Visual Abstract
Keywords: 68Ga-PSMA PET, RTOG, prostate cancer, prostate bed recurrence, salvage radiation
Abstract
The aim of this study was to analyze the patterns of prostate bed (PB) recurrence in prostate cancer patients experiencing prostate-specific antigen (PSA) persistence (BCP) or biochemical recurrence (BCR) after radical prostatectomy using 68Ga-PSMA-11 PET/CT (68Ga-PSMA PET) in relation to the Radiation Therapy Oncology Group (RTOG) clinical target volumes (CTVs). Methods: This single-center, retrospective analysis included patients with BCP or BCR after radical prostatectomy and PB recurrence on 68Ga-PSMA PET. The PB recurrences were delineated by nuclear medicine physicians, the CTVs by radiation oncologists contouring guidelines on the 68Ga-PSMA PET, respectively, masked from each other. The coverage of the 68Ga-PSMA PET recurrence was categorized as PSMA recurrence completely covered, partially covered, or not covered by the RTOG-based CTV. Further, we evaluated the differences in PSMA recurrence patterns among patients with different 68Ga-PSMA PET staging (miTNM). Mann–Whitney U tests, the chi-square test, and Spearman (ρ) correlation analysis were used to investigate associations between CTV coverage and 68Ga-PSMA PET–based tumor volume, serum PSA levels, miTNM, and rectal/bladder involvement. Results: A total of 226 patients were included in the analysis; 127 patients had PSMA recurrence limited to the PB (miTrN0M0), 30 had pelvic nodal disease (miTrN1M0), 32 had extrapelvic disease (miTrN0M1), and 37 had both pelvic nodal disease and extrapelvic disease (miTrN1M1). In the miTrN0M0 cohort, the recurrence involved the rectal and bladder walls in 12 of 127 (9%) and 4 of 127 (3%), respectively. The PSMA-positive PB recurrences were completely covered by the CTV in 68 of 127 patients (53%), partially covered in 43 of 127 (34%), and not covered in 16 of 127 (13%). Full coverage was associated with a smaller tumor volume (P = 0.043), a lack of rectal/bladder wall involvement (P = 0.03), and lower miTNM staging (P = 0.035) but not with lower serum PSA levels (P = 0.979). Conclusion: Our study suggests that 68Ga-PSMA PET can be a valuable tool for guiding salvage radiation therapy (SRT) planning directed to the PB in the setting of postoperative BCR or BCP. These data should be incorporated into the redefinition of PB contouring guidelines.
Approximately one-third of patients undergoing radical prostatectomy for prostate cancer will experience disease progression within 10 y (1–3). Postoperative radiotherapy to the prostate bed (PB) is a potential curative treatment option either in the presence of high-risk factors for local recurrence (adjuvant radiotherapy) or in patients with biochemical and clinical evidence of local recurrence (4).
The definition of the clinical target volume (CTV) for PB radiation has been guided by contouring guidelines mostly based on expert consensus rather than strict anatomic patterns of local recurrence (5–8). In the past decade, PET targeting the prostate specific membrane antigen (PSMA PET) has emerged as an accurate and specific imaging tool for the evaluation of patients experiencing biochemical recurrence (BCR) of prostate cancer (9). Particularly after the FDA approval of the first PSMA-targeting PET radiopharmaceutical in December 2020 (10), 68Ga-PSMA PET has been increasingly implemented in this clinical setting (11) and can serve as an anatomic guide for salvage treatments. Therefore, new imaging modalities like 68Ga-PSMA PET could provide important information for a redefinition of the salvage radiation therapy (SRT) contouring guidelines.
Herein we present a detailed analysis of local recurrences detected by 68Ga-PSMA-11 PET/CT (68Ga-PSMA PET) in men with prostate cancer experiencing prostate-specific antigen (PSA) persistence (BCP) or biochemical recurrence (BCR) after radical prostatectomy. In addition, we assessed the location of these lesions in relation to the CTV recommendations by the Radiation Therapy Oncology Group (RTOG) contouring guidelines (5).
MATERIALS AND METHODS
Patients
We retrospectively screened all 68Ga-PSMA PET scans acquired in our nuclear medicine clinic at UCLA between November 2016 and November 2020 as part of 2 prospective clinical studies enrolling patients with BCR or BCP (hereafter indicated as BCR) of prostate cancer after radical prostatectomy (NCT02940262, NCT03582774). Men with prostate cancer treated with radical prostatectomy undergoing 68Ga-PSMA PET for BCR were included in our analysis if their clinical report described a recurrence in the PB. BCR was defined as a PSA of 0.2 ng/mL or more measured more than 6 wk after prostatectomy (9). The flowchart in Figure 1 shows the patient selection process. Patients’ clinical history and clinical data were collected from electronic medical records. This retrospective analysis was approved by the Ethics Committee (UCLA IRB#20–001948), which waived the necessity for study specific consent.
FIGURE 1.
Flowchart of screening process.
68Ga-PSMA PET
The PSMA-targeting ligand used for 68Ga-PSMA PET was 68Ga-PSMA-11 (Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)]) (12). Images were acquired using a 64-detector PET/CT scanner (2007 Biograph 64 Truepoint or 2010 Biograph mCT 64; Siemens). A diagnostic CT scan (200–240 mAs, 120 kV) was obtained after intravenous or oral contrast administration, unless contraindicated. A whole-body scan was acquired from pelvis to vertex before a dedicated postvoid pelvic scan. The latter was not used for the analysis. All PET images were reconstructed with corrections for attenuation, dead-time, random events, and scatter, using iterative ordered-subsets expectation. The time per bed position was based on patient weight (13).
68Ga-PSMA PET Analysis and PB Recurrence Contouring
The clinical 68Ga-PSMA PET reports were used to screen patients who had a suspected PB recurrence after radical prostatectomy. The description of the miTNM staging on the clinical reports was confirmed by 1 investigator (KN) who retrospectively reviewed all 68Ga-PSMA PET scans. After confirming the presence of a PB recurrence on 68Ga-PSMA PET, 2 board certified nuclear medicine physicians (IS and MB) manually delineated the PB lesions according to the 68Ga-PSMA PET procedure guidelines (14) using the 2- and 3-dimensional brush tools on MIM v 7.7.5 (MIM Software Inc.). A third nuclear medicine physician (JC) was involved in cases deemed difficult and a consensus was achieved between the 3 readers. 68Ga-PSMA PET–based lesion volumes were recorded.
PSMA PET scans were visualized using a default upper SUVmax threshold of 5. Because of the variable intensity of PSMA uptake by different lesions and interference of physiologic urinary bladder uptake, often obscuring visualization of recurrences in its proximity, readers used manual adjustments of the SUVmax window thresholds based on each individual case, to help distinguish physiologic from pathologic uptake (14). Whenever possible and deemed useful by the readers, the fused CT images were used to facilitate lesion delineation.
The CT component of 68Ga-PSMA PET was used to assess the relation between the PSMA-based recurrence and significant anatomic structures (i.e., the rectal and bladder wall). Any PSMA-avid lesions overlapping the rectal or bladder wall on CT was described as involving the rectal or bladder wall. The association between rectal or bladder wall involvement and the PSMA recurrences was assessed. The 68Ga-PSMA PET readers described each patient’s disease spread using the Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) criteria and the molecular imaging TNM (miTNM) (15). Briefly, the presence of a PB recurrence was described as Tr, pelvic lymph node involvement as N1, extrapelvic nodal or distant organs involvement as M1, pelvic nodal and extrapelvic disease as N1M1. The miTNM was used for a subanalysis investigating the correlation between the CTV coverage and the 68Ga-PSMA PET–based disease stage.
RTOG-Based CTV Contouring
The CTV contours were delineated on the CT component of each 68Ga-PSMA PET scan by 4 radiation oncologists (SMY, JD, CS, and AUK) masked with regard to the PSMA component of the imaging scan, and to the lesions contoured on 68Ga-PSMA PET. The delineation of the PB CTV followed the recommendations of the RTOG contouring guidelines (5). In summary, the inferior border of the CTV extended inferiorly 8–12 mm from the vesicourethral anastomosis, and the superior border from the level of the caudal vas deferens remnant. The anterior border of the CTV extended to the posterior border of the pubis up to the top of the pubic symphysis, and the posterior border to the anterior rectal wall. Laterally, at the caudal level, the CTV extended to the levator ani muscle. Above the pubic symphysis, 1–2 cm of the bladder wall was included in the CTV; posteriorly, the CTV extended to the mesorectal fascia, and laterally, to the sacro-recto-genitopubic fascia.
Coverage Analysis and Heat Map
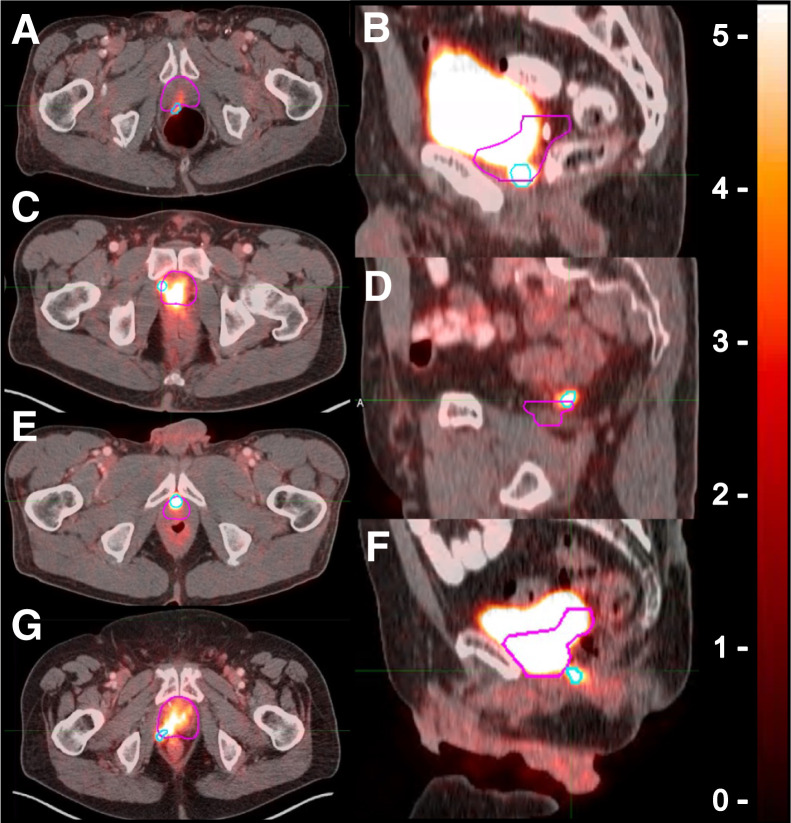
Two of the investigators, a nuclear medicine physician (IS) and a radiation oncologist (ADP), assessed the CTV coverage of the PB 68Ga-PSMA PET–based recurrence in consensus using 3 prespecified outcomes as follows: 68Ga-PSMA PET recurrence completely covered, partially covered, or not covered by the CTV. The 3 outcomes were further combined into: 68Ga-PSMA PET recurrence completely covered by the CTV and not completely covered by the CTV (including the partially covered and not covered by the CTV outcomes). In case the 68Ga-PSMA PET recurrence was not completely covered by the CTV, the border of PB exceeded by the 68Ga-PSMA PET recurrence was described as anterior, posterior, lateral, inferior, superior, and combinations of them. Individual examples of the image analysis findings are shown in Figure 2.
FIGURE 2.
Definition of location of 68Ga-PSMA PET lesion exceeding CTV borders. 68Ga-PSMA PET contours are in light blue, and CTV contours are in pink. 68Ga-PSMA PET lesion extended beyond CTV at posterior (A), inferior (B), lateral (C), superior (D), anterior (E), posteroinferior (F), and posterolateral (G) borders.
A 2-dimensional heat map showing the PB recurrence distribution patterns was created by two of the investigators (DPOC and MC) by mapping all 68Ga-PSMA PET contours onto a template patient’s CT through manual rigid registration for the whole cohort and for each miTNM staging (Fig. 3). A 3-dimensional representation of all recurrences was generated by one of the investigators (ZE) using 3D Slicer (http://www.slicer.org) (16).
FIGURE 3.
Heat map of distribution of prostate bed 68Ga-PSMA PET recurrences in miTrN0Mo cohort (127 patients), mapped on template patient’s anatomy (green contour represents CTV).
As a secondary objective, we conducted a subanalysis evaluating the differences in 68Ga-PSMA PET recurrence patterns among patients with different miTNM staging (i.e., miTrN0M0, miTrN1M0, miTrN0M1, and miTrN1M1).
Statistical Analysis
To assess the association between dichotomized CTV coverage status (fully vs. not fully covered – including the partially covered and not covered outcome) and serum PSA levels we used the Mann–Whitney U test because of the distributional properties of PSA levels. Similarly, we compared PSMA-based tumor volume and rectal/bladder involvement to dichotomized CTV coverage status.
To investigate the association between the miTNM stage and the CTV coverage (e.g., fully, partially, and not covered) we used the chi-square test. Descriptive statistics are expressed as median (IQR), unless stated otherwise. All statistical analyses were conducted using Jamovi version 2.2.5 (The jamovi project (2021) retrieved from https://www.jamovi.org) and P values of less than 0.05 were considered statistically significant.
RESULTS
Patient Population
A total of 2,451 68Ga-PSMA PET scans performed between November 2016 and November 2020 were screened for inclusion criteria. Of the 2,451 scans, 226 (9%) were included in this retrospective analysis. The median (IQR) time interval between radical prostatectomy and 68Ga-PSMA PET was 77 mo (28–128). Table 1 shows the clinical characteristics of all patients and the different cohorts included in the subanalysis. Of 226 patients, 127 (56%) had PB limited disease (miTrN0M0), 30 (13%) were classified as miTrN1M0, 32 (14%) as miTrN0M1, and 37 (16%) as miTrN1M1. The main cohort included 127 patients, whereas patients with spread of disease outside the PB were included in a separate a subanalysis.
TABLE 1.
Clinical and Demographic Characteristics of All Patients and All Cohorts*
| Characteristic | Full cohort (miTrNxMx) | miTrN0M0 cohort | miTrN1M0 cohort | miTrN0M1 cohort | miTrN1M1 cohort | P |
|---|---|---|---|---|---|---|
| No. of patients | 226 | 127 | 30 | 32 | 37 | |
| Median age (y)† | 69.5 (64–73) | 70 (47–89) | 68.5 (63.3–76.3) | 65 (61–74.5) | 72 (68–76) | 0.47‡ |
| Median serum PSA level (ng/mL)† | 1.21 (0.3–0.6) | 1.02 (0.5–2.18) | 1.49 (1–2.25) | 1.5 (0.52–4.21) | 2.8 (1–9.24) | 0.3‡ |
| Median tumor volume (mL)† | 0.88 (0.38–1.35) | 0.72 (0.04–15) | 0.89 (0.44–1.73) | 0. 91 (0.39–1.34) | 1.55 (0.46–4.6) | 0.16‡ |
| NCCN risk group§ | <0.001∥ | |||||
| Low risk | 9 (5) | 8 (6) | 0 (0) | 1 (3) | 0 | |
| Intermediate risk | 82 (44) | 54 (43) | 9 (38) | 132 (41) | 7 (26) | |
| High risk | 52 (28) | 28 (22) | 7 (29) | 10 (34) | 7 (26) | |
| Very high risk | 43 (23) | 16 (13) | 8 (33) | 6 (21) | 13 (48) | |
| Not available | 40 | 21 | 6 | 3 | 10 | |
| Surgical margin involvement§ | 0.08¶ | |||||
| No | 99 (63) | 58 (46) | 16 (84) | 12 (48) | 13 (57) | |
| Yes | 57 (37) | 31 (24) | 3 (16) | 13 (52) | 10 (43) | |
| Not available | 70 | 38 | 11 | 7 | 14 | |
| Outcome§ | 0.35¶ | |||||
| Completely covered by CTV | 107 (46) | 68 (53) | 14 (47) | 11 (34) | 14 (38) | |
| Partially covered by CTV | 91 (41) | 43 (34) | 12 (40) | 17 (53) | 19 (51) | |
| Not covered by CTV | 28 (13) | 16 (13) | 4 (13) | 4 (13) | 4 (11) | |
| Location of recurrence partially or completely exceeding CTV§ | Not applicable | |||||
| Total no. | 119 (100) | 59 (100) | 16 (100) | 21 (100) | 23 (100) | |
| Posterior | 57 (48) | 30 (51) | 5 (31) | 10 (48) | 12 (55) | |
| Posterolateral | 34 (29) | 14 (24) | 8 (50) | 8 (38) | 4 (17) | |
| Posteroinferior | 8 (7) | 3 (5) | 2 (13) | 0 | 3 (13) | |
| Anterior | 1 (1) | 1 (2) | 0 | 0 | 0 | |
| Superior | 4 (3) | 1 (2) | 0 | 2 (10) | 1 (4) | |
| Inferior | 13 (11) | 10 (17) | 1 (6) | 0 | 2 (9) | |
| Lateral | 2 (2) | 0 | 0 | 1 (5) | 1 (4) | |
| Lesion extension§ | 0.23¶ | |||||
| Rectal wall involvement | 19 (8) | 12 (9) | 2 (7) | 1 (3) | 4 (10) | |
| Bladder wall involvement | 10 (4) | 4 (3) | 1 (3) | 0 | 5 (13) |
Location and extension of tumor recurrence on 68Ga-PSMA PET for full cohort and subcohorts. Age and PSA level are at time of PET. Percentages were calculated on basis of total number of patients with data available in selected category.
Values in parentheses are interquartile ranges.
One-way ANOVA.
Reported as number of patients, with percentages in parentheses. NCCN = National Comprehensive Cancer Network.
Spearman (ρ) correlation matrix.
χ2 test.
CTV Coverage of 68Ga-PSMA PET Recurrence
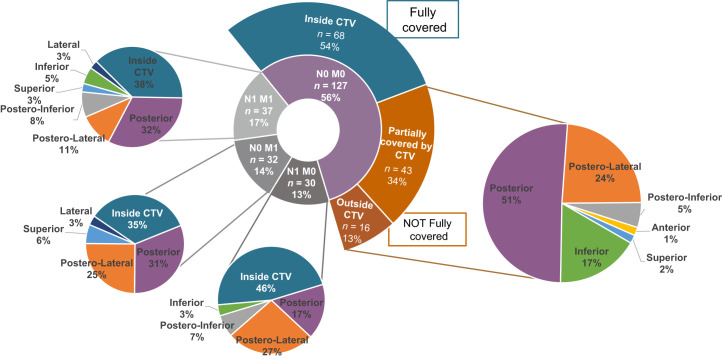
The 68Ga-PSMA PET PB recurrences were fully covered by the CTV in 68 of 127 patients (54%), partially covered in 43 of 127 patients (34%), and not covered in 16 of 127 patients (13%). In the latter 2 groups, the 68Ga-PSMA PET recurrences extended beyond the CTV at the following locations: posteriorly (30/59; 51%), posterolaterally (14/59; 24%), posteroinferiorly (3/59; 5%), anteriorly (1/59; 2%), superiorly (1/59; 2%), and inferiorly (10/59; 17%) (Table 2; Figs. 4 and 5).
TABLE 2.
Clinical Characteristics and Outcome Analysis for miTrN0M0 Cohort*
| Characteristic | miTrN0M0 cohort (n = 127) | Completely covered by CTV (n = 68) | Partially covered by CTV (n = 43) | Not covered by CTV (n = 16) | P |
|---|---|---|---|---|---|
| No. of patients† | 127 (100) | 68 (54) | 43 (34) | 16 (13) | Not applicable |
| Median age (y)‡ | 70 (64–73) | 69.5 (62–72) | 70 (67–75) | 68.5 (66.3–75.3) | 0.14§ |
| Median serum PSA level (ng/mL)‡ | 1.02 (0.5–2.18) | 1.11 (0.5–2.2) | 1.09 (0.46–2.63) | 0.84 (0.62–1.11) | 0.26§ |
| Median tumor volume (mL)‡ | 0.72 (0.38–1.35) | 0.57 (0.36–1.13) | 1.01 (0.49–67) | 0.68 (0.37–0.96) | 0.12§ |
| NCCN risk group∥ | 0.38¶ | ||||
| Low risk | 8 (8) | 7 (10) | 0 (0) | 1 (6) | |
| Intermediate risk | 54 (51) | 26 (38) | 19 (44) | 8 (50) | |
| High risk | 28 (26) | 12 (18) | 12 (28) | 4 (25) | |
| Very high risk | 16 (15) | 9 (13) | 5 (12) | 2 (13) | |
| Not available | 21 | 14 | 7 | 1 | |
| Surgical margin involvement∥ | 0.04# | ||||
| No | 58 (65) | 21 (45) | 20 (69) | 12 (92) | |
| Yes | 31 (35) | 26 (55) | 9 (31) | 1 (8) | |
| Not available | 38 | 21 | 16 | 4 | |
| Location of recurrence partially or completely exceeding CTV∥ | Not applicable | Not applicable | |||
| Total no. | 59 (100) | 43 (100) | 16 (100) | ||
| Posterior | 30 (52) | 25 (58) | 5 (31) | ||
| Posterolateral | 14 (24) | 10 (23) | 4 (25) | ||
| Posteroinferior | 3 (5) | 1 | 2 (13) | ||
| Anterior | 1 (2) | 1 (2) | 0 | ||
| Superior | 1 (2) | 1 (2) | 0 | ||
| Inferior | 10 (14) | 5 (10) | 5 (31) | ||
| Local extension∥ | 0.002# | ||||
| Rectal wall involvement | 12 (9) | 1 (1) | 5 (12) | 6 (38) | |
| Bladder wall involvement | 4 (3) | 4 (6) | 0 | 0 |
Age and PSA level are at time of PET. Percentages were calculated on basis of total number of patients with data available in selected category.
Values in parentheses are percentages of total number of patients.
Values in parentheses are interquartile ranges.
One-way ANOVA.
Reported as number of patients, with percentages in parentheses. NCCN = National Comprehensive Cancer Network.
Spearman (ρ) correlation matrix.
χ2 test.
FIGURE 4.
Three-dimensional rendering of all PB recurrences in relation to RTOG-based CTV (light green), rectum (brown), and urinary bladder (pink) of template patient. PSMA recurrences are in solid colors: green = completely covered, yellow = partially covered, and red = not covered.
FIGURE 5.
Results of coverage analysis and detailed description of location of 68Ga-PSMA PET recurrences exceeding CTV. Two central pie charts show full cohort divided into main cohort (miTrN0M0; purple) and all subcohorts (gray). Full pie charts on sides show location of 68Ga-PSMA PET recurrences exceeding CTV.
Impact of Serum PSA Levels and Tumor Volume on CTV Coverage
The median serum PSA level at time of 68Ga-PSMA PET in the miTrN0M0 cohort was 1.02 ng/mL (IQR, 0.5–2.18; range, 0.10–57.6 ng/mL). In patients with PB recurrences fully covered, partially covered and fully outside the CTV, serum PSA levels were 1.11 ng/mL (0.5–2.2; range, 0.10–28), 1.09 ng/mL (0.46–2.63; range, 0.20–57.63) and 0.84 ng/mL (0.62–1.11; range, 0.23–7.8), respectively.
Thirty-four of 127 patients (27%) had a serum PSA level of less than or equal to 0.5 ng/mL, and 93 of 127 (73%) had a serum PSA level of greater than 0.5 ng/mL. The CTV coverage outcome was not associated with serum PSA levels (P = 0.98) or with PSA levels of less than or equal to 0.5 ng/mL (P = 0.75). In the 34 patients with PSA levels of less than or equal to 0.5 ng/mL, the 68Ga-PSMA PET PB recurrences were fully covered by the CTV in 19 of 34 patients (56%), partially covered in 12 of 34 patients (35%), and not covered in 3 of 34 patients (9%). In the latter 2 groups, the 68Ga-PSMA PET recurrences extended beyond the CTV at the following locations: posteriorly (8/15; 53%), posterolaterally (3/15; 27%), and inferiorly (4/15; 20%).
The median volume of 68Ga-PSMA PET recurrence was 0.72 mL (IQR, 0.04–15; range, 0.04–15). In patients with PB recurrences completely covered, partially covered and not covered by the CTV, tumor volumes were 0.57 mL (0.36–1.13; range, 0.08–11.09), 1.01 mL (0.49–67; range, 0.13–15) and 0.68 mL (0.37–0.96; range, 0.04–4.72), respectively. Complete CTV coverage was significantly associated to smaller tumor volume (P = 0.04).
Impact of Adverse Pathology Features After Surgery on CTV Coverage
In patients with surgical margin involvement (31/127; 24%) the 68Ga-PSMA PET PB recurrences were fully covered by the CTV in 21 of 31 patient (68%), partially covered in 9 of 31 patients (29%), and not covered in 1 of 31 patients (3%). In patients without surgical margins involvement (58/127; 46%), the 68Ga-PSMA PET PB recurrences were fully covered by the CTV in 26 of 58 patients (45%), partially covered in 20 of 58 patients (34%), and not covered in 12 of 58 patients (21%). For 38 of 127 patients (30%), the information regarding surgical margin involvement was not available.
Impact of Rectal/Bladder Wall Involvement on CTV Coverage
PB lesions involved the rectal wall in 12 of 127 patients (9%) and the bladder wall in 4 of 127 patients (3%). Rectal or bladder wall involvement was significantly associated with lack of full CTV coverage (P = 0.03).
Impact of miTNM Stage on CTV Coverage
Clinical characteristics, location of recurrences and CTV coverage patterns of the full cohort (miTrNxMx) are summarized in Table 1. In the miTrNxMx cohort the 68Ga-PSMA PET PB recurrences were completely covered by the CTV in 107 of 226 patients (46%), partially covered in 91 of 226 patients (41%), and not covered in 28 of 226 patients (13%). In the latter 2 groups, the 68Ga-PSMA PET recurrences extended beyond the CTV at the following locations: posteriorly (57/119; 48%), posterolaterally (34/119; 29%), posteroinferiorly (8/119; 7%), anteriorly (1/119; 1%), anteroinferiorly (1/119; 1%), superiorly (4/119; 3%), laterally (2/119; 2%), and inferiorly (12/119; 10%) (Table 1; Suppl. Table 1).
The percentage of 68Ga-PSMA PET recurrences completely covered by the CTV was 46%, 35%, and 38% in the miTrN1M0, miTrN0M1, and miTrN1M1 subcohorts, respectively.
The PB recurrences involved the rectal and bladder walls in 19 of 226 patients (8%) and 10 of 226 patients (4%), respectively. Tumor involvement of the rectal or bladder wall was significantly associated with lack of complete CTV coverage in the full cohort (P = 0.007).
The median serum PSA levels at time of imaging and 68Ga-PSMA PET–based tumor volume were 1.21 ng/mL (IQR, 2.40) and 0.88 mL (IQR, 1.24), respectively. Complete CTV coverage was significantly associated with smaller tumor volume (P = 0.009), and not with lower serum PSA (P = 0.76).
DISCUSSION
Our study showed that in a cohort of 127 patients with prostate cancer local recurrence limited to the PB (N0M0), the RTOG-based CTV fully or partially covered the 68Ga-PSMA PET PB recurrences in 87% of the cases, whereas 13% of the recurrences were not covered by the CTV whatsoever. This work provides important information on patterns of PB recurrence based on novel imaging, indicating areas of potential failures that would not be covered by the current RTOG contouring guidelines for SRT directed to the PB.
It is difficult to define standardized target volumes for radiation treatment after radical prostatectomy. The surgical removal of the prostate alters the anatomy of the pelvic organs, which causes significant challenges to radiation oncologists who contour SRT volumes due to significant patient heterogeneity. To date, the target delineation (CTV) for SRT to the PB is guided by consensus guidelines made by experts, but do not account for data from contemporary imaging, such as 68Ga-PSMA PET. Our study used 68Ga-PSMA PET to generate a 3-dimensional heat map of the PB recurrence patterns after radical prostatectomy in relation with the RTOG-based CTVs with the intent to guide the redefinition of these consensus contours.
Although many groups investigated the patterns of failure after prostatectomy for prostate cancer using 68Ga-PSMA PET, our work represents a large dataset that specifically focuses on the detailed description of patterns of local failure in the PB using 68Ga-PSMA PET (17,18). In 47% of patients from our cohort, the RTOG-based CTVs did not cover completely the PB recurrences identified on 68Ga-PSMA PET. We observed that in most cases without full coverage, recurrences extended beyond the CTVs posteriorly, specifically at the posterior, posterolateral and posteroinferior borders in 80% of cases, and in 17% of cases at the inferior border. The 68Ga-PSMA PET recurrences rarely overlapped the CTVs anteriorly (2%) or superiorly (2%), indicating an overall adequate coverage on these areas. In fact, we believe that the current RTOG recommendations of extending the coverage anteriorly to the top of the pubic symphysis irradiates a large volume of normal bladder tissue unnecessarily given the low probability of recurrences in that area. Therefore, our work provides important information also for a reduction of the CTVs anterosuperiorly to potentially reduce toxicity rates. Further, they provide a potential explanation for why dose escalation to the prostate fossa has failed to improve BCR outcomes in 2 randomized trials in the postoperative setting while consistently improving BCR outcomes in multiple trials in the intact prostate setting (19,20). Although it is true that these trials included patients with serum PSA lower than those of our cohort, a subanalysis of our study specifically looking at CTV coverage in patients with serum PSA levels ≤ 0.5 ng/mL found that the proportions of PB recurrences fully covered and not fully covered were comparable to those of the full cohort. This finding further confirms that PSA levels are not significantly associated with the coverage outcome.
Overall, our results highlight the need for adequate contouring at the posterior border, although RTOG guidelines already recommend extending the posterior treatment volume to the anterior wall of the rectum. This finding can be partly explained by the possible posterior extension of the recurrences to involve the rectal wall, by possible inaccuracies due spill-over effect, as described earlier, and in part by the lack of strict adherence to the guidelines. In addition, a more extensive coverage at the posterolateral angles on both sides of the rectum may be needed. It is important to mention that recurrences outside the CTV could have been adequately covered by the planning target volumes (PTV) margin expansions. However, the PTV is intended to account for uncertainties in planning or treatment delivery rather than uncertainties in the true anatomic location of disease. A more definitive conclusion on the need for target volumes expansions would require validation on a cohort of patients with local failures after salvage radiotherapy.
Our findings are corroborated by a recent study from Australia (21) assessing patterns of failure in relation to the Faculty of Radiation Oncology Genito-Urinary Group and RTOG recommendations. These authors mapped the recurrences relative to vesicourethral anastomosis and showed that the RTOG CTV had better coverage than the Faculty of Radiation Oncology Genito-Urinary Group CTV. They similarly showed the importance of including the posterolateral rectal recesses, and possibly excluding the anterosuperior portion of the CTV.
The results of our subanalysis investigating the patterns of PB recurrences regardless of the N and M status showed that the percentage of 68Ga-PSMA PET recurrences completely covered by the CTV was smaller in patients with wider spread of disease than in those with disease limited to the PB: 46%, 35%, and 38% in the miTrN1M0, miTrN0M1, and miTrN1M1 subcohorts, respectively. The locations of the 68Ga-PSMA PET contours exceeding the CTV were instead similar among the main cohort and the subcohorts, with the vast majority of them extending beyond the posterior, posterolateral, and posteroinferior borders (84%), with a smaller percentage at the inferior border (10%) and a minority at the anterior, superior and lateral borders (6%).
Our study further assessed the correlation of the CTV coverage of PB recurrences with clinical and imaging parameters. The involvement of the rectal or bladder wall, identified in 12 of 127 patients (9%) or 4 of 127 patients (3%), respectively, was found to be significantly associated to worse coverage (P = 0.03). The urinary bladder and the rectum are anatomic landmarks used to delineate the PB and represent crucial organs for the definition of the clinical target volumes used for SRT. Increased dose to normal tissues is associated to acute and late GI and GU side effects that significantly decrease patient’s quality of life. In fact, a retrospective quality assurance analysis from a randomized trial confirmed that an overlap of the CTV with rectal wall at the time of planning is associated with increased toxicity (22).
In our study, PSA and tumor volume were positively correlated with each other, but tumor volume was the only parameter associated with worse coverage. The PSMA-based tumor volumes were significantly associated with PSMA-based recurrence coverage (P = 0.043), whereas serum PSA levels at the time of 68Ga-PSMA PET were not (P = 0.979).
An important aspect that needs to be taken into consideration when interpreting the results of our study is the intrinsic limitation of tumor contouring using PET due to its finite spatial resolution. Tumor delineation done on 68Ga-PSMA PET images inevitably suffers from partial-volume and spillover effects, which can be described as part of the signal coming from the source (tumor) spilling out and being seen outside the location of the source (23). The ultimate result is an over-estimation of the actual tumor volumes, particularly significant for small lesions. In an attempt to overcome this limitation, the nuclear medicine readers used the fused CT images of 68Ga-PSMA PET as an aid for the delineation of the tumor, whenever possible. However, CT does not provide sufficient soft-tissue contrast and its utility is limited (24). This is valid also for the definition of rectal or bladder involvement, which is often not possible based on CT images. Therefore, interpretation of the analysis regarding rectal/bladder wall involvement should take into consideration this limitation. The use of information from MRI, and ideally the use of PET/MRI would have been the best approach to minimize this intrinsic limitation and obtain more accurate tumor extent delineation. Other limitations of our study are the lack of follow up information on our cohort and its retrospective nature. The authors are currently monitoring this cohort, and a study investigating the differences in progression free survival in these patients treated with SRT is planned (25).
Ideally, using a personalized approach with 68Ga-PSMA PET before SRT would allow us to weigh the potential benefits and harms of extending the RT coverage on an individual basis. A recent study found that the use of SUVmax from 68Ga-PSMA PET could identify patients who are at higher risk for progression after SRT and might therefore benefit from a personalized treatment approach (26). However, in practice, when 68Ga-PSMA PET is not available, or when 68Ga-PSMA PET is negative at lower PSA levels, data from studies like ours should be used to redefine the PB contouring guidelines with 2 main objectives: improve coverage of PB recurrences and decrease the unnecessary treatment of healthy tissues much less likely to harbor prostate cancer recurrence.
CONCLUSION
Our study showed that in patients experiencing PSA persistence or BCR after radical prostatectomy with disease limited to the PB on 68Ga-PSMA PET (miTrN0M0), the RTOG contouring guidelines for SRT cover the full extent of disease in 54% of the patients, leaving 34% partially covered and 13% fully uncovered. Our study suggests that 68Ga-PSMA PET can be a valuable tool for SRT planning in the setting of BCR for patients with disease limited to the PB and should be incorporated into a redefinition of SRT contouring guidelines.
DISCLOSURE
Amar U. Kishan reports funding support from grant P50CA09213 from the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence, grant W81XWH-22-1-0044 from the Department of Defense, grant RSD1836 from the Radiologic Society of North America, the STOP Cancer Organization, the Jonsson Comprehensive Cancer Center, and the Prostate Cancer Foundation; personal fees from Varian Medical Systems, Inc., ViewRay Inc., and Intelligent Automation, Inc.; and research support from ViewRay, Inc., the American Society for Radiation Oncology (ASTRO), the Prostate Cancer Foundation, and the Jonsson Comprehensive Cancer Center—all outside of the submitted work. Nicholas G. Nickols reports research support from Lantheus, Janssen, and Bayer, outside of the submitted work, and consulting for PrimeFour, outside of the submitted work. Alan Dal Pra reports research support to institution/sponsor (Veracyte), outside of the submitted work, and advisory board membership (Merck), outside of the submitted work. Jeremie Calais reports prior consulting activities for Advanced Accelerator Applications, Astellas, Blue Earth Diagnostics, Curium Pharma, DS Pharma, EXINI, GE Healthcare, Isoray, IBA RadioPharma, Janssen Pharmaceuticals, Lightpointmedical, Lantheus, Monrol, Novartis, Progenics, POINT biopharma, Radiomedix, Sanofi, and Telix Pharmaceuticals, outside of the submitted work. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Are the CTVs used for salvage radiation treatment of the PB covering the full extent of disease on the basis of 68Ga-PSMA PET findings?
PERTINENT FINDINGS: In patients experiencing PSA persistence or biochemical recurrence after radical prostatectomy with disease limited to the PB on 68Ga-PSMA PET, the RTOG-based CTV for salvage radiation therapy left 13% of the recurrences fully uncovered and 34% partially treated. Most of the recurrences not covered by the CTV extended beyond the posterior and inferior aspects of the PB, whereas the anterior and superior borders were rarely involved—highlighting the need for CTV redefinition with 68Ga-PSMA PET.
IMPLICATIONS FOR PATIENT CARE: Using 68Ga-PSMA PET data to redefine current contouring guidelines has 2 advantages: improve coverage of areas of tumor recurrence and reduce unnecessary exposure of healthy tissues to radiation.
REFERENCES
- 1. Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. [DOI] [PubMed] [Google Scholar]
- 3. Freedland SJ, Rumble RB, Finelli A, et al. Adjuvant and salvage radiotherapy after prostatectomy: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2014;32:3892–3898. [DOI] [PubMed] [Google Scholar]
- 4. Zaorsky NG, Calais J, Fanti S, et al. Salvage therapy for prostate cancer after radical prostatectomy. Nat Rev Urol. 2021;18:643–668. [DOI] [PubMed] [Google Scholar]
- 5. Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poortmans P, Bossi A, Vandeputte K, et al. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol. 2007;84:121–127. [DOI] [PubMed] [Google Scholar]
- 7. Sidhom MA, Kneebone AB, Lehman M, et al. Post-prostatectomy radiation therapy: consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol. 2008;88:10–19. [DOI] [PubMed] [Google Scholar]
- 8. Wiltshire KL, Brock KK, Haider MA, et al. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;69:1090–1099. [DOI] [PubMed] [Google Scholar]
- 9. Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. [DOI] [PubMed] [Google Scholar]
- 10. FDA approves first PSMA-targeted PET drug. J Nucl Med. 2021;62:11N. [PubMed] [Google Scholar]
- 11. Valle L, Shabsovich D, de Meerleer G, et al. Use and impact of positron emission tomography/computed tomography prior to salvage radiation therapy in men with biochemical recurrence after radical prostatectomy: a scoping review. Eur Urol Oncol. 2021;4:339–355. [DOI] [PubMed] [Google Scholar]
- 12. Eder M, Schäfer M, Bauder-Wüst U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–697. [DOI] [PubMed] [Google Scholar]
- 13. Halpern BS, Dahlbom M, Quon A, et al. Impact of patient weight and emission scan duration on PET/CT image quality and lesion detectability. J Nucl Med. 2004;45:797–801. [PubMed] [Google Scholar]
- 14. Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging—version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 15. Eiber M, Herrmann K, Calais J, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. [DOI] [PubMed] [Google Scholar]
- 16. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rowe LS, Harmon S, Horn A, et al. Pattern of failure in prostate cancer previously treated with radical prostatectomy and post-operative radiotherapy: a secondary analysis of two prospective studies using novel molecular imaging techniques. Radiat Oncol. 2021;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calais J, Czernin J, Cao M, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghadjar P, Hayoz S, Bernhard J, et al. Dose-intensified versus conventional-dose salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy: the SAKK 09/10 randomized phase 3 trial. Eur Urol. 2021;80:306–315. [DOI] [PubMed] [Google Scholar]
- 20. Qi X, Li HZ, Gao XS, et al. Toxicity and biochemical outcomes of dose-intensified postoperative radiation therapy for prostate cancer: results of a randomized phase III trial. Int J Radiat Oncol Biol Phys. 2020;106:282–290. [DOI] [PubMed] [Google Scholar]
- 21. Horsley PJ, Koo CM, Eade T, et al. Mapping of local recurrences after radical prostatectomy using 68-gallium-prostate-specific membrane antigen positron emission tomography/computed tomography: implications for postprostatectomy radiation therapy clinical target volumes. Int J Radiat Oncol Biol Phys. 2023;115:106–117. [DOI] [PubMed] [Google Scholar]
- 22. Beck M, Sassowsky M, Schär S, et al. Adherence to contouring and treatment planning requirements within a multicentric trial: results of the quality assurance of the SAKK 09/10 trial. Int J Radiat Oncol Biol Phys. 2022;113:80–91. [DOI] [PubMed] [Google Scholar]
- 23. Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932–945. [DOI] [PubMed] [Google Scholar]
- 24. Daryanani A, Turkbey B. Recent advancements in CT and MR imaging of prostate cancer. Semin Nucl Med. 2022;52:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calais J, Armstrong WR, Kishan AU, et al. Update from PSMA-SRT trial NCT03582774: a randomized phase 3 imaging trial of prostate-specific membrane antigen positron emission tomography for salvage radiation therapy for prostate cancer recurrence powered for clinical outcome. Eur Urol Focus. 2021;7:238–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spohn SKB, Farolfi A, Schandeler S, et al. The maximum standardized uptake value in patients with recurrent or persistent prostate cancer after radical prostatectomy and PSMA-PET-guided salvage radiotherapy: a multicenter retrospective analysis. Eur J Nucl Med Mol Imaging. 2022;50:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]