Abstract
Tumor tissues consist of tumor cells and tumor stroma, which is structured by non-tumor cells and the extracellular matrix. Macrophages are the predominant immune cells in the tumor microenvironment (TME). Based on the intimate interaction between macrophages and tumor cells, macrophages are closely involved in tumor initiation and progression, playing a key role in tumor formation, angiogenesis, metastasis, and immune escape. Extracellular vesicles (EVs) are a group of membrane-enclosed structures secreted by almost all cell types. As crucial mediators of cell-to-cell communication, EVs play a role in various physiological processes and the development of diseases including cancer. According to numerous studies, tumor cell-derived extracellular vesicles (T-EVs) could highly modulate the phenotypes and functions of macrophages, thus promoting tumor development. Herein, we comprehensively introduce the role of T-EVs in regulating the M1/M2 phenotypes and immune functions of macrophages, including cytokine secretion, expression of immune regulatory molecules on the membrane, phagocytosis, and antigen presentation. More importantly, based on the regulatory effects of T-EVs on macrophages, we propose several potential therapeutic approaches that may guide future attempts to increase the effectiveness of cancer therapy.
Keywords: Extracellular vesicles, tumor cell, macrophage, intercellular communication, therapeutic application
Introduction
Tumor tissue consists of tumor cells and tumor stroma. Tumor stroma contains the extracellular matrix and various non-tumor cells, including fibroblasts, endothelial cells, and immune cells (e.g., macrophages and T cells), forming a complex tumor microenvironment (TME) 1, 2. In the TME, tumor cells and stromal cells interact in various ways, including direct cell-to-cell contact, the production of soluble protein-based factors, and the release of extracellular vesicles (EVs) 3. This intercellular communication plays a key role in tumor growth, invasion, and metastasis 1.
As the predominant immune cells within the TME 4-6, through the intimate and extensive interactions with tumor cells, macrophages are closely involved in tumor initiation and progression, playing a key role in tumor formation, angiogenesis, metastasis, and immune escape 7. Macrophages in the TME are mainly recruited from circulating monocytes and are often referred to as tumor-associated macrophages (TAMs). Notably, macrophages are highly plastic and can change their phenotypes and functions in response to environmental stimuli. EVs are a group of membrane-enclosed structures secreted by almost all types of cells 8. As crucial mediators of cell-to-cell communication, EVs play a role in various physiological processes and the development of diseases, including cancer 9, 10. According to numerous studies, tumor cell-derived EVs (T-EVs) could effectively modulate the phenotypes and functions of macrophages through multiple cargos, thus promoting tumor development (Figure 1). Targeting the interactions between tumor cells and macrophages has been a hot area of research in cancer therapy.
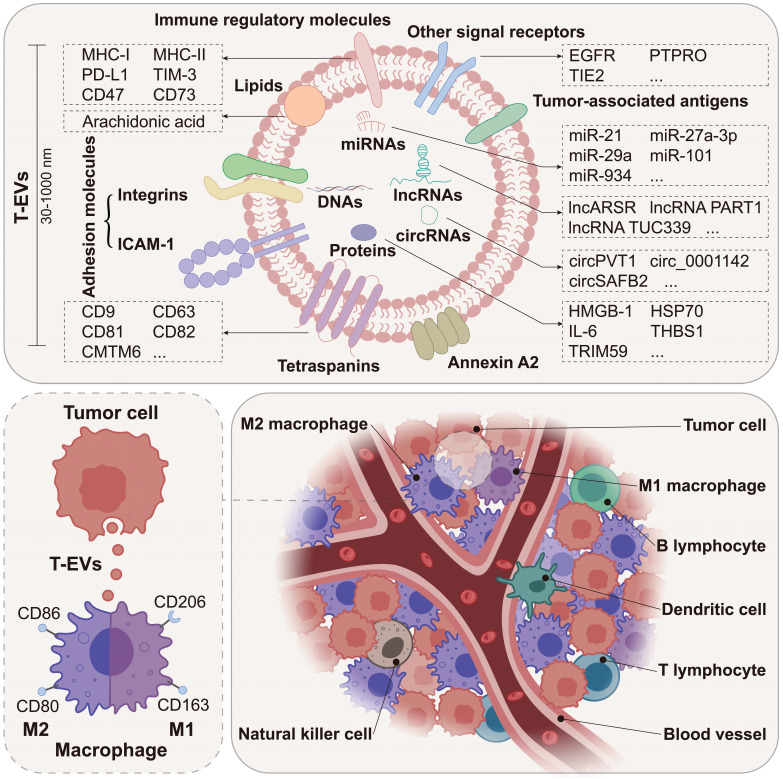
Figure 1.
T-EVs deliver multiple cargos to macrophages within the TME. Macrophages are the predominant immune cells in the TME. T-EVs deliver multiple cargos to macrophages, including DNAs, RNAs, proteins, and lipids. Macrophages are broadly classified into M1 and M2 subtypes.
Herein, we comprehensively introduce the role of T-EVs in regulating the phenotypes (M1/M2) and immune functions of macrophages, including cytokine secretion, expression of immune regulatory molecules on the membrane, phagocytosis, and antigen presentation. Most importantly, given the critical regulatory effects of T-EVs on macrophages and the TME, there is an urgent need for the suppression or elimination of T-EVs to improve the therapy and prognosis of cancer patients. Herein, we propose several potential therapeutic approaches that may guide future attempts to increase the effectiveness of cancer therapy.
Biogenesis and secretion of T-EVs
EVs are a diverse population of membrane vesicles generated via multiple mechanisms. Based on their formation mechanism and size, EVs are generally categorized as exosomes and microvesicles (MVs). Exosomes (30-150 nm in diameter) originate from the endosomal pathway that involves sorted endosomes and intracellular multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs) 9. Due to the fusion of MVBs and the plasma membrane, ILVs could be released into the pericellular space. These ILVs may represent pre-secreted exosomes, but the majority of ILVs may not be released. The process of producing exosomes depends on the endosomal sorting complex required for transport (ESCRT) complexes, which aid in clustering cargos, creating membrane buds, storing cargo within the buds, and facilitating scission 11. According to research, a number of these key regulators are overexpressed and/or hyperactivated in a wide variety of tumor cells, accounting for the high production of T-EVs 12-14. MVs (100-1000 nm in diameter and larger than exosomes) are produced by direct plasma membrane budding 15, 16.
EVs inherit biomolecules (e.g., nucleic acids, proteins, and lipids) from their donor cells, and the level of EV cargos is regulated by a variety of molecules in their donor cells 17. A study conducted by Chen et al., for example, indicated that knockdown of the ESCRT subunit HRS in malignant melanoma cells led to downregulated PD-L1 level in EVs, suggesting that HRS may be an important target in cargo sorting of EVs 18. EVs deliver their cargos to recipient cells, contributing to intercellular communication 17. It is commonly acknowledged that tumor cells generate more EVs than non-tumor cells, and both cell-intrinsic and external signals may influence the EV release of tumor cells 19. Dysregulation of T-EV cargos and T-EV release has a substantial impact on the interactions between tumor cells and macrophages in the TME, as well as in metastatic and premetastatic niches.
Tumor cell-derived EVs affect phenotypes of macrophages
Regulated by the stimuli from the milieu, macrophages are classified into different phenotypes according to their functional orientation. The M1 phenotype and the M2 phenotype are the two major polarization states of macrophages 20. It is generally recognized that M1 macrophages mediate anti-tumor activity, whereas M2 macrophages are linked with pro-tumorigenic functions, including promoting tumor growth, metastasis, and angiogenesis 21. Research suggests that RNAs, proteins, and lipids in T-EVs may help polarize macrophages, mostly toward the M2 phenotype (Figure 2A and Table S1).
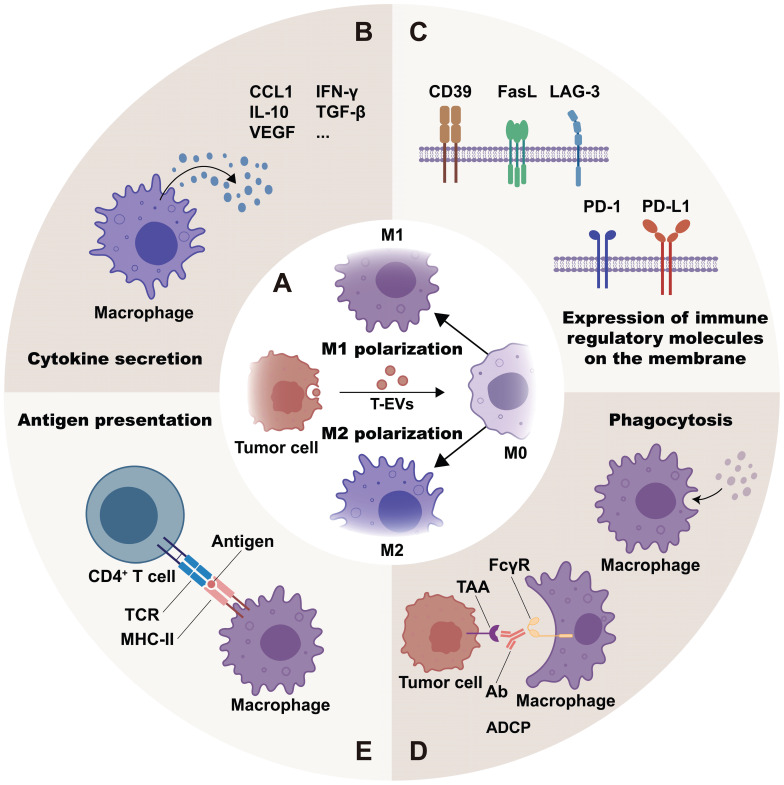
Figure 2.
T-EVs regulate macrophage phenotypes and immune functions: T-EVs may mediate macrophage polarization toward the M1 or M2 phenotype (A) through their contents and surface molecules. Moreover, T-EVs are effective in regulating macrophage immune functions. They do this by regulating cytokine secretion (B), expression of immune regulatory molecules on the membrane (C), phagocytosis (D), and antigen presentation (E) of macrophages.
T-EV-RNAs affect phenotypes of macrophages
RNAs, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), have different effects on cell growth, proliferation, and metabolism through different pathways 22. T-EV-RNAs may alter macrophage polarization through the activation of signaling pathways, signal transduction, transcriptional regulation, and post-transcriptional regulation 23. Specifically, miRNAs silence genes by directly targeting mRNAs, while lncRNAs and circRNAs sponge with miRNAs to indirectly affect protein expression 23.
miRNAs
miRNAs are the most abundant kind of RNAs encapsulated in EVs 24. Under the protection of EV membrane structure, miRNAs can be stably expressed in the extracellular space 25. Valadi et al. found evidence that miRNAs could be functionally transported from donor cells to recipient cells by EVs, which would be a key part of EV-mediated intercellular communication 26. T-EV-encapsulated miRNAs may regulate the phenotypes of macrophages, thus influencing tumor development and formation. In most cases, T-EVs induce macrophages to an M2 phenotype, promoting tumor development. For instance, EVs produced by colorectal cancer (CRC) cells carried miR-934 into macrophages, suppressed PTEN expression and activated the PI3K/AKT signaling pathway to promote M2 macrophage polarization 27. Interestingly, by secreting CXCL13, polarized M2 macrophages enhanced CRC liver metastasis by triggering a positive feedback loop involving CXCL13, CXCR5, NF-κB, p65, and miR-934 in CRC 27.
Hypoxia is a common characteristic of solid tumors. Hypoxia may increase the production of T-EVs that carry miRNAs, and the miRNAs encapsulated in T-EVs may cause M2-like macrophage polarization. Chen et al. demonstrated that hypoxia increased the level of miR-940 in EVs produced by epithelial ovarian cancer (EOC) cells, and that EV-miR-940 delivered to macrophages encouraged M2-phenotype polarization, hence promoting EOC proliferation and migration 28. Wang et al. discovered that miR-301a had a high expression in EVs released by hypoxia-treated pancreatic cancer cells and could be carried to macrophages 29. EV-miR-301a induced macrophage M2 polarization in a HIF-1a or HIF-2-dependent way and subsequently facilitated the invasion, migration, and epithelial-mesenchymal transition (EMT) of pancreatic cancer cells 29. Qian et al. suggested that hypoxic glioma-derived EVs, in comparison to normoxic glioma-derived EVs, markedly triggered M2 macrophage polarization, which promoted the establishment of the immunosuppressive microenvironment 30.
In addition to the pro-tumoral M2 phenotype, specific miRNAs (e.g., miR-9, miR-33, miR-125b, and miR-130) in T-EVs may polarize macrophages towards the anti-tumor M1 phenotype 31-33. Tong et al. reported that miR-9 was abundant in HPV+ head and neck squamous cell carcinoma (HNSCC) cell-derived EVs and could be carried to macrophages and promote M1 polarization by downregulating PPAR, hence promoting HNSCC radiosensitivity 31. Moradi-Chaleshtori et al. revealed that miR-130 and miR-33 overexpression in EVs of human breast cancer cells inhibited tumor development by reprogramming macrophages from the M2 to the M1 phenotype 32. This reprogramming process could be used as a therapeutic method for tumor 32.
Other RNAs
lncRNAs are RNAs that have a length of over 200 nucleotides and have a restricted potential to encode proteins. There is mounting evidence that lncRNAs have a role in a wide range of biological processes, from development and physiology to pathology, including tumorigenesis and tumor progression 34. According to a study by Cao et al., lncRNA-MM2P expression was upregulated during M2 polarization but downregulated during M1 polarization, indicating that lncRNA may influence macrophage polarization 35. Higher amounts of lncRNA TUC339 were found in EVs of hepatocellular carcinoma (HCC) cells, and these EVs could be taken up by macrophages and polarize macrophages to the M2 phenotype 36. Liang et al. revealed that EVs produced by CRC cells carried lncRNA-RPPH1 into macrophages and induced macrophage M2 polarization, increasing CRC cell proliferation and metastasis 37.
circRNAs are a type of non-coding RNA that is generated through a process known as back-splicing, which involves the circularization of exons or introns. Studies have shown that circRNAs could be packaged into EVs and play a key role in the development of tumors. Chen et al. reported that circFARSA in EVs of non-small cell lung cancer cells induced M2 macrophage polarization via PTEN ubiquitination and degradation, which promoted tumor metastasis 38. Pan et al. recently reported that glioma cell-derived EVs transmitted circNEIL3 to infiltrated TAMs, thereby promoting macrophage polarization toward the immunosuppressive phenotype characterized by CD11b and CD163 expression by stabilizing IGF2BP3 protein, which promoted glioma progression 39. Besides, EVs derived from esophageal squamous cell carcinoma (ESCC) cells could transport hsa-circ-0048117 to macrophages and mediate M2 polarization as a miR-140 sponge 40.
T-EV-proteins affect phenotypes of macrophages
Studies have shown that T-EVs may act as trafficking vehicles to transport proteins from tumor cells to macrophages, modulating macrophage polarization. Among these proteins are immune checkpoint molecules and other proteins.
Immune checkpoint molecules
The term "immune checkpoints" describes a group of ligands present on the surface (membrane) of antigen-presenting cells that bind to specific receptor partners on T cells 41. This interaction can result in the activation and differentiation of T cells (stimulatory checkpoints) or the suppression of the immune response (inhibitory checkpoints) 41. Immune checkpoint molecules serve as immune regulators and are critical for maintaining self-tolerance 41. Whereas tumor cells may express high levels of inhibitory checkpoints that inhibit normal anti-tumor immune responses, resulting in immune escape 42.
T-EVs may inherit inhibitory checkpoint molecules from tumor cells and thus limit T-cell activation as well as polarize macrophages towards pro-tumoral M2 phenotype 18, 43, 44. Chen et al. revealed that metastatic melanoma cells generated EVs with PD-L1, which inhibited CD8+ T cells and promoted tumor growth 18. Interestingly, Li et al. discovered that TIM-3 shuttled by human melanoma cell-derived EVs could inhibit the immune function of CD4+ T cells and induce macrophage M2 polarization, which ultimately promoted melanoma cell growth and metastasis 45. According to current studies, T-EVs could transport immune checkpoint molecules (e.g., PD-L1 and TIM-3) to macrophages and induce M2 macrophage polarization. Liu et al. found that xCT inhibitor-treated melanoma cells released EVs with a high expression level of PD-L1, which could upregulate macrophage PD-L1 expression and lead to M2 macrophage polarization 43. Similarly, Cheng et al. found that EVs of osteosarcoma cells induced macrophage M2 polarization through Tim-3, promoting tumor metastasis, invasion, and EMT of osteosarcoma 44.
The blocking of immune checkpoints is an essential component of immunotherapy, the significance of which in treating cancer is well recognized. Ideally, immune checkpoint blockade enables efficient immune responses, and in the meanwhile, maintains sufficient self-tolerance and prevents excessive immune cell activation 46. Given that immune checkpoint blockade not only rescues T cells from immunosuppression and reduces resistance to immunotherapy, but also modifies macrophage phenotypes and substantially affects therapeutic outcomes, immune checkpoint blockade could be regarded as a highly effective cancer treatment method.
Other proteins
In addition to immune checkpoint molecules, studies have identified other T-EV proteins that trigger M1 or M2 macrophage polarization. TEK gene-encoded tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2 (TIE2) is the receptor for angiopoietins and performs essential functions in vascular remodeling during inflammation and tumor angiogenesis 47, 48. TIE2-expressing macrophages (TEMs) are a group of TAMs with characteristics of pro-tumorigenic phenotypes, supporting tumor angiogenesis and being related to cervical cancer progression. The direct transfer of TIE2 protein to macrophages through EVs led to IL-10 upregulation and TNF downregulation in TEMs, which were consistent with M2 macrophages 49. Dong et al. showed that EV-protein tyrosine phosphatase receptor type O (PTPRO) from breast cancer cells inactivated STAT3 and STAT6 in macrophages, polarizing macrophages into an M1-like phenotype and suppressing tumor cell invasion and migration 50. EVs from oral squamous cell carcinoma (OSCC) cells were found to mediate the transfer of THBS1 to macrophages and induce M1-like polarization by activating p38, Akt, and SAPK/JNK signaling 51.
T-EV-lipids affect phenotypes of macrophages
Membrane lipids, especially arachidonic acid (AA), are essential for the fusion of EVs and plasma membranes 52, 53. Linton et al. found that EVs of the ascites-derived AsPC-1 cell line had a much larger proportion of phospholipid-esterified AA than EVs from other pancreatic ductal adenocarcinomas (PDAC) cell lines, which is the most noticeable difference between EVs from various PDAC cell lines 54. They reported that the AA component of phospholipids made it more likely for AsPC-1 EVs to fuse with macrophages 54. More importantly, their results suggested that AA was transported from AsPC-1 cells to macrophages via EVs and would cause a big increase in the production of PGE2 and pro-tumoral molecules (e.g., IL-1, IL-6, MCP-1, MMP-9, TNF, and VEGF), as well as polarize macrophages into an immunosuppressive M2-like phenotype 54.
Tumor cell-derived EVs affect the immune functions of macrophages
It is commonly accepted that the immune system is comprised of two fundamental components: innate immunity and adaptive immunity. The innate immune system is the body's initial line of defense against pathogens (e.g., bacteria and viruses) and tumor cells 55. As the prime immune cells in innate immunity, macrophages engulf bacteria and tumor cells and secrete antimicrobial mediators, playing critical roles in bacterial recognition and elimination, as well as tumor clearance 55. Also, theoretically, the innate immune system's stimulation is required to develop adaptive immune responses against cancer 56.
By regulating macrophages, T-EVs participate in the innate immune response. Epidermal growth factor receptor positive (EGFR+) T-EVs could suppress the activation of IRF3 and type I interferon, thereby impairing the host's innate antiviral immunity 57. Moreover, T-EVs are effective in regulating macrophage immune functions. This is achieved through controlling macrophage activities such as cytokine production, expression of immune regulatory molecules on the membrane, phagocytosis and antigen presentation (Figure 2B-2E).
Cytokine secretion
Cytokines have an important role as mediators of cell-to-cell communication in the TME. Cancer changes the synthesis and function of many cytokines which contribute to host anti-tumor responses 58. Macrophages secrete multiple cytokines that regulate tumor development and anti-tumor immunity. Some cytokines facilitate the polarization of macrophages to an anti-tumor state and promote inflammation to eliminate tumor cells, while others have the opposite effect 59-62. The cytokines that macrophages release also have other functions, like regulating angiogenesis and recruiting macrophages 63.
Certain T-EVs can stimulate the secretion or expression of pro-inflammatory cytokines (e.g., IFN-γ, IL-1β, and TNF-α), anti-inflammatory cytokines (e.g., Arg-1, TGF-β, and IL-10), chemokines (e.g., CCL1, CCL2, and MCP-1) and angiogenic factors (e.g., VEGF). Other T-EVs, however, inhibit the secretion of pro-inflammatory cytokines and co-stimulatory molecules (Figure 2B and Table 1).
Table 1.
T-EVs regulate macrophage cytokine secretion.
| EV source | Key molecules | Mode of action or pathways | Macrophage cell line | Cytokine secretion | Functions | Ref. |
|---|---|---|---|---|---|---|
| Lewis lung cancer cell | EGFR | Activating MEKK2 | Peritoneal macrophage | IFN-β↓ | Suppressing innate antiviral immunity | 57 |
| Lung cancer cell | TRIM59 | Promoting ABHD5 proteasomal degradation and inducing NLRP3 inflammasome activation | THP-1 | IL-1β↑ | Promoting tumor progression | 64 |
| Hypoxic lung tumor cell | miR-101 | CDK8 | THP-1 & U937 | IL-1A↑ IL-6↑ |
Inducing inflammation in the TME | 65 |
| Lewis lung carcinoma cell | / | / | Peritoneal macrophage | IL-6↑ IL-10↑ |
/ | 66 |
| Breast cancer cell | / | gp130/STAT3 | BMDM | IL-6↑ | Establishing a pro-tumorigenic TME | 67 |
| Lung cancer cell | miR-21/miR-29a | TLR8/NF-κB | RAW 264.7 | IL-6↑ TNF-α↑ |
Promoting tumor growth and dissemination | 68 |
| Colorectal cancer cell | IRF-2 | / | RAW 264.7 | VEGFC↑ | Promoting tumor metastasis in sentinel lymph nodes | 69 |
| Breast cancer cell | miR-183-5p | PPP2CA/NF-κB | BMDM | IL-1β↑ IL-6↑ TNF-α↑ |
Promoting tumor progression | 70 |
| Breast cancer cell | Annexin II | p38MAPK/NF-κB/STAT3 | Macrophage | IL-6↑ TNF-α↑ |
Promoting tumor metastasis to brain | 71 |
| Hepatocellular carcinoma cell | lncRNA TUC339 | / | THP-1 | IL-1β↓ TNF-α↓ |
/ | 36 |
| Hypoxic ESCC cell | hsa-circ-0048117 | miR-140/TLR4 | THP-1 | Arg-1↑ IL-10↑ TGF-β↑ |
Promoting tumor invasion and metastasis | 40 |
| Pancreatic cancer cell | AA | / | THP-1 | IL-1β↑ IL-6↑ MCP-1↑ MMP-9↑ PGE2↑ TNF-α↑ VEGF↑ |
Promoting tumor progression | 54 |
| PDAC cell | MIF | / | Human Kupffer cell | TGF-β↑ Fibronectin↑ |
Promoting pre-metastatic niche formation and liver metastasis | 72 |
| Melanoma cell | miR-125b-5p | Lysosomal acid lipase A (LIPA) | THP-1 | IL-1β↑ CCL1↑ CCL2↑ |
/ | 73 |
T-EVs can affect inflammation via regulating the secretion of pro-inflammatory and anti-inflammatory factors by macrophages. Li et al. reported that the inhibition of lung cancer cell EV-miR101 promoted IL-1A and IL-6 expression by macrophages and led to inflammation in the TME by targeting CDK8 65. Ham et al. discovered that bone marrow-derived macrophages produced IL-6 in response to EV-glycoprotein 130 (gp130) of breast cancer cells, which in turn induced pro-survival macrophages through gp130/STAT3 signaling 67. Another study by Liang et al. suggested that EV-TRIM59 generated from lung cancer cells regulated ABHD5 proteasomal degradation to activate macrophage NLRP3 inflammasome signaling pathway, thereby promoting tumor progression via IL-1β production 64. Wei et al. demonstrated that the activation of macrophage NOD1 by CRC cell-derived EVs boosted the secretion of IL-6 and TNF-α, promoting CRC cell proliferation and migration 74. Of note, HCC-derived EV-lncRNA TUC339 could decrease the expression of pro-inflammatory cytokines as well as co-stimulatory molecules after being internalized by THP-1 cells 36.
Chemokines participate in the recruitment and education of macrophages and mediate cancer-related inflammation at the tumor site 75, 76. The release of chemokines by macrophages is also regulated by T-EVs. For instance, Gerloff et al. reported that melanoma EV delivered miR-125b-5p to macrophages and reinforced macrophage M1 activation by inducing CCL1, CCL2, and IL-1β production, hence promoting cancer-associated inflammation and myeloid cell recruitment 73.
The secretion of other cytokines, such as vascular endothelial growth factor C (VEGFC) and transforming growth factors (TGFs), is also influenced by T-EVs. Sun et al. demonstrated that EV-IRF-2 from CT26 cells induced the release of VEGFC by macrophages 69. Costa-Silva et al. found that uptake of PDAC-derived EVs by Kupffer cells induced TGF-β release and fibronectin generation of hepatic stellate cells 72.
Expression of immune regulatory molecules on the membrane
Macrophage surface molecules, including antigens, receptors and other molecules mediate the immune responses of macrophages. Studies have shown that T-EVs regulate the levels of immune regulatory molecules on membrane of macrophages to affect immune suppression and disease progression (Figure 2C and Table 2).
Table 2.
T-EVs regulate the levels of immune regulatory molecules on membrane of macrophages.
| EV source | Key molecules | Mode of action or pathways | Macrophage cell line | Changes in immune regulatory molecules | Functions | Ref. |
|---|---|---|---|---|---|---|
| Ovarian cancer cell | miR-155-5p | miR-155-5p/PD-L1 | THP-1 & PBMC | PD-L1↓ | Suppressing macrophage infiltration and tumor growth, promoting cytotoxic T cell activation | 77 |
| HNSCC cell | CD73 | NF-κB | THP-1 | LAG-3↑ PD-1↑ PD-L1↑ |
Promoting malignant progression | 78 |
| Colorectal cancer cell and multiple myeloma cell | / | TLR4/NF-κB | THP-1 | PD-L1↑ | Creating an immunosuppressive microenvironment | 79 |
| Lung cancer cell | HMGB-1 | TLR2/NF-κB | PBMC & peritoneal macrophage | PD-L1↑ | Promoting tumor metastasis | 66 |
| Intrahepatic cholangiocarcinoma cell | miR-183-5p | miR-183-5p/PTEN/AKT/PD-L1 | Macrophage | PD-L1↑ | Promoting immune suppression and tumor progression | 80 |
| ER-stressed hepatocellular carcinoma cell | miR-23a-3p | miR-23a/PTEN/AKT | THP-1 | PD-L1↑ | Inhibiting T-cell function | 81 |
| Colorectal cancer cell | miR-21-5p and miR-200a | PTEN/AKT and SCOS1/STAT1 | THP-1 & RAW264.7 | PD-L1↑ | Suppressing CD8+ T cell activities | 82 |
| Hepatocellular carcinoma cell | miR-146a-5p | / | Peritoneal macrophage & BMDM & RAW264.7 | MHC-II↓ PD-L1↑ CD80↑ |
Inhibiting T cell response | 83 |
| OSCC cell | CMTM6 | / | THP-1 | PD-L1↑ | Promoting malignant progression | 84 |
| ER-stressed breast cancer cell | miR-27a-3p | MAGI2/PTEN/PI3K /AKT | THP-1 | PD-L1↑ | Inhibiting CD8+ T cells | 85 |
| H. pylori-infected gastric cancer cell | Mesenchymal-epithelial transition factor (MET) | / | THP-1 & PBMC | MET↑ | Promoting tumor growth and progression | 86 |
| Hypoxia pre-challenged ESCC cell | hsa-circ-0048117 | miR-140/TLR4 | THP-1 | TLR4↑ | Promoting tumor metastasis | 40 |
| Follicular lymphoma cell | miR-7e-5p downregulation | / | Peritoneal macrophage | FasL↑ | Inducing stromal M1 macrophage apoptosis | 87 |
| Hepatocellular carcinoma cell | circTMEM181 | miR-488-3p/CD39 | THP-1 | CD39↑ | Causing immunosuppression and anti-PD-1 resistance | 88 |
| Hepatocellular carcinoma cell | / | STAT3 | THP-1 & RAW264.7 | PD-L1↑ | / | 89 |
| Melatonin-treated hepatocellular carcinoma cell | / | STAT3 | THP-1 & RAW264.7 | PD-L1↓ | / | 89 |
| Colorectal cancer cell | miR-934 | PTEN/PI3K/AKT | THP-1 | CD163↑ CD206↑ |
Promoting liver metastasis | 27 |
| Breast cancer cell | Loaded miR-130 | / | Peritoneal macrophage | CD86↑ CD206↓ |
Suppressing migration and invasion | 90 |
The PD-L1/PD-1 signaling axis plays a critical role in tumor immunosuppression since it serves as an immune checkpoint and “don't eat me” signal. This axis inhibits T lymphocyte activation and increases the immunological tolerance of tumor cells, hence enabling tumor immune escape 91. In human and murine studies, macrophages in the TME displayed significant levels of functional PD-L1 expression 92. Several studies have proved that T-EVs foster tumor progression by increasing PD-1/PD-L1 levels in macrophages 80. According to research, intrahepatic cholangiocarcinoma cell-derived EV miR-183-5p upregulated PD-L1-expressing macrophages via the miR-183-5p/PTEN/AKT/PD-L1 pathway 80. Chen et al. revealed that GOLM1 increased PD-L1 expression in macrophages by enabling the transfer of PD-L1 to macrophages via EVs, hence increasing TAM infiltration in HCC 93. Yin et al. reported that HCC-EV therapy suppressed macrophage MHC-II expression while upregulating PD-L1 and CD80 83. As for PD-1, Wang et al. demonstrated that GC cells produced EVs to stimulate PD-1+ TAM production, which impaired CD8+ T-cell function 94.
CD39 functions as a “molecular switch” that regulates the inflammatory reactions of macrophages 95. Specifically, macrophages create immunosuppressive adenosine by hydrolyzing self-released ATP through CD39 96-98. Lu et al. found that EV-circTMEM181 sponged with miR-488-3p to upregulate macrophage CD39 level, which increased adenosine production and thus impaired anti-tumor immunity and caused anti-PD-1 resistance 88.
Furthermore, T-EVs may regulate the expression of a set of M1 (e.g., CD80, CD86, MHC-II, and TLR4) and M2 (e.g., CD163 and CD206) markers 27, 40, 83, 90 on membrane of macrophages. The altered expression levels of these molecules imply macrophage phenotype switch.
So far, a considerable amount of research has been conducted on PD-1/PD-L1 66, 80-82, 84, 94, while limited attention has been paid to other macrophage surface molecules, such as FasL and MET.
Phagocytosis
Phagocytosis is a major characteristic of macrophages. Macrophages engulf and eliminate invading microorganisms and damaged cells, providing an effective immune response and maintaining homeostasis 99. According to research, T-EVs may either stimulate or inhibit macrophage-mediated phagocytosis (Figure 2D).
Macrophage-mediated antibody-dependent cellular phagocytosis (ADCP) is of great importance to tumor cell clearance. The stimulation of macrophage-mediated ADCP seems to be a significant mechanism behind the anti-tumor effects of a variety of therapeutic antibodies 99. Loss or mutation of TP53 is a key mediator of chemoresistance for various malignant tumors, including B-cell malignancies 100, 101. Izquierdo et al. revealed that loss of TP53 function in lymphoma led to increased EV production and that TP53-deficient lymphoma B-cell EVs inhibited Fc receptor-dependent macrophage ADCP and induced chemoimmunotherapy resistance through PD-L1 expression. Notably, neutralizing PD-L1 on the shTP53-EVs restored the anti-tumor potential of macrophages, as measured by ADCP 101. However, according to Su et al., macrophages undergone ADCP suppressed NK cell-mediated antibody-dependent cell-mediated cytotoxicity (ADCC) as well as T cell-mediated cytotoxicity, thereby having a detrimental effect on breast cancer immunosuppression 102. Furthermore, the combination of anti-HER2 antibodies with PD-L1 and IDO inhibitors improved anti-tumor immunity and anti-HER2 therapeutic effectiveness in murine models 102. Consequently, therapeutic antibodies combined with immune checkpoint blockade may have synergistic benefits in the treatment of cancer 102.
The phagocytic ability of macrophages is essential for the clearance of EVs from circulation and is stimulated by “eat me” signals (e.g., PS) and suppressed by “don't eat me” signals (e.g., CD47) 103-105. Yu et al. revealed that the higher level of CD47 and lower level of PS on T-EVs as compared to heterogeneous EVs might explain why T-EVs had a longer lifespan 105. In addition to CD47 and PS, there might be other “eat me” and “don't eat me” signals on T-EVs that can impact the ability of macrophages to phagocytose T-EVs, such as PD-L1, CD24, and CRT, and the interactions between those molecules have yet to be researched.
The phagocytic ability of macrophages could also be evaluated by the phagocytosis activity of the ovalbumin, yeast, or latex beads-rabbit IgG-FITC complex. Wang et al. evaluated phagocytosis levels of PD-1+ macrophages by analyzing the proportion of Ovalbumin+ macrophages. They discovered that gastric cancer cells significantly educated monocytes into PD-1+ macrophages by secreting EVs in vitro and in vivo, and PD-1+ macrophages phagocytosed significantly less ovalbumin in vitro than PD-1- macrophages did 94. Moradi-Chaleshtori et al. revealed that the capacity of macrophages to phagocytose yeast was enhanced by 4T1 breast cancer cell-derived EVs containing miR-130 90. Kamerkar et al. found that after the uptake of HCC cell-derived EVs carrying up-regulated lncRNA TUC339, the phagocytosis of the latex beads-rabbit IgG-FITC complex was inhibited in THP-1 cells (commonly used model cells for macrophages), while the opposite effect was observed upon TUC339 suppression 36.
Antigen presentation
Capture, endocytosis, and antigen presentation are crucial aspects of macrophage functions, which connect innate and adaptive immunity 106. Macrophages engulf pathogens, process their antigens, and present peptide fragments bound to human leukocyte antigen (HLA) molecules on their surface. This process alerts the immune system that pathogens are present 106. The levels of co-stimulatory and antigen-presenting molecules (e.g., MHC-I, MHC-II, CD80, and CD86) are significantly increased when macrophages are activated.
According to studies, T-EVs may facilitate macrophage antigen presentation (Figure 2E). The expression of murine immune region-associated (Ia) antigen on the surface of macrophages is crucial to their ability to present antigens 107. In the presence of Ia antigen plus a suitable “foreign” antigen, macrophages can induce the formation of TH cells. The TH cells may activate both cytotoxic T-lymphocytes and antibody-producing B-lymphocytes subsequently. Therefore, inhibiting Ia antigen expression on macrophages may limit both cellular and humoral immune responses. Taylor and Black discovered that plasma membrane-derived vesicles shed by metastatic variants of the murine B16 melanoma significantly inhibited the stimulation of macrophage Ia antigen expression, the earliest stage in the establishment of an immune response 108. Aspirin was capable of reversing membrane vesicle-induced inhibition, suggesting that this inhibition was caused by an increase in prostaglandin production 108. In addition, MHC-II, a key molecule in macrophage antigen presentation, is highly expressed in M1-polarized macrophages. Since T-EVs have the potential to induce M1 polarization, it can be hypothesized that T-EVs can affect the antigen-presenting function of macrophages by upregulating MHC-II expression 109.
Perspectives
As important cellular mediators, T-EVs regulate macrophage phenotypes and functions by transporting various cargos to macrophages, thereby influencing tumor progression and cancer treatment. T-EVs that target and regulate macrophages are considered promising anti-tumor therapeutic targets, either alone or in combination therapies (Figure 3). A promising way to treat cancer is to explore T-EV's promoting potential and reverse their inhibitory effects on macrophage anti-tumor activities. T-EVs targeting macrophages can be also used for drug delivery, offering multiple advantageous outcomes.
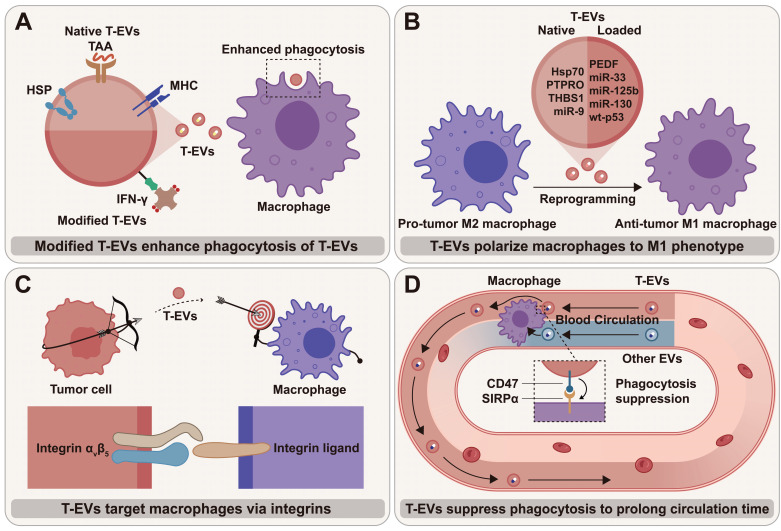
Figure 3.
The therapeutic potential of macrophage-targeted T-EVs: (A) Using modified T-EVs as vaccines that stimulate macrophages to clear T-EVs; (B) T-EVs with native and loaded cargos polarize macrophages toward an anti-tumor M1 phenotype; (C) T-EVs can target macrophages via integrins and their ligands on the membrane; (D) The blood circulation time of T-EVs is likely to be longer than that of non-tumor-cell-derived EVs due to the higher amount of “don't eat me” signal CD47 and the lower level of “eat me” signal PS.
Exploring promoting potential of T-EVs on anti-tumor activities of macrophage
“Eat me” and “don't eat me” signals together control the process by which macrophages phagocytose tumor cells. Since CD47 can competitively occupy SIRPα, the overexpression of CD47 on T-EVs may disrupt CD47/SIRPα signal and lead to enhanced macrophage-mediated phagocytosis of tumor cells 110. Employing CD47-overexpressed T-EVs instead of anti-CD47 antibodies to block CD47/SIRPα signal has the advantage that T-EVs can carry therapeutics to kill tumor cells around macrophages, thus “killing two birds with one stone”. Thus, T-EVs with highly expressed “don't eat me” signals are promising delivery vehicles for cancer therapeutics.
T-EVs inherit tumor antigens from tumor cells, making them a source of tumor antigens that the immune system can identify. Therefore, T-EVs can be employed as tumor vaccines that stimulate immune responses against not only tumor cells but also T-EVs (Figure 3A). Shi et al. modified EVs of prostate cancer cells with interferon-γ (IFN-γ) and created a T-EV vaccine to help suppress tumor development 111. These biotinylated T-EVs contained tumor-associated antigens and immobilized streptavidin (SA)-tagged bioactive cytokines on the surface. They extended the lifespan of prostate cancer mice by increasing the quantity of M1 macrophages and enhancing the ability of M1 macrophages to engulf T-EVs 111. The use of T-EVs to activate the anti-tumor function and phenotype of macrophages is of great importance for tumor therapy, owing to the abundance 112 and phagocytic potential of macrophages. Although the immune response that T-EV vaccine induces is insufficient to kill tumor cells, T-EV vaccine shows adequate ability to stimulate the immune response to eliminate T-EVs, weakening T-EV-mediated immune escape. Thus, as shown in the study by Shi et al. 111, tumor vaccine and T-EV vaccine have a synergistic effect in inducing anti-tumor immunity.
Reversing inhibiting effects of T-EVs on anti-tumor activities of macrophage
Generally, a viable method to treat cancer is to use small molecule inhibitors or antibodies that target specific signaling components to stop the inhibiting effects of T-EVs on macrophages. Given the pro-tumor properties of M2 macrophages, inhibiting macrophage M2 polarization and suppressing TAM infiltration with T-EVs that contain effector molecules can be promising therapeutic strategies against tumors (Figure 3B). For instance, Su et al. modified the cargos inside T-EVs by transfecting Panc-1 cells and successfully achieved stable expression of miR-155 and miR-125b2 in macrophages, which converted macrophages from M2 to M1 subtype 113. Jang et al. extracted EVs from epigallocatechin-3-gallate (EGCG)-treated 4T1 cells and discovered that miR-16 was increased in tumor cells and could be transmitted to TAMs through EVs, thereby suppressing TAM infiltration and M2 polarization 114.
Employing macrophage-targeted T-EVs as drug delivery systems
T-EVs can target macrophages, and integrins are the molecular basis for T-EVs' targeting ability (Figure 3C). According to research, T-EVs express integrin αvβ5 that specifically binds to Kupffer cells and contributes to liver tropism 115. Therefore, strategies targeting T-EV integrins may efficiently block the organ-tropic metastasis of tumors by cutting off the interactions between T-EVs and tissue-resident macrophages (TRMs). More importantly, the targeting ability of T-EVs makes it feasible to deliver therapeutics accurately to macrophages. The results of the studies so far suggest that T-EVs have considerable potential to deliver various therapeutic agents, including small molecule drugs and nucleic acids, to macrophages 116 (Table 3). The surface of T-EVs has been modified to improve transport efficiency and therapeutic effect, and the natural cargos of T-EVs have been discarded in some cases.
Table 3.
Macrophage-targeted T-EVs are employed as drug delivery systems.
| Tumor cell line | Carrier type | Surface modification | Effector molecules | Effects | Ref. |
|---|---|---|---|---|---|
| H22 | T-EVs | / | IONs | Promoting M1 polarization and inhibiting tumor growth | 118 |
| Panc-1 | T-EVs | / | miR-155 and miR-125b | Re-programming macrophages from an M2 phenotype back to an M1 phenotype | 113 |
| CT26 | Exosomes-thermosensitive liposomes hybrid nanovesicles | CD47 overexpression | Photothermal agent ICG and R837 | Improving macrophage-mediated phagocytosis of tumor cells by blocking CD47 signal, promoting tumor-associated antigen generation | 110 |
| RM-1 | T-EVs | IFN-γ modification | / | Increasing M1 macrophages quantity, promoting M1 macrophages to engulf RM-1 cell-derived EVs | 111 |
| 4T1 | Tumor cell apoptotic bodies | / | CpG | Polarizing macrophages to the M1 phenotype, enhancing macrophage antigen presentation and phagocytic ability | 119 |
The macrophage-targeting ability of T-EVs could be enhanced when the “don't eat me” and “eat me” signals are smartly used. In a study by Liu et al., matrix metalloproteinase 2 (MMP2) was exploited to regulate PS externalization on the surface of the PEGylated nanoparticles (NPs) in order to achieve tumor-specific macrophage targeting 117. In non-tumor tissues, the surface-anchored PEG may impede phagocytosis, but in tumors, the increased MMP2 would externalize PS for TAM-targeted phagocytosis 117. Similar techniques may be used to boost the capacity of T-EVs to target macrophages.
Compared to EVs from other cells, T-EVs have a substantial advantage in blood circulation time (Figure 3D). Macrophages are the key to T-EV clearance from systemic circulation 120. As proven by Yu et al., the higher amount of CD47 and the lower level of PS on T-EVs compared to heterogeneous EVs likely result in a longer lifespan 105. The prolonged blood circulation time may lead to reduced toxicity to normal cells and stable drug release.
Despite this, it is still being determined whether native T-EVs can safely serve as drug carriers. The high level of immunosuppressive PD-L1 expression in native T-EVs raises the necessity to consider whether it may promote the progression of tumors 18. Because of this, using T-EVs for medication delivery requires further research to prevent or minimize potential adverse effects.
Conclusions
Overall, this review summarizes the impact of T-EVs on macrophage phenotype and immune function, along with the underlying molecular mechanisms. More importantly, it presents several treatment strategies based on the regulation of T-EVs on macrophages, which are promising methods for treating cancer. Developing new strategies and combination regimens with enhanced specificity profiles is essential to overcome therapy resistance and eradicate cancer. Further research is needed to fully comprehend the complex interactions between T-EVs and macrophages and how these interactions influence cancer treatment.
Supplementary Material
Supplementary table.
Acknowledgments
Funding
This work was funded by the National Key R&D Program of China (2019YFA0210500), National Natural Science Foundation of China (81801842). Planning Project of Innovation and Entrepreneurship Training of National Undergraduate of Wuhan University (202210486126).
Author Contributions
G.C. and Z.-L.Y. conceived the study idea and designed the study. J.-W.T., H.-J.Z., S.-Y.L., Y.-L.G. and Z.-L.Y. drafted the manuscript and drew the figures. J.-W.T., Z.-L.Y. and G.C. discussed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
References
- 1.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobb RJ, Lima LG, Moller A. Exosomes: Key mediators of metastasis and pre-metastatic niche formation. Semin Cell Dev Biol. 2017;67:3–10. doi: 10.1016/j.semcdb.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schroder CP. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat Rev. 2018;70:178–89. doi: 10.1016/j.ctrv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Wagner J, Rapsomaniki MA, Chevrier S, Anzeneder T, Langwieder C, Dykgers A. et al. A Single-Cell Atlas of the Tumor and Immune Ecosystem of Human Breast Cancer. Cell. 2019;177:1330–45. doi: 10.1016/j.cell.2019.03.005. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H. et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell. 2019;35:588–602. doi: 10.1016/j.ccell.2019.02.009. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F. et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 8.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell. 2019;49:347–60. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:640. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Li Y, Gao W, Chen L, Xu W, Zhu X. Exosome-Mediated Crosstalk Between Tumor and Tumor-Associated Macrophages. Front Mol Biosci. 2021;8:764222. doi: 10.3389/fmolb.2021.764222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–9. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyoshima M, Tanaka N, Aoki J, Tanaka Y, Murata K, Kyuuma M. et al. Inhibition of tumor growth and metastasis by depletion of vesicular sorting protein Hrs: its regulatory role on E-cadherin and beta-catenin. Cancer Res. 2007;67:5162–71. doi: 10.1158/0008-5472.CAN-06-2756. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Xu Y, Chen T, Zhang Y, Ma Q, Rauniyar S. et al. TSG101 Promotes the Proliferation, Migration, and Invasion of Human Glioma Cells by Regulating the AKT/GSK3beta/beta-Catenin and RhoC/Cofilin Pathways. Mol Neurobiol. 2021;58:2118–32. doi: 10.1007/s12035-020-02231-7. [DOI] [PubMed] [Google Scholar]
- 14.Fujita K, Kume H, Matsuzaki K, Kawashima A, Ujike T, Nagahara A. et al. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Sci Rep. 2017;7:42961. doi: 10.1038/srep42961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 16.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 17.Maas SLN, Breakefield XO, Weaver AM. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017;27:172–88. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–6. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1–11. doi: 10.1016/j.pharmthera.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Moradi-Chaleshtori M, Hashemi SM, Soudi S, Bandehpour M, Mohammadi-Yeganeh S. Tumor-derived exosomal microRNAs and proteins as modulators of macrophage function. J Cell Physiol. 2019;234:7970–82. doi: 10.1002/jcp.27552. [DOI] [PubMed] [Google Scholar]
- 21.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG. et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–24. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z, Chen Y, Ma L, Chen Y, Liu J, Guo Y. et al. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment. Mol Ther. 2022;30:3133–54. doi: 10.1016/j.ymthe.2022.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G. et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17:147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3:23743. doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y. et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Ying X, Wang X, Wu X, Zhu Q, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep. 2017;38:522–8. doi: 10.3892/or.2017.5697. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T. et al. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kgamma to Promote Pancreatic Cancer Metastasis. Cancer Res. 2018;78:4586–98. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 30.Qian M, Wang S, Guo X, Wang J, Zhang Z, Qiu W. et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-kappaB pathways. Oncogene. 2020;39:428–42. doi: 10.1038/s41388-019-0996-y. [DOI] [PubMed] [Google Scholar]
- 31.Tong F, Mao X, Zhang S, Xie H, Yan B, Wang B. et al. HPV+ HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett. 2020;478:34–44. doi: 10.1016/j.canlet.2020.02.037. [DOI] [PubMed] [Google Scholar]
- 32.Moradi-Chaleshtori M, Bandehpour M, Soudi S, Mohammadi-Yeganeh S, Hashemi SM. In vitro and in vivo evaluation of anti-tumoral effect of M1 phenotype induction in macrophages by miR-130 and miR-33 containing exosomes. Cancer Immunol Immunother. 2021;70:1323–39. doi: 10.1007/s00262-020-02762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trivedi M, Talekar M, Shah P, Ouyang Q, Amiji M. Modification of tumor cell exosome content by transfection with wt-p53 and microRNA-125b expressing plasmid DNA and its effect on macrophage polarization. Oncogenesis. 2016;5:e250. doi: 10.1038/oncsis.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J, Li L, Han ZY, Wang ZX, Qin LX. Long noncoding RNAs, emerging and versatile regulators of tumor-induced angiogenesis. Am J Cancer Res. 2019;9:1367–81. [PMC free article] [PubMed] [Google Scholar]
- 35.Cao J, Dong R, Jiang L, Gong Y, Yuan M, You J. et al. LncRNA-MM2P Identified as a Modulator of Macrophage M2 Polarization. Cancer Immunol Res. 2019;7:292–305. doi: 10.1158/2326-6066.CIR-18-0145. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Lei Y, Wu M, Li N. Regulation of Macrophage Activation and Polarization by HCC-Derived Exosomal lncRNA TUC339. Int J Mol Sci. 2018;19:2958. doi: 10.3390/ijms19102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang ZX, Liu HS, Wang FW, Xiong L, Zhou C, Hu T. et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019;10:829. doi: 10.1038/s41419-019-2077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T, Liu Y, Li C, Xu C, Ding C, Chen J. et al. Tumor-derived exosomal circFARSA mediates M2 macrophage polarization via the PTEN/PI3K/AKT pathway to promote non-small cell lung cancer metastasis. Cancer Treat Res Commun. 2021;28:100412. doi: 10.1016/j.ctarc.2021.100412. [DOI] [PubMed] [Google Scholar]
- 39.Pan Z, Zhao R, Li B, Qi Y, Qiu W, Guo Q. et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol Cancer. 2022;21:16. doi: 10.1186/s12943-021-01485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Q, Wang X, Zhu J, Fei X, Chen H, Li C. Hypoxic Tumor-Derived Exosomal Circ0048117 Facilitates M2 Macrophage Polarization Acting as miR-140 Sponge in Esophageal Squamous Cell Carcinoma. Onco Targets Ther. 2020;13:11883–97. doi: 10.2147/OTT.S284192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orabona C, Mondanelli G, Puccetti P, Grohmann U. Immune Checkpoint Molecules, Personalized Immunotherapy, and Autoimmune Diabetes. Trends Mol Med. 2018;24:931–41. doi: 10.1016/j.molmed.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu N, Zhang J, Yin M, Liu H, Zhang X, Li J. et al. Inhibition of xCT suppresses the efficacy of anti-PD-1/L1 melanoma treatment through exosomal PD-L1-induced macrophage M2 polarization. Mol Ther. 2021;29:2321–34. doi: 10.1016/j.ymthe.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng Z, Wang L, Wu C, Huang L, Ruan Y, Xue W. Tumor-derived Exosomes Induced M2 Macrophage Polarization and Promoted the Metastasis of Osteosarcoma Cells Through Tim-3. Arch Med Res. 2021;52:200–10. doi: 10.1016/j.arcmed.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Liu Y, Yang L, Jiang Y, Qian Q. TIM-3 shuttled by MV3 cells-secreted exosomes inhibits CD4+ T cell immune function and induces macrophage M2 polarization to promote the growth and metastasis of melanoma cells. Transl Oncol. 2022;18:101334. doi: 10.1016/j.tranon.2021.101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brom VC, Burger C, Wirtz DC, Schildberg FA. The Role of Immune Checkpoint Molecules on Macrophages in Cancer, Infection, and Autoimmune Pathologies. Front Immunol. 2022;13:837645. doi: 10.3389/fimmu.2022.837645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12:933–41. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- 48.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–70. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du S, Qian J, Tan S, Li W, Liu P, Zhao J. et al. Tumor cell-derived exosomes deliver TIE2 protein to macrophages to promote angiogenesis in cervical cancer. Cancer Lett. 2022;529:168–79. doi: 10.1016/j.canlet.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Dong H, Xie C, Jiang Y, Li K, Lin Y, Pang X. et al. Tumor-Derived Exosomal Protein Tyrosine Phosphatase Receptor Type O Polarizes Macrophage to Suppress Breast Tumor Cell Invasion and Migration. Front Cell Dev Biol. 2021;9:703537. doi: 10.3389/fcell.2021.703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao M, Zhang J, Chen W, Chen W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2018;37:143. doi: 10.1186/s13046-018-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chattopadhyay S, Sun P, Wang P, Abonyo B, Cross NL, Liu L. Fusion of lamellar body with plasma membrane is driven by the dual action of annexin II tetramer and arachidonic acid. J Biol Chem. 2003;278:39675–83. doi: 10.1074/jbc.M212594200. [DOI] [PubMed] [Google Scholar]
- 53.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–56. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 54.Linton SS, Abraham T, Liao J, Clawson GA, Butler PJ, Fox T. et al. Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages. PLoS One. 2018;13:e0206759. doi: 10.1371/journal.pone.0206759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirayama D, Iida T, Nakase H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int J Mol Sci. 2017;19:92. doi: 10.3390/ijms19010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–74. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 57.Gao L, Wang L, Dai T, Jin K, Zhang Z, Wang S. et al. Tumor-derived exosomes antagonize innate antiviral immunity. Nat Immunol. 2018;19:233–45. doi: 10.1038/s41590-017-0043-5. [DOI] [PubMed] [Google Scholar]
- 58.Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19:237–53. doi: 10.1038/s41571-021-00588-9. [DOI] [PubMed] [Google Scholar]
- 59.Ivashkiv LB. IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018;18:545–58. doi: 10.1038/s41577-018-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 61.Tauriello DVF, Sancho E, Batlle E. Overcoming TGFbeta-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22:25–44. doi: 10.1038/s41568-021-00413-6. [DOI] [PubMed] [Google Scholar]
- 62.Farajzadeh Valilou S, Keshavarz-Fathi M, Silvestris N, Argentiero A, Rezaei N. The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer. Cytokine Growth Factor Rev. 2018;39:46–61. doi: 10.1016/j.cytogfr.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C. et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang M, Chen X, Wang L, Qin L, Wang H, Sun Z. et al. Cancer-derived exosomal TRIM59 regulates macrophage NLRP3 inflammasome activation to promote lung cancer progression. J Exp Clin Cancer Res. 2020;39:176. doi: 10.1186/s13046-020-01688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, Xu P, Wu D, Guan M, Weng X, Lu Y. et al. Hypoxic stress suppresses lung tumor-secreted exosomal miR101 to activate macrophages and induce inflammation. Cell Death Dis. 2021;12:776. doi: 10.1038/s41419-021-04030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C. et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021;33:2040–58. doi: 10.1016/j.cmet.2021.09.002. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ham S, Lima LG, Chai EPZ, Muller A, Lobb RJ, Krumeich S. et al. Breast Cancer-Derived Exosomes Alter Macrophage Polarization via gp130/STAT3 Signaling. Front Immunol. 2018;9:871. doi: 10.3389/fimmu.2018.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R. et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–6. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun B, Zhou Y, Fang Y, Li Z, Gu X, Xiang J. Colorectal cancer exosomes induce lymphatic network remodeling in lymph nodes. Int J Cancer. 2019;145:1648–59. doi: 10.1002/ijc.32196. [DOI] [PubMed] [Google Scholar]
- 70.Guo J, Duan Z, Zhang C, Wang W, He H, Liu Y. et al. Mouse 4T1 Breast Cancer Cell-Derived Exosomes Induce Proinflammatory Cytokine Production in Macrophages via miR-183. J Immunol. 2020;205:2916–25. doi: 10.4049/jimmunol.1901104. [DOI] [PubMed] [Google Scholar]
- 71.Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I. et al. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol Cancer Res. 2017;15:93–105. doi: 10.1158/1541-7786.MCR-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–26. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerloff D, Lutzkendorf J, Moritz RKC, Wersig T, Mader K, Muller LP. et al. Melanoma-Derived Exosomal miR-125b-5p Educates Tumor Associated Macrophages (TAMs) by Targeting Lysosomal Acid Lipase A (LIPA) Cancers (Basel) 2020;12:464. doi: 10.3390/cancers12020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei X, Ye J, Pei Y, Wang C, Yang H, Tian J. et al. Extracellular vesicles from colorectal cancer cells promote metastasis via the NOD1 signalling pathway. J Extracell Vesicles. 2022;11:e12264. doi: 10.1002/jev2.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 76.Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21:27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 77.Li X, Wang S, Mu W, Barry J, Han A, Carpenter RL. et al. Reactive oxygen species reprogram macrophages to suppress antitumor immune response through the exosomal miR-155-5p/PD-L1 pathway. J Exp Clin Cancer Res. 2022;41:41. doi: 10.1186/s13046-022-02244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu T, Zhang Z, Zhang J, Pan X, Zhu X, Wang X. et al. CD73 in small extracellular vesicles derived from HNSCC defines tumour-associated immunosuppression mediated by macrophages in the microenvironment. J Extracell Vesicles. 2022;11:e12218. doi: 10.1002/jev2.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pucci M, Raimondo S, Urzi O, Moschetti M, Di Bella MA, Conigliaro A. et al. Tumor-Derived Small Extracellular Vesicles Induce Pro-Inflammatory Cytokine Expression and PD-L1 Regulation in M0 Macrophages via IL-6/STAT3 and TLR4 Signaling Pathways. Int J Mol Sci. 2021;22:12118. doi: 10.3390/ijms222212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo C, Xin H, Zhou Z, Hu Z, Sun R, Yao N. et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology. 2022;76:982–99. doi: 10.1002/hep.32387. [DOI] [PubMed] [Google Scholar]
- 81.Liu J, Fan L, Yu H, Zhang J, He Y, Feng D. et al. Endoplasmic Reticulum Stress Causes Liver Cancer Cells to Release Exosomal miR-23a-3p and Up-regulate Programmed Death Ligand 1 Expression in Macrophages. Hepatology. 2019;70:241–58. doi: 10.1002/hep.30607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin Y, Liu B, Cao Y, Yao S, Liu Y, Jin G. et al. Colorectal Cancer-Derived Small Extracellular Vesicles Promote Tumor Immune Evasion by Upregulating PD-L1 Expression in Tumor-Associated Macrophages. Adv Sci (Weinh) 2022;9:2102620. doi: 10.1002/advs.202102620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin C, Han Q, Xu D, Zheng B, Zhao X, Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology. 2019;8:1601479. doi: 10.1080/2162402X.2019.1601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pang X, Wang SS, Zhang M, Jiang J, Fan HY, Wu JS. et al. OSCC cell-secreted exosomal CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling pathway. Cancer Immunol Immunother. 2021;70:1015–29. doi: 10.1007/s00262-020-02741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao X, Tu Y, Xu Y, Guo Y, Yao F, Zhang X. Endoplasmic reticulum stress-induced exosomal miR-27a-3p promotes immune escape in breast cancer via regulating PD-L1 expression in macrophages. J Cell Mol Med. 2020;24:9560–73. doi: 10.1111/jcmm.15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Che Y, Geng B, Xu Y, Miao X, Chen L, Mu X. et al. Helicobacter pylori-induced exosomal MET educates tumour-associated macrophages to promote gastric cancer progression. J Cell Mol Med. 2018;22:5708–19. doi: 10.1111/jcmm.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lou X, Fu J, Zhao X, Zhuansun X, Rong C, Sun M. et al. MiR-7e-5p downregulation promotes transformation of low-grade follicular lymphoma to aggressive lymphoma by modulating an immunosuppressive stroma through the upregulation of FasL in M1 macrophages. J Exp Clin Cancer Res. 2020;39:237. doi: 10.1186/s13046-020-01747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu JC, Zhang PF, Huang XY, Guo XJ, Gao C, Zeng HY. et al. Amplification of spatially isolated adenosine pathway by tumor-macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma. J Hematol Oncol. 2021;14:200. doi: 10.1186/s13045-021-01207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng L, Liu J, Liu Q, Liu Y, Fan L, Wang F. et al. Exosomes from Melatonin Treated Hepatocellularcarcinoma Cells Alter the Immunosupression Status through STAT3 Pathway in Macrophages. Int J Biol Sci. 2017;13:723–34. doi: 10.7150/ijbs.19642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moradi-Chaleshtori M, Shojaei S, Mohammadi-Yeganeh S, Hashemi SM. Transfer of miRNA in tumor-derived exosomes suppresses breast tumor cell invasion and migration by inducing M1 polarization in macrophages. Life Sci. 2021;282:119800. doi: 10.1016/j.lfs.2021.119800. [DOI] [PubMed] [Google Scholar]
- 91.Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B. et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. doi: 10.1186/s12943-018-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L. et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest. 2018;128:1708. doi: 10.1172/JCI120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen J, Lin Z, Liu L, Zhang R, Geng Y, Fan M. et al. GOLM1 exacerbates CD8+ T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages. Signal Transduct Target Ther. 2021;6:397. doi: 10.1038/s41392-021-00784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P. et al. Tumor-derived exosomes induce PD1+ macrophage population in human gastric cancer that promotes disease progression. Oncogenesis. 2018;7:41. doi: 10.1038/s41389-018-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood. 2013;122:1935–45. doi: 10.1182/blood-2013-04-496216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR. et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–22. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 97.Zimmermann H. Nucleotides and cd39: principal modulatory players in hemostasis and thrombosis. Nat Med. 1999;5:987–8. doi: 10.1038/12419. [DOI] [PubMed] [Google Scholar]
- 98.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC. et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–92. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 99.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 100.Leskov I, Pallasch CP, Drake A, Iliopoulou BP, Souza A, Shen CH. et al. Rapid generation of human B-cell lymphomas via combined expression of Myc and Bcl2 and their use as a preclinical model for biological therapies. Oncogene. 2013;32:1066–72. doi: 10.1038/onc.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Izquierdo E, Vorholt D, Blakemore S, Sackey B, Nolte JL, Barbarino V. et al. Extracellular vesicles and PD-L1 suppress macrophages, inducing therapy resistance in TP53-deficient B-cell malignancies. Blood. 2022;139:3617–29. doi: 10.1182/blood.2021014007. [DOI] [PubMed] [Google Scholar]
- 102.Su S, Zhao J, Xing Y, Zhang X, Liu J, Ouyang Q. et al. Immune Checkpoint Inhibition Overcomes ADCP-Induced Immunosuppression by Macrophages. Cell. 2018;175:442–57. doi: 10.1016/j.cell.2018.09.007. e23. [DOI] [PubMed] [Google Scholar]
- 103.Belhadj Z, He B, Deng H, Song S, Zhang H, Wang X. et al. A combined "eat me/don't eat me" strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9:1806444. doi: 10.1080/20013078.2020.1806444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matsumoto A, Takahashi Y, Chang HY, Wu YW, Yamamoto A, Ishihama Y. et al. Blood concentrations of small extracellular vesicles are determined by a balance between abundant secretion and rapid clearance. J Extracell Vesicles. 2020;9:1696517. doi: 10.1080/20013078.2019.1696517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu ZL, Liu XC, Wu M, Shi S, Fu QY, Jia J. et al. Untouched isolation enables targeted functional analysis of tumour-cell-derived extracellular vesicles from tumour tissues. J Extracell Vesicles. 2022;11:e12214. doi: 10.1002/jev2.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–6. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 107.Unanue ER, Beller DI, Lu CY, Allen PM. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984;132:1–5. [PubMed] [Google Scholar]
- 108.Taylor DD, Black PH. Inhibition of macrophage Ia antigen expression by shed plasma membrane vesicles from metastatic murine melanoma lines. J Natl Cancer Inst. 1985;74:859–67. [PubMed] [Google Scholar]
- 109.Hu Q, Lyon CJ, Fletcher JK, Tang W, Wan M, Hu TY. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm Sin B. 2021;11:1493–512. doi: 10.1016/j.apsb.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng L, Zhang X, Tang J, Lv Q, Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275:120964. doi: 10.1016/j.biomaterials.2021.120964. [DOI] [PubMed] [Google Scholar]
- 111.Shi X, Sun J, Li H, Lin H, Xie W, Li J. et al. Antitumor efficacy of interferon-gamma-modified exosomal vaccine in prostate cancer. Prostate. 2020;80:811–23. doi: 10.1002/pros.23996. [DOI] [PubMed] [Google Scholar]
- 112.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Su MJ, Aldawsari H, Amiji M. Pancreatic Cancer Cell Exosome-Mediated Macrophage Reprogramming and the Role of MicroRNAs 155 and 125b2 Transfection using Nanoparticle Delivery Systems. Sci Rep. 2016;6:30110. doi: 10.1038/srep30110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M. et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Geng T, Pan P, Leung E, Chen Q, Chamley L, Wu Z. Recent Advancement and Technical Challenges in Developing Small Extracellular Vesicles for Cancer Drug Delivery. Pharm Res. 2021;38:179–97. doi: 10.1007/s11095-021-02988-z. [DOI] [PubMed] [Google Scholar]
- 117.Liu Y, Wang J, Zhang J, Marbach S, Xu W, Zhu L. Targeting Tumor-Associated Macrophages by MMP2-Sensitive Apoptotic Body-Mimicking Nanoparticles. ACS Appl Mater Interfaces. 2020;12:52402–14. doi: 10.1021/acsami.0c15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen H, Jiang S, Zhang P, Ren Z, Wen J. Exosomes synergized with PIONs@E6 enhance their immunity against hepatocellular carcinoma via promoting M1 macrophages polarization. Int Immunopharmacol. 2021;99:107960. doi: 10.1016/j.intimp.2021.107960. [DOI] [PubMed] [Google Scholar]
- 119.Zhao G, Liu H, Wang Z, Yang H, Zhao H, Zhang Y. et al. Exosome transportation-mediated immunosuppression relief through cascade amplification for enhanced apoptotic body vaccination. Acta Biomater. 2022;153:529–39. doi: 10.1016/j.actbio.2022.09.014. [DOI] [PubMed] [Google Scholar]
- 120.Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T. et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238. doi: 10.3402/jev.v4.26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table.