ABSTRACT
Immunogenic cell death (ICD) refers to an immunologically distinct process of regulated cell death that activates, rather than suppresses, innate and adaptive immune responses. Such responses culminate into T cell-driven immunity against antigens derived from dying cancer cells. The potency of ICD is dependent on the immunogenicity of dying cells as defined by the antigenicity of these cells and their ability to expose immunostimulatory molecules like damage-associated molecular patterns (DAMPs) and cytokines like type I interferons (IFNs). Moreover, it is crucial that the host’s immune system can adequately detect the antigenicity and adjuvanticity of these dying cells. Over the years, several well-known chemotherapies have been validated as potent ICD inducers, including (but not limited to) anthracyclines, paclitaxels, and oxaliplatin. Such ICD-inducing chemotherapeutic drugs can serve as important combinatorial partners for anti-cancer immunotherapies against highly immuno-resistant tumors. In this Trial Watch, we describe current trends in the preclinical and clinical integration of ICD-inducing chemotherapy in the existing immuno-oncological paradigms.
KEYWORDS: CAR T cells, antigen-presenting cells; chemotherapy; danger signals; dendritic cell; immune-checkpoint blockers; immunogenic cell death; immunotherapy
Introduction
It has been two decades since the concept of apoptosis being solely immunologically quiet and hence unable to activate the immune system has been overthrown. A large number of studies have substantiated an immunogenic variant of regulated cell death (RCD) programs like apoptosis, called immunogenic cell death (ICD)1–4. Since then, this concept of ICD has been extended to other RCDs, a term that refers to cell death programs that have a known intricate signaling cascade, such as necroptosis, pyroptosis, or ferroptosis5–15.
The most well-known form is Apoptosis. Apoptosis is morphologically defined by the shrinking of cells, fragmentation of the DNA, and blebbing of the cell membrane. In contrast, necroptosis, pyroptosis, and ferroptosis are regulated forms of molecularly defined necrosis. They resemble accidental necrosis in terms of its final morphology (e.g., organelle swelling, plasma membrane rupture, cell lysis, and leakage of intracellular components) but utilize a distinct molecular machinery.
Nevertheless, some degree of caution is required with the ICD-like profile subscribed to some recently discovered RCD pathways, since a full consensus on their immunological impact is still pending16,17. For instance, ferroptosis, a pathway first described in 201218, has been shown to be immuno-modulatory in multiple disease models19–22 including cancer13,22–27. Depending on the temporal stage of ferroptosis (i.e., early vs. late), differences in modulating experimental anti-tumor immunity have been reported13. However, several immunosuppressive mechanisms have also been associated with ferroptosis such as formation of lipid bodies28, oxidized phospholipids (oxPLs)29–33 formation, and cyclo-oxygenase 2 (MT-CO2; also known as COX2)34,35 activation36. Some of the immunosuppressive mechanisms relevant for anti-tumor immunity5 were also attributed to ferroptotic tumor-associated neutrophils resulting even in enhanced tumor growth37. Clinical and immuno-oncology implications of such findings are still pending.
Owing to a lot of research studies published over the last few decades, the molecular and cellular mechanisms behind ICD have been largely deciphered. Organelle and cellular stress, most particularly endoplasmic reticulum (ER) stress induced by reactive oxygen species (ROS) production, is an essential early trigger for the initiation of ICD38–42. Sequentially, ICD enables a time- and space-dependent organized exposure as well as release of damage-associated patterns (DAMPs) or alarmins from dying cancer cells. The main DAMPs associated with ICD include calreticulin exposure on the cell membrane43–49, heat-shock proteins (HSPs), exposure on the cell membrane and/or passively released50,51, passively released high-mobility group box 1 (HMGB1)52–56, surface exposure of annexin A1 (ANXA1)57–60, and adenosine triphosphate (ATP), which can be actively or passively released44,61,62. The binding of these DAMPs to their cognate pattern recognition-receptors (PRRs), present on antigen-presenting cells such as dendritic cells (DCs), eventually leads to the activation of both the innate and the adaptive immune system via a series of cytokine and chemokine networks5,63–66. Dying cancer cells undergoing ICD can autonomously release cytokines as well as induce cytokine production from neighboring immune or stromal cells67–72. Additionally, ICD can also cause the secretion of immunostimulatory and chemotactic cytokines73–75 including, but not limited to, type I interferons (IFNs)76–80 and chemokine (C-X-C) ligand 9 and 10 (CXCL9/10)73,81,82. In the correct context, ICD can also facilitate T cell expansion in a manner that leads to diversification of TCR repertoire,83–91 which can help regress distant (metastatic) tumor lesions via abscopal effect-like immune responses92–96. This abscopal effect is driven by DAMPs (i.e., adjuvanticity) and tumor-associated antigen (TAA) (i.e., antigenicity),97–99 thereby highlighting the importance of ICD100–102. The ability of ICD to initiate an immune response is highly dependent on antigenicity and adjuvanticity103–105. Without antigens, ICD can only induce an antigen-irrelevant inflammatory response without engagement of the adaptive immune system106. Conversely, the presentation of antigens to T cells with poor adjuvanticity actively promotes tolerance107–110.
In general, ICD inducers can be broadly divided into two groups depending on how they initiate ER stress-related pathways relevant for DAMP mobilization111–114. ER stress will lead to unfolded protein response (UPR) activation, leading to an upregulation of pathways including PERK – eukaryotic initiation factor 2 (eIF2a)79. Downstream, this will cause lower amounts of IkB and more activation of nuclear factor-kB (NF-kB)115. The first group includes therapies that induce ICD without directly inducing ER stress. Such type I ICD inducers include radiotherapy as well as chemotherapies like paclitaxel, oxaliplatin, and anthracyclines61,116–120. The second group, i.e., type II ICD inducers, includes treatment modalities that induce ICD by specifically targeting the ER to induce ER stress-driven cell death, e.g., photodynamic therapy (PDT) or oncolytic viruses121–124. Comprehensively, ICD inducers can be part of different treatment classes including not only microbial and chemical but also physical treatments such as irradiation and high hydrostatic pressure (HHP)57.
Importantly, there is little correlation between the specific chemical features of an anti-cancer therapy and their ability to induce ICD. For example, while cisplatin and oxaliplatin are both platinum-based chemotherapies and induce cell death via DNA adduct-formation, yet only oxaliplatin treatment results in ICD125–128. Over the years, at least two pre-clinical criteria have been established to classify an anti-cancer therapy as a potential ICD inducer129,130. First criterion is that a therapy should be able to induce tumor regression in an immuno-competent, but not immuno-deficient, mouse setting131–133. Secondly, the cancer cells treated with an ICD inducer should serve as an anti-cancer vaccine in a prophylactic setting5,134–139. To rephrase, when tumor-naïve mice are injected with cancer cells undergoing ICD upon exposure to the anti-cancer drug, subsequent challenging with live cancer cells of the same type should not result in the formation of a tumor at the vaccination and challenging site. It is important to keep in mind that these approaches of classifying ICD inducers may have clinical translational issues, since they only allow exploration in a mouse setting. For this reason, in addition to these in vivo mice experiments, in vitro or ex vivo settings can also be utilized to assess the immunogenic potency of dying cancer cells treated with a potential ICD inducer57,140. Here, the presence of aforementioned ICD-associated DAMPs can serve as a surrogate marker to confirm ICD in vitro or ex vivo 141–144. Additionally, culturing the dying cancer cells with innate immune cells, such as DCs, to assess ICD-relevant DC functions, is also possible. Phenotypic markers like phagocytic activity145–149, DC activation markers150–154 (CD86 and major histocompatibility complex (CIITA, also known as MHC) Class II molecules) or the secretion of cytokines like interleukin 1 beta (IL-1β)155–157, interleukin 6 (IL-6)158–161, interleukin 12 (IL-12)162,163, and tumor necrosis factor (TNF)164–167, and the ability of DCs to activate T cell proliferation and functional activation168–172 are examples of possible features to assess.
Multiple chemotherapeutics, commonly used in the clinic, have been identified as ICD inducers. The most commonly used chemotherapies with ICD potential are anthracyclines (including doxorubicin, epirubicin, mitoxantrone, and idarubicin), cyclophosphamide, oxaliplatin, paclitaxel, docetaxel, 5-fluorouracil, and targeted therapies like bortezomib126,173–182. In fact, there is concrete evidence supporting the beneficial effects of ICD in cancer patients. For instance, patients with tumors displaying markers of ER stress (like tribbles pseudokinase 3 (TRB3) and DNA damage inducible transcript 3 (DDIT3; also known as CHOP)183–188 or ICD-associated DAMPs, such as calreticulin or HMGB1, have a better prognosis189–197. These findings support the pursuit for an ideal ICD-inducing treatment regimen40. It is important to note that most clinical trials do not choose for specific chemotherapeutics based on their ability to induce ICD. Instead, they are based on their ability to induce tumor response and disease control, without specific knowledge about their potential to induce ICD198,199. In some cases, these design-related decisions can be counterproductive for immune-mediated tumor control, thereby limiting the clinical benefit for cancer patients200–203. Besides chemotherapeutics, there are multiple other treatment options that can induce ICD. Herein, radiation-based modalities are particularly proficient at inducing ICD and associated abscopal effect-like responses204–209. Additionally, some upcoming immunotherapies like oncolytic viruses also operate via ICD induction210–214.
In this Trial Watch edition, we will be focusing on the most recent preclinical and clinical advances around ICD induction by anti-cancer chemotherapy.
Preclinical advances
Since the publication of the previous Trial Watch dealing with chemotherapeutic ICD inducers, several novel preclinical studies on this topic have been published215. Here, we highlight the ones that are of particular importance and/or capture the general trends in this field.
Some papers further contributed to our mechanistic understanding of ICD. Mandula et al. (H. Lee Moffitt Cancer center, Tampa, USA.) established the role of protein kinase R-like reticulum kinase (EIF2AK3; also known as PERK), a well-known ER stress sensor, in mediating ICD via a new RCD sub-routine, i.e., paraptosis. They found that PERK inhibition resulted in an increased T cell activation followed by a reduction in tumor growth via type I IFN responses. These findings encourage the use of PERK-targeting therapies for cancer immunotherapy216. Furthermore, Hayashi et al. (Cedars-Sinai Medical Center, Los Angeles, CA, USA) reported that although gemcitabine stimulates the release of immunostimulatory DAMPs, it also triggers prostaglandin E2 (PGE2) release, which counteracts ICD-relevant immune responses. However, when they combined gemcitabine and PGE2 blockade, an effective DC and T cell activation was induced, which led to tumor regression36. Oresta et al. (Humanitas Clinical and Research Center-IRCCS, Rozzano, Italy) found that mitochondrial metabolic reprogramming is important for ICD occurrence217. This is accompanied by an increased oxidative phosphorylation. Moreover, tumors with low amounts of complex 1 of the respiratory chain expression had a higher chance of recurrence after chemotherapy. Lucarini et al. (Bambino Gesù Children’s Hospital, Rome, Italy) investigated the combination strategy of mitoxantrone and anti-transforming growth factor beta (TGFB1) with programmed cell death-1 (PD-1, also known as PDCD1) blockade in neuroblastoma mouse models. They found that the low dose of mitoxantrone by itself was already able to increase IFNγ and granzyme B (GZMB) in CD8+ T cells, which were further increased upon combination with anti-TGFβ and anti-PD-1 blockades218. Several papers also revealed a novel connection between anti-cancer agents and the ICD pathway125. Humea et al. (Centre de Recherche des Cordeliers, Université de Paris, Paris, France) reported about the induction of immunogenic cell stress and ICD via dactinomycin219, an inhibitor of DNA and RNA transcription220. Marin et al. (Barcelona institute of Science and Technology, Barcelona, Spain) found that senescent cells have a greater immunologic potential that could initiate CD8+ T cell responses. These senescent cells are able to release alarmins and PRR agonists and increase MHCI exposure. Even more so, immunization with these cancer cells caused protection superior to the standard ICD inducers221.
Several papers also focused on increasing tumor-directed drug delivery while decreasing toxicity using nanoparticles222. For example, Zhou et al. (Department of Pharmaceutics, China Pharmaceutical University, Nanjing, China) published that direct delivery of therapeutic proteins, including RNase A, PD-1 antibodies, and photothermal agents, via hydrogels forming membrane pores, increased lactate dehydrogenase (LDHA), HMGB1, and ATP release in multiple murine cancer models223. In due course, the intratumoral hydrogel injections resulted in more CD8+ T cell tumor infiltration and a lower tumor growth compared to the saline treated tumors. Another example is the study by Yang et al. (Wuhan University, Wuhan, China). They focused on decreasing the toxicity of small-molecule inhibitors by creating a prodrug nanomicelle that integrates both a phosphoinositide 3-kinase (PIK3CG; also known as PI3K)/mammalian target of rapamycin (mTOR) inhibitor and a cyclin-dependent kinase (CDK) inhibitor, flavopiridol. With this treatment, they were able to decrease tumor growth via the induction of ICD accompanied by HMGB1 and Gasdermin E (GSDME) release as well as ATP secretion in a breast cancer cell line224. Song et al. (Shanghai Jiaotong University, Shanghai, China) created a porphyrin-cisplatin conjugate (NP@Pt-1) that can be triggered by light225. NP@Pt-1 treatment resulted in an increased ROS production leading to ATP and HMGB1 in murine colon cancer cells release compared to untreated cells. Additionally, NP@Pt-1 treatment resulted in a decreased tumor growth compared to PBS-treated tumors.
Besides nanoparticles, other innovative strategies have been implemented to optimize ICD inducing treatment regimes. Tatarova et al. (OHSU Center for Spatial Systems Biomedicine, Portland, USA) developed a microdevice that could assist with microtargeting-specific regions of the tumor. This implantable chip is able to contain 18 different treatments that can be released in separate regions226. Zawilska et al. (Univeristy of Wroclaw, Wroclaw, Poland) developed a liposomal docetaxel therapy that could overcome the problems of toxicity and poor pharmacokinetics. They saw that the cell growth decreased using their treatment compared to docetaxel alone. Additionally, the half life of docetaxel was significantly increased when liposomal-pegylated227.
A series of studies are also trying to use ICD and its markers as a biomarker modality175. Use of an ICD-associated genetic signature is one of the approaches proposed to exploit ICD as a predicting marker for patient outcome. In high-grade glioma (HGG), such a signature was able to predict responsiveness to immune checkpoint blocker (ICB) therapy including anti-PD-1 and anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)160. This signature, composed of FOXP3, IL6 LY96, MYD88, and PDIA3, was able to distinguish patients with increased immune modulation and immune escape and high expression of human leukocyte antigen (HLA)-related genes. On top of that, multiple papers have reported that even micro-RNAs linked to ICD-relevant DAMPs are able to predict treatment outcome228. Several microRNAs relevant for modulating expression of calreticulin, e.g., miR-27a-3p229,230, or HMGB1231–234 have been characterized.
Finally, optimizing the detection of ICD in response to chemotherapy has also been investigated further since the last Trial Watch publication235. Zhang et al. (Chonnam National University Medical School, Hwasun, Korea) engineered calreticulin-targeting monobodies to detect ICD more accurately236. Via this method, they were able to detect surface expression in multiple cancer cell lines and in mice treated with ICD inducers. Similar to this, Kim et al. (Gyeongsang National University, Jinju, Korea) created a synthetic 18F-labeled peptide that specifically binds calreticulin237. Via this method, they were able to detect calreticulin surface exposure in mouse colon cancer tumors via a small-animal positron emission tomography (PET) scan. This staining was visible in tumors treated with multiple ICD inducers including doxorubicin, oxaliplatin, and radiation.
Finalized clinical studies
All finalized clinical studies published after the previous Trial Watch (June 2019) were gathered using PubMed (http://www.ncbi.nlm.nih.gov/pubmed) with the following search string taking into account to most established ICD inducers (cancer OR tumor OR tumor OR neoplasm) AND (oxaliplatin OR cyclophosphamide OR bortezomib OR doxorubicin OR epirubicin OR idarubicin OR mitoxantrone OR paclitaxel) AND (“danger signal” OR “damage associated molecular pattern” OR “immunogenic cell death” OR “immunogenic cancer cell death” OR immunogenic OR immunogenicity), together with the clinical trial filter. Additionally, articles were filtered manually based on relevance as well as on the presence of measurements of immunological parameters. On November 15, 2022, this query with PubMed resulted in 268 published clinical trial studies. From these papers, 67 studies were investigating immune responses using biomarkers. Of note, many studies reported the blood cell counts of patients during treatment. This kind of publication was not considered for this Trial Watch, since such counts are not valid ICD biomarkers. In this section, we will give a general overview of these published clinical studies (Figure 1) and highlight a few of them.
Figure 1.
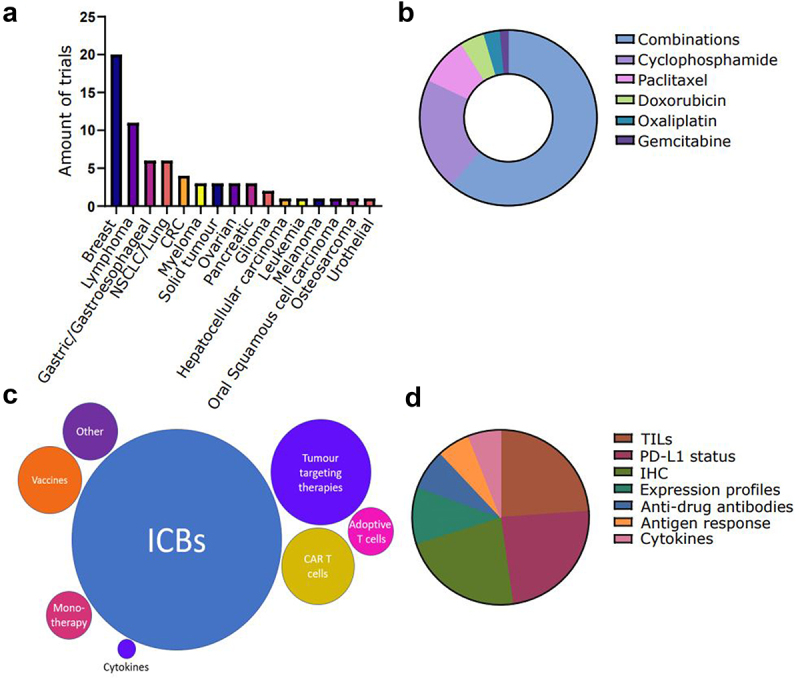
Recently published clinical studies testing immunogenic cell death (ICD)-inducing chemotherapy in oncology that investigate the immunogenic response. Clinical studies were classified on: (a) cancer type, (b) ICD-inducing drug, (c) combinatorial immunotherapy. (d) immunomonitoring approach, CAR, chimeric antigen receptor; CRC, colorectal carcinoma; ICB, immune checkpoint blocker; IHC, immunohistochemistry; PD-L1, programmed death-ligand; TIL, tumor-infiltrating lymphocyte.
Among these studies, we found that there were 15 individual cancer types investigated. A large portion (29.9%) of the studies focused on breast cancer (Figure 1A)238–241. Page et al. (Earle A. Chiles Research Institute, Providence Cancer Institute, Portland, Oregon) reported on the efficiency of cyclophosphamide in combination with a cytokine cocktail including TNF, IL-2, IL-1, IFN-γ, IL-6, IL-8, granulocyte-macrophage colony-stimulating factor (CSF2; also known as GM-CSF), and granulocyte colony stimulating factor (CSF3; also known as G-CSF) with the product name IRX-2 in breast cancer. In this phase 1b study, they demonstrated that after treatment there was a higher T-cell activation profile, based on GZMB, GZMA, IFNG, membrane cofactor protein-4 (CD46; also known as MCP-4), S100, CD184, CC-motif chemokine ligand 21 (CCL21) and perforin-1 (ZNF395; also known as PRF-1) compared to the baseline. Additionally, they found a cyclophosphamide-associated peripheral T-regulatory (Treg) cell depletion242. However, upregulation of the immune-checkpoint ligand, programmed cell death ligand 1 (PD-L1, also known as CD274), assessed by immunohistochemistry (IHC) was also seen in these patients after treatment. Additionally, there were 3 “basket trials” (4.5% of all included published trials), consisting of multiple solid tumor types243–245. Haas et al. (University of Pennsylvania, Philadelphia, PA, USA) analyzed the effects of cyclophosphamide, with and without chimeric antigen receptor (CAR) T cells specific for mesothelin (meso), a protein highly expressed by many cancers246–249. They found that patients pre-treated with cyclophosphamide increased the initial CAR T cell expansion but did not alter the persistence at day 28244. Although both treatment arms were well tolerated, patients showed limited clinical benefit.
Most of the studies included into this Trial Watch, i.e., 41 out of 67, investigated the effect of co-treatment with more than one ICD inducer (Figure 1B). Chemotherapeutic regimes based on paclitaxel, together with other ICD inducers, were very prevalent in the published clinical trials. This was most likely because paclitaxel is regularly applied as part of a multi-modal chemotherapeutic regimen, especially against breast cancer and ovarian cancer241,250–252. In this sense, an immunotherapy-relevant example includes the KEYNOTE-355 trial, where the investigators tested paclitaxel or gemcitabine in combination with carboplatin and pembrolizumab, an anti-PD-1 ICB, in triple-negative breast cancer253. They found that the addition of pembrolizumab resulted in a significant increase in patient overall survival (OS) compared to chemotherapy alone. It is important to note that the combination of ICD inducers together with ICBs is very prevalent (Figure 1C). In general, most studies find that the addition of ICBs increases the overall response rate (ORR) compared to chemotherapy alone254–256. Tumor-targeting passive immunotherapies, such as trastuzumab (anti-human epidermal growth factor receptor 2 (ERBB2; also known as HER2)), are also repeatedly combined with ICD inducers. Similarly, CAR T cells are often combined with ICD inducers like fludarabine and cyclophosphamide257–260. The latter is not per se to promote an immune activation but rather to eliminate the circulating lymphocyte population.
Lastly, another parameter that we assessed in this Trial Watch was the measurement of immune response parameters. In the above studies, the most frequently used immune biomarkers were tumor infiltrating lymphocytes (TILs) analysis and the PD-L1 detection (Figure 1D)239,261–263. For instance, a phase II study for breast cancer investigating the addition of durvalumab, a monoclonal anti-PD-1 antibody, together with anthracycline-taxane-based neoadjuvant therapy included a broad biomarker analysis. The authors found that paclitaxel was able to increase the TILs in both treatments’ arms (with and without durvalumab). Additionally, they found that both arms showed a higher pathological complete response (pCR) rate in the PD-L1-positive tumors compared to PD-L1low tumors264. Finally, they concluded that durvalumab together with anthracycline-/taxane-based neoadjuvant chemotherapy (NACT) was the most optimal treatment regime for increased pCR rates. Additionally, more advanced molecular analysis such as RNA sequencing was also often used as biomarker discovery242,265,266. For instance, Pusztai et al. (Yale Cancer Center, New Haven, CT, USA) found while investigating paclitaxel in combination with durvalumab and the poly-ADP ribose polymerase (PARP) inhibitor olaparib in breast cancer that their dendritic cell signature had a positive correlation with pCR in the treatment arm267. Additionally, they found that their mast cell signature correlated negatively with pCR. However, they did not find any correlation between their T cell signatures and pCR in their clinical trial.
Altogether, these results highlight the promising perspectives and clinical trends in ICD research.
Ongoing clinical studies
In parallel, we also assessed the ClinicalTrials.gov database (http://www.clinicaltrials.gov/) for all the ongoing or active clinical trials using oxaliplatin, cyclophosphamide, bortezomib, doxorubicin, epirubicin, idarubicin, mitoxantrone, or paclitaxel in combination with cancer immunotherapy. With a relevant search string, we found not less than 84 clinical studies that matched the following criteria: (1) they involved at least one ICD-inducing chemotherapeutic agent and (2) they were initiated after June 2019 (when the latest Trial Watch on this topic was published).
In this context, multiple cancer types are being studied (Tables 1 and 2). Like the finalized studies described above, the ongoing clinical trials are predominately focused on breast cancer. This is a trend that has been seen over multiple Trial Watch publications215,268. In contrast to the published clinical trials, recently enlisted clinical studies also have a considerable percentage focusing on hematopoietic cancer types such as lymphoma, leukemia, and multiple myeloma (Table 1). Most likely, this is due to a high increase in CAR T cell studies, a treatment that has been approved for these cancer types in combination with specific chemotherapies that can also induce ICD86. For instance, a single arm clinical study with the aim of observing the tolerance and safety of Fludarabine in combination with CAR natural killer (NK)-CD19 cells in acute lymphoblastic leukemia (NCT05563545). Furthermore, clustering of several different solid tumor types in the same study is also something that is often noticed in these ongoing trials.
Table 1.
Contemporary clinical studies assessing the therapeutic and immunological characteristics of chemotherapeutics.
| Cancer type | ICD inducer | Phase | Status | Combination | Trial number |
|---|---|---|---|---|---|
| Breast cancer | Cyclophosphamide | I | Recruiting | Combined with DPX-Survivac, Letrozole, XRT | NCT04895761 |
| II | Recruiting | Combined with Anthracyclines and P2Et | NCT05007444 | ||
| CRC | Oxaliplatin | I/II | Active, not recruiting | Combined with Leucovorin, 5-FU and Bevacizumab | NCT04068610 |
| II | Recruiting | Combined with Nivolumab | NCT05504252 | ||
| Combined with Capecitabine, Bevacizumab and Pembrolizumab | NCT04262687 | ||||
| Combined with Atezolizumab, Bevacizumab and Capecitabine | NCT04659382 | ||||
| FOLFOX | Withdrawn | Combined with Durvalumab and Oleclumab or Monalizumab | NCT04145193 | ||
| Gastric cancer | Oxaliplatin | II | Recruiting | Combined with 5-FU, Capecitabine, Durvalumab, Trastuzumab, Cisplatin and Pembrolizumab | NCT04379596 |
| Gastric or Gastroesophageal cancer | III | Recruiting | Combined with AK104 and Capecitabine | NCT05008783 | |
| Leukemia | Cyclophosphamide | I | Nyet recruiting | Combined with Fludarabine and CD19/22 targeting T cells | NCT05223686 |
| Recruiting | Combined with Fludarabine and Cl-135 CAR-T cells | NCT05266950 | |||
| Combined with Fludarabine and CAR-NK CD19 cells | NCT05563545 | ||||
| Combined with Fludarabine and pCAR-19B cells | NCT04888442 | ||||
| Completed | Combined with Fludarabine and pCAR-19B cells | NCT04888468 | |||
| II | Recruiting | Combined with Fludarabine and CNCT19 cells | NCT04684147 | ||
| Combined with Fludarabine and pCAR-19B cells | NCT05334823 | ||||
| Leukemia and Lymphoma | Cyclophosphamide | I | Terminated | Combined with Fludarabine and RPM CD19-mbIL 15-CAR-T cells | NCT04844086 |
| Lymphoma | I | Recruiting | Combined with Fludarabine and CB-010 | NCT04637763 | |
| Combined with Fludarabine and LCAR-AIO cells | NCT05318963 | ||||
| I/II | Recruiting | Combined with Fludarabine and CRC01 | NCT04836507 | ||
| Combined with Fludarabine and allogenic CD19-car T cells | NCT05554939 | ||||
| Cyclophosphamide and doxorubicin | I/II | Recruiting | Combined with Rituximab and Doxorubicin | NCT04663347 | |
| Oxaliplatin | II | Recruiting | Combined with Lacutamab and Gemcitabine | NCT04984837 | |
| Multiple myeloma | Bortozomib | I | Recruiting | Combined with REGN5458, Daratumumab, Carfilzomib, Lenalidomide and Dexamethasone | NCT05137054 |
| Cyclophosphamide | I | Recruiting | Combined with ALLO-715/647, Fludarabine and Nirogacestat | NCT04093596 | |
| Rnrolling by invitation | Combined with Fludarabine and − 29 CAR-T cells | NCT04861480 | |||
| II | Recruiting | Combined with Fludarabine and BCMA targeting t cells | NCT05594797 | ||
| Myeloid malignancies | Mitoxantrone | II | Recruiting | Combined with Magrolimab, Etoposide and Cytarabine | NCT04778410 |
| NSCLC | Oxaliplatin | I/II | Recruiting | Combined with Nivolumab and Ipilimumab | NCT04043195 |
| Ovarian cancer | Doxorubicin | I | Recruiting | Combined with SL-172154 | NCT05483933 |
| Cyclophosphamide | I | Recruiting | Combined with Fludarabine and CAR T cells | NCT05225363 | |
| Pancreatic cancer | FOLFIRINOX | I/II | Recruiting | Combined with Mitazalimab | NCT04888312 |
| Rectal cancer | Oxaliplatin | II | Recruiting | Combined with Tislelizumab and Capecitabine | NCT05420584 |
| Combined with Capecitabine and anti-PD1 | NCT05307198 | ||||
| Sarcoma | Doxorubicin | I/II | Not yet recruiting | Combined with YH001 and Envafolimab | NCT05448820 |
| Solid tumor | Cyclophosphamide | I | Recruiting | Combined with neoantigen peptide vaccine, Pembrolizumab and Sargramostim | NCT05269381 |
| Terminated | Combined with GEN-011, IL2 and Fludarabine | NCT04596033 | |||
| Oxaliplatin | I | Recruiting | Combined with HB002.1T and Capecitabine | NCT04802980 | |
| Oxaliplatin or Paclitaxel | I/II | Recruiting | Combined with AK104/AK117, Cisplatin, 5FU | NCT05235542 |
5-FU, 5-fluorouracil; CAR-NK cells, Car natural killer cells; CAR, chimeric antigen receptor; CRC, colorectal cancer; PD-1, programmed death-1; TIL, tumor-infiltrating lymphocyte; NSCLC, non-small cell lung carcinoma.
Table 2.
Contemporary clinical studies assessing the therapeutic and immunological characteristics of paclitaxel.
| Cancer type | ICD inducer | Phase | Status | Combination | Trial number |
|---|---|---|---|---|---|
| Breast cancer | Nab-Paclitaxel | II/III | Not yet recruiting | Combined with EOC202 | NCT05322720 |
| Nab-Paclitaxel | II | recruiting | Combined with SG001 | NCT05068141 | |
| II/III | Not yet recruiting | Combined with B013 | NCT05555706 | ||
| Paclitaxel | I | Not yet recruiting | Combined with Eftilagimob alpha | NCT04252768 | |
| I | Recruiting | Combined with Durvalumab and Trastuzumab Deruxtecan | NCT04556773 | ||
| I/II | Recruiting | Combined with Trastuzumab Deruxtecan | NCT04538742 | ||
| II | Recruiting | Combined with DC vaccines and Trastuzumab and Pertuzumab | NCT05325632 | ||
| III | Recruiting | Combined with Capecitabine and Trastuzumab Deruxtecan | NCT04494425 | ||
| III | Recruiting | Combined with Dato-DXd, Carboplatin, Capecitabine and Eribulin Mesylate | NCT05374512 | ||
| Eribulin and Paclitaxel | IV | Recruiting | NCT05033769 | ||
| Cervical cancer | Paclitaxel | I | Active, not recruiting | Combined with M7824, Carboplatin, Bevacizumab and Cisplatin | NCT04551950 |
| II | Active, not recruiting | Combined with AK104, Bevacizumab, Cisplatin or Carboplatin | NCT04868708 | ||
| II/III | Recruiting | Combined with QL1604 and Cisplatin or Carboplatin | NCT04864782 | ||
| III | Recruiting | Combined with AK104 and Carboplatin, Cisplatin, Bevacizumab | NCT04982237 | ||
| Endometrial neoplasm | Paclitaxel | III | Recruiting | Combined with Olaparib, Durvalumab and Carboplatin | NCT04269200 |
| Gastric cancer | Paclitaxel | II | Recruiting | Combined with IMU-131 and Pembrolizumab | NCT05311176 |
| Gastric or Gastroesophageal cancer | Paclitaxel | II/III | Not yet recruiting | Combined with QL1604 | NCT04435652 |
| III | Recruiting | Combined with Trastuzumab Deruxtecan and Ramucirumab | NCT04704934 | ||
| Head and neck squamous carcinoma | Paclitaxel | I | Recruiting | Combined with SCT-I10A, SCT200 and Docetaxel | NCT05552807 |
| Melanoma and Pancreatic cancer | Nab-Paclitaxel | II | Recruiting | Combined with YH003, Toripalimab and Gemcitabine | NCT05031494 |
| Nasopharyngeal carcinoma | Paclitaxel | I | aAtive, not recruiting | Combined with SHR-1316 and Carboplatin, Gemcitabine and Cisplatin | NCT04282070 |
| NSCLC | Nab-Paclitaxel | III | Not yet recruiting | Combined with Sintilimab, Carboplatin, Cisplatin, Pemetrexed, Docetaxel and Gemcitabine | NCT05116462 |
| Paclitaxel | I/II | Active, not recruiting | Combined with AK104 and carboplatin | NCT04647344 | |
| II | Recruiting | Combined with Carboplatin | NCT04832854 | ||
| III | Recruiting | Combined with SHR-1316 and Carboplatin | NCT04316364 | ||
| III | Terminated | Combined with carboplatin and Bevacizumab or PF-06439535 | NCT04325698 | ||
| III | Unknown | Combined TRS003, Bevacizumab and Carboplatin | NCT04416035 | ||
| III | Completed | Combined with Bp102 or Avastin and Carboplatin | NCT05169801 | ||
| III | Recruiting | Combined with SIBP04, Avastin and Carboplatin | NCT05318443 | ||
| III | Recruiting | Combined with Cisplatin, Carboplatin, Etoposide, Pemetrexed and Ociperlimab or Tislelizumab or Durvalumab | NCT04866017 | ||
| Ovarian cancer | Paclitaxel | I/II | Recruiting | Combined with TILs, interferon and Carboplatin | NCT04072263 |
| Paclitaxel or Doxorubicin | III | Recruiting | Combined with BD0801 | NCT04908787 | |
| Paclitaxel and Doxorubicin | III | Active, not recruiting | Combined with Mirvetuximab Soravtansine and Topotecan | NCT04209855 | |
| Pancreatic cancer | Nab-Paclitaxel | I | Recruiting | Combined with AB680, Zimberelimab and Gemcitabine | NCT04104672 |
| I | Active, not recruiting | Combined with Canakinumab, Spartalizumab and Gemcitabine | NCT04581343 | ||
| II | Recruiting | Combined with NIS793, Spartalizumab and Gemcitabine | NCT04390763 | ||
| Solid tumor | Nab-Paclitaxel | I/II | Recruiting | Combined with DF1001 and Nicolumab | NCT04143711 |
| I/II | Active, not recruiting | Combined with YH003, Toripalimab and Gemcitabine | NCT04481009 | ||
| I/II | Recruiting | Combined with LYT-200, anti-PD1 and Gemcitabine | NCT04666688 | ||
| II | Recruiting | Combined with camrelizumab and famitinib | NCT05214976 | ||
| II | Recruiting | Combined with AZD0171, Durvalumab and Gemcitabine | NCT04999969 | ||
| Paclitaxel | I | Not yet recruiting | Combined with KM257 | NCT05320874 | |
| II | Recruiting | Combined with adoptive T cells | NCT05144698 | ||
| II | Recruiting | Combined with Navicixizumab | NCT05453825 | ||
| Paclitaxel or Oxaliplatin | I | Recruiting | Combined with Ociperlimab, Tislelizumab, Carboplatin, Cisplatin, 5-FU and Capecitabine | NCT04047862 | |
| I/II | Recruiting | Combined with AK104/AK117, Cisplatin, 5FU | NCT05235542 |
5-FU, 5-fluorouracil; TIL, tumor-infiltrating lymphocyte; NSCLC, non-small cell lung carcinoma.
Most of the clinical trials selected for this Trial Watch analysis applied paclitaxel, cyclophosphamide, oxaliplatin, and doxorubicin, not only as a monotherapy but also in combination with other anti-cancer treatments. The most prevalent ICD inducer is paclitaxel as shown in Table 2. However, only a small percentage of the studies assesses ICD as the main study objective. Most of the time, studies include treatment arms of the ICD inducer with and without combinatorial therapies. In general, ICD inducers are mostly combined with (1) other cell death inducers including other chemotherapeutics, such as carboplatin, or radiation therapy; (2) ICBs targeting molecules such as anti-PD-1 or anti-PD-L1 antibodies; (3) tumor-targeting passive immunotherapies such as agents against epidermal growth factor receptor (EGFR) and HER2; (4) T cells that are adoptively transferred or T cells expressing engineered TAA-specific transgenic TCRs; (5) cytokines that further stimulate the immune responses including not only interferon alpha (IFNA) but also mixtures like IRX-2 (IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12, TNF, and IFNγ). These trends are very similar to the previous Trial Watch215 as well as the published clinical trials that we summarized above.
In these selected clinical trials, there are multiple immunological assessment methodologies that were examined with the aim of acquiring a predictive marker for treatment response as or assessing the immune response during treatment. This includes T cell analysis, either via IHC, e.g., to estimate the quantity and phenotypes of tumor-infiltrating T cells or via flow cytometry, e.g., for detailed profiling of the peripheral blood immune cell subsets. For example, clinical trial NCT05033769 is going to assess KI-67 on peripheral T cells. Additionally, in some ongoing clinical trials, such as NCT04868708, PD-L1 expression of the tumor tissue samples is being determined by IHC. In addition, in this clinical trial they will also investigate the development of anti-drug antibodies over 30 days after the last treatment. This latter practice is being performed in many other clinical trials (e.g., NCT04282070, NCT04093596, and NCT04888312). Another biomarker being utilized is antigen-specific immunogenicity especially in CAR T cell or adoptive T cell clinical trials (e.g., NCT04596033). Evaluating the presence of specific or several cytokines is also prioritized in some clinical trials. For example, NCT04895761 aims to assess the IFNγ in breast cancer patients treated with cyclophosphamide or radiotherapy in combination with a neoadjuvant aromatase inhibitor. In each treatment arm, an ELISPOT will be performed in PBMCs. Lastly, multiple ongoing clinical studies are evaluating immunological markers on a larger scale via omics technologies. Omics approaches like RNA sequencing are creating many variables that can give functional patterns associated with T cell status shifting after chemotherapy. So far, a long list of ways to create some sort of immunological output is being practiced in these clinical trials. Yet, there are still many clinical trials that are not planning on assessing any markers for immune response (e.g., NCT04637763, NCT05504252).
Conclusion
Currently, various chemotherapeutics linked to ICD are approved worldwide for use as anti-cancer treatment in patients with multiple cancer subtypes. Approval of most of these chemotherapies was largely preceded by pre-clinical investigations with tumor xenografts269–272 in immunodeficient mice and therefore these were often translated to the clinic without any validation for immune-modulation273–275. Therefore, most of these chemotherapies are currently utilized at doses and treatment schedules that are meant to achieve tumor reduction via maximal tolerable dose276–279 rather than a balance between short-term (tumor reduction) and long-term (anti-cancer immunity) effects. In this sense, overcoming some common side effects of chemotherapies that can also counteract ICD, i.e., neutropenia,280–284 lymphopenia285–289 and intestinal mucositis290–292, should be prioritized via more tumor-targeted delivery of chemotherapies through nanoparticles or other controlled delivery methods293,294.
The ‘first generation’ of anti-cancer immunotherapy has nearly passed. Despite the stunning success of immuno-oncology drugs through a broad spectrum of distinct malignant diseases, it turned out that a large majority of patients do not respond to currently approved immunotherapies, or if they do, responses are mostly transient. Currently, the field of immuno-oncology aims to tackle these immunotherapy-resistant contexts via multi-modal anti-cancer therapies integrating anti-cancer agents, falling into different treatment modalities (radiotherapy, chemotherapy, targeted therapy, and immunotherapy). However, systematically designed as well as multi-arm comparative clinical studies coupled with proper immune biomarkers aimed at identifying correct dosing and treatment scheduling/ordering are pending. Such studies are crucial to maximize the immune-activation relevant synergism between active immunotherapies and ICD-inducing chemotherapies. Simultaneously, pre-selection of patients via specific biomarkers is also necessary to tailor these multi-modal immunotherapies to specific patient sub-groups rather than giving it in a nonspecific fashion thereby contributing to socio-economic healthcare burden.
Acknowledgments
This study was supported by Research Foundation Flanders (FWO) (Fundamental Research Grant, G0B4620N to ADG; Excellence of Science/EOS grant, 30837538, for ‘DECODE’ consortium, for ADG), KU Leuven (C1 grant, C14/19/098; C3 grant, C3/21/037; and POR award funds, POR/16/040 to ADG), Kom op Tegen Kanker (KOTK/2018/11509/1 to ADG; and KOTK/2019/11955/1 to ADG), and VLIR-UOS (iBOF grant, iBOF/21/048, for ‘MIMICRY’ consortium to ADG). I.V. was supported by FWO-SB PhD Fellowship (1S06821N). J.S. was funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish cancer society via Emmanuel van der Schueren (EvDS) PhD fellowship (projectID: 12699). D.M.B. was supported by KU Leuven’s Postdoctoral FWO fellowship (1279223N). R.S. was supported by FWO-SB PhD Fellowship (1S44123N); E.S. was supported by the KOTK (KOTK/2019/7878); O.K. was supported by Institut National du Cancer (INCa) and the DIM ELICIT; D.V.K. was supported by FWO-Flanders (G016221N) and Excellence of Science/EOS grant, 40007488. Research in the Vandenabeele unit was supported by the FWO (research grants G.0C76.18N, G.0B71.18N, G.0B96.20N, G.0A93.22N, EOS MODEL-IDI Grant (30826052), and EOS CD-INFLADIS (40007512)), grants from the Special Research Fund UGent (Methusalem grant BOF16/MET_V/007, iBOF ATLANTIS grant 20/IBF/039), grants from the Foundation against Cancer (F/2016/865, F/2020/1505), CRIG and GIGG consortia, and VIB.
Funding Statement
This study is supported by Research Foundation Flanders (FWO) (Fundamental Research Grant, G0B4620N to ADG; Excellence of Science/EOS grant, 30837538, for ‘DECODE’ consortium, for ADG), KU Leuven (C1 grant, C14/19/098; C3 grant, C3/21/037; and POR award funds, POR/16/040 to ADG), Kom op Tegen Kanker (KOTK/2018/11509/1 and KOTK/2019/11955/1 to ADG) and VLIR-UOS (iBOF grant, iBOF/21/048, for ‘MIMICRY’ consortium to ADG and ST). IV and RSL are supported by FWO-SB PhD Fellowship (1S06821N and 1S44123N). JS is funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish cancer society through the Emmanuel van der Schueren (EvDS) PhD fellowship (projectID: 12699). DMB got support from Senior Postdoctoral FWO fellowship (1279223N) and KU Leuven’s Postdoctoral mandate grant (PDMT1/21/032).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Ahmed A, Tait SWG.. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14:2994–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troitskaya OS, Novak DD, Richter VA, Koval OA. Immunogenic cell death in cancer therapy. Acta Naturae. 2022;14:40–53. doi: 10.32607/actanaturae.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fumet J-D, Limagne E, Thibaudin M, Ghiringhelli F. Immunogenic cell death and elimination of immunosuppressive cells: a double-edged sword of chemotherapy. Cancers (Basel). 2020;12:12. doi: 10.3390/cancers12092637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J ImmunoTher Cancer. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiernicki B, Maschalidi S, Pinney J, Adjemian S, Vanden Berghe T, Ravichandran KS, Vandenabeele P. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat Commun. 2022;13:3676. doi: 10.1038/s41467-022-31218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zi M, Xingyu C, Yang C, Xiaodong S, Shixian L, Shicheng W. Improved antitumor immunity of chemotherapy in OSCC treatment by Gasdermin-E mediated pyroptosis. Apoptosis. 2022;28:348–361. doi: 10.1007/s10495-022-01792-3. [DOI] [PubMed] [Google Scholar]
- 7.Oltean T, Lippens L, Lemeire K, De Tender C, Vuylsteke M, Denys H, Vandecasteele K, Vandenabeele P, Adjemian S. Association of cell death markers with tumor immune cell infiltrates after chemo-radiation in cervical cancer. Front Oncol. 2022;12:892813. doi: 10.3389/fonc.2022.892813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishchenko T, Balalaeva I, Gorokhova A, Vedunova M, Krysko DV. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Disease. 2022;13:455. doi: 10.1038/s41419-022-04851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santofimia-Castaño P, Iovanna J. Combating pancreatic cancer chemoresistance by triggering multiple cell death pathways. Pancreatology. 2021;21:522–529. doi: 10.1016/j.pan.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Wu M, Liu X, Shao S, Huang J, Liu B, Liang T. Pyroptosis remodeling tumor microenvironment to enhance pancreatic cancer immunotherapy driven by membrane anchoring photosensitizer. Adv Sci (Weinh). 2022;9:e2202914. doi: 10.1002/advs.202202914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, Heyndrickx L, Delrue I, Taminau J, Wiernicki B, De Groote P, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15:274–287. doi: 10.1016/j.celrep.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Sprooten J, De Wijngaert P, Vanmeerbeerk I, Martin S, Vangheluwe P, Schlenner S, Krysko DV, Parys JB, Bultynck G, Vandenabeele P, et al. Necroptosis in immuno-oncology and cancer immunotherapy. Cells. 2020;9:1823. doi: 10.3390/cells9081823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C, Bachert C, Coppieters F, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J ImmunoTher Cancer. 2020;8:e001369. doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21:678–695. doi: 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 15.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18:127–136. doi: 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- 16.Kepp O, Kroemer G. Is ferroptosis immunogenic? The devil is in the details! Oncoimmunology. 2022;11:2127273. doi: 10.1080/2162402X.2022.2127273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Dai E, Kang R, Kroemer G, Tang D. The dark side of ferroptosis in pancreatic cancer. Oncoimmunology. 2021;10:1868691. doi: 10.1080/2162402X.2020.1868691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J-Y, Yao Y-M, Y-P T. Ferroptosis: a trigger of proinflammatory state progression to immunogenicity in necroinflammatory disease. Front Immunol. 2021;12:701163. doi: 10.3389/fimmu.2021.701163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demuynck R, Efimova I, Naessens F, Krysko DV. Immunogenic ferroptosis and where to find it? J ImmunoTher Cancer. 2021;9:e003430. doi: 10.1136/jitc-2021-003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D, Zhang F, Ning S, Yao J, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye F, Chai W, Xie M, Yang M, Yu Y, Cao L, Yang L. HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRASQ61L cells. Am J Cancer Res. 2019;9:730–739. [PMC free article] [PubMed] [Google Scholar]
- 23.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong SD, Jung B-K, Lee D, Ha J, Chang H-G, Lee J, Lee S, Yun C-O, Kim Y-C. Enhanced immunogenic cell death by apoptosis/ferroptosis hybrid pathway potentiates PD-L1 blockade cancerimmunotherapy. ACS Biomater Sci Eng. 2022;8:5188–5198. doi: 10.1021/acsbiomaterials.2c00950. [DOI] [PubMed] [Google Scholar]
- 25.Tang X, Liu J, Yao S, Zheng J, Gong X, Xiao B. Ferulic acid alleviates alveolar epithelial barrier dysfunction in sepsis-induced acute lung injury by activating the Nrf2/HO-1 pathway and inhibiting ferroptosis. Pharm Biol. 2022;60:2286–2294. doi: 10.1080/13880209.2022.2147549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He L, Wang B, Wang X, Liu Y, Song X, Zhang Y, Li X, Yang H. Uncover diagnostic immunity/hypoxia/ferroptosis/epithelial mesenchymal transformation-related CCR5. CD86, CD8A, ITGAM, And PTPRC In Kidney Transplantation Patients With Allograft Rejection. Ren Fail. 2022;44:1850–1865. doi: 10.1080/0886022X.2022.2141648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuliani KTK, Grivei A, Nag P, Wang X, Rist M, Kildey K, Law B, Ng MS, Wilkinson R, Ungerer J, et al. Hypoxic human proximal tubular epithelial cells undergo ferroptosis and elicit an NLRP3 inflammasome response in CD1c+ dendritic cells. Cell Death Disease. 2022;13:739. doi: 10.1038/s41419-022-05191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, Hashimoto A, Kapralov A, Amoscato A, Angelini R, et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat Commun. 2017;8:2122. doi: 10.1038/s41467-017-02186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klöditz K, Fadeel B. Three cell deaths and a funeral: macrophage clearance of cells undergoing distinct modes of cell death. Cell Death Discov. 2019;5:65. doi: 10.1038/s41420-019-0146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM, Klein-Seetharaman J, Celis E, et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol. 2014;192:2920–2931. doi: 10.4049/jimmunol.1302801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ugolini A, Tyurin VA, Tyurina YY, Tcyganov EN, Donthireddy L, Kagan VE, Gabrilovich DI, Veglia F. Polymorphonuclear myeloid-derived suppressor cells limit antigen cross-presentation by dendritic cells in cancer. JCI Insight. 2020;5. doi: 10.1172/jci.insight.138581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiernicki B, Dubois H, Tyurina YY, Hassannia B, Bayir H, Kagan VE, Vandenabeele P, Wullaert A, Vanden Berghe T. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Disease. 2020;11:922. doi: 10.1038/s41419-020-03118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J, Zhang Z, Wang X. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol Cell. 2021;81:355–369.e10. doi: 10.1016/j.molcel.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Liu Y, Li K, Yuan D, Yang S, Zhou L, Zhao Y, Miao S, Lv C, Zhao J. COX-2/PGE2 pathway inhibits the ferroptosis induced by cerebral ischemia reperfusion. Mol Neurobiol. 2022;59:1619–1631. doi: 10.1007/s12035-021-02706-1. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Wang J, Chen S, Wu P, Xu S, Wang C, Shi H, Bihl J. MiR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res Ther. 2020;11:330. doi: 10.1186/s13287-020-01836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi K, Nikolos F, Lee YC, Jain A, Tsouko E, Gao H, Kasabyan A, Leung HE, Osipov A, Jung SY, et al. Tipping the immunostimulatory and inhibitory DAMP balance to harness immunogenic cell death. Nat Commun. 2020;11:6299. doi: 10.1038/s41467-020-19970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim R, Hashimoto A, Markosyan N, Tyurin VA, Tyurina YY, Kar G, Fu S, Sehgal M, Garcia-Gerique L, Kossenkov A, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338–346. doi: 10.1038/s41586-022-05443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Zhang Y, Yan S, Zhang G, Wei W, Qi Z, Li B. Alternol triggers immunogenic cell death via reactive oxygen species generation. Oncoimmunology. 2021;10:1952539. doi: 10.1080/2162402X.2021.1952539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galluzzi L, Yamazaki T, Kroemer G. Linking cellular stress responses to systemic homeostasis. Nat Rev Mol Cell Biol. 2018;19:731–745. doi: 10.1038/s41580-018-0068-0. [DOI] [PubMed] [Google Scholar]
- 40.Nayagom B, Amara I, Habiballah M, Amrouche F, Beaune P, de Waziers I. Immunogenic cell death in a combined synergic gene- and immune-therapy against cancer. Oncoimmunology. 2019;8:e1667743. doi: 10.1080/2162402X.2019.1667743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clemen R, Arlt K, von Woedtke T, Bekeschus S. Gas plasma protein oxidation increases immunogenicity and human antigen-presenting cell maturation and activation. Vaccines (Basel). 2022;10:10. doi: 10.3390/vaccines10111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Li J, Wu Y, Xu X, Qian X, Lei Y, Liu H, Zhang Z, Li Y. ROS-Responsive nanocomplex of Apd-L1 and cabazitaxel improves intratumor delivery and potentiates radiation-mediated antitumor immunity. Nano Lett. 2022;22:8312–8320. doi: 10.1021/acs.nanolett.2c03227. [DOI] [PubMed] [Google Scholar]
- 43.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund A-C, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. Embo J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJM, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. Embo J. 2012;31:1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini J-L, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 46.Giglio P, Gagliardi M, Bernardini R, Mattei M, Cotella D, Santoro C, Piacentini M, Corazzari M. Ecto-calreticulin is essential for an efficient immunogenic cell death stimulation in mouse melanoma. Genes Immun. 2019;20:509–513. doi: 10.1038/s41435-018-0047-7. [DOI] [PubMed] [Google Scholar]
- 47.Fucikova J, Kasikova L, Truxova I, Laco J, Skapa P, Ryska A, Spisek R. Relevance of the chaperone-like protein calreticulin for the biological behavior and clinical outcome of cancer. Immunol Lett. 2018;193:25–34. doi: 10.1016/j.imlet.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Venkateswaran K, Verma A, Bhatt AN, Shrivastava A, Manda K, Raj HG, Prasad A, Len C, Parmar VS, Dwarakanath BS. Emerging roles of calreticulin in cancer: implications for therapy. Curr Protein Pept Sci. 2018;19:344–357. doi: 10.2174/1389203718666170111123253. [DOI] [PubMed] [Google Scholar]
- 49.Vaes RDW, Reynders K, Sprooten J, Nevola KT, Rouschop KMA, Vooijs M, Garg AD, Lambrecht M, Hendriks LEL, Rucevic M, et al. Identification of potential prognostic and predictive immunological biomarkers in patients with stage i and stage iii non-small cell lung cancer (NSCLC): a prospective exploratory study. Cancers (Basel). 2021;13:6259. doi: 10.3390/cancers13246259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiu Z, Sun T, Yang Y, He Y, Yang S, Xue X, Yang W, Wang B. Curcumin enhanced ionizing radiation-induced immunogenic cell death in glioma cells through endoplasmic reticulum stress signaling pathways. Oxid Med Cell Longev. 2022;2022:1–17. doi: 10.1155/2022/5424411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao L, Li D, Zhang Y, Huang Q, Zhang Z, Chen C, Xu C-F, Chu X, Zhang Y, Yang X. HSP70-promoter-driven CRISPR/Cas9 system activated by reactive oxygen species for multifaceted anticancer immune response and potentiated immunotherapy. Acs Nano. 2022;16:13821–13833. doi: 10.1021/acsnano.2c01885. [DOI] [PubMed] [Google Scholar]
- 52.Luo Y, Chihara Y, Fujimoto K, Sasahira T, Kuwada M, Fujiwara R, Fujii K, Ohmori H, Kuniyasu H. High mobility group box 1 released from necrotic cells enhances regrowth and metastasis of cancer cells that have survived chemotherapy. Eur J Cancer. 2013;49:741–751. doi: 10.1016/j.ejca.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Kroemer G, Kepp O. Radiochemotherapy-induced elevations of plasma HMGB1 levels predict therapeutic responses in cancer patients. Oncoimmunol. 2021;10:2005859. doi: 10.1080/2162402X.2021.2005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He C, Sun S, Zhang Y, Xie F, Li S. The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer. Oncoimmunol. 2021;10:1897295. doi: 10.1080/2162402X.2021.1897295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turubanova VD, Mishchenko TA, Balalaeva IV, Efimova I, Peskova NN, Klapshina LG, Lermontova SA, Bachert C, Krysko O, Vedunova MV, et al. Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. null. 2021;11:7205. doi: 10.1038/s41598-021-86354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turubanova VD, Balalaeva IV, Mishchenko TA, Catanzaro E, Alzeibak R, Peskova NN, Efimova I, Bachert C, Mitroshina EV, Krysko O, et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J ImmunoTher Cancer. 2019;7:350. doi: 10.1186/s40425-019-0826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G, Galluzzi L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Disease. 2020;11:1013. doi: 10.1038/s41419-020-03221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arai H, Xiao Y, Loupakis F, Kawanishi N, Wang J, Battaglin F, Soni S, Zhang W, Mancao C, Salhia B, et al. Immunogenic cell death pathway polymorphisms for predicting oxaliplatin efficacy in metastatic colorectal cancer. J ImmunoTher Cancer. 2020;8:e001714. doi: 10.1136/jitc-2020-001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baracco EE, Petrazzuolo A, Kroemer G. Assessment of annexin A1 release during immunogenic cell death. Methods Enzymol. 2019;629:71–79. [DOI] [PubMed] [Google Scholar]
- 60.Baracco EE, Stoll G, Van Endert P, Zitvogel L, Vacchelli E, Kroemer G. Contribution of annexin A1 to anticancer immunosurveillance. Oncoimmunology. 2019;8:e1647760. doi: 10.1080/2162402X.2019.1647760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Sun X, Zhao X, Yang C, Shi M, Zhang B, Hu H, Qiao M, Chen D, Zhao X. Combining immune checkpoint blockade with ATP-based immunogenic cell death amplifier for cancer chemo-immunotherapy. Acta Pharm Sin B. 2022;12:3694–3709. doi: 10.1016/j.apsb.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kepp O, Bezu L, Yamazaki T, Di Virgilio F, Smyth MJ, Kroemer G, Galluzzi L. ATP and cancer immunosurveillance. Embo J. 2021;40:e108130. doi: 10.15252/embj.2021108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serrano Del Valle A, Beltrán-Visiedo M, de Poo-Rodríguez V, Jiménez-Alduán N, Azaceta G, Díez R, Martínez-Lázaro B, Izquierdo I, Palomera L, Naval J, et al. Ecto-calreticulin expression in multiple myeloma correlates with a failed anti-tumoral immune response and bad prognosis. Oncoimmunology. 2022;11:2141973. doi: 10.1080/2162402X.2022.2141973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sprooten J, Vanmeerbeek I, Datsi A, Govaerts J, Borras D, Naulaerts S, Laureano R, Calvet A, Kuballa M, Sabel M, et al. A lymph node-to-tumour PD-L1+macrophage circuit antagonizes dendritic cell immunotherapy. bioRxiv. 2023. doi: 10.1101/2023.03.14.532534;. [DOI] [Google Scholar]
- 66.Pan C, Wang Y, Liu Q, Hu Y, Fu J, Xie X, Zhang S, Xi M, Wen J. Phenotypic profiling and prognostic significance of immune infiltrates in esophageal squamous cell carcinoma. Oncoimmunology. 2021;10:1883890. doi: 10.1080/2162402X.2021.1883890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest. 2007;117:3660–3663. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Showalter A, Limaye A, Oyer JL, Igarashi R, Kittipatarin C, Copik AJ, Khaled AR. Cytokines in immunogenic cell death: applications for cancer immunotherapy. Cytokine. 2017;97:123–132. doi: 10.1016/j.cyto.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Martino M, Vanpouille-Box C. Type I interferon induces cancer stem cells-mediated chemotherapy resistance. Oncoimmunology. 2022;11:2127274. doi: 10.1080/2162402X.2022.2127274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanmeerbeek I, Govaerts J, Laureano RS, Sprooten J, Naulaerts S, Borras DM, Laoui D, Mazzone M, Van Ginderachter JA, Garg AD. The interface of tumour-associated macrophages with dying cancer cells in immuno-oncology. Cells. 2022;11(23):3890. doi: 10.3390/cells11233890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murgaski A, Kiss M, Van Damme H, Kancheva D, Vanmeerbeek I, Keirsse J, Hadadi E, Brughmans J, Arnouk SM, Hamouda AEI, et al. Efficacy of CD40 agonists is mediated by distinct cdc subsets and subverted by suppressive macrophages. Cancer Res. 2022;82:3785–3801. doi: 10.1158/0008-5472.CAN-22-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sprooten J, Coosemans A, Garg AD. A first-in-class, non-invasive, immunodynamic biomarker approach for precision immuno-oncology. Oncoimmunology. 2022;11:2024692. doi: 10.1080/2162402X.2021.2024692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garg AD, Vandenberk L, Fang S, Fasche T, Van Eygen S, Maes J, Van Woensel M, Koks C, Vanthillo N, Graf N, et al. Pathogen response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing. Cell Death Differ. 2017;24:832–843. doi: 10.1038/cdd.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castiello L, Zevini A, Vulpis E, Muscolini M, Ferrari M, Palermo E, Peruzzi G, Krapp C, Jakobsen M, Olagnier D, et al. An optimized retinoic acid-inducible gene I agonist M8 induces immunogenic cell death markers in human cancer cells and dendritic cell activation. Cancer Immunol Immunother. 2019;68:1479–1492. doi: 10.1007/s00262-019-02380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lau TS, Chan LKY, Man GCW, Wong CH, Lee JHS, Yim SF, Cheung TH, McNeish IA, Kwong J. Paclitaxel induces immunogenic cell death in ovarian cancer via TLR4/IKK2/SNARE-Dependent exocytosis. Cancer Immunol Res. 2020;8:1099–1111. doi: 10.1158/2326-6066.CIR-19-0616. [DOI] [PubMed] [Google Scholar]
- 76.Forveille S, Sauvat A, Zhang S, Zhao L, Kroemer G, Kepp O. Assessment of type I interferon responses as a feature of immunogenic cell death. Methods Cell Biol. 2022;172:135–143. [DOI] [PubMed] [Google Scholar]
- 77.Sansone C, Bruno A, Piscitelli C, Baci D, Fontana A, Brunet C, Noonan DM, Albini A. Natural compounds of marine origin as inducers of immunogenic cell death (ICD): potential role for cancer interception and therapy. Cells. 2021;10:10. doi: 10.3390/cells10020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126–148. doi: 10.1111/imr.12574. [DOI] [PubMed] [Google Scholar]
- 79.Sprooten J, Garg AD. Type I interferons and endoplasmic reticulum stress in health and disease. Int Rev Cell Mol Biol. 2020;350:63–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sprooten J, Agostinis P, Garg AD. Type I interferons and dendritic cells in cancer immunotherapy. Int Rev Cell Mol Biol. 2019;348:217–262. [DOI] [PubMed] [Google Scholar]
- 81.Klein JC, Wild CA, Lang S, Brandau S. Differential immunomodulatory activity of tumor cell death induced by cancer therapeutic toll-like receptor ligands. Cancer Immunol Immunother. 2016;65:689–700. doi: 10.1007/s00262-016-1828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lopez-Pelaez M, Young L, Vazquez-Chantada M, Nelson N, Durant S, Wilkinson RW, Poon E, Gaspar M, Valge-Archer V, Smith P, et al. Targeting DNA damage response components induces enhanced STING-dependent type-I IFN response in ATM deficient cancer cells and drives dendritic cell activation. Oncoimmunology. 2022;11:2117321. doi: 10.1080/2162402X.2022.2117321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roussot N, Ghiringhelli F, Rébé C. Tumor immunogenic cell death as a mediator of intratumor CD8 T-Cell recruitment. Cells. 2022;11(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radogna F, Diederich M. Stress-induced cellular responses in immunogenic cell death: implications for cancer immunotherapy. Biochem Pharmacol. 2018;153:12–23. [DOI] [PubMed] [Google Scholar]
- 85.Maher J, Adami AA. Antitumor immunity: easy as 1, 2, 3 with monoclonal bispecific trifunctional antibodies? Cancer Res. 2013;73:5613–5617. doi: 10.1158/0008-5472.CAN-13-1852. [DOI] [PubMed] [Google Scholar]
- 86.Pocaterra A, Catucci M, Mondino A. Adoptive T cell therapy of solid tumors: time to team up with immunogenic chemo/radiotherapy. Curr Opin Immunol. 2022;74:53–59. doi: 10.1016/j.coi.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Darmon A, Zhang P, Marill J, Mohamed Anesary N, Da Silva J, Paris S. Radiotherapy-activated NBTXR3 nanoparticles modulate cancer cell immunogenicity and TCR repertoire. Cancer Cell Int. 2022;22:208. doi: 10.1186/s12935-022-02615-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Minute L, Teijeira A, Sanchez-Paulete AR, Ochoa MC, Alvarez M, Otano I, Etxeberrria I, Bolaños E, Azpilikueta A, Garasa S, et al. Cellular cytotoxicity is a form of immunogenic cell death. J ImmunoTher Cancer. 2020;8:8. doi: 10.1136/jitc-2019-000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malviya V, Yshii L, Junius S, Garg AD, Humblet-Baron S, Schlenner SM. Regulatory T cell stability and functional plasticity in health and disease. Immunol Cell Biol. 2022;101:112–129. doi: 10.1111/imcb.12613. [DOI] [PubMed] [Google Scholar]
- 90.Dillard P, Casey N, Pollmann S, Vernhoff P, Gaudernack G, Kvalheim G, Wälchli S, Inderberg EM. Targeting KRAS mutations with HLA class II-restricted TCRs for the treatment of solid tumors. Oncoimmunology. 2021;10:1936757. doi: 10.1080/2162402X.2021.1936757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naulaerts S, Datsi A, Borras DM, Antoranz Martinez A, Messiaen J, Vanmeerbeek I, Sprooten J, Laureano RS, Govaerts J, Panovska D, et al. Multiomics and spatial mapping characterizes human CD8+ T cell states in cancer. Sci Transl Med. 2023;15:eadd1016. doi: 10.1126/scitranslmed.add1016. [DOI] [PubMed] [Google Scholar]
- 92.Luo R, Onyshchenko K, Wang L, Gaedicke S, Grosu A-L, Firat E, Niedermann G. Necroptosis-dependent immunogenicity of cisplatin: implications for enhancing the radiation-induced abscopal effect. Clin Cancer Res. 2022;29(3):667–683. [DOI] [PubMed] [Google Scholar]
- 93.Bian Q, Huang L, Xu Y, Wang R, Gu Y, Yuan A, Ma X, Hu J, Rao Y, Xu D, et al. A facile low-dose photosensitizer-incorporated dissolving microneedles-based composite system for eliciting antitumor immunity and the abscopal effect. Acs Nano. 2021;15:19468–19479. doi: 10.1021/acsnano.1c06225. [DOI] [PubMed] [Google Scholar]
- 94.Franzese O, Torino F, Giannetti E, Cioccoloni G, Aquino A, Faraoni I, Fuggetta MP, De Vecchis L, Giuliani A, Kaina B, et al. Abscopal effect and drug-induced xenogenization: a strategic alliance in cancer treatment? Int J Mol Sci. 2021;22:22. doi: 10.3390/ijms221910672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arabpour M, Paul S, Grauers Wiktorin H, Kaya M, Kiffin R, Lycke N, Hellstrand K, Martner A. An adjuvant-containing cDC1-targeted recombinant fusion vaccine conveys strong protection against murine melanoma growth and metastasis. Oncoimmunology. 2022;11:2115618. doi: 10.1080/2162402X.2022.2115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koerner J, Horvath D, Oliveri F, Li J, Basler M. Suppression of prostate cancer and amelioration of the immunosuppressive tumor microenvironment through selective immunoproteasome inhibition. Oncoimmunology. 2023;12:2156091. doi: 10.1080/2162402X.2022.2156091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yasmin-Karim S, Ziberi B, Wirtz J, Bih N, Moreau M, Guthier R, Ainsworth V, Hesser J, Makrigiorgos GM, Chuong MD, et al. Boosting the abscopal effect using immunogenic biomaterials with varying radiation therapy field sizes. Int J Radiat Oncol Biol Phys. 2022;112:475–486. doi: 10.1016/j.ijrobp.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xing D, Siva S, Hanna GG. The abscopal effect of stereotactic radiotherapy and immunotherapy: fool’s gold or el dorado? Clin Oncol (R Coll Radiol. 2019;31:432–443. doi: 10.1016/j.clon.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 99.Moreau M, Yasmin-Karim S, Kunjachan S, Sinha N, Gremse F, Kumar R, Chow KF, Ngwa W. Priming the abscopal effect using multifunctional smart radiotherapy biomaterials loaded with immunoadjuvants. Front Oncol. 2018;8:56. doi: 10.3389/fonc.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin C, Wang Y, Li Y, Li J, Zhou S, Yu J, Wang Z, Yu Y, Zhang H, Wang D, et al. Doxorubicin-Near infrared dye conjugate induces immunogenic cell death to enhance cancer immunotherapy. Int J Pharm. 2021;607:121027. doi: 10.1016/j.ijpharm.2021.121027. [DOI] [PubMed] [Google Scholar]
- 101.Zhou M, Luo C, Zhou Z, Li L, Huang Y. Improving anti-PD-L1 therapy in triple negative breast cancer by polymer-enhanced immunogenic cell death and CXCR4 blockade. J Control Release. 2021;334:248–262. doi: 10.1016/j.jconrel.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 102.Zheng J, Sun J, Chen J, Zhu S, Chen S, Liu Y, Hao L, Wang Z, Chang S. Oxygen and oxaliplatin-loaded nanoparticles combined with photo-sonodynamic inducing enhanced immunogenic cell death in syngeneic mouse models of ovarian cancer. J Control Release. 2021;332:448–459. doi: 10.1016/j.jconrel.2021.02.032. [DOI] [PubMed] [Google Scholar]
- 103.Galaine J, Turco C, Vauchy C, Royer B, Mercier-Letondal P, Queiroz L, Loyon R, Mouget V, Boidot R, Laheurte C, et al. CD4 T cells target colorectal cancer antigens upregulated by oxaliplatin. Int J Cancer. 2019;145:3112–3125. doi: 10.1002/ijc.32620. [DOI] [PubMed] [Google Scholar]
- 104.Zhou Y, Bastian IN, Long MD, Dow M, Li W, Liu T, Ngu RK, Antonucci L, Huang JY, Phung QT, et al. Activation of NF-κB and p300/CBP potentiates cancer chemoimmunotherapy through induction of MHC-I antigen presentation. Proc Natl Acad Sci USA. 2021;118:118. doi: 10.1073/pnas.2025840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lei X, Khatri I, de Wit T, de Rink I, Nieuwland M, Kerkhoven R, van Eenennaam H, Sun C, Garg AD, Borst J, et al. CD4+ helper T cells endow cDC1 with cancer-impeding functions in the human tumor micro-environment. Nat Commun. 2023;14:217. doi: 10.1038/s41467-022-35615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 107.Xie D, Wang Q, Wu G. Research progress in inducing immunogenic cell death of tumor cells. Front Immunol. 2022;13:1017400. doi: 10.3389/fimmu.2022.1017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23:487–500. doi: 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- 109.Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21:298–312. doi: 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- 110.Vanmeerbeek I, Borras DM, Sprooten J, Bechter O, Tejpar S, Garg AD. Early memory differentiation and cell death resistance in T cells predicts melanoma response to sequential anti-CTLA4 and anti-PD1 immunotherapy. Genes Immun. 2021;22:108–119. doi: 10.1038/s41435-021-00138-4. [DOI] [PubMed] [Google Scholar]
- 111.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 112.Fucikova J, Moserova I, Urbanova L, Bezu L, Kepp O, Cremer I, Salek C, Strnad P, Kroemer G, Galluzzi L, et al. Prognostic and predictive value of DAMPs and DAMP-associated processes in cancer. Front Immunol. 2015;6:402. doi: 10.3389/fimmu.2015.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodrigues MC, Morais JAV, Ganassin R, Oliveira GRT, Costa FC, Morais AAC, Silveira AP, Silva VCM, Longo JPF, Muehlmann LA. An overview on immunogenic cell death in cancer biology and therapy. Pharmaceutics. 2022;14:14. doi: 10.3390/pharmaceutics14081564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 115.Rufo N, Garg AD, Agostinis P. The unfolded protein response in immunogenic cell death and cancer immunotherapy. Trends Cancer. 2017;3:643–658. doi: 10.1016/j.trecan.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 116.Wang J, Zhang H, Yin X, Bian Y, Zhang J. Oxaliplatin induces immunogenic cell death in human and murine laryngeal cancer. J Oncol. 2022;2022:1–12. doi: 10.1155/2022/3760766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shi M, Zhang J, Wang Y, Han Y, Zhao X, Hu H, Qiao M, Chen D. Blockage of the IDO1 pathway by charge-switchable nanoparticles amplifies immunogenic cell death for enhanced cancer immunotherapy. Acta Biomater. 2022;150:353–366. doi: 10.1016/j.actbio.2022.07.022. [DOI] [PubMed] [Google Scholar]
- 118.Lévesque S, Le Naour J, Pietrocola F, Paillet J, Kremer M, Castoldi F, Baracco EE, Wang Y, Vacchelli E, Stoll G, et al. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology. 2019;8:e1657375. doi: 10.1080/2162402X.2019.1657375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vienot A, Pallandre J-R, Renaude E, Viot J, Bouard A, Spehner L, Kroemer M, Abdeljaoued S, van der Woning B, de Haard H, et al. Chemokine switch regulated by TGF-β1 in cancer-associated fibroblast subsets determines the efficacy of chemo-immunotherapy. Oncoimmunology. 2022;11:2144669. doi: 10.1080/2162402X.2022.2144669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim R, Kin T. Current and future therapies for immunogenic cell death and related molecules to potentially cure primary breast cancer. Cancers (Basel). 2021;13:4756. doi: 10.3390/cancers13194756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Y, Xiong T, Zhao X, Du J, Sun W, Fan J, Peng X. Tumor cell-responsive photodynamic immunoagent for immunogenicity-enhanced orthotopic and remote tumor therapy. Adv Healthcare Mater. 2022;12:e2202085. doi: 10.1002/adhm.202202085. [DOI] [PubMed] [Google Scholar]
- 122.Liu X, Liu Y, Li X, Huang J, Guo X, Zhang J, Luo Z, Shi Y, Jiang M, Qin B, et al. ER-Targeting PDT converts tumors into in situ therapeutic tumor vaccines. Acs Nano. 2022;16:9240–9253. doi: 10.1021/acsnano.2c01669. [DOI] [PubMed] [Google Scholar]
- 123.Zeng Q, Yang J, Ji J, Wang P, Zhang L, Yan G, Wu Y, Chen Q, Liu J, Zhang G, et al. PD-L1 blockade potentiates the antitumor effects of ALA-PDT and optimizes the tumor microenvironment in cutaneous squamous cell carcinoma. Oncoimmunology. 2022;11:2061396. doi: 10.1080/2162402X.2022.2061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kudling TV, Clubb JHA, Quixabeira DCA, Santos JM, Havunen R, Kononov A, Heiniö C, Cervera-Carrascon V, Pakola S, Basnet S, et al. Local delivery of interleukin 7 with an oncolytic adenovirus activates tumor-infiltrating lymphocytes and causes tumor regression. Oncoimmunology. 2022;11:2096572. doi: 10.1080/2162402X.2022.2096572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang X, Bian M, Liu L, Yang J, Yang Z, Wang Z, Lu Y, Liu W. Induction of immunogenic cell death by novel platinum-based anticancer agents. Pharmacol Res. 2022;187:106556. doi: 10.1016/j.phrs.2022.106556. [DOI] [PubMed] [Google Scholar]
- 126.Nishimura J, Deguchi S, Tanaka H, Yamakoshi Y, Yoshii M, Tamura T, Toyokawa T, Lee S, Muguruma K, Ohira M. Induction of immunogenic cell death of esophageal squamous cell carcinoma by 5-fluorouracil and cisplatin. Vivo. 2021;35:743–752. doi: 10.21873/invivo.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu P, Chen J, Zhao L, Hollebecque A, Kepp O, Zitvogel L, Kroemer G. PD-1 blockade synergizes with oxaliplatin-based, but not cisplatin-based, chemotherapy of gastric cancer. Oncoimmunology. 2022;11:2093518. doi: 10.1080/2162402X.2022.2093518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bag A, Schultz A, Bhimani S, Stringfield O, Dominguez W, Mo Q, Cen L, Adeegbe D. Coupling the immunomodulatory properties of the HDAC6 inhibitor ACY241 with Oxaliplatin promotes robust anti-tumor response in non-small cell lung cancer. Oncoimmunology. 2022;11:2042065. doi: 10.1080/2162402X.2022.2042065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krackhardt AM, Anliker B, Hildebrandt M, Bachmann M, Eichmüller SB, Nettelbeck DM, Renner M, Uharek L, Willimsky G, Schmitt M, et al. Clinical translation and regulatory aspects of CAR/TCR-based adoptive cell therapies—the German cancer consortium approach. Cancer Immunol Immunother. 2018;67:513–523. doi: 10.1007/s00262-018-2119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krysko DV, Demuynck R, Efimova I, Naessens F, Krysko O, Catanzaro E. In Vitro veritas: from 2D cultures to organ-on-a-chip models to study immunogenic cell death in the tumor microenvironment. Cells. 2022;11:11. doi: 10.3390/cells11223705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun T, Li Y, Yang Y, Liu B, Cao Y, Yang W. Enhanced radiation-induced immunogenic cell death activates chimeric antigen receptor T cells by targeting CD39 against glioblastoma. Cell Death Disease. 2022;13:875. doi: 10.1038/s41419-022-05319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vergato C, Doshi KA, Roblyer D, Waxman DJ. Type-I interferon signaling is essential for robust metronomic chemo-immunogenic tumor regression in murine breast cancer. Cancer Res Commun. 2022;2:246–257. doi: 10.1158/2767-9764.CRC-21-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW, Agostinis P. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med. 2016;8:328ra27. doi: 10.1126/scitranslmed.aae0105. [DOI] [PubMed] [Google Scholar]
- 135.Aaes TL, Vandenabeele P. The intrinsic immunogenic properties of cancer cell lines, immunogenic cell death, and how these influence host antitumor immune responses. Cell Death Differ. 2021;28:843–860. doi: 10.1038/s41418-020-00658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Uscanga-Palomeque AC, Calvillo-Rodríguez KM, Gómez-Morales L, Lardé E, Denèfle T, Caballero-Hernández D, Merle-Béral H, Susin SA, Karoyan P, Martínez-Torres AC, et al. CD47 agonist peptide PKHB1 induces immunogenic cell death in T-cell acute lymphoblastic leukemia cells. Cancer Sci. 2019;110:256–268. doi: 10.1111/cas.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen J, Jin Z, Zhang S, Zhang X, Li P, Yang H, Ma Y. Arsenic trioxide elicits prophylactic and therapeutic immune responses against solid tumors by inducing necroptosis and ferroptosis. Cell Mol Immunol. 2022;20:51–64. doi: 10.1038/s41423-022-00956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martínez-Torres AC, Calvillo-Rodríguez KM, Uscanga-Palomeque AC, Gómez-Morales L, Mendoza-Reveles R, Caballero-Hernández D, Karoyan P, Rodríguez-Padilla C. PKHB1 tumor cell lysate induces antitumor immune system stimulation and tumor regression in syngeneic mice with tumoral T lymphoblasts. J Oncol. 2019;2019:9852361. doi: 10.1155/2019/9852361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fang S, Agostinis P, Salven P, Garg AD. Decoding cancer cell death-driven immune cell recruitment: an in vivo method for site-of-vaccination analyses. Methods Enzymol. 2020;636:185–207. [DOI] [PubMed] [Google Scholar]
- 140.Workenhe ST, Pol J, Kroemer G. Tumor-intrinsic determinants of immunogenic cell death modalities. Oncoimmunology. 2021;10:1893466. doi: 10.1080/2162402X.2021.1893466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oduro PK, Zheng X, Wei J, Yang Y, Wang Y, Zhang H, Liu E, Gao X, Du M, Wang Q. The Cgas-STING signaling in cardiovascular and metabolic diseases: future novel target option for pharmacotherapy. Acta Pharm Sin B. 2022;12:50–75. doi: 10.1016/j.apsb.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim EH, Wong S-W, Martinez J. Programmed necrosis and disease: we interrupt your regular programming to bring you necroinflammation. Cell Death Differ. 2019;26:25–40. doi: 10.1038/s41418-018-0179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Faiz A, Heijink IH, Vermeulen CJ, Guryev V, van den Berge M, Nawijn MC, Pouwels SD. Cigarette smoke exposure decreases CFLAR expression in the bronchial epithelium, augmenting susceptibility for lung epithelial cell death and DAMP release. null. 2018;8:12426. doi: 10.1038/s41598-018-30602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chiang S-F, Huang K-Y, Chen W-L, Chen T-W, Ke T-W, Chao KSC. An independent predictor of poor prognosis in locally advanced rectal cancer: rs867228 in formyl peptide receptor 1 (FPR1). Oncoimmunology. 2021;10:1926074. doi: 10.1080/2162402X.2021.1926074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu P, Zhao L, Loos F, Marty C, Xie W, Martins I, Lachkar S, Qu B, Waeckel-Énée E, Plo I, et al. Immunosuppression by mutated calreticulin released from malignant cells. Mol Cell. 2020;77:748–760.e9. doi: 10.1016/j.molcel.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 146.Brieske C, Lamprecht P, Kerstein-Staehle A. Immunogenic cell death as driver of autoimmunity in granulomatosis with polyangiitis. Front Immunol. 2022;13:1007092. doi: 10.3389/fimmu.2022.1007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Novohradsky V, Pracharova J, Kasparkova J, Imberti C, Bridgewater HE, Sadler PJ, Brabec V. Induction of immunogenic cell death in cancer cells by a photoactivated platinum(IV) prodrug. Inorg Chem Front. 2020;7:4150–4159. doi: 10.1039/D0QI00991A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matsusaka K, Azuma Y, Kaga Y, Uchida S, Takebayashi Y, Tsuyama T, Tada S. Distinct roles in phagocytosis of the early and late increases of cell surface calreticulin induced by oxaliplatin. Biochem Biophys Rep. 2022;29:101222. doi: 10.1016/j.bbrep.2022.101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li F, Zheng X, Wang X, Xu J, Zhang Q. Macrophage polarization synergizes with oxaliplatin in lung cancer immunotherapy via enhanced tumor cell phagocytosis. Transl Oncol. 2021;14:101202. doi: 10.1016/j.tranon.2021.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sequeira GR, Sahores A, Dalotto-Moreno T, Perrotta RM, Pataccini G, Vanzulli SI, Polo ML, Radisky DC, Sartorius CA, Novaro V, et al. Enhanced antitumor immunity via endocrine therapy prevents mammary tumor relapse and increases immune checkpoint blockade sensitivity. Cancer Res. 2021;81:1375–1387. doi: 10.1158/0008-5472.CAN-20-1441. [DOI] [PubMed] [Google Scholar]
- 151.Tomić S, Petrović A, Puač N, Škoro N, Bekić M, Petrović ZL, Čolić M. Plasma-activated medium potentiates the immunogenicity of tumor cell lysates for dendritic cell-based cancer vaccines. Cancers (Basel). 2021;13:1626. doi: 10.3390/cancers13071626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Truxova I, Kasikova L, Salek C, Hensler M, Lysak D, Holicek P, Bilkova P, Holubova M, Chen X, Mikyskova R, et al. Calreticulin exposure on malignant blasts correlates with improved natural killer cell-mediated cytotoxicity in acute myeloid leukemia patients. Haematologica. 2020;105:1868–1878. doi: 10.3324/haematol.2019.223933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laureano RS, Sprooten J, Vanmeerbeerk I, Borras DM, Govaerts J, Naulaerts S, Berneman ZN, Beuselinck B, Bol KF, Borst J, et al. Trial watch: dendritic cell (DC)-based immunotherapy for cancer. Oncoimmunology. 2022;11:2096363. doi: 10.1080/2162402X.2022.2096363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hensler M, Rakova J, Kasikova L, Lanickova T, Pasulka J, Holicek P, Hraska M, Hrnciarova T, Kadlecova P, Schoenenberger A, et al. Peripheral gene signatures reveal distinct cancer patient immunotypes with therapeutic implications for autologous DC-based vaccines. Oncoimmunology. 2022;11:2101596. doi: 10.1080/2162402X.2022.2101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor cell-derived IL1β promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 2020;80:1088–1101. doi: 10.1158/0008-5472.CAN-19-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang AC, Rahavi SM, Fung S-Y, Lu HY, Yang H, Lim CJ, Reid GS, Turvey SE. Combination therapy with proteasome inhibitors and TLR agonists enhances tumour cell death and IL-1β production. Cell Death Disease. 2018;9:162. doi: 10.1038/s41419-017-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Petrovski G, Ayna G, Majai G, Hodrea J, Benko S, Mádi A, Fésüs L. Phagocytosis of cells dying through autophagy induces inflammasome activation and IL-1β release in human macrophages. Autophagy. 2011;7:321–330. doi: 10.4161/auto.7.3.14583. [DOI] [PubMed] [Google Scholar]
- 158.Tang L, Cai D, Qin M, Lu S, Hu M-H, Ruan S, Jin G, Wang Z. Oxaliplatin-based platinum(IV) prodrug bearing toll-like receptor 7 agonist for enhanced immunochemotherapy. ACS Omega. 2020;5:726–734. doi: 10.1021/acsomega.9b03381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beltrán Hernández I, Angelier ML, Del Buono D’Ondes T, Di Maggio A, Yu Y, Oliveira S. The potential of nanobody-targeted photodynamic therapy to trigger immune responses. Cancers (Basel). 2020;12:12. doi: 10.3390/cancers12040978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tang X, Guo D, Yang X, Chen R, Jiang Q, Zeng Z, Li Y, Li Z. Upregulated immunogenic cell-death-associated gene signature predicts reduced responsiveness to immune-checkpoint-blockade therapy and poor prognosis in high-grade gliomas. Cells. 2022;11(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bent EH, Millán-Barea LR, Zhuang I, Goulet DR, Fröse J, Hemann MT. Microenvironmental IL-6 inhibits anti-cancer immune responses generated by cytotoxic chemotherapy. Nat Commun. 2021;12:6218. doi: 10.1038/s41467-021-26407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang D, Cong J, Fu B, Zheng X, Sun R, Tian Z, Wei H. Immunogenic chemotherapy effectively inhibits KRAS-Driven lung cancer. Cancer Lett. 2020;492:31–43. doi: 10.1016/j.canlet.2020.07.043. [DOI] [PubMed] [Google Scholar]
- 163.Ishii K, Shimizu M, Kogo H, Negishi Y, Tamura H, Morita R, Takahashi H. A combination of check-point blockade and α-galactosylceramide elicits long-lasting suppressive effects on murine hepatoma cell growth in vivo. Immunobiology. 2020;225:151860. [DOI] [PubMed] [Google Scholar]
- 164.Varga Z, Rácz E, Mázló A, Korodi M, Szabó A, Molnár T, Szöőr Á, Veréb Z, Bácsi A, Koncz G. Cytotoxic activity of human dendritic cells induces RIPK1-dependent cell death. Immunobiology. 2021;226:152032. doi: 10.1016/j.imbio.2020.152032. [DOI] [PubMed] [Google Scholar]
- 165.Serrano R, Lettau M, Zarobkiewicz M, Wesch D, Peters C, Kabelitz D. Stimulatory and inhibitory activity of STING ligands on tumor-reactive human gamma/delta T cells. Oncoimmunology. 2022;11:2030021. doi: 10.1080/2162402X.2022.2030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sprooten J, Vankerckhoven A, Vanmeerbeek I, Borras DM, Berckmans Y, Wouters R, Laureano RS, Baert T, Boon L, Landolfo C, et al. Peripherally-driven myeloid NFkB and IFN/ISG responses predict malignancy risk, survival, and immunotherapy regime in ovarian cancer. J ImmunoTher Cancer. 2021;9:e003609. doi: 10.1136/jitc-2021-003609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leclercq G, Servera LA, Danilin S, Challier J, Steinhoff N, Bossen C, Odermatt A, Nicolini V, Umaña P, Klein C, et al. Dissecting the mechanism of cytokine release induced by T-cell engagers highlights the contribution of neutrophils. Oncoimmunology. 2022;11:2039432. doi: 10.1080/2162402X.2022.2039432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 169.Liu J, Meng Y, Li B, Wang P, Wan X, Huang W, Li R. Ferroptosis-related biotargets and network mechanisms of fucoidan against colorectal cancer: an integrated bioinformatic and experimental approach. Int J Biol Macromol. 2022;222:1522–1530. doi: 10.1016/j.ijbiomac.2022.09.255. [DOI] [PubMed] [Google Scholar]
- 170.Zhu C, Fang Z, Peng L, Gao F, Peng W, Song F. Curcumin suppresses the progression of colorectal cancer by improving immunogenic cell death caused by irinotecan. Chemotherapy. 2022;67:211–222. doi: 10.1159/000518121. [DOI] [PubMed] [Google Scholar]
- 171.Jeong H, Lee S-Y, Seo H, Kim DH, Lee D, Kim B-J. Potential of Mycobacterium tuberculosis chorismate mutase (Rv1885c) as a novel TLR4-mediated adjuvant for dendritic cell-based cancer immunotherapy. Oncoimmunology. 2022;11:2023340. doi: 10.1080/2162402X.2021.2023340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pipperger L, Riepler L, Kimpel J, Siller A, Stoitzner P, Bánki Z, von Laer D. Differential infection of murine and human dendritic cell subsets by oncolytic vesicular stomatitis virus variants. Oncoimmunology. 2021;10:1959140. doi: 10.1080/2162402X.2021.1959140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rébé C, Demontoux L, Pilot T, Ghiringhelli F. Platinum derivatives effects on anticancer immune response. Biomolecules. 2019;10:10. doi: 10.3390/biom10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lamberti MJ, Montico B, Ravo M, Nigro A, Giurato G, Iorio R, Tarallo R, Weisz A, Stellato C, Steffan A, et al. Integration of miRNA: mRNA co-expression revealed crucial mechanisms modulated in immunogenic cancer cell death. Biomedicines. 2022;10:1896. doi: 10.3390/biomedicines10081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Birmpilis AI, Paschalis A, Mourkakis A, Christodoulou P, Kostopoulos IV, Antimissari E, Terzoudi G, Georgakilas AG, Armpilia C, Papageorgis P, et al. Immunogenic cell death, damps and prothymosin α as a putative anticancer immune response biomarker. Cells. 2022;11:11. doi: 10.3390/cells11091415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cacan E, Ozmen ZC. Regulation of Fas in response to bortezomib and epirubicin in colorectal cancer cells. J Chemother. 2020;32:193–201. doi: 10.1080/1120009X.2020.1740389. [DOI] [PubMed] [Google Scholar]
- 177.Jie Y, Yang X, Chen W. Pulsatilla decoction combined with 5-fluorouracil triggers immunogenic cell death in the colorectal cancer cells. Cancer Biother Radiopharm. 2021;37:945–954. doi: 10.1089/cbr.2020.4369. [DOI] [PubMed] [Google Scholar]
- 178.Jiang M, Zeng J, Zhao L, Zhang M, Ma J, Guan X, Zhang W. Chemotherapeutic drug-induced immunogenic cell death for nanomedicine-based cancer chemo-immunotherapy. Nanoscale. 2021;13:17218–17235. doi: 10.1039/D1NR05512G. [DOI] [PubMed] [Google Scholar]
- 179.Pol JG, Le Naour J, Kroemer G. FLT3LG - a biomarker reflecting clinical responses to the immunogenic cell death inducer oxaliplatin. Oncoimmunology. 2020;9:1755214. doi: 10.1080/2162402X.2020.1755214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Krysko DV, Kaczmarek A, Krysko O, Heyndrickx L, Woznicki J, Bogaert P, Cauwels A, Takahashi N, Magez S, Bachert C, et al. TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell Death Differ. 2011;18:1316–1325. doi: 10.1038/cdd.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kepp O, Cerrato G, Sauvat A, Kroemer G. Nanoparticles releasing immunogenic cell death inducers upon near-infrared light exposure. Oncoimmunology. 2022;11:2131227. doi: 10.1080/2162402X.2022.2131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pol JG, Plantureux C, Pérez-Lanzón M, Kroemer G. PDIA3 as a potential bridge between immunogenic cell death and autoreactivity. Oncoimmunology. 2022;11:2130558. doi: 10.1080/2162402X.2022.2130558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Corazzari M, Rapino F, Ciccosanti F, Giglio P, Antonioli M, Conti B, Fimia GM, Lovat PE, Piacentini M. Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell Death Differ. 2015;22:946–958. doi: 10.1038/cdd.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wen H, Zhong Y, Yin Y, Qin K, Yang L, Li D, Yu W, Yang C, Deng Z, Hong K. A marine-derived small molecule induces immunogenic cell death against triple-negative breast cancer through ER stress-CHOP pathway. Int J Biol Sci. 2022;18:2898–2913. doi: 10.7150/ijbs.70975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li X, Zheng J, Chen S, Meng F-D, Ning J, Sun S-L. Oleandrin, a cardiac glycoside, induces immunogenic cell death via the PERK/elF2α/ATF4/CHOP pathway in breast cancer. Cell Death Disease. 2021;12:314. doi: 10.1038/s41419-021-03605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xu Q, Chen C, Lin A, Xie Y. Endoplasmic reticulum stress-mediated membrane expression of CRT/ERp57 induces immunogenic apoptosis in drug-resistant endometrial cancer cells. Oncotarget. 2017;8:58754–58764. doi: 10.18632/oncotarget.17678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Verfaillie T, van Vliet A, Garg AD, Dewaele M, Rubio N, Gupta S, de Witte P, Samali A, Agostinis P. Pro-apoptotic signaling induced by photo-oxidative ER stress is amplified by Noxa, not Bim. Biochem Biophys Res Commun. 2013;438:500–506. doi: 10.1016/j.bbrc.2013.07.107. [DOI] [PubMed] [Google Scholar]
- 188.Fang C, Weng T, Hu S, Yuan Z, Xiong H, Huang B, Cai Y, Li L, Fu X. IFN-γ-induced ER stress impairs autophagy and triggers apoptosis in lung cancer cells. Oncoimmunology. 2021;10:1962591. doi: 10.1080/2162402X.2021.1962591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yadollahvandmiandoab R, Jalalizadeh M, Buosi K, Garcia-Perdomo HA, Reis LO. Immunogenic cell death role in urothelial cancer therapy. Curr Oncol. 2022;29:6700–6713. doi: 10.3390/curroncol29090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang X, Huang H, Liu X, Li J, Wang L, Li L, Li Y, Han T. Immunogenic cell death-related classifications in breast cancer identify precise immunotherapy biomarkers and enable prognostic stratification. Front Genet. 2022;13:1052720. doi: 10.3389/fgene.2022.1052720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang W, Liu T, Jiang L, Chen J, Li Q, Wang J. Immunogenic cell death-related gene landscape predicts the overall survival and immune infiltration status of ovarian cancer. Front Genet. 2022;13:1001239. doi: 10.3389/fgene.2022.1001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Han Y, Cai Q, Xie X, Gao S, Fan X. Development and validation of prognostic index based on immunogenic cell death-related genes with melanoma. Front Oncol. 2022;12:1011046. doi: 10.3389/fonc.2022.1011046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cao X, Zhou X, Chen C, Wang Z, Sun Q. Identification of tumor antigens and immunogenic cell death-related subtypes for the improvement of immunotherapy of breast cancer. Front Cell Dev Biol. 2022;10:962389. doi: 10.3389/fcell.2022.962389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fu J, Zhang W, Jiang T. Immunogenic cell death mediation patterns reveal novel paradigm for characterizing the immune microenvironment and immunotherapeutic responses in bladder cancer. Front Genet. 2022;13:1035484. doi: 10.3389/fgene.2022.1035484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liao X, Liu H, Zhang Z, Zhang J, Zhang C, Zhao W. An immunogenic cell death-associated classification predictions are important for breast invasive carcinoma prognosis and immunotherapy. Front Genet. 2022;13:1010787. doi: 10.3389/fgene.2022.1010787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ding D, Zhao Y, Su Y, Yang H, Wang X, Chen L. Prognostic value of antitumor drug targets prediction using integrated bioinformatic analysis for immunogenic cell death-related lncRNA model based on stomach adenocarcinoma characteristics and tumor immune microenvironment. Front Pharmacol. 2022;13:1022294. doi: 10.3389/fphar.2022.1022294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kofla G, Radecke C, Frentsch M, Walther W, Stintzing S, Riess H, Bullinger L, Na IK. Conventional amphotericin B elicits markers of immunogenic cell death on leukemic blasts, mediates immunostimulatory effects on phagocytic cells, and synergizes with PD-L1 blockade. Oncoimmunology. 2022;11:2068109. doi: 10.1080/2162402X.2022.2068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Eid M, Ostřížková L, Kunovský L, Brančíková D, Kala Z, Hlavsa J, Janeček P, Kosíková I, Blažková M, Slabý O, et al. Current view of neoadjuvant chemotherapy in primarily resectable pancreatic adenocarcinoma. Neoplasma. 2021;68:1–9. doi: 10.4149/neo_2020_200408N372. [DOI] [PubMed] [Google Scholar]
- 199.Iacoboni G, Zucca E, Ghielmini M, Stathis A. Methodology of clinical trials evaluating the incorporation of new drugs in the first-line treatment of patients with diffuse large B-cell lymphoma (DLBCL): a critical review. Ann Oncol. 2018;29:1120–1129. doi: 10.1093/annonc/mdy113. [DOI] [PubMed] [Google Scholar]
- 200.Wei D, Qi J, Hamblin MR, Wen X, Jiang X, Yang H. Near-infrared photoimmunotherapy: design and potential applications for cancer treatment and beyond. Theranostics. 2022;12:7108–7131. doi: 10.7150/thno.74820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brennan L, Brouwer-Visser J, Nüesch E, Karpova M, Heller A, Gaire F, Schneider M, Gomes B, Korski K. T-Cell heterogeneity in baseline tumor samples: implications for early clinical trial design and analysis. Front Immunol. 2022;13:760763. doi: 10.3389/fimmu.2022.760763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Van Gool SW, Makalowski J, Fiore S, Sprenger T, Prix L, Schirrmacher V, Stuecker W. Randomized controlled immunotherapy clinical trials for GBM challenged. Cancers (Basel). 2020;13:13. doi: 10.3390/cancers13010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cho H, Kim JE, Hong YS, Kim SY, Kim J, Ryu Y-M, Kim S-Y, Kim TW. Comprehensive evaluation of the tumor immune microenvironment and its dynamic changes in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy: from the phase II ADORE study. Oncoimmunology. 2022;11:2148374. doi: 10.1080/2162402X.2022.2148374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huang Z, Wang Y, Yao D, Wu J, Hu Y, Yuan A. Nanoscale coordination polymers induce immunogenic cell death by amplifying radiation therapy mediated oxidative stress. Nat Commun. 2021;12:145. doi: 10.1038/s41467-020-20243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21:120–134. doi: 10.1038/s41590-019-0561-4. [DOI] [PubMed] [Google Scholar]
- 206.Vaes RDW, Hendriks LEL, Vooijs M, De Ruysscher D. Biomarkers of radiotherapy-induced immunogenic cell death. Cells. 2021;10:10. doi: 10.3390/cells10040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Prasit KK, Ferrer-Font L, Burn OK, Anderson RJ, Compton BJ, Schmidt AJ, Mayer JU, Chen C-J, Dasyam N, Ritchie DS, et al. Intratumoural administration of an NKT cell agonist with CpG promotes NKT cell infiltration associated with an enhanced antitumour response and abscopal effect. Oncoimmunology. 2022;11:2081009. doi: 10.1080/2162402X.2022.2081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Patel RB, Hernandez R, Carlson P, Grudzinski J, Bates AM, Jagodinsky JC, Erbe A, Marsh IR, Arthur I, Aluicio-Sarduy E, et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci Transl Med. 2021;13. doi: 10.1126/scitranslmed.abb3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lejeune P, Cruciani V, Berg-Larsen A, Schlicker A, Mobergslien A, Bartnitzky L, Berndt S, Zitzmann-Kolbe S, Kamfenkel C, Stargard S, et al. Immunostimulatory effects of targeted thorium-227 conjugates as single agent and in combination with anti-PD-L1 therapy. J ImmunoTher Cancer. 2021;9:e002387. doi: 10.1136/jitc-2021-002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shekarian T, Sivado E, Jallas A-C, Depil S, Kielbassa J, Janoueix-Lerosey I, Hutter G, Goutagny N, Bergeron C, Viari A, et al. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade. Sci Transl Med. 2019;11:11. doi: 10.1126/scitranslmed.aat5025. [DOI] [PubMed] [Google Scholar]
- 211.Li X, Lu M, Yuan M, Ye J, Zhang W, Xu L, Wu X, Hui B, Yang Y, Wei B, et al. CXCL10-armed oncolytic adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance anti-PD-1 therapy. Oncoimmunology. 2022;11:2118210. doi: 10.1080/2162402X.2022.2118210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang Q, Ma X, Wu H, Zhao C, Chen J, Li R, Yan S, Li Y, Zhang Q, Song K, et al. Oncolytic adenovirus with MUC16-BiTE shows enhanced antitumor immune response by reversing the tumor microenvironment in PDX model of ovarian cancer. Oncoimmunology. 2022;11:2096362. doi: 10.1080/2162402X.2022.2096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tappe KA, Budida R, Stankov MV, Frenz T, Shah R, Volz A, Sutter G, Kalinke U, Behrens GMN. Immunogenic cell death of dendritic cells following modified vaccinia virus Ankara infection enhances CD8+ T cell proliferation. Eur J Immunol. 2018;48:2042–2054. doi: 10.1002/eji.201847632. [DOI] [PubMed] [Google Scholar]
- 214.He T, Hao Z, Lin M, Xin Z, Chen Y, Ouyang W, Yang Q, Chen X, Zhou H, Zhang W, et al. Oncolytic adenovirus promotes vascular normalization and nonclassical tertiary lymphoid structure formation through STING-mediated DC activation. Oncoimmunology. 2022;11:2093054. doi: 10.1080/2162402X.2022.2093054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vanmeerbeek I, Sprooten J, De Ruysscher D, Tejpar S, Vandenberghe P, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L, et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology. 2020;9:1703449. doi: 10.1080/2162402X.2019.1703449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mandula JK, Chang S, Mohamed E, Jimenez R, Sierra-Mondragon RA, Chang DC, Obermayer AN, Moran-Segura CM, Das S, Vazquez-Martinez JA, et al. Ablation of the endoplasmic reticulum stress kinase PERK induces paraptosis and type I interferon to promote anti-tumor T cell responses. Cancer Cell. 2022;40:1145–1160.e9. doi: 10.1016/j.ccell.2022.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Oresta B, Pozzi C, Braga D, Hurle R, Lazzeri M, Colombo P, Frego N, Erreni M, Faccani C, Elefante G, et al. Mitochondrial metabolic reprogramming controls the induction of immunogenic cell death and efficacy of chemotherapy in bladder cancer. Sci Transl Med. 2021;13. doi: 10.1126/scitranslmed.aba6110. [DOI] [PubMed] [Google Scholar]
- 218.Lucarini V, Melaiu O, D’Amico S, Pastorino F, Tempora P, Scarsella M, Pezzullo M, De Ninno A, D’Oria V, Cilli M, et al. Combined mitoxantrone and anti-TGFβ treatment with PD-1 blockade enhances antitumor immunity by remodelling the tumor immune landscape in neuroblastoma. J Exp Clin Cancer Res. 2022;41:326. doi: 10.1186/s13046-022-02525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Humeau J, Sauvat A, Cerrato G, Xie W, Loos F, Iannantuoni F, Bezu L, Lévesque S, Paillet J, Pol J, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med. 2020;12:e11622. doi: 10.15252/emmm.201911622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ioele G, Chieffallo M, Occhiuzzi MA, De Luca M, Garofalo A, Ragno G, Grande F. Anticancer drugs: recent strategies to improve stability profile, pharmacokinetic and pharmacodynamic properties. Molecules. 2022;27:27. doi: 10.3390/molecules27175436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, Ruano I, Attolini C-O, Prats N, López-Domínguez JA, et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 2023;13:410–431. doi: 10.1158/2159-8290.CD-22-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kepp O, Kroemer G. A nanoparticle-based tour de force for enhancing immunogenic cell death elicited by photodynamic therapy. Oncoimmunology. 2022;11:2098658. doi: 10.1080/2162402X.2022.2098658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhou Z, Yang R, Dong J, Di Y, Yang Y, Huang Y, Yang X, Liu W, Wang J, Liu P, et al. Pore forming–mediated intracellular protein delivery for enhanced cancer immunotherapy. Sci Adv. 2022;8:eabq4659. doi: 10.1126/sciadv.abq4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yang Q, Ma X, Xiao Y, Zhang T, Yang L, Yang S, Liang M, Wang S, Wu Z, Xu Z, et al. Engineering prodrug nanomicelles as pyroptosis inducer for codelivery of PI3K/mTOR and CDK inhibitors to enhance antitumor immunity. Acta Pharm Sin B. 2022;12:3139–3155. doi: 10.1016/j.apsb.2022.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Song H, Cai Z, Li J, Xiao H, Qi R, Zheng M. Light triggered release of a triple action porphyrin-cisplatin conjugate evokes stronger immunogenic cell death for chemotherapy, photodynamic therapy and cancer immunotherapy. J Nanobiotechnology. 2022;20:329. doi: 10.1186/s12951-022-01531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tatarova Z, Blumberg DC, Korkola JE, Heiser LM, Muschler JL, Schedin PJ, Ahn SW, Mills GB, Coussens LM, Jonas O, et al. A multiplex implantable microdevice assay identifies synergistic combinations of cancer immunotherapies and conventional drugs. Nat Biotechnol. 2022;40:1823–1833. doi: 10.1038/s41587-022-01379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zawilska P, Machowska M, Wisniewski K, Grynkiewicz G, Hrynyk R, Rzepecki R, Gubernator J. Novel pegylated liposomal formulation of docetaxel with 3-n-pentadecylphenol derivative for cancer therapy. Eur J Pharm Sci. 2021;163:105838. doi: 10.1016/j.ejps.2021.105838. [DOI] [PubMed] [Google Scholar]
- 228.Lamberti MJ, Nigro A, Casolaro V, Rumie Vittar NB, Dal Col J. Damage-associated molecular patterns modulation by microRNA: relevance on immunogenic cell death and cancer treatment outcome. Cancers (Basel). 2021;13:2566. doi: 10.3390/cancers13112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yan X, Yu H, Liu Y, Hou J, Yang Q, Zhao Y. MiR-27a-3p functions as a tumor suppressor and regulates non-small cell lung cancer cell proliferation via targeting HOXB8. Technol Cancer Res Treat. 2019;18:1533033819861971. doi: 10.1177/1533033819861971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 230.Colangelo T, Polcaro G, Ziccardi P, Muccillo L, Galgani M, Pucci B, Milone MR, Budillon A, Santopaolo M, Mazzoccoli G, et al. The miR-27a-calreticulin axis affects drug-induced immunogenic cell death in human colorectal cancer cells. Cell Death Disease. 2016;7:e2108. doi: 10.1038/cddis.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.He S-J, Cheng J, Feng X, Yu Y, Tian L, Huang Q. The dual role and therapeutic potential of high-mobility group box 1 in cancer. Oncotarget. 2017;8:64534–64550. doi: 10.18632/oncotarget.17885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Son G-H, Kim Y, Lee JJ, Lee K-Y, Ham H, Song J-E, Park ST, Kim Y-H. MicroRNA-548 regulates high mobility group box 1 expression in patients with preterm birth and chorioamnionitis. null. 2019;9:19746. doi: 10.1038/s41598-019-56327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lv X, Yao L, Nie YQ, Xu XY. MicroRNA-520a-3p suppresses non-small-cell lung carcinoma by inhibition of high mobility group box 1 (HMGB1). Eur Rev Med Pharmacol Sci. 2018;22:1700–1708. doi: 10.26355/eurrev_201803_14583. [DOI] [PubMed] [Google Scholar]
- 234.Yun Z, Meng F, Jiang P, Yue M, Li S. MicroRNA-548b suppresses aggressive phenotypes of hepatocellular carcinoma by directly targeting high-mobility group box 1 mRNA. Cancer Manag Res. 2019;11:5821–5834. doi: 10.2147/CMAR.S198615. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 235.Tatsuno K, Han P, Edelson R, Hanlon D. Detection of immunogenic cell death in tumor vaccination mouse model. Methods Mol Biol. 2021;2255:171–186. [DOI] [PubMed] [Google Scholar]
- 236.Zhang Y, Thangam R, You S-H, Sultonova RD, Venu A, Min J-J, Hong Y. Engineering calreticulin-targeting monobodies to detect immunogenic cell death in cancer chemotherapy. Cancers (Basel). 2021;13:2801. doi: 10.3390/cancers13112801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kim D-Y, Pyo A, Yun M, Thangam R, You S-H, Zhang Y, Jung Y-R, Nguyen D-H, Venu A, Kim HS, et al. Imaging calreticulin for early detection of immunogenic cell death during anticancer treatment. J Nucl Med. 2021;62:956–960. doi: 10.2967/jnumed.120.245290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chumsri S, Serie DJ, Li Z, Pogue-Geile KL, Soyano-Muller AE, Mashadi-Hossein A, Warren S, Lou Y, Colon-Otero G, Knutson KL, et al. Effects of age and immune landscape on outcome in HER2-positive breast cancer in the NCCTG N9831 (Alliance) and NSABP B-31 (NRG) Trials. Clin Cancer Res. 2019;25:4422–4430. doi: 10.1158/1078-0432.CCR-18-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 240.de Boo L, Cimino-Mathews A, Lubeck Y, Daletzakis A, Opdam M, Sanders J, Hooijberg E, van Rossum A, Loncova Z, Rieder D, et al. Tumour-infiltrating lymphocytes (TILs) and BRCA-like status in stage III breast cancer patients randomised to adjuvant intensified platinum-based chemotherapy versus conventional chemotherapy. Eur J Cancer. 2020;127:240–250. doi: 10.1016/j.ejca.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 241.Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Diéras V, Iwata H, Barrios CH, Nechaeva M, Nguyen-Duc A, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J Natl Cancer Inst. 2021;113:1005–1016. doi: 10.1093/jnci/djab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Page DB, Pucilowska J, Sanchez KG, Conrad VK, Conlin AK, Acheson AK, Perlewitz KS, Imatani JH, Aliabadi-Wahle S, Moxon N, et al. A phase ib study of preoperative, locoregional IRX-2 cytokine immunotherapy to prime immune responses in patients with early-stage breast cancer. Clin Cancer Res. 2020;26:1595–1605. doi: 10.1158/1078-0432.CCR-19-1119. [DOI] [PubMed] [Google Scholar]
- 243.Ishihara M, Kitano S, Kageyama S, Miyahara Y, Yamamoto N, Kato H, Mishima H, Hattori H, Funakoshi T, Kojima T, et al. NY-ESO-1-specific redirected T cells with endogenous TCR knockdown mediate tumor response and cytokine release syndrome. J ImmunoTher Cancer. 2022;10:e003811. doi: 10.1136/jitc-2021-003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Haas AR, Tanyi JL, O’Hara MH, Gladney WL, Lacey SF, Torigian DA, Soulen MC, Tian L, McGarvey M, Nelson AM, et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther. 2019;27:1919–1929. doi: 10.1016/j.ymthe.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Somaiah N, Chawla SP, Block MS, Morris JC, Do K, Kim JW, Druta M, Sankhala KK, Hwu P, Jones RL, et al. A phase 1b study evaluating the safety, tolerability, and immunogenicity of CMB305, a lentiviral-based prime-boost vaccine regimen, in patients with locally advanced, relapsed, or metastatic cancer expressing NY-ESO-1. Oncoimmunology. 2020;9:1847846. doi: 10.1080/2162402X.2020.1847846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ordóñez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 248.Steinbach D, Onda M, Voigt A, Dawczynski K, Wittig S, Hassan R, Gruhn B, Pastan I. Mesothelin, a possible target for immunotherapy, is expressed in primary AML cells. Eur J Haematol. 2007;79:281–286. doi: 10.1111/j.1600-0609.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- 249.Takamizawa S, Yazaki S, Kojima Y, Yoshida H, Kitadai R, Nishikawa T, Shimoi T, Sudo K, Okuma HS, Tanioka M, et al. High mesothelin expression is correlated with non-squamous cell histology and poor survival in cervical cancer: a retrospective study. Bmc Cancer. 2022;22:1215. doi: 10.1186/s12885-022-10277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pusztai L, Yau C, Wolf DM, Han HS, Du L, Wallace AM, String-Reasor E, Boughey JC, Chien AJ, Elias AD, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell. 2021;39:989–998.e5. doi: 10.1016/j.ccell.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yin W, Wang Y, Wu Z, Ye Y, Zhou L, Xu S, Lin Y, Du Y, Yan T, Yang F, et al. Neoadjuvant trastuzumab and pyrotinib for locally advanced HER2-positive breast cancer (NeoATP): primary analysis of a phase II study. Clin Cancer Res. 2022;28:3677–3685. doi: 10.1158/1078-0432.CCR-22-0446. [DOI] [PubMed] [Google Scholar]
- 252.Lee J-Y, Kim B-G, Kim J-W, Lee JB, Park E, Joung J-G, Kim S, Choi CH, Kim HS. Korean Gynecologic Oncology Group (KGOG) investigators. Biomarker-guided targeted therapy in platinum-resistant ovarian cancer (AMBITION; KGOG 3045): a multicentre, open-label, five-arm, uncontrolled, umbrella trial. J Gynecol Oncol. 2022;33:e45. doi: 10.3802/jgo.2022.33.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Cortes J, Rugo HS, Cescon DW, Im S-A, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387:217–226. [DOI] [PubMed] [Google Scholar]
- 254.Jiang Y-Z, Liu Y, Xiao Y, Hu X, Jiang L, Zuo W-J, Ma D, Ding J, Zhu X, Zou J, et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: the FUTURE trial. Cell Res. 2021;31:178–186. doi: 10.1038/s41422-020-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sang W, Wang X, Geng H, Li T, Li D, Zhang B, Zhou Y, Song X, Sun C, Yan D, et al. Anti-PD-1 therapy enhances the efficacy of CD30-directed chimeric antigen receptor T cell therapy in patients with relapsed/refractory CD30+ lymphoma. Front Immunol. 2022;13:858021. doi: 10.3389/fimmu.2022.858021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ozaki Y, Tsurutani J, Mukohara T, Iwasa T, Takahashi M, Tanabe Y, Kawabata H, Masuda N, Futamura M, Minami H, et al. Safety and efficacy of nivolumab plus bevacizumab, paclitaxel for HER2-negative metastatic breast cancer: primary results and biomarker data from a phase 2 trial (WJOG9917B). Eur J Cancer. 2022;171:193–202. doi: 10.1016/j.ejca.2022.05.014. [DOI] [PubMed] [Google Scholar]
- 257.Blumenschein GR, Devarakonda S, Johnson M, Moreno V, Gainor J, Edelman MJ, Heymach JV, Govindan R, Bachier C, Doger de Spéville B, et al. Phase I clinical trial evaluating the safety and efficacy of ADP-A2M10 SPEAR T cells in patients with MAGE-A10+ advanced non-small cell lung cancer. J ImmunoTher Cancer. 2022;10:e003581. doi: 10.1136/jitc-2021-003581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, Landin AM, Mullinax JE, Saller JJ, Saltos AN, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27:1410–1418. doi: 10.1038/s41591-021-01462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sang W, Shi M, Yang J, Cao J, Xu L, Yan D, Yao M, Liu H, Li W, Zhang B, et al. Phase II trial of co-administration of CD19- and CD20-targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma. Cancer Med. 2020;9:5827–5838. doi: 10.1002/cam4.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shah BD, Bishop MR, Oluwole OO, Logan AC, Baer MR, Donnellan WB, O’Dwyer KM, Holmes H, Arellano ML, Ghobadi A, et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: zUMA-3 phase 1 results. Blood. 2021;138:11–22. doi: 10.1182/blood.2020009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, Yuan XL, Chen Y, Yang SJ, Shi JH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30:1479–1486. doi: 10.1093/annonc/mdz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Narayan P, Wahby S, Gao JJ, Amiri-Kordestani L, Ibrahim A, Bloomquist E, Tang S, Xu Y, Liu J, Fu W, et al. FDA approval summary: atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clin Cancer Res. 2020;26:2284–2289. doi: 10.1158/1078-0432.CCR-19-3545. [DOI] [PubMed] [Google Scholar]
- 263.Smith SD, Till BG, Shadman MS, Lynch RC, Cowan AJ, Wu QV, Voutsinas J, Rasmussen HA, Blue K, Ujjani CS, et al. Pembrolizumab with R-CHOP in previously untreated diffuse large B-cell lymphoma: potential for biomarker driven therapy. Br J Haematol. 2020;189:1119–1126. doi: 10.1111/bjh.16494. [DOI] [PubMed] [Google Scholar]
- 264.Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, Grischke EM, Furlanetto J, Tesch H, Hanusch C, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30:1279–1288. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 265.Kohli K, Yao L, Nowicki TS, Zhang S, Black RG, Schroeder BA, Farrar EA, Cao J, Sloan H, Stief D, et al. IL-15 mediated expansion of rare durable memory T cells following adoptive cellular therapy. J ImmunoTher Cancer. 2021;9:e002232. doi: 10.1136/jitc-2020-002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pasqualini C, Rubino J, Brard C, Cassard L, André N, Rondof W, Scoazec J-Y, Marchais A, Nebchi S, Boselli L, et al. Phase II and biomarker study of programmed cell death protein 1 inhibitor nivolumab and metronomic cyclophosphamide in paediatric relapsed/refractory solid tumours: arm G of AcSé-ESMART, a trial of the European innovative therapies for children with cancer consortium. Eur J Cancer. 2021;150:53–62. doi: 10.1016/j.ejca.2021.03.032. [DOI] [PubMed] [Google Scholar]
- 267.Pusztai L. The effectiveness of immune checkpoint inhibitors in the neoadjuvant and post-neoadjuvant breast cancer settings. Clin Adv Hematol Oncol. 2022;20:552–555. [PubMed] [Google Scholar]
- 268.Garg AD, More S, Rufo N, Mece O, Sassano ML, Agostinis P, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology. 2017;6:e1386829. doi: 10.1080/2162402X.2017.1386829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhao Y-Y, Lian J-X, Lan Z, Zou K-L, Wang W-M, Yu G-T. Ferroptosis promotes anti-tumor immune response by inducing immunogenic exposure in HNSCC. Oral Dis. 2021;29:933–941. doi: 10.1111/odi.14077. [DOI] [PubMed] [Google Scholar]
- 270.Ye T, Jiang K, Wei L, Barr MP, Xu Q, Zhang G, Ding C, Meng S, Piao H. Oncolytic Newcastle disease virus induces autophagy-dependent immunogenic cell death in lung cancer cells. Am J Cancer Res. 2018;8:1514–1527. [PMC free article] [PubMed] [Google Scholar]
- 271.Rosato RR, Dávila-González D, Choi DS, Qian W, Chen W, Kozielski AJ, Wong H, Dave B, Chang JC. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018;20:108. doi: 10.1186/s13058-018-1037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Pasquereau-Kotula E, Habault J, Kroemer G, Poyet J-L, Ma W-L. The anticancer peptide RT53 induces immunogenic cell death. PLos One. 2018;13:e0201220. doi: 10.1371/journal.pone.0201220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Nebot-Bral L, Coutzac C, Kannouche PL, Chaput N. Why is immunotherapy effective (or not) in patients with MSI/MMRD tumors? Bull Cancer. 2019;106:105–113. doi: 10.1016/j.bulcan.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 274.Frey B, Rückert M, Deloch L, Rühle PF, Derer A, Fietkau R, Gaipl US. Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol Rev. 2017;280:231–248. doi: 10.1111/imr.12572. [DOI] [PubMed] [Google Scholar]
- 275.Goéré D, Flament C, Rusakiewicz S, Poirier-Colame V, Kepp O, Martins I, Pesquet J, Eggermont A, Elias D, Chaput N, et al. Potent immunomodulatory effects of the trifunctional antibody catumaxomab. Cancer Res. 2013;73:4663–4673. doi: 10.1158/0008-5472.CAN-12-4460. [DOI] [PubMed] [Google Scholar]
- 276.Pasquiers B, Benamara S, Felices M, Nguyen L, Declèves X. Review of the existing translational pharmacokinetics modeling approaches specific to monoclonal antibodies (mAbs) to support the First-In-Human (FIH) dose selection. Int J Mol Sci. 2022;23:23. doi: 10.3390/ijms232112754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kaestner SA, Sewell GJ. Chemotherapy dosing part I: scientific basis for current practice and use of body surface area. Clin Oncol (R Coll Radiol). 2007;19:23–37. doi: 10.1016/j.clon.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 278.Martin JH, Dimmitt S. The rationale of dose-response curves in selecting cancer drug dosing. Br J Clin Pharmacol. 2019;85:2198–2204. doi: 10.1111/bcp.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Flieswasser T, Van Loenhout J, Freire Boullosa L, Van den Eynde A, De Waele J, Van Audenaerde J, Lardon F, Smits E, Pauwels P, Jacobs J. Clinically relevant chemotherapeutics have the ability to induce immunogenic cell death in non-small cell lung cancer. Cells. 2020;9:1474. doi: 10.3390/cells9061474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.McCormick M, Richardson T, Rapkin L, Kalpatthi R. Risk factors for readmission following febrile neutropenia in pediatric oncology patients. J Pediatr Hematol Oncol. 2022;45:e496–e501. doi: 10.1097/MPH.0000000000002585. [DOI] [PubMed] [Google Scholar]
- 281.Xu T, Liu Y, Lu X, Liang J. Toxicity profile of combined immune checkpoint inhibitors and thoracic radiotherapy in esophageal cancer: a meta-analysis and systematic review. Front Immunol. 2022;13:1039020. doi: 10.3389/fimmu.2022.1039020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Panchenko AV, Tyndyk ML, Maydin MA, Baldueva IA, Artemyeva AS, Kruglov SS, Kireeva GS, Golubev AG, Belyaev AM, Anisimov VN. Melatonin administered before or after a cytotoxic drug increases mammary cancer stabilization rates in HER2/Neu mice. Chemotherapy. 2020;65:42–50. doi: 10.1159/000509238. [DOI] [PubMed] [Google Scholar]
- 283.Bawankar PR, Ankar R. Evidence generation of standard nursing protocol on chemotherapy-induced neutropenia among oncology nurses. Cureus. 2022;14:e31217. doi: 10.7759/cureus.31217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gwak H, Lim S-T, Jeon Y-W, Park HS, Kim SH, Suh Y-J. COVID-19 prevention guidance and the incidence of febrile neutropenia in patients with breast cancer receiving TAC Chemotherapy with Prophylactic Pegfilgrastim. J Clin Med. 2022;11(23): 7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Xu Z, Yang L, Yu H, Guo L. A machine learning model for grade 4 lymphopenia prediction during pelvic radiotherapy in patients with cervical cancer. Front Oncol. 2022;12:905222. doi: 10.3389/fonc.2022.905222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ni W, Xiao Z, Zhou Z, Chen D, Feng Q, Liang J, Lv J. Severe radiation-induced lymphopenia during postoperative radiotherapy or chemoradiotherapy has poor prognosis in patients with stage IIB-III after radical esophagectomy: a post hoc analysis of a randomized controlled trial. Front Oncol. 2022;12:936684. doi: 10.3389/fonc.2022.936684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mayr P, Lutz M, Schmutz M, Hoeppner J, Liesche-Starnecker F, Schlegel J, Gaedcke J, Claus R. Progressive multifocal leukoencephalopathy associated with chemotherapy induced lymphocytopenia in solid tumors - case report of an underestimated complication. Front Oncol. 2022;12:905103. doi: 10.3389/fonc.2022.905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hsiehchen D, Naqash AR, Espinoza M, Von Itzstein MS, Cortellini A, Ricciuti B, Owen DH, Laharwal M, Toi Y, Burke M, et al. Association between immune-related adverse event timing and treatment outcomes. Oncoimmunology. 2022;11:2017162. doi: 10.1080/2162402X.2021.2017162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yang S-H, Lu L-C, Kao H-F, Chen B-B, Kuo T-C, Kuo S-H, Tien Y-W, Bai L-Y, Cheng A-L, Yeh K-H. Negative prognostic implications of splenomegaly in nivolumab-treated advanced or recurrent pancreatic adenocarcinoma. Oncoimmunology. 2021;10:1973710. doi: 10.1080/2162402X.2021.1973710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kaczmarek A, Brinkman BM, Heyndrickx L, Vandenabeele P, Krysko DV. Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways. J Pathol. 2012;226:598–608. doi: 10.1002/path.3009. [DOI] [PubMed] [Google Scholar]
- 291.Dahlgren D, Sjöblom M, Hellström PM, Lennernäs H. Chemotherapeutics-induced intestinal mucositis: pathophysiology and potential treatment strategies. Front Pharmacol. 2021;12:681417. doi: 10.3389/fphar.2021.681417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Roberti MP, Yonekura S, Duong CPM, Picard M, Ferrere G, Tidjani Alou M, Rauber C, Iebba V, Lehmann CHK, Amon L, et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med. 2020;26:919–931. doi: 10.1038/s41591-020-0882-8. [DOI] [PubMed] [Google Scholar]
- 293.Morano WF, Aggarwal A, Love P, Richard SD, Esquivel J, Bowne WB. Intraperitoneal immunotherapy: historical perspectives and modern therapy. Cancer Gene Ther. 2016;23:373–381. doi: 10.1038/cgt.2016.49. [DOI] [PubMed] [Google Scholar]
- 294.Aghanejad A, Bonab SF, Sepehri M, Haghighi FS, Tarighatnia A, Kreiter C, Nader ND, Tohidkia MR. A review on targeting tumor microenvironment: the main paradigm shift in the mAb-based immunotherapy of solid tumors. Int J Biol Macromol. 2022;207:592–610. doi: 10.1016/j.ijbiomac.2022.03.057. [DOI] [PubMed] [Google Scholar]


