Abstract
Background
Bacteria colonizing the nasopharynx play a key role as gatekeepers of respiratory health. Yet, dynamics of early life nasopharyngeal (NP) bacterial profiles remain understudied in low- and middle-income countries (LMICs), where children have a high prevalence of risk factors for lower respiratory tract infection. We investigated longitudinal changes in NP bacterial profiles, and associated exposures, among healthy infants from low-income households in South Africa.
Methods
We used short fragment (V4 region) 16S rRNA gene amplicon sequencing to characterize NP bacterial profiles from 103 infants in a South African birth cohort, at monthly intervals from birth through the first 12 months of life and six monthly thereafter until 30 months.
Results
Corynebacterium and Staphylococcus were dominant colonizers at 1 month of life; however, these were rapidly replaced by Moraxella- or Haemophilus-dominated profiles by 4 months. This succession was almost universal and largely independent of a broad range of exposures. Warm weather (summer), lower gestational age, maternal smoking, no day-care attendance, antibiotic exposure, or low height-for-age z score at 12 months were associated with higher alpha and beta diversity. Summer was also associated with higher relative abundances of Staphylococcus, Streptococcus, Neisseria, or anaerobic gram-negative bacteria, whilst spring and winter were associated with higher relative abundances of Haemophilus or Corynebacterium, respectively. Maternal smoking was associated with higher relative abundances of Porphyromonas. Antibiotic therapy (or isoniazid prophylaxis for tuberculosis) was associated with higher relative abundance of anerobic taxa (Porphyromonas, Fusobacterium, and Prevotella) and with lower relative abundances of health associated-taxa Corynebacterium and Dolosigranulum. HIV-exposure was associated with higher relative abundances of Klebsiella or Veillonella and lower relative abundances of an unclassified genus within the family Lachnospiraceae.
Conclusions
In this intensively sampled cohort, there was rapid and predictable replacement of early profiles dominated by health-associated Corynebacterium and Dolosigranulum with those dominated by Moraxella and Haemophilus, independent of exposures. Season and antibiotic exposure were key determinants of NP bacterial profiles. Understudied but highly prevalent exposures prevalent in LMICs, including maternal smoking and HIV-exposure, were associated with NP bacterial profiles.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-023-01563-5.
Keywords: 16S rRNA gene, Birth cohort, Microbiome, High-throughput sequencing, Infant, Longitudinal, Low- and middle-income country, Microbiota, Nasopharyngeal, Upper respiratory tract
Background
A growing body of evidence describes the importance of the microbiota of the respiratory tract as gatekeepers of respiratory health [1, 2]. Bacteria colonizing the nasopharynx play a key role in respiratory tract infection (RTI) in children, by protecting the airway lining against air-transmitted pathogenic infections [3]. Commensals such as Corynebacterium and Dolosigranulum may benefit the ecosystem balance and respiratory health by excluding pathogenic bacteria [4–7]. However, the nasopharynx is also the natural niche for several potentially pathogenic species (pathobionts), including Streptococcus, Haemophilus, and Moraxella species.
Data from high-income settings indicate that early life nasopharyngeal (NP) bacterial communities transition from profiles dominated by Staphylococcus early in life towards Corynebacterium- and Dolosigranulum-enriched profiles, followed by enrichment with Moraxella later in infancy [8]. Temporal dynamics of these developmental stages could be important for respiratory health. For example, early enrichment with Moraxella and oral species, such as Prevotella, has been associated with an increased susceptibility to RTI during the first year of life [7]. Similarly, early life bacterial profiles dominated by Haemophilus or Streptococcus have been associated with respiratory virus infection and increased risk of bronchiolitis during infancy [9–11].
Exposures such as mode of delivery, feeding practices, and antimicrobials shape NP bacterial communities and may impact long-term respiratory health [4, 7]. For example, when compared to infants born by vaginal delivery, cesarean-section delivery has been associated with delayed succession of NP bacterial profiles and reduced colonization with health-associated taxa such as Corynebacterium and Dolosigranulum [12]. The protective role of breastfeeding against lower respiratory tract infection (LRTI) may be related, in part, to the presence of Corynebacterium spp. in breast milk [13], which could enhance the growth of commensals such as Dolosigranulum pigrum and protect against pathobionts such as Staphylococcus [14, 15]. Reduced relative abundance of Corynebacterium and shifts in NP bacterial community profiles have been associated with antibiotic administration [7, 16]. Other factors modulating early life NP bacterial profiles, also associated with LRTI, include indoor air pollution [17], tobacco smoke exposure [17], and seasonal changes [18, 19].
Data on longitudinal changes in NP bacterial profiles early in life, and factors influencing these profiles remain scarce—particularly from low- and middle-income countries (LMICs) [4, 7, 8, 12, 14, 16, 18, 20, 21]. Children in LMICs have a high prevalence of risk factors for LRTI. For example, malnutrition and HIV exposure have been associated with more severe LRTI and poorer outcomes [22–24]. Other risk factors for LRTI, common in LMICs, include short duration of exclusive breastfeeding [25], tobacco smoke exposure [22], indoor air pollution [26], lack of immunization, and suboptimal clinical care [26, 27]. Nonetheless, the impact of these exposures on early life NP bacterial communities in infants from LMICs is not well studied.
We therefore characterized succession of NP bacterial communities from children without LRTI living in a low-income setting at monthly intervals from birth for 12 months and six monthly thereafter until 30 months of life. We investigated how host and environmental factors influenced bacterial diversity and community composition.
Methods
Study setting
This study was nested within the Drakenstein Child Health Study (DCHS) [28], a birth cohort in South Africa, which longitudinally followed mother–child dyads through childhood to investigate the impact of early life exposures on child health [28]. Enrolment of consenting pregnant women (> 18 years of age) took place during their second trimester at public sector primary health care clinics. All births and hospital care occurred at Paarl Hospital (60 km outside Cape Town, South Africa), while all children received primary health care at these clinics [28].
The local community of approximately 200,000 people is of low socioeconomic status, and most residents live in informal housing and crowded conditions [28]. Participants experience high levels of unemployment, food insecurity, and other poverty-related exposures including tobacco smoke exposure, and indoor air pollution, as described [29, 30]. More than 90% of the population access health care, including antenatal services and HIV treatment and prevention of mother-to-child transmission programs (PMTCT), in the public sector [28]. LRTI [31], tuberculosis [32], and HIV exposure (HIV-uninfected infants born to HIV-infected mothers) [29] are common among children enrolled in the DCHS.
Participants included in this substudy had NP specimens collected at two weekly intervals during the first year of life [28], with additional 6 monthly study visits from 12 to 30 months [28].
Measures
Antenatal ultrasound from the second trimester was used to calculate gestational age at delivery. Preterm was defined as < 37 weeks of gestation. Birth weight, length, and head circumference were measured at the time of delivery. Weight-for-age z (WAZ) scores at birth and at 12 months were calculated using the revised Fenton preterm growth charts [33, 34]. Low- and high-WAZ scores were defined as scores less and greater than 1 standard deviation (SD) below the mean weight-for-age value, respectively. Using previously published classifications of South African seasonal patterns, “birth season” and “specimen collection season” were categorized into autumn (April to May), winter (June to August), spring (September to November), and summer (December to March) [35, 36]. Feeding practices were longitudinally reported through infancy.
Maternal smoking was self-reported antenatally and 10 weeks postnatally. Monthly household income and maternal educational attainment were self-reported.
Children who experienced a LRTI were excluded from this analysis. Tuberculin skin tests were done six monthly and isoniazid (INH) prophylaxis provided at primary health clinics to those testing positive.
Maternal HIV infection was confirmed in pregnancy; all HIV-infected mothers received antiretroviral therapy (ART) as per local guidelines [37]. HIV-exposed children were tested for HIV by polymerase chain reaction (PCR) at 6 weeks, enzyme-linked immunosorbent assay (ELISA) or rapid antibody testing at 9 months, and rapid antibody testing at 18 months, as per guidelines [37]. HIV-exposed children who tested negative for HIV were classified as HIV-exposed, uninfected.
Specimen collection and selection for 16S rRNA gene amplicon sequencing
NP flocked swabs (FLOQSwab™, Copan Diagnostics, CA, USA) were collected and immediately suspended in PrimeStore® Molecular Transport medium (Longhorn Vaccines & Diagnostics, Bethesda, MD, USA), transported on ice and stored at −80 °C until further processing. We included NP specimens collected at monthly intervals (using a window of ± 15 days) during the first year of life and then at 18, 24, and 30 months of life (using a window of ± 60 days).
16S rRNA gene amplicon library preparation and sequencing
Each sequencing run consisted of four 96-well plates (384 reactions). A comprehensive set of sequencing controls was included alongside NP specimens on each 96-well plate [38] (Supplementary material, Section A, Fig. S1). Nucleic acid extraction steps have been described in detail elsewhere [38, 39]. Briefly, we transferred 400 µl of homogenized NP specimen to ZR BashingBead™ Lysis Tubes containing 0.5 mm bashing beads (catalogue no. ZR S6002-50, Zymo Research Corp., Irvine, CA, USA) for mechanical lysis at 50 Hz for 5 min using the TissueLyser LT™ (Qiagen, FRITSCH GmbH, Idar-Oberstein, Germany). We loaded 250 μl of the supernatant to the QIAsymphony® SP instrument (Qiagen, Hombrechtikon, Switzerland) for automated nucleic acid extraction using the DSP Virus/Pathogen Mini Kit® (catalogue no. 937036, Qiagen GmbH, Hilden, Germany) [38] with an elution volume of 60 µl. We quantified total 16S rRNA gene copy numbers from each nucleic acid extract using quantitative polymerase chain reaction (qPCR) [18]. Amplicon library preparation steps have been described in detail elsewhere [38, 39] (Supplementary material, Section A). We sequenced the libraries on the Illumina® MiSeq™ platform using the MiSeq Reagent Kit v3 (600-cycle) Reagent Cartridge (Illumina, San Diego, CA, USA).
A detailed description of the bioinformatics approach is provided in Supplementary material, Section A. In brief, we first assessed the quality of demultiplexed paired-end reads via FastQC [40] and MultiQC [41]. We then used the DADA2 pipeline [42] (wrapped in the Nextflow algorithm [43]) to filter and trim reads, infer amplicon sequence variants (ASVs), and assign taxonomy to ASVs. We assigned taxonomy to each of the ASVs using the RDP [44] classifier implementation for DADA2 [45] and SILVA version 138 [46] (Supplementary Tables 1 and 2). We removed ASVs classified as Eukaryota and ASVs with unassigned taxonomy at Kingdom level from the dataset.
Participant selection for downstream analyses
We used a step-wise in silico quality control approach [38] to ensure the inclusion of high-quality gene amplicon data (Supplementary material, Section A). We subsequently excluded HIV-infected participants (n = 1) and participants with ≥ 4 missing NP specimens from the first year of life.
Statistical analyses
Details of statistical methods are described in Supplementary material, Section A. We used R software version 3.6.3 and RStudio software version 1.3.1056 for data analysis and visualization [47, 48]. We applied a one-way analysis of variance (ANOVA) to compare Shannon diversity indices [49] between timepoints and implemented Tukey’s Honest Significant Difference test to compare each pair of timepoints simultaneously pairwise. We computed Aitchison distances [50, 51] between specimens at each of the timepoints and across all timepoints. We used principal coordinate analysis (PCoA) plots to visually represent between-specimen beta diversity with 90%-bags [52] enclosing the inner 90% of the observations from each of the timepoints under study.
We used the R function [gm] in the package robCompositions [53] to calculate compositional mean relative abundances [54, 55] and the R function [barplot] in base R to generate compositional mean relative abundance barplots. Bootstrap confidence intervals of compositional mean relative abundances were computed at the 95% confidence level with the R function [boot.ci] in the package boot [56, 57]. We used the R package vioplot [58] to construct violin plots of the relative abundances of the most abundant bacterial genera. Each NP specimen was assigned to a “bacterial profile group” based on the most abundant bacterial genus detected from the specimen. We generated alluvial plots of NP bacterial trajectories using the package plotly in Python [59].
We investigated associations between covariates and alpha diversity, and covariates and bacterial taxa, across three specimen collection intervals [interval A: 1 to 3 months (M01-M03), interval B: 4 to 6 months (M04-M06), and interval C: 7 to 12 months (M07-M12)] (Supplementary material, Section A). We elected to separately compare covariates across these intervals since microbiota profiles are strongly age-dependent over the first year of life and since not all participants had samples collected at each timepoint. For the analysis of association with covariates, we excluded participants where specimen collection was incomplete for the respective interval (A, B, or C). We performed differential abundance testing using Microbiome Multivariable Associations with Linear Models (MaAsLin2) [60] and Analysis of Composition of Microbiomes (ANCOM 2) [61]. We applied a random effects model to each of the three specimen collection intervals to account for repeated sampling.
Since statistical methods for beta diversity were not able to account for multiple sampling per participant (random effects could not be modelled), we investigated associations between covariates and beta diversity cross-sectionally at five timepoints in the first year (M01, M03, M06, M09, and M12) (Supplementary material, Section A). In addition, we used compositional tensor factorization (CTF) [62], which uses dimensionality reduction to incorporate information from participant-level patterns in microbiome composition across multiple samples, to identify associations between exposure variables and microbial composition across the full 12-month period. We visualized these differences over time using volatility control plots and used univariate linear models to explore associations between exposures and the three major ordination axes derived from CTF.
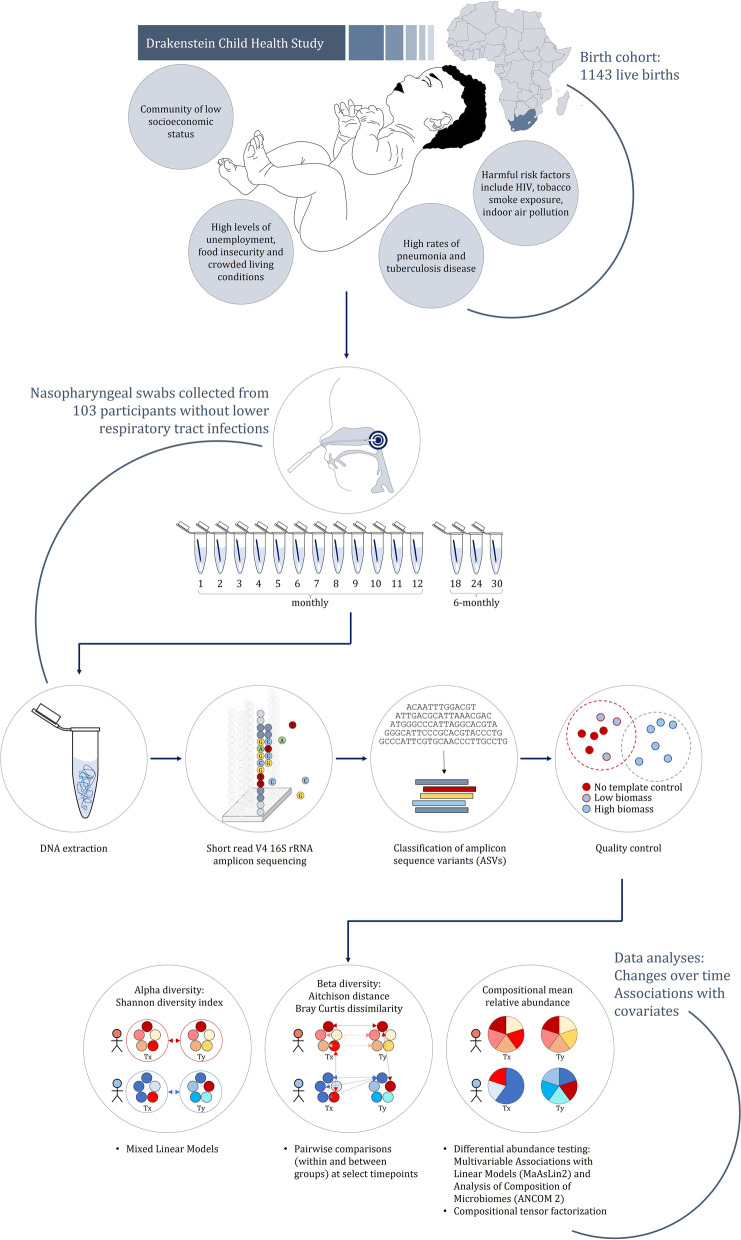
Covariates tested included specimen collection season, sex, mode of delivery, gestational age, WAZ at birth, duration of exclusive breastfeeding, maternal smoking, pets in the home during the first 3 months of life, older siblings, day-care attendance, total monthly household income, maternal educational attainment, antibiotic administration, isoniazid (INH) prophylaxis, WAZ and height-for-age z score (HAZ) at 12 months, and HIV exposure. We categorized NP specimens as INH- and/or antibiotic-exposed if exposed < 100 days prior to collection (Supplementary Tables 3 and 4). The analysis code has been posted at https://github.com/yxia-code/hcm_analysis. The overall study design and analysis strategy is summarized in Fig. 1.
Fig. 1.
Summary of overall study design and analysis approach
Results
Nasopharyngeal specimens, amplicon sequence variants, and participants
Bacterial profiles from the 24 mock community controls included in the five sequencing runs were reproducible and comparable to the theoretical compositions provided by the manufacturer (Supplementary material, Section B). We also observed high sequencing reproducibility from NP specimens randomly selected for repeat processing (Supplementary material, Section B).
Following bioinformatic processing and quality control processes, a total of 1358 NP specimens (and 1031 ASVs) from 103 participants were included to investigate changes in NP bacterial profiles over time. The median read count from NP specimens included was 20,900 (IQR: 16,719–26,036). The reasons for sample exclusion and ASV removal are detailed in Supplementary material Section B, Fig. S7.
To investigate associations between early life exposures and NP bacterial profiles during the first year of life, we excluded a further four participants due to incomplete specimen collection across intervals A, B, and C (Fig. S7). A total of 70/99 (71%), 85/99 (86%), or 77/99 (78%) participants had complete specimen collections across intervals A, B, or C, respectively.
Participant characteristics
Characteristics of the participants are summarized in Table 1. Of the 103 children included in the study of longitudinal changes in the microbiota, most were female (59%) and were delivered by vaginal delivery (80%) and full term [median gestational age: 39 weeks (interquartile range (IQR): 38–40)] with normal weight-for-age z scores (WAZ) [median WAZ: − 0.28 (IQR: − 1.06–0.33)]). One quarter (24%) of infants were HIV-exposed but uninfected. Duration of exclusive breastfeeding was short, with 69% of participants exclusively breastfed for at least 1 month and only 20% for more than 4 months. Maternal smoking occurred in 15% of participants. Poor socioeconomic status was reflected by low total monthly income [less than 5000 ZAR (USD 320) per month for 87% of households] and low maternal educational attainment (69% of mothers had primary level education only).
Table 1.
Participant characteristics
| Study of NP microbiota over time | Study of association between NP microbiota and exposures | |
|---|---|---|
| N = 103 | N = 99 | |
| Participant characteristics | n (%) | n (%) |
| Perinatal factors | ||
| Birth season | ||
| Autumn (April–May) | 10 (10) | 10 (10) |
| Winter (June–August) | 29 (28) | 29 (29) |
| Spring (September–November) | 26 (25) | 23 (23) |
| Summer (December–March) | 38 (37) | 37 (38) |
| Sex | ||
| Female | 61 (59) | 59 (60) |
| Mode of delivery | ||
| Cesarean-section delivery | 21 (20) | 21 (21) |
| Gestational age | ||
| Preterm (< 37 weeks) | 14 (13) | 13 (13) |
| 37 to 40 weeks | 76 (74) | 74 (75) |
| > 40 weeks | 13 (13) | 12 (12) |
| WAZ at birth | ||
| Low WAZ (< -1 SD below the mean WAZ score) | 29 (28) | 29 (29) |
| Medium WAZ (-1 to 1 SD of the mean WAZ score) | 65 (63) | 61 (62) |
| High WAZ (> 1 SD above the mean WAZ score) | 10 (9) | 9 (9) |
| Duration of exclusive breastfeeding | ||
| < 1 month or none | 32 (31) | 30 (31) |
| Until 1 month | 29 (28) | 28 (28) |
| Until 2–3 months | 21 (20) | 21 (21) |
| Until 4–6 months | 21 (20) | 20 (20) |
| Environmental exposures | ||
| Maternal tobacco use (self-reported) | ||
| Yes | 15 (15) | 15 (15) |
| Pets in the home during the 1st 3 months of life | ||
| Yes | 23 (22) | 23 (23) |
| Older siblings | ||
| Yes | 70 (68) | 66 (67) |
| First day-care attendance (prior to specimen collection) | ||
| At 0–6 months of age (yes) | 7 (7) | 6 (6) |
| At 7–12 months of age (yes) | 12 (12) | 12 (12) |
| Sociodemographic variables | ||
| Total household income | ||
| < 1000 ZAR (< 64 USD) per month | 48 (47) | 46 (47) |
| 1000–5000 ZAR (64–320 USD) per month | 42 (41) | 41 (41) |
| > 5000 ZAR (> 320 USD) per month | 13 (12) | 12 (12) |
| Maternal educational attainment | ||
| Completed primary level education | 71 (69) | 68 (69) |
| Completed secondary level education | 32 (31) | 31 (31) |
| Infant exposures | ||
| Immunization cover | ||
| Bacille Calmette-Guérin at birth | 103 (100) | 99 (100) |
| Diphtheria, tetanus, and acellular pertussis: 3 doses | 103 (100) | 99 (100) |
| Pneumococcal conjugate vaccine: 3 doses | 102 (99) | 98 (99) |
| Measles, mumps, rubella at 9 months | 102 (99) | 98 (99) |
| Oral polio vaccine: 3 doses | 103 (100) | 99 (100) |
| Antibiotic administration | ||
| At 0–6 months of age (yes) | 15 (15) | 15 (15) |
| At 7–12 months of age (yes) | 29 (28) | 29 (29) |
| INH prophylaxis | ||
| At 6–12 months of age (yes) | 9 (9) | 9 (9) |
| WAZ at 12 months of age | ||
| Low WAZ (≤ 1 SD below the mean WAZ score) | 17 (17) | 17 (17) |
| Medium WAZ (− 1 to 1 SD of the mean WAZ score) | 68 (66) | 66 (67) |
| High WAZ (> 1 SD above the mean WAZ score) | 18 (17) | 16 (16) |
| HAZ at 12 months of age | ||
| Low HAZ (≤ 1 SD below the mean HAZ score) | 31 (30) | 31 (31) |
| Medium HAZ (− 1 to 1 SD of the mean HAZ score) | 64 (62) | 61 (62) |
| High HAZ (> 1 SD above the mean HAZ score) | 8 (8) | 7 (7) |
| HIV-exposure | ||
| HIV-exposed, uninfected | 26 (25) | 23 (23) |
HAZ Height-for-age z score, HIV Human immunodeficiency virus, TB-INH Tuberculosis isoniazid, SD Standard deviation, USD US dollar, WAZ Weight-for-age z score, ZAR South African Rand
There was high immunization coverage for all expanded program on immunization vaccines (Table 1). Ten participants had a positive tuberculin skin test over the first year of life, nine of whom received INH prophylaxis. Hospitalization or antibiotic administration (for reasons other than LRTI) was recorded for 15% or 43% of infants, respectively.
Dynamics of NP bacterial profiles during the first 30 months of life
Bacterial density (16S rRNA gene copies/μl) was lowest at 1 month of life, but there were no significant differences through 30 months (Fig. 2A). Within-specimen bacterial diversity (Shannon diversity) measured at each timepoint showed an overall decrease after 4 months of life; however, no statistically significant differences were observed between consecutive timepoints throughout the study period (Fig. 2B). Changes in between-specimen bacterial diversity (Aitchison distance) with age are shown in Fig. 2C.
Fig. 2.
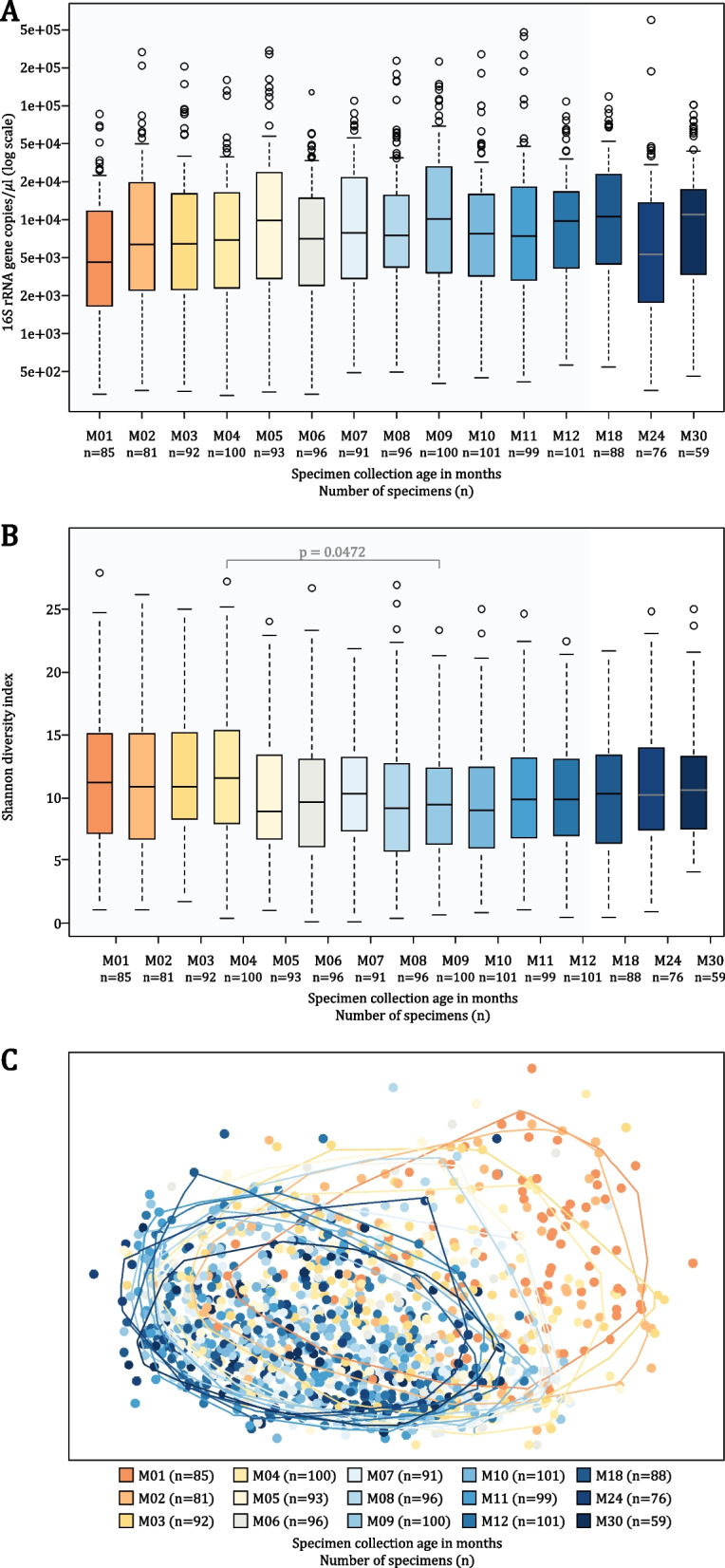
Bacterial density and diversity measured during the first 30 months of life. A Bacterial density (16S rRNA gene copies/μl) compared across timepoints. B Within-specimen bacterial diversity (Shannon diversity) compared across timepoints. One-way analysis of variance (ANOVA) was used to compare alpha diversity indices between timepoints. Tukey’s Honest Significant Difference test was implemented to compare each pair of timepoints simultaneously pairwise. Median values are presented by horizontal lines within each of the boxplots while upper and lower ranges of the boxplots represent the 75% and 25% quartiles, respectively. Maximum and minimum values, excluding outliers, are presented by whiskers. C Principal coordinate analysis of between-specimen bacterial diversity (Aitchison distance). Alpha bags (90%) are used to enclose observations from each of the timepoints, excluding the 10% of the observations at the extremes of each cluster. Specimen collection age (in months) and the number of specimens included at each timepoint are shown at the bottom of each panel
We used CTF to summarize the microbial trajectory of each child over 12 months. Each child’s time series was then represented as a single point on a compositional biplot, using the top two ordination axes, and showing the feature loadings (Fig. S8). Trajectory analysis (Fig. S9) for Axis 1 showed significant variation between participants at early timepoints, with convergence and decrease along this axis over time. Axis 1 was driven along the positive axis by ASV_ 30 (Fusobacterium spp.), ASV_20 (Neisseria spp.), and ASV_8 (Corynebacterium spp.), and along the negative axis by ASV_1 (Moraxella spp.), ASV_2, and ASV_3 (both Haemophilus spp.). In contrast, Axis 2 showed an increase with age, with convergence at 3 months, and was driven along the positive axis by ASV_ 24 (Streptococcus spp.), ASV_4 (Corynebacterium spp.), and ASV_20 (Neisseria spp.), and along the negative axis by ASV_8 (Corynebacterium spp.) and ASV_ 30 (Fusobacterium spp.).
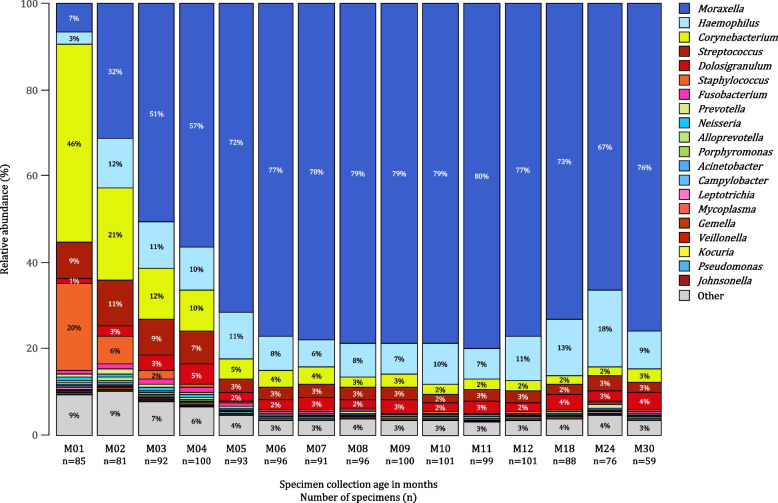
Nasopharyngeal bacterial profiles during the first 30 months of life were dominated by six bacterial genera, including Moraxella, Haemophilus, Corynebacterium, Streptococcus, Dolosigranulum, and Staphylococcus (Fig. 3).
Fig. 3.
Barplots of compositional mean relative abundances of the 20 most abundant nasopharyngeal (NP) bacterial genera detected in 103 participants during the first 30 months of life. Each barplot represents compositional mean relative abundances for each of the 20 most abundant NP bacterial genera at each of the timepoints. The 20 most abundant bacterial genera were identified as the 20 bacterial genera with the highest sum of mean relative abundances across the first 30 months of life. Specimen collection age (in months) and the number of specimens analyzed at each of the timepoints are shown on the X-axis. Shades of colors are used to present phylum-level classification [shades of blue: Proteobacteria (and Campilobacterota, previously Epsilonproteobacteria), shades of yellow: Actinobacteria, shades of red: Firmicutes, shades of pink: Fusobacteriota, shades of green: Bacteroidetes]
Staphylococcus and Corynebacterium were detected at highest compositional mean relative abundances at 1 month of life, with a tenfold decrease observed by 3 and 5 months of life for Staphylococcus and Corynebacterium, respectively (Fig. 3). Corynebacterium was a dominant colonizer in 32% (27/85), 21% (17/81), 13% (12/92), and 11% (11/100) of NP bacterial profiles at 1 to 4 months of life. Staphylococcus dominated 21% (18/85), 12% (10/81), 5% (5/92), and 6% (6/100) of NP bacterial profiles at 1 to 4 months of life.
Compositional mean relative abundance of Moraxella increased fivefold by 2 months of age and 12-fold by 6 months whereafter it remained relatively constant (Figs. 3 and 4). Moraxella had highest relative abundance among 18% (15/85), 35% (28/81), 41% (38/92), and 55% (55/100) of NP bacterial profiles at 1 to 4 months of life, respectively. Haemophilus compositional mean relative abundance plateaued around two months of age. At 1 to 4 months of life, Haemophilus dominated 13% (11/85), 22% (18/81), 28% (26/92), and 19% (19/100) of NP bacterial profiles, respectively.
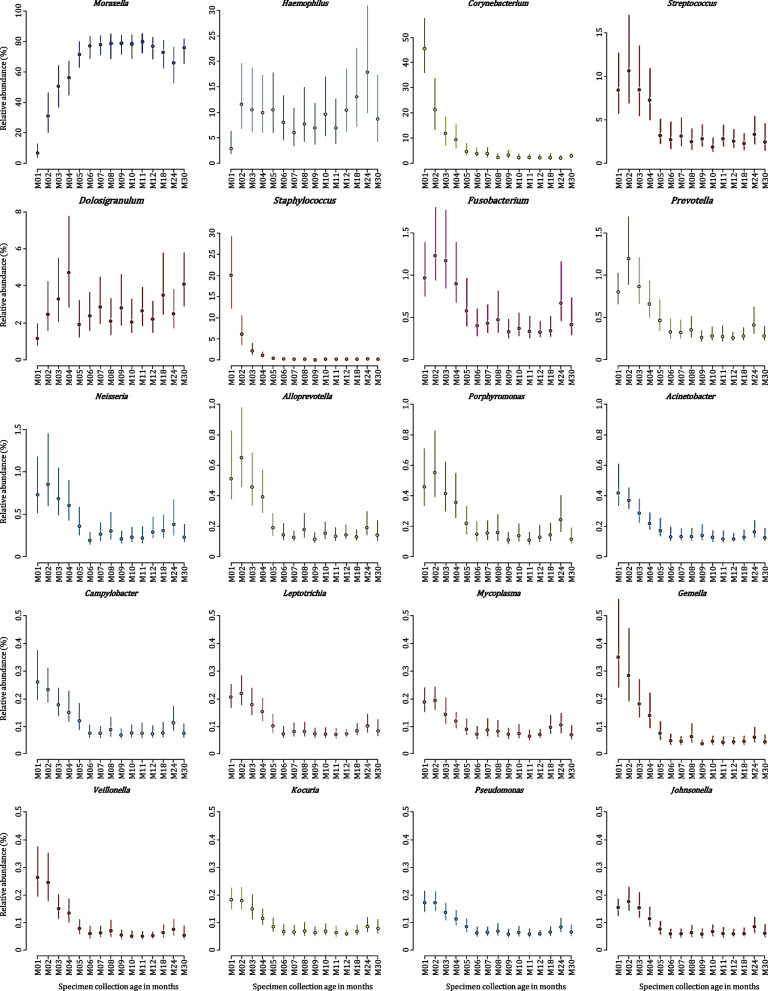
Fig. 4.
Bootstrap confidence intervals of estimates of compositional mean relative abundances of the 20 most abundant nasopharyngeal (NP) bacterial genera detected in 103 participants during the first 30 months of life. Bootstrap confidence intervals were computed at the 95% confidence level (vertical lines) for each of the 20 most abundant NP bacterial genera at each of the timepoints under study. Compositional mean relative abundances at each of the timepoints are presented by the dot on each bootstrap confidence interval line. Specimen collection age (in months) is shown on the X-axis. Shades of colors are used to present phylum-level classification [shades of blue: Proteobacteria (and Campilobacterota, previously Epsilonproteobacteria), shades of yellow: Actinobacteria, shades of red: Firmicutes, shades of pink: Fusobacteriota, shades of green: Bacteroidetes]. Note that the Y-axis scale varies
Moraxella and Haemophilus were detected at higher compositional mean relative abundances than any other bacterial genera at each of the timepoints after 4 months of age. However, the distributions of relative abundances for these two genera across children were different. Moraxella represented approximately 77% of the compositional mean relative abundances at each timepoint after 4 months and was the dominant genus in a similar proportion of specimens (60–70%) at each of these timepoints. In contrast, although Haemophilus only accounted for approximately 10% of the compositional mean relative abundances at each timepoint, it was the dominant genus in 20–30% of specimens at each timepoint after 4 months.
Streptococcus compositional mean relative abundance decreased threefold by 4 months; whereafter, it remained relatively constant (Figs. 3 and 4). Streptococcus was most abundant among 8% (7/85), 4% (3/81), 8% (7/92), and 2% (2/100) of NP bacterial profiles at 1 to 4 months of life, respectively. Streptococcus was dominated by two ASVs (ASV_7 and ASV_10). Mean relative abundance of ASV_10 decreased from 1.7% at 1 month of life to < 0.1% at 4 months, whilst mean relative abundances of ASV_7 remained relatively stable, between 0.3% and 1.4% across all timepoints.
Most (99%) NP specimens were dominated (sum of relative abundances ≥ 90%) by 10 or fewer bacterial genera, with 93% NP specimens dominated by 5 or fewer bacterial genera (Supplementary Fig. S10).
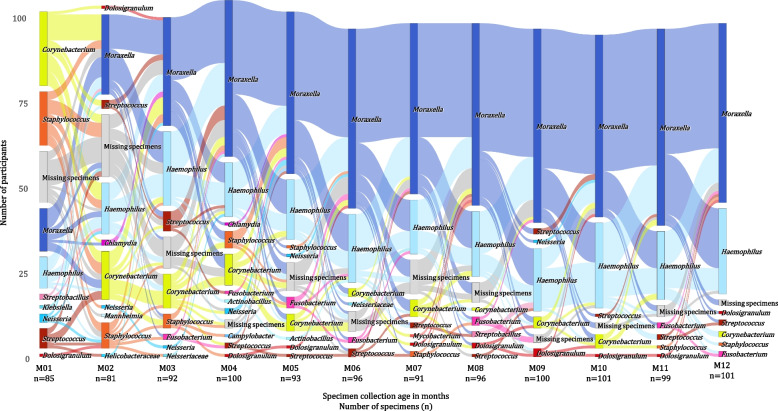
Following participants over time, we observed substantial instability of profiles during the first 4 months of life (Fig. 5). We observed more stable profiles after 4 months of life with most shifts in profiles occurring between Moraxella- and Haemophilus-dominated profiles. Profiles dominated by Streptococcus, Staphylococcus, Corynebacterium, and Neisseria diminished over time.
Fig. 5.
Alluvial plot showing changes in nasopharyngeal (NP) bacterial profiles among the 103 participants based on the most abundant bacterial genus detected at each preceding timepoint during the first year of life. Vertical bars at each timepoint represent the number of participants from which respective bacterial genera (shades of yellow: Actinobacteria; shades of red: Firmicutes, shades of blue: Proteobacteria, shades of pink: Fusobacteriota) were identified as most abundant. The proportion of participants with missing specimens at each timepoint is shown in gray. Specimen collection age (in months) and the number of specimens analyzed at each of the timepoints are denoted on the X-axis. Each participant is represented by a ribbon moving along the X-axis according to specimen collection age and Y-axis according to the most abundant bacterial genus detected at each of the timepoints. The color assigned to each participant ribbon is based on the most abundant bacterial genus detected at each preceding timepoint, highlighting sequential changes
Determinants of infant nasopharyngeal bacterial profiles
Associations between covariates and alpha diversity, beta diversity, and relative abundances of bacterial taxa (genus and ASV level) were investigated across three specimen collection intervals [interval A: one to three months (M01-M03), interval B: 4 to 6 months (M04-M06), and interval C: 7 to 12 months (M07-M12)]. Differential abundance testing was performed on 54, 44, and 47 bacterial genera and 95, 76, and 74 ASVs across intervals A, B, and C, respectively (Supplementary Table S5).
Association between season of specimen collection and bacterial profiles
Apart from age, season of specimen collection was the exposure most consistently associated with microbiota profiles. Specimens collected during summer yielded higher within-specimen diversity compared to most other collection seasons (Table 2; Fig. S11A). Similarly, specimens collected during summer had the highest between-specimen diversity across most timepoints (Aitchison distance and Bray-Curtis dissimilarity) while specimens collected during autumn had the lowest between-specimen diversity across four of the five timepoints studied (Figs. S12A and S13A).
Table 2.
Early life exposures associated with nasopharyngeal bacterial alpha (Shannon) diversity
| Covariate | Interval A: M01-M03 | P value | Interval B: M04-M06 | P value | Interval C: M07-M12 | P value |
|---|---|---|---|---|---|---|
| Specimen collection season | Summer > Autumn | 0.008** | Summer > Autumn | 0.315 | Summer > Autumn | 0.150 |
| Summer > Winter | 0.261 | Summer > Winter | 0.925 | Summer > Winter | 0.083* | |
| Summer > Spring | 0.415 | Summer < Spring | 0.358 | Summer > Spring | 0.570 | |
| Summer > Autumn | 0.268 | Summer > Autumn | 0.005** | Summer > Autumn | 0.071* | |
| Summer < Winter | 0.388 | Summer > Winter | 0.009** | Summer > Winter | < 0.001** | |
| Summer < Spring | 0.525 | Summer > Spring | 0.891 | Summer > Spring | 0.004** | |
| Gestational age | More than 40 weeks > Less than 37 weeks | 0.903 | More than 40 weeks < Less than 37 weeks | 0.057* | More than 40 weeks < Less than 37 weeks | 0.241 |
| More than 40 weeks < 37–40 weeks | 0.439 | More than 40 weeks < 37–40 weeks | 0.093* | More than 40 weeks < 37–40 weeks | 0.372 | |
| More than 40 weeks > Less than 37 weeks | 0.659 | More than 40 weeks < Less than 37 weeks | 0.097* | More than 40 weeks < Less than 37 weeks | 0.211 | |
| More than 40 weeks < 37–40 weeks | 0.339 | More than 40 weeks < 37–40 weeks | 0.254 | More than 40 weeks < 37–40 weeks | 0.114 | |
| Duration of exclusive breastfeeding | None/less than 1 month < Until 1 month | 0.437 | None/less than 1 month < Until 1 month | 0.789 | None/less than 1 month < Until 1 month | 0.599 |
| None/less than 1 month < Until 2 months | 0.905 | None/less than 1 month < Until 2 months | 0.671 | None/less than 1 month < Until 2 months | 0.325 | |
| None/less than 1 month < Until 3 months | 0.426 | None/less than 1 month > Until 3 months | 0.182 | None/less than 1 month < Until 3 months | 0.658 | |
| None/less than 1 month < Until 4 months | < 0.001** | None/less than 1 month < Until 4 months | 0.049** | |||
| None/less than 1 month > Until 5 months | 0.905 | None/less than 1 month > Until 5 months | 0.762 | |||
| None/less than 1 month > Until 6 months | 0.581 | None/less than 1 month < Until 6 months | 0.499 | |||
| None/less than 1 month < Until 1 month | 0.740 | None/less than 1 month > Until 1 month | 0.691 | None/less than 1 month < Until 1 month | 0.004** | |
| None/less than 1 month < Until 2 months | 0.697 | None/less than 1 month > Until 2 months | 0.927 | None/less than 1 month < Until 2 months | 0.006** | |
| None/less than 1 month < Until 3 months | 0.912 | None/less than 1 month > Until 3 months | 0.809 | None/less than 1 month < Until 3 months | 0.358 | |
| None/less than 1 month < Until 4 months | 0.008** | None/less than 1 month < Until 4 months | 0.050* | |||
| None/less than 1 month > Until 5 months | 0.918 | None/less than 1 month < Until 5 months | 0.815 | |||
| None/less than 1 month > Until 6 months | 0.279 | None/less than 1 month < Until 6 months | 0.425 | |||
| Maternal smoking | Yes > No | 0.350 | Yes > No | 0.317 | Yes > No | 0.420 |
| Yes > No | 0.089* | Yes > No | 0.113 | Yes > No | 0.209 | |
| Pets in the home during the 1st 3 months of life | Yes < No | 0.116 | Yes > No | 0.651 | Yes > No | 0.095* |
| Yes < No | 0.491 | Yes > No | 0.279 | Yes > No | 0.084* | |
| Older siblings | Yes > No | 0.405 | Yes > No | 0.764 | Yes > No | 0.090* |
| Yes > No | 0.600 | Yes > No | 0.030** | Yes > No | 0.362 | |
| Maternal educational attainment | Primary level education < Secondary level education | 0.936 | Primary level education < Secondary level education | 0.003** | Primary level education < Secondary level education | 0.779 |
| Primary level education < Secondary level education | 0.918 | Primary level education < Secondary level education | 0.198 | Primary level education < Secondary level education | 0.346 | |
| Antibiotics prior to specimen collection | Not tested | - | Yes > No | 0.991 | Yes > No | 0.239 |
| Not tested | - | Yes < No | 0.623 | Yes > No | 0.077* | |
| HIV-exposure | HIV-unexposed > HIV-exposed, uninfected | 0.560 | HIV-unexposed > HIV-exposed, uninfected | 0.050* | HIV-unexposed > HIV-exposed, uninfected | 0.341 |
| HIV-unexposed < HIV-exposed, uninfected | 0.423 | HIV-unexposed > HIV-exposed, uninfected | 0.281 | HIV-unexposed > HIV-exposed, uninfected | 0.071* | |
| Height-for-age z-score (HAZ) at 12 months | Higha HAZ scores < Lowb HAZ scores | 0.026** | Higha HAZ scores < Lowb HAZ scores | 0.381 | Higha HAZ scores < Lowb HAZ scores | 0.202 |
| Higha HAZ scores < Mediumc HAZ scores | 0.009** | Higha HAZ scores < Mediumc HAZ scores | 0.694 | Higha HAZ scores < Mediumc HAZ scores | 0.250 | |
| Higha HAZ scores < Lowb HAZ scores | 0.068* | Higha HAZ scores > Lowb HAZ scores | 0.851 | Higha HAZ scores < Lowb HAZ scores | 0.461 | |
| Higha HAZ scores < Mediumc HAZ scores | 0.058* | Higha HAZ scores > Mediumc HAZ scores | 0.505 | Higha HAZ scores < Mediumc HAZ scores | 0.689 |
Within-specimen (alpha) diversity p values were adjusted for specimen collection age and season
HIV Human immunodeficiency virus, TB-INH Tuberculosis isoniazid
*p values < 0.10; **p values < 0.05
aHigh HAZ: > 1 standard deviation above the mean HAZ score
bLow HAZ: ≤ 1 standard deviation below the mean HAZ score
cMedium HAZ score: − 1 to 1 standard deviation of the mean HAZ score
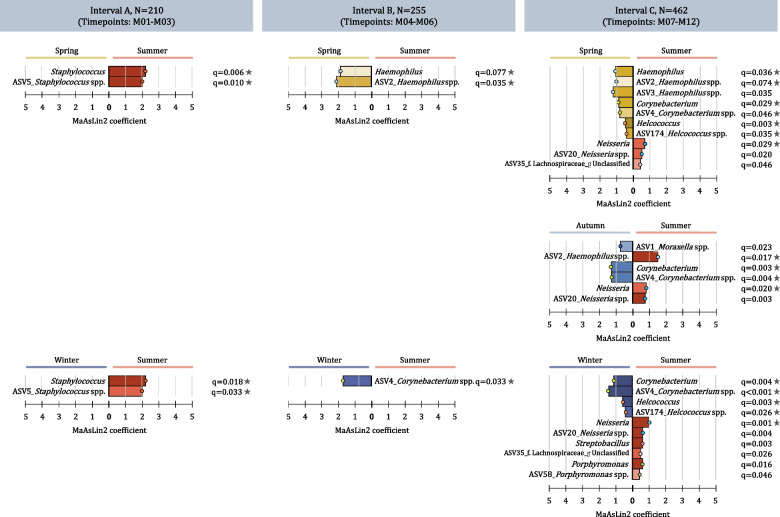
We detected higher relative abundances of Staphylococcus from specimens collected in summer compared to spring and winter (interval A) (Fig. 6). We found higher relative abundances of Haemophilus from specimens collected in spring compared to summer (intervals B and C). We observed higher relative abundances of Corynebacterium from specimens collected in winter compared to summer (interval B and C) and autumn compared to summer (interval C). Streptococcus, Neisseria, Fusobacterium, Streptobacillus, unclassified ASV_35 (family Lachnospiraceae), Porphyromonas, Alloprevotella, and Gemella were detected at higher relative abundances from specimens collected during summer compared to other seasons (interval C).
Fig. 6.
Differential abundance testing for specimen collection season and perinatal factors. Differential abundance testing results are presented for specimen collection season across three specimen collection intervals (interval A: M01-M03, interval B: M04-M06, and interval C: M07-M12). Differentially abundant taxa are shown at genus- and ASV-level. Taxa with q values < 0.10 were deemed differentially abundant using Microbiome Multivariable Associations with Linear Models (MaAsLin2). The color intensity of each taxon bar represents the level of significance (darker shades represent smaller q values). The horizontal length of each taxon bar shows the MaAsLin2 coefficient. Stars next to q values show differentially abundant taxa as per Analysis of Composition of Microbiomes (ANCOM 2) (W statistic > 0.6)
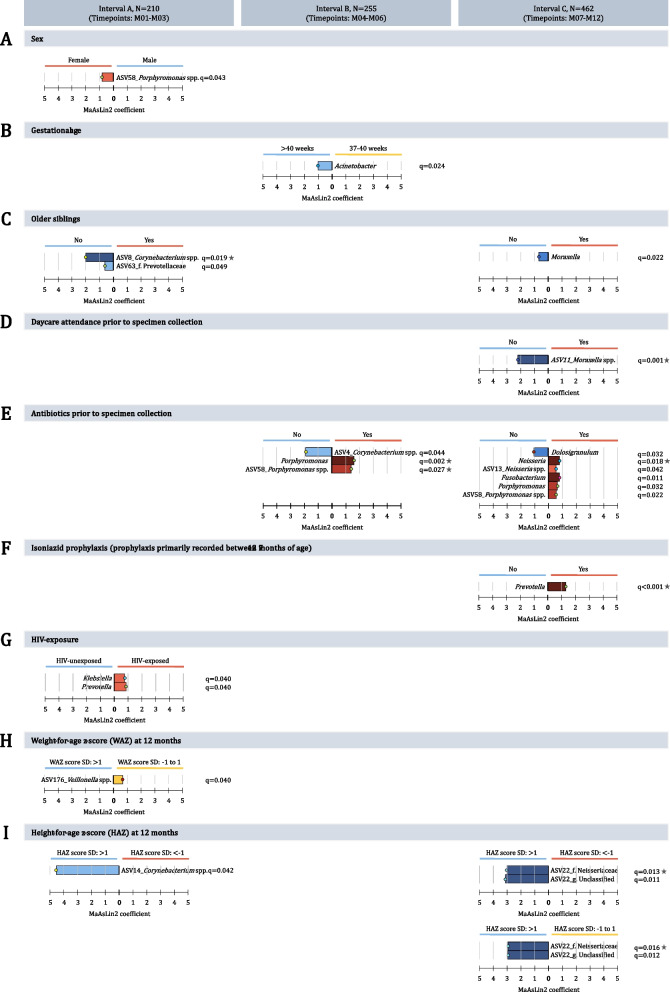
Association between perinatal factors and bacterial profiles
Sex, gestational age, and mode of delivery were not consistently associated with diversity or relative abundance of taxa (Fig. 7, S11B-D). Between-specimen diversity (Bray–Curtis dissimilarity) was higher among infants born < 37 weeks of gestation compared to infants born > 40 weeks of gestation, from 3 months of age onwards (Fig. S13D). Our ability to detect associations with exclusive breastfeeding was limited by the overall short duration of exclusive breastfeeding; however, we did note that very short duration of exclusive breastfeeding (< 1 month) was associated with lower within-specimen diversity across several time periods (Table 2; Fig. S11F).
Fig. 7.
Differential abundance testing for environmental or sociodemographic variables. Differential abundance testing results are presented for A sex, B gestational age, C older siblings, D daycare attendance (prior to specimen collection), E first report of antibiotic administration (prior to specimen collection), F tuberculosis isoniazid prophylaxis, G HIV exposure, H weight-for-age z score measured at 12 months, and I height-for-age z score measured at 12 months across three specimen collection intervals (interval A: M01-M03, interval B: M04-M06, and interval C: M07-M12). Differentially abundant taxa are shown at genus- and ASV-level. Taxa with q values < 0.10 were deemed differentially abundant using Microbiome Multivariable Associations with Linear Models (MaAsLin2). The color intensity of each taxon bar represents the level of significance (darker shades represent smaller q values). The horizontal length of each taxon bar shows the MaAsLin2 coefficient. Stars next to q values show differentially abundant taxa as per Analysis of Composition of Microbiomes (ANCOM 2) (W statistic > 0.6)
Association between environmental or sociodemographic exposures and bacterial profiles
At 6 months of age, between-specimen diversity (Bray–Curtis dissimilarity) was higher between specimens from infants whose mothers smoked when compared to specimens from infants whose mothers did not (Fig. S16A), with a similar trend observed at nine months of age.
Infants without older siblings had higher relative abundances of ASV_8 (Corynebacterium spp.) across interval A and Moraxella in interval C, compared to infants with older siblings (Fig. 7C).
We identified consistently higher (but individually non-significant) within-specimen diversity among infants not attending day-care compared to infants attending day-care (Fig. S14D). We also observed higher between-specimen diversity (Bray Curtis dissimilarity) among infants not attending day-care (Fig. S16D). Relative abundance of Moraxella ASV_11 was higher across interval C for infants not attending day-care (Fig. 7D).
Infants with no antibiotic exposure in the three months prior to specimen collection had higher relative abundances of Corynebacterium (interval B) and Dolosigranulum (interval C) (Fig. 7E), compared to infants who had received antibiotics. Conversely, infants who received antibiotics prior to specimen collection had higher Porphyromonas (intervals B and C) and Neisseria and Fusobacterium (interval C). Infants who received INH prophylaxis had higher relative abundances of Prevotella at interval C (Fig. 7F).
HIV-unexposed infants had a trend to consistently higher (but individually non-significant) within-specimen diversity when compared to HIV-exposed infants (Table 2; Fig. S17C). At interval A, HIV-exposed infants had higher relative abundances of Klebsiella and Prevotella (Fig. 7G).
Infants with lower HAZ scores measured at 12 months of age had higher within-specimen diversity compared to infants with higher HAZ scores (Table 2, Fig. S17E). At 6, 9, and 12 months of age infants with low HAZ scores (measured at 12 months of age) had higher between-specimen diversity (Bray–Curtis dissimilarity) compared to infants with higher HAZ scores (Figs. S18E and S19E). High HAZ scores at 12 months of age were associated with higher relative abundances of ASV_14 (Corynebacterium) over interval A, and ASV_22 (family Neisseriaceae) over interval C (Fig. 7I).
In addition to analyzing associations between exposures separately across each of the three time intervals, we explored associations between exposures and the overall microbial trajectory of each child, summarized using CTF (Fig. S9). Axis 1 was significantly negatively associated with low gestational age (p < 0.001) and HIV-exposure (p = 0.05) and positively associated with low income (p < 0.001), pets in the household (p = 0.012) and low or middle (vs. high) WAZ score at birth (p < 0.001). Axis 2 was significantly negatively associated with vaginal delivery (p < 0.001) and HIV-exposure (p = 0.05) and middle (vs high) income (p = 0.016).
Discussion
Our study investigating NP bacterial profiles in an intensively sampled and well-phenotyped South African birth cohort showed similarities in NP bacterial trajectories over time when compared to most previous reports from high-income countries. Dominant colonizers during the first 2 months of life included Corynebacterium and Staphylococcus, whilst Moraxella dominated profiles at 6 months [4, 7, 8, 12, 16, 21, 63]. A striking difference in NP bacterial trajectories observed from our cohort, compared to previous reports, was the rapid loss of Corynebacterium and Dolosigranulum with early transition to profiles dominated by Moraxella and Haemophilus [4, 7, 8, 12, 16, 21, 63]. Studies from high-income countries generally reported higher mean relative abundances for Corynebacterium, Dolosigranulum, Streptococcus, and Staphylococcus at 6 months of age when compared to our study, with mean relative abundances of Moraxella below 50% [4, 8, 12, 16, 21]. These findings may have implications for child health, since Moraxella, Haemophilus, and Streptococcus have been associated with LRTI in childhood, whilst Corynebacterium and Dolosigranulum are considered health-associated taxa in early life [4, 8, 20, 64, 65]. Indeed, infants in the broader DCHS cohort have a very high incidence of LRTI during the first year of life [66, 67].
Early colonization with a Moraxella- or Dolosigranulum/Corynebacterium-dominated profile have been associated with more stable bacterial colonization patterns during the first 2 years of life [4]. Conversely, Streptococcus- and Haemophilus-dominated profiles early in life were marked by high levels of change and dispersion of profiles over time [4]. Early life NP bacterial profiles may therefore drive NP bacterial succession patterns which may have important implications for respiratory health [4]. In our study of healthy children, instability in NP bacterial profiles was highest throughout the first four months of life. Thereafter, more stable profiles (with shifts primarily occurring between Moraxella- and Haemophilus-dominated profiles) occurred. Results from our study highlight the importance of high frequency of sampling to understand the developmental dynamics of NP bacterial profiles.
In our study, mean relative abundances of bacterial genera identified at 12 months of age did not differ significantly from those at 6 months. Conversely, studies from Australia and the Netherlands have reported an increase in mean relative abundance of Moraxella, Haemophilus, and Streptococcus and a decrease in Corynebacterium when comparing profiles at 12 months to 6- or 9-month timepoints [4, 7, 8]. One other study from The Gambia showed higher relative abundance of Streptococcus in early life NP bacterial communities; however, children were primarily recruited from communities unvaccinated with pneumococcal conjugate vaccine [68].
To date, most studies investigating NP bacterial dynamics during infancy have been done in high-income countries [4, 7, 12, 16, 20, 63, 68–71] where sociodemographic conditions and environmental exposures may be very different compared to LMICs. These exposures could impact on the dynamics of NP bacterial profiles [17] and susceptibility to LRTI [29–31]. A striking finding from our study, using multiple analytical approaches, was that only a few of the broad range of exposures measured had a consistent impact on microbial profiles across time intervals, and the effect size of these exposures was relatively modest.
Specimen collection season was an important determinant of NP bacterial diversity [72], and relative abundance of several bacterial taxa. Staphylococcus (ASV_5) was detected at significantly higher relative abundance during summer in the first 3 months of life. Although short fragment 16S rRNA gene amplicon sequencing does not allow for species-level identification, previous culture and genotyping data on the acquisition of Staphylococcus aureus within the DCHS [73] suggests that ASV_5 may represent S. aureus. In support of these findings, a review evaluating seasonality in S. aureus colonization and infection of different body sites reported trends towards increased S. aureus carriage and infections during warmer weather [74]. However, a further study of NP specimens from 72 infants without upper respiratory tract infection (URTI), sampled during the first 6 months of life, showed a significantly higher prevalence of S. epidermidis during summer [75]. Across four to 12 months of life, Haemophilus relative abundance was highest during spring, consistent with findings from a previous study of infants in Perth, Australia [69], which is on the same latitude and has similar seasonal climate to Cape Town.
The effect of cesarean-section delivery on infant bacterial communities, particularly in the gastrointestinal tract, has been widely studied [76]. Unlike previous reports from high-income countries [7, 12], our study which used MaAsLin2 and the relatively conservative ANCOM 2 method for testing differential abundance [61] did not find statistically significant differences in relative abundances of genera previously associated with mode of delivery. However, there were relatively small numbers of children born by cesarean section in our study. Studies performed in the Netherlands reported limited direct impact of mode of delivery on NP bacterial profiles at the time of delivery, with differences emerging over subsequent months [12]. These findings suggest that changes in NP bacterial profiles among vaginal and cesarean-section delivered infants may be mediated by other early life exposures such as feeding practices [4, 7, 12] or antibiotic use [7].
Several studies have reported associations between early life feeding practices and NP bacterial communities [4, 7, 20, 63], yet our data did not show similar associations. However, duration of exclusive breastfeeding was very short in our cohort. A study conducted in the Netherlands reported that exclusively breastfed infants demonstrate higher relative abundances of Corynebacterium and Dolosigranulum, but reduced abundances of Staphylococcus, Prevotella, and Veillonella compared to formula-fed infants at 6 weeks of age [63].
Co-habiting with older siblings has been associated with lower abundances of Staphylococcus [69] and higher abundances of Haemophilus [69] (H. influenzae [77]), Streptococcus [69] (S. pneumoniae [77]), and Moraxella [69] during infancy. Our study suggests that exposure to older children may be a key factor associated with early loss of health-associated Corynebacterium; however, Moraxella relative abundance at 7–12 months of age was lower in children with older siblings. Exposure to older children may also occur during day-care attendance. We found that infants attending day-care had lower relative abundance of ASV_11 (Moraxella spp.). Moraxella has been previously reported at higher relative abundances from infants attending day-care [69]; however, ASV_11 was not the dominant Moraxella sequence variant in our study.
Prior antibiotic therapy was clearly associated with changes in the microbiota. Relative abundances of health-associated Corynebacterium and Dolosigranulum were reduced in infants with recent antibiotic exposure, as previously reported [7, 16, 69]. In addition, we found that gram-negative anaerobes (Porphyromonas, Fusobacterium, and Prevotella) had higher relative abundances in infants exposed to antibiotics, including INH, used for tuberculosis preventative therapy. Nasopharyngeal enrichment of oral anaerobes has been reported from hospitalized children with invasive pneumococcal disease following antibiotic treatment [78]. Longitudinal studies should investigate whether repopulation of the nasopharynx following antibiotic exposure results from expansion of oral anaerobic bacteria and whether such repopulation could be manipulated through oral hygiene and pre- or probiotics [78].
Despite associations between HIV and LRTI, few studies have compared NP bacterial community composition in HIV-exposed and HIV-unexposed infants. A study performed in Botswana reported lower relative abundances of Dolosigranulum from HIV-infected children but higher relative abundances of Klebsiella from HIV-exposed children [79]. In support of this, we found that Klebsiella and Prevotella ASVs had higher relative abundance among HIV-exposed compared to HIV-unexposed infants, but only across the first 3 months of life. Studies of lower airway microbial communities have previously reported enrichment of select oral commensal bacteria including Veillonella, Prevotella, and Streptococcus among HIV-infected patients [80]. Enrichment of these oral commensals has been associated with pneumonia, pulmonary tuberculosis, and chronic obstructive pulmonary disease [80].
Longitudinal modeling of associations between the microbiota and covariates is complex, particularly in early life, when rapid changes in composition occur. We used CTF to reduce each child’s microbial profile over the time series to a point in three-dimensional space, and then modeled the association between each ordination axis and exposure variables. This analysis revealed several additional potential associations, for example between HIV-exposure or low gestational age and a microbial trajectory driven by Moraxella and Haemophilus genera. However, interpretation of these associations is not straightforward, and further validation of these findings is needed.
The strengths of our study include intensive sampling and careful and detailed phenotyping of participants who experienced a range of relevant and previously understudied exposures. We identified relatively few consistent associations between exposures and microbial profiles. This may be because the ability to track associations over time allows us to exclude spurious findings observed at a single timepoint. Our study has several limitations. The use of short fragment 16S rRNA gene amplicon sequencing precluded species-level taxonomic assignment and does not provide information on absolute abundance of bacteria. The sample size was relatively modest, which may have limited our ability to detect subtle associations; however, there were few instances in which consistent, but non-significant, effects were observed over time, suggesting that increased sample size would not substantially alter our findings. Direct comparisons of NP bacterial profiles across studies may be complicated by several factors. These include 16S rRNA gene amplicon library preparation protocols [81–83], bioinformatic pipelines [84, 85], in silico quality control approaches [38], and the manner in which authors report their findings (for example, arithmetic versus compositional means of relative abundances of bacterial taxa or assignment of specimen clusters using varying definitions). We did not address mechanistic relationships between bacterial taxa and exposures.
In conclusion, our study is the first to longitudinally investigate NP bacterial dynamics and their determinants from healthy infants without LRTI residing in a in a low-resource setting with high LRTI incidence. NP bacterial profiles followed similar trajectories to those reported from high-income countries, but with rapid loss of health associated taxa Corynebacterium and Dolosigranulum and early replacement with Moraxella and Haemophilus [28]. Season and antibiotic exposure were key determinants of NP bacterial profiles early in life; however, succession of profiles appeared to be robust to a broad range of other exposures. Stochastic events, such as acquisition of a new taxon, and microbial interactions, should be further explored as important determinants of microbial succession in this niche.
Supplementary Information
Additional file 1: Section A. Extended methods (Figure S1). Section B. Extended results - sequencing controls and sample selection (Figures S2-S7). Section C. Extended results - Figures S8-S19. Section D. Extended references. Table S1. RDP classifier implementation for DADA2 and SILVA version: ASV table. Table S2. RDP classifier implementation for DADA2 and SILVA version: taxonomic classification. Table S3. Metadata file: NP specimens. Table S4. Metadata file: Participants. Table S5. Differential abundance testing.
Acknowledgements
We thank participants and their families, and the study's clinical, laboratory, and data team staff. We thank the staff of the Western Cape Government Health Department at Paarl Hospital and at the clinics for support of the study. We acknowledge facilities provided by the University of Cape Town’s ICTS High Performance Computing team (http://hpc.uct.ac.za). We thank Prof. Debby Bogaert’s team at the Centre for Inflammation Research, University of Edinburgh, for guidance related to their qPCR protocol.
Authors’ contributions
Conception and design of the parent study (DCHS): HJZ, MPN. Conception and design of this study: MPN and HJZ. Funding acquisition: MPN, WCN and HJZ. 16S rRNA amplicon sequencing training: SHM. Laboratory experiments: SC. Bioinformatics processing: KSM. Data analysis and interpretation: SG, SC, LW and YX. Initial draft of the manuscript: SC. Major contributors to writing of the manuscript: SC and MPN. Manuscript revisions: SC, SG, YX, KSM, HJZ, MPN. All authors read and approved the final manuscript.
Funding
This work was supported by an H3Africa U01 award from the National Institutes of Health of the USA (1U01AI110466-01A1), the Bill and Melinda Gates Foundation (OPP1017641; OPP1017579), the National Research Foundation South Africa, and the South African Medical Research Council. SC was supported by the Drakenstein Child Health Study, funded by Bill and Melinda Gates Foundation (OPP1017641), the National Research Foundation South Africa, and L’Oréal-UNESCO For Women in Science (South African Young Talents Award). SHM and WCN were supported by the Bill and Melinda Gates Foundation. MPN was supported by an Australian National Health and Medical Research Council Investigator Grant (APP1174455). HJZ was supported by the South African Medical Research Council. Funding bodies had no role in the design of the study and collection, analysis, and interpretation of the data and in writing the manuscript and the decision to publish this report.
Availability of data and materials
Sequence data and subject characteristics are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the BioProject ID PRJNA790843 and PRJNA548658. The ASV table, taxonomic classification, sample metadata, and participant metadata are available in Supplementary Tables 1–4.
Declarations
Ethics approval and consent to participate
Both this study (585/2015) and parent study (401/2009) received ethical approval from the Faculty of Health Sciences, Human Research Ethics Committee (HREC) of the University of Cape Town, South Africa. The relevant guidelines and regulations were followed during the performance of all experiments. Mothers participating in the parent study provided informed, written consent for enrolment of their infants at the time of delivery and annually.
Consent for publication
All authors of this work concur with this submission, and the data presented have not been previously reported nor are they under consideration for publication elsewhere.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shantelle Claassen-Weitz, Email: tellafiela@gmail.com.
Sugnet Gardner-Lubbe, Email: slubbe@sun.ac.za.
Yao Xia, Email: xiayao0125@outlook.com.
Kilaza S. Mwaikono, Email: kilazasmsn24@gmail.com
Stephanie Harris Mounaud, Email: smounaud@gmail.com.
William C. Nierman, Email: wnierman@jcvi.org
Lesley Workman, Email: lesley.workman@uct.ac.za.
Heather J. Zar, Email: heather.zar@uct.ac.za
Mark P. Nicol, Email: mark.nicol@uwa.edu.au
References
- 1.Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019;17:1–20. doi: 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Man WH, De Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Z, Kang Y, Yu J, Ren L. Human pharyngeal microbiome may play a protective role in respiratory tract infections. Genomics Proteomics Bioinforma. 2014;12:144–150. doi: 10.1016/j.gpb.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biesbroek G, Tsivtsivadze E, Sanders EAM, Montijn R, Veenhoven RH, Keijser BJF, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190:1283–1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 5.Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. Microbial communities of the upper respiratory tract and otitis media in children. MBio. 2011;2:6. doi: 10.1128/mBio.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettigrew MM, Laufer AS, Gent JF, Kong Y, Fennie KP, Metlay JP. Upper respiratory tract microbial communities, acute otitis media pathogens, and antibiotic use in healthy and sick children. Appl Environ Microbiol. 2012;78:6262–6270. doi: 10.1128/AEM.01051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch AATM, De Steenhuijsen Piters WAA, Van Houten MA, Chu MLJN, Biesbroek G, Kool J, et al. Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. Am J Respir Crit Care Med. 2017;196:1582–1590. doi: 10.1164/rccm.201703-0554OC. [DOI] [PubMed] [Google Scholar]
- 8.Teo SM, Tang HHF, Mok D, Judd LM, Watts SC, Pham K, et al. Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and allergy in childhood respiratory disease. Cell Host Microbe. 2018;24:341–352.e5. doi: 10.1016/j.chom.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luna PN, Hasegawa K, Ajami NJ, Espinola JA, Henke DM, Petrosino JF, et al. The association between anterior nares and nasopharyngeal microbiota in infants hospitalized for bronchiolitis. Microbiome. 2018;6:1–14. doi: 10.1186/s40168-017-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vissing NH, Chawes BLK, Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am J Respir Crit Care Med. 2013;188:1246–1252. doi: 10.1164/rccm.201302-0215OC. [DOI] [PubMed] [Google Scholar]
- 11.Claassen-Weitz S, Lim KYL, Mullally C, Zar HJ, Nicol MP. The association between bacteria colonizing the upper respiratory tract and lower respiratory tract infection in young children: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:1262–1270. doi: 10.1016/j.cmi.2021.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch AATM, Levin E, Van Houten MA, Hasrat R, Kalkman G, Biesbroek G, et al. Development of upper respiratory tract microbiota in infancy is affected by mode of delivery. EBioMedicine. 2016;9:336–345. doi: 10.1016/j.ebiom.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojo-Okunola A, Claassen-Weitz S, Mwaikono KS, Gardner-Lubbe S, Stein DJ, Zar HJ, et al. Influence of socio-economic and psychosocial profiles on the human breast milk bacteriome of south african women. Nutrients. 2019;11:1–19. doi: 10.3390/nu11061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biesbroek G, Bosch AA, Wang X, Keijser BJF, Veenhoven RH, Sanders EA, et al. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Ajrccm. 2014;190:1–44. doi: 10.1164/rccm.201401-0073OC. [DOI] [PubMed] [Google Scholar]
- 15.Brugger S, Eslami S, Pettigrew M, Escapa I, Henke M, Kong Y, et al. Dolosigranulum pigrum cooperation and competition in human nasal microbiota. mSphere. 2020;5(5):e00852–20. [DOI] [PMC free article] [PubMed]
- 16.Prevaes SMPJ, De Winter-De Groot KM, Janssens HM, De Steenhuijsen Piters WAA, Tramper-Stranders GA, Wyllie AL, et al. Development of the nasopharyngeal microbiota in infants with cystic fibrosis. Am J Respir Crit Care Med. 2016;193:504–15. doi: 10.1164/rccm.201509-1759OC. [DOI] [PubMed] [Google Scholar]
- 17.Vanker A, Nduru PM, Barnett W, Dube FS, Sly PD, Gie RP, et al. Indoor air pollution and tobacco smoke exposure: impact on nasopharyngeal bacterial carriage in mothers and infants in an African birth cohort study. ERJ Open Res. 2019;5:00052–2018. doi: 10.1183/23120541.00052-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogaert D, Keijser B, Huse S, Rossen J, Veenhoven R, van Gils E, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One. 2011;6(2):e17035. doi: 10.1371/journal.pone.0017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mika M, Mack I, Korten I, Qi W, Aebi S, Frey U, et al. Dynamics of the nasal microbiota in infancy: a prospective cohort study. J Allergy Clin Immunol. 2015;135:905–912.e11. doi: 10.1016/j.jaci.2014.12.1909. [DOI] [PubMed] [Google Scholar]
- 20.Man WH, van Houten MA, Mérelle ME, Vlieger AM, Chu MLJN, Jansen NJG, et al. Bacterial and viral respiratory tract microbiota and host characteristics in children with lower respiratory tract infections: a matched case-control study. Lancet Respir Med. 2019;7:417–426. doi: 10.1016/S2213-2600(18)30449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Man WH, Clerc M, de Steenhuijsen Piters WAA, van Houten MA, Chu MLJN, Kool J, et al. Loss of microbial topography between oral and nasopharyngeal microbiota and development of respiratory infections early in life. Am J Respir Crit Care Med. 2019;200:760–770. doi: 10.1164/rccm.201810-1993OC. [DOI] [PubMed] [Google Scholar]
- 22.le Roux DM, Myer L, Nicol MP, Zar HJ. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: the Drakenstein Child Health Study. Lancet Glob Heal. 2015;3:e95–103. doi: 10.1016/S2214-109X(14)70360-2. [DOI] [PubMed] [Google Scholar]
- 23.Ngari MM, Fegan G, Mwangome MK, Ngama MJ, Mturi N, Scott JAG, et al. Mortality after inpatient treatment for severe pneumonia in children: a cohort study. Paediatr Perinat Epidemiol. 2017;31:233–242. doi: 10.1111/ppe.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iroh Tam P, Wiens M, Kabakyenga J, Kiwanuka J, Kumbakumba E, Moschovis P. Pneumonia in HIV-exposed and infected children and association with malnutrition. Pediatr Infect Dis J. 2018;37:1011–1013. doi: 10.1097/INF.0000000000001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamberti LM, Zakarija-Grković I, Fischer Walker CL, Theodoratou E, Nair H, Campbell H, et al. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta-analysis. BMC Public Health. 2013;13 SUPPL.3(Suppl 3):S18. doi: 10.1186/1471-2458-13-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonego M, Pellegrin MC, Becker G, Lazzerini M. Risk factors for mortality from acute lower respiratory infections (ALRI) in children under five years of age in low and middle-income countries: a systematic review and meta-analysis of observational studies. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0116380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliwa JN, Marais BJ. Vaccines to prevent pneumonia in children – a developing country perspective. Paediatr Respir Rev. 2017;22:23–30. doi: 10.1016/j.prrv.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zar HJ, Barnett W, Myer L, Stein DJ, Nicol MP. Investigating the early-life determinants of illness in Africa: the Drakenstein Child Health Study. Thorax. 2014;0:1–3. doi: 10.1136/thoraxjnl-2014-206242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zar HJ, Pellowski JA, Cohen S, Barnett W, Vanker A, Koen N, et al. Maternal health and birth outcomes in a South African birth cohort study. PLoS One. 2019;14:1–16. doi: 10.1371/journal.pone.0222399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanker A, Gie R, Zar H. Early-life exposures to environmental tobacco smoke and indoor air pollution in the Drakenstein Child Health Study: impact on child health. S Afr Med J. 2018;108:71–72. doi: 10.7196/SAMJ.2018.v108i2.13088. [DOI] [PubMed] [Google Scholar]
- 31.Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, Nicol MP. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med. 2016;4:463–472. doi: 10.1016/S2213-2600(16)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez L, Zar HJ. Tuberculin conversion and tuberculosis disease in infants and young children from the drakenstein child health study: a call to action. S Afr Med J. 2018;108:247–248. doi: 10.7196/SAMJ.2018.v108i4.13169. [DOI] [PubMed] [Google Scholar]
- 33.Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013;13:92. doi: 10.1186/1471-2431-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Walt AJ, Fitchett JM. Statistical classification of South African seasonal divisions on the basis of daily temperature data. S Afr J Sci. 2020;116:1–15. [Google Scholar]
- 36.Roffe SJ, Fitchett JM, Curtis CJ. Quantifying rainfall seasonality across South Africa on the basis of the relationship between rainfall and temperature. Clim Dyn. 2021;56:2431–2450. doi: 10.1007/s00382-020-05597-5. [DOI] [Google Scholar]
- 37.Gray DM, Wedderburn CJ, MacGinty RP, McMillan L, Jacobs C, Stadler JAM, et al. Impact of HIV and antiretroviral drug exposure on lung growth and function over 2 years in an African Birth Cohort. Aids. 2020;34:549–58. doi: 10.1097/QAD.0000000000002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claassen-Weitz S, Gardner-Lubbe S, Mwaikono KS, du Toit E, Zar HJ, Nicol MP. Optimizing 16S rRNA gene profile analysis from low biomass nasopharyngeal and induced sputum specimens. BMC Microbiol. 2020;20:113. doi: 10.1186/s12866-020-01795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claassen-Weitz S, Gardner-Lubbe S, Nicol P, Botha G, Mounaud S, Shankar J, et al. HIV-exposure, early life feeding practices and delivery mode impacts on faecal bacterial profiles in a South African birth cohort. Sci Rep. 2018;8:1–15. doi: 10.1038/s41598-018-22244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010. http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- 41.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di TP, Chatzou M, Floden EW, Barja PP, Palumbo E, Notredame C. Nextflow enables reproducible computational workflows. Nat Biotechnol. 2017;35:316–319. doi: 10.1038/nbt.3820. [DOI] [PubMed] [Google Scholar]
- 44.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, et al. Ribosomal Database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:633–642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callahan BJ. RDP taxonomic training data formatted for DADA2 (RDP trainset 16/release 11.5). Zenodo. 2017. https://zenodo.org/record/801828#.X7VWKs7itdg.
- 46.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41 Database issue:D590–6. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2021. https://www.r-project.org/.
- 48.RStudio Team. RStudio: integrated development environment for R. 2021. http://www.rstudio.org/.
- 49.Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- 50.Aitchison J. The statistical analysis of compositional data. J R Stat Soc. 1982;44:139–160. [Google Scholar]
- 51.Aitchison J, Greenacre M. Biplots of compositional data. J R Stat Soc Ser C Appl Stat. 2002;51:375–92. doi: 10.1111/1467-9876.00275. [DOI] [Google Scholar]
- 52.Gower J, Lubbe S, Le Roux N. Understanding biplots. Chichester: Wiley; 2011. [Google Scholar]
- 53.Templ M, Hron K, Filzmoser P. robCompositions: an R-package for Robust statistical analysis of compositional data. In: Compositional data analysis: theory and applications. New York: Wiley; 2011. p. 341–55. 10.1002/9781119976462.ch25.
- 54.Pawlowsky-Glahn V, Egozcue JJ. BLU estimators and compositional data. Math Geol. 2002;34:259–274. doi: 10.1023/A:1014890722372. [DOI] [Google Scholar]
- 55.Filzmoser P, Hron K, Templ M. Applied compositional data analysis: with worked examples in R. Springer Series in Statistics. Cham: Springer; 2018. 10.1007/978-3-319-96422-5_8.
- 56.Canty A, Ripley B. boot: Bootstrap R (S-Plus) functions. 2021.
- 57.Davison A, Hinkley D. Bootstrap methods and their applications. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- 58.Adler D, Kelly ST. vioplot: violin plot. 2021. https://github.com/TomKellyGenetics/vioplot.
- 59.Plotly Technologies Inc. Collaborative data science. 2015. https://plot.ly.
- 60.Mallick H, Rahnavard A, McIver LJ, Ma S, Zhang Y, Nguyen LH, et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol. 2021;17:1–27. doi: 10.1371/journal.pcbi.1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Heal Dis. 2015;26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martino C, Shenhav L, Marotz C, Armstrong G, Mcdonald D, Vázquez-Baeza Y, et al. Context-aware dimensionality reduction deconvolutes gut microbial community dynamics HHS Public Access Author manuscript. Nat Biotechnol. 2021;39:165–168. doi: 10.1038/s41587-020-0660-7.Context-aware. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biesbroek G, Bosch AATM, Wang X, Keijser BJF, Veenhoven RH, Sanders EAM, et al. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am J Respir Crit Care Med. 2014;190:298–308. doi: 10.1164/rccm.201401-0073OC. [DOI] [PubMed] [Google Scholar]
- 64.De Steenhuijsen Piters WAA, Heinonen S, Hasrat R, Bunsow E, Smith B, Suarez-Arrabal MC, et al. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2016;194:1104–1115. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chonmaitree T, Jennings K, Golovko G, Khanipov K, Pimenova M, Patel JA, et al. Nasopharyngeal microbiota in infants and changes during viral upper respiratory tract infection and acute otitis media. PLoS One. 2017;12:e0180630. doi: 10.1371/journal.pone.0180630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zar HJ, Nduru P, Stadler JAM, Gray D, Barnett W, Lesosky M, et al. Early-life respiratory syncytial virus lower respiratory tract infection in a South African birth cohort: epidemiology and effect on lung health. Lancet Glob Health. 2020;8:e1316–e1325. doi: 10.1016/S2214-109X(20)30251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Roux DM, Nicol MP, Myer L, Vanker A, Stadler JAM, Von Delft E, et al. Lower respiratory tract infections in children in a well-vaccinated South African birth cohort: spectrum of disease and risk factors. Clin Infect Dis. 2019;69:1588–1596. doi: 10.1093/cid/ciz017. [DOI] [PubMed] [Google Scholar]
- 68.Kwambana-Adams B, Hanson B, Worwui A, Agbla S, Foster-Nyarko E, Ceesay F, et al. Rapid replacement by non-vaccine pneumococcal serotypes may mitigate the impact of the pneumococcal conjugate vaccine on nasopharyngeal bacterial ecology. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-08717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:1–12. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biesbroek G, Wang X, Keijser BJF, Eijkemans RMJ, Trzciński K, Rots NY, et al. Seven-valent pneumococcal conjugate vaccine and nasopharyngeal microbiota in healthy children. Emerg Infect Dis. 2014;20:201–210. doi: 10.3201/eid2002.131220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salter SJ, Turner C, Watthanaworawit W, de Goffau MC, Wagner J, Parkhill J, et al. A longitudinal study of the infant nasopharyngeal microbiota: the effects of age, illness and antibiotic use in a cohort of South East Asian children. PLoS Negl Trop Dis. 2017;11:1–17. doi: 10.1371/journal.pntd.0005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoos AM, Kragh M, Ahrens P, Kuhn KG, Rasmussen MA, Chawes BL, et al. Season of birth impacts the neonatal nasopharyngeal microbiota. Children. 2020;7:45. doi: 10.3390/children7050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdulgader SM, Robberts L, Ramjith J, Nduru PM, Dube F, Gardner-Lubbe S, et al. Longitudinal population dynamics of Staphylococcus aureus in the nasopharynx during the first year of life. Front Genet. 2019;10 MAR:1–10. doi: 10.3389/fgene.2019.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leekha S, Diekema DJ, Perencevich EN. Seasonality of staphylococcal infections. Clin Microbiol Infect. 2012;18:927–933. doi: 10.1111/j.1469-0691.2012.03955.x. [DOI] [PubMed] [Google Scholar]
- 75.Harrison LM, Morris JA, Telford DR, Brown SM, Jones K. The nasopharyngeal bacterial flora in infancy: effects of age, gender, season, viral upper respiratory tract infection and sleeping position. FEMS Immunol Med Microbiol. 1999;25:19–28. doi: 10.1111/j.1574-695X.1999.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 76.Hoang DM, Levy EI, Vandenplas Y. The impact of caesarean section on the infant gut microbiome. Acta Paediatr. 2021;110:60–67. doi: 10.1111/apa.15501. [DOI] [PubMed] [Google Scholar]
- 77.Vives M, Garcia ME, Saenz P, De Los Angles Mora M, Mata L, Sabharwal H, et al. Nasopharyngeal colonization in Costa Rican children during the first year of life. Pediatr Infect Dis J. 1997;16:852–8. doi: 10.1097/00006454-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 78.Henares D, Rocafort M, Brotons P, de Sevilla MF, Mira A, Launes C, et al. Rapid increase of oral bacteria in nasopharyngeal microbiota after antibiotic treatment in children with invasive pneumococcal disease. Front Cell Infect Microbiol. 2021;11 October:1–12. doi: 10.3389/fcimb.2021.744727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelly MS, Surette MG, Smieja M, Pernica JM, Rossi L, Luinstra K, et al. The nasopharyngeal microbiota of children with respiratory infections in Botswana. Pediatr Infect Dis J. 2017;36:e211–e218. doi: 10.1097/INF.0000000000001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shenoy MK, Lynch SV. Role of the lung microbiome in HIV pathogenesis. Curr Opin HIV AIDS. 2018;13:45–52. doi: 10.1097/COH.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 81.Brooks JP, Edwards DJ, Harwich MD, Rivera MC, Fettweis JM, Serrano MG, et al. The truth about metagenomics: quantifying and counteracting bias in 16S rRNA studies. Ecological and evolutionary microbiology. BMC Microbiol. 2015;15:1–14. doi: 10.1186/s12866-015-0351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walker AW, Martin JC, Scott P, Parkhill J, Flint HJ, Scott KP. 16S rRNA gene-based profiling of the human infant gut microbiota is strongly influenced by sample processing and PCR primer choice. Microbiome. 2015;3:1–11. doi: 10.1186/s40168-015-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clooney AG, Fouhy F, Sleator RD, O’Driscoll A, Stanton C, Cotter PD, et al. Comparing apples and oranges?: next generation sequencing and its impact on microbiome analysis. PLoS One. 2016;11:1–16. doi: 10.1371/journal.pone.0148028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xue Z, Kable ME, Marco ML. Impact of DNA sequencing and analysis methods on 16S rRNA gene bacterial community analysis of dairy products. mSphere. 2018;3(5):e00410–18. doi: 10.1128/mSphere.00410-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Callahan BJ, Mcmurdie PJ, Holmes SP. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017;11:2639–2643. doi: 10.1038/ismej.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Section A. Extended methods (Figure S1). Section B. Extended results - sequencing controls and sample selection (Figures S2-S7). Section C. Extended results - Figures S8-S19. Section D. Extended references. Table S1. RDP classifier implementation for DADA2 and SILVA version: ASV table. Table S2. RDP classifier implementation for DADA2 and SILVA version: taxonomic classification. Table S3. Metadata file: NP specimens. Table S4. Metadata file: Participants. Table S5. Differential abundance testing.
Data Availability Statement
Sequence data and subject characteristics are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the BioProject ID PRJNA790843 and PRJNA548658. The ASV table, taxonomic classification, sample metadata, and participant metadata are available in Supplementary Tables 1–4.