Abstract
Objectives:
Visceral leishmaniasis remains a deadly parasitic disease with diagnostic complexities. Currently, point-of-care chest imaging is gaining momentum in the diagnosis of infectious diseases. Respiratory symptoms are common in visceral leishmaniasis. Here we aimed to systematically synthesize the evidence on the utility of chest imaging on the diagnosis and management of patients with visceral leishmaniasis.
Methods:
We searched PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar databases for studies reporting chest imaging findings in patients with visceral leishmaniasis, published in English from database inception to November 2022. We used the Joanna Briggs Institute checklists to evaluate the risk of bias. The protocol of this systematic review was registered with the Open Science Framework: https://doi.org/10.17605/OSF.IO/XP24W.
Results:
Of 1792 studies initially retrieved, 17 studies with 59 participants were included. Of the 59 patients, 51% (30) had respiratory symptoms and 20% (12) were human immunodeficiency virus co-infected. Chest X-ray, high-resolution computed tomography, and chest ultrasound findings were available for 95% (56), 93% (55), and 2% (1) of the patients, respectively. The most common findings were pleural effusion (20%; 12), reticular opacities (14%; 8), ground-glass opacities (12%; 7), and mediastinal lymphadenopathies (10%; 6). High-resolution computed tomography was more sensitive than chest X-ray and detected lesions that were lost on chest X-ray, 62% (37) versus 29% (17). In almost all cases, regression of the lesions was observed with treatment. Microscopy of pleural or lung biopsy detected amastigotes. Polymerase chain reaction yield was better in pleural and bronchoalveolar lavage fluids. A parasitological diagnosis from pleural and pericardial fluid was possible in AIDS patients. Overall, the risk of bias was low.
Conclusions:
Visceral leishmaniasis patients frequently had abnormal findings on high-resolution computed tomography. Chest ultrasound is a useful alternative in resource-limited settings to aid in diagnosis and subsequent treatment follow-up, especially when routine tests yield negative results despite clinical suspicion.
Keywords: Visceral leishmaniasis, tropical diseases, diagnostic imaging, chest X-ray, high-resolution computed tomography, chest ultrasound, point-of-care ultrasound
Introduction
Visceral leishmaniasis (VL), a parasitic disease caused by Leishmania donovani and Leishmania infantum,1–4 is almost always fatal if left untreated and is the second leading cause of parasitic deaths after malaria.5,6 It is estimated that 50,000–90,000 new cases of VL occur worldwide each year, with many cases going unreported. 5 The highest disease burden and human immunodeficiency virus (HIV)-VL co-infection rate is found in East Africa, India, and Brazil.5,7,8 East Africa, the worst affected region, accounted for 57% of VL cases reported to WHO in 2020. 7 The highest co-infection rate between 20% and 45% is reported from north-western Ethiopia.9–11 HIV-VL co-infection poses a challenge for early diagnosis and treatment, and negatively impacts efforts to control and eliminate VL. 7 So far, East African countries rely on early diagnosis and effective treatment to reduce disease prevalence and mortality rates due to a lack of robust vector control measures.5,9
In endemic settings, diagnosis of VL is made based on clinical criteria and serological tests, mainly rk39 immunochromatographic test.1,9–11 Definitive diagnosis depends on tissue detection of the amastigote form.12,13 Currently available serological tests are reliable for use in India.10,14 However, they have reduced sensitivity in East Africa, significantly less in HIV-VL co-infected patients.9,11,14–17 These tests cannot also be used to diagnose relapse and confirm response to treatment.13,14 More sensitive molecular tests such as polymerase chain reaction (PCR) are not currently available for routine use. 4 Therefore, microscopic examination of invasive specimens is required for diagnosis. 9 Tissue sampling, especially splenic aspiration, can lead to serious complications in inexperienced hands.20,28,29 Clinical findings in VL are nonspecific12,16 and immunosuppressed patients, AIDS patients, transplant recipients, and children with primary immunodeficiency can have atypical presentations.18–20 These all add to a complex diagnosis of VL.
The importance of ultrasound (US) in diagnosing tropical infectious diseases, particularly in resource-poor settings with high HIV infection, has been studied with remarkable detail.21–24 Studies found that a diagnostic US protocol focused assessment with sonography for HIV/tuberculosis (FASH), hastened treatment initiation, and provided exclusive support for TB treatment in 50–72% of study participants.22,25,26 In this protocol, patients are scanned in six different positions to identify pathologies in the chest, abdomen, and fluid collections in pelvis. 27 In the case of VL, studies have elaborated the abdominal US findings taking into account typical features of the disease.28–30 One study showed that abdominal US findings, hepatosplenomegaly, lymph node enlargement, and nodular splenic lesions, have unclear diagnostic accuracy. 21 A study conducted in Ethiopia reported that there is no evidence to support the routine use of abdominal US in the management of VL except to detect subclinical complications of splenic aspiration. 29
However, previous studies have given limited attention to chest imaging findings, which are not uncommon, with a sparse attempt to review the diagnostic yield of specimens from the identified lesions. To the best of our knowledge, this is the first systematic review done on this subject. This study evaluates chest imaging, namely chest X-ray (CXR), chest US, and high-resolution computed tomography (HRCT) findings in patients with VL and appraises the diagnostic yield of specimens collected from identified lesions. This could help identify the role of chest imaging in the diagnosis and management of VL patients.
Methods
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020) guidelines. 29 The protocol of this systematic review was registered with the Open Science Framework: https://doi.org/10.17605/OSF.IO/XP24W.
Eligibility criteria
Participants
Human patients with VL,
Diagnosed for VL based on laboratory criteria set by WHO, 9 and
No restrictions based on age groups, sex, immune, and co-infection status.
Intervention
Chest imaging with any of the diagnostic imaging modalities: CXR, chest US, or HRCT.
Radiology reports written or images available.
Comparator
With or without a comparator.
Outcomes
Early diagnosis and improved prognosis.
Study designs
No restrictions,
Published in English language.
Data sources
To find eligible studies, we searched PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar databases from their inception to November 25, 2022. Additional relevant studies were identified during forward citation searches and reference lists reviewed.
Search strategy
The search was run in incognito mode to reduce personalization bias. MeSH terms were identified from PubMed and supplemented with free text to enable a comprehensive search (Supplemental Material 1). The search strategies used in PubMed for the MeSH terms and text words was: “Visceral leishmaniasis” OR “Kala Azar” OR “kala-azar” OR “Fever, Black” OR “Black Fever” OR “Leishmaniasis, Visceral”[Mesh] AND Imaging* OR Radiography OR “X-Ray*” OR “X RAY” OR Ultrasound* OR Ultrasonographic OR ultrasonic OR CT OR Tomography OR “Diagnostic Imaging”[Mesh] AND “Thorax”[Mesh] OR “Respiratory System”[MeSH] OR “Heart”[MeSH] OR chest OR Pleur* OR Lung OR Pulmonary OR Pericardi* OR Trachea* OR Bronchi* OR mediastin*.
Study selection
The search results from the five databases were merged into an Excel spreadsheet and duplicates were removed. The title and abstract screening of the retrieved results was performed independently by three authors (TH, TA, and YM) based on the eligibility criteria. Papers for which the three reviewers did not reach a consensus were considered for full-text screening. Studies eligible for full-text review were retrieved and selection criteria were reapplied. The three reviewers performed the screening independently, and disagreements were resolved through discussion.
Data extraction
We developed a structured data extraction form and created a template in systematic review software (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia). The form was pilot tested by TH, TA, and YM in four randomly selected studies and then adjusted as appropriate. The three reviewers independently extracted data from eligible studies, and differences were negotiated through discussion. The information extracted are as follows:
The report: Author, journal, and year of publication.
Study characteristics: Study type, number of participants, and setting and country the study was conducted.
Patient characteristics: Age, sex, immune status, and comorbidities.
Clinical presentations: Clinical history and physical examinations, laboratory test results, and whether the case was new or relapsed.
Chest imaging findings: Details of chest imaging findings, the sample taken from pathologies as applicable, and results of tests performed.
Assessment of risk of bias
We assessed the risk of bias in the included studies using Joanna Briggs Institute (JBI) risk assessment tools for case reports and prevalence studies31,32 based on the study type. Three reviewers (TH, TA, and YM) independently scored individual items of the checklists. Any discrepancies were dealt with discussion. An overall score was calculated by adding the score given to each item, and calculating a percentage. The papers were then classified as low risk (score > 70%), medium risk (score: 50–69%), or high (score < 49%) risk of bias.
Results
Study characteristics and patient demographics
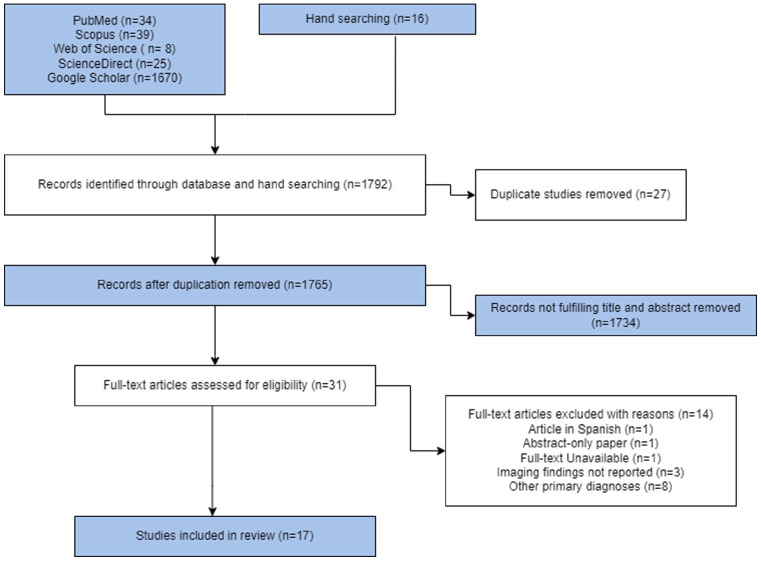
A total of 1792 search results were retrieved from the five databases and 27 duplicates were removed before screening. After title and abstract screening, 1734 papers were removed as they did not meet the selection criteria. Full-text screening was performed for 31 articles and 17 studies were eligible and thus included in the review (Figure 1).
Figure 1.
PRISMA flow diagram of the study.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Most of the included studies were case reports and one study followed a cross-sectional design.33–49 The majority of the studies came from India, southern European countries, and Brazil. The studies included 59 patients with a mean age (standard deviation) of 40.3 (±5.9) years, with males predominating (74.5%; 44) and 20% (12) patients co-infected with HIV/AIDS and immunocompromised. Other reported comorbidities were hepatitis B virus, hepatitis C virus, diabetes mellitus, and tuberculosis, each present in two patients. Patient demographics and clinical presentation are summarized in Table 1.
Table 1.
Demographic characteristics and clinical presentation of patients in the included studies.
| Characteristics | Frequency (N = 59) | Percentage |
|---|---|---|
| Sex | ||
| Male | 44 | 74.6 |
| Female | 15 | 25.4 |
| Presentation | ||
| Respiratory symptoms | 30 | 50.8 |
| Non respiratory symptoms | 29 | 49.2 |
| Respiratory symptoms | ||
| Cough | 21 | 35.6 |
| Sputum production (productive) | 13 | 22 |
| Sputum production (dry) | 8 | 13.5 |
| Shortness of breath | 4 | 6.8 |
| Chest pain | 3 | 5 |
| Hemoptysis | 2 | 3.4 |
| Typical symptoms (reported for 16 patients) | ||
| Splenomegaly | 8 | 50 |
| Fever | 9 | 56 |
| Hepatomegaly | 6 | 37.5 |
| Pancytopenia | 5 | 31 |
| Immune status | ||
| Immunocompetent | 47 | 79.7 |
| Immunocompromised | 12 | 20.3 |
| Comorbidity | ||
| HIV/AIDS | 12 | 20.3 |
| HCV | 2 | 3.4 |
| HBV | 2 | 3.4 |
| TB | 2 | 3.4 |
| Episode (reported for 17 patients) | ||
| Initial | 14 | 82.3 |
| Relapse | 3 | 17.7 |
HBV: hepatitis B virus; HCV hepatitis C virus; TB: tuberculosis; HIV: human immunodeficiency virus.
Clinical presentation of patients
The number of patients with or without respiratory symptoms was comparable, 50.8% and 49.1%, respectively. Cough was reported by 21 (35.6%) patients, shortness of breath by 4 (6.8%) patients, chest pain by 3 (5%) patients, and hemoptysis by 2 (3.4%) patients (Table 1). In the 42 patients included in the cross-sectional study, splenomegaly was the most common finding. However, among the case reports with a full description of physical examinations, it was reported that splenomegaly was present in only eight (50%) of included patients, with five (62.5%) of these being diagnosed with VL-HIV co-infection.
Chest imaging findings
The studies used CXR, HRCT, or chest US as diagnostic imaging modalities. The cross-sectional study required participants to undergo both CXR and HRCT, and a full report of the results was available. Among the case reports, no CXR and HRCT findings were available for three and four studies, respectively. Echocardiography was performed on a single patient (Table 2).
Table 2.
Chest imaging modalities utilized and findings reported from included studies.
| Study | Imaging | Description | No. of patients |
|---|---|---|---|
| Bispo et al. 33 | HRCT | Reticular opacities | 8 |
| Ground-glass opacities | 7 | ||
| Alveolar opacities | 3 | ||
| Tree in bud opacities | 2 | ||
| Interstitial thickening | 2 | ||
| Interstitial infiltrates | 1 | ||
| Bronchiectasis/bronchioloectasis | 2 | ||
| Atelectasis | 2 | ||
| Other parenchymal abnormalities | 2 | ||
| Pleural effusion | 5 | ||
| Pleural thickening | 2 | ||
| CXR | Consolidation ± air bronchogram | 2 | |
| Air bronchogram | 1 | ||
| Other parenchymal abnormalities | 1 | ||
| Pleural effusion | 1 | ||
| Kotsifas et al. 43 | HRCT | Mediastinal lymphadenopathy | 1 |
| CXR | Not reported | ||
| Herrejón et al. 42 | HRCT | Lung nodules Tree in bud opacities |
1 |
| CXR | Not reported | ||
| Escribano et al. 41 | HRCT | Cystic mediastinal mass | 1 |
| CXR | Enlarged right cardiac silhouette | 1 | |
| Viglianesi et al. 49 | HRCT | A diffuse annular ↑ of trachea wall thickness | 1 |
| CXR | Not reported | ||
| Raina et al. 48 | HRCT | Pleural effusion Atelectasis |
1 |
| CXR | Pleural effusion | 1 | |
| Nigro et al. 47 | HRCT | Cavitary lung lesion | 1 |
| CXR | Cavitary lung lesion | 1 | |
| Mofredj et al. 46 | HRCT | Not reported | |
| CXR | Cardiomegaly Pleural effusion |
1 | |
| Chest US | Pericardial effusion | 1 | |
| Matheron et al. 45 | HRCT | Lung nodules Plural effusion Mediastinal lymphadenopathy |
1 |
| CXR | Lung nodules Pleural effusion Mediastinal lymphadenopathy |
1 | |
| Marshall et al. 44 | HRCT | Mediastinal lymphadenopathy | 1 |
| CXR | Not reported | ||
| Diehl et al. 40 | HRCT | Interstitial infiltrates | 1 |
| CXR | Interstitial infiltrates Pleural effusion |
1 | |
| Dehghani et al. 39 | HRCT | Interstitial infiltrates Lung nodules Mediastinal lymphadenopathy |
1 |
| CXR | Interstitial infiltrates | 1 | |
| Dasgupta et al. 38 | HRCT | Not reported | |
| CXR | Pleural effusion | 1 | |
| Das et al. 37 | HRCT | Not reported | |
| CXR | A non-homogeneous opaque shadow with infiltration in right upper lobe—first patient Pleural effusion—second patient |
1 | |
| Choudhary et al. 36 | HRCT | Mediastinal lymphadenopathy | 1 |
| CXR | Not reported | ||
| Chenoweth et al. 35 | HRCT | Not reported | |
| CXR | Pleural effusion | 1 | |
| Casado et al. 34 | HRCT | Lung nodules Mediastinal lymphadenopathy |
1 |
| CXR | Lung nodules | 1 |
CXR: chest X-ray; HRCT: high-resolution computed tomography; US: ultrasound.
The most common chest imaging findings were pleural effusion in 20% (12), reticular opacities in 14% (8), ground-glass opacities in 12% (7), and mediastinal lymphadenopathies in 6(10%) of patients. Most of these findings, including but not limited to pulmonary interstitial changes and mediastinal lymphadenopathies, were diagnosed by HRCT. CXR of two patients showed an enlarged cardiac silhouette identified as a pericardial effusion on US in one patient and a pleuropericardial cyst on HRCT in another patient. Among patients with confirmed pulmonary VL, one patient had a bronchoapneumonia pattern on CXR and another patient had bilateral cavitary lung lesions on CXR and HRCT.
The prevalence of lung lesions detected by HRCT tends to increase with disease duration. The mean time from symptom onset to hospital admission was 58 days and 34 days in patients with and without HRCT pathology findings, respectively, in 71% (42) of patients. Interestingly, HRCT findings consistent with progressive lung injury were absent, even in patients who presented late. Overall, imaging findings were found to resolve with effective treatment. The chest imaging results reported from the included studies are summarized in Table 3.
Table 3.
A summary of HRCT and CXR imaging findings of patients included in the studies.
| Chest imaging findings | HRCT, N (59) % | CXR, N (59) % | No. of patients, N (59) % |
|---|---|---|---|
| Pleural effusion | 7 (12%) | 8 (14%) | 12 (20%) |
| Reticular opacities | 8 (14%) | — | 8 (14%) |
| Ground-glass opacities | 7 (12%) | — | 7 (12%) |
| Mediastinal lymphadenopathies | 6 (10%) | 1 (2%) | 6 (10%) |
| Lung nodules | 4 (7%) | 2 (3%) | 4 (7%) |
| Interstitial infiltrates | 3 (5%) | 3 (5%) | 4 (7%) |
| Alveolar opacities | 3 (5%) | — | 3 (5%) |
| Atelectasis | 3 (5%) | — | 3 (5%) |
| Other parenchymal abnormalities | 2 (3%) | 1 (2%) | 3 (5%) |
| Tree in bud opacities | 2 (3%) | — | 2 (3%) |
| Interstitial thickening | 2 (3%) | — | 2 (3%) |
| Bronchiectasis/bronchioloectasis | 2 (3%) | — | 2 (3%) |
| Consolidation ± air bronchogram | — | 2 (3%) | 2 (3%) |
| Pleural thickening | 2 (3%) | — | 2 (3%) |
| Enlarged cardiac silhouette | — | 2 (3%) | 2 (3%) |
| Air bronchogram | — | 1 (2%) | 1 (2%) |
| Cavitary lung lesion | 1 (2%) | 1 (2%) | 1 (2%) |
| Increased tracheal wall thickness | 1 (2%) | — | 1 (2%) |
| Cystic mediastinal mass | 1 (2%) | — | 1 (2%) |
N: absolute frequency; %: percentage; No: number; CXR: chest X-ray; HRCT: high-resolution computed tomography.
VL diagnostic tests
In all, 12 studies have reported leishmaniasis tests, microscopy, culture, or PCR performed on clinical specimens collected from identified chest pathologies. Microscopic examination was the most commonly performed test, and mediastinal lymph nodes were examined relatively more frequently. The test results exclusively informed the diagnosis of VL in eight patients, two of them had culture-negative bone marrow aspirates, and microscopic examination or culture of the bronchoalveolar lavage (BAL) fluid was also negative. To make a diagnosis, either a PCR test was performed on the same samples or microscopic examination of more invasive samples was done. A pleural fluid or pericardial fluid microscopy examination was performed on three patients and tested positive. Notably, they were AIDS patients. Leishmania amastigotes have been identified at atypical sites, including BAL fluid, tracheal or bronchial tissue, mediastinal lymph nodes, and a cystic mediastinal mass in apparently immunocompetent patients. A list of reported leishmaniasis diagnostic tests is summarized in Table 4.
Table 4.
VL diagnostic tests done on samples taken from identified pathologies and their results.
| Study | Microscopy | Culture | PCR | Remark |
|---|---|---|---|---|
| Kostifas et al. 43 | Bronchial biopsy Mediastinal lymph node biopsy |
– | – | Spleen and liver aspirates were positive for amastigotes |
| Herrejón et al. 42 | Transbronchial biopsy | – | – | VL was diagnosed exclusively based on this result |
| Escribano et al. 41 | Mediastinal cystic mass biopsy | – | – | VL was diagnosed exclusively based on this result |
| Viglianesi et al. 49 | Tracheal biopsy | – | – | VL was diagnosed exclusively based on this result |
| Nigro et al. 47 | BAL fluid | – | – | Amastigotes were identified from peripheral blood and oral mucosal ulcer |
| Mofredi et al. 46 | Pericardial fluid | – | – | A relapse of VL was diagnosed exclusively based on this result |
| Matheron et al. 45 | Pleural biopsy Lung biopsy Mediastinal Lymph node biopsy *BAL fluid |
Pleural biopsy | – | Bone marrow aspirate microscopy and culture were negative |
| Marshall et al. 44 | Not performed |
*Bronchial biopsy *BAL fluid |
BAL fluid Bronchial biopsy |
Bone marrow aspirate culture showed no growth |
| Diehl et al. 40 | Pleural fluid | – | Pleural fluid | VL was diagnosed exclusively based on this result |
| Dehghani et al. 39 | Lung biopsy | – | – | Positive peripheral blood PCR and IFAT titer |
| Choudhary et al. 36 | Mediastinal lymph node biopsy | – | – | VL was diagnosed exclusively based on this result |
| Chenoweth et al. 35 | Pleural fluid | Pleural fluid | – | Bone marrow aspirate was positive for amastigotes |
BAL: bronchoalveolar lavage; VL: visceral leishmaniasis; PCR: polymerase chain reaction; IFAT: indirect fluorescence antibody test.
Test results were negative.
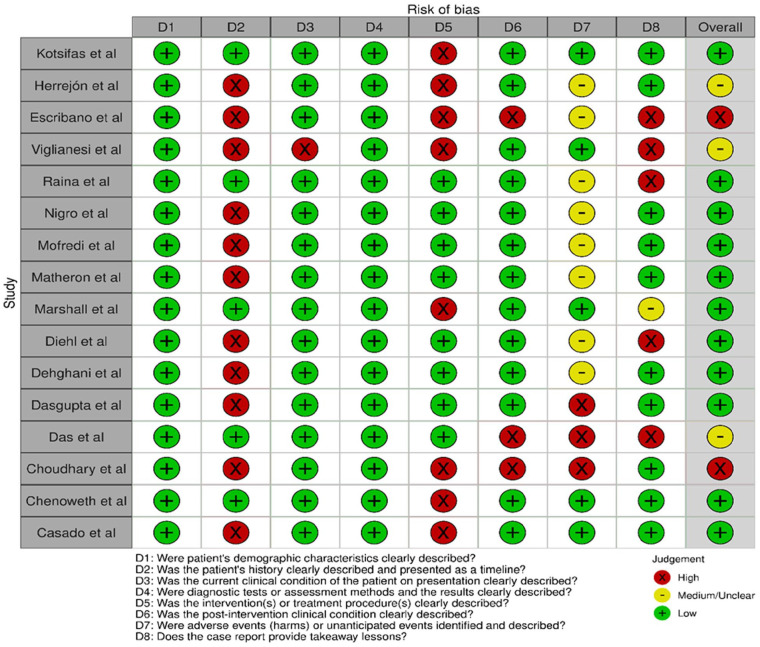
Quality assessment and risk of bias
Based on the JBI quality appraisal tools, five case reports were assessed to be high/moderate risk. The other included studies were low risk. Overall, the studies were deemed to be low risk (Figure 2).
Figure 2.
“Risk of bias” summary: review authors’ judgment about each “risk of bias” item and overall appraisal for case reports.
Discussion
We included 17 articles in our review, 16 case reports, and one cross-sectional study. VL has heterogeneous imaging findings of the chest that occur more frequently than previously thought. 33 Pleura, lung parenchyma, mediastinal lymph nodes, airways, and the pericardium can be affected.33,44,46,49 Pleural effusion and pulmonary interstitial changes were the predominant imaging findings, and the pulmonary vessels were relatively spared. This is in contrast to chronic pulmonary schistosomiasis (CPS), in which pulmonary hypertension can develop over time, whereas the pleura and pericardium are spared.50,51 A possible explanation for this difference could be chronic inflammation in periarteriolar regions induced by migrating eggs in CPS and systemic spread of the parasite in VL.
Immune dysregulation associated with VL may predispose patients to develop pulmonary infections.43,52 However, respiratory symptoms should be considered signs of lung involvement in both immunocompetent and immunocompromised patients.38,39,43,45 In addition, patients with no respiratory symptoms may still have abnormalities on chest imaging, and the parasite can be detected.36,40,41,46 The sum of imaging findings combined with the clinical picture and epidemiology of the disease can help in reaching a diagnosis, even if individual imaging abnormalities are nonspecific and uncommon.50,53 A parasitologic and imaging response to treatment was observed in almost all cases, in line with response to treatment seen in CPS, including some cases of schistosomiasis-associated pulmonary hypertension.50,54 This observation suggests that follow-up imaging could confirm disease resolution and spare the patient from invasive testing.
HRCT is a more sensitive imaging modality and detects lesions that are lost on CXR, 62% (37) and 29% (17) of patients, respectively.33,34,39 This is consistent with what was found in previous studies on tropical lung disease, CPS and pulmonary hydatid cysts.50,55,56 CXR can serve as the primary imaging modality in low-resource settings, as only subtle or complicated imaging findings go undetected, and it is convenient for use in patient follow-up.50,57 However, our findings support HRCT as the imaging modality of choice in VL patients.
Chest US is a viable alternative to detect abnormal findings in the chest. 33 Man et al. 58 found that transthoracic lung US is a useful tool for the diagnosis and follow-up of patients with interstitial lung disease. Coley et al. 59 stated that US can reliably diagnose chest pathologies in children. US-guided biopsy can be safely performed in numerous pulmonary and mediastinal pathologies.60,61 Recently, it has been suggested that point-of-care lung US-based algorithms are useful for triage of patients with symptoms of COVID-19, especially when resources are limited.62,63 A study also found the importance of lung US to diagnose pneumocystis jirovecii pneumonia in HIV patients. 64 US is an appealing imaging modality for use in resource-constrained countries as it is safe, inexpensive, portable, and can be used by a range of users. 65
Diagnosis of VL can be made based on demonstration of the parasite in pleural fluid,35,40 BAL fluid, 47 transbronchial or open lung biopsy,39,42,45 pleural biopsy 45 mediastinal lymph node biopsy,36,43,45 pericardial fluid 46 tracheal, or bronchial biopsy.43,49 PCR on respiratory samples has also been used to diagnose leishmaniasis with increased sensitivity.40,44 Patients with lung involvement, lung biopsy, and BAL fluid have a better diagnostic yield for amastigotes than pleural fluid. 40 However, microscopic examination of pleural and pericardial fluid was particularly sensitive in AIDS patients.35,46 This can be due to a high parasite load seen in HIV-VL co-infected patients.9,66 It is important to note that negative microscopic exam results of any respiratory specimen cannot rule out pulmonary involvement.44,45,52 PCR detection of the parasite from pleural fluid and BAL fluid is a promising diagnostic test.40,44 Of note, newly developed PCR assays have improved diagnostic accuracy and potential to be used as point-of-care tests in the future. 14
We acknowledge the following limitations of the study. All the included studies, except one, have not utilized US, thus typical or suggestive US findings are not available. Most of the included studies were case reports, which naturally include a higher risk of publication bias 67 despite the overall low risk of bias assessment. The differential diagnoses commonly considered for the above imaging findings were not discussed. However, a diagnosis of VL should be suspected in a patient with abnormal chest imaging findings and a favorable clinical presentation, regardless of the patient’s immune status.
Conclusion
IHRCT was abnormal in more than half of the VL patients included in the review, although the findings were nonspecific and heterogeneous. Chest US may be developed for low-resource settings as an alternative to HRCT and used when routinely available diagnostic tests are negative despite clinical suspicion of VL. PCR has improved sensitivity to identify the parasite from respiratory samples, and parasitological tests give more positive results in AIDS patients.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121231177812 for Utility of chest imaging in the diagnosis and management of patients with visceral leishmaniasis: A systematic review by Tesfahunegn Hailemariam, Yonas Mebratu, Tsion Andrias, Fikremariam Melkeneh, Abel Abebe, Biruk Mulualem, Zewdu Abadi, Wazema Desta, Selamawit Bedasso, Fekadu Belay, Abibual Sileshi, Elilta Desta, Felipe Velsaquez-Botero, Rahel Birhane, Francesco Marinucci and Tsegahun Manyazewal in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_20503121231177812 for Utility of chest imaging in the diagnosis and management of patients with visceral leishmaniasis: A systematic review by Tesfahunegn Hailemariam, Yonas Mebratu, Tsion Andrias, Fikremariam Melkeneh, Abel Abebe, Biruk Mulualem, Zewdu Abadi, Wazema Desta, Selamawit Bedasso, Fekadu Belay, Abibual Sileshi, Elilta Desta, Felipe Velsaquez-Botero, Rahel Birhane, Francesco Marinucci and Tsegahun Manyazewal in SAGE Open Medicine
Supplemental material, sj-docx-3-smo-10.1177_20503121231177812 for Utility of chest imaging in the diagnosis and management of patients with visceral leishmaniasis: A systematic review by Tesfahunegn Hailemariam, Yonas Mebratu, Tsion Andrias, Fikremariam Melkeneh, Abel Abebe, Biruk Mulualem, Zewdu Abadi, Wazema Desta, Selamawit Bedasso, Fekadu Belay, Abibual Sileshi, Elilta Desta, Felipe Velsaquez-Botero, Rahel Birhane, Francesco Marinucci and Tsegahun Manyazewal in SAGE Open Medicine
Acknowledgments
The authors thank the staff of CDT-Africa who facilitated the study.
Footnotes
Authors’ contributions: TH developed the protocol with the advice from TM. TH, YM, and TA reviewed the reference list and extracted the data. TH and RB conducted the analysis. TH, TA, YM, RB, FB, FM (Francesco Marinucci), and TM evaluated the quality and comprehensiveness of the study and underlying data. TH developed the first draft of the manuscript and TM critically reviewed it. TH, YM, TA, FM, AA, BM, ZA, WD, SB, FB, AS, ED, FV-B, RB, FM, and TM read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-AFRICA), College of Health Sciences, Addis Ababa University, Ethiopia.
Ethics approval: Not applicable.
Consent for publication: Not applicable.
ORCID iDs: Tesfahunegn Hailemariam  https://orcid.org/0000-0003-4304-804X
https://orcid.org/0000-0003-4304-804X
Fikremariam Melkeneh https://orcid.org/0000-0003-3581-5464
https://orcid.org/0000-0003-3581-5464
Felipe Velsaquez-Botero  https://orcid.org/0000-0002-1353-0428
https://orcid.org/0000-0002-1353-0428
Availability of data and materials: The datasets analyzed during the current study will be available from the corresponding author on reasonable request.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Yimer M, Nibret E, Yismaw G.Updates on prevalence and trend status of visceral leishmaniasis at two health facilities in Amhara regional state, northwest Ethiopia: a retrospective study. Biochem Res Int 2022; 2022: 3603892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarpini S, Dondi A, Totaro C, et al. Visceral leishmaniasis: epidemiology, diagnosis, and treatment regimens in different geographical areas with a focus on pediatrics. Microorganisms 2022; 10(10): 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moirano G, Ellena M, Mercogliano P, et al. Spatio-temporal pattern and meteo-climatic determinants of visceral leishmaniasis in Italy. Trop Med Infect Dis 2022; 7(11): 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mewara A, Gudisa R, Padhi BK, et al. Visceral leishmaniasis outbreak in Kenya—a setback to the elimination efforts. New Microbes New Infect 2022; 49: 101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Leishmaniasis factsheet. Geneva: World Health Organization, 2020. [Google Scholar]
- 6.Burki T.East African countries struggle with visceral leishmaniasis. Lancet 2009; 374(9687): 371–372. [DOI] [PubMed] [Google Scholar]
- 7.Mondiale de la Santé O. and World Health Organization. Global leishmaniasis surveillance: 2021, assessing the impact of the COVID-19 pandemic–surveillance mondiale de la leishmaniose: 2021, évaluation de l’impact de la pandémie de COVID-19. Wkly Epidemiol Rec 2022; 97(45): 575–590. [Google Scholar]
- 8.Wamai RG, Kahn J, McGloin J, et al. Visceral leishmaniasis: a global overview. J Glob Health Sci 2020; 2(1): e3. [Google Scholar]
- 9.Burki T.Guidelines for visceral leishmaniasis and HIV co-infection. Lancet Infect Dis 2022; 22(8): 1124–1125. [DOI] [PubMed] [Google Scholar]
- 10.Sundar S, Reed SG, Singh VP, et al. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet 1998; 351(9102): 563–565. [DOI] [PubMed] [Google Scholar]
- 11.Kiros YK, Regassa BF.The role of rk39 serologic test in the diagnosis of visceral leishmaniasis in a Tertiary Hospital, Northern Ethiopia. BMC Res Notes 2017; 10(1): 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 2016; 63(12): e202–e264. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava P, Dayama A, Mehrotra S, et al. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg 2011; 105(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundar S, Singh OP.Molecular diagnosis of visceral leishmaniasis. Mol Diagn Ther 2018; 22(4): 443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundar S, Rai M.Laboratory diagnosis of visceral leishmaniasis. Clin Vaccine Immunol 2002; 9(5): 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boelaert M, Bhattacharya S, Chappuis F, et al. Evaluation of rapid diagnostic tests: visceral leishmaniasis. Nat Rev Microbiol 2007; 5(11): S31–S39. [DOI] [PubMed] [Google Scholar]
- 17.Schallig HD, Canto-Cavalheiro M, da Silva ES.Evaluation of the direct agglutination test and the rK39 dipstick test for the sero-diagnosis of visceral leishmaniasis. Mem Inst Oswaldo Cruz 2002; 97: 1015–1018. [DOI] [PubMed] [Google Scholar]
- 18.Prestes-Carneiro LE, Spir PRN, Fontanesi M, et al. Unusual manifestations of visceral leishmaniasis in children: a case series and its spatial dispersion in the western region of São Paulo state, Brazil. BMC Infect Dis 2019; 19(1): 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurissa Z, Gebre-Silassie S, Hailu W, et al. Clinical characteristics and treatment outcome of patients with visceral leishmaniasis and HIV co-infection in northwest Ethiopia. Trop Med Int Health 2010; 15(7): 848–855. [DOI] [PubMed] [Google Scholar]
- 20.Ramos JM, León R, Merino E, et al. Is visceral leishmaniasis different in immunocompromised patients without human immunodeficiency virus? A comparative, multicenter retrospective cohort analysis. Am J Trop Med Hyg 2017; 97(4): 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bélard S, Tamarozzi F, Bustinduy AL, et al. Point-of-care ultrasound assessment of tropical infectious diseases—a review of applications and perspectives. Am J Trop Med Hyg 2016; 94(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobbio F, Di Gennaro F, Marotta C, et al. Focused ultrasound to diagnose HIV-associated tuberculosis (FASH) in the extremely resource-limited setting of South Sudan: a cross-sectional study. BMJ Open 2019; 9(4): e027179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heller T, Goblirsch S, Bahlas S, et al. Diagnostic value of fash ultrasound and chest X-ray in Hiv-co-infected patients with abdominal tuberculosis [notes from the field]. Int J Tuberc Lung Dis 2013; 17(3): 342–344. [DOI] [PubMed] [Google Scholar]
- 24.Kaminstein D, Heller T, Tamarozzi F.Sound around the world: ultrasound for tropical diseases. Infect Dis Clin 2019; 33(1): 169–195. [DOI] [PubMed] [Google Scholar]
- 25.Heller T, Mtemang’ombe EA, Huson MA, et al. Ultrasound for patients in a high HIV/tuberculosis prevalence setting: a needs assessment and review of focused applications for Sub-Saharan Africa. Int J Infect Dis 2017; 56: 229–236. [DOI] [PubMed] [Google Scholar]
- 26.Kahn D, Pool KL, Phiri L, et al. Diagnostic utility and impact on clinical decision making of focused assessment with sonography for HIV-associated tuberculosis in malawi: a prospective cohort study. Glob Health Sci Pract 2020; 8(1): 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heller T, Wallrauch C, Goblirsch S, et al. Focused assessment with sonography for HIV-associated tuberculosis (FASH): a short protocol and a pictorial review. Crit Ultrasound J 2012; 4(1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bélard S, Stratta E, Zhao A, et al. Sonographic findings in visceral leishmaniasis–a narrative review. Travel Med Infect Dis 2021; 39: 101924. [DOI] [PubMed] [Google Scholar]
- 29.Mohammed R, Gebrewold Y, Schuster A, et al. Abdominal ultrasound in the diagnostic work-up of visceral leishmaniasis and for detection of complications of spleen aspiration. PLoS Negl Trop Dis 2021; 15(2): e0009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdalla EA, Ayad CE, Ahmed AMF, et al. Ultrasound findings in patients with visceral leishmaniasis. Int J Med Imaging 2014; 2(1): 5. [Google Scholar]
- 31.Joanna Briggs Institute. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews: checklist for prevalence studies. The University of Adelaide: The Joanna Briggs Institute, https://joannabriggs.org/assets/docs/sumari/ReviewersManual_2017-The-Systematic-Review-of-Prevalence-and-Incidence-Data_v2.pdf (2017, accessed 9 February 2019).
- 32.Joanna Briggs Institute. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic review: checklists for case reports. Joanna Briggs Institute, 2019. [Google Scholar]
- 33.Bispo AJB, Almeida MLD, de Almeida RP, et al. Pulmonary involvement in human visceral leishmaniasis: clinical and tomographic evaluation. PLoS One 2020; 15(1): e0228176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casado JL, Cuesta C, Sanchez JA, et al. Solitary pulmonary nodule due to Leishmania in a patient with AIDS. Clin Infect Dis 1998; 26(2): 532–534. [DOI] [PubMed] [Google Scholar]
- 35.Chenoweth CE, Singal S, Pearson RD, et al. Acquired immunodeficiency syndrome-related visceral leishmaniasis presenting in a pleural effusion. Chest 1993; 103(2): 648–649. [DOI] [PubMed] [Google Scholar]
- 36.Choudhary NS, Kataria S, Guleria M, et al. Leishmaniasis presenting as small isolated mediastinal lymphadenopathy diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Endoscopy 2015; 47(S 1): E147–E148. [DOI] [PubMed] [Google Scholar]
- 37.Das V, Pandey K, Kumar N, et al. Case report. Visceral leishmaniasis and tuberculosis in patients with HIV co-infection. Southeast Asian J Trop Med Public Health 2006; 37(1): 18. [PubMed] [Google Scholar]
- 38.Dasgupta S, Saha M, Chakrabarti S, et al. Visceral leishmaniasis with pleural effusion in an immunocompetent patient. Lung India 2014; 31(1): 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dehghani M, Monabati A, Sanei M, et al. Visceral Leishmaniasis with massive pulmonary involvement: a case report. Int J Infect 2019; 6(1): e87993. [Google Scholar]
- 40.Diehl AR, Dos Santos RP, Zimmerman R, et al. Microscopy and polymerase chain reaction detection of Leishmania chagasi in the pleural and ascitic fluid of a patient with AIDS: case report and review of diagnosis and therapy of visceral leishmaniasis. Can J Infect Dis Med Microbiol 2004; 15(4): 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Escribano MDA, Gómez MJG, Hurtado EJS. Mediastinal cystic mass as atypical location of visceral leishmaniasis. Med Clin 2018; 151(9): 379–380. [DOI] [PubMed] [Google Scholar]
- 42.Herrejón A, Cervera A, Maciá M, et al. Bronchioloalveolar adenoma associated with bronchiolitis obliterans and leishmaniasis with lung involvement in acquired immunodeficiency syndrome. Arch Bronconeumol 2005; 41(4): 233–235. [DOI] [PubMed] [Google Scholar]
- 43.Kotsifas K, Metaxas E, Koutsouvelis I, et al. Visceral leishmaniasis with endobronchial involvement in an immunocompetent adult. Case Rep Med 2011; 2011: 561985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall BG, Kropf P, Murray K, et al. Bronchopulmonary and mediastinal leishmaniasis: an unusual clinical presentation of Leishmania donovani infection. Clin Infect Dis 2000; 30(5): 764–769. [DOI] [PubMed] [Google Scholar]
- 45.Matheron S, Cabié A, Parquin F, et al. Visceral leishmaniasis and HIV infection: unusual presentation with pleuropulmonary involvement, and effect of secondary prophylaxis. AIDS 1992; 6(2): 238–240. [PubMed] [Google Scholar]
- 46.Mofredj A, Guerin JM, Leibinger F, et al. Visceral leishmaniasis with pericarditis in an HIV-infected patient. Scand J Infect Dis 2002; 34(2): 151–153. [DOI] [PubMed] [Google Scholar]
- 47.Nigro L, Montineri A, La Rosa R, et al. Visceral leishmaniasis and HIV co-infection: a rare case of pulmonary and oral localization. Infez Med 2003; 11(2): 93–96. [PubMed] [Google Scholar]
- 48.Raina S, Kaul R, Kashyap R, et al. Atypical presentation of visceral leishmaniasis from non-endemic region. Online J Health Allied Sci 2010; 9(2): 13. [Google Scholar]
- 49.Viglianesi A, Di Mauro D, Petrillo G.Multidetector computed tomography aspects of tracheal mucosal leishmaniasis localization. Jpn J Radiol 2011; 29(1): 59–62. [DOI] [PubMed] [Google Scholar]
- 50.Foti G, Gobbi F, Angheben A, et al. Radiographic and HRCT imaging findings of chronic pulmonary schistosomiasis: review of 10 consecutive cases. BJR Case Rep 2019; 5(3): 20180088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoette S, Figueiredo C, Dias B, et al. Pulmonary artery enlargement in schistosomiasis associated pulmonary arterial hypertension. BMC Pulm Med 2015; 15(1): 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duarte MIS, da Matta VL, Corbett CE, et al. Interstitial pneumonitis in human visceral leishmaniasis. Trans R Soc Trop Med Hyg 1989; 83(1): 73–76. [DOI] [PubMed] [Google Scholar]
- 53.Kuzucu A.Parasitic diseases of the respiratory tract. Curr Opin Pulm Med 2006; 12(3): 212–221. [DOI] [PubMed] [Google Scholar]
- 54.Papamatheakis DG, Mocumbi AOH, Kim NH, et al. Schistosomiasis-associated pulmonary hypertension. Pulm Circ 2014; 4(4): 596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garg MK, Sharma M, Gulati A, et al. Imaging in pulmonary hydatid cysts. World J Radiol 2016; 8(6): 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saksouk FA, Fahl MH, Rizk GK.Computed tomography of pulmonary hydatid disease. J Comput Assist Tomogr 1986; 10(2): 226–232. [DOI] [PubMed] [Google Scholar]
- 57.Emlik D, Ödev K, Poyraz N, et al. Radiological characteristics of pulmonary hydatid cysts. In: Rodriguez-Morales AJ. (ed.) Current Topics in Echinococcosis. IntechOpen, 2015, pp. 148–152. [Google Scholar]
- 58.Man MA, Dantes E, Domokos Hancu B, et al. Correlation between transthoracic lung ultrasound score and HRCT features in patients with interstitial lung diseases. J Clin Med 2019; 8(8): 1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coley BD.Pediatric chest ultrasound. Radiol Clin 2005; 43(2): 405–418. [DOI] [PubMed] [Google Scholar]
- 60.Middleton WD, Teefey SA, Dahiya N.Ultrasound-guided chest biopsies. Ultrasound Q 2006; 22(4): 241–252. [DOI] [PubMed] [Google Scholar]
- 61.von Groote-Bidlingmaier F, Koegelenberg CF. A practical guide to transthoracic ultrasound. Sheffield: European Respiratory Society, 2012. [Google Scholar]
- 62.Abrams ER, Rose G, Fields JM, et al. Point-of-care ultrasound in the evaluation of COVID-19. J Emerg Med 2020; 59(3): 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haak SL, Renken IJ, Jager LC, et al. Diagnostic accuracy of point-of-care lung ultrasound in COVID-19. Emerg Med J 2021; 38(2): 94–99. [DOI] [PubMed] [Google Scholar]
- 64.Giordani MT, Tamarozzi F, Kaminstein D, et al. Point-of-care lung ultrasound for diagnosis of Pneumocystis jirovecii pneumonia: notes from the field. Crit Ultrasound J 2018; 10(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gopaul R, Bearman G, Stevens MP.Ultrasound use in resource-limited settings: a systematic review. J Glob Radiol 2018; 4(1): 5. [Google Scholar]
- 66.Lindoso JAL, Moreira CHV, Cunha MA, et al. Visceral leishmaniasis and HIV coinfection: current perspectives. HIV/AIDS (Auckland, NZ) 2018; 10: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naik H, Abuabara K.Systematic reviews of case reports and case series: from anecdote to evidence. Br J Dermatol 2018; 178(2): 317–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121231177812 for Utility of chest imaging in the diagnosis and management of patients with visceral leishmaniasis: A systematic review by Tesfahunegn Hailemariam, Yonas Mebratu, Tsion Andrias, Fikremariam Melkeneh, Abel Abebe, Biruk Mulualem, Zewdu Abadi, Wazema Desta, Selamawit Bedasso, Fekadu Belay, Abibual Sileshi, Elilta Desta, Felipe Velsaquez-Botero, Rahel Birhane, Francesco Marinucci and Tsegahun Manyazewal in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_20503121231177812 for Utility of chest imaging in the diagnosis and management of patients with visceral leishmaniasis: A systematic review by Tesfahunegn Hailemariam, Yonas Mebratu, Tsion Andrias, Fikremariam Melkeneh, Abel Abebe, Biruk Mulualem, Zewdu Abadi, Wazema Desta, Selamawit Bedasso, Fekadu Belay, Abibual Sileshi, Elilta Desta, Felipe Velsaquez-Botero, Rahel Birhane, Francesco Marinucci and Tsegahun Manyazewal in SAGE Open Medicine
Supplemental material, sj-docx-3-smo-10.1177_20503121231177812 for Utility of chest imaging in the diagnosis and management of patients with visceral leishmaniasis: A systematic review by Tesfahunegn Hailemariam, Yonas Mebratu, Tsion Andrias, Fikremariam Melkeneh, Abel Abebe, Biruk Mulualem, Zewdu Abadi, Wazema Desta, Selamawit Bedasso, Fekadu Belay, Abibual Sileshi, Elilta Desta, Felipe Velsaquez-Botero, Rahel Birhane, Francesco Marinucci and Tsegahun Manyazewal in SAGE Open Medicine