Abstract
Background
Pain is a common symptom in people with cancer; 30% to 50% of people with cancer will experience moderate‐to‐severe pain. This can have a major negative impact on their quality of life. Opioid (morphine‐like) medications are commonly used to treat moderate or severe cancer pain, and are recommended for this purpose in the World Health Organization (WHO) pain treatment ladder. Pain is not sufficiently relieved by opioid medications in 10% to 15% of people with cancer. In people with insufficient relief of cancer pain, new analgesics are needed to effectively and safely supplement or replace opioids.
Objectives
To evaluate the benefits and harms of cannabis‐based medicines, including medical cannabis, for treating pain and other symptoms in adults with cancer compared to placebo or any other established analgesic for cancer pain.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 26 January 2023.
Selection criteria
We selected double‐blind randomised, controlled trials (RCT) of medical cannabis, plant‐derived and synthetic cannabis‐based medicines against placebo or any other active treatment for cancer pain in adults, with any treatment duration and at least 10 participants per treatment arm.
Data collection and analysis
We used standard Cochrane methods. The primary outcomes were 1. proportions of participants reporting no worse than mild pain; 2. Patient Global Impression of Change (PGIC) of much improved or very much improved and 3. withdrawals due to adverse events. Secondary outcomes were 4. number of participants who reported pain relief of 30% or greater and overall opioid use reduced or stable; 5. number of participants who reported pain relief of 30% or greater, or 50% or greater; 6. pain intensity; 7. sleep problems; 8. depression and anxiety; 9. daily maintenance and breakthrough opioid dosage; 10. dropouts due to lack of efficacy; 11. all central nervous system adverse events. We used GRADE to assess certainty of evidence for each outcome.
Main results
We identified 14 studies involving 1823 participants. No study assessed the proportions of participants reporting no worse than mild pain on treatment by 14 days after start of treatment.
We found five RCTs assessing oromucosal nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) or THC alone involving 1539 participants with moderate or severe pain despite opioid therapy. The double‐blind periods of the RCTs ranged between two and five weeks. Four studies with a parallel design and 1333 participants were available for meta‐analysis.
There was moderate‐certainty evidence that there was no clinically relevant benefit for proportions of PGIC much or very much improved (risk difference (RD) 0.06, 95% confidence interval (CI) 0.01 to 0.12; number needed to treat for an additional beneficial outcome (NNTB) 16, 95% CI 8 to 100). There was moderate‐certainty evidence for no clinically relevant difference in the proportion of withdrawals due to adverse events (RD 0.04, 95% CI 0 to 0.08; number needed to treat for an additional harmful outcome (NNTH) 25, 95% CI 16 to endless). There was moderate‐certainty evidence for no difference between nabiximols or THC and placebo in the frequency of serious adverse events (RD 0.02, 95% CI −0.03 to 0.07). There was moderate‐certainty evidence that nabiximols and THC used as add‐on treatment for opioid‐refractory cancer pain did not differ from placebo in reducing mean pain intensity (standardised mean difference (SMD) −0.19, 95% CI −0.40 to 0.02).
There was low‐certainty evidence that a synthetic THC analogue (nabilone) delivered over eight weeks was not superior to placebo in reducing pain associated with chemotherapy or radiochemotherapy in people with head and neck cancer and non‐small cell lung cancer (2 studies, 89 participants, qualitative analysis). Analyses of tolerability and safety were not possible for these studies.
There was low‐certainty evidence that synthetic THC analogues were superior to placebo (SMD −0.98, 95% CI −1.36 to −0.60), but not superior to low‐dose codeine (SMD 0.03, 95% CI −0.25 to 0.32; 5 single‐dose trials; 126 participants) in reducing moderate‐to‐severe cancer pain after cessation of previous analgesic treatment for three to four and a half hours (2 single‐dose trials; 66 participants). Analyses of tolerability and safety were not possible for these studies.
There was low‐certainty evidence that CBD oil did not add value to specialist palliative care alone in the reduction of pain intensity in people with advanced cancer. There was no difference in the number of dropouts due to adverse events and serious adverse events (1 study, 144 participants, qualitative analysis).
We found no studies using herbal cannabis.
Authors' conclusions
There is moderate‐certainty evidence that oromucosal nabiximols and THC are ineffective in relieving moderate‐to‐severe opioid‐refractory cancer pain. There is low‐certainty evidence that nabilone is ineffective in reducing pain associated with (radio‐) chemotherapy in people with head and neck cancer and non‐small cell lung cancer. There is low‐certainty evidence that a single dose of synthetic THC analogues is not superior to a single low‐dose morphine equivalent in reducing moderate‐to‐severe cancer pain. There is low‐certainty evidence that CBD does not add value to specialist palliative care alone in the reduction of pain in people with advanced cancer.
Keywords: Adult; Humans; Analgesics, Opioid; Analgesics, Opioid/adverse effects; Cancer Pain; Cancer Pain/drug therapy; Cannabis; Carcinoma, Non-Small-Cell Lung; Carcinoma, Non-Small-Cell Lung/drug therapy; Codeine; Lung Neoplasms; Lung Neoplasms/drug therapy; Medical Marijuana; Medical Marijuana/adverse effects; Morphine; Randomized Controlled Trials as Topic
Plain language summary
Cannabis‐based medicines for cancer pain
Do medicines based on cannabis help adults with cancer pain?
Key messages
Cannabis‐based medicines (CbMs) did not relieve cancer pain that did not respond to morphine‐like medicines.
The studies analysed did not allow any statement to be made on the place of these medications in the World Health Organization (WHO) analgesic ladder for cancer pain.
Trials with CbMs in cancer need to be very much better designed than those conducted so far.
Pain in cancer and its treatment
One person in two or three who gets cancer will have pain that becomes moderate or severe in intensity. The pain tends to get worse as the cancer progresses.
The WHO recommends taking morphine‐like medicines for moderate‐to‐severe pain from cancer, but 1 in 6 to 10 people with cancer pain do not experience sufficient pain relief from morphine‐like medicines. Several products based on the cannabis plant have been suggested as treatment for cancer pain. These products include inhaled or orally ingested herbal cannabis, and various oils, sprays or tablets containing active cannabis ingredients obtained from the plant, or made synthetically. Some people with cancer pain have reported that CbMs are effective for them, and that is often highlighted in the media.
What did we want to find out?
If CbMs relieved cancer pain in people living with cancer.
If CbMs were associated with any unwanted or harmful effects.
What did we do?
We searched for clinical trials that examined CbMs compared to other medications to treat cancer pain in adults.
We summarised the results of the studies and rated our confidence in the evidence, based on factors such as the methods and size of studies.
What did we find?
We found 14 studies involving 1823 people. The biggest study included 399 people and the smallest study included 10 people.
Studies were conducted in countries around the world; most (six) were based in North America.
Five studies used one dose of CbM and lasted less than one day. Other studies lasted between two and eight weeks.
Pharmaceutical companies funded seven studies.
Six studies compared a mouth spray with a plant‐derived combination of tetrahydrocannabinol (THC), the principal psychoactive constituent of cannabis, and cannabidiol (CBD), an anti‐inflammatory ingredient of cannabis, against a fake medication (placebo). Seven studies compared an artificial cannabinoid mimicking the effects of THC against placebo. Of these seven studies, two studies compared against a morphine‐like medication (codeine), too. One study compared CBD against placebo.
We did not find studies with herbal cannabis.
Main results
Mouth spray with a plant‐derived combination of THC and CBD was probably not better than placebo in reducing pain in people with moderate‐to‐severe cancer pain despite opioid treatment. Thirty‐two out of 100 people reported to be much or very much improved by cannabis‐based mouth spray and 23 out of 100 people with mouth spray with placebo. A total of 19 out of 100 people withdrew early because of side effects by cannabis‐based mouth spray and 16 out of 100 people by mouth spray with placebo. There was no difference in serious side effects between the cannabis‐based mouth spray and a placebo mouth spray.
Artificial cannabinoid mimicking the effects of THC may not be better than a fake medication in reducing pain associated with chemotherapy or radiochemotherapy in people with head and neck cancer and a certain type of lung cancer.
A single dose of an artificial cannabinoid mimicking the effects of THC may be better than a single dose of placebo, but may not differ from a single small dose of a morphine‐like medication in reducing moderate‐to‐severe cancer pain after cessation of previous analgesic treatment for three to four and a half hours.
CBD may not add value to specialist palliative care alone in the reduction of pain in people with advanced cancer.
We found no studies with medical cannabis.
What are the limitations of the evidence?
We are moderately confident in the evidence that a mouth spray with a plant‐derived combination of THC and CBD does not reduce severe cancer pain despite opioid treatment because studies did not provide information about everything that we could have used.
We have little confidence in the evidence that an artificial cannabinoid mimicking the effects of THC (nabilone) does not reduce pain associated with chemotherapy or radiochemotherapy because the studies did not provide data about everything that we could have used, and because the studies were small.
We have little confidence in the evidence that artificial cannabinoids mimicking the effects of THC reduce cancer pain after the previous pain‐relieving medication was stopped some hours before because the studies did not provide data about everything that we could have used, and because the studies were small.
We have little confidence in the evidence that CBD added to standard palliative care does not reduce cancer pain because there was only one study available.
How up to date is the evidence?
The evidence is up to date to January 2023.
Summary of findings
Summary of findings 1. Cannabis‐based medicines compared with placebo medication for cancer pain.
| Cannabis‐based medicines compared with placebo medication for cancer pain | ||||||
|
Patient or population: adults with cancer pain Settings: outpatient study centres and hospitals in Europe and North America Intervention: oromucosal THC with or without CBD Comparison: oromucosal placebo | ||||||
| Outcomes | Observed outcome (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Oromucosal placebo | Oromucosal THC with or without CBD | |||||
| Proportion of participants reporting no worse than mild pain by 14 days after start of treatment | No data for this outcome were reported. | — | — | — | — | |
| PGIC of much improved or very much improved | 230 per 1000 | 320 per 1000 (95% CI 290 to 350 per 1000) | RD 0.06 (0.01 to 0.12) | 996 (3) | ⊕⊕⊕⊝ Moderatea | NNTB 16 (95% CI 8 to 100) |
| Withdrawals due to adverse events | 160 per 1000 | 190 per 1000 (95% CI 170 to 210 per 1000) |
RD 0.04 (0.00 to 0.08) |
1332 (4) | ⊕⊕⊕⊝ Moderatea | NNTH 25 (95% CI 12 to endless) |
| Mean pain intensity (Numeric Rating Scale 0–10) | The mean pain intensity at baseline was 5.6 (SD 1.2)b | The mean pain intensity in the intervention group was 0.19 SDs lower (0.40 lower to 0.02 higher) | SMD −0.19 (−0.40 to 0.02) | 1315 (4) | ⊕⊕⊕⊝ Moderatea | — |
| Daily maintenance opioid dosage (mg morphine equivalent) | The mean dosage at baseline was 159.7 (SD 121.2) mg/dayb | The mean dosage in the intervention group was 0.08 SDs higher (0.10 lower to 0.27 higher) | SMD 0.08 (−0.10 to 0.27) | 970 (3) | ⊕⊕⊝⊝ Lowc | — |
| Daily breakthrough opioid dosage (mg morphine equivalent) | The mean dosage at baseline was 26.4 (SD 40.4) mg/dayb | The mean dosage in the intervention group was 0.08 SDs lower (0.23 lower to 0.07 higher) | SMD −0.08 (−0.23 to 0.07) | 957 (3) | ⊕⊕⊕⊝ Moderatea | — |
| Participants experiencing any serious adverse event | 210 per 1000 | 240 per 1000 (95% CI 220 to 260 per 1000) |
RD 0.02 (−0.03 to 0.07) |
1330 (4) | ⊕⊕⊕⊝ Moderatea | — |
| CBD: cannabidiol; CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; PGIC: Patient Global Impression of Change; RD: risk difference; SD: standard deviation; SMD: standardised mean difference; THC: tetrahydrocannabinol. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level due to limitations of study. bLichtman 2018. c Downgraded two levels due to limitations of study design and imprecision of results.
Background
Description of the condition
Cancer is the second leading cause of death globally, accounting for an estimated 9.6 million deaths, or one in six deaths, in 2018 (WHO 2021). Lung, prostate, colorectal, stomach and liver cancer are the most common types of cancer in men, while breast, colorectal, lung, cervical and thyroid cancer are the most common amongst women (WHO 2021). Pain is one of the most feared symptoms associated with cancers, and can occur at any time during the course of the disease. The frequency and intensity of pain tend to increase as the cancer advances (van den Beuken‐van Everdingen 2016). One systematic review has shown that approximately 40% of people living with cancer experienced pain after curative treatment, 55% during cancer treatment, and 66% in advanced disease (van den Beuken‐van Everdingen 2016). Pain may be specifically related to the cancer (direct tumour effects, systemic tumour effects), the effects of cancer treatments (e.g. radiation or chemotherapy) or due to some other comorbid disease (Swarm 2019). In this review, we defined cancer pain as pain arising as a direct consequence of the cancer or of cancer therapy (or both), and not due to another condition.
The World Health Organization (WHO) analgesic ladder advocates a stepwise approach to analgesia for cancer pain. It recommends that opioids be used as first‐line treatment for moderate‐to‐severe cancer pain (WHO 2019). An overview of Cochrane Reviews found the quantity and quality of evidence supporting the use of opioids for cancer pain to be low (Wiffen 2017). In clinical practice, most people with cancer will achieve adequate pain relief with opioids. However, wide interpatient variability in the response to opioids has been reported and 10% to 15% of people with cancer pain are defined as opioid non‐responders (Corli 2016). Therefore, there is a substantial need for new analgesics that can effectively and safely supplement or replace opioids in people with insufficient relief of cancer pain.
Description of the intervention
The cannabinoid (CB) system is ubiquitous in the animal kingdom and is said to perform multiple functions that move the organism back to equilibrium. A large body of evidence currently supports the presence of CB receptors and ligands in the peripheral and central nervous system, but also in other tissues such as bone and in the immune system (Owens 2015; Soliman 2019). The endocannabinoid system is said to have three broad and overlapping functions in mammals. The first is a stress recovery role, operating in a feedback loop in which endocannabinoid signalling is activated by stress and functions to return endocrine, nervous and behavioural systems to homeostatic balance. The second is to control energy balance through regulation of the intake, storage and utilisation of food. The third involves immune regulation; endocannabinoid signalling is activated by tissue injury and modulates immune and inflammatory responses (Hillard 2012). Thus, the endocannabinoid neuromodulatory system is assumed to be involved in multiple physiological functions, such as antinociception, cognition and memory, endocrine function, nausea and vomiting, inflammation and immune recognition (De Vries 2014; Hillard 2012).
Cannabis is a genus of the flowering plant in the family Cannabaceae. The number of species within the genus is disputed. Three species are recognised, Cannabis sativa, Cannabis indica and Cannabis ruderalis. These plants, commonly known as marijuana, have been used for pain relief for millennia, and have additional effects on appetite, sleep and mood (Kalant 2001). Because of the multiple mechanisms of action of cannabis in the human organism, cannabis has the potential to modulate some of the most common and debilitating symptoms of cancer and its treatments, including nausea and vomiting, loss of appetite and pain (Kleckner 2019).
How the intervention might work
Cannabis contains over 450 compounds, with at least 120 classified as phytocannabinoids. Two are of particular medical interest. Delta 9‐tetrahydrocannabinol (delta 9‐THC) is the main active constituent, with psychoactive (e.g. reduction of anxiety) and pain‐relieving properties. The second molecule of interest is cannabidiol (CBD), which has lower affinity for the CB receptors and the potential to counteract the negative effects of tetrahydrocannabinol (THC) on memory, mood and cognition, but may also have an effect on pain modulation due to anti‐inflammatory properties. The specific roles of currently identified cannabis‐based medicines (CbM) that act as ligands at CB receptors within the nervous system (primarily but not exclusively CB1 receptors) and in the periphery (primarily but not exclusively CB2 receptors) are only partially elucidated, but there are many preclinical data to support their influence on nociception (Owens 2015; Soliman 2019). It is also hypothesised that cannabis reduces alterations in cognitive and autonomic processing in chronic pain states. The frontal‐limbic distribution of CB receptors in the brain suggests that cannabis may preferentially target the affective qualities of pain (Lee 2013).
Terminology and definitions of CbMs vary in the literature. A terminology based on the proposals of the task forces of the European Pain Federation (EFIC) (Häuser 2018), and the International Association for the Study of Pain (IASP) (Soliman 2019) is listed in Appendix 1.
CbMs are available in different forms.
Licenced medical drugs or products currently being tested for medical use are as follows.
Plant‐derived CBs: oromucosal THC and CBD (nabiximols; Sativex) or oral CBD (Epidiolex). Nabiximols is approved in some countries for the treatment of refractory spasticity in people with multiple sclerosis (Krcevski‐Skvarc 2018). Oral CBD is approved by the European Medicines Agency for the management of Dravet syndrome and Lennox‐Gastaut syndrome, two rare forms of epilepsy in children (European Medicines Agency 2019).
Synthetic CBs: nabilone (Cesamet or Canemes), a synthetic THC, is approved in some countries for the management of refractory nausea/emesis in people with cancer (Abuhasira 2018; Krcevski‐Skvarc 2018). Dronabinol (Marinol or Syndros), a synthetic THC, is approved for similar therapeutic use in some countries (Abuhasira 2018; Krcevski‐Skvarc 2018). Levonantradol, a potent synthetic THC is used in research, but is not available as a licensed therapeutic drug in any country.
Magistral preparations (i.e. any medicinal product prepared in a pharmacy in accordance with a medical prescription for an individual patient) of cannabis plant derivatives as follows.
Defined CBs such as plant‐derived dronabinol (THC) or plant‐derived CBD
Herbal cannabis, resins and extracts, such as oil or tinctures with defined content of THC or CBD (or both), together with other active ingredients (phytocannabinoids other than CBD/THC, such as terpenes and flavonoids)
The main forms of administration are as follows.
Oromucosal: spray (nabiximols)
Oral: capsules (dronabinol, nabilone), oil (CBD), extracts (dronabinol, herbal cannabis)
Smoke or vapour inhalation: CBD, dronabinol, herbal cannabis, resins
Topical or rectal: CBD, herbal cannabis, resins, extracts
There is a great variability in European countries with regard to the availability of the different CbMs and medical cannabis and their reimbursement by health statutory companies (Krcevski‐Skvarc 2018).
In addition, CBD and extracts of cannabis flowers (THC content less than 0.2%) are available in many countries as nutritional supplements (Radbruch 2020).
CB receptor antagonists and negative allosteric modulators (e.g. rimonabant (SR141716A)) and modulators that increase or enhance endocannabinoid system activity (e.g. fatty acid amide hydrolase inhibitors) are experimental medications which have been not yet been approved for use in pain therapy outside clinical studies (Ye 2019).
Why it is important to do this review
Contrary to the usual path of drug approval, CbMs in an increasing number of European countries have bypassed traditional approval by drug agencies and have been made available by legislative bodies as therapeutic products for pain management (Krcevski‐Skvarc 2018). Propelled by public advocacy and the media, medical cannabis in particular has been promoted as an effective and safe treatment for cancer pain (Blake 2017). Other benefits that are quoted include the potential reduction of harm related to opioid use, and the purported benefits for sleep disturbance as well as mood disorders (Vyas 2018). The worldwide surge in use of cannabis in the management of people with cancer is illustrated by the prevalent use of medical cannabis and illegal cannabis by up to 40% of people with cancer in Canada and Israel, countries where legal access to medical cannabis is available (Bar‐Lev Schneider 2018; Martell 2018).
At the time of writing this review, the amount and quality of evidence for CbMs for chronic pain has been low, with the evidence compromised by studies of short duration and small numbers of participants (Fisher 2021; Stockings 2018). In addition, a systematic overview of systematic reviews has pointed out that non‐Cochrane systematic reviews of CBs for pain are of overwhelmingly low or very low quality (Moore 2021). A 2020 systematic review of randomised controlled trials (RCTs) of CbMs for chronic pain concluded that studies in this field have unclear or high risk of bias, and outcomes had GRADE ratings of low‐ or very low‐certainty evidence, with little confidence in the estimates of effect (Fisher 2021). The systematic review found no benefit of nabiximols compared to placebo, for at least 30% pain relief (two RCTs delivering treatment of two to five weeks) and mean change of pain from baseline (four RCTs delivering treatment of two to five weeks). Another systematic review analysed the same RCTs as Fisher 2021 and found no benefit of nabiximols when compared to placebo for reducing pain and sleep problems (Häuser 2019). However, this review found patient impression of change to be much or very much improved in the group receiving nabiximols (Häuser 2019).
Additional outcomes have gained importance to assess the efficacy and safety of CbMs for cancer pain. The US Food and Drug Administration (FDA) has suggested new combined responder outcomes for cancer pain trials: participants are only considered responders if they experience a clinically significant decrease in pain intensity compared with baseline at the primary analysis time point, and overall analgesic use is either decreased or stable compared with baseline (Basch 2014). Moreover, Cochrane Reviews of the use of opioids for cancer pain have favoured the primary outcome of mild or no pain at 14 days (Wiffen 2017). Our review will look for that outcome to allow comparability with opioids for cancer pain, as it was not an outcome reported in Fisher 2021.
Potential positive effects of CbMs for people with cancer have to be balanced against potential adverse effects. One systematic review with pooled analysis of studies of CbMs for chronic pain emphasised the high rate of adverse effects with low (unfavourable) numbers needed to harm for central nervous system and psychiatric adverse effects (Stockings 2018). Fisher 2021 has combined all adverse effects in one analysis.
In view of these considerable uncertainties, we have seen the need to update the literature and to assess the efficacy, tolerability and safety of CbMs compared to placebo or conventional medications for cancer pain. We concentrated on:
additional participant‐reported outcomes beyond pain, such as sleep problems and mood;
opioid‐sparing effects;
central nervous system and psychiatric adverse effects.
Objectives
To evaluate the benefits and harms of cannabis‐based medicines, including medical cannabis, for treating pain and other symptoms in adults with cancer compared to placebo or any other established analgesic for cancer pain.
Methods
Criteria for considering studies for this review
Types of studies
RCTs are the best design to minimise bias when evaluating the effectiveness of an intervention. We considered randomised, double‐blind (participants and physicians), controlled trials comparing CbMs and medical cannabis with placebo or any other established analgesic for cancer pain, according to the ladder scheme of the WHO (WHO 2019). Trials must have included participant‐reported pain outcomes. We included RCTs of any duration. The emphasis of the review was studies of two weeks or longer to try to obtain the efficacy outcome used in a previous overview of Cochrane Reviews on opioids for cancer pain (Wiffen 2017). The clinical importance of experimental studies (one to three days' duration) and very short‐term studies (four to 13 days' duration) in chronic pain is limited. In addition, we considered studies in which CbMs are used as add‐on therapy to established analgesics, compared to these established analgesics without CbMs, and with participant‐reported pain outcomes. Studies had to include at least 10 participants per treatment arm (we made an ad hoc decision to change the method described in our protocol, which required 20 participants per treatment arm; Differences between protocol and review; Häuser 2022). We included RCTs reporting at least one of our primary outcomes.
Types of participants
Eligible studies included men and women (aged 18 years or older) of any race with cancer‐related pain (cancer pain or cancer therapy‐related pain, or both). We included all types and stages of cancer, in all settings, and receiving any type of cancer therapy. We included studies with mixed pain conditions, if the results for people with cancer‐related pain were reported separately.
Types of interventions
We included CbMs (plant‐based CBs (CBD, dronabinol, nabiximols)), or synthetic CBs (nabilone) or medical cannabis (cannabis flowers or full spectrum cannabis extracts) at any dose or by any route that were administered for the relief of cancer pain.
The comparison groups received placebo or other established analgesic medication for cancer pain.
We did not consider experimental and non‐registered drugs such as CB receptor antagonists and negative allosteric modulators (e.g. rimonabant (SR141716A)) and modulators that increase or enhance endocannabinoid system activity (e.g. fatty acid amide hydrolase inhibitors) or synthetic CBs (e.g. levonantradol).
Types of outcome measures
Primary outcomes
The proposed primary outcomes are the same as those used by Wiffen 2017 in the overview review of opioids for cancer pain.
Proportion of participants reporting no worse than mild pain by 14 days after start of treatment (typically below 30/100 mm on a 100‐mm Visual Analogue Scale (VAS) or below 3 on an 11‐point Numeric Rating Scale (NRS)) as an acceptable outcome when their pain was moderate or severe (Moore 2013).
Patient Global Impression of Change (PGIC) of much improved or very much improved.
Withdrawals due to adverse events.
Secondary outcomes
Combined responder: number of participants who reported pain relief of 30% or greater and overall opioid use reduced or stable compared to baseline for parallel and cross‐over design studies and loss of this therapeutic response for studies with an enriched enrolment randomised withdrawal (EERW) design.
Number of participants who reported pain relief of 30% or greater.
Number of participants who reported pain relief of 50% or greater.
Mean pain intensity: we preferentially extracted outcomes of numeric over visual pain scales.
Sleep problems: we preferentially extracted outcomes of multidimensional questionnaires over single‐item questionnaires.
Depression: we preferentially extracted outcomes of multidimensional questionnaires over single‐item questionnaires.
Anxiety: we preferentially extracted outcomes of multidimensional questionnaires over single‐item questionnaires.
Daily maintenance opioid dosage (mg morphine equivalent).
Daily breakthrough opioid dosage (mg morphine equivalent).
Number of participants dropping out due to lack of efficacy.
All central nervous system adverse events according to the Medical Dictionary for Regulatory Activities (International Council for Harmonisation 2020).
All psychiatric adverse events according to the Medical Dictionary for Regulatory Activities (International Council for Harmonisation 2020).
Participants experiencing any serious adverse event.
Search methods for identification of studies
Electronic searches
We searched the following databases originally on 3 March 2022 and performed an updated search on 26 January 2023, without language or date restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Library, Issue 1, 2023.
MEDLINE (via Ovid) (1946 to 25 January 2023).
Embase (via Ovid) (1974 to 25 January 2023).
The search strategies used are outlined in Appendix 2. The MEDLINE search strategy was independently peer‐reviewed when it was developed. We checked for retractions of included studies using the Retraction Watch database (retractiondatabase.org/).
Searching other resources
We reviewed the bibliographies of any RCTs identified. We searched the following clinical trials databases to identify additional published or unpublished data (all to 3 March 2022 and updated 27 January 2023).
US National Institutes of Health ClinicalTrials.gov (www.ClinicalTrials.gov)
EU Clinical Trials Register (www.clinicaltrialsregister.eu)
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/clinical-trials-registry-platform)
In addition, we searched grey literature, checked reference lists of reviews and retrieved articles for additional studies, and performed citation searches on key articles. We contacted experts in the field for unpublished and ongoing trials. We did not contact study authors for additional information.
Data collection and analysis
Selection of studies
Three review authors (EF, LR, WH) independently determined eligibility by reading the abstract and title of each study identified by the search. They eliminated studies that clearly did not satisfy the inclusion criteria, and obtained full copies of the remaining studies. Two review authors (RFB, WH) independently read these studies and reached agreement by discussion. Consulting a third review author (PW) was not necessary because there was no disagreement on the inclusion and exclusion of studies. We did not anonymise the studies before assessment. We created a PRISMA flow chart (Moher 2009).
Data extraction and management
Two review authors (PW, WH) independently extracted data using a prepiloted standard form and checked for agreement before entering data into Review Manager 5 (Review Manager 2020). Three review authors (PW, RFB, WH) independently extracted information about the study funding sources and study author conflicts of interest, the cancer condition, number of participants treated, study setting, inclusion and exclusion criteria, demographic and clinical characteristics of the study samples (age, gender, race, pain baseline), prior recreational cannabis use, drug and dosing regimen, cotherapies allowed, rescue medication, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event or serious adverse effect). We analysed the nature of all serious adverse events. We analysed the nature of all adverse events, but concentrated on those that are regarded to be most relevant adverse events of CbMs and MC, namely central nervous system and psychiatric adverse events.
Assessment of risk of bias in included studies
Two review authors (RFB, WH) independently assessed risk of bias for each study using the Cochrane RoB 1 tool, using the criteria outlined in the 2011 edition of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We also used criteria adapted from those used by the Pain, Palliative and Supportive Care Review Group (group was closed in 2023) for reviews on medication therapy for cancer pain, with any disagreements resolved by discussion. Consulting a third review author (PW) was not necessary because there was no disagreement on the risk of bias assessment.
We assessed the following risks of bias for each study as follows.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (i.e. any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (when the method used to generate the sequence was not clearly stated); high risk of bias (studies used a non‐random process (e.g. odd or even date of birth; hospital or clinic record number)).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); unclear risk of bias (when method was not clearly stated). We excluded studies that did not conceal allocation and were, therefore, at high risk of bias (e.g. open list).
Blinding of participants and personnel/treatment providers (systematic performance bias). We assessed the methods used to blind participants and personnel/treatment providers from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, e.g. identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved); high risk of bias (blinding of participants was not ensured, e.g. tablets different in form or taste).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that outcome assessors were blinded to the intervention or exposure status of participants); unclear risk of bias (study stated that the outcome assessors were blinded but did not provide an adequate description of how it was achieved); high risk of bias (outcome assessors knew the intervention or exposure status of participants).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (i.e. less than 10% participant dropout or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); or high risk of bias (used 'completer' analysis).
Reporting bias due to selective outcome reporting (reporting bias). We checked if a study protocol before the start of the study was available and if all outcomes of the study protocol were reported in the publications of the study. There was low risk of reporting bias if the study protocol was available and all the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way, or if the study protocol was not available, but it was clear that the published reports included all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). We judged high risk of reporting bias if not all the study's prespecified primary outcomes were reported; one or more primary outcomes was reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); one or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis; the study report did not include results for a key outcome that would be expected to have been reported for such a study. We judged unclear risk of bias if there was insufficient information to permit judgement of 'low risk' or 'high risk'.
In addition to the original risk of bias criteria outlined in the 2011 edition of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we assessed 'Group similarity at baseline' (selection bias) as another risk of bias. We assessed similarity of the study groups at baseline for the most important prognostic clinical and demographic indicators. We judged low risk of bias if groups were similar at baseline for demographic factors, value of main outcome measure(s) and important prognostic factors. We judged unclear risk of bias if important prognostic clinical and demographic indicators were not reported. We judged high risk of bias if groups were not similar at baseline for demographic factors, value of main outcome measure(s) and important prognostic factors.
We also assessed overall risk of bias in each trial according to guidance in the current edition of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Low risk of bias: the trial was judged to be at low risk of bias for all domains for this result.
Some concerns: the trial was judged to raise some concerns in at least one domain for this result, but not to be at high risk for any domain for this result.
High risk of bias: the trial was judged to be at high risk of bias in at least one domain for this result or the judged to raise some concerns in multiple domains for this result in a way that substantially lowers confidence in the result.
Measures of treatment effect
We calculated numbers needed to treat for an additional beneficial outcome (NNTB) as the reciprocal of the absolute risk reduction (ARR) (McQuay 1998). For unwanted effects, the NNTB becomes the number needed to treat for an additional harmful outcome (NNTH) and is calculated in the same manner. We used dichotomous data to calculate risk differences (RD) with 95% confidence intervals (CIs) using a fixed‐effect model unless we found significant statistical or clinical heterogeneity (see below). We set the threshold for a clinically relevant benefit or a clinically relevant harm for categorical variables by an NNTB or NNTH less than 10 (Moore 2008).
We calculated standardised mean differences (SMD) with 95% CIs for continuous variables, using a random‐effects model. We used Cohen's categories to evaluate the magnitude of the effect size, calculated by SMD, with Hedges' g value of 0.2 = small, 0.5 = medium and 0.8 = large (Cohen 1988). We labelled a g value less than 0.2 to be a 'not substantial' effect size. We assumed a minimally important difference if the Hedges' g value was 0.2 or greater (Fayers 2014). To increase interpretability, we analysed the mean difference of mean pain intensity. If needed, we converted 0 to 10 and 0 to 100 NRS or VAS to a single scale.
Unit of analysis issues
For studies with more than two arms, we split the control treatment arm between active treatment arms in a single study if the active treatment arms could not to be combined for analysis. We included studies with a cross‐over design where separate data from the two periods were reported, data were presented that excluded a significant carry‐over effect or statistical adjustments were carried out in case of a significant carry‐over effect. We did not anticipate cluster trials for this intervention.
Dealing with missing data
We used intention‐to‐treat (ITT) analysis where the ITT population consisted of participants who were randomised, took at least one dose of the assigned study medication and provided at least one postbaseline assessment. Where means or standard deviations (SDs) were missing, we attempted to obtain these data through contacting trial authors. Where SDs were not available from trial authors, we calculated them from t‐values, P values, CIs or standard errors, where reported by the studies (Higgins 2020a). Where rates of pain relief of 30% or greater and of 50% or greater were not reported or provided on request, we calculated them from means and SDs using a validated imputation method (Furukawa 2005).
Assessment of heterogeneity
We dealt with clinical heterogeneity by combining studies that examined similar conditions. We assessed statistical heterogeneity using the I2 statistic. Where the I2 value was greater than 50%, we considered possible reasons for this.
Assessment of reporting biases
We assessed publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNTB of 10 or higher) (Moore 2008).
Data synthesis
We used a random‐effects model, using the inverse variance method in Review Manager 5 for meta‐analysis, because we expected clinical heterogeneity due to the different types of cancer pain conditions (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
We intended to perform subgroup analyses for the primary outcomes according to the following, where there were at least two studies available.
Different types of CbMs.
Different dosages of the same CbM and study duration. We distinguished between short‐term (four to 12 weeks), intermediate‐term (13 to 26 weeks) and long‐term (more than 26 weeks) studies (Chaparro 2013), as well as experimental studies (one to three days) and very short‐term (three to 13 days) studies.
Types of controls (placebo; established analgesic).
Types of cancer‐related pain (pain directly caused by cancer, e.g. by bone metastases versus pain caused by cancer treatment, e.g. chemotherapy‐induced polyneuropathy).
These subgroup analyses were predefined due to the many uncertainties about CbMs for chronic pain, such as the selection of the type of CbM (cannabis flowers versus CBs), optimal dosage for efficacy, duration of efficacy, and comparative efficacy and safety to established medications (Fisher 2021; Häuser 2018).
Because of the relevant differences of study designs and purposes of the studies, we decided not to pool all studies. Instead, we conducted four separate analyses.
Sensitivity analysis
We performed a sensitivity analysis by excluding studies with imputed rates of pain relief of 30% or greater. We did not conduct the planned sensitivity analysis by excluding studies with imputed rates of pain relief of 50% or greater because all rates of pain relief of 50% or greater had to be calculated by an imputation method. The planned sensitivity analysis excluding studies with less than 14 days' duration was not necessary because we did not pool studies with a duration of less and of more than 14 days.
Summary of findings and assessment of the certainty of the evidence
Two review authors (EF, WH) independently rated the certainty of the body of evidence for the outcomes. We resolved discrepancies by consulting a third review author (RAM). We used the GRADE system to rank the certainty of the evidence using the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2020) and the GRADE Handbook (Schünemann 2013).
The GRADE system considers study design as a marker of quality. It uses the following criteria for assigning a certainty level to the body of evidence for a given outcome.
High: randomised trials without downgrading or double‐upgraded observational studies
Moderate: downgraded randomised trials or upgraded observational studies
Low: double‐downgraded randomised trials or observational studies without downgrading
Very low: triple‐downgraded randomised trials, downgraded observational studies or case series/case reports
Factors that may decrease the certainty level of a body of evidence are as follows.
Limitations in the design and implementation of available studies, suggesting high likelihood of bias. We assumed that there were limitations in study design if more than 50% of participants were from studies with high risk of bias, as defined by the Cochrane RoB 1 tool (Higgins 2011).
Indirectness of evidence (indirect population, intervention, control, outcomes). We assessed if the study population was different from the population in routine clinical care by assessing if studies excluded participants with relevant medical conditions (cardiovascular, hepatic, renal and endocrine system). If exclusion of participants with clinically relevant medical conditions resulted in 50% or more of the total number of participants, we decreased the certainty of evidence.
Unexplained heterogeneity (I2 greater than 50%) or inconsistency of results.
Imprecision of results (wide CIs; low number of events).
High probability of publication bias. We assumed a potential publication bias if all studies were initiated and funded by the manufacturer of the drug tested in the trial.
We used the GRADE system criteria for assigning the grade of evidence (Schünemann 2013).
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We created one summary of findings table to present in a transparent and simple tabular format the main findings for comparisons of CbMs and medical cannabis with placebo or any established analgesic. In particular, we included key information concerning the certainty of evidence, the magnitude of effect of the interventions examined and the sum of available data on these outcomes:
proportion of participants reporting no worse than mild pain by 14 days after start of treatment;
PGIC of much improved or very much improved;
withdrawals due to adverse events;
mean pain intensity;
daily maintenance opioid dosage (mg morphine equivalent);
daily breakthrough opioid dosage (mg morphine equivalent);
participants experiencing any serious adverse event.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
The searches (performed 3 March 2022, updated 26 January 2023) produced 966 records We identified 145 potentially relevant studies in CENTRAL, 433 in MEDLINE, 246 in Embase, 121 in the ClinicalTrials.gov, nine in the EU Clinical Trials Register and 12 in the WHO ICTRP. A search for studies in the International Association for Cannabinoid Medicines (IACM) databank was not possible because the database was no longer available. We identified 38 additional records through other sources. After removing duplicates, we read the titles and abstracts of 297 articles and excluded studies that were clearly irrelevant. We read the full text of 18 potentially eligible articles and included 14 studies in the review (Côté 2016; Fallon 2017a; Fallon 2017b; Hardy 2023; Jochimsen 1978; Johnson 2010; Lichtman 2018; Lynch 2014; Noyes 1975a; Noyes 1975b; Portenoy 2012; Staquet 1978a; Staquet 1978b; Turcott 2018) (see Figure 1). We excluded one study with reasons (see Characteristics of excluded studies table) and identified five ongoing studies (see Characteristics of ongoing studies table). No studies are awaiting classification.
1.
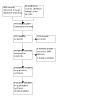
Study flow diagram.
Four studies with nabiximols for opioid refractory cancer pain (Fallon 2017a; Johnson 2010; Lichtman 2018; Portenoy 2012), and four experimental studies with a synthetic THC analogue (Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b), were suited for quantitative analysis. Six studies were included only in qualitative analysis: one study employed a withdrawal design (Fallon 2017b). The heterogeneity of the aims of four studies (reducing chemotherapy induced neuropathic pain (Lynch 2014), improving health‐related quality of life (Côté 2016), reducing cachexia (Turcott 2018), and total symptom burden (Hardy 2023)), and the different medications used prohibited quantitative synthesis (Lynch 2014: nabiximols; Côté 2016; Turcott 2018: synthetic THC analogue (nabilone); Hardy 2023: CBD). The reported outcomes of one experimental study with synthetic THC analogue was not suited for quantitative analysis (Jochimsen 1978).
Included studies
Characteristics of the studies
We included 14 studies with 20 treatment arms involving 1823 participants into the analysis. The studies of Noyes 1975a and Noyes 1975b involved different populations. Four studies involving different participants were reported in two publications (Fallon 2017a; Fallon 2017b; Staquet 1978a; Staquet 1978b).
Aims of the studies
Five studies tested if nabiximols was effective as an add‐on therapy for people with cancer pain not adequately relieved by opioids (Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018; Portenoy 2012). Five studies tested if a single dose of synthetic THC analogue relieved moderate‐to‐severe cancer pain after stopping other analgesics four hours before the intake of synthetic THC analogue (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b). One study tested nabiximols for chemotherapy‐induced neuropathic pain (Lynch 2014). Two studies tested a synthetic THC analogue (nabilone) to reduce cancer and radiochemotherapy‐related symptoms (Côté 2016; Turcott 2018). One study tested CBD to reduce total symptom burden in advanced cancer (Hardy 2023). Thus, the aim of one study was to reduce cancer therapy‐related pain (Lynch 2014), of two studies to reduce cancer‐related and cancer therapy‐related pain (Côté 2016; Turcott 2018), and of the remaining studies to reduce cancer‐related pain.
Study setting
We found eight studies used a single‐centre recruitment strategy (Côté 2016; Jochimsen 1978; Lynch 2014; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b; Turcott 2018), the remaining studies were multicentre. The number of study centres ranged between 20 and 114. Six studies were conducted in North America (Côté 2016; Jochimsen 1978; Lynch 2014; Noyes 1975a; Noyes 1975b; Portenoy 2012), two in Belgium (Staquet 1978a; Staquet 1978b), one in Australia (Hardy 2023), and one in Mexico (Turcott 2018). The remaining studies were conducted across two continents: Fallon 2017a; Johnson 2010; and Lichtman 2018 included participants from North America and Europe and Fallon 2017b from Europe and Asia.
Study design
Seven studies used a parallel design (Côté 2016; Fallon 2017a; Hardy 2023; Johnson 2010; Lichtman 2018; Portenoy 2012; Turcott 2018), six studies had a cross‐over design (Jochimsen 1978; Lynch 2014; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b), and one study had a withdrawal design (Fallon 2017b). The one‐day studies tested two and three dosages of a synthetic THC analogue (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b). One study had one THC and one THC/CBD arm (Johnson 2010). One study had three THC/CBD arms with different dosages (Portenoy 2012).
Study duration
We found five experimental studies with one dose lasting less than one day (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b). The double‐blind period was two weeks in one study (Johnson 2010), five weeks in four studies (Fallon 2017a; Fallon 2017b; Lichtman 2018; Portenoy 2012), and eight weeks in three studies (Côté 2016; Lynch 2014; Turcott 2018).
Sample sizes
The sample sizes ranged between 10 and 399. Eight studies had treatment group sizes below 50 participants (Côté 2016; Jochimsen 1978; Lynch 2014; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b; Turcott 2018). The remaining six were between 50 and 200 participants in each treatment group (Fallon 2017a; Fallon 2017b; Hardy 2023; Johnson 2010; Lichtman 2018; Portenoy 2012). Treatment group sizes were of the order of 200 participants or more in two studies (Fallon 2017a; Lichtman 2018).
Study period
Two studies reported the study period which was 2005 to 2007 (Côté 2016) and 2017 to 2019 (Hardy 2023).
Study funding
Four studies received public funding (Côté 2016; Hardy 2023; Noyes 1975a; Noyes 1975b). Pharmaceutical companies funded four studies (Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018). Two studies received public and pharmaceutical company funding (Jochimsen 1978; Portenoy 2012). Two studies received no funding (Lynch 2014; Turcott 2018). Two studies did not report on funding (Staquet 1978a; Staquet 1978b).
Conflicts of interest
Authors of seven studies reported that they had no conflicts of interest (Côté 2016; Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018; Lynch 2014; Turcott 2018). Authors of six studies did not report on conflicts of interest (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Portenoy 2012; Staquet 1978a; Staquet 1978b). Authors of one study reported on conflicts of interest (Hardy 2023).
Characteristics of participants
Age
The mean age of the participants was between 55 and 60 years. Two studies reported the range of age which was 21 to 75 years (Staquet 1978a; Staquet 1978b).
Gender
The percentage of men was between 17% and 93%. Two studies did not report gender (Staquet 1978a; Staquet 1978b).
Types of cancer and of cancer pain
The studies included mainly participants with carcinoma. One study included participants with squamous cell carcinoma of the head and neck (Côté 2016), and one study included participants with non‐small cell lung cancer (Turcott 2018). Four studies reported the percentage of participants with different types of cancer pain (e.g. nociceptive, neuropathic, visceral) (Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018). One study included participants with chemotherapy‐induced neuropathic pain (Lynch 2014).
Inclusion criteria
One study did not report on a required pain intensity for inclusion (Jochimsen 1978). The inclusion criteria of three studies were not based on pain intensity. Of these, one study did not report baseline pain values (Côté 2016). Two studies indicated a moderate pain intensity with a large SD and thus included some participants with a lower pain intensity (Hardy 2023; Turcott 2018). The remaining studies required at least moderate pain intensity for inclusion, of which four studies required moderate pain intensity despite opioid therapy (Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018).
Exclusion criteria
Eight studies excluded people with major internal diseases (e.g. of the heart, liver) (Côté 2016; Fallon 2017a; Fallon 2017b; Jochimsen 1978; Johnson 2010; Lichtman 2018; Lynch 2014; Portenoy 2012). Five studies excluded people with major psychiatric disorders (e.g. psychosis, substance‐use disorder) (Côté 2016; Hardy 2023; Jochimsen 1978; Johnson 2010; Portenoy 2012). Five studies excluded people with cannabis use (Fallon 2017a; Fallon 2017b; Lichtman 2018; Portenoy 2012; Turcott 2018). Five studies excluded people with "large dosages of narcotics" without defining the type of narcotic and the threshold of a large dosage (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b).
Previous experience of participants with herbal cannabis
Two studies reported on previous cannabis experience (12% of participants in both studies) without making a distinction between recreational and medical use (Johnson 2010; Portenoy 2012).
Characteristics of the treatment delivered
Types and doses of cannabis‐based medicines and comparators
Five studies used flexible oromucosal nabiximols with 'medium' dosages (THC/CBD up to 27/24 mg/day) (Fallon 2017a; Fallon 2017b; Lichtman 2018; Lynch 2014; Portenoy 2012). One study included a treatment arm with 'low‐dose' THC/CBD (up to 10.8/10 mg/day). One study included an arm with 'high‐dose' THC/CBD (up to 42.2/40 mg/day) (Portenoy 2012). One study arm used a 'medium' dosage of THC (up to 27 mg/day) (Johnson 2010). The experimental studies tested different fixed dosages of synthetic THC analogue orally (4 mg/day, 5 mg/day, 10 mg/day and 20 mg/day) (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b). The fixed dosages of nabilone orally were within the recommended range with 1 mg/day (Côté 2016), and 2 mg/day (Turcott 2018). The median daily dose in the study with oral CBD oil was 400 mg/day. All studies compared CbMs to placebo. The experimental studies also compared CbMs to codeine 50 mg/day, 60 mg/day and 120 mg/day (Noyes 1975a; Noyes 1975b; Staquet 1978a).
Rescue medication
Three studies used opioids as rescue medication. The study authors did not report the dosages of rescue medication used in the study groups (Fallon 2017a; Fallon 2017b; Lichtman 2018). The single dosage studies did not use rescue medication (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b). The remaining studies did not report on the type of rescue medication (Côté 2016; Hardy 2023; Johnson 2010; Portenoy 2012; Turcott 2018).
Excluded studies
We excluded one study after full‐text review because it did not include a study arm as required by our inclusion criteria (placebo or other active medication) (Zylla 2021).
Ongoing studies
We identified five ongoing studies with unpublished results; two studies used medical cannabis, either by oral liquid (ACTRN12619001534178) or by inhalation (NCT04042545) application, and three studies used THC/CBD, either by orobuccal (ACTRN12621001302842) or oral liquid application (EudraCT 001382‐32; Hardy 2020).
Risk of bias in included studies
We judged risk of bias across most domains as unclear (Figure 2; Figure 3; see Characteristics of included studies table for detailed information regarding risk of bias assessments of each study). We rated the overall risk of bias according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). Five studies were at unclear overall risk of bias (Côté 2016; Fallon 2017a; Fallon 2017b; Lichtman 2018; Lynch 2014), and the overall risk of bias was high in the remaining studies (Hardy 2023; Jochimsen 1978; Johnson 2010; Noyes 1975a; Noyes 1975b; Portenoy 2012; Staquet 1978a; Staquet 1978b; Turcott 2018). No trial was at low risk of bias for all categories examined.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation (selection bias)
We judged one study at low risk of random sequence generation (Portenoy 2012). There were concerns for the remaining studies, which did not report the details of random sequence generation (unclear risk).
Allocation concealment (selection bias)
No studies described allocation concealment adequately. Therefore, we judged all studies at unclear risk of bias.
Blinding
We judged blinding of participants and personnel as low risk of bias in six studies (Côté 2016; Fallon 2017a; Fallon 2017b; Jochimsen 1978; Staquet 1978a; Staquet 1978b). There were some concerns for the studies for seven studies, which reported no details of the blinding of participants and personnel; we judged these at unclear risk of bias (Hardy 2023; Lichtman 2018; Lynch 2014; Noyes 1975a; Noyes 1975b; Portenoy 2012; Turcott 2018). One study was at high risk of bias, as it did not report if nabiximols and placebo were identical in taste (Johnson 2010).
No study reported on details of the blinding of the outcome assessor and therefore we judged all studies at unclear risk of detection bias.
Incomplete outcome data
Five studies had high risk of attrition bias, which used completer analysis (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b). There were some concerns for the remaining studies, which used ITT analysis by LOCF. We judged these studies at unclear risk of bias.
Selective reporting
We judged reporting bias as low in the nabiximols studies because there was a study protocol (Fallon 2017a; Fallon 2017b; Hardy 2023; Johnson 2010; Lichtman 2018; Portenoy 2012). The risk of bias was unclear for the remaining studies because they did not publish a study protocol.
Other potential sources of bias
Four studies were at high risk of other bias due to significant differences in demographic or clinical variables (or both) at baseline between the study groups (Hardy 2023; Johnson 2010; Portenoy 2012; Turcott 2018). We found group similarity at baseline in the remaining studies, which were at low risk of other bias.
Effects of interventions
See: Table 1
In total, we analysed 14 studies with 20 treatment arms involving 1823 participants. See Table 1 for the main comparison.
Cannabis‐based medicines as add‐on for opioid refractory cancer pain
Studies with a parallel design
We found four studies with seven study arms including 1334 participants that used a parallel design for nabiximols in participants with opioid‐refractory cancer pain. We report the meta‐analyses below.
Primary outcomes
Proportion of participants reporting no worse than mild pain by 14 days after start of treatment
No study assessed this outcome.
Patient Global Impression of Change of much improved or very much improved
We analysed three studies with five treatment arms. A total of 179/561 (31.9%) participants in the nabiximols and 100/434 (23.0%) participants in the placebo group reported being much or very much improved (RD 0.06, 95% CI 0.01 to 0.12; P = 0.03; I² = 0%; NNTB 16, 95% CI 8 to 100; Analysis 1.1). According to the predefined categories, there was no clinically relevant benefit by nabiximols. We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (one study was at high risk of bias).
1.1. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 1: Patient Global Impression of Change (PGIC) of much improved or very much improved
Withdrawals due to adverse events
We analysed four studies with seven treatment arms and 1332 participants. We found 148/785 (18.6%) participants in the nabiximols and 85/547 (15.5%) participants in the placebo group dropped out due to adverse events (RD 0.04, 95% CI 0 to 0.08; P = 0.04; I² = 0%; NNTH 25, 95% CI 12 to indefinite; Analysis 1.2). According to the predefined categories, there was no clinically relevant harm. We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (two studies were at high risk of bias).
1.2. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 2: Withdrawal due to adverse events
Secondary outcomes
Combined responder
No study assessed this outcome.
Number of participants who reported pain relief of 30% or greater
We analysed four studies with seven treatment arms and 1332 participants. We found 217/785 (26.8%) participants receiving nabiximols and 145/547 (26.5%) participants in the placebo group reported pain relief of 30% or greater (RD 0.02, 95% CI −0.03 to 0.07; P = 0.51; I² = 0%; Analysis 1.3). We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (two studies at high risk of bias).
1.3. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 3: Pain relief ≥ 30%
Number of participants who reported pain relief of 50% or greater
We analysed four studies with seven treatment arms and 1333 participants. We found 104/786 (13.2%) participants receiving nabiximols and 50/547 (9.1%) participants in the placebo group reported pain relief of 50% or greater (RD 0.01, 95% CI −0.02 to 0.05; P = 0.38; I² = 1%; Analysis 1.4). We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (two studies at high risk of bias).
1.4. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 4: Pain relief ≥ 50%
Mean pain intensity
We analysed four studies with seven treatment arms and 1315 participants. There was no evidence of a difference in mean pain intensity on a 0 to 10 scale (MD −0.19, 95% CI −0.40 to 0.02; P = 0.08, I² = 21%; Analysis 1.5). We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (two studies at high risk of bias).
1.5. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 5: Mean pain intensity
Sleep problems
We analysed four studies with seven treatment arms and 1314 participants. We found no benefit of nabiximols for improving sleep (SMD −0.06, 95% CI −0.19 to 0.06; P = 0.31, I² = 11%; Analysis 1.6). We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (two studies at high risk of bias).
1.6. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 6: Sleep problems
Depression
In one study including 360 participants, we found no difference between placebo and the low‐dose THC/CBD group (P = 0.48), the medium‐dose THC/CBD group (P = 0.08) and the high‐dose THC/CBD group (P = 0.15) on the Montgomery Åsberg Depression Rating Scale (Portenoy 2012). We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (two studies at high risk of bias), and imprecision of results (only one study analysed).
Anxiety
No studies assessed anxiety.
Daily maintenance opioid dosage (mg morphine equivalent)
We analysed three studies with four treatment arms and 970 participants. We found no difference in opioid dose between groups (SMD 0.08, 95% CI −0.10 to 0.27; P = 0.38, I² = 43%; Analysis 1.7). We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (two studies at high risk of bias), and imprecision of results (CIs included zero). Portenoy 2012 reported that there were no differences between the three dosages arms of THC and CBD (P values not reported).
1.7. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 7: Daily maintenance opioid dosage
Daily breakthrough opioid dosage (mg morphine equivalent)
We analysed three studies with four treatment arms and 957 participants. We found no difference between groups (SMD −0.08, 95% CI −0.23 to 0.07; P = 0.29, I² = 19%; Analysis 1.8). Portenoy 2012 reported that there was no difference between the three dosages arms of THC and CBD (P values not reported). We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (two studies at high risk of bias).
1.8. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 8: Daily breakthrough opioid dosage
Number of participants dropping out due to lack of efficacy
No study assessed this outcome.
All central nervous system adverse events
We analysed four studies with seven treatment arms and 1331 participants. We found 202/785 (25.7%) participants receiving nabiximols and 57/546 (10.4%) participants in the placebo group reported nervous system disorders adverse events (RD 0.11, 95% CI 0.05 to 0.17; P < 0.001; I² = 43%; NNTH 9, 95% CI 6 to 25; Analysis 1.9). According to the predefined categories there was a clinically relevant harm by nabiximols. We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (two studies at high risk of bias).
1.9. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 9: All central nervous system adverse events
All psychiatric adverse events
We analysed four studies with seven treatment arms and 1331 participants. We found 75/785 (9.6%) participants receiving nabiximols and 17/546 (3.1%) participants in the placebo group reported psychiatric disorders adverse events (RD 0.01, 95% CI −0.01 to 0.04; P = 0.24; I² = 35%; Analysis 1.10). We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (two studies at high risk of bias).
1.10. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 10: All psychiatric adverse events
Participants experiencing any serious adverse event
We analysed four studies with seven treatment arms and 1330 participants. We found 187/784 (23.9%) participants receiving nabiximols and 116/546 (21.2%) participants in the placebo group reported serious adverse events (RD 0.02, 95% CI −0.03 to 0.07; P = 0.43; I² = 9%; Analysis 1.11). We judged the certainty of evidence as moderate, downgraded one level due to limitations of study design (two studies at high risk of bias).
1.11. Analysis.

Comparison 1: Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain, Outcome 11: Any serious adverse event
Studies with a withdrawal design
We found one study with 206 participants that used a withdrawal design (Fallon 2017b). We could not meta‐analyse the results, so we describe the double‐blind period only below.
Primary outcomes
Proportion of participants reporting no worse than mild pain 14 days after start of treatment
The study did not assess this outcome.
Patient Global Impression of Change of much improved or very much improved
There was no evidence of a difference on the mean PGIC (0.33, 95% CI –0.35 to 0.41; P = 0.41). We judged the certainty of evidence as moderate, downgraded one level due to imprecision of results (only one study available).
Withdrawals due to adverse event
We found 21/103 of participants receiving nabiximols and 13/103 in placebo group withdrew due to adverse events (P = 0.05). We judged the certainty of evidence as low, downgraded one level to low due to imprecision of results (only one study available).
Secondary outcomes
Combined responder
The study did not assess this outcome.
Number of participants who reported pain relief of 30% or greater
The study did not assess this outcome.
Number of participants who reported pain relief of 50% or greater
The study did not assess this outcome.
Mean pain intensity
There was no evidence of a difference in mean pain intensity (MD −0.02, 95% CI –0.42 to 0.38; P = 0.92). We judged the certainty of evidence as moderate, downgraded one level due to and imprecision of results (only one study available).
Sleep problems
There was no evidence of a difference in sleep problems (MD 0.06, 95 CI –0.28 to 0.39; P = 0.73). We judged the certainty of evidence as moderate, downgraded one level due to imprecision of results (only one study available).
Depression
The study did not assess this outcome.
Anxiety
The study did not assess this outcome.
Daily maintenance opioid dosage (mg morphine equivalent)
There was no evidence of a difference in daily maintenance opioid dosage (MD –8.93, 95% CI –19.69 to 1.84; P = 0.10). We judged the certainty of evidence as low, downgraded one level due to imprecision of results (only one study available).
Daily breakthrough opioid dosage (mg morphine equivalent)
There was no evidence of a difference in daily breakthrough opioid dosage (MD 1.81, 95% CI –10.34 to 13.69; P = 0.77). We judged the certainty of evidence as moderate, downgraded one level due to imprecision of results (only one study available).
Number of participants dropping out due to lack of efficacy
One participant in each group withdraw due to lack of efficacy. We judged the certainty of evidence as moderate, downgraded one level due to imprecision of results (low number of events).
All central nervous system adverse events
Six participants in the nabiximols and one in the placebo group reported dizziness or somnolence. We judged the certainty of evidence as moderate, downgraded one level due to imprecision of results (only one study available).
All psychiatric adverse events
There were no treatment‐emergent suicidal ideations or behaviour in either group. We judged the certainty of evidence as moderate, downgraded one level due to imprecision of results (low number of events).
Participants experiencing any serious adverse event
There were treatment‐related serious adverse events in 33/103 of nabiximols‐treated and 16/103 of placebo‐treated participants (P = 0.13). We judged the certainty of evidence as moderate, downgraded one level due to imprecision of results (low number of events).
Nabiximols for cancer therapy‐induced neuropathic pain
We found one study with 16 participants that delivered nabiximols for cancer therapy‐induced neuropathic pain (Lynch 2014). This could not be meta‐analysed, so we described the results below.
Primary outcomes
Proportion of participants reporting no worse than mild pain by 14 days after start of treatment
The study did not assess this outcome.
Patient Global Impression of Change of much improved or very much improved
The study did not assess this outcome.
Withdrawals due to adverse event
The study did not report why two participants dropped out.
Secondary outcomes
Combined responder
The study did not assess this outcome.
Number of participants who reported pain relief of 30% or greater
We found 5/18 participants with nabiximols and 3/18 participants with placebo reported pain relief of 30% or greater (P = 0.16). We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (study with high risk of bias) and imprecision of results (only one study with low number of participants available).
Number of participants who reported pain relief of 50% or greater
We found 2/18 participants with nabiximols and 3/18 participants with placebo reported pain relief of 50% or greater (P = 0.47). We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (study with high risk of bias) and imprecision of results (only one study with low number of participants available).
Main pain intensity
The mean pretreatment score was 6.75 and was 6.00 in the nabiximols and 6.38 in the placebo group. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (study with high risk of bias) and imprecision of results (only one study with low number of participants available).
Sleep problems
The study did not assess this outcome.
Depression
The study did not assess this outcome.
Anxiety
The study did not assess this outcome.
Daily maintenance opioid dosage (mg morphine equivalent)
The study did not assess this outcome.
Daily breakthrough opioid dosage (mg morphine equivalent)
The study did not assess this outcome.
Number of participants dropping out due to lack of efficacy
The study did not assess this outcome.
All central nervous system adverse events
Nine participants with nabiximols (six dizziness, one confusion, two "foggy brain") and none with placebo reported nervous system adverse events (P = 0.02). We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (study with high risk of bias) and imprecision of results (only one study with low number of participants available).
All psychiatric adverse events
Three participants with nabiximols and none with placebo reported on psychiatric disorders adverse events (feeling "stoned", anxiety and panic attack, one each) (P = 0.23). We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (study with high risk of bias) and imprecision of results (only one study with low number of participants available).
Participants experiencing any serious adverse event
The study reported "There were no serious medication‐related events."
Studies with synthetic tetrahydrocannabinol analogue (nabilone) compared to placebo to improve health‐related quality of life of people undergoing radiation or radiochemotherapy
We found two studies with 89 participants that delivered nabilone to improve health‐related quality of life of people undergoing radiation or radiochemotherapy (Côté 2016; Turcott 2018). The presentation of the outcomes did not allow quantitative synthesis, so we described the results below.
Primary outcomes
Proportion of participants reporting no worse than mild pain by 14 days after start of treatment
The studies did not assess this outcome.
Patient Global Impression of Change of much improved or very much improved
The studies did not assess this outcome.
Withdrawals due to adverse events
Neither study reported withdrawal due to adverse effects in detail. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (study with high risk of bias) and imprecision of results (low number of participants).
Secondary outcomes
Combined responder
The studies did not assess this outcome.
Number of participants who reported pain relief of 30% or greater
Neither study reported this outcome. We could not use the imputation method because Côté 2016 did not report baseline pain intensity. By imputation, Turcott 2018 reported 7/14 participants in the nabilone and 7/19 participants in the placebo group experienced pain relief of 30% or greater (P = 0.45). We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (one study with high risk of bias) and imprecision of results (low number of participants).
Number of participants who reported pain relief of 50% or greater
Neither study reported this outcome. We could not use the imputation method because Côté 2016 did not report baseline pain intensity. By imputation, Turcott 2018 reported 5/14 participants in the nabilone and 5/19 participants in the placebo group experienced pain relief of 50% or greater (P = 0.56). We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (one study with high risk of bias) and imprecision of results (low number of participants).
Mean pain intensity
Côté 2016 reported no difference between nabilone and placebo in mean pain intensity (P = 0.61). Turcott 2018 reported the change in mean pain intensity score in the nabilone group from baseline to the end of treatment was 13 in the nabilone group and 6.6 in the control group on a 0 to 100 scale. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (one study with high risk of bias) and imprecision of results (low number of participants).
Sleep problems
Côté 2016 reported no difference between nabilone and placebo in sleep problems (P = 0.44). Turcott 2018 reported change in mean insomnia score in the nabilone group from baseline to the end of treatment was −40.7 in the nabilone group and −9.9 in the control group on a 0 to 100 scale. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (one study with high risk of bias) and imprecision of results (low number of participants).
Depression
Côté 2016 reported no difference between nabilone and placebo on mood (P = 0.32). Turcott 2018 did not assess this outcome. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (one study with high risk of bias) and imprecision of results (low number of participants).
Anxiety
The studies did not assess this outcome.
Daily maintenance opioid dosage (mg morphine equivalent)
The studies did not assess this outcome.
Daily breakthrough opioid dosage (mg morphine equivalent)
The studies did not assess this outcome.
Number of participants dropping out due to lack of efficacy
Neither study reported withdrawals due to lack of efficacy in detail. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (one study with high risk of bias) and imprecision of results (low number of participants).
All central nervous system adverse events
Côté 2016 reported that there was no difference between nabilone and placebo in the prevalence of drowsiness (P = 0.32). Turcott 2018 did not assess this outcome. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (one study with high risk of bias) and imprecision of results (low number of participants).
All psychiatric adverse events
Côté 2016 reported that there was no difference between nabilone and placebo in the prevalence of anxiety (P = 0.91). Turcott 2018 did not assess this outcome. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (one study with high risk of bias) and imprecision of results (low number of participants).
Participants experiencing any serious adverse event
Neither study explicitly mentioned serious adverse events.
Experimental (single dosage) studies to reduce cancer pain: synthetic THC analogue versus placebo
We found five studies with 126 participants that delivered a single dosage of a synthetic THC analogue (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b).
Primary outcomes
Proportion of participants reporting no worse than mild pain by 14 days after start of treatment
The studies did not assess this outcome.
Patient Global Impression of Change of much improved or very much improved
The studies did not assess this outcome.
Withdrawals due to adverse events
The studies did not assess this outcome.
Secondary outcomes
Combined responder
The studies did not assess this outcome.
Number of participants who reported pain relief of 30% or greater
The studies did not assess this outcome.
Number of participants who reported pain relief of 50% or greater
Jochimsen 1978 reported that 23% of participants with 4 mg of a synthetic THC analogue, 40% of participants with 2 mg of a synthetic THC analogue and 43% of participants with placebo reported pain relief of 50% or greater. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (all studies with high risk of bias) and imprecision of results (low number of participants).
Mean pain intensity
We analysed three studies with four treatment arms and 301 participants. There was a difference in mean pain intensity in favour of THC analogue (SMD of pain reduction −0.98, 95% CI −1.36 to −0.60; P < 0.001, I² = 54%; Analysis 2.1). The effect size was large according to Cohen's categories. The criterion of a clinically relevant effect was met. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (all studies with high risk of bias) and inconsistency (high heterogeneity). Jochimsen 1978 stated that "reductions of pain intensity occurred in a larger proportion of patients than pain relief, but the reductions were small and clearly without clinical significance." A total of 19/35 participants with 2 mg synthetic THC analogue, 20/35 participants with 4 mg synthetic THC analogue and 25/35 participants with placebo reported a pain reduction.
2.1. Analysis.

Comparison 2: Experimental studies with synthetic tetrahydrocannabinol (THC) analogue versus placebo for individuals with cancer pain, Outcome 1: Mean pain intensity
Sleep problems
The studies did not assess this outcome.
Depression
The studies did not assess this outcome.
Anxiety
The studies did not assess this outcome.
Daily maintenance opioid dosage (mg morphine equivalent)
The studies did not assess this outcome.
Daily breakthrough opioid dosage (mg morphine equivalent)
The studies did not assess this outcome.
Number of participants dropping out due to lack of efficacy
The studies did not assess this outcome.
All central nervous system adverse events
Jochimsen 1978 did not report nervous system adverse events in the placebo group. Noyes 1975a reported 94% of participants with THC 20 mg, 71% of participants with THC 10 mg and 29% of participants with placebo reported sedation. Noyes 1975b reported 14 nervous system adverse events (drowsiness, dizziness) with 5 mg, 19 with 10 mg, 26 with 15 mg, and 36 with 20 mg synthetic THC analogue, and 21 with placebo. Staquet 1978b and Staquet 1978a pooled the data of both studies and reported that 40% of participants reported drowsiness with synthetic THC analogue and 21% of participants reported drowsiness with placebo. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (all studies with high risk of bias) and imprecision of results (low number of participants).
All psychiatric adverse events
Jochimsen 1978 reported that "psychiatric interview, failed to reveal any consistent changes which could be ascribed to any drug or to the test period as a whole." Noyes 1975a reported 62 psychiatric adverse events (mental clouding, disorientation thought, slurred speech) in the 20 mg synthetic THC analogue group, 32 in the 10 mg synthetic THC analogue group and 15 in the placebo group. Noyes 1975b reported 17 psychiatric adverse events (slurred speech, blurred vision, mental clouding, dreaminess, disconnected thought, euphoria, visual hallucinations) in the 5 mg, 19 in the 10 mg, 33 in the 15 mg and 37 in the 20 mg synthetic THC analogue groups and five in the placebo group. Staquet 1978b and Staquet 1978a pooled the data of both studies and reported there was no euphoria reported by participants with synthetic THC analogue and placebo. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (all studies with high risk of bias) and imprecision of results (low number of participants).
Participants experiencing any serious adverse event
The studies did not assess this outcome.
Experimental (single dosage) studies to reduce cancer pain: synthetic THC analogue versus codeine
We found four studies with 116 participants which compared a single dose of a synthetic THC analogue with a single dose of codeine.
Primary outcomes
Proportion of participants reporting no worse than mild pain by 14 days after start of treatment
The studies did not assess this outcome.
Patient Global Impression of Change of much improved or very much improved
The studies did not assess this outcome.
Withdrawals due to adverse events
The studies did not assess this outcome.
Secondary outcomes
Combined responder
The studies did not assess this outcome.
Number of participants who reported pain relief of 30% or greater
The studies did not assess this outcome.
Number of participants who reported pain relief of 50% or greater
Jochimsen 1978 reported that 23% of participants with 4 mg of a synthetic THC analogue, 40% of participants with 2 mg of a synthetic THC analogue, 57% of participants with codeine 120 mg and 49% of participants with codeine 60 mg reported pain relief of 50% or greater. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (all studies with high risk of bias) and imprecision of results (low number of participants).
Mean pain intensity
We analysed two studies with three treatment arms and 194 participants. There was no evidence of a difference in pain intensity (SMD 0.03, 95% CI −0.25 to 0.32; P = 0.82, I² = 0%; Analysis 3.1). We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (both studies with high risk of bias) and imprecision of results (CIs included zero). In Jochimsen 1978, we found 19/35 participants receiving synthetic THC analogue 2 mg, 20/35 participants receiving synthetic THC analogue 4 mg, 25/35 participants receiving codeine 60 mg and 31/35 participants receiving codeine 120 mg reported a pain reduction.
3.1. Analysis.

Comparison 3: Experimental studies with synthetic tetrahydrocannabinol (THC) analogue versus codeine for individuals with cancer pain, Outcome 1: Mean pain intensity
Sleep problems
The studies did not assess this outcome.
Depression
The studies did not assess this outcome.
Anxiety
The studies did not assess this outcome.
Daily maintenance opioid dosage (mg morphine equivalent)
The studies did not assess this outcome.
Daily breakthrough opioid dosage (mg morphine equivalent)
The studies did not assess this outcome.
Number of participants dropping out due to lack of efficacy
The studies did not assess this outcome.
All central nervous system adverse events
Jochimsen 1978 reported that the sedation induced by synthetic THC analogue in doses of 2 mg and 4 mg was of the same order as that induced by the two doses of codeine, "although this was not marked for either drug." Noyes 1975a reported that 94% of participants with THC 20 mg, 71% of participants with THC 10 mg, 47% of participants with codeine 60 mg and 50% of participants with codeine 120 mg reported sedation. Staquet 1978b and Staquet 1978a pooled the data of both studies and reported that 40% of participants with synthetic THC analogue and 44% of participants treated with codeine reported drowsiness. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (all studies with high risk of bias) and imprecision of results (low number of participants).
All psychiatric adverse events
Jochimsen 1978 reported that "psychiatric interview, failed to reveal any consistent changes which could be ascribed to any drug or to the test period as a whole". Noyes 1975a reported 62 psychiatric adverse events (mental clouding, disorientation thought, slurred speech) in the 20 mg, 32 in the 10 mg, 11 in the codeine 60 mg and seven in the codeine 120 mg group. Staquet 1978b and Staquet 1978a pooled the data of both studies; participants reported no euphoria with synthetic THC analogue. We judged the certainty of evidence as low, downgraded two levels due to limitations of study design (all studies with high risk of bias) and imprecision of results (low number of participants).
Participants experiencing any serious adverse event
The studies did not assess this outcome.
Cannabidiol added to specialist palliative care to reduce pain in advanced cancer
We found one study with 142 participants (Hardy 2023). We report the outcomes at day 28.
Primary outcomes
Proportion of participants reporting no worse than mild pain by 14 days after start of treatment
The study did not assess this outcome.
Patient Global Impression of Change of much improved or very much improved
About 70% of participants in the CBD group and 64% in the placebo group reported feeling better or much better.
Withdrawals due to adverse events
A total of 10/70 participants withdrew with CBD and 8/72 participants withdrew with placebo.
Secondary outcomes
Combined responder
The study did not assess this outcome.
Number of participants who reported pain relief of 30% or greater
A total of 26/70 in the CBD group and 29/72 in the placebo group reported pain relief of 30% or greater (calculated by imputation method).
Number of participants who reported pain relief of 50% or greater
A total of 19/70 in the CBD group and 20/72 in the placebo group reported pain relief of 50% or greater (calculated by imputation method).
Mean pain intensity
In the CBD group, baseline pain score (VAS 0 to 100) was 40.34 (SD 26.26) and at 28 days was 37.80 (SD 28.14). In the placebo group, baseline pain score (VAS 0 to 100) was 50.94 (SD 30.20) and at 28 days was 43.56 (SD 30.54).
Sleep problems
The study did not assess this outcome.
Depression
The mean change in depression in the CBD group was −0.50 (SD 0.46) and in the placebo group was −0.63 (SD 0.405).
Anxiety
The mean change in the CBD group was −1.10 (SD 0.44) and in the placebo group was −0.79 (SD 0.43).
Daily opioid dosage (mg morphine equivalent)
The study did not make a distinction between maintenance and breakthrough opioid doses. At day 28, 3/42 (7.1%) participants in the CBD group and 6/4 (13.6%) participants in the placebo group had a morphine dose reduction from baseline; 20/42 (47.6%) participants in the CBD group and 21/42 (50%) participants in the placebo group had no change; and 18/42 (42.9%) participants in the CBD group and 16/42 (38.1%) participants in the placebo group had an increase in total opioid dose.
Number of participants dropping out due to lack of efficacy
The study did not assess this outcome.
All central nervous system adverse events
A total of 30/66 (45%) participants in the CBD group and 21/68 (31%) participants in the placebo group reported new or worse days with somnolence; 16/66 (24%) participants in the CBD group and 14/68 (11%) participants in the placebo group reported new or worse days with dizziness; and 9/70 (12.8%) participants in the CBD group and 7/72 (9.7%) participants in the placebo group reported new or worse days with fatigue.
All psychiatric adverse events
A total of 3/70 (4.3%) participants in the CBD group and 1/72 (1.4%) participants in the placebo group reported new or worse days with anxiety.
Participants experiencing any serious adverse event
There were eight serious adverse events resulting in hospitalisations (five in the CBD group and three in the placebo group).
Subgroup analysis
We conducted a subgroup analysis comparing the effects of lower dosages of synthetic THC analogue (5 mg or 10 mg) versus placebo (four studies with 193 participants) compared to higher dosages of synthetic THC analogue (15 mg or 20 mg) versus placebo (two studies with 108 participants) on mean pain intensity. The P value for the subgroup comparison was 0.16 (see Analysis 2.1).
We conducted a subgroup analysis comparing the effects of lower dosages of synthetic THC analogue (4 mg or 10 mg) versus codeine 50 mg or 60 mg (two studies with 126 participants) compared to higher dosages of synthetic THC analogue (20 mg) versus codeine 120 mg. The P value of subgroup comparison was 0.51 (see Analysis 3.1).
We did not perform the other predefined subgroup analyses because most subgroups included only one study or treatment arm.
Sensitivity analysis
By removing one study with one treatment arm with imputed rates for pain relief of 30% or greater, the RD of the remaining study with two study arms was 0.03 (95% CI −0.03 to 0.09; P = 0.31).
Publication bias
Analysis 1.1 for PGIC much or very much improved had an NNTB of 16. This precluded any sensitivity analysis for publication bias as the NNTB of 16 was already higher than the preset utility boundary of an NNTB of 10. Increasing the preset boundary to an NNTB of 20 would mean that results from 249 participants in zero treatment effect trials would be required to that higher level. That is about the size of one of the larger trials included in the analysis.
Discussion
Summary of main results
We identified five RCTs delivering oromucosal nabiximols or THC to 1539 participants with moderate and severe pain despite opioid therapy (Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018; Portenoy 2012). The double‐blind periods of the RCTs ranged between two and five weeks. We found four studies that used a parallel design and included 1333 participants that could be included in a meta‐analysis (Fallon 2017a; Johnson 2010; Lichtman 2018; Portenoy 2012). The certainty of evidence was moderate for all comparisons except one, which was low. Nabiximols and THC did not differ from placebo in reducing pain, sleep problems, and opioid maintenance and breakthrough dosages. There was no clinically relevant benefit of nabiximols for the number of participants who reported that they were much or very much improved (NNTB 16, 95% CI 6 to 100). There was no difference between nabiximols and THC versus placebo with regards to the dropout rates due to adverse events and to the frequency of psychiatric disorders as adverse events. There was a clinically relevant harm by nabiximols and THC in the frequency of nervous system adverse events compared to placebo (NNTH 9, 95% CI 6 to 20).
We found low‐certainty evidence for two RCTs of eight weeks' duration that delivered nabilone (compared to placebo) in 89 participants (Côté 2016; Turcott 2018). We found that nabilone did not reduce pain associated with chemotherapy or radiochemotherapy in people with head and neck cancer and non‐small cell lung cancer.
We found low‐certainty evidence across five single‐dose RCTs with 126 participants that synthetic THC analogue was superior to placebo and not superior to codeine in reducing moderate‐to‐severe cancer pain after cessation of previous analgesic treatment for three to 4.5 hours (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b).
Overall completeness and applicability of evidence
The evidence for all types of CbMs and medical cannabis is not complete because we did not analyse studies with medical cannabis only. The ongoing studies might change the findings and conclusions of our review (ACTRN12619001534178; ACTRN12621001302842; EudraCT 001382‐32; Hardy 2020; NCT04042545). The usefulness of the available evidence is limited because the quality of studies was overall poor by current standards. The results of the studies analysed can be mainly applied to routine clinical care because the participants included in the clinical studies were largely representative for people with cancer in routine clinical care. However, the studies with nabiximols excluded studies with advanced hepatic and renal failure (Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018; Portenoy 2012).
Quality of the evidence
We found the evidence for the outcomes of the studies with synthetic THC analogues to be low quality because of limitations of study design and imprecision (Côté 2016; Jochimsen 1978; Johnson 2010; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b; Turcott 2018), and the outcomes of the studies with nabiximols to be moderate quality because of limitations of study design (Fallon 2017a; Johnson 2010; Lichtman 2018; Portenoy 2012). Our confidence in the effect estimates is limited; the true effect may be substantially different from the estimate of the effect.
In addition, the studies with synthetic THC analogues might have overestimated the effect size due to their small sample size (Côté 2016; Jochimsen 1978; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b; Turcott 2018).
Six studies used a cross‐over design (Jochimsen 1978; Lynch 2014; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b), which has methodological issues that could lead to bias (Elbourne 2002).
The experimental studies with synthetic THC analogue were single‐dose studies (Jochimsen 1978; Noyes 1975a; Noyes 1975b; Portenoy 2012; Staquet 1978a; Staquet 1978b). We do not know if the effects on pain relief can be maintained. Another concern is the short study duration of the nabiximols and dronabinol studies (Côté 2016; Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018; Lynch 2014; Portenoy 2012; Turcott 2018) and CBD study (Hardy 2023), with no intermediate (12 to 26 weeks) or long‐term (greater than 26 weeks) randomised study. However, long‐term studies are difficult to conduct in people with advanced cancer because of the limited life expectancy.
There are different types of cancer pain (bone, neuropathic, visceral, somatic). Although the nabiximols studies reported the percentages of these categories of cancer pain in the baseline characteristics (Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018; Portenoy 2012), no study has conducted subgroup analyses according to these cancer pain characteristics.
Potential biases in the review process
We conducted a broad search for studies and believe that it is unlikely that significant amounts of relevant data have been overlooked. We cannot exclude the possibility that early studies including synthetic THC analogues have not been published before registration of a study protocol was required for approval by ethical committees or drug agencies.
We did not analyse other relevant symptoms associated with cancer pain such as fatigue and did not analyse other relevant adverse effects of CbMs such as gastrointestinal disorders.
We had to estimate some missing SDs from P values. We calculated most 50% responder rates of the nabiximols studies using imputation methods.
All studies used statistical methods (LOCF, completer analysis) that bias results towards exaggerating the efficacy of the medication.
The influence of allowed co‐interventions (e.g. rescue medication) on positive effects and adverse events was unclear because type and dosage of co‐interventions were neither clearly reported nor controlled.
Adverse events were not systematically assessed or reported (or both) by most studies. Therefore, we may have underestimated the prevalence of adverse events.
It is possible that we have overestimated risk of bias for studies that failed to report some details of methodology (e.g. randomisation and treatment allocation).
The negative results of the nabiximols trials could be due to a relatively high number of patient withdrawals and high mortality rate (Boland 2020). The intensity of cancer pain can increase in the end stage of the disease and might have counterbalanced the positive effects of nabiximols. In addition, there is a high degree of variability in pharmacokinetic parameters between participants as well as within participants following single and repeat dosing of oromucosal nabiximols. When nabiximols is administered oromucosally, plasma levels of THC and other CBs are lower compared with the levels achieved following inhalation of CBs at a similar dose (GW Pharmaceuticals 2020). Insufficient plasma concentrations of nabiximols might be a potential cause of therapeutic failure in some participants of the studies analysed.
However, analyses for CbMs as add‐on for opioid refractory cancer pain were dominated (70% or greater weighting) by two large trials that each had about 200 participants in the treatment and placebo groups. The results were negative, and while there was some uncertainty, we have considered that this constitutes no lower than moderate confidence. This is especially the case when the effects of poor quality are almost universally to inflate treatment effect. So the lack of effect in large, reasonably well‐designed studies leads to a conclusion that benefits of CBs tested to date for opioid refractory cancer pain is unlikely.
Finally, this systematic review included 1823 participants. To capture rare and potentially severe adverse events a much larger data set is necessary. For example, to capture an adverse event with a frequency of 1 in 100,000 population, 300,000 participants' observations would be required (Andersohn 2008). We did not look at other data from observational studies for safety evaluations within the scope of our review.
Agreements and disagreements with other studies or reviews
There are numerous reviews available on the efficacy and safety of CbMs and medical cannabis for chronic pain in general. Here, we compare our results only to systematic reviews that included separate analyses for studies on cancer pain. The results and conclusions of our review are not in line with the ones of Aviram 2017 and Wang 2021. Aviram 2017 analysed three RCTs with 10 arms that were also included into our analyses and found an SMD for a fixed‐effect model of −0.62 Hedge's g (95% CI −0.80 to −0.44) for mean pain reduction (Johnson 2010; Noyes 1975a; Noyes 1975b; Staquet 1978a; Staquet 1978b). The divergent results can be explained as follows: Aviram 2017 pooled experimental studies with only one of the studies with negative results with nabiximols in opioid‐refractory cancer pain (Johnson 2010). Wang 2021 concluded that moderate to high certainty evidence showed that, compared with placebo, non‐inhaled medical cannabis or CBs results in a very small to small increase in the proportion of people living with chronic pain. The authors pooled studies with cancer and non‐cancer pain. They included only one study with cancer pain in their analysis of mean pain reduction, which was also included into our review (Portenoy 2012).
The findings on nabiximols for opioid‐refractory cancer pain are in line with two systematic reviews (Boland 2020; Häuser 2019), which included the same studies as we did (Fallon 2017a; Fallon 2017b; Johnson 2010; Lichtman 2018; Portenoy 2012). Nabiximols was not effective in relieving opioid‐refractory cancer and did not reduce maintenance and breakthrough opioid medication. In addition, the use of CbMs was associated with a higher frequency of nervous system and psychiatric disorders compared to placebo. However, serious adverse events did not differ from placebo. These findings were supported by a subgroup analyses of the systematic review of Fisher 2021 on participants with cancer pain. It found no superiority of nabiximols over placebo in the two studies that reported the number of participants with pain relief of 30% or greater (Johnson 2010; Portenoy 2012). In addition, Fisher 2021 did not find a benefit of nabiximols compared to placebo for mean pain intensity as reported by four studies (MD on a 0 to 10 scale 0.22, 95% CI −0.49 to 0.06) as we did.
Authors' conclusions
Implications for practice.
The potential importance of cannabis‐based medicines (CbMs) and medical cannabis in the management of the different types and stages of cancer pain cannot be defined by our review because of the lack of any good evidence of efficacy or harm. The studies analysed do not allow any statement to be made on the place of these medications in the World Health Organization (WHO) analgesic ladder for cancer pain (e.g. if they can be used first, second or third line or as an adjunct). We do not know if the efficacy of single‐dose synthetic tetrahydrocannabinol (THC) analogue in analgesic‐naive people can be maintained. In addition, synthetic THC analogue was not superior to codeine 50 mg to 120 mg which corresponds to 7 mg, 5 mg and 18 mg morphine equivalent (Oregon Health Authority 2022). There was moderate‐certainty evidence against the use of oromucosal nabiximols for cancer pain that is not sufficiently relieved by strong opioids and low‐certainty evidence against the use of nabilone to improve health‐related quality of life in people undergoing (radio‐)chemotherapy. In consideration of adverse effects, CbMs are not as well tolerated as is often claimed by some authors. Nervous system and psychiatric adverse events are prevalent and may limit the clinical usefulness of CbMs.
Of note, we did not find a randomised controlled trial that could confirm the positive effects of medical cannabis on multiple symptoms of people with cancer (pain, sleep problems, psychological distress) described by Israeli observational studies (Aviram 2020; Bar‐Lev Schneider 2018). We hope that the ongoing trials with THC and cannabidiol (CBD)‐rich medical cannabis will provide high‐certainty evidence of medical cannabis in the treatment of cancer pain, as currently the certainty is low (ACTRN12619001534178; ACTRN12621001302842; EudraCT 001382‐32; Hardy 2020; NCT04042545). Currently, the European Pain Federation recommends clinicians should consider the use of CbMs only on a case‐to‐case basis. Taking patient preferences into consideration, an individual therapeutic trial in opioid‐naive people with cancer can be considered. An individual therapeutic trial may also be considered in people with moderate‐to‐severe cancer pain despite optimised pharmacological therapy including co‐analgesics (Häuser 2018). Smoking of herbal cannabis is not recommended for people with cancer‐related pain as there is no easily defined dosage of cannabis ingredients and smoking presents dangers for physical health. In the event that a person insists on the use of cannabis flowers used for medical reasons, it is prudent for the healthcare professional to recommend inhalation using a vaporiser and oral intake as cannabis oil extract (Fitzcharles 2019).
Implications for research.
Studies should clearly define if the medication aims to relieve pain arising as a direct consequence of the cancer or of cancer therapy, or both.
Pain mechanisms underlying the cancer pain (e.g. nociceptive, neuropathic) should be reported to enable subgroup analyses of efficacy according to pain mechanisms.
Placebo‐controlled studies without absence of any established analgesic for cancer pain are unlikely to be ethically feasible. WHO analgesic ladder‐recommended medication as comparator would allow the assessment of comparative efficacy and safety.
Studies with different CbMs arms (e.g. THC‐rich, CBD‐rich, balanced THC/CBD ratio) are necessary to define the optimal ratio of THC and CBD for cancer pain.
Many of the studies were very small, and, combined with cross‐over design and consequent attrition, resulted in reporting on very few participants. Much larger studies of at least several hundred participants are needed.
Prospective cohort studies incorporating initial randomisation but a pragmatic design in order to provide immediately relevant information on effectiveness and costs should complement randomised controlled trials.
It is preferable that study protocols define that treating people with CbMs who do not have pain relief is unacceptable, so that there would be built‐in stopping rules linked to pain relief after an adequate trial of therapy.
Reporting the details of the assessment of adverse events (spontaneous reports, open questions, symptom questionnaires) is mandatory because the type and frequency of adverse events is influenced by the modes of assessment.
Reporting of mean pain changes should be complemented using responder analyses (pain relief of 50% or greater or participants experiencing mild or no pain).
Imputation method are to be abandoned, as the outcome desired is that of adequate pain relief in the longer term, and for that people have to continue on therapy. Withdrawal for any reason has to be classified as treatment failure.
Study data have to be made available to review authors for individual participant data analyses.
Systematic reviews should not pool experimental and clinical studies, or studies aimed to relieve pain arising as a direct consequence of the cancer with studies aimed to relieve cancer therapy‐related pain.
History
Protocol first published: Issue 2, 2022
Acknowledgements
We thank Joanne Abbott, Pain, Palliative and Supportive Care Review Group (PaPaS) Information Specialist, for conducting the searches.
Cochrane Review Group funding acknowledgement: this project was funded by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane PaPaS. The views expressed are those of the review author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Editorial and peer‐reviewer contributions
The Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS) supported the authors in the development of this review.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Dr Neil O'Connell, PaPaS Co‐ordinating Editor, and Reader at Brunel University London.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Jessica Thomas (review), Anna Erskine (protocol and review), Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK.
Contact Editor (editorial and methods input): Prof McKenzie Ferguson PharmD, BCPS, Southern Illinois University Edwardsville, USA.
Information Specialist (preparing search strategy and running searches): Joanne Abbott, Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK.
Copy‐editing (initial copy‐edit and final proofread): Anne Lawson, Central Production Service, Cochrane.
Peer reviewers (provided comments and recommended an editorial decision): Dr Carole A Paley, Academic Unit of Palliative Care, University of Leeds, UK (Clinical Reviewer); Catherine Hofstetter, Consumer; Juliana Esther Martin‐Lopez, Andalusian Public Foundation Progress and Health‐FPS (Consumer); Janet Hardy, Senior Research Fellow, Mater Research – University of Queensland (Clinical Reviewer).
Appendices
Appendix 1. Terminology
| Term | Definition | Examples/typical products |
| (Herbal) Cannabis, marijuana | The whole plant or parts or material from the plant (e.g. flowers, buds, resin, leaves). |
Cannabis sativa, hashish |
| Medical or medicinal cannabis | The terms 'medical/medicinal cannabis' (or 'medical/medicinal marijuana') is used for cannabis plants, plant material or full plant extracts used for medical purposes. | Bedrocan, Bedrobinol, Tilray 10THC/10CBD |
| Cannabinoids | Cannabinoids are biologically active constituents of cannabis, or synthetic compounds, usually having affinity for, and activity at, cannabinoid receptors. | THC, CBD, CP55, 940, WIN55, 212‐2, HU210 |
| Phytocannabinoid | A cannabinoid found in the cannabis plant or purified/extracted from plant material. | THC, CBD |
| Endocannabinoid | An endogenous ligand found in the body of humans and other animals and which has affinity for, and activity at, cannabinoid receptors. | Anandamide, 2‐AG |
| Endocannabinoid system modulators | In addition to individual phytocannabinoids, cannabis‐derived or cannabis‐based medicines, and cannabis extracts, other pharmacological approaches under development for manipulation of the endocannabinoid system include selective synthetic cannabinoid receptor agonists or antagonists, and inhibitors of the catabolism (e.g. fatty acid amide hydrolase (FAAH) inhibitors) or reuptake of endocannabinoids. | PF‐04457845, URB597, Rimonabant |
| Cannabis‐based (or cannabis‐derived) medicines | Registered, regulatory body approved medicinal cannabis extracts with defined and standardized phytocannabinoid content, particularly THC and THC/CBD. | Nabiximols (Sativex), Dronabinol, Marinol, Epidiolex |
Soliman 2019, adapted from Häuser 2018.
CBD: cannabidiol; THC: tetrahydrocannabinol.
Appendix 2. Search strategies
CENTRAL
#1 MeSH descriptor: [Cannabis] this term only
#2 ((cannabis or hemp or marijuana or ganja or hashish or marihuana or bhang or cannabinoid*)):ti,ab,kw (Word variations have been searched)
#3 MeSH descriptor: [Cannabinoids] explode all trees
#4 ((dronabinol or marinol or nabilone or cesamet or "HU 211" or dexanabinol or nabiximols or sativex or dronabinol or tetrahydrocannabinol)):ti,ab,kw (Word variations have been searched)
#5 (CANNABIDIOL):ti,ab,kw (Word variations have been searched)
#6 (cannabinol):ti,ab,kw (Word variations have been searched)
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Neoplasms] explode all trees
#9 ((cancer* or neoplas* or tumo* or carcinoma* or hodgkin* or nonhodgkin* or adenocarcinoma* or leuk?emia* or metasta* or malignan* or lymphoma* or sarcoma* or melanoma* or myeloma* or oncolog*)):ti,ab,kw (Word variations have been searched)
#10 #8 or #9
#11 MeSH descriptor: [Pain] explode all trees
#12 (pain):ti,ab,kw (Word variations have been searched)
#13 #11 or #12
#14 #7 and #10 and #13
MEDLINE (Ovid)
1 Cannabis/
2 (cannabis or hemp or marijuana or ganja or hashish or marihuana or bhang or cannabinoid*).tw.
3 exp Cannabinoids/
4 (dronabinol or marinol or nabilone or cesamet or "HU 211" or dexanabinol or nabiximols or sativex or dronabinol or tetrahydrocannabinol).tw.
5 CANNABIDIOL.tw.
6 cannabinol.tw.
7 1 or 2 or 3 or 4 or 5 or 6
8 exp Neoplasms/
9 (cancer* or neoplas* or tumo* or carcinoma* or hodgkin* or nonhodgkin* or adenocarcinoma* or leuk?emia* or metasta* or malignan* or lymphoma* or sarcoma* or melanoma* or myeloma* or oncolog*).tw.
10 8 or 9
11 exp Pain/
12 pain.tw.
13 11 or 12
14 7 and 10 and 13
15 randomized controlled trial.pt.
16 controlled clinical trial.pt.
17 randomized.ab.
18 placebo.ab.
19 drug therapy.fs.
20 randomly.ab.
21 trial.ab.
22 or/15‐21
23 exp animals/ not humans.sh.
24 22 not 23
25 14 and 24
Embase (Ovid)
1 Cannabis/
2 (cannabis or hemp or marijuana or ganja or hashish or marihuana or bhang or cannabinoid*).tw.
3 exp Cannabinoid/
4 (dronabinol or marinol or nabilone or cesamet or "HU 211" or dexanabinol or nabiximols or sativex or dronabinol or tetrahydrocannabinol).tw.
5 CANNABIDIOL.tw.
6 cannabinol.tw.
7 1 or 2 or 3 or 4 or 5 or 6
8 exp Neoplasm/
9 (cancer* or neoplas* or tumo* or carcinoma* or hodgkin* or nonhodgkin* or adenocarcinoma* or leuk?emia* or metasta* or malignan* or lymphoma* or sarcoma* or melanoma* or myeloma* or oncolog*).tw.
10 8 or 9
11 exp Pain/
12 pain.tw.
13 11 or 12
14 7 and 10 and 13
15 random$.tw.
16 factorial$.tw.
17 crossover$.tw.
18 cross over$.tw.
19 cross‐over$.tw.
20 placebo$.tw.
21 (doubl$ adj blind$).tw.
22 (singl$ adj blind$).tw.
23 assign$.tw.
24 allocat$.tw.
25 volunteer$.tw.
26 Crossover Procedure/
27 double‐blind procedure.tw.
28 Randomized Controlled Trial/
29 Single Blind Procedure/
30 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31 (animal/ or nonhuman/) not human/
32 30 not 31
33 14 and 32
Data and analyses
Comparison 1. Nabiximols (tetrahydrocannabinol (THC) and cannabidiol (CBD)) versus placebo for individuals with opioid‐refractory cancer pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Patient Global Impression of Change (PGIC) of much improved or very much improved | 3 | 996 | Risk Difference (IV, Random, 95% CI) | 0.06 [0.01, 0.12] |
| 1.1.1 THC/CBD 1–4 sprays (2.7 mg/2.5 mg to 10.8 mg/10 mg) | 1 | 94 | Risk Difference (IV, Random, 95% CI) | 0.11 [‐0.12, 0.34] |
| 1.1.2 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 3 | 805 | Risk Difference (IV, Random, 95% CI) | 0.06 [0.00, 0.12] |
| 1.1.3 THC/CBD 11–16 sprays (THC 29.7–43.2 mg/CBD 27.5–40 mg) | 1 | 97 | Risk Difference (IV, Random, 95% CI) | 0.02 [‐0.20, 0.24] |
| 1.2 Withdrawal due to adverse events | 4 | 1332 | Risk Difference (IV, Random, 95% CI) | 0.04 [0.00, 0.08] |
| 1.2.1 THC/CBD 1–4 sprays (2.7 mg/2.5 mg to 10.8 mg/10 mg) | 1 | 122 | Risk Difference (IV, Random, 95% CI) | ‐0.05 [‐0.21, 0.11] |
| 1.2.2 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 4 | 1002 | Risk Difference (IV, Random, 95% CI) | 0.05 [‐0.00, 0.09] |
| 1.2.3 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 88 | Risk Difference (IV, Random, 95% CI) | 0.05 [‐0.07, 0.18] |
| 1.2.4 THC/CBD 11–16 sprays (THC 29.7–43.2 mg/CBD 27.5–40 mg) | 1 | 120 | Risk Difference (IV, Random, 95% CI) | 0.08 [‐0.09, 0.25] |
| 1.3 Pain relief ≥ 30% | 4 | 1332 | Risk Difference (IV, Random, 95% CI) | 0.02 [‐0.03, 0.07] |
| 1.3.1 THC/CBD 1–4 sprays (2.7 mg/2.5 mg to 10.8 mg/10 mg) | 1 | 122 | Risk Difference (IV, Random, 95% CI) | ‐0.02 [‐0.19, 0.16] |
| 1.3.2 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 4 | 1003 | Risk Difference (IV, Random, 95% CI) | 0.03 [‐0.04, 0.09] |
| 1.3.3 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 87 | Risk Difference (IV, Random, 95% CI) | 0.00 [‐0.18, 0.18] |
| 1.3.4 THC/CBD 11–16 sprays (THC 29.7–43.2 mg/CBD 27.5–40 mg) | 1 | 120 | Risk Difference (IV, Random, 95% CI) | ‐0.02 [‐0.20, 0.16] |
| 1.4 Pain relief ≥ 50% | 4 | 1333 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 1.4.1 THC/CBD 1–4 sprays (THC/CBD 2.7 mg/2.5 mg to 10.8 mg/10 mg) | 1 | 122 | Risk Difference (IV, Random, 95% CI) | 0.13 [‐0.01, 0.28] |
| 1.4.2 THC/CBD 6–10 sprays (THC 16.2–27 mg/ CBD 15–25 mg) | 4 | 1004 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 1.4.3 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 87 | Risk Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 1.4.4 THC/CBD 11–16 sprays (THC 29.7–43.2 mg/CBD 27.5–40 mg) | 1 | 120 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.13, 0.15] |
| 1.5 Mean pain intensity | 4 | 1315 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.40, 0.02] |
| 1.5.1 THC/CBD 1–4 sprays (THC/CBD 2.7 mg/2.5 mg to 10.8 mg/10 mg) | 1 | 122 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.57, ‐0.03] |
| 1.5.2 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 4 | 993 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.42, 0.11] |
| 1.5.3 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.84, 0.44] |
| 1.5.4 THC/CBD 11–16 sprays (THC 29.7–43.2 mg/CBD 27.5–40 mg) | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.85, 0.65] |
| 1.6 Sleep problems | 4 | 1314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.19, 0.06] |
| 1.6.1 THC/CBD 1–4 sprays (THC/CBD 2.7 mg/2.5 mg to 10.8 mg/10 mg) | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.74, 0.08] |
| 1.6.2 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 4 | 993 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.12] |
| 1.6.3 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.47, 0.44] |
| 1.6.4 THC/CBD 11–16 sprays (THC/CBD 29.7–43.2 mg/CBD 27.5–40 mg) | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.37, 0.46] |
| 1.7 Daily maintenance opioid dosage | 3 | 970 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.10, 0.27] |
| 1.7.1 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 3 | 883 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.14, 0.19] |
| 1.7.2 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.05, 0.85] |
| 1.8 Daily breakthrough opioid dosage | 3 | 957 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.23, 0.07] |
| 1.8.1 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 3 | 877 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.01] |
| 1.8.2 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.21, 0.71] |
| 1.9 All central nervous system adverse events | 4 | 1331 | Risk Difference (IV, Random, 95% CI) | 0.11 [0.05, 0.17] |
| 1.9.1 THC/CBD 1–4 sprays (THC/CBD 2.7 mg/2.5 mg to 10.8 mg/10 mg) | 1 | 122 | Risk Difference (IV, Random, 95% CI) | 0.07 [‐0.10, 0.25] |
| 1.9.2 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 4 | 1002 | Risk Difference (IV, Random, 95% CI) | 0.10 [0.03, 0.17] |
| 1.9.3 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 87 | Risk Difference (IV, Random, 95% CI) | 0.12 [‐0.07, 0.31] |
| 1.9.4 THC/CBD 11–16 sprays (THC 29.7–43.2 mg/CBD 27.5–40 mg) | 1 | 120 | Risk Difference (IV, Random, 95% CI) | 0.24 [0.06, 0.43] |
| 1.10 All psychiatric adverse events | 4 | 1330 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 1.10.1 THC/CBD 1–4 sprays (THC/CBD 2.7 mg/2.5 mg to 10.8 mg/10 mg) | 1 | 122 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.15] |
| 1.10.2 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 4 | 1001 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 1.10.3 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 87 | Risk Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 1.10.4 THC/CBD 11–16 sprays (THC 29.7–43.2 mg/CBD 27.5–40 mg) | 1 | 120 | Risk Difference (IV, Random, 95% CI) | 0.18 [0.04, 0.32] |
| 1.11 Any serious adverse event | 4 | 1330 | Risk Difference (IV, Random, 95% CI) | 0.02 [‐0.03, 0.07] |
| 1.11.1 THC/CBD 1–4 sprays (2.7 mg/2.5 mg to 10.8 mg/10 mg) | 1 | 122 | Risk Difference (IV, Random, 95% CI) | 0.13 [‐0.06, 0.31] |
| 1.11.2 THC/CBD 6–10 sprays (THC 16.2–27 mg/CBD 15–25 mg) | 4 | 1001 | Risk Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 1.11.3 THC 6–10 sprays (THC 16.2–27 mg) | 1 | 87 | Risk Difference (IV, Random, 95% CI) | 0.12 [‐0.03, 0.27] |
| 1.11.4 THC/CBD 11–16 sprays (THC 29.7–43.2 mg/CBD 27.5–40 mg) | 1 | 120 | Risk Difference (IV, Random, 95% CI) | 0.04 [‐0.14, 0.23] |
Comparison 2. Experimental studies with synthetic tetrahydrocannabinol (THC) analogue versus placebo for individuals with cancer pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mean pain intensity | 4 | 301 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.36, ‐0.60] |
| 2.1.1 Synthetic THC analogue 5 mg and 10 mg | 4 | 193 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.19, ‐0.35] |
| 2.1.2 Synthetic THC analogue 15 mg and 20 mg | 2 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.23, ‐0.62] |
Comparison 3. Experimental studies with synthetic tetrahydrocannabinol (THC) analogue versus codeine for individuals with cancer pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mean pain intensity | 2 | 194 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.25, 0.32] |
| 3.1.1 Synthetic THC analogue 4 mg or 10 mg versus codeine 50 mg or 60 mg | 2 | 126 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.25, 0.45] |
| 3.1.2 Synthetic THC analogue 20 mg versus codeine 120 mg | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.57, 0.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Côté 2016.
| Study characteristics | ||
| Methods |
Purpose of the study: improving quality of life, especially pain, appetite and nausea, of people treated by radiotherapy or radiochemotherapy for head and neck squamous cell carcinomas Study setting: 1 university centre in Canada Study period: May 2005 to August 2007 Study design: double‐blind, randomised, placebo‐controlled, parallel‐group design Study duration: 8 weeks (no data on washout period reported); 4 weeks' double‐blind individually titrated dose, 4 weeks' follow‐up |
|
| Participants |
Type of cancer: head and neck squamous cell carcinoma Inclusion criteria: histological diagnosis of squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, larynx, or a combination of these; treated by radiotherapy alone, postoperative radiotherapy, radiochemotherapy alone, or postoperative radiochemotherapy; aged 18–80 years; no other cancer diagnosis in past 5 years, except for basal cell and squamous cell carcinoma of the skin Exclusion criteria: metastatic disease; history of radiotherapy in the head and neck region; Karnofsky score < 60; cognitive impairment; hepatic insufficiency; pregnant or breastfeeding woman; history of hypersensitivity or adverse reactions to marijuana or other CBs; history of schizophrenia or any other form of psychosis Nabilone: 28 participants; 93% men; mean age 63.5 years; race: not reported; type of cancer pain: not reported; previous cannabis use: not reported Placebo: 28 participants; 71% mean; mean age 63.8 years; race: not reported; type of cancer pain: not reported; previous cannabis use: not reported |
|
| Interventions |
Nabilone, PO, flexible dosage up to 2 mg/day: administration began the day before the first radiotherapy treatment, with 1 tablet (0.5 mg PO at bedtime). The same dose was maintained for the entire first week (0.5 mg). For the second week, the dose was increased to 2 tablets a day (0.5 mg PO twice daily). From the third week until the end of radiotherapy treatments, the dose was adjusted by the radio‐oncologist to a maximum of 4 tablets a day. Placebo Rescue medication: not reported Allowed cotherapies: antiemetics (metoclopramide only) and analgesics (only paracetamol/codeine, hydromorphone or transdermal fentanyl) allowed. Dosages not reported |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Participant impression to be much or very much improved: not assessed Withdrawal due to adverse events: no details of assessment reported Combined responder: not assessed Pain relief ≥ 30%: VAS 0–10, time frame not reported. Imputation method could not be used because baseline values were not reported. Pain relief ≥ 50%: VAS 0–10, time frame not reported. Imputation method could not be used because baseline values were not reported. Mean pain intensity: VAS 0–10, time frame not reported. No means and SDs reported. Sleep problems: EORTC QLQ‐C30 subscale sleep. No means and SDs reported. Depression: EORTC QLQ‐C30 subscale mood. No means and SDs reported. Anxiety: not assessed Daily maintenance opioid therapy dose: not assessed Daily breakthrough opioid therapy dose: not assessed Withdrawals due to lack of efficacy: not reported in detail Nervous system disorders adverse effects: no details of assessment reported Psychiatric disorders adverse effects: no details of assessment reported Any serious adverse event: no details of assessment reported |
|
| Notes |
Funding: research grants from the Canadian Institutes of Health Research and the Fonds de Recherche en Santé du Québec. ICN Valeant Pharmaceuticals provided the nabilone and the placebo tablets during the trial. Conflicts of interest: authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The physicians, nurse, and subjects were blinded: the hospital pharmacist was the only one who knew patients' grouping." Comment: no details of blinding reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Nabilone and placebo "both look identical." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details reported if ITT was applied. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Selection bias | Low risk | No differences in demographic and clinical parameters between the study groups at baseline. |
Fallon 2017a.
| Study characteristics | ||
| Methods |
Purpose of the study: reducing cancer‐related pain that was unalleviated by an optimised maintenance dose of Step 3 opioid therapy Study setting: 101 centres in Belgium, Bulgaria, Czech Republic, Estonia, Germany, Hungary, Latvia, Lithuania, Poland, Romania, UK and US Study period: not reported Study design: double‐blind, randomised, placebo‐controlled, parallel‐group design Study duration: 6 weeks (2 weeks' double‐blind individual titration, 3 weeks' double‐blind stable individual dose, 1‐week follow‐up) |
|
| Participants |
Type of cancer: not reported Inclusion criteria: aged ≥ 18 years; cancer‐related pain that was unalleviated by an optimised maintenance dose of Step 3 opioid therapy. Opioid therapy was considered optimised if 1. a dose increase was clinically inappropriate due to opioid‐related adverse effects or 2. further efficacy benefit was not expected at higher doses (for the second definition, participants had to be receiving ≥ 90 mg morphine equivalents/day, inclusive of maintenance and breakthrough opioids). ≤ 4 opioid breakthrough analgesic episodes per day (mean over the 3 days), a stable maintenance opioid therapy dose, mean pain ≥ 4 and ≤ 8 on a 0–10 NRS and mean pain scores on the NRS that did not change by > 2 points from the beginning to end of screening (i.e. no more than a 2‐point difference between the highest and lowest scores, with all scores remaining between 4 and 8). Exclusion criteria: baseline use of morphine at > 500 mg morphine equivalents/day (inclusive of maintenance and breakthrough opioids), current use of > 1 type of breakthrough opioid analgesic, planned clinical interventions that would affect pain, and history of schizophrenia or substance abuse including recreational use of cannabis product. Any planned clinical interventions that would have affected their pain (e.g. chemotherapy or radiotherapy where, in the clinical judgement of the investigator, these would be expected to affect pain). The participant was using or had used cannabis or CB‐based medications within 30 days of study entry and was unwilling to abstain for the duration of the study. The participant had experienced myocardial infarction or clinically significant cardiac dysfunction within the last 12 months or had a cardiac disorder that, in the opinion of the investigator, would have put the participant at risk of a clinically significant arrhythmia or myocardial infarction. Impaired renal or hepatic function. THC/CBD: 200 participants; 53% men; mean age 60.0 (SD 11.0) years; Caucasian 97%; type of cancer pain: neuropathic (13.5%), somatic (4.5%), visceral (10.5%), mixed (55.5%), bone (16.0%), other (0%); mean pain baseline 5.7 (SD 1.2); daily morphine equivalent maintenance 170.4 (SD 118.7) mg/day; daily breakthrough morphine equivalent 28.8 (SD 40.2) mg/day; previous cannabis use: not reported Placebo: 199 participants; 49% men; mean age 59.6 (SD 11.0) years; Caucasian 91%; type of cancer pain: neuropathic (11.6%), somatic (8.5%), visceral (11.1%), mixed (58.3%), bone (10.6%), other (0%); mean pain 5.8 (SD 1.1); daily morphine equivalent maintenance 182.4 (SD 124.3) mg/day; daily breakthrough morphine equivalent 25.3 (SD 38.1) mg/day; previous cannabis use: not reported |
|
| Interventions |
THC/CBD extract, oromucosal spray; flexible dosage: THC 2.7 mg and CBD 2.5 mg, both per 100 µL (which equalled 1 pump action). Treatment was initiated as a single spray in the evening of the first day of treatment and was gradually increased by 1 additional spray/day (15 minutes apart) according to a prespecified dose escalation protocol until participants experienced/ unacceptable adverse effects, received acceptable pain relief or reached the maximum allowed daily dosage of 10 sprays/day (participants were advised to reach ≥ 3 sprays/day). Mean 6.3 sprays/day Placebo, oromucosal spray: mean 7.4 sprays/day Rescue medication: opioids Allowed cotherapies: not reported |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Participant impression to be much or very much improved: Subject Global Impression of Change Withdrawal due to adverse events: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Combined responder: not assessed Pain relief ≥ 30%: NRS 0–10, last 24 hours. Calculated by imputation method Pain relief ≥ 50%: NRS 0–10, last 24 hours. Calculated by imputation method Mean pain intensity: NRS 0–10, last 24 hours Sleep problems: sleep disruption score 0–10, last 24 hours Depression: not assessed Anxiety: not assessed Maintenance opioid therapy dose: – Breakthrough opioid therapy dose: – Withdrawals due to lack of efficacy: not reported in detail Nervous system disorders adverse effects: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Psychiatric disorders adverse effects: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Any serious adverse event: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. |
|
| Notes |
Funding: Otsuka Pharmaceutical Development & Commercialisation, Inc, Rockville, Maryland, USA Conflicts of interest: authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active product peppermint flavoured, placebo coloured and peppermint flavoured. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis; no details reported. |
| Selective reporting (reporting bias) | Low risk | Data reported as outlined in NCT01361607. |
| Selection bias | Low risk | No differences in demographic and clinical variables between the study groups at baseline. |
Fallon 2017b.
| Study characteristics | ||
| Methods |
Purpose of the study: reducing cancer‐related pain that was unalleviated by an optimised maintenance dose of Step 3 opioid therapy Study setting: 65 centres in Australia, Bulgaria, Germany, Hungary, India, Israel, Italy, Lithuania, Poland, Romania, Spain, Taiwan and UK Study period: not reported Study design: double‐blind, placebo‐controlled, enriched enrolment randomised withdrawal design Study duration: 7 weeks (2 weeks' single‐blind individual titration, 5 weeks' randomised withdrawal, 2 weeks' follow‐up for safety evaluation) |
|
| Participants |
Type of cancer: not reported Inclusion criteria: aged ≥ 18 years; cancer‐related pain that was unalleviated by an optimised maintenance dose of Step 3 opioid therapy. Opioid therapy was considered optimised if 1. a dose increase was clinically inappropriate due to opioid‐related adverse effects or 2. further efficacy benefit was not expected at higher doses (for the second definition, participants had to be receiving ≥ 90 mg morphine equivalent/day, inclusive of maintenance and breakthrough opioids). ≤ 4 opioid breakthrough analgesic episodes/day (mean of the 3 days), a stable maintenance opioid therapy dose, mean pain ≥ 4 and ≤ 8 on a 0–10 NRS and mean pain scores on the NRS that did not change by > 2 points from the beginning to end of screening (i.e. ≤ 2‐point difference between the highest and lowest scores, with all scores remaining between 4 and 8). Exclusion criteria: baseline use of morphine at > 500 mg morphine equivalents/day (inclusive of maintenance and breakthrough opioids), current use of > 1 type of breakthrough opioid analgesic, planned clinical interventions that would affect pain and any history of schizophrenia or substance abuse including recreational use of cannabis product. Any planned clinical interventions that would have affected their pain (e.g. chemotherapy or radiotherapy where, in the clinical judgement of the investigator, these would be expected to affect pain). The participant was using or had used cannabis or CB‐based medications within 30 days of study entry and was unwilling to abstain for the duration of the study. The participant had experienced myocardial infarction or clinically significant cardiac dysfunction within the last 12 months or had a cardiac disorder that, in the opinion of the investigator, would have put the participant at risk of a clinically significant arrhythmia or myocardial infarction. Impaired renal or hepatic function. THC/CBD: 103 participants; 61.2% men; mean age 61.4 (SD 10.9) years; Caucasian 99.1%; type of cancer pain: neuropathic (10.7%), somatic (8.7%), visceral (11.7%), mixed (54.4%), bone (13.6%), other (1.0%); mean pain baseline 5.6 (SD 1.1); daily morphine equivalent maintenance 185.5 (SD 123.7) mg/day; daily breakthrough morphine equivalent 26.8 (SD 36.1) mg/day; previous cannabis use: not reported Placebo: 103 participants; 53.4% men; mean age 61.6 (SD 11.8) years; Caucasian 98.1%; type of cancer pain: neuropathic (11.7%), somatic (5.8%), visceral (7.8%), mixed (52.4%), bone (19.4%), other (2.9%); mean pain 5.6 (SD 1.2); daily morphine equivalent maintenance 175.3 (SD 106.5) mg/day; daily breakthrough morphine equivalent 34.0 (SD 48.5) mg/day; previous cannabis use: not reported |
|
| Interventions |
THC/CBD extract, oromucosal spray; flexible dosage: THC 2.7 mg and CBD 2.5 mg, both per 100 µL (which equalled 2 pump action). Treatment was initiated as a single spray in the evening of the first day of treatment and was gradually increased by 1 additional spray/day (15 minutes apart) according to a prespecified dose escalation protocol until participants experienced unacceptable adverse effects, received acceptable pain relief or reached the maximum allowed daily dosage of 10 sprays/day (participants were advised to reach ≥ 3 sprays/day). Mean 6.5 sprays/day in double‐blind phase Placebo, oromucosal spray: mean 6.3 sprays/day in double‐blind period Rescue medication: opioids Allowed cotherapies: not reported |
|
| Outcomes |
Loss of proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Loss of therapeutic response of patient impression to be much or very much improved: authors reported mean Subject Global Impression of Change scores. Not suited for meta‐analysis. Withdrawal due to adverse events: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Combined responder: not assessed Loss of pain relief ≥ 30%: not assessed Pain relief ≥ 50%: not assessed Mean pain intensity: NRS 0–10, last 24 hours Sleep problems: Sleep Disruption Score 0–10, last 24 hours Depression: not assessed Anxiety: not assessed Maintenance opioid therapy dose: reported Breakthrough opioid therapy dose: reported Withdrawals due to lack of efficacy: not reported in detail Nervous system disorders adverse effects: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Psychiatric disorders adverse effects: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Any serious adverse event: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. |
|
| Notes |
Funding: Otsuka Pharmaceutical Development & Commercialisation, Inc, Rockville, Maryland, USA Conflicts of interest: authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active product peppermint flavoured, placebo coloured and peppermint flavoured. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis; no details reported. |
| Selective reporting (reporting bias) | Low risk | Data reported as outlined in NCT01424566. |
| Selection bias | Low risk | No differences in demographic and clinical variables between the study groups at baseline. |
Hardy 2023.
| Study characteristics | ||
| Methods |
Purpose of the study: to determine whether CBD oil can improve symptom distress in people with advanced cancer receiving palliative care. Study setting: 5 tertiary medical centres within south‐east Queensland, Australia Study period: February 2019 to November 2021 Study design: double‐blind, randomised, placebo‐controlled, parallel‐group design Study duration: no information on baseline period reported, 4 weeks' double‐blind |
|
| Participants |
Type of cancer: prostate 21%, breast 16%, colorectal 15%, gynaecological 13%, lung 9%, haematological 5%, others 22% Inclusion criteria: aged > 18 years, with advanced cancer who had a TSDS as measured using an ESAS 8 of ≥ 10/90 (with ≥ 1 score ≥ 3), a negative baseline THC urine test, Australian‐modified Karnofsky Performance Scale ≥ 30, adequate cognitive function (as assessed using the St Louis University Mental Status Examination) and were able to take oral medications. Exclusion criteria: severe hepatic or renal dysfunction, history of significant psychiatric or substance use disorder (as assessed by the Alcohol, Smoking and Substance Involvement Screening Test) and the potential for drug diversion or a new anticancer therapy or radiotherapy within 7 days. CBD: 70 participants; 56% men; mean age 63.6 (SD 14.0) years; race not reported; type of cancer pain: not reported; pain medication: background opioid dose 45 mg (OMEs) median (range 0–590); previous cannabis use: not reported Placebo: 72 participants; 50% men; mean age 65.5 (SD 11.4) years; race not reported; type of cancer pain: not reported; pain medication: background opioid dose 40 mg (OMEs) median (range 0–555); previous cannabis use: not reported |
|
| Interventions |
CBD: 50–600 mg/day PO, flexible dosage (median dose 400 mg/day, range 50–600 mg/day) Placebo: PO, flexible dosage Rescue medication: no details reported Allowed cotherapies: antipsychotics: CBD group 19%; placebo group 22%; benzodiazepines: CBD group 30%; placebo group 39% |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: assessed Withdrawal due to adverse events: no details of assessment reported Combined responder: not assessed Pain relief ≥ 30%: EORTC QLQ‐C15, score 0–100, time frame last week; calculated by imputation method Pain relief ≥ 50%: EORTC QLQ‐C15, score 0–100, time frame last week; calculated by imputation method Mean pain intensity: EORTC QLQ‐C15, score 0–100, time frame last week; calculated by imputation method Sleep problems: EORTC QLQ‐C15, score 0–100, time frame last week; scores at baseline and end of treatment not reported Depression: Depression Anxiety Stress Scale 0–42 Anxiety: Depression Anxiety Stress Scale 0–42 Daily maintenance and breakthrough opioid therapy dose combined: assessed Withdrawals due to lack of efficacy: not reported Nervous system disorders adverse effects: only somnolence and dizziness reported Psychiatric disorders adverse effects: not reported Any serious adverse event: reported |
|
| Notes |
Funding: Commonwealth of Australia–Medical Research Future Fund Grant No. APP1152232 Conflicts of interest: 2 authors received funding from GD Pharma Ltd (Inst). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Any effect of dropouts was evaluated using Cox proportional hazards regression." |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as outlined in ACTRN 126180001220257. |
| Selection bias | High risk | Quote: "Those randomly assigned to placebo had a higher baseline total symptom distress score than those on CBD oil." |
Jochimsen 1978.
| Study characteristics | ||
| Methods |
Purpose of the study: to assess the analgesic activity of a synthetic THC analogue at 2–4 mg Study setting: 1 university hospital in USA Study period: not reported Study design: double‐blind, randomised, placebo‐controlled, cross‐over design Study duration: 1 day each. Regular medication was stopped 4 hours before the intake of the medication |
|
| Participants |
Type of cancer: no details reported Inclusion criteria: pain related to malignancies and history of frequent analgesic use, though 1 had received large doses of narcotics Exclusion criteria: conditions interfering with drug metabolism, severe organic disease other than cancer, pregnancy or major psychiatric disorders Synthetic THC analogue: 35 participants; 17% men; aged 38–77 years; race: not reported; type of cancer pain: not reported; pain medication: not reported; previous cannabis use: not reported |
|
| Interventions |
Synthetic nitrogen‐containing benzopyran derivative (modification of delta‐I‐trans‐THC): 2 mg and 4 mg single, fixed dosage PO Codeine: 60 mg and 120 mg single, fixed dosage PO Placebo: PO Rescue medication: none Allowed cotherapies: no details reported |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: not assessed Withdrawal due to adverse events: no details of assessment reported Combined responder: not assessed Pain relief ≥ 30%: not reported; imputation method not applicable because baseline pain scores not reported. Number of participants with moderate pain relief reported Pain relief ≥ 50%: reported Mean pain intensity: hourly ratings of the severity of pain (0, absent; 1, mild; 2, moderate; 3, severe) were used to determine hourly pain reduction scores Sleep problems: not assessed Depression: not assessed Anxiety: not assessed Daily maintenance opioid therapy dose: not assessed Daily breakthrough opioid therapy dose: not assessed Withdrawals due to lack of efficacy: not reported Nervous system disorders adverse effects: at hourly intervals, the participant completed an 11‐item subjective effects questionnaire, designed to quantify certain psychic manifestations of the test preparations. Data provided not suited for analysis. Psychiatric disorders adverse effects: at hourly intervals, the participant completed an 11‐item subjective effects questionnaire, designed to quantify certain psychic manifestations of the test preparations. Data provided not suited for analysis. Any serious adverse event: not assessed |
|
| Notes |
Funding: grant RR‐59 from the General Clinical Research Canters Program Division of Research Resources, National Institutes of Health and Abbott Laboratories. Conflicts of interest: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical appearing capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Responder analysis. |
| Selective reporting (reporting bias) | Unclear risk | No prepublished study protocol available. |
| Selection bias | Low risk | Identical baseline parameters of the study groups due to cross‐over design. |
Johnson 2010.
| Study characteristics | ||
| Methods |
Purpose of the study: reducing moderate‐to‐severe cancer‐related pain despite therapy with strong opioids Study setting: 20 study centres in UK and Romania Study period: not reported Study design: double‐blind, randomised, placebo‐controlled, parallel‐group design Study duration: 2 days' baseline, 2 weeks' double‐blind |
|
| Participants |
Type of cancer: breast, prostate, lung Inclusion criteria: adults using strong opioids ≥ 1 week to relieve pain associated with incurable malignancy, pain severity score ≥ 4 on a 0–10 NRS on both days of the baseline period Exclusion criteria: cancers affecting the oral cavity; radiotherapy to the floor of the mouth; major psychiatric or cardiovascular disorders; epilepsy; renal or hepatic impairment; or pregnant, lactating or not using adequate contraception. Participants who received therapies expected to confound the study outcome (epidural analgesia within 48 hours of screening; palliative radio‐, chemo‐, or hormonal therapy within 2 weeks of screening; or CBs within 7 days of randomisation). Participants taking levodopa, sildenafil or fentanyl, or participants with a hypersensitivity to CBs THC/CBD: 60 participants; 55% men; mean age 59.4 (SD 12.1) years; Caucasian 98%; type of cancer pain: neuropathic (18%), somatic (11%), visceral (23%), mixed (52%), bone (27%), other (0%): mean pain 5.7 (SD 1.2); daily morphine equivalent maintenance 258 (789) mg/day; daily breakthrough morphine equivalent: not reported; previous cannabis use: 10% THC: 58 participants; 52% men; mean age 61.3 (SD 12.5) years; Caucasian 98%; type of cancer pain: neuropathic (19%), somatic (9%), visceral (21%), mixed (48%), bone (41%), other (0%): mean pain 5.6 (SD 1.2); daily morphine equivalent maintenance 188.2 (243.5) mg/day; daily breakthrough morphine equivalent: not reported; previous cannabis use: 10% Placebo: 59 participants; 54% men; mean age 60.1 (SD 12.3) years; Caucasian 98%; type of cancer pain: neuropathic (29%), somatic (10%), visceral (19%), mixed (51%), bone (42%), other (0%): mean pain 5.6 (SD 1.2); daily morphine equivalent maintenance 367 (886.4) mg/day; daily breakthrough morphine equivalent: not reported; previous cannabis use: 12% |
|
| Interventions |
THC/CBD extract; oromucosal spray; flexible dosage: THC 2.7 mg and CBD 2.5 mg, both per 100 µL (which equalled 1 pump action). Mean 8.8 sprays/day THC extract (plant‐based), oromucosal spray flexible dosage: THC 2.7 mg/100 µL. Mean 8.4 sprays/day Placebo, oromucosal spray: mean 9.1 sprays/day Rescue medication: no details reported Allowed cotherapies: usual breakthrough analgesia as required (recorded) maintained background medication as necessary |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: not assessed Withdrawal due to adverse events: adverse events as reported by the participant Combined responder: not assessed Pain relief ≥ 30%: NRS 0–10, last 24 hours (mean of 3 ratings). Pain relief ≥ 30%: NRS 0–10, last 24 hours. Extracted from figure Mean pain intensity: NRS 0–10, last 24 hours (mean of 3 ratings) Sleep problems: Sleep Disruption Score 0–10, last 24 hours Depression: not assessed Anxiety: not assessed Maintenance opioid therapy dose: mean change from baseline Breakthrough opioid therapy dose: baseline to end of week 2 (last 3 days of treatment) Withdrawals due to lack of efficacy: not reported in detail Nervous system disorders adverse effects: participant‐reported adverse events Psychiatric disorders adverse effects: participant‐reported adverse events Any serious adverse event: participant‐reported adverse events. Study physicians determined the intensity of adverse events |
|
| Notes |
Funding: Otsuka Pharmaceutical Development & Commercialisation, Inc, Rockville, Maryland, USA Conflicts of interest: study authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo was only coloured, no taste blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis; no details reported. |
| Selective reporting (reporting bias) | Low risk | Data reported as outlined in NCT00674609. |
| Selection bias | High risk | Higher morphine dose in THC and placebo compared to THC/CBD group at baseline. |
Lichtman 2018.
| Study characteristics | ||
| Methods |
Purpose of the study: reducing cancer‐related pain that was unalleviated by an optimised maintenance dose of Step 3 opioid therapy Study setting: 114 centres in the Czech Republic, Estonia, Germany, Hungary, Latvia, Lithuania, Poland, Romania, UK, and US Study period: not reported Study design: double‐blind, randomised, placebo‐controlled, parallel‐group design Study duration: 5–14 days' screening, 4 weeks' double‐blind (2 weeks' titration, 3 weeks' stable dosage) |
|
| Participants |
Type of cancer: not reported Inclusion criteria: advanced cancer, aged ≥ 18 years, clinical diagnosis of cancer‐related pain that was unalleviated by an optimised maintenance dose of Step 3 opioid therapy. Opioid therapy was considered optimised if: 1. a dose increase was clinically inappropriate due to opioid‐related adverse effects or 2. further efficacy benefit was not expected at higher doses (for the second definition, participants had to be receiving ≥ 90 mg morphine equivalents/day, inclusive of maintenance and breakthrough opioids). The maintenance opioid was preferably a sustained‐release formulation, but a 24‐hour immediate‐release formulation was acceptable. To be eligible, participants also had to fulfil the following criteria on each of 13 consecutive days during the screening period: ≤ 4 opioid breakthrough analgesic episodes/day (mean over 3 days); a stable maintenance opioid therapy dose; mean pain ≥ 4 and ≤ 8 on a 0–10 NRS; and mean pain scores on the NRS that did not change by > 2 points (i.e. ≤ 2‐point difference between the highest and lowest scores), with all scores remaining between 4 and 8. Exclusion criteria: baseline use of morphine at > 500 mg morphine equivalents/day (inclusive of maintenance and breakthrough opioids), current use of > 1 type of breakthrough opioid analgesic, planned clinical interventions that would affect pain, and any history of schizophrenia or substance abuse. Any planned clinical interventions that would have affected their pain (e.g. chemotherapy or radiotherapy) where, in the clinical judgement of the investigator, these would be expected to affect pain. The participant was using or had used cannabis or CB‐based medications within 30 days of study entry and was unwilling to abstain for the duration of the study. The participant had experienced myocardial infarction or clinically significant cardiac dysfunction within the last 12 months or had a cardiac disorder that, in the opinion of the investigator, would have put the participant at risk of a clinically significant arrhythmia or myocardial infarction, impaired renal or hepatic function. THC/CBD: 199 participants; 55.8% men; mean age 59.2 (SD 12.0) years; Caucasian 93.0%; type of cancer pain: neuropathic (13.1%), somatic (5.0%), visceral (13.1%), mixed (48.2%), bone (19.6%), other (1.0%); mean pain 5.6 (SD 1.2); daily morphine equivalent maintenance 167.5 (SD 118.8) mg/day; daily breakthrough morphine equivalent 25.4 (SD 38.3); previous cannabis use: not reported Placebo: 198 participants; 52.0% men; mean age 60.7 (SD 11.1) years; Caucasian 93.4%; type of cancer pain: neuropathic (12.6%), somatic (3.0%), visceral (14.1%), mixed (54.0%), bone (16.2%), other (0%): mean pain 5.6 (SD 1.2); daily morphine equivalent maintenance 159.7 (SD 121.2) mg/day; daily breakthrough morphine equivalent 26.4 (SD 40.4); previous cannabis use: not reported |
|
| Interventions |
THC/CBD extract, oromucosal spray, flexible dosage: THC 2.7 mg and CBD 2.5 mg, both per 100 µL (which equalled 1 pump action). Mean spray in nabiximols group: 6.4 sprays/day Placebo, oromucosal spray: mean spray in nabiximols group: 7.3 sprays/day Rescue medication: opioids Allowed cotherapies: (quote) "Whenever possible, stable doses of other prescribed pain medications were continued during the study period." |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: PGIC Withdrawal due to adverse events: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Combined responder: not assessed Pain relief ≥ 30%: NRS 0–10, last 24 hours Pain relief ≥ 30%: NRS 0–10, last 24 hours. Calculated by imputation method Mean pain intensity: NRS 0–10, last 24 hours Sleep problems: Sleep Disruption Score 0–10, last 24 hours Depression: not assessed Anxiety: not assessed Maintenance opioid therapy dose: mg/day Breakthrough opioid therapy dose: mg/day. SD calculated from P value Withdrawals due to lack of efficacy: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Nervous system disorders adverse effects: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Psychiatric disorders adverse effects: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. Any serious adverse event: no details of assessment reported except that participants completed the Columbia Suicide Severity Rating Scale at every visit and that laboratory tests and vital signs reading were performed at every study visit. |
|
| Notes |
Sponsor: Otsuka Pharmaceutical Development & Commercialisation, Inc, Rockville, Maryland, USA. The efforts of AH Lichtman were supported by the Virginia Commonwealth University School of Pharmacy start‐up funds. Conflicts of interest: authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis by last observation carried forward method. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as outlined in NCT01262651. |
| Selection bias | Low risk | No differences in demographic and clinical variables between the study groups at baseline. |
Lynch 2014.
| Study characteristics | ||
| Methods |
Purpose of the study: reducing chemotherapy‐induced neuropathic pain Study setting: participants were recruited through advertisements in the local paper and posters in oncology clinics at the university teaching hospital (Capital District Health Authority, Halifax, Nova Scotia, Canada) Study period: not reported Study design: double‐blind, randomised, placebo‐controlled, cross‐over design Study duration: no information on baseline period reported, 4 weeks each study period separated by a 2‐week wash‐out period |
|
| Participants |
Type of cancer: ovary cancer (27.8%), uterus cancer (16.7%), lung cancer (16.7%), cervix cancer (11.1%), breast cancer (11.1%), blood/lymphoma (5.6%), lung cancer (5.6%), testicle cancer (5.6%) Inclusion criteria: neuropathic pain based on history and general physical examination along with specific quantitative sensory testing of the painful area; neuropathic pain persisting for 3 months after completing chemotherapy with paclitaxel, vincristine or cisplatin; mean 7‐day intensity of pain had to be ≥ 4 on an 11‐point NRS; concurrent analgesics had to be stable for 14 days before entry into the trial. Exclusion criteria: ischaemic heart disease, ongoing epilepsy, a personal or family history of schizophrenia, or psychotic disorder or substance abuse or dependency within the previous 2 years, pregnancy or other medical condition that might compromise safety in the trial. Nabiximols and placebo: 18 participants; 17% men; mean age 55 years; race not reported; type of cancer pain: neuropathic pain induced by chemotherapy; co‐medication: antidepressants (5.6%), NSAIDs (11.1%), opioids (11.1%); previous cannabis use: 27.8% |
|
| Interventions |
Oromucosal spray THC/CBD extract, flexible dosage: THC 2.7 mg and CBD 2.5 mg, both per 100 µL (which equalled 1 pump action). Individually titration, maximum 12 pumps/day; mean 8 sprays/day (range 8–12) Oromucosal placebo spray, flexible dosage: individual titration, maximum 12 pumps/ day; mean 11 sprays/day Rescue medication: no details reported |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: not assessed Withdrawal due to adverse events: no details of assessment reported Combined responder: not assessed Pain relief ≥ 30%: neuropathic pain score 7‐day mean; calculated by imputation method Pain relief ≥ 50%: neuropathic pain score 7‐day mean; calculated by imputation method Mean pain intensity: neuropathic pain score 7‐day mean Sleep problems: not assessed Depression: not assessed Anxiety: not assessed Daily maintenance opioid therapy dose: not assessed Daily breakthrough opioid therapy dose: not assessed Withdrawals due to lack of efficacy: not reported Nervous system disorders adverse effects: no details of assessment reported Psychiatric disorders adverse effects: no details of assessment reported Any serious adverse event: no details of assessment reported |
|
| Notes |
Funding: none Conflicts of interest: authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Last observation carried forward analysis. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol reported. |
| Selection bias | Low risk | Identical baseline data of the study groups due to cross‐over design. |
Noyes 1975a.
| Study characteristics | ||
| Methods |
Purpose of the study: reducing moderate‐to‐severe cancer pain Study setting: 1 university hospital in USA Study period: not reported Study design: double‐blind, randomised, placebo‐controlled, cross‐over design Study duration: 1 day each. Regular medication was stopped 4.5 hours before the intake of the medication |
|
| Participants |
Type of cancer: 13 breast, 7 non‐Hodgkin's lymphoma, 3 Hodgkin's disease, 2 each lung, colon, prostate and malignant melanoma, and 1 each cervix, carcinoid, leiomyosarcoma, parotid gland and anaplastic carcinoma of unknown origin Inclusion criteria: continuous pain of moderate severity Exclusion criteria: none Synthetic THC: 36 participants; 28% men; mean age 51 years; race: not reported; type of cancer pain: not reported; pain medication: (quote) "None were receiving large doses of narcotics;" previous cannabis use: not reported |
|
| Interventions |
Synthetic THC, fixed dosage: 10 mg and 20 mg single dosage PO Codeine, fixed dosage: 120 mg single dosage PO Placebo Rescue medication: none Allowed cotherapies: no details reported |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: not assessed Withdrawal due to adverse events: no details of assessment reported Combined responder: not assessed Pain relief ≥ 30%: not reported; imputation method not applicable because baseline pain scores not reported Pain relief ≥ 50%: not reported; imputation method not applicable because baseline pain scores not reported. Number of participants with substantial pain relief reported Mean pain intensity: hourly ratings of the severity of pain (0, absent; 1, mild; 2, moderate; 3, severe) were used to arrive at hourly pain reduction scores. The sum of hourly pain reduction or relief scores for a given 7‐hour observation period (total reduction or relief scores) was used as a basis for statistical analysis. Sleep problems: not assessed Depression: not assessed Anxiety: not assessed Daily maintenance opioid therapy dose: not assessed Daily breakthrough opioid therapy dose: not assessed Withdrawals due to lack of efficacy: not reported Nervous system disorders adverse effects: the nurse's observations, including evident or reported adverse effects, were recorded on a pain chart designed for that purpose. The same observer also administered an 11‐item subjective effects questionnaire hourly and an adverse effects inventory at the end of each 7‐hour observation period. Psychiatric disorders adverse effects: the nurse's observations, including evident or reported adverse effects, were recorded on a pain chart designed for that purpose. The same observer also administered an 11‐item subjective effects questionnaire hourly and an adverse effects inventory at the end of each 7‐hour observation period. Any serious adverse event: not reported |
|
| Notes |
Funding: grant RR‐59 from the General Clinical Research Canters Program Division of Research Resources. National Institute of Health. Conflicts of interest: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis. |
| Selective reporting (reporting bias) | Unclear risk | No pre‐published protocol available. |
| Selection bias | Low risk | Identical baseline data of the study groups due to cross‐over design. |
Noyes 1975b.
| Study characteristics | ||
| Methods |
Purpose of the study: reducing moderate‐to‐severe cancer pain Study setting: 1 university hospital in USA Study period: not reported Study design: double‐blind, randomised, placebo‐controlled, cross‐over design Study duration: 1 day each. Regular medication was stopped 4.5 hours before the intake of the medication |
|
| Participants |
Type of cancer: 5 breast, 2 malignant lymphoma, 1 cervix, 1 colon and 1 lymphoepithelioma Inclusion criteria: continuous pain of moderate severity Exclusion criteria: participants receiving large doses of narcotics Synthetic THC analogue: 10 participants; 20% men; mean age 51 years; race not reported; type of cancer pain: not reported; pain medication: (quote) "None were receiving large doses of narcotics." 7 participants had received methadone as part of their regular analgesic regimen. Previous cannabis use: not reported |
|
| Interventions |
Synthetic THC analogue, fixed dosage: 5 mg, 10 mg, 15 mg and 20 mg single dosage PO Placebo: single dose, PO Rescue medication: none Allowed cotherapies: no details reported |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: not assessed Withdrawal due to adverse events: no details of assessment reported Combined responder: not assessed Pain relief ≥ 30%: not reported; imputation method not applicable because baseline pain scores not reported Pain relief ≥ 50%: not reported; imputation method not applicable because baseline pain scores not reported. Number of participants with substantial pain relief reported Mean pain intensity: hourly ratings of the severity of pain (0, absent; 1, mild; 2, moderate and 3, severe) were used to arrive at hourly pain reduction scores. The sum of hourly pain reduction or relief scores for a given 7‐hour observation period (total reduction or relief scores) was used as a basis for statistical analysis. Sleep problems: not assessed Depression: not assessed Anxiety: not assessed Daily maintenance opioid therapy dose: not assessed Daily breakthrough opioid therapy dose: not assessed Withdrawals due to lack of efficacy: not reported Nervous system disorders adverse effects: the nurse's observations, including evident or reported adverse effects, were recorded on a pain chart designed for that purpose. The same observer also administered an 11‐item subjective effects questionnaire hourly and an adverse effects inventory at the end of each 7‐hour observation period. Psychiatric disorders adverse effects: the nurse's observations, including evident or reported adverse effects, were recorded on a pain chart designed for that purpose. The same observer also administered an 11‐item subjective effects questionnaire hourly and an adverse effects inventory at the end of each 7‐hour observation period. Any serious adverse event: not reported |
|
| Notes |
Funding: grant RR‐59 from the General Clinical Research Canters Program Division of Research Resources. National Institutes of Health Conflicts of interest: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis. |
| Selective reporting (reporting bias) | Unclear risk | No prepublished protocol available. |
| Selection bias | Low risk | Identical baseline data of the study groups due to cross‐over design. |
Portenoy 2012.
| Study characteristics | ||
| Methods |
Purpose of the study: to reduce moderate‐to‐severe cancer pain despite stable opioid regimen Study setting: USA, number of study centres not reported Study period: not reported Study design: randomised, double‐blind, placebo‐controlled, parallel group, graded‐dose design Study duration: 5‐ to 14‐day baseline period, a 5‐week double‐blind titration and treatment period, and a poststudy visit after 2 weeks. Maximum duration 9 weeks |
|
| Participants |
Inclusion criteria: active cancer and chronic pain that was moderate or severe despite a stable opioid regimen that could not be made more effective by further opioid dose titration Type of cancer: breast, gastrointestinal, lung, prostate, other Exclusion criteria: receiving long‐term methadone therapy for pain, major psychiatric or cardiovascular disorder, epilepsy, or significant renal or hepatic impairment, pregnancy, lactating or not using adequate contraception, had received or were to receive radiotherapy, chemotherapy or hormonal therapy, usage of marijuana, CB‐based medications or rimonabant within 30 days of study entry unwilling to abstain for the duration of the study Oromucosal spray; THC:CBD extract: THC 2.7 mg and CBD 2.5 mg Low‐dose group: 91 participants; 49.4% men; mean age 59 (SD 12.3) years; Caucasian 73.6%, Black 12.1%, Hispanic 11.0%, Asian 0%, other 3.3%; type of cancer pain: neuropathic (8.8%), somatic (1.1%), visceral (22.0%), mixed (46.2%), bone (22.0%), other (0%); mean pain 5.8 (SD 1.3); daily morphine equivalent maintenance 120 mg/day; daily breakthrough morphine equivalent not reported; previous cannabis use: 12.1% Medium‐dose group: 88 participants; 55.7% men; mean age 59 (SD 13,1) years; Caucasian 84.1%, Black 6.8%, Hispanic 8.0%, Asian 1.1%, other 0%. Type of cancer pain: neuropathic (13.6%), somatic (14.8%), visceral (12.5%), mixed (42.0%), bone (17.0%), other (0%); mean pain 5.8 (SD 1.2); daily morphine equivalent maintenance 120 mg/day; daily breakthrough morphine equivalent not reported; previous cannabis use 12.5% High‐dose group: 90 participants; 53.3% men; mean age 58 (SD 11.2) years; Caucasian 75.6%, Black 11.1%, Hispanic 7.8%, Asian 1.1%, Other 4.4%; type of cancer pain: neuropathic (7.8%), somatic (7.8%), visceral (11.1%), mixed (35.6%), bone (37.8%), other (0%); mean pain 5.8 (SD 1.2); daily morphine equivalent maintenance 180 mg/day; daily breakthrough morphine equivalent not reported; previous cannabis use: 11.1% Placebo: 91 participants, 48.3% men; mean age 56 (SD 12.2) years; Caucasian 75.8%, Black 6.6%, Hispanic 13.2%, Asian 0%, Other 4.4%; type of cancer pain: neuropathic (12.1%), somatic (12.1%), visceral (14.3%), mixed (42.9%), bone (18.7%), other (0%); mean pain 5.7 (SD 1.2); daily morphine equivalent maintenance 120 mg/day; daily breakthrough morphine equivalent not reported; previous cannabis use: 6.6% |
|
| Interventions |
THC/CBD extract 2.7 mg/2.5 mg, oromucosal spray, flexible dosage:low‐dose group: 1–4 actuations/day; medium‐dose group: 6–10 actuations/day, high‐dose group: 11–16 actuations/day Placebo, oromucosal spray: 1–16 actuations/day; flexible dosage Rescue medication: not reported Allowed cotherapies: all opioids typically used for severe cancer pain except for methadone were allowed. |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: PGIC Withdrawal due to adverse events: adverse events as reported by the participant Combined responder: not assessed Pain relief ≥ 30%: BPI‐SF NRS 0–10, daily Pain relief ≥ 50%: BPI‐SF NRS 0–10, daily. Calculated by imputation method. Mean pain intensity: BPI‐SF NRS 0–10, daily Sleep problems: Sleep Disruption Score 0–10, last 24 hours Depression: Montgomery Åsberg Depression Rating Scale Anxiety: not assessed Maintenance opioid therapy dose: assessed, but not reported in detail. Data not suited for meta‐analysis Breakthrough opioid therapy dose: assessed, but not reported in detail. Data not suited for meta‐analysis Withdrawals due to lack of efficacy: not reported Nervous system disorders adverse effects: participant‐reported adverse events Psychiatric disorders adverse effects: participant‐reported adverse events Any serious adverse event: participant‐reported serious adverse events |
|
| Notes |
Funding: in part by the Huntsman Cancer Foundation (S.W.). GW Pharmaceuticals produces nabiximols, which is licenced in Canada as an adjunctive analgesic treatment in adults with advanced cancer. GW Pharmaceuticals and Otsuka funded the study. Conflicts of interest: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer using a block approach. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo was similar colour, no adjustment for taste described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis by last observation carried forward. |
| Selective reporting (reporting bias) | Low risk | NCT00530764. |
| Selection bias | High risk | Number of participants with previous cannabis use double in all nabiximols groups as in placebo groups. |
Staquet 1978a.
| Study characteristics | ||
| Methods |
Purpose of the study: reducing moderate‐to‐severe cancer pain Study setting: 1 university hospital in Belgium Study period: not reported Study design: double‐blind, randomised, placebo‐controlled, cross‐over design Study duration: 1 day each. Regular medication was stopped 3 hours before the intake of the medication |
|
| Participants |
Type of cancer: no details reported Inclusion criteria: continuous moderate‐to‐severe pain for ≥ 3 days at time of admission to study Exclusion criteria: receiving large doses of narcotics, insufficient mental clarity to judge discomfort or relief, serious gastrointestinal pathology, renal and hepatic diseases susceptible to interfere with drug metabolism or excretion Synthetic THC: 30 participants; gender not reported; aged 21–75 years; race not reported; type of cancer pain: not reported; pain medication: not reported; previous cannabis use: not reported |
|
| Interventions |
Synthetic nitrogen‐containing benzopyran derivative (which is a modification of delta‐I‐trans‐THC): 4 mg single dose, fixed dosage PO Codeine: 50 mg single dose, fixed dosage Placebo Rescue medication: none Allowed cotherapies: no details reported |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: not assessed Withdrawal due to adverse events: no details of assessment reported Combined responder: not assessed Pain relief ≥ 30%: not reported; imputation method not applicable because baseline pain scores not reported Pain relief ≥ 50%: not reported; imputation method not applicable because baseline pain scores not reported Mean pain intensity: hourly ratings of the severity of pain (0, absent; 1, mild; 2, moderate; 3, severe) were used to arrive at hourly pain reduction scores. The sum of hourly pain reduction or relief scores for a given 6‐hour observation period (total reduction or relief scores) was used as a basis for statistical analysis. Sleep problems: not assessed Depression: not assessed Anxiety: not assessed Daily maintenance opioid therapy dose: not assessed Daily breakthrough opioid therapy dose: not assessed Withdrawals due to lack of efficacy: not reported Nervous system disorders adverse effects: reports on drowsiness without providing information how the symptoms were assessed Psychiatric disorders adverse effects: not assessed Any serious adverse event: not assessed |
|
| Notes |
Funding: not reported Conflicts of interest: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis. |
| Selective reporting (reporting bias) | Unclear risk | No prepublished study protocol available. |
| Selection bias | Low risk | Identical baseline data of the study groups due to cross‐over design. |
Staquet 1978b.
| Study characteristics | ||
| Methods |
Purpose of the study: reducing moderate‐to‐severe cancer pain Study setting: 1 university hospital in Belgium Study period: not reported Study design: double‐blind, randomised, placebo‐controlled, cross‐over design Study duration: 1 day each. Regular medication was stopped 3 hours before the intake of the medication |
|
| Participants |
Type of cancer: no details reported Inclusion criteria: continuous moderate‐to‐severe pain for ≥ 3 days at time of admission to study Exclusion criteria: receiving large doses of narcotics, insufficient mental clarity to judge discomfort or relief, serious gastrointestinal pathology, renal and hepatic diseases susceptible to interfere with drug metabolism or excretion Synthetic THC: 15 participants; gender not reported; aged 21–75 years; race not reported; type of cancer pain: not reported; pain medication: not reported; previous cannabis use: not reported |
|
| Interventions |
Synthetic nitrogen‐containing benzopyran derivative (modification of delta‐I‐trans‐THC): 4 mg single dose, fixed dosage PO Secobarbital: 50 mg single dose, fixed dosage (not used for comparison, because secobarbital is not used for cancer pain treatment) Placebo Rescue medication: none Allowed cotherapies: no details reported |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: not assessed Withdrawal due to adverse events: no details of assessment reported Combined responder: not assessed Pain relief ≥ 30%: not reported; imputation method not applicable because baseline pain scores not reported Pain relief ≥ 50%: not reported; imputation method not applicable because baseline pain scores not reported Mean pain intensity: hourly ratings of the severity of pain (0, absent; 1, mild; 2, moderate; 3, severe) were used to arrive at hourly pain reduction scores. The sum of hourly pain reduction or relief scores for a given 6‐hour observation period (total reduction or relief scores) was used as a basis for statistical analysis Sleep problems: not assessed Depression: not assessed Anxiety: not assessed Daily maintenance opioid therapy dose: not assessed Daily breakthrough opioid therapy dose: not assessed Withdrawals due to lack of efficacy: not reported Nervous system disorders adverse effects: reports on drowsiness without information how the symptoms were assessed Psychiatric disorders adverse effects: not assessed Serious adverse events: not reported |
|
| Notes |
Funding: not reported Conflicts of interest: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis. |
| Selective reporting (reporting bias) | Unclear risk | No prepublished study protocol available. |
| Selection bias | Low risk | Identical baseline data of the study groups due to cross‐over design. |
Turcott 2018.
| Study characteristics | ||
| Methods |
Purpose of the study: improvement of appetite, nutritional status and quality of life in people with lung cancer undergoing chemotherapy or targeted therapy Study setting: 1 outpatient clinic at the National Institute of Cancer Mexico Study period: December 2013 to December 2015 Study design: double‐blind, randomised, placebo‐controlled, parallel‐group design Study duration: no information on baseline period reported, 8 weeks' double‐blind |
|
| Participants |
Type of cancer: histologically confirmed advanced NSCLC Inclusion criteria: adults with histologically confirmed advanced NSCLC, regardless of current therapeutic scheme, with a good performance status (ECOG 0–2), diagnosed with anorexia Exclusion criteria: known allergy or contraindication for receiving CBs, previously received treatment with CBs, and previously received any other pharmacological treatment for anorexia Nabilone: 14 participants; 21% men; mean age 61.1 (SD 10.6) years; race not reported; type of cancer pain: not reported; pain medication: not reported; previous cannabis use: not reported Placebo: 19 participants; 21% men; mean age 52.6 (SD 11.8) years; race not reported; type of cancer pain: not reported; pain medication: not reported; previous cannabis use: not reported |
|
| Interventions |
Nabilone: 1.0 mg/day PO, fixed dosage Placebo: PO, fixed dosage Rescue medication: no details reported Allowed cotherapies: no details reported |
|
| Outcomes |
Proportion of participants reporting no worse than mild pain on treatment at 14 days after start of treatment (typically < 30/100 mm on a 100‐mm VAS or < 3 on an 11‐point NRS): not assessed Patient impression to be much or very much improved: not assessed Withdrawal due to adverse events: no details of assessment reported Combined responder: not assessed Pain relief ≥ 30%: VAS 0–100, time frame not reported; calculated by imputation method Pain relief ≥ 50%: VAS 0–100, time frame not reported; calculated by imputation method Mean pain intensity: VAS 0–100, time frame not reported Sleep problems: EORTC‐QLQ‐C30 and EORTC‐QLQ‐LC13. Scores for the multi‐item functional, symptom scales and the single‐item scales were calculated using a linear transformation of raw scores to produce a range from 0 to 100, as described by EORTC. Subscale Insomnia Depression: not assessed Anxiety: not assessed Daily maintenance opioid therapy dose: not assessed Daily breakthrough opioid therapy dose: not assessed Withdrawals due to lack of efficacy: not reported Nervous system disorders adverse effects: not assessed Psychiatric disorders adverse effects: not assessed Any serious adverse event: not reported |
|
| Notes |
Funding: nabilone and placebo were donated by Valeant pharmaceutical without any further participation in the trial. Conflicts of interest: authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as outlined in NCT02802540 |
| Selection bias | High risk | Significant differences between nabilone and control group with regard to ECOG status and age at baseline, |
BPI‐SF: Brief Pain Inventory – Short Form; CB: cannabinoid; CBD: cannabidiol; EORTC‐QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaires – Cancer; EORTC‐QLQ‐LC: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaires – Lung Cancer; ESAS: Edmonton Symptom Assessment Scale; ITT: intention to treat; NRS: Numeric Rating Scale; NSAID: non‐steroidal anti‐inflammatory drug; NSCLC: non‐small cell lung cancer; PGIC: Patient Global Impression of Change; PO: oral; SD: standard deviation; THC: tetrahydrocannabinol; TSDS: Total Symptom Distress Score; VAS: Visual Analogue Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Zylla 2021 | 30 participants with stage IV cancer requiring opioid randomised 1:1 to early cannabis (15 participants) vs delayed start cannabis (15 participants) for 12 weeks. No control group with placebo or other non‐cannabis based medication. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12619001534178.
| Study name | A phase I/II double‐blind, randomised controlled trial assessing effect of medicinal cannabis on quality of life and symptom control in advanced cancer |
| Methods | Phase 1/2 Purpose: treatment Allocation: randomised controlled trial Blinding: yes Assignment: parallel Type of endpoint: safety/efficacy |
| Participants | People with incurable advanced cancer, of any histological subtype; target sample size: 116 |
| Interventions | CGL002 (medicinal cannabis) vs placebo CGL002 2.5–30 mg administered 1–3 times a day, oral liquid administered via syringe, daily for a maximum of 168 days/6 months. |
| Outcomes |
Primary outcomes Phase 1 (composite)
Phase 2
Secondary outcome Phase 1
Phase 2
|
| Starting date | 25 September 2020 |
| Contact information | Name: Dr Jodie Palmer Address: Olivia Newton‐John Cancer Research Institute Level 5, ONJWRC 145 Studley Road Heidelberg VIC 3084 Australia Telephone: +61 3 9496 3573 E‐mail: trials@onjcri.org.au |
| Notes | Funding: Victorian Cancer Agency |
ACTRN12621001302842.
| Study name | NanaBis™ an oro‐buccal administered delta9‐tetrahydrocannabinol (d9‐THC) and cannabidiol (CBD) medicine for the management of chronic pain from metastatic bone cancer |
| Methods | Phase 3 Purpose: treatment Allocation: randomised controlled trial Blinding: yes Assignment: parallel Type of endpoint: safety/efficacy |
| Participants | Metastatic bone pain from a cancer diagnosis as the only major cause of pain Target sample size: 360 |
| Interventions | NanaBis vs placebo or oxycodone NanaBis: a nanoparticle water soluble equimolar solution of delta 9‐THC and CBD administered through the oro‐buccal membrane. 1 dose is equivalent to 2 actuations of the pump delivering 280 µL volume containing delta 9‐THC 2.5 mg and CBD 2.5 mg. The dose administered will be 2 to 3.5 doses (2–7 sprays) per 4 hours unless asleep. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 1 October 2021 |
| Contact information | Name: Mrs Larah Hall Address: Medlab Clinical Ltd, Unit 5‐6/11 Lord St, Botany, NSW Australia 2019 Australia Telephone: +61 02 8188 0311 Email: larah_hall@medlab.co |
| Notes | Funding: Medlab Clinical Ltd |
EudraCT 001382‐32.
| Study name | A double‐blind, randomized phase 1/2 study to assess the efficacy and safety of BCT‐521 versus placebo for pain associated with cancer in patients already receiving standard of care treatment with opioids |
| Methods | Phase 1/2 Purpose: treatment Allocation: randomised controlled trial Blinding: yes Assignment: parallel Type of endpoint: safety/efficacy |
| Participants | Any type of active cancer at any stage; cancer‐related pain that is not wholly alleviated with their current opioid treatment and whose mean pain NRS score over 24 hours is ≥ 4 but < 8 during the last 3 days of screening, with ≤ 2‐point difference between the highest and lowest scores, with all scores remaining between 4 and 8; target sample size 173 |
| Interventions | CBD 3.5 mg/THC 2.5 mg soft capsules twice a day vs placebo up to 5 weeks |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 26 November 2019 |
| Contact information | Beckley Canopy Therapeutics Ltd; Head of Clinical Operations Address: Beckley Park, Oxford, UK Telephone: +44 7545 923519 E‐mail: kalpana@beckley‐canopy.com |
| Notes | Funding: Beckley Canopy Therapeutics Ltd |
Hardy 2020.
| Study name | Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double‐blind, placebo‐controlled, randomised clinical trial of efficacy and safety of 1:1 delta‐9‐tetrahydrocannabinol (THC) and cannabidiol (CBD) |
| Methods | Phase 2 Purpose: treatment Allocation: randomised controlled trial Blinding: yes Assignment: parallel Type of endpoint: safety/efficacy |
| Participants | People with advanced histologically confirmed cancer (metastatic or locally advanced) known to the palliative care team of the recruiting centre; target sample size 144 participants |
| Interventions | THC/CBD 10 mg/mL oral oily liquid (dose range 2.5 mg/2.5 mg–30 mg/30 mg/day) vs placebo |
| Outcomes |
Primary outcome Change from baseline of total ESAS TSDS (assessed at baseline and day 14) Secondary outcomes
|
| Starting date | 9 September 2019 |
| Contact information |
Name: Ms Georgie Cupples Address: Clinical Trial Coordinator Palliative and Supportive Care Mater Misericordiae Ltd Raymond Terrace South Brisbane, Qld 4101 Australia Telephone: +61 7 3163 6057 Email: Georgie.Cupples@mater.org.au |
| Notes | Funding: National Health and Medical Research Council (NHMRC)/Medical Research Future Fund (MRFF) and Mater Misericordiae Ltd |
NCT04042545.
| Study name | Safety and efficacy of inhaled cannabis for the uncontrolled pain relief in patients with advanced cancer (PLENITUDE) |
| Methods | Phase 2 Allocation: randomised Primary purpose: treatment Blinding: participant, care provider, investigator, outcomes assessor Assignment: parallel |
| Participants | 78 participants with uncontrolled cancer pain relief |
| Interventions | 1 cannabis dosing capsule inhaled 3 times a day with a vaporiser device vs 1 placebo dosing capsule inhaled 3 times a day with a vaporiser device |
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | 30 July 2020 |
| Contact information | Contact: Suzanne Sisley, MD Scottsdale Research Institute Address: Cave Creek, Arizona, 85331, USA |
| Notes | Sponsors and collaborators: Tetra Bio‐Pharma |
BPI: Brief Pain Inventory; CBD: cannabidiol; CR: controlled release; CTCAE: Common Terminology Criteria for Adverse Events; delta 9‐THC: delta 9‐tetrahydrocannabinol; DASS‐21: 21‐item Depression, Anxiety and Stress Scale; EORTC‐QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaires – Cancer; EORTC QLQ PAL‐Q30/EORTC‐QLQ‐C15‐PAL: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Palliative; ESAS TSDS: Edmonton Symptom Assessment System Total Symptom Distress Score; ESAS‐r‐CS: Revised Edmonton Symptom Assessment System – Core Symptoms; NPRS: Numeric Pain Rating Scale; NRS: Numeric Rating Scale; OME: oral morphine equivalent; UKU: Udvalg for Kliniske Undersøgelser.
Differences between protocol and review
We made the following changes from our protocol (Häuser 2022).
We changed the order of the secondary outcomes with outcomes of efficacy first and outcomes of tolerability and safety second.
The protocol required a minimum of 20 participants per arm because of growing evidence of bias in small studies (Dechartres 2014; Moore 1998). We amended this into 10 participants per treatment arm in order to review all available information and maximise results.
The outcome "proportion of participants reporting a pain relief of 30% or greater and overall opioid use reduced or stable compared to baseline" could not be included in the summary of findings table because no study analysed this outcome. Instead, we included the outcome "mean pain intensity" in the summary of findings table.
A search in the IACM databank was not possible because it was no longer accessible.
Contributions of authors
All authors participated in writing the protocol.
WH developed the search strategy together with Joanne Abbott (PaPaS Information Specialist).
EF, LR, RFB, PW and WH selected studies for inclusion and extracted data from the studies.
RFB and WH assessed risk of bias.
PW and WH entered data into Review Manager 5 and carried out the analyses.
EF, RAM and WH rated the certainty of the body of evidence.
All review authors interpreted the analysis.
WH drafted the final review.
All authors commented on the draft.
Sources of support
Internal sources
None, Other
External sources
-
National Institute for Health Research (NIHR), UK
Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS)
Declarations of interest
WH was a member of the PaPaS Editorial Board and had no input into the editorial decisions or processes for this review. WH is treating people with cannabis‐based medicines.
PW is treating people with cannabis‐based medicines.
EF was a member of the PaPaS Editorial Board and had no input into the editorial decisions or processes for this review.
LR is treating people with cannabis‐based medicines.
RFB: none.
RAM was a member of the PaPaS Editorial Board and had no input into the editorial decisions or processes for this review.
New
References
References to studies included in this review
Côté 2016 {published data only}
- Côté M, Trudel M, Wang C, Fortin A. Improving quality of life with nabilone during radiotherapy treatments for head and neck cancers: a randomized double-blind placebo-controlled trial. Annals of Otology, Rhinology, and Laryngology 2016;125(4):317-24. [DOI] [PubMed] [Google Scholar]
Fallon 2017a {published data only}
- Fallon MT, Albert Lux E, McQuade R, Rossetti S, Sanchez R, Sun W, et al. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. British Journal of Pain 2017;11:119-33. [DOI: 10.1177/2049463717710042] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fallon 2017b {published and unpublished data}
- Fallon MT, Albert Lux E, McQuade R, Rossetti S, Sanchez R, Sun W, et al. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. British Journal of Pain 2017;11:119-33. [DOI: 10.1177/2049463717710042] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardy 2023 {published data only}
- Hardy J, Greer R, Hugegett G, Kearney A, Gurgenic T, Good P. Phase IIb randomized, placebo-controlled, dose-escalating, double-blind study of cannabidiol oil for the relief of symptoms in advanced cancer (MedCan1-CBD). Journal of Clinical Oncology 2023;41(7):1444-52. [DOI: 10.1200/JCO.22.] [DOI] [PubMed] [Google Scholar]
Jochimsen 1978 {published data only}
- Jochimsen PR, Lawton RL, VerSteeg K, Noyes R Jr. Effect of benzopyranoperidine, a delta-9-THC congener, on pain. Clinical Pharmacology & Therapeutics 1978;24:223-7. [DOI: 10.1002/cpt1978242223] [DOI] [PubMed] [Google Scholar]
Johnson 2010 {published data only}
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. Journal of Pain and Symptom Management 2010;39:167-79. [DOI: 10.1016/j.jpainsymman.2009.06.008] [DOI] [PubMed] [Google Scholar]
Lichtman 2018 {published and unpublished data}
- Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W, et al. Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. Journal of Pain and Symptom Management 2018;55(2):179-88.e1. [DOI] [PubMed] [Google Scholar]
Lynch 2014 {published data only}
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. Journal Pain and Symptom Management 2014;47:166-73. [DOI: 10.1016/j.jpainsymman.2013.02.018] [DOI] [PubMed] [Google Scholar]
Noyes 1975a {published data only}
- Noyes R Jr, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clinical Pharmacology & Therapy 1975;18:84-9. [DOI: 10.1002/cpt197518184] [DOI] [PubMed] [Google Scholar]
Noyes 1975b {published data only}
- Noyes R Jr, Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. Journal of Clinical Pharmacology 1975;15:139-43. [DOI: 10.10.1002/j.1552-4604.1975.tb02348.x] [DOI] [PubMed] [Google Scholar]
Portenoy 2012 {published and unpublished data}
- Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. Journal of Pain 2012;13:438-49. [DOI: 10.1016/j.jpain.2012.01.003] [DOI] [PubMed] [Google Scholar]
Staquet 1978a {published data only}
- Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clinical Pharmacology & Therapeutics 1978;23:397-401. [DOI: 10.1002/cpt1978234397] [DOI] [PubMed] [Google Scholar]
Staquet 1978b {published data only}
- Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clinical Pharmacology & Therapeutics 1978;23:397-401. [DOI: 10.1002/cpt1978234397] [DOI] [PubMed] [Google Scholar]
Turcott 2018 {published data only}
- Turcott JG, Del Rocío Guillen Núñez M, Flores-Estrada D, Oñate-Ocaña LF, Zatarain-Barrón ZL, Barrón F, et al. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: a randomized, double-blind clinical trial. Support Care Cancer 2018;26(9):3029-38. [DOI: 10.1007/s00520-018-4154-9] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Zylla 2021 {published data only}
- Zylla DM, Eklund J, Gilmore G, Gavenda A, Guggisberg J, VazquezBenitez G, et al. A randomised trial of medical cannabis in patients with stage IV cancers to assess feasibility, dose requirements, impact on pain and opioid use, safety, and overall patient satisfaction. Supportive Care in Cancer 2021;29(12):7471-8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12619001534178 {published data only}trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12619001534178
- ACTRN12619001534178. A phase I/II double-blind, randomised controlled trial assessing effect of medicinal cannabis on quality of life and symptom control in advanced cancer. anzctr.org.au/ACTRN12619001534178.aspx (first received 30 September 2019). [anzctr.org.au/ACTRN12619001534178.aspx]
ACTRN12621001302842 {published data only}trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12621001302842
- ACTRN12621001302842. NanaBis™ an oro-buccal administered delta9-tetrahydrocannabinol (d9-THC) and cannabidiol (CBD) medicine for the management of chronic pain from metastatic bone cancer. anzctr.org.au/ACTRN12621001302842.aspx (first received 14 April 2021). [anzctr.org.au/ACTRN12621001302842.aspx]
EudraCT 001382‐32 {published data only}
- EudraCT 001382-32. A double-blind, randomized phase 1/2 study to assess the efficacy and safety of BCT-521 versus placebo for pain associated with cancer inpatients already receiving standard of care treatment with opioids. www.clinicaltrialsregister.eu/ctr-search/trial/2019-001382-32/PL/ (first received 1 August 2019).
Hardy 2020 {published data only}
- Hardy J, Haywood A, Gogna G, Martin J, Yates P, Greer R, et al. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo-controlled, randomised clinical trial of efficacy and safety of 1:1 delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Trials 2020;21:611. [DOI: 10.1186/s13063-020-04541-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04042545 {published data only}
- NCT04042545. Safety and efficacy of inhaled cannabis for the uncontrolled pain relief in patients with advanced cancer (PLENITUDE). clinicaltrials.gov/ct2/show/NCT04042545 (first received 2 August 2019). [clinicaltrials.gov/ct2/show/NCT04042545]
Additional references
Abuhasira 2018
- Abuhasira R, Shbiro L, Landschaft Y. Medical use of cannabis and cannabinoids containing products – regulations in Europe and North America. European Journal of Internal Medicine 2018;49:2-6. [DOI: 10.1016/j.ejim.2018.01.001] [DOI] [PubMed] [Google Scholar]
Andersohn 2008
- Andersohn F, Garbe E. Pharmacoepidemiological research with large health databases. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2008;51:1135-55. [DOI: 10.1007/s00103-008-0648-9] [DOI] [PubMed] [Google Scholar]
Aviram 2017
- Aviram J, Samuelly-Leichtag G. Efficacy of cannabis based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician 2017;20:E755-96. [PMID: 28934780] [PubMed] [Google Scholar]
Aviram 2020
- Aviram J, Lewitus GM, Vysotski Y, Uribayev A, Procaccia S, Cohen I, et al. Short-term medical cannabis treatment regimens produced beneficial effects among palliative cancer patients. Pharmaceuticals (Basel) 2020;13:435. [DOI: 10.3390/ph13120435] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bar‐Lev Schneider 2018
- Bar-Lev Schleider L, Mechoulam R, Lederman V, Hilou M, Lencovsky O, Betzalel O, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. European Journal of Internal Medicine 2018;49:37-43. [DOI: 10.1016/j.ejim.2018.01.023] [DOI] [PubMed] [Google Scholar]
Basch 2014
- Basch E, Trentacosti AM, Burke LB, Kwitkowski V, Kane RC, Autio KA, et al. Pain palliation measurement in cancer clinical trials: the US Food and Drug Administration perspective. Cancer 2014;106:dju244. [DOI: 10.1093/jnci/dju244] [DOI] [PubMed] [Google Scholar]
Blake 2017
- Blake A, Wan BA, Malek L, DeAngelis C, Diaz P, Lao N, et al. A selective review of medical cannabis in cancer pain management. Annals Palliative Medicine 2017;6:S215-22. [DOI: 10.21037/apm.2017.08.05] [DOI] [PubMed] [Google Scholar]
Boland 2020
- Boland EG, Bennett MI, Allgar V, Boland JW. Cannabinoids for adult cancer-related pain: systematic review and meta-analysis. BMJ Supportive and Palliative Care 2020;10:14-24. [DOI: 10.1136/bmjspcare-2019-002032] [DOI] [PubMed] [Google Scholar]
Chaparro 2013
- Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004959. [DOI: 10.1002/14651858.CD004959.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale (NJ): Lawrence Erlbaum Associates, 1988. [Google Scholar]
Corli 2016
- Corli O, Floriani I, Roberto A, Montanari M, Galli F, Greco MT, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV "real life" trial on the variability of response to opioids. Annals of Oncology 2016;27:1107-15. [DOI: 10.1093/annonc/mdw097] [DOI] [PubMed] [Google Scholar]
De Vries 2014
- De Vries M, Rijckevorsel DC, Wilder-Smith OH, Goor H. Dronabinol and chronic pain: importance of mechanistic considerations. Expert Opinion on Pharmacotherapy 2014;15:1525-34. [DOI: 10.1517/14656566.2014.918102] [DOI] [PubMed] [Google Scholar]
Dechartres 2014
- Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA 2014;312:623-30. [DOI: 10.1001/jama.2014.8166] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31:140-9. [DOI: 10.1093/ije/31.1.140] [DOI] [PubMed] [Google Scholar]
European Medicines Agency 2019
- European Medicines Agency. Epydiolex (2019). Available from www.ema.europa.eu/en/medicines/human/EPAR/epidyolex (accessed 21 June 2021).
Fayers 2014
- Fayers PM, Hays RD. Don't middle your MIDs: regression to the mean shrinks estimates of minimally important differences. Quality of Life Research 2014;23:1-4. [DOI: 10.1007/s11136-013-0443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2021
- Fisher E, Moore RA, Fogarty AE, Finn DP, Finnerup NB, Gilron I, et al. Cannabinoids, cannabis, and cannabis-based medicine for pain management: a systematic review of randomised controlled trials. Pain 2021;162(Suppl 1):S45-66. [DOI: 10.1097/j.pain.0000000000001929] [DOI] [PubMed] [Google Scholar]
Fitzcharles 2019
- Fitzcharles MA, Niaki OZ, Hauser W, Hazlewood G, Canadian Rheumatology Association. Position statement: a pragmatic approach for medical cannabis and patients with rheumatic diseases. Journal of Rheumatology 2019;46:532-8. [DOI: 10.3899/jrheum.181120] [DOI] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta-analyses. International Clinical Psychopharmacology 2005;20:49-52. [DOI: 10.1097/00004850-200501000-00010] [DOI] [PubMed] [Google Scholar]
GW Pharmaceuticals 2020
- GW Pharmaceuticals. Sativex oromucosal spray. www.medicines.org.uk/emc/product/602/smp (accessed prior to 3 May 2023).
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020a
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from handbook: training.cochrane.org/handbook/archive/v6.1.
Higgins 2021
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Hillard 2012
- Hillard CJ, Weinlander KM, Stuhr KL. Contributions of endocannabinoid signalling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience 2012;204:207-29. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Häuser 2018
- Häuser W, Finn DP, Kalso E, Krcevski-Skvarc N, Kress HG, Morlion B, et al. European Pain Federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. European Journal of Pain 2018;22:1547-64. [DOI: 10.1002/ejp.1297] [DOI] [PubMed] [Google Scholar]
Häuser 2019
- Häuser W, Welsch P, Klose P, Radbruch L, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain. A systematic review with meta-analysis of randomised controlled trials. Schmerz 2019;33:424-36. [DOI: 10.1007/s00482-019-037] [DOI] [PubMed] [Google Scholar]
International Council for Harmonisation 2020
- International Council for Harmonisation. Medical dictionary for regulatory activities version 23.1 (2020). Available from www.meddra.org/how-to-use/support-documentation/english (accessed 21 June 20217).
Kalant 2001
- Kalant H. Medicinal use of cannabis: history and current status. Pain Research & Management 2001;6:80-91. [DOI: 10.1155/2001/469629] [DOI] [PubMed] [Google Scholar]
Kleckner 2019
- Kleckner AS, Kleckner IR, Kamen CS, Tejani MA, Janelsins MC, Morrow GR, et al. Opportunities for cannabis in supportive care in cancer. Therapeutic Advances in Medical Oncology 2019;11:1758835919866362. [DOI: 10.1177/1758835919866362] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krcevski‐Skvarc 2018
- Krcevski-Skvarc N, Wells C, Häuser W. Availability and approval of cannabis-based medicines for chronic pain management and palliative/supportive care in Europe: a survey of the status in the chapters of the European Pain Federation. European Journal of Pain 2018;22:440-54. [DOI: 10.1002/ejp.1147] [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee MC, Ploner M, Wiech K, Bingel U, Wanigasekera V, Brooks J, et al. Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain 2013;134:123-34. [DOI: 10.1016/j.pain.2012.09.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martell 2018
- Martell K, Fairchild A, LeGerrier B, Sinha R, Baker S, Liu H, et al. Rates of cannabis use in patients with cancer. Current Oncology 2018;25:219-25. [DOI: 10.3747/co.25.3983] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An Evidence-Based Resource for Pain Relief. Oxford (UK): Oxford University Press, 1998. [ISBN:0–19–263048–2] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLOS Medicine 2009;6:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything – large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78:209-16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: Systematic Reviews in Pain Research: Methodology Refined. Washington (DC): IASP Press, 2008:15-24. [ISBN: 978–0–931092–69–5] [Google Scholar]
Moore 2013
- Moore RA, Straube S, Aldington D. Pain measures and cut-offs – no worse than mild pain' as a simple, universal outcome. Anesthesia 2013;68:400-12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2021
- Moore RA, Fisher E, Finn DP, Finnerup NB, Gilron I, Haroutounian S, et al. Cannabinoids, cannabis, and cannabis-based medicines for pain management: an overview of systematic reviews. Pain 2021;62(Suppl 1):S67-79. [DOI: 10.1097/j.pain.0000000000001941] [DOI] [PubMed] [Google Scholar]
Oregon Health Authority 2022
- Oregon Health Authority. Opioid calculator. www.oregonpainguidance.org/opioidmedcalculator/ (accessed prior to 3 May 2023).
Owens 2015
- Owens B. Drug development: the treasure chest. Nature 2015;525:S6-8. [DOI: 10.1038/525S6a] [DOI] [PubMed] [Google Scholar]
Radbruch 2020
- Radbruch L, Häuser W. Cannabidiol. Schmerz 2020;34:115-6. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Elie AE, Skoetz N, et al. Chapter 14. Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from handbook: training.cochrane.org/handbook/archive/v6.1.
Soliman 2019
- Soliman N, Hohmann AG, Haroutounian S, Wever K, Rice ASC, Finn D. A protocol for the systematic review and meta-analysis of studies in which cannabinoids were tested for antinociceptive effects in animal models of pathological or injury-related persistent pain. Pain Reports 2019;4:e766. [DOI: 10.1097/PR9.000000000000076] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockings 2018
- Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, et al. Cannabis and cannabinoids for the treatment of people with chronic non-cancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 2018;158:1932-54. [10.1097/j.pain.0000000000001293] [DOI] [PubMed] [Google Scholar]
Swarm 2019
- Swarm RA, Paice JA, Anghelescu DL, Are M, Bruce JY, Buga S. Adult Cancer Pain, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2019;17:977-1007. [DOI: 10.6004/jnccn.2019.0038] [DOI] [PubMed] [Google Scholar]
van den Beuken‐van Everdingen 2016
- den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. Journal of Pain and Symptom Management 2016;51:1070-90. [DOI: 10.1016/j.jpainsymman.2015.12.340] [DOI] [PubMed] [Google Scholar]
Vyas 2018
- Vyas MB, LeBaron VT, Gilson AM. The use of cannabis in response to the opioid crisis: a review of the literature. Nursing Outlook 2018;66:56-65. [DOI: 10.1016/j.outlook.2017.08.012] [DOI] [PubMed] [Google Scholar]
Wang 2021
- Wang L, Hong PJ, May C, Rehman Y, Oparin Y, Hong CJ, et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: a systematic review and meta-analysis of randomised clinical trials. BMJ 2021;374:n1034. [DOI: 10.1136/bmj.n1034] [DOI] [PubMed] [Google Scholar]
WHO 2019
- World Health Organization. WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents, 2019. Available from www.who.int/ncds/management/palliative-care/cancer-pain-guidelines/en (accessed 21 June 2021). [PubMed]
WHO 2021
- World Health Organization. Cancer, 2021. Available from www.who.int/health-topics/cancer#tab=tab_1 (accessed 21 June 2021).
Wiffen 2017
- Wiffen PJ, Wee B, Derry S, Bell RF, Moore RA. Opioids for cancer pain – an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD012592. [DOI: 10.1002/14651858.CD012592] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 2019
- Ye L, Cao Z, Wang W, Zhou N. New insights in cannabinoid receptor structure and signaling. Current Molecular Pharmacology 2019;12:239-48. [DOI: 10.2174/1874467212666190215112036] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Häuser 2022
- Häuser W, Welsch P, Radbruch L, Fisher E, Bell RF, Moore RA. Cannabis-based medicines and medical cannabis for adults with cancer pain. Cochrane Database of Systematic Reviews 2022, Issue 2. Art. No: CD014915. [DOI: 10.1002/14651858.CD014915] [DOI] [PMC free article] [PubMed] [Google Scholar]


