ABSTRACT
Pancreatic β-cell dysfunction is a key factor in the development of type 2 diabetes. Pancreatic β-cell senescence accelerates abnormal glucose metabolism, which decreases insulin secretion and cell regeneration ability, eventually leading to diabetes. A cholesterol oxidation product, 7-ketocholesterol (7-KC) can affect pancreatic β-cell function. However, its role in pancreatic β-cell senescence has not been reported. We investigated the role of 7-KC in pancreatic β-cell senescence and its underlying molecular mechanism in MIN6 cells. MIN6 cells were treated with 25 μmol/L 7-KC for 24 h and the proportion of senescent cells was detected based on senescence-associated β-galactosidase (SA-β-gal) activity. The cell cycle, DNA damage, and the senescence-associate secretory phenotype (SASP) and protein expression were detected by flow cytometry, immunofluorescence, and western blotting, respectively. 7-KC can significantly increase SA-β-gal activity, promoted G0/G1 arrest, DNA damage, and interleukin-1β expression in MIN6 cells and significantly inhibited insulin synthesis. Further studies indicated that 7-KC induced β-cell senescence by inhibiting the SIRT1/CDK4–Rb – E2F1 signaling pathway.
KEYWORDS: 7-Ketocholesterol, cell cycle, diabetes mellitus, lipotoxicity, pancreatic β-cells, senescence, SIRT1
Graphical abstract
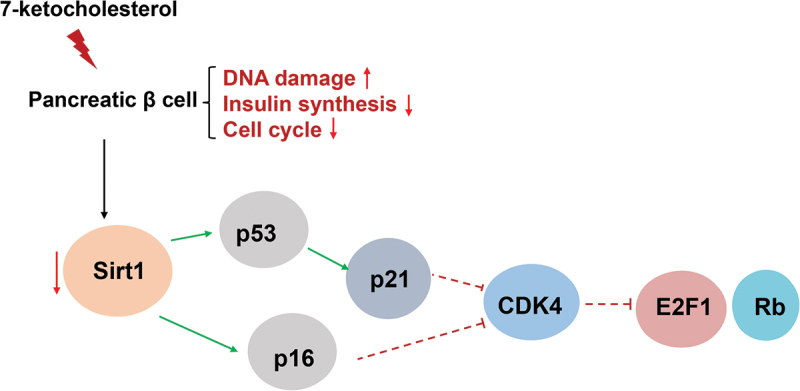
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease characterized by insulin resistance and pancreatic β-cell dysfunction. Obesity and abnormal glucose and lipid metabolism can cause pancreatic β-cell senescence and lead to β-cell dysfunction.1 Increased numbers of senescent pancreatic β-cells accelerate the gradual deterioration of β-cell function and cell secretion dysfunction.2 Moreover, senescence-associated secretory phenotype (SASP) secreted by senescent cells can trigger chronic, low-level, systemic inflammation that accelerates the pancreatic β-cell senescence process.3
Pancreatic β-cell lipotoxicity and glucolipotoxicity may contribute to pancreatic β-cell senescence. Previously, we demonstrated that cholesterol metabolism disorder inhibited glucose-stimulated pancreatic β-cell insulin secretion.4,5 Excess cholesterol reduced glucose transporter activity to influence glucose uptake, reduce glucokinase activity, and inhibit glycolysis.6–9 Cholesterol is converted to other products via oxidation/reduction in vivo, where 7-ketocholesterol (7-KC) is the main oxidation product following auto-oxidation. 7-KC demonstrates extensive toxicity and is difficult to metabolize for most cells. Consequently, 7-KC accumulation in organisms can damage cell and tissue functional activity10–13 and is directly related to many diseases, including metabolic disease, atherosclerosis, heart failure, age-related macular degeneration, and Alzheimer disease.14,15 Non-alcoholic fatty liver disease is caused by hepatocyte ballooning, inflammation, and steatosis due to the accumulation of oxysterols, specifically 7-KC.16,17 7-KC is toxic to macrophages, where it promotes inflammation via the LXR and hepatocyte nuclear factor 4 (HNF-4) signaling pathway in atherosclerotic plaques16,18 7-KC induces peroxisomal disorders in glial, microglial, and neuronal cells and induces mitochondrial dysfunctions, oxidative stress, and inflammation, which promote neurodegeneration.19,20 However, the role of 7-KC in pancreatic β-cell senescence has not been reported.
Based on the above reports, we examined whether 7-KC affects pancreatic β-cell senescence and elucidated the mechanism of β-cell senescence. Our results indicated that 7-KC accelerated pancreatic β-cell senescence, which occurred via SIRT1/CDK4–Rb – E2F1 signaling pathway inhibition.
Materials and methods
Reagents and antibodies
The hydrogen peroxide (H2O2) was from Sigma-Aldrich (St. Louis, MO, USA). The 7-KC was from Sigma-Aldrich and dissolved in 45% (w/v) 2-hydroxypropyl-β-cyclodextrin solution (Sigma-Aldrich). Type-V collagenase (C9263) was fromSigma-Aldrich (St. Louis, MO, USA). SA-β-gal activity kit was from GenMed Scientific (Shanghai, China). Cell cycle and apoptosis analysis kit was from Beyotime (Shanghai, China). The antibodies against CDKN2A/p16INK4a (ab211542), γ-H2A.X (ab81299), p21 (ab88224), SIRT1 (ab189494), CDK4 (ab68266), and phosphorylated retinoblastoma (p-Rb) protein (ab184796) were from Abcam (Cambridge, UK). The antibodies against E2F1 (A19579), insulin (a19066) were from ABclonal (Wuhan, China). The anti-p53 antibody (10442–1-AP) was from Proteintech (Wuhan, China). The anti-p-p38MAPK antibody (4511) was from Cell Signaling Technology (Boston, MA, USA). The anti-β-gal antibody (A11132) was from Thermo Fisher Scientific (Waltham, MA, USA).
Cell culture
The 35th to 38th generation MIN6 cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium containing 15% fetal bovine serum, 1% penicillin/streptomycin, and 0.5‰ β-mercaptoethanol at 37°C in a humidified atmosphere under 5% CO2 for 16 h. Then, the cells were exposed to 25 μmol/L 7-KC according to our previous study before they were harvested. The positive control was 150 μmol/L H2O2.21
Islet isolation
The mice were anesthetized and immediately process for pancreatic perfusion with 3 mL fresh type-V collagenase dissolved in HBSS (Hank’s Buffered Salt Solution). The perfused pancreas was quickly incubated at 37°C for 28 min, and terminated by adding HBSS buffer containing 10% FBS, then the islets were purified by density gradient centrifugation. The islets were picked by hand under an anatomical microscope. After washing with HBSS, the islets were cultured in RPMI-1640 medium with 10% FBS overnight. Then, the islets were exposed to different concentrations 7-KC for 24 h.
SA-β-gal assay
MIN6 cells were seeded on 6-well plates and incubated as described in the Cell Culture section. After 24-h incubation in the fixative solution provided in the commercial kit, cell senescence was assessed by X-gal staining for detecting β-gal activity. Images were recorded with an optical microscope (Carl Zeiss, USA), where stained cells in 10 different fields were counted and the percentage of positive cells was calculated by Image J software.
Cell cycle determination
MIN6 cells were seeded on 6-well plates and incubated as described in the Cell Culture section. Subsequently, cycle analysis was performed as per the manufacturer’s instructions using cell cycle and apoptosis detection reagents. The percentage of apoptotic cells was quantified by measuring G1 cell levels using flow cytometry (BD bioscience, USA) and ModFit LT software.
Immunofluorescence assay
MIN6 cells were plated on cell slides in 6-well plates and incubated as described in the Cell Culture section. Then, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100/phosphate-buffered saline (PBS) and blocked with 5% bovine serum albumin (BSA) for 60 min. The cells on coverslips were incubated overnight at 4°C with the primary antibodies. After several washes in PBS, the samples were incubated with the fluorescent secondary antibody (1:200 dilution; Invitrogen, Carlsbad, CA, USA) at room temperature for 60 min. After rinsing with PBS, the samples were stained for 15–30 min with 4′, 6-diamidino-2-phenylindole (DAPI) working solution. After mounting with fluorescence decay-resistant medium, the cells were observed and photographed under a confocal microscope (IX83-FV3000, Olympus, Tokyo, Japan).
Western blotting
The MIN6 cells or islets were lysed on ice with cell lysis buffer and the protein concentration was determined by a bicinchoninic acid kit (FdBio Science, Hangzhou, China). Then, the sample underwent 10% or 15% sodium dodecyl sulfate – polyacrylamide gel electrophoresis and were transferred to polyvinylidene fluoride membranes. After incubation with BSA, primary antibodies were added to the sample and incubated at 4°C overnight. Subsequently, the sample was incubated for 2 h with horseradish peroxidase-conjugated secondary antibodies at room temperature. Then, the protein was detected by a chemiluminescence ECL kit (FdBio Science) and the protein bands were analyzed by ImageJ.
Statistical analysis
The experimental data are expressed as the mean ± SEM. The data were statistically analyzed with SPSS 20 and plotted with GraphPad Prism 5. The experiment in each group was repeated at least three times independently, and the statistical significance of the difference between the experimental and control groups was determined by an independent sample t-test. P < 0.05 was set as the threshold for statistical significance.
Results
7-KC significantly induced senescence in pancreatic β cell
As β-gal is a characterized senescence marker, we detected its activity in 7-KC-treated MIN6 cells. Aguayo-Mazzucato et al. demonstrated that H2O2 induced β-cell senescence and led to the loss of β-cell identity22 . Accordingly, we used H2O2 as the positive control in our study. In this study, the positive rate of SA-β-gal in the normal control (NC), 7-KC, and H2O2 groups was 8.1% ± 1.4%, 98.7% ± 4.6%, and 58.6% ± 3.6%, respectively (Figures 1A, B). Compared with the NC group, the 7-KC and H2O2 groups exhibited obviously enhanced SA-β-gal staining (P < 0.01), where the 7-KC group had more significantly enhanced staining than the H2O2 group (P < 0.05). Moreover, we detected the β-gal expression in islets. The results showed the β-gal expression was increased in 25 and 50 μmol/L 7-KC treatment group (Figures 1G, H).
Figure 1.
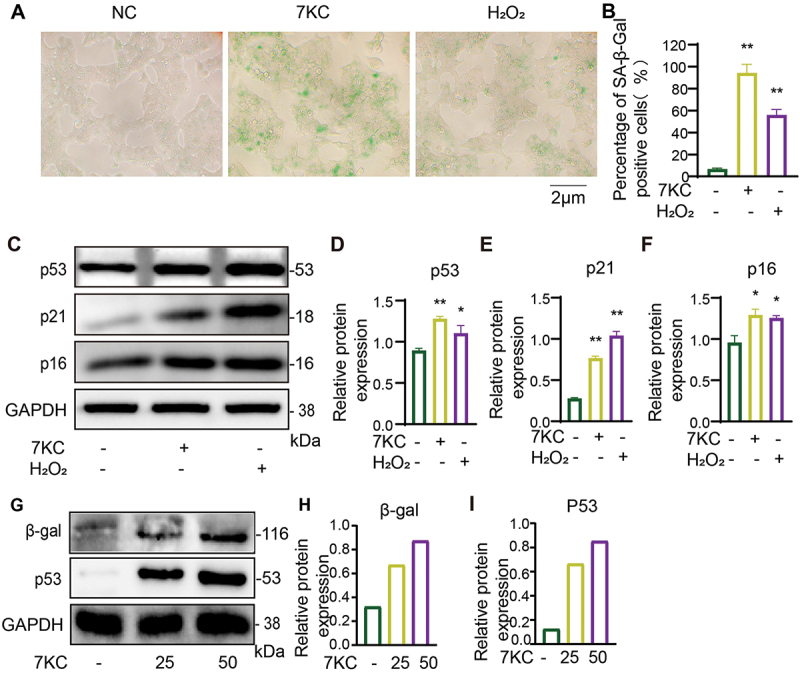
7-KC significantly induced senescence in pancreatic β cell. (A, B) SA-β-gal activity detection after 7-KC treatment in MIN6 cell. (C – F) p53, p21, and p16 expression after 7-KC or H2O2 treatment in MIN6 cell. (G – I) β-gal and p53 expression after 25 and 50 μmol/L 7-KC treatment in islets. **P < 0.01, *P < 0.05 vs. Normal control.
The senescence staining, senescence markers p53, p21 and p16 were detected by SA-gal staining and Western blot after 7-KC or H2O2 treatment in pancreatic β cell for 24 h. (A, B) SA-β-gal activity detection after 7-KC or H2O2 treatment in MIN6 cell for 24 h. (C – F) p53, p21, and p16 expression after 7-KC or H2O2 treatment in MIN6 cell for 24 h. Normal control (2-hydroxypropyl-β-cyclodextrin solution); 7-KC (25 μmol/L 7-KC in 2-hydroxypropyl-β-cyclodextrin solution); H2O2(150 μmol/L H2O2+2-hydroxypropyl-β-cyclodextrin solution). (G – I) β-gal and p53 expression after 25 and 50 μmol/L 7-KC treatment in islets for 24 h. **P < 0.01, *P < 0.05 vs. Normal control.
The p53–p21 and p16INK4a – Rb axes establish and maintain cell senescence.23 The p53–p21 axis initiates the aging process while p16INK4a activation maintains the aging state.22 Senescent cells contain increased levels of the senescence-related proteins p16, p53, and p21.24 To study the effect of 7-KC on pancreatic β-cell senescence, we detected the expression of senescence-related proteins in each group with western blotting. 7-KC significantly upregulated p53, p21, and p16 expression in the pancreatic MIN6 cells as compared with that in the NC group (Figure 1C-F). And the p53 expression in islets was increased at 25 and 50 μmol/L 7-KC treatment group, the result accorded with in MIN6 cell (Figures 1G, I).
7-KC significantly arrested the cell cycle of MIN6 cells
Cellular senescence is a terminal state of growth arrest where cells cannot reenter the cell cycle despite mitogenic growth signals, which is termed cell cycle arrest.25 To verify the effect of 7-KC on the cell cycle, we detected the proportion of G1-, G2-, and S-phase MIN6 cells by flow cytometry. Compared with the NC, 7-KC significantly increased G0/G1 cells and decreased the proportion of S-phase cells. The proportion of G1 MIN6 cells was increased in both the 7-KC and H2O2 groups as compared with those in the NC group, but there was no significant difference between the 7-KC and H2O2 groups (Figures 2A, B). These findings suggested that 7-KC inhibited cell proliferation by inhibiting the cell cycle.
Figure 2.
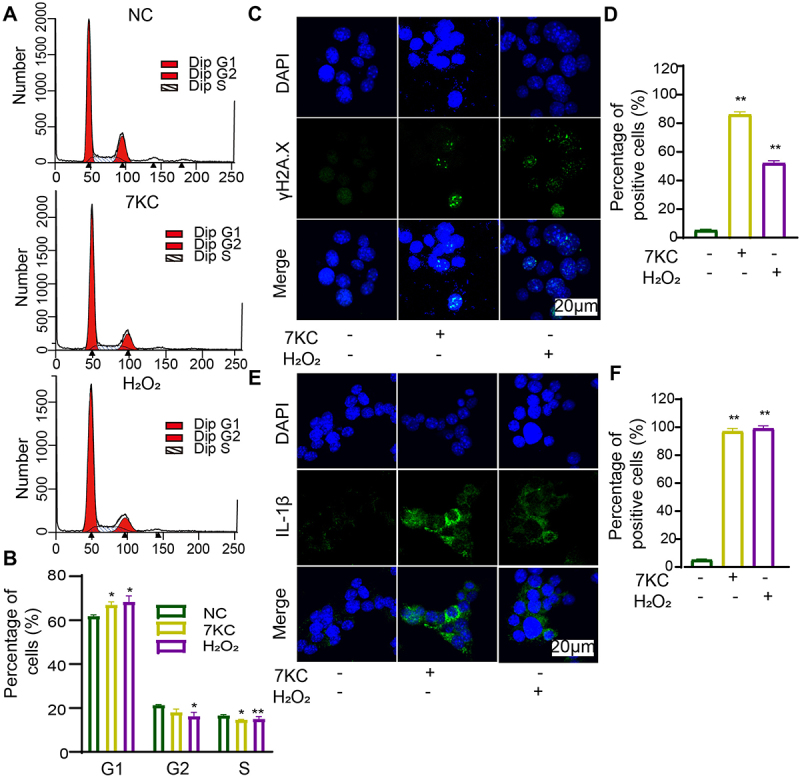
7-KC or H2O2 significantly promoted the senescence phenotype in MIN6 cells. (A, B) Flow cytometry cell cycle analysis after 7-KC or H2O2 treatment. (C, D) Immunofluorescence detection of γ-H2A.X expression. (E, F) Immunofluorescence detection of IL-1β expression. **P < 0.01, *P < 0.05 vs. Normal control.
The cell cycle was inhibited, γ-H2A.X and IL-1β expression were increased after 7-KC or H2O2 treatment. (A, B) Flow cytometry cell cycle analysis after 7-KC or H2O2 treatment. (C, D) Immunofluorescence detection of γ-H2A.X expression. (E, F) Immunofluorescence detection of IL-1β expression. Normal control (2-hydroxypropyl-β-cyclodextrin solution); 7-KC (25 μmol/L 7-KC in 2-hydroxypropyl-β-cyclodextrin solution); H2O2(150 μmol/L H2O2+2-hydroxypropyl-β-cyclodextrin solution). **P < 0.01, *P < 0.05 vs. Normal control.
7-KC significantly increased the phosphorylation of histone variant H2A.X (γ-H2A.X) in MIN6 cells
Cell senescence can also be characterized by increased the phosphorylation of histone variant H2A.X (γ-H2A.X).26 The γ-H2A.X level can clearly reflect the degree of DNA damage and DNA repair. (Figures 2C , 2D) depict the γ-H2A.X expression level detected by immunofluorescence staining. The proportion of γ-H2A.X-positive cells in the NC, 7-KC, and H2O2 groups was 5.2% ± 1.2%, 86% ± 3.4%, and 52% ± 3.1%, respectively. Our findings demonstrated that 7-KC exposure significantly promoted DNA double-strand break in pancreatic MIN6 cells.
7-KC significantly promoted inflammatory cytokine expression in MIN6 cells
The SASP accompanies the permanent cell cycle arrest of senescent cells and consists of proinflammatory factors such as cytokines, chemokines, and extracellular matrix remodeling factors. The proinflammatory cytokines consist of interleukin IL-1α, IL-1β, IL-6, and IL-8.5 The above results demonstrated that 7-KC arrested the cell cycle of β-cells. Subsequently, we studied the effect of 7-KC on inflammatory cytokine expressed from MIN6 cells, where the proportion of IL-1β-positive cells was detected by immunofluorescence. The percentages of IL-1β-positive cells among the NC-, 7-KC-, and H2O2-treated MIN6 cells were 4.7% ± 1.1%, 97% ± 3.8%, and 99% ± 3.5%, respectively (Figures 2E, F). The results demonstrated that 7-KC significantly promoted IL-1β expression in the pancreatic β-cells.
7-KC significantly inhibited MIN6 cell insulin expression
The earlier results demonstrated that 7-KC caused β-cell senescence and attenuated β-cell insulin secretion (unpublished data). To confirm the effect of 7-KC on insulin secretion, we detected β-cell insulin expression with immunofluorescence. The positive rates of insulin expression in the NC, 7-KC, and H2O2 groups were 99.2% ± 4.2%, 67% ± 3.7%, and 65% ± 3.4%, respectively (Figure 3). Compared with the NC, 7-KC significantly downregulated insulin expression, but there was no significant difference between the effects of 7-KC and that of H2O2.
Figure 3.
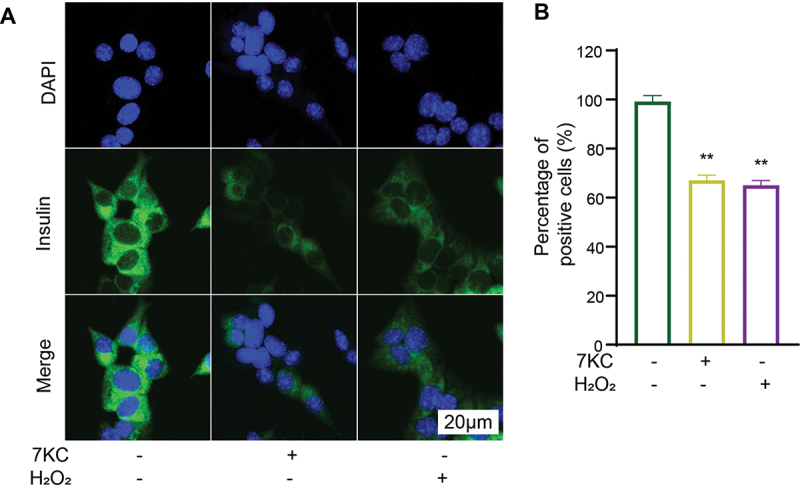
7-KC or H2O2 significantly inhibited insulin expression in MIN6 cells. (A, B) Immunofluorescence detection of insulin expression. **P < 0.01 vs. Normal control.
The immunofluorescence staining showed the 7-KC or H2O2 significantly inhibited insulin expression in MIN6 cells. (A, B) Immunofluorescence detection of insulin expression. Normal control (2-hydroxypropyl-β-cyclodextrin solution); 7-KC (25 μmol/L 7-KC in 2-hydroxypropyl-β-cyclodextrin solution); H2O2(150 μmol/L H2O2+2-hydroxypropyl-β-cyclodextrin solution). **P < 0.01 vs. Normal control.
7-KC caused MIN6 cell senescence possibly through the SIRT1–CDK4–Rb – E2F1 signaling pathway
SIRT1 plays a major role in metabolic disease attenuation, lifespan extension, antioxidation, and cellular senescence inhibition.27 SIRT1 activation protected pancreatic β-cells against palmitate-induced dysfunction.28 Zhang et al. reported that SIRT1 deficiency impaired normal cell cycle progression and that cyclin B1 and p-CHK2 expression was increased in SIRT1−/− brains.29 Many CDK and cyclin complexes control the cell cycle. The cyclin D – CDK4/6 complex phosphorylates the Rb C-terminal to prevent active transcription. When stimulated by mitosis, cyclin D – CDK4/6 initiates the phosphorylation of pocket proteins, leading to the destruction of the E2F/pocket protein inhibitory complex and the nuclear output of E2F.30 Figure 4 demonstrates that 7-KC significantly inhibited SIRT1 expression, which agreed with the β-gal staining results. Moreover, CDK4, EZH2, and E2F1 expression and Rb phosphorylation were inhibited while γ-H2A.X expression and p38MAPK phosphorylation (Figure 4) were upregulated in the MIN6 cells. These results suggested that 7-KC regulated the MIN6 cell senescence process through the SIRT1–CDK4–Rb – E2F1 signal pathway.
Figure 4.
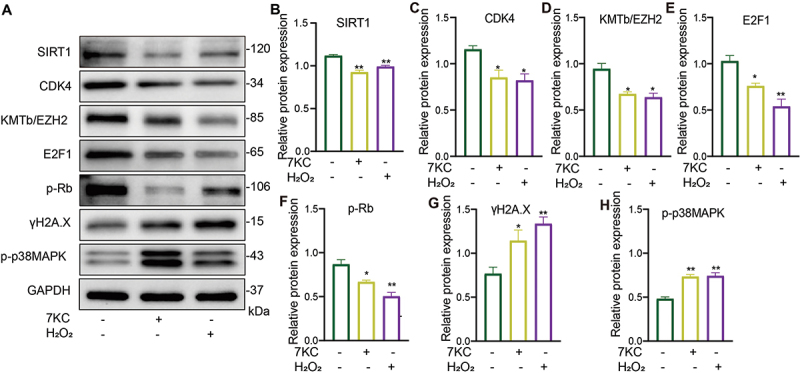
7-KC or H2O2 caused MIN6 cell senescence through the SIRT1–CDK4–Rb – E2F1 signaling pathway. (A – H) MIN6 cells were treated with 7-KC or H2O2 for 24 h, then SIRT1 (B), CDK4 (C), KMTb/EZH2 (D), E2F1 (E), p-Rb (F), γ-H2A.X (G), and p-p38MAPK (H) expression was detected by western blotting (A). *P < 0.05, **P < 0.01 vs. Normal control.
The protein expression showed that 7-KC or H2O2 caused MIN6 cell senescence through the inhibiting SIRT1 and CDK4–Rb – E2F1 signaling pathway. (A – H) MIN6 cells were treated with 7-KC or H2O2 for 24 h, then SIRT1 (B), CDK4 (C), KMTb/EZH2 (D), E2F1 (E), p-Rb (F), γ-H2A.X (G), and p-p38MAPK (H) expression was detected by western blotting (A). Normal control (2-hydroxypropyl-β-cyclodextrin solution); 7-KC (25 μmol/L 7-KC in 2-hydroxypropyl-β-cyclodextrin solution); H2O2(150 μmol/L H2O2 in 2-hydroxypropyl-β-cyclodextrin solution). *P < 0.05, **P < 0.01 vs. Normal control.
Discussion
The loss of functional β-cells is the key mechanism leading to diabetes. Senescent β-cells accumulate during obesity and aging. Pancreatic β-cell senescence was observed in a high-fat-fed mouse model and was followed by insufficient insulin release.31 Using oral ABT263 to remove senescent β-cells improved glucose metabolism and β-cell function in high-fat diet C57BL/6 mice.22 These studies suggested that lipotoxicity caused the β-cell senescence. Previous studies also demonstrated that cholesterol accumulation damaged β-cell function. Cholesterol accumulation affects the metabolic process of glucose-stimulated insulin release. Patients with T2DM present a cluster of lipid abnormalities associated with cholesterol and fatty acid accumulation in pancreatic β-cells, which may contribute to islet degeneration.6 7-KC is the main cholesterol auto-oxidation product, where accumulated 7-KC can exert various adverse effects and is associated with many diseases. Our results demonstrated that 7-KC clearly increased the proportion of β-gal cells and the senescence markers p16, p21, and p53 were increased in the 7-KC group. Many studies demonstrated that H2O2 is the classical model for inducing cell senescence22,32,33 and β-gal staining and protein expression confirmed that H2O2 induced cell senescence. Compared with H2O2, 7-KC accelerated β-cell senescence in an obvious manner. The results suggested that 7-KC affects β-cell function and may accelerates the T2DM process.
Senescent cells are characterized by increased cell size and lysosome content, which upregulate β-gal activity and DNA damage, inhibiting cell proliferation, arresting the cell cycle, and upregulating CDKN2A/1A (encoding p16INK4a and p21, respectively), anti-apoptotic molecules (Bcl-2, Bcl-xL, Bcl-w), and SASP factors.22,34–36 The DNA damage level increases with senescence while DNA repair ability decreases, rendering β-cells more prone to senescence-related cell cycle arrest and the DNA damage response.37 Multiple proteins regulate the cell cycle. p16 is a cyclin kinase inhibitor that participates in cell cycle regulation and competes with cyclin D1 (cyclin D1) to bind CDK4 and CDK6, specifically inhibiting CDK4 activity, preventing S phase entry and DNA synthesis initiation, and inhibiting Rb protein phosphorylation.38 Unphosphorylated Rb binds to E2F1 to inhibit the activation of E2F1 and cell proliferation.39,40 EZH2 downregulation in aging pancreatic β-cells leads to the activation of p16INK4a and p19ARF sites and decreased pancreatic β-cell function.41 Our results demonstrated that 7-KC treatment of MIN6 cells promoted G0/G1 arrest, which was regulated by inhibiting CDK4, E2F1, and EZH2, and Rb phosphorylation. Furthermore, the phosphorylation level of the histone γ-H2A.X, a DNA damage marker, was increased in an obvious manner. Senescent cells trigger profound phenotypic changes, such as the production of a bioactive secretome, referred to as the SASP.42 Our results demonstrated that 7-KC treatment increased IL-1β levels. The results are in accordance with reports that IL-1β was increased in β-cells and was an independent predictor of diabetes.21,43,44
SIRT1 is strongly implicated in obesity and diabetes. SIRT1 decreases the inflammatory process by affecting the NF-κB pathway. In Sirt1 knockout mice, high-fat diet lead to upregulated insulin resistance.45 SIRT1 activation promoted β-cell regeneration by activating AMPK signaling-mediated fatty acid oxidation.46 The islets from Sirt1 knockout mice exhibited reduced glucose-stimulated insulin secretion.47 In rats, resveratrol attenuated β-cell senescence induced by excessive ethanol exposure via the SIRT1–p38MAPK – p16 pathway.27 Our results are consistent with the previous studies in which Sirt1 was obviously decreased after 7-KC treatment.45,46
In summary, our experimental data demonstrated that DNA damage induced p21, p16, and p53 expression, which was accompanied by decreased DNA repair (SIRT1 downregulation), CDK4 inhibition, decreased Rb phosphorylation, and the limitation of E2F1 activation. These changes promoted G1 arrest in MIN6 β-cells. In this study, we not only elucidated the potential mechanism of 7-KC in pancreatic β-cell senescence, but may also suggested potential therapeutic targets for preventing and treating pancreatic β-cell senescence-related diseases such as T2DM.
Our study indicated that 7-KC led to pancreatic β-cell senescence possibly by regulating SIRT1/CDK4–Rb – E2F1 signal pathway activation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grant 81870562, 82270857 and 41906095; the National Key Technology R&D Program of China under Grant 2009BAI80B02; and the Zhejiang Provincial Natural Science Foundation of China under Grant LZ22H070002 and LY22D060003.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Li N, Liu F, Yang P, Xiong F, Yu Q, Li J, Zhou Z, Zhang S, Wang CY.. Aging and stress induced β cell senescence and its implication in diabetes development. Aging. 2019;11(21):9947–10. PMID: 31721726. doi: 10.18632/aging.102432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizukami H, Takahashi K, Inaba W, Osonoi S, Kamata K, Tsuboi K, Yagihashi S. Age-associated changes of islet endocrine cells and the effects of body mass index in Japanese. J Diabetes Invest. 2014;5(1):38–47. PMID: 24843735. doi: 10.1111/jdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908(1):244–254. PMID: 10911963. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 4.Kong FJ, Wu JH, Sun SY, Zhou JQ. The endoplasmic reticulum stress/autophagy pathway is involved in cholesterol-induced pancreatic β-cell injury. null. 2017;7(1):44746. PMID: 28294183. doi: 10.1038/srep44746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamsanathan S, Gurkar AU. Lipids as regulators of cellular senescence. Front Physiol. 2022;13:796850. PMID: 35370799. doi: 10.3389/fphys.2022.796850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perego C, Da Dalt L, Pirillo A, Galli A, Catapano AL, Norata GD. Cholesterol metabolism, pancreatic β-cell function and diabetes. Biochimica et biophysica acta molecular basis of disease. Biochim Biophys Acta, Mol Basis Dis. 2019;1865(9):2149–2156. PMID: 31029825. 10.1016/j.bbadis.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Imai Y, Cousins RS, Liu S, Phelps BM, Promes JA. Connecting pancreatic islet lipid metabolism with insulin secretion and the development of type 2 diabetes. Ann Ny Acad Sci. 2020;1461(1):53–72. PMID: 30937918. doi: 10.1111/nyas.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta-cell dysfunction. Diabetes. 2007;56(9):2328–2338. PMID: 17575085. doi: 10.2337/db07-0056. [DOI] [PubMed] [Google Scholar]
- 9.Brunham LR, Kruit JK, Verchere CB, Hayden MR. Cholesterol in islet dysfunction and type 2 diabetes. J Clin Invest. 2008;118(2):403–408. PMID: 18246189. doi: 10.1172/jci33296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vejux A, Lizard G. Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Aspects Med. 2009;30(3):153–170. PMID: 19248805. doi: 10.1016/j.mam.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Zarrouk A, Vejux A, Mackrill J, O’Callaghan Y, Hammami M, O’Brien N, Lizard G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res Rev. 2014;18:148–162. PMID: 25305550. doi: 10.1016/j.arr.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Trompier D, Vejux A, Zarrouk A, Gondcaille C, Geillon F, Nury T, Savary S, Lizard G. Brain peroxisomes. Biochimie. 2014;98:102–110. PMID: 24060512. doi: 10.1016/j.biochi.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Ravi S, Duraisamy P, Krishnan M, Martin LC, Manikandan B, Raman T, Sundaram J, Arumugam M, Ramar M. An insight on 7- ketocholesterol mediated inflammation in atherosclerosis and potential therapeutics. Steroids. 2021;172:108854. PMID: 33930389. doi: 10.1016/j.steroids.2021.108854. [DOI] [PubMed] [Google Scholar]
- 14.Anderson A, Campo A, Fulton E, Corwin A, Jerome WG 3rd, O’Connor MS. 7-Ketocholesterol in disease and aging. Redox Biol. 2020;29:101380. PMID: 31926618. doi: 10.1016/j.redox.2019.101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nury T, Yammine A, Ghzaiel I, Sassi K, Zarrouk A, Brahmi F, Samadi M, Rup-Jacques S, Vervandier-Fasseur D, de Barros J P P, et al. Attenuation of 7-ketocholesterol- and 7β-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: potential for the prevention of age-related diseases. Ageing Res Rev. 2021;68:101324. doi: 10.1016/j.arr.2021.101324. PMID: 33774195. [DOI] [PubMed] [Google Scholar]
- 16.Nury T, Zarrouk A, Ragot K, Debbabi M, Riedinger JM, Vejux A, Aubourg P, Lizard G. 7-Ketocholesterol is increased in the plasma of X-ALD patients and induces peroxisomal modifications in microglial cells: potential roles of 7-ketocholesterol in the pathophysiology of X-ALD. J Steroid Biochem Mol Biol. 2017;169:123–136. PMID: 27041118. doi: 10.1016/j.jsbmb.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Serviddio G, Bellanti F, Villani R, Tamborra R, Zerbinati C, Blonda M, Ciacciarelli M, Poli G, Vendemiale G, Iuliano L. Effects of dietary fatty acids and cholesterol excess on liver injury: a lipidomic approach. Redox Biol. 2016;9:296–305. PMID: 27639112. doi: 10.1016/j.redox.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.G NA, Castrillo A. Liver X receptors as regulators of macrophage inflammatory and metabolic pathways. Biochim Biophys Acta. 2011;1812(8):982–994. PMID: 21193033. doi: 10.1016/j.bbadis.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Debbabi M, Zarrouk A, Bezine M, Meddeb W, Nury T, Badreddine A, M KE, Sghaier R, Bretillon L, Guyot S, et al. Comparison of the effects of major fatty acids present in the Mediterranean diet (oleic acid, docosahexaenoic acid) and in hydrogenated oils (elaidic acid) on 7-ketocholesterol-induced oxiapoptophagy in microglial BV-2 cells. Chem Phys Lipids. 2017;207:151–170. doi: 10.1016/j.chemphyslip.2017.04.002. PMID: 28408132. [DOI] [PubMed] [Google Scholar]
- 20.Nury T, Yammine A, Menetrier F, Zarrouk A, Vejux A, Lizard G. 7-Ketocholesterol- and 7β-Hydroxycholesterol-induced peroxisomal disorders in glial. Microglial And Neuronal Cells: Potential Role In Neurodegeneration: 7-Ketocholesterol And 7β-Hydroxycholesterol-Induced Peroxisomal Disorders And Neurodegeneration Advances In Experimental Medicine And Biology. 2020;1299:31–41. PMID: 33417205. doi: 10.1007/978-3-030-60204-8_3. [DOI] [PubMed] [Google Scholar]
- 21.Wu N, Jin W, Zhao Y, Wang H, He S, Zhang W, Zhou J. Sulfated fucogalactan from laminaria japonica ameliorates β-cell failure by attenuating mitochondrial dysfunction via SIRT1–PGC1-α signaling pathway activation. Front Endocrinol. 2022;13:881256. PMID: 35909530. doi: 10.3389/fendo.2022.881256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguayo-Mazzucato C, Andle J, T B L, Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019;30(1):129–142.e124. PMID: 31155496. doi: 10.1016/j.cmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Zamudio RI, Robinson L, Roux PF, Bischof O. SnapShot: cellular senescence pathways. Cell. 2017;170(4):816–816.e811. PMID: 28802049. doi: 10.1016/j.cell.2017.07.049. [DOI] [PubMed] [Google Scholar]
- 24.Burton DGA, Faragher RGA. Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology. 2018;19(6):447–459. PMID: 30054761. doi: 10.1007/s10522-018-9763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pack LR, Daigh LH, Meyer T. Putting the brakes on the cell cycle: mechanisms of cellular growth arrest. Curr Opin Cell Biol. 2019;60:106–113. PMID: 31252282. doi: 10.1016/j.ceb.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamad Kamal NS, Safuan S, Shamsuddin S, Foroozandeh P. Aging of the cells: insight into cellular senescence and detection Methods. Eur J Cell Biol. 2020;99(6):151108. PMID: 32800277. doi: 10.1016/j.ejcb.2020.151108. [DOI] [PubMed] [Google Scholar]
- 27.Luo G, Xiao L, Wang D, Wang N, Luo C, Yang X, Hao L. Resveratrol attenuates excessive ethanol exposure-induced β-cell senescence in rats: a critical role for the NAD(+)/SIRT1-p38MAPK/p16 pathway. J Nutr Biochem. 2021;89:108568. PMID: 33326842. doi: 10.1016/j.jnutbio.2020.108568. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Zhou L, Lu Y, Zhang J, Jian F, Liu Y, Li F, Li W, Wang X, Li G. Activation of SIRT1 protects pancreatic β-cells against palmitate-induced dysfunction. Biochim Biophys Acta. 2012;1822(11):1815–1825. PMID: 22968147. doi: 10.1016/j.bbadis.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Feng Y, Guo Q, Guo W, Xu H, Li X, Yi F, Guan Y, Geng N, Wang P, et al. SIRT1 modulates cell cycle progression by regulating CHK2 acetylation−phosphorylation. Cell Death Differ. 2020;27(2):482–496. doi: 10.1038/s41418-019-0369-7. PMID: 31209362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Liu Y, Ma X, Hu H. The influence of cell cycle regulation on chemotherapy. IJMS. 2021;22(13):6923. PMID: 34203270. doi: 10.3390/ijms22136923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia. 2005;48(1):58–67. PMID: 15624098. doi: 10.1007/s00125-004-1605-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Chen M, Zhou N, Qi Y. Metformin prevents H₂O₂-Induced senescence in human lens epithelial B3 cells. Med Sci Monit Basic Res. 2020;26:e923391. PMID: 32336745. doi: 10.12659/msmbr.923391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khemais-Benkhiat S, Belcastro E, Idris-Khodja N, H PS, Amoura L, Abbas M, Auger C, Kessler L, Mayoux E, Toti F, et al. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J Cell Mol Med. 2020;24(3):2109–2122. doi: 10.1111/jcmm.14233. PMID: 30929316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15(7):397–408. PMID: 26105537. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 35.Aguayo-Mazzucato C, van Haaren M, Mruk M, Lee T B C Jr., Hollister-Lock C, J SBA, W JJ, Ebrahimi A, M DJ, Dreyfuss JM, et al. β cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab. 2017;25(4):898–910.e895. doi: 10.1016/j.cmet.2017.03.015. PMID: 28380379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguayo-Mazzucato C. Functional changes in beta cells during ageing and senescence. Diabetologia. 2020;63(10):2022–2029. PMID: 32894312. doi: 10.1007/s00125-020-05185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niedernhofer LJ, Gurkar AU, Wang Y, Vijg J, Hoeijmakers JHJ, Robbins PD. Nuclear Genomic Instability and Aging. Annu Rev Biochem. 2018;87(1):295–322. PMID: 29925262. doi: 10.1146/annurev-biochem-062917-012239. [DOI] [PubMed] [Google Scholar]
- 38.Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2(10):731–737. PMID: 11584300. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- 39.Zhao R, Choi BY, Lee MH, Bode AM, Dong Z. Implications of genetic and epigenetic alterations of CDKN2A (p16 INK4a) in Cancer. EBioMedicine. 2016;8:30–39. PMID: 27428416. doi: 10.1016/j.ebiom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkar D, Fisher PB. Regulation of Myc function by ARF: checkpoint for Myc-induced oncogenesis. Cancer Biol Ther. 2006;5(6):693–695. PMID: 16775430. doi: 10.4161/cbt.5.6.2939. [DOI] [PubMed] [Google Scholar]
- 41.Kuzmichev A, Jenuwein T, Tempst P, Reinberg D. Different EZH2-containing complexes target methylation of histone H1 or nucleosomal histone H3. Mol Cell. 2004;14(2):183–193. PMID: 15099518. doi: 10.1016/s1097-2765(04)00185-6. [DOI] [PubMed] [Google Scholar]
- 42.Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34(23–24):1565–1576. PMID: 33262144. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama. 2001;286(3):327–334. PMID: 11466099. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 44.Spranger J, Kroke A, Mőhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European prospective investigation into cancer and nutrition (EPIC)-potsdam study. Diabetes. 2003;52(3):812–817. PMID: 12606524. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 45.Singh R, Chandel S, Dey D, Ghosh A, Roy S, Ravichandiran V, Ghosh D. Epigenetic modification and therapeutic targets of diabetes mellitus. Biosci Rep. 2020;40(9). PMID: 32815547. doi: 10.1042/bsr20202160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu SY, Liang J, Yang BC, Leung PS. SIRT1 activation promotes β-cell regeneration by activating endocrine progenitor cells via AMPK signaling-mediated fatty acid oxidation. Stem Cells (Dayton, Ohio). 2019;37(11):1416–1428. PMID: 31400234. doi: 10.1002/stem.3073. [DOI] [PubMed] [Google Scholar]
- 47.Pinho AV, Bensellam M, Wauters E, Rees M, Giry-Laterriere M, Mawson A, Ly le Q, Biankin AV, Wu J, Laybutt DR, et al. Pancreas-specific sirt1-deficiency in mice compromises beta-cell function without development of hyperglycemia. PLos One. 2015;10(6):e0128012. doi: 10.1371/journal.pone.0128012. PMID: 26046931. [DOI] [PMC free article] [PubMed] [Google Scholar]


