Abstract
Background:
Trichomonas vaginalis (TV) is a sexually transmitted parasite associated with multiple adverse outcomes in women. Estimating TV incidence is challenging because of its largely asymptomatic presentation.
Methods:
Per-capita prevalence was estimated using the National Health and Nutrition Examination Survey, 2013 to 2018. Incidence was estimated using ordinary differential equations assuming static incidence at steady state and fit using Bayesian techniques. Model inputs included estimates of proportion of asymptomatic cases, natural clearance, and time to symptomatic treatment seeking. Posterior distributions were drawn, and uncertainty was reported, from 25th (Q1) to 75th (Q3) percentiles. Aggregated measures were estimated by combining component distributions.
Results:
Among 15- to 59-year-olds in 2018, the number of prevalent TV infections was 2.6 (Q1, 2.4; Q3, 2.7) million overall, 470,000 (Q1, 414,000; Q3, 530,000) among men, and 2.1 (Q1, 2.0; Q3, 2.2) million among women; the numbers of incident infections were 6.9 (Q1, 6.2; Q3, 7.6) million, 3.3 (Q1, 2.8; Q3, 3.8) million, and 3.5 (Q1, 3.1; Q3, 4.0) million among all persons, men, and women, respectively. Persons aged 15 to 24 years comprised 15.6% and 16.3% of all prevalent and incident infections, respectively; prevalence and incidence in both sexes increased with age. Incidences in both sexes were highly dependent on estimates of natural clearance, which were based on few data.
Conclusions:
Prevalence and incidence of TV are substantial in the United States, particularly among those 25 years or older. Although estimated prevalence is higher in women, estimated incidence is similar in men and women. Data on key parameters of TV infection are limited; future research should focus on clarifying the natural history of TV.
Trichomonas vaginalis (TV) is a sexually transmitted parasite that has been associated with multiple adverse outcomes in women, including pelvic inflammatory disease, increased human immunodeficiency virus acquisition and transmission, and pre-term birth.1,2 Although at least 80% of cases are asymptomatic, clinical presentation of TV in females can include unpleasant odor, discharge, dysuria, genital irritation or itching, and pain during intercourse; males may experience symptoms such as dysuria, urethral itch, and discharge.3–5 In 2008, the estimated cost of TV to the US health care system was $24.0 ($12.0–$36.0) million6; more recently, costs per case of outpatient diagnosis and treatment of TV were estimated to be $220 for women and $153 for men, with a total cost to the health care system in 2018 of $173.74 million.7,8
Trichomonas vaginalis is thought to be the most prevalent curable sexually transmitted infection globally9; however, it is challenging to estimate TV incidence because of its high rate of asymptomatic infection. Furthermore, TV surveillance data are limited, as it is not a reportable disease in the United States.10 To our knowledge, there have been only 2 studies that have attempted incidence estimates for TV in the United States that are based on validated, nationally representative data.11,12 In this analysis, we provide updated estimates of the prevalence and incidence of TV in 2018 using recently developed modeling methods (Supplemental Materials, http://links.lww.com/OLQ/A633). Notably, we also provide uncertainty intervals around prevalence and incidence estimates by combining the uncertainty of multiple input data sources.
MATERIALS AND METHODS
We estimated the number of prevalent and incident TV infections in the United States in 2018. All estimates are provided both stratified by sex (male vs. female) and age (15–24, 25–39, and 40–59 years), as well as aggregated over sex and age. After first estimating per-capita prevalence and incidence separately for each subpopulation, we estimated the number of prevalent and incident TV infections by multiplying those estimates by the American Community Survey full resident population estimates for 2018.13 Our estimates represent the number of prevalent and incident infections rather than the number of people with an infection in 2018, because it is possible for a single person to have a repeat TV infection in 2018. All estimates are rounded to the nearest thousand infections. Our primary estimates of prevalence and incidence are summarized by the median (50th percentile) from the posterior distributions of prevalence and incidence. Uncertainty intervals around primary estimates are described by the 25th (Q1) and 75th (Q3) percentiles of the posterior. Aggregated measures (i.e., total male incidence, total female incidence, total 15- to 24-year-old incidence, etc.) were estimated by combining component distributions, similar to the methods used in the overview article in this special issue.14
Prevalence
The point prevalence and unweighted sample sizes of TV were estimated using 3 data cycles (2013–2014, 2015–2016, and 2017–2018) of the National Health and Nutrition Examination Survey (NHANES).15–17 The NHANES uses the Hologic Aptima nucleic acid amplification test (NAAT) for detection of TV infection; indeterminate test results for TV in NHANES were counted as negatives in our analysis. To increase the stability of our estimates, we combined data from multiple cycles of NHANES. Although NHANES analytic guidelines18 recommend interpreting measures of prevalence at the midpoint of the combined cycles, we found minimal differences in overall TV prevalence among 14- to 59-year-olds between the 2013–2014, 2015–2016, and 2017–2018 cycles (data not shown). We assumed TV prevalence was at steady state; therefore, these data are reflective of prevalence across all cycles from 2013 to 2018.
We then generated a distribution of TV prevalence around the weighted NHANES point prevalence estimate for each subpopulation to estimate the median TV prevalence by sampling from a beta distribution with shape parameters defined by the NHANES weighted prevalence and the unweighted sample size of each subpopulation.
Incidence
To estimate TV incidence, we used a methodology similar to that used to estimate chlamydial and gonococcal incidence19 (Supplemental Materials, http://links.lww.com/OLQ/A633). This relied on the SymPy package20 in Python21 to solve ordinary differential equations assuming static incidence at steady state. All people were classified as uninfected, asymptomatically infected, or symptomatically infected. This distinction was made because symptomatic people differ from asymptomatically infected people by seeking treatment to resolve their TV infection; however, both categories of infected people are assumed to be able to naturally clear their infections.22–26
Data Inputs
A summary of the parameters used in this model, stratified by sex and age (where appropriate),22 is shown in Table 1 and includes population size, the proportion of cases that are asymptomatic, the time to natural clearance of infection, and time to treatment seeking among symptomatic persons. Unlike chlamydia and gonorrhea, we assumed there was no background screening for TV. There is evidence to suggest that both men9,22,23,25,26,28 and women9,24,26 are able to naturally clear infection. However, we estimated natural clearance rates for men and women only from studies that used NAAT for the diagnosis of infection and included a determination of time from last sex.23,24 These estimated rates were bootstrapped to generate distributions. We relied on assumptions, informed by clinical plausibility, to determine the following parameters: asymptomatic proportion of cases and rate of symptomatic treatment seeking. The range of possible values generated in this manner was broad, and we sampled from uniform distributions for both treatment seeking and asymptomatic proportion of cases. To further constrain our input parameter values to real-world data, we used diagnosis codes for TV contained in the MarketScan Commercial Claims and Encounters database for 2016 to 2018. The MarketScan database contains commercial medical insurance claims from employment-based health insurance plans with at least 100 enrollees including large employers and health plans; overall, the database comprises data from 43.6 million persons.27 We assumed that TV diagnosis codes reflected treated TV infections. MarketScan only represents a fraction of all nationally diagnosed and treated TV infections; for the purposes of this analysis, we called this fraction the sampling fraction and assumed it was the same for both Chlamydia trachomatis and TV. We then fit the annual number of model-estimated treated TV infections to achieve a sampling fraction that was within the same bounds as C. trachomatis in separate analyses.19,29 This fitting process is described more below.
TABLE 1.
Model Parameter Descriptions, Prior Distributions, and Posterior (i.e., Fitted) Distributions
| Parameter | Population | Prior Distribution, Median (25th–75th Percentile) | Posterior Distribution, Median (25th–75th Percentile) | Distribution Form | References |
|---|---|---|---|---|---|
|
| |||||
| Population size, N | Men | ||||
| 15–24y | 22,076,120* | 22,076,120* | NA (unvaried) | (13) | |
| 25–39 y | 33,757,505* | 33,757,505* | |||
| 40–59 y | 40,919,254* | 40,919,254* | |||
| Women | |||||
| 15–24y | 21,025,717* | 21,025,717* | |||
| 25–39 y | 33,171,851* | 33,171,851* | |||
| 40–59 y | 42,274,644* | 42,274,644* | |||
| Percent of cases that are | Men | 71.8% (60.8%–83.6%) | 91.9% (88.5%–93.8%) | Uniform | Assumption |
| asymptomatic | Women | 60.5% (42.9%–77.6%) | 80.8% (77.1%–83.8%) | ||
| Natural clearance rate | Men | 7.10 (6.03–8.35) | 6.70 (5.56–8.03) | Bootstrapped empirical data | (23,24) |
| (1/years)† | Women | 1.45 (1.21 –1.70) | 1.40 (1.17–1.66) | ||
| Time to natural clearance, d† | Men | 51.6 (43.7–60.6) | 54.5 (45.5–65.7) | Bootstrapped empirical data | (23,24) |
| Women | 252 (215–302) | 260 (221–312) | |||
| Symptomatic treatment seeking | Men | 10.1 (5.67–14.8) | 4.46 (2.42–8.69) | Uniform | Assumption |
| rate (1/years)‡ | Women | 10.5 (6.26–15.0) | 9.89 (5.54–14.5) | ||
| Time from infection to | Men | 36.0 (24.7–64.4) | 81.8 (42.0–151) | Uniform | Assumption |
| symptomatic treatment seeking‡, d | Women | 34.7 (24.3–58.3) | 36.9 (25.2–65.9) | ||
| Sampling fraction of treated | Men | 0.182%–0.445%§ | 0.182%–0.445%§ | NA (unvaried) | (27) |
| infections (from MarketScan) | Women | 0.261%–0.422%§ | 0.261%–0.422%§ | ||
Population size not varied. These numbers reflect the American Community Survey estimates used.
The natural clearance rate and time to natural clearance rate reflect the same mechanistic parameter as they are inverses of one another. Both are shown in this table, although with dfferent temporal units, because different readers may prefer different formulations.
The symptomatic treatment seeking rate and time from infection to symptomatic treatment seeking reflect the same mechanistic parameter as they are inverses of one another. Both are shown in this table, although with different temporal units, because different readers may prefer different formulations.
The sampling fraction bounds determine the range of acceptability for the number of annual treated infections. These bounds were estimated by calculating the treated infection sampling fraction for chlamydia, limiting the bounds to the middle 99.9% of sample estimates. Given 243.3 male and 1798 female MarketScan TV-treated infections, this translates to an acceptability region for model-estimated treated TV infections of (54,644–133,889) in men and (425,760–688,573) in women.
Model Fitting
Our model was fit using a simple Bayesian accept/reject algorithm similar to one used in previously published work.30 Prior distributions were drawn for each parameter using Monte Carlo simulations. The posterior distribution consisted of parameter sets where the number of treated TV infections lay within an acceptable interval. By assuming the chlamydia sampling fraction also applied to TV, we were able to define acceptability bounds for the number of model-estimated treated infections by dividing the number of TV diagnoses in MarketScan by the sampling fraction distribution. The posterior distribution consisted of 10,000 parameter sets, each drawn independently, that were fit to the annual number of model-estimated treated TV infections, as described previously for each subpopulation.
Ranked Uncertainty Impact Analysis
Because of uncertainty in the model input parameters, there is also output uncertainty in the TV incidence estimates generated. To evaluate the impact of input uncertainty, we generated tornado plots summarizing the distributions of incidence that would be expected by varying each input parameter to its 25th and 75th percentiles while keeping all other input parameters constant at their median (50th percentile).
RESULTS
Prevalence
The prevalence of TV infection is shown in Table 2. The median numbers of prevalent TV infections in 2018 among 15- to 59-year-olds were 2.6 (Q1, 2.4; Q3, 2.7) million overall, 470,000 (Q1, 414,000; Q3, 530,000) among men, and 2.1 (Q1, 2.0; Q3, 2.2) million among women. Persons aged 15 to 24 years comprised only 15.6% of all prevalent infections, with 402,000 (Q1, 356,000; Q3, 449,000) overall, 83,000 (Q1, 63,000; Q3, 107,000) among men, and 314,000 (Q1, 275,000; Q3, 356,000) among women. The age distribution of prevalent infections was relatively similar for males and females. In males compared with females, 15- to 24-year-olds comprised 17.7% versus 14.9% of sex-stratified TV infections, 25- to 39-year-olds comprised 34.9% versus 41.9%, and 40- to 59-year-olds comprised 45.1% versus 42.7%. Overall, women comprised 81.6% of all prevalent infections; this percent was also relatively similar in each age category: 78.1%, 84.1%, and 80.4% in 15 to 24, 25 to 39, and 40 to 59 years, respectively.
TABLE 2.
Estimated Number of Prevalent Trichomonas vaginalis Infections in Persons Aged 15 to 59 Years, by Sex and Age Group, United States, 2018
| Median Prevalence (25th–75th percentile)* | |||
|---|---|---|---|
|
|
|||
| Age, y | Men | Women | Total |
|
| |||
| 15–24 | 83,000 (63,000–107,000) | 314,000 (275,000–356,000) | 402,000 (356,000–449,000) |
| 25–39 | 164,000 (130,000–203,000) | 882,000 (805,000–965,000) | 1,049,000 (965,000–1,143,000) |
| 40–59 | 212,000 (176,000–255,000) | 897,000 (821,000–978,000) | 1,115,000 (1,029,000–1,206,000) |
| 15–59 | 470,000 (414,000–530,000) | 2,103,000 (1,982,000–2,225,000) | 2,576,000 (2,446,000–2,713,000) |
Prevalence estimates represent the 2018 point prevalence calculated from pooling 2013–2018 cycles of the National Health and Nutrition Examination Survey. All counts are rounded to the nearest thousand infections. The uncertainty interval surrounding each point estimate represents the 25th and 75th percentiles of the empirical frequency distribution of the estimate for each infection. The number of prevalent infections was calculated by multiplying the per-capita prevalence estimates by the American Community Survey full population estimates for 2018. Total estimates are not the sum of individual estimates but rather descriptions of multiple distributions that have been combined.
Incidence
The median numbers of incident TV infections were estimated to be 6.9 (Q1, 6.2; Q3, 7.6) million, 3.3 (Q1, 2.8; Q3, 3.8) million, and 3.5 (Q1, 3.1; Q3, 4.0) million among all persons, men, and women aged 15 to 59 years in 2018, respectively (Table 3). Persons aged 15 to 24 years comprised only 16.3% of all incident infections, with 1.1 (Q1, 914,000; Q3, 1.4 million) million infections overall, 568,000 in men (Q1, 411,000; Q3, 771,000) among men, and 520,000 (Q1, 411,000; Q3, 644,000) among women. The age distribution of the incident cases was relatively similar for males and females: in males compared with females, 15 to 24 years comprised 17.3% versus 14.7% of all sex-stratified TV infections, 25 to 39 years comprised 34.3% versus 41.6%, and 40–59 years comprised 44.6% versus 42.3%. Overall, women comprised 51.5% of all incident infections, with 46.6%, 55.6%, and 49.8% occurring in the 15 to 24, 25 to 39, and 40 to 59 years of age, respectively.
TABLE 3.
Estimated Number of Incident Trichomonas vaginalis Infections in Persons Aged 15 to 59 years, by Sex and Age Group, United States, 2018
| Median Incidence (25th–75th percentile)* | |||
|---|---|---|---|
|
|
|||
| Age, y | Men | Women | Total |
|
| |||
| 15–24 | 568,000 (411,000–771,000) | 520,000 (411,000–644,000) | 1,115,000 (914,000–1,350,000) |
| 25–39 | 1,124,000 (839,000–1,469,000) | 1,471,000 (1,196,000–1,773,000) | 2,648,000 (2,235,000–3,100,000) |
| 40–59 | 1,462,000 (1,131,000–1,863,000) | 1,495,000 (1,222,000–1,795,000) | 3,005,000 (2,562,000–3,510,000) |
| 15–59 | 3,278,000 (2,780,000–3,834,000) | 3,536,000 (3,112,000–3,975,000) | 6,861,000 (6,209,000–7,563,000) |
Ordinary differential equation-based modeling assuming equilibrium and static incidence was used to estimate Trichomonas vaginalis incidence. Incidence estimates represent the cumulative number of incident infections in 2018. All counts are rounded to the nearest thousand infections. The uncertainty interval surrounding each point estimate represents the 25th and 75th percentiles of the empirical frequency distribution of the estimate for each infection. The number of incident infections was calculated by multiplying the per-capita incidence estimates by the American Community Survey full population estimates for 2018. Total estimates are not the sum of individual estimates but rather descriptions of multiple distributions that have been combined.
Ranked Uncertainty Impact Analysis of Incidence Estimate
Uncertainty in prevalence and natural clearance had a big impact on our incidence estimates (Fig. 1). For example, when TV prevalence was varied from the 25th to the 75th percentiles of its empirical frequency distribution among men aged 40 to 59 years while holding all other parameters constant at their median values, the incidence of TV ranged from 1.22 to 1.76 million. When natural clearance was similarly varied among men aged 40 to 59 years, the incidence of TV ranged from 1.22 to 1.75 million cases. Among women, natural clearance made more of an impact on incidence than prevalence. Among women aged 40 to 59 years, when natural clearance was varied from the 25th to the 75th percentiles of its input distribution, the incidence of TV ranged from 1.27 to 1.78 million cases. When prevalence was similarly varied among women aged 40 to 59 years, the incidence of TV ranged from 1.38 to 1.65 million. The 2 remaining parameters, the proportion of new TV infections that are asymptomatic and the symptomatic treatment seeking rate, had input uncertainty that impacted TV incidence much less, regardless of sex or age group. These effects were similar in all age groups.
Figure 1.
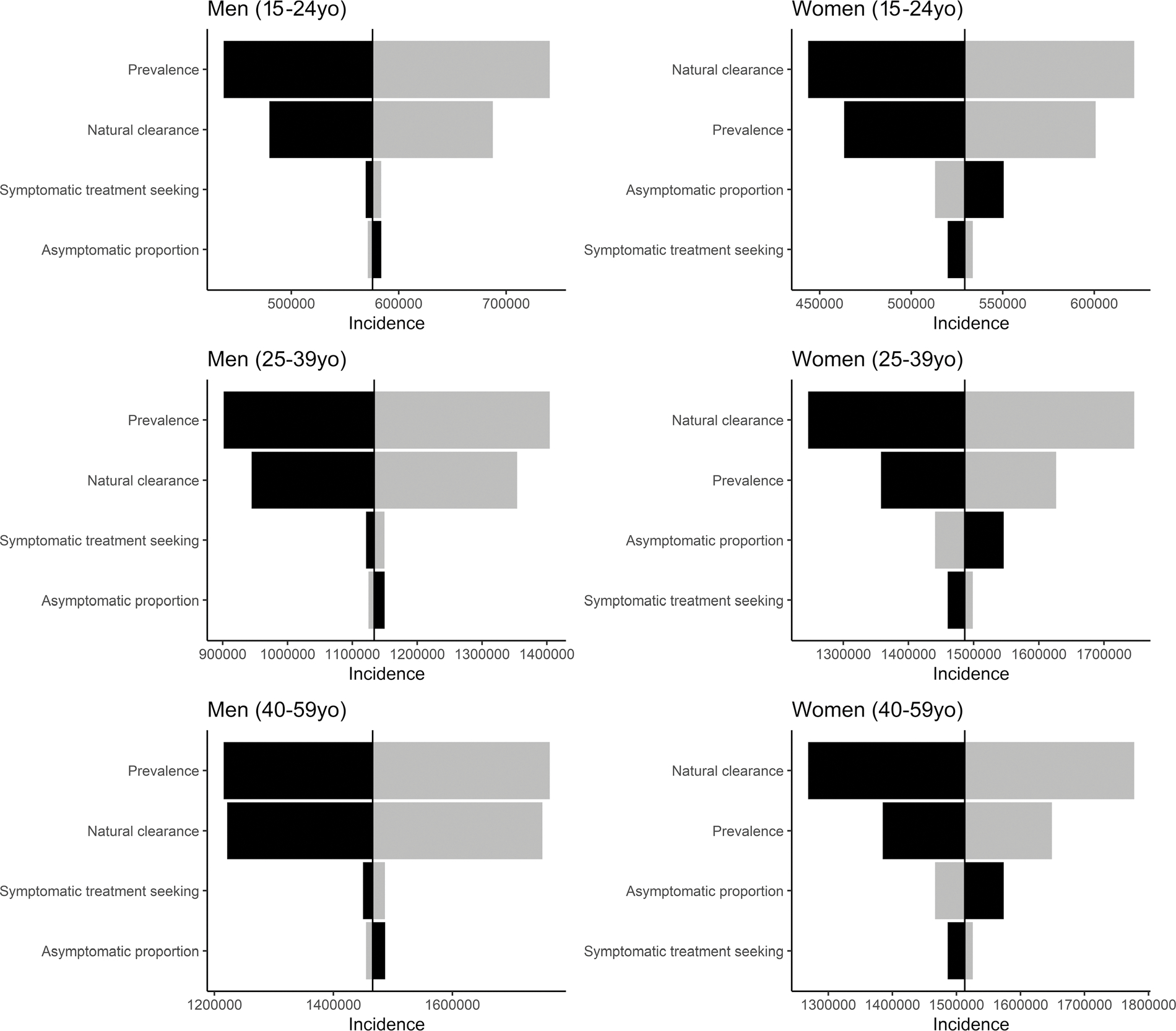
Tornado plot summarizing the ranked impact of individual model parameters on Trichomonas vaginalis incidence, by age and sex, United States, 2018. Distributions of incidence were generated by varying each input parameter to its 25th and 75th percentiles while keeping all other input parameters constant at their median (50th percentile). Darker versus lighter bars represent lower versus higher values of a given parameter; higher parameter values do not always translate to higher incidence estimates.
DISCUSSION
We estimate that there were 2.1 million prevalent TV infections among women and 470,000 among men aged 15 to 59 years in the United States in 2018. Estimates among those aged 15 to 24 years remained low, with 83,000 among males and 314,000 among females, respectively. There is less TV infection in younger age groups when compared with older groups1,12,31,32; in men, there was a steady increase with increasing age, whereas for women, prevalence sharply increased after age 24 years and did not seem to differ in the 2 older age groups. We found that overall incidence was similar in men and women, but prevalence was lower in men compared with women. This results from a natural clearance in men that is assumed to be greater than 4 times faster than in women (Table 1).
Prevalence estimates for TV are greatly affected by the characteristics of the diagnostic tests used. The false-positive rate (FPR; 1 − specificity) of a test describes the minimum proportion of people that would be expected to test positive in a disease-free population. The specificity of urine-based NAATs for TV in males has been estimated to be 96.3% (confidence interval, 93.2–98.1)33; therefore, the FPR for TV NAATs in males is 3.7. Interestingly, this FPR is higher than the TV prevalence estimated from the NHANES in all age-specific male subpopulations; each male subpopulation had a test prevalence <1 (data not shown). This phenomenon was also present in 2 of the subgroups of women, although less pronounced than in men. This could indicate that the test specificity is better than previously ascertained (indicating a lower FPR), among other possibilities. Other less likely possibilities include issues related to internal validity of testing or data collection. In the current analysis, had we accounted for imperfect testing, our results would have been uninterpretable, resulting in negative values of prevalence in multiple subpopulations. For this reason, we ignored these issues and assumed perfect test characteristics to generate estimates of TV prevalence and therefore incidence.
Our incidence estimates differ from prior estimates.11,12 The most recent estimates reported by Satterwhite et al12 for 2008 estimated the incidence of TV using the weighted proportion of women diagnosed with trichomoniasis in the NHANES during the period 2001–2004, projections from the National Disease and Therapeutic Index, and the assumed symptomatic proportion of cases. This resulted in an estimate of 415,000 and 680,000 incident TV infections among men and women, respectively. The estimates by Weinstock et al.11 for 2000 were not stratified by sex; used nationally notifiable disease reports, literature reviews, and World Health Organization estimates; and reported a TV incidence of 1.9 million and 7.4 million for 15- to 24- and 15- to 59-year-olds, respectively. In our analysis, we used an ordinary differential equation based model to estimate incidence, using the best available evidence to inform model parameters; our analysis resulted in estimates of 3.3 million and 3.5 million incident infections among men and women aged 15 to 59 years, respectively. Some of the differences in estimates may be due to the improvement in molecular testing methods for TV since 20009; however, because prevalence estimates have not changed as extensively, the differences in incidence are more likely due to assumptions inherent to our model design, particularly those about natural clearance. Unlike previous analyses,12 we incorporated estimated rates of natural clearance of TV among males and females and found it to be a key determinant of incidence in our model (as explained below). The methods we used to estimate incidence were explicit mathematical formulations of our assumptions related to infection and recovery; all inputs to the model are theoretically measurable, but most were not available in the literature. We used Monte Carlo simulations to describe uncertainty in the factors involved in TV infection and clearance. Moreover, we anchored our model to real-world data by replicating an expected number of treated infections that corresponded to TV diagnoses documented by MarketScan. However, it is possible that a small proportion of persons with a TV diagnosis code may not have been treated, or had delayed treatment, for TV. Furthermore, if providers are less likely to record a diagnosis of TV in an administrative claims database than we expected, it may result in an underestimate of treated TV infections and therefore an underestimate of model-estimated incidence.
Among both males and females, modeled incidence rates were highly affected by differences in TV natural clearance (Fig. 1). There is very little available evidence to inform this parameter. To inform the natural clearance rate in males, we used a single randomized controlled trial23 that followed 16 TV infected men over time. For the natural clearance rate in females, we again used a single longitudinal study24 in which 37 TV infections in adolescent women were identified. Both of these studies used NAAT for TV diagnosis and accounted for time since last sexual exposure to their infected partner. The few other studies that have attempted to define male natural clearance used culture as a primary means of diagnosis22,25,28; because culture is far less sensitive than NAAT for detection of TV infection,33,34 we did not include them in our analysis.
Although we based our estimates on the best available data, our findings highlight the need for a better understanding of the natural history of TV infection. We were unable to find data on the percent of TV infections that are asymptomatic, time from TV exposure to treatment in males and females, and differences in treatment seeking by age and sex, although all of these would have altered the outcomes of our model (Fig. 1). More importantly for our model, however, the clearance rate of TV in both men and women is based on small numbers of patients. For women, the patients were aged 14 to 17 years. Our model assumes that all patients who receive treatment completely clear infection and that clearance of TV infection does not vary by age. However, persistence of infection after single-dose metronidazole treatment (defined as a positive test result after exclusion of reinfection or early retesting) is suspected to occur in at least 3% to 19% of women, particularly in women older than 25 years.31,35,36 One recent randomized, controlled trial of single-dose versus multidose metronidazole for treatment of TV in human immunodeficiency virus–negative women demonstrated that 19% of women treated with single-dose versus 11% of women treated with multidose metronidazole had persistent TV infection 28 days after therapy despite similar reported sexual behavior, probably indicating a dose-response effect on true persistence of infection rather than reinfection.35 If there is increased time to TV clearance among treated women or if there is slower natural clearance in older women, our model demonstrates that TV incidence in women would be lower, particularly in older age groups. More research in this area is urgently needed.
An additional limitation of our analysis is that we did not determine the prevalence or incidence of TV stratified by race/ethnicity. This is due to small sample sizes among some of our stratifications and would have resulted in unstable estimates. In many studies, rates of TV have been determined to be far higher in Black or African American women.1,32 Moreover, by using 2018 estimates of total population, rather than the civilian noninstitutionalized population, we assumed that TV prevalence and incidence are similar in active military and institutionalized populations, including incarcerated persons. Therefore, our estimates may not be applicable to significant subpopulations or groups. Furthermore, the analysis is subject to the inherent limitations of the NHANES, which include the presumption of stability of prevalence within cycles, that infection data are only available for those aged 15 to 59 years, and that indeterminate test results for TV were counted as negatives.
Despite the uncertainties inherent in the analysis, however, we estimate a high prevalence and incidence of TV in the United States, particularly among women and men 25 years or older. We found that, although TV prevalence is higher in women, TV incidence is similar in both sexes. Given the high disease burden caused by TV, research should focus on clarifying its natural history, because this affects incidence estimates. Public health efforts should be directed toward increasing screening and treatment efforts for this very common infection.
Supplementary Material
Footnotes
Conflict of Interest and Sources of Funding: All authors report no conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Patel E, Gaydos C, Packman Z, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis 2018; 67:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobbs MA, Seña AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infect 2013; 89:434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allsworth JE, Ratner JA, Peipert JF. Trichomoniasis and other sexually transmitted infections: Results from the 2001–2004 National Health and Nutrition Examination Surveys. Sex Transm Dis 2009; 36:738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton M, Sternberg M, Koumans EH, et al. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45:1319–1326. [DOI] [PubMed] [Google Scholar]
- 5.Ryan CM, de Miguel N, Johnson PJ. Trichomonas vaginalis: Current understanding of host-parasite interactions. Essays Biochem 2011; 51:161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owusu-Edusei K Jr., Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013; 40:197–201. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Chesson HW, Gift TL. Estimating the direct medical outpatient costs of diagnosis and treatment of trichomoniasis among commercially insured patients in the United States, 2016–2018. Sex Transm Dis 2021; 48:e45–e47. doi: 10.1097/OLQ.0000000000001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesson HW, Spicknall IH, Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis 2021; 48:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poole DN, McClelland RS. Global epidemiology of Trichomonas vaginalis. Sex Transm Infect 2013; 89:418–422. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2020; Available at: https://wwwn.cdc.gov/nndss/conditions/notifiable/2020/. Accessed October 16, 2020.
- 11.Weinstock H, Berman S, Cates W. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004; 36:6–9. [DOI] [PubMed] [Google Scholar]
- 12.Satterwhite L, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis 2013; 40:187–193. [DOI] [PubMed] [Google Scholar]
- 13.US Census Bureau. American Community Survey, 2018. American Community Survey 1-Year Estimates, Table S0201. Generated by Kristen Kreisel using American FactFinder. Available at: data.census.gov. Accessed February 18, 2020. [Google Scholar]
- 14.Kreisel KM, Spicknall IH, Gargano J, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2018. Sex Transm Dis 2021; 48:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Available at: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2013. Accessed July 1, 2020.
- 16.Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey. Available at: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2015. Accessed July 1, 2020.
- 17.Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey. Available at: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2017. Accessed July 1, 2020.
- 18.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2016. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention. Available at: https://wwwn.cdc.gov/nchs/data/nhanes/analyticguidelines/11-16-analytic-guidelines.pdf. Accessed July 1, 2020. [Google Scholar]
- 19.Kreisel KM, Weston E, St Cyr S, et al. Estimates of the prevalence and incidence of chlamydia and gonorrhea among US men and women, 2018. Sex Transm Dis 2021; 48:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meurer A, Smith C, Paprocki M, et al. SymPy: symbolic computing in Python. Peer J Comput Sci 2017; 3:e103. [Google Scholar]
- 21.Van Rossum G, Drake F. Python 3 Reference Manual. Scotts Valley, CA: CreateSpace, 2009. [Google Scholar]
- 22.Watt L, Jennison R. Incidence of Trichomonas vaginalis in marital partners. Br J Vener Dis 1960; 36:163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwebke JR, Rompalo A, Taylor SN, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens—a randomized clinical trial. Clin Infect Dis 2011; 52:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Pol B, Williams J, Orr D, et al. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J Infect Dis 2005; 192:2039–2044. [DOI] [PubMed] [Google Scholar]
- 25.Krieger JN, Verdon M, Siegel N, et al. Natural history of urogenital trichomoniasis in men. J Urol 1993; 149:1455–1458. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. 2011. Available at: https://apps.who.int/iris/bitstream/handle/10665/44735/9789241502450_eng.pdf;jsessionid=9F1944920C4DC9671D38D8176B3327BB?sequence=1. Accessed December 27, 2020. [Google Scholar]
- 27.IBM. MarketScan Research Databases 2019. Available at: https://www.ibm.com/products/marketscan-research-databases. Accessed July 15, 2020.
- 28.Price MA, Zimba D, Hoffman IF, et al. Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: A randomized clinical trial. Sex Transm Dis 2003; 30:516–522. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Chesson HW, Spicknall IH, et al. The estimated lifetime medical cost of chlamydia, gonorrhea, and trichomoniasis in the United States, 2018. Sex Transm Dis 2021; 48:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spicknall I, Mayer K, Aral S, et al. Assessing uncertainty in an anatomical site-specific gonorrhea transmission model of men who have sex with men. Sex Transm Dis 2019; 46:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterman T, Tian L, Metcalf C, et al. , RESPECT-2 Study Group. Persistent, undetected Trichomonas vaginalis infections? Clin Infect Dis 2009; 48:259–260. [DOI] [PubMed] [Google Scholar]
- 32.Flagg E, Meites E, Phillips C, et al. Prevalence of Trichomonas vaginalis among civilian, noninstitutionalized male and female population aged 14 to 59 years: United States, 2013 to 2016. Sex Transm Dis 2019; 46:e93–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nye M, Schwebke J, Body B. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200:188e1–e7. [DOI] [PubMed] [Google Scholar]
- 34.Hobbs MM, Lapple DM, Lawing LF, et al. Methods for detection of Trichomonas vaginalis in the male partners of infected women: Implications for control of trichomoniasis. J Clin Microbiol 2016; 44:3994–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: An open-label, randomised controlled trial. Lancet Infect Dis 2018; 18:1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazenby GB, Hill A, Tarleton J, et al. Diagnosis, treatment, follow-up, and persistence of Trichomonas vaginalis in women 45 years and older according to HIV status: a 10-year retrospective cohort. Sex Transm Dis 2020; 47:332–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


