Abstract
Reactive oxygen species (ROS) are critical for plant biological processes. As signaling molecules, ROS regulate plant growth and development through cell expansion, elongation, and programmed cell death. Furthermore, ROS production is induced by microbe-associated molecular patterns (MAMPs) treatment and biotic stresses, and contributes to plant resistance to pathogens. Thus, MAMP-induced ROS production has been an indicator for plant early immune responses or stress responses. One of widely used methods for the measurement is a luminol-based assay to measure extracellular ROS production with a bacterial flagellin epitope (flg22) as a MAMP elicitor. Nicotiana benthamiana is susceptible to a wide variety of plant pathogenic agents and therefore commonly used for ROS measurements. On the other hand, Arabidopsis thaliana, many of genetical lines of which are available, is also conducted to ROS measurements. Tests in an asterid N. benthamiana and a rosid A. thaliana can reveal conserved molecular mechanisms in ROS production. However, the small size of A. thaliana leaves requires many seedlings for experiments. This study examined flg22-induced ROS production in another member of the Brassicaceae family, Brassica rapa ssp. rapa (turnip), which has large and flat leaves. Our experiments indicated that 10 nM and 100 nM flg22 treatments induced high ROS levels in turnip. Turnip tended to have a lower standard deviation in multiple concentrations of flg22 treatment. Therefore, these results suggested that turnip can be a good material from the rosid clade for ROS measurement.
Keywords: Arabidopsis, Brassica rapa ssp. rapa (turnip), luminol-based assay, Nicotiana benthamiana, ROS
Generation of reactive oxygen species (ROS) are required for plant biological processes of development and adaptation to stressful conditions (Huang et al. 2019; Sandalio and Romero-Puertas 2015). ROS, including Singlet oxygen (1O2), superoxide anion (O2·−), hydrogen peroxide (H2O2), and hydroxyl radical (OH·), are induced by aerobic metabolism and stressful conditions. The recognition of microbe-associated molecular patterns (MAMPs) induces immune responses including ROS production (Couto and Zipfel 2016; Qi et al. 2017). One of representative MAMP elicitors is an epitope of bacterial flagellin, such as the 22-amino acid peptide flg22. The recognition of flg22 by the receptor-like kinase FLAGELLIN SENSING2 (FLS2) triggers ROS production in apoplast by NADPH oxidase through the signaling (Kadota et al. 2014). This signaling pathway is conserved across divergent plant taxa (Takai et al. 2008). Since ROS production contributes to the resistance to bacterial and fungal pathogens (Kadota et al. 2014; Yoshioka et al. 2003), monitoring ROS production allows us to probe or tag plant systems involved in early immune responses or stress situations. Hence, measurement of ROS production is important to understand the plant adaptation to stressful environment. For this reason, many studies attempt to track the ROS forms in vivo as well as in vitro. H2O2 is more stable in biological systems and has a longer half-life time than its typical precursor O2·− (Smirnoff and Arnaud 2019; Waszczak et al. 2018). The most basic and widely used method for measurement of ROS production is luminol-based chemiluminescence, in which H2O2 reacts with luminol in the presence of horseradish peroxidase, and produces an unstable intermediate that emits a photon of light (Smith and Heese 2014; Zhu et al. 2016). The photon emission is then measured by using a microplate reader. Most luminol-based assay studies are conducted in leaf discs of Nicotiana benthamiana or Arabidopsis thaliana (Arabidopsis). However, the experimental variability is a common problem that require increasing the number of replications each sample, usually up to 24 replicates per sample (Sang and Macho 2017) or modifying the experimental method to reduce variability while maintaining reliable results (Melcher and Moerschbacher 2016). Although Arabidopsis has been said to demonstrate less intra-experimental variation than N. benthamiana (Sang and Macho 2017), many seedlings are required for reliable measurements because of the small leaf size. These considerations have led to efforts to identify other plant species with large leaf size and low variability as in Arabidopsis. Turnip (Brassica rapa ssp. rapa) is one of the most commercially important vegetables in Europe and Asia and belong to the Brassicaceae family, which includes Arabidopsis. Turnips have various cultivars through breeding, and the history of breeding was inferred from molecular genetics and ancient literature (Kawakatsu et al. 2021; Kubo et al. 2019). Many turnip cultivars have simple and flat leaves without leafy head (Ren et al. 2018), and are larger than Arabidopsis, allowing for the preparation of many leaf discs from a single leaf (Figure 1A). Furthermore, there is much available information about the genetic variation and morphological characteristics of turnip today (Wang et al. 2011; Zhang et al. 2014), which is helpful for further in-depth research.
Figure 1. The overview of this study. (A) Leaf images of N. benthamiana, Arabidopsis and turnip. A biopsy punch with a diameter of 3 mm was used in this study. Bars, 1 cm. Morphological characteristics of each plant species are shown in bottom. (B) A procedure for measuring flg22-induced ROS production with a luminol-based assay.
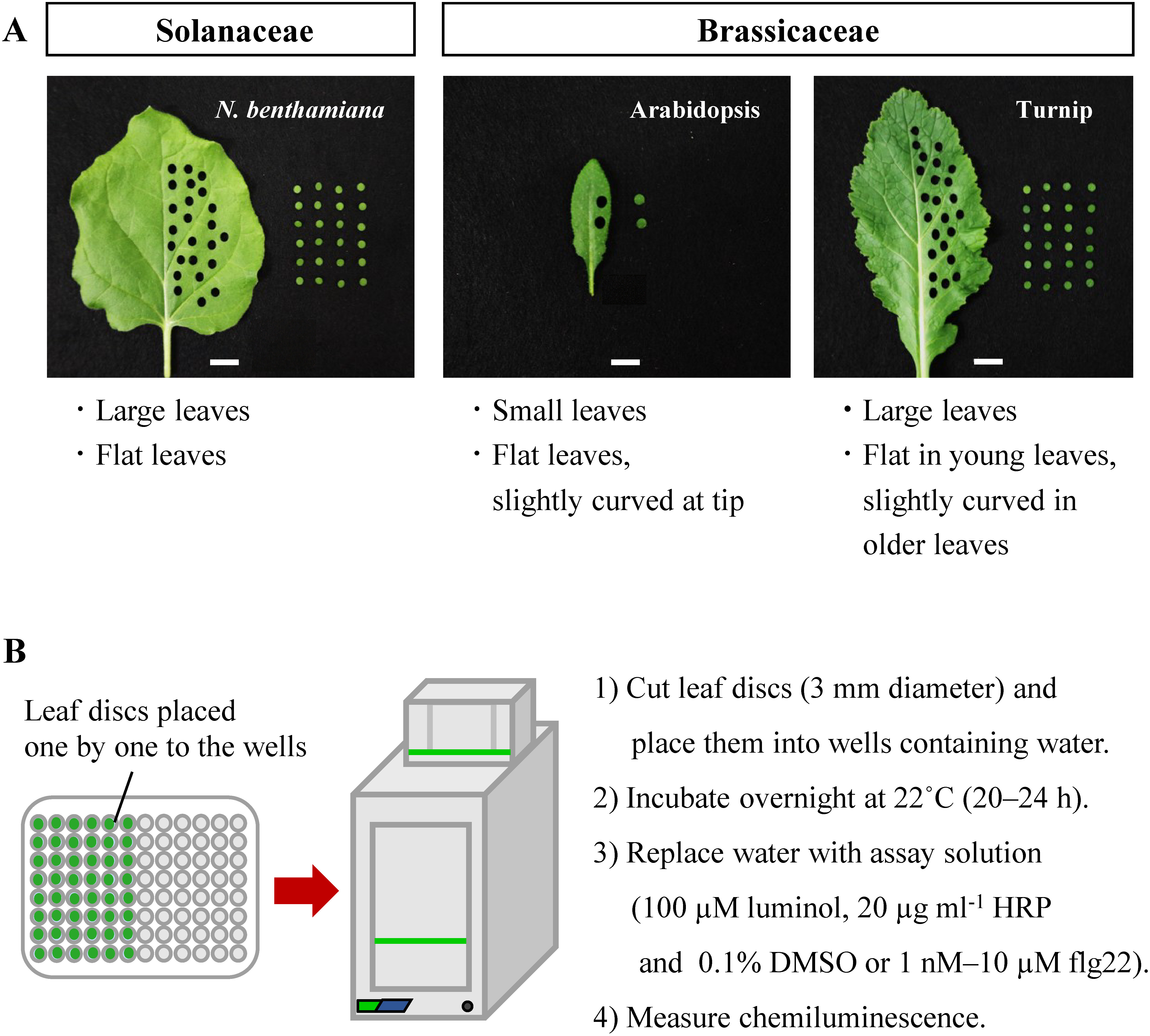
In this study, N. benthamiana (lab strain) and Arabidopsis (Col-0) seeds were sterilized with 6% sodium hypochlorite and 0.02% Triton X-100 for 3 min. Then, seeds were rinsed 5 times with sterilized distilled water. N. benthamiana seeds were sown in a half strength Murashige and Skoog medium containing 3 µg ml−1 thiamine hydrochloride, 5 µg ml−1 nicotinic acid, 0.5 µg ml−1 pyridoxine hydrochloride, 0.5% sucrose and 0.4% gellan gum. Arabidopsis seeds were replaced from the water to 0.1% agar. After that, both seeds were incubated for 3 days at 4°C under the dark. N. benthamiana were grown at 27°C for 7 days on the plate before the transplanting to pots (size: 7.5 cm×7.5 cm) containing the soil mixture of Hana-chan Potting Soil (Hanagokoro Co., Ltd., Aichi, Japan) and vermiculite (Nittai Co., Ltd., Osaka, Japan) in 1 : 1 ratio, whereas Arabidopsis seeds were sown directly in pots (size: 6 cm diameter) containing the soil mixture. Turnip seeds (Murasakihime) was purchased from Nohara Seed Co., Ltd. (Saitama, Japan). These seeds were sown in pots (size: 6 cm diameter) containing the soil mixture without sterilization. All plant species were grown in a growth chamber under continuous light conditions (around 60–70 µmol m−2 s−1): Arabidopsis and turnip were grown at 22°C and 60% humidity, while N. benthamiana was grown at 27°C and 60% humidity. Nutrient water, 1,000-fold diluted solution of WSF Professional Hyponex (HYPONeX JAPAN Co., Ltd., Osaka, Japan), was added to the tray until the soil being wet thoroughly three times a week until use. ROS production was measured with a luminol-based assay, as previously described (Bach-Pages and Preston 2018; Sang and Macho 2017; Smith and Heese 2014), with a few modifications for our tests. Leaf discs (3 mm diameter) were collected from 3rd-4th order leaves from the top of 4-week-old N. benthamiana, 3rd-5th order leaves from the top of 3-week-old Arabidopsis and 2nd-3rd order from the top of 3-week-old turnip. These leaves are flat and do not curl at the leaf margin. These leaf discs were placed in a 96-well plate containing 150 µl of ultrapure water each well. The plate was covered with a plastic lid to prevent water evaporation, and incubated in 22°C overnight. The water was replaced with fresh one after incubation (20–24 h) to remove the injury-indued secondary metabolites, and incubated at room temperature for 1 h. After that, the water was replaced with 100 µl of assay solution (containing 100 µM luminol, 20 µg ml−1 horseradish peroxidase, and 0.1% DMSO or flg22 concentration (0.1% DMSO final concentration) ranging from 1 nM to 10 µM); Figure 1B depicts a brief procedure. Luminescence was measured using the VICTOR Nivo Multimode Plate Reader (PerkinElmer, Inc.) for 60 min with a 1-s measurement time and a 2-min interval time. Eight biological replicates were measured for each treatment, and the experiments were repeated more than three times independently. ROS levels were represented as relative luminescence units (RLU) each time point.
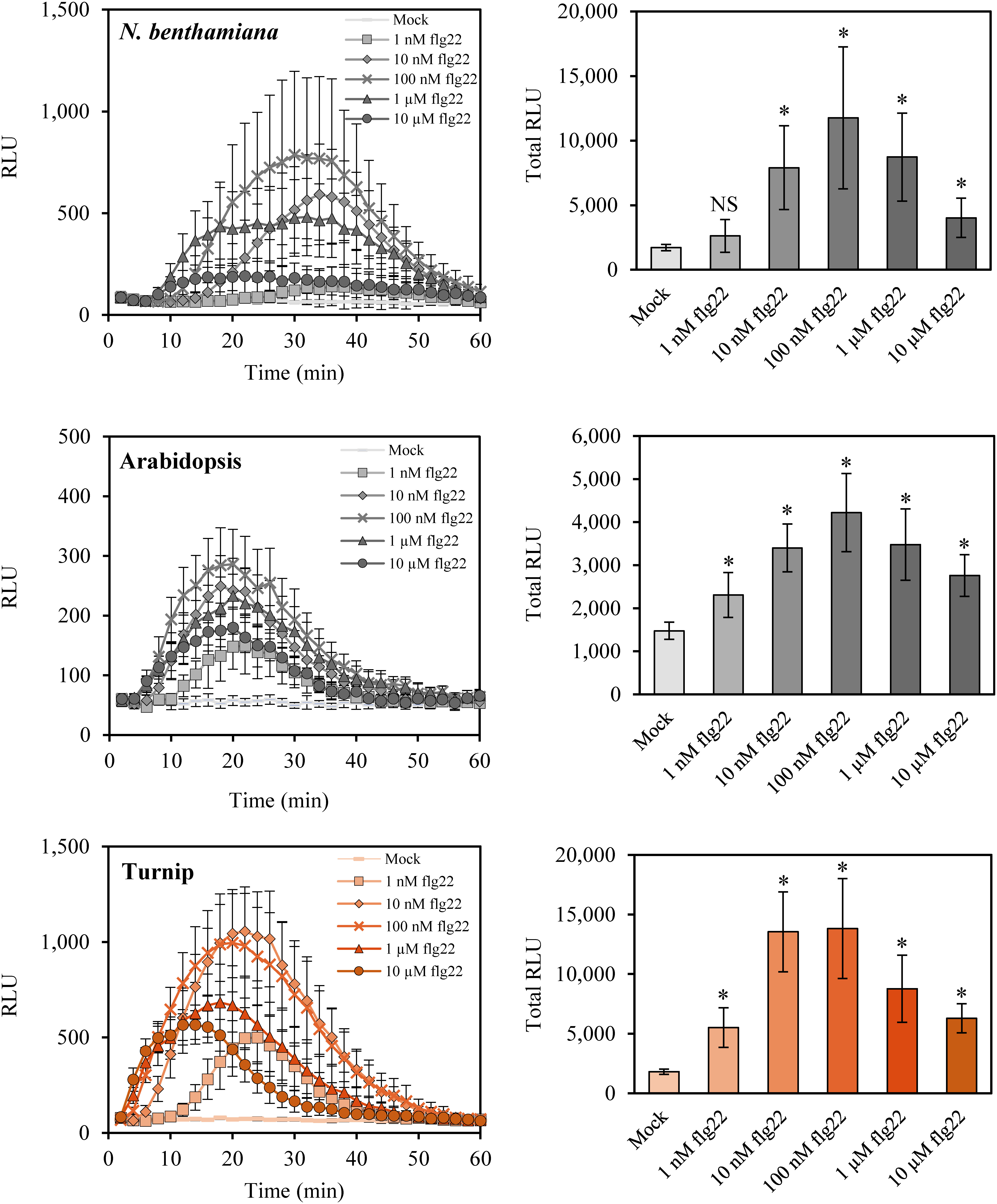
To determine the sensibility of N. benthamiana, Arabidopsis and turnip to flg22, we examined dose-dependent flg22-induced ROS production in these species. The highest RLU and total RLU were detected in 100 nM flg22 treatment in N. benthamiana and Arabidopsis (Figure 2). This result is consistent with previous studies in N. benthamiana and Arabidopsis (Chung et al. 2014; Koller and Bent 2014; Roux et al. 2011; Segonzac et al. 2011; Yeh et al. 2016). In turnip, the peak of RLU was slightly higher in 10 nM than 100 nM flg22 treatment and similar values of total RLU were detected in 10 nM and 100 nM flg22 treatment (Figure 2). In 1 nM lower concentration of flg22 treatment, significant increase of total RLU was detected in Arabidopsis and turnip, but not in N. benthamiana (Figure 2, right). It is noted that the time to increase the RLUs was faster in turnip as the flg22 concentration increased (the time to reach the peak value of RLU after flg22 treatment was earlier in the order of 10 µM, 1 µM, 100 nM, 10 nM, 1 nM). These results suggest that turnip is highly sensitive to flg22 peptide.
Figure 2. ROS production by dose-dependence treatment of flg22 in N. benthamiana, Arabidopsis, and turnip. Left panels show schematic RLU for 60 min after mock or flg22 treatment. Right panels show total RLU for 60 min after mock or flg22 treatment. Concentrations of flg22 are indicated. Data are presented as means±SD (n=8, biological replicates). Asterisks (Student’s t-test, * p<0.01) indicate statistically significant differences to mock treatment. NS, not significant.
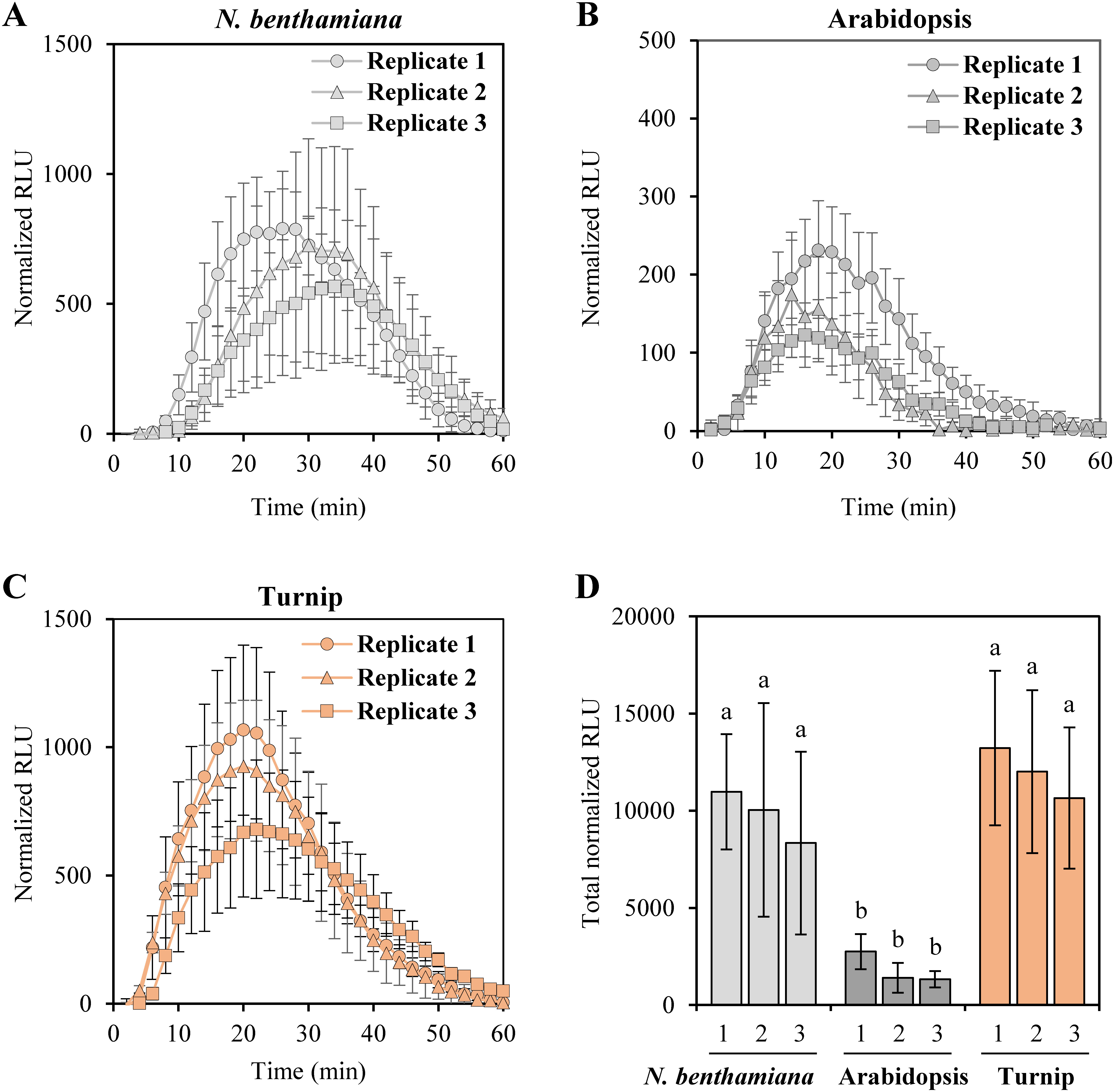
Mock treatment provided the background level of total RLU in all tested species. The background RLU values were varied in individual experiments (Supplementary Figure S1). Therefore, the background RLU value was subtracted from that of flg22 treatment in order to compare the responsibility for the flg22 peptide among species hereafter. Figure 3 shows results from three biological replicates when 100 nM flg22 was treated to N. benthamiana, Arabidopsis and turnip. The data are represented as a normalized RLU (=RLUflg22−RLUmock) and a total normalized RLU. In considering the measurement time for the maximum normalized RLU (t max), it is interesting to note that the t max is 26–34 min in N. benthamiana (Figure 3A), but 14–22 min in Arabidopsis and turnip (Figure 3B, C). This may imply that in N. benthamiana needs more time to induce maximized ROS levels than that in these Brassicaceae species. Treatment of 100 nM flg22 induced high ROS levels in N. benthamiana and turnip, relative to Arabidopsis in three individual tests (Figure 3D). In addition, treatment of 1 nM flg22 induced significantly high ROS levels in turnip compared to N. benthamiana and Arabidopsis (Supplementary Figure S2). This result confirms the high sensibility to flg22 peptide in turnip. Turnip has higher ROS levels and higher standard deviations than Arabidopsis in the overall results (Figure 3D, Supplementary Figure S2). Important notion is that, even with higher ROS levels than N. benthamiana in 10 nM or 100 nM, turnip appeared to have low standard deviations (Figure 3D, Supplementary Figure S2). Hence, turnip is a potential useful plant to measure flg22-induced ROS production.
Figure 3. Normalized RLU after removal of background when 100 nM flg22 was treated to N. benthamiana, Arabidopsis, and turnip. (A–C) Normalized RLU for 60 min after 100 nM flg22 treatment in triplicate experiments of N. benthamiana (A), Arabidopsis (B), and turnip (C). For each time point, normalized RLU value was obtained by subtraction of the background RLU value of mock treatment from the RLU value of 100 nM flg22 treatment. Each graph shows three individual tests. (D) Total normalized RLU of the three plant species. Each number under the horizontal axis indicates the replicate number of (A–C). Data are presented as average±SD (n=8, biological replicates). Significance was analyzed by one-way ANOVA and Tukey HSD test. Different letters indicate significant differences (p<0.05).
The luminol-based assay are severely affected by environment factors related with plant growth condition. Although previous studies suggested that N. benthamiana is preferred to grow under long day conditions and Arabidopsis is preferred to grow under short-day conditions (Nie et al. 2021; Sang and Macho 2017; Segonzac et al. 2011), all three species were grown under the same coninuous light conditions in this study for comparison. But, in future studies, each plant can be grown in preferable light conditions respectively. Another caring point is that the instruments used to measure chemiluminescence, the type of microplate reader used by each researcher, impact the efficacy of the results (Mott et al. 2018). In our experiments, some significant level of RLU background was detected in no flg22 (mock) treatment.
In our study, turnip showed more senstivity to flg22 and higher ROS elevation than Arabidopsis of the same family (Figures 2, 3, Supplementary Figure S2). Testing other Brassicaceae species may exhibit more different sensitivity to flg22 and be useful as experimental materials. The different sensibility to flg22 among different species was previously reported in Leguminosae family (Wei et al. 2020). In addition, although different sensitivity was also found in tomato accessions, high sensitibility to flg22 was not correlated with the resistance to pathogens (Roberts et al. 2019). Since ROS production is one output of flg22-induced defense responses, the measurement of other defense responses is needed to evaluate the effect of ROS production on resistance to pathogen in turnip.
Furthermore, it also appeared that turnip tends to have low data variances in ROS measurement despite high ROS production in our experiments. This nature is favorable when we investigate the effect of various biomolecular substances or gene knockout of interest on flg22-induced ROS accumulation. In conclusion, turnip from Brassicaceae family can be used as a plant material for measuring flg22-induced ROS production.
Acknowledgments
We thank Dr. Shuta Asai for technical advices on ROS measurment.
Abbreviations
- flg22
flagellin epitope
- MAMPs
microbe-associated molecular patterns
- RLU
relative luminescence units
- ROS
reactive oxygen species
Conflict of interest
The authors declare no conflict of interest.
Author contribution
L.J., Y.K., and M.N. conceived this study. Y.K. selected plant materials. L.J. designed and conducted the main experiments with supports from K.O. and K.K. L.J. and K.O. wrote a draft manuscript. K.K. and M.N. edited and prepared the paper.
Funding
This work was supported by grants from the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (20H03273 to K.K. and M.N., 21H00368 and 21H05657 to M.N.), the Japan Science and Technology Agency (JPMJTR194G to M.N.), and the New Energy and Industrial Technology Development Organization (JPNP20004 to M.N.).
Supplementary Data
References
- Bach-Pages M, Preston GM (2018) Methods to quantify biotic-induced stress in plants. Methods Mol Biol 1734: 241–255 [DOI] [PubMed] [Google Scholar]
- Chung EH, El-Kasmi F, He Y, Loehr A, Dangl JL (2014) A plant phosphoswitch platform repeatedly targeted by type III effector proteins regulates the output of both tiers of plant immune receptors. Cell Host Microbe 16: 484–494 [DOI] [PubMed] [Google Scholar]
- Couto D, Zipfel C (2016) Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol 16: 537–552 [DOI] [PubMed] [Google Scholar]
- Huang H, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10: 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, et al. (2014) Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 54: 43–55 [DOI] [PubMed] [Google Scholar]
- Kawakatsu Y, Sakamoto T, Nakayama H, Kaminoyama K, Igarashi K, Yasugi M, Kudoh H, Nagano AJ, Yano K, Kubo N, et al. (2021) Combination of genetic analysis and ancient literature survey reveals the divergence of traditional Brassica rapa varieties from Kyoto, Japan. Hortic Res 8: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller T, Bent AF (2014) FLS2-BAK1 extracellular domain interaction sites required for defense signaling activation. PLoS One 9: e111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo N, Ueoka H, Satoh S (2019) Genetic relationships of heirloom turnip (Brassica rapa) cultivars in Shiga Prefecture and other regions of Japan. The Horticulture Journal 88: 471–480 [Google Scholar]
- Melcher RL, Moerschbacher BM (2016) An improved microtiter plate assay to monitor the oxidative burst in monocot and dicot plant cell suspension cultures. Plant Methods 12: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott GA, Desveaux D, Guttman DS (2018) A high-sensitivity, microtiter-based plate assay for plant pattern-triggered immunity. Mol Plant Microbe Interact 31: 499–504 [DOI] [PubMed] [Google Scholar]
- Nie J, Zhou W, Liu J, Tan N, Zhou JM, Huang L (2021) A receptor-like protein from Nicotiana benthamiana mediates VmE02 PAMP-triggered immunity. New Phytol 229: 2260–2272 [DOI] [PubMed] [Google Scholar]
- Qi J, Wang J, Gong Z, Zhou JM (2017) Apoplastic ROS signaling in plant immunity. Curr Opin Plant Biol 38: 92–100 [DOI] [PubMed] [Google Scholar]
- Ren W, Wang H, Bai J, Wu F, He Y (2018) Association of microRNAs with types of leaf curvature in Brassica rapa. Front Plant Sci 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Mainiero S, Powell AF, Liu AE, Shi K, Hind SR, Strickler SR, Collmer A, Martin GB (2019) Natural variation for unusual host responses and flagellin-mediated immunity against Pseudomonas syringae in genetically diverse tomato accessions. New Phytol 223: 447–461 [DOI] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalio LM, Romero-Puertas MC (2015) Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann Bot (Lond) 116: 475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y, Macho AP (2017) Analysis of PAMP-triggered ROS burst in plant immunity. Methods Mol Biol 1578: 143–153 [DOI] [PubMed] [Google Scholar]
- Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen JP (2011) Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol 156: 687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221: 1197–1214 [DOI] [PubMed] [Google Scholar]
- Smith JM, Heese A (2014) Rapid bioassay to measure early reactive oxygen species production in Arabidopsis leave tissue in response to living Pseudomonas syringae. Plant Methods 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai R, Isogai A, Takayama S, Che FS (2008) Analysis of flagellin perception mediated by flg22 receptor OsFLS2 in rice. Mol Plant Microbe Interact 21: 1635–1642 [DOI] [PubMed] [Google Scholar]
- Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun JH, Bancroft I, Cheng F, et al.; Brassica rapa Genome Sequencing Project Consortium (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43: 1035–1040 [DOI] [PubMed] [Google Scholar]
- Waszczak C, Carmody M, Kangasjärvi J (2018) Reactive oxygen species in plant signaling. Annu Rev Plant Biol 69: 209–236 [DOI] [PubMed] [Google Scholar]
- Wei Y, Balaceanu A, Rufian JS, Segonzac C, Zhao A, Morcillo RJL, Macho AP (2020) An immune receptor complex evolved in soybean to perceive a polymorphic bacterial flagellin. Nat Commun 11: 3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YH, Panzeri D, Kadot Y, Huang YC, Huang PY, Tao CN, Roux M, Chien HC, Chin TC, Chu PW, et al. (2016) The arabidopsis malectin-like/LRR-RLK IOS1 is critical for BAK1-dependent and BAK1-independent pattern-triggered immunity. Plant Cell 28: 1701–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15: 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Zhao J, Lens F, de Visser J, Menamo T, Fang W, Xiao D, Bucher J, Basnet RK, Lin K, et al. (2014) Morphology, carbohydrate composition and vernalization response in a genetically diverse collection of Asian and European turnips (Brassica rapa subsp. rapa). PLoS One 9: e114241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Jia Z, Trush MA, Li YR (2016) A highly sensitive chemiluminometric assay for real-time detection of biological hydrogen peroxide formation. React Oxyg Species (Apex) 1: 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


