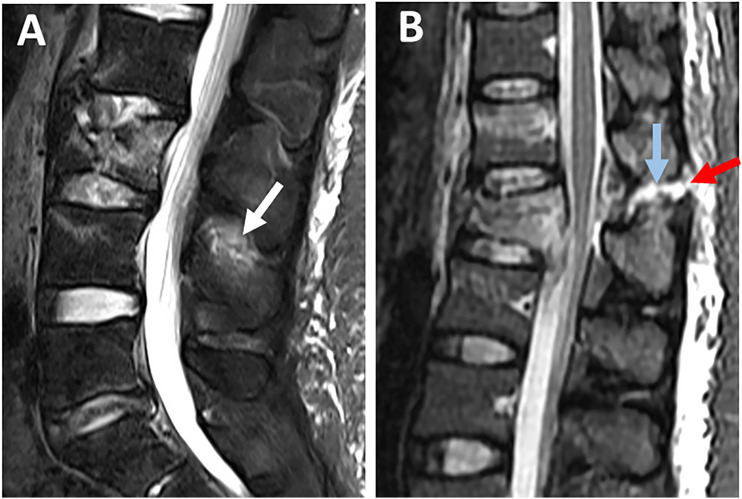
ISL is best evaluated using sagittal STIR or T2 image, which may show hyperintensity for both ISL edema and rupture. ISL edema can be differentiated from ISL rupture by the ill-defined hyperintensity as opposed to the sharp horizontal line across the ligament. 31 However, the low contribution of ISL to PLC competence makes the distinction between ISL edema and rupture of little practical importance (Figure 7A and B). 35
Figure 7.
Magnetic resonance imaging findings of interspinous ligament injury. Sagittal STIR images show (A) ill-defined high signal intensity indicating interspinous ligament edema (white arrow); (B) high signal intensity as a sharp horizontal line indicating interspinous ligament rupture (blue arrow) associated with supraspinous ligament rupture (red arrow).
Facet Joint Capsule
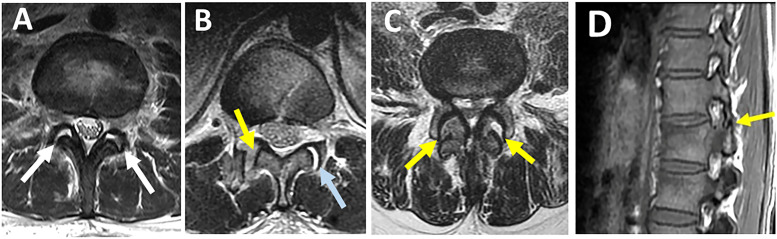
Facet joint effusion and widening represent partial capsular ligamnt injury and can be seen as hyperintensity on axial STIR or T2 images (Figures 8A and B). In contrast, facet dislocations, which indicate complete injury, are better visualized in T1 axial/sagittal images because of their high anatomical resolution (Figures 8B and D). 31 Thoracic facets' effusion is most easily seen on STIR sagittal sequences, which are perpendicular to the thoracic facets' coronal orientation. T2 axial scans, on the other hand, were the most helpful in identifying a sagittal-orientated lumbar facet injury. 26 Although FC is a major contributor to PLC competence, less emphasis was put on its MRI assessment due to low specificity (52%, Table 3). 19
Figure 8.
Magnetic resonance imaging findings of facet capsule ligament injury. Axial T2-weighted images (A-C) show (A) high signal intensity due to bilateral facet joint effusion with the preserved alignment of articular surfaces (two white arrows); (B) left facet joint effusion (blue arrow) and right facet subluxation indicating rupture of facet capsule (YELLOW arrow); (C) Bilateral dislocated facets (two YELLOW arrows); (D) Sagittal T1-weighted images are the best to depict the anatomy of subluxed facet (YELLOW arrow).
Thoracolumbar Fascia
While most research uses a "four-structure" definition of the PLC, Vaccaro et al report the thoracolumbar fascia as a fifth component. The fact that thoracolumbar fascia is the least reliable and specific (53%, Table 3) component of PLC may account for its unpopularity.18,20
Definition of PLC Injury in MRI
There is no agreement on the definition of PLC injury in MRI regarding the number of components damaged or the degree of damage (rupture vs edema). 1 There is a debate whether to define PLC injury as “black stripe discontinuity” due to SSL or LF rupture or High-signal intenisty (HSI), a broader criterion that includes any hyperintensity whether or not associated with “black stripe discontinuity”. Earlier research found HSI to be highly sensitive in detecting PLC injury when using intraoperative findings as a reference.17,36 Recent research suggests HSI may overread PLC injury due to a high false positive rate. 18 On the other hand, black stripe discontinuity demonstrated high specificity in detecting PLC injury (Table 3).19,26 Furthermore, biomechanical evidence that SSL and LF are the key components of PLC competence further supports the use of “black stripe discontinuity” to define disturbed PLC.35,37 Despite low specificity, the HSI criterion is still widely used in research, most likely to increase the sample size. 1 HSI may be easier to assess than black stripe discontinuity, which requires examining each PLC component for partial vs complete injury.6,12
MRI’s Pitfalls
A significant drawback of MRI's PLC evaluation is the reported moderate to poor interobserver reliability. 38 Differences in experience or training level could explain the moderate reliability. Another possibility is that radiologists and spine surgeons have different perspectives on the MRI sequence or interpretation. 26 MRI parameters are more challenging to standardize due to their large numbers and device-specific specifications. 25 while black stripe discontinuity is a more specific criterion than HSI; it is still imperfect. Notably, all MRI accuracy studies use intraoperative findings as a reference, restricted to the more severe surgical cases as a spectrum bias. 32 Therefore, MRI findings of PLC injury should be integrated with other findings, including X-ray and CT finidngs. 18
CT Assessment of PLC Injury
CT assessment of PLC injury depends on detecting secondary signs. 12 Table 4 summarizes CT findings suggestive of PLC injury and their accuracy measures. Following is a summary of the most important CT findings for diagnosing PLC injury.
Table 4.
Proposed Definitions, Accuracy, and Reliability of Computed Tomography Findings of Thoracolumbar Posterior Ligamentous Complex Injury.
| CT criteria | Definition | Best imaging plane/Additional | PPV | NPV | Interobserver K |
Pearls /pitfalls |
|---|---|---|---|---|---|---|
| Vertebral body translation | Relative listhesis of a vertebra compared to infreadjacent vertebra in the sagittal or coronal plane. 39 | Midsagittal /coronal | 100 40 | 79 | .623 sagittal translation, .612 coronal translation 10 | Distinguish between translation and retropulsion fragment involving inferior endplate look at the facet management |
| Facet joint Malalignment | Unilateral or bilateral Facet (s) that were perched, dislocated, or fractured.12,24 | All planes | 94 12 | 76 | .656 dislocation, 10 .57 12 | Must be reviewed in all imaging planes. |
| Facet joint widening | Unilateral or bilateral widening ≥ 3 mm in the axial CT with no evidence of facet malalignment12,24 | Axial | 36 12 | 67 | .49 axial, .36 sagittal, 10 .45 12 | It should be distinguished as it has little predictive value for PLC injury |
| Horizontal laminar fracture | Horizontally-oriented fracture (in the coronal plane) of the lamina, pars /or pedicles, whether unilateral or bilateral 35 | Coronal | 91 12 | 81 | .76 34 | Distinguish between horizontal and vertical laminar fracture best in coronal view |
| Vertical laminar fracture | Vertically oriented fracture (in the coronal plane) of the lamina located between the medial borders of both pedicles, with or with or without spinous process involvement.35,41 | Coronal | 29 12 | 65 | .91 35 | Sometimes vertical split involves the base of the spinous process: this should not be considered a spinous process fracture |
| Spinous process fracture | Avulsion or transverse fracture of the spinous process12,24 | Midsagittal/coronal | 86 12 | 81 | .89 12 | Avoid missing a tiny avulsion fracture |
| Interspinous widening | Widening of interspinous distance > 4 mm compared to the adjacent levels.12,24 | Midsagittal | 59 12 | 78 | .303, 10 .63 12 | In case of coronal plane deformity, measure it in the coronal plane |
Abbreviations: CT, Computed tomography; PPV, Positive predictive value; NPV, Negative predictive value; K, Kappa Coefficient.
CT Findings of PLC Injury
Vertebral Body Translation
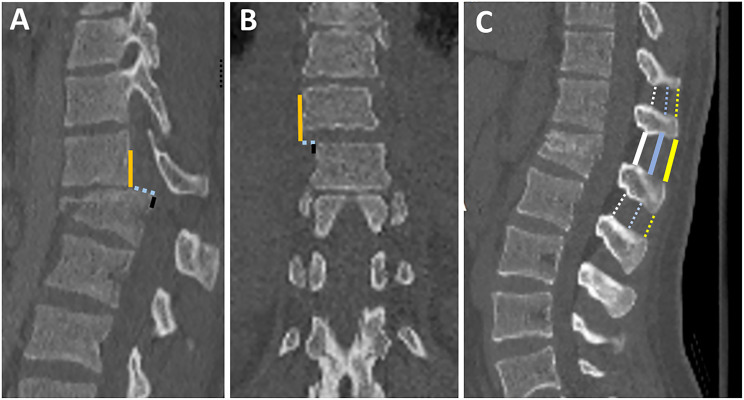
Vertebral body translation (VBT) is the anterior or lateral translation of a cranial vertebra relative to a fractured vertebra, implying severe damage to PLC and the intervertebral disc.4,39 While a standardized method for measuring VBT was reported, there is no agreed-upon threshold for VBT in TLFs (Figure 9A and B). 39 A possible explanation is that any translation in thoracolumbar region, regardless of magnitude, strongly indicates instability. 39 When a severe burst fracture involves the inferior endplate, the retropulsed fragment may migrate posteriorly relative to the caudal vertebra, mimicking translation. In that case, a key point for differentiation is to examine the facet joint to see if it is dislocated or not.
Figure 9.
Assessment of vertebral translation and interspinous widening in CT. (A) Midsagittal CT image shows the method of measurement of vertebral translation by the distance between the most cephalad, posterior corner of the caudal vertebral body (black line), and a tangent line to the posterior vertebral body of the cranial vertebra (yellow line);(B) Coronal CT image shows the method of measurement of vertebral translation by the distance between the most cephalad, lateral corner of the caudal vertebral body (black line), and a tangent line to the lateral vertebral body of the cranial vertebra (yellow line). 39 ; (C) midsagittal CT shows various methods of measurement of interspinous widening 42 (1) Supraspinous distance; the distance between the inferior edge spinous process of the upper adjacent vertebra and the superior edge spinous process of the fractured vertebra (solid yellow line) minus the average interspinous distance of the upper and lower adjacent vertebra or the lower vertebrae (2 dashed yellow lines), (2) Interspinous distance measured at the middle of the spinous process (blue lines), (2) interlaminar distance measured between the lamina (white lines).
Facet Joint Diastasis
In a survey of expert spine surgeons, facet diastasis was rated as one of the most predictive and reliable CT signs of PLC injury.7,12,14 Facet diastasis refers to a wide spectrum of injuries that includes: facet dislocation (complete uncoverage of articular surfaces), subluxed (partial uncoverage), fractured (displaced or non-displaced), or facet joint widening (FJW) (Figure 10; A-H).12,23 Controversy exists regarding the diagnostic value of subtle facet injuries such as FJW for PLC injury. Two recent studies have demonstrated that FJW > 3mm on axial CT is not a predictor of PLC injury.12,23 Facet subluxation is the most likely morphology to be overlooked for various reasons. First, facet subluxation is classically diagnosed by the naked facet sign that occurs when the inferior articular facets of the cephalad vertebra do not appear adjacent to the superior facets of the caudal vertebra in axial CT images (Figures 11A-D). However, in case of slight vertical distraction, a naked facet sign may appear in only one or few axial cuts and could be easily overlooked. 43 (Figures 12A-D) Additionally, Harris et al pointed out that the naked facet sign can occur due to minor angulation or flexion and does not always indicate the presence of PLC injury. 44 Thoracolumbar facets have a variety of sagittal/coronal orientations, so they may dislocate in any plane and be easily missed unless all imaging planes are carefully examined (Figures 12A-D). 45 Second, facet diastasis may reduce in supine position; however, even when reduced, there is an asymmetry in the facets that can be observed on a careful review of the axial CT images. 7
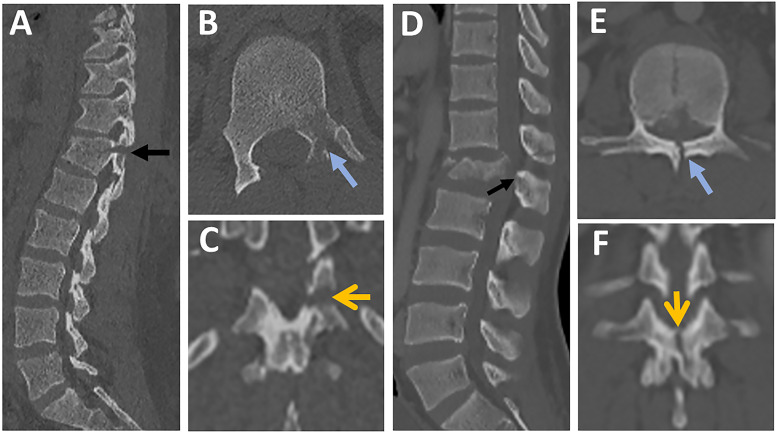
Figure 10.
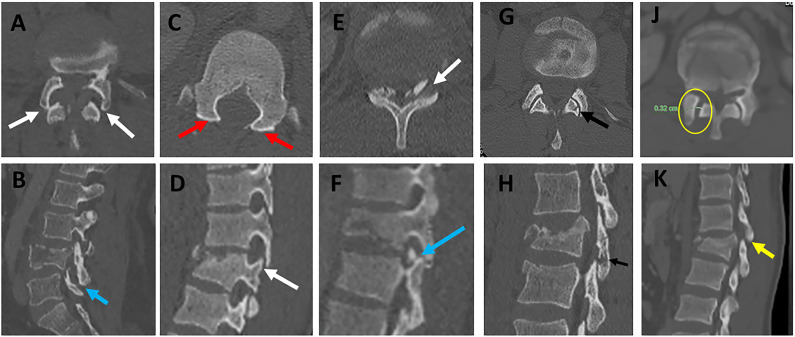
Various patterns of facet diastasis in CT. Facet dislocation (A, B ) seen in CT axial images show (2 white arrows, A) and sagittal CT image (blue arrow, B); Facet Subluxation (C, D ) seen in CT axial images as bilateral naked facet sign (2 red arrows, C) and sagittal CT image as a vertical distraction (white arrow, D); Displaced facet fracture (E, F) seen in CT axial images (white arrows, E) and sagittal CT image as a displaced fracture of the left superior articular facet (blue arrow, F); Non-displaced facet fracture (G, H) is seen in CT axial images (black arrow, E) and sagittal CT images as a non-displaced fracture of the left inferior articular facet (black arrow, F); Facet joint widening (J, K) is seen in CT axial images as widening of the facet joint > 3mm with the preserved alignment of articular surfaces (yellow circle, J) and sagittal CT images (green arrow, K). Abbreviations: CT, computed tomography.
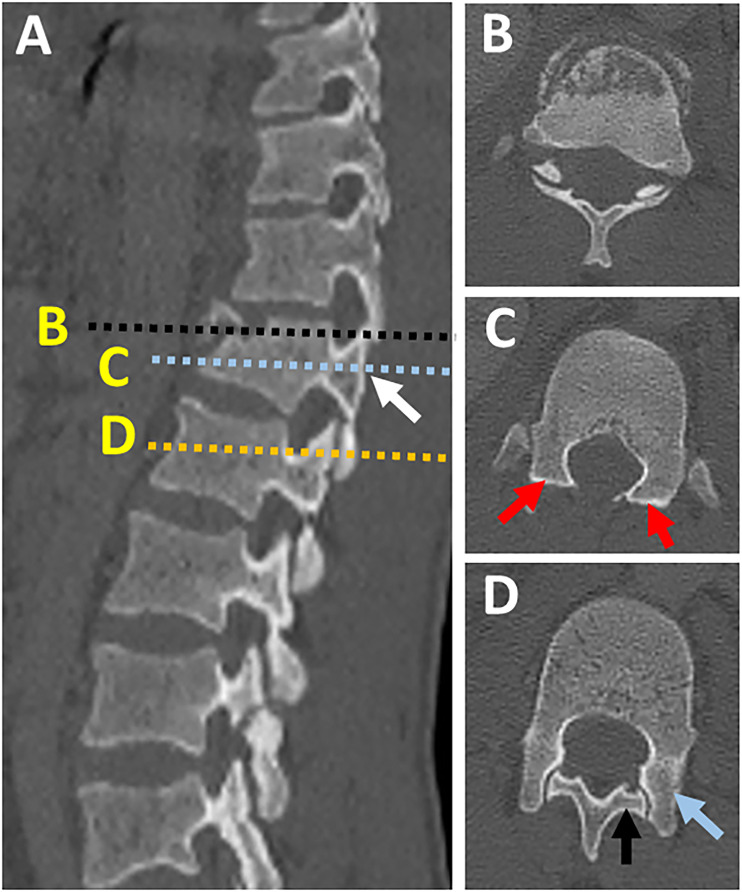
Figure 11.
Naked facet sign. (A) CT parasagittal images showing vertical facet distraction with partial uncoverage of superior articular process (white arrow); (B) Axial CT cut at the level of overlapping facets ( black dashed line in image A) shows the overlap of superior and inferior articular process, (C) axial CT images at the level of uncovered superior articular facet (dashed blue line in image A) shows naked facet sign (two red arrows), (D) axial CT images at the normal level below (dashed yellow line in image) shows the normal alignment of the facet joint with the superior facet of the level blow directed posteromedially (black arrow) and inferior articular facet of the same level directed anterolaterally (blue arrow). Abbreviations: CT, computed tomography.
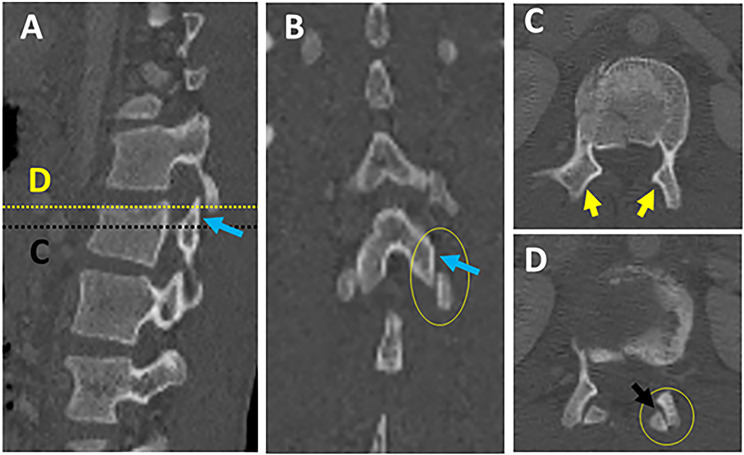
Figure 12.
Multiplanar CT assessment of facet subluxation (A-B). (A-B) CT parasagittal and coronal reconstruction images showing vertical facet distraction with partial uncoverage of superior articular facet (blue arrows) ; (C) axial CT images at the level of uncovered superior articular facet (dashed black line in image A) show naked facet sign (2 yellow arrows); (D) axial CT images at the level of uncovered superior articular Facet (dashed yellow line in image A) shows the overlap of superior and inferior articular facet with posterior subluxation of an inferior articular facet (black arrow). Abbreviations: CT, computed tomography.
Laminar Fracture
The AOSpine has recognized two types of laminar fractures based on their distinct pathomechanics. 4 Horizontal laminar fracture (HLF) implies a flexion-distraction mechanism of injury, indicating AO type B. 4 Vertical laminar fracture (VLF), on the other hand, results from severe axial loading with the transfer of energy to posterior elements and vertical split fractures. VLF does not constitute PLC damage; consequently, it is a feature of burst fractures (Figures 13A-K).4,46 According to a recent study, HLF, but not VLF, was independently predictive of PLC injury (PPV of 91% vs 48%). 34 The following features of HLF were highly associated with PLC injury: displacement > 2mm, bilateral laminar fractures, and laminar, and pedicle fractures. 34 A common pitfall is failing to differentiate between HLF and VLF when images are read only in axial images without carefully reviewing the coronal reconstruction images.34,41 Indeed, because of their lower prevalence and horizontal orientation, HLF is more likely to be missed, resulting in missing PLC injury. 34 The 3- columns Denis' concept, which remains popular, implies that all burst fractures with laminar fractures, regardless of type, are unstable, hence undermining the importance of distinguishing between vertical and horizontal laminar fractures. 47 Understanding that HLF and VLF, while morphologically similar, are the footprints of distinct injury mechanisms may help to reduce misinterpretation. 48
Figure 13.
The distinction between horizontal and vertical laminar fractures in CT. (A-C) images from the same patient showing (A) Parasagittal CT image showing a displaced fracture of lamina and pedicle ( black arrow); (B) Axial CT image showing a fracture of the left lamina and pedicle ( blue arrow); (C) Coronal reconstruction images showing left horizontal lamina fracture (yellow arrow); (D-F) images from the same patient showing (D) Parasagittal CT image showing a fracture of lamina and base of the spinous process (black arrow); (E) Axial CT image showing a vertical fracture of the lamina ( blue arrow); (F) Coronal reconstruction images showing the vertical orientation of the laminar fracture (yellow arrow). Abbreviations: CT, computed tomography.
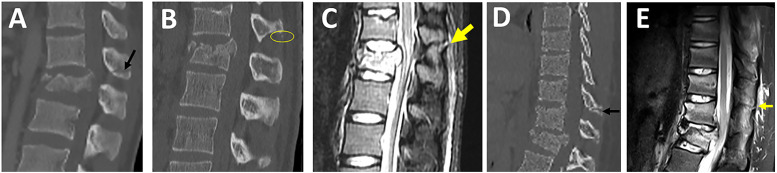
Spinous Process Fractures
Avulsion, transverse, or oblique spinous process fracture (SPF) is one of the most reliable and predictive signs of PLC injury. 12 SPF is best seen in sagittal images, but fractures can be seen in axial, coronal, or sagittal images depending on the orientation of the fracture lines (Figures 14A-D). The most common pitfall is missing or ignoring a tiny avulsion SPF, which is highly predictive of PLC injury (Figures 14B and C). Spinous process base fracture may be caused by a vertical split associated with axial loading, but this does not constitute PLC. It is crucial to differentiate SPF from non-united ossification centers within the spinous process's superior or inferior corner, typically with smooth and well-corticated margins. 49 In osteoporotic patients, SPF can occur even with low-energy trauma and hence are less predictive of PLC injury.
Figure 14.
Evaluation of spinous process fractures in CT. Midsagittal CT image showing (A) transverse spinous process fracture (black arrow), (B-C) images from the same patient showing a tiny avulsion spinous process fracture (yellow circle, B) with corresponding supraspinous ligament rupture seen in Sagittal-T2-STIR (yellow arrow, C); (D-E) images from the same patient showing a midsagittal CT shows a spinous process fracture two levels above the burst fracture (black arrow, D) with corresponding supraspinous ligament rupture seen in Sagittal-T2-STIR (yellow arrow, E). Abbreviations: CT, computed tomography; STIR: short tau inversion-recovery.
Interspinous Widening
ISW is defined as widening of interspinous distance compared to the adjacent levels, best seen in the midsagittal plane (Figure 9C). 12 Although ISW is considered an important sign of PLC injury; it is the most contentious finding regarding variations in measurement technique or threshold and measurement errors (Figure 9C).7,8,48A wide range of ISW's thresholds (2-7 mm) can be attributed to interindividual and regional differences.50,51 Another explanation is that ISW may vary between supine CT and 48 standing X-ray. Rjasakaran et al reported various ISW's thresholds trade-off sensitivity and specificity and found that > 4 mm yielded a specificity of 90%.7,8,12,50 ISW may be difficult to measure in the presence of out-of-plane coronal deformity; in this case, ISW measurements in coronal images may be beneficial. 39
CT Criteria to Define PLC Injury
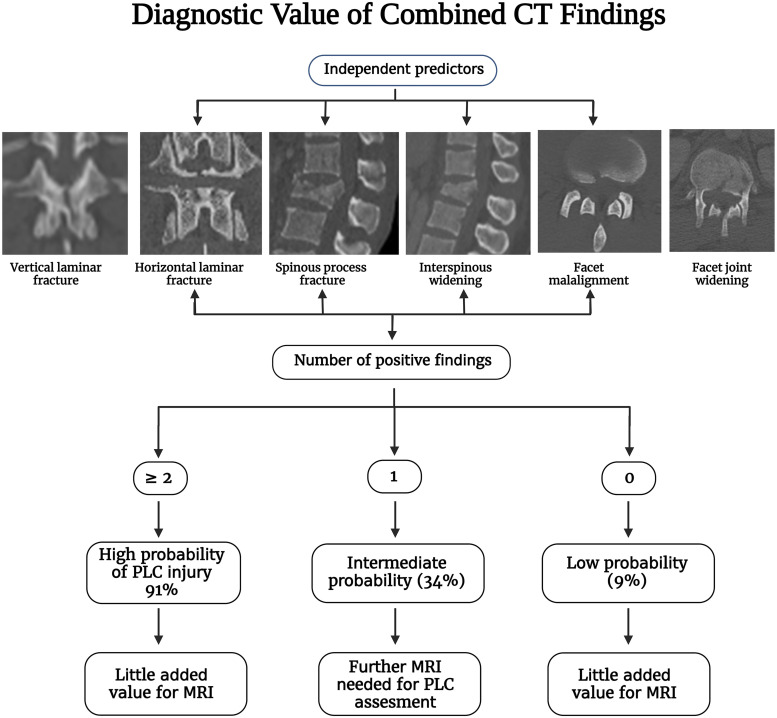
The greatest challenge in CT's evaluation of PLC assessment is the lack of agreement on CT criteria for PLC injury. 1 The typical flexion distraction morphology, i.e., horizontal osteoligamentous disruption, is used to diagnose PLC injury in CT.3,4 However, this morphology is frequently lacking, leading to disagreement about PLC status in CT.13,14 Two recent studies have proposed to define PLC injury in CT based on the diagnostic value of combined CT findings.12,23 Such combined analysis reflects the different clinical scenarios encountered in clinical practice; patients may present with one or more CT findings.12,23 Vertebral translation, facet joint malalignment, spinous process fracture, horizontal laminar fracture, and interspinous widening were independent predictors of PLC injury. FJW and VLF were not associated with PLC injury and are most likely responsible for most falsely identified PLC injuries in CT. At least two CT findings have a PPV of 88-91% for PLC injury, suggesting its use as a CT criterion for PLC injury and little added value for MRI testing (Figure 15).12,23 In addition, the absence of all four CT findings provided the best ability to rule out PLC injury (A negative predictive value of 91%).12,23 The presence of single CT findings lacked sufficient PPV or NPV to rule in or rule out PLC injury; hence it can be used to indicate indeterminate PLC injury in CT and the need for further MRI testing. Currently, there are no clear guidelines on when to do an MRI for PLC assessment. 5 MRI has been shown in previous studies to change TLF classification or treatment decisions in up to 30% of cases.22,24
Figure 15.
Diagnostic value of combined computed tomography findings for thoracolumbar posterior ligamentous complex assessment.
Pitfalls of Interpretations of PLC Injury in CT
Osteoporosis
Osteoporosis may reduce the accuracy of CT in detecting HLF or SPF. Furthermore, osteoporosis may cause osseous injuries out of proportion to PLC injury, lowering the PPV of CT findings for PLC injury. 32 For instance, in osteoporotic patients, SPF can occur even with low-energy trauma and hence are less predictive of PLC injury. However, the impact of osteoporosis on CT accuracy is difficult to assess because not all patients have DEXA scans in the trauma setting. 6
Multiple Injuries
In case of multiple contiguous injuries, HLF or SPF may occur proximal to the most severe fracture level depending on the injury vector and thus go unnoticed (Figures 14A-D). Non-contiguous fractures should be assessed independently because PLC injury at a second level may be treated separately or lead to instrumentation extension.
Supine CT vs. Standing X-Ray
Supine CT may be less sensitive than standing X-ray for detecting ISW and facet subluxation due to reduced bone displacement in the supine position. This may explain why even a subtle subluxation of the facet joints on CT may be highly predictive of PLC injury. 7
Interpretation Morphology vs Mechanism Based
Because CT interpretation of PLC necessitates a thorough understanding of 3D imaging anatomy and pathomechanics, it may vary depending on experience and training. 34 Furthermore, in addition to the morphological interpretation of CT findings, interpretation based on the presumed mechanism of injury is critical.34,48 The distinction between HLF and VLF is a perfect example based on their distinct pathomechanics: flexion-distraction vs axial compression.
Interregional Variations
Most studies evaluating CT accuracy for detecting PLC injury include a mixed cohort of thoracic and lumbar fractures without accounting for anatomical and biomechanical variations of the regions.52,53 Aly et al have shown that CT may be highly accurate (95%) in detecting PLC injury in lower lumbar fractures (L3-L5) because of the lumbar lordosis and inherent stability of this region. 40
Suggested Step-by-step Algorithm for MRI and CT Assessment of PLC Injury
MRI Assessment of PLC Injury
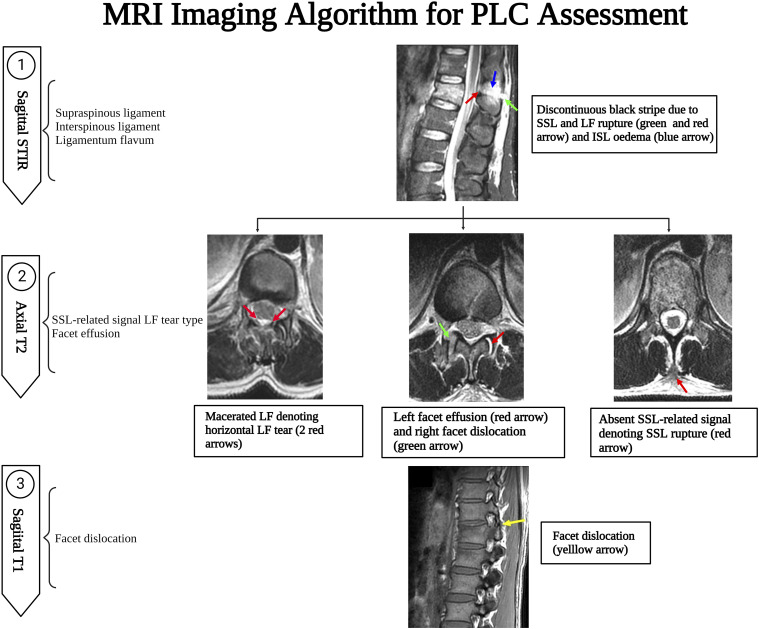
A suggested algorithm for MRI PLC assessment is provided in Figure 16. Assessing the four PLC structures in all imaging sequences and planes may be time-consuming, which could explain the low reliability. As a result, focusing on the key PLC components and the most sensitive sequences is critical for developing a simple, yet efficient algorithm for MRI interpretation. 26 SSL and LF are the key PLC structures because of their biomechanical importance as well as the high accuracy of MRI. In contrast, ISL and FC are less critical because of limited biomechanical importance or poor specificity of MRI, respectively. 24 The sagittal STIR is the most useful in screening for SSL, ISL, and LF injuries, which are sagittally oriented due to their high sensitivity to oedema (Figure 16). 26 T2 axial is the second most crucial sequence as it can incorporate the discontinuous black stripe in sagittal STIR with the absence of SSL associated with diffusely macerated LF. 7 In most cases, these two sequences adequately evaluate PLC damage. T1 axial /sagittal is the most sensitive to detect malalignment of facet joints due to its superb ability to detect anatomical details. 24 However, facet malignment is difficult to assess in MRI and may be the least reliable and sensitive finding of PLC injury.
Figure 16.
Magnetic resonance imaging imaging algorithm for thoracolumbar posterior ligamentous complex assessment.
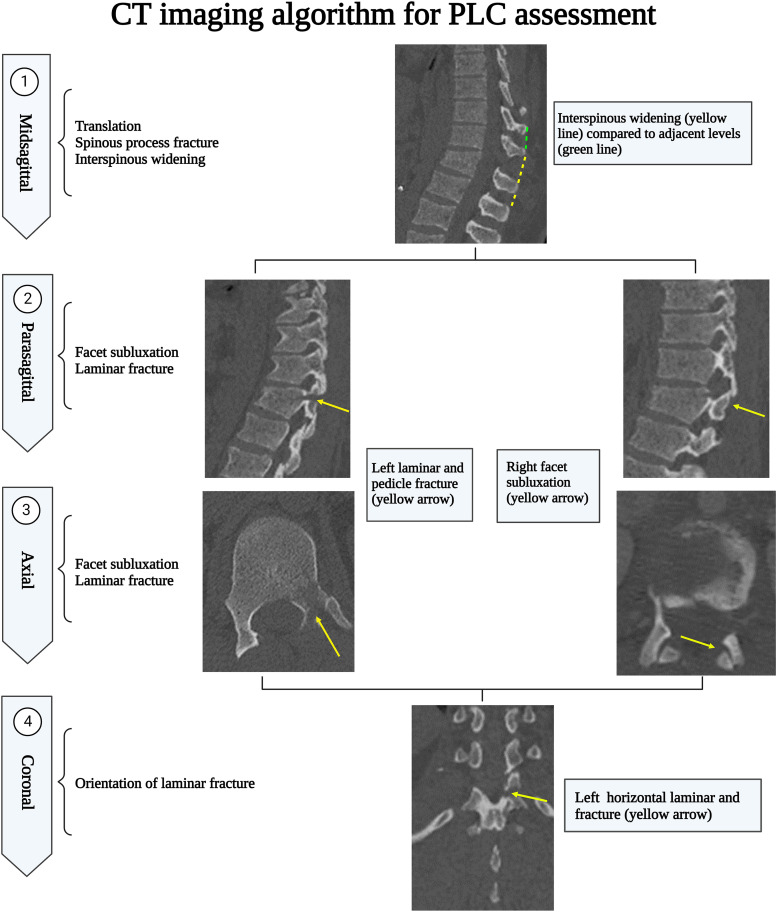
CT Assessment of PLC Injury
A suggested algorithm for MRI PLC assessment is provided in Figure 17. A CT algorithm focuses on the CT findings independently associated with PLC injury and the best plane for detecting them. The midsagittal CT plane is the most useful in screening for VBT, ISW, and SPF. Then a parasagittal plane helps screen laminar fracture and facet misalignment. However, in case of a laminar fracture, coronal reconstruction images are the best to display the fracture line orientation to distinguish VLF from HLF, which is clinically significant. 34 Facet subluxations are the most likely to be overlooked; hence all image planes should be checked for malalignment of any facet articular surfaces. Finally, axial CT may be the most useful to measure FJW because of its orientation. 23 FJW and VLF are the most likely findings to identify PLC injury falsely and, therefore, should be distinguished from HLF and other types of facet malalignment. 12
Figure 17.
Computed tomography imaging algorithm for thoracolumbar posterior ligamentous complex assessment.
Limitations
The systematic review was limited by data heterogeneity, precluding a meta-analysis from being performed. Another limitation of data heterogeneity is the inconsistent definitions of reference standards because some studies used operative findings to define PLC injury while others used MRI findings or surgeon consensus. Moreover, there is still a need for studies that provide a higher level of evidence to help guide decision-making utilizing available imaging options. While only English articles were included, which may have limited the global generalizability of these studies, there is no evidence of systematic biases due to language restrictions in medical meta-analyses. Finally, the proposed CT and MRI algorithms were developed based on the authors’ own experience. As a result, before being widely implemented in clinical practice, those algorithms should be validated in a multicenter prospective agreement study.
Conclusion
We developed a systematic imaging algorithm for detecting PLC injury in CT and MRI. The pitfalls of image interpretation, as well as pearls for avoiding them, were thoroughly discussed. Furthermore, the predictive value of various CT findings and their morphological variations were discussed based on the available literature. A consensus-based definition of CT findings and a more detailed analysis of their predictive value for PLC injury are required. The implementation of those CT and MRI algorithm will potentially improve the accuracy and reliability of PLC assessment. However, before being widely implemented in clinical practice, those algorithms should be validated in a multicenter prospective reliability study.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Mohamed M. Aly https://orcid.org/0000-0001-5221-8288
Tariq Ziad Issa https://orcid.org/0000-0002-0978-5225
References
- 1.Van Middendorp JJ, Patel AA, Schuetz M, Joaquim AF. The precision, accuracy and validity of detecting posterior ligamentous complex injuries of the thoracic and lumbar spine: A critical appraisal of the literature. Eur Spine J. 2013;22(3):461-474. doi: 10.1007/s00586-012-2602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaccaro AR, Lim MR, Hurlbert RJ, et al. Surgical decision making for unstable thoracolumbar spine injuries. J Spinal Disord Tech. 2006;19(1):1-10. doi: 10.1097/01.bsd.0000180080.59559.45. [DOI] [PubMed] [Google Scholar]
- 3.Vaccaro AR, Lehman RA, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: The importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa1976). 2005;30(20):2325-2333. doi: 10.1097/01.brs.0000182986.43345.cb. [DOI] [PubMed] [Google Scholar]
- 4.Vaccaro AR, Oner C, Kepler CK, et al. AOSpine thoracolumbar spine injury classification system: Fracture description, neurological status, and key modifiers. Spine (Phila Pa 1976). 2013;38(23):2028-2037. doi: 10.1097/BRS.0b013e3182a8a381. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi S, Dhall SS, Anderson PA, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the evaluation and treatment of patients with thoracolumbar spine trauma: Radiological evaluation. Clin Neurosurg. 2019;84(1):E28-E31. doi: 10.1093/neuros/nyy373. [DOI] [PubMed] [Google Scholar]
- 6.Aly MM, Al-Shoaibi AM, Abduraba Ali S, Al Fattani A, Eldawoody H. How often would MRI change the thoracolumbar fracture classification or decision-making compared to CT alone? Glob spine J . Published online April. 2022;5:219256822210895. doi: 10.1177/21925682221089579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JY, Vaccaro AR, Schweitzer KM, et al. Assessment of injury to the thoracolumbar posterior ligamentous complex in the setting of normal-appearing plain radiography. Spine J. 2007;7(4):422-427. doi: 10.1016/j.spinee.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Rajasekaran S, Maheswaran A, Aiyer SN, Kanna R, Dumpa SR, Shetty AP. Prediction of posterior ligamentous complex injury in thoracolumbar fractures using non-MRI imaging techniques. Int Orthop. 2016;40(6):1075-1081. doi: 10.1007/s00264-016-3151-1. [DOI] [PubMed] [Google Scholar]
- 9.Kepler CK, Vaccaro AR, Koerner JD, et al. Reliability analysis of the AOSpine thoracolumbar spine injury classification system by a worldwide group of naïve spinal surgeons. Eur Spine J. 2016;25(4):1082-1086. doi: 10.1007/s00586-015-3765-9. [DOI] [PubMed] [Google Scholar]
- 10.Barcelos ACES, Joaquim AF, Botelho RV. Reliability of the evaluation of posterior ligamentous complex injury in thoracolumbar spine trauma with the use of computed tomography scan. Eur Spine J. 2016;25(4):1135-1143. doi: 10.1007/s00586-016-4377-8. [DOI] [PubMed] [Google Scholar]
- 11.Aly MM, Elemam R, El-Sharkawi M, Hurlbert JR. Injury of the thoracolumbar posterior ligamentous complex : a bibliometric literature review. World Neurosurg . 2022;161:21-33. doi: 10.1016/J.WNEU.2022.01.041. [DOI] [PubMed] [Google Scholar]
- 12.Aly MM, Al-Shoaibi AM, Alzahrani AJ, Al Fattani A. Analysis of the combined computed tomography findings improves the accuracy of computed tomography for detecting posterior ligamentous complex injury of the thoracolumbar spine as defined by magnetic resonance imaging. World Neurosurg. 2021;151:e760-e770. doi: 10.1016/j.wneu.2021.04.106. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder GD, Kepler CK, Koerner JD, et al. A worldwide analysis of the reliability and perceived importance of an injury to the posterior ligamentous complex in AO type a fractures. Glob Spine J. 2015;5(5):378-382. doi: 10.1055/s-0035-1549034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweitzer KM, Vaccaro AR, Harrop JS, et al. Interrater reliability of identifying indicators of posterior ligamentous complex disruption when plain films are indeterminate in thoracolumbar injuries. J Orthop Sci. 2007;12(5):437-442. doi: 10.1007/s00776-007-1155-9. [DOI] [PubMed] [Google Scholar]
- 15.Lewkonia P, Paolucci EO, Thomas K. Reliability of the thoracolumbar injury classification and severity score and comparison with the denis classification for injury to the thoracic and lumbar spine. Spine (Phila Pa 1976). 2012;37(26):2161-2167. doi: 10.1097/BRS.0b013e3182601469. [DOI] [PubMed] [Google Scholar]
- 16.Ratliff J, Anand N, Vaccaro AR, et al. Regional variability in use of a novel assessment of thoracolumbar spine fractures: United States versus international surgeons. World J Emerg Surg. 2007;2(1):1-9. doi: 10.1186/1749-7922-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haba H, Taneiciu H, Kotani Y, et al. Diagnostic accuracy of magnetic resonance imaging for detecting posterior ligamentous complex injury associated with thoracic and lumbar fractures. J Neurosurg. 2003;99(1 suppl L):20-26. doi: 10.3171/spi.2003.99.1.0020. [DOI] [PubMed] [Google Scholar]
- 18.Vaccaro AR, Rihn JA, Saravanja D, et al. Injury of the posterior ligamentous complex of the thoracolumbar spine: A prospective evaluation of the diagnostic accuracy of magnetic resonance imaging. Spine (Phila Pa 1976). 2009;34(23):841-847. doi: 10.1097/BRS.0b013e3181bd11be. [DOI] [PubMed] [Google Scholar]
- 19.Javier P, Sánchez-Mariscal F, Zúñiga L, Patricia Álvarez EI. Prospective analysis of magnetic resonance imaging accuracy in diagnosing traumatic injuries of the posterior ligamentous complex of the thoracolumbar spine. Spine (Phila Pa 1976). 2013;38(9):745-751. [DOI] [PubMed] [Google Scholar]
- 20.Mehta G, Shetty UC, Meena D, Tiwari AK, Nama KG, Aseri D. Evaluation of diagnostic accuracy of magnetic resonance imaging in posterior ligamentum complex injury of thoracolumbar spine. Asian Spine J. 2021;15(3):333-339. doi: 10.31616/ASJ.2020.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee GY, Lee JW, Choi SW, et al. MRI inter-reader and intra-reader reliabilities for assessing injury morphology and posterior ligamentous complex integrity of the spine according to the thoracolumbar injury classification system and severity score. Korean J Radiol. 2015;16(4):889-898. doi: 10.3348/kjr.2015.16.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winklhofer S, Thekkumthala-Sommer M, Schmidt D, et al. Magnetic resonance imaging frequently changes classification of acute traumatic thoracolumbar spine injuries. Skeletal Radiol. 2013;42(6):779-786. doi: 10.1007/s00256-012-1551-x. [DOI] [PubMed] [Google Scholar]
- 23.Khurana B, Prevedello LM, Bono CM, et al. CT for thoracic and lumbar spine fractures: Can CT findings accurately predict posterior ligament complex injury? Eur Spine J. 2018;27(12):3007-3015. doi: 10.1007/s00586-018-5712-z. [DOI] [PubMed] [Google Scholar]
- 24.Pizones J, Izquierdo E, Álvarez P, et al. Impact of magnetic resonance imaging on decision making for thoracolumbar traumatic fracture diagnosis and treatment. Eur Spine J. 2011;20(suppl 3):390-396. doi: 10.1007/s00586-011-1913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedetti PF, Fahr LM, Kuhns LR, Hayman LA. MR imaging findings in spinal ligamentous injury. Am J Roentgenol. 2000;175(3):661-665. doi: 10.2214/ajr.175.3.1750661. [DOI] [PubMed] [Google Scholar]
- 26.Crosby CG, Even JL, Song Y, Block JJ, Devin CJ. Diagnostic abilities of magnetic resonance imaging in traumatic injury to the posterior ligamentous complex: The effect of years in training. Spine J. 2011;11(8):747-753. doi: 10.1016/j.spinee.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Pizones J, Castillo E. Assessment of acute thoracolumbar fractures: Challenges in multidetector computed tomography and added value of emergency MRI. Semin Musculoskelet Radiol. 2013;17(4):389-395. doi: 10.1055/s-0033-1356468. [DOI] [PubMed] [Google Scholar]
- 28.Khurana B, Karim SM, Zampini JM, et al. Is focused magnetic resonance imaging adequate for treatment decision making in acute traumatic thoracic and lumbar spine fractures seen on whole spine computed tomography? Spine J. 2019;19(3):403-410. doi: 10.1016/j.spinee.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Tins B. Technical aspects of CT imaging of the spine. Insights Imaging. 2010;1(5–6):349-359. doi: 10.1007/s13244-010-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sixta S, Moore FO, Ditillo MF, et al. Screening for thoracolumbar spinal injuries in blunt trauma: An eastern association for the surgery of trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 suppl 4). doi: 10.1097/TA.0b013e31827559b8. [DOI] [PubMed] [Google Scholar]
- 31.Pizones J, Izquierdo E, Sánchez-Mariscal F, Zúñiga L, Álvarez P, Gómez-Rice A. Sequential damage assessment of the different components of the posterior ligamentous complex after magnetic resonance imaging interpretation: Prospective study 74 traumatic fractures. Spine (Phila Pa 1976). 2012;37(11). doi: 10.1097/BRS.0b013e3182422b2b. [DOI] [PubMed] [Google Scholar]
- 32.Pizones J, Zúniga L, Sánchez-Mariscal F, Lvarez PA, Gómez-Rice A, Izquierdo E. MRI study of post-traumatic incompetence of posterior ligamentous complex: Importance of the supraspinous ligament. Prospective study of 74 traumatic fractures. Eur Spine J. 2012;21(11):2222-2231. doi: 10.1007/s00586-012-2403-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson GM, Zhang M. Regional differences within the human supraspinous and interspinous ligaments: A sheet plastination study. Eur Spine J. 2002;11(4):382-388. doi: 10.1007/s00586-001-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aly MM, Al-Shoaibi AM, Al Fattani A, AlJuzair AH. Diagnostic value of various morphological features of horizontal and vertical laminar fractures for posterior ligamentous complex injury of the thoracolumbar spine as defined by MRI. World Neurosurg. 2021;153:e290-e299. doi: 10.1016/j.wneu.2021.06.109. [DOI] [PubMed] [Google Scholar]
- 35.Wu CC, Jin HM, Yan YZ, et al. Biomechanical role of the thoracolumbar ligaments of the posterior ligamentous complex: A finite element study. World Neurosurg. 2018;112(2018):e125-e133. doi: 10.1016/j.wneu.2017.12.171. [DOI] [PubMed] [Google Scholar]
- 36.Vaccaro AR, Lee JY, Schweitzer KM, et al. Assessment of injury to the posterior ligamentous complex in thoracolumbar spine trauma. Spine J. 2006;6(5):524-528. doi: 10.1016/j.spinee.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Shen Z, Huang M, Wang X. Stepwise resection of the posterior ligamentous complex for stability of a thoracolumbar compression fracture. Med (United States). 2017;96(35). doi: 10.1097/MD.0000000000007873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rihn JA, Yang N, Fisher C, et al. Using magnetic resonance imaging to accurately assess injury to the posterior ligamentous complex of the spine: A prospective comparison of the surgeon and radiologist. J Neurosurg Spine. 2010;12(4):391-396. doi: 10.3171/2009.10.SPINE08742. [DOI] [PubMed] [Google Scholar]
- 39.Keynan O, Fisher CG, Vaccaro A, et al. Radiographic measurement parameters in thoracolumbar fractures: A systematic review and consensus statement of the spine trauma study group. Spine (Phila Pa 1976). 2006;31(5):156-165. doi: 10.1097/01.brs.0000201261.94907.0d. [DOI] [PubMed] [Google Scholar]
- 40.Aly MM, Al-Shoaibi AM, Abduraba S, Alzahrani AJ, Eldawoody H. Traumatic low lumbar fractures: How often MRI changes the fracture classification or clinical decision-making compared to CT alone? Eur Spine J. Published online October. 2021;8:37-45. doi: 10.1007/S00586-021-06987-X. [DOI] [PubMed] [Google Scholar]
- 41.Xu JX, Zhou CW, Tang Q, et al. The correlation between vertical laminar fractures and the severity of associated burst fractures. World Neurosurg. 2018;109:e829-e834. doi: 10.1016/j.wneu.2017.10.107. [DOI] [PubMed] [Google Scholar]
- 42.Jiang L, Zhang H, Chen H, Wu Q. Kyphotic angle of the motion segment most accurately predicts injury to the ligamentous complex on computed tomography scan of thoracolumbar fractures. World Neurosurg. 2018;118:e405-e413. doi: 10.1016/j.wneu.2018.06.202. [DOI] [PubMed] [Google Scholar]
- 43.O’Callaghan JP, Ullrich CG, Yuan HA, Kieffer SA. CT of facet distraction in flexion injuries of the thoracolumbar spine: The “naked” facet. Am J Neuroradiol. 1980;1(1):97-102. [DOI] [PubMed] [Google Scholar]
- 44.Harris MB, Stelly MV, Villarraga ML, Schroeder AC, Thomas KA. Modeling of the naked facet sign in the thoracolumbar spine. J Spinal Disord. 2001;14(3):252-258. doi: 10.1097/00002517-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Manaster BJ, Osborn AG. CT patterns of facet fracture dislocations in the thoracolumbar region. Am J Roentgenol. 1987;148(2):335-340. doi: 10.2214/ajr.148.2.335. [DOI] [PubMed] [Google Scholar]
- 46.Aly MM, Al-Shoaibi AM, Al-Aithan A, et al. Can vertical laminar fracture further discriminate fracture severity between thoracolumbar AO type A3 and A4 fractures? World Neurosurg. 2021;155:e177-e187. doi: 10.1016/J.WNEU.2021.08.035. [DOI] [PubMed] [Google Scholar]
- 47.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8(8):817-831. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Daffner RH, Deeb ZL, Rothfus WE. Fingerprints” of vertebral trauma-a unifying concept based on mechanisms. Skeletal Radiol. 1986;15(7):518-525. doi: 10.1007/BF00361047. [DOI] [PubMed] [Google Scholar]
- 49.Mellado JM, Larrosa R, Martín J, Yanguas N, Solanas S, Cozcolluela MR. MDCT of variations and anomalies of the neural arch and its processes: Part 1 - Pedicles, pars interarticularis, laminae, and spinous process. Am J Roentgenol. 2011;197(1):104-113. doi: 10.2214/AJR.10.5803. [DOI] [PubMed] [Google Scholar]
- 50.Jackson ER, Lador R, Ben-Galim PJ, Reitman CA, Hipp JA. Reference data for interpreting widening between spinous processes in the lumbar spine. Spine J. 2011;11(4):336-339. doi: 10.1016/j.spinee.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Chen JX, Goswami A, Xu DL, et al. The radiologic assessment of posterior ligamentous complex injury in patients with thoracolumbar fracture. Eur Spine J. 2017;26(5):1454-1462. doi: 10.1007/s00586-016-4687-x. [DOI] [PubMed] [Google Scholar]
- 52.Schroeder GD, Kepler CK, Koerner JD, et al. Can a thoracolumbar injury severity score be uniformly applied from T1 to L5 or are modifications necessary? Glob Spine J. 2015;5(4):339-345. doi: 10.1055/s-0035-1549035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore TA, Bransford RJ, France JC, et al. Low lumbar fractures: Does thoracolumbar injury classification and severity score work? Spine (Phila Pa 1976). 2014;39(17):1021-1025. doi: 10.1097/BRS.0000000000000415. [DOI] [PubMed] [Google Scholar]