Abstract
OBJECTIVE.
To determine the effect of mandatory and nonmandatory influenza vaccination policies on vaccination rates and symptomatic absenteeism among healthcare personnel (HCP).
DESIGN.
Retrospective observational cohort study.
SETTING.
This study took place at 3 university medical centers with mandatory influenza vaccination policies and 4 Veterans Affairs (VA) healthcare systems with nonmandatory influenza vaccination policies.
PARTICIPANTS.
The study included 2,304 outpatient HCP at mandatory vaccination sites and 1,759 outpatient HCP at nonmandatory vaccination sites.
METHODS.
To determine the incidence and duration of absenteeism in outpatient settings, HCP participating in the Respiratory Protection Effectiveness Clinical Trial at both mandatory and nonmandatory vaccination sites over 3 viral respiratory illness (VRI) seasons (2012–2015) reported their influenza vaccination status and symptomatic days absent from work weekly throughout a 12-week period during the peak VRI season each year. The adjusted effects of vaccination and other modulating factors on absenteeism rates were estimated using multivariable regression models.
RESULTS.
The proportion of participants who received influenza vaccination was lower each year at nonmandatory than at mandatory vaccination sites (odds ratio [OR], 0.09; 95% confidence interval [CI], 0.07–0.11). Among HCP who reported at least 1 sick day, vaccinated HCP had lower symptomatic days absent compared to unvaccinated HCP (OR for 2012–2013 and 2013–2014, 0.82; 95% CI, 0.72–0.93; OR for 2014–2015, 0.81; 95% CI, 0.69–0.95).
CONCLUSIONS.
These data suggest that mandatory HCP influenza vaccination policies increase influenza vaccination rates and that HCP symptomatic absenteeism diminishes as rates of influenza vaccination increase. These findings should be considered in formulating HCP influenza vaccination policies.
Immunization is a primary influenza prevention strategy.1 Since 1984, the Advisory Committee for Immunization Practices (ACIP) has recommended annual influenza vaccination for healthcare personnel (HCP).2 This recommendation is endorsed by the Healthcare Infection Control Practices Advisory Committee (HICPAC).2,3 In 2008, the Centers for Disease Control and Prevention (CDC) estimated that only 49% of HCP in the United States had received the influenza vaccine.4 By the 2015–2016 season, that number increased to 79%, and vaccination rates were highest (>95%) at sites where employers required its use.5 Studies suggest that HCP vaccination decreases patient mortality and healthcare-associated influenza in certain settings,6,7 leading the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) to support mandatory HCP vaccination programs, and the IDSA, SHEA, and the Pediatric Infectious Diseases Society (PIDS) to support universal HCP immunization against vaccine-preventable disease.8–10 Reasons cited for the IDSA and SHEA influenza immunization recommendation and the joint IDSA, SHEA, and PIDS vaccine-preventable disease recommendations were the direct benefit of protecting HCP from disease, the possible secondary benefits of protecting patients from infection by HCP, and reducing HCP illness, which negatively impacts patient care. Several states, municipalities, federal departments or agencies, as well as many healthcare organizations have adopted mandatory influenza immunization policies.10–15
The secondary benefits and rationale for mandatory HCP vaccination policies remain controversial. A 2015 Cochrane Collaborative Review (covering 90 studies between January 1966 and May 2013) reported that the efficacy of influenza vaccine in preventing laboratory-confirmed influenza in healthy adults was 60%, and when the vaccine strain closely matched the circulating strain, efficacy rose to 62%.16 Several cohort studies and clinical trials have suggested that vaccination of HCP may be important in protecting vulnerable patients from influenza.7,17,18 Van Buynder determined that influenza immunization of HCP in British Columbia reduced absenteeism during the 2012–2013 season.19 Conversely, 2 separate Cochrane Collaborative reviews showed little or no secondary benefit from influenza vaccination of HCP in reducing absenteeism or protecting patients in long-term care facilities, respectively.16,20 A recent publication faulted some analytical methods and the conclusion that HCP influenza vaccination substantially protected residents of long-term care facilities, while another defended that conclusion.21,22
The Respiratory Protection Effectiveness Clinical Trial (ResPECT: NCT-01240625) was conducted over 3 influenza seasons at geographically distributed outpatient healthcare sites, including 3 that mandated annual HCP influenza vaccination and 4 that did not.23 We retrospectively examined these prospectively collected data to assess the impact of mandatory influenza vaccination policies in populations at risk for influenza and other respiratory viral diseases. In contrast to reports24 aggregating sick and/or vacation days and all-cause sick days, our analysis only included self-reported HCP absence due to symptomatic influenza-like illness (SILI), “symptomatic absenteeism.” We hypothesized that SILI-related absenteeism would be lower at sites mandating HCP influenza vaccination.
METHODS
ResPECT, a cluster-randomized study designed to evaluate protection from viral respiratory illness (VRI), including influenza, among HCP wearing N95 respirators and medical masks, was conducted at 3 university healthcare systems (Johns Hopkins Health System, Denver Health Medical Center, and Children’s Hospital Colorado) and 4 Veterans Affairs (VA) health systems (VA Eastern Colorado Healthcare System, Michael E. DeBakey VA Medical Center, Washington DC VA Medical Center, and VA New York Harbor Healthcare System).23 Outpatient HCP were enrolled annually in primary care, emergency departments, and specialty clinics where they were regularly exposed to outpatients with acute VRI. Recruited HCP included physicians, medical students, nurses, nursing students, dentists, social workers, environmental management personnel, administrative, and clinic clerical staff. Active surveillance was conducted over a 12-week peak period of each VRI season at each site, based on a predictive tool designed for ResPECT.25
Children’s Hospital Colorado began mandating annual influenza vaccination for HCP (except those with medical or religious exemptions) in 2010, while Johns Hopkins Health System and Denver Health Medical Center began similar mandated programs with similar exemptions in 2011. The VA facilities did not mandate annual HCP influenza vaccination. All sites provided free, onsite influenza vaccine for HCP. In addition, 1 mandatory and all nonmandatory sites authorized paid sick leave for nonprobationary employees. The remaining 2 mandatory vaccination sites required employees to use vacation for paid sick leave or had policies that provided a block of time to be used for vacation, illness, or excused absence. All sites prohibited febrile employees from working with patients.
Study participants reported vaccination status at enrollment and weekly throughout each 12-week study period. Participants completed daily online surveys recording whether they had worked that day and/or experienced any SILI (defined as a temperature ≥ 37.8°C [100°F] plus cough and/or sore throat). They also completed weekly surveys recording days of work missed due to SILI (ie, symptomatic absenteeism) and their specific influenza-like illness (ILI) symptoms using Research Electronic Data Capture (REDCap, Vanderbilt University, Nashville, TN). These data were used to calculate (1) the number of sick days due to SILI per participant per season, and (2) a sick-day ratio (SDR) determined by dividing the total number of symptomatic days absent at a site by the total number of participants at the same site.
To assess the rate of systemwide symptomatic absenteeism, SDRs for health system types were compared in unadjusted analyses. In the adjusted analysis, absenteeism rates were determined using a 2-stage hurdle model, first to analyze the risk of sick days within a season, and second to analyze the average number of sick days among those HCP who reported ≥1 sick day.
Unadjusted and adjusted analyses were conducted to assess the hypothesis that mandated influenza vaccination was associated with reduced HCP symptomatic absenteeism. Unadjusted analyses compared site-level outcomes. Wilcoxon rank-sum tests were used to compare sites with mandatory or nonmandatory policies. We chose the Wilcoxon rank-sum test because it is an appropriate test for small sample sizes and does not assume a normal distribution. Poisson regression was used to test whether vaccination status affected number of absent days due to upper respiratory illness with year included as a covariate. In these analyses, significance was defined as P < .05.
For adjusted analyses, we used individual-level HCP data. We fit generalized linear regression models to estimate effect sizes of interest while adjusting for possible confounders including number of children in the home, age, vaccination policy, and type of healthcare setting (VA or other). First, a logistic regression model with vaccination status as the outcome was fit to assess whether mandatory vaccination policy was associated with increased vaccination. Second, we fit a 2-stage regression model with the number of sick days reported as the outcome, to determine whether vaccination was associated with (1) HCP reported sick leave within each season, and (2) the number of sick days reported among HCP who reported any sick leave. We used a Poisson hurdle regression model to account for the large number of HCP reporting zero sick days.26 Hurdle models account for the possibility that different mechanisms may produce more zeros than produce nonzero outcomes. In this case, the mechanism that produces an HCP not taking any sick days may be not be the same as that producing the number of sick days an HCP takes. The hurdle model was fit using the pscl package for R.27 In both the logistic and hurdle models, we included the following fixed effects: year, sex, family members under 5 years old, self-reported upper respiratory infection (URI) exposure, age, and whether the site had a mandatory influenza vaccination policy. The hurdle model also included an interaction term that compared vaccinated to nonvaccinated individuals in the 2012–2013 VRI season (year 1) and the 2013–2014 VRI season (year 2) to the 2014–2015 (year 3) VRI season. Terms were considered statistically significant when 95% confidence intervals did not include zero. All statistical analyses were performed using R version 3.3.1 statistical software (R Foundation for Statistical Computing, Vienna, Austria).28
RESULTS
During 3 years of data collection at 7 health systems, 2,304 participants were enrolled at mandatory sites and 1,759 were enrolled at nonmandatory sites. Most demographic and occupational risk (of direct exposure to patients with acute respiratory infections) characteristics at both mandatory and nonmandatory vaccination sites were similar, but some differed (Table 1). At mandatory vaccine sites, 11.0% of subjects were male compared to 19.2% at nonmandated sites (P ≤ .001). Over the 3 studied VRI seasons, mean ages of study participants were 35.1, 39.8, and 39.8 years at mandatory vaccination sites compared to 46.1, 47.2, and 48.1 years at nonmandatory sites, respectively (P ≤ .001). Overall, 97.1%, 96.3%, and 92.1% of participants reported being vaccinated during the 3 study years at mandatory sites, while 67.9%, 63.3%, and 60.4% reported vaccination at nonmandatory sites, respectively (odds ratio [OR], 0.09; 95% confidence interval [CI], 0.07–0.11).
TABLE 1.
Characteristics of ResPECT Participants
| Characteristic | VRI Season | Mandatory Influenza Vaccine Sites (n = 3) | NonMandatory Influenza Vaccine Sites (n = 4) |
|---|---|---|---|
| Numbera | 2012–2013 | 689 | 384 |
| 2013–2014 | 775 | 600 | |
| 2014–2015 | 840 | 775 | |
| Total | 2,304 | 1,759 | |
| Sex ratio, male:femaleb | 2012–2013 | 90:599 | 78:306 |
| 2013–2014 | 80:695 | 110:490 | |
| 2014–2015 | 83:57 | 150:625 | |
| Age, range y (mean)c | 2012–2013 | 21–71 (35.1) | 23–74 (46.1) |
| 2013–2014 | 21–67 (39.8) | 24–73 (47.2) | |
| 2014–2015 | 18–67 (39.8) | 24–71 (48.1) | |
| Occupation risk | 2012–2013 | L: 195 (28); M: 65 (10); H: 430 (62) | L: 65 (17); M: 34 (9); H: 285 (74) |
| level, no. (%)d | 2013–2014 | L: 224 (29); M: 124 (16); H: 427 (55) | L: 175 (29); M: 64 (11); H: 361 (60) |
| 2014–2015 | L: 216 (26); M: 163 (19); H: 459 (55) | L: 251 (32); M: 68 (9); H: 456 (59) | |
| Family members <5 y old in household, no. (%)e | 2012–2013 | No: 475 (69) | No: 315 (82) |
| Yes: 214 (31) | Yes: 68 (17) No report: 1 |
||
| 2013–2014 | No: 564 (73) | No: 451 (75) | |
| Yes: 211 (27) | Yes: 102 (17) No report: 47 (8) |
||
| 2014–2015 | No: 606 (72) | No: 576 (74) | |
| Yes: 233 (28) | Yes: 138 (18) | ||
| No report: 1 | No report: 61 (8) | ||
| Received flu vaccine, no. (%) | 2012–2013 | 669 (97.1) | 261 (67.9) |
| 2013–2014 | 747 (96.3) | 380 (63.3) | |
| 2014–2015 | 774 (92.1) | 468 (60.4) |
NOTE. VRI, viral respiratory illness; Logistic regression was used to compare demographics between the mandatory vaccination policy and nonmandatory vaccination sites with year included as a covariate.
Individuals (n = 7) who chose not to report their vaccination status were excluded from the analysis.
More men participated in the study at the nonmandatory sites (OR, 2.04; 95% CI, 0.53–0.91; P < .001).
The participants at nonmandatory sites were older (OR, 1.06; 95% CI, 0.05–0.06; P < .001).
L, low exposure risk occupation (eg, clerical and administrative personnel); M, medium exposure risk occupation (eg, psychologist, social worker, occupational therapist, triage personnel); H, high exposure risk occupation (eg, physician, dentist, nurse practitioner, nurse, physician assistant, emergency medicine personnel, respiratory or physical therapist); U, unknown occupation. Per protocol cohort.
There was no significant difference between the populations with respect to whether or not the participants had household members under 5 years old (OR, 0.88; 95% CI, −0.29 to 0.04; P = .14).
The proportion of HCPs claiming any sick days at mandatory sites was estimated to be 5.9% lower than at nonmandatory sites (95% CI, −12.5 to −1.4; P = .02) (Table 2). Among HCP reporting at least 1 sick day, the median number of symptomatic sick days at mandatory sites was 0.74 lower than nonmandatory sites (95% CI, −1.37 to −0.37; P< .01) (Table 3).
TABLE 2.
ResPECT Participants Taking 1 or More Sick Days at Mandatory and Nonmandatory Influenza Vaccination Sites
| Mandatory Influenza Vaccination Sites | Nonmandatory (NM) Influenza Vaccination Sites | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| VRI Season | Site 1, No. (%) | Site 2, No. (%) | Site 3, No. (%) | Mean, No. (%) | NM Site 1, No. (%) | NM Site 2, No. (%) | NM Site 3, No. (%) | NM Site 4, No. (%) | NM Mean, No. (%) |
| 2012–2013 | 70 (17.95) | 60 (23.72) | 11 (23.91) | 47.0 (20.46) | 48 (36.36) | 18 (28.12) | 29 (29.59) | 30 (33.33) | 31.3 (32.55) |
| 2013–2014 | 57 (13.23) | 51 (20.56) | 29 (30.21) | 45.7 (17.68) | 54 (24.66) | 48 (37.50) | 35 (26.52) | 31 (25.62) | 42.0 (28.00) |
| 2014–2015 | 85 (17.56) | 50 (20.83) | 37 (31.90) | 57.3 (20.48) | 59 (23.14) | 52 (33.55) | 48 (21.43) | 33 (23.40) | 48.0 (24.77) |
| 3-year mean | 50.0 (19.53) | 40.4 (27.57) | |||||||
NOTE. VRI, viral respiratory illness. The percentage of HCP taking any sick days at mandatory sites was estimated to be 5.9% lower than at nonmandatory sites (95% CI, −12.5 to −1.4; P = .02).
TABLE 3.
Unadjusted Mean Number of Sick Days Reported by ResPECT Participants with ≥ 1 Sick Days at Mandatory and Nonmandatory Influenza Vaccination Sites
| Mandatory Influenza Vaccination Sites, Mean (10th, 90th Percentiles) | Nonmandatory (NM) Influenza Vaccination Sites, Mean (10th, 90th Percentiles) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| VRI Season | Site 1 | Site 2 | Site 3 | Overall | NM Site 1 | NM Site 2 | NM Site 3 | NM Site 4 | NM Overall |
| 2012–2013 | 2.43 (1.0–4.1) | 2.62 (1.0–5.0) | 1.45 (1.0–2.0) | 2.43 (1.0–5.0) | 3.38 (1.0–7.0) | 2.72 (1.0–5.0) | 2.76 (1.0–5.4) | 2.97 (1.0–5.2) | 3.04 (1.0–6.6) |
| 2013–2014 | 2.49 (1.0–5.0) | 2.69 (1.0–7.0) | 1.72 (1.0–3.0) | 2.40 (1.0–5.0) | 4.30 (1.0–8.0) | 2.44 (1.0–6.0) | 3.00 (1.0–6.0) | 3.90 (1.0–8.0) | 3.42 (1.0–7.0) |
| 2014–2015 | 2.41 (1.0–5.0) | 2.64 (1.0–4.1) | 1.70 (1.0–3.0) | 2.33 (1.0–4.0) | 3.07 (1.0–7.0) | 3.06 (1.0–7.0) | 3.29 (1.0–4.6) | 2.91 (1.0–7.2) | 3.09 (1.0–7.0) |
| Mean (10th, 90th percentiles) | 2.38 (1.0–5.0) | 3.19 (1.0, 7.0) | |||||||
NOTE. VRI, viral respiratory illness. Mean number of sick days at each site was compared using a Wilcoxon rank-sum test. Among HCP who reported at least 1 sick day, the median number of sick days taken by HCP at mandatory sites was estimated to be 0.74 lower than at nonmandatory sites (95% CI, −1.37 to −0.37; P < .01).
Mean SDRs were lower at all 3 mandatory influenza vaccination sites (year 1: 0.47 mandatory vs 0.95 nonmandatory; year 2: 0.47 mandatory vs 0.94 nonmandatory; year 3: 0.51 mandatory vs 0.78 nonmandatory) (Table 4). Unadjusted median difference in SDRs at mandatory vaccination sites was 0.39 days per HCP lower than at nonmandatory sites (95% CI, −0.55 to −0.26; P ≤ .001) (Table 4).
TABLE 4.
Unadjusted Sick Day Ratios of ResPECT Participants at Mandatory and Nonmandatory Influenza Vaccination Sites
| Mandatory Influenza Vaccination Sites, Sick Day Ratio | Nonmandatory (NM) Influenza Vaccination Sites, Sick Day Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| VRI Season | Site 1 | Site 2 | Site 3 | Mean | NM Site 1 | NM Site 2 | NM Site 3 | NM Site 4 | NM Mean |
| 2012–2013 | 0.44 | 0.62 | 0.35 | 0.47 | 1.23 | 0.77 | 0.82 | 0.99 | 0.95 |
| 2013–2014 | 0.33 | 0.55 | 0.52 | 0.47 | 1.06 | 0.91 | 0.80 | 1.00 | 0.94 |
| 2014–2015 | 0.43 | 0.55 | 0.54 | 0.51 | 0.71 | 1.03 | 0.71 | 0.68 | 0.78 |
| 3-year mean | 0.48 | 0.89 | |||||||
NOTE. VRI, viral respiratory illness; SDR, sick day ratio. SDR was determined by dividing the total number of symptomatic days absent at a site by the total number of participants at that site. SDR at each site for each year was compared using a Wilcoxon rank-sum test. The estimated median SDR among HCP at mandatory sites was estimated to be 0.39 lower than at nonmandatory sites (95% CI, −0.55 to −0.26; P < .001).
Stratified by year but not site, unadjusted SDRs of vaccinated HCP were 29% lower than those of unvaccinated HCP (relative risk [RR], 0.71; 95% CI, 0.67–0.74; P ≤ .001) (Table 5). During the year 3 of data collection (when the match between the vaccine and circulating influenza strains was lower and vaccine efficacy was 19%), SDRs were more nearly similar (0.60 in vaccinated HCP participants vs 0.66 in unvaccinated HCP participants).
TABLE 5.
Unadjusted Sick Day Ratio of Influenza Vaccinated and Nonvaccinated ResPECT Participants
| VRI Season | CDC Estimated Influenza Vaccine Efficacy for the Season, %39 | Vaccinated Subjects, No. (SDR) | Unvaccinated Subjects, No. (SDR) |
|---|---|---|---|
| 2012–2013 | 49 | 930 (0.62) | 143 (1.03) |
| 2013–2014 | 52 | 1,127 (0.58) | 248 (1.00) |
| 2014–2015 | 19 | 1,242 (0.60) | 373 (0.66) |
NOTE. VRI, viral respiratory illness, SDR, sick day ratio; HCP, healthcare personnel. Poisson regression was used to test the effects of vaccination on the number of sick days reported with year included as a covariate. The vaccinated HCP were estimated to have a 3-year mean reduction of 29% in absenteeism rate compared to unvaccinated HCP (relative rate, 0.71; 95% CI, 0.67–0.74; P ≤ .001).
After adjusting for possible confounders, HCP at nonmandatory compared to mandatory sites reported significantly lower vaccination rates (odds ratio [OR], 0.09; 95% CI, 0.07–0.11). Self-reported factors that significantly increased the odds an HCP was vaccinated were (1) being male, (2) having household children under 5 years of age, (3) being exposed to upper respiratory tract infections, and (4) being older (Figure 1, panel A).
FIGURE 1.
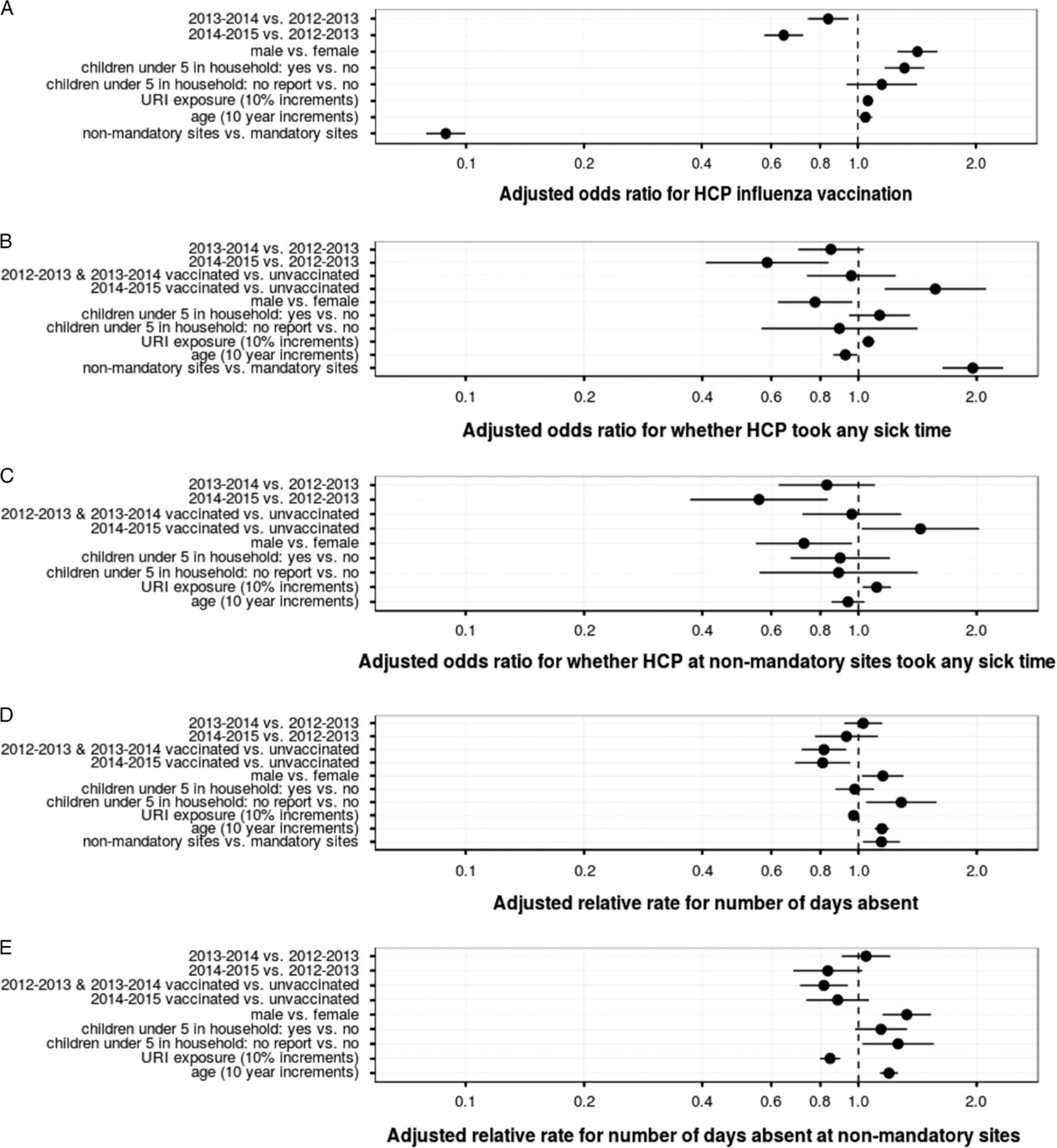
(Panel A) Adjusted odds ratio for influenza vaccination in ResPECT HCP. Adjusted for study year (2012–2013; 2013–2014; 2014–2015), sex of participants, number of children <5years of age at home, age, reported exposure to patients with symptomatic respiratory illness in workplace, and work at mandatory or nonmandatory vaccination policy at workplace. (Panel B) Adjusted odds ratio for 1 or more SILI days absent during observation period. Adjusted for study year (2012–2013; 2013–2014; 2014–2015), sex of participants, number of children <5 years of age at home, age, reported exposure to patients with symptomatic respiratory illness in workplace, and work at mandatory or nonmandatory vaccination policy at workplace. (Panel C) Adjusted odds ratio for 1 or more symptomatic influenza-like illness (SILI) days absent during observation period in study participants at the sites without a mandatory vaccination policy. Adjusted for study year (2012–2013; 2013–2014; 2014–2015), sex of participants, number of children <5 years of age at home, age, and reported exposure to patients with symptomatic respiratory illness in workplace. (Panel D) Adjusted relative rate of SILI-associated days of absenteeism during observation period. Adjusted for study year (2012–2013; 2013–2014; 2014–2015), sex of participants, number of children <5 years of age at home, age, reported exposure to patients with symptomatic respiratory illness in workplace, and work at mandatory or mandatory vaccination policy at workplace. (Panel E) Adjusted relative rate of SILI-associated days of absenteeism during observation period in study participants at the sites without a mandatory vaccination policy. Adjusted for study year (2012–2013; 2013–2014; 2014–2015), sex of participants, number of children <5 years of age at home, age, and reported exposure to patients with symptomatic respiratory illness in the workplace. In all panels, dot represents the point estimate, whiskers the 95% confidence intervals.
After similar adjustments, vaccinated HCP were as likely to have at least 1 symptomatic absence as unvaccinated individuals in years 1 and 2 when vaccine efficacy was higher (OR for 2012–2013 and 2013–2014, 0.96; 95% CI, 0.74–1.24) (Figure 1, panel B). In year 3, when vaccine efficacy was lower, vaccinated HCP were significantly more likely to have at least 1 symptomatic absence compared to unvaccinated individuals (OR for 2014–2015, 1.57; 95% CI, 1.17–2.11). In contrast, working at a nonmandatory site increased the adjusted odds of any sick leave during all seasons studied (OR, 1.95; 95% CI, 1.64–2.33) (Figure 1, panel B). The HCP who were male or younger had significantly decreased odds of any sick leave (Figure 1, panel B). However, among the HCP who reported at least 1 sick day, vaccinated HCP had lower symptomatic absenteeism (total number of symptomatic days absent) compared to unvaccinated HCP (OR for 2012–2013 and 2013–2014, 0.82; 95% CI, 0.72–0.93; OR for 2014–2015, 0.81; 95% CI, 0.69–0.95) (Figure 1, panel D). However, these results were sensitive to the effects of outliers with excess absenteeism. The HCP who were male, older, or working at nonmandatory vaccination sites had longer durations of sick leave.
We examined the effect of “VHA culture” on our results by making similar adjustments in the subset of HCP at the nonmandatory sites and comparing the results to those of the entire cohort. The adjusted odds ratios for taking any sick time in the nonmandatory cohort of HCP (Figure 1, panel C) were similar to the entire HCP cohort, with the exception of having children <5 in the household (panel B). In addition, the adjusted relative rate for number of symptomatic days absent in the nonmandatory cohort (panel E) and the entire group of HCP (panel D) were similar.
DISCUSSION
This multisite observational study, embedded in an outpatient comparative effectiveness trial, provided a unique opportunity to examine whether influenza vaccination policies influenced HCP absenteeism. Sites with mandatory influenza vaccination policies had higher vaccination rates and lower symptomatic absenteeism during 3 viral respiratory infection (VRI) seasons, even when controlling for factors contributing to influenza infection. In 2 study seasons, 2012–2013 and 2013–2014, vaccination had little impact on HCP sick leave, and in the third season, 2014–2105, vaccinated individuals were significantly more likely to claim some sick leave. However, among HCP who reported at least 1 day of sick leave, those at mandatory vaccination sites reported 0.74 fewer days. Irrespective of site, influenza vaccination was significantly associated with reduced absenteeism duration even after controlling for confounders.
During the first 2 VRI seasons, when vaccine and circulating influenza strains matched more closely, the SDR of vaccinated and unvaccinated subjects were different (0.62 vs 1.03; 0.58 vs 1.00, respectively). In the third VRI season, when the influenza vaccine and circulating strains did not match well, lowering vaccine efficacy, the SDRs of the 2 groups were strikingly more similar (0.60 in vaccinated subjects and 0.68 in unvaccinated subjects).
Our findings have implications for healthcare institutions, governmental agencies, and others whose goal is to provide the safest work environment. This study suggests that mandatory vaccination policies were associated with increased HCP vaccination rates and with reduced occurrence and duration of symptomatic absences. Reducing symptomatic absenteeism among HCP could help maintain the staffing and functioning of healthcare organizations during patient surges, including those occurring in an influenza epidemic.
The ACIP and HICPAC recommend that HCP without medical contraindications receive the influenza vaccine annually.3 More than 600 professional organizations, healthcare systems, cities, states, and federal agencies now mandate influenza vaccination for HCP.14 Mandatory vaccination policies substantially increase rates of HCP vaccination.5,29,30,31 However, HCP acceptance of voluntary vaccination continues to be problematic.32
Vaccination of HCP has previously been advocated as an effective means to control the spread of influenza and prevent absenteeism.33–35 Absenteeism poses a threat to hospital effectiveness during influenza outbreaks when increased patient volume creates greater demands.36–38 Our data demonstrate that the duration of absenteeism was modestly reduced at sites where annual influenza vaccination was mandated.
Many investigators have studied the influence of HCP influenza vaccination on patients. Potter et al7 studied influenza vaccination of patients and HCP at long-term-care geriatric facilities and found that vaccination of the HCP was associated with reductions in all-cause patient mortality and in symptomatic influenza-like illness (ILI). Similarly, Lemaitre17 studied influenza vaccination of HCP in nursing homes and showed a trend toward lower resident SILI in facilities where staff were vaccinated against influenza. Ahmed et al18 conducted a systematic review designed to evaluate the effectiveness of HCP influenza vaccination on patient mortality, hospitalization rates, and nosocomial influenza cases in patients. These authors concluded that there was moderate evidence for secondary benefit for protecting patients against influenza-like illness and all-cause mortality.
We believe this study focused more specifically on HCP SILI-related absenteeism than previous studies that combined vacation and sick days, lost workdays due to SILI in non-healthcare settings, or “all cause” sick days, regardless of symptoms experienced.39–41 This study targeted “symptomatic absenteeism,” defined as work shifts missed specifically due to influenza-like symptoms.
The Healthy People collaborative in the United States set a target for 90% of HCP to receive annual influenza vaccination by 2020.35 Our data suggest that this may be difficult to achieve by relying on voluntary vaccination policies. Vaccination rates at the nonmandatory sites decreased during the study. Other data suggest voluntary HCP vaccination policies do not meet desired rates and put them at excess risk. A CDC study reported that during the 2014–2015 flu season, healthcare sites that mandated influenza vaccination achieved an average of 96% vaccination, those that provided vaccine on site for >1 day but did not mandate it had an average vaccination rate of 84%, while those that neither promoted nor provided influenza vaccination had an average vaccination rate of 44%.36 Incentives to encourage and/or mandate influenza vaccination for HCP include the Joint Commission’s adoption of a standard for phased improvement of employee vaccination rates in healthcare organizations and public reporting of these data in some states.42
This study has several limitations. First, the data used were self-reported. Influenza vaccination status and documented absenteeism were not independently verified because confidentiality was guaranteed to participants. However, the fact that absenteeism rates were similar during the 2014–2015 season, when the vaccine match was low, suggests that any self-reporting bias was minimal. Second, assignment of the influenza vaccination policy at the sites and of the HCP within sites who received the vaccine was not random. Third, we could not exclude the possibility that HCP at mandatory sites without paid sick leave policies worked despite illness because “presenteeism” is known to be common among HCP.43 Finally, the 4 sites with nonmandatory influenza vaccination policies were Veterans Health Administration (VHA) systems. These VHA facilities served veteran patients only, had older HCP participants, and had similar human resource policies that allowed for paid sick leave accrued independently of vacation leave. Furthermore, several outlier observations among the VHA HCP reported SILI-related absenteeism substantially more than others, and these may have affected the sensitivity of our analyses. However, adjustment for confounders in nonmandatory HCP (comprising 85% of the unvaccinated but only 33% of the entire vaccinated study cohort) showed odd ratios and relative rates of absenteeism that were strikingly similar to the entire group. Thus, these data strongly suggest that vaccinated and unvaccinated subjects at the nonmandatory sites in our study behaved like their counterparts at mandatory sites in terms of SILI-related absenteeism and that vaccination policy was associated with absenteeism.
This study is unique and demonstrates that mandatory influenza vaccination policies were associated with increased HCP vaccination. Additionally, we demonstrated a benefit to individual HCP and facilities in that the total number of symptomatic ILI days absent was lower if individuals were vaccinated or worked in settings with highly vaccinated employee populations. These findings suggest that rates of workplace symptomatic ILI absenteeism may be reduced as HCP influenza vaccination rates increase, and they should be considered in the development of HCP influenza vaccination policies.
ACKNOWLEDGMENTS
The findings and conclusions in this manuscript are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention, the National Institute for Occupational Safety and Health, the Veterans Health Administration, the Biomedical Advanced Research and Development Authority, or affiliated organizations.
Financial support:
This study was supported by the Centers for Disease Control and Prevention (CDC), the Veterans Health Administration, and the Biomedical Advanced Research and Development Authority.
Footnotes
Potential conflicts of interest: Drs Perl and Cummings are coinvestigators on a grant from Medimmune awarded to Johns Hopkins Medical Institutes and not related to influenza or influenza vaccine research.
ResPECT Team members: Trish Perl, MD, MSc, Johns Hopkins University, Baltimore, MD; Justin Getka, BA, Johns Hopkins University, Baltimore, MD; Tina Hoang, MS, Johns Hopkins University, Baltimore, MD; Rose Kajih, PharmD, Johns Hopkins University, Baltimore, MD; Amanda Krosche, BS, Johns Hopkins University, Baltimore, MD; Meghan Kubala, MS, MD, Johns Hopkins University, Baltimore, MD; Jenna Los, MLA, Johns Hopkins University, Baltimore, MD; Liandra Presser, MD, Johns Hopkins University, Baltimore, MD; Kathleen Pulice, MS, Johns Hopkins University, Baltimore, MD; Margaret Spach, DDS, Johns Hopkins University, Baltimore, MD; Michael S. Simberkoff, MD, VA New York Harbor Healthcare System, New York, NY; Cynthia Akagbosu, BA, MA, VA New York Harbor Healthcare System, New York, NY; Madeline Dansky, BA, VA New York Harbor Healthcare System, New York, NY; Benedict J. Frederick, BA, VA New York Harbor Healthcare System, New York, NY; Marilyn Last, RN, VA New York Harbor Healthcare System, New York, NY; Scott Laverie, RN, VA New York Harbor Healthcare System, New York, NY; Courtney Pike, BA, VA New York Harbor Healthcare System, New York, NY; Shefali Rikhi, BS, VA New York Harbor Healthcare System, New York, NY; Nicole Spector, RN, VA New York Harbor Healthcare System, New York, NY; Christine A. Reel-Brander, RN, VA New York Harbor Healthcare System, New York, NY; Connie Price, MD, Denver Health and Hospital Authority, Denver, CO; Katie Gorman, BS, Denver Health and Hospital Authority, Denver, CO; Amy Irwin, DNP, RN, Denver Health and Hospital Authority, Denver, CO; Sean O’Malley, Denver Health and Hospital Authority, Denver, CO; Kevin Silva, BS, Denver Health and Hospital Authority, Denver, CO; Trish M. Perl, MD, MSc, University of Texas Southwestern Medical Center, Dallas, TX; Deepa Raj, MPH, University of Texas Southwestern Medical Center, Dallas, TX; Mary Bessesen, MD, VA Eastern Colorado Healthcare System, Denver, CO; Jill C. Adams BSN, BA, VA Eastern Colorado Healthcare System, Denver, CO; Shannon Kingery, BS, VA Eastern Colorado Healthcare System, Denver, CO; Stefanie Tuder, BS, VA Eastern Colorado Healthcare System, Denver, CO; Erron Fruchter-Palmer, MPH, VA Eastern Colorado Healthcare System, Denver, CO; Ann-Christine Nyquist, MD, MSPH, Children’s Hospital Colorado, Denver, CO; Megan Brocato, BA, Children’s Hospital Colorado, Denver, CO; Cynthia Gibert, MD, MSc; Laura Chopko, BA, Washington DC VA Medical Center, Washington, DC; Kathy Haines, MSW, MPH, Washington DC VA Medical Center, Washington, DC; Caitlin Langhorne, MPH, Washington DC VA Medical Center, Washington, DC; Dana Silver, BA, Washington DC VA Medical Center, Washington, DC; Courtney Southard, MPH, Washington DC VA Medical Center, Washington, DC; Maria C. Rodriguez-Barradas, MD; Barbara Kertz, MS; Mahwish Mushtaq, MD, MPH, VA Michael C. DeBakey Medical Center, Houston, TX; Blanca Vargas, MD, VA Michael C. DeBakey Medical Center, Houston, TX; Edward Fisher, MS, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Pittsburgh, PA; Ronald Shaffer, PhD, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Pittsburgh, PA; Lewis J. Radonovich, MD, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Pittsburgh, PA; Aaron Eagan, MPH, RN, Veterans Health Administration Office of Public Health, Gainesville, FL; Melanie Lipka, BS, HandyMetrics, Toronto, Ontario, Canada; Michael Tsang, PhD, HandyMetrics, Toronto, Ontario, Canada; Charlotte Gaydos, DrPH, Laboratory Core at Johns Hopkins University, Baltimore, MD; Jeffrey Holden, MA, Laboratory Core at Johns Hopkins University, Baltimore, MD; Alexandra Valsamakis, MD, PhD, Laboratory Core at Johns Hopkins University, Baltimore, MD; Geoffrey J. Gorse, MD, Laboratory Core at VA St Louis Healthcare System and St Louis University School of Medicine, St Louis, MO; Michelle Mitchell, BS, Laboratory Core at VA St Louis Healthcare System and St Louis University School of Medicine, St Louis, MO; Gira B. Patel, MS, Laboratory Core at VA St Louis Healthcare System and St Louis University School of Medicine, St Louis, MO; Yinyi Yu, BS, Laboratory Core at VA St Louis Healthcare System and St Louis University School of Medicine, St Louis, MO; Andre Hackman, BA, REDCap Core at Johns Hopkins University, Baltimore, MD; Michael Sherman, BS, REDCap Core at Johns Hopkins University, Baltimore, MD; Derek Cummings, PhD, MPH, MSc, Statistical and Epidemiologic Core, University of Florida, Gainesville, FL; Alexandria C. Brown, PhD, University of Massachusetts, Amherst, MA; Nicholas G. Reich, PhD, Statistical and Epidemiologic Core, University of Florida, Gainesville, FL; Justin Lesser, PhD, MHS, MS, Johns Hopkins University, Baltimore, MD.
REFERENCES
- 1.Groshkopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2017–2018. Morb Mortal Wkly Rep Recomm Rep 2017;66(RR-2):1–20. [Google Scholar]
- 2.Centers for Disease Control (CDC). Adult immunization: recommendations of the Immunization Practices Advisory Committee (ACIP). Morb Mortal Wkly Rep 1984;33(Suppl 1):1S–68S. [PubMed] [Google Scholar]
- 3.Pearson ML, Bridges CB, Harper SA. Healthcare Infection Control Practices Advisory Committee (HICPAC), Advisory Committee on Immunization Practices (ACIP). Influenza vaccination of health-care personnel: recommendations of the Healthcare Infection Control Practices Advisory Committee (HICPAC) and the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep Recomm 2006;55(RR-2):1–16. [PubMed] [Google Scholar]
- 4.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep Recomm 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 5.Black CL, Yun X, Ball SW, et al. Influenza vaccination coverage among health care personnel—United States, 2015–16 influenza season. Morb Mortal Wkly Rep 2016;65:1009–1015. [DOI] [PubMed] [Google Scholar]
- 6.Talbot TR, Babcock H, Caplan AL, et al. Revised SHEA position paper: influenza vaccination of healthcare personnel. Infect Control Hosp Epidemiol 2010;31:987–995. [DOI] [PubMed] [Google Scholar]
- 7.Potter J, Stott DJ, Roberts MA, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis 1997;175:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IDSA policy on mandatory immunization revision. Infectious Diseases Society of America website. https://www.idsociety.org/uploadedFiles/IDSA/Policy_and_Advocacy/Current_Topics_and_Issues/Immunizations_and_Vaccines/Health_Care_Worker_Immunization/Statements/IDSA%20Policy%20on%20Mandatory%20Immunization%20Revision%20083110.pdf. Updated 2010. Accessed January 19, 2017.
- 9.IDSA/SHEA/PIDS policy on mandatory immunization of healthcare personnel according to the ACIP-recommended vaccine schedule. Infectious Diseases Society of America website. https://www.idsociety.org/uploadedFiles/IDSA/Policy_and_Advocacy/Current_Topics_and_Issues/Immunizations_and_Vaccines/Health_Care_Worker_Immunization/Statements/IDSA_SHEA_PIDS%20Policy%20on%20Mandatory%20Immunization%20of%20HCP.pdf. Published 2013. Accessed January 19, 2017.
- 10.Babcock HM, Gemeinhart N, Jones M, Dunagan WC, Woeltje KF. Mandatory influenza vaccination of health care workers: translating policy to practice. Clin Infect Dis 2010; 50:459–464. [DOI] [PubMed] [Google Scholar]
- 11.Stewart AM, Cox MA. State law and influenza vaccination of health care personnel. Vaccine 2013;31:827–832. [DOI] [PubMed] [Google Scholar]
- 12.Caplan A, Shah NR. Managing the human toll caused by seasonal influenza: New York State’s mandate to vaccinate or mask. JAMA 2013;310:1797–1798. [DOI] [PubMed] [Google Scholar]
- 13.Rakita RM, Hagar BA, Crome P, Lammert JK. Mandatory influenza vaccination of healthcare workers: a 5-year study. Infect Control Hosp Epidemiol 2010;31:881–888. [DOI] [PubMed] [Google Scholar]
- 14.Honor roll: honorees with influenza vaccination mandates. Immunization Action Coalition website. http://www.immunize.org/honor-roll/influenza-mandates/honorees.asp. Updated 2018. Accessed January 16, 2018. [Google Scholar]
- 15.Sullivan SJ, Jacobson R, Poland GA. Mandating influenza vaccination for healthcare workers. Expert Rev Vaccines 2009;8:1469–1474. [DOI] [PubMed] [Google Scholar]
- 16.Demicheli V, Jefferson T, Al-Ansary LA, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2014:CD001269. doi: 10.1002/14651858.CD001269.pub5. [DOI] [PubMed] [Google Scholar]
- 17.Lemaitre M, Meret T, Rothan-Tondeur M, et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster-randomized trial. J Am Geriatr Soc 2009; 57:1580–1586. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed F, Lindley MC, Allred N, Weinbaum CM, Grohskopf L. Effect of influenza vaccination of healthcare personnel on morbidity and mortality among patients: systematic review and grading of evidence. Clin Infect Dis 2014;58:50–57. [DOI] [PubMed] [Google Scholar]
- 19.Van Buynder PG, Konrad S, Kersteins F, et al. Healthcare worker influenza immunization vaccinate or mask policy: strategies for cost effective implementation and subsequent reductions in staff absenteeism due to illness. Vaccine 2015; 33:1625–1628. [DOI] [PubMed] [Google Scholar]
- 20.Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions. Cochrane Database Syst Rev 2016:CD005187. doi: 10.1002/14651858.CD005187.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serres GD, Skowronski DM, Ward BJ, et al. Influenza vaccination of healthcare workers: critical analysis of the evidence for patient benefit underpinning policies of enforcement. PLoS One 2017;12:e0163586. doi: 10.1371/journal.pone.0163586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayward AC. Influenza vaccination of healthcare workers is an important approach for reducing transmission of influenza from staff to vulnerable patients. PLoS One 2017;12:e0169023. doi: 10.1371/journal.pone.0169023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radonovich LJ, Bessesen MT, Cummings DA, et al. The Respiratory Protection Effectiveness Clinical Trial (ResPECT): a cluster-randomized comparison of respirator and medical mask effectiveness against respiratory infections in healthcare personnel. BMC Infect Dis 2016;16:243. doi: 10.1186/s12879-016-1494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abstract: Influenza Vaccination for Hospital Employees (IDWeek 2012 Meeting). Infectious Disease Society of America website. https://idsa.confex.com/idsa/2012/webprogram/Paper36658.html. Published 2012. Accessed January 19, 2017.
- 25.Reich NG, Cummings DAT, Lauer SA, et al. Triggering interventions for influenza: the ALERT algorithm. Clin Infect Dis 2015;60:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadfield J. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Statist Soft 2010;33(2). doi: 10.18637/jss.v033.i02. [DOI] [Google Scholar]
- 27.Zeileis A, Kleiber C, Jackman S. Regression models for count data in R | Zeileis |. J Statist Soft 2008;27(8). doi: 10.18637/jss.v027.i08. [DOI] [Google Scholar]
- 28.R Studio Team. RStudio: integrated development for R. R Studio website. http://www.rstudio.com/. Published 2015. Accessed January 16, 2018. [Google Scholar]
- 29.Frenzel E, Chemaly RF, Ariza-Heredia E, et al. Association of increased influenza vaccination in health care workers with a reduction in nosocomial influenza infections in cancer patients. Am J Infect Control 2016;44:1016–1021. [DOI] [PubMed] [Google Scholar]
- 30.Wang TL, Jing L, Bocchini JA. Mandatory influenza vaccination for all healthcare personnel: a review on justification, implementation and effectiveness. Curr Opin Pediatr 2017; 29:606–615. [DOI] [PubMed] [Google Scholar]
- 31.Maltezou HC, Poland GA. Immunization of health-care providers: necessity and public health policies. Healthcare 2016;4:47. doi: 10.3390/healthcare4030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheema S, Vinnard C, Foster-Chang S, Linkin DR. A time off incentive was not associated with influenza vaccination acceptance among healthcare workers. Influenza Res Treat 2013;2013:209491. doi: 10.1155/2013/209491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anikeeva O, Braunack-Mayer A, Rogers W. Requiring influenza vaccination for health care workers. Am J Public Health 2009;99:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steckel CM. Mandatory influenza immunization for health care workers—an ethical discussion. AAOHN J 2007; 55:34–39. [DOI] [PubMed] [Google Scholar]
- 35.Immunization and infectious diseases. US Office of Disease Prevention and Health Promotion website. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases. Accessed January 19, 2017.
- 36.Black CL, Yue X, Ball SW, et al. Influenza vaccination coverage among health care personnel—United States, 2014–15 influenza season. Morb Mortal Wkly Rep 2015;64:993–999. [DOI] [PubMed] [Google Scholar]
- 37.Wilde JA, McMillan JA, Serwint J, Butta J, O’Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA 1999;281:908–913. [DOI] [PubMed] [Google Scholar]
- 38.Derber CJ, Shankaran S. Health-care worker vaccination for influenza: strategies and controversies. Curr Infect Dis Rep 2012;14:627–632. [DOI] [PubMed] [Google Scholar]
- 39.Abstract: Universal Employee Influenza Vaccination Decreases Employee Sick Days (IDWeek 2012 Meeting). Infectious Diseases Society of America website. https://idsa.confex.com/idsa/2012/webprogram/Paper37051.html. Published 2012. Accessed January 20, 2017.
- 40.McKevitt C, Morgan M, Dundas R, Holland WW. Sickness absence and “working through” illness: a comparison of two professional groups. J Public Health Med 1997;19:295–300. [DOI] [PubMed] [Google Scholar]
- 41.Krane L, Larsen EL, Nielsen CV, Stapelfeldt CM, Johnsen R, Risør MB. Attitudes towards sickness absence and sickness presenteeism in health and care sectors in Norway and Denmark: a qualitative study. BMC Public Health 2014;14:880. doi: 10.1186/1471-2458-14-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Influenza. The Joint Commission website. http://www.jointcommission.org/topics/hai_influenza.aspx. Accessed January 20, 2017.
- 43.Chiu S, Black CL, Yue X, et al. Working with influenza-like illness: presenteeism among US health care personnel during the 2014–2015 influenza season. Am J Infect Control 2017;45:1254–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]


